Abstract
Introduction
Currently, flap operation (FOP) using REGROTH® (0.3% basic fibroblast growth factor [FGF-2]) is the standard treatment for periodontal regenerative therapy in Japan. However, the periodontal tissue regenerative effect with REGROTH® monotherapy is inadequate for severe alveolar bone defects. Therefore, in this study, we evaluated the safety and effectiveness of periodontal regenerative therapy for patients with severe periodontitis using REGROTH® (test medicine) combined with Cytrans® Granules (test device: carbonated apatite granules), which is a new artificial bone.
Methods
The study participants included 10 patients with severe periodontitis (mean age: 47.4 years). All participants provided written informed consents. In each patient, the intrabony defect site (mean bone defect depth: 5.7 mm) was defined as the test site. FOP was performed for the test site after the baseline investigation; moreover, the test medicine and test device were administered simultaneously. Furthermore, the observation of subjects’ general condition and test sites was conducted and the blood, urine, and periodontal tissue tests were performed up to 36 weeks after FOP. The rate of bone increase (%), clinical attachment level (CAL), probing pocket depth (PPD), bleeding on probing (BOP), tooth mobility (Mo), width of keratinized gingiva (KG), gingival recession (REC), gingival index (GI), and plaque index (PlI) were evaluated during the periodontal tissue investigation.
Results
As the primary endpoint, no adverse events related to the test medicine and test device occurred during the entire observation period of this study. Regarding the secondary endpoints, there was a significant increase in new alveolar bone (p = 0.003) and CAL acquisition (p = 0.001) as well as decrease in PPD (p = 0.002) and BOP (p = 0.016) at 36 weeks after administration of the test medicine and test device compared with the preoperative values. Furthermore, at 36 weeks after surgery, the Mo, GI, and PlI decreased to preoperative levels at 40%, 60%, and 30% of sites, respectively. However, at 36 weeks after surgery, there was no difference in KG and REC compared with their preoperative values.
Conclusions
The safety of periodontal regenerative therapy using the test medicine in combination with the abovementioned test device was confirmed. In addition, it was suggested that this periodontal regenerative therapy is effective for tissue regeneration in severe alveolar bone defects.
This clinical trial was conducted after registering and publicizing as a specified clinical trial in the Japan registry of clinical trials (jRCTs051190045).
Keywords: Periodontal regeneration, Basic fibroblast growth factor, Carbonated apatite, Flap operation, Intrabony defect
Abbreviations: FGF-2, Basic fibroblast growth factor; FOP, Flap operation; EMD, Emdogain®Gel
Graphical abstract
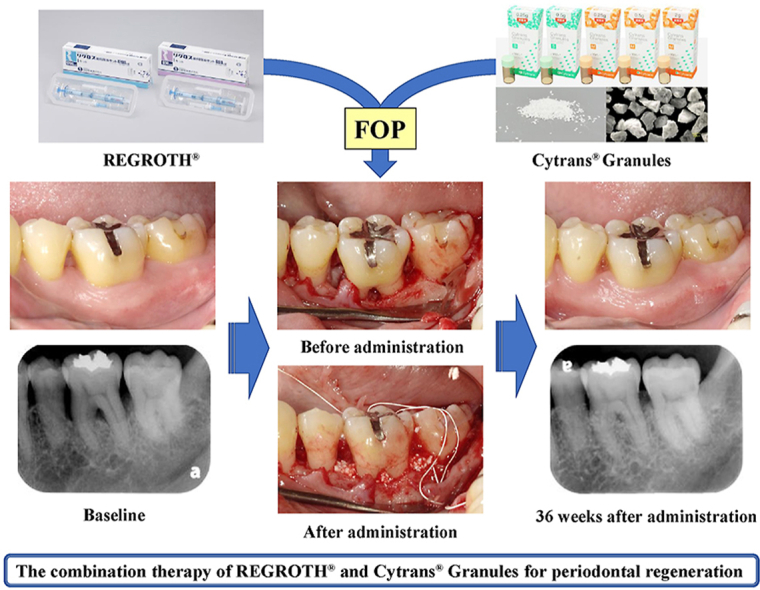
Highlights
-
•
The safety of flap operation using 0.3% FGF-2 and carbonated apatite was confirmed.
-
•
The administration of 0.3% FGF-2 and carbonated apatite improved periodontitis.
-
•
Combining 0.3% FGF-2 and carbonated apatite for severe alveolar bone defects.
-
•
Periodontal regenerative therapy combining both could be effective.
1. Introduction
In Japan, the guided tissue regeneration technique (GTR) [1], whereby an artificial membrane is placed in the defective periodontal tissue area during flap operation (FOP), and the administration of Emdogain® gel (EMD) [2,3] made from proteins extracted from the tooth germ of juvenile swine, have long since been used as periodontal regenerative therapy. To establish the next generation of periodontal regenerative therapy, since the beginning of the 1990s, with the aim of developing a safer and more effective agent for periodontal tissue regeneration, we have undertaken efforts to develop a new agent to induce periodontal tissue regeneration using basic fibroblast group factor (FGF-2), which has a potent neovascularization action and the ability to induce mesenchymal cell proliferation [[4], [5], [6], [7], [8], [9]]. In clinical trials, it has been confirmed that local administration of 0.3% FGF-2 significantly induces periodontal tissue regeneration [[10], [11], [12]], and in 2016, REGROTH® became available in the Japanese market as the world's first periodontal regenerative medicine containing FGF-2, a human recombinant protein, as the active ingredient. Moreover, in a comparative trial of REGROTH® and EMD, it has been found that the periodontal tissue regenerative effect of REGROTH® is superior to that of EMD [12].
REGROTH® is indicated for periodontal pockets with a depth of 4 mm or more and intrabony defects of 3 mm or more; however, the regenerative effect for severe intrabony defects, such as 1-wall and 4-wall bone defects, is limited as per conventional periodontal regenerative therapy [12]. Therefore, to improve the regenerative effect in severe alveolar bone defects, the combination therapy with GTR therapy or EMD and various prosthetic bone materials has been attempted. As a result, the combination therapy with EMD and some prosthetic bone materials has been shown to be more effective than EMD alone [3], but the combined effect of the GTR method and prosthetic bone materials has hardly been found [1]. Accordingly, even for large periodontal tissue defects in which it is difficult to obtain sufficient clinical effects with REGROTH® alone, it is conceivable to achieve periodontal tissue regeneration by combining REGROTH® and a prosthetic bone material that exceeds the periodontal tissue regeneration obtained with the use of REGROTH® alone.
Therefore, in the present study, to examine the safety and efficacy of the periodontal regenerative therapy combining REGROTH® and the newly developed Cytrans® granules, which is a prosthetic bone material (artificial bone) containing carbonated apatite [[13], [14], [15]], we evaluated the clinical outcomes of FOP by applying both test medicine and test device for the intrabony defect with severe periodontitis, with safety as the primary endpoint and effectiveness as the secondary endpoint.
2. Methods
2.1. Study subjects and the test site
The study included 10 patients with periodontitis aged 20 years or older in whom the test tooth corresponded to the inclusion and exclusion criteria presented in Table 1. Patients with periodontitis who satisfied the exclusion criteria presented in Table 2 were excluded from the subject sample. The present study was conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and upon obtaining informed consent for study participation from the study participants using an informed consent form.
Table 1.
Criteria for selecting and excluding test teeth.
| Inclusion criteria |
|---|
| 1. Teeth received the initial periodontal therapy |
| 2. Teeth diagnosed with periodontitis and determined to require flap operation (FOP) by the dentist |
| 3. Teeth with probing pocket depth (PPD) of 4 mm or more and vertical bone defects of 3 mm or more in-depth on its mesial or distal site |
| 4. Teeth with tooth mobility of 2° [16] or less |
| 5. Teeth with keratinized gingiva that are judged to be capable of FOP |
| Exclusion criteria |
| 1. Teeth that have other complicated diseases except for periodontitis (apical periodontitis, tooth root fracture, etc.) that may affect the periodontal tissue healing |
| 2. Teeth that are expected to undergo treatment that will affect the evaluation of safety or efficacy within 36 weeks after FOP |
| 3. Teeth whose restoration, abnormal eruption, and other complications may interfere with accurate measurement of the clinical attachment level |
Table 2.
Criteria for excluding subjects.
| Exclusion criteria |
|---|
| 1. Persons who have a complicated malignant tumor or have its history |
| 2. Persons who have used bisphosphonate drugs or have osteoporosis |
| 3. Persons with abnormal gingival overgrowth or its history |
| 4. Persons with malignant tumor, precancerous lesions, or findings that they are suspected in the oral cavity |
| 5. Persons with severe blood disorder or bone target hormone metabolic disorders |
| 6. Persons suspected of collagenosis and the abnormalities of calcium metabolism organs such as kidney and gastrointestinal tract |
| 7. Persons under dialysis treatment or steroid use |
| 8. Persons with uncontrollable complications who are restricted from observing the protocol of this clinical trial, such as severe disease (infection, immunodeficiency, and heart disease) or psychiatric disorder |
| 9. Persons with alcohol/drug addiction |
| 10. Patients with uncontrolled diabetes mellitus who do not have adequate glycemic control (HbA1c < 6.5%) |
| 11. Persons who are pregnant, wishing to get pregnant during this clinical study, may be pregnant, or breastfeeding |
| 12. Persons who are considered difficult for follow-up visits such as residents staying in remote places |
| 13. Persons who are restricted from observing the protocol of this clinical trial for social or domestic environments |
| 14. Persons who participated in clinical studies with interventions for other medical devices or medicines within the past 3 months |
| 15. Persons who remained affected by the treatment performed in the oral cavity within the past 3 months |
| 16. For other reasons; persons who are considered inappropriate as subjects for this clinical study or combination therapy of REGROTH® and Cytrans® Granules by the clinical research director or registered dentist |
The characteristics of the study subjects and test sites at baseline are presented in Table 3. The 10 subjects (1 male and 9 females) had a mean age of 47.4 (31–61) years. Regarding the bone defect morphology of the 10 test sites, 1-wall, 2-wall, 3-wall, 4-wall, and crater-like defect were found in 1 site, 4 sites, 3 sites, 1 site, and 1 site, respectively. The mean bone defect depth evaluated on X-ray investigation was 5.7 (3.65–9.71) mm. The average values of clinical attachment level (CAL), probing pocket depth (PPD), gingival recession (REC), and width of keratinized gingiva (KG) at the test site were 8.20 mm, 7.30 mm, 0.90 mm, and 4.50 mm, respectively. Bleeding on probing (BOP) of all the test sites was positive.
Table 3.
Characteristics of the subjects and test sites at baseline.
| Subject |
Tooth |
Site |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Age (years) | Sex | Tooth No. | Tooth mobility | Pulp (Vital/Non-vital) | Restorative or prosthetic treatment | furcation involvementa | Site | Morphology of bone defect | Depth of bone defect on X-ray examination (mm) | CAL | PPD | REC | KG | BOP | GI | PlI |
| 1 | 58 | Female | 44 | 0 | Non-vital | Presence | – | Mesial-buccal | 2-wall | 5.27 | 7 | 7 | 0 | 2 | + | 1 | 1 |
| 2 | 45 | Female | 37 | 1 | Vital | Absence | III | Distal-buccal | 3-wall | 9.71 | 10 | 8 | 2 | 2 | + | 1 | 1 |
| 3 | 61 | Female | 13 | 0 | Vital | Absence | – | Distal-labial | Crater-like | 3.95 | 8 | 6 | 2 | 3 | + | 1 | 0 |
| 4 | 31 | Male | 36 | 1 | Vital | Presence | III | Distal-buccal | 2-wall | 3.65 | 9 | 7 | 2 | 4 | + | 1 | 1 |
| 5 | 49 | Female | 27 | 1 | Vital | Presence | – | Mesial-lingual | 1-wall | 5.40 | 9 | 6 | 3 | 4 | + | 1 | 0 |
| 6 | 46 | Female | 46 | 0 | Vital | Presence | – | Mesial-buccal | 3-wall | 4.75 | 8 | 6 | 2 | 3 | + | 1 | 1 |
| 7 | 44 | Female | 36 | 1 | Non-vital | Presence | III | Mesial-buccal | 4-wall | 6.72 | 10 | 10 | 0 | 5 | + | 2 | 1 |
| 8 | 44 | Female | 26 | 1 | Vital | Presence | I | Mesial-lingual | 2-wall | 4.10 | 8 | 8 | 0 | 3 | + | 1 | 0 |
| 9 | 50 | Female | 24 | 1 | Vital | Presence | – | Mesial-lingual | 2-wall | 9.71 | 6 | 9 | −3 | 15 | + | 2 | 1 |
| 10 | 46 | Female | 25 | 1 | Vital | Presence | – | Distal-buccal | 3-wall | 3.76 | 7 | 6 | 1 | 4 | + | 1 | 1 |
| 47.4 (8.2)b | 5.70 (2.19)b | 8.20 (1.32)b | 7.30 (1.42)b | 0.90 (1.73)b | 4.50 (3.81)b | ||||||||||||
CAL: clinical attachment level.
PPD: probing pocket depth.
BOP: bleeding on probing.
Mo: tooth mobility.
KG: width of keratinized gingiva.
REC: gingival recession.
GI: gingival index.
PlI: plaque index.
Reference No. 21.
Mean (standard deviation).
2.2. Test medicine and test device
2.2.1. Test medicine
REGROTH® (Active ingredient: Trafermin (genetical recombination), 0.3% basic Fibroblast Growth Factor: FGF-2, Kaken Pharmaceutical Co., LTD., Tokyo, Japan).
Purchased from Kaken Pharmaceutical Co., LTD.
2.2.2. Test device
Cytrans® Granules (carbonated apatite granules, GC Corporation, Tokyo, Japan).
Purchased from GC Corporation.
2.2.3. Administration of REGROTH® and Cytrans® granules
A 0.2-ml solution of REGROTH® (containing 600 μg FGF-2) and 0.25 g Cytrans® Granules were mixed in a stainless steel mixing cup (GC Corporation, Tokyo, Japan) to prepare a mixture. Following the application of a small amount of REGROTH® to the bottom of the bone defect, the mixture was applied to the bone defect using a small stainless steel spatula (GC Corporation, Tokyo, Japan).
2.3. Study design
The present study was conducted as a single-center exploratory clinical trial (non-blinded/single-arm trial). The study schedule is presented in Table 4. After obtaining informed consent in writing for study participation, screening tests, case enrollment, and preoperative tests were performed. Thereafter, FOP was performed for the test tooth [[10], [11], [12]], and the test medicine and test device were administered to the periodontal tissue defect area. Furthermore, the observation of the test tooth and the subject's general condition, as well as each test, were performed on days 1–4, and at 1 week, 2 weeks, 4 weeks, 12 weeks, 24 weeks, and 36 weeks after FOP.
Table 4.
Schedule of this clinical trial.
2.4. Endpoints
2.4.1. Primary endpoint
The primary endpoint was adverse events with which a causal relationship to the test medicine or test device could not be ruled out. In addition to the observation of systemic and test site subjective symptoms and objective findings, blood and urine tests were performed, and adverse events were evaluated. Blood testing included red blood cell count, white blood cell count, hemoglobin, hematocrit, platelet count, and hemogram (neutrophil, lymphocyte, monocyte, eosinophil, and basophil count). Regarding blood biochemistry, serum levels of sodium, potassium, chlorine, blood urea nitrogen, creatinine, uric acid, aspartate aminotransferase, alanine transaminase, creatinine phosphokinase, C-reactive protein, alkaline phosphatase, lactate dehydrogenase, total cholesterol, total bilirubin, total protein, blood sugar, hemoglobin A1, albumin, and human chorionic gonadotropin (pregnancy test) were measured. Furthermore, as urinalysis, protein, sugar, urobilinogen, and occult blood in urine were measured.
Adverse events included all unfavorable medical events (regardless of the presence or absence of a causal relationship with the protocol treatment) that occurred in the subjects who underwent FOP. Furthermore, symptoms caused by concurrent illness, and abnormal laboratory tests values and test findings existing before study commencement, were not included as adverse events. The outcomes of all adverse events were recorded, and the causal relationship with the procedure (FOP), test medicine, and test device was evaluated.
The causal relation was evaluated according to five levels (“not related,” “unlikely related,” “possibly related,” “probably related,” and “definitely relate”), and “possibly related,” “probably related,” and “definitely related” were defined as “cannot be ruled out.”
2.4.2. Secondary endpoints
2.4.2.1. Rate of bone increase (%)
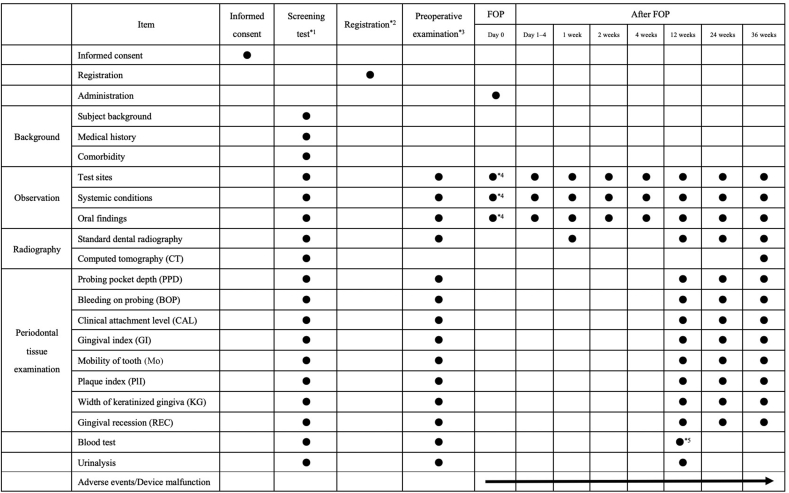
X-ray assessment of the evaluation site (interdental intrabony defect) was performed in the same manner as that mentioned in earlier clinical studies [10,11]. Thus, for uniformity in the scan position at the time of each scan, the X-ray test for each test site was conducted under standardized conditions using individual photographic indicators (Cone Indicator-II; Hanshin Technical Laboratory, Nishinomiya, Japan). However, some error is thought to occur in each X-ray scan, and we therefore corrected measurements based on two-dimensional lengths thought to be unchanged on each X-ray image (e.g., the length between the cement–enamel junction (CEJ) and apex, or the length of the apex from the repair margin). The series of X-ray evaluations were all completed by one specialist (TN) who was not involved in the administration of the test medicine or test device for the treatment of periodontal disease. Fig. 1 presents a case in which the rate of bone increase was measured for an intrabony defect in the distal area of the left mandibular first molar using X-ray images obtained before and after administration. That is, first, the CEJ (A), apex (B), residual alveolar bone crest (C), and bone defect base (D) were confirmed on X-ray images obtained preoperatively (1-a), and at 36 weeks after administration (1-b), the distance between each point with each point projected along the tooth axis was measured (1-c and 1-d), and the rate of bone increase was calculated using the following formula:
-
•
Corrected AD at 36 weeks after administration = measured AD after administration × (measured AB before administration/measured AB after administration)
-
•
Amount of increase in the height of the alveolar bone = AD before administration − corrected AD at 36 weeks after administration
-
•
Depth of the bone defect before administration = AD before administration − AC before administration
-
•
Rate of bone increase (%) = (amount of increase in the height of the alveolar bone/depth of the bone defect before administration) × 100
Fig. 1.
Measured points of alveolar bone height using standardized radiographs. A standardized X-ray image taken using photographic indicators of a certain test site (of distal-buccal site #36 in a male, 31 years of age) (1-a: preoperative image, 1-b: after 36 weeks of administration of the test medicine and the test device). Each point shown in figure (A: CEJ, B: root apex, C: residual alveolar bone crest, and D: bone defect base) was confirmed on each X-ray image, and the distance between each point with each point projected along the tooth axis was measured (1-c and 1-d). Furthermore, the rate of bone increase was calculated upon correction of the error between each X-ray image based on the two-dimensional length thought to be unchanged on each X-ray image (length between the CEJ and root apex). The rate of bone increase of the test site was 85.3%.
2.4.2.2. Periodontal tissue investigation
In the present study, as per earlier clinical studies [[10], [11], [12]], the periodontal tissue investigation items included the CAL, PPD, BOP, tooth mobility (Mo) [16], width of KG, REC, gingival index (GI) [17], and plaque index (PlI) [18]. The CAL and PPD were measured using a probing pressure of 25 g with a PCP-UNC-15 periodontal probe (Hu-Friedy, Chicago, IL), and the measurement was standardized upon performing calibration between measurers before the trial. For BOP, the presence or absence of bleeding was evaluated 10 s after measuring PPD. REC was calculated as the difference between the CAL and PPD.
2.5. Statistical analyses
The rate of alveolar bone increase, CAL, PPD, KG, and REC were compared preoperatively to those at 12 weeks, 24 weeks, and 36 weeks after surgery using a one-sample t-test. When normality was not satisfied, a one-sample Wilcoxon signed-rank test was performed. The positive rates for BOP before surgery and at 36 weeks after surgery were compared using McNemar's test. The rate of improvement (%) in Mo, GI, and PlI was determined at 36 weeks after surgery, and the bilateral 95% confidence interval was calculated using the Clopper–Pearson method.
2.6. Ethical considerations, enrollments, and publication for this study
The present study was conducted after enrollment and publication as a specified trial (jRCTs051190045) upon being examined and approved by the Osaka University Clinical Research Review Committee.
3. Results
3.1. Primary endpoint
Table 5 shows the rate of incidence of adverse events that occurred during the study period. Following the administration of the test medicine and test device, no abnormalities were observed in healing at the test sites, whereas pain, inflammation, redness, swelling, and stomach ache were noted elsewhere in the body. Furthermore, in the blood test and urinalysis, no changes in the test results that were related to the test medicine and test device were found. Based on the results above, we found no adverse event with which a causal relationship with the test medicine and test device could not be ruled out, i.e., the primary endpoint, throughout the observation period of the present study up to 36 weeks after administration of the both. On the other hand, temporary postoperative pain associated with the FOP of all test sites was observed; however, its degree did not differ considerably with respect to postoperative pain caused by FOP and could be alleviated by normal oral analgesics.
Table 5.
Rate of the incidence of adverse events.
| Adverse events | Frequencya (%) |
|---|---|
| Test sites | |
| Postoperative pain | 10 (100%) |
| Other parts in the body excluding the test sites and test teeth | |
| Pain | 1 (10%) |
| Inflammation | 3 (30%) |
| Redness | 1 (10%) |
| Swelling | 1 (10%) |
| Stomach ache | 1 (10%) |
| Blood test | |
| Red blood cell count increased | 2 (20%) |
| Hemoglobin decreased | 1 (10%) |
| Hematocrit increased | 2 (20%) |
| White blood cell count increased | 1 (10%) |
| White blood cell count decreased | 1 (10%) |
| Neutrophil (%) increased | 1 (10%) |
| Neutrophil (%) decreased | 1 (10%) |
| Lymphocyte (%) decreased | 1 (10%) |
| Eosinophil (%) increased | 1 (10%) |
| Potassium decreased | 1 (10%) |
| Uric acid increased | 1 (10%) |
| ALT increased | 2 (20%) |
| LDH decreased | 1 (10%) |
| Urine test | |
| White blood cell present | 1 (10%) |
ALT: alanine transaminase.
LDH: lactate dehydrogenase.
number.
3.2. Secondary endpoints
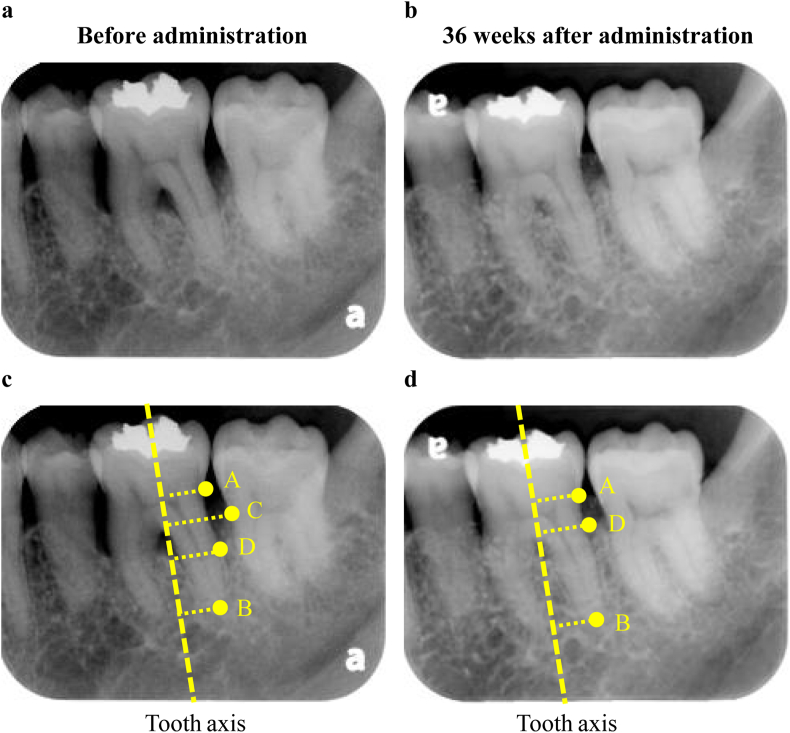
Table 6 presents the evaluation results of the secondary endpoints obtained from the period spanning the preoperative evaluation to 36 weeks after administration of the test medicine and test device. Regarding subject number 9, it was difficult to differentiate X-ray permeation by standard X-ray imaging of the test site, and the rate of bone increase (%) thus could not be evaluated. Therefore, study subject number 9 was excluded and the rate of bone increase (%) was evaluated in nine subjects (nine sites). The X-ray opacity of the bone defect area as an indicator of alveolar bone increased over time, and a significant increase in new alveolar bone (mean 53.1%) was observed at 36 weeks after administration compared with its preoperative value (p = 0.003, one-sample t-test). Fig. 2 shows the standardized left maxillary second molar radiographs before and 36 weeks after the administration of REGROTHⓇ and CytransⓇ Granules. Alveolar bone regeneration can be observed around the left maxillary second molar 36 weeks after administration. Furthermore, after the combined use of the test medicine and test device, a reduction in PPD and an increase in CAL were observed. Moreover, at 36 weeks after administration compared with preoperatively, there was a significant reduction in PPD (p = 0.002, one-sample t-test) and increase in CAL (p = 0.001, one-sample t-test). Moreover, the BOP-positive sites decreased after the combined use of the test medicine and the test device, and there was a significant decrease in BOP compared with preoperatively at 36 weeks after administration (p = 0.016, McNemar's test). In addition, at 36 weeks after surgery, the levels of Mo, GI and PlI had significantly decreased compared with preoperatively in 40%, 60%, and 30% of test sites, respectively. In contrast, there was no difference observed in KG and REC at 36 weeks after surgery compared with preoperatively (Table S1).
Table 6.
Changes in clinical measures between baseline and 36 weeks after administration.
| Item | Classification | Baseline | 12 weeks | 24 weeks | 36 weeks |
|---|---|---|---|---|---|
| Rate of bone increase (%)a Mean (SD) |
0 | 36.9 (40.3) | 45.5 (42.6) | 53.1b (38.0) | |
| PPD reduction (mm) Mean (SD) |
0 | 2.10 (1.20) | 2.50 (1.43) | 2.30b (1.64) | |
| CAL gain (mm) Mean (SD) |
0 | 2.20 (1.14) | 2.70 (1.64) | 2.50b (1.58) | |
| KG (mm) Mean (SD) |
4.50 (3.81) | 4.25 (3.55) | 4.35 (3.56) | 4.35 (3.59) | |
| REC reduction (mm) Mean (SD) |
0 | 0.1 (1.29) | 0.2 (1.69) | 0.2 (1.81) | |
| BOP | + | 100 | 50 | 20 | 30 |
| (%. of sites) | - | 0 | 50 | 80 | 70c |
| Mobility of tooth | 0 | 30 | 50 | 60 | 70 |
| (%. of sites) | 1 | 70 | 50 | 40 | 30 |
| Improvement rate (%) | 40 | ||||
| Gingival index | 0 | 0 | 40 | 60 | 50 |
| (%. of sites) | 1 | 80 | 40 | 40 | 50 |
| 2 | 20 | 20 | 0 | 0 | |
| Improvement rate (%) | 60 | ||||
| Plaque index | 0 | 30 | 30 | 50 | 50 |
| (%. of sites) | 1 | 70 | 50 | 50 | 50 |
| 2 | 0 | 20 | 0 | 0 | |
| Improvement rate (%) | 30 |
PPD: Probing pocket depth.
CAL: Clinical attachment level.
KG: Width of keratinized gingiva.
REC: Gingival recession.
BOP: Bleeding on probing.
Analysis results of nine cases, excluding one case that could not be evaluated by X-ray.
The mean at 36 weeks after administration was significantly higher than tht at baseline (one-sample t-test, Rate of bone increase: P = 0.003, PPD: P = 0.002, CAL: P = 0.001).
BOP was significantly improved 36 weeks after administration compared with baseline. (McNemar's test, P = 0.016).
Fig. 2.
Comparison by standardized radiographs between before and 36 weeks after administration of REGROTH® and Cytrans® Granules. A standardized X-ray image taken using photographic indicators preoperatively (2-a) and at 36 weeks after treatment (2-b) of cases administered with REGROTH® and Cytrans® granules for bone defect in tooth #27 (Subject No. 5, female, 49 years of age, 1-wall bone defect, depth of the bone defect: 5.40 mm). After 36 weeks of administration, alveolar bone regeneration can be confirmed.
4. Discussion
In “The 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions,” periodontitis with CALs of 5 mm or more is classified as severe stage (“stage 3” or “stage 4”) [19]. In addition, the Japanese Society of Periodontology defines the sites with a PPD of 6 mm or more as severe periodontitis sites [20]. As CAL and PPD of all test sites in this study were ≥6 mm, they were categorized as severe periodontitis sites.
REGROTH®, the world's first periodontal regenerative agent containing FGF-2, a human recombinant protein, as the active ingredient, has many benefits in terms of its technical simplicity, clinical effectiveness, and safety compared with periodontal regenerative therapy using GTR method and EMD. At present, it has become the standard treatment in Japan as part of periodontal regenerative therapy. However, REGROTH® uses hydroxypropyl cellulose as the base material, which does not have a space-making function, and the indication for severe alveolar bone defects is thus difficult as is conventional periodontal regenerative therapy. Previously, combination therapy has been attempted using EMD and a bone prosthetic material has been used as a scaffold to broaden the indication for severe alveolar bone defects, which have shown beneficial effects [3]. Therefore, in the present study, we evaluated the safety and efficacy of the combination therapy using REGROTH® (the test medicine), and Cytrans® Granules (test device), a newly developed artificial bone, in patients with periodontitis indicated for FOP.
Throughout observation period of this clinical trial, there were no poor quality products or defects with the test medicine and no malfunction with the test device observed. All 10 study subjects received the treatment according to the protocol and were able to complete the subsequent follow-up schedule. After administration of the test medicine and device, 10%–20% of the subjects showed minor fluctuations in the blood and urine test (Table 5). However, several fluctuations were observed in the placebo group of previous clinical trials that evaluated the efficacy and safety of FGF-2 medicine [10,12]; thus, it was considered that these changes were caused by surgical procedures (FOP) and/or physiological fluctuations. In addition, pain, inflammation, redness, swelling, and stomach ache were observed in places other than the surgical sites during the observation period up to 36 weeks. No causal relationship between these symptoms and the combination therapy was observed. Furthermore, the postoperative pain degree in the combination therapy was similar to that in the conventional FOP alone. Therefore, we consider that the administration of the combination therapy we examined did not lead to severe postoperative pain. Throughout the observation period of this clinical trial, there was no adverse event with which a causal relationship with the test medicine and test device could not be ruled out, which was the primary endpoint, and the safety of combination therapy using REGROTH® and Cytrans® Granules was thus confirmed.
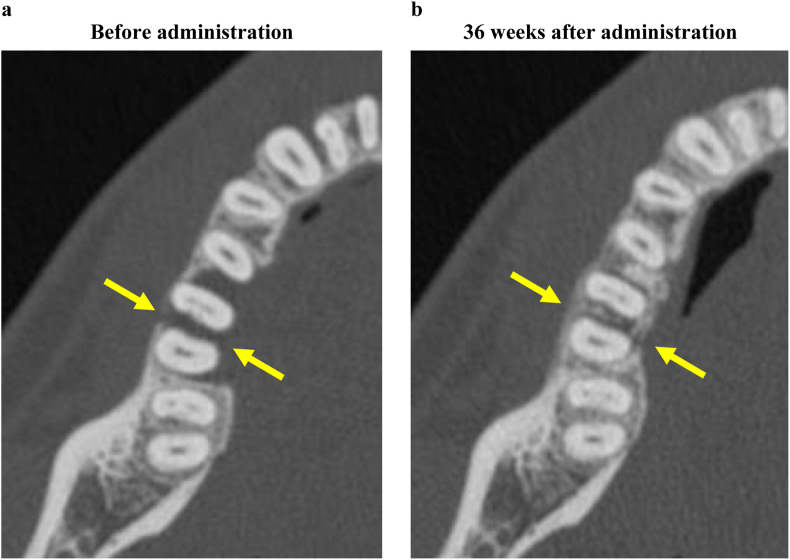
In one subject (subject No: 9), it was difficult to interpret the X-ray image of the alveolar bone defect. Therefore, this subject was excluded, and the rate of alveolar bone increase in nine subjects (nine sites) was evaluated as an indicator of periodontal tissue regeneration. As a result, the alveolar bone increased over time from preoperatively for 36 weeks after administration of the test medicine and test device, and the rate of alveolar bone increase was significantly higher (mean of 53.1%) at 36 weeks after operation compared with preoperatively (p = 0.003, one-sample t-test) (Table 6). The bone increase rate (53.1%) in this clinical study, which targeted the bone defects deeper (5.70 mm) than that (4.991 mm) in the phase III clinical trial [12], was higher than that (37.08%) in the phase III clinical trial. This suggests that the additional use of carbonated apatite with FGF-2 is more effective. Furthermore, the regeneration of the alveolar bone at degree III furcation involvement [21], in which the regeneration of the alveolar bone is unlikely with REGROTH® monotherapy, was observed on computed tomography (CT) after the combination therapy using REGROTH® with Cytrans® Granules (Fig. 3). In the present study, we did not assess the bone increase rate (%) at furcation involvements because of the limitations of standardized dental X-ray imaging. However, as shown in Fig. 3, combination therapy may be effective at furcation involvements, and the efficacy needs to be evaluated by a different evaluation procedure, such as CT, in future studies. Furthermore, on continuous observation of X-ray opacity of the bone defect, which is an indicator of increase in new alveolar bone, a highly opaque area—believed to be Cytrans® Granules—was observed 1 week after administration. Subsequently, the highly opaque area tended to slowly decrease until 3 months after administration, following which the highly opaque area gradually increased again. This is considered to indicate that the Cytrans® Granules administered to the bone defect area were gradually absorbed and turned into bone. Cytrans® Granules, whose combination effect with REGROTH® was examined in the present study, is an artificial bone consisting of carbonate apatite, which is an inorganic component of human bone [[13], [14], [15]]. Bone grafting is a method of treatment involving the grafting of various types of bones in alveolar bone defects and is classified into autologous bone graft, allograft of bone, xenograft of bone, and alloplastic bone graft depending on the type of bone grafted. Till date, autogenous bone graft has been considered the gold standard for bone grafting because osteogenesis, osteoinduction, and osteoconduction can be expected. However, invasion of healthy sites for bone harvesting cannot be avoided in autogenous bone grafting, and the amount of bone that can be harvested is also limited. In contrast, human demineralized free-dried bone allograft (DFDBA) has gained popularity in the United States of America as an alternative to autogenous bone; however, DFDBA has problems concerning safety and ethics and has thus not been approved for usage in Japan. Moreover, as xenograft, bovine sintered bone has been clinically applied; however, a considerable number of patients would refuse the use of animal-derived xenografts. A typical example of artificial bone is hydroxyapatite (HA), and β-tricalcium phosphate (β-TCP). HA is a biological non-absorbable material with properties similar to those of living bone; therefore, when used as a bone prosthetic material, it carries the risk of exposure and infection. In contrast, β-TCP has the advantage in that it is an absorbable material that can substitute autologous bone; however, depending on the case, there is concern that sufficient mechanical strength cannot be expected. Therefore, in this clinical trial, Cytrans® Granules were selected from the perspective that it is absorbable, can act as a replacement in vivo, and has appropriate strength; in addition, there is no risk of infection as is feared in case of animal-derived materials because it is an entirely artificial product.
Fig. 3.
Comparison by computed tomography (CT) between before and 36 weeks after administration of REGROTH® and Cytrans® Granules. Axial images obtained by CT for the medical department preoperatively (3-a) and at 36 weeks after treatment (3-b) of cases administered with REGROTH® and Cytrans® granules for bone defect in tooth #36 (Subject No. 4, male, 31 years of age, 2-wall bone defect, depth of the bone defect: 3.65 mm, the same case as in the graphical abstract). After 36 weeks of administration, regeneration of the alveolar bone can be confirmed with degree III [21] furcation involvement in the furcation area (arrow).
There was a significant increase in CAL (mean of 2.5 mm) and reduction in PPD (mean of 2.3 mm) (Table 6) at 36 weeks after test medicine and test device administration compared with preoperatively. Furthermore, an improvement was observed in the BOP, GI, and Mo; therefore, it is believed that the combined administration of REGROTH® and Cytrans® Granules reduced the pocket, caused inflammation to disappear, and contributed to the reconstruction of healthy periodontal tissue that is easy to maintain. The combination of clinically relevant CAL gain ≥3 mm and post-surgery pocket closure (PPD ≤4 mm) was reported as one of the assessments for a regenerative periodontal treatment outcome [22]. According to this assessment, only 30% of the tested sites were regarded as successful in this clinical study (Table S1). This suggests that the new attachment may not be sufficiently promoted by the combination therapy of REGROTH® and Cytrans® Granules at 9 months after the treatment, although the therapy stimulates alveolar bone renewal. A larger clinical study will be needed to clarify the efficacy and clinical relevance of this combination therapy for alveolar bone level increase, CAL gain, and post-surgery pocket closure.
In contrast, KG and REC showed no significant difference between the 36-week period after the administration of the test medicine and test device and pre-operation, and there was no major change in these variables throughout the study period (Table 6). Therefore, it was believed that the combined administration of the test medicine and the test device at FOP would maintain KG and cause no gingival recession. Furthermore, the PlI of the test sites tended to increase somewhat after 12 weeks following the operation; however, it was also found to decrease later. PlI in 3 out of 10 sites (30%) was reduced at 36 weeks after administration compared with preoperatively (Table 6). Therefore, because there was no increasing tendency in PlI during the study period, the plaque adhesion in the test site may have had no effect on the prognosis of periodontal tissue regeneration in this clinical trial.
REGROTH®, whose vehicle is hydroxypropyl cellulose, does not have a space-making function. Therefore, the attempts that increase the periodontal tissue regenerative effect of REGROTH® by combining REGROTH® with a bone prosthetic material have been underway since the development stage of REGROTH®. Previously, our group reported that there was significant regeneration of alveolar bone, periodontal ligament, and cementum upon the administration of 0.3% FGF-2 (REGROTH® equivalent) and β-TCP to 1-wall bone defect created in beagles compared with that the β-TCP monotherapy group [23]. Furthermore, in a clinical trial conducted in the United States of America examining the combined effect of FGF-2 and β-TCP in patients with periodontitis, it was reported that the combined administration of 0.3% FGF-2 and β-TCP to 1-wall and 2-wall bone defects significantly induced alveolar bone regeneration compared with that in the β-TCP monotherapy group [24]. Moreover, after REGROTH® became commercially available in Japan, significant alveolar bone regeneration was reportedly observed upon combining bovine sintered bone [25,26] and autologous bone [27] with REGROTH® compared with REGROTH® monotherapy. In this clinical trial, we found that periodontal regenerative therapy combining REGROTH® and Cytrans® Granules was safe, and the combined use of the two could effectively enable periodontal tissue regeneration even for severe alveolar bone defects in which sufficient alveolar bone regeneration is unlikely by REGROTH® monotherapy.
However, the present study has some limitations. First, the subject sample was small at 10 patients, and to evaluate the safety and efficacy of the test medicine and the test device more clearly, further examination with a larger subject sample is needed. In addition, a larger number of cases is needed to evaluate the efficacy of this combination therapy for various bone defects. Second, there was no control group; therefore, it is possible that the efficacy of combination therapy with the two could not be closely evaluated. In the future, a clinical study is needed to evaluate the effectiveness of combination therapy using REGROTH® and Cytrans® Granules compared with monotherapy using these two agents.
Despite the few limitations noted above, in the present study, the safety of combination therapy using REGROTH® and Cytrans® Granules was ensured, and it was suggested that the indication of the combination therapy could be expanded to include patients with severe alveolar bone defects. This study has made it possible to develop a large clinical trial with a control group for severe alveolar bone defects, such as 1-wall or 4-wall defect and furcation involvement for which a space-making function is necessary, to examine the effectiveness of periodontal regenerative therapy combining the both.
5. Conclusions
The safety of FOP combining REGROTH® and Cytrans® Granules was confirmed, and it was suggested that periodontal regenerative therapy combining the two could be effective for tissue regeneration of severe alveolar bone defects.
Funding
The present study was conducted with funding (Joint research project with Osaka University and GC Corporation; J180801017) provided by GC Corporation.
Author contributions
SM, MK, KY, HM, and HI conceived this project and developed the protocol of this clinical trial. MK, MY, KM, KI, MT, and YK performed flap operations and collected the data in addition to the administration of the test medicine and test device. TN performed X-ray assessments of the evaluation sites. MK, SM, HM, and YI interpreted and analyzed the data. MK primarily wrote the paper with the assistance of HM, YI, and KY, and SM had primary responsibility for the final content of the manuscript. All authors read and approved the final manuscript.
Declaration of competing interest
The funding for this clinical trial was provided by GC Corporation, which developed Cytrans® Granules. SM is a consultant for GC Corporation and Kaken Pharmaceutical Co., Ltd., which developed REGROTH and received a research grant. KY, HM, and YI are employees of GC Corporation. MK is an adviser for Kaken Pharmaceutical Co., Ltd. MY, KM, KI, MT, YK, and TN have no declarations of interest.
Acknowledgments
We would like to express our gratitude to Professor Tomomi Yamada and Specially Appointed Researcher Akimitsu Miyake of the Data Coordinating Center of the Department of Medical Innovation in Osaka University Hospital as well as to Associate Professor Kento Asano of the Academic Clinical Research Center for their support in the statistical analyses and implementation of this clinical trial.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reth.2022.06.001.
Contributor Information
Masahiro Kitamura, Email: kitamura.masahiro.dent@osaka-u.ac.jp.
Motozo Yamashita, Email: yamashita.motozou.dent@osaka-u.ac.jp.
Koji Miki, Email: miki.koji.dent@osaka-u.ac.jp.
Kuniko Ikegami, Email: ikegami.kuniko.dent@osaka-u.ac.jp.
Masahide Takedachi, Email: takedachi.masahide.dent@osaka-u.ac.jp.
Yoichiro Kashiwagi, Email: kashiwagi.yoichiro.dent@osaka-u.ac.jp.
Takenori Nozaki, Email: nozaki.takenori.dent@osaka-u.ac.jp.
Katsuyuki Yamanaka, Email: katsuyuki.yamanaka@gc.dental.
Hijiri Masuda, Email: hijiri.masuda@gc.dental.
Yoko Ishihara, Email: yoko.ishihara@gc.dental.
Shinya Murakami, Email: murakami.shinya.dent@osaka-u.ac.jp.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Needleman I., Tucker R., Giedrys-Leeper E., Worthington H. Guided tissue regeneration for periodontal intrabony defects–a cochrane systematic review. Periodontal 2000. 2005;37:106–123. doi: 10.1111/j.1600-0757.2004.37101.x. [DOI] [PubMed] [Google Scholar]
- 2.Koop R., Merheb J., Quirynen M. Periodontal regeneration with enamel matrix derivative in reconstructive periodontal therapy: a systematic review. J Periodontal. 2012;83:707–720. doi: 10.1902/jop.2011.110266. [DOI] [PubMed] [Google Scholar]
- 3.Miron J., Sculean A., Cochran L., Froum S., Zucchelli G., Nemcovsky C., et al. Twenty years of enamel matrix derivative: the past, the present and the future. J Clin Periodontal. 2016;43:668–683. doi: 10.1111/jcpe.12546. [DOI] [PubMed] [Google Scholar]
- 4.Murakami S., Takayama S., Ikezawa K., Shimabukuro S., Kitamura M., Nozaki T., et al. Regeneration of periodontal tissues by basic fibroblast growth factor. J Periodontal Res. 1999;34:425–430. doi: 10.1111/j.1600-0765.1999.tb02277.x. [DOI] [PubMed] [Google Scholar]
- 5.Takayama S., Murakami S., Shimabukuro Y., Kitamura M., Okada H. Periodontal regeneration by FGF-2 (bFGF) in primate models. J Dent Res. 2001;80:2075–2079. doi: 10.1177/00220345010800121001. [DOI] [PubMed] [Google Scholar]
- 6.Murakami S., Takayama S., Kitamura M., Shimabukuro Y., Yanagi K., Ikezawa K., et al. Recombinant human basic fibroblast growth factor (bFGF) stimulates periodontal regeneration in class II furcation defects created in beagle dogs. J Periodontal Res. 2003;38:97–103. doi: 10.1034/j.1600-0765.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- 7.Murakami S., Yamada S., Nozaki T., Kitamura M. Fibroblast growth factor-2 stimulates periodontal tissue regeneration. Clin Adv Periodontics. 2011;1:95–99. [Google Scholar]
- 8.Murakami S. Periodontal tissue regeneration by signaling molecule(s): what role does basic fibroblast growth factor (FGF-2) have in periodontal therapy? Periodontol. 2011;56:188–208. doi: 10.1111/j.1600-0757.2010.00365.x. 2000. [DOI] [PubMed] [Google Scholar]
- 9.Ninomiya M., Azuma T., Kido J., Murakami S., Nagata T. Successful case of periodontal tissue repair with fibroblast growth factor-2: long- term follow-up and comparison to enamel matrix derivative. Clin Adv Periodontics. 2013;3:215–221. [Google Scholar]
- 10.Kitamura M., Nakashima K., Kowashi Y., Fujii T., Shimauchi H., Sasano T., et al. Periodontal tissue regeneration using fibroblast growth factor-2: randomized controlled phase Ⅱ clinical traial. PLoS One. 2008;3:e26. doi: 10.1371/journal.pone.0002611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitamura M., Akamatsu M., Machigashira M., Hara Y., Sakagami R., Hirofuji T., et al. FGF-2 stimulates periodontal regeneration: results of a multi-center randomized clinical trial. J Dent Res. 2011;90:35–40. doi: 10.1177/0022034510384616. [DOI] [PubMed] [Google Scholar]
- 12.Kitamura M., Akamatsu M., Kawanami M., Furuichi Y., Fujii T., Mori M., et al. Randomized placebo-controlled and controlled non-inferiority phase Ⅲ trials comparing trafermin, a recombinant human fibroblast growth factor 2, and enamel matrix derivative in periodontal regeneration in intrabony defects. J Bone Miner Res. 2016;1:806–814. doi: 10.1002/jbmr.2738. [DOI] [PubMed] [Google Scholar]
- 13.Kudoh K., Fukuda N., Kasugai S., Tachikawa N., Koyano K., Matsushita Y., et al. Maxillary sinus floor augmentation using low-crystalline carbonate apatite granules with simultaneous implant installation: first-in-human clinical trial. J Oral Maxillofac Surg. 2019;77:985. doi: 10.1016/j.joms.2018.11.026. 985.e11. [DOI] [PubMed] [Google Scholar]
- 14.Nakagawa T., Kudoh K., Fukuda N., Kasugai S., Tachikawa N., Koyano K., et al. Application of low-crystalline carbonate apatite granules in 2-stage sinus floor augmentation: a prospective clinical trial and histomorphometric evaluation. J Periodontal Implant Sci. 2019;49:382–396. doi: 10.5051/jpis.2019.49.6.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagata K., Fuchigami K., Kitami R., Okuhama Y., Wakamori K., Sumitomo H., et al. Comparison of the performances of low-crystalline carbonate apatite and Bio-Oss in sinus augmentation using three-dimensional image analysis. Int J Implant Dent. 2021;7:24. doi: 10.1186/s40729-021-00303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller S. 3rd ed. Blakiston Co Inc.; Philadelphia: 1950. Textbook of periodontia; p. 125. [Google Scholar]
- 17.Loë H., Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 18.Silness J., Loë H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 19.Papapanou P.N., Sanz M., Buduneli N., Dietrich T., Feres M., Fine D.H., et al. Periodontitis: consensus report of workgroup 2 of the 2017 world Workshop on the classification of periodontal and peri-implant Diseases and conditions. J Periodontol. 2018;(Suppl 1):S173–S182. doi: 10.1002/JPER.17-0721. [DOI] [PubMed] [Google Scholar]
- 20.Kurihara H. Ishiyaku Publishers, Inc.; Tokyo: 2015. JSP clnical practice guideline for the periodontal lreatment; p. 26. 2017. [Google Scholar]
- 21.Lindhe J., Nyman S. The effect of plaque control and surgical pocket elimination on the establishment and maintenance of periodontal health. A longitudinal study of periodontal therapy in cases of advanced disease. J Clin Periodontol. 1975;2:67–79. doi: 10.1111/j.1600-051x.1975.tb01727.x. [DOI] [PubMed] [Google Scholar]
- 22.Trombelli L., Farina1 R., Vecchiatini R., Maietti E., Simonelli A. A simplified composite outcome measure to assess the effect of periodontal regenerative treatment in intraosseous defects. J Periodontol. 2020;91:723–731. doi: 10.1002/JPER.19-0127. [DOI] [PubMed] [Google Scholar]
- 23.Anzai J., Kitamura M., Nozaki T., Nagayasu T., Terashima A., Asano T., et al. Effects of concomitant use of fibroblast growth factor (FGF)-2 with beta-tricalcium phosphate (β-TCP) on the beagle dog 1-wall periodontal defect model. Biochem Biophys Res Commun. 2010;403:345–350. doi: 10.1016/j.bbrc.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 24.Cochran D., Oh T.-J., Mills M., Clem D., McClain P., Schallhorn R., et al. A randomized clinical trial evaluating rh-FGF-2/β-TCP in periodontal defects. J Dent Res. 2016;95:523–530. doi: 10.1177/0022034516632497. [DOI] [PubMed] [Google Scholar]
- 25.Saito A., Bizenjima T., Takeuchi T., Suzuki E., Sato M., Yoshikawa K., et al. Treatment of intrabony periodontal defects using rhFGF-2 in combination with deproteinized bovine bone mineral or rhFGF-2 alone: a 6-month randomized controlled trial. J Clin Periodontal. 2019;46:332–341. doi: 10.1111/jcpe.13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aoki H., Bizenjima T., eshima F., Sato M., Irokawa D., Yoshikawa K., et al. Kouki Periodontal surgery using rhFGF-2 with deproteinized bovine bone mineral or rhFGF-2 alone: 2-year follow-up of a randomized controlled trial. J Clin Periodontal. 2021;48:92–100. doi: 10.1111/jcpe.13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakayama Y., Matsuda H., Itoh S., Iwai Y., Takai H., Mezawa M., et al. Impact of adjunctive procedures on recombinant human fibroblast growth factor (rhFGF-2) mediated periodontal regeneration therapy: a retrospective study. J Periodontal. 2021;92:983–994. doi: 10.1002/JPER.20-0481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.