Abstract
An autopsy case of sporadic amyotrophic lateral sclerosis (ALS) with lower urinary tract (LUT) and bowel dysfunction is reported. The dysfunction occurred simultaneously with motor neuron symptoms in the early stages of the illness. A 75-year-old man developed exertional dyspnea and constipation following weight loss. Subsequently, he developed swallowing disturbance, fecal incontinence, and urinary retention. Neurological examination showed dysphagia, muscle weakness of the upper limbs, and prominent fasciculation affecting all four limbs and the tongue. All deep tendon reflexes were diminished, but the left plantar response was extensor. Orthostatic hypotension (OH) and the anal reflex were absent. Neuropathological findings did not show neuronal loss and gliosis in the thoracic and sacral intermediolateral nucleus (IML) and in Onuf's nucleus, whereas gliosis was observed in the periaqueductal gray (PAG) and striatum. Therefore, urinary retention may have resulted from involvement of the PAG. Phosphorylated TAR DNA binding protein 43 kDa (p-TDP-43)-positive inclusions were present in the peripheral nerves within the thoracic sympathetic ganglia, as well as the IML of the thoracic spinal cord. However, considering the lack of OH, the IML and peripheral sympathetic nerves unlikely played major roles. Furthermore, neuronal loss or p-TDP-43-immunoreactive deposits were absent in the Auerbach and Meissner plexuses of the rectum, suggesting that the responsible anatomical sites for fecal incontinence could not be found. Although it is difficult to elucidate the precise neuropathological lesions corresponding to LUT and bowel dysfunction, physicians need to recognize that neurogenic bladder and bowel dysfunction can occur in patients with ALS.
Keywords: ALS, Urinary retention, Fecal incontinence, TDP-43, Lower urinary tract dysfunction, And bowel dysfunction
Highlights
-
•
An autopsy case of sporadic ALS with lower urinary tract (LUT) and bowel dysfunction is described.
-
•
The simultaneous occurrence of dysautonomia and motor-neuron symptoms could be unusual in ALS with TDP-43 pathology.
-
•
It is difficult to elucidate the precise neuropathological lesions corresponding to LUT and bowel dysfunction.
-
•
Physicians need to recognize that LUT and bowel dysfunction can occur in ALS with TDP-43 pathology.
1. Introduction
Autonomic symptoms in Amyotrophic lateral sclerosis (ALS) are considered to be rare, but research has shown a relatively high prevalence of bladder and bowel problems in patients with ALS [[1], [2], [3], [4]]. An autopsy case of sporadic ALS with the simultaneous occurrence of severe dysautonomia and motor neuron symptoms is presented.
1.1. Case presentation
A 75-year-old Japanese man had lost 8 kg with little loss of appetite over 8 months. Subsequently, he developed exertional dyspnea and constipation. Radiological and endoscopic examinations did not show any abnormal findings. One month later, he developed swallowing disturbance, fecal incontinence, and urinary urge incontinence (UUI). Post-void residual volume was 120 ml, and benign prostatic enlargement was ruled out by ultrasound measurements. Lumbar magnetic resonance imaging (MRI) showed a small solitary intradural cauda equina mass at the L3 level (Fig. 1A). He presented with dyspnea and heart palpitations (over 120 beats per minute) after walking about 10 m, but electrocardiogram and echocardiogram was normal. Subsequently, he developed loss of bladder sensation and urinary retention. A urinary catheter drained more than 800 ml of urine. Thereafter, he was transferred to our hospital. He had no past medical history or family history of neurological or psychiatric disorders. Neurological examination showed muscle atrophy and weakness of both upper limbs. Prominent fasciculations were present in all 4 limbs and the tongue. There was slight spasticity of the left lower extremity, and the left plantar response was extensor, but all deep tendon reflexes were diminished. The anal reflex was absent. His mini mental state examination score and frontal assessment battery score were 29/30 and 17/18, respectively. Extrapyramidal symptoms, sensory impairment, and ataxia were not seen. Cerebrospinal fluid analysis showed nothing remarkable except for an elevated protein level (74 mg/dL). Nerve conduction studies showed normal sensory responses and reduced compound motor action potentials in all motor nerves. Needle electromyography showed active and chronic denervation in the upper and lower extremities. A respiratory function test demonstrated a restrictive pattern (vital capacity 26.6%). Orthostatic hypotension (OH) was not seen in the Schellong test. Cranial and cervicothoracic MRI was unremarkable. A skin biopsy was performed, but polyglucosan bodies or other inclusions were not found. Anti-ganglioside, anti-voltage-gated potassium channel, and paraneoplastic antibodies were all negative. Three courses of intravenous methylprednisolone pulse therapy (1000 mg/day for 3 days) were also performed, but he did not respond to it. Based on these findings, he was diagnosed as having clinically probable-laboratory-supported ALS according to the revised El Escorial criteria. The patient died of respiratory failure 16 months after disease onset. The clinical characteristics of the patient are shown in Table 1.
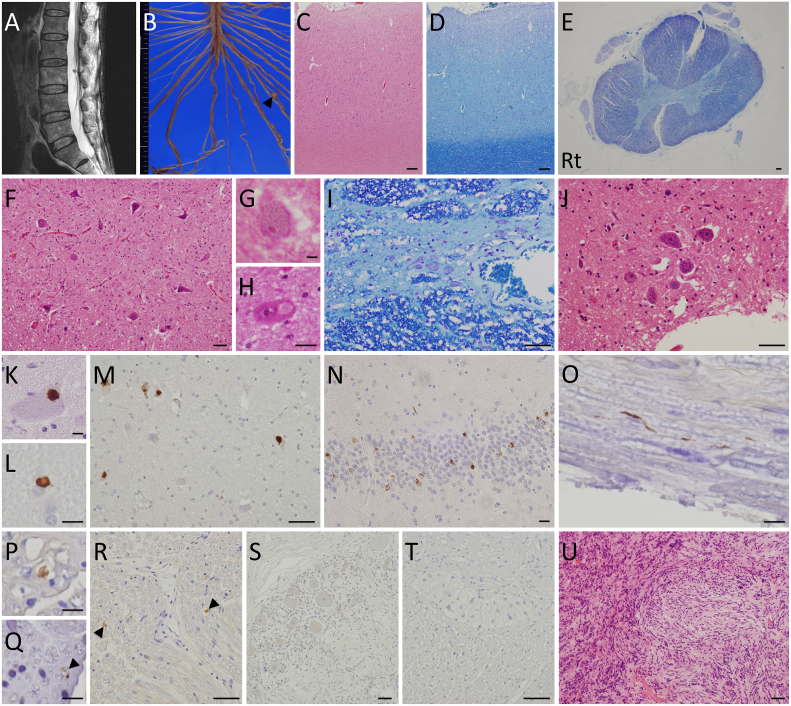
Fig. 1.
MRI (A), macroscopic (B), and microscopic (C-U) findings.
A: Sagittal T2-weighted images on lumbar magnetic resonance imaging show a small solitary intradural cauda equina mass at the L3 level. B: Macroscopic findings of the spinal cord show a small nodule (arrowhead) within the left L5 root. C-D: At a low magnification of the motor cortex, hematoxylin and eosin (HE) staining shows mild neuronal loss and reactive gliosis in the motor cortex (C). Klüver-Barrera (KB) staining shows no apparent myelin pallor in the white matter (D). E: KB staining of a cross-section of the thoracic spinal cord shows right-dominant myelinated fiber loss in the corticospinal tract. F: Neuronal loss and gliosis are shown in the anterior horn cells of the lumbar spinal cord. G-H: In the anterior horn cells of the cervical spinal cord, a Bunina body (G) and round hyalin inclusion (H) are identified by HE staining. I: The number of neurons in the intermediolateral nucleus of thoracic spinal cord is preserved. J: Onuf's nucleus in the anterior horn cells of the sacral spinal cord is well preserved. K: A p-TDP-43-immunopositive inclusion is found in the cytoplasm of an anterior horn cell of the lumbar spinal cord. L: A p-TDP-43 immunoreactive glial cytoplasmic inclusion is present in the white matter of the motor cortex. M-N: In the hypoglossal nucleus (M) and hippocampal granular cell layer (N), p-TDP-43-positive neuronal cytoplasmic inclusions are observed. O-Q: A small number of p-TDP-43-immunoreactive deposits are observed in the lumbar (O—P) and cervical anterior roots (Q, arrowhead). R-S: p-TDP-43 immunoreactivity is seen in the peripheral nerves in the thoracic sympathetic ganglia (R, arrowheads), but not in the neurons of thoracic sympathetic ganglia (S). T: p-TDP-43-immunoreactive deposits are absent in Auerbach's myenteric nerve plexus of the rectum. U: Histopathology of the tumor shows fascicular proliferation of spindle-shaped or oval-shaped cells. Scale bars = 10 μm (G-H, K-L, N-Q), 50 μm (F, I-J, M, R-U), and 200 μm (C-E).
Table 1.
Summary of clinical findings.
| Clinical sign | Duration to appearance (months) | ||
|---|---|---|---|
| Initial symptom | Weight loss | + | 0 |
| Autonomic symptoms | Constipation | + | 9 |
| Fecal incontinence | + | 10 | |
| Urinary incontinence | + | 10 | |
| Tachycardia | + | 11 | |
| Urinary retention | + | 12 | |
| Increased sweating | ー | ||
| Reduced sweating | ー | ||
| Heat intolerance | ー | ||
| Blurred vision | ー | ||
| Orthostatic hypotension | ー | ||
| Orthostatic dizziness | ー | ||
| Blood pressure fluctuations | ー | ||
| Motor symptoms | Dyspnea | + | 9 |
| Dysphagia | + | 10 | |
| Upper limb weakness | + | 11 | |
| Wheelchair | + | 12 | |
| Dysarthria | + | 14 | |
| Lower limb weakness | + | 14 | |
| Bedridden | + | 15 | |
The pathological features are summarized in Table 2. The brain weighed 1300 g before fixation. Macroscopic examination showed mild frontal lobe atrophy. The coronal sections showed no abnormalities in the cerebrum, cerebellum, and brainstem. The spinal cord was atrophic, and a small nodule was present within the left L5 root (Fig. 1B). Microscopically, the loss of Betz cells and gliosis in the motor cortex were mild (Fig. 1C—D). There was no significant neuronal loss in the pyramidal layer of the hippocampus or in the dentate gyrus. The striatum and thalamus showed gliosis. In the midbrain, some neurons of the oculomotor nucleus were slightly atrophic, but their total number was preserved. The substantia nigra and locus coeruleus were unremarkable. Moderate neuronal loss and gliosis were observed in the hypoglossal nucleus, whereas there was no neuronal loss and gliosis in the dorsal vagal nucleus, nucleus ambiguus, and solitary tract nucleus. Myelin pallor was not evident in the corticospinal tracts in the brainstem, but mildly present in the spinal cord (Fig. 1E). Neuronal loss and gliosis were seen in the anterior horn at all levels of the spinal cord (Fig. 1F). Bunina bodies or round hyalin inclusions were occasionally seen in the remaining anterior horn cells (AHCs) of the spinal cord (Fig. 1G-H). Neuronal loss was absent in the intermediolateral nucleus (IML) of the thoracic spinal cord and the IML and Onuf's nucleus of the sacral spinal cord (Fig. 1I-J). Immunohistochemically, phosphorylated TAR DNA binding protein 43 kDa (p-TDP-43)-immunoreactive neuronal cytoplasmic inclusions (NCIs) were detected in the hypoglossal nuclei and AHCs of the spinal cord (Fig. 1K, M). Only a few p-TDP-43-positive glial cytoplasmic inclusions (GCIs) were present in the white matter of the motor cortex (Fig. 1L). In addition, p-TDP-43-positive NCIs were observed in the striatum, pontine nucleus, inferior olivary nucleus, IML of the thoracic spinal cord, hippocampus, and oculomotor nucleus (Fig. 1N), indicating stage 5 p-TDP-43 ALS pathology in Brettschneider's staging. Furthermore, a small number of p-TDP-43-immunoreactive deposits were found in the spinal anterior nerve roots (Fig. 1O-Q) and peripheral nerves within the thoracic sympathetic ganglia (Fig. 1R), but not in neurons of thoracic sympathetic ganglia (Fig. 1S), in the Auerbach and Meissner plexuses of the esophagus or rectum (Fig. 1T), and in nerve fibers of the bladder wall.
Table 2.
Summary of neuropathological findings.
| Anatomical region | p-TDP-43 inclusions | |||
|---|---|---|---|---|
| Frontal lobe | Cortex | NL | + | + |
| White matter | GL | + | ー | |
| Striatum | Caudate nucleus | NL/GL | ー/+ | + |
| Putamen | NL/GL | ー/+ | + | |
| Thalamus | NL/GL | ー/+ | + | |
| Hypothalamus | NL/GL | NA/NA | NA | |
| Midbrain | Oculomotor nucleus | NL/GL | ー/ー | + |
| Substantia nigra | NL/GL | ー/ー | ー | |
| Periaqueductal gray | NL/GL | ー/+ | + | |
| Pons | Locus coeruleus | NL/GL | ー/ー | + |
| Lateral dorsal tegmental nucleus | NL/GL | ー/ー | ー | |
| Pontine nuclei | NL/GL | ー/ー | + | |
| Medulla oblongata | Hypoglossal nucleus | NL/GL | +/+ | + |
| Dorsal vagal nucleus | NL/GL | ー/ー | + | |
| Ambiguous nucleus | NL/GL | ー/ー | ー | |
| Solitary tract nucleus | NL/GL | ー/ー | ー | |
| Inferior olivary nucleus | NL/GL | ー/ー | + | |
| Cerebellum | Cerebellar cortex | NL/GL | ー/ー | ー |
| Dentate nucleus | NL/GL | ー/+ | ー | |
| Cervical cord | Anterior horn cells | NL/GL | +/+ | + |
| Lateral corticospinal tract | Myel.f.loss | + | ー | |
| Anterior corticospinal tract | Myel.f.loss | + | ー | |
| Posterior funiculus | Myel.f.loss | ー | ー | |
| Posterior spinocerebellar tract | Myel.f.loss | ー | ー | |
| Thoracic/Lumbar cord | Anterior horn cells | NL/GL | +/+ | + |
| Intermediolateral nucleus | NL/GL | ー/ー | + | |
| Lateral corticospinal tract | Myel.f.loss | + | ー | |
| Anterior corticospinal tract | Myel.f.loss | + | ー | |
| Posterior funiculus | Myel.f.loss | ー | ー | |
| Posterior spinocerebellar tract | Myel.f.loss | ー | ー | |
| Sacral cord | Intermediolateral nucleus | NL/GL | ー/ー | ー |
| Onuf's nucleus | NL/GL | ー/ー | ー | |
| Sympathetic ganglia | NL/GL | ー/ー | ー | |
p-TDP-43: phosphorylated TAR DNA binding protein 43 kDa; NL: neuronal loss; GL: gliosis; NA: not available; +: present; −: none; Myel: Myelinated; f: fiber.
Several phosphorylated tau-positive neurofibrillary tangles were detected in restricted regions consistent with Braak AT8 stage III. No β-amyloid pathology was seen. There were also no Lewy bodies and GCIs identified by phosphorylated α-synuclein immunostaining. The histopathological features of a nodule were consistent with schwannoma (Fig. 1U).
2. Discussion
Clinically, this patient showed autonomic symptoms, as well as lower-dominant motor neuron signs, from the early stages of the illness. The neuropathological findings included Bunina bodies, moderate loss of AHCs, and mild corticospinal tract degeneration, which were consistent with the neuropathological diagnosis of ALS. Furthermore, there was widespread distribution of p-TDP-43-immunoreactive NCIs and GCIs in the central and peripheral nervous systems (CNS and PNS).
Lower urinary tract (LUT) dysfunction has been reported in patients with ALS, but its prevalence varied widely among studies, ranging from 4% to 44%. [[1], [2], [3], [4]]. Most patients complained of both storage and voiding symptoms, caused by detrusor overactivity (DO) combined with detrusor sphincter dyssynergia (DSD) [4]. Generally, suprapontine lesions cause storage symptoms secondary to DO without DSD [5]. Suprasacral lesions result in both storage and voiding symptoms secondary to DSD [5]. On the other hand, sacral/infrasacral lesions cause voiding symptoms [5]. Although urodynamic testing was not performed, the current patient also presented with storage and voiding symptoms (UUI, reduced bladder sensation, and urinary retention). Concerning bowel dysfunction, it has been reported that constipation had a high prevalence (46%), whereas fecal incontinence was rare (9%) [2]. The present patient showed constipation and subsequently fecal incontinence. Furthermore, the anal reflex was absent. These findings suggest that LUT and bowel dysfunction were most likely caused by sacral/infrasacral lesions. Neuropathological examination did not show neuronal loss and gliosis in the thoracic and sacral IMLs of the spinal cord, as well as in Onuf's nucleus of the sacral spinal cord, whereas gliosis was observed in the periaqueductal gray (PAG) and striatum, which are among the structures controlling micturition and defecation. The PAG receives information from the bladder and then sends a signal to the pontine micturition center, which provides descending output to the sacral spinal cord [6]. Therefore, urinary retention and decreased bladder sensation may result from involvement of the PAG, as one possible explanation. In addition, p-TDP-43-positive inclusions were present in the peripheral nerves within the thoracic sympathetic ganglia, as well as the IML of the thoracic spinal cord. However, considering the lack of OH, the IML and peripheral sympathetic nerves unlikely played major roles.
The enteric nervous system (ENS) is large, complex, and uniquely able to orchestrate gastrointestinal behavior independently of the CNS [7]. Neuronal loss or p-TDP-43- or α-synuclein-immunoreactive deposits were absent in the Auerbach and Meissner plexuses of the esophagus and rectum, indicating that the ENS was not very damaged in the present patient. Fecal incontinence would result from damage to the CNS and PNS, which regulate the anal sphincter, but the responsible anatomical sites for this dysfunction could not be found.
To date, there have been very few reports of the neuropathological findings of SOD1-related ALS patients with LUT or bowel dysfunction [[8], [9], [10]]. Common pathological features were neuronal loss and gliosis in the thalamus, substantia nigra, PAG, ventrolateral medulla, IML, and Onuf's nucleus, besides the degeneration of upper and lower motor neurons, as well as the posterior column of the spinal cord. However, in the present case, degeneration was not evident in regions relevant to the observed LUT dysfunction, such as the IML and Onuf's nucleus, which contain sympathetic and parasympathetic preganglionic neurons, respectively. These results suggest that TDP-43- and SOD1-related ALS must have a different pathomechanism as the cause of autonomic failure. In any case, it would be difficult to elucidate the precise neuropathological lesions corresponding to LUT and bowel dysfunction. Further neuropathological studies will be needed to identify the anatomic correlates of LUT and bowel dysfunction in TDP-43-related ALS.
In conclusion, an interesting autopsy case of sporadic ALS with TDP-43 pathology who presented with LUT and bowel dysfunction, in which the dysfunction occurred simultaneously with motor symptoms in the early stage of illness, was described. Although the underlying pathomechanisms are currently uncertain, physicians need to recognize that LUT and bowel dysfunction can occur in a subset of patients with ALS.
Consent for publication
Written, informed consent was obtained from the patient for publication.
Funding
This work was supported by internal funds.
Declaration of Compeitng Interest
The authors declare there is no conflict of interest.
References
- 1.Baltadzhieva R., Gurevich T., Korczyn A.D. Autonomic impairment in amyotrophic lateral sclerosis. Curr. Opin. Neurol. 2005;18:487–493. doi: 10.1097/01.wco.0000183114.76056.0e. [DOI] [PubMed] [Google Scholar]
- 2.Nübling G.S., Mie E., Bauer R.M., Hensler M., Lorenzl S., Hapfelmeier A., Irwin D.E., Borasio G.D., Winkler A.S. Increased prevalence of bladder and intestinal dysfunction in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemp. Degener. 2014;15:174–179. doi: 10.3109/21678421.2013.868001. [DOI] [PubMed] [Google Scholar]
- 3.Lopes de Carvalho M.L., Motta R., Battaglia M.A., Brichetto G. Urinary disorders in amyotrophic lateral sclerosis subjects. Amyotroph. Lateral Scler. 2011;12:352–355. doi: 10.3109/17482968.2011.574141. [DOI] [PubMed] [Google Scholar]
- 4.Arlandis S., Vázquez-Costa J.F., Martínez-Cuenca E., Sevilla T., Boronat F., Broseta E. Urodynamic findings in amyotrophic lateral sclerosis patients with lower urinary tract symptoms: results from a pilot study. Neurourol. Urodyn. 2017;36:626–631. doi: 10.1002/nau.22976. [DOI] [PubMed] [Google Scholar]
- 5.Panicker J.N., Fowler C.J., Kessler T.M. Lower urinary tract dysfunction in the neurological patient: clinical assessment and management. Lancet Neurol. 2015;14:720–732. doi: 10.1016/S1474-4422(15)00070-8. [DOI] [PubMed] [Google Scholar]
- 6.Zare A., Jahanshahi A., Rahnama’i M.S., Schipper S., van Koeveringe G.A. The role of the periaqueductal gray matter in lower urinary tract function. Mol. Neurobiol. 2019;56:920–934. doi: 10.1007/s12035-018-1131-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao M., Gershon M.D. The bowel and beyond: the enteric nervous system in neurological disorders. Nat. Rev. Gastroenterol. Hepatol. 2016;13:517–528. doi: 10.1038/nrgastro.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimizu T., Kawata A., Kato S., Hayashi M., Takamoto K., Hayashi H., Hirai S., Yamaguchi S., Komori T., Oda M. Autonomic failure in ALS with a novel SOD1 gene mutation. Neurology. 2000;54:1534–1537. doi: 10.1212/wnl.54.7.1534. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi K., Mochizuki Y., Koide R., Kawata A., Homma T., Shimizu T., Komori T., Isozaki E. A Japanese familial ALS patient with autonomic failure and a p.Cys146Arg mutation in the gene for SOD1 (SOD1) Neuropathology. 2016;36:551–555. doi: 10.1111/neup.12303. [DOI] [PubMed] [Google Scholar]
- 10.Hineno A., Oyanagi K., Nakamura A., Shimojima Y., Yoshida K., Ikeda S. Lower urinary tract dysfunction and neuropathological findings of the neural circuits controlling micturition in familial amyotrophic lateral sclerosis with L106V mutation in the SOD1 gene. Rinsho Shinkeigaku. 2016;56:69–76. doi: 10.5692/clinicalneurol.cn-000767. [DOI] [PubMed] [Google Scholar]