Abstract
The global incidence and prevalence of paediatric inflammatory bowel disease (IBD) is increasing, with a notable emergence in developing countries with historically low rates. This suggests that environmental and epigenetic factors may play an important role in the pathogenesis and progression of IBD. Epigenetics refers to the study of biological mechanisms that result in a change of phenotype, without an change in the underlying DNA sequence. Epigenetic mechanisms drive many biological processes that occur in health, such as development and ageing, and are also implicated in disease, including cancer and other inflammatory diseases. Importantly, identification of cell-type-specific epigenetic mechanisms could lead to the identification of molecular disease subtypes allowing a personalised treatment approach. In this short review, we provide a summary of epigenetic mechanisms operative in mammals, and their potential involvement in IBD pathogenesis. Furthermore, we discuss key challenges associated with investigating epigenetics in IBD and provide potential strategies to overcome these, such as through the use of ‘omics’ and organoid technologies.
Keywords: PAEDIATRIC GASTROENTEROLOGY, IBD
Key points.
The global incidence and prevalence of paediatric inflammatory bowel disease (IBD) is increasing, with a notable emergence in developing countries that are becoming ‘Westernised’.
Epigenetics refers to the study of heritable changes in phenotype, such as gene expression, that are not attributed to alterations in the fundamental DNA sequence.
There are three mechanisms of epigenetic control: DNA methylation, post-translational histone modifications and expression of non-coding RNAs.
Epigenetic mechanisms are crucial in normal development and ageing, and provide a plausible framework for many aspects of IBD pathogenesis and progression, including variation in the timing of disease onset.
Key challenges associated with investigating epigenetic mechanisms in humans include the limited value of analysing tissue samples with heterogeneous cell composition, which could potentially be overcome by using novel single cell molecular profiling techniques.
Epigenetics signatures could serve as potential diagnostic, prognostic, predictive or therapeutic biomarkers.
Patient-derived human intestinal organoids provide an elegant experimental approach to investigate the role of environmental factors on the host cell epigenome, and test for stable, disease associated epigenetic marks, in a cell-type-specific manner.
Introduction
Paediatric inflammatory bowel disease (IBD) is a chronic debilitating condition with a rising global incidence and prevalence, and a notable emergence in countries with historically low rates such as the Middle East and Asia.1–3 IBD is an umbrella term that includes Crohn’s disease (CD), ulcerative colitis (UC) and IBD-unclassified. The pathogenesis of this systemic, relapse-remitting disorder is complex and is hypothesised to result from the disruption in homoeostasis between the intestinal epithelium, microbiome and mucosal immune system.4
Family history of IBD is a strong independent risk factor to develop IBD. Indeed, twin and family studies have shown that there is a genetic predisposition for the development of IBD. Furthermore, genome-wide association studies (GWAS) have identified more than 200 risk loci for IBD, many of which involve genes that correspond to loss-of-function variants in immune components.5 6 While IBD is more likely polygenic, very early onset IBD (VEO-IBD), defined as IBD in children diagnosed under 6 years of age, has been linked with monogenetic variations and are associated with primary immunodeficiencies.7–9 Although genetic variation undoubtedly plays a role in determining the risk of developing IBD in some individuals, the rising incidence of IBD in recent decades, particularly in low/middle-income countries that are becoming increasingly ‘Westernised’, suggest that environmental and epigenetic factors may be as or even more important in the pathogenesis of IBD.1–3 Furthermore, epigenetic mechanisms could be the driver for determining disease onset and progression, especially in the absence of genetic changes.
The principles of epigenetics
The ‘epigenetic landscape’ was first described by Conrad Hall Waddington in 1942.10 As a developmental biologist, Waddington used the term ‘epigenetics’ to explain interactions between the environment and genes ultimately leading to the development of phenotype. Today, epigenetics can be defined as the study of heritable changes in phenotype, such as gene expression, that are not attributed to alterations in the fundamental DNA sequence. This is in contrast to genetics, which is the study of heritable changes in gene expression or function due to the direct changes to the genetic code, including point mutations, insertions, deletions and translocations. Epigenetic marks can be heritable, enabling changes in the epigenome to be passed onto daughter cells during mitosis, or from one generation to the next—a phenomenon described as transgenerational epigenetic inheritance.11 A major role for epigenetic mechanisms has been demonstrated for several fundamental biological processes including cellular differentiation, cell-type-specific gene transcription and cellular function, as well as immunological memory.4 12 13 With technological advances enabling researchers to study epigenetics on a genome wide scale in high resolution, there is increasing evidence for the role of epigenetics is health and in a multitude of diseases, including those with complex processes such as cancers, autoimmune and inflammatory diseases.
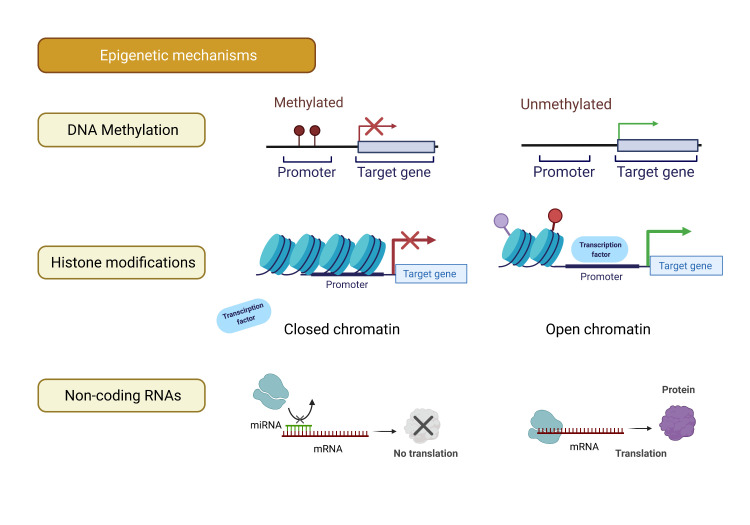
There are three main mechanisms of epigenetic control known to be operative in mammals: DNA methylation, post-translational histone modifications and expression of non-coding RNAs (ncRNAs) (figure 1).
Figure 1.
Summary of the main epigenetic mechanisms in mammals—DNA methylation, histone modifications and non-coding RNAs. Created with BioRender.com. miRNA, microRNA.
DNA methylation is the most extensively studied epigenetic mechanism, and refers to the addition of a methyl group onto the 5’ position of cytosine, forming a 5-methylcytosine. This usually occurs in the context of a CpG site, in which a cytosine immediately precedes a guanine nucleotide and is a process catalysed by enzymes called DNA methyltransferases.14 In the human genome, most CpGs are methylated, with an exception being CpG islands, a region usually within promoter regions with a high proportion of CpG motifs. In principle, hypermethylation of a promoter region results in reduced gene expression and vice versa. Interestingly, DNA methylation is a process that is dependent on cofactors, dietary substrates and nutrients. For instance, folate and vitamin B12 act as methyl donors and cofactors, contributing to DNA methylation.15 16
Chromatin refers to the complex of DNA and histone proteins. Post-transcriptional histone modifications enable chromatin to be either tightly packed (ie, heterochromatin) or more accessible (ie, euchromatin). Consequently, the degree of chromatin accessibility determines transcriptional activity and hence gene expression and phenotype.17
ncRNAs include housekeeping ncRNAs (eg, tRNAs, rRNAs and snRNAs) and regulatory ncRNAs. The latter group can be further subdivided based on nucleotide length—transcripts shorter than 200 nucleotides are termed short ncRNAs (sncRNAs) and those exceeding 200 nucleotides in length are termed long ncRNAs. Among the sncRNAs, microRNAs are of increasing interest in their role in post-transcriptional regulation of gene expression in response to cellular or environmental changes through complex gene regulatory networks.18
Epigenetics as a conceptual framework for IBD pathogenesis
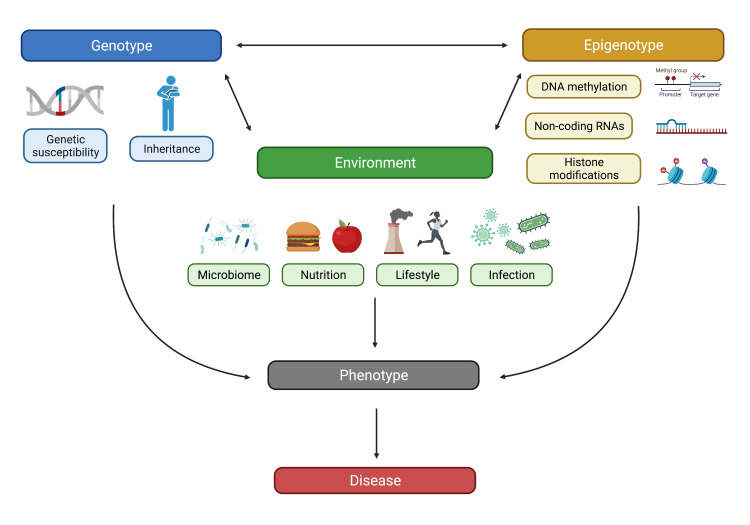
Development of the human intestine relies on exposure to a wide range of environmental factors. These include nutrients, microbes, as well as other antigens capable of activating the mucosal immune system. In addition to allowing immune tolerance to develop, such environmental factors are also critical for the successful colonisation of the intestine by a healthy, diverse gut microbiome. The list of factors that have the potential of altering the physiological process of gut and intestinal microbiome development is much longer. Examples include exposure to pathogens (including enteric bacterial and/or viral infections), toxins (eg, food emulsifiers or preservatives) or medical drugs such as antibiotics. Any of these factors, if encountered during a critical time of development, has the potential to alter physiological progression and may even predispose to disease (figure 2).
Figure 2.
Schematic illustrating factors (genotype, epigenotype and environment) contributing to IBD pathogenesis. Created with BioRender.com. IBD, inflammatory bowel disease.
The complex interaction between genetic, immune and environmental factors has been implicated in in the disease pathogenesis of IBD. Large-scale genome GWAS has been successful at identifying disease susceptibility gene loci, such is IL23R, IL12B, JAK2 and STAT3, as well as, pathways involving T-helper (Th)17 and interleukin (IL)-12/IL-23.7 19 While genetic changes may account for modest proportion of the disease variance, epigenetic factors could potentially account for ‘hidden heritability’ in IBD.7 Assuming the human genome to be highly stable even over centuries, the major increase in the incidences of IBDs in recent decades could be seen as further evidence in support for a key role of environmental factors in IBD pathogenesis. Lastly, the rapid emergence of IBDs in countries adapting to a western lifestyle, also referred to undergoing ‘Westernisation’, further illustrates the striking correlation between environmental changes and the rising global incidence of IBD.1–3
Although these concepts have long been established based on clinical observations and epidemiological studies, our understanding of underlying molecular mechanisms capable of explaining these observations remains limited. Epigenetics provides a plausible framework by being capable of mediating exposure to environmental factors into stable, heritable changes of cellular phenotypes without changing the underlying DNA sequence.20 The potential impact of environmental factors on cellular epigenome as well as their stability depends on several factors. Perhaps the most important among them is the degree of epigenetic plasticity, or in other words the ability for the cellular epigenome to be modifiable. Epigenetic plasticity has been shown to be directly linked to the developmental stage of a cell type or an entire organism.21 As such pluripotent stem cells display a high degree of epigenetic plasticity allowing them to develop into any cell or tissue type.22 23 Once this process is complete, epigenetic mechanisms become less modifiable ensuring cellular identity is retained. Other factors determining the degree of cellular epigenetic changes include the type of environmental factors and their duration. Applying these concepts to the development of the human intestine, exposure at early developmental stages, for example, in utero or in the early postnatal period, might represent particularly vulnerable period during which specific environmental factors have the potential to alter the epigenetic programming of the intestine. Either prolonged and or repeat exposure to certain environmental factors could lead to the development of stable epigenetic changes, which predispose to an individual to the development IBD.11 24
The concept of repeat and or prolonged exposure to environmental factors causing stable epigenetic changes, ultimately leading to altered cellular function may also explain the variation in the timing of disease onset. Approximately one-third of patients with IBD manifest symptoms during childhood or early adulthood, although the majority are diagnosed in adolescence. Excluding rare forms of VEO, monogenic IBD, genetic risk alone does not sufficiently explain this distinct difference in the age of disease onset. As such, we hypothesise that variations in the timing, duration and specific nature of environmental factors might determine the occurrence of epigenetic changes and ultimately the onset of disease.
Howell et al demonstrated that in primary human epithelial cells obtained from mucosal biopsies from paediatric IBD compared with non-IBD control patients, genome wide analysis showed gut segment and disease specific differences DNA methylation and transcriptional changes.25 Furthermore, these DNA methylation marks remained stable over time in an ex-vivo organoid model.25 Studies have also supported the developmental origin of IBD—methylation analysis on the human epithelium of paediatric IBD patients compared with fetal and paediatric controls, showed an overlap significantly with those undergoing methylation changes during the development of the fetal intestine, including key genes in intestinal epithelial immune defence.26
IBD is also known to increase the risk of colorectal cancer (CRC), and this process could also be in part epigenetically driven. Rajamäki et al showed that in IBD associated CRC (IBD-CRCs) compared with sporadic CRC clustered separately, and there was global hypermethylation in IBD-CRCs. This was not found to be associated with younger age at diagnosis or differential expression of the enzymes regulating methylation, hence suggesting these methylation changes are driven by inflammation.27
Overall, these examples lend support to the role of epigenetics in the pathogenesis of IBD, and these signatures potentially serve as new diagnostic and prognostic biomarkers.
Challenges in demonstrating the role of epigenetics in IBD
Despite the highly promising existing evidence in support of epigenetic mechanisms in IBD pathogenesis, reports on the identification of disease associated, cell-type-specific epigenetic alterations and their impact on causing stable changes of cellular function remain scarce. This is likely to be at least in part due to the many challenges that researchers and clinicians face when investigating epigenetics in IBD. Examples include the identification of relevant cell types, choosing specific epigenetic marks while considering that epigenetic mechanisms are closely connected, the timing of sampling and the limited access to human tissue. Outlining these aspects in detail is far beyond the scope of this article, and we will therefore focus on highlighting selected aspects. Perhaps the most important challenge, and often the most difficult to overcome, is the confounding factor that tissue samples have a heterogenous cellular composition, and hence, making it difficult to dissect cell-type-specific epigenetic changes. As a result, performing epigenetic profiling on mixed cell tissues, such as mucosal biopsies or whole blood samples, will not provide information of actual cell-type-specific epigenetic changes. Instead, epigenetic signatures are a ‘merged’ summary of all cell types present and therefore vary vastly according to cellular composition.28 29 This is important as a comparison between tissue samples with differences in cellular composition will yield differences in epigenetic profiles that are driven by difference in cellular composition. Even the application of in-silico cellular decomposition tools is unable to reliably detect cell-type-specific, disease-associated epigenetic changes.
One approach to overcome this challenge is to purify specific cell types of interest.25 However, such methods are extremely labour intensive, and could still be potentially confounded by cellular contamination. Single cell profiling could also circumvent this problem and recent cutting-edge methodologies have demonstrated the possibly of performing genome wide epigenetic profiling on a single cell level such single cell Assay for Transposase Accessible Chromatin sequencing or single cell DNA methylation profiling.30 Indeed, the possibility of performing simultaneous genome wide transcriptional and epigenetic profiling on a single cell level is hugely exciting and has great potential of delivering novel insights.
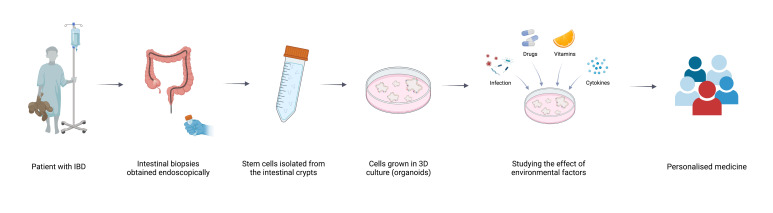
Another elegant way of investigating epigenetic mechanisms in human cells is the use of patient-derived human organoids. Since the development of the human intestinal epithelial organoid (IEO) culture model by Hans Clevers just over a decade ago, these versatile translational research tools have become widely used in the field of gastrointestinal research including IBD.31 IEOs can be generated from stem cells obtained from mucosal samples, and expanded in culture into three dimensional structures which closely mimic the intestinal epithelium in vivo (figure 3). Our group and others have demonstrated that organoids retain epigenetic (specifically DNA methylation) profiles and are key to determining patient and gut segment specific cellular function.32 Furthermore, comparing DNA methylation profiles of IEOs derived from human fetal with paediatric gut revealed striking differences in their epigenetic plasticity with fetal cell displaying major epigenetic changes in culture.32 Taken together, these early studies demonstrate the utility of patient-derived organoids as a reductionist model to investigate epigenetic mechanisms in human gut health and IBD.
Figure 3.
Schematic illustrating the generation of intestinal organoids from patient-derived biopsies and potential applications. Created with BioRender.com. 3D, three dimensions; IBD, inflammatory bowel disease.
Future directions in epigenetics of IBD research
Despite all the challenges associated with investigating the role of epigenetic mechanisms in IBD pathogenesis, the future potential for major findings remains high. The emergence of novel high throughput and high-resolution technologies, in particular single cell molecular profiling, spatial transcriptomics and the use of patient-derived organoids are capable of addressing some of the key questions in this area. For example, are there stable, cell type specific epigenetic marks that can either cause or contribute to the chronic, relapse-remitting gut inflammation in IBD? If so, what are the specific cell types are affected, and which environmental factors are directly involved in causing these epigenetic changes? Another important and promising area of research refers to the use of epigenetic signatures as clinical biomarkers—diagnostic, prognostic, predictive or therapeutic. For example, Ventham et al have demonstrated hypomethylation of ribosomal protein S6 kinase A2 (RPS6KA2) could predict complicated structuring and/or penetrating CD and extensive UC.7 In particular, stable epigenetic marks such as DNA methylation, which can reliably be analysed on extracted DNA has some advantages over other, less stable molecular profiles (eg, gene transcription). Furthermore, in the right context, performing epigenetic profiling of mixed cell tissue with the aim of generating clinical biomarkers may be justifiable. However, the identification of underlying mechanisms will still require the demonstrating of cell-type-specific stable epigenetic marks. Importantly, demonstrating epigenetic, cell-type-specific variation among patients diagnosed with the same condition (ie, UC or CD) could provide an opportunity to develop novel, molecular disease subtypes that could explain the major phenotypic variation, thereby allowing a more personalised treatment approach.33
Conclusion
Epigenomics is an emerging field, that a could mediate interactions between the genome and environment. Epigenetic mechanisms provide a plausible and attractive framework that is capable of filling in the gaps in our understanding of IBD pathogenesis, and in particular, aspects specific to patients diagnosed in childhood. Despite the challenges in performing translational epigenetic research, the availability of novel, cutting-edge research tools, including single cell epigenetic profiling and patient-derived gut organoids, is indeed exciting and holds huge potential to deliver compelling evidence in support of this largely theoretical framework. Overall, identifying epigenetic signatures in IBD could pave way for the development of new clinical biomarkers of IBD, identify new drug targets and hence could ultimately lead to major advances in IBD patient care.
Footnotes
Contributors: NG and MZ designed and wrote the article together.
Funding: MZ was funded by a Medical Research Council (MRC) New Investigator Research Grant (MR/T001917/1). The funder had no role in the design, creation or decision to submit this work for publication.
Competing interests: None declared.
Provenance and peer review: Commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142:46–54. e42; quiz e30. 10.1053/j.gastro.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 2. Roberts SE, Thorne K, Thapar N, et al. A systematic review and meta-analysis of paediatric inflammatory bowel disease incidence and prevalence across Europe. J Crohns Colitis 2020;14:1119–48. 10.1093/ecco-jcc/jjaa037 [DOI] [PubMed] [Google Scholar]
- 3. Kuenzig ME, Fung SG, Marderfeld L, et al. Twenty-first century trends in the global epidemiology of pediatric-onset inflammatory bowel disease: systematic review. Gastroenterology 2022;162:1147–59. 10.1053/j.gastro.2021.12.282 [DOI] [PubMed] [Google Scholar]
- 4. Petronis A, Petroniene R. Epigenetics of inflammatory bowel disease. Gut 2000;47:302–6. 10.1136/gut.47.2.302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jostins L, Ripke S, Weersma RK, et al. Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012;491:119–24. 10.1038/nature11582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu JZ, van Sommeren S, Huang H, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet 2015;47:979–86. 10.1038/ng.3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ventham NT, Kennedy NA, Nimmo ER, et al. Beyond gene discovery in inflammatory bowel disease: the emerging role of epigenetics. Gastroenterology 2013;145:293–308. 10.1053/j.gastro.2013.05.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ouahed J, Spencer E, Kotlarz D, et al. Very early onset inflammatory bowel disease: a clinical approach with a focus on the role of genetics and underlying immune deficiencies. Inflamm Bowel Dis 2020;26:820–42. 10.1093/ibd/izz259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Loddo I, Romano C. Inflammatory bowel disease: genetics, epigenetics, and pathogenesis. Front Immunol 2015;6:551. 10.3389/fimmu.2015.00551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Waddington CH. The epigenotype. 1942. Int J Epidemiol 2012;41:10–13. 10.1093/ije/dyr184 [DOI] [PubMed] [Google Scholar]
- 11. Zilbauer M, Zellos A, Heuschkel R. Epigenetics in paediatric gastroenterology, hepatology, and nutrition: present trends and future perspectives. J Pediatr Gastroenterol Nutr 2016;62:521–9. [DOI] [PubMed] [Google Scholar]
- 12. Xu J, Xu H-M, Yang M-F, et al. New insights into the epigenetic regulation of inflammatory bowel disease. Front Pharmacol 2022;13:813659. 10.3389/fphar.2022.813659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Däbritz J, Menheniott TR. Linking immunity, epigenetics, and cancer in inflammatory bowel disease. Inflamm Bowel Dis 2014;20:1638–54. 10.1097/MIB.0000000000000063 [DOI] [PubMed] [Google Scholar]
- 14. Hornschuh M, Wirthgen E, Wolfien M, et al. The role of epigenetic modifications for the pathogenesis of Crohn’s disease. Clin Epigenetics 2021;13:108. 10.1186/s13148-021-01089-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Navarro E, Funtikova AN, Fíto M, et al. Prenatal nutrition and the risk of adult obesity: long-term effects of nutrition on epigenetic mechanisms regulating gene expression. J Nutr Biochem 2017;39:1–14. 10.1016/j.jnutbio.2016.03.012 [DOI] [PubMed] [Google Scholar]
- 16. Aleksandrova K, Romero-Mosquera B, Hernandez V. Diet, gut microbiome and epigenetics: emerging links with inflammatory bowel diseases and prospects for management and prevention. Nutrients 2017;9:962. 10.3390/nu9090962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kouzarides T. Chromatin modifications and their function. Cell 2007;128:693–705. 10.1016/j.cell.2007.02.005 [DOI] [PubMed] [Google Scholar]
- 18. Kalla R, Ventham NT, Kennedy NA. MicroRNAs: new players in inflammatory bowel disease. Gut 2015;64:1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Anderson CA, Boucher G, Lees CW, et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet 2011;43:246–52. 10.1038/ng.764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jenke AC, Zilbauer M. Epigenetics in inflammatory bowel disease. Curr Opin Gastroenterol 2012;28:577–84. 10.1097/MOG.0b013e328357336b [DOI] [PubMed] [Google Scholar]
- 21. Socha-Banasiak A, Pawłowska M, Czkwianianc E, et al. From intrauterine to extrauterine Life—The role of endogenous and exogenous factors in the regulation of the intestinal microbiota community and gut maturation in early life. Front Nutr 2021;8:696966. 10.3389/fnut.2021.696966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meir Y-JJ, Li G. Somatic Reprogramming—Above and beyond pluripotency. Cells 2021;10:2888. 10.3390/cells10112888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paksa A, Rajagopal J. The epigenetic basis of cellular plasticity. Curr Opin Cell Biol 2017;49:116–22. 10.1016/j.ceb.2018.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kellermayer R, Balasa A, Zhang W, et al. Epigenetic maturation in colonic mucosa continues beyond infancy in mice. Hum Mol Genet 2010;19:2168–76. 10.1093/hmg/ddq095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Howell KJ, Kraiczy J, Nayak KM, et al. DNA Methylation and Transcription Patterns in Intestinal Epithelial Cells From Pediatric Patients With Inflammatory Bowel Diseases Differentiate Disease Subtypes and Associate With Outcome. Gastroenterology 2018;154:585–98. 10.1053/j.gastro.2017.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kraiczy J, Nayak K, Ross A, et al. Assessing DNA methylation in the developing human intestinal epithelium: potential link to inflammatory bowel disease. Mucosal Immunol 2016;9:647–58. 10.1038/mi.2015.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rajamäki K, Taira A, Katainen R, et al. Genetic and epigenetic characteristics of inflammatory bowel Disease–Associated colorectal cancer. Gastroenterology 2021;161:592–607. 10.1053/j.gastro.2021.04.042 [DOI] [PubMed] [Google Scholar]
- 28. Zilbauer M, Rayner TF, Clark C, et al. Genome-wide methylation analyses of primary human leukocyte subsets identifies functionally important cell-type–specific hypomethylated regions. Blood 2013;122:e52–60. 10.1182/blood-2013-05-503201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jenke AC, Postberg J, Raine T, et al. DNA methylation analysis in the intestinal epithelium-effect of cell separation on gene expression and methylation profile. PLoS One 2013;8:e55636. 10.1371/journal.pone.0055636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Clark SJ, Lee HJ, Smallwood SA, et al. Single-cell epigenomics: powerful new methods for understanding gene regulation and cell identity. Genome Biol 2016;17:72. 10.1186/s13059-016-0944-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kraiczy J, Zilbauer M. Intestinal epithelial organoids as tools to study epigenetics in gut health and disease. Stem Cells Int 2019;2019:7242415. 10.1155/2019/7242415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kraiczy J, Nayak KM, Howell KJ, et al. DNA methylation defines regional identity of human intestinal epithelial organoids and undergoes dynamic changes during development. Gut 2019;68:49–61. 10.1136/gutjnl-2017-314817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zilbauer M, Heuschkel R. Disease prognostic biomarkers in inflammatory bowel Diseases—A reality check. J Crohns Colitis 2022;16:162–5. 10.1093/ecco-jcc/jjab118 [DOI] [PubMed] [Google Scholar]