Abstract
Background and purpose
We aimed to determine whether young adults (<50 years) with acute ischaemic stroke (AIS) are more likely to receive intravenous tissue plasminogen activator (IV tPA) and have shorter time to treatment than older patients with stroke.
Methods
We analysed data from the Chinese Stroke Center Alliance registry for patients with AIS hospitalised between August 2015 and July 2019. Patients were classified into two groups according to age: young adults (<50 years of age) and older adults (≥50 years of age).
Results
Of 793 175 patients with AIS admitted to 1471 hospitals, 9.1% (71 860) were young adults. Compared with older adults, a higher proportion of young adults received IV tPA among patients without contraindicaitons (7.2% vs 6.1%, adjusted OR (aOR) 1.13, 95% CI 1.10 to 1.17) and among patients without contraindications and with onset-to-door time ≤3.5 hours (23.6% vs 19.3%, aOR 1.20, 95% CI 1.15 to 1.24). We did not observe differences in onset-to-needle time (median hours 2.7 hours) or door-to-needle time (DNT) (median minutes 60 min) between young and older adults. The proportion of DNT ≤30 min, DNT ≤45 min and DNT ≤60 min in young and older IV tPA-treated patients were 16.9% vs 18.8%, 30.2% vs 32.8% and 50.2% vs 54.2%, respectively. Compared with older adults, young adults treated with IV tPA had lower odds of in-hospital mortality (0.5% vs 1.3%, aOR 0.54, 95% CI 0.35 to 0.82) and higher odds of independent ambulation at discharge (61.0% vs 53.6%, aOR 1.15, 95% CI 1.08 to 1.22), and the associations may be partly explained by stroke severity measured by the National Institutes of Health Stroke Scale score.
Conclusion
Young adults with AIS were more likely to receive IV tPA than older adults, although there was no difference between the two groups in time to treatment. Compared with older adults, young adults may had better in-hospital outcomes.
Keywords: adult neurology, stroke, vascular medicine
Strengths and limitations of this study.
We used data from a large-scale, nationwide, hospital-based, multicentre quality improvement initiative.
Multiple regression models adjusted for different levels of covariates were used to check the robustness of the results.
Data on intra-arterial therapies, puncture times, door-to-imaging and follow-up outcomes after discharge were not collected and reported.
Introduction
Stroke incidence among adults under 50 years of age has risen in recent years.1–5 This creates a significant socioeconomic burden due to high healthcare costs and loss of labour productivity.5 6 According to the 2019 Chinese Stroke Statistics, 81.9% of patients with stroke had ischaemic strokes,7 of which young adults constituted 15%–18%.8 Given this trend, researchers must carefully consider the clinical features and best practices of treatment for strokes in young adults.8
Ischaemic stroke is a common, preventable and treatable disease that typically results from thrombotic or thromboembolic blockage of a cerebral artery.9 Revascularisation therapy plays a major role in the process of saving penumbral tissue from infarction.10 Tissue plasminogen activator (tPA) is the main intravenous drug approved for the treatment of acute ischaemic stroke.11 Treatment of acute occlusive stroke with IV tPA is considered the most regular and important method when given within 4.5 hours of occlusion.12 13 While epidemiological studies have been done in other countries regarding IV tPA treatment in young patients with AIS, there is limited research on this topic in China.13–17
The purpose of this study was to compare the characteristics, IV tPA treatment rates, onset-to-needle time, door-to-needle time (DNT) and in-hospital outcomes of young (<50 years of age) and older (≥50 years of age) patients with AIS in the Chinese Stroke Center Alliance (CSCA). We hypothesised that young patients with AIS would be treated more frequently with IV tPA, have shorter treatment time and better in-hospital outcomes compared with older adults.
Methods
Data source
CSCA is a national, hospital-based, multicentre, voluntary, multifaceted intervention and continuous quality improvement initiative. The data coordinating centre of CSCA resides at the China National Clinical Research Center for Neurological Diseases, Beijing Tiantan Hospital.18 Trained personnel collected patient demographics, medical history, medications, DNT and in-hospital outcomes, then entered this information into a database using a web-based Patient Management Tool (Medicine Innovation Research Center, Beijing, China). The tool is characterised by predefined logic features, range checks and user alerts to identify a potentially invalid format or value entries and to optimise data quality at the time of entry. Training in the use of the tool was provided online and onsite for all users. However, data collected by hospitals were not independently audited by external chart review. In addition, The China National Clinical Research Center for Neurological Diseases serves as the data analysis centre and has an agreement to analyse the aggregate deidentified data for care quality feedback and research purposes. We abstracted 838 229 cases and identified 793 175 patients admitted with ischaemic strokes from 2015 to 2019.
Study population
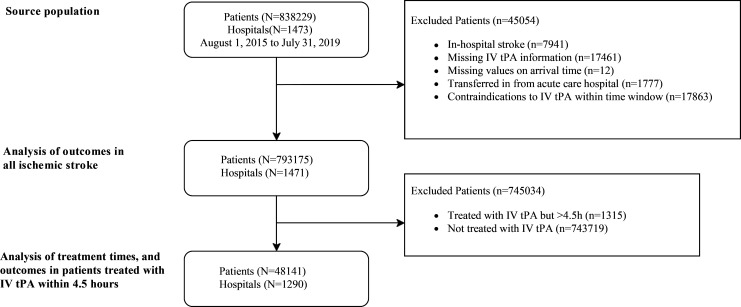
In the first stage, our analyses included patients admitted with AIS within 7 days of the onset of symptoms between 1 August 2015 and 31 July 2019 from 1473 hospitals (online supplemental table Ⅰ). We excluded patients who had in-hospital strokes (n=7941, 0.95%), were missing IV tPA information (n=17 461, 2.08%), had imprecise or undocumented arrival times (n=12), transferred in from an acute care hospital (n=1777, 0.212%) or had contraindications to venous thrombolysis within the time window (n=17 863, 2.13%). This yielded a population of patients with ischaemic strokes with indications for thrombolysis (n=793 175). To analyse DNT and in-hospital outcomes of ischaemic stroke, we excluded patients treated with IV tPA >4.5 hours after stroke onset (n=1315, 0.17%) and patients who were not treated with IV tPA (n=743 719, 93.76%). This yielded a subset of the study population that consisted of 48 141 patients with AIS from 1290 hospitals (figure 1).
Figure 1.
Study flow chart for patient identification. IV tPA, intravenous tissue plasminogen activator.
bmjopen-2021-055055supp001.pdf (151.3KB, pdf)
Outcomes
The IV tPA treatment rate was assessed among two populations. First, IV tPA rate among patients without contraindications to thrombolytic therapy was calculated as the number of IV tPA cases divided by the total number of ischaemic stroke cases without any contraindications to thrombolytic therapy. Then IV tPA rate among patients without contraindication to thrombolytic therapy and with onset-to-door time ≤3.5 hours was calculated as the number of IV tPA cases divided by the total number of ischaemic stroke cases without any contraindications to thrombolytic therapy and arrived at a hospital within 3.5 hours after stroke onset. The contraindications were defined according to guidelines for the early management of patients with acute ischaemic stroke from the Heart Association/American Stroke Association and Chinese Society of Neurology. DNT was defined as the time between arrival at the emergency department and time of intravenous thrombolysis, and is an important metric in AIS treatment.19 20 We analysed DNT as a binary outcome three times with a different cut-off point each time (≤30, ≤45 or ≤60 min, respectively).
In-hospital outcomes included symptomatic intracranial haemorrhage (sICH), in-hospital mortality and independent ambulation at discharge. sICH was defined as intracranial haemorrhage (ICH) within 36 hours of admission, documented by CT or MRI, with the treating physician’s notes indicating clinical deterioration attributable to haemorrhage. Patients who were able to walk <48 hours after hospital admission were considered to have independent ambulation at discharge.
Statistical analysis
Continuous variables with normal distribution were expressed as mean and SD, and those with skewed/non-normal distribution as medians and IQRs. Categorical variables were summarised as frequencies and percentages. Because of the large sample size, some statistically significant differences may not be clinically meaningful. We used absolute standardised differences (ASD) or Hodges-Lehmann estimator to compare differences in baseline characteristics between young and older adults independent of sample size. An ASD >10% was considered meaningful imbalance.21
For outcomes such as IV tPA treatment and in-hospital outcomes, logistic regression models were performed to determine adjusted ORs (aORs) and 95% CIs. Multivariable models with different level of adjustment were used to check the robustness of the results. Covariates in multivariable models including gender, insurance status, body mass index (BMI), medical history of prior stroke or transient ischaemic attack (TIA), coronary artery disease (CAD) or prior myocardial infarction (MI), diabetes mellitus, hypertension, smoking status, atrial fibrillation/flutter, glycated haemoglobin, diastolic blood pressure, systolic blood pressure, medication history (hypoglycaemic drugs, antihypertensive drugs, antiplatelet drugs and lipid-lowering drugs) and hospital grade.
There were few missing data for most variables, with the exceptions of the in-hospital National Institutes of Health Stroke Scale (NIHSS) score (missing 19.4%), DNT (6.3%), glycated haemoglobin (10.6%) and BMI (1.6%). For continuous variables missing <15%, the median was used for imputation. Since the NIHSS score is a widely used tool for assessing stroke severity22 and contributes important information to AIS prognosis,23 sensitivity analyses adjusting for the NIHSS score were conducted. In addition, results from multiple imputation were also provided.
All statistical analyses were performed using SAS V.9.4 (SAS Institute, Cary, North Carolina, USA) and the %ggBaseline SAS macro.24 Two-sided p values of <0.05 were considered statistically significant.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Results
Of 838 229 patients with AIS in the CSCA, 793 175 patients enrolled from 1471 hospitals were eligible for inclusion in this study. The mean age was 66.1±12.0 years and 62.7% were men. A total of 261 760 (33.0%) patients had previous stroke/TIA, 69 810 (8.8%) had CAD/prior MI, 170 638 (21.5%) had diabetes, 510 928 (64.4%) had hypertension, 294 708 (37.2%) were smokers and 40 231 (5.1%) had atrial fibrillation/flutter.
Clinical characteristics
A total of 71 860 (9.1%) patients were young adults (<50 years) and 721 315 (90.9%) were older adults (≥50 years). The mean age among young adults was 43.8±5.3 years and 68.3±10.0 years among older adults. A larger proportion of the young adults were men (76.3% vs 61.3%) and did not have health insurance coverage (12.9% vs 5.7%), compared with older adults. Young adults had a lower prevalence of cardiovascular risk factors compared with older adults, including history of stroke or TIA (22.5% vs 34.0%), CAD/prior MI (4.0% vs 9.3%), diabetes mellitus (15.3% vs 22.1%), hypertension (53.9% vs 65.5%) and atrial fibrillation (1.2% vs 5.5%). Young adults had a lower rate of medication use than older adults, including hypoglycaemic drugs (10.9% vs 17.4%), antihypertensive drugs (33.5% vs 48%), antiplatelet drugs (14.6% vs 21.5%) and lipid-lowering drugs (11.4% vs 15.3%). However, diastolic blood pressure in young adults was significantly higher than that of older adults (mean±SD 92.3±16.1 vs mean±SD 86.5±13.5), and young adults had a statistically higher mean BMI than older adults (mean±SD 24.9±4.8 vs mean±SD 23.9±4.2). The proportion of young adults who smoked was also higher than that of older adults (49.9% vs 35.9%) (table 1).
Table 1.
Baseline characteristics of young and old patients with ischaemic stroke
| Baseline characteristics | Total (n=793 175 (100%)) |
Young adults (<50 years) (n=71 860 (9.1%)) |
Old adults (≥50 years) (n=721 315 (90.9%)) |
ASD (%)/H-L estimator* |
| Patient characteristics | ||||
| Age, years | 66.1±12.0 | 43.8±5.3 | 68.3±10.0 | 306.1 |
| Male, n (%) | 496 960 (62.7) | 54 850 (76.3) | 442 110 (61.3) | 32.8 |
| Insurance status, n (%) | ||||
| UEBMI | 225 940 (28.5) | 19 160 (26.7) | 206 780 (28.7) | 4.5 |
| URBMI | 149 839 (18.9) | 12 393 (17.2) | 137 446 (19.1) | 4.9 |
| NRCMS | 333 979 (42.1) | 27 587 (38.4) | 306 392 (42.5) | 8.4 |
| Self-pay | 50 727 (6.4) | 9263 (12.9) | 41 464 (5.7) | 25.0 |
| Other | 32 690 (4.1) | 3457 (4.8) | 29 233 (4.1) | 3.4 |
| Arrive mode, n (%) | ||||
| Ambulance | 89 484 (11.3) | 7170 (10.0) | 82 314 (11.4) | 4.5 |
| Private car | 372 727 (47.0) | 33 340 (46.4) | 339 387 (47.1) | 1.4 |
| Taxi | 68 801 (8.7) | 6743 (9.4) | 62 058 (8.6) | 2.8 |
| Bicycle or tricycle | 7237 (0.9) | 577 (0.8) | 6660 (0.9) | 1.1 |
| Helicopter | 338 (0.0) | 27 (0.0) | 311 (0.0) | |
| Mobile stroke unit | 246 (0.0) | 24 (0.0) | 222 (0.0) | |
| Other | 254 342 (32.1) | 23 979 (33.4) | 230 363 (31.9) | 3.2 |
| Medical history, n (%) | ||||
| Previous stroke/TIA | 261 760 (33.0) | 16 197 (22.5) | 245 563 (34.0) | 25.8 |
| CAD/prior MI | 69 810 (8.8) | 2906 (4.0) | 66 904 (9.3) | 21.4 |
| Diabetes | 170 638 (21.5) | 10 985 (15.3) | 159 653 (22.1) | 17.5 |
| Peripheral vascular disease | 13 512 (1.7) | 718 (1.0) | 12 794 (1.8) | 6.8 |
| Hypertension | 510 928 (64.4) | 38 722 (53.9) | 472 206 (65.5) | 23.8 |
| Smoking† | 294 708 (37.2) | 35 848 (49.9) | 258 860 (35.9) | 28.6 |
| Atrial fibrillation/flutter | 40 231 (5.1) | 886 (1.2) | 39 345 (5.5) | 24.1 |
| Dyslipidaemia | 60 605 (7.6) | 5861 (8.2) | 54 744 (7.6) | 2.2 |
| Carotid stenosis | 10 161 (1.3) | 509 (0.7) | 9652 (1.3) | 6.0 |
| Medication history, n (%) | ||||
| Anticoagulants | 31 326 (3.9) | 2394 (3.3) | 28 932 (4.0) | 3.7 |
| Hypoglycaemic drugs | 133 244 (16.8) | 7802 (10.9) | 125 442 (17.4) | 18.7 |
| Antihypertensive drugs | 370 017 (46.7) | 24 065 (33.5) | 345 952 (48.0) | 29.8 |
| Antiplatelet drugs | 165 771 (20.9) | 10 482 (14.6) | 155 289 (21.5) | 18.0 |
| Lipid-lowering drugs | 118 827 (15.0) | 8171 (11.4) | 110 656 (15.3) | 11.5 |
| NIHSS score in hospital‡ | 3.0 (2.0–6.0) | 3.0 (1.0–5.0) | 3.0 (2.0–6.0) | |
| Biochemical indicators | ||||
| Glycated haemoglobin§, % | 5.8 (5.3–6.5) | 5.7 (5.2–6.1) | 5.8 (5.3–6.5) | |
| BMI¶ | 24.0±4.3 | 24.9±4.8 | 23.9±4.2 | 22.2 |
| Homocysteine**, µmol/L | 13.9 (10.4–19.1) | 13.4 (10.0–19.0) | 13.9 (10.5–19.1) | |
| Systolic blood pressure††, mm Hg | 150.0±23.0 | 147.9±24.4 | 150.2±22.8 | 9.7 |
| Diastolic blood pressure‡‡, mm Hg | 87.0±13.9 | 92.3±16.1 | 86.5±13.5 | 39.0 |
| Hospital characteristics, n (%) | ||||
| Hospital level | ||||
| Secondary hospital | 303 790 (38.3) | 23 993 (33.4) | 279 797 (38.8) | 11.3 |
| Tertiary hospital | 489 385 (61.7) | 47 867 (66.6) | 441 518 (61.2) | 11.3 |
| Hospital region | ||||
| Eastern China | 365 579 (46.1) | 32 744 (45.6) | 332 835 (46.1) | 1.0 |
| Central China | 262 618 (33.1) | 24 477 (34.1) | 238 141 (33.0) | 2.3 |
| Western China | 164 978 (20.8) | 14 639 (20.4) | 150 339 (20.8) | 1.0 |
*H-L estimator; an absolute standardised difference (%) >10% indicates meaningful imbalance between two groups.
†Smoking: having smoking experience or behaviours.
‡Information was missing for n=154 052 patients (19.4%).
§Information was missing for n=84 393 patients (10.6%); median was used for imputation.
¶Information was missing for n=12 698 patients (1.6%); median was used for imputation.
**Information was missing for n=35 781 patients (4.5%).
††Information was missing for n=245 patients (≈0.0%); median was used for imputation.
‡‡Information was missing for n=251 patients (≈0.0%); median was used for imputation.
ASD, absolute standardised difference; CAD, coronary artery disease; H-L, Hodges-Lehmann; MI, myocardial infarction; NIHSS, National Institutes of Health Stroke Scale; NRCMS, New Rural Cooperative Medical Scheme; TIA, transient ischaemic attack; UEBMI, Urban Employee Basic Medical Insurance; URBMI, Urban Resident Basic Medical Insurance.
IV tPA treatment rates
Young adults were treated more frequently with IV tPA than older adults among patients without contraindications to thrombolysis (7.2% vs 6.1%, aOR 1.13, 95% CI 1.10 to 1.17) and among patients without contraindication and with onset-to-door time ≤3.5 hours (23.6% vs 19.3%, aOR 1.20, 95% CI 1.15 to 1.24). Data from sensitivity analyses showed consistent results (table 2).
Table 2.
Multivariable analysis of IV tPA treatment by age group
| Treatment | Rate of IV tPA | aOR (95% CI) from model 1 | aOR (95% CI) from model 2 | aOR (95% CI) from model 3 | aOR (95% CI) from model 4 |
| IV tPA among patients without contraindications | 49 456/793 175 (6.2) | ||||
| Young adults | 5181/71 860 (7.2) | 1.19 (1.15 to 1.22) | 1.13 (1.10 to 1.17) | 1.20 (1.16 to 1.24) | 1.19 (1.15 to 1.22) |
| Old adults | 44 275/721 315 (6.1) | 1.00 | 1.00 | 1.00 | 1.00 |
| IV tPA among patients without contraindication and with onset-to-door time ≤3.5 hours | 45 842/232 905 (19.7) | ||||
| Young adults | 4768/20 191 (23.6) | 1.29 (1.25 to 1.34) | 1.20 (1.15 to 1.24) | 1.24 (1.20 to 1.29) | 1.23 (1.19 to 1.28) |
| Old adults | 41 074/212 714 (19.3) | 1.00 | 1.00 | 1.00 | 1.00 |
Model 1: logistic regression model without adjustment.
Model 2: adjusted for gender, insurance, BMI, previous stroke/TIA, previous CAD/prior MI, diabetes, hypertension, have smoking experience or behaviour, atrial fibrillation/flutter, glycated, haemoglobin, diastolic blood pressure, hypoglycaemic drugs, antihypertensive drugs, antiplatelet drugs, lipid-lowering drugs and hospital level.
Model 3: adjusted for were in-hospital NIHSS score, gender, insurance, BMI, previous stroke/TIA, previous CAD/prior MI, diabetes, hypertension, have smoking experience or behaviour, atrial fibrillation/flutter, glycated haemoglobin, diastolic blood pressure, hypoglycaemic drugs, antihypertensive drugs, antiplatelet drugs, lipid-lowering drugs and hospital level. Patients (n=154 052) with missing values on NIHSS score were not included in this analysis.
Model 4: results from multiple imputation.
aOR, adjusted OR; BMI, body mass index; CAD, coronary artery disease; MI, myocardial infarction; NIHSS, National Institutes of Health Stroke Scale; IV tPA, intravenous tissue plasminogen activator.
Treatment time
While young adults were more likely to receive IV tPA, there was no significant difference in onset-to-needle time (median 2.7 hours, IQR 2.0–3.5 for both groups) and DNT (median 60.0 min, IQR 42.0–90.0 vs median 60.0 min, IQR 36.0–84.0) among young and older adults. DNT was also analysed as a binary outcome at three levels: DNT ≤30, DNT ≤45 and DNT ≤60 min. There were no significant differences between the two groups on any DNT level (16.9% vs 18.8%; 30.2% vs 32.8%; 50.2% vs 54.2%) (table 3).
Table 3.
Treatment time in young and old patients with ischaemic stroke treated with IV tPA
| Measures | Total (n=48 141 (100%)) |
Young adults (<50 years) (n=5044 (10.5%)) |
Old adults (≥50 years) (n=43 097 (89.5%)) |
ASD (%)/H-L estimator* |
| Onset-to-needle time†, hour | 2.7 (2.0–3.5) | 2.7 (2.0–3.5) | 2.7 (2.0–3.5) | |
| DNT‡, min | 60.0 (36.0–84.0) | 60.0 (42.0–90.0) | 60.0 (36.0–84.0) | |
| Treatment time | ||||
| DNT ≤30 min | 8938 (18.6) | 850 (16.9) | 8088 (18.8) | 5.0 |
| DNT ≤45 min | 15 637 (32.5) | 1521 (30.2) | 14 116 (32.8) | 5.6 |
| DNT ≤60 min | 25 884 (53.8) | 2531 (50.2) | 23 353 (54.2) | 8.0 |
*H-L estimator; an absolute standardised difference (%) >10% indicates meaningful imbalance between two groups.
†Onset-to-needle time was missing for 3818 (7.9%) patients, with 431 (8.5%) in age <50 years and 3387 (7.9%) in age ≥50 years groups.
‡DNT was missing for 3027 (6.3%) patients, with 342 (6.8%) in age <50 years and 2685 (6.2%) in age ≥50 years groups.
ASD, absolute standardised difference; DNT, door-to-needle time; H-L, Hodges-Lehmann; IV tPA, intravenous tissue plasminogen activator.
In-hospital outcomes
In-hospital outcomes including sICH, in-hospital mortality and independent ambulation at discharge are summarised in table 4. Multiple logistic regression with adjustments of unbalanced covariates (ASD% >10% in online supplemental table Ⅱ) showed that young adults had non-significantly lower rates of sICH (0.5% vs 0.9%, aOR=0.74, 95% CI 0.49 to 1.11) than older adults. However, young adults had significantly lower rates of in-hospital mortality (0.5% vs 1.3%, aOR=0.54, 95% CI 0.35 to 0.82) and were more likely to be independently ambulating at discharge (61.0% vs 53.6%, aOR=1.15, 95% CI 1.08 to 1.22).
Table 4.
In-hospital outcomes in young and old patients with ischaemic stroke treated with IV tPA
| Outcome | Rate of outcomes | aOR (95% CI) from model 1 | aOR (95% CI) from model 2 | aOR (95% CI) from model 3 | aOR (95% CI) from model 4 |
| sICH | 414/48 141 (0.9) | ||||
| Young adults | 26/5044 (0.5) | 0.57 (0.38 to 0.85) | 0.74 (0.49 to 1.11) | 0.77 (0.5 to 1.18) | 0.79 (0.52 to 1.20) |
| Old adults | 388/43 097 (0.9) | 1.00 | 1.00 | 1.00 | 1.00 |
| In-hospital mortality | 579/48 141 (1.2) | ||||
| Young adults | 24/5044 (0.5) | 0.37 (0.24 to 0.55) | 0.54 (0.35 to 0.82) | 0.70 (0.46 to 1.09) | 0.65 (0.43 to 1.00) |
| Old adults | 555/43 097 (1.3) | 1.00 | 1.00 | 1.00 | 1.00 |
| Independent ambulation at discharge | 26 175/48 141 (54.4) | ||||
| Young adults | 3079/5044 (61.0) | 1.36 (1.28 to 1.44) | 1.15 (1.08 to 1.22) | 1.00 (0.93 to 1.08) | 1.02 (0.96 to 1.10) |
| Old adults | 23 096/43 097 (53.6) | 1.00 | 1.00 | 1.00 | 1.00 |
Model 1: logistic regression model without adjustment.
Model 2: adjusted for gender, insurance, BMI, previous stroke/TIA, previous CAD/prior MI, diabetes, hypertension, have smoking experience or behaviour, atrial fibrillation/flutter, glycated haemoglobin, diastolic blood pressure, systolic blood pressure, hypoglycaemic drugs, antihypertensive drugs, antiplatelet drugs and lipid-lowering drugs.
Model 3: adjusted for in-hospital NIHSS score, gender, insurance, BMI, previous stroke/TIA, previous CAD/prior MI, diabetes, hypertension, have smoking experience or behaviour, atrial fibrillation/flutter, glycated haemoglobin, diastolic blood pressure, blood pressure, hypoglycaemic drugs, antihypertensive drugs, antiplatelet drugs and lipid-lowering drugs. Patients (n=2503) with missing values on NIHSS score were not included in this analysis.
Model 4: results from multiple imputation.
aOR, adjusted OR; BMI, body mass index; CAD, coronary artery disease; MI, myocardial infarction; NIHSS, National Institutes of Health Stroke Scale; sICH, symptomatic intracranial haemorrhage; IV tPA, intravenous tissue plasminogen activator.
In sensitivity analyses adjusted for NIHSS scores, young adults had non-significantly lower odds of sICH (aOR=0.77, 95% CI 0.5 to 1.18), in-hospital mortality (aOR=0.70, 95% CI 0.46 to 1.09) and a neutral association with independent ambulation at discharge (aOR=1.00, 95% CI 0.93 to 1.08). Sensitivity analysis using different age cut-off point (<35 years and ≥35 years) showed consistent results with primary analysis (online supplemental table III). But when we used another age cut-off point (<45 years and ≥45 years), we had a lower sICH rate (aOR=0.44, 95% CI 0.19 to 0.99) in the young group (online supplemental table IV).
Discussion
In this hospital-based observational study of 793 175 patients with AIS, we found that young adults (<50 years of age) were more likely to be treated with IV tPA than older adults (7.2% vs 6.1%), which was consistent with our hypothesis. Our study demonstrated that young patients with stroke had fewer comorbidities (previous stroke/TIA, previous CAD/prior MI, diabetes, hypertension and atrial fibrillation/flutter) at baseline, which may compel providers to feel more secure in administering IV tPA to this group of patients. Even though we did not observe a difference in DNT between age groups, we found that young adults had more favourable in-hospital outcomes than older adults, including lower odds of sICH, in-hospital mortality and higher odds of independent ambulation at discharge. However, after adjusting for NIHSS scores, the differences were not significant. Therefore, the benefit among young adults may be explained by stroke severity measured by NIHSS score.
We chose 50 as the cut-off age because several important international studies on thrombolytic therapy in young people also set the cut-off age at 50 years.15 16 Using the same cut-off age value would benefit the comparison with other studies and also have advantages for potential systematic review and meta-analysis.
IV tPA thrombolytic therapy is considered to be the standard therapy in patients with acute ischaemic stroke. However, its use has been studied primarily in adults over age 50 years.16 Given increasing evidence in the literature that thrombolytic therapy rarely causes haemorrhages in patients with stroke-mimicking conditions, providers may also feel more comfortable administering IV tPA to young adults when it is uncertain whether a stroke or a stroke mimic had occurred.25 Our research also supports this conclusion. Young adults had similar time to treatment and were more likely to be treated than older adults, which may reflect increased awareness among patients and clinicians that stroke is a potentially fatal disease affecting people of all ages.
The relationship between time to treatment and mortality was first recognised in clinical studies.26 27 The importance of this metric was re-emphasised in the 2004 American College of Cardiology/American Heart Association practice guidelines, which stated that DNT targets ‘should not be perceived as an average performance standard but as a goal that an early treatment system in every hospital should seek for every appropriate patient’.28 Consistent with a prior analysis of The Safe Implementation of Thrombolysis in Stroke-International Stroke Thrombolysis Register (SITS-ISTR) showing no difference in median door-to-needle times between patients 18–50 and >50 years of age, there was also not an obvious differentiation in treatment among young or older adults in our study.15 However, a prior study by Dodds et al found that young adults (18–40 years) with AIS were more likely to experience a delay in evaluation and treatment.13 Our definition of young adults (age <50 years) differs from theirs (age 18–40 years) and is more applicable in China, which may also explain why we did not find differences in treatment times between the two groups.29 Another explanation for the lack of differences in treatment times between the two groups may be that DNT mainly depends on hospital level and treatment process rather than age.
In our study, we observed a higher rate of sICH and in-hospital mortality among older adults treated with IV tPA, although the difference was not significantly adjusted for NIHSS scores. We cannot entirely separate young age from lower NIHSS score, as young patients are highly correlated with lower NIHSS scores in clinical practice (4.0±4.5 vs 4.9±5.3, the Rank Biserial correlation coefficient in this study is 0.10, p<0.001). Therefore, the associations may be partly explained by stroke severity measured by NIHSS score. Although we do not find significant differences between these two groups, we do see a trend towards better outcomes in young people. One possible explanation is that younger patients with stroke have fewer comorbidities in their medical history, such as previous stroke or TIA, CAD/MI, diabetes mellitus, hypertension and atrial fibrillation, as well as milder strokes, all of which are associated with better outcomes.30 Other factors favouring recovery in young patients with stroke include greater brain plasticity and more robust family and social support.31
There are several limitations in our study. Since hospital participation in the CSCA is voluntary, participating hospitals are more likely to be larger, tertiary centres with a myriad of resources that smaller hospitals do not have access to. We recognise that findings from CSCA may not be generalisable to patients with AIS admitted to hospitals outside of the CSCA. Second, we did not report data on intra-arterial therapies and puncture times because only a very small number of patients in our study received thrombectomy. We also did not have door-to-imaging data to support the study and there was a relatively high proportion of missing DNT values (6.3% overall, 6.8% in young and 6.2% in older patients). However, we did not observe a significant difference in DNTs between young and older patients with AIS. Lastly, although we provided some possible explanation for the differences between young and old adults group, further researches are needed to explorer the detailed mechanism.
Conclusion
In summary, young adults with AIS are more likely to receive IV tPA than older adults, but there is no difference between the two groups in time to treatment. Young adults may have better in-hospital outcomes compared with older adults, suggesting favourable effects of treatment with IV tPA.
Supplementary Material
Acknowledgments
We thank the staff and participants of the Chinese Stroke Center Alliance studies for their contribution.
Footnotes
Twitter: @chelsealiu96
H-YW and H-QG contributed equally.
Contributors: Conceived and led the project: X-QZ, Y-LW, Y-JW and YJ. Conception and design: H-YW, H-QG, Y-JW and YJ. Data collection and analysis: H-YW, XY, C-JW, H-QG and QZ. Interpret the data: L-PL, XM and HL. Drafting the article and revising the content: H-YW, H-QG, CL, QZ, Y-YJ and Z-XL. All authors approved the final version of the manuscript. YJ is the guarantor of the article.
Funding: The Chinese Stroke Center Alliance programme was supported by grants from the Ministry of Science and Technology and the Ministry of Health of the People’s Republic of China (National Key R&D Program of China, Code: 2016YFC0901001, 2017YFC1310901, 2016YFC0901002, 2017YFC1307905, 2015BAI12B00), Beijing Talents Project (2018A13, 2018000021223ZK03), CAMS Innovation Fund for Medical Sciences (2019-I2M-5-029), Beijing Municipal Committee of Science and Technology (Z201100005620010), Beijing Natural Science Foundation (Z200016).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was approved by the Institutional Review Board/Ethics Committee of Beijing Tiantan Hospital, Capital Medical University (ID: KY 2018-061-02). Participating hospitals received research approval to collect data in CSCA without requiring individual patient informed consent under the common rule or a waiver of authorisation and exemption from their Institutional Review Board.
References
- 1.Béjot Y, Bailly H, Durier J, et al. Epidemiology of stroke in Europe and trends for the 21st century. Presse Med 2016;45:e391–8. 10.1016/j.lpm.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 2.Hathidara MY, Saini V, Malik AM. Stroke in the young: a global update. Curr Neurol Neurosci Rep 2019;19:91. 10.1007/s11910-019-1004-1 [DOI] [PubMed] [Google Scholar]
- 3.Rutten-Jacobs LCA, Maaijwee NAM, Arntz RM, et al. Risk factors and prognosis of young stroke. The future study: a prospective cohort study. study rationale and protocol. BMC Neurol 2011;11:109. 10.1186/1471-2377-11-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.George MG. Risk factors for ischemic stroke in younger adults: a focused update. Stroke 2020;51:729–35. 10.1161/STROKEAHA.119.024156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maaijwee NAMM, Rutten-Jacobs LCA, Schaapsmeerders P, et al. Ischaemic stroke in young adults: risk factors and long-term consequences. Nat Rev Neurol 2014;10:315–25. 10.1038/nrneurol.2014.72 [DOI] [PubMed] [Google Scholar]
- 6.Feigin VL, Roth GA, Naghavi M, et al. Global burden of stroke and risk factors in 188 countries, during 1990-2013: a systematic analysis for the global burden of disease study 2013. Lancet Neurol 2016;15:913–24. 10.1016/S1474-4422(16)30073-4 [DOI] [PubMed] [Google Scholar]
- 7.Wang Y-J, Li Z-X, Gu H-Q, et al. China stroke statistics 2019: a report from the National center for healthcare quality management in neurological diseases, China national clinical research center for neurological diseases, the Chinese stroke association, National center for chronic and non-communicable disease control and prevention, Chinese center for disease control and prevention and Institute for global neuroscience and stroke collaborations. Stroke Vasc Neurol 2020;5:211–39. 10.1136/svn-2020-000457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singhal AB, Biller J, Elkind MS, et al. Recognition and management of stroke in young adults and adolescents. Neurology 2013;81:1089–97. 10.1212/WNL.0b013e3182a4a451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peña-Martínez C, Durán-Laforet V, García-Culebras A, et al. Pharmacological modulation of neutrophil extracellular traps reverses thrombotic stroke tPA (tissue-type plasminogen activator) resistance. Stroke 2019;50:3228–37. 10.1161/STROKEAHA.119.026848 [DOI] [PubMed] [Google Scholar]
- 10.Martínez-Sánchez P, Díez-Tejedor E, Fuentes B, et al. Systemic reperfusion therapy in acute ischemic stroke. Cerebrovasc Dis 2007;24 Suppl 1:143–52. 10.1159/000107390 [DOI] [PubMed] [Google Scholar]
- 11.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart Association/American stroke association. Stroke 2019;50:e344–418. 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 12.Berge E, Whiteley W, Audebert H, et al. European stroke organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J 2021;6:I–LXII. 10.1177/2396987321989865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dodds JA, Xian Y, Sheng S, et al. Thrombolysis in young adults with stroke: findings from get with the Guidelines-Stroke. Neurology 2019;92:e2784–92. 10.1212/WNL.0000000000007653 [DOI] [PubMed] [Google Scholar]
- 14.Papavasileiou V, Goldstein LB. Thrombolysis in young adults with acute ischemic stroke: quicker may be better. Neurology 2019;92:1129–30. 10.1212/WNL.0000000000007651 [DOI] [PubMed] [Google Scholar]
- 15.Toni D, Ahmed N, Anzini A, et al. Intravenous thrombolysis in young stroke patients: results from the SITS-ISTR. Neurology 2012;78:880–7. 10.1212/WNL.0b013e31824d966b [DOI] [PubMed] [Google Scholar]
- 16.Poppe AY, Buchan AM, Hill MD. Intravenous thrombolysis for acute ischaemic stroke in young adult patients. Can J Neurol Sci 2009;36:161–7. 10.1017/S031716710012027X [DOI] [PubMed] [Google Scholar]
- 17.Shi J, Cao Y, You S, et al. Young stroke patients treated with intravenous thrombolysis have a more favorable outcome and mortality compared with older patients. Curr Neurovasc Res 2017;14:141–8. 10.2174/1567202614666170328095431 [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Li Z, Wang Y, et al. Chinese stroke center alliance: a national effort to improve healthcare quality for acute stroke and transient ischaemic attack: rationale, design and preliminary findings. Stroke Vasc Neurol 2018;3:256–62. 10.1136/svn-2018-000154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsieh C-Y, Chen W-F, Chen C-H, et al. Efforts to reduce the door-to-needle time of thrombolysis in acute ischemic stroke: video-assisted therapeutic risk communication. J Formos Med Assoc 2014;113:929–33. 10.1016/j.jfma.2013.11.012 [DOI] [PubMed] [Google Scholar]
- 20.van Tuijn CFJ, Luitse JS, van der Valk M, et al. Reduction of the door-to-needle time for administration of antibiotics in patients with a severe infection: a tailored intervention project. Neth J Med 2010;68:123–7. [PubMed] [Google Scholar]
- 21.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083–107. 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Habib M, ASMR K, Hossain MM. Assessment of initial stroke severity by National Institute health stroke scale (NIHSS) score at admission. Journal of Dhaka Medical College 2018;26:90–3. [Google Scholar]
- 23.Chen X, Zhan X, Chen M, et al. The prognostic value of combined NT-pro-BNP levels and NIHSS scores in patients with acute ischemic stroke. Intern Med 2012;51:2887–92. 10.2169/internalmedicine.51.8027 [DOI] [PubMed] [Google Scholar]
- 24.Gu H-Q, Li D-J, Liu C, et al. %ggBaseline: a SAS macro for analyzing and reporting baseline characteristics automatically in medical research. Ann Transl Med 2018;6:326. 10.21037/atm.2018.08.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zinkstok SM, Engelter ST, Gensicke H, et al. Safety of thrombolysis in stroke mimics: results from a multicenter cohort study. Stroke 2013;44:1080–4. 10.1161/STROKEAHA.111.000126 [DOI] [PubMed] [Google Scholar]
- 26.Cannon CP, Gibson CM, Lambrew CT, et al. Relationship of symptom-onset-to-balloon time and door-to-balloon time with mortality in patients undergoing angioplasty for acute myocardial infarction. JAMA 2000;283:2941–7. 10.1001/jama.283.22.2941 [DOI] [PubMed] [Google Scholar]
- 27.Schull MJ, Vermeulen M, Donovan L, et al. Can the wrong statistic be bad for health? Improving the reporting of door-to-needle time performance in acute myocardial infarction. Am Heart J 2005;150:583–7. 10.1016/j.ahj.2005.03.061 [DOI] [PubMed] [Google Scholar]
- 28.Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). Circulation 2004;110:588–636. 10.1161/01.CIR.0000134791.68010.FA [DOI] [PubMed] [Google Scholar]
- 29.Xiaobing ZSH, Li'an H. Clinical investigation of stroke in young and middle-aged adults. Cerebrovascular Diseases Foreign Medical Sciences 2004;012:354–7. [Google Scholar]
- 30.Arnold M, Halpern M, Meier N, et al. Age-Dependent differences in demographics, risk factors, co-morbidity, etiology, management, and clinical outcome of acute ischemic stroke. J Neurol 2008;255:1503–7. 10.1007/s00415-008-0949-9 [DOI] [PubMed] [Google Scholar]
- 31.Popa-Wagner A, Carmichael ST, Kokaia Z, et al. The response of the aged brain to stroke: too much, too soon? Curr Neurovasc Res 2007;4:216–27. 10.2174/156720207781387213 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-055055supp001.pdf (151.3KB, pdf)
Data Availability Statement
Data are available on reasonable request.