Abstract
Objectives
To investigate German physicians’ attitudes towards and experiences with voluntary disclosure of payments by pharmaceutical companies in a public database and their impact on future decisions for or against disclosure.
Design
A national cross-sectional survey conducted in 2018 among physicians who voluntarily disclosed at least one payment in the German transparency regulation.
Setting
Retrospective paper-pencil questionnaire about attitudes towards and experiences with voluntary payment disclosures in the first (2015) and second (2016) years of the German transparency regulation.
Participants
German physicians who disclosed either in the first year only, the second year only, or in both years of the transparency regulation.
Primary outcomes
(1) The probability to disclose in 2016, predicted by physicians’ experience of reactions from others in 2015, descriptive norms and attitudes towards transparency; (2) Frequency and (3) Content of reactions from others in 2015 compared with 2016.
Results
Data of 234 respondents were analysed (n=42, 45 and 147 physicians who disclosed in 2015, 2016 or both years, respectively). The probability to disclose in 2016 was not predicted by perceived reactions, norms or attitudes towards transparency (p>0.01). Most participants reported not to have received any reactions by patients (190/234, 81%), colleagues (128/234, 55%) or the private environment (153/234, 65%). Neither frequency nor content of reactions differed between the first and second years (scale 1–5; frequency: Mdn2015,2016 = 1.33 vs 1.00, rb=−0.17, p>0.01; content: Mdn2015,2016 = 3.00 vs 3.00, rb=0.19, p>0.01). However, media reporting, fear of reputational damage and a feeling of being defamed were mentioned as reasons for non-disclosure.
Conclusions
While confirmatory analyses did not provide significant results, descriptive analyses showed that participants who voluntarily disclose payments mainly do not experience any reactions towards their disclosures but report fears about losing their reputation due to disclosures.
Keywords: health policy, protocols & guidelines, medical ethics
Strengths and limitations of this study.
This study is the first survey of attitudes and experiences of physicians who voluntarily disclosed payments by pharmaceutical companies in a nationwide transparency database.
The sample takes into account whether physicians disclosed only in 1 year or in 2 consecutive years.
The study was preregistered and provides qualitative and quantitative data on reasons for non-disclosure in this database.
The questionnaire used in this study was only constructed for this purpose, so a direct comparison with other data is not possible.
Introduction
The services sector of the health industry has a long tradition of close ties to the pharmaceutical industry.1 2 Such ties have been shown to potentially lead to systematic biases in research and daily patient care.3–5 Situations in which a secondary interest (eg, financial gain) creates a risk that a primary interest (eg, patient welfare) is unduly influenced are defined as conflicts of interest (COI).1 6 Several approaches have been established to meet the challenge of COI in medicine, among which transparency regulations are very popular.7–10 Transparency regulations have been introduced to shed light on formerly unknown information,7 8 in this case: information about payments from pharmaceutical companies to healthcare professionals (HCPs). They differ in their coverage and implementation. In the USA, payments are fully transparent since the introduction of the Physician Payments Sunshine Act (PPSA) and publicly disclosed on the Open Payments website.11 In Europe, transparency of payments to HCPs is mandatory only in some countries whereas in others such as Germany, it is regulated on a voluntary level.7 9 12 13 However, disclosing COI may have unintended effects (eg, loss of patient trust14 15), which may interact with the mode of the transparency regulation. This study explores the effects of Germany’s voluntary transparency regulation of payments by pharmaceutical companies to HCPs, and investigates factors that lead HCPs to decide against disclosing payments in this database voluntarily.
Effects of transparency guidelines
An intended effect of transparency guidelines is that publicly disclosing COI could motivate conflicted persons to change their behaviour in the sense that they decrease industry contacts in the future.16 Thus, transparency regulations affect those who disclose information. In focus groups about experiences with the PPSA conducted in 2015,17 physicians reported to be frustrated with the administrative process, to feel treated unfairly and to worry the disclosures might mislead patients.17 For voluntary regulations, there is only anecdotal evidence: In a newspaper article18 about physicians who decided against disclosure in the German transparency database, the interviewees stated to approve of transparency in general, but also said the current regulation was unfair, the disclosed information was misleading and patients’ trust would suffer.18
Public awareness thus appears to be a relevant element of transparency regulations.16 Research has shown that patients would like their physicians to disclose financial COI, since they were concerned about biased clinical judgement.19 20 However, at least in the USA, public awareness of the Open Payments website was low, as shown by citizen surveys in 2014 and 2015: only 9%–12% knew about the disclosed information.21 22 Accordingly, US physicians believed patients were uninterested in the data.17 In Germany, physicians reported to fear negative effects on patients and therefore decided against disclosure.18 The interaction between disclosing HCPs and the public and its effects on disclosing behaviour in a voluntary transparency database has not been systematically investigated yet.
Another important factor when discussing the effects of voluntary transparency regulations is the descriptive norm (ie, behaviour that most of the peers show is considered ‘normal’ behaviour23 24) and thus, the moment when area-wide information about the frequency of behaviour becomes available. From then on, information is available about how many HCPs voluntarily disclose payments, which forms a new reference frame for whether it is considered ‘normal’ to disclose payments. An HCP’s decision to voluntarily disclose payments may depend on the subjectively estimated number of disclosing HCPs. Additionally, HCPs themselves will consider the fact that HCPs receive payments by pharmaceutical companies relatively ‘normal’, while most of the public will only learn about it with the first disclosure round and judge the behaviour as ‘abnormal’—an impression which will decline over time. Therefore, reactions by the public may be more pronounced in the first year of a transparency database than in the following.
Germany’s transparency regulation
In Germany, transparency of payments to HCPs is self-regulated by the pharmaceutical industry: 54 pharmaceutical companies organised in the ‘association of voluntary self-regulation in the pharmaceutical industry’ passed a transparency codex which requires HCPs’ consent for the respective financial interaction to be disclosed on each company’s website.12 13 25 First data were disclosed 2016 for payments from 2015. The investigative newsroom CORRECTIV gathered this data from each company’s website and established the ‘Euros for Doctors’ database—a searchable platform that provided, per HCP, an overview of all payments they had received. The database started in 2016, but it was discontinued after only 2 years, making the investigation of long-term changes of disclosing rates difficult.26 The kick-off was accompanied with investigative articles,27 collaborating with the popular German online news magazine SPIEGEL ONLINE. They criticised the undifferentiated way of disclosing (eg, the designated use of the money was not disclosed) and the large number of HCPs who did not disclose information.18 28 An analysis of the 2015 and 2016 data of this database by our group13 showed that about 28% and 24% of all HCPs who had received payments agreed to disclose payments in 2015 and 2016, respectively. Of all disclosing HCPs, 26% disclosed payments in both years, 44% disclosed only in 2015, and 29% only in 2016. The total number of disclosing HCPs decreased by 21%.
Study aims and research questions
This study investigated HCPs’ attitudes towards and experiences with the voluntary transparency database, and reasons for non-disclosure. Main research question 1 was: Do the reactions physicians experienced to their disclosed information or their perception of how normal it is to disclose predict the decision to disclose in the following year? Does a positive attitude moderate this effect? We hypothesised that the probability for deciding against disclosure in the subsequent year was higher the more unpleasant reactions were experienced and the lower the descriptive norm to disclose was estimated, and that a positive attitude towards transparency moderates this relationship. Research questions 2 and 3 were: Do physicians experience a higher number of reactions and more negative reactions in the first than in the second year of the regulation? We hypothesised that reactions were more frequent and more negative in the first compared with the following year.
Methods
Sample
Our sample was drawn from the population of 28 230 HCPs who disclosed at least one financial interaction with a pharmaceutical company in 2015 or 2016 in the German transparency regulation.13 We built our survey sample of three groups: HCPs who disclosed only 2015 (group 1), only 2016 (group 2), and HCPs who disclosed both 2015 and 2016 (group 3). For further analyses in the underlying dissertation,29 the third group was split up and analysed in three subgroups. Therefore, group 3 is bigger than groups 1 and 2. To enhance the probability that we survey HCPs who receive payments annually, we excluded HCPs who disclosed an annual payment sum <€1000. This was based on the observation that the median disclosed annual payments of HCPs who disclosed in both years was €899 in 2015, compared with the median disclosed sum of HCPs who disclosed only once, which was €452.13 Based on that, we excluded 19 267 HCPs with annual payment sums <€1000. From the remaining 8963 HCPs, possible participants were selected (see below). Further, we only included HCPs who worked as physicians at the time point of the survey. This criterion was evaluated after selection: for each chosen HCP, we verified by internet research whether they currently worked as a physician. If they did not or no information was available, another HCP was randomly selected, and it was checked whether they worked as physicians. This was repeated until the determined sample size was reached.
Procedure and sample size
For the planned regression model, an analysable sample of 150 participants was estimated based on Green’s rule of thumb.30 Expecting a response rate of 30%–50%, we formulated a detailed sample plan: starting in August 2018, we sent out questionnaires in waves of 50 questionnaires per group. Questionnaires were sent by mail, accompanied by a cover letter and a reply envelope. A reminder letter was sent after 2 weeks. Two weeks after that, we phoned those with a publicly available phone number. If the planned sample size was not reached a month after the last contact attempt, the next wave was started: the next 50 physicians were randomly selected and contacted as described above. We stopped this procedure for each group after the thirtieth questionnaire was received, which was after we had sent the third wave of questionnaires in February 2019. All examinable questionnaires that we received afterwards were also included in the data analysis. Study procedures were preregistered at www.osf.io/ztvur.
Questionnaire
The two-page questionnaire contains questions about demographics, disclosure and attitude towards transparency in German language. Response formats include five-level Likert items, default categories and open formats. Responses were given by ticking boxes or writing text onto the questionnaire. It was clarified in the cover letter that sending back completed questionnaires implies that data will be analysed anonymously. All items and response options can be found in online supplemental file A.
bmjopen-2021-055963supp001.pdf (55.4KB, pdf)
Main outcomes
The items to investigate research questions 1–3 are listed in table 1. Physicians were asked about the frequency, content and pleasantness of reactions that they experienced. Those questions could be answered separately for the reactions of patients, colleagues and the private environment. For the analyses of the main research questions, an average value was calculated across the three groups of people. Participants of group 2 were asked about reactions to their disclosure 2016; all other participants were asked about reactions to their disclosure 2015.
Table 1.
Translated list of relevant questionnaire items with response format
| Variable | Item Response format |
| Research question 1 | |
| Pleasantness of reactions | ‘If there were reactions, how did you perceive them?’ 1–5: very unpleasant, rather unpleasant, neutral, rather pleasant, very pleasant |
| Descriptive norm | ‘What percentage of German physicians do you estimate consented to disclose in the database?’ ___ % (open format in per cent) |
| Attitude | ‘To what extent do you agree with the following statement: In principle, I approve of transparency.’ 1–5: strongly disagree, disagree, neutral, agree, strongly agree |
| Research question 2 | |
| Frequency of reactions | ‘How many reactions did you get from patients / colleagues / your private environment?’ 1–5: none, very few, rather few, rather many, many |
| Research question 3 | |
| Content of reactions | ‘If there were reactions, how was their content?’ 1–5: very negative, somewhat negative, neutral, somewhat positive, very positive |
Note. The original questionnaire was in German; the translated complete questionnaire can be found in online supplemental file A.
Analysis
To investigate hypothesis 1, a multiple logistic regression with the outcome variable disclosure 2016 (0=no disclosure, 1=disclosure) and the main predictors X1: pleasantness of reactions, and X2: descriptive norm was conducted. To investigate the moderating role of X3: attitude, two interactions terms were added as predictors: X3*X1 and X3*X2. To test hypotheses 2 and 3, the frequency and content of reactions 2015 were compared with the frequency and content of reactions 2016. Directed tests for independent samples were conducted (more frequent/more negative reactions in 2015 than 2016). To test for normal distribution, the Shapiro-Wilk test was used. Data in all groups were not normally distributed on the respective dependent variable, therefore Wilcoxon tests were conducted. Effect sizes with 95% CIs are given as rank-biserial correlations (rb). A conservative α level of 0.01 was used for all tests.
Exploratively, we performed a content analysis31 of answers to the question ‘Was there anything that bothered you about the reactions?’. All answers were reviewed by two researchers independently and categories were suggested. From the suggested categories, 10 final categories were decided based on mutual consensus. Then, each answer was categorised independently (overall inter-rater agreement: 93%). Diverging ratings were discussed until consensus was reached. Statistical analyses were performed in JASP (Jeffrey's Amazing Statistics Program) V.0.10.2,32 RStudio, R V.3.6.133 and Microsoft Excel V.2011.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Results
Sample
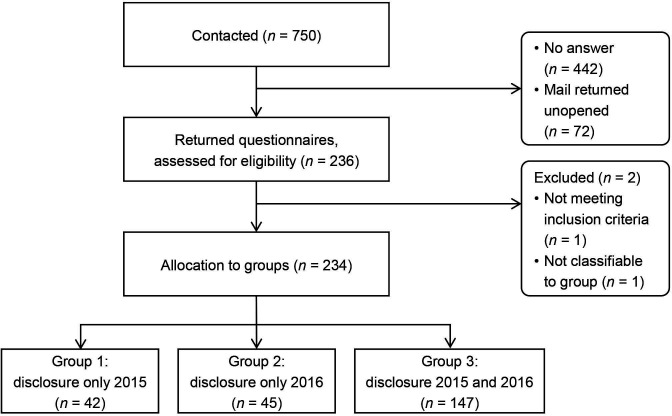
We contacted n=750 physicians and received 236 filled-in questionnaires (figure 1). The response rate was 35% (236/678; 72 questionnaires were undeliverable). Two questionnaires needed to be excluded: one was missing a page and could not be allocated to a group; another contained a note that the participant was not a medical doctor but a biologist. The remaining 234 questionnaires were allocated to the groups and analysed. Mean and median age of participants was 53 years (SD=8.29; IQR=10; range: 31–75; 48/234 (21%) female, 185/234 (79%) male, 1/234 (0%) missing). Further sample characteristics are listed in online supplemental file B.
Figure 1.
Participant flow chart.
bmjopen-2021-055963supp002.pdf (15.1KB, pdf)
Physicians’ experiences with the transparency database
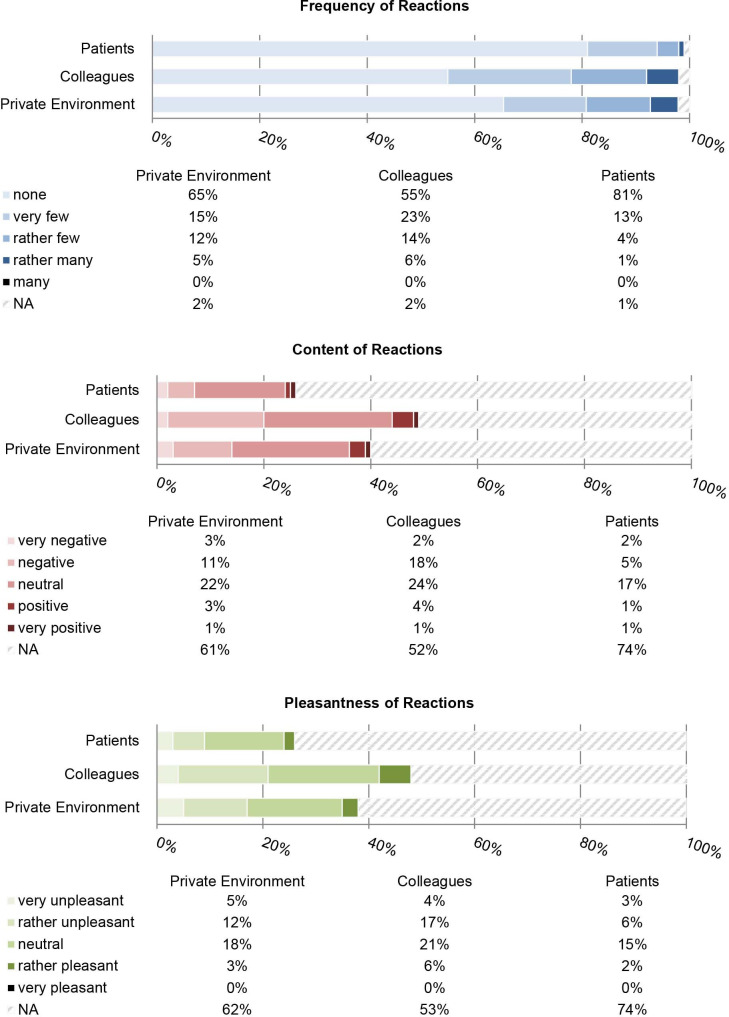
Of the 234 participants, 87 (37%) stated they had not looked at the database and 131 (56%) reported to have at least somewhat followed media coverage about the database. Most participants said they did not know whether their payments had been correctly reported: of 189 participants who agreed to disclose payments in 2015, 91 (48%) did not know; 70 (37%) said their payments had been correctly reported and 24 (13%) said they had been incorrectly reported. Of 192 participants who agreed to disclose payments in 2016, 105 (55%) did not know, 60 (31%) said their payments had been correctly reported and 23 (12%) said they had been incorrectly reported. Most participants stated they had not received any reactions from patients (190/234, 81%), colleagues (128/234, 55%) or the private environment (153/234, 65%). Response rates for items of content and pleasantness of reactions were between 26% (60/234, pleasantness of patients’ reactions) and 48% (113/234, content of colleagues’ reactions). See figure 2 for detailed results.
Figure 2.
Relative frequencies of item answers for frequency, content and pleasantness of reactions from recipients, n=234.
Descriptive norm
For investigating how high participants estimated the percentage of German physicians who disclosed in the database in 2015 and 2016, data were available from 216 and 218 participants and ranged between 0% and 100%. For 2015, participants estimated on average that 33% of German physicians had agreed to disclose (SD=21, Mdn=30, IQR=30) and for 2016, participants estimated on average that 31% of German physicians agreed to disclose (SD=20, Mdn=25, IQR=25).
Investigating non-disclosure
To answer research question 1, we investigated data of those participants who disclosed in 2015 (groups 1, 3; n=189) to predict whether they disclosed again in 2016 (group 3; n=147) or did not disclose again in 2016 (group 1; n=42). Neither regression model 1 with the three predictor variables X1: pleasantness of reactions, X2: descriptive norm and X3: attitude significantly improved the model fit compared with the null model (χ2=1.0, p=0.792) nor regression model 2, in which the interaction terms X3*X2 and X3*X1 were added (χ2=12.66, p=0.027). A more detailed description of regression models 1 and 2 can be seen in online supplemental file C.
bmjopen-2021-055963supp003.pdf (27.9KB, pdf)
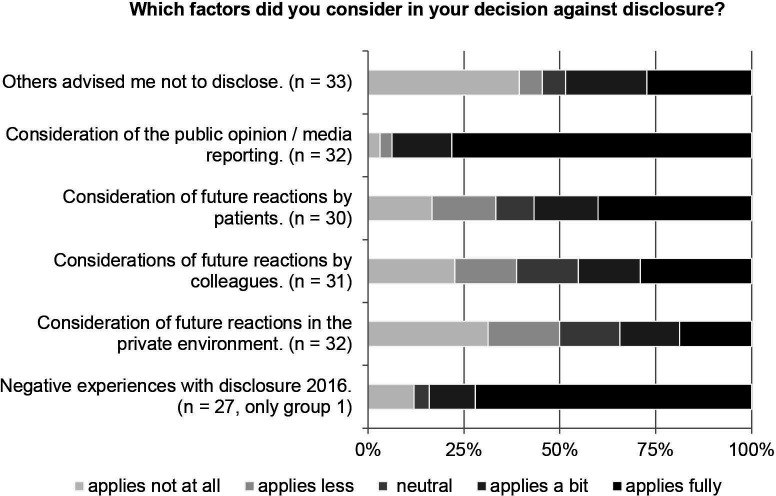
We additionally explored the reasons for participants’ non-disclosure in general. In our sample, two groups did not disclose payments in 1 year: participants of group 1 had an entry in 2015 but not in 2016 (n=42), and participants of group 2 had no entry in 2015 but in 2016 (n=45). We asked these participants for the reason for the missing entry (table 2). The most frequently chosen reason in group 1 was that they had consciously decided against disclosure (50%, vs 18% in group 2). The most frequently chosen answer in group 2 was that they were not asked for their consent to disclose (36%, vs 7% in group 1). We further asked how several statements applied to the participants in case they consciously decided against disclosure. Most participants reported that considerations of public opinion or media reporting led to the decision against disclosure (25/32, 78%) (figure 3).
Table 2.
Reasons for non-disclosure
| You don’t have an entry in the year 2015 (2016). Why? | Group 1 | Group 2 | ||
| Abs. frequency | (%) | Abs. frequency | (%) | |
| I have not received any payments | 14/42 | (33%) | 10/45 | (22%) |
| I was not asked for my consent to disclose | 3/42 | (7%) | 16/45 | (36%) |
| I forgot to answer the inquiry for disclosure consent | 1/42 | (2%) | 2/45 | (4%) |
| I consciously decided against disclosure | 21/42 | (50%) | 8/45 | (18%) |
| No reply | 3/42 | (7%) | 9/45 | (20%) |
Note. Participants were asked to choose one of the four options. Group 1=disclosure in 2015, but not in 2016; group 2=no disclosure in 2015, but in 2016.
Figure 3.
Factors considered for decision against disclosure.
Year of disclosure
To investigate research questions 2 and 3, we compared the frequency and content of reactions to participants who disclosed for the first time in 2015 (groups 1, 3) with data of participants who disclosed for the first time in 2016 (group 2). Data for frequency of reactions were available for 2015 from 187/189 (99%) and for 2016 from 44/45 (98%) participants; data for content of reactions were available for 2015 from 110/189 (58%) and for 2016 from 19/45 (42%) participants. All variables were significantly non-normal (all W=0.71–0.90, all p<0.01). Testing hypothesis 2, we found no statistically significant difference between frequency of reactions 2015 and 2016 (2015: M=1.54, SD=0.66, Mdn=1.33, IQR=1; and 2016: M=1.36, SD=0.53, Mdn=1.00, IQR=0.67), as evidenced by a Wilcoxon rank-sum test (W=3410, rb=−0.17, 95% CI −∞ to −0.01, p=0.031). Testing hypothesis 3, we found no statistically significant difference between negativity of reactions 2015 and 2016 (2015: M=2.69, SD=0.71, Mdn=3.00, IQR=1; and 2016: M=2.96, SD=0.67, Mdn=3.00, IQR=0.33), as indicated by a Wilcoxon rank-sum test (W=1243, rb=0.19; 95% CI −0.05 to ∞, p=0.085).
Further exploratory investigations
Participants were asked to indicate their agreement with statements about attitude towards disclosure in general and in research. The statements that participants agreed with most strongly were that disclosure of payments should be more nuanced, that the undifferentiated display of the disclosures brings science into disrepute and that disclosure leads to a wrong impression in the public (table 3).
Table 3.
Attitudes towards transparency
| N | Strongly disagree | Disagree | Neutral | Agree | Strongly agree | |
| Payments by pharmaceutical companies are a risk for the independence of clinical practice and research | 233 | 26/233 (11%) | 41/233 (18%) | 35/233 (15%) | 90/233 (39%) | 41/233 (18%) |
| In principle, I approve of transparency | 233 | 4/233 (2%) | 3/233 (1%) | 16/233 (7%) | 39/233 (17%) | 171/233 (73%) |
| Collaboration with pharmaceutical companies and receiving payments by those companies is part of the medical profession | 230 | 19/230 (8%) | 35/230 (15%) | 66/230 (28%) | 71/230 (31%) | 39/230 (17%) |
| Disclosure of payments should be more nuanced | 233 | 8/233 (3%) | 7/233 (3%) | 43/233 (18%) | 51/233 (22%) | 124/233 (53%) |
| Disclosure of payments increases patients' trust in me | 233 | 72/233 (31%) | 45/233 (19%) | 75/233 (32%) | 32/233 (14%) | 9/233 (4%) |
| Disclosure leads to a wrong impression in the public | 233 | 9/233 (4%) | 24/233 (10%) | 31/233 (13%) | 78/233 (33%) | 91/233 (39%) |
| In case you are working in research | ||||||
| Transparency guidelines impede my scientific work | 154 | 45/154 (29%) | 40/154 (26%) | 29/154 (19%) | 32/154 (21%) | 8/154 (5%) |
| I have been confronted with disclosures within the context of a published study at least once | 154 | 56/154 (36%) | 17/154 (11%) | 22/154 (14%) | 24/154 (16%) | 35/154 (23%) |
| My research results were criticised because of my disclosures at least once | 152 | 119/152 (78%) | 11/152 (7%) | 13/152 (9%) | 5/152 (3%) | 4/152 (3%) |
| The undifferentiated displaying of the disclosures brings science into disrepute | 155 | 10/155 (6%) | 5/155 (3%) | 16/155 (10%) | 37/155 (24%) | 87/155 (56%) |
Sixty-eight participants answered the question ‘Was there anything that bothered you about the reactions?’. The content categories with respective frequencies are:
Negative media reporting (20/68, 29%)
Defamation/criminalisation (17/68, 25%)
Unknown cases of undisclosed information (12/68, 18%)
Disclosed information is not put into context with services rendered in return (12/68, 18%)
Misleading data representation (7/68, 10%)
Contacted by lawyer who aimed a class action against CORRECTIV (7/68, 10%)
Feeling of being dragged into the public eye (5/68, 7%)
Feeling of being treated unfairly (5/68, 7%)
Involvement of employer (4/68, 6%)
Others expressed lack of understanding (2/68, 3%).
Discussion
Principal findings
The aim of this study was to gain insight into physicians’ attitudes towards and experiences with the voluntary German transparency regulation. Research question 1 aimed to investigate how these experiences affect future disclosure behaviour, but no significant prediction model was found. Research questions 2 and 3 aimed to investigate whether reactions to disclosures between the first and the second year of the database differed. No significant difference in the frequency or content reactions was found on the α level of 0.01, which might be related to the fact that most participants in our sample had not received any reactions towards their disclosure. The fewest reactions came from patients. Only every fifth physician stated they had received at least ‘very few’ reactions by patients.
We observed that the reasons for non-disclosure in our sample differed depending on the time point of non-disclosure: participants who had disclosed in the first but not in the second year more often said they had consciously decided against disclosure than those who had not disclosed in the first year but in the second year. The latter more often said that they had not been asked for consent by the respective pharmaceutical company. Most physicians who had consciously decided against disclosure said it was because of public opinion and media reporting. We also found that nearly half of the participating physicians had not looked at the database and did not know whether their disclosed payment sum was correct. However, more than half of them at least somewhat followed the media coverage about the database and some reported high objections to public exposure. This can be interpreted according to the spotlight effect which describes that people overestimate the attention they receive by others.16 Several participants stated concerns about the public opinion and a feeling about being denunciated, which is in line with the observation that physicians are concerned that COI disclosure may damage their reputation.17 This tendency relates to the psychological heuristic that people do not like to be viewed as biased. Studies show that if people are able to avoid COI, they may be motivated to avoid such conflicts so that they can disclose the absence of conflicts.34 In case of voluntary disclosure, however, people can simply avoid being viewed as biased by deciding against disclosure.
Strengths and weaknesses
The strength of this study is that it provides quantitative and qualitative data on physicians’ experiences with COI disclosure in a national database. To our knowledge, no such evidence exists for any European transparency regulation in medicine. The investigated sample was stratified to their disclosing behaviour. Due to the otherwise random selection of participants, our sample comprises a great bandwidth of age, disciplines and workplaces. The study, however, also has several limitations. A common problem in survey methods, answers may be skewed by social desirability.35 The answers to a controversially discussed subject may be even more skewed: physicians may be more motivated to respond to the survey if they have strong opinions on transparency, or if they experienced extreme reactions towards their disclosure. We tried to counter this by our efforts to increase the response rate. Additionally, the questionnaire we used was only constructed for this study, so our data cannot be directly compared with other data.
Meaning of the study
Physicians in our sample reported to be concerned about reputational damage and public exposure. Those who did not disclose payments had various reasons. Mandatory transparency could approach these issues: first, if disclosure is mandatory, it will no longer feel ‘unfair’ that some disclose information and some hide this information. Second, if conducted in a standardised form, everyone’s information is available, and therefore the disclosed information is easier to compare and better to interpret, which will lessen the risk of unfair reputational damage and might enable a fair discussion between pharmaceutical companies, physicians, researchers and the public.
Currently, the consent rate to disclose payments by pharmaceutical companies in Germany is low, compared with other countries.12 13 In our study we observed that even if physicians consented to disclosure, our participants mainly appear not to have used the database nor checked their entries. Therefore, we propose that disclosers need to be educated about the background of transparency regulations and the concept of COI to raise commitment.
For the management of financial COI in medicine, transparency is by now seen as a necessary, but not sufficient, measure.7 10 36 Managing the influence of COI involves further higher action, for example, people with relevant COI being excluded from guideline development groups.1 36 Voluntary transparency regulations do not serve this aim, but may paint a distorted picture of the actual situation. The voluntary database investigated in this study is a good example: only 24% of HCPs decided to disclose information about pharmaceutical payments in 2016,13 which means that the publicly visible amount of payments and number of HCPs who receive payments very probably greatly underestimates the actual amount of payments and the actual number of HCPs. Voluntary transparency regulations may fuel discussion and raise awareness for the interaction of pharmaceutical companies with HCPs, however this may backfire if information is not contextualised, and the regulation is not driven forward.
Unanswered questions and future research
In this sample, reasons for non-disclosure were heterogeneous. More research is needed about the motives for and against voluntary disclosure to improve current transparency policies. Our data show that there are more issues that need to be considered about the experiences with transparency guidelines, such as the fear of reputational damage. Broad evaluations of transparency guidelines including all involved persons are needed to get a full picture of the current situation.
Conclusion
The study at hand was the first survey of physicians who disclosed voluntarily in a nationwide transparency database. We found no significant predictors for future disclosure behaviour and no statistically significant difference in the reactions to disclosures between the first year and the second year of the database. The exploratory results of this study show preliminary evidence that although German HCPs experienced only few reactions by patients, colleagues or in private, they are concerned that disclosing payments in a public database will result in reputational damage. Considering public opinion and media exposure was the most frequent reason for non-disclosure in this subsample. We propose that mandatory disclosure could be a solution to this problem by creating a standardised environment for an open discussion.
Supplementary Material
Acknowledgments
The authors thank CORRECTIV for providing the database. The authors also thank Jasmin Peifer and Marc Himmelmann for their help with the database and the paper questionnaire, and Alexander Mancini for discussing the free answers.
Footnotes
Twitter: @starlene_moll
Contributors: MS, CK, KL and BE were responsible for the study conception and design. MS, KL and BE were responsible for the title, abstract and full-text review. MS and LH were responsible for data extraction and validation. MS, CK and BE analysed and interpreted results. MS drafted the manuscript. All authors provided a critical review and approved the final manuscript. MS is the guarantor.
Funding: Funded by Volkswagen Foundation (grant no. A118085 (ref.91498) to KL).
Competing interests: CK, MS and LH declared that they had received salary from the Volkswagen Foundation to conduct the project. KL declared that he had received a research grant from the Volkswagen Foundation to conduct the project. BE declared that he has no conflict of interest.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants but the ethics committee of the State Chamber of Physicians of Rhineland-Palatinate decided that further consultation is not necessary since no personal but anonymous data were processed (appl. no. 2018-13295-Epidemiologie).
References
- 1.Institute of Medicine Committee on Conflict of Interest in Medical Research . The National academies collection: reports funded by National Institutes of Health. In: Lo B, Field B, eds. Conflict of interest in medical research, education, and practice. Washington, DC: National Academies Press, 2009. [PubMed] [Google Scholar]
- 2.Chimonas S, Mamoor M, Zimbalist SA, et al. Mapping conflict of interests: scoping review. BMJ 2021;375:e066576. 10.1136/bmj-2021-066576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lundh A, Lexchin J, Mintzes B, et al. Industry sponsorship and research outcome: systematic review with meta-analysis. Intensive Care Med 2018;44:1603–12. 10.1007/s00134-018-5293-7 [DOI] [PubMed] [Google Scholar]
- 4.Mitchell AP, Trivedi NU, Gennarelli RL, et al. Are financial payments from the pharmaceutical industry associated with physician prescribing?: a systematic review. Ann Intern Med 2021;174:353–61. 10.7326/M20-5665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nejstgaard CH, Bero L, Hróbjartsson A, et al. Association between conflicts of interest and favourable recommendations in clinical guidelines, advisory committee reports, opinion pieces, and narrative reviews: systematic review. BMJ 2020;371:m4234. 10.1136/bmj.m4234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson D. Understanding financial conflicts of interest. N Engl J Med 1993. [DOI] [PubMed] [Google Scholar]
- 7.Fabbri A, Santos Ala, Mezinska S, et al. Sunshine policies and murky shadows in Europe: disclosure of pharmaceutical industry payments to health professionals in nine European countries. Int J Health Policy Manag 2018;7:504–9. 10.15171/ijhpm.2018.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grundy Q, Habibi R, Shnier A, et al. Decoding disclosure: comparing conflict of interest policy among the United States, France, and Australia. Health Policy 2018;122:509–18. 10.1016/j.healthpol.2018.03.015 [DOI] [PubMed] [Google Scholar]
- 9.Europe M-MH . Shedding light on transparent cooperation in healthcare. The way forward for sunshine and transparency laws across Europe, 2020. Available: https://mhe-sme.org/wp-content/uploads/2019/01/MHE-SHEDDING-LIGHT-REPORT-Final.pdf
- 10.Bradley SH, DeVito NJ, Lloyd KE, et al. Reducing bias and improving transparency in medical research: a critical overview of the problems, progress and suggested next steps. J R Soc Med 2020;113:433–43. 10.1177/0141076820956799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Open payments [online]. Available: https://www.cms.gov/openpayments [Accessed 19 Nov 2021].
- 12.Mulinari S, Martinon L, Jachiet P-A, et al. Pharmaceutical industry self-regulation and non-transparency: country and company level analysis of payments to healthcare professionals in seven European countries. Health Policy 2021;125:915–22. 10.1016/j.healthpol.2021.04.015 [DOI] [PubMed] [Google Scholar]
- 13.Stoll M, Hubenschmid L, Koch C, et al. Voluntary disclosures of payments from pharmaceutical companies to healthcare professionals in Germany: a descriptive study of disclosures in 2015 and 2016. BMJ Open 2020;10:e037395. 10.1136/bmjopen-2020-037395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loewenstein G, Sah S, Cain DM. The unintended consequences of conflict of interest disclosure. JAMA 2012;307:669–70. 10.1001/jama.2012.154 [DOI] [PubMed] [Google Scholar]
- 15.Sah S, Loewenstein G, Cain D. Insinuation anxiety: concern that advice rejection will signal distrust after conflict of interest disclosures. Pers Soc Psychol Bull 2019;45:1099–112. 10.1177/0146167218805991 [DOI] [PubMed] [Google Scholar]
- 16.Loewenstein G, Sunstein CR, Golman R. Disclosure: psychology changes everything. Annu Rev Econom 2014;6:391–419. 10.1146/annurev-economics-080213-041341 [DOI] [Google Scholar]
- 17.Chimonas S, DeVito NJ, Rothman DJ. Bringing transparency to medicine: exploring physicians' views and experiences of the sunshine act. Am J Bioeth 2017;17:4–18. 10.1080/15265161.2017.1313334 [DOI] [PubMed] [Google Scholar]
- 18.Boytchev WB. Warum Ärzte schweigen, 2016. Available: https://correctiv.org/aktuelles/euros-fuer-aerzte/2016/07/17/warum-aerzte-schweigen [Accessed 19 Nov 2021].
- 19.Licurse A, Barber E, Joffe S, et al. The impact of disclosing financial ties in research and clinical care: a systematic review. Arch Intern Med 2010;170:675–82. 10.1001/archinternmed.2010.39 [DOI] [PubMed] [Google Scholar]
- 20.Riedl EM, König J, Koch C, et al. Einstellungen und Erwartungen von Patienten in Bezug auf Interessenkonflikte ihrer behandelnden Ärzte. Z Evid Fortbild Qual Gesundheitswes 2016;110-111:45–53. 10.1016/j.zefq.2015.12.002 [DOI] [PubMed] [Google Scholar]
- 21.Pham-Kanter G, Mello MM, Lehmann LS, et al. Public awareness of and contact with physicians who receive industry payments: a national survey. J Gen Intern Med 2017;32:767–74. 10.1007/s11606-017-4012-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young PD, Xie D, Schmidt H. Towards patient-centered conflicts of interest policy. Int J Health Policy Manag 2017;7:112–9. 10.15171/ijhpm.2017.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung A, Rimal RN. Social norms: a review. Rev Commun Res 2016;4:1–28. 10.12840/issn.2255-4165.2016.04.01.008 [DOI] [Google Scholar]
- 24.Lapinski MK, Rimal RN. An explication of social norms. Commun Theory 2005;15:127–47. 10.1111/j.1468-2885.2005.tb00329.x [DOI] [Google Scholar]
- 25.Freiwillig Selbstkontrolle für die Arzneimittelindustrie e.V. FSA-transparenzkodex, 2019. Available: https://www.fsa-pharma.de/de/kodizes/sk_fsa_transparenzkodex_13.03.2019.pdf [Accessed 19 Nov 2021].
- 26.Richter F. “Euros für Ärzte“-Datenbank beendet, 2021. Available: https://correctiv.org/aktuelles/2021/01/14/euros-fuer-aerzte-datenbank-beendet/ [Accessed 19 Nov 2021].
- 27.CORRECTIV . Euros für Ärzte, 2021. Available: https://correctiv.org/aktuelles/euros-fuer-aerzte [Accessed 19 Nov 2021].
- 28.Elmer C, Stotz P. Warum Ärzte schweigen, 2016. Available: https://www.spiegel.de/gesundheit/diagnose/euros-fuer-aerzte-datenbank-wie-viel-hat-mein-arzt-bekommen-a-1102819.html [Accessed 19 Nov 2021].
- 29.Stoll M. Unintended consequences of conflict of Interest disclosure: a psychological perspective [online]. Johannes Gutenberg-Universität Mainz 2021. https://openscience.ub.uni-mainz.de/handle/20.500.12030/5731 [Google Scholar]
- 30.Green SB. How many subjects does it take to do a regression analysis. Multivariate Behav Res 1991;26:499–510. 10.1207/s15327906mbr2603_7 [DOI] [PubMed] [Google Scholar]
- 31.Mayring P. Qualitative content analysis: theoretical foundation, basic procedures and software solutions [online]. Social Science Open Access Repository 2014. https://nbn-resolving.org/urn:nbn:de:0168-ssoar-395173 [Google Scholar]
- 32.JASP Team . JASP (Version 0.10.2), 2019. [Google Scholar]
- 33.R Core Team . R: a language and environment for statistical computing, 2019. Available: https://www.R-project.org
- 34.Sah S, Loewenstein G. Nothing to declare: mandatory and voluntary disclosure leads advisors to avoid conflicts of interest. Psychol Sci 2014;25:575–84. 10.1177/0956797613511824 [DOI] [PubMed] [Google Scholar]
- 35.Barclay S, Todd C, Finlay I, et al. Not another questionnaire! maximizing the response rate, predicting non-response and assessing non-response bias in postal questionnaire studies of GPs. Fam Pract 2002;19:105–11. 10.1093/fampra/19.1.105 [DOI] [PubMed] [Google Scholar]
- 36.Lexchin J, Fugh-Berman A. A ray of sunshine: transparency in physician-industry relationships is not enough. J Gen Intern Med 2021;36:3194–8. 10.1007/s11606-021-06657-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-055963supp001.pdf (55.4KB, pdf)
bmjopen-2021-055963supp002.pdf (15.1KB, pdf)
bmjopen-2021-055963supp003.pdf (27.9KB, pdf)
Data Availability Statement
Data are available upon reasonable request.