Abstract
This study aimed to explore the moderating effects of the frequently used cognitive reserve (CR) proxies [i.e., education, premorbid intelligence quotient (pIQ), occupational complexity (OC), and lifetime cognitive activity (LCA)] on the relationships between various in vivo Alzheimer’s disease (AD) pathologies and cognition. In total, 351 [268 cognitively unimpaired (CU), 83 cognitive impaired (CI)] older adults underwent multi-modal brain imaging to measure AD pathologies and cognitive assessments, and information on CR proxies was obtained. For overall participants, only education moderated the relationship between Aβ deposition and cognition. Education, pIQ, and LCA, but not OC, showed moderating effect on the relationship between AD-signature cerebral hypometabolism and cognition. In contrast, only OC had a moderating effect on the relationship between cortical atrophy of the AD-signature regions and cognition. Such moderation effects of the CR proxies were similarly observed in CI individuals, but most of them were not in CU individuals. The findings suggest that the proposed CR proxies have different moderating effects on the relationships between specific AD pathologies and cognition.
Keywords: Education, Intelligence, Occupation, Cognitive activity, Cognitive reserve, Alzheimer pathology
1. Introduction
The concept of cognitive reserve (CR) has been introduced to explain how some people can tolerate more brain pathologies or age-related brain changes than others (Stern, 2002). It is defined as the capacity to preserve cognitive function better in the presence of brain pathology and it acts as a moderator between brain changes and the degree of cognitive impairment (Stern, 2012, 2002; Stern et al., 2018). Various measures have been proposed as proxies of CR, including education, the premorbid intelligence quotient (pIQ), occupational complexity (OC), and lifetime cognitive activity (LCA), and have been used to estimate the CR of an individual indirectly (Arenaza-Urquijo et al., 2015; Jones et al., 2011).
Among CR proxies, education was reported to moderate the relationships of Aβ senile plaques (Bennett et al., 2005, 2003), cerebrospinal fluid (CSF) amyloid-beta Aβ) (Yaffe et al., 2011), cerebral Aβ-positive status on positron emission tomography (PET) (Roe et al., 2008), or medial temporal atrophy on Magnetic Resonance Imaging (MRI) (Perneczky et al., 2009) with cognitive function, but not that of neurofibrillary tangles (Bennett et al., 2005, 2003) and Alzheimer’s disease (AD) pathology composite (Bauer et al., 2020). 2 studies showed that pIQ, estimated using an adult reading test, interacted with inferior temporal tau deposition (Rentz et al., 2017) and precuneus Aβ deposition on PET (Rentz et al., 2010) to predict cognitive function, while another study suggested that pIQ does not moderate the relationship of various AD biomarkers with cognition, including CSF Aβ, t-tau, and structural abnormalities on MRI (Vemuri et al., 2011). A study showed that composite measure of education and occupation was associated with baseline cognitive performance but did not moderate Aβ-related cognitive decline in cognitively normal older adults (Vemuri et al., 2015). However, no study has yet investigated the independent moderating effects of OC and LCA on the relationship between AD pathologies and cognition.
Therefore, we explored the moderating effects of education, pIQ, OC, and LCA on the relationships between various in vivo AD pathologies and global cognition to compare their appropriateness as CR proxies for specific pathologies. We used 3 AD neuroimaging biomarkers to measure different in vivo AD pathologies: cerebral Aβ deposition on 11C-Pittsburg compound B (PiB)-PET, AD-signature region cerebral glucose metabolism (AD-CM) on 18F-fluoro-2-deoxyglucose (FDG)-PET, and AD-signature region cortical thickness (AD-CT) on structural brain MRI (Jack et al., 2018, 2016).
2. Methods
2.1. Participants
This study is part of the Korean Brain Aging Study for the Early Diagnosis and Prediction of AD (KBASE), an ongoing prospective cohort study designed to identify novel AD biomarkers and to investigate various lifetime experiences contributing to AD-related brain changes. Details on the KBASE study have been described previously (Byun et al., 2017). The current study included 351 middle-aged and older individuals with age between 55 and 90 years. The participants consisted of 232 cognitively unimpaired (CU) with no Aβ deposition on PiB-PET (CU−), 36 CU with Aβ deposition (CU+), 44 mild cognitive impairment (MCI) with Aβ deposition (MCI+), 39 AD dementia with Aβ deposition (ADD+). CU individuals had no diagnosis of mild cognitive impairment or dementia, and a global Clinical Dementia Rating (CDR) score of 0 (Lee et al., 2002; Morris, 1993). All individuals with MCI met the current consensus criteria for amnestic MCI, which are as follows: (1) memory complaints confirmed by an informant, (2) objective memory impairments, (3) preserved global cognitive function, (4) independence in functional activities, and (5) no dementia. Regarding criterion 2, the age-, education-, and sex adjusted z-scores for at least 1 of 4 episodic memory tests was <−1.5. The 4 memory tests were the Word List Memory, Word List Recall, Word List Recognition, and Constructional Recall tests, which are included in the Korean version of the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) neuropsychological assessment battery (Lee et al., 2004, 2002). All individuals with MCI had a CDR score of 0.5. All individuals with AD dementia meet both the Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV) criteria (American Psychiatric Association, 2000) for dementia and National Institute on Aging and Alzheimer’s Association (NIA-AA) criteria (McKhann et al., 2011) for probable AD dementia, and had a global CDR score of 0.5 or 1. Individuals with medical, psychiatric, or neurological conditions or a history of conditions that may affect brain structures or functions, such as stroke, head trauma, depression, hydrocephalus, or focal brain lesions on MRI were excluded. Each participant was diagnosed at a consensus conference of board-certified psychiatrists and neuropsychologists before inclusion in the study. The study protocol was approved by the Institutional Review Board of Seoul National University Hospital and Seoul National University-Seoul Metropolitan Government Boramae Center (Seoul, South Korea) and conducted in accordance with the recommendations of the current version of the Declaration of Helsinki. All participants or their caregivers gave informed consent to participate in this study.
2.2. Clinical and neuropsychological assessments
All participant underwent comprehensive clinical and neuropsychological assessments following the KBASE assessment protocol (Byun et al., 2017), which incorporated the Korean version of the CERAD assessment packet (Lee et al., 2004, 2002). CERAD-total score (CERAD-TS) (Chandler et al., 2005; Seo et al., 2010), which is calculated by summing the scores of 6 tests included in the CERAD neuropsychological battery (i.e., Semantic Verbal Fluency Tasks, 15-item Boston Naming Test, Word List Memory, Word List Recall, Word List Recognition, and Constructional Praxis), was used as an established composite measure of global cognition. CERAD-TS has a maximum of 100 points.
2.3. CR proxies
The years of formal education, pIQ, OC, and LCA participation frequency were included as candidate CR proxies. Years of formal education was self-reported by the participants and confirmed by reliable informants. Formal education was defined as “education which takes place in education and training institutions, leading to recognized diplomas or qualifications” (Commission of the European Communities, 2000). pIQ was estimated by the performance on the Korean Adult Reading Test (Yi et al., 2017), a word-reading test for Korean-speaking adults using 50 irregularly pronounced words that is useful for estimating premorbid intelligence. The Korean Adult Reading Test-predicted Full-Scale IQ, derived from the Korean Adult Reading Test error score, was used as the pIQ of an individual. Information about occupation was self-reported by the participant and confirmed by reliable informants. Only the longest-held occupation was considered. We then classified OC into 4 levels based on the skill levels described in the International Standard Classification of Occupations (ISCO-08) (Darwish et al., 2018; International Labor Office (ILO), 2012). Occupations at skill level 1 typically involve the simple and routine physical or manual tasks (e.g., office cleaners, freight handlers or garden laborers). Occupations at skill level 2 typically involve the performance of tasks, such as operating machinery and electronic equipment; driving vehicles; maintenance and repair of electrical and mechanical equipment; and manipulation, ordering and storage of information (e.g., butchers, bus drivers or secretaries). Occupations classified at skill level 3 typically involve the performance of complex technical and practical tasks that require complex problem solving, reasoning, and decision making in a specialized field (e.g., shop managers, medical laboratory technicians or diagnostic medical radiographers). Occupations classified at skill level 4 typically involve the performance of tasks that require complex problem-solving, decision-making and creativity based on an extensive body of theoretical and factual knowledge in a specialized field (e.g., sales and marketing managers, civil engineers or secondary school teachers). The LCA participation frequency was measured with a 39-item structured questionnaire (Wilson et al., 2007). The included items were relatively common activities with few barriers to participation, such as reading newspapers, magazines, or books; visiting a museum or library; attending a concert, play, or musical; writing letters; and playing games. The frequency of participation was rated from 1 (once a year or less) to 5 (approximately daily). There were nine items for current, 11 items for childhood (6–12 years of age), 10 items for young adulthood (18 years of age), and nine items for midlife (40 years of age) cognitive activities. Item scores for childhood, young adulthood, and midlife periods were averaged to yield a separate value for the corresponding age period. We then calculated the composite LCA score to use in the subsequent analysis, which was an average of the 3 epoch values. The items for current activities were not included to calculate LCA score to avoid the influence of current cognitive impairment state on LCA score.
2.4. Measurement of cerebral Aβ deposition
All participants underwent simultaneous 3-dimensional [11C]-PiB-PET and T1-weighted MRI using a 3.0 T Biograph mMR scanner (Siemens; Washington DC, WC, USA), according to the manufacturer’s guidelines. After intravenous administration of 555 MBq of [11C] PiB (range, 450-610 MBq), a 30-minute emission scan was obtained 40 minutes after injection. The PiB-PET data collected in list mode were processed for routine corrections such as uniformity, UTE-based attenuation, and decay corrections, and were reconstructed into a 256 × 256 image matrix using iterative methods (6 iterations with 21 subsets). The details of PiB-PET acquisition and preprocessing were described previously (Park et al., 2019). An automatic anatomic labeling algorithm and a region-combining method (Reiman et al., 2009) were applied to determine the regions of interest (ROIs) for characterizing the PiB retention levels in the frontal, lateral parietal, posterior cingulate–precuneus, and lateral temporal regions. The standardized uptake value ratio (SUVR) value for each ROI was calculated by dividing the mean value for all voxels within each ROI by the mean cerebellar grey matter uptake value for the same image. A global cortical ROI consisting of the 4 ROIs was also defined and a global Aβ retention value was generated by dividing the mean value for all voxels of the global cortical ROI by the mean cerebellar uptake value in the same image (Choe et al., 2014; Reiman et al., 2009). Each participant was classified as Aβ-positive if the SUVR value was > 1.4 in at least 1 of 4 ROIs or as Aβ-negative if the SUVR values of all 4 ROIs was ≤ 1.4 (Jack et al., 2014; Reiman et al., 2009).
2.5. Measurement of AD-CM
All participants underwent [18F]-FDG-PET imaging using the machine described above. The participants fasted for at least 6 hours and rested in a waiting room for 40 minutes prior to the scans after intravenous administration of 0.1 mCi/Kg of [18F] FDG radioligands. The PET data collected in list mode (5 minutes x 4 frames) were processed for routine corrections such as uniformity, UTE-based attenuation, and decay corrections. The details of FDG-PET acquisition and preprocessing were described previously (Park et al., 2019). AD-signature FDG ROIs, such as the angular gyri, posterior cingulate cortex, and inferior temporal gyri, which are sensitive to the changes associated with AD (Jack et al., 2014), were determined. AD-CM was defined as the voxel-weighted mean SUVR extracted from the AD-signature FDG ROIs.
2.6. Measurement of AD-CT
All T1-weighted MR images were acquired in the sagittal orientation using the same PET-MR machine. The parameters were as follows: repetition time = 1670 ms, echo time = 1.89 ms, field of view 250 mm, and 256 × 256 matrix with 1.0 mm slice thickness. MRI acquisition and preprocessing were described previously (Park et al., 2019). AD-CT was defined as the mean cortical thickness obtained from AD-signature regions, including the inferior frontal sulcus, superior frontal gyrus, temporal pole, inferior temporal gyrus, medial temporal cortex, precuneus, superior parietal lobule, angular gyrus, and supramarginal gyrus, based on Desikan-Killany atlas (Desikan et al., 2006; Dickerson et al., 2009).
2.7. Statistical analysis
To analyze the associations of CR proxies each other, Kendall’s tau correlation was conducted due to non-normal distribution of CR proxies. To analyze the moderating effects of the CR proxies on the relationships between in vivo AD pathologies and cognition, we performed multiple linear regression models with CERAD-TS as the dependent variable, each CR proxy, each AD neuroimaging biomarker, and CR proxy × AD neuroimaging biomarker interaction term as independent variables controlling for age, sex, and apolipoprotein ε4 (APOE4) carrier status. Additonal subgroup analyses were conducted for each of CU and cognitively impaired (CI: MCI plus AD dementia) group using the same models, considering that the effect of CR measured by proxy variable may differently observed by cognitive status (Pettigrew and Soldan, 2019; Soldan et al., 2017; Stern, 2012). All analyses were done with SPSS 23.0 (IBM Corporation, New York) and results were examined at p < 0.05.
3. Results
3.1. Participant characteristics
The characteristics of the participants are presented in Table 1. CU− subsamples were younger than CU+ and MCI+ subsamples, as well as less depressed compared to MCI+ or ADD+ subsamples.
Table 1.
Participant characteristics
| Allparticipants (n = 351) | CU−(n = 232) | CU+(n = 36) | MCI+(n = 44) | ADD+(n = 39) | p a | |
|---|---|---|---|---|---|---|
| Age, y | 69.60 (7.84) | 68.09 (7.88) | 74.00 (6.77) | 72.39 (6.55) | 71.38 (7.33) | <0.001 |
| Female (%) | 195 (55.56) | 124 (53.45) | 16 (44.44) | 31 (70.45) | 24 (61.54) | 0.081 |
| APOE ε4 carriersb (%) | 105 (29.91) | 37 (15.95) | 11 (30.56) | 29 (65.90) | 28 (71.79) | <0.001 |
| GDS, score | 5.53 (5.14) | 4.60 (4.96) | 6.53 (5.31) | 8.00 (5.32) | 7.33 (4.37) | <0.001 |
| CR proxies | ||||||
| Education, years | 11.36 (4.75) | 11.60 (4.79) | 12.17 (4.42) | 10.52 (4.46) | 10.13 (4.90) | 0.13 |
| pIQ, score | 114.59 (11.05) | 115.87 (9.87) | 117.13 (9.04) | 112.01 (11.44) | 107.54 (15.32) | <0.001 |
| OC, score | 2.35 (1.39) | 2.44 (1.35) | 2.61 (1.36) | 1.86 (1.52) | 2.15 (1.41) | 0.040c |
| LCA, score | 2.27 (0.69) | 2.33 (0.70) | 2.26 (0.57) | 2.08 (0.66) | 2.13 (0.70) | 0.092 |
| Cognitive measures | ||||||
| CERAD-TS, score in vivo AD biomarkers | 66.62 (15.50) | 73.20 (9.94) | 72.03 (9.51) | 50.50 (10.30) | 40.64 (11.13) | <0.001 |
| Global Aβ, SUVR | 1.38 (0.45) | 1.11 (0.06) | 1.67 (0.35) | 1.96 (0.32) | 2.07 (0.41) | <0.001 |
| AD-CM, SUVR | 1.38 (0.14) | 1.42 (0.12) | 1.39 (0.12) | 1.27 (0.15) | 1.20 (0.13) | <0.001 |
| AD-CT, mm | 2.50 (0.20) | 2.58 (0.14) | 2.49 (0.15) | 2.36 (0.20) | 2.22 (0.19) | <0.001 |
Key: Aβ, amyloid-beta; CU−, Aβ-negative cognitively unimpaired; CU+, Aβ-positive cognitively unimpaired; MCI+, Aβ-positive mild cognitive impairment; ADD+, Aβ-positive Alzheimer’s disease dementia; APOE ε4, apolipoprotein E ε4; GDS, Geriatric Depression Scale; CR, cognitive reserve; pIQ premorbid intelligence quotient; OC, occupational complexity; LCA, lifetime cognitive activity; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; CERAD-TS, Total Score of CERAD neuropsychological assessment battery; SUVR, standardized uptake value ratio; AD-CM, AD-signature region cerebral glucose metabolism; AD-CT, AD-signature region cortical thickness.
Note: Data are presented as mean (SD) unless otherwise indicated.
Estimated by 1-way analysis of variance (continuous variable) and chi-square test (categorical variable).
APOE ε4 carriers are the percentage of individuals with at least one APOE4 allele.
Post-hoc test (Bonferroni) of 1-way analysis of variance did not show any significant group differences.
For the group difference of CR proxies by clinical diagnosis and biomarker classification, AD subsamples showed lower pIQ compared to CU− and CU+. Other proxy measures did not show any group differences. Group differences were also observed in CERAD-TS, APOE ε4 and in vivo AD biomarkers by clinical diagnosis and biomarker classification as expected.
3.2. Correlations between CR proxies
All CR proxies showed significant positive correlations with each other (Table S1). Correlation between education and pIQ was the strongest (Kendall’s tau = 0.67, p < .01) and correlation between OC and LCA was the weakest (Kendall’s tau = 0.26, p < .01).
3.3. Moderating effects of CR proxies on the relationship between Aβ deposition and cognition
Of the four CR proxies, only education had a significant moderating effect on the relationship between global Aβ deposition and CERAD-TS (Table 2). Individuals with higher education showed shallower slope of decline in CERAD-TS when Aβ deposition increased compared to those with lower education. The result remained unchanged when we re-examined the model using the education as groups stratified into tertiles [≥ 13 years, 10 ~12 years, and < 10 years] instead of a continuous value, for illustrative purpose (Fig. 1A).
Table 2.
Moderating effect of each CR proxy on the relationship between global Aβ and cognition
| Model term | B | SE | β | p |
|---|---|---|---|---|
| Model 1 | ||||
| Education | 1.14 | 0.13 | 0.35 | <.001b |
| Global Aβ | −17.94 | 1.44 | −0.52 | <.001b |
| Education × global Aβ | 0.80 | 0.28 | 0.11 | .004a |
| Model 2 | ||||
| pIQ | 0.55 | 0.06 | 0.39 | <.001b |
| Global Aβ | −16.14 | 1.41 | −0.46 | <.001b |
| pIQ × global Aβ | 0.07 | 0.10 | 0.03 | .45 |
| Model 3 | ||||
| OC | 2.12 | 0.49 | 0.19 | <.001b |
| Global Aβ | −17.55 | 1.55 | −0.50 | <.001b |
| OC × global Aβ | 1.83 | 0.97 | 0.07 | .061 |
| Model 4 | ||||
| LCA | 5.85 | 0.89 | 0.26 | <.001b |
| Global Aβ | −17.01 | 1.52 | −0.49 | <.001b |
| LCA × global Aβ | 2.38 | 2.01 | 0.05 | .24 |
Note: Age, sex, and APOE4 carrier status were treated as covariates. B: unstandardized coefficient, β: standardized coefficient.
Key: CR, cognitive reserve; Aβ, amyloid-beta; pIQ premorbid Intelligence Quotient; OC, occupational complexity; LCA, lifetime cognitive activity; SE, standard error; APOE4, apolipoprotein E ε4.
p < 0.01.
p < 0.001.
Fig. 1.
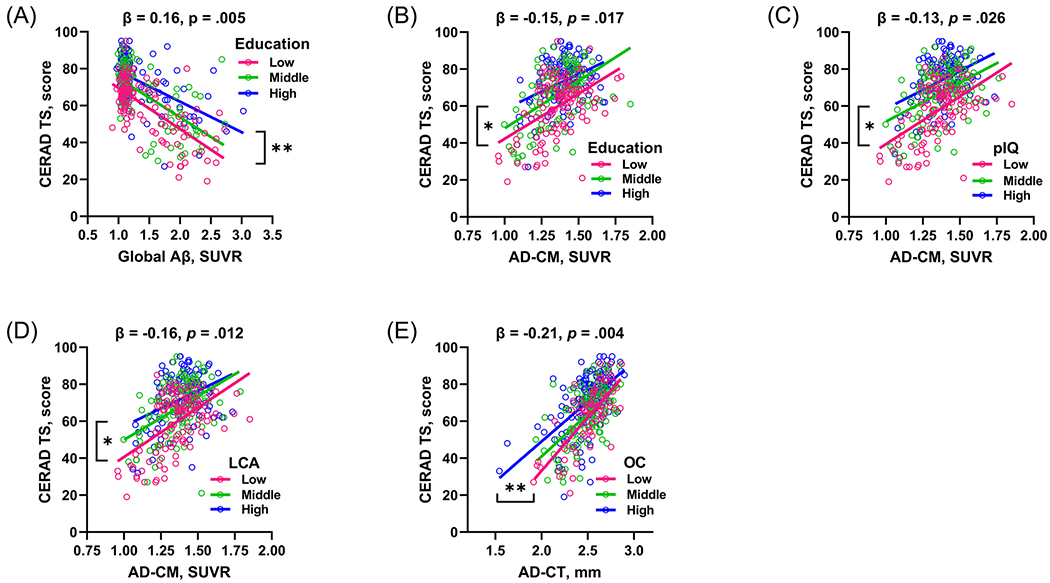
Visual representations showing the relationships between CR proxies, AD pathologies, and cognition. (A) Moderation of education for the relationship between Aβ deposition and CERAD TS. Moderation of (B) education, (C) pIQ, and (D) LCA for the relationship between AD-CM and CERAD-TS. (E) Moderation of OC for the relationship between AD-CT and CERAD-TS. Each of education, pIQ, and LCA was stratified into tertiles: Educational level was stratified into high (≥ 13 years), middle (10~12 years), and low (≤ 9) levels; pIQ was divided into high (> 120), middle (115~120), and low (≤ 114) levels; and LCA was stratified into high (> 2.6), middle (2.0~2.6), and low (≤ 1.9) levels. OC was stratified into high (3~4), middle (2), low (0~1) levels. Age, sex, and APOE ε4 carrier status are controlled as covariates. *p < 0.05; **p < 0.05; ***p < 0.001. Key: CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; CERAD TS, Total Score of CERAD neuropsychological assessment battery; Aβ, amyloid-beta; AD-CM, AD-signature region glucose metabolism; AD-CT, AD-signature region cortical thickness; pIQ, premorbid Intelligence Quotient; LCA, lifetime cognitive activity; OC, occupational complexity; APOE ε4, apolipoprotein E ε4.
3.4. Moderating effects of CR proxies on the relationship between AD-CM and cognition
For the association between AD-CM and CERAD-TS, education, pIQ, and the LCA score showed significant moderating effects, except for the OC (Table 3). For the 3 significant proxies, the higher CR group showed shallower slope of decline of CERAD-TS associated with AD-CM compared with the lower CR group (Fig. 1B–1D).
Table 3.
Moderating effect of each CR proxy on the relationship between AD-CM and cognition
| Model term | B | SE | β | p |
|---|---|---|---|---|
| Model 1 | ||||
| Education | 1.04 | 0.14 | 0.32 | <.001b |
| AD-CM | 38.84 | 4.37 | 0.36 | <.001b |
| Education × AD-CM | −2.23 | 1.00 | −0.09 | .026a |
| Model 2 | ||||
| pIQ | 0.53 | 0.06 | 0.38 | <.001b |
| AD-CM | 34.24 | 4.20 | 0.32 | <.001b |
| pIQ × AD-CM | −0.91 | 0.38 | −0.10 | .016a |
| Model 3 | ||||
| OC | 1.85 | 0.52 | 0.17 | <.001b |
| AD-CM | 38.75 | 4.66 | 0.36 | <.001b |
| OC × AD-CM | −2.77 | 3.25 | −0.04 | .40 |
| Model 4 | ||||
| LCA | 5.52 | 0.94 | 0.25 | <.001b |
| AD-CM | 36.92 | 4.48 | 0.35 | <.001b |
| LCA × AD-CM | −15.63 | 6.33 | −0.10 | .014a |
Note: Age, sex, and APOE4 carrier status were treated as covariates. B: unstandardized coefficient, β: standardized coefficient.
Key: CR, cognitive reserve; AD-CM, AD-signature region cerebral glucose metabolism; pIQ premorbid Intelligence Quotient; OC, occupational complexity; LCA, lifetime cognitive activity; SE, standard error; APOE4, apolipoprotein E ε4.
p < 0.05.
p < 0.001.
3.5. Moderating effects of CR proxies on the relationship between AD-CT and cognition
Of the four proxies, only OC showed significant moderating effect on the relationship between AD-CT and CERAD-TS (Table 4). The higher OC subgroup showed shallower slope of decline in CERAD-TS when AD-CT decreased compared to the lower OC subgroup (Fig. 1E).
Table 4.
Moderating effect of each CR proxy on the relationship between AD-CT and cognition
| Model term | B | SE | β | p |
|---|---|---|---|---|
| Model 1 | ||||
| Education | 0.98 | 0.14 | 0.30 | <.001b |
| AD-CT | 39.74 | 3.41 | 0.51 | <.001b |
| Education × AD-CT | −0.61 | 0.63 | −0.04 | .33 |
| Model 2 | ||||
| pIQ | 0.55 | 0.05 | 0.40 | <.001b |
| AD-CT | 37.27 | 3.24 | 0.48 | <.001b |
| pIQ × AD-CT | −0.18 | 0.24 | −0.03 | .45 |
| Model 3 | ||||
| OC | 1.93 | 0.49 | 0.17 | <.001b |
| AD-CT | 39.18 | 3.56 | 0.50 | <.001b |
| OC × AD-CT | −6.28 | 2.19 | −0.11 | .004a |
| Model 4 | ||||
| LCA | 5.81 | 0.89 | 0.26 | <.001b |
| AD-CT | 40.03 | 3.50 | 0.51 | <.001b |
| LCA × AD-CT | 0.59 | 4.20 | 0.01 | .89 |
Note: Age, sex, and APOE4 carrier status were treated as covariates. B: unstandardized coefficient, β: standardized coefficient.
Key: CR, cognitive reserve; AD-CT, AD-signature region cortical thickness; pIQ, premorbid Intelligence Quotient; OC, occupational complexity; LCA, lifetime cognitive activity; SE, standard error; APOE4, apolipoprotein E ε4.
p < 0.01.
p < 0.001.
3.6. Subgroup analyses for CU and CI
For CI individuals, the findings were similar with full sample except that the moderation effects of education, pIQ, and LCA on AD-CM to cognition were not significant (Table S2). For CU individuals, only LCA significantly moderated the relationship between AD-CT and cognition (Table S3).
4. Discussion
We found that various CR proxies showed different moderating effects on the relationships between in vivo AD pathologies and cognition for overall participants. First, for the relationship of cerebral Aβ deposition with cognition, only education had a significant moderating effect. Secondly, education, pIQ, and LCA had significant moderating effects on the association between AD-CM and cognition. Thirdly, only OC had significant moderating effect on the relationship between AD-CT and cognition. Additional subgroup analyses showed that such moderation effects of the CR proxies were similarly observed in CI individuals, but most of them were not in CU individuals.
Our finding on the significant moderating effect of education on the association between Aβ deposition and cognitive decline is in line with the findings from 2 postmortem neuropathological studies (Bennett et al., 2005, 2003) and an amyloid PET study (Roe et al., 2008). However, for the other CR proxies, we did not find presence of moderating effect influencing the effect of Aβ on cognition. Similar to our result, a previous study with smaller sample (133 CN, 17 MCI, and 6 AD dementia) showed that pIQ did not moderate the relationship between global Aβ deposition on PET and cognition, whereas it moderated the association of tau deposition with cognition (Rentz et al., 2017). There are no prior reports on the moderating effects of OC and LCA on the relationship between Aβ pathology and cognitive decline.
Although it is not easy to explain why only education moderated the negative effect of Aβ pathology on cognition, a possible explanation is that education level is not only associated with the level of cognitive activity (Landau et al., 2012; Wilson et al., 2007) but also with establishing healthy lifestyle (e.g., regular exercise, restricted caloric intake, avoiding harmful substance use, etc.) for maintaining brain health (Mirowsky and Ross, 2003), whereas pIQ, OC, and LCA may largely reflect intellectual ability, demand, or activity. Such difference between education and other CR proxies may be one of the reasons for the differential moderation of Aβ-related cognitive decline. Other possibility is that formal education is mainly achieved during critical period of early-life brain development compared to other CR proxies reflecting long adulthood period as well as early-life. Early-life lifestyle enrichment is associated with better late-life cognition (Wilson et al., 2013) and increased cerebral glucose metabolism in AD-vulnerable regions (Ko et al., 2018). In addition, although not directly examined in the currently study, education may moderate the relationship between Aβ and cognitive decline by delaying or lowering tau pathology, given that brain tau deposition is a key mediator of Aβ-driven cognitive decline (Hanseeuw et al., 2019; Jack et al., 2018). A previous study reported that education was related to lower CSF t-tau concentrations at similar CSF Aβ42 levels (Rolstad et al., 2010). Another study also showed that education could mitigate age-related changes in CSF tau biomarkers (Almeida et al., 2015).
We observed significant moderating effect of education, pIQ, and LCA on the relationship between AD-CM and CERAD-TS. The findings for education and pIQ were consistent with previous reports that indicated individuals with higher education or pIQ can better tolerate the influence of hypometabolism on cognitive decline (Alexander et al., 1997; Ewers et al., 2013; Garibotto et al., 2008; Kemppainen et al., 2008). The finding for LCA also suggests that participating in cognitive activity, as a modifiable lifestyle factor, may be beneficial to preserve cognition in late life by resilience to hypometabolism in AD-vulnerable regions. Previous literatures focused on the effect of cognitive activity on cognition (Gidicsin et al., 2015; Wilson et al., 2013) or AD pathologies (Gidicsin et al., 2015; Ko et al., 2018; Landau et al., 2012), but whether cognitive activity moderates the negative effect of AD pathology on cognition was unclear yet.
In contrast to other CR proxies, OC moderated the relationship between AD-CT and cognition, whereas it did not change the effect of AD-CM. The finding for the significant moderation of OC for the negative influence of cortical atrophy is in line with a prior report that showed greater OC is associated with increased brain atrophy in a cohort at risk for AD while holding cognition constant (Boots et al., 2015). However, the mechanisms by which OC moderate the effect of AD-CT on cognitive decline differentially from other proxies are not clear. Some explanation might be possible. OC may encapsulate interpersonal or social component as well as intellectual one (Boots et al., 2015; Smart et al., 2014), whereas other CR proxies are mainly related to intellectual abilities or activities. A previous study showed that when OC was broken down into components (i.e., complexity of work with people, complexity of work with data, and complexity of work with things), only the complexity of work with people had a significant impact on brain volume (Boots et al., 2015). Social interaction, which could be related to complexity of work with people is known to delay progression of AD (Fratiglioni et al., 2000; Zuelsdorff et al., 2013). In addition, previous studies demonstrated that occupation and participating in social activities exerted similar protective effects on cognition (Adam et al., 2013; Andel et al., 2015).
As suggested from previous reports (Pettigrew and Soldan, 2019; Soldan et al., 2017; Stern, 2012), the results from subgroup analyses revealed that the moderation effects of CR proxies were different between CU and CI group. While the findings from CI subgroup were similar to those from the whole group, we did not find significant result for most cases except the moderation of LCA on the relationship between AD-CT and cognition. The results for CU subgroup were consistent with previous reports that there was no significant moderating effect of CR on the relationship between AD pathology and cognition in cognitively intact individuals (Rentz et al., 2017; Vemuri et al., 2015). As negative effect of AD pathology on cognition is probably subtle and variance of cognition itself is very small, it is not likely to detect easily the moderation effect of CR in CU status.
Our study has several strengths: First, we used 3 different neuroimaging modalities including amyloid PET, FDG-PET, and 3D MRI to analyze the abilities of CR proxies to moderate the influence by various in vivo AD pathologies. In addition, we included relatively large number of participants with diverse cognitive spectrum from CU, MCI to dementia. Moreover, we included only Aβ-positive MCI and AD dementia individuals selected by using amyloid PET to investigate specifically the effect of AD pathologies on cognition moderated by candidate CR proxies considering that 29~63% of MCI and 7~21% of AD dementia patients do not actually have Aβ deposition in brain (Jansen et al., 2015; Ossenkoppele et al., 2015).
Nevertheless, several limitations need to be mentioned. First, due to its cross-sectional design, moderation for the causal relationships of brain pathologies with cognitive decline could not be assessed precisely. Longitudinal follow-up studies are required to confirm the findings. Second, the cognitive activity questionnaire used to assess LCA was based on retrospective recall and might be influenced by recall bias related to cognitive impairment or depression. To minimize the recall bias, we excluded the individuals with major depressive disorder or advanced dementia (CDR > 1). Remote memory for early or midlife experience is usually maintained in mild AD dementia patients with CDR 0.5 or 1 (Morris, 1993). Third, we did not assess brain tau pathology, although it is one of the key AD pathologies. Further studies including tau PET or CSF tau measurement could provide additional insight into the differential moderation of CR proxies. Lastly, as the current study was conducted for exploratory purposes, we did not strictly control the possibility of increased type I error due to multiple tests. To confirm the findings, therefore, further studies with larger sample size and more strict threshold for statistical significance are needed.
5. Conclusions
In conclusion, the present findings suggest that the proposed CR proxies have different moderating effects on the relationships between specific AD pathologies and cognition. To investigate CR or related issues, it may be necessary to select proper CR proxies considering the target brain pathology. For Aβ pathology, education seem the best CR proxy. Education, pIQ, and LCA are suitable for evaluating the moderating effect on the relationship of AD-related hypometabolism with cognition, while OC may be the best proxy for the influence of AD-related brain atrophy with cognition.
This material is original research, has not been previously published and has not been submitted for publication elsewhere while under consideration at Neurobiology of Aging.
The Methods section of this manuscript includes a statement that the Institutional Review Board of, Seoul National University Hospital and SNU-SMG Boramae Center in Seoul, South Korea approved the present study. The authors declare no conflict of interest. This original material has been approved, by each author.
All authors have reviewed the contents of the manuscript being submitted, approve of its contents and validate the accuracy of the data.
Supplementary Material
Acknowledgements
We thank all study participants and their families for their contribution. This study was supported by a grant from the Ministry of Science, ICT, and Future Planning, Republic of Korea (Grant No: NRF-2014M3C7A1046042) and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant No: HI18C0630 & HI19C0149), a grant from the Seoul National University Hospital, Republic of Korea (No. 3020200030) and a grant from the National Institute of Aging, United States of America (U01AG072177). The funding source had no role in the study design, data collection, data analysis, data interpretation, writing of the manuscript, or decision to submit it for publication.
Abbreviations:
- Aβ
Amyloid-beta
- AD
Alzheimer’s disease
- AD-CM
AD-signature region cerebral glucose metabolism
- AD-CT
AD-signature region cortical thickness
- ADD+
AD dementia with Aβ deposition
- APOE4
Apolipoprotein ε4
- CDR
Clinical Dementia Rating
- CERAD-TS
Consortium to Establish a Registry for Alzheimer’s Disease-total score
- CI
Cognitively impaired
- CR
Cognitive Reserve
- CSF
Cerebrospinal fluid
- CU
Cognitively unimpaired (CU−, CU without Aβ deposition
- CU+
CU with Aβ deposition
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders IV
- FDG
18 F-fluoro-2-deoxyglucose
- KBASE
Korean Brain Aging Study for the Early Diagnosis and Prediction of AD
- LCA
Lifetime cognitive activity
- MCI
Mild cognitive impairment (MCI+, MCI with Aβ deposition)
- MRI
Magnetic resonance imaging
- NIA-AA
National Institute on Aging and Alzheimer’s Association
- OC
Occupational complexity
- PET
Positron emission tomography
- PiB
11 C-Pittsburg compound B
- pIQ
Premorbid intelligence quotient
- ROI
Regions of interest
- SUVR
Standardized uptake value ratio
Footnotes
Disclosure statement
The authors have no competing interest to declare.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neurobiolaging.2021.10.005.
CRediT authorship contribution statement
Kang Ko: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Writing – original draft, Writing – review & editing, Visualization. Dahyun Yi: Investigation, Data curation, Resources. Min Soo Byun: Investigation, Data curation, Resources. Jun Ho Lee: Investigation, Resources. So Yeon Jeon: Investigation, Resources. Woo Jin Kim: Investigation, Resources. Gihwan Byeon: Investigation, Resources. Younghwa Lee: Investigation, Resources. Haejung Joung: Investigation, Resources. Gijung Jung: Investigation, Resources. Jun-Young Lee: Data curation, Project administration. Heejung Kim: Data curation, Project administration. Yu Kyeong Kim: Data curation, Project administration. Koung Mi Kang: Investigation, Data curation, Resources. Chul-Ho Sohn: Data curation, Project administration. Dong Young Lee: Conceptualization, Methodology, Validation, Formal analysis, Writing – original draft, Writing – review & editing, Funding acquisition, Project administration, Supervision.
References
- Adam S, Bonsang E, Grotz C, Perelman S, 2013. Occupational activity and cognitive reserve: Implications in terms of prevention of cognitive aging and Alzheimer’s disease. Clin. Interv. Aging 8, 377–390. doi: 10.2147/CIA.S39921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Furey ML, Grady CL, Pietrini P, Brady DR, Mentis MJ, Schapiro MB, 1997. Association of Premorbid Intellectual Function With Cerebral Metabolism in Alzheimer’s Disease: Implications for the Cognitive Reserve Hypothesis. Am J Psychiatry 154, 165–172. doi: 10.1176/ajp.154.2.165. [DOI] [PubMed] [Google Scholar]
- Almeida RP, Schultz SA, Austin BP, Boots EA, Dowling NM, Gleason CE, Bendlin BB, Sager MA, Hermann BP, Zetterberg H, Carlsson CM, Johnson SC, Asthana S, Okonkwo OC, 2015. Effect of cognitive reserve on age-related changes in cerebrospinal fluid biomarkers of Alzheimer disease. JAMA Neurol 72, 699–706. doi: 10.1001/jamaneurol.2015.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association, 2000. Diagnostic criteria from DSM-IV TR. American Psychiatric Association, Washington, D.C. [Google Scholar]
- Andel R, Silverstein M, Kareholt I, 2015. The role of midlife occupational complexity and leisure activity in late-life cognition. Journals Gerontol. - Ser. B Psychol. Sci. Soc. Sci 70, 314–321. doi: 10.1093/geronb/gbu110. [DOI] [PubMed] [Google Scholar]
- Arenaza-Urquijo EM, Wirth M, Chételat G, 2015. Cognitive reserve and lifestyle: Moving towards preclinical Alzheimer’s disease. Front. Aging Neurosci 7. doi: 10.3389/fnagi.2015.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CE, Brown CA, Gold BT, 2020. Education does not protect cognitive function from brain pathology in the ADNI 2 cohort. Neurobiol. Aging 90, 147–149. doi: 10.1016/j.neurobiolaging.2019.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE, 2005. Education modifies the association of amyloid but not tangles with cognitive function. Neurology 65, 953–955. doi: 10.1212/01.wnl.0000176286.17192.69. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Mendes de Leon CF, Arnold SE, Barnes LL, Bienias JL, 2003. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology 60, 1909–1915. doi: 10.1212/01.WNL.0000069923.64550.9F. [DOI] [PubMed] [Google Scholar]
- Boots EA, Schultz SA, Almeida RP, Oh JM, Koscik RL, Dowling MN, Gallagher CL, Carlsson CM, Rowley HA, Bendlin BB, Asthana S, Sager MA, Hermann BP, Johnson SC, Okonkwo OC, 2015. Occupational complexity and cognitive reserve in a middle-aged cohort at risk for Alzheimer’s Disease. Arch. Clin. Neuropsychol 30, 634–642. doi: 10.1093/arclin/acv041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun MS, Yi D, Lee JH, Choe YM, Sohn BK, Lee J-Y, Choi HJ, Baek H, Kim YK, Lee Y-S, Sohn C-H, Mook-Jung I, Choi M, Lee YJ, Lee DW, Ryu S-H, Kim SG, Kim JW, Woo JI, Lee DY, 2017. Korean brain aging study for the early diagnosis and prediction of Alzheimer’s disease: methodology and baseline sample characteristics. Psychiatry Investig 14, 851. doi: 10.4306/pi.2017.14.6.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler MJ, Lacritz LH, Hynan LS, Barnard HD, Allen G, Deschner M, Weiner MF, Cullum CM, 2005. A total score for the CERAD neuropsychological battery. Neurology 65, 102–106. doi: 10.1212/01.wnl.0000167607.63000.38. [DOI] [PubMed] [Google Scholar]
- Choe YM, Sohn BK, Choi HJ, Byun MS, Seo EH, Han JY, Kim YK, Yoon EJ, Lee J-M, Park J, Woo JI, Lee DY, 2014. Association of homocysteine with hippocampal volume independent of cerebral amyloid and vascular burden. Neurobiol. Aging 35, 1519–1525. doi: 10.1016/j.neurobiolaging.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Commission of the European Communities, 2000. A Memorandum on Lifelong Learning. Commission Staff Working Paper, Brussels: Directorate General Education and Culture. 1–36. [Google Scholar]
- Darwish H, Farran N, Assaad S, Chaaya M, 2018. Cognitive reserve factors in a developing country: education and occupational attainment lower the risk of dementia in a sample of lebanese older adults. Front. Aging Neurosci 10, 1–10. doi: 10.3389/fnagi.2018.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ, 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HD, Sperling RA, Atri A, Growdon JH, Hyman BT, Morris JC, Fischl B, Buckner RL, 2009. The cortical signature of Alzheimer’s disease: Regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb. Cortex 19, 497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers M, Insel PS, Stern Y, Weiner MW, 2013. Cognitive reserve associated with FDG-PET in preclinical Alzheimer disease. Neurology 80, 1194–1201. doi: 10.1212/WNL.0b013e31828970c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratiglioni L, Wang HX, Ericsson K, Maytan M, Winblad B, 2000. Influence of social network on occurrence of dementia: A community-based longitudinal study. Lancet 355, 1315–1319. doi: 10.1016/S0140-6736(00)02113-9. [DOI] [PubMed] [Google Scholar]
- Garibotto V, Borroni B, Kalbe E, Herholz K, Salmon E, Holtoff V, Sorbi S, Cappa SF, Padovani A, Fazio F, Perani D, 2008. Education and occupation as proxies for reserve in aMCI converters and AD: FDG-PET evidence. Neurology 71, 1342–1349. doi: 10.1212/01.wnl.0000327670.62378.c0. [DOI] [PubMed] [Google Scholar]
- Gidicsin CM, Maye JE, Locascio JJ, Pepin LC, Philiossaint M, Becker JA, Younger AP, Dekhtyar M, Schultz AP, Amariglio RE, Marshall GA, Rentz DM, Hedden T, Sperling RA, Johnson KA, 2015. Cognitive activity relates to cognitive performance but not to Alzheimer disease. Neurology 85, 48–55. doi: 10.1212/WNL.0000000000001704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanseeuw BJ, Betensky RA, Jacobs HIL, Schultz AP, Sepulcre J, Becker JA, Cosio DMO, Farrell M, Quiroz YT, Mormino EC, Buckley RF, Papp KV, Amariglio RA, Dewachter I, Ivanoiu A, Huijbers W, Hedden T, Marshall GA, Chhatwal JP, Rentz DM, Sperling RA, Johnson K, 2019. Association of amyloid and tau with cognition in preclinical alzheimer disease: a longitudinal study. JAMA Neurol 76, 915–924. doi: 10.1001/jamaneurol.2019.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Labor Office (ILO), 2012. International Standard Classification of Occupations 1–420. doi: 10.13140/RG.2.1.1419.3126, Isco-08 I. [DOI] [Google Scholar]
- Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Elliott C, Masliah E, Ryan L, 2018. NIA-AA Research framework: toward a biological definition of Alzheimer’s disease. Alzheimer’s dement. Silverberg, N, 14, 535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Hampel HJ , Universities S, Cu M, Petersen RC, 2016. A new classification system for AD, independent of cognition A /T / N : An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 0, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Wiste HJ, Weigand SD, Rocca WA, Knopman DS, Mielke MM, Lowe VJ, Senjem ML, Gunter JL, Preboske GM, Pankratz VS, Vemuri P, Petersen RC, 2014. Age-specific population frequencies of cerebral β-amyloidosis and neurodegeneration among people with normal cognitive function aged 50-89 years: A cross-sectional study. Lancet Neurol 13, 997–1005. doi: 10.1016/S1474-4422(14)70194-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FRJ, Visser PJ, Aalten P, Aarsland D, Alcolea D, Alexander M, Almdahl IS, Arnold SE, Baldeiras I, Barthel H, Van Berckel BNM, Bibeau K, Blennow K, Brooks DJ, Van Buchem MA, Camus V, Cavedo E, Chen K, Chetelat G, Cohen AD, Drzezga A, Engelborghs S, Fagan AM, Fladby T, Fleisher AS, Van Der Flier WM, Ford L, Forster S, Fortea J, Foskett N, Frederiksen KS, Freund-Levi Y, Frisoni GB, Froelich L, Gabryelewicz T, Gill KD, Gkatzima O, Gomez-Tortosa E, Gordon MF, Grimmer T, Hampel H, Hausner L, Hellwig S, Herukka SK, Hildebrandt H, Ishihara L, Ivanoiu A, Jagust WJ, Johannsen P, Kandimalla R, Kapaki E, Klimkowicz-Mrowiec A, Klunk WE, Kohler S, Koglin N, Kornhuber J, Kramberger MG, Van Laere K, Landau SM, Lee DY, De Leon M, Lisetti V, Lleo A, Madsen K, Maier W, Marcusson J, Mattsson N, De Mendonca A, Meulenbroek O, Meyer PT, Mintun MA, Mok V, Molinuevo JL, Mollergard HM, Morris JC, Mroczko B, Van Der Mussele S, Na DL, Newberg A, Nordberg A, Nordlund A, Novak GP, Paraskevas GP, Parnetti L, Perera G, Peters O, Popp J, Prabhakar S, Rabinovici GD, Ramakers IHGB, Rami L, De Oliveira CR, Rinne JO, Rodrigue KM, Rodriguez-Rodriguez E, Roe CM, Rot U, Rowe CC, Ruther E, Sabri O, Sanchez-Juan P, Santana I, Sarazin M, Schroder J, Schutte C, Seo SW, Soetewey F, Soininen H, Spiru L, Struyfs H, Teunissen CE, Tsolaki M, Vandenberghe R, Verbeek MM, Villemagne VL, Vos SJB, Van Waalwijk Van Doorn LJC, Waldemar G, Wallin A, Wallin AK, Wiltfang J, Wolk DA, Zboch M, Zetterberg H, 2015. Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. JAMA - J. Am. Med. Assoc 313, 1924–1938. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RN, Manly J, Glymour MM, Rentz DM, Jefferson AL, Stern Y, 2011. Conceptual and measurement challenges in research on cognitive reserve. J. Int. Neuropsychol. Soc. 17, 593–601. doi: 10.1017/S1355617710001748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemppainen NM, Aalto S, Karrasch M, Någren K, Savisto N, Oikonen V, Viitanen M, Parkkola R, Rinne JO, 2008. Cognitive reserve hypothesis: Pittsburgh compound B and fluorodeoxyglucose positron emission tomography in relation to education in mild Alzheimer’s disease. Ann. Neurol. 63, 112–118. doi: 10.1002/ana.21212. [DOI] [PubMed] [Google Scholar]
- Ko K, Byun MS, Yi D, Lee JH, Kim CH, Lee DY, 2018. Early-life cognitive activity is related to reduced neurodegeneration in Alzheimer signature regions in late life. Front. Aging Neurosci. 10, 70. doi: 10.3389/fnagi.2018.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Marks SM, Mormino EC, Rabinovici GD, Oh H, O’Neil JP, Wilson RS, Jagust WJ, 2012. Association of lifetime cognitive engagement and low β-amyloid deposition. Arch. Neurol. 69, 623–629. doi: 10.1001/archneurol.2011.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DY, Lee KU, Lee JH, Kim KW, Jhoo JH, Kim SY, Yoon JC, Woo SI, Ha J, Woo JI, 2004. A normative study of the CERAD neuropsychological assessment battery in the Korean elderly. J. Int. Neuropsychol. Soc. 10, 72–81. doi: 10.1017/S1355617704101094. [DOI] [PubMed] [Google Scholar]
- Lee JH, Lee KU, Lee DY, Kim KW, Jhoo JH, Kim JH, Lee KH, Kim SY, Han SH, Woo JI, 2002. Development of the Korean version of the Consortium to Establish a Registry for Alzheimer’s Disease Assessment Packet (CERAD-K): Clinical and neuropsychological assessment batteries. J Gerontol. - Ser. B Psychol. Sci. Soc. Sci 57, P47–P53. doi: 10.1093/geronb/57.1.P47. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH, 2011. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers. Dement 7, 263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirowsky J, Ross CE, 2003. Education, Social Status, and Health (1st ed.). Routledge; 10.4324/9781351328081. [DOI] [Google Scholar]
- Morris JC, 1993. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43, 2412–2414. [DOI] [PubMed] [Google Scholar]
- ell. Ossenkoppele R, Jansen WJ, Rabinovici GD, Knol DL, van der Flier WM, van Berckel BNM, Scheltens P, Visser PJ, Verfaillie SCJ, Zwan MD, Adriaanse SM, Lammertsma AA , Barkhof F, Jagust WJ, Miller BL, Rosen HJ, Landau SM, Villemagne VL, Rowe CC, Lee DY, Na DL, Seo SW, Sarazin M, Roe CM, Sabri O, Barthel H, Koglin N, Hodges J, Leyton CE, Vandenberghe R, van Laere K, Drzezga A, Forster S, Grimmer T, Sánchez-Juan P, Carril JM, Mok V, Camus V, Klunk WE, Cohen AD, Meyer PT, Hellwig S, Newberg A, Frederiksen KS, Fleisher AS, Mintun MA, Wolk DA, Nordberg A, Rinne JO, Chételat G, Lleo A, Blesa R, Fortea J, Madsen K, Rodrigue KM, Brooks DJ, 2015. Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. Jama 313, 1939–1949. doi: 10.1001/jama.2015.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JC, Han SH, Yi D, Byun MS, Lee JH, Jang S, Ko K, Jeon SY, Lee YS, Kim YK, Lee DY, Mook-Jung I, 2019. Plasma tau/amyloid-β 1-42 ratio predicts brain tau deposition and neurodegeneration in Alzheimer’s disease. Brain 142, 771–786. doi: 10.1093/brain/awy347. [DOI] [PubMed] [Google Scholar]
- Perneczky R, Wagenpfeil S, Lunetta KL, Cupples LA, Green RC, Decarli C, Farrer LA, Kurz A, 2009. Education attenuates the effect of medial temporal lobe atrophy on cognitive function in alzheimer’s disease: The mirage study. J. Alzheimer’s Dis. 17, 855–862. doi: 10.3233/JAD-2009-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew C, Soldan A, 2019. Defining cognitive reserve and implications for cognitive aging. Curr. Neurol. Neurosci. Rep 19. doi: 10.1007/s11910-019-0917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, Ayutyanont N, Keppler J, Reeder SA, Langbaum JBS, Alexander GE, Klunk WE, Mathis CA, Price JC, Aizenstein HJ, DeKosky ST, Caselli RJ, 2009. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A. 106, 6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentz DM, Locascio JJ, Becker JA, Moran EK, Eng E, Buckner RL, Sperling RA, Johnson KA, 2010. Cognition, reserve, and amyloid deposition in normal aging. Ann. Neurol. 67, 353–364. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentz DM, Mormino EC, Papp KV, Betensky RA, Sperling RA, Johnson KA, 2017. Cognitive resilience in clinical and preclinical Alzheimer’s disease: the Association of Amyloid and Tau Burden on cognitive performance. Brain Imaging Behav 11, 383–390. doi: 10.1007/s11682-016-9640-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe CM, Mintun MA, D’Angelo G, Xiong C, Grant EA, Morris JC, 2008. Alzheimer disease and cognitive reserve: variation of education effect with carbon 11–labeled pittsburgh compound B uptake. Arch. Neurol. 65, 1467–1471. doi: 10.1001/archneur.65.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolstad S, Nordlund A, Eckerström C, Gustavsson MH, Blennow K, Olesen PJ, Zetterberg H, Wallin A, 2010. High education may offer protection against tauopathy in patients with mild cognitive impairment. J. Alzheimer’s Dis. 21, 221–228. doi: 10.3233/JAD-2010-091012. [DOI] [PubMed] [Google Scholar]
- Seo EH, Lee DY, Lee JH, Choo IH, Kim JW, Kim SG, Park SY, Shin JH, Do YJ, Yoon JC, Jhoo JH, Kim KW, Woo JI, 2010. Total scores of the CERAD neuropsychological assessment battery: Validation for mild cognitive impairment and dementia patients with diverse etiologies. Am. J. Geriatr. Psychiatry 18, 801–809. doi: 10.1097/JGP.0b013e3181cab764. [DOI] [PubMed] [Google Scholar]
- Smart EL, Gow AJ, Deary IJ, 2014. Occupational complexity and lifetime cognitive abilities. Neurology 83, 2285–2291. doi: 10.1212/WNL.0000000000001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldan A, Pettigrew C, Cai Q, Wang J, Wang MC, Moghekar A, Miller MI, Albert M, 2017. Cognitive reserve and long-term change in cognition in aging and preclinical Alzheimer’s disease. Neurobiol. Aging 60, 164–172. doi: 10.1016/j.neurobiolaging.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, 2012. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 11, 1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, 2002. What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 8, 448–460. doi: 10.1017/S1355617702813248. [DOI] [PubMed] [Google Scholar]
- Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, Belleville S, Cantilon M, Chetelat G, Ewers M, Franzmeier N, Kempermann G, Kremen WS, Okonkwo O, Scarmeas N, Soldan A, Udeh-Momoh C, Valenzuela M, Vemuri P, Vuoksimaa E, Arenaza Urquiljo EM, Bartrés-Faz D, Belleville S, Cantillon M, Chetelat G, Clouston SAP, Estanga A, Ewers M, Franzmeier N, Gold B, Habeck C, Jones R, Kempermann G, Kochhann R, Kremen W, Lim YY, Martínez-Lage P, Morbelli S, Okonkwo O, Ossenkoppele R, Pettigrew C, Rosen AC, Scarmeas N, Soldan A, Song X, Udeh-Momoh C, Stern Y, Valenzuela M, Van Loenhoud AC, Vemuri P, Vuoksimaa E, 2018. Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer’s Dement 1–7. doi: 10.1016/j.jalz.2018.07.219. [DOI] [PMC free article] [PubMed]
- Vemuri P, Lesnick TG, Przybelski SA, Knopman DS, Preboske GM, Kantarci K, Raman MR, Machulda MM, Mielke MM, Lowe VJ, Senjem ML, Gunter JL, Rocca WA, Roberts RO, Petersen RC, Jack CR, 2015. Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain 138, 761–771. doi: 10.1093/brain/awu393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri P, Weigand SD, Przybelski SA, Knopman DS, Smith GE, Trojanowski JQ, Shaw LM, Decarli CS, Carmichael O, Bernstein MA, Aisen PS, Weiner M, Petersen RC, Jack CR, 2011. Cognitive reserve and Alzheimer’s disease biomarkers are independent determinants of cognition. Brain 134, 1479–1492. doi: 10.1093/brain/awr049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Boyle PA, Yu L, Barnes LL, Schneider JA, Bennett DA, 2013. Lifespan cognitive activity, neuropathologic burden, and cognitive aging. Neurology 81, 314–321. doi: 10.1212/WNL.0b013e31829c5e8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA, 2007. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology 69, 1911–1920. doi: 10.1212/01.wnl.0000271087.67782.cb. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Weston A, Graff-Radford NR, Satterfield S, Simonsick EM, Younkin SG, Younkin LH, Kuller L, Ayonayon HN, Ding J, Harris TB, 2011. Association of plasma beta-amyloid level and cognitive reserve with subsequent cognitive decline. Jama 305, 261–266. doi: 10.1001/jama.2010.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi D, Seo EH, Han JY, Sohn BK, Byun MS, Lee JH, Choe YM, Ahn S, Woo JI, Jun J, Lee DY, 2017. Development of the Korean Adult Reading Test (KART) to estimate premorbid intelligence in dementia patients. PLoS One 12, e0181523. doi: 10.1371/journal.pone.0181523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuelsdorff ML, Engelman CD, Friedman EM, Koscik RL, Jonaitis EM, Rue A.La, Sager MA, 2013. Stressful events, social support, and cognitive function in middle-aged adults with a family history of Alzheimer’s disease. J. Aging Health 25, 944–959. doi: 10.1177/0898264313498416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


