Abstract
Background
Bearberry (Arctostaphylos uva-ursi) leaf is available as a treatment of uncomplicated cystitis in several European countries. The antimicrobial activity of its extracts and some of its individual constituents has been observed in vitro; however, the efficacy of bearberry compared with standard antimicrobial therapy has not been assessed yet.
Objective
The objective of the study is to assess the safety and non-inferiority of bearberry as an alternative therapy in the treatment of acute uncomplicated cystitis in comparison with standard antibiotic therapy (fosfomycin).
Methods and analysis
This is a randomised controlled double-blinded multicentre trial. Eligible patients will be premenopausal women with a sum score of ≥6 for the typical acute uncomplicated cystitis symptoms (frequency, urgency, painful urination, incomplete emptying, suprapubic pain and visible haematuria) reported on the Acute Cystitis Symptom Score (ACSS) typical domain and pyuria. Patients will be randomly assigned to receive 3 g single dose of fosfomycin powder and two placebo tablets three times a day for 7 days or B a single dose of placebo powder and two tablets containing a dry extract of Uvae ursi folium. At least 504 patients (allocated as 1:1) will need to be enrolled to access non-inferiority with a non-inferiority limit of 14% for the primary endpoint.
Improvement of symptoms of uncomplicated cystitis (based on the ACSS score) at day 7 is defined as the primary endpoint, whereas several secondary endpoints such as the number and ratio of patients with bacteriuria at day 7, frequency and severity of side effects; recurrence of urinary tract infection, concurrent use of other over the counter (OTC) medications and food supplements will be determined to elucidate more detailed differences between the groups. The number of recurrences and medications taken for treatment will be monitored for a follow-up period of 90 days (80–100 days).
Ethics and dissemination
This study has been approved by the Scientific and Research Ethics Committee of the Hungarian Medical Research Council (IV/4225-1/2021/EKU). The results will be disseminated by publication of peer-reviewed manuscripts.
Trial registration number
Keywords: Urinary tract infections, Interstitial cystitis, Clinical trials
Strengths and limitations of this study.
This is the first randomised controlled trial to examine the efficacy of bearberry in the treatment of uncomplicated cystitis.
The efficacy will be compared with fosfomycin, a first-line drug.
This is a multicentre, prospective, randomised, non-inferiority study.
A validated score will be used to assess efficacy.
One limitation of this study is that the study population is limited to non-pregnant adult women before menopause.
Introduction
Urinary tract infections (UTIs) are the most frequent occurring infections in women, and one of the major reasons for antibiotic prescriptions.1 UTIs are classified as uncomplicated if anatomical or functional abnormalities are not observed in the urinary system. Regarding UTIs, Escherichia coli is the most frequent pathogen, followed by Staphylococcus saprophyticus and Enterococcus faecalis.2 3 In clinical practice, acute uncomplicated cystitis is diagnosed and treated based on the clinical signs and symptoms, microbiologic investigations are not performed routinely. According to the available guidelines, first-line drugs include fosfomycin trometamol, trimethoprim-sulfamethoxazole, nitrofurantoin, nitroxolin and pivmecillinam.4–7
Fosfomycin, a first-line medication of acute uncomplicated cystitis possesses a wide spectrum of antibacterial activity against both Gram-negative and Gram-positive pathogens, including E. coli.8 9 Based on a large scale surveillance study, E. coli, the most frequently isolated pathogen from UTI samples, is highly susceptibility to fosfomycin with a susceptibility rate of 98.5%.6 In the majority of the published trials, the achieved short-term (7–9 days) microbial cure rate was more than 80%.10–12In a more recent study, bacteriological success through day 14 after treatment with fosfomycin was 73%, whereas clinical success was 66%.13 Based on the published evidence, a single 3 g dose of fosfomycin trometamol is considered to be sufficient for the treatment of uncomplicated lower UTIs.
Bacterial resistance is one of the major limiting factors of antibiotic use. Despite fosfomycin’s activity against resistant strains (eg, multidrug resistant E. coli), fosfomycin-resistance is an increasing problem.14 In Spain, the frequency of fosfomycin-resistance increased from 4% to 11% between 1997 and 2009.15
Bearberry leaves have been used in traditional medicine for the treatment of acute cystitis. The European Medicines Agency acknowledged the traditional medicinal use of the herbal tea, the powdered plant material as well as defined extracts for treatment of symptoms of mild recurrent lower UTIs such as burning sensation during urination and/or frequent urination in women.16 The antimicrobial effect of bearberry leaf extracts and hydroquinone derivatives present in the plant have been confirmed in several in vitro studies, including several uropathogens. A bearberry leaf extract (extraction solvent ethanol 70%) exhibited antimicrobial activity towards a variety of organisms, including E. coli.17 The pharmacokinetics of hydroquinones have also been studied, confirming the secretion of these compounds into the urine.18 Unfortunately, no pharmacokinetic data are available on hydroquinone derivatives in patients with acute uncomplicated cystitis and also no data are available on the antimicrobial activities of secondary metabolites of bearberry leaves other than hydroquinone derivatives. Overall, although there are data suggesting the antimicrobial activity of bearberry components against E. coli, there is no direct evidence for their clinical efficacy in humans.
Herbal preparations of bearberry leaf have been accepted as a traditional medicine, however, their efficacy has not been confirmed in clinical trials in comparison with antibiotic treatment. So far, no resistance has been reported for bearberry. The lack of resistance, together with the cost-effectiveness of the treatment might be the major advantages of the use of this plant as a medicine in the treatment of acute uncomplicated cystitis. In one study, the efficacy was compared with ibuprofen in terms of symptom improvement,19 and in one ongoing trial fosfomycin is used as comparator.20 The aim of our study is to assess the non-inferiority of a dry extract of bearberry leaves in terms of clinical efficacy and safety in comparison with standard antibiotics used in acute uUTI.
Methods and analysis
Trial design
The study protocol is structured following the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) statement 2013.21 This study is a multicentre, randomised, controlled, double-blind trial assessing non-inferiority. The allocation ratio is 1:1. Eligible patients will be randomly assigned to groups A (single dose of fosfomycin powder and two placebo tablets three times a day) or B (single dose of placebo powder and two bearberry tablets three times a day). The trial will be carried out in 2022–2023.
The trial organisation, committees and boards
The corresponding centre and designer of the BRUMI (Bearberry in the treatment of acute uncomplicated cystitis) trial is the Centre for Translational Medicine at the Medical School, University of Pécs (coordinating institution and sponsor, www.tm-centre.org) and the Hungarian Phytotherapy Study Group.
The steering committee (SC) will be led by Péter Hegyi (University of Pécs, Hungary). The members will be Dezső Csupor, András Jávorházy (University of Pécs, Hungary) and Péter Nyirády (Semmelweis University, Budapest). SC will make decisions concerning all relevant questions including the drop-outs during the study. There will be independent members as well, and the SC will include a patient representative. The SC will supervise the trial primarily and will make decisions regarding all critical questions (protocol deviations, drop-outs, etc.)
The International Translational Advisory Board (ITAB) will include urologists, microbiologists and experts in phytotherapy. ITAB will continuously monitor the progress of the study and will give advice to the SC. The study was designed by the SC and ITAB. The study is financially sponsored by the University of Pécs, the Hungarian Academy of Sciences and the National Research, Development and Innovation Office. Neither sponsors were involved in the design of the study, and they will have no access to the database management or to the randomisation code.
Sponsor
The sponsor of the BRUMI study is the University of Pécs, Medical School. The sponsor was not involved in the design of the study and will have no access to the database.
Patient and public involvement
Patients or the public were not involved in the design, conduct, reporting or dissemination plans of our research.
Participating centres
Study participants are recruited from two centres in Hungary: Urology Clinic, University of Pécs, Pécs and Department of Urology, Semmelweis University, Budapest. Participants will be recruited from patients visiting the study centres with the symptoms of acute uncomplicated cystitis.
Study population
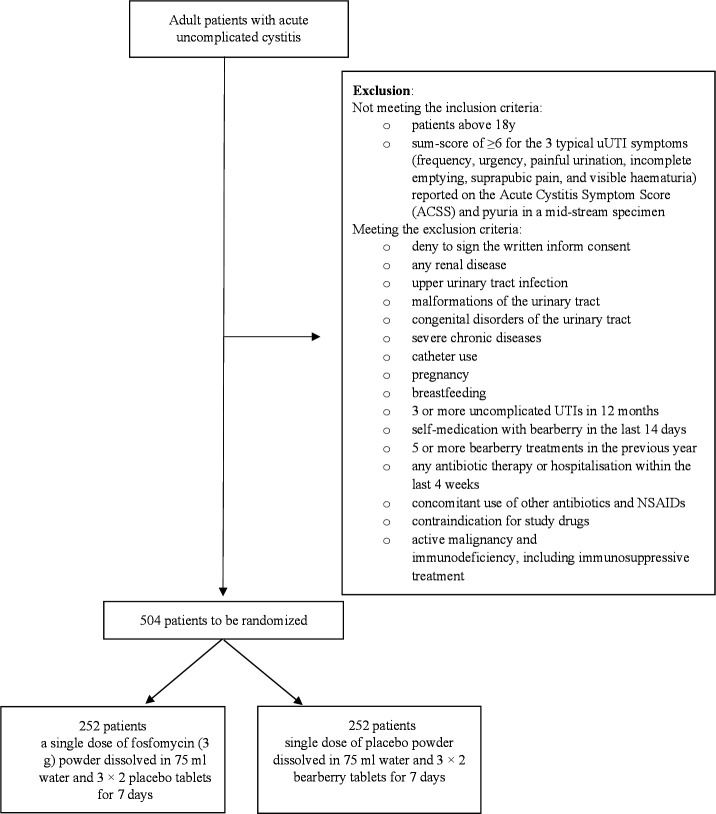
All premenopausal female patients diagnosed with uncomplicated UTI presenting to the participating centres will be informed of the possibility of taking part in the BRUMI study. After the consent form is signed, a computer using a block randomisation protocol will randomise the patients (figure 1).
Figure 1.
Flow chart of participants (SPIRIT 2013 statement). NSAID, non-steroidal anti-inflammatory drugs; SPIRIT, Standard Protocol Items: Recommendations for Interventional Trials; UTIs, upper urinary tract infections.
Inclusion and exclusion criteria
Non-pregnant adult women before menopause (absence of menses for 1 year) having acute uncomplicated cystitis will be included to the study. Acute uncomplicated (simple) cystitis is defined as acute UTI with the symptoms of dysuria, increased frequency and urgency of urination and lower abdominal pain (suprapubic pain), that is, presumed to be confined to the bladder; with no signs or symptoms that suggest an upper tract or systemic infection. Acute uncomplicated (simple) cystitis lacks signs or symptoms that suggest an infection extending beyond the bladder, which include: (1) elevated body temperature (>37.7°C) or fever; (2) other signs or symptoms of systemic illness (including chills or rigours, significant fatigue or malaise beyond baseline); (3) flank pain; (4) costovertebral angle tenderness.22 Eligible patients will be premenopausal women with a sum-score of ≥6 for the typical uUTI symptoms (frequency, urgency, painful urination, incomplete emptying, suprapubic pain and visible haematuria) reported on the Acute Cystitis Symptom Score (ACSS) typical domain and pyuria (10 white blood cells/mm3 in a mid-stream specimen) at day 023–25
The exclusion criteria are: (1) any renal disease; (2) upper UTI; (3) malformations of the urinary tract; (4) congenital disorders of the urinary tract; (5) catheter use; (6) pregnancy; (7) breast feeding; (8) self-medication with bearberry in the last 14 days; (9) five or more bearberry treatments in the previous year; (10) any antibiotic therapy or hospitalisation within the last 4 weeks (11) concomitant use of other antibiotics and NSAIDs (non-steroidal anti-inflammatory drugs); (12) contraindication for study drugs; (13) active malignancy and (14) immunodeficiency, including immunosuppressive treatment.
Randomisation and blinding
Participants will be divided into two groups (1:1) receiving one of the two study treatments in each centre. Randomisation will be performed by using a predefined list with balanced blocked allocation size of four, stratified by recruiting centres, using a computer-generated random sequence. The medical staff (eg, those who will take the patients’ history, examine the patients, collect the specimen of urine, distributing the study drugs, diaries, and questionnaires), statisticians performing data analyses and the patients receiving the study drugs will be blinded regarding treatment assignment. PB (who will be not involved in the clinical study) will coordinate the preparation of medication packages. The packages will be prepared for each patients separately and will be labelled with individual codes only.
Interventions
All eligible patients will be randomised to receive either a single dose of fosfomycin (3 g) powder dissolved in 75 mL water and two placebo tablets three times a day for 7 days, or single dose of placebo powder dissolved in 75 mL water and two bearberry tablets three times a day for 7 days.
The study drugs are the following: (1) Fosfomycin trometamol as Monural, containing 3 g fosfomycin (in the form of fosfomycin trometamol (5.631 g))/sachet); (2) Bearberry tablet as Urzinol containing dry extract of Arctostaphylos uva-ursi (L.) Spreng leaf extract (DER 3.5–5.5:1, extracting solvent 60% v/v ethanol), 238.7–297.5 mg extract/tablet, corresponding to 70 mg hydroquinone derivatives, expressed as anhydrous arbutin. Both bearberry leaf extract and the fosfomycin trometamol would be used according to the recommendations of the SPCs. Placebo products will be identical in appearance (and in case of the placebo of the antibiotic powder also in colour and taste) with the active treatments.
The doses of the study drugs (fosfomycin trometamol and bearberry leaf extract) were chosen based on either the international guidelines on the treatment of uncomplicated UTIs and on the European Union herbal monograph on Arctostaphylos uva-ursi (L.) Spreng., folium.4 5 16
Patients will be scheduled for follow-up visits at day 7. At the beginning of the study (day 0) and on day 7, patients will be asked to fill in a self-reported questionnaire ACSS.23 At the beginning of the study and at the visit at day 7, midstream urine specimens will be collected to evaluate bacteriuria (presence and colony forming unit (CFU) number of pathogens) and the pH of urine. Urine samples collected at day 0 will also be used to rule out pregnancy.
Outcomes
The primary endpoint of our trial is the improvement of symptoms of uncomplicated cystitis after 7 days of treatment. The improvement of symptoms will be determined by using the validated Hungarian version of the ACSS on day 0 and day 7 according to the following predefined thresholds: summary score of the typical symptoms≤5 scores, no item >1 (mild), and ‘visible blood in urine’ negative.26 27
Secondary endpoints include the (1) number of patients with urine with <103 CFU/mL on day 7 in patients with significant bacteriuria (CFU>105 /mL) at D0; (2) average number of CFU of pathogens (7 days after the start of the therapy) in urine; (3) frequency and severity of side effects; (4) recurrence of UTI (follow-up after better during 90 days; severity and diagnostics of recurrences to be assessed by using the ACSS) and (5) concurrent use of other OTC medications and food supplements that are started taking during the 7-day treatment trial.
Statistical analysis and sample size calculation
Sample size estimation
Sample size calculation suggests that 504 patients (1:1) will need to be enrolled to confirm or reject the hypothesis for the primary endpoint, that is, improvement of symptoms of uncomplicated cystitis based on the ACSS score at day 7 (80% vs 77%; non-inferiority margin: 14%) with a 15% drop-out, and power 80%. For this calculation we considered the clinical cure rates of different antibiotic treatments in UTI (79%–92%).28 Sample size calculation was performed for the dichotomous primary endpoint by using Stata V.16 (StataCorp).
Statistical analysis
Descriptive statistics—mean, median, SD, quartiles and relative frequency—relative risk (dichotomous variables) for the primary endpoint. Statistical analyses will be performed with an error probability of 0.0294 (type-I error probability). A safety analysis will be performed after reaching 10% of the planned sample size. OR will be calculated for the primary endpoint. Statistical analysis will be performed by using R Core Team (2022; R Foundation for Statistical Computing, Vienna, Austria).
Interim analysis
A predefined interim analysis will be performed after reaching 50% of the planned sample size. We will calculate statistical power for the primary endpoint which will decide whether additional subjects should be enrolled or not. If 80% statistical power is reached, no more subjects will be needed, and early stopping will be applied. We will test our hypotheses first in an interim analysis, and at the end of the study, in the final analysis. For this reason, the p value should be adjusted to diminish the probability of type I error; therefore, the corrected level of significance (p value) will be 0.0294.
Study duration
The planned starting date of the study is 1 October 2022, and the planned finishing date of the study is 1 September 2023.
Flow and timing
Enrolment
Patients presenting with symptoms referring to uncomplicated UTI will be examined by a study physician and assessed for eligibility to participate in the trial in the participating clinical centres. Eligible patients will be asked to give an informed consent to the study. Patients will be asked to complete ACSS symptom questionnaire, and along with detailed medical history, duration and severity of symptoms and activity impairment will be documented. Symptom evaluation will cover the typical symptoms according to the ACSS questionnaire, such as dysuria, urgency, frequency and lower abdominal pain, each scored from 0 (none) to 3 (strong). UTI-related activity impairment covers impairment by the symptom (see above), scored as well from 0 (none) to 3 (strong). Data will be transferred to electronic Case Report Forms (eCRF) by practice staff. On enrolment, midstream urine specimens will be collected (one sample per participant) to detect pyuria, to perform microbial analysis of the urine and to rule out pregnancy. After performing the above-mentioned tasks, participants will be divided into two groups receiving one of the two study treatments in each centre (figure 1).
Course of the study and follow-up
After randomisation, study drugs, questionnaires for recording adverse events and concurrent use of other medications (pain killers, antibiotics or other OTC drugs) and food supplements that were started during the 7-day treatment trial will be distributed by a study nurse (table 1).
Table 1.
SPIRIT flow chart: schedule of enrolment, interventions and assessments
| Timepoint | Study period | ||||||||
| Enrolment | Allocation | Postallocation | Close-out | Follow-up | |||||
| Day 0 | Day 0 | Day 1 | Day2 | Day3 | Day4 | Day 5 | Day 7 | Day 90 | |
| Enrolment: | |||||||||
| Eligibility screen | X | ||||||||
| Informed consent | X | ||||||||
| Assessment of inclusion/exclusion criteria | X | ||||||||
| Allocation, randomisation | X | ||||||||
| Interventions: | |||||||||
| Pharmacotherapy |

|
||||||||
| Assessments: | |||||||||
| ACSS score | X | X | |||||||
| Patient diaries | X | ||||||||
| Urine culture | X | X | |||||||
| Follow-up interview | X | ||||||||
ACSS, Acute Cystitis Symptom Score; SPIRIT, Standard Protocol Items: Recommendations for Interventional Trials.
Patients will be asked to take the study drugs as follows: 1 sachet at the first evening after the last urination before going to sleep, and two tablets three times a day for seven consecutive days. Patients will also be asked to record adverse events and concurrent use of other medications (pain medications, antibiotics or other OTC drugs) and food supplements for seven consecutive days in diaries. The diaries will be collected on day 7 by the assigned study nurses.
Patients will be asked to return the centre for a follow-up at day 7 to complete an ACSS questionnaire and to return the diaries. Clinical efficacy will be defined as a summary score of the six typical symptoms of the ACSS no more than 5, with no item >1 and visible haematuria absent. Moreover, to evaluate the efficacy of the treatment arms midstream urine specimens will also be collected on day 7(one sample per patient). Microbiological efficacy will be determined in the subgroups of patients with significant bacteriuria (CFU >105/ml) versus those with CFU values of 103–104/ml vs <103/mL at day 0 and elimination of bacteriuria (CFU <103/ml) at day 7 according to EMA (European Medicines Agency) and FDA (United States Food and Drug Administration) guidelines.24 25 ACSS questionnaires (10 questionnaire/patient) will be distributed to patients with the request to fill in a questionnaire each time when there is a recurrence of UTI.
There will be a long-term follow-up documentation on day 90, when patients will be contacted to collect ACSS questionnaires and will be and interviewed for numbers of UTI recurrences, UTI-related consultations, days of sick leave and medications and food supplements taken for UTI. The aforementioned data will be transferred to the eCRF by study nurses.
Data management
Data handling
Data handling will be performed confidentially and anonymously and by the data monitoring committee (DMC). eCRFs will be used. Principal investigator will have full access for the database. Accuracy, completeness and legibility of the data in the eCRF will be ensured by the investigator. Detailed data flow will be described in a data management plan. Data from completed eCRFs will be validated at DMC according to a data cleaning plan under the direction of the data manager. All changes to eCRFs will be documented.
Discussion
Although herbal preparations of bearberry leaves are available as a medicine, their clinical efficacy and safety has not been confirmed in acute uncomplicated cystitis. The assessment of their efficacy is of primary importance: if it does not have a sufficient clinical efficacy, it should not be considered as an alternative to antibiotic treatment; however, if it is non-inferior to fosfomycin, it could be used as an alternative to fosfomycin, a traditional antibiotic. The study was designed to assess non-inferiority of bearberry leaf compared with fosfomycin for patients with acute uncomplicated cystitis.
Results of this clinical trial will help to revise the recommendations on the treatment of uncomplicated cystitis. If bearberry leaf proves to be non-inferior to fosfomycin, the therapeutic approach of uncomplicated cystitis may be changed in a long term, which might lead to fewer antibiotic prescriptions and subsequent lower antibiotic resistance rates.
Ethics and dissemination
The study has been approved by the Scientific and Research Ethics Committee of the Hungarian Medical Research Council (IV/4225-1/2021/EKU).
This clinical study will be conducted following the Declaration of Helsinki. It will be conducted in compliance with the protocol, good clinical practice (GCP) (2001/20/EEC, CPMP/ICH/135/95), designated standard operating procedures, and local laws and regulations relevant to the country of conduct. The study has been approved by the Scientific and Research Ethics Committee of the Hungarian Medical Research Council (IV/4225-1/2021/EKU). All patients voluntarily sign the informed consent form, indicating that they agree to participate.
During the study, all investigators will report adverse or serious adverse events on a separate form which must be sent to both SC and DMC. If any adverse or serious adverse effects emerge, SC will discuss and, if the adverse effect is confirmed, it will be reported to the relevant institutional and national ethical committee (http://www.ett.hu/tukeb.htm).
The results of this study will be disseminated by publication of peer-reviewed manuscripts and presentation at national and international scientific meetings.
Supplementary Material
Footnotes
Contributors: BT: conceptualisation, writing the protocol. NV: writing the protocol, methodology. AJ, SV and NG: methodology, critical revising of the protocol. PB, GZ, KN, RL, BC-L, PH, PN and KN: critical revising of the protocol. DC: conceptualisation, writing the protocol, supervision.
Funding: Centre costs (IT, biostatistics, trial organisation, etc) are covered by the University of Pécs, Momentum Grant of the Hungarian Academy of Sciences (LP2014-10/2014); and Economic Development and Innovation Operative Programme Grant and Highly Cited Publication Grant of the National Research, Development and Innovation Office (GINOP-2.3.2-15-2016-00015, KH-125678). Since no additional treatment is necessary for the study, the general healthcare costs are covered by the National Healthcare System (grant number not applicable). This study was designed with help of the Centre for Translational Medicine at the University of Pécs. This centre is committed to improve patients’ life with research activities (http://www.tm-pte.org/).
Disclaimer: The contribution of the author R. Länger does not necessarily represent the views of the AGES Medizinmarktaufsicht / BASG in Austria nor of the (committees of the) European Medicines Agency.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Naber KG, Schito G, Botto H, et al. Surveillance study in Europe and Brazil on clinical aspects and antimicrobial resistance epidemiology in females with cystitis (ARESC): implications for empiric therapy. Eur Urol 2008;54:1164–78. 10.1016/j.eururo.2008.05.010 [DOI] [PubMed] [Google Scholar]
- 2.Keating GM. Fosfomycin trometamol: a review of its use as a single-dose oral treatment for patients with acute lower urinary tract infections and pregnant women with asymptomatic bacteriuria. Drugs 2013;73:1951–66. 10.1007/s40265-013-0143-y [DOI] [PubMed] [Google Scholar]
- 3.Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the infectious diseases Society of America and the European Society for microbiology and infectious diseases. Infect Dis 2011;52:e103–20. 10.1093/cid/ciq257 [DOI] [PubMed] [Google Scholar]
- 4.Kranz J, Schmidt S, Lebert C, et al. The 2017 update of the German clinical guideline on epidemiology, diagnostics, therapy, prevention, and management of uncomplicated urinary tract infections in adult patients. Part II: therapy and prevention. Urol Int 2018;100:271–8. 10.1159/000487645 [DOI] [PubMed] [Google Scholar]
- 5.Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the infectious diseases Society of America and the European Society for microbiology and infectious diseases. Clin Infect Dis 2011;52:561–4. 10.1093/cid/cir102 [DOI] [PubMed] [Google Scholar]
- 6.Kranz J, Schmidt S, Lebert C, et al. [Epidemiology, diagnostics, therapy, prevention and management of uncomplicated bacterial outpatient acquired urinary tract infections in adult patients : Update 2017 of the interdisciplinary AWMF S3 guideline]. Urologe A 2017;56:746–58. 10.1007/s00120-017-0389-1 [DOI] [PubMed] [Google Scholar]
- 7.EAU guidelines: urological infections | Uroweb, (n.d.). Available: https://uroweb.org/guideline/urological-infections/ [Accessed 10 Mar 2020].
- 8.Gardiner BJ, Stewardson AJ, Abbott IJ, et al. Nitrofurantoin and fosfomycin for resistant urinary tract infections: old drugs for emerging problems. Aust Prescr 2019;42:14–19. 10.18773/austprescr.2019.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falagas ME, Vouloumanou EK, Samonis G, et al. Fosfomycin. Clin Microbiol Rev 2016;29:321–47. 10.1128/CMR.00068-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pienbroek E, Hermans J, Kaptein AA, et al. Fosfomycin trometamol in a single dose versus seven days nitrofurantoin in the treatment of acute uncomplicated urinary tract infections in women. Pharm World Sci 1993;15:257–62. 10.1007/BF01871127 [DOI] [PubMed] [Google Scholar]
- 11.Stein GE. Comparison of single-dose fosfomycin and a 7-day course of nitrofurantoin in female patients with uncomplicated urinary tract infection. Clin Ther 1999;21:1864–72. 10.1016/S0149-2918(00)86734-X [DOI] [PubMed] [Google Scholar]
- 12.Minassian MA, Lewis DA, Chattopadhyay D, et al. A comparison between single-dose fosfomycin trometamol (Monuril®) and a 5-day course of trimethoprim in the treatment of uncomplicated lower urinary tract infection in women. Int J Antimicrob Agents 1998;10:39–47. 10.1016/S0924-8579(98)00021-1 [DOI] [PubMed] [Google Scholar]
- 13.Huttner A, Kowalczyk A, Turjeman A, et al. Effect of 5-day nitrofurantoin vs single-dose fosfomycin on clinical resolution of uncomplicated lower urinary tract infection in women. JAMA 2018;319:1781–9. 10.1001/jama.2018.3627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loras C, Mendes AC, Peixe L, et al. Escherichia coli resistant to fosfomycin from urinary tract infections: detection of the fosA3 gene in Spain. J Glob Antimicrob Resist 2020;21:414–6. 10.1016/j.jgar.2020.01.023 [DOI] [PubMed] [Google Scholar]
- 15.Oteo J, Bautista V, Lara N, et al. Parallel increase in community use of fosfomycin and resistance to fosfomycin in extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli. J Antimicrob Chemother 2010;65:2459–63. 10.1093/jac/dkq346 [DOI] [PubMed] [Google Scholar]
- 16.E. medicines Agency, European Union herbal monograph on Arctostaphylos uva-ursi (L.) Spreng., folium, 2009. Available: www.ema.europa.eu/contact [Accessed 23 Feb 2020].
- 17.Moskalenko SA. Preliminary screening of far-eastern ethnomedicinal plants for antibacterial activity. J Ethnopharmacol 1986;15:231–59. 10.1016/0378-8741(86)90163-7 [DOI] [PubMed] [Google Scholar]
- 18.E. Medicines Agency, Uvae ursi folium - AR, n.d.. Available: www.ema.europa.eu/contact [Accessed 23 Feb 2020].
- 19.Moore M, Trill J, Simpson C, et al. Uva-ursi extract and ibuprofen as alternative treatments for uncomplicated urinary tract infection in women (ATAFUTI): a factorial randomized trial. Clin Microbiol Infect 2019;25:973–80. 10.1016/j.cmi.2019.01.011 [DOI] [PubMed] [Google Scholar]
- 20.Afshar K, Fleischmann N, Schmiemann G, et al. Reducing antibiotic use for uncomplicated urinary tract infection in general practice by treatment with uva-ursi (REGATTA) - a double-blind, randomized, controlled comparative effectiveness trial. BMC Complement Altern Med 2018;18:203. 10.1186/s12906-018-2266-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan A-W, Tetzlaff JM, Altman DG, et al. Spirit 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acute simple cystitis in women - UpToDate, (n.d.). Available: https://www.uptodate.com/contents/acute-simple-cystitis-in-women [Accessed 24 Feb 2020].
- 23.Alidjanov JF, Abdufattaev UA, Makhsudov SA, et al. New self-reporting questionnaire to assess urinary tract infections and differential diagnosis: acute cystitis symptom score. Urol Int 2014;92:230–6. 10.1159/000356177 [DOI] [PubMed] [Google Scholar]
- 24.EMA . Guideline on the evaluation of medicinal products indicated for treatment of bacterial infections, Rev. 3. London, 2018. [Google Scholar]
- 25.Uncomplicated urinary tract infections: developing drugs for treatment guidance for industry, Rockville, 2019. Available: https://www.fda.gov/drugs/guidance-compliance-regulatory-information/guidances-drugs [Accessed 10 Aug 2021].
- 26.Magyar A, Alidjanov J, Pilatz A, et al. The role of the acute cystitis symptom score questionnaire for research and antimicrobial stewardship. validation of the Hungarian version. Cent European J Urol 2018;71:134–41. 10.5173/ceju.2018.1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alidjanov JF, Naber KG, Pilatz A, et al. Additional assessment of acute cystitis symptom score questionnaire for patient-reported outcome measure in female patients with acute uncomplicated cystitis: Part II. World J Urol 2020;38:1977–88. 10.1007/s00345-019-02948-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huttner A, Verhaegh EM, Harbarth S, et al. Nitrofurantoin revisited: a systematic review and meta-analysis of controlled trials. J Antimicrob Chemother 2015;70:2456–64. 10.1093/jac/dkv147 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.