Abstract
As global studies report varying trends in antibiotic susceptibility of uropathogens, it is necessary to have current and constant information on the prevalence of urinary tract infections, the causative pathogens, and their susceptibility profiles, for effective management in specific geographical settings. This prospective cross-sectional study focused on the prevalence of urinary tract infections, etiological agents, and their antibiogram in a secondary and tertiary care hospital in Northern Ghana. Urine samples collected from 219 patients of all age groups were cultured on cysteine lactose electrolyte deficient agar. Pathogens were identified following standard microbiological methods, and their susceptibility to antibiotics was determined by the Kirby-Bauer disk diffusion method. Approximately 34% of the patients had significant bacteria, but the prevalence was slightly higher (P = .763) in the Tertiary care hospital (37.3%) than in the Secondary hospital (30.3%). Patients who were 60 years and above (27.0%) were commonly found with UTIs followed by the year group 20 to 29 years (20.3%). Although all the diagnoses had a positive relationship with urinary tract infection except Pyelonephritis, none of the underlying conditions was a significant (P > .05) predictor of urinary tract infection, with the odds ratio indicating that patients with hyperparathyroidism and dysuria had 2.606 times more likely increased risk or predictor of urinary tract infection. Ten different pathogens were identified, but Escherichia coli and Staphylococcus saprophyticus were frequently encountered. Gram-negative isolates generally showed more resistance. High resistance against ampicillin (100%), trimethoprim-sulfamethoxazole (88.5%), chloramphenicol (84.6%), augmentin (69.2%), ceftriaxone (69.2%), and ciprofloxacin (61.5%) were recorded. Amikacin was relatively effective against isolated pathogens. The high records of resistance among uropathogens and the occurrence of multidrug resistance (92%) reiterate the urgent call for rigorous surveillance of antimicrobial resistance among infectious pathogens in Ghana.
Keywords: Urinary tract infection, resistance, Tamale, Ghana
Introduction
Urinary tract infection (UTI) is a significant health problem caused by the growth of a large number of microbes in the urinary tract. It is a common clinical finding in most hospitals around the world, with an estimated episode of 150 million encounters each year. 1 UTI is a sequel of morbidity and high health care expenditures in all gender and age groups. 2 The infection can occur in 40% to 50% of women and 5% of men, attributable to elements of hormonal changes during pregnancy and anatomical differences, highlighting the higher incidence in women than in men.3 -5 The prevalence may also advance with old age, diabetes, catheterization, sexual activity, menopause, lack of circumcision, and prostate problems. Predisposing to anatomical, functional, or metabolic abnormalities is part of the complicating factors of UTI. Individuals with spinal cord injury have a greater possibility of acquiring urinary tract infection due to chronic use of catheters and voiding dysfunction. 6
The notable organisms responsible for UTIs are Enterobacterales, especially E. coli, accounting for 80% to 85% of cases, although fungal and viral pathogens have also been mentioned. 7 Most UTIs are self-limiting, but untreated and poorly managed infections can pose a serious health risk through the spread of pathogens into the ureters and the kidneys resulting in pyelonephritis; capable of claiming lives. 8
The global upsurge in antibiotic resistance is a public health threat, and in the developing world, the situation is important. This is because, in developing regions, several factors contribute to the problem of drug resistance. The readily available over-the-counter drugs, illiteracy, and fake and cheap substandard drugs are part of the reasons that promote the misuse and abuse of drugs. 9
In Ghana, effective treatment of UTIs is often hampered by inadequate facilities for isolation and antimicrobial susceptibility testing. This can sometimes lead to urologic complications due to untreated, undetected, and improperly treated UTIs. 10
Northern Ghana is a region of limited resources in terms of health care. Microbiological services (culture and susceptibility test) are occasionally available at the only teaching hospital in Tamale, the capital. The published data on UTIs are generally scarce and are mostly reported from other regions of the country, revealing striking resistance among isolates to commonly prescribed antibiotics.11 -14
Insignificant data remain on UTIs in this study area as there is no publication describing the prevalence, etiologies, and susceptibility profile of uropathogens. Therefore, it is mandatory to carry out this research to provide information on prevalence, type of microbial isolates, and susceptibility to relevant antibiotics in secondary and tertiary care hospitals for the practical management of infections.
Materials and Methods
Study design
This is a prospective cross-sectional study that was carried out from 16th April 2018 to 10th September 2018 at the Tamale Teaching Hospital (TTH) and the Tamale Central Hospital (TCH). The Tamale Teaching Hospital is an 800-bed capacity tertiary care facility affiliated with the School of Medicine of the University for Development Studies. It is the only tertiary care hospital that provides referral services to the 5 regions in the northern sector of Ghana. The Tamale Central Hospital is a 186-bed capacity secondary care facility that supports the teaching hospital in providing healthcare services to the people of Tamale, and its surrounding towns and villages.
Recruitment of study participants
A total of 219 patients of all age groups seeking medical attention for urinary tract conditions at these 2 facilities were approached and those who gave their consent were eligible and therefore recruited. Consent of the babies and children was obtained from their parents. Patients who did not present with UTI conditions, as well as those who had UTI conditions but refused to participate, were excluded from the study. Verbal informed consent was obtained because most participants were unwilling to give their written consent.
Specimen collection and processing
In this study, clean urine samples from consent patients submitted to the TTH bacteriology laboratory for analysis were used. Urine samples collected in sterile screwed urine containers were transported on ice packs within 4 hours after collection to the Spanish laboratory of the University for Development Studies for processing and analysis.
However, patients with suspected cases of UTI at the TCH were given sterile urine containers and the procedure for clean-catch midstream urine was demonstrated, as the facility rarely performs urine culture. Approximately 10 and 5 ml of urine were obtained, respectively, from the adult and child participants into sterile screwed urine containers. Urine samples were transported on ice packs within 4 hours after collection to the Spanish laboratory of the University for Development Studies for processing and analysis.
Urine culture and identification
Urine samples were cultured on cysteine lactose electrolyte-deficient agar (CLED) using a standardized (0.01 ml) wire loop. Plates were incubated at 37℃ and read after 24 hours for significant bacteriuria. Plates with significant microbial growth were defined as a quantitative count of ⩾1 × 105 cfu/ml. 15 Pathogens were identified using standard biochemical tests (indole, citrate), mannitol salt agar, and sugar fermentation using Triple Sugar Iron agar from 24 hours pure culture colonies.
Confirmation of S. aureus by Mannitol salt fermentation test
Colonies of gram-positive cocci catalase-positive strains were inoculated on mannitol salt agar and incubated at 37 ± 2℃ for 24 hours. Colonies appearing yellow with yellow zones were classified as Staphylococcus aureus, while colonies with pink coloration were classified as coagulase-negative Staphylococcus.
The susceptibility to Novobiocin was used to confirm isolates of Staphylococcus saprophyticus and Staphylococcus epidermidis from the coagulase-negative Staphylococcus.
Triple sugar iron (TSI) test
Suspected colonies were stabbed in the butt and streaked on the slants of TSI agar and incubated at 37 ± 2℃ for 24 hours. The organisms were identified based on the acid and alkaline reactions, hydrogen sulfide, and gas production in the medium.
Indole test
A loop full of distinct colonies was inoculated into a tryptone soya broth, incubated at 37 ± 2℃ for 24 hours, then 3 drops of the indole reagent were added after incubation. The appearance of a red ring was indicative of Indole positive isolate.
Citrate test
Citrate agar tubes were stabbed with suspected colonies and incubated at 37 ± 2℃ for 24 hours. A blue coloration of media was indicative of Citrate positive isolate.
Susceptibility testing of bacterial isolates
The Kirby-Bauer disk diffusion method was adopted for the sensitivity test in this study. Mueller-Hinton agar plates were inoculated with 0.5 McFarland standard saline suspension using a sterile swab stick. The antibiotics were applied to the inoculated plates and incubated at 37℃ for 24 hours. Antibiotics tested included ciprofloxacin 10 µg, gentamicin 10 µg, erythromycin 15 µg, ceftriaxone 30 µg, chloramphenicol 30 µg, nitrofurantoin 50 µg, tetracycline 30 µg, ampicillin 10 µg, clindamycin 10 µg, vancomycin 30 µg, cefoxitin 30 µg, amikacin 30 µg, trimethoprim-sulfamethoxazole 25 µg, imipenem 10 µg, amoxicillin clavulanic acid 30 µg, and norfloxacin 10 µg. The recorded inhibition zones were interpreted using CLSI breakpoints. 16 Quality control strains of Escherichia coli (ATCC 25922) and Staphylococcus aureus (ATCC 25923) were used. 16 Multidrug resistance in this study was defined as the resistance of isolates to 3 or more classes of antibiotics.
Data management
The data were entered into SPSS version 20. Descriptive statistics, such as frequencies and percentages, were used. The data was presented in tables and graphs. Associations between categorical outcome variables were conducted using the Pearson-Chi square test at the 95% significant level. A 2-tailed P-value of ⩽.05 was considered statistically significant. A binary logistic regression analysis was used to determine which diagnosis is the most predictor of UTI. Odds ratios (OR) were used as a prediction measure in the 95% confidence interval and P-values of ⩽.05 were considered significant.
Ethical consideration
The approval for the study was obtained from the Ethics Review Committee of the Tamale Teaching Hospital (TTHERC/25/06/19/14). Patients were asked for their informed consent verbally after sufficient information was provided on the study.
Results
The occurrence and demographic characteristics of patients with UTI in TTH and TCH
Of the 219 samples, 110 came from Tamale Teaching Hospital (TTH) and 109 from Tamale Central Hospital (TCH). One hundred thirteen samples 113 (51.6%) out of the 219 samples did not show significant bacterial growth and 32 (14.6%) were negative (no growth). Two (0.9%) samples had mixed cultures of S. aureus and Candida sp. but were added to the negative samples because they were considered contaminants. The overall prevalence of UTI was 33.8% (74), but a rate of 37.3% (41) and 30.3% (33) were recorded, respectively, in TTH and TCH, Table 1.
Table 1.
Prevalence of UTI and demographic characteristics of patients with UTI in TTH and TCH.
| Prevalence | Tamale teaching hospital | Tamale central hospital | P-value | |
|---|---|---|---|---|
| (n = 110) | (n = 109) | |||
| (%) | (%) Total (%) | |||
| Significant microbial growth | 41 (37.3) | 33 (30.3) | 74 (33.8) | .763 |
| Insignificant microbial growth | 55 (50) | 58 (53.2) | 113 (51.6) | |
| No microbial growth | 14 (12.7) | 18 (16.5) | 32 (14.6) | |
| Demographic characteristics | Frequency of UTI (%) | Frequency of UTI (%) | ||
| Age | ||||
| 1-9 | 5 (12.2) | 1 (3.0) | 6 (8.1) | .06 |
| 10-19 | 1 (2.4) | 3 (9.1) | 4 (5.4) | |
| 20-29 | 5 (12.2) | 10 (30.3) | 15 (20.3) | |
| 30-39 | 4 (9.8) | 7 (21.2) | 11 (14.9) | |
| 40-49 | 6 (14.6) | 6 (18.2) | 12 (16.2) | |
| 50-59 | 3 (7.3) | 3 (9.1) | 6 (8.1) | |
| 60 and above | 17 (41.5) | 3 (9.1) | 20 (27.0) | |
| Sex | ||||
| Male | 26 (63.4) | 10 (30.3) | 36 (48.6) | .958 |
| Female | 15 (36.6) | 23 (69.7) | 38 (51.4) | |
| Total | 41 (100) | 33 (100) | 74 (100) | |
The population of female patients suffering from UTIs was 51.4% versus 48.6% of male patients. However, in the various hospitals, UTIs in males (63.4%) were more than in females (36.6%) in TTH, and more females (69.7%) than males (30.3%) were recorded in TCH. But these observed differences were not statistically significant, P = .958. The age of the patients ranged from 1 to 86 years with an average age of 39.18. At TTH, most (41.5%) of the UTI patients came from the age group 60 years and older, contrasting records from TCH where 51.5% of the patients were within the age group 20 to 39 years, Table 1. Age (P = .495) and sex (P = .166) did not have a significant association with UTI.
Of the 41 and 33 UTI-positive cases from TTH and TCH, respectively, 29 and 10 of the patients, respectively, had underlying conditions. The main condition of TTH was benign prostatic hyperplasia (BPH) (12/29) as HIV infection was common among UTI patients in TCH. None of the underlying conditions was a significant (P > .05) predictor of UTI, though all the diagnoses had a positive relationship with UTI except Pyelonephritis, With the odds ratio indicating that patients with hyperparathyroidism, dysuria, and benign prostatic hyperplasia had 2.606, 2.606, and 1.694 times, respectively, more likely increased risk or predictor of UTI, Table 2.
Table 2.
Binary logistic regression on diagnoses of UTI in both Tamale teaching and Tamale central hospitals.
| Diagnosis | Coefficient | P-value | OR | 95% CI |
|---|---|---|---|---|
| HIV | 0.137 | .812 | 1.146 | 0.371-3.547 |
| Benign prostate hyperplasia | 0.527 | .385 | 1.694 | 0.516-5.562 |
| Diabetes | 0.041 | .963 | 1.042 | 0.182-5.958 |
| Dysuria | 0.958 | .390 | 2.606 | 0.294-23.131 |
| Hyperparathyroidism | 0.958 | .390 | 2.606 | 0.294-23.131 |
| Pyelonephritis | −0.246 | .792 | 0.782 | 0.125-4.887 |
| Urine retention | 0.304 | .590 | 1.355 | 0.449-4.092 |
| Vesicovaginal fistula | 0.447 | .703 | 1.563 | 0.157-15.559 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Distribution of uropathogens among gender in TTH and TCH
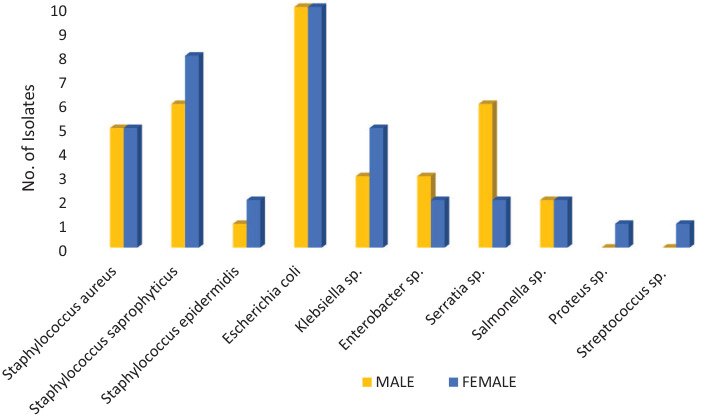
A total of 10 pathogens were isolated, all 10 were recovered from females but 8 were obtained from men. Escherichia coli was the most common pathogen, followed by S. saprophyticus, S. aureus, Klebsiella sp., and Serratia sp. Proteus sp. and Streptococcus sp. were recovered only from females. More Serratia sp. and Enterobacter sp. were found in males than in females, whereas greater numbers of S. saprophyticus and Klebsiella sp., were seen in females. The difference in pathogen isolation between genders was not statistically significant (P = .817), Figure 1.
Figure 1.
Distribution of microbial isolates among gender of patients with UTI at TTH and TCH.
 Microbial isolates from female.
Microbial isolates from female.  Microbial isolates
from male.
Microbial isolates
from male.
P value = .817.
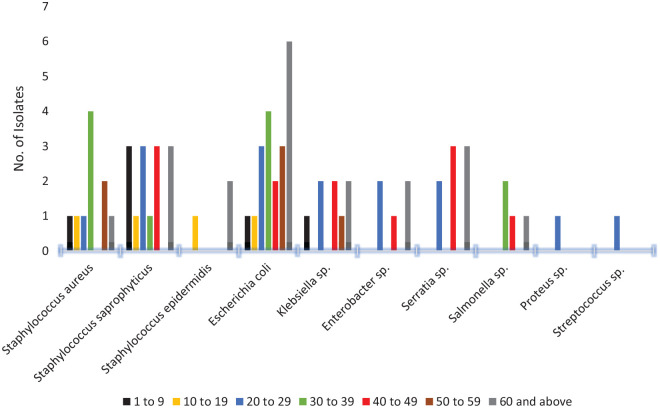
Pathogen distribution among the various age groups in both hospitals
The main pathogens found among age groups 1 to 9 years, and 50 years and older were respectively, Staphylococcus saprophyticus and E. coli. In the 30 to 39 year age group, the main pathogens were S. aureus and E. coli while S. saprophyticus and Serratia sp. were seen primarily among the 40 to 49 year group. Staphylococcus epidermidis was only isolated from the age group 10 to 19 years, Figure 2.
Figure 2.
Isolates distribution among various age groups of patients with UTI at TTH and TCH.
 Isolates from patients aged 1 to 9 years,
Isolates from patients aged 1 to 9 years,  10 to 19,
10 to 19,
 20
to 29,
20
to 29,  30 to 39,
30 to 39,  40 to 49,
40 to 49,
 50
to 59, and
50
to 59, and  60 years and
older.
60 years and
older.
Resistance profile of uropathogens isolated from TTH and TCH
Gram-negative pathogens isolated from both hospitals showed significantly (P = .000) greater resistance than Gram-positive strains. The Teaching hospital strains were also more resistant than Central hospital isolates, P = .000. The Gram positives were moderately resistant to amikacin, gentamicin (0%-26.7%), and ciprofloxacin (15.4%-20%). Except for ampicillin, where Gram-positive resistance was up to 80%, general resistance of less than 55% was recorded against the remaining antibiotics. In Gram-negatives, the resistance of up to 30% was recorded against amikacin (10%-23.1%) and Imipenem (10%-30.8%) while a range of 20% to 100% was observed against the remaining drugs, Table 3.
Table 3.
Resistance profile of uropathogens recovered from Tamale teaching and Tamale central hospitals.
| Antibiotic | Tamale teaching hospital | Tamale central hospital | P-value | ||
|---|---|---|---|---|---|
| Resistance % | |||||
| Gram-positive 15 | Gram-negative 26 | Gram-positive 13 | Gram-negative 20 | ||
| Ciprofloxacin | 20.0 | 61.5 | 15.4 | 25.0 | .000 |
| SXT | 46.7 | 88.5 | 23.1 | 75.0 | |
| Gentamicin | 26.7 | 57.7 | 15.4 | 20.0 | |
| Amikacin | 26.7 | 23.1 | 0.0 | 10.0 | |
| Ampicillin | 80.0 | 100 | 53.8 | 100 | |
| Augmentin | 46.7 | 69.2 | 0.0 | 45.0 | |
| Chloramphenicol | 53.3 | 84.6 | 30.8 | 55.0 | |
| Nitrofurantoin | 33.3 | 88.5 | 38.5 | 85.0 | |
| Vancomycin | 33.3 | NT | 30.8 | NT | |
| Ceftriaxone | 46.7 | 69.2 | 38.5 | 60.0 | |
| Erythromycin | 40.0 | 88.5 | 30.8 | 70.0 | |
| Tetracycline | 40.0 | 84.6 | 23.1 | 75.0 | |
| Cefoxitin | 46.7 | NT | 7.7 | NT | |
| Norfloxacin | 26.7 | 61.5 | 38.5 | 35.0 | |
| Clindamycin | 40.0 | NT | 30.8 | NT | |
| Imipenem | NT | 30.8 | NT | 10.0 | |
Abbreviations: NT, not tested; SXT, trimethoprim-sulfamethoxazole.
Distribution of resistant strains among sex
Generally, isolates from males showed significantly more resistance than isolates from females, P = .000. The resistance of Gram-positive isolates to the antibiotics tested ranged from 0% to 56.3% in the isolates recovered from females versus 25% to 83.3% in the isolates recovered from males. In Gram-negative isolates, the recorded resistance was between 22.7% and 95.5% in the isolates recovered from females versus 12.5% to 100% in the isolates recovered from males. The aminoglycosides (gentamicin, amikacin) and ciprofloxacin were relatively effective against the female Gram-positive isolates, with respective resistance of 0%, 6.3%, and 6.3%, while Gram-negative isolates obtained from females were moderately susceptible to amikacin and imipenem with only 22.7% being resistant to imipenem these drugs. Both Gram-positive and negative isolates obtained from males showed resistance of below 30% to amikacin and imipenem, Table 4.
Table 4.
Distribution of resistant pathogens among gender in both Tamale teaching and Tamale central hospitals.
| Antibiotic | Female | Male | P-value | ||
|---|---|---|---|---|---|
| Resistance (%) | |||||
| Gram-positive 16 | Gram-negative 22 | Gram-positive 12 | Gram-negative 24 | ||
| Ciprofloxacin | 6.3 | 31.8 | 41.7 | 58.3 | .000 |
| SXT | 18.8 | 77.3 | 58.3 | 87.5 | |
| Gentamicin | 0.0 | 31.8 | 50.0 | 50.0 | |
| Amikacin | 6.3 | 22.7 | 25.0 | 12.5 | |
| Ampicillin | 56.3 | 95.5 | 83.3 | 100.0 | |
| Augmentin | 12.5 | 45.5 | 41.7 | 58.3 | |
| Chloramphenicol | 25.0 | 63.6 | 66.7 | 79.2 | |
| Nitrofurantoin | 37.5 | 95.5 | 33.3 | 79.2 | |
| Vancomycin | 25.0 | NT | 41.7 | NT | |
| Ceftriaxone | 31.3 | 68.2 | 58.3 | 62.5 | |
| Erythromycin | 31.3 | 81.8 | 41.7 | 79.2 | |
| Tetracycline | 18.8 | 77.3 | 50.0 | 83.3 | |
| Cefoxitin | 18.8 | NT | 41.7 | NT | |
| Norfloxacin | 18.8 | 40.9 | 50.0 | 58.3 | |
| Clindamycin | 31.3 | NT | 41.7 | NT | |
| Imipenem | NT | 22.7 | NT | 20.8 | |
Abbreviations: NT, not tested; SXT, trimethoprim-sulfamethoxazole.
Multidrug resistance among isolated uropathogens in TTH and TCH
Multidrug resistance was the typical occurrence among all the recovered pathogens. Of the 41 pathogens recovered from patients from the Tamale Teaching Hospital, 38 (92.7%) showed multiple resistance to the tested drugs. At the Tamale Central hospital, 29 (87.9%) of the 33 isolated pathogens were multidrug-resistant, Table 5.
Table 5.
Multidrug resistance among UTI pathogens in Tamale teaching and Tamale central hospitals.
| Isolates | Tamale teaching hospital | Tamale central hospital | P-value | ||
|---|---|---|---|---|---|
| No of isolates | MDR (%) | No of Isolates | MDR (%) | ||
| Staphylococcus aureus | 4 | 4 (100) | 6 | 5 (83.3) | .483 |
| Staphylococcus saprophyticus | 8 | 7 (87.5) | 6 | 3 (50.0) | |
| Staphylococcus epidermidis | 2 | 1 (50.0) | 1 | 1 (100) | |
| Escherichia coli | 10 | 10 (100) | 10 | 10 (100) | |
| Klebsiella sp. | 5 | 5 (100) | 3 | 3 (100) | |
| Enterobacter sp. | 3 | 3 (100) | 2 | 2 (100) | |
| Serratia sp. | 6 | 6 (100) | 2 | 2 (100) | |
| Salmonella sp. | 2 | 2 (100) | 2 | 2 (100) | |
| Proteus sp. | NI | NI | 1 | 1 (100) | |
| Streptococcus sp. | 1 | 0 (0) | NI | NI | |
| Total | 41 | 38 (92.7) | 33 | 29 (87.9) | |
Abbreviations: MDR, multidrug resistance; NI, not isolated. .
Discussion
This study addressed antibiotic resistance of pathogens associated with urinary tract infections in patients seeking medical care in a secondary and tertiary care facility, intending to provide data to improve the efficient treatment of infections. The overall prevalence of UTI was 33.8%, which is comparable to the reported rates of 31.6% in Accra, Ghana, 11 and 32.2% in Uganda, 17 but sharply contrasts 90.1% rate in Ethiopia. 18 The main sources of UTIs recounted in similar studies in the West African sub-region suggest cross-infection from spouses, poor personal hygiene, pregnancy, and reinfection due to poor antibiotic compliance.18,19
The higher prevalence of UTIs at the Tamale Teaching Hospital could be due to its status as a tertiary care hospital and a referral center where advanced levels of care and diagnostics are available compared to the Tamale Central Hospital which is a secondary care facility. This disparity in the level of healthcare may have contributed to the difference in observed prevalence.
The general proportions of females with UTI were slightly higher than males, however, in the respective hospitals, more males with UTI were recorded in the TTH while the opposite was observed in the TCH. This occurrence possibly reveals the gender distribution in both hospitals. About 41% of the study patients at the Teaching Hospital were men of 60 years and above, and men in this age bracket are at increased risk of contracting UTI due to elements of kidney stones or prostate complications. 6 On the other hand, 51.5% of the study population in the Central hospital were women in the age range of 20 to 39 years and they are identified as a susceptible group to UTI infections due to the female pelvic anatomy, increased sexual activity, poor hygiene, and use of contraceptives, among other factors.18,20 In a related study at the Ghana Police Hospital in Accra, more males than females presenting with UTI infections were also described. 11 Many UTI studies usually report a common occurrence of higher prevalence in females,3,19,21,22 which contradicts our record in the Teaching hospital; however, the greater recovery of pathogens from the urine of our female patients corroborates these literature reports.
Numerous organisms can colonize and cause infection in the urinary tract, but Gram-negative bacteria can account for about 85% of all UTI cases because they are normal flora of the rectum, which is close to the urethral orifice. 23 Escherichia coli was the leading pathogen among isolated Gram-negatives (Klebsiella sp, Enterobacter sp, Serratia sp, Salmonella sp., and Proteus sp.) in this study, which is consistent with studies in Ghana and other countries.11,17,18 Quite some Serratia sp. were isolated from our patients. These species, according to reports, are gradually gaining notoriety for catheter-associated infections, urologic obstructions, recurrent, and nosocomial infections. 24 Most of Serratia sp. came from males in the Teaching Hospital with underlying conditions of chronic urine retention, diabetes mellitus, hyperparathyroidism, benign prostate hyperplasia, and appendicitis. Although none of the screened female patients at this hospital had the pathogen; at the Central hospital, 2 of the species were isolated from 2 females with no underlying disease.
Gram-positive uropathogens are mostly recovered from young females and are responsible for about 10% to 15% of acute symptomatic UTIs. 6 Consistent with this observation, close to 42% of the significant microbial pathogens were Gram positives isolated from females with respective frequencies of 21.1% S. saprophyticus, 13.2% S. aureus, and 5.3% S. epidermidis, and 2.6% Streptococcus sp.
Information on the susceptibility of etiological agents of UTI does not only guide clinicians to select appropriate drugs for treatment, but also offers resilient recommendations in the empirical treatment of infections 25 ; since timely treatment, and intervention can prevent complications. Antibiotics including cefuroxime, amoxicillin/clavulanic acid, trimethoprim-sulfamethoxazole (SXT), and fluoroquinolones have been mentioned as part of the drugs of choice for UTIs.26,27 Comparing the resistance patterns of our Gram-positive to Gram-negative; greater resistance was generally observed among the Gram-negative strains and male strains also showed higher resistance. The Teaching hospital pathogens were more resistant to the antibiotics tested. Against augmentin, ciprofloxacin, trimethoprim-sulfamethoxazole (SXT), and ceftriaxone, the recorded resistance was <50% among Gram-positive strains and up to 89% in Gram-negative strains. The height of resistance, particularly among Gram-negatives in this study, corresponds to a national investigation of antibiotic resistance in Ghana that documented resistance of more than 50% among members of Enterobacterales in 2015. 12
Amikacin was relatively active against all uropathogens, which is related to other studies that demonstrated the high efficacy of this antibiotic against ESBL-producing and quinolone-resistant E. coli.11,14 Although amikacin appeared to be appropriate as an empirical drug for the treatment of UTIs in this study, the nephrotoxicity associated with the drug and other aminoglycosides makes it unsuitable, particularly in patients with renal impairment.28,29 Therefore, aminoglycosides are prescribed when it is necessary. 30 This partly explains the relatively higher susceptibility of pathogens to aminoglycosides compared to the other antibiotics frequently used, as evidenced in various epidemiological studies in Ghana.11,12,31 Multiple drug resistance (87.9%) was prevalent among uropathogens, which is of concern due to the limited treatment options associated with this phenomenon. Considerable resistance to vancomycin (30%-33%) and imipenem (10%-31%) was recorded, which is worrying, as these drugs are part of the last-line drugs for the management of complicated and multidrug-resistant infections in these hospitals (personal communication). Thus, the failure of these last-line drugs could result in greater healthcare costs for the patient and the state.
Conclusions
Urinary tract infection cases were marginally higher at the Tamale Teaching Hospital. The study revealed that Escherichia coli and Staphylococcus saprophyticus are the main uropathogens. Resistance was particularly high among Gram-negative uropathogens to most of the antibiotics tested. Although amikacin appeared suitable for the treatment of UTI in this study, it should be prescribed with caution due to its nephrotoxic potency, especially in renal-infirm patients. It is necessary to consistently conduct studies at each level of healthcare to establish the prevalence, causative pathogens, and their susceptibility pattern to antibiotics to achieve optimal empirical therapy of UTI infections and to help the antibiotic stewardship program of hospitals.
Acknowledgments
The authors thank the laboratory staff of TTH and TCH for the assistance in patient sample collection.
Footnotes
Author Contributions: All authors read and consented to the final draft of the manuscript. ABK was involved in the conception, study design and drafting of the manuscript. CKSS contributed in conception and drafting of the manuscript. DYY was involved in sample collection and processing as well as data analysis.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of Data: We consider our data private, but the corresponding author will make it available upon reasonable request.
Ethics and Consent to Participants: The approval for this study was obtained from the Ethics Review Committee of the Tamale Teaching Hospital (TTHERC/25/06/19/14). Patients were asked for their informed consent verbally after sufficient information was provided on the study.
Significance Statement: The epidemiology of uropathogens is changing as coagulase-negative Staphylococcus and Serratia sp. are emerging top pathogens. The resistance of uropathogens to last-line drugs; vancomycin and imipenem is steadily rising, making them empirically unreliable.
ORCID iD: David Y. Yamik  https://orcid.org/0000-0001-9612-6430
https://orcid.org/0000-0001-9612-6430
References
- 1. Gales AC, Jones RN, Gordon KA, et al. Activity and spectrum of 22 antimicrobial agents tested against urinary tract infection pathogens in hospitalized patients in Latin America: report from the second year of the SENTRY antimicrobial surveillance program (1998). J Antimicrob Chemother. 2000;45:295-303. [DOI] [PubMed] [Google Scholar]
- 2. Orenstein R, Wong ES. Urinary tract infections in adults. Am Fam Physician. 1999;59:1225-NaN34, 1237. [PubMed] [Google Scholar]
- 3. Uehling DT, Hopkins WJ, Balish E, Xing Y, Heisey DM. Vaginal mucosal immunization for recurrent urinary tract infection: phase II clinical trial. Urol J. 1997;157:2049-2052. [PubMed] [Google Scholar]
- 4. Kolawole AS, Kolawole Om, et al. Prevalence of urinary tract infections (UTI) among patients attending Dalhatu Araf specialist hospital, Lafia, Nasarawa State, Nigeria. Int J Med Med Sci. 2010;1:163-167. [Google Scholar]
- 5. Totsika M, Moriel DG, Idris A, et al. Uropathogenic Escherichia coli mediated urinary tract infection. Curr Drug Targets. 2012;13:1386-1399. [DOI] [PubMed] [Google Scholar]
- 6. Nicolle LE. Uncomplicated urinary tract infection in adults including uncomplicated pyelonephritis. Urol Clin North Am. 2008;35:1-12. [DOI] [PubMed] [Google Scholar]
- 7. Amdekar S, Singh V, Singh DD. Probiotic therapy: immunomodulating approach toward urinary tract infection. Curr Microbiol. 2011;63:484-490. [DOI] [PubMed] [Google Scholar]
- 8. Lane DR, Takhar SS. Diagnosis and management of urinary tract infection and pyelonephritis. Emerg Med Clin North Am. 2011;29:539-552. [DOI] [PubMed] [Google Scholar]
- 9. Fagan M, Lindbæk M, Grude N, et al. Antibiotic resistance patterns of bacteria causing urinary tract infections in the elderly living in nursing homes versus the elderly living at home: an observational study. BMC Geriatr. 2015;15:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brown BJ, Asinobi AO, Fatunde OJ, Osinusi K, Fasina NA. Antimicrobial sensitivity pattern of organisms causing urinary tract infection in children with sickle cell anaemia in Ibadan, Nigeria. West Afr J Med. 2003;22:110-113. [DOI] [PubMed] [Google Scholar]
- 11. Gyansa-Lutterodt M, Afriyie D, Asare G, Amponsah S, Abutiate H, Darko D. Antimicrobial use and susceptibility pattern of uropathogens associated with urinary tract infections at the Ghana Police Hospital. Glob J Pharmacol. 2014;8:306-315. [Google Scholar]
- 12. Opintan JA, Newman MJ, Arhin RE, Donkor ES, Gyansa-Lutterodt M, Mills-Pappoe W. Laboratory-based nationwide surveillance of antimicrobial resistance in Ghana. Infect Drug Resist. 2015;8:379-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Agyepong N, Govinden U, Owusu-Ofori A, Essack SY. Multidrug-resistant gram-negative bacterial infections in a teaching hospital in Ghana. Antimicrob Resist Infect Control. 2018;7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Donkor ES, Horlortu PZ, Dayie NT, Obeng-Nkrumah N, Labi A-K. Community acquired urinary tract infections among adults in Accra, Ghana. Infect Drug Resist. 2019;12:2059-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mwaka AD, Mayanja-Kizza H, Kigonya E, Kaddu-Mulindwa D. Bacteriuria among adult non-pregnant women attending Mulago hospital assessment center in Uganda. Afr Health Sci. 2011;11:182-189. [PMC free article] [PubMed] [Google Scholar]
- 16. Clinical and Laboratory Standards Institute (CLSI). M100S Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute; 2016. [Google Scholar]
- 17. Odoki M, Almustapha Aliero A, Tibyangye J, et al. Prevalence of bacterial urinary tract infections and associated factors among patients attending hospitals in Bushenyi District, Uganda. Int J Microbiol. 2019;2019:4246780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seifu WD, Gebissa AD. Prevalence and antibiotic susceptibility of uropathogens from cases of urinary tract infections (UTI) in Shashemene referral hospital, Ethiopia. BMC Infect Dis. 2018;18:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johnson JR, Tiu FS, Stamm WE. Direct antimicrobial susceptibility testing for acute urinary tract infections in women. J Clin Microbiol. 1995;33:2316-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scholes D, Hooton TM, Roberts PL, Stapleton AE, Gupta K, Stamm WE. Risk factors for recurrent urinary tract infection in young women. J Infect Dis. 2000;182:1177-1182. [DOI] [PubMed] [Google Scholar]
- 21. Randrianirina F, Soares JL, Carod JF, et al. Antimicrobial resistance among uropathogens that cause community-acquired urinary tract infections in Antananarivo, Madagascar. J Antimicrob Chemother. 2007;59:309-312. [DOI] [PubMed] [Google Scholar]
- 22. Abubakar EM. Antimicrobial susceptibility pattern of pathogenic bacteria causing urinary tract infection in a specialist hospital in Yola Adamawa state. J Clin Med Res. 2009;1:001-008. [Google Scholar]
- 23. Obiogbolu CH, Okonko IO, Anyamere CO, et al. Incidence of urinary tract infections (UTIs) among pregnant women in Akwa metropolis, Southeastern Nigeria. Sci Res Essays. 2009;4:820-824. [Google Scholar]
- 24. Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious diseases society of America: Guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40:643-654. [DOI] [PubMed] [Google Scholar]
- 25. Moyo SJ, Aboud S, Kasubi M, Lyamuya EF, Maselle SY. Antimicrobial resistance among producers and non-producers of extended spectrum beta-lactamases in urinary isolates at a tertiary hospital in Tanzania. BMC Res Notes. 2010;3:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Adjei O, Opoku C. Urinary tract infections in African infants. Int J Antimicrob Agents. 2004;24:S32-S34. [DOI] [PubMed] [Google Scholar]
- 27. Jha N, Bapat SK. A study of sensitivity and resistance of pathogenic micro-organisms causing UTI in Kathmandu valley. Kathmandu Univ Med J. 2005;3:123-129. [PubMed] [Google Scholar]
- 28. Cho SY, Choi SM, Park SH, Lee DG, Choi JH, Yoo JH. Amikacin therapy for urinary tract infections caused by extended-spectrum β-lactamase-producing Escherichia coli. Korean J Intern Med. 2016;31:156-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ramirez MS, Tolmasky ME. Amikacin: uses, resistance, and prospects for inhibition. Molecules. 2017;22:2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martínez-Salgado C, López-Hernández FJ, López-Novoa JM. Glomerular nephrotoxicity of aminoglycosides. Toxicol Appl Pharmacol. 2007;223:86-98. [DOI] [PubMed] [Google Scholar]
- 31. Donkor ES, Newman MJ, Yeboah-Manu D. Epidemiological aspects of non-human antibiotic usage and resistance: implications for the control of antibiotic resistance in Ghana. Trop Med Int Health. 2012;17:462-468. [DOI] [PubMed] [Google Scholar]