Abstract
The COVID-19 pandemic exacerbated the decline in Hepatitis C Virus (HCV) screening and treatment globally in part due to lockdowns and restrictions at healthcare centers. The goal of this retrospective cohort study was to assess the effectiveness of an updated workflow implemented at Boston Medical Center (BMC) HCV clinics. Revised workflow incorporated appointments via telemedicine, transitioning to blood test-based fibrosis scoring, and delivering medication by mail to mitigate the lack of in-person services. We compared 2 cohorts of patients who attended at least the initial intake appointment at BMCHCV clinics: 170 before the pandemic and 133 after the pandemic. Outcome variables included treatment starts, fibrosis lab tests completed, appointment attendance, and SVR achievement. Proportions for outcome variables were compared between groups by use of χ2 and 2-sample t-tests where appropriate. Our results showed a 14.43% decrease in completing fibrosis scoring tests (P-value: <.001) and a 15.21% decrease in medication initiation (P-value: <.001) among the patients who initiated care during the pandemic (modified workflow group). Furthermore, we found a 18.56% decrease in sustained virologic response (SVR) among the modified workflow group when compared to the controls. Overall, these results align with current trends of patients’ decreasing engagement in HCV care but show higher retention than other published data. Furthermore, these figures support how appointments via telemedicine, transitioning to blood test-based fibrosis scoring, and medication delivery by mail can serve as tools to increase access to HCV care and successful HCV treatment completion even after COVID restrictions are lifted.
Keywords: COVID, primary care, prevention, underserved communities, community health, access to care
Introduction
The COVID-19 pandemic has had deleterious effects the detection and treatment of Hepatitis C Virus (HCV). HCV remains a major public health concern with an estimated 2.1 million persons living with the virus in the United States. 1 Throughout the pandemic, health centers across the globe were ill equipped to continue HCV screening and treatment. Globally, 88% of HCV prevention, care and treatment centers experienced disrupted HCV treatment in 2020, with 80% indicating lower patient volumes than pre-COVID numbers. 2 HCV testing at 1 urban medical center decreased by 49.6% after March 2020 and new patient identification decreased by 42.1% hospital wide. 3 Some clinics saw treatment initiation decline between 25% and 40% since the onset of the COVID 19 pandemic. 4 Additionally, interventions and surveillance of cirrhosis and hepatocellular carcinoma were disrupted as elective procedures like Fibroscan and abdominal ultrasounds were delayed or suspended. In-person outpatient clinic visits rapidly decreased due to both patient and clinic cancelations.1,5,6 Global models predict a delay in HCV intervention and treatment by even 1 year during COVID-19 would result in 121 000 excess infections and 906 000 missed diagnoses by 2030. 7 This rapid disengagement demonstrates the great need for health care centers to reevaluate traditional treatment approaches during times when in-person appointments are limited.
Telemedicine has been identified a useful technology that is poised to handle the challenges of these problems. Prior to the pandemic telemedicine has been a successful tool in coordinating testing and treatment of HCV. A study at the Ottawa Hospital–General Campus, a Canadian hospital, showed that treatment uptake and achievement of sustained virologic response (SVR) was similar between outpatient and telemedicine groups. 8 Another study for the ECHO program, which implements tele-education at a New Mexico health sciences center, found similar success in SVR among rural and underserved populations when compared to those treated by specialists. 9 These findings indicate that telemedicine eliminates structural barriers to treatment for some populations and is a potentially effective tool for healthcare centers to use for HCV intervention and treatment during and beyond the COVID-19 pandemic. More recently, some studies have built on the existing evidence of using telemedicine to treat HCV patients during COVID-19.4,10,11
These existing studies, however, predominantly focused on treating patients in remote communities or in countries outside of the United States where the impact of COVID-19 on health care facilities may have looked different. Furthermore, while telemedicine can help bridge the gap in access to provider visits, typical pre-treatment evaluations for Hepatitis C still consist of lengthy, in-person processes including lab specimen collections, liver fibrosis staging, and medication administration and monitoring.12,13 The purpose of this study is to evaluate solutions to these problems, while providing further evidence on the effectiveness of telemedicine on HCV treatment in a large urban safety net hospital in the Unites States. This was done by assessing the effectiveness of unique modifications to HCV treatment, including shifts in fibrosis screening and transition to telemedicine, over the months following the start of the COVID-19 pandemic. The secondary aim is to describe the implications of these modifications on patient linkage to care for similar health care centers in the years to come.
Methods
Study Setting and Intervention
Boston Medical Center is a large urban “safety-net” hospital that receives approximately 1 150 000 patient visits per year. 14 More than 70% of patients seen at the medical center identify as a racial minority while approximately 25% are homeless. 14 Historically, the prevalence of HCV RNA+ patients is 3.94%, which is greater than the national average. 14 BMC has a comprehensive HCV screening and linkage to care program and collocated HCV treatment programs embedded in multiple ambulatory care clinics across the hospital.3,12 Each clinic has a multidisciplinary team including physicians and nurse practitioners clinically trained to treat HCV. Most clinics also work with specialty pharmacists, pharmacy liaisons, patient navigators, and social work case managers.
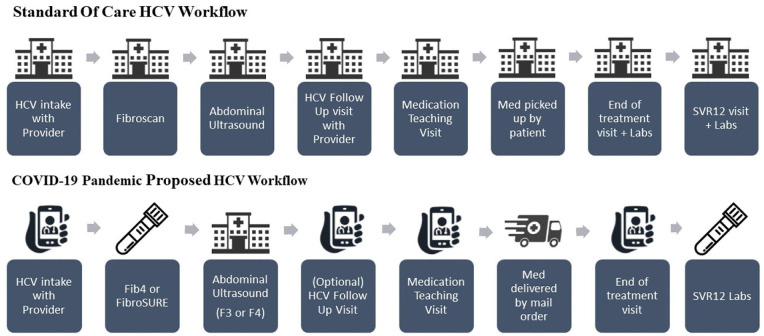
During the early days of the COVID-19 pandemic, in-person appointments at BMC were limited to urgent care and essential monitoring visits only. As a result, BMC saw a 71.9% decrease in testing across ambulatory clinics, and a 63.3% decrease in newly identified HCV+ cases after March 16 2020, even considering hospital-wide implementation of universal 1 time HCV testing in line with the April 2020 United States Preventative Services Taskforce guidelines. 3 Elective procedures were put on hold, meaning that access to HCV care would be delayed for many patients. In response to the challenges posed by the pandemic, HCV clinics at BMC recommended a modified workflow for treating HCV patients (Figure 1).
Figure 1.
Workflow of Boston Medical Center HCV clinics both pre and during COVID-19 pandemic for patients with hepatitis C (March 2020).
First, most appointments with HCV providers and all medication teaching visits with pharmacists were converted to telemedicine. Second, tests for liver fibrosis staging shifted from using in-person Fibroscan® tests to FibroSURE or FIB-4 laboratory tests that could be completed at the medical center or at another location based on patient preference. Both FibroSURE and Fib-4 have been demonstrated to be effective methods for evaluating severe fibrosis.7,15 Fibrosis test results are defined on a scale between F0 and F4 in which F4 indicates the most severe fibrosis of the liver. 7 In keeping with national guidelines, patients with a fibrosis score of F3 or F4 were still recommended to complete an abdominal ultrasound to monitor for hepatocellular carcinoma. 7 Third, medication was dispensed by mail delivery, if possible, to further reduce the patients’ need to come in-person. BMC changed the workflow beginning in March 2020 when an HCV clinic newsletter was circulated to all HCV providers with the above recommendations.
Population Data
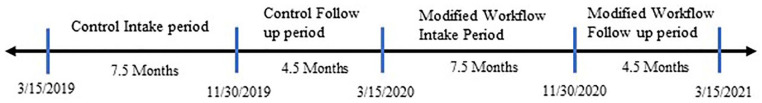
Our study population included HCV positive patients who completed an HCV clinic intake appointment in the adult primary care, family medicine, or infectious diseases departments between March 1, 2019 and July 23, 2021. We assigned patients who had an HCV intake appointment between March 15th, 2019, and November 30th, 2019, as the control group, and those between March 15th, 2020, and November 30th, 2020, as the modified workflow group. We excluded patients who had an intake appointment between December 1st, 2019, and March 14th, 2020, in the control group since these patients’ HCV care may extend beyond the first COVID pandemic lockdown period and become affected by modified workflow (Figure 2). Patients from this control group with follow-up appointments (follow-up with a provider, medication teaching with a pharmacist) after March 14th, 2020, were also excluded to ensure that the control group was not affected by the modified workflow. To ensure equal observation times, similar restrictions were applied to the modified workflow group 1 year later. All clinical data was retrieved from BMC’s HealthCloud network.
Figure 2.
Timeline of observation periods for outcome variables in both the control and modified workflow group.
Outcome Variables
The primary variables of interest include appointment type, fibrosis staging method used, abdominal ultrasound for hepatocellular carcinoma screening (yes; no), appointment attendance, treatment initiation (yes; no), and SVR status (confirmed, unconfirmed). Only patients eligible for SVR tests were included in SVR status; Patient’s SVR eligibility was determined to be 12 weeks after treatment ended. Patients who cleared their original infection of HCV and were later re-infected with another genotype of HCV were designated confirmed SVR. We collected demographic information such as gender (male, female), ethnicity (Hispanic, Non-Hispanic), race (White, Black, Asian, Other, decided not to Answer), homelessness status, any recorded substance use, any recorded alcohol use, primary insurance type (Medicaid, Medicare, private, missing), and age. Other variables collected include medication type (sofosbuvir/velpatasvir 400/100 mg (Epclusa 400/100 mg), ledipasvir/sofosbuvir 90/400 mg (Harvoni 90/400 mg), glecaprevir/pibrentasvir 100/40 mg (Mayvret 100/40 mg), other), and reason for unconfirmed SVR.
Statistical Analysis
All data were analyzed using SAS software (Version 9.4; SAS Institute Inc. Cary, NC). Statistical comparisons of variables between modified workflow and control groups were made by χ2 for categorical variables and 2-sample t-tests for continuous variables. Statistical significance was performed at the 5% level.
Results
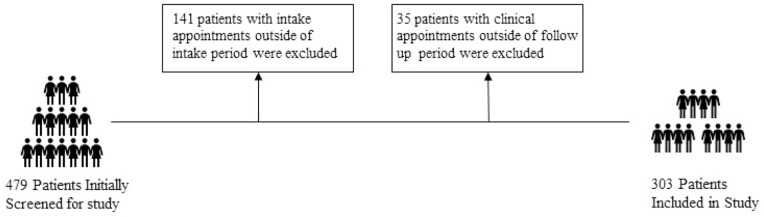
A total of 479 patients were screened who attended their intake appointments at BMCHCV clinics between March 15th, 2019 and July 17th, 2021. These patients were then separated into control group or modified exposure group based on intake date. A final study population of 303 patients was reached: 170 were the controls and 133 were under the modified workflow group (Figure 3).
Figure 3.
Exclusion criteria for study population based on initially screened patients from Boston Medical Center HCV clinics, March 1, 2019 to July 23, 2021.
Patients were middle-aged, with a mean age of 47 years in the control group and 42.5 years in the modified workflow group. Patients in the modified workflow group were 4.88 years younger on average than the control group (P-value: <.001). In addition, 70.8% of patients from the control group had Medicaid as their primary insurance compared to 84.1% in the modified workflow group (P value = .025). Patients in each group did not differ by gender, race, housing status, and substance use (Table 1).
Table 1.
Demographic Statistics Comparing BMC HCV Clinic Patients Between Modified Workflow Group and Control Group.
| Demographic variables | Control group (n = 170) | Modified workflow group (n = 133) | P-value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Gender | — | — | — | — | .41 |
| Male | 115 | 67.65% | 84 | 63.16%% | — |
| Female | 55 | 32.35% | 49 | 36.84% | — |
| Ethnicity | .18 | ||||
| Not Hispanic/Latino | 139 | 84.76% | 104 | 78.79% | — |
| Hispanic/Latino | 25 | 15.24% | 28 | 21.21% | — |
| Missing | (6) | — | (1) | — | — |
| Race | — | — | — | — | .54 |
| White | 91 | 53.84% | 70 | 52.33% | — |
| Black | 53 | 31.36% | 33 | 25.19% | — |
| Asian | 21 | 12.43% | 22 | 16.79% | — |
| Decided not to Answer | 4 | 2.37% | 6 | 4.58% | — |
| Missing | (1) | — | (2) | — | — |
| Homeless | — | — | — | — | .70 |
| Yes | 49 | 28.82% | 41 | 30.93% | — |
| No | 121 | 71.18% | 92 | 69.17% | — |
| Substance use** | — | — | — | — | .98 |
| Yes | 85 | 50.30% | 65 | 48.87% | — |
| No | 84 | 49.70% | 64 | 48.12% | — |
| Missing | (1) | — | (4) | — | — |
| Alcohol use** | — | — | — | — | .22 |
| Yes | 30 | 17.65% | 16 | 12.50% | — |
| No | 140 | 82.35% | 112 | 87.50% | — |
| Primary insurance* | — | — | — | — | .025 |
| Medicaid | 114 | 70.81% | 111 | 84.09% | — |
| Medicare | 34 | 21.12% | 14 | 10.61% | — |
| Private | 13 | 8.07% | 7 | 5.30% | — |
| Missing | (9) | — | (1) | — | — |
| Mean | Std | Mean | Std | ||
| Age* | 47.33 | 13.28 | 42.45 | 12.53 | .001 |
Indicates significant statistical difference at the 5% level between modified workflow and control groups.
Patient has ever indicated use since intake appointment.
Between the 2 groups, fibrosis staging, appointment attendance, and medication initiation differed across most variables (Table 2). For fibrosis staging, we see that number of Fibroscan performed decreased from control group to modified workflow group by 41.95%, FibroSURE tests increased by 25.83%, and any fibrosis test decreased by 14.43%. Appointment attendance decreased across the board between groups as follow up appointment attendance decreased by 18.24% and medication teaching appointments decreased by 11.00%. Medication initiation decreased as 39 less patients started medication and percent of patients who did decreased by 13.29.
Table 2.
Appointment Attendance and HCV Interventions Among Modified Workflow Group and Control Group in BMC HCV Clinics.
| Control group (n = 170) | Modified workflow group (n = 133) | Percent difference** | P value | |||
|---|---|---|---|---|---|---|
| N | % | N | % | % | ||
| Intake appointment type* | <.0001 | |||||
| In-person | 170 | 100 | 16 | 12.03 | −87.97 | |
| Telemedicine | 0 | 0 | 116 | 87.21 | 87.21 | |
| Fibrosis staging method* | <.0001 | |||||
| Fibroscan | 125 | 73.53 | 42 | 31.58 | −41.95 | |
| FibroSURE or FIB-4 | 20 | 11.76 | 50 | 37.59 | 25.83 | |
| Severe fibrosis (F3-F4)* | 31 | 18.24 | 7 | 5.3 | −12.94 | <.0001 |
| Received any fibrosis test* | 137 | 80.59 | 88 | 66.16 | −14.43 | <.0001 |
| Abdominal ultrasound done | <.0001 | |||||
| All | 132 | 77.65 | 48 | 36.09 | −41.56 | |
| Patients with severe fibrosis* | 30 | 96.77 | 7 | 100 | .63 | |
| Attended follow-up* | 109 | 64.1 | 61 | 45.86 | −18.24 | .0019 |
| Attended medication teaching appointment | 89 | 52.35 | 55 | 41.35 | −11.00 | .065 |
| Started medication | 98 | 57.65 | 59 | 44.36 | −13.29 | .013 |
Indicates significant statistical difference at the 5% level between modified workflow group and control group.
Calculated by modified workflow group minus control group.
Patients who had received treatment were analyzed for treatment result variables (Table 3). Medication prescription was generally the same between groups (P-value = .82). SVR confirmation was higher in the control group with a SVR confirmation proportion of 85% compared to 66.04% (18.56% different) in the modified workflow group. The primary reason for unconfirmed SVR data was loss to follow up.
Table 3.
Treatment Results Among Modified Workflow Group and Control Group in BMC HCV Clinics Who Started Treatment.
| Treatment variables | Control (n = 98) | Modified workflow group (n = 59) | P value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Medication prescribed | .82 | ||||
| Sofosbuvir/velpatasvir | 61 | 62.24 | 33 | 56.90 | |
| Ledipasvir/sofosbuvir | 13 | 13.27 | 11 | 17.24 | |
| Glecaprevir/pibrentasvir | 21 | 21.43 | 14 | 24.14 | |
| Other | 4 | 3.06 | 1 | 1.72 | |
| SVR eligible* | |||||
| Eligible | 96 | 97.96 | 53 | 89.83 | .025 |
| Non-eligible | 2 | 2.04 | 6 | 10.17 | |
| SVR result of eligible* | .0069 | ||||
| Unconfirmed | 13 | 13.54 | 17 | 32.08 | |
| Confirmed | 83 | 86.46 | 36 | 67.92 | |
| Reason for unconfirmed | .59 | ||||
| Loss to follow up | 8 | 57.14 | 14 | 80.00 | |
| Detected | 5 | 35.71 | 3 | 15.00 | |
Indicates significant statistical difference at the 5% level between modified workflow group and control group.
Discussion
Across clinic interface and treatment initiation, we observe a net decrease across most variables and a general decrease in engagement with HCV prevention and treatment. As expected, less patients received a Fibroscan test and more received a FibroSURE or FIB-4 test which aligns with updated clinic guidelines. Even with telemedicine and updated protocols, however, overall patient engagement decreased in terms of fibrosis screening, appointment attendance, and treatment uptake. These are similar results as other healthcare settings during the pandemic. One study that used national estimates of dispensed prescriptions for HCV treatment saw that prescriptions decreased 43% in May, 37% in June, and 38% in July when compared to the same months in 2018 and 2019. 16 A recently published article showed that in rural communities in Canada, telemedicine for HCV treatment was associated with lower no-show rates compared to in-person appointments and still saw a 30% decline in pre-pandemic treatment starts. 4 Furthermore, a study in the republic of Georgia found 59% fewer people with HCV infection were treated and 46% fewer achieved SVR when comparing data from 2020 to that of 2019. 17 Our results only show a 15% decline in treatment starts which is nearly half the percentage decrease that these other 2 studies estimated. Although these studies were not assessing the same populations, this comparison indicates that the clinic protocols implemented at BMC were effective in mitigating the effects of the pandemic.
These findings become even more relevant when considering World Health Organization’s goal to eliminate HCV by 2030. National estimates prior to the pandemic proposed that the USA would not reach HCV elimination until 2037, with some states not reaching HCV elimination until 2050. 18 Nationwide, there is no single, standardized protocol for hepatitis C treatment and AASLD guidelines leave room for healthcare organizations to implement the methods and approaches that are available to them. Increasing access to treatment by streamlining the fibrosis staging process and utilizing telemedicine may be an effective and accessible way to help increase treatment engagement across the board and work toward that goal, even as health care operations begin to return to pre-pandemic functioning. 19
Looking at treatment result data, we see that SVR confirmation was statistically higher among the control group when compared to the modified workflow group (85.42% and 66.02% respectfully). These data are acceptable results given the length of time patients were observed and shows both protocols are effective in achieving SVR. Loss to follow up was the largest factor for patients with unconfirmed SVR. The high substance usage and homeless status of our hospital population may contribute to why SVR has been historically difficult to track at BMC. This is not unusual given our patient demographics. A study of HCV treatment in an internal medicine clinic in Seattle, Washington with very similar patient demographics also reported as high as 46% of patients did not return for treatment labs and patients who completed SVR labs took anywhere from 12 weeks to 1 year after completing treatment. 20
The modified workflow group showed lower confirmation of SVR, this could be attributed to the study design and limitations in observation period for this variable. Patients in the control group were given a larger observation period for SVR confirmation than the modified workflow and may indicate that those treated and eligible for SVR is higher than the data suggests. The difference in SVR rates may also be indicative of patients’ ongoing hesitancy in leaving their homes during the COVID-19 pandemic, even if offered to complete labs at a more convenient location, suggesting areas for improvement in outreach and education to increase rates of SVR testing after treatment.
Conclusion
The pandemic impacted the effectiveness of health care centers worldwide to screen and treat HCV and forced many facilities to put non-urgent treatment on hold or adapt approaches to care. Even with streamlined treatment protocol to mitigate the impact of the pandemic on access to HCV care, BMC HCV clinics still saw a reduction in patient retention, treatment initiation and SVR in 2020 compared to 2019. Rates for liver fibrosis screening, treatment initiation, and SVR confirmation decreased after the pandemic began and will have negative health consequences on those living with chronic HCV. This is not to say that telemedicine and offering remote services is not effective in the HCV clinic, however, it appears that a lack of in-person services has had a negative impact on the clinic’s ability to address this public health issue. We do see promising results in updated clinic workflow as increased services in telemedicine and medication delivery by mail appear to have mitigated the effect of the pandemic on clinic engagement when compared to other health centers. The added flexibility for treatment and screening options are important tools that would be best included in other similar healthcare settings. Our research indicates that in-person patient engagement is still important, however, and a combination of both in-person and telemedicine options will quite possibly lead to the more optimal health outcomes for those with HCV.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Matthew O’Brien  https://orcid.org/0000-0002-5630-1369
https://orcid.org/0000-0002-5630-1369
References
- 1. Hofmeister MG, Rosenthal EM, Barker LK, et al. Estimating prevalence of hepatitis C virus infection in the United States, 2013-2016. Hepatology. 2019;69(3):1020-1031. doi: 10.1002/hep.30297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Laury J, Hiebert L, Ward JW. Impact of COVID-19 response on hepatitis prevention care and treatment: results from Global Survey of Providers and Program Managers. Clin Liver Dis (Hoboken). 2021;17:41-46. doi: 10.1002/cld.1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sperring H, Ruiz-Mercado G, Schechter-Perkins EM. Impact of the 2020 COVID-19 pandemic on ambulatory hepatitis C testing. J Prim Care Community Health. 2020;11:2150132720969554. doi: 10.1177/2150132720969554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee SS, Williams SA, Pinto HI, Liu H. Treating hepatitis C during the COVID-19 pandemic in Alberta. Canadian Liver Journal. 2021;4.2:79-81. doi: 10.3138/canlivj-2021-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tapper EB, Arsani SK. The COVID-19 pandemic will have a long-lasting impact on the quality of cirrhosis care. Journal of Hepatology. 2020;73:441-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Toyoda H, Yasuda S, Kiriyama S, et al. Impact of COVID-19 pandemic on surveillance of hepatocellular carcinoma: a study in patients with chronic hepatitis C after sustained virologic response. GastroHep. 2020;2(5):247-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ghany MG, Morgan TR. Hepatitis C guidance 2019 update: American Association for the Study of Liver Diseases–Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology. 2020;71(2):686-721. doi: 10.1002/hep.31060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lepage C, Garber G, Corrin R, Galanakis C, Leonard L, Cooper C. Telemedicine successfully engages marginalized rural hepatitis C patients in curative care. J Assoc Med Microbiol Infect Dis Can. 2020;5(2):87-97. doi: 10.3138/jammi-2019-0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arrese M. Telemedicine, COVID-19 and liver diseases: revamping remote care initiatives in hepatology. Ann Hepatol. 2020;19(4):339-340. doi: 10.1016/j.aohep.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guarino M, Cossiga V, Fiorentino A, Pontillo G, Morisco F. Use of telemedicine for chronic liver disease at a single care center during the COVID-19 pandemic: prospective observational study. J Med Internet Res. 2020;22(9):e20874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Doica IP, Florescu DN, Oancea CN, et al. Telemedicine chronic viral hepatitis C treatment during the lockdown period in Romania: a pilot study. Int J Environ Res Public Health. 2021;18:3694. doi: 10.3390/ijerph18073694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee KS, Quintiliani L, Heinz A, et al. A financial incentive program to improve appointment attendance at a safety-net hospital-based primary care hepatitis C treatment program. PLoS One. 2020;15:e0228767. doi: 10.3138/canlivj-2021-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lasser KE, Heinz A, Battisti L, et al. A hepatitis C treatment program based in a safety-net hospital patient-centered medical home. Ann Fam Med. 2017;15(3):258-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Calner P, Sperring H, Ruiz-Mercado G, et al. HCV screening, linkage to care, and treatment patterns at different sites across one academic medical center. PLoS One. 2019;14(7):e0218388. doi: 10.1371/journal.pone.0218388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. United States Department of Veteran Affairs. Viral hepatitis and liver disease. Published October 18, 2018. Accessed June 28, 2021. https://www.hepatitis.va.gov/hcv/liver-fibrosis.asp#S12X
- 16. Kaufman HW, Bull-Otterson L, Meyer WA, et al. Decreases in hepatitis C testing and treatment during the COVID-19 pandemic. Am J Prev Med. 2021;61(3):369-376. doi: 10.1016/j.amepre.2021.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gamkrelidze A, Handanagic S, Shadaker S, et al. The impact of COVID-19 pandemic on the 2020 hepatitis C cascade of care in the Republic of Georgia. Public Health. 2022;205:182-186. doi: 10.1016/j.puhe.2022.01.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sulkowski M, Cheng WH, Marx S, Sanchez Gonzalez Y, Strezewski J, Reau N. Estimating the year each state in the United States will achieve the World Health Organization’s elimination targets for hepatitis C. Adv Ther. 2021;38(1):423-440. doi: 10.1007/s12325-020-01535-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Di Marco L, La Mantia C, Di Marco V. Hepatitis C: standard of treatment and what to do for global elimination. Viruses. 2022;14(3):505. doi: 10.3390/v14030505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ngo BV, James JR, Blalock KL, Jackson SL, Chew LD, Tsui JI. Hepatitis C treatment outcomes among patients treated in co-located primary care and addiction treatment settings. J Subst Abuse Treat. 2021;131:108438. doi: 10.1016/j.jsat.2021.108438 [DOI] [PubMed] [Google Scholar]