Abstract
This review explores the evolution of the use of hydrogels for craniofacial soft tissue engineering, ranging in complexity from acellular injectable fillers to fabricated, cell-laden constructs with complex compositions and architectures. Addressing both in situ and ex vivo approaches, tissue restoration secondary to trauma or tumor resection is discussed. Beginning with relatively simple epithelia of oral mucosa and gingiva, then moving to more functional units like vocal cords or soft tissues with multilayer branched structures, such as salivary glands, various approaches are presented toward the design of function-driven architectures, inspired by native tissue organization. Multiple tissue replacement paradigms are presented here, including the application of hydrogels as structural materials and as delivery platforms for cells and/or therapeutics. A practical hierarchy is proposed for hydrogel systems in craniofacial applications, based on their material and cellular complexity, spatial order, and biological cargo(s). This hierarchy reflects the regulatory complexity dictated by the Food and Drug Administration (FDA) in the United States prior to commercialization of these systems for use in humans. The wide array of available biofabrication methods, ranging from simple syringe extrusion of a biomaterial to light-based spatial patterning for complex architectures, is considered within the history of FDA-approved commercial therapies. Lastly, the review assesses the impact of these regulatory pathways on the translational potential of promising pre-clinical technologies for craniofacial applications.
Statement of Significance
While many commercially available hydrogel-based products are in use for the craniofacial region, most are simple formulations that either are applied topically or injected into tissue for aesthetic purposes. The academic literature previews many exciting applications that harness the versatility of hydrogels for craniofacial soft tissue engineering. One of the most exciting developments in the field is the emergence of advanced biofabrication methods to design complex hydrogels systems that can promote the functional or structural repair of tissues. To date, no clinically available hydrogel-based therapy takes full advantage of current pre-clinical advances. This review surveys the increasing complexity of the current landscape of available clinical therapies and presents a framework for future expanded use of hydrogels with an eye toward translatability and U.S. regulatory approval for craniofacial applications.
Keywords: Hydrogels, craniofacial repair, soft tissue, tissue engineering, biofabrication, biomaterials, regulatory path, device approval
Graphical Abstract

1. Introduction
Hydrogels are three-dimensional (3-D) assemblies of a hydrophilic network (e.g., polymers, proteins, or self-assembled small molecules), swollen with a high proportion of water. These systems retain their physical structure through physical or chemical crosslinking of the network, but remain in a dynamic equilibrium between the solid and liquid phases [1-4]. While they have been used commercially for several decades in a broad range of applications, the use of hydrogels for tissue engineering is rapidly growing, with a projected global market size of over $1.1 billion by 2022. [5] Regeneration of lower-modulus tissues below 10 kPa [6] remains an unmet need, as these tissues often have irreplaceable functions, and could be served well by the tunable modulus range of soft hydrogels. In the case of craniofacial tissues, needs exist both for simple agents that provide structural reinforcement, and for complex whole soft organs such as salivary glands that provide essential lubrication to the entire oral cavity.
Craniofacial applications have been a unique proving ground for simpler hydrogel-based therapies, in particular those used cosmetically as external creams or injectable fillers. As technological breakthroughs emerge to create more precisely structured hydrogel-based tissue constructs, opportunities arise in parallel to generate higher-order craniofacial neotissues with architectures that mimic those of human tissues. Such needs span a spectrum from primarily structural to functional/secretory, but in all cases, recent advances in biofabrication enabled new methods to create multilayer, 3-D tissues with spatial hierarchy.[7] Over the past decade, a new cadre of researchers has emerged with training in advanced biofabrication methods such as 3-D printing, and commercially-available manufacturing tools have emerged in parallel, advancing such efforts beyond homegrown systems. Such methods have captured the imaginations of clinical investigators [8,9]. Yet, there remains a chasm between research success in proof-of-concept studies and true commercial translation and clinical adoption. Currently, no 3D-printed soft tissue devices or implants exist on the market in the United States for any craniofacial application. One study analyzed the FDA internal database and found 80 3D-printed devices that received 510(k) clearance so far, with a majority of these intended for orthopedic (hard tissue) applications. [10] Broadly, the tissue engineering market as a whole generated an estimated $9 billion in U.S. sales in 2017. [11]
Craniofacial applications are an inviting beachhead use, particularly within the oral cavity, as it is an easily accessible region, with regenerative/reconstructive needs across the human lifespan: congenital malformations at birth, trauma throughout life, and progressive decay/dysfunction in later life. Although the field has a primary foundation in tooth and bone restorations, advances in soft tissue regeneration are equally necessary and potentially within reach through today’s technologies. But can they advance to the clinic? In the present review, we highlight needs for craniofacial soft tissue engineering and identify the potential of hydrogels to address those needs. We stage these needs across levels of complexity, identify the solutions that have advanced through FDA approval, and consider future opportunities for developing complex, multifunctional soft craniofacial tissues.
2. Craniofacial soft tissue needs – issues in disease, trauma, and repair
Craniofacial soft tissue spans a broad range of tissue types with different mechanical properties and moduli ranging from 1 kPa in the case of brain to tens of kPa for skeletal muscle, and hundreds of kPa in the case of skin. [1,12] Here, soft tissue is defined broadly as human tissue that is not mineralized and has a maximum stiffness of approximately 1 MPa. Many living tissues, including craniofacial soft tissue, behave simultaneously as an elastic material and as a viscous fluid, and therefore are characterized as viscoelastic. In an important recent study, it was shown that cells encapsulated in 3D hydrogels respond not only to the elastic but also the viscoelastic properties of their surrounding matrix. [13] This has an important implication for designing synthetic hydrogels, particularly for craniofacial soft tissue applications. Soft tissue injuries are among the most common traumatic craniofacial injuries and account for nearly 10% of all emergency department visits. [14] Recent reports within specific contexts (e.g., electric scooter use [15-17], professional sports [18]) identify soft tissue lacerations as the most common injuries requiring expert repair. The tools available for such repairs vary, both with the urgency (i.e., emergent vs. planned reconstruction) and the extent of the correction. As one example, autologous fat grafting (AFG) has emerged as a common surgical method to optimize long term aesthetics, symmetry, and functional outcomes such as volume supplementation for improved facial contouring [19,20]. However, these and other tissue-based approaches face limitations in the predictability of their stability/shrinkage, while more traditional flap transfers provide a more stable tissue result at the expense of longer surgical time with a potential aesthetic mismatch. While autologous fat grafting is the current gold standard for liposuction and lipofilling, the long-term results of fat grafting are often disappointing. [21] Similarly, issues of craniofacial muscle loss or dysfunction because of congenital abnormalities or trauma can be corrected via autologous muscle grafts, but at the expense of donor site scarring or morbidity [22]. Finally, in the clinical care of patients with severe burn injuries, autologous split thickness skin grafting is still the gold standard, because no skin substitute has proven successful to date. [23,24] In all levels of soft tissue repair, the extent to which clinical intervention can recreate and restore the complex 3D structure that is unique to each patient and procedure could be enhanced by a more predictable suite of off-the-shelf products.
In more complex cases, advanced biomanufacturing methods could reconstruct muscle, glands, and other tissues that might be unavailable from donors, or inaccessible by other means. In many of these types of cases, there is no widely accepted gold standard method, and this presents an opportunity for the use of hydrogels to facilitate personalized regenerative approaches that can more accurately mimic native geometry of soft tissues by using patient image-guided scaffold design to achieve desirable outcomes. [25] Craniofacial congenital defects, such as cleft lip and palate, represent one such opportunity, as these affect 1 in 1,000 children born each year in the United States and are among the most common birth defects. [26] The cleft lip and palate is a combination of soft and hard tissue defects involving the lip and the maxilla. Surgical correction of these defects often involves bone grafting and multiple surgical procedures and requires extensive healing time for the child. Novel hydrogel solutions continue to have great potential in this use, as they are the best suited to space-filling needs, when used around a stable high modulus bone or polymer support and offer the necessary bridge from hard to soft tissue through the combination of hard scaffold and soft hydrogel. A recent thorough review describes craniofacial tissue engineering applications that span the interface of soft and hard tissue, as well as the spectrum of tissue engineering approaches, beyond hydrogels. [27]
3. Use of hydrogels for craniofacial soft tissue restoration
The application of hydrogels in craniofacial soft tissue repair can be divided into several different use-cases: delivery of fillers for tissue repair or broad cosmetic “repair”, delivery of restorative agents post trauma or for congenital defects, and delivery of biologics for regenerative medicine treatments.
For drug delivery applications, hydrogels are used widely, both as transdermal delivery systems and as vehicles for localized sustained release to anatomic sites, particularly in the temporomandibular joint. [28-30] The shear-thinning behavior of some hydrogel systems is particularly attractive, as it enables these delivery vehicles to be spread across skin for easy application.
Within the tissue restoration paradigm, hydrogels have been integral to the development of craniofacial soft tissue therapies, ranging from acute treatment to long-term regeneration. Hydrogels are particularly well-suited for soft tissue applications because of their mechanical properties (elastic modulus and viscoelasticity) that can be tuned to align closely with those of native soft tissue. [31] As they are viable extracellular matrix (ECM) surrogates, hydrogels can serve as scaffolds for multiple tissue types required for clinical applications, namely skin, muscle, blood vessels, and nerves, all needed in craniofacial repair applications. As noted above, hydrogel scaffolds used in congenital defect applications can fill irregular contours in the interface between bone and soft tissue, and can serve as delivery vehicles for encapsulated cells and/or cytokines. [32-36] Substantial advancements in bioprinting technologies over the past decade offer far greater control over geometric placement of cells and cargo, particularly for advance laboratory preparation of an implant [37]. Bioprinting of hydrogels has lagged efforts with thermoplastics and resins, due to inherent challenges in creating stable, self-supporting structures from low-modulus gels. However, multiple solutions were commercialized recently, through innovations in printing hardware or hydrogel crosslinking chemistry [38-40], Although such innovations may not have originated in the craniofacial space, they offer great potential to pattern more closely the complex architecture of native soft tissues and expand the slate of options for engineered tissue replacements. While the bioprinting of skin and other soft tissues is still at an early stage of development, multiple promising studies have employed hydrogels as scaffolds for advanced biofabrication of soft tissues for craniofacial applications. [41]
Because of this broad range of hydrogel-based applications in craniofacial therapies, we apply a similarly broad definition to “tissue engineered” therapies in this review while maintaining a focus on the materials and material combinations. Whether employed as a base for a lotion, a substrate for an injectable particulate-laden filler, or a scaffold for cell-based therapies, the same hyaluronic acid (HA) or poly(ethylene glycol) (PEG) hydrogel might be selected. From the perspective of a therapeutic developer, the goals of delivering a drug, protein, or cell to a tissue site might favor the use of an off-the-shelf hydrogel with a known FDA master file, rather than the considerable time and expense of developing a new material. Similarities in materials across applications require a recognition that the simplest use cases often resemble the most complex. In that sense, we continually return to the hydrogel itself in this review and demonstrate the versatility of our current array of options. The subsections below provide some recent literature examples of tissue engineered elements, of increasing complexity, that have relevance to restoration of the structure or function of craniofacial soft tissues. Later, in Section 4, we turn our focus to commercial examples, also categorized by their levels of complexity.
Recent advances in hydrogel chemistry produced a new generation of hydrogel-based biomaterials that can better mimic the behavior (mechanical, chemical, biological) of native tissues in the body. [1] Four important design criteria for hydrogels for biological applications include: 1) composition, 2) cross-linking strategy, 3) delivery methods, and 4) biological agents. Each of these four design criteria include multiple factors to consider in the engineering design process, and inform, or in certain instances, dictate, the fabrication method. Additionally, further considerations might be more relevant to clinicians, such as the degradation profile once implanted, the surface/bulk properties of the material, and release kinetics for conventional drugs (e.g., analgesics, antibiotics, and so forth). Some common methods for fabricating hydrogels for regenerative medicine applications include microscale photo-patterning, 3D bioprinting, photo-crosslinking, self-assembling or self-integrating hydrogels, and microfluidic-based fabrication. [42] Often, the target tissue/organ and end use case can best inform the fabrication method. The types of craniofacial soft tissue discussed here include dermal fillers, gingiva, facial muscle, ocular, and glandular tissue. Instead of serving as an exhaustive list of tissue types, this highlights a broad range of applications with some exciting commercial examples. Furthermore, these five tissue types showcase the diversity of hydrogels, given the large range of stiffness and other design parameters.
3.1. Fillers for tissue repair
Soft tissue lesions, caused by either trauma or disease, present an opportunity for hydrogels to be used as soft tissue fillers, both for structural integrity and replacement of tissue that was damaged or lost. Biologically derived fillers have a long history of use, both as facial fillers and in a variety of soft tissue applications and include collagens and other extractable ECM components. In a recent example, decellularized porcine ECM hydrogel was used in a rat model of stroke to preserve brain tissue. [43] The ECM gel was injected into the brain and formed a hydrogel in situ. The injected hydrogel decreased brain tissue cavitation and was retained in situ over the course of 12 weeks. For aesthetic applications, similar methods are used in adipose tissue engineering as a filler material. The design specifications of biomaterials for adipose tissue regeneration involve mimicking the soft and rubbery characteristics of native tissue, and its ample cytokine production. Another study recently described the modification of collagen hydrogels to affect adipocyte gene expression through tuning of elastic modulus, with subsequent impacts on intracellular actin organization and reprogramming toward a fibrotic phenotype. [44] This manuscript, and another describing adipocyte-endothelium crosstalk [45], serve to improve both in vitro models of adipose regeneration, and in vivo applications. The wide range of hydrogels in development for adipose tissue regeneration is highlighted in a thorough recent review. [46]
A notable division in options exists for contemporary dermal fillers, and this is reflected below in the description of FDA-approved products in Section 5. Broadly, these categorize as hydrogels intended for near-complete resorption, and those that carry particulates or fibers that drive local collagen production in response. A report by Li et al. describes a system in the latter category: electrospun poly(ε-caprolactone) (PCL) fibers were incorporated into a HA polymer hydrogel to chemically mimic native ECM. [47] The porosity of the hydrogel was intended to encourage cellular infiltration of macrophages and endothelial cells, while the PCL fibers impart mechanical structure independent of hydrogel crosslink density. In a rat model, the authors demonstrated that this composite promoted infiltration of host macrophages, association with the PCL fibers, and induction of a “pro-regenerative” phenotype. Unlike current models of particulate-hydrogel facial fillers, this work focused primarily on influencing cell invasion, rather than collagen deposition, and targeted larger space-filling needs (e.g., mammary tissues). However, such a system matches the design of particulate-hydrogel facial fillers, and with an analogous tissue-level response, has a similar end-result to translate to craniofacial applications where a localized increase in collagen is desired.
3.2. Gingiva
Wound healing in the periodontal space presents an important need for novel therapeutic approaches, since periodontal disease is a major public health issue and the periodontal wound healing process for gingiva is different than that of cutaneous wound healing in several ways, namely the lack of significant scar formation. [48] Oral epithelium also heals much faster than dermal skin [49,50] These factors, combined with the warm, moist environment with copious oral flora, present unique opportunities for tissue engineering and developing bridge materials. Gingiva, also referred to as gums, is the soft tissue that surrounds the base of the tooth, and gingiva recession is a major health concern and active area of research. [51] Through a greater understanding of periodontal wound healing, the treatment of periodontal disease has gradually shifted away from resective surgical procedures to regenerative options. [48] Here, hydrogel-based therapies provide some promising examples. In a recent pre-clinical study, alginate gelatin methacrylate (GelMA) hydrogels were used to encapsulate gingival mesenchymal stem cells and demonstrated expedited wound healing in a mouse model. [52] In a clinical study of intraoral grafting, lab-produced tissue-engineered human oral mucosa equivalents (EVPOME) were used to assess efficacy in producing a well-integrated surface epithelium. AlloDerm® was used as the base substrate onto which cells were seeded. The clinical end use case, where the grafts were to be surgically implanted and sutured into place underneath a flap of skin, necessitated a stiff ECM matrix that could maintain its integrity after threading with a needle and suture. [53]
3.3. Facial muscle
Dysfunction of facial muscle can result from injury to facial nerves and can be a disfiguring condition with both aesthetic and functional impacts. Transplantation of a peripheral muscle to the dysfunctional site is a common treatment but risks poor reinnervation at the new site. Within the broad scope of craniofacial soft tissues, skeletal muscle occupies the higher end of both mechanical potential and observed phenotype preference for its constitutive cell, the myocyte. This range, typically >10 kPa, spans an interface between the upper limits of hydrogels and the lower limits of biodegradable thermoplastics and elastomers; synthetic material choices are therefore often hybrid models, employing both categories of materials [54]. Decellularized biologically derived extracellular matrices span a similar range of mechanical properties and are a preferred muscle regeneration substrate by some investigators. For this soft tissue, hydrogels may serve either as a primary substrate for myofiber restoration, or a secondary support that encourages necessary vascular or nerve integration.
In a recent study, Raimondo et al. demonstrated the use of ionic-crosslinked alginate hydrogels to deliver vascular endothelial growth factor (VEGF) and insulin-like growth factor-1 (IGF-1) in a rabbit model of facial nerve damage. [55] Cytokine-laden alginate gels were spaced periodically along the length of a transplanted gracilis (thigh) muscle, at the time of transplantation to the recipient site, and were reinforced every 3-4 weeks with further injections. Over the course of 3 months, the cytokine treatment from the hydrogel carrier demonstrated significant functional improvements in the response of the transplanted facial muscle.
In a separate study of muscle regeneration, photo-crosslinked chitosan hydrogels were used to deliver re-programmed stem cells to promote myogenic differentiation in vitro. [56] A methacrylated chitosan framework enabled blue light-based photo-crosslinking via a riboflavin initiator, mitigating cell viability concerns inherent in UV-based crosslinking systems using the Irgacure® 2959 initiator. Incorporation of type I collagen further improved cell viability, and the hybrid chitosan/collagen hydrogel ultimately provided an optimal microenvironment to promote differentiation of the encapsulated cells toward a myogenic lineage. Increased expression of phenotypic myogenic genes over time, as well as assembly into structures resembling myotubes, indicated strong potential for reconstituting these tissues with high relevance to craniofacial use.
Furthermore, in considering tissue engineering approaches for generating functional skeletal muscle, the generation of intrafusal muscle fibers is highly relevant as is the interaction with sensory neurons in vivo. A recent excellent review discusses this in depth. [57]
3.4. Ocular
While not strictly considered craniofacial, many ocular applications of hydrogels are informative for other soft tissues in the craniofacial space. Ocular interventions require some of the softest hydrogel materials to match native tissue mechanics. As with oral and other maxillofacial applications, ocular uses span from transient contact-based delivery of therapeutics (e.g., eyedrops, now often containing dilute amounts of common hydrogel components) to extended-use removable devices (e.g., daily-wear contact lenses, one of the broadest use cases for hydrogels [58] over the past several decades). Intraocular lenses (IOLs) are one of the few exceptions, as these tend to be sourced from comparatively rigid and hydrophobic polymers. [59] Extending this use to tissue engineering and 3D bioprinting of cells within hydrogels, a recent detailed review of the challenges and achievements in ocular bioprinting and tissue engineering was contributed by Fenton, et al. [60]
Of the current FDA-approved injectable hydrogel-based systems, the majority employ either HA, PEG, or collagen as a hydrogel base. [61,62] For the present review, we highlight one example of a hydrogel system that is less frequently used in oral/craniofacial applications, but has been described recently for multiple ocular uses. Poly(N-isopropylacrylamide) (pNIPAAm) has been the subject of extensive research in biotechnology, for its self-healing properties, as a drug-delivery system, and as a sensor and actuator. It is particularly well-suited to biomedical applications because its lower critical solution temperature (LCST) is very close to body temperature, meaning that it can be delivered as a liquid at room temperature, and at approximately 32 deg C it undergoes a volume phase transition, becomes hydrophobic, and stiffens into a gel. [63-65] In addition to leveraging the versatility of hydrogels in delivering cells or bioactive compounds, the hydrogel system can be engineered to reversibly change viscosity in response to temperature. Because of these properties, and the ease of placement and removal if necessary, pNIPAAm is particularly well-suited for craniofacial applications.
A recent implementation of pNIPAAm as an ocular sealant applied to the eye sclera after cataract surgery was described. [66] In this composition, pNIPAAm was crosslinked with butylacrylate to match the LCST to local physiologic temperature and improve mechanical resilience. This preliminary study demonstrated basic safety and efficacy in preserving intraocular pressures after injury and led to early-stage commercialization efforts of similar technology by AesculaTech. In their envisioned use, this hydrogel system can be used to treat dry eye, by injecting as a plug into a tear duct, restricting tear drainage from the eye surface. Although still early-stage, this technology has potential for other ocular uses, such as extended drug delivery.
3.5. Glandular
The salivary gland has been an area of clinical need for functional tissue replacement for some time, as highlighted in past reviews. [67,68] Previous strategies combined cell transplantation and gene transfer with engineered scaffolds to generate an artificial salivary gland substitute. [69-74] Scaffold selection takes cues from materials used for surgical replacements of other tubular structures in the body, namely intestine, vasculature, ureter, and trachea. Previously tested materials include poly(L-lactic acid) (PLLA), poly(glycolic acid) (PGA), PEG derivatives, chitosan, and collagen/Matrigel®. [75,76] Our own lab has used HA-based hydrogels to support 3D culture of primary human salivary derived cells [77-79], with customizations to enable gradient delivery of growth factors [80] or hydrogel tuning to promote spheroid assembly. [81] In a study by Miyake et al., a gelatin-based hydrogel was used in a rat model of submandibular gland (SMG) resection to demonstrate regeneration of newly formed acinar cells. [82] A particularly elegant study from the Okano and Baker groups used temperature responsive pNIPAAm polymer to form cell sheets of mouse SMG cells, which were able to polarize and differentiate in vitro [83]. Furthermore, the authors demonstrated that double layer cell sheets form a glandular-like appearance in vitro, offering a promising new therapeutic strategy.
3.6. Focus on the transition to commercial implementations
Of course, these and many other discoveries can only have an impact on human life if they can transition out of laboratories and into the hands of oral surgeons, dentists, and other clinical practitioners. Which technologies advance to commercial translation? How does new research cross the chasm to clinical use? In the following sections, we consider these questions, and suggest that the answer may lie in a combination of market demand, interdisciplinary education of clinically focused faculty within tissue engineering curricula, and ease of regulatory approval by governing agencies like FDA. As new technologies mature within laboratories, the need to translate these to clinical use remains paramount.
4. Proposal for a hierarchical categorization of hydrogel-based therapies
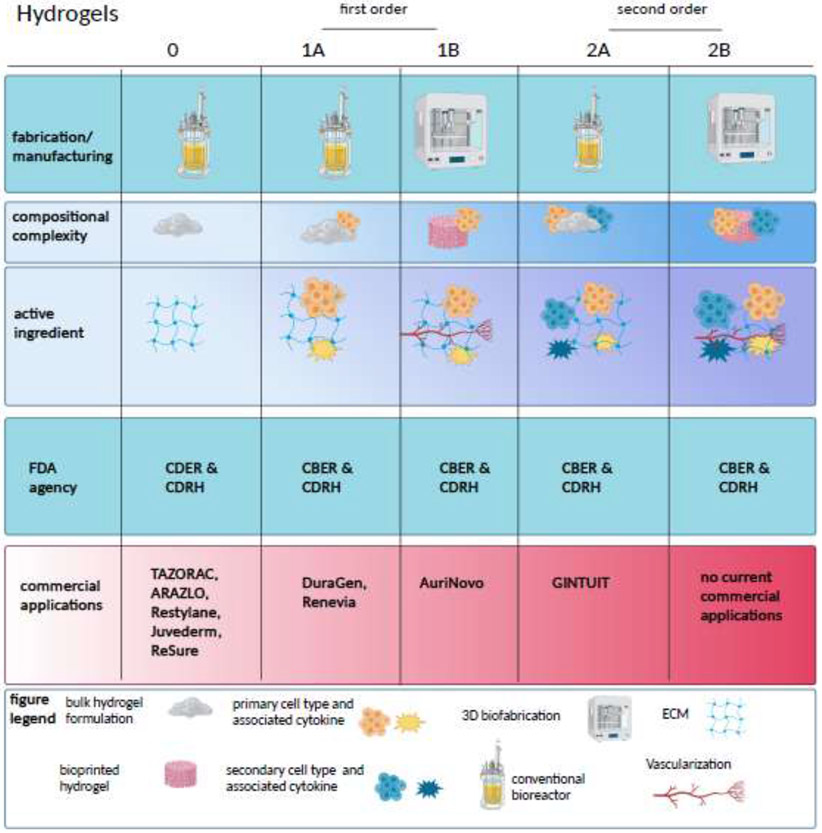
In this review, we propose a hierarchy for describing hydrogel systems with increasing degrees of complexity, from simple hydrogel-based delivery vehicles with minimal internal order, to complex structures with intricate internal order and/or multiple biological cargos, as graphically depicted in Figure 1. These range from the simplest applications, designated as 0 order systems, to the most complex use cases, termed 2nd order systems. (Table 1) Contributing factors used for classification include composition, spatial order, clinical translatability and efficacy, manufacturing considerations, and ultimately, regulatory categorization. Progressive complexity from the simplest 0 order hydrogels also represents the progression through the complexity of the regulatory pathway, from topically applied gels that are not FDA regulated to products appropriate for the Biologic License Application (BLA) designation. [84]
Figure 1.
In this figure, the categories of hydrogel systems for craniofacial applications are classified by manufacturing processes, structure and composition, and mechanism of action. In each case, complexity builds from left to right.
Table 1.
Proposed hierarchical categorization of hydrogel-based therapies
| 0 Order Systems: Hydrogels that have no deliberate molecular orientation, macroscale design, or CBER/CDER-regulated biologics embedded (e.g., dermal fillers) | |
| 1st Order Systems: | |
| 1A: Hydrogel systems that include a single CBER/CDER-regulated biologic, either living cells or cell products, or a function-driven structure (alignment, orientation, etc.) (e.g., injectable HA gel with stem cells) | 1B: Hydrogel systems from 1A that rely on a manufacturing method that requires further validation (e.g., 3D bioprinting) |
| 2nd Order Systems: | |
| 2A: Hydrogel systems that employ multiple mechanisms of action, any combination of cells, cell components, and drugs, or one of those and a structural mode of action (orientation, channels, gradient, etc.) (e.g., allogeneic cellularized scaffold) | 2B: Hydrogel systems from 2A that rely on a manufacturing method that requires further validation (e.g., 3D bioprinting) |
The hierarchical framework and the regulatory considerations in this review are focused on the U.S. market and the U.S. FDA in order to provide examples of regulatory processes and pathways that may be expected to be similar in other markets worldwide. We acknowledge that there are significant differences in regulatory processes worldwide, particularly as it relates to regenerative medicine therapies. The FDA is organized into 9 center-level organizations, of which three are particularly relevant in the context of this review: the Center for Biologics Evaluation and Research (CBER), the Center for Devices and Radiological Health (CDRH), and the Center for Drug Evaluation and Research (CDER). CDRH is primarily focused on medical devices, such as those marketed through the 510(k) pathway. CBER has offices within it that focus specifically on biologics quality, blood research, vaccines research, and tissues and advanced therapies, representing the major categories of novel products that would be routed through CBER for regulatory approval. CDER is responsible for therapeutic drugs in product classes that include chemically synthesized molecules, antibiotics, hormone products, and bioactive proteins.
Historically, as the production of biologics (such as small molecule inhibitors) became more standardized and commonplace, the interagency agreement and jurisdiction for the review of certain biologic agents transferred from CBER to CDER. [85] More often than not, CBER reviewed applications involving novel agents with mechanisms of action that were poorly understood. Furthermore, CBER reviewers historically tended to focus more on clinical outcomes as primary endpoints, and as part of a BLA, assess for safety, purity, and potency, as compared to a New Drug Application (NDA) routed to CDER that would merely need to show safety and efficacy. [85] Notably, a BLA can be directed to either CBER or CDER for review. Biologics including cell and gene therapy products, as well as vaccines and blood products, would be directed to CBER, while most bioactive proteins, such as antibodies, growth factors, cytokines, and enzymes, would be reviewed by CDER. Given the added complexity in the BLA versus the NDA, the proposed hierarchical categorization indexes on the requirement for a BLA, as well as the number of active biologics in the product.
Beyond the center-level classification, many commercial examples discussed in this review may receive one or more other notable designations: HCT/P and/or RMAT. Human cells, tissues, and cellular and tissue-based products (HCT/P) are subject to specific requirements in 21 CFR 1271, largely related to public health, avoidance of disease transmission, and quality controls. HCT/Ps can receive a primary classification either through CBER or CDRH, depending on the specific tissue type that is employed, and whether or not it is a combination product. The majority of these HCT/Ps are classified under CBER, and FDA provides multiple Guides and other tools to assist manufacturers in determining their product’s likely designation. [86] Certain HCT/P categories are also exempt from a PMA or BLA; that exemption hinges largely on whether the HCT/P is considered “minimally manipulated” and intended for “homologous use,” concepts which are described well in a recent review. [87] Conversely, HCT/P sources which exceed these boundaries, either through extraordinary processing (e.g., decellularization, ECM digestion, lyophilization, etc.) or a non-homologous use (e.g., bladder-derived matrix as a wound therapy) require additional FDA approval for safety and efficacy. The relevance of this provision will be more apparent below, when considering human-derived ECM.
The Regenerative Medicine Advanced Therapy (RMAT) designation was introduced in 2016 as part of the 21st Century Cures Act, as a means to expedite the development, review, and ultimate approval of novel regenerative medicine therapies. Qualifying RMAT therapies are either a cell-based therapy, therapeutic tissue engineering product, human cell and tissue product, or any combination of these. Furthermore, the therapy has to be intended to treat a serious or life-threatening disease, and preliminary clinical evidence has to indicate that the therapy has potential to address unmet clinical needs for that condition. RMAT designation includes all of the benefits of the Fast-Track and Breakthrough Therapy designations, particularly more frequent interactions with the FDA. [88] A tissue engineering product, Humacyl, received one of the earliest RMAT designations in 2017. [89]
Alternatively, there is another commercialization pathway for simple, topically applied hydrogel creams and lotions, which are commonly marketed in the cosmetic category. Because they are not classified as Class III products and include components that are substantially equivalent to an existing legally marketed device, they are subjected to fewer regulatory controls. The Federal Food, Drug, and Cosmetic Act (FD&C Act) and the Fair Packaging and Labeling Act (FPLA) are two major statutes pertaining to cosmetics on the market in the United States. The FD&C defines cosmetics by their intended use, which is a product intended to be topically applied to the body for cleansing or altering the appearance. The item becomes a ‘drug’ if it is intended to treat or prevent disease. This is the legal mechanism by which many products containing hydrogel formulations avoid the FDA approval process and are merely FDA regulated (rather than “approved”), to ensure they are not making a therapeutic claim. The FPLA, on the other hand, is responsible for regulations requiring that all consumer commodities be labeled to disclose the net contents (the ingredients), the name of the distributor, and the net quantity of contents. Therefore, aside from regulations that restrict the use of certain hazardous chemicals and require warning statements for others, there is no requirement for specific tests to demonstrate safety or efficacy of these products. The distinction between a topical treatment that requires a prescription and one that is available over the counter could be the concentration of the active ingredient, or sometimes, the decision of the manufacturer to apply for a 510k filing. [90]
Many of the most popular hydrogels for clinical use are synthetic (e.g., PEG), or derived from non-mammalian sources (e.g., alginate, HA). These all are viewed reasonably as injectable “devices” within which drugs or biologics can be added. However, ECM-derived hydrogels serve as a notable exception, due to their inherent content of potentially active structural and signaling proteins, and therefore cannot be classified as a monolith. If the hydrogel is derived from human cells or tissues (i.e., HCT/P), it would be CBER-regulated (except for a select few tissues that are CDRH-regulated), and is classified in this review as a 1st order product. Alternatively, if the ECM is derived from a non-human animal tissue, and does not contain any human biologics, then it typically would be regulated by CDRH. Fine intermediate divisions remain in these cases; for example, secreted human products are not HCT/Ps, but an organized ECM matrix deposited by human cells onto a scaffold may begin to resemble a “combination” device, and require review by both CDRH and CBER. In all cases, the addition of human cells to the hydrogel would automatically classify the product as CBER-regulated. Therefore, ECM-derived hydrogels could conceivably range in complexity from 0th order to 2nd order, depending on whether human cells are present, and whether or not the matrix itself requires a BLA.
In the Sections 5-7 that follow, we describe examples in each category of our proposed hierarchical organization (Table 1), with intentional focus on commercialized products. In the more complex cases, there may be no example of a tissue engineering product that has been transitioned successfully from the lab to the clinic; for these, we offer the closest pre-clinical examples for soft tissues in the craniofacial space. Section 8 considers the outlook for all of these potential therapies, given the above regulatory requirements.
5.0. order applications: Hydrogels with simple structure and composition
0 order hydrogels are those with no deliberate molecular orientation, macroscale design, or embedded CBER/CDER-regulated biologics. These acellular hydrogels can be applied non-surgically, either topically as transdermal lotions and creams, or via injection, commonly as fillers. [91] For example, Juvederm® and other similar dermal fillers would be classified as 0 order systems. 0 order hydrogel systems encompass seminal contributions in the field of craniofacial tissue engineering and provide examples of some of the earliest clinically approved engineered substances, like HA. Examples highlighted here as 0 order systems have a more immediate clinical translatability because of their ease of application and broad use cases.
5.1. Topical lotions and creams
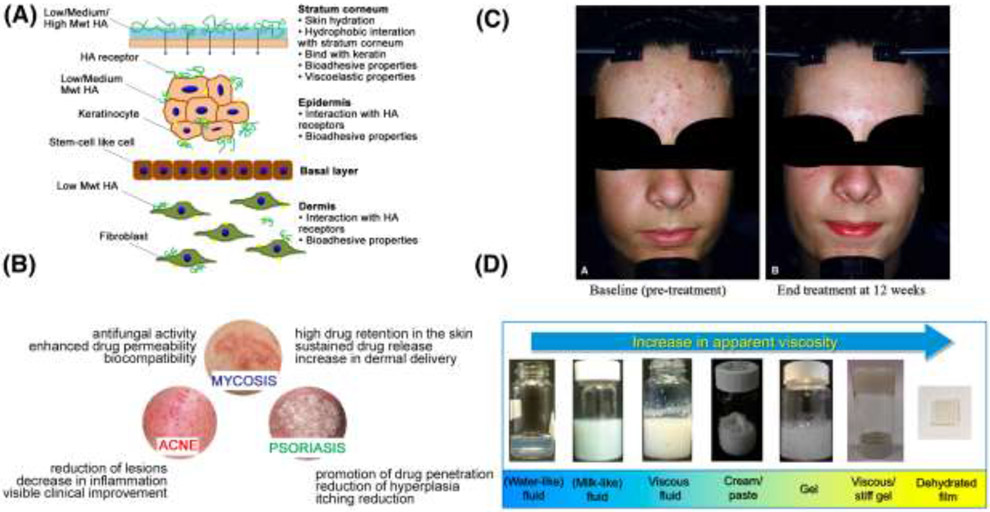
The most fundamental examples of hydrogel systems for soft tissue applications involve topical lotions and creams, which are non-invasive and have a quicker pathway to global commercialization. For example, certain dermal fillers and creams that require a prescription in the US can be legally obtained without a prescription in the UK. As noted in Section 3, these represent the very simplest examples of tissue engineering/restoration, yet their use of very similar hydrogels to those in traditional tissue engineering scaffolds warrants their inclusion here. Some relevant design criteria for a manufacturer of topical hydrogel-based formulations are highlighted in Figure 2. Figure 2A describes examples of absorption rates of hydrogel components (e.g., multiple molecular weights of HA) through the skin, which have relevance to the target layers for treatment. Although hydrogel properties vary widely, based on composition, charge, and total amphiphilicity, multiple studies found that hydrogels enable deeper penetration and increased absorption of drug compounds into more layers of the skin as compared to other formulations, such as oil-based emulsions and ointments. [92-94] One possible explanation for this is that compounds in hydrogels are immediately available for diffusion into the skin, compared to oil-based formulations, where the compound must first release from the lipid phase before it can penetrate through the skin. Figure 2B demonstrates the broad range of types of skin diseases that can be treated with hydrogel creams topically applied to the face. These include most commonly acne, mycosis, and psoriasis, and represent an important precursor to current tissue engineering approaches. Acne represents the biggest commercial opportunity as it accounts for the second-highest global disease burden among all skin disorders (second only to eczema). [95] Figure 2C depicts improvement in acne vulgaris symptoms following application of a hydrogel delivering a combination of therapeutics. In two multi-center clinical trials, patients demonstrated significant improvement in total lesion counts at 12 weeks using a hydrogel based tretinoin gel. [96] Finally, Figure 2D demonstrates the broad range of potential formulations for topical products, including gels, ointments, emulsions, films, creams, pastes, lotions, and patches. [28,29,96-107] As shown in Figure 2C and described in ([106],[108]) and other reviews, the “simplicity” of 0 order hydrogel systems reflects only their path to commercial use, and not the effort involved in deriving acceptable features in the marketplace. [103] Fine adjustments in the balance among base polymers, emulsifiers, and water content can greatly impact key elements, such as perceived “feel” on the skin and long-term shelf stability. These formulations are the product of decades of industry expertise. However, the availability of hydrogel precursors with existing FDA Master Files simplifies their implementation in final formulations, and their ultimate approval.
Figure 2.
The set of design criteria for a manufacturer of topical hydrogel-based formulations are highlighted. Panel (A) is a schematic of the proposed mechanisms for the skin permeability of hyaluronic acid. [106] High MW HA primarily interacts with the stratum corneum through hydrophobic interactions, while some low MW HA can even permeate into dermis This greatly informs design choices for topical facial applications. (B) is a schematic illustration of the effects of hydrogels in the treatment of selected skin diseases that affect the head and face. [107] These treatments are commercially available as hydrogel formulations that can be topically applied. (C) is a depiction of a typical patient before and after treatment with a combination clindamycin/tretinoin hydrogel for acne vulgaris [96] (D) is a depiction of a range in the apparent viscosity and appearance of lipid-based systems and emulsion for cosmetic applications. Apparent viscosity increases from a water-like liposome solution to a dehydrated film of a stiff gel. [97]
Beyond these design considerations, some examples of hydrogel formulations that are used for very specific applications include tazarotene, which is available in a gel under the brand name TAZORAC® and as a lotion under the brand name ARAZLO™. While there exist slight differences in the formulations that are beyond the scope of this review, it is noteworthy that retinoids, which are a class of compounds related to vitamin A, have been used commercially in hydrogel formulations for the treatment of acne for over 20 years, and are a classic example of a topical 0 order hydrogel system. [109] However, an important distinction with tazarotene derivatives such as ARAZLO™, as compared to other topically applied products mentioned previously, is that ARAZLO™ underwent CDER approval because of the active drug ingredient, tazarotene. A key inactive ingredient in these formulations is carbomer homopolymer type B, which is produced by Lubrizol for pharmaceutical applications under the brand names Carbopol, Pemulen, and Noveon. Specifically, Carbopol homopolymers are polymers of acrylic acid crosslinked with allyl sucrose or allylpentaerythritol. The networks swell 1000x when exposed to water to form a hydrogel, and can be tuned to provide the following functionality for clinical use: controlled release properties, bioadhesion in buccal and ophthalmic applications, rheology modification to provide a wide array of viscosities for end use as a lotion, cream, or gel, and suspension of insoluble active ingredients. [110] Interestingly, MuGard® represents a different application of Carbopol – this loose hydrogel solution is used as an oral swish for management of oral mucositis and all types of oral wounds. [111]
In contrast to the small molecule delivery paradigms used in ARAZLO™ and TAZORAC®, the company NOVAN has a proprietary technology for binding a nitric oxide precursor to a polymer (berdazimer sodium), co-delivered with carboxymethylcellulose hydrogel, for the purpose of using NO release to treat acne vulgaris and other skin conditions. [112,113] A final notable commercial example of a 0 order hydrogel systems is Geliperm, a semi-rigid hydrogel patch for use in corneal protection during general anesthesia for nonocular surgery [114,115]. Although this material is not used as either a lotion or cream for drug delivery and stretches or exceeds the category of “soft tissue craniofacial engineering,” its simple composition addresses a key clinical need and demonstrates the utility of such materials across the spectrum of use.
5.2. Dermal fillers
Acellular absorbable dermal fillers in the cosmetic market began with bovine collagen formulations in the 1970s, with Allergan products (Zyderm and Zyplast) capturing a significant portion of the overall market over the last few decades. The approval of Restylane, brought to market by Medicis, in 2003, marked the first FDA-approved dermal filler made from biodegradable HA from non-animal sources. [116,117] With reduced immunogenicity and an in vivo lifespan of up to 18 months, it demonstrated superior results, and Allergan and Anika quickly followed with Juvederm® and Elevess™, respectively. [118,119] Of note, Elevess™ contains 0.3% lidocaine, but none of the products contained any other types of biological materials, such as cells or growth factors. [120]
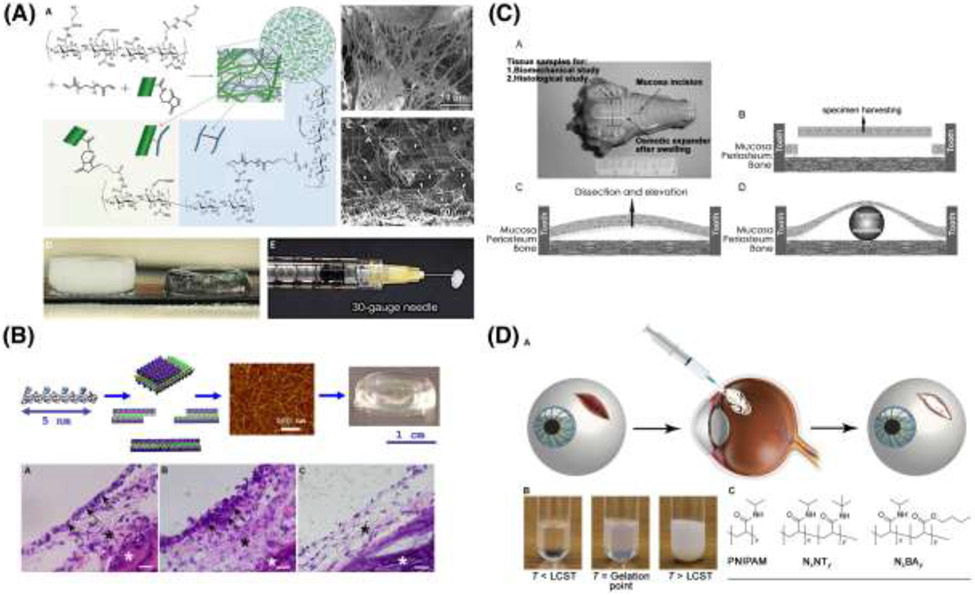
0 order injectable hydrogel formulations are presented in Figure 3. In a second category of FDA approved dermal fillers, represented with a demonstrative academic example in Figure 3A, similar hydrogels include suspended microparticles that augment volume by inducing collagen accumulation. Sculptra Aesthetic consists of poly(L-lactic acid) (PLLA) microparticles suspended within a carboxymethylcellulose support. The PLLA microparticles initiate a foreign body response, which in turn results in collagen type I deposition in the ECM. [121] Other similar agents used for these aesthetic purposes include poly(ε-caprolactone) (PCL) (used in Sinclair Ellansé) [122], poly(methyl methacrylate) (PMMA) (used in Bellafill, formerly Artefill) [122,123] and calcium hydroxyapatite (used in Merz Radiesse), [124] all suspended within injectable hydrogels, and all with the same intended mechanism of collagen production at the injection site.
Figure 3.
0th order hydrogel systems are summarized. (A) depicts a nanofiber-hydrogel composite that mimics soft tissue ECM through covalent interfacial bonding between electrospun PCL fibers and a HA hydrogel. [47] (B) Self-assembling peptide gels represent an injectable option [134], and have been used for mucosal regeneration and are sold commercially under the name PuraStat and PuraSinus. [126] C) Depicts Osmotic tissue expanders, sold under the trade name OSMED, which have been used for soft tissue expansion of the palate cleft. [138] (D) Outlines the design of a thermoresponsive gel for use in patients with ocular trauma. [66]
Another example of an injectable hydrogel for soft tissue use is PuraMatrix™, by 3D-Matrix, as shown in Figure 3B. PuraMatrix™ is a synthetic 16-amino acid peptide with alternating charged residues and is characterized by a stable beta-sheet structure that enables its pH-triggered self-assembly into hydrogels in aqueous conditions. In the presence of physiological concentrations of cations, it forms nanofibers with a mesh size similar to native ECM and provides a suitable scaffold for encapsulated cells. [125,126] Its analogous clinical products, PuraStat® and PuraSinus®, are approved for some soft-tissue applications, and are in multiple clinical trials for several others. [127,128] PuraSinus® is of particular interest for craniofacial use, as it has been approved by the FDA as a Class II medical device for use in both hemostasis and adhesion prevention in otorhinolaryngeal applications. [129] Outside of the United States, PuraStat® has been used for hemostasis in multiple similar applications, in gastrointestinal, colorectal, hepatic, and other resections. By our definitions above, both PuraSinus® and PuraStat® would be classified as 0 order hydrogels. PuraMatrix™ is more often described in the literature as a support matrix for cells in tissue regeneration, and would be considered as a 1st order product; however, it is not yet marketed for clinical use. [130-134]
Osmotic tissue expanders are examples of 0 order hydrogels that are self-inflating and eliminate the need for repeated injections in the process of soft tissue reconstruction. Highlighted in Figure 3C, Osmed™ hydrogel soft tissue expanders consist of a cross-linked hydrogel of methyl-methacrylate (MMA) and N-vinylpyrrolidone co-polymers, enclosed within a thin silicone shell. The hydrogel is engineered to swell at a predetermined, controlled rate and maintenance by the surgeon is not necessary. [135-139]
5.3. Ocular sealants
Other hydrogel systems have found valuable use as sealants, particularly in ocular applications, which is depicted in Figure 3D. [140] In 2014, FDA provided Premarket Approval (PMA) through CDRH for ReSure® (Ocular Therapeutix), a poly(ethylene glycol) (PEG)-based sealant for use in closing incisions resulting from cataract surgery. The two-part compound consists of an amine-functional tri-lysine, and a 4-arm PEG, terminated in amine-reactive N-hydroxysuccinimide (NHS). The two components are reconstituted in sterile water and mixed at the time of use, reacting to form a stable hydrogel in under a minute. This system offers advantages over cyanoacrylate sealants, which are comparably fast and mechanically robust, but overly rigid for ocular use, non-biodegradable, and inflammatory. Ocular Therapeutix has other PEG-based hydrogel systems in clinical trials now, as dehydrated injectable fibers that enable sustained release of small molecules (e.g., tyrosine kinase inhibitor axitinib [141], prostaglandin analog travoprost [142], or cyclosporine [143] for dry eye). Another interesting ocular application includes Vitargus®, which is a vitreous gel substitute for the eye. [144] The natural vitreous gel is composed of collagen and HA, and Vitargus® is similarly based on chemically-modified HA, functionalized with either aldehyde or hydrazide groups. [145] These react quickly when mixed, enabling a self-supporting gel to form, with appropriate refractive index to match the native vitreous, while maintaining low cytotoxicity in vitro. In practice, Vitargus® sets as a stable semisolid gel adhering to the retina. There remains an ongoing need for such vitreous substitutes within the field of ophthalmology, [146] and Vitargus® is among other comparable engineering substitutes (e.g., Healaflow) in the field. [147]
5.4. ECM-based hydrogels
Another approach relies on the production of ECM-derived hydrogels from decellularized animal tissues. [148] In this case, the ECM would not be an HCT/P or a CBER/CDER-regulated biologic since it is animal tissue-derived. This approach has seen some early success in Phase I clinical trials and because animal tissue can easily be sourced and processed, manufacturing processes can be optimized and performed in GMP facilities. The process involves two key steps: 1) solubilization of the ECM source material into its constitutive protein components, and 2) temperature and pH-controlled neutralization to ensure spontaneous reformation into a hydrogel. [148-150] Protein components are obtained through enzymatic digestion of the ECM in a dilute acid solution. After neutralizing to physiologic pH and adding salt buffers, the ECM digest forms a gel in a process dominated by collagen kinetics.
The most prominent examples of these hydrogel systems have used ECM derived from bladder tissue or small intestine, but these have not yet reached a critical commercial application in craniofacial use. A recent alternate example of how such hydrogels could be employed in other tissue systems is found in Ventrigel, an injectable, porcine myocardium-derived hydrogel. Ventrigel is intended for injection near the site of cardiac muscle injury in myocardial infarction patients. Its creators suggest that its derivation from myocardial tissue yields a tissue-specific ECM composition that uniquely mimics the native tissue, and could better support repair than non-matched ECM. It has successfully completed phase I trials and was demonstrated safe in a first-in-human study of treatment. [149]
5.5. Cosmeceuticals
In contrast to these examples, some hydrogel-based products may contain biologics, but still would be considered 0 order, as they are not CBER/CDER-regulated. Cosmetic products that contain bioactive ingredients, but are not marketed to generate a biologically-driven response, have been dubbed “cosmeceuticals,” as they are subject to fewer regulatory controls. [103,105,151] AIVITA Biomedical is testing a technology to deliver epidermal growth factor (EGF) and other human cell secreted growth factors in a serum or lotion format with the use of filler-grade HA to significantly improve skin morphology and collagen type I content. [152,153] Cosmeceuticals are not recognized by current legislation governing the regulation of other products, such as the Federal Food, Drug, and Cosmetic Act. Therefore, while claims of efficacy are strictly limited by law, the FDA does not require approval for topically applied products intended for aesthetic purposes or to alter appearance. In this case, the more challenging consideration is the ability to manufacture pure, bioactive recombinant growth factors at scale for improved scientific analysis, which could provide safety and efficacy data and lay the groundwork for injectable, therapeutic uses in the future.
6. 1st order applications: Hydrogels with a single level of function-driven complexity
In our hydrogel categorization, 1st order systems are positioned at a higher level of complexity than 0 order systems. As shown in Table 1, we define 1st order hydrogel systems as those that add a single level of function-driven complexity, either as a specific internal organization (alignment, orientation, etc.) that is required to drive a function, or through inclusion of a CBER/CDER-regulated biologic (e.g., living cells or cell products). We further subcategorize these into groups 1A and 1B: 1B systems have the same characteristics as 1A, however they are produced using 3D bioprinting, or a similar manufacturing method that requires independent validation. [8,37] Presently, the overwhelming majority of 1st order systems belong to subcategory 1A; however, we identify the 1B subcategory as an outlook to future technologies, and their need for additional validation.
Because of their added complexity and potential for cell incorporation, the hydrogel selection criteria for 1A order applications are likely different than those for 0 order uses. These 1A order systems are more likely to be implanted or injected, and therefore their skin penetration profile is less of a concern than in situ aspects, such as: native support for the encapsulated cell type, tailored degradation profiles, desired local response (tissue integration, angiogenesis, etc.), cargo release if applicable (e.g., cytokines) and all at a site-specific timescale. We highlight these elements throughout the examples that follow.
1A and 1B order hydrogel systems build complexity with the addition of a CBER/CDER-regulated biologic, or with a function-driven structure. Significant progress was made recently in the regulatory approval of products in this category, however significant hurdles remain to be overcome before these types of products gain widespread clinical use in the United States.
6.1. Hydrogels delivering cells
Craniofacial injury and trauma were major sources of motivation for developing early tissue engineering approaches to regenerate damaged tissues as an alternative to reconstructive surgeries. Mesenchymal stem cells (MSCs) have been widely studied for craniofacial applications, because they are multipotent and have the capacity to differentiate into multiple soft-tissue types that are damaged in craniofacial disease, including fat, bone, and muscle. [154] Various types of natural and synthetic hydrogels were designed to serve as delivery vehicles for MSCs and serve as prominent early academic examples of this category of therapy. This approach is described in detail in a review by Salinas et al. [154]
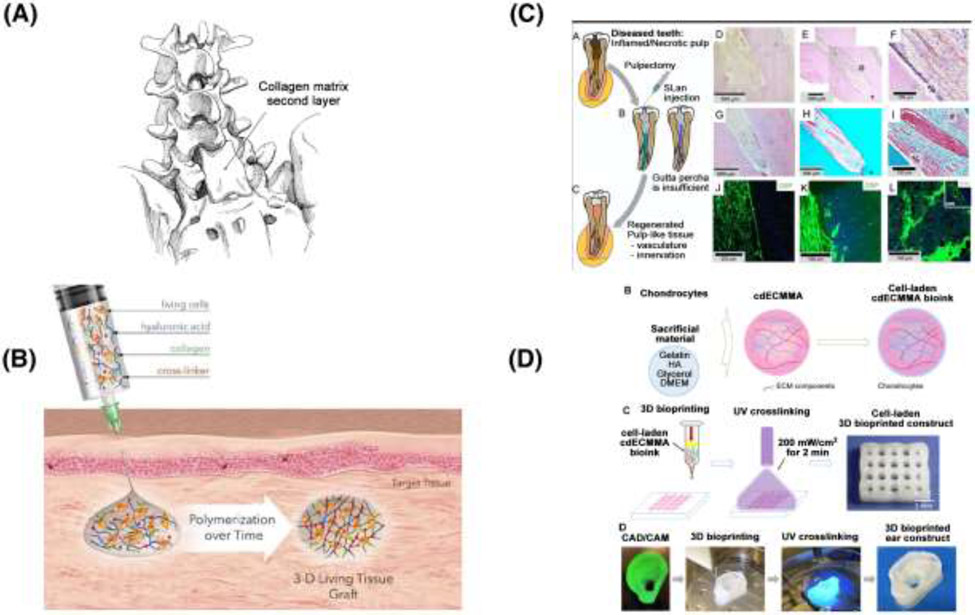
As an example of a novel product that builds complexity upon an already approved earlier technology, DuraGen® is a commercial product designed for the repair of dura mater and to limit cerebrospinal fluid (CSF) leakage. [155] Figure 4A depicts the location in which the collagen matrix could be used for duraplasty in an effort to repair CSF leakage. In this application, the material essentially serves as collagen sponges, which would be classified as a 0 order material, and it has been used in this manner for several decades. However, there remains some residual CSF leakage with this approach, so further studies are underway to assess strategies to improve outcomes. Currently a clinical trial is underway to assess the effect of an adipose stem cell (ASC)-seeded DuraGen® hydrogel on CSF leak rates following skull base surgery. [156] Depending on the extent of external cell/tissue manipulation, this potentially would advance the therapy to a 1A order use. According to official FDA guidance, adipose tissue is considered structural tissue, and using adipose stem cells would not meet the definition of minimal manipulation if the processing of the tissue alters an original relevant characteristic of the tissue. [157] It is difficult to exactly determine the level of manipulation based on the information provided in the clinical trial. Based on previous published work from the sponsor of the clinical trial [158,159], it can be inferred that adipose stem cells need to be expanded, enriched, and otherwise processed. This would lead that product to be classified as a biologic and fall under CBER/CDER BLA regulations, thus classifying it as a 1A order hydrogel system.
Figure 4.
1st order hydrogels, which contain a CBER/CDER-regulated biologic, or a function-driven structure, but are manufactured using previously validated methods. (A) Depicts the surgical placement of a collagen patch for dura repair, an early example motivating the need for a current clinical trial for the use of Duragen seeded with ASCs for skull base surgery [155,156] (B) Depicts the mode of action of Biotime’s Renevia®, demonstrating how a living tissue graft is established in vivo. [160] (C) A schematic depicting supramolecular peptide-based hydrogels for dental pulp revascularization. [163] (D) Outlines the development of a decellularized ECM based bioink for auricular reconstruction, a variation of which is currently in clinical trials [167,168]
Another prominent example in clinical development is Renevia® (Premvia in the US) by Lineage Cell Therapeutics, formerly BioTime [160]; this is shown in Figure 4B. In 2019, Renevia® received a Conformitè Europëenne (CE) mark from the European Union as a Class III medical device intended for use in adults for the treatment of facial lipoatrophy. In its final form, Renevia® is a crosslinked HA-gelatin hydrogel, based on the HyStem platform and intended to mimic extracellular matrix for the delivery of adipocytes to augment facial soft tissue volume secondary to HIV lipodystrophy. [161] However, unlike the above example of DuraGen®, Renevia® is reconstituted as a solution of hydrogel components, which are mixed with a cell fraction and injected as a suspension. These hydrogel precursors solidify in situ, with cells suspended within the resultant matrix. Lineage Cell Therapeutics has 510k clearance in the US for a very similar product, named Premvia®, for wound management. The thiolated carboxymethyl HA developed by BioTime effectively mimics ECM and enhances viability and engraftment post-injection. [162]
6.2. Hydrogels delivering other biologics
NangioTx is commercializing angiogenic peptide self-assembling hydrogels with a nanofiber architecture that mimics native ECM. One of their clinical indications is dental pulp revascularization. Shown in Figure 4C is a depiction of a preclinical study using a very similar hydrogel system to promote angiogenesis in vivo in a canine model. [163] This self-assembling shear-thinning hydrogel has an ECM-mimetic ultrastructure by virtue of the nanofibers that form, as well as a vascular endothelial growth factor (VEGF)-mimicking bioactive domain. The authors report a low materials synthesis cost, a potential benefit for widespread clinical use, particularly for patients who do not have access to stem-cell based and transplant therapies. It is noteworthy that a VEGF-mimicking peptide sequence was designed specifically to avoid introduction of whole growth factor, which in the setting of a hydrogel delivery system faces a more complex regulatory pathway. [164] Therefore, while the product currently described by NangioTx would not classify as a 1st order system, earlier work by this group where pro-angiogenic placental growth factor (PIGF-1) was loaded as cargo in peptide hydrogels leads to the conclusion that NangioTx gels have the potential to be 1A hydrogel systems in the near future. [165] While it is not intended for craniofacial use and is outside this scope of this review, REGRANEX® gel, which is a sodium carboxymethylcellulose-based (CMC) hydrogel containing Becaplermin (a human platelet-derived growth factor), has a noteworthy regulatory history and serves as a key example for future hydrogel products containing growth factors, especially when administered to cancer patients. [166] Briefly, it was the first FDA-approved recombinant platelet-derived growth factor therapy for the treatment of the lower extremity diabetic neuropathic ulcers. A boxed warning was added to the label in 2008 following a post-marketing retrospective cohort study due to reports of an increased rate of mortality secondary to malignancy in cancer patients. Ultimately, 10 years later, the FDA removed the boxed warning after multiple post-market studies demonstrated no increased safety risk with use of the gel.
6.3. 3D printed cellularized hydrogels
In each of the above examples, the final hydrogel solution does not rely on specific manufacturing or assembly techniques to achieve its final form. As we noted in Section 6 above, one of the most common current examples of these methods is 3D bioprinting, and we separate out such examples in this review. The field of tissue engineering is rife with examples of hard polymeric scaffolds for repair and regeneration of hard tissues such as bone. Thermoplastics and resins compatible with 3D printing are readily available to manufacture complex porous scaffolds for hard tissues. However, for soft tissue, far fewer hydrogel analogues exist. When considering therapeutic solutions that are in preclinical development and are manufactured using advanced biofabrication (1B systems), only a select few solutions address craniofacial needs. One example currently undergoing clinical testing is AuriNovo™ for auricular cartilage reconstruction. [167] Developed by 3DBio Therapeutics, AuriNovo™ is a 3D-printed collagen hydrogel scaffold encapsulating autologous auricular chondrocytes. The construct is indicated for patients with congenital microtia, a deformity where the external ear is underdeveloped. A proprietary, therapeutic grade bioink (ColVivo™) is used to print the constructs. While little is publicly available on the specifics of ColVivo™, the use of ECM-derived bioinks for auricular cartilage tissue engineering has been explored by other groups, notably in Visscher et al. [168] Figure 4D is a schematic diagram of the bioink development process referenced here. In this study, a collagen-based hydrogel was formed into a bioink by processing cartilage-derived decellularized ECM into a photo-crosslinkable hydrogel using methacrylation. This specific type of hydrogel is useful in this application because prior to crosslinking, constructs can be printed that are fitted to each patient, and after photo-crosslinking, the constructs provide structural integrity and mechanical stability.
Of note, Precise Bio is an early-stage start-up that is seeking to commercialize similar techniques for 3D printed corneal grafts, based on a recent patent application. [169] Based on this published application, the hydrogel crosslinked collagen/gelatin. The corneal graft is comprised of a support layer of either collagen methacrylate, poly(ethylene glycol) diacrylate (PEGDA), or gelatin methacrylate, and a second hydrogel layer encapsulating human endothelial cells. These polymers are subsequently crosslinked using UV light and a photo-initiator such as Irgacure®. In terms of the 3D printing technique, it is based on Laser Induced Forward Transfer (LIFT), wherein a microfluidic chip is used as the print head to deposit droplets of hydrogel, in some cases with encapsulated human corneal endothelial cells, based on previous published work. [170,171] While no clinical trials are currently listed for this company, Precise Bio recently partnered with Lineage Cell Therapeutics (formerly BioTime) for the development of a bio-retinal patch. [172]
Outside of these examples, there is one promising proof-of-concept academic example for 3D-printing craniofacial soft tissue, in developing a corneal stroma equivalent [173]. In this study, sodium alginate-based bioinks were used, with primary human corneal keratocytes as the cell type used. A noteworthy aspect to this study was the digital corneal model that enabled patient-specific 3D molds.
6.4. 3D printed hydrogels containing other biologics
Finally, decellularized ECM (dECM), when resolubilized, can also serve as a bioink for 3D bioprinting. Furthermore, there is a recent trend toward using tissue-specific dECM for 3D-printed bioinks [174] and away from more generic dECM scaffolds that were used in earlier applications. [175] Since recent applications are leveraging the growth factors and cytokines already present in the dECM to develop more tailored therapeutics, this would classify these types of therapies as 1B hydrogel systems, because the human-derived ECM would be an HCT/P, and most likely a CBER-regulated biologic. Some of the challenges that face expanded use of ECM in regenerative medicine include preserving the original ECM composition and developing decellularization methods without the use of harsh chemicals. Furthermore, scaling up for larger tissues and organs remains a challenge currently. [176]
7. 2nd order applications – Hydrogel systems with multiple levels of function-driven complexity
As a parallel to 1st order systems, 2nd order systems can be subdivided similarly into 2A and 2B. 2A hydrogel systems employ multiple mechanisms of action, with any combination of cells, cell components, and drugs, or one of those and a structural mode of action (orientation, channels, gradient, etc.). 2B hydrogel systems, on the other hand, include any system from 2A that relies upon a manufacturing method that requires further independent validation, like 3D bioprinting. Notably, both 2A and 2B systems are the targets of many imaginative laboratories, and the research literature contains many examples from the past 2 decades of so-called combination therapies. Conversely, rare examples exist, if at all, in the commercial realm. We propose that the challenges of regulatory approval, despite the advent of Regenerative Medicine Advanced Therapy (RMAT) designations, may be a key reason.
7.1. Hydrogels supporting multiple types of biologics
In 2012, Organogenesis received a landmark FDA approval for GINTUIT™, which was the first approved regenerative medicine product for dental care. It was approved for topical application to a surgically created vascular wound bed in the treatment of mucogingival conditions in adults. The regulatory pathway was aided by long-term safety data on Apligraf®, an identical product approved for wound healing in 1998 as a Class III medical device. GINTUIT™ (and Apligraf®) consists of allogeneic cultured keratinocytes and fibroblasts, derived from neonatal foreskin, delivered on a bovine collagen scaffold. It is intended for topical use (but still sutured into place) in a surgically created vascular wound bed in the treatment of mucogingival conditions in adults. [177] Of note, despite over two decades of clinical studies, there remains a dearth of guidelines on how the age of patients, their general health conditions, and co-morbidities may affect the success of these therapies. Furthermore, a lack of studies persists to demonstrate the mechanism of action, the fate of transplanted cells, and dose response of transplanted cells. These factors will be just as important in a dental application with GINTUIT™, given it is packaged as a small construct, identical in size to Apligraf®, only a 75mm diameter circular construct with 0.75 mm thickness. [178,179] Considering that GINTUIT™ was approved in 2012 and was promoted in a large amount of press releases given its historic status as the first biologic of its kind approved through CBER [84], it is noteworthy that no recent studies have been published on the clinical efficacy of GINTUIT™. While the financial and business details of these decisions are beyond the scope of this review, according to Organogenesis’ 10-K filing in 2018, the company made a business decision to stop commercializing GINTUIT™ in 2014, and is seeking to continue marketing GINTUIT™ in the future via a partnership in the oral surgery market. [180] As a relevant aside, sponsors of products for which the FDA has approved a BLA are obligated by the Pediatric Research Equity Act (PREA) [181] to conduct clinical trials in pediatric patients post approval unless a waiver is granted, which was not granted in the case of GINTUIT™. This is noteworthy because GINTUIT™ was never intended to be used in pediatric populations, and it was denied (upon appeal) a waiver for the requirement to comply with PREA. While the final denial for the waiver ultimately came in 2017, after the company had already suspended commercialization of GINTUIT™, it is possible the product could face further regulatory hurdles due to PREA that could limit its future clinical use.
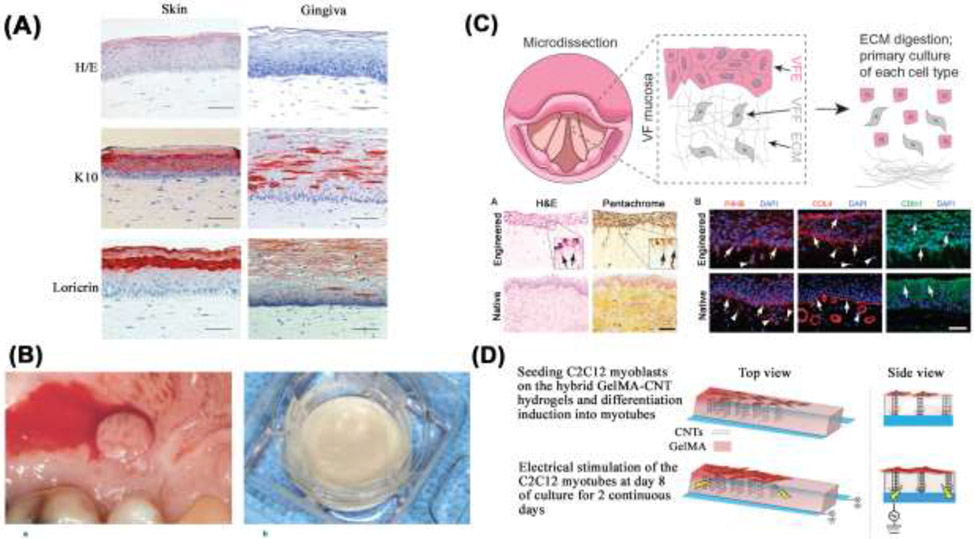
In contrast to GINTUIT™, which is intended for topical use on a surgical mucosal defect, not as a permanent tissue graft, another example of a 2A hydrogel is the development of tissue-engineered, full-thickness gingiva equivalents comprising a fibroblast-populated collagen hydrogel. [182] In Figure 5, several demonstrative examples of 2A hydrogel systems are depicted. Figure 5A depicts a histological comparison of full-thickness tissue engineered skin and gingiva, illustrating the different representative characteristics of skin and gingiva equivalents. [183] Similar to GINTUIT™, immortalized human donor cell lines are used, and immunohistological analysis with keratin 10 demonstrates the different representative characteristics of skin and gingiva equivalents. Furthermore, cytokine secretion showed differential proinflammatory secretion from skin and gingiva-derived fibroblasts. A start-up called A-SkinBV has attempted to commercialize this technology with a product called Tiscover. It is an autologous full thickness skin graft cultured from 3mm biopsies from the patient’s own healthy skin. [184,185] The authors of these studies were shareholders in A-SkinBV. In a very similar regulatory pathway to that which Organogenesis pursued, A-SkinBV first pursued approval for chronic wound healing in the extremities, and previously enrolled patients in a phase II trial in Europe. [186] While the company ceased to exist in its original form in 2019, the original co-founders are still active in the field of reconstructed human skin (RhS), recently publishing a study demonstrating a RhS model containing neopapillae. [187] In another contrast to GINTUIT™, Figure 5B depicts EVPOME, a tissue-engineered human ex vivo-produced oral mucosa equivalent intended for periodontal soft tissue augmentation. [53,188] Unlike GINTUIT™, which uses fibroblasts and keratinocytes, EVPOME uses keratinocytes alone (seeded onto an Alloderm construct) and is meant to be a permanent tissue graft. The construct upon implantation exhibits a function-driven structure through a well-differentiated epidermis on the outer surface of the construct. This, combined with the single cell type, classifies it as another example of a 2A hydrogel system, alongside GINTUIT™. This group continues to work on co-cultures of oral and skin keratinocytes, for use in tissue-engineered products, applied at delicate interfaces. As noted in Section 3.2, oral epithelium and epidermal skin heal differently, and efforts at these interfaces merit increased attention.
Figure 5.
2A hydrogel systems, which have a secondary mechanism of action and no need for manufacturing validation, are summarized. (A) depicts stained sections of tissue engineered skin and gingiva formed using collagen gels [183] (B) depicts a biopsy specimen harvested from the mucosa inside a patient’s mouth, as well as the EVPOME construct pre-grafting, which consists of an Alloderm construct seeded with oral keratinocytes. [53] (C) outlines a tissue-engineered human vocal-fold mucosa using a collagen scaffold and demonstrates similarity to native tissue. [190] (D) Depicts GelMA hydrogels infused with vertically aligned carbon nanotubes to support skeletal muscle differentiation and myofiber formation. [194]
A different potential approach involves the use of human cells embedded in a human tissue-derived ECM-based hydrogel, which has recently been demonstrated with the use of dental pulp matrix. [189] Decellularized pulp matrices were adapted to regenerative endodontic applications where acellular pulp bioscaffolds and MSCs were examined for their ability to promote mineralization needed for repair of bony defects. Given the presence of human cells and a human tissue-derived ECM hydrogel, this would be categorized as a 2A hydrogel system.
Beyond the above examples that have reached either clinical trials or full commercial use, the following examples are in a similar vein, and represent other craniofacial soft tissues with strong pre-clinical evidence. Figure 5C illustrates an engineered vocal fold mucosa using a polymerized type I collagen hydrogel that supports two different cell types, human focal fold fibroblasts and epithelial cells. [190] Hundreds of proteins have been identified in vocal fold lamina ECM, and yet most synthetic biomaterials consist of only a few types of macromolecules, which could limit therapeutic efficacy. Nevertheless, several examples of synthetic hydrogels are in use for vocal cord therapy, including thiol-modified HA and electrospun nanofibrous scaffolds that are a combination of thermoplastic polyurethane and poly(glycerol sebacate). These materials effectively mimic the vocal fold lamina propria ECM in terms of mechanical properties. [191-193] Finally, hybrid hydrogels can be well suited for muscle myofiber fabrication as well. Figure 5D is a schematic representation of the procedure used to produce and electrically stimulate C2C12 myotubes, which originate from an immortalized mouse myoblast cell line. [194] The hybrid hydrogels were composed of vertically aligned carbon nanotubes (CNTs) with methacrylated gelatin (GelMA) hydrogels. GelMA hydrogels provide a suitable milieu for the alignment of CNTs due to its low ion concentration and viscosity.
In summary, notable examples of 2A hydrogel systems range in application from oral mucosa and vocal fold regeneration to muscle myofiber formation, and range in degree of clinical translation from early-stage translational studies in the case of myofiber regeneration to FDA approved products as in the case of GINTUIT™.
7.2. 3D printed hydrogels containing multiple biologics
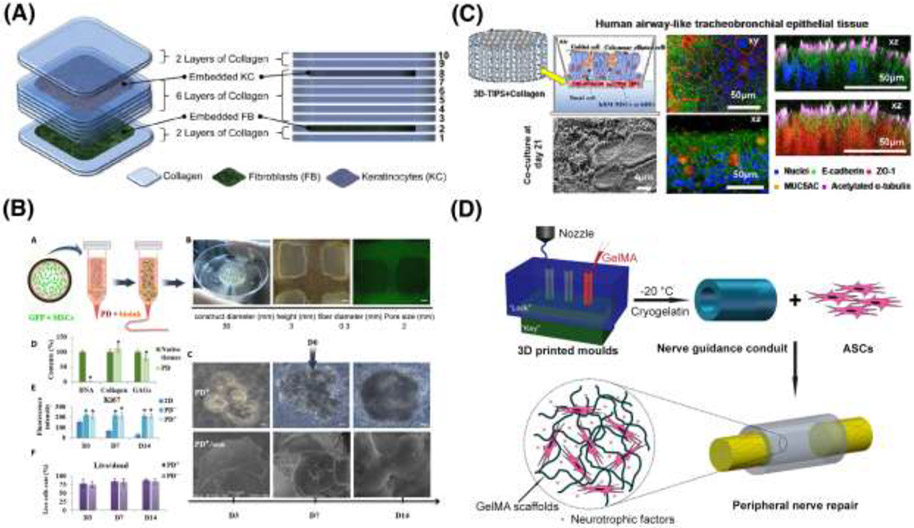
In contrast to 2A hydrogels, the additional defining characteristic of a 2B system is its implementation of a manufacturing method that requires further validation (such as 3D printing). Given that, to date, no examples of 2B hydrogels are in clinical trials, Figure 6 highlights promising examples in development with translational potential, to give an indication of what a future 2B hydrogel in clinical development could resemble.
Figure 6.
2B hydrogel systems, which have a secondary mechanism of action, and need manufacturing validation, are summarized. (A) This schematic outlines an extrusion-based method of collagen 3D printing with alternating layers of keratinocytes and fibroblasts. [197] (B) A schematic of the approach to alginate-based 3D printing of MSCs along with sweat gland ECM containing growth factors for sweat gland regeneration. [198] (C) This study develops a 3D printed human airway epithelium using thermoresponsive collagen hybrid gel embedded with human bronchial epithelial cells and human bronchial fibroblasts. [199] (D) This depicts 3D-printing of GelMA along with ASCs that secrete neurotrophic factors in a secondary mechanism of action as nerve guidance conduits that promote peripheral nerve regeneration. [200]
Regeneration of the periodontium complex is one application in which 2B hydrogels are described in the literature. In a study by Lee et al., PCL/HA scaffolds were fabricated by additive extrusion-based deposition using a 3D-Bioplotter® printer (EnvisionTec), and the fabricated scaffold consisted of three phases with different channel sizes; amelogenin, connective tissue growth factor, and bone morphogenetic protein-2 (BMP-2) were spatially tethered to their respective phase and underwent sustained release. [195] These findings illustrate a strategy for the regeneration of multiphase periodontal tissue by spatiotemporal delivery of multiple biologics.
In a similar application, Athirasala et al. targeted the regeneration of soft, dental pulp tissue, using a bioink composed of demineralized and digested dentin ECM from human molars and sodium alginate solution. [196] These blends were crosslinked with CaCl2 to encapsulate human stem cells from the apical papilla (SCAP) as well as OD21 cells, a mouse odontoblast-like cell line. Furthermore, the dentin matrix used also contains growth factors, which the authors claim could provide an additional mechanism of action through their osteogenic/odontogenic differentiation potential. Flow rate through the 3D printing extrusion nozzle and cross-linking time were modulated to optimize fiber formation. This blended material yielded desirable physical and biological properties and sustained high cell viability. This bioink leverages 3D bioprinting to precisely control the spatial presentation of signaling factors to engineer a physiologically relevant pulp dentin complex.
Beyond regenerative dentistry, 2B hydrogel systems provide a compelling way to design engineered skin tissues, with 3D printing enabling fabrication of a multi-layer composite that mimics native skin layers. Figure 6A depicts a schematic of this approach, with fibroblasts and keratinocytes embedded in layers of a 3D printed collagen hydrogel. [197] Type I collagen from rat tail was used as the hydrogel precursor, and upon deposition, sodium bicarbonate was nebulized. Crosslinking was achieved via surface coating of the newly printed layer. This process was repeated for the second cell type, with up to 8 layers printed in this fashion.
A clinical application that is particularly suited for biofabrication of cell-laden hydrogels with internal architecture is for exocrine glands. These secretory glands release substances onto an epithelial surface through a duct, have a complex structure with various cell types along the length of the gland, and cannot be easily recapitulated with standard methods of gel casting and patterning. Figure 6B depicts a recent study by Yao et al. and highlights the translational potential of a 3D-printed alginate hydrogel for guiding stem cell differentiation toward functional sweat gland regeneration. In this study, the authors combined MSCs with a specific sweat gland ECM containing growth factors to generate 3D-printed microenvironments that promote sweat gland differentiation of MSCs. [198]
Figure 6C depicts another recent study, which developed tissue-engineered human airway epithelium that was 3D-printed using a collagen gel embedded with human bronchial epithelial cells and human bronchial fibroblasts. [199] In this study the authors employed an indirect method of fabrication by 3D printing sacrificial molds that incorporated thermally induced phase separation (3D-TIPS), allowing for controlled porosity and hierarchically interconnected pores.
In another compelling example that holds promise for craniofacial applications, Hu et al. developed a bio-conduit for peripheral nerve regeneration consisting of a cryopolymerized gelatin methacryloyl gel cellularized with adipose-derived stem cells (ADSCs). [200] The authors suggest that neurotrophic factors also are released from these scaffolds, which provides another mechanism of action through a secondary biologic. Depicted in Figure 6D, the conduit is biodegradable and supported significant re-innervation in a small animal model. The nerve conduits were customized through the integration of magnetic resonance neurography with computer-aided design (CAD) models and subsequent 3D bioprinting. Through this fabrication technique the authors were able to incorporate relatively low doses of cells and complex geometries, with bifurcating structures unique to each patient.
Finally, a noteworthy proof-of concept study recently demonstrated 3D printing of patient-specific nasal cartilage. [201] In this study, primary human adipose-derived stem cells were used, and the bioink was derived from human cartilage-decellularized ECM. Given that the human-derived bioink itself as well as the human cells used are both CBER regulated, this is a 2B hydrogel system that is also noteworthy for using 3D facial models to generate custom-designed implants to mimic native facial features.
In summary, multiple exciting examples of 2B hydrogel systems exist in early-stage development, a snapshot of which is provided here, some of which could form the basis for a RMAT designated tissue engineered product in the future. Beyond the technical and biological challenges in recapitulating native ECM and growth factors, the broader industry challenge involves regulatory hurdles, since advanced biofabrication methods often are customized and require validation prior to regulatory approval. The regulatory requirement for manufacturing validation of engineered tissues inevitably adds complexity to an already challenging path to commercialization.
8. Conclusion and Future Outlook
The history of the use of hydrogels as regenerative or tissue engineering tools for craniofacial applications is a cross-section of all levels of complexity in terms of hydrogel composition, bioactive components, and internal vascular structure and hierarchy. Early work initiated several decades ago began with topically applied animal-derived proteins for purely cosmetic purposes. Since then, the field has evolved tremendously. Current state-of-the-art is to use 3D printing and/or other advanced fabrication methods to design multiphase, spatially oriented structures that closely mimic native tissues and can restore functions. Another promising area of research in regenerative medicine is the development of hydrogel microparticles, or granular hydrogels [202], as a platform for tissue repair and drug delivery that has greater porosity and easier delivery through a syringe compared to bulkier hydrogel scaffolds. Given its complex hierarchical structure, the salivary gland is a prime clinical target for the application of 3D printed hydrogels. Recent work in the field of salivary gland tissue engineering has increased our understanding of the dynamic assembly and differentiation of salivary gland components and could inform future work in biofabrication. [79,203,204] Recent work in our group has highlighted the importance of HA based hydrogels in recapitulating intact eccrine gland structure for preclinical testing as well as regenerative medicine applications. [205]
A key consideration is that no FDA-approved and clinically available 3D printed hydrogel-based products exist for tissue engineering applications. While many promising developmental and pre-clinical examples of hydrogel systems exist that leverage the advantages of 3D-printing for tissue engineering and regenerative medicine applications, most of these are recent developments that have not progressed through the long process of regulatory approval. Beyond that, however, structural challenges in the current regulatory landscape remain, where current regulations require independent validation of each step in the manufacturing process, and declaration of one primary mode of action (e.g., as a device, drug, or biologic). Current regulations are not optimized to assess the complexity and synergistic effects of the latest-generation of 3D printed, cell laden hydrogels with a function-driven structure, or a secondary biologic embedded. Of note, of the more than 50 and counting currently approved therapies with RMAT designations [206], only a handful appear to integrate traditional tissue engineering, that is, have a scaffold or employ hydrogel-based delivery of biologics. These include AmnioFix (a dehydrated, injectable human amnion/chorion membrane allograft), Avance (nerve guide comprising decellularized human cadaveric peripheral nerve allograft [207]), Humacyl (deposited ECM from human vascular cells seeded onto a PLGA scaffold), StrataGraft (allogeneic cultured keratinocytes and dermal fibroblasts in a murine collagen scaffold), and TTAX02 (cryopreserved human umbilical cord tissue). Many of the currently approved RMATs are cell therapy agents, which could be partially explained by the fact that RMAT is intended for the potential to address a serious life-threatening disease or condition, such as cancer, which is the target indication for most of the RMATs currently in clinical use. A notable example of an RMAT-designated cellular therapy for regenerative medicine is Cook Myosite, an injectable, autologous muscle derived cell (AMDC) therapy. Current Myosite applications include clinical trials for AMDC-mediated sphincter repair in cases of stress urinary incontinence [208], and treatment of tongue dysphagia [209]. The current disposition of RMAT designees toward cell-only therapies suggests that, despite the availability of a combination therapy designation, the technology of combination therapy development still outpaces its application to clinical use. Future regulatory efforts could require additional streamlining to improve time to market or reduce burden in clinical trials.
In summary, this review assesses hydrogel systems based on the various regulatory hurdles a given therapy might encounter on the way to FDA approval. As each technology builds in complexity from 0 order to 2nd order systems, the regulatory process also increases in complexity. Simultaneously, the number of clinically approved, commercially available products declines significantly. On an optimistic note, the pipeline of 1st and 2nd order hydrogels in later stages of preclinical testing and development continues to grow, and outcomes from those trials foster future development of even more advanced tissue engineering therapies.
Acknowledgements
This work was supported by the National Institutes of Health, grants R03DE028988 (to DAH) and R56DE026530 (to MCFC), grant funding from the Oral and Maxillofacial Surgery Foundation (to MCFC), and institutional support from The University of Texas Health Science Center at Houston. The authors are grateful to Dr. Stephen E. Feinberg, University of Michigan, for insightful discussions that improved the manuscript. Figure 1 was created with BioRender.com. In other figures, original images may have been slightly modified for clarity and legibility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Foyt DA, Norman MDA, Yu TTL, Gentleman E, Exploiting Advanced Hydrogel Technologies to Address Key Challenges in Regenerative Medicine, Adv. Healthc. Mater 7 (2018) 1700939. 10.1002/adhm.201700939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Brown TE, Anseth KS, Spatiotemporal hydrogel biomaterials for regenerative medicine, Chem. Soc. Rev 46 (2017) 6532–6552. 10.1039/c7cs00445a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Highley CB, Prestwich GD, Burdick JA, Recent advances in hyaluronic acid hydrogels for biomedical applications, Curr. Opin. Biotechnol 40 (2016) 35–40. 10.1016/j.copbio.2016.02.008. [DOI] [PubMed] [Google Scholar]
- [4].Annabi N, Tamayol A, Uquillas JA, Akbari M, Bertassoni LE, Cha C, Camci-Unal G, Dokmeci MR, Peppas NA, Khademhosseini A, 25th anniversary article: Rational design and applications of hydrogels in regenerative medicine, Adv. Mater. Deerfield Beach Fla 26 (2014) 85–123. 10.1002/adma.201303233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Singh V, Hydrogels: Applications and Global Markets to 2022, BCC Research, 2017. https://www.bccresearch.com/market-research/advanced-materials/hydrogels-applications-and-global-markets-report.html. [Google Scholar]
- [6].McKee CT, Last JA, Russell P, Murphy CJ, Indentation Versus Tensile Measurements of Young’s Modulus for Soft Biological Tissues, Tissue Eng. Part B Rev 17 (2011) 155–164. 10.1089/ten.teb.2010.0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tao O, Kort-Mascort J, Lin Y, Pham HM, Charbonneau AM, ElKashty OA, Kinsella JM, Tran SD, The Applications of 3D Printing for Craniofacial Tissue Engineering, Micromachines Basel. 10 (2019). 10.3390/mi10070480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sigaux N, Pourchet L, Breton P, Brosset S, Louvrier A, Marquette CA, 3D Bioprinting: principles, fantasies and prospects, J. Stomatol. Oral Maxillofac. Surg 120 (2019) 128–132. 10.1016/j.jormas.2018.12.014. [DOI] [PubMed] [Google Scholar]
- [9].Zhong N, Zhao X, 3D printing for clinical application in otorhinolaryngology, Eur. Arch. Oto-Rhino-Laryngol. Off. J. Eur. Fed. Oto-Rhino-Laryngol. Soc. EUFOS Affil. Ger. Soc. Oto-Rhino-Laryngol. - Head Neck Surg 274 (2017) 4079–4089. 10.1007/s00405-017-4743-0. [DOI] [PubMed] [Google Scholar]
- [10].Ricles LM, Coburn JC, Prima MD, Oh SS, Regulating 3D-printed medical products, Sci. Transl. Med 10 (2018). 10.1126/scitranslmed.aan6521. [DOI] [PubMed] [Google Scholar]
- [11].Kim YS, Smoak MM, Melchiorri AJ, Mikos AG, An Overview of the Tissue Engineering Market in the United States from 2011 to 2018, Tissue Eng. Part A 25 (2019) 1–8. 10.1089/ten.TEA.2018.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Guimarães CF, Gasperini L, Marques AP, Reis RL, The stiffness of living tissues and its implications for tissue engineering, Nat. Rev. Mater 5 (2020) 351–370. 10.1038/s41578-019-0169-1. [DOI] [Google Scholar]
- [13].Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, Huebsch N, Lee H, Lippens E, Duda GN, Mooney DJ, Hydrogels with tunable stress relaxation regulate stem cell fate and activity, Nat. Mater 15 (2016) 326–334. 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kretlow JD, McKnight AJ, Izaddoost SA, Facial Soft Tissue Trauma, Semin. Plast. Surg 24 (2010) 348–356. 10.1055/s-0030-1269764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Faraji F, Lee JH, Faraji F, MacDonald B, Oviedo P, Stuart E, Baxter M, Vuong CL, Lance SH, Gosman AA, Castillo EM, Hom DB, Electric scooter craniofacial trauma, Laryngoscope Investig. Otolaryngol 5 (2020) 390–395. 10.1002/lio2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Einy S, Goldman S, Radomislensky I, Israel Trauma Group (ITG), Bodas M, Peleg K, Maxillofacial trauma following road accidents-An 11-year multi-center study in Israel, Dent. Traumatol. Off. Publ. Int. Assoc. Dent. Traumatol (2020). 10.1111/edt.12639. [DOI] [PubMed] [Google Scholar]
- [17].Trivedi B, Kesterke MJ, Bhattacharjee R, Weber W, Mynar K, Reddy LV, Craniofacial Injuries Seen With the Introduction of Bicycle-Share Electric Scooters in an Urban Setting, J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg 77 (2019) 2292–2297. 10.1016/j.joms.2019.07.014. [DOI] [PubMed] [Google Scholar]
- [18].Cohn JE, Melley LE, Lafferty D, Othman S, Stucker FJ, Bundrick P, Adult Maxillofacial Trauma Patterns in American Football, J. Craniofac. Surg Publish Ahead of Print (2021). 10.1097/SCS.0000000000007399. [DOI] [PubMed] [Google Scholar]
- [19].Anderson C, Hamidian Jahromi A, Miller EJ, Konofaos P, The Current Status of the Autologous Fat Grafting for Pediatric Craniofacial Patients, Ann. Plast. Surg 85 (2020) 568–573. 10.1097/SAP.0000000000002286. [DOI] [PubMed] [Google Scholar]
- [20].Denadai R, Raposo-Amaral CA, Buzzo CL, Raposo-Amaral CE, Autologous Free Fat Grafting for Management of the Facial Contour Asymmetry, J. Craniofac. Surg 29 (2018) 878–886. 10.1097/SCS.0000000000004369. [DOI] [PubMed] [Google Scholar]
- [21].Simonacci F, Bertozzi N, Grieco MP, Grignaffini E, Raposio E, Procedure, applications, and outcomes of autologous fat grafting, Ann. Med. Surg 20 (2017) 49–60. 10.1016/j.amsu.2017.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Deshpande MV, West AJ, Bernacki SH, Luan K, King MW, Poly(ε-Caprolactone) Resorbable Auxetic Designed Knitted Scaffolds for Craniofacial Skeletal Muscle Regeneration, Bioeng. Basel Switz 7 (2020). 10.3390/bioengineering7040134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schlottmann F, Bucan V, Vogt PM, Krezdorn N, A Short History of Skin Grafting in Burns: From the Gold Standard of Autologous Skin Grafting to the Possibilities of Allogeneic Skin Grafting with Immunomodulatory Approaches, Medicina (Mex.). 57 (2021) 225. 10.3390/medicina57030225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hosseini M, Shafiee A, Engineering Bioactive Scaffolds for Skin Regeneration, Small Weinh. Bergstr. Ger (2021) e2101384. 10.1002/smll.202101384. [DOI] [PubMed] [Google Scholar]
- [25].Zhang W, Yelick PC, Craniofacial Tissue Engineering, Cold Spring Harb. Perspect. Med 8 (2018). 10.1101/cshperspect.a025775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mai CT, Isenburg JL, Canfield MA, Meyer RE, Correa A, Alverson CJ, Lupo PJ, Riehle-Colarusso T, Cho SJ, Aggarwal D, Kirby RS, National population-based estimates for major birth defects, 2010–2014, Birth Defects Res. 111 (2019) 1420–1435. 10.1002/bdr2.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Emara A, Shah R, Recent update on craniofacial tissue engineering, J. Tissue Eng 12 (2021) 20417314211003736. 10.1177/20417314211003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pastore MN, Roberts MS, Selection of Topically Applied Chemical Candidates: Transdermal Drug Delivery Systems, in: Sugibayashi K (Ed.), Skin Permeat. Dispos. Ther. Cosmeceutical Compd, Springer Japan, Tokyo, 2017: pp. 251–262. 10.1007/978-4-431-56526-0_21. [DOI] [Google Scholar]
- [29].Kathe K, Kathpalia H, Film forming systems for topical and transdermal drug delivery, Asian J. Pharm. Sci 12 (2017) 487–497. 10.1016/j.ajps.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Talaat WM, Haider M, Kawas SA, Kandil NG, Harding DRK, Chitosan-Based Thermosensitive Hydrogel for Controlled Drug Delivery to the Temporomandibular Joint, J. Craniofac. Surg 27 (2016) 735–740. 10.1097/SCS.0000000000002588. [DOI] [PubMed] [Google Scholar]
- [31].Varma DM, Gold GT, Taub PJ, Nicoll SB, Injectable carboxymethylcellulose hydrogels for soft tissue filler applications, Acta Biomater. 10 (2014) 4996–5004. 10.1016/j.actbio.2014.08.013. [DOI] [PubMed] [Google Scholar]
- [32].Zuk PA, Tissue Engineering Craniofacial Defects With Adult Stem Cells? Are We Ready Yet?, Pediatr. Res 63 (2008) 478–486. 10.1203/PDR.0b013e31816bdf36. [DOI] [PubMed] [Google Scholar]
- [33].Naudot M, Davrou J, Djebara A-E, Barre A, Lavagen N, Lardière S, Azdad SZ, Zabijak L, Lack S, Devauchelle B, Marolleau J-P, Le Ricousse S, Functional Validation of a New Alginate-based Hydrogel Scaffold Combined with Mesenchymal Stem Cells in a Rat Hard Palate Cleft Model, Plast. Reconstr. Surg. Glob. Open 8 (2020) e2743. 10.1097/GOX.0000000000002743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hong C, Song D, Lee D-K, Lin L, Pan HC, Lee D, Deng P, Liu Z, Hadaya D, Lee H-L, Mohammad A, Zhang X, Lee M, Wang C-Y, Ho D, Reducing posttreatment relapse in cleft lip palatal expansion using an injectable estrogen-nanodiamond hydrogel, Proc. Natl. Acad. Sci. U. S. A 114 (2017) E7218–E7225. 10.1073/pnas.1704027114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mostafa NZ, Talwar R, Shahin M, Unsworth LD, Major PW, Doschak MR, Cleft palate reconstruction using collagen and nanofiber scaffold incorporating bone morphogenetic protein in rats, Tissue Eng. Part A 21 (2015) 85–95. 10.1089/ten.tea.2014.0075. [DOI] [PubMed] [Google Scholar]
- [36].Swan MC, Bucknall DG, Goodacre TEE, Czernuszka JT, Synthesis and properties of a novel anisotropic self-inflating hydrogel tissue expander, Acta Biomater. 7 (2011) 1126–1132. 10.1016/j.actbio.2010.10.017. [DOI] [PubMed] [Google Scholar]
- [37].Choi Y-J, Park H, Ha D-H, Yun H-S, Yi H-G, Lee H, 3D Bioprinting of In Vitro Models Using Hydrogel-Based Bioinks, Polymers. 13 (2021) 366. 10.3390/polym13030366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cleversey C, Robinson M, Willerth SM, 3D Printing Breast Tissue Models: A Review of Past Work and Directions for Future Work, Micromachines. 10 (2019). 10.3390/mi10080501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Abelseth E, Abelseth L, De la Vega L, Beyer ST, Wadsworth SJ, Willerth SM, 3D Printing of Neural Tissues Derived from Human Induced Pluripotent Stem Cells Using a Fibrin-Based Bioink, ACS Biomater. Sci. Eng 5 (2019) 234–243. 10.1021/acsbiomaterials.8b01235. [DOI] [PubMed] [Google Scholar]
- [40].Lee C, Abelseth E, de la Vega L, Willerth SM, Bioprinting a novel glioblastoma tumor model using a fibrin-based bioink for drug screening, Mater. Today Chem 12 (2019) 78–84. 10.1016/j.mtchem.2018.12.005. [DOI] [Google Scholar]
- [41].Visscher DO, Farré-Guasch E, Helder MN, Gibbs S, Forouzanfar T, van Zuijlen PP, Wolff J, Advances in Bioprinting Technologies for Craniofacial Reconstruction, Trends Biotechnol. 34 (2016) 700–710. 10.1016/j.tibtech.2016.04.001. [DOI] [PubMed] [Google Scholar]
- [42].Yang JM, Olanrele OS, Zhang X, Hsu CC, Fabrication of Hydrogel Materials for Biomedical Applications, in: Chun HJ, Park K, Kim C-H, Khang G (Eds.), Nov. Biomater. Regen. Med, Springer, Singapore, 2018: pp. 197–224. 10.1007/978-981-13-0947-2_12. [DOI] [PubMed] [Google Scholar]
- [43].Ghuman H, Gerwig M, Nicholls FJ, Liu JR, Donnelly J, Badylak SF, Modo M, Long-term retention of ECM hydrogel after implantation into a sub-acute stroke cavity reduces lesion volume, Acta Biomater. 63 (2017) 50–63. 10.1016/j.actbio.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Di Caprio N, Bellas E, Collagen Stiffness and Architecture Regulate Fibrotic Gene Expression in Engineered Adipose Tissue, Adv. Biosyst 4 (2020) 1900286. 10.1002/adbi.201900286. [DOI] [PubMed] [Google Scholar]
- [45].Hammel JH, Bellas E, Endothelial cell crosstalk improves browning but hinders white adipocyte maturation in 3D engineered adipose tissue, Integr. Biol 12 (2020) 81–89. 10.1093/intbio/zyaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Van Nieuwenhove I, Tytgat L, Ryx M, Blondeel P, Stillaert F, Thienpont H, Ottevaere H, Dubruel P, Van Vlierberghe S, Soft tissue fillers for adipose tissue regeneration: From hydrogel development toward clinical applications, Acta Biomater. 63 (2017) 37–49. 10.1016/j.actbio.2017.09.026. [DOI] [PubMed] [Google Scholar]
- [47].Li X, Cho B, Martin R, Seu M, Zhang C, Zhou Z, Choi JS, Jiang X, Chen L, Walia G, Yan J, Callanan M, Liu H, Colbert K, Morrissette-McAlmon J, Grayson W, Reddy S, Sacks JM, Mao H-Q, Nanofiber-hydrogel composite–mediated angiogenesis for soft tissue reconstruction, Sci. Transl. Med 11 (2019). 10.1126/scitranslmed.aau6210. [DOI] [PubMed] [Google Scholar]
- [48].Cho Y-D, Kim K-H, Lee Y-M, Ku Y, Seol Y-J, Periodontal Wound Healing and Tissue Regeneration: A Narrative Review, Pharmaceuticals. 14 (2021). 10.3390/ph14050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Turabelidze A, Guo S, Chung AY, Chen L, Dai Y, Marucha PT, DiPietro LA, Intrinsic Differences between Oral and Skin Keratinocytes, PLoS ONE. 9 (2014) e101480. 10.1371/journal.pone.0101480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Waasdorp M, Krom BP, Bikker FJ, van Zuijlen PPM, Niessen FB, Gibbs S, The Bigger Picture: Why Oral Mucosa Heals Better Than Skin, Biomolecules. 11 (2021) 1165. 10.3390/biom11081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Jati AS, Furquim LZ, Consolaro A, Gingival recession: its causes and types, and the importance of orthodontic treatment, Dent. Press J. Orthod 21 (2016) 18–29. 10.1590/2177-6709.21.3.018-029.oin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ansari S, Pouraghaei Sevari S, Chen C, Sarrion P, Moshaverinia A, RGD-Modified Alginate–GelMA Hydrogel Sheet Containing Gingival Mesenchymal Stem Cells: A Unique Platform for Wound Healing and Soft Tissue Regeneration, ACS Biomater. Sci. Eng (2021). 10.1021/acsbiomaterials.0c01571. [DOI] [PubMed] [Google Scholar]
- [53].Izumi K, Neiva RF, Feinberg SE, Intraoral Grafting of Tissue-Engineered Human Oral Mucosa, Int. J. Oral Maxillofac. Implants 28 (2013) e295–e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Carnes ME, Pins GD, Skeletal Muscle Tissue Engineering: Biomaterials-Based Strategies for the Treatment of Volumetric Muscle Loss, Bioengineering. 7 (2020). 10.3390/bioengineering7030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Raimondo TM, Li H, Kwee BJ, Kinsley S, Budina E, Anderson EM, Doherty EJ, Talbot SG, Mooney DJ, Combined delivery of VEGF and IGF-1 promotes functional innervation in mice and improves muscle transplantation in rabbits, Biomaterials. 216 (2019) 119246. 10.1016/j.biomaterials.2019.119246. [DOI] [PubMed] [Google Scholar]
- [56].Yang P, Li C, Lee M, Marzvanyan A, Zhao Z, Ting K, Soo C, Zheng Z, Photopolymerizable Hydrogel-Encapsulated Fibromodulin-Reprogrammed Cells for Muscle Regeneration, Tissue Eng. Part A (2020). 10.1089/ten.tea.2020.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Barrett P, Quick TJ, Mudera V, Player DJ, Generating intrafusal skeletal muscle fibres in vitro: Current state of the art and future challenges, J. Tissue Eng 11 (2020) 2041731420985205. 10.1177/2041731420985205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Moreddu R, Vigolo D, Yetisen AK, Contact Lens Technology: From Fundamentals to Applications, Adv. Healthc. Mater 8 (2019) 1900368. 10.1002/adhm.201900368. [DOI] [PubMed] [Google Scholar]
- [59].Princz MA, Lasowski FJR, Sheardown H, 16 - Advances in intraocular lens materials, in: Chirila TV, Harkin DG (Eds.), Biomater. Regen. Med. Ophthalmol. Second Ed, Woodhead Publishing, 2016: pp. 401–417. 10.1016/B978-0-08-100147-9.00016-X. [DOI] [Google Scholar]
- [60].Fenton OS, Paolini M, Andresen JL, Müller FJ, Langer R, Outlooks on Three-Dimensional Printing for Ocular Biomaterials Research, J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther 36 (2020) 7–17. 10.1089/jop.2018.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wang K, Han Z, Injectable hydrogels for ophthalmic applications, J Control Release. 268 (2017) 212–224. 10.1016/j.jconrel.2017.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Mandal A, Clegg JR, Anselmo AC, Mitragotri S, Hydrogels in the clinic, Bioeng. Transl. Med 5 (2020). 10.1002/btm2.10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hacker MC, Klouda L, Ma BB, Kretlow JD, Mikos AG, Synthesis and characterization of injectable, thermally and chemically gelable, amphiphilic poly(N-isopropylacrylamide)-based macromers, Biomacromolecules. 9 (2008) 1558–70. 10.1021/bm8000414. [DOI] [PubMed] [Google Scholar]
- [64].Gulyuz U, Okay O, Self-healing poly(N-isopropylacrylamide) hydrogels, Eur. Polym. J 72 (2015) 12–22. 10.1016/j.eurpolymj.2015.09.002. [DOI] [Google Scholar]
- [65].Chatterjee S, Hui PC, Kan CW, Thermoresponsive Hydrogels and Their Biomedical Applications: Special Insight into Their Applications in Textile Based Transdermal Therapy, Polym. Basel 10 (2018). 10.3390/polym10050480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Bayat N, Zhang Y, Falabella P, Menefee R, Whalen JJ, Humayun MS, Thompson ME, A reversible thermoresponsive sealant for temporary closure of ocular trauma, Sci Transl Med. 9 (2017). 10.1126/scitranslmed.aan3879. [DOI] [PubMed] [Google Scholar]
- [67].Harrington DA, Martinez M, Wu D, Pradhan-Bhatt S, Farach-Carson MC, Salivary Gland Tissue Engineering and Future Diagnostics, in: Streckfus CF (Ed.), Adv. Salivary Diagn, Springer Berlin Heidelberg, Berlin, Heidelberg, 2015: pp. 157–185. 10.1007/978-3-662-45399-5_8. [DOI] [Google Scholar]
- [68].Pradhan-Bhatt S, Cannon K, Zakheim D, Harrington DA, Duncan RL, Jia X, Farach-Carson MC, Witt RL, Chapter 46 - Salivary Gland Tissue Engineering and Repair, in: Vishwakarma A, Sharpe P, Shi S, Ramalingam M (Eds.), Stem Cell Biol. Tissue Eng. Dent. Sci, Academic Press, Boston, 2015: pp. 613–623. 10.1016/B978-0-12-397157-9.00050-3. [DOI] [Google Scholar]
- [69].Wang Z, Benza RL, Zourelias L, Sanguino A, Geguchadze R, Shields KJ, Wu C, Highland KB, Passineau MJ, In vivo Endocrine Secretion of Prostacyclin Following Expression of a Cyclooxygenase-1/Prostacyclin Fusion Protein in the Salivary Glands of Rats Via Nonviral Gene Therapy, Hum. Gene Ther 28 (2017) 681–689. 10.1089/hum.2017.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Lombaert IMA, Patel VN, Jones CE, Villier DC, Canada AE, Moore MR, Berenstein E, Zheng C, Goldsmith CM, Chorini JA, Martin D, Zourelias L, Trombetta MG, Edwards PC, Meyer K, Ando D, Passineau MJ, Hoffman MP, CERE-120 Prevents Irradiation-Induced Hypofunction and Restores Immune Homeostasis in Porcine Salivary Glands, Mol. Ther. Methods Clin. Dev 18 (2020) 839–855. 10.1016/j.omtm.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Samuni Y, Baum BJ, Gene delivery in salivary glands: from the bench to the clinic, Biochim. Biophys. Acta 1812 (2011) 1515–1521. 10.1016/j.bbadis.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Baum BJ, Alevizos I, Chiorini JA, Cotrim AP, Zheng C, Advances in salivary gland gene therapy - oral and systemic implications, Expert Opin. Biol. Ther 15 (2015) 1443–1454. 10.1517/14712598.2015.1064894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Baum BJ, Radiation-induced salivary hypofunction may become a thing of the past, Oral Dis. 22 (2016) 81–84. 10.1111/odi.12388. [DOI] [PubMed] [Google Scholar]
- [74].Baum BJ, Afione S, Chiorini JA, Cotrim AP, Goldsmith CM, Zheng C, Gene Therapy of Salivary Diseases, Methods Mol. Biol. Clifton NJ 1537 (2017) 107–123. 10.1007/978-1-4939-6685-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Scheller EL, KREBSBACH PH, KOHN DH, Tissue engineering: state of the art in oral rehabilitation, J. Oral Rehabil 36 (2009) 368–389. 10.1111/j.1365-2842.2009.01939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Alsberg E, Hill EE, Mooney DJ, Craniofacial Tissue Engineering, Crit. Rev. Oral Biol. Med 12 (2001) 64–75. 10.1177/10454411010120010501. [DOI] [PubMed] [Google Scholar]
- [77].Pradhan-Bhatt S, Harrington DA, Duncan RL, Jia X, Witt RL, Farach-Carson MC, Implantable three-dimensional salivary spheroid assemblies demonstrate fluid and protein secretory responses to neurotransmitters., Tissue Eng. Part A 19 (2013) 1610–1620. 10.1089/ten.tea.2012.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Ozdemir T, Srinivasan PP, Zakheim DR, Harrington DA, Witt RL, Farach-Carson MC, Jia X, Pradhan-Bhatt S, Bottom-up assembly of salivary gland microtissues for assessing myoepithelial cell function., Biomaterials. 142 (2017) 124–135. 10.1016/j.biomaterials.2017.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Wu D, Witt RL, Harrington DA, Farach-Carson MC, Dynamic Assembly of Human Salivary Stem/Progenitor Microstructures Requires Coordinated α1β1 Integrin-Mediated Motility, Front. Cell Dev. Biol 7 (2019) 224. 10.3389/fcell.2019.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Hubka KM, Carson DD, Harrington DA, Farach-Carson MC, Perlecan domain I gradients establish stable biomimetic heparin binding growth factor gradients for cell migration in hydrogels, Acta Biomater. 97 (2019) 385–398. 10.1016/j.actbio.2019.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Ozdemir T, Fowler EW, Liu S, Harrington DA, Witt RL, Farach-Carson MC, Pradhan-Bhatt S, Jia X, Tuning Hydrogel Properties to Promote the Assembly of Salivary Gland Spheroids in 3D, ACS Biomater. Sci. Eng 2 (2016) 2217–2230. 10.1021/acsbiomaterials.6b00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Miyake Y, Shimizu O, Shiratsuchi H, Tamagawa T, Tonogi M, Wound healing after applying a gelatin-based hydrogel sheet to resected rat submandibular gland, J. Oral Sci 62 (2020) 222–225. 10.2334/josnusd.19-0244. [DOI] [PubMed] [Google Scholar]
- [83].Nam K, Kim K, Dean SM, Brown CT, Davis RS, Okano T, Baker OJ, Using cell sheets to regenerate mouse submandibular glands, NPJ Regen. Med 4 (2019) 16. 10.1038/s41536-019-0078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Mendicino M, Fan Y, Griffin D, Gunter KC, Nichols K, Current state of U.S. Food and Drug Administration regulation for cellular and gene therapy products: potential cures on the horizon, Cytotherapy. 21 (2019) 699–724. 10.1016/j.jcyt.2019.04.002. [DOI] [PubMed] [Google Scholar]
- [85].Schwieterman WD, Regulating biopharmaceuticals under CDER versus CBER: an insider’s perspective, Drug Discov. Today 11 (2006) 945–951. 10.1016/j.drudis.2006.08.011. [DOI] [PubMed] [Google Scholar]
- [86].C. for B.E. and Research, FDA Regulation of Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/P’s) Product List, FDA. (2019). https://www.fda.gov/vaccines-blood-biologics/tissue-tissue-products/fda-regulation-human-cells-tissues-and-cellular-and-tissue-based-products-hctps-product-list (accessed September 30, 2021). [Google Scholar]
- [87].Belsky K, Smiell J, Navigating the Regulatory Pathways and Requirements for Tissue-Engineered Products in the Treatment of Burns in the United States, J. Burn Care Res. Off. Publ. Am. Burn Assoc 42 (2021) 774–784. 10.1093/jbcr/iraa210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].C. for B.E. and Research, Regenerative Medicine Advanced Therapy Designation, FDA. (2021). https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/regenerative-medicine-advanced-therapy-designation (accessed September 30, 2021). [Google Scholar]
- [89].Humacyte Receives FDA Regenerative Medicine Advanced Therapy (RMAT) Expedited Review Designation for HUMACYL® in Vascular Access for Hemodialysis, (2017). https://www.businesswire.com/news/home/20170320005777/en/Humacyte-Receives-FDA-Regenerative-Medicine-Advanced-Therapy-RMAT-Expedited-Review-Designation-for-HUMACYL%C2%AE-in-Vascular-Access-for-Hemodialysis (accessed September 30, 2021).
- [90].Draelos ZD, Prescription Versus Over-the-counter Moisturizers: Unraveling the Mystery, Cosmet. Dermatol 24 (2011) 256–259. [Google Scholar]
- [91].Zhao P, Zhao W, Zhang K, Lin H, Zhang X, Polymeric injectable fillers for cosmetology: Current status, future trends, and regulatory perspectives, J. Appl. Polym. Sci 137 (2020) 48515. 10.1002/app.48515. [DOI] [Google Scholar]
- [92].Negi P, Singh B, Sharma G, Beg S, Raza K, Katare OP, Phospholipid microemulsion-based hydrogel for enhanced topical delivery of lidocaine and prilocaine: QbD-based development and evaluation, Drug Deliv. 23 (2016) 941–957. 10.3109/10717544.2014.923067. [DOI] [PubMed] [Google Scholar]
- [93].Cal K, Skin Penetration of Terpenes from Essential Oils and Topical Vehicles, Planta Med. 72 (2006) 311–316. 10.1055/s-2005-916230. [DOI] [PubMed] [Google Scholar]
- [94].Haltner-Ukomadu E, Sacha M, Richter A, Hussein K, Hydrogel increases diclofenac skin permeation and absorption, Biopharm. Drug Dispos 40 (2019) 217–224. 10.1002/bdd.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Vos T, Flaxman A, Naghavi M, Murray CJ, Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010, Lancet. 380 (2012) 2163–96. 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Leyden JJ, Krochmal L, Yaroshinsky A, Two randomized, double-blind, controlled trials of 2219 subjects to compare the combination clindamycin/tretinoin hydrogel with each agent alone and vehicle for the treatment of acne vulgaris, J. Am. Acad. Dermatol 54 (2006) 73–81. 10.1016/j.jaad.2005.04.046. [DOI] [PubMed] [Google Scholar]
- [97].Kulkarni CV, Lipid Self-Assemblies and Nanostructured Emulsions for Cosmetic Formulations, Cosmetics. 3 (2016) 37. 10.3390/cosmetics3040037. [DOI] [Google Scholar]
- [98].Lee J, Hlaing SP, Cao J, Hasan N, Ahn H-J, Song K-W, Yoo J-W, In Situ Hydrogel-Forming/Nitric Oxide-Releasing Wound Dressing for Enhanced Antibacterial Activity and Healing in Mice with Infected Wounds, Pharmaceutics. 11 (2019) 496. 10.3390/pharmaceutics11100496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Egambaram OP, Pillai SK, Ray SS, Materials Science Challenges in Skin UV Protection: A Review, Photochem. Photobiol 96 (2020) 779–797. 10.1111/php.13208. [DOI] [PubMed] [Google Scholar]
- [100].Haney B, Indications and Placement of Temporary Dermal Fillers, in: Haney B (Ed.), Aesthetic Proced. Nurse Pract. Guide Cosmet. Dermatol, Springer International Publishing, Cham, 2020: pp. 149–176. 10.1007/978-3-030-19948-7_17. [DOI] [Google Scholar]
- [101].Bloemen MCT, Van Der Wal MBA, Verhaegen PDHM, Nieuwenhuis MK, Van Baar ME, Van Zuijlen PPM, Middelkoop E, Clinical effectiveness of dermal substitution in burns by topical negative pressure: A multicenter randomized controlled trial, Wound Repair Regen. 20 (2012) 797–805. 10.1111/j.1524-475X.2012.00845.x. [DOI] [PubMed] [Google Scholar]
- [102].Sundaram H, Cegielska A, Wojciechowska A, Delobel P, Prospective, Randomized, Investigator-Blinded, Split-Face Evaluation of a Topical Crosslinked Hyaluronic Acid Serum for Post-Procedural Improvement of Skin Quality and Biomechanical Attributes, J Drugs Dermatol. 17 (2018) 442–450. https://doi.org/jddonline.com/articles/dermatology/S1545961618P0442X. [PubMed] [Google Scholar]
- [103].Malerich S, Berson D, Next generation cosmeceuticals: the latest in peptides, growth factors, cytokines, and stem cells, Dermatol Clin. 32 (2014) 13–21. 10.1016/j.det.2013.09.003. [DOI] [PubMed] [Google Scholar]
- [104].Hussain M, Phelps R, Goldberg DJ, Clinical, histologic, and ultrastructural changes after use of human growth factor and cytokine skin cream for the treatment of skin rejuvenation, J Cosmet Laser Ther. 10 (2008) 104–9. 10.1080/14764170701885392. [DOI] [PubMed] [Google Scholar]
- [105].Draelos ZD, Cosmeceuticals: undefined, unclassified, and unregulated, Clin Dermatol. 27 (2009) 431–4. 10.1016/j.clindermatol.2009.05.005. [DOI] [PubMed] [Google Scholar]
- [106].Zhu J, Tang X, Jia Y, Ho C-T, Huang Q, Applications and delivery mechanisms of hyaluronic acid used for topical/transdermal delivery – A review, Int. J. Pharm 578 (2020) 119127. 10.1016/j.ijpharm.2020.119127. [DOI] [PubMed] [Google Scholar]
- [107].Zagórska-Dziok M, Sobczak M, Hydrogel-Based Active Substance Release Systems for Cosmetology and Dermatology Application: A Review, Pharmaceutics. 12 (2020). 10.3390/pharmaceutics12050396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Ahsan A, Tian W-X, Farooq MA, Khan DH, An overview of hydrogels and their role in transdermal drug delivery, Int. J. Polym. Mater. Polym. Biomater 0 (2020) 1–11. 10.1080/00914037.2020.1740989. [DOI] [Google Scholar]
- [109].Kakita L, Tazarotene versus tretinoin or adapalene in the treatment of acne vulgaris, J. Am. Acad. Dermatol 43 (2000) S51–54. 10.1067/mjd.2000.108322. [DOI] [PubMed] [Google Scholar]
- [110].Parente ME, Ochoa Andrade A, Ares G, Russo F, Jiménez-Kairuz Á, Bioadhesive hydrogels for cosmetic applications, Int. J. Cosmet. Sci 37 (2015) 511–518. 10.1111/ics.12227. [DOI] [PubMed] [Google Scholar]
- [111].Velez I, Spielholz NI, Siegel MA, Gonzalez T, MuGard, an oral mucoadhesive hydrogel, reduces the signs and symptoms of oral mucositis in patients with lichen planus: a double-blind, randomized, placebo-controlled pilot study, Oral Surg. Oral Med. Oral Pathol. Oral Radiol 118 (2014) 657–664.e2. 10.1016/j.oooo.2014.07.007. [DOI] [PubMed] [Google Scholar]
- [112].Baldwin H, Blanco D, McKeever C, Paz N, Vasquez YN, Quiring J, Enloe C, De León E, Stasko N, Results of a Phase 2 Efficacy and Safety Study with SB204, an Investigational Topical Nitric Oxide-releasing Drug for the Treatment of Acne Vulgaris, J. Clin. Aesthetic Dermatol 9 (2016) 12–18. [PMC free article] [PubMed] [Google Scholar]
- [113].Eichenfield LF, Gold LS, Nahm WK, Cook-Bolden FE, Pariser DM, Results of a Phase 2, Randomized,Vehicle-Controlled Study Evaluating the Efficacy,Tolerability, and Safety of Daily or Twice Daily SB204 for the Treatment of Acne Vulgaris, J. Drugs Dermatol. JDD 15 (2016) 1496–15027. [PubMed] [Google Scholar]
- [114].Grixti A, Sadri M, Watts MT, Corneal protection during general anesthesia for nonocular surgery, Ocul. Surf 11 (2013) 109–118. 10.1016/j.jtos.2012.10.003. [DOI] [PubMed] [Google Scholar]
- [115].Kamoun EA, Kenawy E-RS, Chen X, A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings, J. Adv. Res 8 (2017) 217–233. 10.1016/j.jare.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Attenello NH, Maas CS, Injectable Fillers: Review of Material and Properties, Facial Plast. Surg 31 (2015) 29–34. 10.1055/s-0035-1544924. [DOI] [PubMed] [Google Scholar]
- [117].Lemperle G, Morhenn V, Charrier U, Human Histology and Persistence of Various Injectable Filler Substances for Soft Tissue Augmentation, Aesthetic Plast. Surg 44 (2020) 1348–1360. 10.1007/s00266-020-01827-7. [DOI] [PubMed] [Google Scholar]
- [118].Knapp TR, Kaplan EN, Daniels JR, Injectable collagen for soft tissue augmentation, Plast Reconstr Surg. 60 (1977) 398–405. 10.1097/00006534-197709000-00013. [DOI] [PubMed] [Google Scholar]
- [119].Narins RS, Brandt F, Leyden J, Lorenc ZP, Rubin M, Smith S, A randomized, double-blind, multicenter comparison of the efficacy and tolerability of Restylane versus Zyplast for the correction of nasolabial folds, Dermatol Surg. 29 (2003) 588–95. 10.1046/j.1524-4725.2003.29150.x. [DOI] [PubMed] [Google Scholar]
- [120].Newman J, Review of soft tissue augmentation in the face, Clin Cosmet Investig Dermatol. 2 (2009) 141–50. 10.2147/ccid.s3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Fitzgerald R, Bass LM, Goldberg DJ, Graivier MH, Lorenc ZP, Physiochemical Characteristics of Poly-L-Lactic Acid (PLLA), Aesthet. Surg. J 38 (2018) S13–S17. 10.1093/asj/sjy012. [DOI] [PubMed] [Google Scholar]
- [122].de Melo F, Carrijo A, Hong K, Trumbic B, Vercesi F, Waldorf HA, Zenker S, Minimally Invasive Aesthetic Treatment of the Face and Neck Using Combinations of a PCL-Based Collagen Stimulator, PLLA/PLGA Suspension Sutures, and Cross-Linked Hyaluronic Acid, Clin. Cosmet. Investig. Dermatol 13 (2020) 333–344. 10.2147/CCID.S248280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Lehman A, Pilcher B, Roberts WE, Schlesinger TE, Vachon G, Postmarket Experience of Polymethylmethacrylate-Collagen Gel Dermal Filler, Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. Al 46 (2020) 1086–1091. 10.1097/DSS.0000000000002222. [DOI] [PubMed] [Google Scholar]
- [124].Wollina U, Goldman A, Long lasting facial rejuvenation by repeated placement of calcium hydroxylapatite in elderly women, Dermatol. Ther 33 (2020) e14183. 10.1111/dth.14183. [DOI] [PubMed] [Google Scholar]
- [125].Zhang S, Holmes TC, DiPersio CM, Hynes RO, Su X, Rich A, Self-complementary oligopeptide matrices support mammalian cell attachment, Biomaterials. 16 (1995) 1385–93. 10.1016/0142-9612(95)96874-y. [DOI] [PubMed] [Google Scholar]
- [126].Akiyama N, Yamamoto-Fukuda T, Takahashi H, Koji T, In situ tissue engineering with synthetic self-assembling peptide nanofiber scaffolds, PuraMatrix, for mucosal regeneration in the rat middle-ear, Int J Nanomedicine. 8 (2013) 2629–40. 10.2147/IJN.S47279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Uraoka T, Ochiai Y, Fujimoto A, Goto O, Kawahara Y, Kobayashi N, Kanai T, Matsuda S, Kitagawa Y, Yahagi N, A novel fully synthetic and self-assembled peptide solution for endoscopic submucosal dissection-induced ulcer in the stomach, Gastrointest Endosc. 83 (2016) 1259–64. 10.1016/j.gie.2015.11.015. [DOI] [PubMed] [Google Scholar]
- [128].Giritharan S, Salhiyyah K, Tsang GM, Ohri SK, Feasibility of a novel, synthetic, self-assembling peptide for suture-line haemostasis in cardiac surgery, J Cardiothorac Surg. 13 (2018) 68. 10.1186/s13019-018-0745-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Rameau A, Hong RS, Djalilian H, Erbele ID, Phillips KM, Capasso R, Rose AS, Brenner MJ, Santa Maria PL, New Medical Device and Therapeutic Approvals in Otolaryngology: State of the Art Review of 2019, OTO Open. 4 (2020). 10.1177/2473974X20932506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Holmes TC, de Lacalle S, Su X, Liu G, Rich A, Zhang S, Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds, Proc Natl Acad Sci U A. 97 (2000) 6728–33. 10.1073/pnas.97.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Semino CE, Merok JR, Crane GG, Panagiotakos G, Zhang S, Functional differentiation of hepatocyte-like spheroid structures from putative liver progenitor cells in three-dimensional peptide scaffolds, Differentiation. 71 (2003) 262–70. 10.1046/j.1432-0436.2003.7104503.x. [DOI] [PubMed] [Google Scholar]
- [132].Wang S, Nagrath D, Chen PC, Berthiaume F, Yarmush ML, Three-dimensional primary hepatocyte culture in synthetic self-assembling peptide hydrogel, Tissue Eng Part A. 14 (2008) 227–36. 10.1089/tea.2007.0143. [DOI] [PubMed] [Google Scholar]
- [133].Koutsopoulos S, Zhang S, Long-term three-dimensional neural tissue cultures in functionalized self-assembling peptide hydrogels, matrigel and collagen I, Acta Biomater. 9 (2013) 5162–9. 10.1016/j.actbio.2012.09.010. [DOI] [PubMed] [Google Scholar]
- [134].Koutsopoulos S, Self-assembling peptide nanofiber hydrogels in tissue engineering and regenerative medicine: Progress, design guidelines, and applications, J Biomed Mater Res A. 104 (2016) 1002–16. 10.1002/jbm.a.35638. [DOI] [PubMed] [Google Scholar]
- [135].Abrahamsson P, Walivaara DA, Isaksson S, Andersson G, Periosteal expansion before local bone reconstruction using a new technique for measuring soft tissue profile stability: a clinical study, J Oral Maxillofac Surg. 70 (2012) e521–30. 10.1016/j.joms.2012.06.003. [DOI] [PubMed] [Google Scholar]
- [136].Uijlenbroek HJJ, YuelianLiu D Wismeijer, Soft tissue expansion: principles and inferred intraoral hydrogel tissue expanders, Dent. Oral Craniofacial Res 1 (2016). 10.15761/docr.1000140. [DOI] [Google Scholar]
- [137].Asa’ad F, Bellucci G, Ferrantino L, Trisciuoglio D, Taschieri S, Del Fabbro M, Preaugmentation Soft Tissue Expansion: A Report of Four Pilot Cases, Case Rep Dent. 2018 (2018) 3162617. 10.1155/2018/3162617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Wysocki M, Kobus K, Szotek S, Kobielarz M, Kuropka P, Będziñski R, Biomechanical effect of rapid mucoperiosteal palatal tissue expansion with the use of osmotic expanders, J. Biomech 44 (2011) 1313–1320. 10.1016/j.jbiomech.2011.01.012. [DOI] [PubMed] [Google Scholar]
- [139].Chummun S, Addison P, Stewart KJ, The osmotic tissue expander: a 5-year experience, J. Plast. Reconstr. Aesthetic Surg. JPRAS 63 (2010) 2128–2132. 10.1016/j.bjps.2010.02.002. [DOI] [PubMed] [Google Scholar]
- [140].Trujillo-de Santiago G, Sharifi R, Yue K, Sani ES, Kashaf SS, Alvarez MM, Leijten J, Khademhosseini A, Dana R, Annabi N, Ocular adhesives: Design, chemistry, crosslinking mechanisms, and applications, Biomaterials. 197 (2019) 345–367. 10.1016/j.biomaterials.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Ocular Therapeutix, Inc., A Phase 1 Open-Label, Dose Escalation Study of OTX-TKI for Intravitreal Use in Subjects With Neovascular Age-Related Macular Degeneration (AMD), clinicaltrials.gov, 2020. https://clinicaltrials.gov/ct2/show/NCT03630315 (accessed February 10, 2021). [Google Scholar]
- [142].Ocular Therapeutix, Inc., A Prospective, Multicenter, Open-Label Study to Evaluate the Safety, Tolerability and Efficacy of OTX-TIC (Travoprost) Implant in Subjects With Primary Open-Angle Glaucoma or Ocular Hypertension, clinicaltrials.gov, 2020. https://clinicaltrials.gov/ct2/show/NCT04360174 (accessed February 10, 2021). [Google Scholar]
- [143].Ocular Therapeutix, Inc., A Randomized, Multi-center, Double-masked, Vehicle-controlled, Phase 1/2 Study to Evaluate the Safety, Tolerability, and Efficacy of OTX-CSI (Cyclosporine Ophthalmic Insert) for Intracanalicular Use for the Treatment of Subjects With Dry Eye Disease (DED), clinicaltrials.gov, 2020. https://clinicaltrials.gov/ct2/show/NCT04362670 (accessed February 10, 2021). [Google Scholar]
- [144].BioFirst Corporation, A Phase I, Safety and Tolerability Study of Vitargus® in Vitrectomy Surgery, clinicaltrials.gov, 2020. https://clinicaltrials.gov/ct2/show/NCT04481386 (accessed April 7, 2021). [Google Scholar]
- [145].Su W-Y, Chen K-H, Chen Y-C, Lee Y-H, Tseng C-L, Lin F-H, An injectable oxidated hyaluronic acid/adipic acid dihydrazide hydrogel as a vitreous substitute, J. Biomater. Sci. Polym. Ed 22 (2011) 1777–1797. 10.1163/092050610X522729. [DOI] [PubMed] [Google Scholar]
- [146].Kleinberg TT, Tzekov RT, Stein L, Ravi N, Kaushal S, Vitreous Substitutes: A Comprehensive Review, Surv. Ophthalmol 56 (2011) 300–323. 10.1016/j.survophthal.2010.09.001. [DOI] [PubMed] [Google Scholar]
- [147].Schulz A, Rickmann A, Wahl S, Germann A, Stanzel BV, Januschowski K, Szurman P, Alginate- and Hyaluronic Acid-Based Hydrogels as Vitreous Substitutes: An In Vitro Evaluation, Transl. Vis. Sci. Technol 9 (2020) 34. 10.1167/tvst.9.13.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Saldin LT, Cramer MC, Velankar SS, White LJ, Badylak SF, Extracellular matrix hydrogels from decellularized tissues: Structure and function, Acta Biomater. 49 (2017) 1–15. 10.1016/j.actbio.2016.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Traverse JH, Henry TD, Dib N, Patel AN, Pepine C, Schaer GL, DeQuach JA, Kinsey AM, Chamberlin P, Christman KL, First-in-Man Study of a Cardiac Extracellular Matrix Hydrogel in Early and Late Myocardial Infarction Patients, JACC Basic Transl Sci. 4 (2019) 659–669. 10.1016/j.jacbts.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Cramer MC, Badylak SF, Extracellular Matrix-Based Biomaterials and Their Influence Upon Cell Behavior, Ann. Biomed. Eng 48 (2020) 2132–2153. 10.1007/s10439-019-02408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Draelos ZD, Cosmeceuticals: What’s Real, What’s Not, Dermatol Clin. 37 (2019) 107–115. 10.1016/j.det.2018.07.001. [DOI] [PubMed] [Google Scholar]
- [152].Draelos ZD, The Effect of a Combination of Recombinant EGF Cosmetic Serum and a Crosslinked Hyaluronic Acid Serum as Compared to a Fibroblast-Conditioned Media Serum on the Appearance of Aging Skin, J Drugs Dermatol. 15 (2016) 738–41. https://doi.org/jddonline.com/articles/dermatology/S1545961616P0738X. [PubMed] [Google Scholar]
- [153].Nistor G, Poole AJ, Draelos Z, Lupo M, Tzikas T, Liu JH, Keirstead HS, Human Stem Cell-Derived Skin Progenitors Produce Alpha 2-HS Glycoprotein (Fetuin): A Revolutionary Cosmetic Ingredient, J Drugs Dermatol. 15 (2016) 583–98. https://doi.org/jddonline.com/articles/dermatology/S1545961616P0583X. [PubMed] [Google Scholar]
- [154].Salinas CN, Anseth KS, Mesenchymal Stem Cells for Craniofacial Tissue Regeneration, J. Dent. Res 88 (2009) 681–692. 10.1177/0022034509341553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Narotam PK, José S, Nathoo N, Taylon C, Vora Y, Collagen matrix (DuraGen) in dural repair: analysis of a new modified technique, Spine. 29 (2004) 2861–2867; discussion 2868–2869. 10.1097/01.brs.0000148049.69541.ad. [DOI] [PubMed] [Google Scholar]
- [156].Gurtner GC, The Effect of an ASC-seeded Collagen Hydrogel on Cerebrospinal Fluid Leak Rates Following Skull Base Surgery, clinicaltrials.gov, 2021. https://clinicaltrials.gov/ct2/show/NCT04503161 (accessed April 7, 2021). [Google Scholar]
- [157].FDA, Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue-Based Products: Minimal Manipulation and Homologous Use; Guidance for Industry and Food and Drug Administration Staff, Food Drug Adm. (2020). https://www.fda.gov/media/109176/download. [Google Scholar]
- [158].Barrera J, Trotsyuk A, Maan ZN, Bonham CA, Larson MR, Mittermiller PA, Henn D, Chen K, Mays CJ, Mittal S, Mermin-Bunnell AM, Sivaraj D, Jing S, Rodrigues M, Kwon SH, Noishiki C, Padmanabhan J, Jiang Y, Niu S, Inayathullah M, Rajadas J, Januszyk M, Gurtner GC, Adipose-derived stromal cells seeded in pullulan-collagen hydrogels improve healing in murine burns, Tissue Eng. Part A (2021). 10.1089/ten.TEA.2020.0320. [DOI] [PubMed] [Google Scholar]
- [159].Kosaraju R, Rennert RC, Maan ZN, Duscher D, Barrera J, Whittam AJ, Januszyk M, Rajadas J, Rodrigues M, Gurtner GC, Adipose-Derived Stem Cell-Seeded Hydrogels Increase Endogenous Progenitor Cell Recruitment and Neovascularization in Wounds, Tissue Eng. Part A 22 (2016) 295–305. 10.1089/ten.tea.2015.0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].I. BioTime, BioTime Securities and Exchange Commission Form 10-K, Form 10-K. (2017). https://www.sec.gov/Archives/edgar/data/876343/000149315218003416/form10-k.htm (accessed September 24, 2020). [Google Scholar]
- [161].Blackshear CP, Flacco JS, Vistnes SM, Chung NN, Irizarry D, Brett EA, Yen DJ, Momeni A, Longaker MT, Wan DC, Cell-Based Soft Tissue Reconstruction in a Hydrogel Scaffold, Ann Plast Surg. 79 (2017) 618–622. 10.1097/SAP.0000000000001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM, Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT), Cytotherapy. 15 (2013) 641–8. 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Siddiqui Z, Sarkar B, Kim K-K, Kadincesme N, Paul R, Kumar A, Kobayashi Y, Roy A, Choudhury M, Yang J, Shimizu E, Kumar VA, Angiogenic hydrogels for dental pulp revascularization, Acta Biomater. (2021). 10.1016/j.actbio.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Kumar VA, Liu Q, Wickremasinghe NC, Shi S, Cornwright TT, Deng Y, Azares A, Moore AN, Acevedo-Jake AM, Agudo NR, Pan S, Woodside DG, Vanderslice P, Willerson JT, Dixon RA, Hartgerink JD, Treatment of hind limb ischemia using angiogenic peptide nanofibers, Biomaterials. 98 (2016) 113–119. 10.1016/j.biomaterials.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Wickremasinghe NC, Kumar VA, Shi S, Hartgerink JD, Controlled Angiogenesis in Peptide Nanofiber Composite Hydrogels, ACS Biomater. Sci. Eng 1 (2015) 845–854. 10.1021/acsbiomaterials.5b00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].FDA approves removal of boxed warning from REGRANEX◊ (becaplermin) gel, 0.01% as multiple studies demonstrate product safety ∣ Smith & Nephew, (n.d.). https://www.smith-nephew.com/news-and-media/media-releases/news/fda-approves-removal-of-boxed-warning-from-regranex-becaplermin-gel-001-as-multiple-studies-demonstrate-product-safety/ (accessed July 1, 2021). [Google Scholar]
- [167].3DBio Therapeutics, A Multicenter, Single Arm, Prospective, Open-Label, Staged Study of the Safety and Efficacy of the AuriNovo Construct for Auricular Reconstruction in Subjects With Unilateral Microtia, clinicaltrials.gov, 2021. https://clinicaltrials.gov/ct2/show/NCT04399239 (accessed April 14, 2021). [Google Scholar]
- [168].Visscher DO, Lee H, van Zuijlen PPM, Helder MN, Atala A, Yoo JJ, Lee SJ, A photo-crosslinkable cartilage-derived extracellular matrix bioink for auricular cartilage tissue engineering, Acta Biomater. 121 (2021) 193–203. 10.1016/j.actbio.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Khanh N, Wu Y, Eitan A, SHAV L, EISENBACH A, Hayardeni Y, Batt A, Bioengineered corneal graft and methods of preparation thereof, WO2019198086A1, 2019. https://patents.google.com/patent/WO2019198086A1/en?q=cornea&assignee=%22Precise+Bio%22&oq=%22Precise+Bio%22+cornea (accessed April 16, 2021).
- [170].Batt A, Eitan A, EISENBACH A, Microfluidic head for laser induced forward transfer, WO2018193454A1, 2018. https://patents.google.com/patent/WO2018193454A1/en (accessed April 23, 2021).
- [171].Zhang Z, Niu G, Choi JS, Giegengack M, Atala A, Soker S, Bioengineered multilayered human corneas from discarded human corneal tissue, Biomed. Mater. Bristol Engl 10 (2015) 035012. https://doi.org/2020022709454827. [DOI] [PubMed] [Google Scholar]
- [172].Lineage Cell Therapeutics, Lineage Cell Therapeutics Awarded Grant From Israel Innovation Authority for Development of a Bio-Retinal Patch for Treatment of Retinal Diseases in Partnership With Precise Bio, (2020). https://www.businesswire.com/news/home/20200908005333/en/Lineage-Cell-Therapeutics-Awarded-Grant-From-Israel-Innovation-Authority-for-Development-of-a-Bio-Retinal-Patch-for-Treatment-of-Retinal-Diseases-in-Partnership-With-Precise-Bio (accessed April 23, 2021).
- [173].Isaacson A, Swioklo S, Connon CJ, 3D bioprinting of a corneal stroma equivalent, Exp. Eye Res 173 (2018) 188–193. 10.1016/j.exer.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Kim BS, Das S, Jang J, Cho D-W, Decellularized Extracellular Matrix-based Bioinks for Engineering Tissue- and Organ-specific Microenvironments, Chem. Rev 120 (2020) 10608–10661. 10.1021/acs.chemrev.9b00808. [DOI] [PubMed] [Google Scholar]
- [175].Hussey GS, Dziki JL, Badylak SF, Extracellular matrix-based materials for regenerative medicine, Nat. Rev. Mater 3 (2018) 159–173. 10.1038/s41578-018-0023-x. [DOI] [Google Scholar]
- [176].García-Gareta E, Abduldaiem Y, Sawadkar P, Kyriakidis C, Lali F, Greco KV, Decellularised scaffolds: just a framework? Current knowledge and future directions, J. Tissue Eng 11 (2020) 2041731420942903. 10.1177/2041731420942903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Schmidt C, Gintuit cell therapy approval signals shift at US regulator, Nat Biotechnol. 30 (2012) 479. 10.1038/nbt0612-479. [DOI] [PubMed] [Google Scholar]
- [178].Domaszewska-Szostek A, Krzyzanowska M, Siemionow M, Cell-Based Therapies for Chronic Wounds Tested in Clinical Studies: Review, Ann Plast Surg. 83 (2019) e96–e109. 10.1097/SAP.0000000000001947. [DOI] [PubMed] [Google Scholar]
- [179].C. for B.E. and FDA, GINTUIT (Allogeneic Cultured Keratinocytes and Fibroblasts in Bovine Collagen), FDA. (2019). https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/gintuit-allogeneic-cultured-keratinocytes-and-fibroblasts-bovine-collagen (accessed April 23, 2021). [Google Scholar]
- [180].Organogenesis, Organogenesis Holdings Inc 10-K Filing, (n.d.). https://www.sec.gov/Archives/edgar/data/1661181/000119312519078332/d716594d10k.htm (accessed April 23, 2021).
- [181].C. for D.E. and FDA, Pediatric Research Equity Act ∣ PREA, FDA. (2019). https://www.fda.gov/drugs/development-resources/pediatric-research-equity-act-prea (accessed April 23, 2021). [Google Scholar]
- [182].Buskermolen JK, Reijnders CM, Spiekstra SW, Steinberg T, Kleverlaan CJ, Feilzer AJ, Bakker AD, Gibbs S, Development of a Full-Thickness Human Gingiva Equivalent Constructed from Immortalized Keratinocytes and Fibroblasts, Tissue Eng Part C Methods. 22 (2016) 781–91. 10.1089/ten.TEC.2016.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Kosten IJ, Buskermolen JK, Spiekstra SW, de Gruijl TD, Gibbs S, Gingiva Equivalents Secrete Negligible Amounts of Key Chemokines Involved in Langerhans Cell Migration Compared to Skin Equivalents, J Immunol Res. 2015 (2015) 627125. 10.1155/2015/627125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Vriens AP, Waaijman T, van den Hoogenband HM, de Boer EM, Scheper RJ, Gibbs S, Comparison of autologous full-thickness gingiva and skin substitutes for wound healing, Cell Transpl. 17 (2008) 1199–209. 10.3727/096368908787236521. [DOI] [PubMed] [Google Scholar]
- [185].Moharamzadeh K, Colley H, Murdoch C, Hearnden V, Chai WL, Brook IM, Thornhill MH, Macneil S, Tissue-engineered oral mucosa, J Dent Res. 91 (2012) 642–50. 10.1177/0022034511435702. [DOI] [PubMed] [Google Scholar]
- [186].Varkey M, Ding J, Tredget EE, Advances in Skin Substitutes-Potential of Tissue Engineered Skin for Facilitating Anti-Fibrotic Healing, J Funct Biomater. 6 (2015) 547–63. 10.3390/jfb6030547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Vahav I, van den Broek LJ, Thon M, Monsuur HN, Spiekstra SW, Atac B, Scheper RJ, Lauster R, Lindner G, Marx U, Gibbs S, Reconstructed human skin shows epidermal invagination towards integrated neopapillae indicating early hair follicle formation in vitro, J. Tissue Eng. Regen. Med 14 (2020) 761–773. 10.1002/term.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].McGuire MK, Tavelli L, Feinberg SE, Rasperini G, Zucchelli G, Wang H, Giannobile WV, Living cell-based regenerative medicine technologies for periodontal soft tissue augmentation, J. Periodontol 91 (2020) 155–164. 10.1002/JPER.19-0353. [DOI] [PubMed] [Google Scholar]
- [189].Lee DJ, Miguez P, Kwon J, Daniel R, Padilla R, Min S, Zalal R, Ko C-C, Shin HW, Decellularized pulp matrix as scaffold for mesenchymal stem cell mediated bone regeneration, J. Tissue Eng 11 (2020) 2041731420981672. 10.1177/2041731420981672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Ling C, Li Q, Brown ME, Kishimoto Y, Toya Y, Devine EE, Choi K-O, Nishimoto K, Norman IG, Tsegyal T, Jiang JJ, Burlingham WJ, Gunasekaran S, Smith LM, Frey BL, Welham NV, Bioengineered vocal fold mucosa for voice restoration, Sci. Transl. Med 7 (2015) 314ra187–314ra187. 10.1126/scitranslmed.aab4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Li L, Stiadle JM, Lau HK, Zerdoum AB, Jia X, Thibeault SL, Kiick KL, Tissue engineering-based therapeutic strategies for vocal fold repair and regeneration, Biomaterials. 108 (2016) 91–110. 10.1016/j.biomaterials.2016.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Jiang L, Jiang Y, Stiadle J, Wang X, Wang L, Li Q, Shen C, Thibeault SL, Turng LS, Electrospun nanofibrous thermoplastic polyurethane/poly(glycerol sebacate) hybrid scaffolds for vocal fold tissue engineering applications, Mater Sci Eng C Mater Biol Appl. 94 (2019) 740–749. 10.1016/j.msec.2018.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Xu CC, Mau T, A tissue-specific, injectable acellular gel for the treatment of chronic vocal fold scarring, Acta Biomater. 99 (2019) 141–153. 10.1016/j.actbio.2019.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Ahadian S, Ramón-Azcón J, Estili M, Liang X, Ostrovidov S, Shiku H, Ramalingam M, Nakajima K, Sakka Y, Bae H, Matsue T, Khademhosseini A, Hybrid hydrogels containing vertically aligned carbon nanotubes with anisotropic electrical conductivity for muscle myofiber fabrication, Sci. Rep 4 (2014) 4271. 10.1038/srep04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Lee CH, Hajibandeh J, Suzuki T, Fan A, Shang P, Mao JJ, Three-dimensional printed multiphase scaffolds for regeneration of periodontium complex, Tissue Eng Part A. 20 (2014) 1342–51. 10.1089/ten.TEA.2013.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Athirasala A, Tahayeri A, Thrivikraman G, Franca CM, Monteiro N, Tran V, Ferracane J, Bertassoni LE, A dentin-derived hydrogel bioink for 3D bioprinting of cell laden scaffolds for regenerative dentistry, Biofabrication. 10 (2018) 024101. 10.1088/1758-5090/aa9b4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Lee W, Debasitis JC, Lee VK, Lee J-H, Fischer K, Edminster K, Park J-K, Yoo S-S, Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication, Biomaterials. 30 (2009) 1587–1595. 10.1016/j.biomaterials.2008.12.009. [DOI] [PubMed] [Google Scholar]
- [198].Yao B, Wang R, Wang Y, Zhang Y, Hu T, Song W, Li Z, Huang S, Fu X, Biochemical and structural cues of 3D-printed matrix synergistically direct MSC differentiation for functional sweat gland regeneration, Sci. Adv 6 (2020) eaaz1094. 10.1126/sciadv.aaz1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Wu L, Magaz A, Huo S, Darbyshire A, Loizidou M, Emberton M, Birchall M, Song W, Human airway-like multilayered tissue on 3D-TIPS printed thermoresponsive elastomer/collagen hybrid scaffolds, Acta Biomater. 113 (2020) 177–195. 10.1016/j.actbio.2020.07.013. [DOI] [PubMed] [Google Scholar]
- [200].Hu Y, Wu Y, Gou Z, Tao J, Zhang J, Liu Q, Kang T, Jiang S, Huang S, He J, Chen S, Du Y, Gou M, 3D-engineering of Cellularized Conduits for Peripheral Nerve Regeneration, Sci Rep. 6 (2016) 32184. 10.1038/srep32184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Yi H-G, Choi Y-J, Jung JW, Jang J, Song T-H, Chae S, Ahn M, Choi TH, Rhie J-W, Cho D-W, Three-dimensional printing of a patient-specific engineered nasal cartilage for augmentative rhinoplasty, J. Tissue Eng 10 (2019) 2041731418824797. 10.1177/2041731418824797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Qazi TH, Burdick JA, Granular hydrogels for endogenous tissue repair, Biomater. Biosyst 1 (2021) 100008. 10.1016/j.bbiosy.2021.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].May AJ, Cruz-Pacheco N, Emmerson E, Gaylord EA, Seidel K, Nathan S, Muench MO, Klein OD, Knox SM, Diverse progenitor cells preserve salivary gland ductal architecture after radiation-induced damage, Dev. Camb. Engl 145 (2018). 10.1242/dev.166363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Weng P-L, Aure MH, Maruyama T, Ovitt CE, Limited Regeneration of Adult Salivary Glands after Severe Injury Involves Cellular Plasticity, Cell Rep. 24 (2018) 1464–1470.e3. 10.1016/j.celrep.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Hubka KM, Wu D, Harrington DA, Langer JC, Pocard T, Jammayrac O, Farach-Carson MC, Pradhan-Bhatt S, Dissociative and Nondissociative Models for Culture of Human Eccrine Glands for Toxicology Testing and Tissue Engineering Applications, Appl. Vitro Toxicol 1 (2015) 187–197. 10.1089/aivt.2015.0013. [DOI] [Google Scholar]
- [206].Bioinformant, What Is An RMAT? List of RMAT Designations, BioInformant. (2021). http://https%253A%252F%252Fbioinformant.com%252Frmat%252F (accessed April 18, 2021). [Google Scholar]
- [207].Means KR, Rinker BD, Higgins JP, Payne SH, Merrell GA, Wilgis EFS, A Multicenter, Prospective, Randomized, Pilot Study of Outcomes for Digital Nerve Repair in the Hand Using Hollow Conduit Compared With Processed Allograft Nerve, Hand N. Y. N 11 (2016) 144–151. 10.1177/1558944715627233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Jankowski RJ, Tu LM, Carlson C, Robert M, Carlson K, Quinlan D, Eisenhardt A, Chen M, Snyder S, Pruchnic R, Chancellor M, Dmochowski R, Kaufman MR, Carr L, A double-blind, randomized, placebo-controlled clinical trial evaluating the safety and efficacy of autologous muscle derived cells in female subjects with stress urinary incontinence, Int. Urol. Nephrol 50 (2018) 2153–2165. 10.1007/s11255-018-2005-8. [DOI] [PubMed] [Google Scholar]
- [209].Belafsky P, Muscle Cell Mediated Therapy for Tongue Dysphagia: An Investigation of Cook MyoSite Autologous Muscle Derived Cells, clinicaltrials.gov, 2020. https://clinicaltrials.gov/ct2/show/NCT02838316 (accessed April 22, 2021). [Google Scholar]