Abstract
A model cocontaminated system was developed to determine whether a metal-complexing biosurfactant, rhamnolipid, could reduce metal toxicity to allow enhanced organic biodegradation by a Burkholderia sp. isolated from soil. Rhamnolipid eliminated cadmium toxicity when added at a 10-fold greater concentration than cadmium (890 μM), reduced toxicity when added at an equimolar concentration (89 μM), and had no effect at a 10-fold smaller concentration (8.9 μM). The mechanism by which rhamnolipid reduces metal toxicity may involve a combination of rhamnolipid complexation of cadmium and rhamnolipid interaction with the cell surface to alter cadmium uptake.
Forty percent of hazardous waste sites on the U.S. Environmental Protection Agency's National Priority List are cocontaminated with organic and metal pollutants. Previous studies have shown that biodegradation of organic contaminants is often severely inhibited by toxic metals, such as cadmium (19, 20). Increasing interest in bioremediation warrants development of strategies that can be successfully implemented in cocontaminated sites, yet few efforts have been made to develop such strategies. Effective strategies to enhance organic biodegradation in the presence of toxic metals include reducing the bioavailable concentration of the toxic metal and reducing interactions of the toxic metal with the cell.
Attempts to reduce bioavailable metal concentrations in cocontaminated soils have included amendment with kaolinite and montmorillonite clays (2, 3, 11), wherein reductions in metal toxicity were observed. Recently, modified clay complexes (metal-chelating ligands bound to clay particles via a cationic surfactant) and a chelating resin (Chelex) were found to reduce cadmium toxicity during biodegradation of naphthalene by Pseudomonas putida ppo200 (14). Reductions in toxicity were assumed to be related to the metal-complexing characteristics of both the modified clay and the resin, despite the fact that metal chelators, such as EDTA, can alter cell surface properties through the release of lipopolysaccharide (LPS) (5–7, 12). Because LPS confers a considerable negative charge upon the cell surface (18) which favors electrostatic interactions with cations, removal of LPS may reduce the magnitude of the negativity of the cell surface charge, thus reducing interactions with cations, such as cadmium. The mechanism by which metal-chelating agents reduce toxicity clearly warrants further exploration.
We have previously studied a rhamnolipid biosurfactant produced by various Pseudomonas aeruginosa strains capable of selectively complexing cationic metal species, such as cadmium (Cd2+), lead (Pb2+), and zinc (Zn2+) (8, 17, 21, 22), increasing the bioavailability of substrates with limited aqueous solubilities (9, 24–27), and increasing cell surface hydrophobicity (1, 26). Delivery of a biosurfactant into cocontaminated sites for in situ treatment may be more environmentally compatible and more economical than using modified clay complexes or metal chelators, such as EDTA. For these reasons, the objective of this research was to develop a model system to determine the effect of rhamnolipid on the capability of a metal-sensitive microorganism to degrade an organic contaminant. Naphthalene was chosen as the model organic contaminant because of its ubiquity at hazardous waste sites and its demonstrated biodegradability (16). Cadmium, the second most common metal found at Superfund sites (4), was chosen as the model metal. The biosurfactant used in this study was a monorhamnolipid produced by P. aeruginosa 9027, prepared as previously described (24, 25). A naphthalene-degrading bacterium was obtained from an uncontaminated loamy sand (Hayhook soil) by serial enrichment in 50 ml of mineral salts medium (MSM) containing 15 mg of naphthalene, monobasic potassium phosphate (1 g/liter), dibasic sodium phosphate (1 g/liter), ammonium nitrate (0.5 g/liter), ammonium sulfate (0.5 g/liter), magnesium sulfate (0.2 g/liter), calcium chloride (0.02 g/liter), iron chloride (0.002 g/liter), and manganese sulfate (0.002 g/liter). Enrichment flasks were maintained at 23°C on a rotary shaker at 200 rpm. Metabolic (tetrazolium redox technology; BIOLOG, Hayward, Calif.) and 16S ribosomal DNA sequence analyses (23) were used to identify the naphthalene-degrader as a Burkholderia sp. (NCBI U37342). The Burkholderia sp. was maintained on the same media.
Cadmium complexation.
Experiments were performed to determine the effect of the concentration of rhamnolipid on cadmium complexation. Rhamnolipid was added at concentrations of 0, 8.9, 89, and 890 μM to polypropylene beakers containing 89 μM cadmium in 10% MSM and stirred at 150 rpm for 15 min. MSM was used at only 10% of the normal concentration to minimize interactions between the rhamnolipid and salts in the medium. Concentrations of free cadmium were determined using an ion-selective cadmium electrode (model 94-48; Orion Research, Cambridge, Mass.). The ion-selective electrode measures only free, uncomplexed cadmium. An ionic strength adjuster (ISA), 5 M NaNO3, typically used in this type of analysis, was not employed in these experiments. In preliminary studies, it was found that the addition of ISA to solutions containing rhamnolipid produced inaccurate measurements of the amount of complexed cadmium. It is probable that the large quantities of added sodium cations were exchanging cadmium cations from cation binding sites on the rhamnolipid molecules. Omission of ISA in these studies was appropriate for the following reasons. (i) The ionic strength of all treatments was nearly identical. (ii) The electrode functioned properly and accurately without ISA, as demonstrated by the characteristic calibration slope obtained (Orion Research, Inc. [Technical Services], personal communication). Of the three rhamnolipid concentrations tested, the 890 μM rhamnolipid treatment reduced the concentration of free cadmium to 11.2 ± 0.47 μM, while 8.9 and 89 μM concentrations of rhamnolipid did not significantly reduce the free cadmium concentration.
Growth on naphthalene in the presence of cadmium.
Initial experiments were performed to determine the concentration of cadmium that is most toxic to the Burkholderia sp. In these experiments, a cadmium nitrate (0, 0.89, 8.9, 45, 89, and 450 μM) solution was added to 125-ml Nalgene flasks containing 50 ml of MSM and 15 mg of crystalline naphthalene. Potassium nitrate was added as necessary to equalize nitrate concentrations among the flasks. All flasks were inoculated with approximately 5 × 106 CFU of the Burkholderia sp. and then incubated on a rotary shaker at 200 rpm and 23°C. Samples (1 ml each) were taken periodically for protein determination by the method of Lowry et al. (13) as a measure of naphthalene biodegradation.
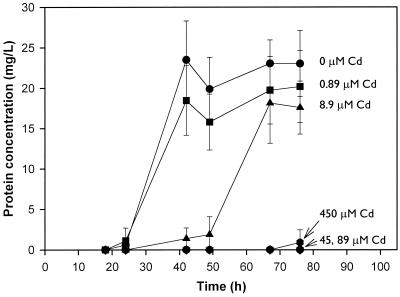
The effect of cadmium on the growth of the Burkholderia sp. on naphthalene is shown in Fig. 1. As the cadmium concentration increased, cadmium toxicity increased, resulting in a delay or complete inhibition of growth. For example, in the presence of 8.9 μM cadmium, the onset of exponential growth was delayed. At 45, 89, and 450 μM concentrations of cadmium, no measurable growth occurred during the 76-h experiment.
FIG. 1.
Effect of cadmium concentration on the growth of a Burkholderia sp. on naphthalene. Each point represents the mean protein concentration for triplicate flasks. Error bars represent standard deviations.
Effect of rhamnolipid on growth.
To determine the effect of rhamnolipid on growth in the presence of a toxic level of cadmium, 89 μM cadmium was added to 125-ml flasks containing 10% MSM, 15 mg of crystalline naphthalene, and 0, 8.9, 89, or 890 μM rhamnolipid to yield a final volume of 50 ml. Each flask was inoculated and incubated as described above. One-milliliter samples were removed periodically to determine the protein content.
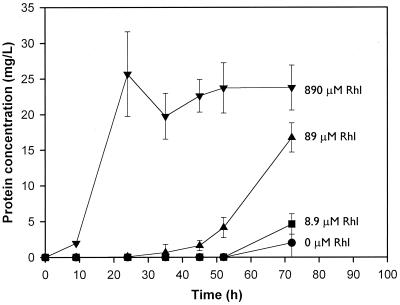
The effect of increasing concentrations of rhamnolipid on naphthalene biodegradation in the presence of 89 μM cadmium is shown in Fig. 2. As expected, in the absence of rhamnolipid, essentially no growth was observed. Rhamnolipid added at a 10-fold-smaller concentration than cadmium (8.9 μM rhamnolipid versus 89 μM cadmium) also had no effect on growth. This was expected, since there was essentially no cadmium complexation at this rhamnolipid level. Rhamnolipid added at an equimolar concentration resulted in substantial growth, but growth was delayed. Rhamnolipid added at a 10-fold-higher concentration (890 μM rhamnolipid; 89 μM cadmium) eliminated the effects of cadmium toxicity.
FIG. 2.
Effect of the rhamnolipid (Rhl) concentration on the growth of a Burkholderia sp. on naphthalene in the presence of 89 μM cadmium. Each point represents the mean protein concentration for triplicate flasks. Error bars represent standard deviations.
In contrast to the conclusions of Malakul et al. (14), these results cannot be explained by cadmium complexation alone. Only 890 μM rhamnolipid significantly reduced the bioavailable cadmium concentration (to 11.2 ± 0.47 μM). This level of cadmium should be inhibitory to the Burkholderia sp., which is sensitive to as little as 8.9 μM cadmium (Fig. 1). In addition, 89 μM rhamnolipid did not significantly reduce the concentration of bioavailable cadmium and should therefore completely inhibit growth (Fig. 1). The inability of the complexation data to completely explain the reductions in cadmium toxicity suggested that an additional mechanism(s) of toxicity reduction was involved. Several possibilities were considered and are discussed in the following sections.
Utilization of naphthalene and rhamnolipid as carbon sources.
Growth studies with 890 μM rhamnolipid in MSM showed that the Burkholderia sp. did not grow on rhamnolipid as a sole source of carbon and energy (data not shown). The effect of rhamnolipid (0, 8.9, 89, or 890 μM) on the growth of the degrader on naphthalene in the absence of cadmium was also investigated. While the growth rates were similar in the absence and presence of rhamnolipid, a decrease in the lag period and an increase in the cell yield were associated with greater concentrations of rhamnolipid (data not shown). This was likely due to rhamnolipid increasing the bioavailability of naphthalene, as has been shown previously for octadecane, hexadecane, and phenanthrene (9, 24–27). Thus, rhamnolipid had a stimulative effect on degradation of naphthalene by the Burkholderia sp. in both the presence and absence of cadmium.
To further differentiate whether the effects of rhamnolipid are to reduce cadmium toxicity or to enhance naphthalene bioavailability, the effect of rhamnolipid on the biodegradation of glucose (a substrate with high bioavailability) in the presence of cadmium was determined. In this experiment, conditions were identical to those described above, except that glucose (300 mg/liter) rather than naphthalene was used as the sole source of carbon and energy. Rhamnolipid mitigated cadmium toxicity during biodegradation of glucose in a manner similar to that observed for naphthalene (data not shown). This suggests that the effect of rhamnolipid in systems containing cadmium is to reduce cadmium toxicity and that enhanced bioavailability may be a secondary effect that plays a minor role in changing the lag period or the cell yield.
Effect of rhamnolipid on LPS release.
In addition to increasing organic solubility and complexing metals, rhamnolipid has recently been shown to increase cell surface hydrophobicity in P. aeruginosa by inducing the release of LPS from the outer cell membrane (1). We hypothesized that rhamnolipid would similarly cause a loss of LPS from the Burkholderia sp. used in this study. In this case, the uptake of cadmium would be reduced due to an overall reduction in the negative charge on the cell surface that decreases the interaction of the cationic cadmium form (Cd2+) with the cell surface. It is also possible that released LPS molecules could bind cationic cadmium via charged functional groups, such as 2-keto-3-deoxyoctonic acid. To test this hypothesis, the release of LPS was measured using sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis. Cells were grown in 10% MSM containing 300 mg of naphthalene/liter for 48 h, adjusted to an optical density at 600 nm of 1.0, and then centrifuged at 12,100 × g for 10 min and resuspended in 0, 8.9, 89, or 890 μM rhamnolipid in 10% MSM. Each suspension was vortexed and incubated on a rotary shaker (200 rpm) at 25°C for 24 h. Cell suspensions were then centrifuged, and the supernatants were removed and concentrated to 10× by lyophilization and resuspension in sterile double-distilled water. Each concentrated supernatant preparation (10 μl) was electrophoresed on a 4% stacker and 12.5% vertical resolving gel (16 by 18 by 0.15 cm) against 1 and 10 μg of P. aeruginosa serotype 10 LPS (Sigma, St. Louis, Mo.) for comparison. Two hundred volts were applied until the samples had migrated approximately 14 cm. Gels were run at 4°C in a Tris-Tricine running buffer (Bio-Rad, Hercules, Calif.). LPS were visualized by silver staining (10), and the density of LPS bands was analyzed using the SpotDenso feature of AlphaImager Software (Alpha Innotech, San Leandro, Calif.).
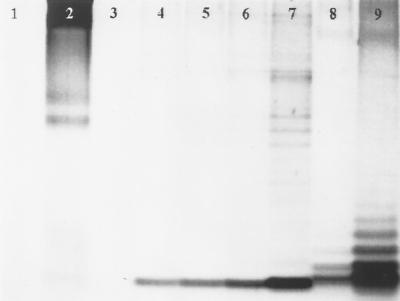
The gel in Fig. 3 shows a background level of LPS release in cells not treated with rhamnolipid (lane 4). LPS release increased with increasing concentrations of added rhamnolipid (lanes 5 to 7). Based on a densitometric analysis of the bands, 8.9 μM rhamnolipid increased LPS release over background levels by a factor of 1.3, 89 μM rhamnolipid increased release by a factor of 1.5, and 890 μM rhamnolipid doubled release. As shown in the control lanes, rhamnolipid was not stained (lane 3), while protein, represented by bovine serum albumin (BSA), was stained (lane 2). Since protein is a significant component of the outer membrane, it is reasonable to assume that a release of LPS may be accompanied by a release of LPS-associated proteins. For this reason, the bands from the samples (lanes 4 to 7) may represent both protein and LPS; however, previous work (10) has shown that only LPS molecules migrate to the bottom of the gel. The results presented here support this finding: BSA (lane 2) failed to migrate one-half of the length of the gel, while LPS standards from P. aeruginosa serotype 10 (lanes 8 and 9) migrated nearly the entire length of the gel. As such, the bands near the bottom of the gel from supernatants of Burkholderia sp. (lanes 4 to 7) represent LPS.
FIG. 3.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of concentrated (10×) supernatants of suspensions of a Burkholderia sp. The gel was stained using a silver-staining procedure for LPS. Lanes: 1, buffer; 2, 5 μg of BSA; 3, 4485.6 μg (890 μM) of rhamnolipid; 4, supernatant of the Burkholderia sp. treated only with MSM; 5, supernatant of the Burkholderia sp. treated with 8.9 μM rhamnolipid; 6, supernatant of the Burkholderia sp. treated with 89 μM rhamnolipid; 7, supernatant of the Burkholderia sp. treated with 890 μM rhamnolipid; 8, 1 μg of P. aeruginosa serotype 10 LPS; 9, 10 μg of P. aeruginosa serotype 10 LPS. The gel was imaged and the band density (integrated density value) was determined using the SpotDenso function of AlphaImager (Alpha Innotech, San Leandro, Calif.).
Summary.
This appears to be the first report of the use of a biosurfactant to reduce metal toxicity during the biodegradation of an organic contaminant in a cocontaminated system. In the model cocontaminated system studied herein, reductions in cadmium toxicity were observed for 89 and 890 μM rhamnolipid treatments. At an 890 μM concentration of rhamnolipid, both metal complexation and increased LPS release were observed. In this system, naphthalene biodegradation occurred at normal rates. At 89 μM rhamnolipid, very little cadmium complexation was measured, but LPS release increased. In this case, naphthalene degradation occurred, but with a longer lag period and at a slower rate. At 8.9 μM rhamnolipid, no cadmium complexation occurred, and only a slight amount of LPS was released. In this case, no naphthalene degradation occurred. These data suggest that rhamnolipid reduces cadmium-induced inhibition of naphthalene degradation through a combination of cadmium complexation and release of LPS from the cell. This is in contrast to previous work with modified clay complexes and chelating resins that focused solely on metal complexation to reduce metal toxicity (14). The fact that rhamnolipid reduced cadmium toxicity during biodegradation of both naphthalene (a substrate with limited aqueous solubility) and glucose (a substrate with essentially unlimited aqueous solubility) suggests that the ability of rhamnolipid to increase substrate bioavailability does not play an important role in reducing cadmium toxicity. Finally, this research demonstrates that rhamnolipid can induce the release of LPS from bacteria of a gram-negative genus (Burkholderia) that does not produce rhamnolipid. This suggests that rhamnolipid may be able to reduce metal toxicity to microbial consortia in cocontaminated soils through a combination of metal complexation and cell surface alteration, resulting in enhanced rates of bioremediation. This has been verified for two cocontaminated soil systems (15).
Acknowledgments
This work was supported by U.S. Department of Energy grant DE-FGD3-97ER62470 and the U.S. Environmental Protection Agency's Science to Achieve Results Fellowship Program.
We extend our gratitude to A. A. Bodour for providing 16S ribosomal DNA sequence data for the degrader used in this study.
REFERENCES
- 1.Al-Tahhan R, Sandrin T, Bodour A, Maier R. Cell surface hydrophobicity of P. aeruginosa: effect of monorhamnolipid on fatty acid and lipopolysaccharide content. Appl Environ Microbiol. 2000;66:3262–3268. doi: 10.1128/aem.66.8.3262-3268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babich H, Stotzky G. Reductions in toxicity of cadmium to microorganisms by clay minerals. Appl Environ Microbiol. 1977;33:696–705. doi: 10.1128/aem.33.3.696-705.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babich H, Stotzky G. Effect of cadmium on microbes in vitro and in vivo: influence of clay minerals. In: Babich H, Stotzky G, editors. Microbial ecology. Berlin, Germany: Springer-Verlag; 1978. pp. 412–415. [Google Scholar]
- 4.Enger E D, Smith B F. Hazardous and toxic wastes. In: Enger E D, Smith B F, editors. Environmental science: the study of interrelationships. Dubuque, Iowa: Wm. C. Brown Publishers; 1992. [Google Scholar]
- 5.Gilleland H E, Jr, Stinnett J D, Roth I L, Eagon R G. Freeze-etch study of Pseudomonas aeruginosa—localization within the cell wall of an ethylenediaminetetraacetate-extractable component. J Bacteriol. 1973;113:417–432. doi: 10.1128/jb.113.1.417-432.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg S S, Cordeiro M N, Silva Pereira A A, Mares-Guia M L. Release of lipopolysaccharide (LPS) from cell surface of Trypanosoma cruzi by EDTA. Int J Parasitol. 1983;13:11–18. doi: 10.1016/s0020-7519(83)80062-9. [DOI] [PubMed] [Google Scholar]
- 7.Gray G W, Wilkinson G. The action of ethylenediaminetetraacetic acid on Pseudomonas aeruginosa. J Appl Bacteriol. 1965;28:153–164. [Google Scholar]
- 8.Herman D C, Artiola J F, Miller R M. Removal of cadmium, lead, and zinc from soil by a rhamnolipid biosurfactant. Environ Sci Technol. 1995;29:2280–2285. doi: 10.1021/es00009a019. [DOI] [PubMed] [Google Scholar]
- 9.Herman D C, Zhang Y M, Miller R M. Rhamnolipid (biosurfactant) effects on cell aggregation and biodegradation of residual hexadecane under saturated flow conditions. Appl Environ Microbiol. 1997;63:3622–3627. doi: 10.1128/aem.63.9.3622-3627.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hitchcock P J, Brown T M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983;154:269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamel Z. Toxicity of cadmium to two Streptomyces species as affected by clay minerals. Plant Soil. 1986;93:193–205. [Google Scholar]
- 12.Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965;21:290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- 13.Lowry O H, Rosenbrough N J, Farr R L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 14.Malakul P, Srinivasan K R, Wang H Y. Metal toxicity reduction in naphthalene biodegradation by use of metal-chelating adsorbents. Appl Environ Microbiol. 1998;64:4610–4613. doi: 10.1128/aem.64.11.4610-4613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maslin, P. M., and R. M. Maier. Rhamnolipid-enhanced mineralization of phenanthrene in organic-metal co-contaminated soils. Biorem. J., in press.
- 16.Mihelcic J R, Luthy R G. Degradation of polycyclic aromatic hydrocarbon compounds under various redox conditions in soil-water systems. Appl Environ Microbiol. 1988;54:1182–1187. doi: 10.1128/aem.54.5.1182-1187.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ochoa, F. J., J. F. Artiola, and R. M. Maier. Stability constants for the complexation of various metals with a rhamnolipid biosurfactant. J. Environ. Qual., in press. [DOI] [PubMed]
- 18.Remacle J. The cell wall and metal binding. In: Volesky B, editor. Biosorption of heavy metals. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 83–92. [Google Scholar]
- 19.Roane T M, Pepper I L. Proceedings of the 12th Annual Conference on Hazardous Waste Research: Building Partnership for Innovative Technologies. 1997. Microbial remediation of soils co-contaminated with 2,4-dichlorophenoxy acetic acid and cadmium; pp. 343–356. Kansas City, Mo. [Google Scholar]
- 20.Said W A, Lewis D L. Quantitative assessment of the effects of metals on microbial degradation of organic chemicals. Appl Environ Microbiol. 1991;57:1498–1503. doi: 10.1128/aem.57.5.1498-1503.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan H, Champion J T, Artiola J F, Brusseau M L, Miller R M. Complexation of cadmium by a rhamnolipid biosurfactant. Environ Sci Technol. 1994;28:2402–2406. doi: 10.1021/es00062a027. [DOI] [PubMed] [Google Scholar]
- 22.Torrens J L, Herman D C, Miller-Maier R M. Biosurfactant (rhamnolipid) sorption and the impact on rhamnolipid-facilitated removal of cadmium from various soils under saturated flow conditions. Environ Sci Technol. 1998;32:776–781. [Google Scholar]
- 23.Weisburg W G, Barns S M, Pelletier D A, Lane D J. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y M, Maier W J, Miller R M. Effect of rhamnolipids on the dissolution, bioavailability and biodegradation of phenanthrene. Environ Sci Technol. 1997;31:2211–2217. [Google Scholar]
- 25.Zhang Y M, Miller R M. Enhanced octadecane dispersion and biodegradation by a Pseudomonas rhamnolipid surfactant (biosurfactant) Appl Environ Microbiol. 1992;58:3276–3282. doi: 10.1128/aem.58.10.3276-3282.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y M, Miller R M. Effect of a Pseudomonas rhamnolipid biosurfactant on cell hydrophobicity and biodegradation of octadecane. Appl Environ Microbiol. 1994;60:2101–2106. doi: 10.1128/aem.60.6.2101-2106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y M, Miller R M. Effect of rhamnolipid (biosurfactant) structure on solubilization and biodegradation of n-alkanes. Appl Environ Microbiol. 1995;61:2247–2251. doi: 10.1128/aem.61.6.2247-2251.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]