Abstract
Background: Drug resistance and recurrence often develop during the treatment of muscle-invasive bladder cancer (MIBC). The existence of cancer stem cells (CSCs) in MIBC makes the formulation of effective treatment strategies extremely challenging. We aimed to use single-cell RNA sequencing approaches to identify CSCs and evaluate their molecular characteristics and to discover possible therapeutic measures.
Methods: GEO data sets GSE130001 and GSE146137 were used to construct an expression matrix. After cells were identified by type, malignant epithelial cells inferred by InferCNV were extracted for stemness evaluation. The subset of cells with the highest stemness was subjected to weighted gene coexpression network analysis (WGCNA) and pseudotime analysis to identify key genes. In addition, we predicted drug sensitivity relationships for key genes in CTD and predicted the correlation between drugs and survival through siGDC.
Results: We found that there were some CSCs in MIBC samples. The CSC population was heterogeneous during tumor development and was divided into quiescent and proliferating CSCs. We identified DBI as the key gene in quiescent CSCs. Analysis of a TCGA data set showed that higher DBI expression indicated higher histological grade. In addition, we predicted that acetaminophen can reduce DBI expression, thereby reducing the stemness of CSCs. Thus, we identified a potential new use of acetaminophen.
Conclusion: We systematically explored CSCs in tumors and determined that DBI may be a key gene and potential therapeutic target in quiescent CSCs. In addition, we confirmed that acetaminophen may be a candidate drug targeting CSCs, improving our understanding of CSC-targeting therapeutic strategies.
Keywords: cancer stem cells, bladder cancer, scRNA-seq, acetaminophen, DBI
Introduction
Muscle-invasive bladder cancer (MIBC) is an aggressive disease with high mortality and a propensity for metastatic dissemination (Bochner et al., 2006; Lenis et al., 2020). Neoadjuvant chemotherapy (NAC) followed by radical cystectomy is the standard of care for patients with MIBC (Bellmunt and Petrylak, 2012). However, even in patients who receive optimal treatment with surgery and chemotherapy, the 5-years overall survival rate is only 60% due to recurrence and metastasis (Soloway, 2013).
Cancer stem cells (CSCs) have received attention as a small population of highly malignant cells within liquid and solid tumors responsible for tumor initiation, self-renewal, chemo- and radiotherapeutic resistance, and evasion of immune surveillance to accelerate recurrence and metastasis (Magee et al., 2012; Nguyen et al., 2012; Visvader and Lindeman, 2012; Marquardt et al., 2018). Therefore, specific targeting of CSCs may improve the efficiency of cancer treatment (Du et al., 2019; Duan et al., 2021). However, CSCs constitute only a small fraction of the total tumor cell population (Reya et al., 2001), and a broad consensus on approaches to identify CSCs in clinical specimens is lacking. Furthermore, CSCs may be more heterogeneous than previously recognized, complicating their identification and eradication (Batlle and Clevers, 2017). Studies have shown that environmental signals can temporarily induce the proliferation of cells, but CSCs can reenter a quiescent state (Cai et al., 2017). However, many cell surface markers and signaling pathways differ between quiescent and proliferating CSCs. In addition, drug sensitivity and resistance may differ between quiescent and proliferating cells. Studies have shown that drugs therapeutically targeting the Wnt signaling pathway may eliminate proliferating CSCs but not quiescent CSCs (Choi et al., 2013). Unfortunately, quiescent CSCs have a strong ability for self-renewal, leading to tumor recurrence. Therefore, more attention should be devoted to quiescent CSCs, as the more recalcitrant subpopulation of CSCs.
Single-cell RNA sequencing (scRNA-seq) has been widely used to measure gene expression in individual cells. This approach can allow more comprehensive exploration of the tumor microenvironment and characterization of rare cells, thus overcoming the limitations of traditional bulk RNA sequencing (Li et al., 2012; Gawad et al., 2016; Plass et al., 2018). Through scRNA-seq, we can conduct a more accurate analysis to identify important pathways and genes related to CSCs. In previous studies, researchers found quiescent CSCs in tumors, which may be the cause of tumor recurrence and drug resistance, using scRNA-seq. These CSCs have lower proliferative activity and higher self-renewal ability than proliferating CSCs (Wang et al., 2021). Therefore, it is particularly important to define the molecular characteristics of quiescent CSCs and find targeted drugs.
Drug discovery and development is an expensive and time-consuming task. Currently, the FDA has approved only three targeted drugs (vismodegib, ivosidenib, and venetoclax) for CSCs (Clarke, 2019), but none of these target bladder CSCs. Repurposing of older drugs has become an alternative strategy to overcome the considerable costs and time required for drug development. Old drugs can be successfully used for new purposes because these compounds have been clinically tested in humans and have acceptable known side effects. Thus, some old drugs may be able to be reused to treat CSCs.
Here, we used scRNA-seq to demonstrate that diazepam binding inhibitor (DBI) is an important molecule in bladder CSCs that can maintain their stemness. In addition, we demonstrated that acetaminophen can reduce the expression of DBI and inhibit tumor proliferation. In our research, we revealed the importance of DBI in CSCs and identified a new use of acetaminophen to therapeutically target CSCs.
Methods and Materials
Acquisition, Quality Control, and Analysis of scRNA-Seq Data
The data used for our research were downloaded from an online public database and present no ethical issues. We obtained single-cell MIBC data from publicly available GEO data sets (GSE130001, GSE146137), and only human data were obtained from GSE146137 (Sfakianos et al., 2020; Wang et al., 2020). According to the original articles, all samples had been sorted to obtain the CD45-negative subpopulation. A total of four specimens and 7,979 cells were used for this study.
We used criteria for filtering the single cells to exclude low‐quality cells (<500 genes/cell and >15% mitochondrial genes). Based on the Seurat manual (Hao et al., 2020), gene expression levels were normalized and scaled for downstream analyses. The four samples were integrated by the canonical correlation analysis (CCA) method using 2000 anchors. A total of 2000 highly variable genes identified by the FindVariableFeatures function in the Seurat package were used for principal component analysis (PCA)-based dimensionality reduction with RunPCA. t-distributed stochastic neighbor embedding (t-SNE) was utilized to visualize single-cell clustering using principal components (PCs) 1 to 30.
Identification of Cell Clusters and Prediction of Copy Number Variations
To identify the population of epithelial cells in each sample, we used SingleR (Aran et al., 2019), an R package designed to identify different cell types. We used the dataset function in the celldex package and call HumanPrimaryCellAtlasData as the reference database to infer the cell type of each single cell.
We extracted the count matrix of epithelial cells and used it for CNV prediction. InferCNV (https://github.com/broadinstitute/inferCNV), an available method to identify evidence of somatic large-scale chromosomal copy number alterations with reference to “normal cells”, was used to predict the CNVs in scRNA-seq data (Ortega et al., 2017). We used the following settings: cutoff = 0.1 (this value generally works well with 10X Genomics data), denoising = TRUE, and reference cells = endothelial cells, NK cells, and tissue stem cells. A heatmap showing the relative expression intensities across each chromosome was generated. We extracted cells with obvious CNVs and defined them as malignant epithelial cells.
Calculation of the mRNA Expression-Based Stemness Index
An innovative one-class logistic regression (OCLR) machine learning algorithm was used to perform multiplatform analysis of the transcriptome, methylome and transcription factor binding sites of stem cells; the mRNAsi, an independent stemness index, was thus obtained (Malta et al., 2018). The OCLR machine learning algorithm is an effective method of quantifying the cancer stemness index using two independent indices. Calculation of stemness of tumor cells using the OCLR algorithm has been effectively applied in a variety of malignant tumors. We identified one cluster of 279 cells with the highest mRNAsi values as the CSC cluster. The stemness index was calculated via the OCLR algorithm by utilizing the scCancer (version 2.2.1) package in R (Guo et al., 2020).
Pseudotime Analysis
Monocle two was applied to generate single-cell pseudotime trajectories from scRNA-seq data assuming that the one-dimensional quantity “time” can describe high-dimensional expression values. Briefly, we selected genes that were differentially expressed (p-value < 0.05) among t-SNE clusters as candidate genes. And converted the Seurat object into CellDataSet through setOrderingFilter, and estimated the size factor and dispersion. Set the dimensionality reduction parameter in reduceDimension to “DDRTree” to perform dimensionality reduction for all epithelial cells. And through the orderCells function, cell sorting and trajectory construction are performed.
Weighted Gene Coexpression Network Analysis
We selected 279 cells from the CSC cluster to construct a gene expression matrix for WGCNA (Zhang and Horvath, 2005; Langfelder and Horvath, 2008). A signed network was constructed using 2000 genes selected with the FindVariableFeatures function in the Seurat package. After constructing the adjacency matrix, we chose the soft-thresholding power (β = 3) to obtain the topological overlap matrix (TOM). Genes were grouped using average linkage hierarchical clustering based on the high similarity of coexpression relationships.
pySCENIC
Single-cell regulatory network inference and clustering (SCENIC) is a new computational method to map genes regulatory networks and identify stable cell states by evaluating the activity of each cell from scRNA-seq data (Aibar et al., 2017). pySCENIC was performed on all cells, and the regulons were calculated based on transcription factors (TFs) or their target genes. Only regulons significantly upregulated or downregulated were involved in further analysis.
Gene Ontology Analysis and Single-Sample Gene Set Enrichment Analysis
ClusterProfiler (version 3.18.1) was applied to analyze differences among clusters (Yu et al., 2012). Based on the GO database, functional enrichment analysis of marker genes (log2FC ≥ 1, adjusted p ≤ 0.05) was performed on each cell to explore their potential biological functions. ssGSEA was applied to calculate the relationship between the stemness index of each cell in the CSC cluster and the “Hallmark” gene sets (version 7.3) using the R package GSVA (1.38.2) with the “ssgsea” option for the method argument (Hänzelmann et al., 2013).
Drug Selection
The Comparative Toxicogenomics Database (CTD, http://ctd.mdibl.org/) is a public resource that constructs chemical-gene-disease networks and predicts novel relationships using different data. This database facilitates the generation of testable hypotheses about the molecular mechanisms linking drugs and genes (Davis et al., 2011). Combining the search results with the relevant literature reports, we selected acetaminophen as the target drug for CSC therapy.
Survival interaction of Genes, Cells and Drugs in human cancers (siGCD, http://sigcd.idrug.net.cn) is a web server for exploration of the interactions of genes, cells and drugs with survival in human cancers. We predicted the correlation between acetaminophen and survival by using the “Cell & Drug” module in this database. We defined “Cell” as “Cancer stem cell-Bladder” and “Drug” as “acetaminophen”.
Results
Single-Cell Analysis Identified Cellular Subtypes and Inferred Malignant Epithelial Cells
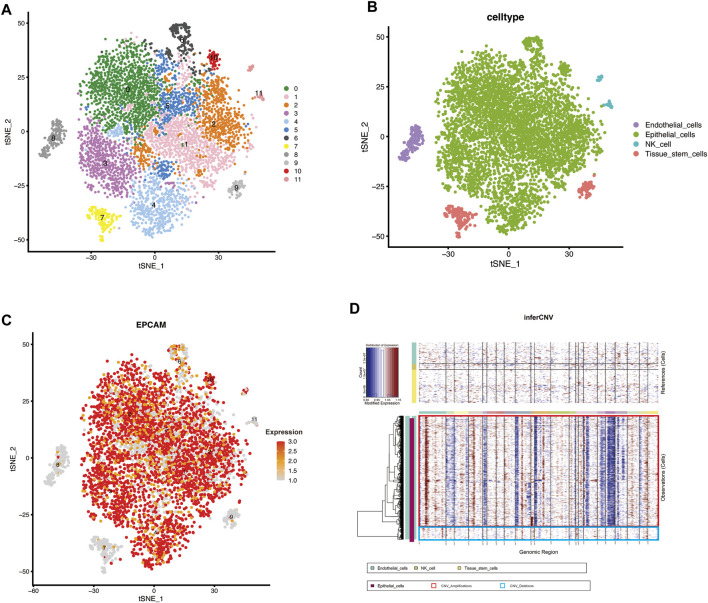
The scRNA-seq data were screened with strict QC criteria (Supplementary Figure S1A), and 7,228 cells were obtained for follow-up analysis after QC. To determine the components of tumor cells, we first set the resolution at 0.5, resulting in the identification of 12 cell clusters, and visualized the clusters utilizing a t-SNE plot (Figure 1A).
FIGURE 1.
scRNA-seq identified the cellular components in MIBC and inferred malignant epithelial cells (A,B) t-SNE plot showing clustering information in MIBC (C) FeaturePlot was used to demonstrate the identity of epithelial cells through the expression of a well-known specific marker (EPCAM) (D) Heatmap showing the chromosomal landscape of inferred large-scale copy number variations (inferCNVs) distinguishing individual tumor (malignant) cells from nontumor cells. Red box: Amplifications of CNV, Blue box: Deletions of CNV.
To identify different genes in each cluster, we used the FindAllMarkers function in Seurat to obtain the key genes in each cluster. The ten genes with the highest expression levels in each cluster are shown in the heatmap (Supplementary Figure S1B). Based on the expression of the key genes, the 12 cell clusters were divided into four different cell types (Figure 1B), which were identified with the SingleR (version 1.4.1) R package. Because the included data were only from cells without expression of CD45, which is recognized as the marker gene of lymphocytes (Clement, 1992), lymphocytes were almost completely absent from this analysis. The common epithelial cell marker EPCAM was expressed in the “Epithelial_cells” subpopulation, verifying the accuracy of the identification (Figure 1C). Finally, we extracted the cells defined as “Epithelial_cells” for subsequent analysis.
To reveal the changes in epithelial cells during malignant progression of MIBC, we estimated the CNV of epithelial cells with inferCNV (version 1.6.0) and set the reference group to nonmalignant cells (endothelial cells, NK cells, and tissue stem cells) (Xiong et al., 2020; Rindler et al., 2021; Wu et al., 2021). We clustered cells according to the degree of CNV; blue indicates deletions, and red indicates amplifications (Figure 1D). In Figure 1D, the epithelial cells in the red box have a significantly higher CNV degree than the cells in the blue box. Finally, the cells in the red box were extracted and defined as malignant epithelial cells.
Stemness of Malignant Epithelial Cells in Each Cell Cluster Based on mRNAsi
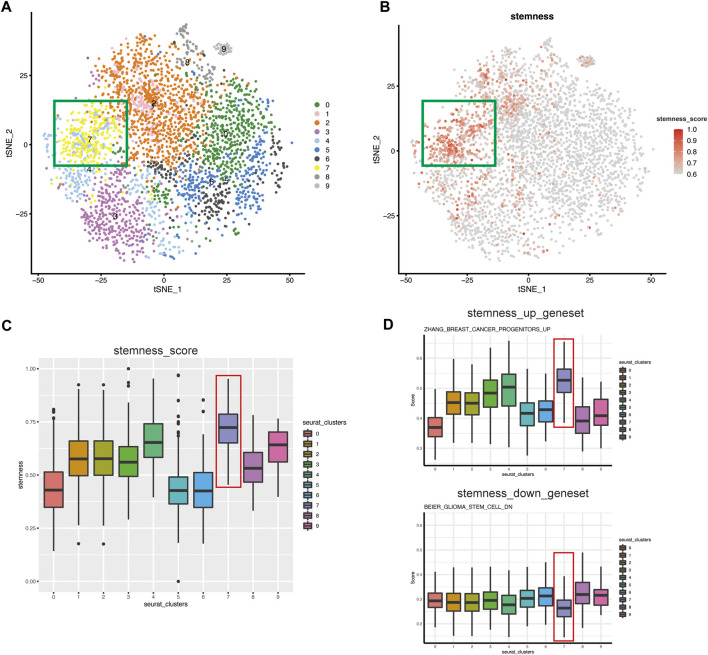
We reclustered the malignant cells with a resolution of 0.8 and obtained ten clusters (Figure 2A). The t-SNE plot shows that the tumor epithelial cells formed distinct clusters, indicating that the gene expression pattern of epithelial cells gradually changes during the progression of MIBC. To detect CSCs, we used the OCLR model (Malta et al., 2018) to estimate the mRNAsi of each individual tumor cell and found that the cells of clusters-7 in the green box have higher stemness than other clusters (Figure 2B). The box plot intuitively shows that the stemness of cluster-7 was higher than that of the other clusters (Figure 2C).
FIGURE 2.
The CSC subgroup was identified and verified by evaluation of stemness (A) t-SNE plot of malignant epithelial cells isolated across all specimens, colored and labeled by cluster. The cells of clusters-7 in the green box have higher stemness (B) t-SNE plot showing the stemness score for each cell. The color of the dots represents the stemness, and the darker the color, the higher the stemness. The cells in the green box have the highest stemness (C) Box plot showing the stemness score for each cluster. Cluster-7 has the highest stemness (D) ssGSEA score of CSCs-related gene set in each seurat_clusters. Top: Represent the gene set of stem genes upregulation. Bottom: Represent the gene set of stem genes downregulation.
We performed ssGSEA to verify the features of cell stemness. “Hallmark” gene sets were significantly enriched, and the results showed that as stemness increased, hallmark gene sets such as DNA_REPAIR (Zhang et al., 2017), MYC_TARGET (Hanahan and Weinberg, 2011; Bahr et al., 2018), and OXIDATIVE_PHOSPHORYLATION were activated (Supplementary Figure S1F). These results were consistent with the enrichment results in the original article and suggested the strong self-renewal ability of CSCs (Malta et al., 2018). To further identify the CSC-like cluster, we selected CSCs-related up-regulated gene sets and down-regulated gene sets from MsigDB for evaluation of all clusters. And the results showed that, cluster-7 had a higher score in the gene set of stem genes upregulation, while lower scores in the gene set of stem genes downregulation (Figure 2D). It further confirmed that cluster-7 had the highest stemness. Therefore, we tentatively defined cluster-7 as a CSC-like cluster.
A Stemness Gene Set in CSCs Was Identified by WGCNA
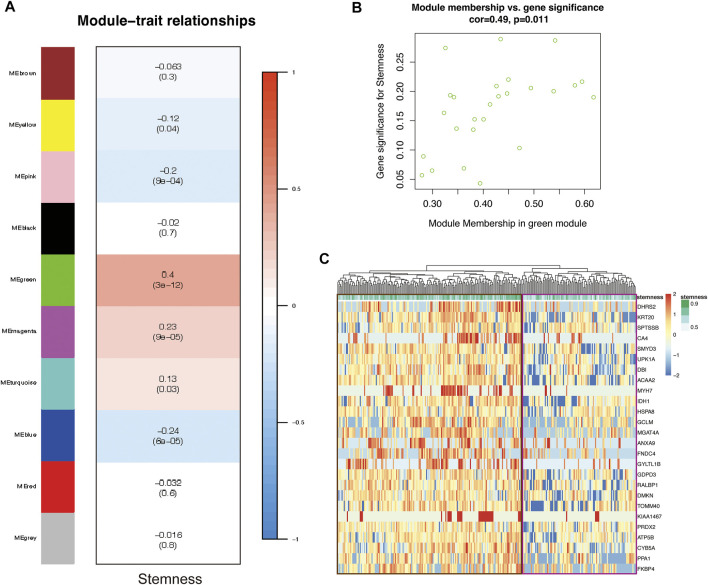
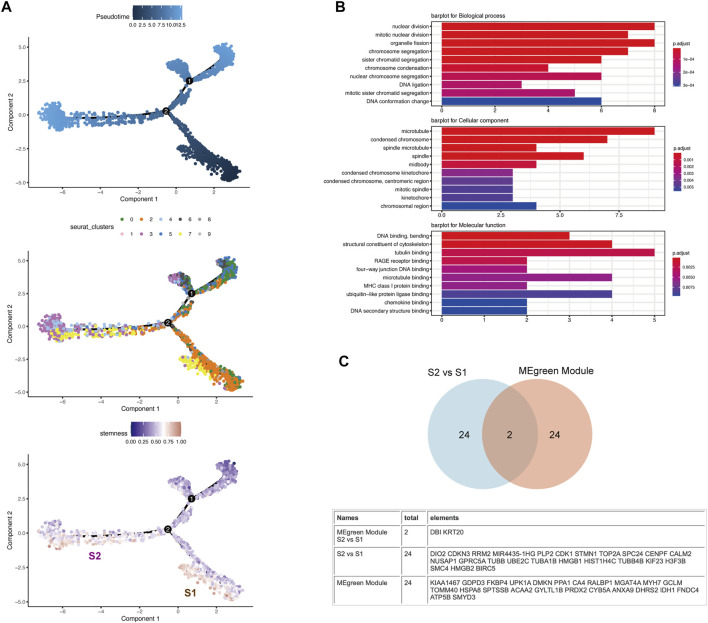
WGCNA is a systems biology method for describing correlation patterns among genes in RNA sequencing data (Zhang and Horvath, 2005; Langfelder and Horvath, 2008). We exploited this powerful tool to identify the hub genes that induce the stemness of tumor cells. Then, we extracted 279 cells in cluster-7 for WGCNA. After we chose the soft-thresholding power (β = 3), the algorithm for gene network construction and module identification was run, and 10 modules were identified (Figure 3A). There was a significant correlation between the MEgreen module and the stemness of cells (Figure 3A). To better prove the correlation between gene significance for CSCs and module membership of the MEgreen module, a scatter plot was generated (Figure 3B). These results indicated that the MEgreen module could accurately represent the CSC subpopulation.
FIGURE 3.
The CSC gene-network module was identified by WGCN A. (A) Heatmap showing module associations. Each row corresponds to a module eigengene; each column, to stemness. In addition, each box contains the corresponding correlation and p-value (B) Scatter plot of gene significance (GS) for CSCs vs. module membership (MM) in the MEgreen module. There is a significant correlation between CSCs and the MEgreen module (C) Heatmap showing the relationship between 26 genes in the MEgreen module and stemness (cells with higher stemness are in the brown box; cells with lower stemness are in the purple box).
The MEgreen module contained 26 genes. To further support the correlation between the expression of these genes and cell stemness, we generated a Heatmap to visualize the relationship (Figure 3C). The cells in the brown box had higher stemness, and the expression of these genes in these cells was significantly higher than that in the cells in the purple box (which had lower stemness). This pattern proved that high expression of these genes was positively correlated with enhanced stemness of CSCs.
pySCENIC Analysis Revealed Abnormally Activated Transcription Factors in CSCs
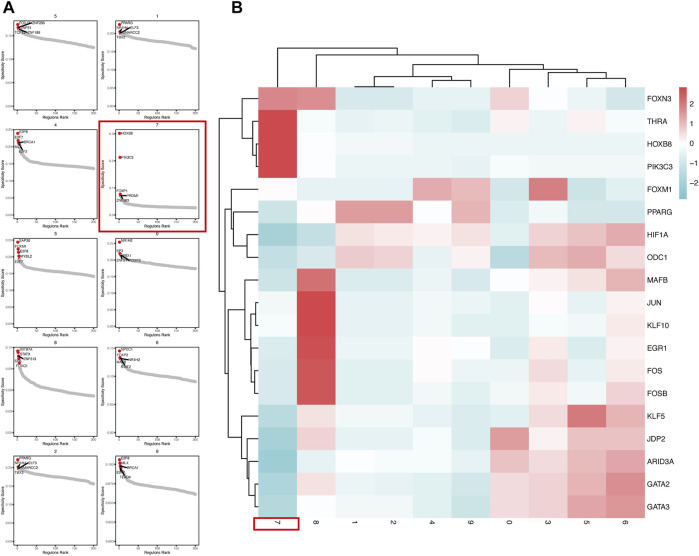
In order to explore the transcriptional regulation inside the CSCs cluster (cluster-7), we then used pySCENIC to infer transcription factors (TFs) regulatory information underlying each cluster. SCENIC analysis revealed that some TFs had obvious differential activation in the CSC. We then compared and detected five up-regulated TFs in each cluster (Figure 4A).
FIGURE 4.
pySCENIC analysis revealed abnormally activated transcription factors in CSCs (A) Top5 transcription factor abnormally activated in each tumor cell cluster (B) The Heatmap showed the activation of transcription factors activity in different tumor cell clusters.
In the CSCs cluster (cluster-7), we can clearly see that the PIK3C3, HOXB8 and THRA are highly activated (Figure 4B). The Class III phosphoinositide 3-kinase (PIK3C3), also known as vacuolar protein sorting 34 (Vps34), plays an important role in the control of autophagy, which are critical in a wide range of cellular processes. The suppression of PIK3C3 inhibits the activation of SGK3, a CSCs promoter induced by PI3K inhibitors. And PIK3C3 inhibitors inhibit liver CSCs by activating AMP-activated kinase (AMPK) (Liu et al., 2020). HOXB8 has been shown to be associated with the development of various cancers, such as colorectal, hepatocellular and gastric cancers (Kanai et al., 2010; Ding et al., 2017). In vitro studies by Vider et al. showed that HOXB8 is upregulated in colorectal cancer cell lines. Furthermore, upregulated expression of HOXB8 was observed in all stages of colorectal cancer (Vider et al., 2000). At the same time, HOXB8 also upregulates STAT3 expression, which induces the development of EMT. In addition, the activation of the miR-133b/HOXB8 axis also promotes tumor stemness, proliferation and invasion (Jiang et al., 2020).
Pseudotime Analysis Identified the Quiescent CSCs
To better understand the functional states and relationship of CSCs, we next determined their developmental trajectories with Monocle 2 (Supplementary Table S4). Pseudotime analysis showed that the cells in cluster-7 were divided into early and late groups on the time trajectory (Figure 5A). The differentially expressed genes (DEGs) with higher expression in S1 than S2 were DBI and KRT20, and the rest of the DEGs were highly expressed in S2. We extracted the highly expressed DEGs in S2 for GO functional enrichment analysis to compare the late cluster-7 cell group (S2) with the early cluster-7 cell group (S1), and we found that the S2 cell group were involved in more functions, such as nuclear division and chromosomal division (Figure 5B). This result suggested that S2 cells have high proliferative activity and might be a group of cells that increase tumor malignancy. In contrast, the S1 cells were in a relatively quiescent state. We defined the S1 cells as “quiescent CSCs” and S2 cells as “proliferating CSCs” (Clarke, 2019). Furthermore, tumor recurrence and metastasis are closely related to quiescent CSCs (Wang et al., 2021). Therefore, we identified the differentially expressed genes (DEGs) between the quiescent CSCs and proliferating CSCs (Supplementary Table S1).
FIGURE 5.
Pseudotime analysis revealed the heterogeneity of CSCs along their developmental trajectory (A) The distribution of pseudotime (top), cluster (middle) and stemness score (bottom) exhibits a continuous pattern. Top: Pseudotime is shown colored along a gradient from dark to light blue, and the start of pseudotime is indicated. Middle: Clusters are color-coded by subpopulation. Bottom: CSCs are divided into two subsets (S1 and S2) along their developmental trajectory (B) The figure shows that the differentially expressed genes with higher expression in S2 than S1 were extracted and screened for GO analysis. The results of the GO analysis suggests that the S2 subgroup shows higher division and proliferation abilities than the S1 subgroup (C) Venn diagram depicting the intersection between the DEGs of S2 vs. S1 and the gene set corresponding to the MEgreen module in WGCNA.
By comparing the gene set corresponding to the MEgreen module in WGCNA and the DEGs between S2 and S1, we found that DBI and KRT20, which are closely related to stemness, appeared in both gene sets (Figure 5C). By searching the CellMarker database (http://biocc.hrbmu.edu.cn/CellMarker/), we found that DBI and KRT20 are closely related to embryonic cells, fetal cells, and CSCs (Supplementary Table S2). We evaluated the correlations among KRT20, DBI and common CSC markers (PROM1/CD133 (Aghajani et al., 2019) and CD24 (Ooki et al., 2018)) in the TCGA database and found that the expression of these molecules showed a trend toward a positive correlation (Supplementary Figure S1C). And with the progress of the pseudotime, the expression of DBI and KRT20 shows a downward trend (Supplementary Figure S1D). Meanwhile, we evaluated the correlation of DBI and KRT20 expression with cell stemness and plotted the scatter plot (Supplementary Figure S1E).DBI, KRT20 and cell stemness were all positively correlated, with the strongest correlation between DBI and stemness (R = 0.37, p < 2.2e-16). Therefore, we deemed DBI and KRT20 to be closely associated with quiescent CSCs, and DBI as a follow-up study.
Acetaminophen Decreased DBI Expression and Inhibited Tumor Proliferation
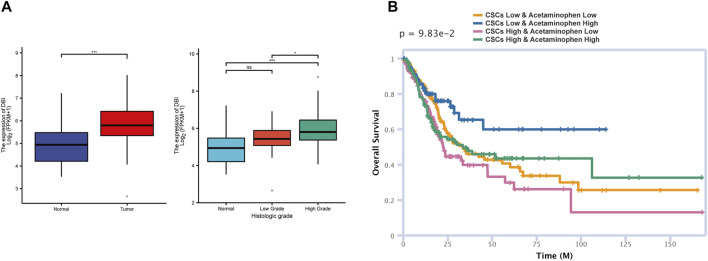
As a marker of bladder cancer CSCs newly discovered in our research, DBI has rarely been reported in previous articles. Higher stemness is related to a higher degree of active tumor dedifferentiation, suggesting that stemness is related to histopathological grade (Malta et al., 2018). We analyzed the relationship between DBI expression and clinical histological grade in the TCGA database and found that the higher the expression of DBI, the higher was the histological grade (Figure 6A), which means that DBI may enhance the stemness of CSCs, leading to increased tumor heterogeneity (Reddy, 2020; Zeng et al., 2021). Therefore, we focused on DBI molecules in our research.
FIGURE 6.
Molecule and drug prediction in the database (TCGA) (A) Analysis of the TCGA BLCA data of matched tumor–normal tissue pairs showing that DBI expression is higher in tumor tissue than in normal tissue (left). The higher the DBI expression, the higher is the histological grade (right) (*p < 0.05; **p < 0.001) (B) The siGCD database was used to predict the effects of acetaminophen and CSCs on patient survival.
Through the CTD database, we screened targeted drugs for interactions with DBI. Among the candidate drugs and compounds, acetaminophen can reduce the expression of DBI (Supplementary Table S3). Acetaminophen and its metabolites have been proven to kill CSCs in previous studies (Ahmed et al., 2019; Pingali et al., 2021). Then, we used the siGCD database to predict the relationships among drugs, cells and survival, defining “Cell” as “Cancer stem cell-Bladder” and “Drug” as “acetaminophen”. The prediction results indicated that under the same dose of acetaminophen, a higher CSCs count indicates a poorer prognosis (Figure 6B), which is closely related to tumor recurrence and drug resistance mediated by CSCs (Bayik and Lathia, 2021). Moreover, a high dose of acetaminophen predicted a good prognosis in both the high and low CSC groups, suggesting that acetaminophen exerts great therapeutic effects against CSCs. The above analysis results indicated that acetaminophen may kill CSCs by inhibiting the expression of DBI, thereby inhibiting tumor growth and recurrence. We speculate that acetaminophen may be a candidate for the treatment of quiescent CSCs.
Discussion
Drug resistance and recurrence of bladder cancer, which strongly affect the therapeutic efficacy and survival rate of patients, are highly correlated with CSCs. However, CSCs are rare cells with stem cell characteristics within the tumor cell population, accounting for only a small proportion of the total cell population in tumor tissue. Therefore, accurately sorting and killing CSCs is difficult. It is difficult to identify and sort CSCs from traditional bulk RNA sequencing data because the cell population is mixed. scRNA-seq overcomes this limitation and has become one of the most important tools for studying CSCs. Therefore, we analyzed CSCs using scRNA-seq and found that DBI is an important prognostic marker and potential target for quiescent CSCs. In addition, our data suggested that acetaminophen can reduce the stemness of CSCs by reducing the expression of DBI.
Clinical translational research on CSCs has bright prospects but is only in its infancy. Several problems need to be solved before the clinical potential of CSCs can be realized (Clarke, 2019). First and foremost, it is necessary to further define the molecular and cytological characteristics of CSCs. Obviously, not all carcinogenic mutations can pass stemness to CSCs. Second, therapeutic drugs should be able to target both quiescent and proliferating CSCs. By targeting the microenvironment of CSCs, drugs abolish the self-renewal ability of CSCs, thus eliminating the root causes of tumor development. In this study, we isolated the CSC population by evaluating the stemness of each cell and identified a stemness gene set through WGCNA. Then, this CSC population was divided into two subsets, S1 and S2, by pseudotime analysis, and enrichment analysis suggested that S1 was quiescent CSCs and S2 was proliferating CSCs. Studies have shown that the CSC population is heterogeneous during tumor development. A small subset of CSCs, called quiescent CSCs, have a greater ability for self-renewal than for proliferation. These cells are less sensitive to treatment and maintain the renewal of tumor cells, leading to tumor recurrence. DBI was highly expressed in quiescent CSCs and was highly correlated with the stemness gene set in WGCNA. To verify this result, we used the TCGA database to increase the number of samples. DBI expression was significantly higher in tumors; moreover, higher expression of DBI suggested higher histopathological grade (Figure 6A). This finding also suggests that DBI may maintain the stemness of CSCs, thereby promoting tumor heterogeneity.
DBI (also named acyl-CoA-binding protein, ACBP) is a 10-kD protein that binds, buffers and transports acyl-coenzyme A molecules, contributing to lipid metabolism (Knudsen et al., 1993; Lebrun et al., 2021). A previous study showed that this metabolic reprogramming mediated by DBI can promote tumor development (Duman et al., 2019). Interestingly, several studies have shown that DBI can maintain the proliferation of stem and progenitor cells (Alfonso et al., 2012; Dumitru et al., 2017). Interestingly, DBI expression is not restricted to gliomas but is also present in most tumor types pointing to the possibility of a general mechanism of DBI in other cancers. Indeed, in non-small cell lung cancer, DBI has been shown to promote tumor cell proliferation in vitro by regulating FAO, and high DBI expression predicts a poorer prognosis for lung cancer patients (Harris et al., 2014). Considering these results collectively with our findings, we believe that DBI, as an important molecule for stemness maintenance, can serve as both a marker and therapeutic target for quiescent CSCs.
We speculated that acetaminophen can reduce the expression of DBI, suggesting that acetaminophen may be an important candidate for CSC treatment. In previous studies, acetaminophen has been shown effective in treating recurrent tumors and CSCs (Wu et al., 2013; Ahmed et al., 2019; Pingali et al., 2021). We thus believe that acetaminophen can be used as a DBI-targeting drug to reduce the CSC population, hence suppressing tumor recurrence and the development of drug resistance.
Our future research will address the following topics: 1) The specific mechanism by which DBI maintains CSCs needs further clarification by multiomics analysis, which will facilitate our understanding of CSCs; 2) The possible mechanisms by which DBI is inhibited by acetaminophen need verification through additional molecular biology experiments, which will improve treatments against CSCs; and 3) The possibility of combining acetaminophen with antidotes to combat its considerable side effects when used at high doses needs further exploration.
In summary, we demonstrated through scRNA-seq that quiescent CSCs are maintained by DBI and revealed that acetaminophen can be used as an inhibitor of DBI and CSCs. These findings are highly clinically relevant and provide a theoretical basis for a new therapeutic approach of combining acetaminophen with other drugs to treat incurable and recurrent tumors.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
CF and XC designed the study and revised the manuscript. JY and YL preprocessed and interpreted the data, and drafted the manuscript. JY, ML, SL, BZ, RY, and YZ assisted with the statistical analysis of data. CF and XC conducted the experimental guidance. All authors contributed to the article and approved the submitted version.
Funding
The study received funding from the Education Department of Liaoning Province (FWZR2020003), the Scientific Research Funding Project of Education Department of Liaoning Province (No. JCZR2020010), the National Key R&D Program of China (Grant No.2016YFC1303800), and China Postdoctoral Science Foundation (2018 M630310).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.904536/full#supplementary-material
References
- Aghajani M., Mansoori B., Mohammadi A., Asadzadeh Z., Baradaran B. (2019). New Emerging Roles of CD133 in Cancer Stem Cell: Signaling Pathway and miRNA Regulation. J. Cell. Physiol. 234, 21642–21661. 10.1002/jcp.28824 [DOI] [PubMed] [Google Scholar]
- Ahmed M., Jinks N., Babaei-Jadidi R., Kashfi H., Castellanos-Uribe M., May S. T., et al. (2019). Repurposing Antibacterial AM404 as a Potential Anticancer Drug for Targeting Colorectal Cancer Stem-like Cells. Cancers (Basel) 12. 106. 10.3390/cancers12010106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aibar S., González-Blas C. B., Moerman T., Huynh-Thu V. A., Imrichova H., Hulselmans G., et al. (2017). SCENIC: Single-Cell Regulatory Network Inference and Clustering. Nat. Methods 14, 1083–1086. 10.1038/nmeth.4463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso J., Le Magueresse C., Zuccotti A., Khodosevich K., Monyer H. (2012). Diazepam Binding Inhibitor Promotes Progenitor Proliferation in the Postnatal SVZ by Reducing GABA Signaling. Cell Stem Cell 10, 76–87. 10.1016/j.stem.2011.11.011 [DOI] [PubMed] [Google Scholar]
- Aran D., Looney A. P., Liu L., Wu E., Fong V., Hsu A., et al. (2019). Reference-based Analysis of Lung Single-Cell Sequencing Reveals a Transitional Profibrotic Macrophage. Nat. Immunol. 20, 163–172. 10.1038/s41590-018-0276-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr C., von Paleske L., Uslu V. V., Remeseiro S., Takayama N., Ng S. W., et al. (2018). A Myc Enhancer Cluster Regulates Normal and Leukaemic Haematopoietic Stem Cell Hierarchies. Nature 553, 515–520. 10.1038/nature25193 [DOI] [PubMed] [Google Scholar]
- Batlle E., Clevers H. (2017). Cancer Stem Cells Revisited. Nat. Med. 23, 1124–1134. 10.1038/nm.4409 [DOI] [PubMed] [Google Scholar]
- Bayik D., Lathia J. D. (2021). Cancer Stem Cell-Immune Cell Crosstalk in Tumour Progression. Nat. Rev. Cancer 21 (8), 526–536. 10.1038/s41568-021-00366-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellmunt J., Petrylak D. P. (2012). New Therapeutic Challenges in Advanced Bladder Cancer. Seminars Oncol. 39, 598–607. 10.1053/j.seminoncol.2012.08.007 [DOI] [PubMed] [Google Scholar]
- Bochner B. H., Bochner B. H., Kattan M. W., Vora K. C. (2006). Postoperative Nomogram Predicting Risk of Recurrence after Radical Cystectomy for Bladder Cancer. J. Clin. Oncol. 24, 3967–3972. 10.1200/JCO.2005.05.3884 [DOI] [PubMed] [Google Scholar]
- Cai S., Kalisky T., Sahoo D., Dalerba P., Feng W., Lin Y., et al. (2017). A Quiescent Bcl11b High Stem Cell Population is Required for Maintenance of the Mammary Gland. Cell Stem Cell 20, 247–260. 10.1016/j.stem.2016.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. S., Zhang Y., Xu M., Yang Y., Ito M., Peng T., et al. (2013). Distinct Functions for Wnt/β-Catenin in Hair Follicle Stem Cell Proliferation and Survival and Interfollicular Epidermal Homeostasis. Cell Stem Cell 13, 720–733. 10.1016/j.stem.2013.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M. F. (2019). Clinical and Therapeutic Implications of Cancer Stem Cells. N. Engl. J. Med. 380, 2237–2245. 10.1056/nejmra1804280 [DOI] [PubMed] [Google Scholar]
- Clement L. T. (1992). Isoforms of the CD45 Common Leukocyte Antigen Family: Markers for Human T-Cell Differentiation. J. Clin. Immunol. 12, 1–10. 10.1007/bf00918266 [DOI] [PubMed] [Google Scholar]
- Davis A. P., King B. L., Mockus S., Murphy C. G., Saraceni-Richards C., Rosenstein M., et al. (2011). The Comparative Toxicogenomics Database: Update 2011. Nucleic Acids Res. 39, D1067–D1072. 10.1093/nar/gkq813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W.-J., Zhou M., Chen M.-M., Qu C.-Y. (2017). HOXB8 Promotes Tumor Metastasis and the Epithelial-Mesenchymal Transition via ZEB2 Targets in Gastric Cancer. J. Cancer Res. Clin. Oncol. 143, 385–397. 10.1007/s00432-016-2283-4 [DOI] [PubMed] [Google Scholar]
- Du F.-Y., Zhou Q.-F., Sun W.-J., Chen G.-L. (2019). Targeting Cancer Stem Cells in Drug Discovery: Current State and Future Perspectives. World J. Stem Cells 11, 398–420. 10.4252/wjsc.v11.i7.398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H., Liu Y., Gao Z., Huang W. (2021). Recent Advances in Drug Delivery Systems for Targeting Cancer Stem Cells. Acta Pharm. Sin. B 11, 55–70. 10.1016/j.apsb.2020.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman C., Yaqubi K., Hoffmann A., Acikgöz A. A., Korshunov A., Bendszus M., et al. (2019). Acyl-CoA-Binding Protein Drives Glioblastoma Tumorigenesis by Sustaining Fatty Acid Oxidation. Cell Metab. 30, 274–289. 10.1016/j.cmet.2019.04.004 [DOI] [PubMed] [Google Scholar]
- Dumitru I., Neitz A., Alfonso J., Monyer H. (2017). Diazepam Binding Inhibitor Promotes Stem Cell Expansion Controlling Environment-dependent Neurogenesis. Neuron 94, 125–137. 10.1016/j.neuron.2017.03.003 [DOI] [PubMed] [Google Scholar]
- Gawad C., Koh W., Quake S. R. (2016). Single-cell Genome Sequencing: Current State of the Science. Nat. Rev. Genet. 17, 175–188. 10.1038/nrg.2015.16 [DOI] [PubMed] [Google Scholar]
- Guo W., Wang D., Wang S., Shan Y., Liu C., Gu J. (2020). scCancer: A Package for Automated Processing of Single-Cell RNA-Seq Data in Cancer. Brief. Bioinform. 10.1093/bib/bbaa127 [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R. A. (2011). Hallmarks of Cancer: The Next Generation. Cell 144, 646–674. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- Hänzelmann S., Castelo R., Guinney J. (2013). GSVA: Gene Set Variation Analysis for Microarray and RNA-Seq Data. BMC Bioinform. 14, 7. 10.1186/1471-2105-14-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y., Hao S., Andersen-Nissen E., Mauck W. M., Zheng S., Butler A., et al. (2020). Integrated Analysis of Multimodal Single-Cell Data. Cell 184 (13), 3573–3587.e29. 10.1186/1471-2105-14-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris F. T., Rahman S. M. J., Hassanein M., Qian J., Hoeksema M. D., Chen H., et al. (2014). Acyl-coenzyme A-Binding Protein Regulates Beta-Oxidation Required for Growth and Survival of Non-small Cell Lung Cancer. Cancer Prev. Res. 7, 748–757. 10.1158/1940-6207.capr-14-0057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Li J., Wang W., Hu Z., Guan C., Zhao Y., et al. (2020). AR-induced ZEB1-AS1 Represents Poor Prognosis in Cholangiocarcinoma and Facilitates Tumor Stemness, Proliferation and Invasion through Mediating miR-133b/HOXB8. Aging 12, 1237–1255. 10.18632/aging.102680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai M., Hamada J., Takada M., Asano T., Murakawa K., Takahashi Y., et al. (2010). Aberrant Expressions of HOX Genes in Colorectal and Hepatocellular Carcinomas. Oncol. Rep. 23, 843–851. [PubMed] [Google Scholar]
- Knudsen J., Mandrup S., Rasmussen J. T., Andreasen P. H., Poulsen F., Kristiansen K. (1993). The Function of Acyl-CoA-Binding Protein (ACBP)/diazepam Binding Inhibitor (DBI). Mol. Cell Biochem. 123, 129–138. 10.1007/bf01076484 [DOI] [PubMed] [Google Scholar]
- Langfelder P., Horvath S. (2008). WGCNA: An R Package for Weighted Correlation Network Analysis. BMC Bioinforma. 9, 559. 10.1186/1471-2105-9-559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun B., Barbot M., Tonon M. C., Prévot V., Leprince J., Troadec J. D. (2021). Glial Endozepines and Energy Balance: Old Peptides with New Tricks. Glia 69, 1079–1093. 10.1002/glia.23927 [DOI] [PubMed] [Google Scholar]
- Lenis A. T., Lec P. M., Chamie K., Mshs M. (2020). Bladder Cancer: A Review. JAMA 324, 1980–1991. 10.1001/jama.2020.17598 [DOI] [PubMed] [Google Scholar]
- Li Y., Xu X., Song L., Hou Y., Li Z., Tsang S., et al. (2012). Single-cell Sequencing Analysis Characterizes Common and Cell-lineage-specific Mutations in a Muscle-Invasive Bladder Cancer. GigaSci 1, 12. 10.1186/2047-217x-1-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Wu X., Qian Y., Jiang X., Wang Y., Gao J. (2020). PIK3C3 Regulates the Expansion of Liver CSCs and PIK3C3 Inhibition Counteracts Liver Cancer Stem Cell Activity Induced by PI3K Inhibitor. Cell Death Dis. 11, 427. 10.1038/s41419-020-2631-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee J. A., Piskounova E., Morrison S. J. (2012). Cancer Stem Cells: Impact, Heterogeneity, and Uncertainty. Cancer Cell 21, 283–296. 10.1016/j.ccr.2012.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malta T. M., Sokolov A., Gentles A. J., Burzykowski T., Poisson L., Weinstein J. N., et al. (2018). Machine Learning Identifies Stemness Features Associated with Oncogenic Dedifferentiation. Cell 173, 338–354. 10.1016/j.cell.2018.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt S., Solanki M., Spitschak A., Vera J., Pützer B. M. (2018). Emerging Functional Markers for Cancer Stem Cell-Based Therapies: Understanding Signaling Networks for Targeting Metastasis. Seminars Cancer Biol. 53, 90–109. 10.1016/j.semcancer.2018.06.006 [DOI] [PubMed] [Google Scholar]
- Nguyen L. V., Vanner R., Dirks P., Eaves C. J. (2012). Cancer Stem Cells: an Evolving Concept. Nat. Rev. Cancer 12, 133–143. 10.1038/nrc3184 [DOI] [PubMed] [Google Scholar]
- Ooki A., Vanden Bussche C. J., Kates M., Hahn N. M., Matoso A., McConkey D. J., et al. (2018). CD24 Regulates Cancer Stem Cell (CSC)-like Traits and a Panel of CSC-Related Molecules Serves as a Non-invasive Urinary Biomarker for the Detection of Bladder Cancer. Br. J. Cancer 119, 961–970. 10.1038/s41416-018-0291-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega M. A., Poirion O., Zhu X., Huang S., Wolfgruber T. K., Sebra R., et al. (2017). Using Single-Cell Multiple Omics Approaches to Resolve Tumor Heterogeneity. Clin. Transl. Med. 6, 46. 10.1186/s40169-017-0177-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingali P., Wu Y. J., Boothello R., Sharon C., Li H., Sistla S., et al. (2021). High Dose Acetaminophen Inhibits STAT3 and has Free Radical Independent Anti-cancer Stem Cell Activity. Neoplasia 23, 348–359. 10.1016/j.neo.2021.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plass M., Solana J., Wolf F. A., Ayoub S., Misios A., Glažar P., et al. (2018). Cell Type Atlas and Lineage Tree of a Whole Complex Animal by Single-Cell Transcriptomics. Science 360, eaaq1723. 10.1126/science.aaq1723 [DOI] [PubMed] [Google Scholar]
- Reddy K. B. (2020). Stem Cells: Current Status and Therapeutic Implications. Genes. (Basel) 11. 1372. 10.3390/genes11111372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T., Morrison S. J., Clarke M. F., Weissman I. L. (2001). Stem Cells, Cancer, and Cancer Stem Cells. Nature 414, 105–111. 10.1038/35102167 [DOI] [PubMed] [Google Scholar]
- Rindler K., Bauer W. M., Jonak C., Wielscher M., Shaw L. E., Rojahn T. B., et al. (2021). Single-Cell RNA Sequencing Reveals Tissue Compartment-specific Plasticity of Mycosis Fungoides Tumor Cells. Front. Immunol. 12, 666935. 10.3389/fimmu.2021.666935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfakianos J. P., Daza J., Hu Y., Anastos H., Bryant G., Bareja R., et al. (2020). Epithelial Plasticity Can Generate Multi-Lineage Phenotypes in Human and Murine Bladder Cancers. Nat. Commun. 11, 2540. 10.1038/s41467-020-16162-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloway M. S. (2013). Bladder Cancer: Lack of Progress in Bladder Cancer-What are the Obstacles? Nat. Rev. Urol. 10, 5–6. 10.1038/nrurol.2012.219 [DOI] [PubMed] [Google Scholar]
- Vider B.-Z., Zimber A., Chastre E., Gespach C., Halperin M., Mashiah P., et al. (2000). Deregulated Expression of Homeobox-Containing Genes, HOXB6, B8, C8, C9, and Cdx-1, in Human Colon Cancer Cell Lines. Biochem. Biophys. Res. Commun. 272, 513–518. 10.1006/bbrc.2000.2804 [DOI] [PubMed] [Google Scholar]
- Visvader J. E., Lindeman G. J. (2012). Cancer Stem Cells: Current Status and Evolving Complexities. Cell Stem Cell 10, 717–728. 10.1016/j.stem.2012.05.007 [DOI] [PubMed] [Google Scholar]
- Wang L., Sebra R. P., Sfakianos J. P., Allette K., Wang W., Yoo S., et al. (2020). A Reference Profile-free Deconvolution Method to Infer Cancer Cell-Intrinsic Subtypes and Tumor-type-specific Stromal Profiles. Genome Med. 12, 24. 10.1186/s13073-020-0720-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Gong P., Chen T., Gao S., Wu Z., Wang X., et al. (2021). Colorectal Cancer Stem Cell States Uncovered by Simultaneous Single‐Cell Analysis of Transcriptome and Telomeres. Adv. Sci. 8, 2004320. 10.1002/advs.202004320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. J., Neuwelt A. J., Muldoon L. L., Neuwelt E. A. (2013). Acetaminophen Enhances Cisplatin- and Paclitaxel-Mediated Cytotoxicity to SKOV3 Human Ovarian Carcinoma. Anticancer Res. 33, 2391–2400. [PMC free article] [PubMed] [Google Scholar]
- Wu F., Fan J., He Y., Xiong A., Yu J., Li Y., et al. (2021). Single-cell Profiling of Tumor Heterogeneity and the Microenvironment in Advanced Non-small Cell Lung Cancer. Nat. Commun. 12, 2540. 10.1038/s41467-021-22801-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Z., Yang Q., Li X. (2020). Effect of Intra‐ and Inter‐tumoral Heterogeneity on Molecular Characteristics of Primary IDH‐wild Type Glioblastoma Revealed by Single‐cell Analysis. CNS Neurosci. Ther. 26, 981–989. 10.1111/cns.13396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G., Wang L.-G., Han Y., He Q.-Y. (2012). clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS A J. Integr. Biol. 16, 284–287. 10.1089/omi.2011.0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X., Liu C., Yao J., Wan H., Wan G., Li Y., et al. (2021). Breast Cancer Stem Cells, Heterogeneity, Targeting Therapies and Therapeutic Implications. Pharmacol. Res. 163, 105320. 10.1016/j.phrs.2020.105320 [DOI] [PubMed] [Google Scholar]
- Zhang B., Horvath S. (2005). A General Framework for Weighted Gene Co-expression Network Analysis. Stat. Appl. Genet. Mol. Biol. 4, Article17. 10.2202/1544-6115.1128 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Zhuang J., Deng Y., Yang L., Cao W., Chen W., et al. (2017). miR34a/GOLPH3 Axis Abrogates Urothelial Bladder Cancer Chemoresistance via Reduced Cancer Stemness. Theranostics 7, 4777–4790. 10.7150/thno.21713 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.