Allergen immunotherapy (AIT) used for patients with allergic rhinitis has been a mainstay of treatment for more than a century.1 The mechanisms underlying the efficacy of AIT, or lack thereof, are only partially understood. For several decades, one hypothesis has been that immunotherapy induces IgG antibody production to suppress the patient’s response to allergenic proteins. It is thought that these IgG antibodies bind to epitopes on allergens that in turn block the allergen’s access to cell surface IgE on mast cells and basophils.2,3 This behaviour has led to the term blocking antibodies. However, the ability of an IgG antibody to interfere with allergen binding to cell-bound IgE is only one way that IgG could potentially inhibit cell secretion. IgG-allergen complexes could interact with the inhibitory receptor (CD32b, FcgRIIb) on basophils and mast cells, co-crosslinking with surface IgE and down-regulating the allergen-driven signalling reaction.4,5
Recently, we examined the question of whether CD32b interacted with the subclasses of IgG differently.6 Early studies working with mouse IgG subclasses had uncovered distinct interactions between the various subclasses and murine CD32b,7 so we examined this behaviour in the context of the inhibition of the human basophil response, a leukocyte that expresses CD32b. Our studies showed that human IgG1 has very little functional interaction with CD32b, IgG4 had none1 and only IgG2 and IgG3 allow CD32b to act as a strong inhibitor of IgE-mediated secretion, with IgG3 being 10-fold more efficacious than IgG2 in blocking the IgE-mediated response.
Historically, studies of immunotherapy only measure IgG4 or both IgG1 and IgG4 and rarely examine IgG2 or IgG3 (e.g. see the early studies of8,9). The collective decision to focus on IgG1/4 largely took place before there was knowledge of CD32 inhibitory receptors that could modulate the IgE-mediated reaction. There were early studies examining all subclasses, although these assays had limitations. In addition, while there have been a few more recent studies (e.g. see10) examining either IgG2 or IgG3 subclasses in mechanistic studies of allergic subjects or AIT, most studies do not. In light of the functional studies in which IgG2/3 subclasses are most effective in engaging CD32b, this oversight is unfortunate. To study the efficacy of immunotherapy without knowing the changes that occur in the levels of these two subclasses, which are potentially important for suppressing the IgE-mediated response at the cellular level, could be problematic.
A critical question is whether the fraction of allergen-specific IgG2 and IgG3 in a subject’s serum can be predicted from knowledge of IgG1 and IgG4 subclass fractions. For example, if there were a constant proportion of IgG2 and IgG3 antibodies relative to IgG1 or IgG4, then it would be possible to extrapolate from prior studies as to the potential levels of IgG2 and IgG3. Therefore, a first step is to determine the population variability in the relative proportions of the 4 subclasses of IgG to allergens. Two metrics were of particular interest, the constancy of the ratio of IgG2/3 to IgG1/4 and the absolute concentration of IgG2 and IgG3. These values are relevant because these are the two metrics that most influence the role of CD32b in inhibiting IgE-mediated secretion.6 The following study was not designed to answer questions about the role of IgG2 or IgG3 in AIT but to promote further interest in whether future studies should examine these two subclasses.
An ELISA methodology was developed using established WHO-approved anti-subclass antibodies to make measurements of allergen-specific subclasses. Pilot studies described in the online repository (see Supplementary Material) addressed several issues with allergen-specific IgG assays. In particular, subclass transfectoma antibodies were used to cross-calibrate the subclasses and establish a quantitative assessment of the presence of the four subclasses in a particular serum.
Two ELISAs were developed, ragweed (Ambrosia artemisiifolia) and dust mite (for dust mite, a combination Dermatophagoides farinae [Df] and Dermatophagoides pteronyssinus [Dp]). The dust mite assay was included to also partially address whether the relationship between IgG2/3 and IgG1/4 was similar for two distinct allergens. Dust mite allergens may shape a different immune response due to differences in their content of proteolytic enzymes intrinsic to this allergen compared with the proteolytic enzymes intrinsic to ragweed allergens.
Figure 1 shows results from 30 AIT-naïve atopic subjects (without a prior history of subcutaneous or sublingual allergen immunotherapy), nine AIT-treated atopic subjects and 13 subjects who did not report allergies. The range of proportionality is large for IgG2 and IgG3; min -> max for the ragweed-specific IgG2 proportion of the total ragweed-specific IgG was 0.00->0.91 and for ragweed-specific IgG3 was 0.00->0.21 (Figure 1A). Figure 1A separates the AIT-naïve atopic, AIT-treated and non-atopic subjects as three distinct distributions. To make a point about functional equivalency, an additional distribution is added to the IgG3 columns of symbols. As noted, IgG3 is 10-fold more efficacious than IgG2, so the fractional IgG3 levels are expanded by 10-fold and the result shows that the “functional” IgG3 distribution is similar to the range of proportions for IgG2. Figure 1A also shows the proportions for subjects with a history of AIT (i.e. “post ITX A”). There is a suggestion that ragweed-specific IgG1 increases at the expense of the other subclasses but this did not replicate with dust mite (see below). A future study will be necessary to confirm this observation. Nonetheless, the overall subclass distribution remains broad in this group; IgG2 ranges from 0.02 to 0.40 (and 0.03–0.73 for dust mite, discussed next).
FIGURE 1.
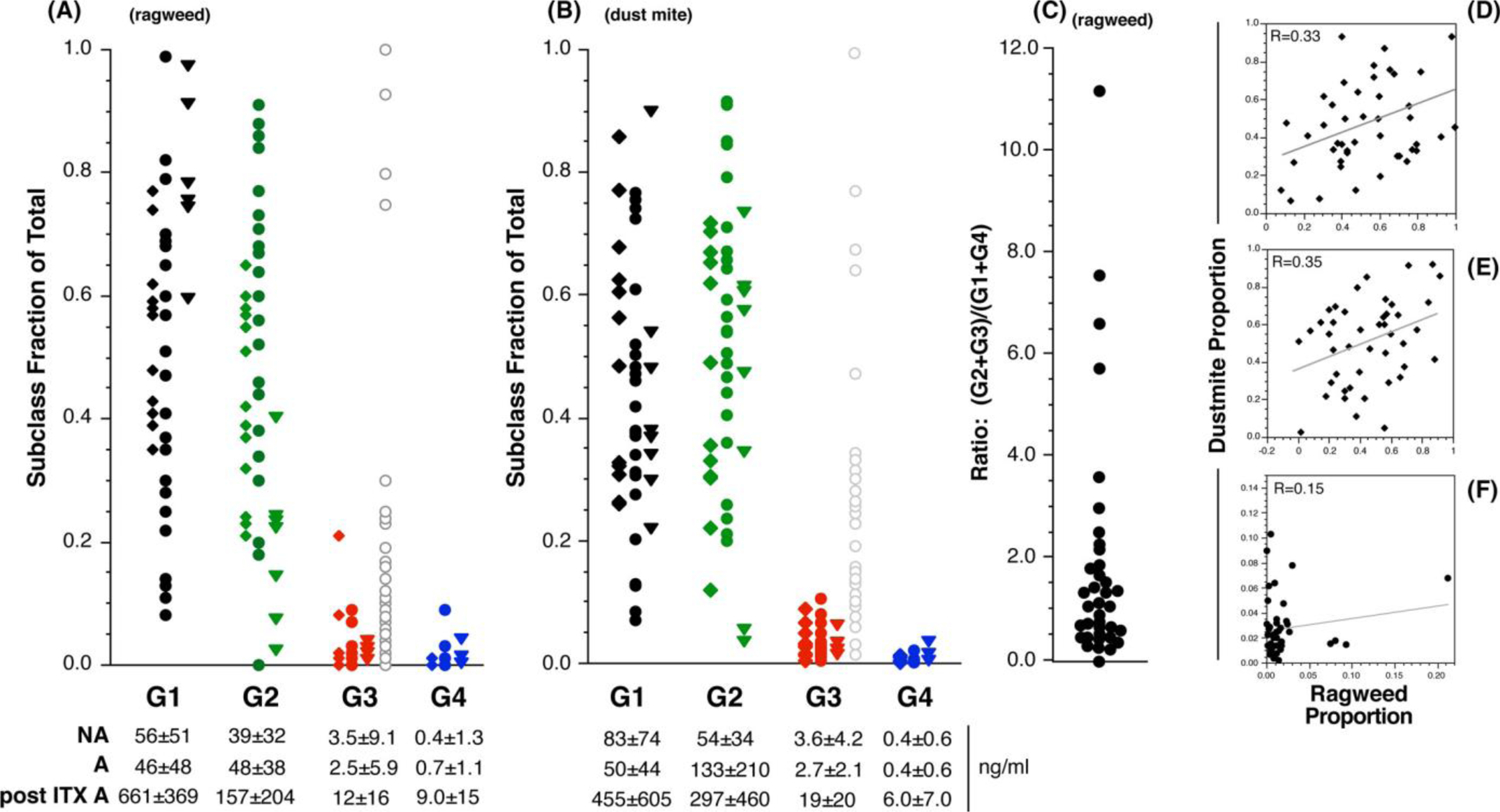
Subclasses of allergen-specific IgG: Serum IgG2 and IgG3 levels are not predicted by IgG1/IgG4 levels
Subclass proportion—population distribution. Panel A; proportions of IgG1, IgG2, IgG3 and IgG4 in atopic and non-atopic subjects for ragweed-specific IgG. The diamond symbols represent non-atopic subjects, the circle symbols represent atopic subjects and the triangle symbols represent atopic subjects with a reported history of AIT. For the IgG3 data set, a fourth column (represented by the grey open circle symbols), the proportion data were transformed by multiplying by 10 to account the higher functional effect of IgG3. Absolute average ragweed- or dust mite-specific subclass concentrations (± S.D.) are shown below the x-axis where A = allergic, NA = non-allergic and post ITX A = allergics with a reported history of AIT. Panel B shows subclass proportion distributions for dust mite-specific IgG in the three categories of subjects. Panel C; Distribution of the ratio of ragweed-specific (IgG2+IgG3)/(IgG1+IgG4) for the non-atopic and AIT-naïve atopics. Panels D-F show the correlation between ragweed- vs. dust mite-specific IgG1, 2 and 3 proportions, respectively; R = .331, p = .028; R = .353, p = .019; R = .151, p = .312, respectively
The range of proportionality for dust mite-specific IgG2 and IgG3 (Figure 1B) was similar to ragweed. Figure 1A,B also show the average levels of the various IgG subclasses. Based on our in vitro studies of CD32b-mediated inhibition in basophils, the average absolute levels in AIT-naïve subjects are not high enough to mediate significant inhibition. However, the high end of these distributions in AIT-naïve subjects and AIT-treated subjects are capable of mediating inhibition. Generally, 500 ng/ml and 50 ng/ml for IgG2 and IgG3, respectively, can drive CD32b-mediated inhibition in in vitro basophil assays. It is also notable that the functional levels of IgG3 are very similar to IgG2 because of the 10-fold difference in efficacy. A future immunotherapy study will determine whether these treatment-based elevations attain levels sufficient to deduce that interaction with CD32b is likely to inhibit IgE-mediated secretion.
To emphasize that IgG2 and IgG3 levels cannot be predicted from IgG1 and IgG4 information, Figure 1C summarizes the broad distribution in the (G2+3)/(G1+4) ratio. Figure 1D–F, respectively, shows the correlation between IgG1, IgG2 and IgG3 proportions specific for ragweed vs. dust mite. The IgG1 and IgG2 correlations are relatively weak (r2≈.10), and these data suggest that for the IgG3 response that the proportional response to one allergen does not predict the proportional response to another allergen (there was also no correlation for IgG4 but the IgG4 concentrations were quite low).
Our use of allergens or allergen components that were not highly purified for the ELISA plating could introduce a bias in the results since our reagents may contain substances not necessarily relevant to the IgE-mediated reaction, that is selective off-target IgG subclass antibodies may skew the subclass profiles. Absent this possibility, our results suggest that it is not possible to extrapolate from IgG1/4 measurements to IgG2/3 levels. The proportionality of IgG2/3 is significantly variable, ranging from near 0%–90% of the total IgG. For IgG3, the proportionality also differs considerably between the two allergens tested, making it more challenging to extrapolate from one allergen to another. The large range of proportionalities for IgG2 and IgG3 further suggest that a proper understanding of the role of blocking antibodies in future mechanistic studies of AIT requires measurements of IgG2 and IgG3 subclasses to ascertain the role of CD32b inhibitory receptors in the biology of allergen immunotherapy.
*In our published study,6 IgG4 concentrations were limited but studies in mice also demonstrate the absence of IgG4 binding to CD32b.
Supplementary Material
Key messages.
The study suggests that without explicitly measuring allergen-specific IgG2 and IgG3 subclass antibodies, one cannot extrapolate from IgG1 or IgG4 their concentrations in serum.
Because IgG2 and IgG3 may be important for CD32b-mediated inhibition of basophil and mast cell secretion, future studies of “blocking” antibodies should include their measurement.
ACKNOWLEDGEMENTS
These studies were supported by NIH grants AI100952 and AI139326.
Footnotes
1In our published study 6, IgG4 concentrations were limited but studies in mice also demonstrate the absence of IgG4 binding to CD32b.
CONFLICT OF INTEREST
There is no conflict of Interest to declare.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Noon L. Prophylactic inoculation against hay fever. Lancet. 1911;4:1572. [DOI] [PubMed] [Google Scholar]
- 2.Huber S, Lang R, Steiner M, et al. Does clinical outcome of birch pollen immunotherapy relate to induction of blocking antibodies preventing IgE from allergen binding? a pilot study monitoring responses during first year of AIT. Clin Transl Allergy. 2018;8:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vizzardelli C, Gindl M, Roos S, et al. Blocking antibodies induced by allergen-specific immunotherapy ameliorate allergic airway disease in a human/mouse chimeric model. Allergy. 2018;73:851–861. [DOI] [PubMed] [Google Scholar]
- 4.Cady CT, Powell MS, Harbeck RJ, et al. IgG antibodies produced during subcutaneous allergen immunotherapy mediate inhibition of basophil activation via a mechanism involving both FcgammaRIIA and FcgammaRIIB. Immunol Lett. 2010;130:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strait RT, Morris SC, Finkelman FD. IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and Fc gamma RIIb cross-linking. J Clin Invest. 2006;116:833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacGlashan D Jr., Hamilton RG. Parameters determining the efficacy of CD32 to inhibit activation of FcepsilonRI in human basophils. J Allergy Clin Immunol. 2016;137(4):1256–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warmerdam PA, van de Winkel JG, Vlug A, Westerdaal NA, Capel PJ. A single amino acid in the second Ig-like domain of the human Fc gamma receptor II is critical for human IgG2 binding. J Immunol. 1991;147:1338–1343. [PubMed] [Google Scholar]
- 8.Djurup R, Osterballe O. IgG subclass antibody response in grass pollen-allergic patients undergoing specific immunotherapy. Prognostic value of serum IgG subclass antibody levels early in immunotherapy. Allergy. 1984;39:433–441. [DOI] [PubMed] [Google Scholar]
- 9.Søndergaard I, Djurup R, Weeke B. Characterization of IgG-subclass antibodies to individual allergens by crossed radioimmunoelectro-phoresis. Allergy. 1984;39:622–629. [DOI] [PubMed] [Google Scholar]
- 10.Sugimoto M, Kamemura N, Nagao M, et al. Differential response in allergen-specific IgE, IgGs, and IgA levels for predicting outcome of oral immunotherapy. Pediatr Allergy Immunol. 2016;27:276–282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


