Highlights
-
•
The first meta-analysis to comprehensively evaluate the effects of taurine supplementation on diabetic patients.
-
•
Statistical significance in HbA1C, Fasting Blood Sugar, HOMA-IR after oral supplemental of taurine by diabetic patients than that of placebo.
-
•
Taurine is expected to be a new option for the management of diabetes.
Keywords: Taurine, Diabetes mellitus, Meta-analysis
Abstract
Objective
The ameliorative effect of taurine on diabetes has received extensive attention in recent years. Despite promising data from animal studies, the efficacy of taurine supplementation in human studies has been inconsistent. We thus did a meta-analysis of randomized controlled trials to assess the effect of taurine supplement on glycemic indices, serum lipids, blood pressure, body composition in patients with diabetes.
Methods
We systematically searched PubMed, Embase, Cochrane, Web of Science, FDA.gov, and ClinicalTrials.gov for randomized controlled trials (published from inception to January 15, 2022; no language restrictions) about the effect of taurine supplement on diabetes. Values of Standardized Mean Differences (SMD) were determined for continuous outcomes.
Results
Of 2206 identified studies, 5 randomized controlled trials were eligible and were included in our analysis (N = 209 participants). Compared with the control group, taurine could significantly reduce HbA1c (SMD −0.41[95% CI: −0.74, −0.09], p = 0.01), Fasting Blood Sugar (SMD − 1.28[95% CI: −2.42, −0.14], p = 0.03) and HOMA-IR (SMD − 0.64[95% CI: −1.22, −0.06], p = 0.03). In addition, taurine also reduced Insulin (SMD −0.48 [95% CI: −0.99, 0.03], p = 0.06) and TG (SMD −0.26 [95% CI: −0.55, 0.02], p = 0.07), but did not reach statistical significance.
Conclusions
Taurine supplementation is beneficial in reducing glycemic indices, such as HbA1c, Fasting Blood Sugar, HOMA-IR in diabetic patients, but has no significant effect on serum lipids, blood pressure and body composition in diabetic patients. Taurine emerges as a new option for the management of patients with diabetes. Further studies are needed to understand the potential effect of taurine in diabetic patients.
1. Introduction
Diabetes is one of the most common metabolic diseases in the world, of which type 1 diabetes is an autoimmune disease characterized by hyperinsulinemia and hyperglycemia (Maahs et al., 2010, Turner and La Gruta, 2022), Type 2 diabetes is a progressive disease characterized by insulin resistance. According to the International Diabetes Federation (IDF), around 693 million adults will be affected by diabetes by 2045 (Cho et al., 2018). Diabetic complications are common in people with type 1 or type 2 diabetes and are a significant cause of morbidity and mortality. The chronic complications of diabetes are roughly divided into two categories: microvascular and macrovascular, in which the incidence of microvascular is much higher than that of macrovascular. Microvascular complications include neuropathy, nephropathy, and retinopathy. Macrovascular complications include cardiovascular disease, stroke, and peripheral arterial disease. Diabetic foot syndrome is the leading cause of lower extremity amputations (Papatheodorou, Banach, Bekiari, Rizzo, & Edmonds, 2018). Diabetes imposes a huge burden on the health care system while seriously affecting the overall life quality of patients (Shi, Fonseca, & Childs, 2021). As a complex metabolic disease, researchers are always looking for new treatments that can effectively slow down the progression of diabetes while minimizing toxic side effects.
Taurine is a sulfonate-containing beta-amino acid isolated from bovine bile (Lourenço & Camilo, 2002). Taurine is widely distributed in various tissues and organs, especially in excitable tissues, where the content is more abundant, such as the brain, heart and skeletal muscle (De Luca et al., 2015, Kp and Martin, 2022, Schuller-Levis and Park, 2003).As a naturally occurring amino acid, taurine has few side effects, and current studies have not found any genotoxic, carcinogenic, or teratogenic effects (Ripps & Shen, 2012). Due to its good safety, taurine is widely used in functional drinks (Jagim et al., 2022), infant formula (Almeida et al., 2021) and other products. Meat, especially seafood products, are rich in taurine (Mendivil, 2021). Taurine plays beneficial roles in a variety of metabolic and physiological processes, such as glucose and lipid regulation, energy metabolism, anti-inflammatory regulation and antioxidation (Kim et al., 2012). Taurine has certain functions in cell development, nutrition and survival (Ripps & Shen, 2012), the depletion of taurine leads to a wide range of pathological conditions, including severe cardiomyopathy (Zulli, 2011), renal dysfunction (Yamori et al., 2010), pancreatic β cell malfunction (L'Amoreaux et al., 2010), and loss of retinal photoreceptors (Schmidt, Berson, & Hayes, 1976). Taurine has been used as a potential energy enhancer to improve exercise performance. It is worth noticing that several factors such as taurine intake time, delivery mode and exercise program will affect the effect of taurine on exercise performance (Kurtz, VanDusseldorp, Doyle, & Otis, 2021). Taurine has a wide range of anti-inflammatory effect (Park, Quinn, Wright, & Schuller-Levis, 1993). Taurine supplements are beneficial to epilepsy (Oja & Saransaari, 2013), heart disease (Wójcik, Koenig, Zeleniuch-Jacquotte, Costa, & Chen, 2010), cystic fibrosis (“Taurine supplementation in cystic fibrosis,” 1988) and diabetes (Caine & Geracioti, 2016). Taurine is a major antioxidant that scavenges reactive oxygen species and protects organs, including the brain (Oja & Saransaari, 2017), from oxidative stress. It has neuroprotective effects and has been shown in animal studies to prevent neurotoxic damage caused by alcohol, ammonia, lead and other substances. Taurine is considered to be a modulator of neuronal activity. It is structurally similar to the main inhibitory neurotransmitters in the brain γ- Aminobutyric acid (Caine & Geracioti, 2016).
The protective effect of taurine on diabetes and its complications has been demonstrated in many animal model (El Zahraa et al., 2012, Kim et al., 2012, Mohamed and Gawad, 2017). Multiple studies have found that plasma taurine concentration is inversely correlated with fasting blood sugar (FBS) and diabetic complications, suggesting that taurine has a protective role in the progression of diabetes (Franconi et al., 1994, Sak et al., 2019). A large number of animal experiments have shown that taurine can improve various diabetic complications, including endothelial dysfunction, diabetic nephropathy, diabetic retinopathy, diabetic cataract, diabetic neuropathy, diabetic cardiomyopathy, and so on (Ito, Schaffer, & Azuma, 2012). Although a number of controlled clinical trials have been conducted to study the effects of taurine supplementation in patients with DM (diabetes mellitus), the efficacy of taurine supplementation for DM in human studies has been inconsistent. Recognizing that individual studies might not be able to provide sufficient data on clinical practice, we sought to objectively assess the potential role of taurine in the management of diabetes. There is no systematic review on RCTs (randomized controlled trials) of the effect of taurine on various indicators of diabetes mellitus in human studies. Therefore, we conducted a systematic review and meta-analysis to examine the effects of taurine on glycemic indices, serum lipids, blood pressure and body composition in patients with diabetes.
2. Methods
2.1. Search strategy and selection criteria
This meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement and was registered at International Prospective Register of Systematic Reviews (number CRD42022307677).
We selected relevant studies published from inception to January 15, by searching Embase, PubMed, Cochrane, Web of Science and ClinicalTrials.gov. We used the following combined text and MeSH terms: “taurine” and “diabetes Mellitus”. The complete search used for Web of Science was: “TS = (Taurine OR Tauphon OR Taufon OR Taurine Hydrochloride)” and “TS = (Diabetes Mellitus OR Diabetes Insipidus OR Prediabetic State OR Scleredema Adultorum OR Glucose Intolerance OR Gastroparesis)”. We considered all potentially eligible studies for review, irrespective of the primary outcome or language.
Two independent investigators reviewed study titles and abstracts, and studies that satisfied the inclusion criteria were retrieved for full-text assessment. Trials selected for detailed analysis and data extraction were analyzed by two investigators, and the differences were resolved by consensus or the discussion with the third independent author. Inclusion criteria: RCTs on the efficacy of taurine in the treatment of DM patients. Only original articles were included. Exclusion criteria were as follows: non-human studies, non-RCTs, systematic reviews or meta-analyses.
2.2. Study selection and data extraction
Two authors independently extracted relevant data from each study into a pre-designed Excel spreadsheet, which included country of origin, year of publication, first author, trial design, inclusion criteria, study duration, study population, intervention and duration, participant gender and age, baseline patient information, and treatment outcomes. The outcomes included was serum lipids (TG, TC, HDL, LDL), glycemic indices (HbA1c, FBS, Insulin, HOMA-IR), Body Composition (Weight, BMI, Energy Intake, Protein, Fat, Carbohydrate, Waist Circumference) and blood pressure (SBP, DBP). For continuous variables, we extracted the mean and Standard Deviation (SD). In the absence of means and SD, the data were transformed according to the existing formulae. Two independent reviewers assessed risk for bias according to the PRISMA recommendations. Differences are resolved independently by the third author.
2.3. Statistical analysis
Review Manager 5.4.1 was used for statistical analysis. For continuous variables, we used Standardized Mean Difference (SMD) and 95% CI for analysis. The I2 statistic was used to evaluate heterogeneity. I2 values of 25%, 50% and 75% were considered low, medium and high heterogeneity, respectively. Random effects models were used to pool measures in all studies. P values less than 0.05 were considered statistically significant. Risk of publication bias for studies will be assessed using Funnel plot. Quality of the RCT was assessed using the Cochrane Risk of Bias Assessment Tool, including the following seven criteria: random sequence generation, allocation concealment, blinding of patients, trial lists, blinding of outcome assessors, incomplete outcome data, selective reporting, and other biases. Each item was assessed for risk of bias as 'low risk', 'high risk' or 'unclear risk' according to the recommendations of the Cochrane Handbook.
3. Results
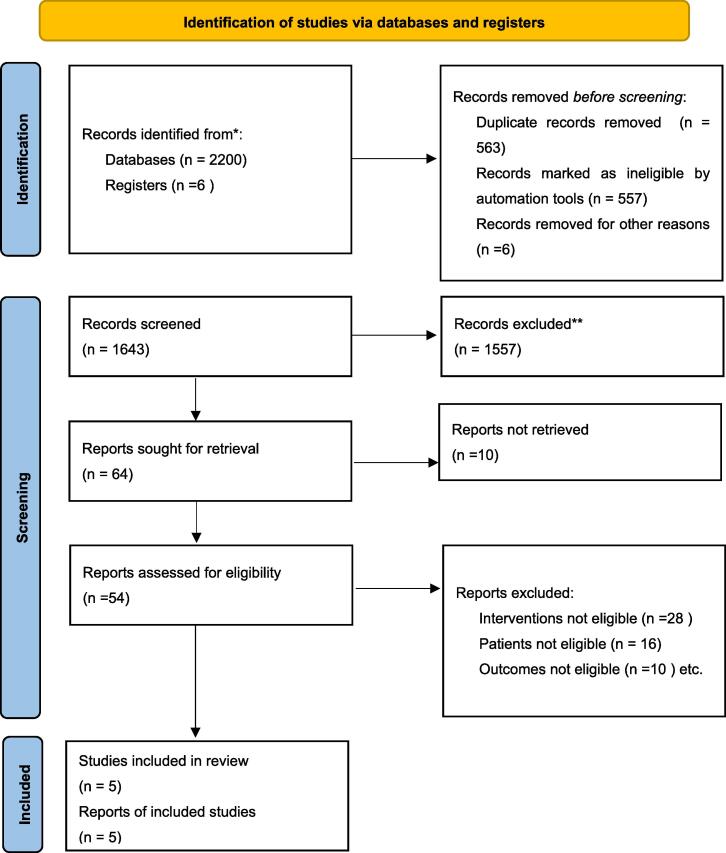
The literature search identified 2206 studies, of which 471 studies were from PubMed, 37 were from the Cochrane Library, 902 were from Embase, 790 were from the Web of Science and 6 were from clinical trials. After excluding 563 duplicates, reviewing 1643 titles and abstracts, 1557 outcomes were excluded, and the remaining 86 outcomes were sought for retrival, The full text of 10 articles can not be obtained, 54 articles were assessed in detain, of which 28 studies were excluded for interventions not eligible, 16 studies were excluded for patients not eligible, 10 studies were excluded for outcomes not eligible, resulting in a total of 5 randomized controlled studies of taurine for diabetes included in the meta-analysis (Esmaeili et al., 2021, Moloney et al., 2010, Premanath et al., 2019, Samadpour Masouleh et al., 2021, Svarovskaya and Garganeeva, 2018). The study selection process is summarized in the PRISMA flow chart (Fig. 1).
Fig. 1.
PRISMA flow chart.
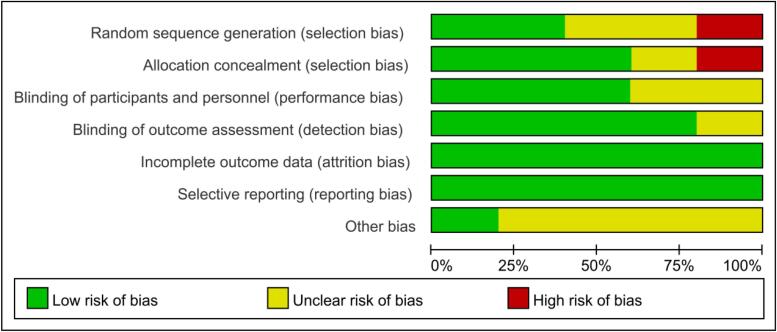
Five studies were conducted in 2010–2021, all five studies were RCTs with a total of 219 participants (trial group 123 participants used taurine and control group 96 participants used placebo), all patients were diagnosed with DM (Four of the studies patients had Type 2 Diabetes and one was Type 1 Diabetes), two of the included studies were conducted in Iran and the remaining three were conducted in India, Ireland, Russia, the duration of interventions ranged from 2 to 16 weeks (Supplementary 1). All trials were parallel group studies with high quality according to the Cochrane Risk of Bias tool. Quality assessment results of the included studies are summarized in Fig. 2.
Fig. 2.
Quality assessment of included studies.
3.1. The effect of taurine on serum lipids
3.1.1. Triglyceride (TG)
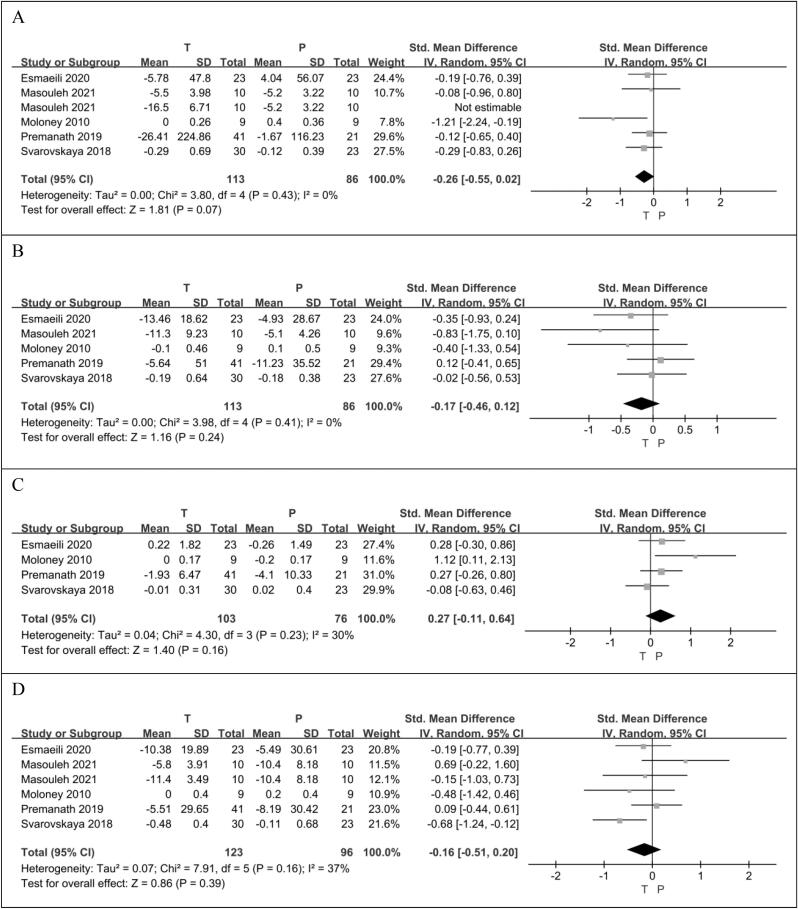
One group of data in the RCT article published by Masouleh.S et al. results in a high heterogeneity (p = 0.02, I2 = 61%) of the overall analysis results (SMD −0.51 [95% CI: −0.98, −0.04], p = 0.04). After excluding this group of data, the heterogeneity was reduced to zero, so this group of data was not included. A total of five RCTs (Esmaeili et al., 2021, Moloney et al., 2010, Premanath et al., 2019, Samadpour Masouleh et al., 2021, Svarovskaya and Garganeeva, 2018), reported, in a total of 199 patients, 113 taurine users and 86 non-users, that taurine had the potential to reduce TG compared to controls (SMD −0.26 [95% CI: −0.55, 0.02], p = 0.07), but the difference was not statistically significant. There was no heterogeneity between studies (p = 0.43, I2 = 0%) (Fig. 3A).
Fig. 3.
The effect of taurine on TG (A); the effect of taurine on TC (B); the effect of taurine on HDL (C);the effect of taurine on LDL (D).
3.1.2. Total cholesterol (TC)
A total of five RCTs (Esmaeili et al., 2021, Moloney et al., 2010, Premanath et al., 2019, Samadpour Masouleh et al., 2021, Svarovskaya and Garganeeva, 2018), reported, in a total of 199 patients, 113 taurine users and 86 non-users, that taurine had the potential to reduce TC compared to controls, but the difference was not statistically significant (SMD −0.17 [95% CI: −0.46, 0.12], p = 0.24). There was no heterogeneity between studies (p = 0.41, I2 = 0%) (Fig. 3B).
3.1.3. High-Density lipoprotein (HDL)
Four RCTs (Esmaeili et al., 2021, Moloney et al., 2010, Premanath et al., 2019, Svarovskaya and Garganeeva, 2018) reported the HDL levels of 179 patients, 103 taurine users and 76 non-users. There was no significant difference in HDL level increase between the taurine group and the control group. (SMD 0.27 [95%CI: −0.11, 0.64], p = 0.16). There was no significant difference in heterogeneity between the included studies (p = 0.23, I2 = 30%) (Fig. 3C).
3.1.4. Low-Density lipoprotein (LDL)
A total of five RCTs (Esmaeili et al., 2021, Moloney et al., 2010, Premanath et al., 2019, Samadpour Masouleh et al., 2021, Svarovskaya and Garganeeva, 2018), reported on LDL in 219 patients, 123 taurine users and 96 non-users. Compared with the control group, taurine was more likely to reduce LDL levels, but the statistical difference was not significant (SMD −0.16 [95% CI: −0.51, 0.20], p = 0.39). There was no significant difference in heterogeneity between the included studies (p = 0.16, I2 = 37%) (Fig. 3D).
3.2. The effect of taurine on glycemic indices
3.2.1. Glycosylated hemoglobin A1c (HbA1c)
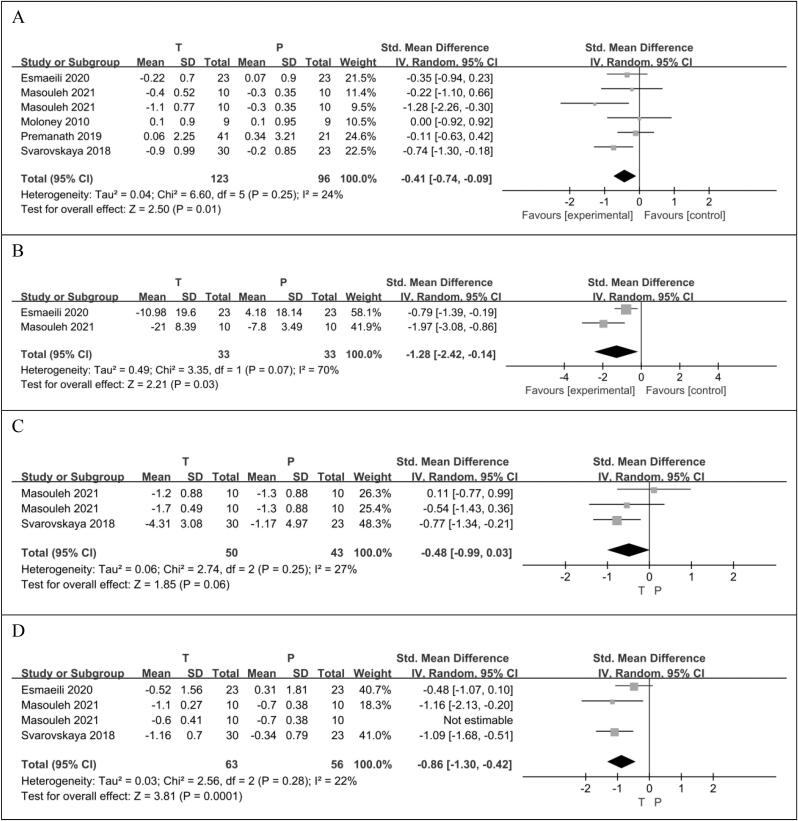
Five RCTs (Esmaeili et al., 2021, Moloney et al., 2010, Premanath et al., 2019, Samadpour Masouleh et al., 2021, Svarovskaya and Garganeeva, 2018) reported HbA1c in 219 patients, 123 taurine users and 96 taurine non-users. There was statistically significant difference in HbA1c reduction between the taurine group and the control group (SMD −0.41 [95%CI: −0.74, −0.09], p = 0.01). There was no significant difference in heterogeneity between the included studies (p = 0.25, I2 = 24%) (Fig. 4A).
Fig. 4.
The effect of taurine on HbA1c (A); the effect of taurine on FBS (B); the effect of taurine on insulin (C); the effect of taurine on HOMA-IR (D).
3.2.2. Fasting blood Sugar (FBS)
Two RCTs (Esmaeili et al., 2021, Samadpour Masouleh et al., 2021) reported on FBS levels in 66 patients, 33 taurine users and 33 taurine non-users. There was statistically significant difference in FBS reduction between the taurine group and the control group (SMD −1.28 [95% CI: −2.42, −0.14], p = 0.03). There was significant difference in heterogeneity between the included studies (p = 0.07, I2 = 70%) (Fig. 4B).
3.2.3. Insulin
Two RCTs (Samadpour Masouleh et al., 2021, Svarovskaya and Garganeeva, 2018) reported insulin levels in 93 patients, 50 taurine users and 43 non-users. Compared with the control group, taurine may reduce insulin levels, but the difference was not statistically significant (SMD −0.48 [95%CI: −0.99,0.03], p = 0.06). There was no significant difference in heterogeneity between the included studies (p = 0.25, I2 = 27%) (Fig. 4C).
3.3. Homeostatic model assessment for homeostatic model assessment for insulin resistance (HOMA-IR)
One group of data in the RCT article published by Masouleh et al. results in a high heterogeneity (p = 0.06, I2 = 60%) of the overall analysis results (SMD-0.64 [95% CI: −1.22,-0.06], p = 0.03). After excluding this group of data, the heterogeneity was reduced to 22%, so this group of data was not included. Three RCTs (Esmaeili et al., 2021, Samadpour Masouleh et al., 2021, Svarovskaya and Garganeeva, 2018) reported the HOMA-IR levels of 119 patients, 63 taurine users and 56 non-users. There was statistically significant difference in HOMA-IR reduction between the taurine group and the control group (SMD-0.86 [95% CI: −1.30,-0.42], p = 0.0001). There was no significant difference in heterogeneity between the included studies (p = 0.28, I2 = 22%) (Fig. 4D).
3.4. The effect of taurine on body composition
3.4.1. Weight
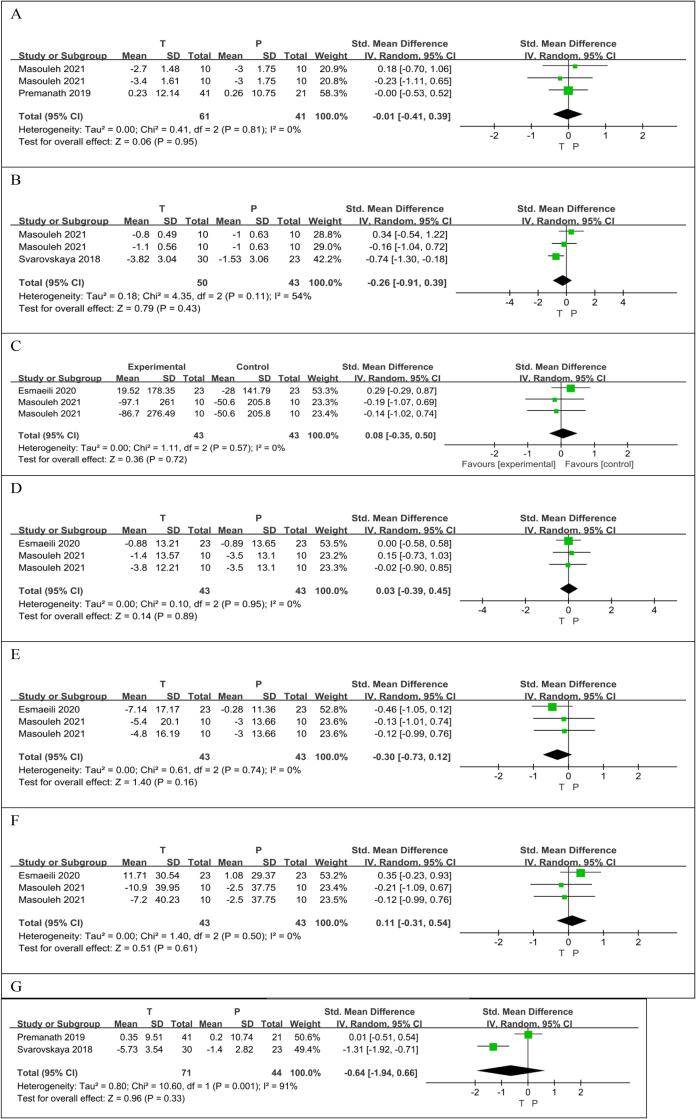
Two RCTs (Premanath et al., 2019, Samadpour Masouleh et al., 2021) reported on 102 patients, 61 taurine users and 41 non-users. There was no significant difference in weight loss between the taurine group and the control group (SMD −0.01[95% CI: −0.41, 0.39], p = 0.95). There was no heterogeneous difference between studies (p = 0.81, I2 = 0%) (Fig. 5A).
Fig. 5.
The effect of taurine on weight (A); the effect of taurine on BMI (B);the effect of taurine on Energy Intake (C);the effect of taurine on Protein (D);the effect of taurine on Fat (E);the effect of taurine on Carbohydrate (F);the effect of taurine on waist circumference (G).
3.4.2. Body mass index (BMI)
Two RCTs (Samadpour Masouleh et al., 2021, Svarovskaya and Garganeeva, 2018) reported BMI levels in 93 patients, 50 taurine users and 43 non-users. Compared with the control group, taurine may reduce BMI, but the difference was not statistically significant (SMD −0.26 [95% CI: −0.91, 0.39], p = 0.43). Significant heterogeneity was found between studies (p = 0.11, I2 = 54%) (Fig. 5B).
3.4.3. Energy Intake
Two RCTs (Esmaeili et al., 2021, Samadpour Masouleh et al., 2021) reported BMI levels in 86 patients, 43 taurine users and 43 non-users. There was no significant difference in energy intake between the taurine group and the control group (SMD 0.08 [95% CI: −0.35, 0.50], p = 0.72). There was no heterogeneous difference between studies (p = 0.57, I2 = 0%) (Fig. 5C).
3.4.4. Protein
Two RCTs (Esmaeili et al., 2021, Samadpour Masouleh et al., 2021) reported protein levels in 86 patients, 43 taurine users and 43 non-users. There was no significant difference in Protein level between the taurine group and the control group (SMD 0.03 [95% CI: −0.39, 0.45], p = 0.89). There was no heterogeneous difference between studies (p = 0.95, I2 = 0%) (Fig. 5D).
3.4.5. Fat
Two RCTs (Esmaeili et al., 2021, Samadpour Masouleh et al., 2021) reported fat levels in 86 patients, 43 taurine users and 43 non-users. Compared with the control group, taurine may reduce fat levels, but the difference was not statistically significant (SMD −0.30 [95% CI: −0.73, 0.12], p = 0.16). There was no heterogeneous difference between studies (p = 0.74, I2 = 0%) (Fig. 5E).
3.4.6. Carbohydrate
Two RCTs (Esmaeili et al., 2021, Samadpour Masouleh et al., 2021) reported carbohydrate levels in 86 patients, 43 taurine users and 43 non-users. There was no significant difference in Carbohydrate level between the taurine group and the control group (SMD 0.11 [95% CI: −0.31, 0.54], p = 0.61). There was no heterogeneous difference between studies (p = 0.50, I2 = 0%) (Fig. 5F).
3.4.7. Waist circumference (WC)
Two RCTs (Premanath et al., 2019, Svarovskaya and Garganeeva, 2018) reported carbohydrate levels in 115 patients, 71 taurine users and 44 non-users. Compared with the control group, taurine may reduce WC levels, but the difference was not statistically significant (SMD −0.64 [95% CI: −1.94, 0.66], p = 0.33). Significant heterogeneity was found between studies (p = 0.001, I2 = 91%) (Fig. 5G).
3.5. The effect of taurine on blood pressure
3.5.1. Systolic blood pressure (SBP)
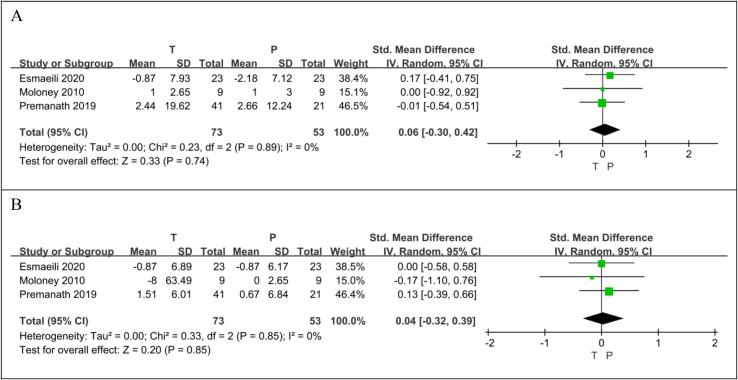
Three RCTs (Esmaeili et al., 2021, Moloney et al., 2010, Premanath et al., 2019) reported on 126 patients, 73 taurine users and 53 non-users. There was no significant difference in SBP level between the taurine group and the control group (SMD 0.06[95% CI: −0.30, 0.42], p = 0.74). There was no heterogeneous difference between studies (p = 0.89, I2 = 0%) (Fig. 6A).
Fig. 6.
The effect of empagliflozin on SBP (A); the effect of empagliflozin on DBP (B).
3.5.2. Diastolic blood pressure (DBP)
Three RCTs (Esmaeili et al., 2021, Moloney et al., 2010, Premanath et al., 2019) reported on 126 patients, 73 taurine users and 53 non-users. There was no significant difference in DBP level between the taurine group and the control group (SMD 0.04[95% CI: −0.32, 0.39], p = 0.85). There was no heterogeneous difference between studies (p = 0.85, I2 = 0%) (Fig. 6B).
4. Discussion
A meta-analysis of 5 studies was conducted to evaluate the effect of taurine on various indicators in patients with diabetes. The 5 included studies were all RCT studies in patients with diabetes, of which 4 were for patients with type 2 diabetes, and 1 was for type 2 diabetes. This meta-analysis comprehensively evaluated the effects of taurine supplementation on multidimensional indicators of diabetes, such as blood lipids, blood glucose, blood pressure, body weight, and body composition. Our analysis results show that taurine has a significant reducing effect on HbA1c, FBS, HOMA-IR, and taurine may have a reducing effect on TG, TC, LDL, Insulin, BMI, Fat, Waist circumference, but there is no statistical significance difference. Taurine has no effect on other diabetes related indicators such as HDL, Weight, Energy Intake, Protein, Carbohydrate, SBP, DBP.
Taurine has been used as a dietary supplement to improve health in humans and animal models of metabolic syndrome (El Idrissi, 2019, Ra et al., 2019). In vitro study on human erythrocytes shows that the increase in glycated hemoglobin levels was blocked significantly when erythrocytes were pretreated with taurine (Selvaraj, Bobby, & Sathiyapriya, 2006). A single administration of taurine at a dose of 200 mg per kg increased the insulin-like activity in blood plasma, elevated two-fold the content of glycogen in liver tissue, decreased content of sugars in blood (Dokshina, Silaeva, & Lartsev, 1976).
Animal experiments showed that supplementation of taurine could improve hyperglycemia and insulin resistance in non-insulin-dependent diabetes mellitus rats (Harada et al., 2004). Supplementing the fructose-fed rats with taurine has ameliorated the rise in HOMA by 56%, triglycerides (TGs) by 22.5%, total cholesterol (T-Chol) by 11%, and low density lipoprotein cholesterol (LDL-C) by 21.4% (El Mesallamy, El-Demerdash, Hammad, & El Magdoub, 2010). Taurine can improve insulin signaling and insulin resistance in rat liver caused by intravenous infusion of fatty acids (Wu et al., 2010).
Taurine also plays a significant role in the metabolic process. The concentration of taurine in plasma was 25% lower in diabetic patients than that in normal subjects (Sak et al., 2019). In fact, most of the major targets of diabetes (kidney, retina and neurons) undergo hyperglycemic-mediated reductions in taurine content (Schaffer and Kim, 2018, Schaffer et al., 2009). Ingestion of 0.4–6 g taurine per day for various days improved metabolic profiles in blood (including reductions in total cholesterol and low-density-lipoprotein cholesterol), and cardiovascular functions in healthy subjects, as well as in patients with overweight, diabetes, hypertension, or congestive heart failure (Militante and Lombardini, 2002, Xu et al., 2008), which means that both healthy volunteers and diabetic patients can benefit from taurine supplements. In healthy humans, administration of a commercial energy drink which contained taurine caused a decrease in serum glucose and an elevation in the HOMA-IR (P < 0.001) (Basrai et al., 2019). Taurine could improve whole-body insulin sensitivity in volunteers with hyperglycemia (Sarkar, Basak, Ghosh, Kundu, & Sil, 2017). Based on Seven-hundred eleven overweight or obese participants on a weight-loss diet for 2 years, baseline taurine levels significantly altered the effect of diabetic genetic risk score on fasting glucose, insulin, and HOMA-IR. Plasma taurine levels may differentially modulate the effect of diabetes-related genes on insulin sensitivity during weight-loss diets in overweight/obese patients (Zheng et al., 2016). Oral supplementation of taurine could ameliorate lipid-induced functional beta cell decompensation and insulin resistance in humans (Xiao, Giacca, & Lewis, 2008). RCT study showed that taurine rich diet reduced HOMA-IR value compared with the control group (P = 0.032) (Díaz-Rizzolo et al., 2021).
As shown in Fig. 4, our study has showed taurine supplement could reduce the glycemic indices. Indeed, this consistency is apparent despite the fact that these studies differ in several ways, including the background anti-diabetic drugs, the extra interventions like Physical exercise, and the dosage of taurine supplement. Taken together, these studies showed beneficial effects of taurine supplement for glycemic indices of diabetes.
The improvement of taurine supplement on HbA1c, FBS, HOMA-IR of diabetic patients may attribute to the molecular mechanism of taurine, including anti-oxidative effect, anti-inflammatory effect, protection of pancreatic cells, improvement of insulin resistance, and promotion of bile acid production, especially antioxidant and anti-inflammatory effects.
Oxidative stress plays a vital role in diabetes because of the increased synthesis of Reactive Oxygen Species (ROS). Glycosylation of non-enzymatic protein and glucose auto-oxidation are major causes of production of free radicals that cause tissue injuries in diabete (Brownlee, 2001, Wolff and Dean, 1987). High glucose levels or hyperglycemia is linked to ROS and plays a key function in such ailments (Winiarska, Szymanski, Gorniak, Dudziak, & Bryla, 2009).
Several mechanisms may contribute to the protection effect of taurine against oxidative stress in diabetes. First, Taurine reduces AGEs production, which plays a vital role in the formation of diabetes complications. The reaction between taurine and aldehydes was supposed to perform as a scavenging element against glycation and protect intracellular production of AGE and carbonyl compounds (Devamanoharan et al., 1998, Nandhini et al., 2004). Tau prevented protein glycation and lipid peroxidation in red blood cells during exposure to high glucose (Nandhini & Anuradha, 2003). Second, taurine regulates the PI3 kinase/AKT pathway. Studies on the mechanism of apoptosis showed that alloxan accelerated the markers of mitochondrial dependent apoptotic pathway (enhanced cytochrome C release in cytosol from mitochondria, altered the expression of Bax, Bcl-2, Apaf-1, caspase-9, caspase-3). Treatment with taurine could restore all the alteration caused by alloxan. Moreover, taurine activates hepatic PI3Kinase, Akt, hexokinase and augments the translocation of GLUT 2 to hepatic membrane in diabetic rats, which may benefit diabetic liver injury (Inam et al., 2018, Rashid et al., 2013). Third, taurine affects the mitochondrial apoptosis pathway and energy metabolism. Taurine increases the rate of ATP production, the ratio of ATP:ADP, the activity of pyruvate dehydrogenase, protects glucose oxidation, respiratory chain function and energy metabolism, effects that could increase beta-cell secretory function (Oprescu et al., 2007, Schaffer et al., 2016).
The anti-inflammatory mechanism of taurine is mainly by interacting with hypochlorous acid. Neutrophils can release hypochlorous acid, which is a strong oxidant promoting the occurrence of inflammation. Taurine reacts with hypochlorite and acquires chloride to its N–H group, producing taurine chloramine, which plays an anti-inflammatory role (Grisham et al., 1984, Kim and Cha, 2014).
Taurine may have effects on serum lipids, but in our study, there is no statistical difference. The effect of taurine on blood lipids needs more research to confirm.
To our knowledge, this is the first meta-analysis to comprehensively evaluate the effects of taurine supplementation on various indicators in patients with diabetes.
However, this analysis has several limitations: (a) Due to the limited number of large clinical trials and only a few RCTs eligible, only 5 RCTs were included in this paper. Most RCTs have small sample sizes and therefore produce results that are not significant. (b) The short follow-up period, and relatively short duration of the included studies, warrants further study.
Although further studies are needed to establish the optimal approach to the application of taurine in practice, our findings clearly lend support to the supplementation of taurine has ameliorative effect on diabetes.
5. Conclusions
In conclusion, our results suggest that taurine supplement has a significant effect in reducing the HbA1c, FBS, HOMA-IR of diabetic patients. However, the beneficial effects of taurine did not achieve statistical significance in TG, TC, HDL, LDL, Insulin, Weight, BMI, Energy Intake, Protein, Fat, Carbohydrate, Waist circumference, SBP, DBP. More RCTs with longer duration and larger sample sizes are needed to determine the effects of taurine on patients with diabetes and to better guide clinical practice.
Funding information
This work was supported by the National Natural Science Foundation of China [grant numbers 82074061]; and Beijing Hospitals Authority Innovation Studio of Young Staff Funding Support [code:202115].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochms.2022.100106.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Almeida C.C., Mendonça Pereira B.F., Leandro K.C., Costa M.P., Spisso B.F., Conte-Junior C.A. Bioactive compounds in infant formula and their effects on infant nutrition and health: A systematic literature review. International Journal of Food Science. 2021;2021:8850080. doi: 10.1155/2021/8850080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basrai M., Schweinlin A., Menzel J., Mielke H., Weikert C., Dusemund B.…Bischoff S.C. Energy drinks induce acute cardiovascular and metabolic changes pointing to potential risks for young adults: A randomized controlled trial. Journal of Nutrition. 2019;149(3):441–450. doi: 10.1093/jn/nxy303. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- Caine J.J., Geracioti T.D. Taurine, energy drinks, and neuroendocrine effects. Cleveland Clinic Journal of Medicine. 2016;83(12):895–904. doi: 10.3949/ccjm.83a.15050. [DOI] [PubMed] [Google Scholar]
- Cho N.H., Shaw J.E., Karuranga S., Huang Y., da Rocha Fernandes J.D., Ohlrogge A.W., Malanda B. IDF diabetes atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Research and Clinical Practice. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- De Luca A., Pierno S., Camerino D.C. Taurine: The appeal of a safe amino acid for skeletal muscle disorders. Journal of Translational Medicine. 2015;13:243. doi: 10.1186/s12967-015-0610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devamanoharan P.S., Ali A.H., Varma S.D. Oxidative stress to rat lens in vitro: Protection by taurine. Free Radical Research. 1998;29(3):189–195. doi: 10.1080/10715769800300221. [DOI] [PubMed] [Google Scholar]
- Díaz-Rizzolo D.A., Serra A., Colungo C., Sala-Vila A., Sisó-Almirall A., Gomis R. Type 2 diabetes preventive effects with a 12-months sardine-enriched diet in elderly population with prediabetes: An interventional, randomized and controlled trial. Clinical Nutrition. 2021;40(5):2587–2598. doi: 10.1016/j.clnu.2021.03.014. [DOI] [PubMed] [Google Scholar]
- Dokshina G.A., Silaeva T., Lartsev E.I. Insulin-like effects of taurine. Voprosy Meditsinskoi Khimii. 1976;22(4):503–507. [PubMed] [Google Scholar]
- El Idrissi A. Taurine Regulation of Neuroendocrine Function. Advances in Experimental Medicine and Biology. 2019;1155:977–985. doi: 10.1007/978-981-13-8023-5_81. [DOI] [PubMed] [Google Scholar]
- El Mesallamy H.O., El-Demerdash E., Hammad L.N., El Magdoub H.M. Effect of taurine supplementation on hyperhomocysteinemia and markers of oxidative stress in high fructose diet induced insulin resistance. Diabetology & Metabolic Syndrome. 2010;2:46. doi: 10.1186/1758-5996-2-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Zahraa Z.E.A.F., Mahmoud M.F., El Maraghy N.N., Ahmed A.F. Effect of Cordyceps sinensis and taurine either alone or in combination on streptozotocin induced diabetes. Food and Chemical Toxicology. 2012;50(3–4):1159–1165. doi: 10.1016/j.fct.2011.12.020. [DOI] [PubMed] [Google Scholar]
- Esmaeili F., Maleki V., Kheirouri S., Alizadeh M. The effects of taurine supplementation on metabolic profiles, pentosidine, soluble receptor of advanced glycation end products and methylglyoxal in adults with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Canadian Journal of Diabetes. 2021;45(1):39–46. doi: 10.1016/j.jcjd.2020.05.004. [DOI] [PubMed] [Google Scholar]
- Franconi F., Bennardini F., Mattana A., Miceli M., Ciuti M., Milan M.…Seghieri G. Taurine levels in plasma and platelets in insulin-dependent and non-insulin-dependent diabetes mellitus: Correlation with platelet aggregation. Advances in Experimental Medicine and Biology. 1994;359:419–424. doi: 10.1007/978-1-4899-1471-2_45. [DOI] [PubMed] [Google Scholar]
- Grisham M.B., Jefferson M.M., Melton D.F., Thomas E.L. Chlorination of endogenous amines by isolated neutrophils. Ammonia-dependent bactericidal, cytotoxic, and cytolytic activities of the chloramines. Journal of Biological Chemistry. 1984;259(16):10404–10413. [PubMed] [Google Scholar]
- Harada N., Ninomiya C., Osako Y., Morishima M., Mawatari K., Takahashi A., Nakaya Y. Taurine alters respiratory gas exchange and nutrient metabolism in type 2 diabetic rats. Obesity Research. 2004;12(7):1077–1084. doi: 10.1038/oby.2004.135. [DOI] [PubMed] [Google Scholar]
- Inam U.L., Piao F., Aadil R.M., Suleman R., Li K., Zhang M.…Ahmed Z. Ameliorative effects of taurine against diabetes: A review. Amino Acids. 2018;50(5):487–502. doi: 10.1007/s00726-018-2544-4. [DOI] [PubMed] [Google Scholar]
- Ito T., Schaffer S.W., Azuma J. The potential usefulness of taurine on diabetes mellitus and its complications. Amino Acids. 2012;42(5):1529–1539. doi: 10.1007/s00726-011-0883-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagim A.R., Harty P.S., Barakat A.R., Erickson J.L., Carvalho V., Khurelbaatar C.…Kerksick C.M. Prevalence and amounts of common ingredients found in energy drinks and shots. Nutrients. 2022;14(2) doi: 10.3390/nu14020314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C., Cha Y.N. Taurine chloramine produced from taurine under inflammation provides anti-inflammatory and cytoprotective effects. Amino Acids. 2014;46(1):89–100. doi: 10.1007/s00726-013-1545-6. [DOI] [PubMed] [Google Scholar]
- Kim K.S., Oh D.H., Kim J.Y., Lee B.G., You J.S., Chang K.J.…Jeong I.K. Taurine ameliorates hyperglycemia and dyslipidemia by reducing insulin resistance and leptin level in Otsuka Long-Evans Tokushima fatty (OLETF) rats with long-term diabetes. Experimental & Molecular Medicine. 2012;44(11):665–673. doi: 10.3858/emm.2012.44.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kp A.D., Martin A. Recent insights into the molecular regulators and mechanisms of taurine to modulate lipid metabolism: A review. Critical Reviews in Food Science and Nutrition. 2022;1–13 doi: 10.1080/10408398.2022.2026873. [DOI] [PubMed] [Google Scholar]
- Kurtz J.A., VanDusseldorp T.A., Doyle J.A., Otis J.S. Taurine in sports and exercise. Journal of the International Society of Sports Nutrition. 2021;18(1):39. doi: 10.1186/s12970-021-00438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Amoreaux W.J., Cuttitta C., Santora A., Blaize J.F., Tachjadi J., El Idrissi A. Taurine regulates insulin release from pancreatic beta cell lines. Journal of Biomedical Science. 2010;17(Suppl 1):S11. doi: 10.1186/1423-0127-17-s1-s11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenço R., Camilo M.E. Taurine: A conditionally essential amino acid in humans? An overview in health and disease. Nutricion Hospitalaria. 2002;17(6):262–270. [PubMed] [Google Scholar]
- Maahs D.M., West N.A., Lawrence J.M., Mayer-Davis E.J. Epidemiology of type 1 diabetes. Endocrinology and Metabolism Clinics of North America. 2010;39(3):481–497. doi: 10.1016/j.ecl.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendivil, C. O. (2021). Fish consumption: A review of its effects on metabolic and hormonal health. Nutrition and Metabolic Insights, 14, 11786388211022378. 10.1177/11786388211022378. [DOI] [PMC free article] [PubMed]
- Militante J.D., Lombardini J.B. Treatment of hypertension with oral taurine: Experimental and clinical studies. Amino Acids. 2002;23(4):381–393. doi: 10.1007/s00726-002-0212-0. [DOI] [PubMed] [Google Scholar]
- Mohamed N., Gawad H.S. Taurine dietary supplementation attenuates brain, thyroid, testicular disturbances and oxidative stress in streptozotocin-induced diabetes mellitus in male rats. Beni-Suef University Journal of Basic and Applied Sciences. 2017;6 doi: 10.1016/j.bjbas.2017.04.006. [DOI] [Google Scholar]
- Moloney M.A., Casey R.G., O'Donnell D.H., Fitzgerald P., Thompson C., Bouchier-Hayes D.J. Two weeks taurine supplementation reverses endothelial dysfunction in young male type 1 diabetics. Diabetes and Vascular Disease Research. 2010;7(4):300–310. doi: 10.1177/1479164110375971. [DOI] [PubMed] [Google Scholar]
- Nandhini A.T., Thirunavukkarasu V., Anuradha C.V. Stimulation of glucose utilization and inhibition of protein glycation and AGE products by taurine. Acta Physiologica Scandinavica. 2004;181(3):297–303. doi: 10.1111/j.1365-201X.2004.01287.x. [DOI] [PubMed] [Google Scholar]
- Nandhini T.A., Anuradha C.V. Inhibition of lipid peroxidation, protein glycation and elevation of membrane ion pump activity by taurine in RBC exposed to high glucose. Clinica Chimica Acta. 2003;336(1–2):129–135. doi: 10.1016/s0009-8981(03)00337-1. [DOI] [PubMed] [Google Scholar]
- Oja S.S., Saransaari P. Taurine and epilepsy. Epilepsy Research. 2013;104(3):187–194. doi: 10.1016/j.eplepsyres.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Oja S.S., Saransaari P. Significance of taurine in the brain. Advances in Experimental Medicine and Biology. 2017;975(Pt 1):89–94. doi: 10.1007/978-94-024-1079-2_8. [DOI] [PubMed] [Google Scholar]
- Oprescu A.I., Bikopoulos G., Naassan A., Allister E.M., Tang C., Park E.…Giacca A. Free fatty acid-induced reduction in glucose-stimulated insulin secretion: Evidence for a role of oxidative stress in vitro and in vivo. Diabetes. 2007;56(12):2927–2937. doi: 10.2337/db07-0075. [DOI] [PubMed] [Google Scholar]
- Papatheodorou K., Banach M., Bekiari E., Rizzo M., Edmonds M. Complications of Diabetes 2017. Journal of Diabetes Research. 2018;2018:1–4. doi: 10.1155/2018/3086167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E., Quinn M.R., Wright C.E., Schuller-Levis G. Taurine chloramine inhibits the synthesis of nitric oxide and the release of tumor necrosis factor in activated RAW 264.7 cells. Journal of Leukocyte Biology. 1993;54(2):119–124. doi: 10.1002/jlb.54.2.119. [DOI] [PubMed] [Google Scholar]
- Premanath M., Mahesh M., Babu M., Bhanukumar M., Devegowda D. Can N acetyl cysteine – Taurine provide additional reduction in microalbuminuria in type 2 diabetic patients already on optimum doses of Angiotensin converting enzyme inhibitors? International Journal of Health & Allied Sciences. 2019;8:236. doi: 10.4103/ijhas.IJHAS_27_19. [DOI] [Google Scholar]
- Ra S.G., Choi Y., Akazawa N., Kawanaka K., Ohmori H., Maeda S. Effects of taurine supplementation on vascular endothelial function at rest and after resistance exercise. Advances in Experimental Medicine and Biology. 2019;1155:407–414. doi: 10.1007/978-981-13-8023-5_38. [DOI] [PubMed] [Google Scholar]
- Rashid K., Das J., Sil P.C. Taurine ameliorate alloxan induced oxidative stress and intrinsic apoptotic pathway in the hepatic tissue of diabetic rats. Food and Chemical Toxicology. 2013;51:317–329. doi: 10.1016/j.fct.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Ripps H., Shen W. Review: Taurine: A “very essential” amino acid. Mol Vis. 2012;18:2673–2686. [PMC free article] [PubMed] [Google Scholar]
- Sak D., Erdenen F., Müderrisoglu C., Altunoglu E., Sozer V., Gungel H.…Uzun H. The relationship between plasma taurine levels and diabetic complications in patients with type 2 diabetes mellitus. Biomolecules. 2019;9(3) doi: 10.3390/biom9030096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samadpour Masouleh S., Bagheri R., Ashtary-Larky D., Cheraghloo N., Wong A., Yousefi Bilesvar O.…Siahkouhian M. The Effects of TRX suspension training combined with taurine supplementation on body composition, glycemic and lipid markers in women with type 2 diabetes. Nutrients. 2021;13(11) doi: 10.3390/nu13113958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar P., Basak P., Ghosh S., Kundu M., Sil P.C. Prophylactic role of taurine and its derivatives against diabetes mellitus and its related complications. Food and Chemical Toxicology. 2017;110:109–121. doi: 10.1016/j.fct.2017.10.022. [DOI] [PubMed] [Google Scholar]
- Schaffer S., Kim H.W. Effects and mechanisms of taurine as a therapeutic agent. Biomolecules & Therapeutics (Seoul) 2018;26(3):225–241. doi: 10.4062/biomolther.2017.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer S.W., Azuma J., Mozaffari M. Role of antioxidant activity of taurine in diabetes. Canadian Journal of Physiology and Pharmacology. 2009;87(2):91–99. doi: 10.1139/y08-110. [DOI] [PubMed] [Google Scholar]
- Schaffer S.W., Shimada-Takaura K., Jong C.J., Ito T., Takahashi K. Impaired energy metabolism of the taurine-deficient heart. Amino Acids. 2016;48(2):549–558. doi: 10.1007/s00726-015-2110-2. [DOI] [PubMed] [Google Scholar]
- Schmidt S.Y., Berson E.L., Hayes K.C. Retinal degeneration in cats fed casein. I. Taurine deficiency. Investigative Ophthalmology. 1976;15(1):47–52. [PubMed] [Google Scholar]
- Schuller-Levis G.B., Park E. Taurine: New implications for an old amino acid. FEMS Microbiology Letters. 2003;226(2):195–202. doi: 10.1016/s0378-1097(03)00611-6. [DOI] [PubMed] [Google Scholar]
- Selvaraj N., Bobby Z., Sathiyapriya V. Effect of lipid peroxides and antioxidants on glycation of hemoglobin: An in vitro study on human erythrocytes. Clinica Chimica Acta. 2006;366(1–2):190–195. doi: 10.1016/j.cca.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Shi L., Fonseca V., Childs B. Economic burden of diabetes-related hypoglycemia on patients, payors, and employers. Journal of Diabetes and Its Complications. 2021;35(6) doi: 10.1016/j.jdiacomp.2021.107916. [DOI] [PubMed] [Google Scholar]
- Svarovskaya, A., & Garganeeva, A. (2018). Evaluation of the effect of taurine on the course of coronary artery disease associated with diabetes of the 2nd type, in patients undergoing coronary revascularization. Medical Council, 94-99. 10.21518/2079-701X-2018-16-94-99.
- Taurine supplementation in cystic fibrosis. (1988). Nutrition Reviews, 46(7), 257-258. 10.1111/j.1753-4887.1988.tb05445.x. [DOI] [PubMed]
- Turner S.J., La Gruta N.L. A subset of immune-system T cells branded as seeds for type 1 diabetes. Nature. 2022 doi: 10.1038/d41586-021-03800-z. [DOI] [PubMed] [Google Scholar]
- Winiarska K., Szymanski K., Gorniak P., Dudziak M., Bryla J. Hypoglycaemic, antioxidative and nephroprotective effects of taurine in alloxan diabetic rabbits. Biochimie. 2009;91(2):261–270. doi: 10.1016/j.biochi.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Wójcik O.P., Koenig K.L., Zeleniuch-Jacquotte A., Costa M., Chen Y. The potential protective effects of taurine on coronary heart disease. Atherosclerosis. 2010;208(1):19–25. doi: 10.1016/j.atherosclerosis.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff S.P., Dean R.T. Glucose autoxidation and protein modification. The potential role of 'autoxidative glycosylation' in diabetes. The Biochemical Journal. 1987;245(1):243–250. doi: 10.1042/bj2450243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N., Lu Y., He B., Zhang Y., Lin J., Zhao S.…Han P. Taurine prevents free fatty acid-induced hepatic insulin resistance in association with inhibiting JNK1 activation and improving insulin signaling in vivo. Diabetes Research and Clinical Practice. 2010;90(3):288–296. doi: 10.1016/j.diabres.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Xiao C., Giacca A., Lewis G.F. Oral taurine but not N-acetylcysteine ameliorates NEFA-induced impairment in insulin sensitivity and beta cell function in obese and overweight, non-diabetic men. Diabetologia. 2008;51(1):139–146. doi: 10.1007/s00125-007-0859-x. [DOI] [PubMed] [Google Scholar]
- Xu Y.J., Arneja A.S., Tappia P.S., Dhalla N.S. The potential health benefits of taurine in cardiovascular disease. Experimental & Clinical Cardiology. 2008;13(2):57–65. [PMC free article] [PubMed] [Google Scholar]
- Yamori Y., Taguchi T., Hamada A., Kunimasa K., Mori H., Mori M. Taurine in health and diseases: Consistent evidence from experimental and epidemiological studies. Journal of Biomedical Science. 2010;17(Suppl 1):S6. doi: 10.1186/1423-0127-17-s1-s6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Ceglarek U., Huang T., Wang T., Heianza Y., Ma W.…Qi L. Plasma taurine, diabetes genetic predisposition, and changes of insulin sensitivity in response to weight-loss diets. Journal of Clinical Endocrinology and Metabolism. 2016;101(10):3820–3826. doi: 10.1210/jc.2016-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulli A. Taurine in cardiovascular disease. Current Opinion in Clinical Nutrition & Metabolic Care. 2011;14(1):57–60. doi: 10.1097/MCO.0b013e328340d863. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.