Abstract
Neutrophils are the first line of defense against tissue injury and play an important role in tumor progression. Tumor-associated neutrophils (TANs) mediate pro-tumor immunosuppressive activity and their infiltration into tumors is associated with poor outcome in a variety of malignant diseases. The tumor cell-neutrophil crosstalk is mediated by small extracellular vesicles (sEVs) also referred to as exosomes which represent a major mechanism for intercellular communication. This review will address the role of neutrophil-derived sEVs (NEX) in reprogramming the TME and on mechanisms that regulate the dual potential of NEX to promote tumor progression on one hand and suppress tumor growth on the other. Emerging data suggest that both, NEX and tumor-derived sEVs (TEX) carry complex molecular cargos which upon delivery to recipient cells in the tumor microenvironment (TME) modulate their behavior and reprogram them to mediate pro-inflammatory or immunosuppressive responses. Although it remains unknown how the balance between the often conflicting signaling of TEX and NEX is regulated, this review is an attempt to provide insights into mechanisms that underpin this complex bidirectional crosstalk. A better understanding of the signals NEX process or deliver in the TME might lead to the development of novel approaches to the control of tumor progression in the future.
Keywords: neutrophils, small extracellular vesicles, tumor-derived exosomes (TEX), tumor-associated neutrophils (TAN), tumor microenvironment (TME)
Introduction
The tumor microenvironment (TME) is a structurally complex, tumor-driven milieu of various cells, all programmed to promote tumor growth. In addition to tumor cells, blood vessels, extracellular matrix (ECM) components and other non-malignant cells, including fibroblasts, epithelial cells, and immune cells are components of the TME (Figure 1A/2A). Neutrophils are also components of the TME in most human tumors, although for many years, their presence in human tumors was a subject of considerable controversy [1]. More recent studies confirm that neutrophils are a component of the TME in most, if not all, human tumors and suggest that infiltration of neutrophils into the tumor is linked to poor prognosis [2].
Figure 1.

Neutrophil mobilization and functions in cancer. (A) Cells in the TME release cytokines, such as G-CSF, which induces granulopoiesis in the bone marrow, and chemokines which stimulate the migration of different neutrophil subpopulations. Some factors, such as TGF-β, are involved in promoting a pro-tumor phenotype in neutrophils. (B) Granulopoiesis is the formation and maturation of neutrophils, which is also important for granule production. Neutrophils are attracted to the bloodstream through cytokines and chemokines, especially by the release of IL-8 and their activation via CXCR2. Neutrophil primes with the first stimuli and enhanced expression of adhesion molecules, which allows diapedesis. When neutrophils reach the tumor site, they are exposed to second stimuli, such as DAMPs, and accomplish their major functions: NETosis, degranulation and extracellular vesicle release.
Abbreviations: G-CSF - granulocyte colony-stimulating factor, IFNγ – interferon gamma, TGF-β - transforming growth factor beta, IL-8 - interleukin-8, CXCR2 - CXC chemokine receptor 2, DAMPs - damage-associated molecular patterns, PMN - polymorphonuclear, MPO - myeloperoxidase, NE – neutrophil elastase, MMP-9 – metalloproteinase 9, NGAL – neutrophil gelatinase-associated lipocalin
Figure 2:
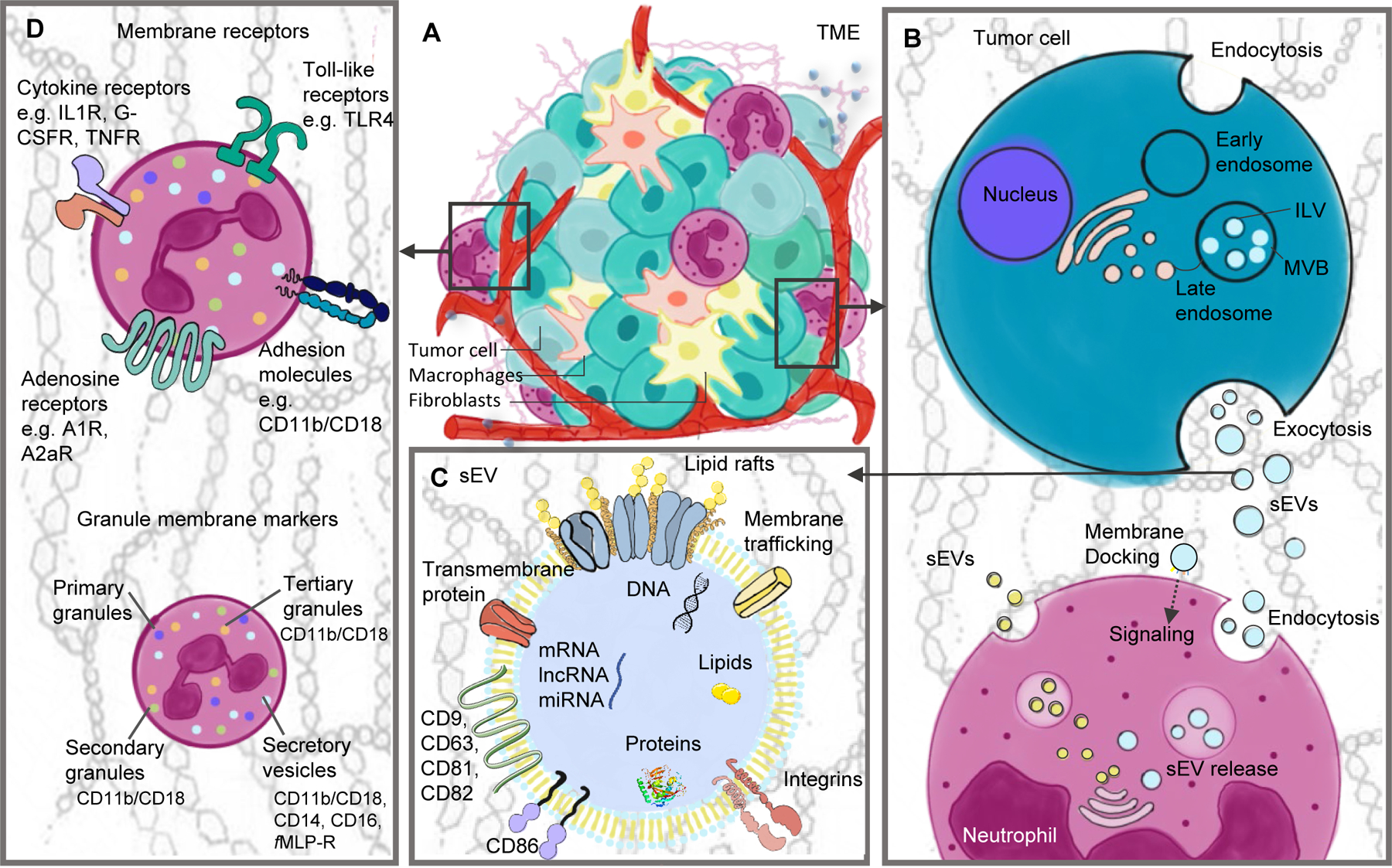
The role neutrophils in the tumor microenvironment. (A) The composition of the TME is complex and includes, in addition to tumor cells, surrounding blood vessels, the ECM, and non-malignant cells. (B) Tumor cells are capable of producing sEVs as a mean of cell communication. The biogenesis of these sEVs occurs via initial endocytosis, which forms early endosomes and later MVBs with ILVs, followed by exocytosis and sEV release. sEVs can be internalized by recipient cells and reprogram its phenotype and functions. Neutrophils can internalize sEVs via endocytosis. Also, neutrophils are capable of producing their own sEVs (NEX), which are released to the extracellular space and interact with a variety of different cells types. (C) General cargo composition of sEVs: the cargo changes according to the metabolism of the parent cell and each sEV can carry different molecules even if derived from the same cell. (D) Neutrophils express a variety of cell surface proteins: chemokine receptors, for responding to chemokines; toll-like receptors, which recognize tumor molecules, such as HMGB1; adhesion molecules for migration and diapedesis; activation markers; adenosine receptors, which bind ADO and enhance immunosuppressive functions.
Abbreviations: TME - tumor microenvironment, ECM - extracellular matrix, HMGB1 - high mobility group box 1, ADO – adenosine,
Neutrophils are a component of the innate immune system, representing 50 to 70 % of circulating leukocytes in humans under normal physiological conditions [3]. They are the first cell type recruited to the site of tissue injury. After neutrophil granulopoiesis and mobilization, their recruitment is mainly mediated by CXCR1/CXCR2 in response to pathogen-associated or damage-associated molecular patterns (PAMPs and DAMPs, respectively) [4]. Neutrophils effectively participate in immune/inflammatory responses, utilizing various mechanisms such as phagocytosis, degranulation and/or extracellular trap (NET) formation (Figure 1B) [5]. They also secrete a variety of chemokines, which promote the recruitment of other immune cells, including macrophages [6]. Neutrophils are characterized by phenotypic and functional plasticity, that enables them to play multiple roles in immune/inflammatory responses. It is in this context that the concept of neutrophil heterogeneity has been introduced [7]. Indeed, the existence of the neutrophil polarization spectrum characterized by N1 and N2 phenotypes, similar to those described for macrophages, has been proposed [8]. N1 neutrophils are inflammatory and N2 neutrophils are involved in tissue reconstitution/regeneration and angiogenesis, which are especially important for the support of tumor growth. This functional polarization does not exclude the likely possibility that the same neutrophil is capable of performing a spectrum of functions depending on the conditions in the microenvironment.
The participation of neutrophils in tumor progression has been controversial and the neutrophil plasticity has been used to explain their dual role in the TME [9]. The ability of neutrophils to communicate with other immune cells, such as macrophages, lymphocytes or natural killer (NK) cells has been recognized as a key feature of their biology. The ability of both tumors and neutrophils to produce and release small extracellular vesicles (sEVs) to the extracellular environment is an important mechanism for communication with other cells [10]. It is likely that sEVs may be responsible for neutrophil capabilities to “sense” and respond to external signals in the TME as well as tumor cells to modulate and promote a convenient environment through the release of tumor-derived small extracellular vesicles (TEX). sEVs have been in the limelight as a major intercellular communication system in health and disease [11]. Therefore, this review will address the role of neutrophil-derived small extracellular vesicles (NEX) and TEX in reprogramming the TME and on mechanisms that regulate the potential of sEVs to promote tumor progression.
The role of neutrophils in cancer
Neutrophil infiltration in solid tumors has been associated with worse prognosis [12]. The number and phenotypic as well as functional attributes of tumor-infiltrating neutrophils can serve as biomarkers of response to chemotherapy or immunotherapy and survival in patients with cancer [2,13]. Although interactions of neutrophils with tumor or non-malignant cells in the TME are not yet fully understood, their engagement in sophisticated bidirectional interactions with these cells has been reported [14,15]. Emerging evidence associates increase in the number of circulating neutrophils with poor prognosis in different types of malignancies, including glioblastoma [16] and colorectal cancer [17]. Prognostic relevance of neutrophils is usually based on calculations of the neutrophil to lymphocyte ratio (NLR). The NLR has been used as a prognostic factor of overall survival (OS) in many types of solid tumors, including gastric [18], lung [19], and head and neck [20] cancers. The high NLR values associate with worse prognosis for cancer patients [21]. Moreover, it has been shown to correlate with the abundance of neutrophils infiltrating the TME [22]. Importantly, not only the number but also the location of neutrophils within the TME appears to impact their activation and functions and can, therefore, have prognostic relevance. Also, OS varies, depending on whether neutrophils are localized in the tumor nests (intratumoral) [23], around the tumor nests (peritumoral) [24], or in the tumor-associated stroma [2,25].
Tumor-associated neutrophils (TANs) are predominantly classified based on their phenotypes, as discussed below. Recent studies report the existence in the TME of different subpopulations of neutrophils, which are characterized by distinct functions during tumor progression [26]. One hallmark of neutrophils is their phenotypic plasticity which can be modulated by environmental conditions. Thus, activation of neutrophils largely depends on the surrounding microenvironment [27]. TANs are known to act either as tumor promotors or as tumor suppressors, depending on the microenvironmental context, which clearly changes as the tumor develops and progresses. The dual role of TANs reflects the constantly changing complexity of the TME and of TAN interactions with different cell types, such as tumor-associated macrophages [28]. Today, the nomenclature of TANs still needs to be established and the definition of phenotypes for different TAN subsets in the TME awaits further studies [9].
Current evidence indicates that TANs promote tumor growth, invasion, angiogenesis, and metastasis in various types of cancer [8,29,30]. TANs are exposed to a variety of stimuli in the TME, such as granulocyte colony-stimulating factor (G-CSF), interleukin (IL)-8, and transforming growth factor β (TGF-β), which alter their maturation and polarization states [29]. Neutrophils mediating anti-tumor activities are known for their cytolytic activity directed at tumor cells and, at the same time, for displaying an immunostimulatory profile (i.e., TNF-αhigh, CCL3high, ICAM-1high, Arginaselow). These TANs produce higher levels of superoxide and hydrogen peroxide, in contrast to pro-tumor TANs, which mediate immunosuppressive functions and upregulate expression of chemokines CCL2, 3, 4, 8, 12, and 17 as well as CXCL1, 2, 8 and 16 [31]. According to Jaillon et al. human pro-tumor TANs are characterized by expression of LOX-1+, CD170high and PD-L1+ [32], while anti-tumor TANs express CD54+, HLA-DR+, CD86+, and CD15high. Immature TANs express CD117+, CD16int/low, LOX-1+, CD84+, and JAML+ (Figure 1A). Besides, mouse pro-tumor TANs express Ly6G+, CD11b+, PD-L1+, CD170high and mouse anti-tumor neutrophils express Ly6G+, CD170low, CD177+, CD54+, and CD16+. Immature mouse TANs are known for expressing Ly6G+, CD11b+, CD117+, CD170low, CD101−, CD84+, and JAML+ [33].
Another immune cell subset accumulating in tumors are the myeloid-derived suppressor cells (MDSCs). Their major function is the suppression of anti-tumor T cell functions and promotion of tumor progression. In cancer and in other pathological conditions, G-CSF is overproduced and favors MDSC generation, as extensively reviewed [34,35]. MDSC activity in the TME is closely related to the neutrophil (G-MDSC) or monocyte (M-MDSC) differentiation [34,36]. It is currently under debate whether the origin of the MDSC population is the same as that of neutrophils. However, just recently, G-MDSCs, also known as polymorphonuclear MDSCs (PMN-MDSCs), were described as “neutrophils with proven immunosuppressive activity” [33,37]. Numbers of PMN-MDSCs are elevated in the blood of cancer patients and they are thought to exert tumor-promoting functions, including suppression of the adaptive immune responses, stimulation of angiogenesis, and shaping of pre-metastatic niches [38]. It has also been discovered that the protein expression profile of pro-tumor G-MDSCs includes CD11b+, CD15+, CD66+, CD33dim, and HLA-DRneg [39]. To discriminate neutrophils from PMN-MDSCs, a panel of several markers, including expression of the lectin-type oxidized LDL receptor 1 (LOX-1) are currently used. LOX-1+ neutrophils are potent suppressors of T cell proliferation and genomically resemble MDSCs, while LOX-1− neutrophils do not mediate immunosuppressive effects [40].
Cell interactions involved in the neutrophil-tumor crosstalk that takes place in the TME are illustrated in Figure 1. First, the figure presents cytokine-mediated effects on granulopoiesis in the bone marrow, maturation of neutrophils, and their diapedesis and entry into the TME. Next, in Figure 2, the capability of TANs to produce and release sEVs is presented. TANs have recently emerged as a major producer of sEVs that are engaged in promoting tumor growth and impacting disease outcome. Therefore, TANs have acquired clinical relevance, and understanding of the mechanisms that are responsible for TAN-mediated tumor-promoting activities are of great importance.
Small extracellular vesicles (sEVs) and their role in cancer
Small extracellular vesicles (sEVs) or also referred to as exosomes are currently in the lime light as potential mediators of intercellular crosstalk [41,42]. sEVs, a subset of EVs derived from multivesicular bodies (MVBs) and sized at 30 to 150 nm in diameter are of particular interest, because of their unique biogenesis and the surface membrane topography that mimics that of parental cells [43,44]. This EV subset is distinct from larger (~500 nm) microvesicles and even larger (~1,000 nm) apoptotic bodies (Figure 2B) [45,46]. The biogenesis of sEVs has been extensively investigated and has been recently reviewed [41]. The cargo of sEVs released by parent cells is complex, reflects the content of parent cells and consists of proteins, nucleic acids, lipids and glycans, forming glycoconjugates or lipid rafts on the sEV surface. The protein content of sEVs is enriched in membrane trafficking molecules, integrins, transmembrane proteins and tetraspanins (CD9, CD63, CD81). The latter are often used as sEV surface markers (Figure 2C) [47]. The overall cargo of sEVs can shift depending on the local microenvironment of the parent cells, and various environmental stimuli such as tissue hypoxia, oxidative stress or chemotherapeutics are known to alter the content of sEVs [45,48].
Today, understanding of the sEV biology is in its infancy, but their capability to induce reprogramming of recipient cells has been noted and followed [49,50]. The effects sEVs induce in recipient cells vary depending on their molecular cargo and probably also on the molecular make up of recipient cells, resulting in functional heterogeneity [45,48]. Interactions between sEVs and recipient cells usually involve delivery of the sEV cargo to recipient cells, but it can also occur without any cargo delivery. This happens when sEVs interact directly with receptors on the surface of recipient cells. The mechanism(s) underpinning membrane docking of sEVs is still unclear; however, commonly described surface proteins, such as integrins appear to be involved [51]. Cells internalize sEVs using various endocytic pathways, including clathrin-dependent endocytosis, and clathrin-independent pathways such as caveolin-mediated uptake, macropinocytosis, phagocytosis, and lipid raft-mediated internalization (Figure 2B) [52].
sEVs entering the recipient cell, such as an immune cell, exert various effects that are biologically relevant and include alterations in functional competence. Specifically, tumor cell-derived small extracellular vesicles (TEX) impair anti-tumor functions of immune cells [53]. Cancer patients have elevated levels of circulating sEVs decorated with immunosuppressive proteins on the surface membrane and able to suppress immune cell functions [54,55]. The presence of the immunosuppressive sEVs in patients’ plasma was associated with poor prognosis in colorectal cancer and recently also in non-small cell lung cancer (NSCLC) [53,56]. Breast cancer-derived sEVs were reported to carry proteins and miRNA that correlated with tumor recurrence and metastasis [57,58]. These preliminary findings indicate that sEVs have a potential to serve as a component of non-invasive liquid tumor biopsy along with circulating tumor cells (CTC) and cell free DNA (cfDNA) [59].
Neutrophil-derived small extracellular vesicles
Neutrophils, like all other cells, produce and release sEVs that we have identified as “NEX”. Initially, NEX were considered to be neutrophil granules, but more careful scrutiny indicated that NEX have features that distinguish them from granules and other secretory vesicles. Neutrophil granules are formed during neutrophil maturation in the bone marrow and are fully formed, pre-packed and ready for release in response to stimuli encountered during a neutrophil life span [60]. In contrast, the biogenesis of NEX is initiated in mature neutrophils in response to intracellular metabolic changes and/or extracellular, environmental stress. NEX are formed and released on the “as needed” basis and their molecular/genetic composition, reflecting that of the parent cell, is tailored to meet the existing physiological or pathological situation. NEX may be especially useful in pathological conditions, where they are “first responders”, effectively signaling the presence of danger and/or summoning help. In line with this, NEX activities mirror those of the parent cell. As indicated above, neutrophils might be functionally polarized into inflammatory N1 and regenerative N2 subtypes. If so, then it would be expected that N1 neutrophils produce N1 NEX, while N2 neutrophils release N2 NEX, each NEX subtype functionally recapitulating the parent cell [61]. Figure 3 illustrates the potential of the individual neutrophil to produce and release NEX subtypes packaged in the mother cell to carry inflammatory or regenerative cargos. This scenario fits well with the above described plasticity of neutrophils and their capacity for swift activation and responsiveness to incoming signals. Figure 3 also presents phenotypic profiles of N1 and N2 NEX as well as their cargo profiles and functions associated with each NEX subtype.
Figure 3.
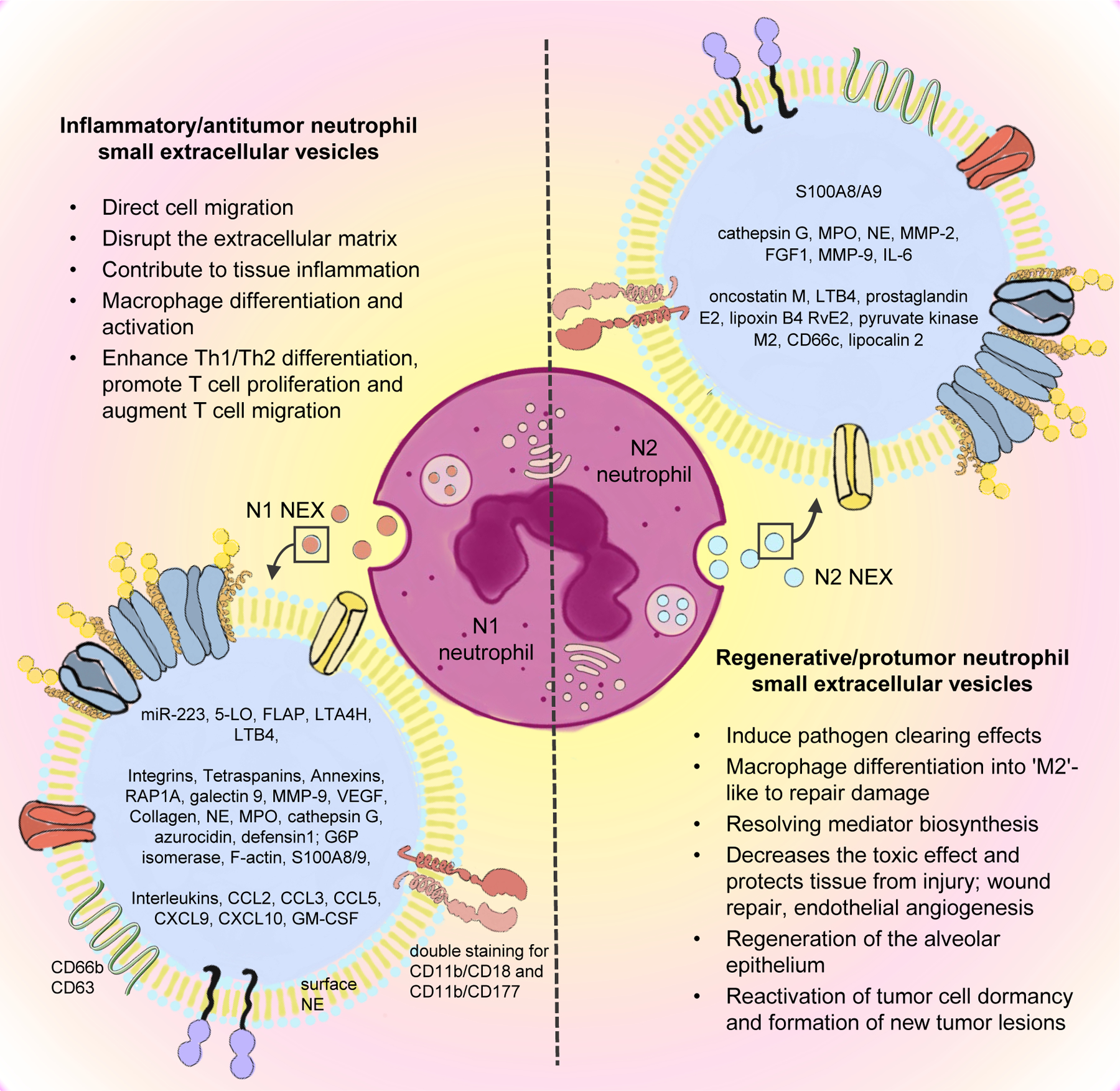
Differential neutrophil polarization as source of N1 and N2 NEX. The phenotypes and biological functions of neutrophils are heterogenous and it is most likely that this heterogeneity is reflected in NEX. The molecular content of NEX was found to be varying depending on the microenvironmental conditions of the parent cells, thus N1 and N2 neutrophils might produce N1 NEX and N2 NEX, respectively. N1 NEX have the potential to promote anti-tumor effects and might enhance the anti-tumor immune response with special regards to lymphocytes. N2 NEX carry tumor-promoting cargo components, which are potentially involved in promoting tumor growth, survival, angiogenesis and escape of immune surveillance.
Abbreviations: NEX – neutrophil-derived small extracellular vesicles, A2MG – alpha-2-macroglobulin, MPO - myeloperoxidase, NE – neutrophil elastase, MMP-2 – mettaloproteinase-2, FGF1 - fibroblast growth factor 1, MMP-9 – mettaloproteinase-9, HSPs - heat shock proteins, IL-6 – interleukin-6, LTB4 - leukotriene B4, 5-LO - 5-lipoxygenase, FLAP - 5-lipoxygenase-activating protein, LTA4H - leukotriene A4 Hydrolase, VEGF - vascular endothelial growth factor
NEX can be isolated from supernatants of neutrophil cultures using the same methods employed for sEV isolation [62]. Despite the same methodology for isolation and characterization, there are few studies focusing on NEX and even fewer experiments relating to the role of NEX in cancer. Neutrophils have recently emerged as a key player in the TME and little is known about their tumor-promotion mechanisms. Studies have proven the complexity of neutrophil interactions among malignant and non-malignant cells [1,63–65], therefore, further studies are needed to fully comprehend the tumor-neutrophil crosstalk mediated by NEX.
The role of neutrophil-derived small extracellular vesicles (NEX) in cancer
It is well established that neutrophils secrete NEX under different physiologic and pathologic conditions and that secreted NEX are functionally capable of mediating a variety of biological effects. Studies of the NEX involvement in inflammation, tissue repair, and autoimmune diseases [66,67] indicate that neutrophils promote a positive feedback in order to enhance their migration and activation in different disease settings by releasing NEX [68]. Therefore, NEX appear to have a heterogeneous role, by mediating both inflammatory and anti-inflammatory states, which reflects their plasticity: They can act as inflammatory or regulatory vesicles, as shown in Table 1 [69–71]. It is unclear whether NEX also play a functional role in malignant diseases. The finding that TANs have an immunosuppressive profile and thus might promote tumor progression suggests that NEX also contribute to changes that occur in the TME during tumorigenesis.
Table 1:
Cargo components of NEX and their functional role according to neutrophil activation state.
| NEX type | Function | Cargo | Description | Ref |
|---|---|---|---|---|
| N1 inflammatory | N0 mobilization | miR-223 | crucial functions in myeloid lineage development | [74] |
| Cell migration | 5-LO | 5-LO catalyzes two steps in biosynthesis of leukotrienes contributing to innate immunity | [77] | |
| FLAP | FLAP is necessary for the activation of 5-LO and synthesis of leukotriene, both lipid mediators of inflammation | [76] | ||
| LTA4H | converts leukotriene A4 to leukotriene B4 | [76] | ||
| LTB4 | induce the adhesion and activation of leukocytes on the endothelium; potent neutrophil chemoattractant | [76] | ||
| F-Actin | cell motility | [116] | ||
| S100A8/9 | stimulates leukocyte recruitment and induces cytokine secretion | [111] | ||
| Integrins | facilitate cell-cell and cell-extracellular matrix adhesion; mediate cellular signals such as regulation of the cell cycle, organization of the intracellular cytoskeleton, and movement of new receptors to the cell membrane | [51,78] | ||
| RAP1A | regulates signaling pathways that affect cell proliferation and adhesion; Rap1 activation has been implicated in preventing solid tumor metastasis | [78] | ||
| Cell metabolism | G6P Isomerase | involved in glycolysis and gluconeogenesis, as well as the pentose phosphate pathway | [116] | |
| Galectin 9 | may affect recipient cell metabolism through its cytoplasmic action in control of AMPK | [78] | ||
| Cell modulation | Proinflammatory cytokines | recruitment and activation of immune cells | [80] | |
| Tissue lesion | NE | play a role in degenerative and inflammatory diseases by its proteolysis of collagen-IV and elastin of the extracellular matrix | [84] | |
| MPO | produces HOCl during the neutrophil’s respiratory burst; causes tissue lesions | [111] | ||
| Cathepsin G | important role in breaking down tissues at inflammatory sites | [81] | ||
| Azurocidin | important multifunctional inflammatory mediator | [116] | ||
| Defensin 1 | recruitment of T lymphocytes | [116] | ||
| MMP-9 | involved in the degradation of the extracellular matrix | [86] | ||
| Tetraspanins | may interact with and regulate other platelet receptors | [47] | ||
| Annexins | inhibits leukocyte (specifically neutrophils) extravasation and downregulates the magnitude of the inflammatory response | [78] | ||
| Collagen | structural | [78] | ||
| N2 regenerative | Tumor growth / progression | LTB4 | acts on non-immune cells via BLT1 to initiate and/or amplify pathological inflammation; LTB4-BLT1 axis in cancer, either tumor inhibitory or tumor-promoting, depending on the different target cells | [76] |
| MPO | excessive generation of MPO-derived oxidants has been linked to tissue damage and in the initiation and progression of cancer | [83] | ||
| NE | can directly stimulate proliferative pathways by extracellular transactivation of membrane receptors by MAPK signaling | [81] | ||
| E2 | tissue remodeling, angiogenesis, cell proliferation, contribution to tumor growth and invasion; immunosuppressive factor | [70] | ||
| FGF1 | regulation of cell survival, cell division, angiogenesis, cell differentiation, and cell migration | [85] | ||
| pyruvate kinase M2 | regulates the rate-limiting step of glycolysis in tumor cells; contributes to tumorigenesis | [117] | ||
| Evasion / Metastasis | MMP-2 | contributes to cell migration by interacting with collagen | [85] | |
| CD66c | adhesion protein on cell surface | [81] | ||
| Lipocalin 2 | abnormal expression serves critical roles in the epithelial-to-mesenchymal transition process, angiogenesis, and cell migration and invasion | [87] | ||
| MMP-9 | angiogenesis | [86] | ||
| TME regulation | Oncostatin M | induces collagen production and proliferation | [88] | |
| IL6 | promotes tumorigenesis by regulating signaling pathways, which includes apoptosis, cell survival, proliferation and metabolism, angiogenesis, invasiveness and metastasis | [89] | ||
| Cathepsin G | anti-inflammatory response | [81] | ||
| Immunosuppressors | Lipoxin B4 | inflammation resolving endogenous lipid mediators | [70] | |
| RvE2 | regulates chemotaxis of human neutrophils, enhances phagocytosis and anti-inflammatory cytokine production | [70] | ||
| S100A8/9 | recruitment of MDSCs and stimulation of their immunosuppressive functions | [111] |
Abbreviations: NEX: neutrophil-derived small extracellular vesicles; N1: anti tumor neutrophil; N0: inactivated neutrophil; miR-223: microRNA-223; 5-LO: arachidonate 5-lipoxygenase; FLAP: 5-lipoxygenase-activating protein; LTA4H: leukotriene-A4 hydrolase; LTB4: leukotrine B4; F-actin: actin filaments; S100A8/A9: calcium-binding protein A8/A9; RAP1A: ras-related protein; G6P isomerase: glucose-6-phosphate isomerase; AMPK: 5’ adenosine monophosphate-activated protein kinase; NE: neutrophil elastase; MPO: myeloperoxidase; HOCl: hypochlorous acid; MMP-9: matrix metallopeptidase 9; N2: pro-tumor neutrophil; E2: regularory protein E2; FGF1: fibroblast growth factor 1; MMP-2: matrix metallopeptidase 2; CD66c: cell adhesion molecule 6; IL-6: interleukin 6; RvE2: resolvin E2.
Chronic inflammatory processes often preceed malignancy, and the involvement of neutrophils in inflammation suggests that NEX with the N1-like phenotype (Figure 3) may be involved as well. The N1 NEX carrying factors that increase the recruitment of neutrophils along with other immune cells to inflamed tissues are the first to arrive at the TME. The N1 NEX carry miR-223 which plays a crucial role in the development of the myeloid lineage cells [72–74]. It acts directly at granulopoiesis and mobilizes neutrophils to transition from the bone marrow to blood stream [74]. Delivered in N1 NEX to the TME, it promotes immune cell recruitment (Figure 3). It has been observed that down-regulation of miR-223 in the TME is associated with tumor aggressiveness and worse prognosis [75]. Several other factors known to be involved in cell migration were also detected in N1 NEX, including 5-lipoxygenase-activating protein (FLAP), 5-lipoxygenase (5-LO), and leukotriene B4 (LTB4) [68,76,77]. Moreover, it was shown that N1 NEX carry factors such as RAP1A and integrins [78] that modulate adhesion of rolling neutrophils to endothelial cells. Interestingly, RAP1A has also been reported to prevent metastasis [79]. N1 NEX can also carry pro-inflammatory cytokines, such as IL-1β, IL-2 and IL-4 [80]. Overall, N1 NEX have the potential to mediate anti-tumor effects and might enhance anti-tumor immune responses in the TME.
Once malignancy is established and inflammatory milieu is replaced by the ECM supporting tumor growth, infiltrating neutrophils assume a different role [29]. It is possible that the developing tumor induces recruitment of different neutrophil populations into the TME and that NEX participate in this recruitment. Elevated numbers of circulating and infiltrating TANs with the N2 phenotype might also be associated with the initiation of the production of regenerative N2 NEX. N2 NEX carry factors responsible for tumor progression and mediate immunosuppression (Figure 3). For instance, excessive MPO levels in the TME delivered by N2 NEX have been related to tumor progression [81,82]. MPO promotes metabolism of carcinogenic chemicals that compromise DNA repair and contributes to the appearance of new mutations [83]. Also, NE, another cargo component of N2 NEX [81], stimulates oncogenic signaling and activates proliferation pathways driven by MAPK [84]. N2 NEX also carry numerous components which are involved in accelerating angiogenesis, tumor invasion, and metastasis, such as fibroblast growth factor-1 (FGF-1), matrix metalloproteinase 2 and 9 (MMP-2, MMP-9) [85,86], CD66c, and lipocalin 2 [81,87]. Finally, the cargo of N2 NEX contributes to the immunosuppressive profile of the TME. N2 NEX can carry anti-inflammatory cytokines and antiproteases that maintain an immunosuppressive environment [88–90]. To conclude, N2 NEX carry tumor-promoting cargo components, which appear to regulate all aspects of tumor growth and metastasis, including angiogenesis and immune suppression.
Despite some similar content in both N1 and N2 NEX, they express a dual response regarding the environmental stimuli. For instance, MPO and NE are described as cargo of both N1 and N2 NEX, since the same molecular pathway that supports inflammation is also operating in support of tumor growth. This dual activity enhances immunity [82], but simultaneously facilitates tumor progression and metastasis through upregulating MMP activity [91] and imposes tumor cell mutation by MPO enzymatic activity [92]. Moreover, the controversial role of reactive oxygen species (ROS) is widely discussed in cancer [93,94]. Cancer cells increase their rate of ROS production by adapting to the TME. However, excessive levels of ROS can increase oxidative stress and induce cancer cell death [95,96]. Tumor cells boost their antioxidant capacity in order to avoid ROS escalation and maintain redox balance. Thus, it is suggested that – in comparison to non-neoplastic cells - tumor cells have an altered redox environment, therefore, an increased sensitivity to alterations in ROS levels in the TME [97]. This dual role supports that cargo components of NEX can either contribute to or inhibit tumor progression depending on the microenvironmental milieu.
Neutrophil-derived small extracellular vesicles (NEX) carry different messages in early vs late stages of tumor growth
Observations indicating that TANs found in the TME are no longer inflammatory cells but have assumed different phenotypic and functional characteristics implies that NEX produced in the TME are also different. The shift from N1 to N2 NEX is more conspicuous in advanced tumors, and it has been proposed that the developing tumors acquire capabilities to educate, alter and reprogram functions of immune and non-immune cells in the TME [98]. The implication of these experiments is that tumors communicate with surrounding and distant cells via sEVs. The intercellular communication of neutrophils with malignant and non-malignant cells is not exclusive for tumor cells in the TME as it also involves various non-malignant cells. Therefore, cell communication via sEVs appears to be an important modulator of neutrophil responses [15].
It was demonstrated that TEX from glioblastoma cells reprogram macrophage subsets. In this regard, TEX reprogram naïve macrophages or shift M1- into M2-like macrophages, but also educate M2 macrophages into strong immunosuppressive TAMs [99]. Accordingly, TANs are different from neutrophils in normal healthy or even inflamed tissues because of the influence of the TME. Similar to macrophages, TANs may acquire either an anti-tumor (N1) or a pro-tumor (N2) activity [9]. N1 TANs exhibit higher production of ROS and, thus are cytotoxic to tumor cells [94]. On the other hand, N2 TANs endorse tumor growth and dissemination by inducing ECM remodeling and angiogenesis [100,101]. As good inflammatory cells, neutrophils send signals via NEX to the emerging tumor. The first events probably release N1 NEX with anti-tumor signals. However, if tumor prevails and grows, it assumes control and reprograms every cell around. At this point, neutrophils might become N2, release N2 NEX, and help promote tumor growth. This shift is well documented for macrophages [99] and only limited experimental data is available for TANs. However, a recent publication from Kolonics et al. is proposing a similar mechanism for neutrophils and NEX. The authors showed, that neutrophils produce a wide range of EVs, mostly depending on the environmental conditions [61].
Tumor-derived small extracellular vesicles (TEX) as drivers of pro-tumor neutrophil responses
It is well recognized that non-tumor cells in the TME contribute to tumor progression and that the TME is organized to promote tumor growth. The TME is characterized by the presence of a variety of factors, including VEGF, colony stimulating factor 1 (CSF1), and platelet-derived growth factor (PDGF) that induce angiogenesis, recruitment of macrophages and neutrophils, and promote tumor cell proliferation, respectively [102]. Moreover, the presence of immunomodulatory molecules in the TME has been described as a key factor for tumor progression and is one of the major mechanism of the tumor cell escape from immune surveillance. For instance, tumor-associated endothelial cells secrete CXCL1/2 which promotes the recruitment of neutrophils into the TME [103]. However, the factors which are released by tumor cells do not only exist in their soluble forms, they can also be associated with sEVs. TEX deriving from breast cancer cells induced NETs release and accelerated cancer-associated thrombosis [104]. Beyond that, HMGB1 appears to be an important cargo component of TEX. HMGB1, a sterile inflammatory molecule and DAMP released from various cells during stress has been implicated in inflammation and it is considered as a neutrophil recruiter [105]. TEX isolated from gastric cancer cells carried HMGB1 and prolonged neutrophil survival. HMGB1-positive TEX also activated the Toll-like receptor 4/NF-κB cascade, inducing a pro-tumor phenotype in neutrophils [15]. Besides, HMGB1 modulated neutrophils in vitro to suppress T cell proliferation, activation, and function by inducing PD-L1 expression [106]. Indeed, CD66+ neutrophil infiltration correlated with decreased CD8+ T cell infiltration, leading to tumor progression [107]. In oral squamous cell carcinoma, TEX isolated from cells cultured in hypoxic conditions carried miR-21 and enhanced the suppressive effect of CD11b+Gr-1+ MDSCs in a PTEN/PD-L1-axis-dependent manner, which, in turn, suppressed anti-tumor functions of γδ T cells. γδ T cells are a diverse subgroup of T cells involved in both innate and adaptive responses and they have been demonstrated to be involved in immune regulatory and tumor surveillance activities [108]. In several malignant entities it was shown that TEX carry heat shock protein (Hsp)-70 and Hsp72 which suppressed the activity of CD11b+Gr-1+ MDSCs via TLR2/STAT3 and, thus, stimulated tumor progression [109].
Neutrophils have been directly associated with tumor metastasis in several in vivo and clinical analyses [9]. In this regard, HMGB1-positive TEX induced the expression of inflammatory factors in neutrophils, which promoted gastric cancer cell migration [15]. Indeed, TEX modulate the ECM, and TEX-induced fibronectin deposition was shown to initiate a pre-metastatic niche formation [110]. Interestingly, stress-associated adrenergic hormones were described to induce a fast S100A8/9 release from neutrophils unrelated to either degranulation or NETs formation. These proteins result in an accumulation of oxidized lipids in dormant tumor cells through MPO activity and seem to engage their reactivation and initiation of new cancer lesions [111]. Moreover, a recent study has demonstrated that a TEX from melanoma were able to recruit pro-tumor neutrophils to a pre-metastatic niche [112]. This data indicates the importance of the role of neutrophils throughout the carcinogenic process, as demonstrated by Tyagi et al. in breast cancer with a premetastatic pulmonary site [113].
Neutrophils play a relevant role in both innate immunity and triggering adaptive immunity. Especially, pro-tumor neutrophils or TANs promote tumor progression by accelerating angiogenesis, metastasis formation, CD8+ T cell exclusion, a loss of immune surveillance, and reactivation of dormant cells (Figure 4). There is evidence in the literature that the recruitment and activation of neutrophils and especially the reprogramming towards TANs might be mediated by TEX (Table 2). Understanding the sEV-mediated crosstalk between tumor cells and neutrophils shows the potential to increase the overall understanding of neutrophil biology.
Figure 4.
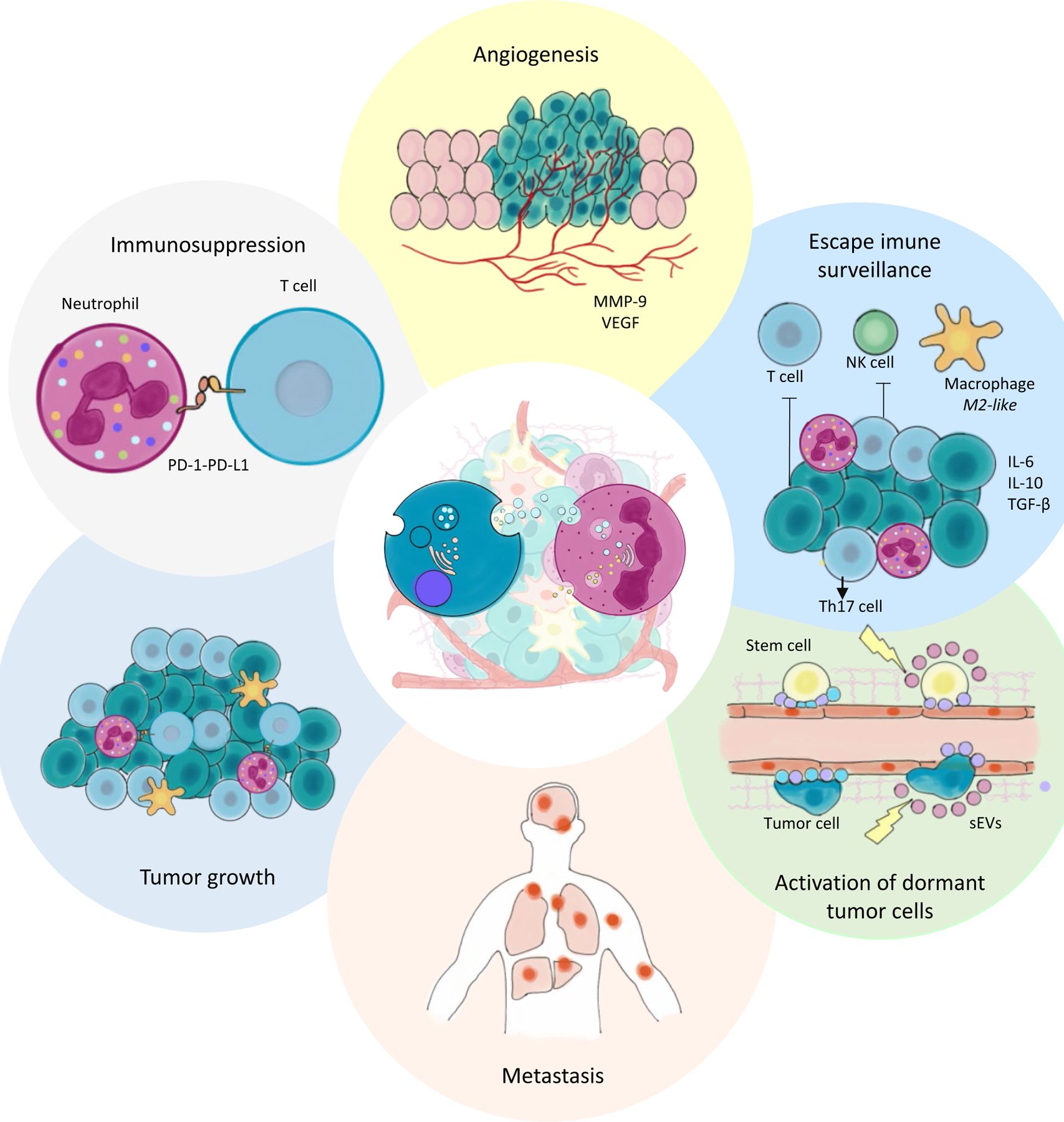
Tumor cells release TEX which interact with a variety of cell types, including TANs in the TME and neutrophils at distant sites. TEX were shown to alter the phenotype and functional behavior of neutrophils. This figure summarizes the tumor-promoting effects of neutrophils reprogrammed by the tumor.
Abbreviations: TEX – tumor-derived small extracellular vesicles, TANs – tumor-associated neutrophils, TME – tumor microenvironment, MMP-9 – mettaproteinase-9, VEGF - vascular endothelial growth factor , PD1 - programmed cell death protein 1, PDL1 - programmed cell death ligand 1, IL – interleukin
Table 2:
TEX as modulators of neutrophil response in cancer.
| Disease | Experimental model | Source of sEVs | Cargo components | Induced biological effects | Ref. |
|---|---|---|---|---|---|
| Lung/ovarian cancer | in vivo and clinical | neutrophils | S100A8/A9 | reactivation of dormant tumor cells and formation of new tumor lesions | [111] |
| Breast cancer | in vivo | 4T1 cell line | unknown | NETs release, cancer-related thrombosis | [104] |
| Colorectal cancer | in vivo | CT26 cell line | unknown | stimulation of neutrophils | [118] |
| Colorectal cancer | in vitro, in vivo | HCT15, HT29, and CT26 cell lines | miRNA-146a | increase of tumor-infiltrating neutrophils and decrease of tumor-infiltrating T cells | [107] |
| Colon cancer | in vivo | CT26 cell line | HSP72 | immunosuppressive activity of MDSCs | [109] |
| Renal cell carcinoma | in vitro, in vivo | Renca cell | HSP70 | immunosuppressive activity of MDSCs | [119] |
| Oral squamous cell carcinoma | in vivo | Cal-27 and SCCVII cell lines | miR-21 | immunosuppressive activity of MDSCs | [108] |
| Breast, lung, and ovarian cancer | ex vivo, in vivo | breast, lung, and ovarian cancer | HSP70 | immunosuppressive activity of MDSCs | [120] |
| Gastric cancer | in vitro | cell line BGC-823, HGC-27, MGC-803, and SGC-7901 | HMGB1, HSP70, fibronectin | angiogenesis, metastasis | [15] |
| Gastric cancer | in vitro | gastric cancer cell lines | HMGB1 | induction of PD-L1 expression on neutrophils | [106] |
| Pancreatic ductal adenocarcinoma | in vivo | pancreatic ductal adenocarcinomas | migration inhibitory factor (MIF) | migration of bone marrow-derived macrophages and neutrophils, metastatic niche formation | [110] |
Abbreviations: sEVs: small extracellular vesicles; S100A8/A9: calcium-binding protein A8/A9; 4T1: breast cancer cell line; NETs: neutrophil extracellular traps; CT26: colon carcinoma cell line; HCT15: colon carcinoma cell line; HT29: colon carcinoma cell line; miRNA-146a: micro-RNA 146-a; HSP72: heat shock protein 72; MDSC: myeloid derived suppressor cell; HSP70: heat shock protein 70; Cal-27: oral squamous carcinoma cell line; SCC VII: oral squamous carcinoma cell line; miR-21: microRNA-21; BGC-823: gastric cancer cell line; HGC-27: gastric cancer cell line; MGC-803: gastric cancer cell line; SGC-7901: gastric cancer cell line; HMGB1: high mobility group box 1; PD-L1: programmed death-ligand 1
Concluding remarks
The understanding of the multifaceted role of neutrophils in cancer is of current interest and bears the potential to uncover important biological aspects, which may become important for the development of future therapeutic targets. Neutrophils are equipped and ready to shift from one mode of surveillance to completely different mode of tumor-promoting activity when tumor appears. sEVs are a crucial mechanism for intercellular communication and the investigation of their cargo components and ability to reprogram recipient cells are of great current interest. Recently, it was shown that the crosstalk between tumor cells and neutrophils is mediated by sEVs either released by tumor cells (TEX) or by neutrophils (NEX). There is a lot of uncertainty about these complex interactions and how they modulate the TME, ultimately creating a tumor-promoting microenvironment. However, the role of neutrophils in cancer proved to be extremely important and so far underestimated. Studies have shown their close relation to cancer metastasis [86], cancer-associated thrombosis through NETs release [104], and neutrophil uptake of oncogenic EVs [114].
Further in vivo investigations are necessary to evaluate whether the effects of NEX and TEX in the TME are as central as they are currently presented in the literature. Multiple studies attempted to deplete cancer cells, or mice of the effectors of the sEV biogenesis and in many instances cancer progression was not abrogated, or the changes were subtle [115]. An enhanced understanding of the sEV biogenesis and the cargo components and functions of NEX and TEX in the TME are necessary to validate the concept that sEV-based intercellular communication is a driver of tumor progression. Most recent publications focus on sEVs as one subset of EVs, however, also other subsets such as exomeres, microvesicles, and apoptotic bodies were shown to play functional roles in the TME. It will be necessary to validate how central sEVs are to the EV-mediated intercellular communication in comparison to other EV subsets. Determination of the exact contributions of NEX and TEX to malignant entitites will be the aim of future studies which are expected to: 1) provide more details about the complex biology of the TME; 2) give further insights into neutrophil biology; and 3) show the potential to discover new therapeutic strategies which will be based on targeting neutrophils with a tumor-promoting phenotype.
Highlights.
Tumor-associated neutrophils (TANs) mediate pro-tumor activities
The tumor cell-neutrophil crosstalk is mediated by small extracellular vesicles (sEVs)
sEVs carry complex molecular cargos and reprogram neutrophils
Reprogrammed neutrophils mediate pro-inflammatory or immunosuppressive responses
Acknowledgments:
The authors would like to thank the Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS - 19/2551-0000663-2; 21/2551-0000078-3), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; code 001), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq - 312187/2018-1; 400882/2019-1; 400882/2019-1), HCPA (FIPE - 2019-0446), and UFCSPA. D.S. Rubenich, N. Omizzollo, and E. Braganhol are recipients of CNPq fellowships. N. Ludwig was supported by the Walter Schulz Foundation (info@walter-schulz-stiftung.de). Partial support was provided by the NIH grant U01-DE029759 to Theresa L. Whiteside.
Footnotes
Conflict of interest
Authors declare no conflict of interest.
References
- [1].Fridlender ZG, Albelda SM, Tumor-associated neutrophils: Friend or foe?, Carcinogenesis. 33 (2012) 949–955. 10.1093/carcin/bgs123. [DOI] [PubMed] [Google Scholar]
- [2].Shaul ME, Fridlender ZG, Tumour-associated neutrophils in patients with cancer, Nat. Rev. Clin. Oncol 16 (2019) 601–620. 10.1038/s41571-019-0222-4. [DOI] [PubMed] [Google Scholar]
- [3].Lawrence SM, Corriden R, Nizet V, The Ontogeny of a Neutrophil: Mechanisms of Granulopoiesis and Homeostasis, Microbiol. Mol. Biol. Rev 82 (2018) 1–22. 10.1128/mmbr.00057-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Filippi MD, Neutrophil transendothelial migration: Updates and new perspectives, Blood. 133 (2019) 2149–2158. 10.1182/blood-2018-12-844605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Silvestre-Roig C, Fridlender ZG, Glogauer M, Scapini P, Neutrophil Diversity in Health and Disease, Trends Immunol. 40 (2019) 565–583. 10.1016/j.it.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].D. A Sadik CD, Kim ND, & Luster, Neutrophils cascading their way to inflammation, Trends Immunol. 32 (2011) 452–460. https://doi.org/ 10.1016/j.it.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hellebrekers P, Vrisekoop N, Koenderman L, Neutrophil phenotypes in health and disease, 48 (2018). 10.1111/eci.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Coffelt SB, Wellenstein MD, De Visser KE, Neutrophils in cancer: Neutral no more, Nat. Rev. Cancer 16 (2016) 431–446. 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- [9].Giese MA, Hind LE, Huttenlocher A, Neutrophil plasticity in the tumor microenvironment, Blood. 133 (2019) 2159–2167. 10.1182/blood-2018-11-844548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Soehnlein O, Steffens S, Hidalgo A, Weber C, Neutrophils as protagonists and targets in chronic inflammation, Nat. Rev. Immunol 17 (2017) 248–261. 10.1038/nri.2017.10. [DOI] [PubMed] [Google Scholar]
- [11].Cassatella MA, Östberg NK, Tamassia N, Soehnlein O, Biological Roles of Neutrophil-Derived Granule Proteins and Cytokines, Trends Immunol. 40 (2019) 648–664. 10.1016/j.it.2019.05.003. [DOI] [PubMed] [Google Scholar]
- [12].Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, Nair VS, Xu Y, Khuong A, Hoang CD, Diehn M, West RB, Plevritis SK, Alizadeh AA, The prognostic landscape of genes and infiltrating immune cells across human cancers, Nat. Med 21 (2015) 938–945. 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].He G, Zhang H, Zhou J, Wang B, Chen Y, Kong Y, Xie X, Wang X, Fei R, Wei L, Chen H, Zeng H, Peritumoural neutrophils negatively regulate adaptive immunity via the PD-L1/PD-1 signalling pathway in hepatocellular carcinoma, J. Exp. Clin. Cancer Res 34 (2015) 1–11. 10.1186/s13046-015-0256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kim J, Bae JS, Tumor-associated macrophages and neutrophils in tumor microenvironment, Mediators Inflamm. (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhang X, Shi H, Yuan X, Jiang P, Qian H, Xu W, Tumor-derived exosomes induce N2 polarization of neutrophils to promote gastric cancer cell migration, Mol. Cancer 17 (2018) 1–16. 10.1186/s12943-018-0898-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rahbar A, Cederarv M, Wolmer-Solberg N, Tammik C, Stragliotto G, Peredo I, Fornara O, Xu X, Dzabic M, Taher C, Skarman P, Söderberg-Nauclér C, Enhanced neutrophil activity is associated with shorter time to tumor progression in glioblastoma patients, Oncoimmunology. 5 (2016). 10.1080/2162402X.2015.1075693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ye L, Zhang T, Kang Z, Guo G, Sun Y, Lin K, Huang Q, Shi X, Ni Z, Ding N, Zhao KN, Chang W, Wang J, Lin F, Xue X, Tumor-infiltrating immune cells act as a marker for prognosis in colorectal cancer, Front. Immunol 10 (2019) 1–12. 10.3389/fimmu.2019.02368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hirahara T, Arigami T, Yanagita S, Matsushita D, Uchikado Y, Uenosono Y, Ishigami S, Natsugoe S, Combined neutrophil-lymphocyte ratio and platelet-lymphocyte ratio predicts chemotherapy response and prognosis in patients with advanced gastric cancer, BMC Cancer. 2014 (2019) 1–7. 10.1186/s12885-019-5903-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kang J, Chang Y, Ahn J, Oh S, Koo D, Lee Y, Shin H, Ryu S, mortality in a low-risk population : A cohort study, 3275 (2019) 3267–3275. 10.1002/ijc.32640. [DOI] [PubMed] [Google Scholar]
- [20].Yoon J, Roh J, Kim S, Choi S, Yuhl S, Yoon S, Journal of Geriatric Oncology Prognostic value of neutrophil-to-lymphocyte ratio in older patients with head and neck cancer, J. Geriatr. Oncol (2019). 10.1016/j.jgo.2019.06.013. [DOI] [PubMed] [Google Scholar]
- [21].Caldeira P, Vieira E, Sousa A, Teixeira A, de Aguiar M, Immunophenotype of neutrophils in oral squamous cell carcinoma patients, J. Oral Pathol. Med 46 (2017) 703–709. 10.1111/jop.12575. [DOI] [PubMed] [Google Scholar]
- [22].Takakura K, Ito Z, Suka M, Kanai T, Matsumoto Y, Odahara S, Matsudaira H, Haruki K, Fujiwara Y, Saito R, Gocho T, Nakashiro KI, Hamakawa H, Okamoto M, Kajihara M, Misawa T, Ohkusa T, Koido S, Comprehensive assessment of the prognosis of pancreatic cancer: Peripheral blood neutrophil-lymphocyte ratio and immunohistochemical analyses of the tumour site, Scand. J. Gastroenterol 51 (2016) 610–617. 10.3109/00365521.2015.1121515. [DOI] [PubMed] [Google Scholar]
- [23].Gao Q, Zhao YJ, Wang XY, Qiu SJ, Shi YH, Sun J, Yi Y, Shi JY, Shi GM, Bin Ding Z, Xiao YS, Zhao ZH, Zhou J, He XH, Fan J, CXCR6 upregulation contributes to a proinflammatory tumor microenvironment that drives metastasis and poor patient outcomes in hepatocellular carcinoma, Cancer Res. 72 (2012) 3546–3556. 10.1158/0008-5472.CAN-11-4032. [DOI] [PubMed] [Google Scholar]
- [24].Kuang DM, Zhao Q, Wu Y, Peng C, Wang J, Xu Z, Yin XY, Zheng L, Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma, J. Hepatol 54 (2011) 948–955. 10.1016/j.jhep.2010.08.041. [DOI] [PubMed] [Google Scholar]
- [25].Carus A, Ladekarl M, Hager H, Pilegaard H, Nielsen PS, Donskov F, Tumor-associated neutrophils and macrophages in non-small cell lung cancer: No immediate impact on patient outcome, Lung Cancer. 81 (2013) 130–137. 10.1016/j.lungcan.2013.03.003. [DOI] [PubMed] [Google Scholar]
- [26].Liew PX, Kubes P, The Neutrophil’s role during health and disease, Physiol. Rev 99 (2019) 1223–1248. 10.1152/physrev.00012.2018. [DOI] [PubMed] [Google Scholar]
- [27].Ng LG, Ostuni R, Hidalgo A, Heterogeneity of neutrophils, Nat. Rev. Immunol 19 (2019) 255–265. 10.1038/s41577-019-0141-8. [DOI] [PubMed] [Google Scholar]
- [28].Uribe-Querol E, Rosales C, Neutrophils in cancer: Two sides of the same coin, J. Immunol. Res 2015 (2015). 10.1155/2015/983698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Worthen GS, Albelda SM, Polarization of TAN phenotype by TGFb: “N1” versus “N2” TAN, Cancer Cell. 16 (2010) 183–194. 10.1016/j.ccr.2009.06.017.Polarization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yang Y.S. Li, DeBusk Laura M. Fukuda Koari, Fingleton Barbara, Green-Jarvis Brenda, C DP. and L PC. Matrisian Lynn M., Expansion of myeloid immune suppressor Gr_CD11b_ cells in tumor-bearing host directly promotes tumor angiogenesis, (2004) 6(4), 409–421. [DOI] [PubMed] [Google Scholar]
- [31].Fridlender ZG, Sun J, Mishalian I, Singhal S, Cheng G, Kapoor V, Horng W, Fridlender G, Bayuh R, Worthen GS, Albelda SM, Transcriptomic analysis comparing tumor-associated neutrophils with granulocytic myeloid-derived suppressor cells and normal neutrophils, PLoS One. 7 (2012). 10.1371/journal.pone.0031524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wang TT, Zhao YL, Peng LS, Chen N, Chen W, Lv YP, Mao FY, Zhang JY, Cheng P, Teng YS, Fu XL, Yu PW, Guo G, Luo P, Zhuang Y, Zou QM, Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway, Gut. 66 (2017) 1900–1911. 10.1136/gutjnl-2016-313075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jaillon S, Ponzetta A, Di Mitri D, Santoni A, Bonecchi R, Mantovani A, Neutrophil diversity and plasticity in tumour progression and therapy, Nat. Rev. Cancer 20 (2020) 485–503. 10.1038/s41568-020-0281-y. [DOI] [PubMed] [Google Scholar]
- [34].Filippo D. Veglia; Michela Perego; Gabrilovich, Myeloid-derived suppressor cells coming of age, Nat. Immunol 176 (2018) 100–106. 10.1038/s41590-017-0022-x.Myeloid-derived. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gabrilovich DI, Ostrand-Rosenberg S, Bronte V, Coordinated regulation of myeloid cells by tumours, Nat. Rev. Immunol 12 (2012) 253–268. 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].R. AD Bayne Lauren J., Beatty Gregory L., Jhala Nirag, Clark Carolyn E., and V. RH Stanger Ben Z., Tumor-derived granulocyte-macrophage colony stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer, Cancer Cell. 21 (2012) 822–835. 10.1016/j.ccr.2012.04.025.Tumor-derived. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, Rodriguez PC, Sica A, Umansky V, Vonderheide RH, Gabrilovich DI, Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards, Nat. Commun 7 (2016) 1–10. 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sprouse ML, Welte T, Boral D, Liu HN, Yin W, Vishnoi M, Goswami-Sewell D, Li L, Pei G, Jia P, Glitza-Oliva IC, Marchetti D, PMN-MDSCs enhance CTC metastatic properties through reciprocal interactions via ROS/notch/nodal signaling, Int. J. Mol. Sci 20 (2019). 10.3390/ijms20081916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Si Y, Merz SF, Jansen P, Wang B, Bruderek K, Altenhoff P, Mattheis S, Lang S, Gunzer M, Klode J, Squire A, Brandau S, Multidimensional imaging provides evidence for down-regulation of T cell effector function by MDSC in human cancer tissue, Sci. Immunol 4 (2019). 10.1126/SCIIMMUNOL.AAW9159. [DOI] [PubMed] [Google Scholar]
- [40].Kumar V, Cheng P, Condamine T, Mony S, Languino LR, Mccaffrey JC, Hockstein N, Guarino M, Masters G, Denstman F, Xu X, Altieri DC, Du H, Yan C, CD45 Phosphatase Inhibits STAT3 Transcription Factor Activity in Myeloid Cells and Promotes Tumor-Associated Macrophage Differentiation, Immunity. 44 (2016) 303–315. 10.1016/j.immuni.2016.01.014.CD45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ludwig N, Azambuja JH, Rao A, Gillespie DG, Jackson EK, Whiteside TL, Adenosine receptors regulate exosome production, Purinergic Signal. (2020). https://doi.org/ 10.1007/s11302-020-09700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringnér M, Mörgelin M, Bourseau-Guilmain E, Bengzon J, Belting M, Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development., Proc. Natl. Acad. Sci. U. S. A 110 (2013) 7312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gurunathan S, Kang M-H, Jeyaraj M, Qasim M, Kim J-H, Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes, Cells. 8 (2019) 307. 10.3390/cells8040307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].György B, Módos K, Pállinger É, Pálóczi K, Pásztói M, Misják P, Deli MA, Sipos Á, Szalai A, Voszka I, Polgár A, Tóth K, Csete M, Nagy G, Gay S, Falus A, Kittel Á, Buzás EI, Detection and isolation of cell-derived microparticles are compromised by protein complexes resulting from shared biophysical parameters, Blood. 117 (2011). 10.1182/blood-2010-09-307595. [DOI] [PubMed] [Google Scholar]
- [45].Kalluri R, LeBleu VS, The biology, function, and biomedical applications of exosomes, Science (80-. ). 367 (2020). 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H, Mark MT, Molina H, Martin AB, Bojmar L, Fang J, Rampersaud S, Hoshino A, Matei I, Kenific CM, Nakajima M, Mutvei AP, Sansone P, Buehring W, Wang H, Jimenez JP, Cohen-Gould L, Paknejad N, Brendel M, Manova-Todorova K, Magalhães A, Ferreira JA, Osório H, Silva AM, Massey A, Cubillos-Ruiz JR, Galletti G, Giannakakou P, Cuervo AM, Blenis J, Schwartz R, Brady MS, Peinado H, Bromberg J, Matsui H, Reis CA, Lyden D, Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation, Nat. Cell Biol 20 (2018) 332–343. 10.1038/s41556-018-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, Liebler DC, Ping J, Liu Q, Evans R, Fissell WH, Patton JG, Rome LH, Burnette DT, Coffey RJ, Reassessment of Exosome Composition, Cell. 177 (2019) 428–445.e18. 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chevillet JR, Kang Q, Ruf IK, Briggs HA, Vojtech LN, Hughes SM, Cheng HH, Arroyo JD, Meredith EK, Gallichotte EN, Pogosova-Agadjanyan EL, Morrissey C, Stirewalt DL, Hladik F, Yu EY, Higano CS, Tewari M, Quantitative and stoichiometric analysis of the microRNA content of exosomes, Proc. Natl. Acad. Sci. U. S. A 111 (2014) 14888–14893. 10.1073/pnas.1408301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Głuszko A, Szczepanski MJ, Ludwig N, Shafag M, Olejarz W, Exosomes in Cancer: Circulating Immune-Related Biomarkers, Biomed Res. Int (2019) 1–9. 10.1155/2019/1628029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Escrevente C, Keller S, Altevogt P, Costa J, Interaction and uptake of exosomes by ovarian cancer cells, BMC Cancer. 11 (2011). 10.1186/1471-2407-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Mathieu M, Martin-Jaular L, Lavieu G, Théry C, Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication, Nat. Cell Biol 21 (2019) 9–17. 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- [52].Mulcahy LA, Pink RC, Carter DRF, Routes and mechanisms of extracellular vesicle uptake., J. Extracell. Vesicles 3 (2014) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, Yang J, Wang B, Sun H, Xia H, Man Q, Zhong W, Antelo LF, Wu B, Xiong X, Liu X, Guan L, Li T, Liu S, Yang R, Lu Y, Dong L, McGettigan S, Somasundaram R, Radhakrishnan R, Mills G, Lu Y, Kim J, Chen YH, Dong H, Zhao Y, Karakousis GC, Mitchell TC, Schuchter LM, Herlyn M, Wherry EJ, Xu X, Guo W, Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response, Nature. 560 (2018) 382–386. 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Whiteside TL, Exosomes carrying immunoinhibitory proteins and their role in cancer, Clin. Exp. Immunol 189 (2017) 259–267. 10.1111/cei.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ludwig S, Floros T, Theodoraki MN, Hong CS, Jackson EK, Lang S, Whiteside TL, Suppression of lymphocyte functions by plasma exosomes correlates with disease activity in patients with head and neck cancer, Clin. Cancer Res 23 (2017) 4843–4854. 10.1158/1078-0432.CCR-16-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Vetsika E, Sharma P, Samaras I, Markou A, Georgoulias V, Whiteside TL, Kotsakis A, Small Extracellular Vesicles in Pre-Therapy Plasma Predict Clinical Outcome in Non-Small-Cell Lung Cancer Patients, Cancers (Basel). 13 (2021) 1–16. https://doi.org/ 10.3390/cancers13092041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wang M, Ji S, Shao G, Zhang J, Zhao K, Wang Z, Wu A, Effect of exosome biomarkers for diagnosis and prognosis of breast cancer patients, Clin. Transl. Oncol 20 (2018) 906–911. 10.1007/s12094-017-1805-0. [DOI] [PubMed] [Google Scholar]
- [58].Huang Q, Gumireddy K, Schrier M, le Sage C, Nagel R, Nair S, Egan DA, Li A, Huang G, Klein-Szanto AJ, Gimotty PA, Katsaros D, Coukos G, Zhang L, Puré E, Agami R, The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis, Nat. Cell Biol 10 (2008) 202–210. 10.1038/ncb1681. [DOI] [PubMed] [Google Scholar]
- [59].Ko SY, Lee WJ, Kenny HA, Dang LH, Ellis LM, Jonasch E, Lengyel E, Naora H, Cancer-derived small extracellular vesicles promote angiogenesis by heparin-bound, bevacizumab-insensitive VEGF, independent of vesicle uptake, Commun. Biol 2 (2019) 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ramadass M, Catz SD, Molecular mechanisms regulating secretory organelles and endosomes in neutrophils and their implications for inflammation, Immunol. Rev 273 (2016) 249–265. 10.1111/imr.12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kolonics F, Szeifert V, Timár CI, Ligeti E, Lőrincz ÁM, The Functional Heterogeneity of Neutrophil-Derived Extracellular Vesicles Reflects the Status of the Parent Cell, Cells. 9 (2020). 10.3390/cells9122718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Allen ER, Lempke SL, Miller MM, Bush DM, Braswell BG, Estes CL, Benedict EL, Mahon AR, Sabo SL, Greenlee-Wacker MC, Effect of extracellular vesicles from S. aureus-challenged human neutrophils on macrophages, J. Leukoc. Biol 108 (2020) 1841–1850. 10.1002/JLB.3AB0320-156R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Michaeli J, Shaul ME, Mishalian I, Hovav AH, Levy L, Zolotriov L, Granot Z, Fridlender ZG, Tumor-associated neutrophils induce apoptosis of non-activated CD8 T-cells in a TNFα and NO-dependent mechanism, promoting a tumor-supportive environment, Oncoimmunology. 6 (2017). 10.1080/2162402X.2017.1356965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, Scheidmann MC, Donato C, Scherrer R, Singer J, Beisel C, Kurzeder C, Heinzelmann-Schwarz V, Rochlitz C, Weber WP, Beerenwinkel N, Aceto N, Neutrophils escort circulating tumour cells to enable cell cycle progression, Nature. 566 (2019) 553–557. 10.1038/s41586-019-0915-y. [DOI] [PubMed] [Google Scholar]
- [65].Németh T, Sperandio M, Mócsai A, Neutrophils as emerging therapeutic targets, Nat. Rev. Drug Discov 19 (2020) 253–275. 10.1038/s41573-019-0054-z. [DOI] [PubMed] [Google Scholar]
- [66].Genschmer KR, Russell DW, Lal C, Szul T, Bratcher PE, Noerager BD, Abdul Roda M, Xu X, Rezonzew G, Viera L, Dobosh BS, Margaroli C, Abdalla TH, King RW, McNicholas CM, Wells JM, Dransfield MT, Tirouvanziam R, Gaggar A, Blalock JE, Activated PMN Exosomes: Pathogenic Entities Causing Matrix Destruction and Disease in the Lung, Cell. 176 (2019) 113–126.e15. 10.1016/j.cell.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Rahat MA, Shakya J, Parallel Aspects of the Microenvironment in Cancer and Autoimmune Disease, Mediators Inflamm. 2016 (2016) 1–17. 10.1155/2016/4375120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Majumdar R, Tavakoli Tameh A, Parent CA, Exosomes Mediate LTB4 Release during Neutrophil Chemotaxis, PLoS Biol. 14 (2016) 1–28. 10.1371/journal.pbio.1002336. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [69].Li L, Zuo X, Xiao Y, Liu D, Luo H, Zhu H, Neutrophil-derived exosome from systemic sclerosis inhibits the proliferation and migration of endothelial cells, Biochem. Biophys. Res. Commun 526 (2020) 334–340. 10.1016/j.bbrc.2020.03.088. [DOI] [PubMed] [Google Scholar]
- [70].Dalli J, Serhan CN, Specific lipid mediator signatures of human phagocytes: Microparticles stimulate macrophage efferocytosis and pro-resolving mediators, Blood. 120 (2012) 60–72. 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Bui TM, Mascarenhas LA, Sumagin R, Extracellular vesicles regulate immune responses and cellular function in intestinal inflammation and repair, Tissue Barriers. 6 (2018) 1–14. 10.1080/21688370.2018.1431038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Neudecker V, Brodsky KS, Clambey ET, Schmidt EP, Packard TA, Davenport B, Standiford TJ, Weng T, Fletcher AA, Barthel L, Masterson JC, Furuta GT, Cai C, Blackburn MR, Ginde AA, Graner MW, Janssen WJ, Zemans RL, Evans CM, Burnham EL, Homann D, Moss M, Kreth S, Zacharowski K, Henson PM, Eltzschig HK, Neutrophil transfer of miR-223 to lung epithelial cells dampens acute lung injury in mice, Sci. Transl. Med 9 (2017). 10.1126/scitranslmed.aah5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Brook AC, Jenkins RH, Clayton A, Kift-Morgan A, Raby AC, Shephard AP, Mariotti B, Cuff SM, Bazzoni F, Bowen T, Fraser DJ, Eberl M, Neutrophil-derived miR-223 as local biomarker of bacterial peritonitis, Sci. Rep 9 (2019) 1–12. 10.1038/s41598-019-46585-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Fazi F, Rosa A, Fatica A, Gelmetti V, De Marchis ML, Nervi C, Bozzoni I, A minicircuitry comprised of microRNA-223 and transcription factors NFI-A and C/EBPα regulates human granulopoiesis, Cell. 123 (2005) 819–831. 10.1016/j.cell.2005.09.023. [DOI] [PubMed] [Google Scholar]
- [75].Citron F, Segatto I, Vinciguerra GLR, Musco L, Russo F, Mungo G, D’Andrea S, Mattevi MC, Perin T, Schiappacassi M, Massarut S, Marchini C, Amici A, Vecchione A, Baldassarre G, Belletti B, Downregulation of miR-223 expression is an early event during mammary transformation and confers resistance to CDK4/6 inhibitors in luminal breast cancer, Cancer Res. 80 (2020) 1064–1077. 10.1158/0008-5472.CAN-19-1793. [DOI] [PubMed] [Google Scholar]
- [76].Afonso PV, Janka-Junttila M, Lee YJ, McCann CP, Oliver CM, Aamer KA, Losert W, Cicerone MT, Parent CA, LTB4 Is a Signal-Relay Molecule during Neutrophil Chemotaxis, Dev. Cell 22 (2012) 1079–1091. 10.1016/j.devcel.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Szatmary AC, Nossal R, Parent CA, Majumdar R, Modeling neutrophil migration in dynamic chemoattractant gradients: Assessing the role of exosomes during signal relay, Mol. Biol. Cell 28 (2017) 3457–3470. 10.1091/mbc.E17-05-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Vargas A, Roux-Dalvai F, Droit A, Lavoie JP, Neutrophil-derived exosomes: A new mechanism contributing to airway smooth muscle remodeling, Am. J. Respir. Cell Mol. Biol 55 (2016) 450–461. 10.1165/rcmb.2016-0033OC. [DOI] [PubMed] [Google Scholar]
- [79].Huang D, Anand Miller, Murphy Sudarshan, Desgrosellier, Stupack Jay, Shattil Dwayne, Schlaepfer Sanford, Cheresh David, EGFR-dependent pancreatic carcinoma cell metastasis via Rap1 activation, Oncogene. 176 (2012) 139–148. 10.1038/onc.2011.450.EGFR-dependent. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Gao K, Jin J, Huang C, Li J, Luo H, Li L, Huang Y, Jiang Y, Exosomes derived from septic mouse serum modulate immune responses via exosome-associated cytokines, Front. Immunol 10 (2019) 1–11. 10.3389/fimmu.2019.01560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Bekeschus S, Lackmann JW, Gümbel D, Napp M, Schmidt A, Wende K, A neutrophil proteomic signature in surgical trauma wounds, Int. J. Mol. Sci 19 (2018). 10.3390/ijms19030761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Reber LL, Gillis CM, Starkl P, Jönsson F, Sibilano R, Marichal T, Gaudenzio N, Bérard M, Rogalla S, Contag CH, Bruhns P, Galli SJ, Neutrophil myeloperoxidase diminishes the toxic effects and mortality induced by lipopolysaccharide, J. Exp. Med 214 (2017) 1249–1258. 10.1084/jem.20161238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Meng Q, Wu S, Wang Y, Xu J, Sun H, Lu R, Gao N, Yang H, Li X, Tang B, Aschner M, Chen R, Mpo promoter polymorphism rs2333227 enhances malignant phenotypes of colorectal cancer by altering the binding affinity of AP-2a, Cancer Res. 78 (2018) 2760–2769. 10.1158/0008-5472.CAN-17-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Lerman I, De La Luz Garcia-Hernandez M, Rangel-Moreno J, Chiriboga L, Pan C, Nastiuk KL, Krolewski JJ, Sen A, Hammes SR, Infiltrating myeloid cells exert protumorigenic actions via neutrophil elastase, Mol. Cancer Res 15 (2017) 1138–1152. 10.1158/1541-7786.MCR-17-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Paris AJ, Liu Y, Mei J, Dai N, Guo L, Spruce LA, Hudock KM, Brenner JS, Zacharias WJ, Mei HD, Slamowitz AR, Bhamidipati K, Beers MF, Seeholzer SH, Morrisey EE, Worthen GS, Neutrophils promote alveolar epithelial regeneration by enhancing type ii pneumocyte proliferation in a model of acid-induced acute lung injury, Am. J. Physiol. - Lung Cell. Mol. Physiol 311 (2016) L1062–L1075. 10.1152/ajplung.00327.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Deryugina EI, Zajac E, Juncker-Jensen A, Kupriyanova TA, Welter L, Quigley JP, Tissue-Infiltrating Neutrophils Constitute the Major In Vivo Source of Angiogenesis-Inducing MMP-9 in the Tumor Microenvironment, Neoplasia (United States). 16 (2014) 771–788. 10.1016/j.neo.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Gomez-Chou Z, S.; Swidnicka-Siergiejko A; Chavez-Tomar M; Lesinski G; Bekaii-Saab T; Cruz-Monserrate, Lipocalin-2 Promotes Pancreatic Ductal Adenocarcinoma by Regulating Inflammation in the Tumor Microenvironment, Cancer Res. 77 (2017) 2647–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Lörchner H, Pöling J, Gajawada P, Hou Y, Polyakova V, Kostin S, Adrian-Segarra JM, Boettger T, Wietelmann A, Warnecke H, Richter M, Kubin T, Braun T, Myocardial healing requires Reg3β-dependent accumulation of macrophages in the ischemic heart, Nat. Med 21 (2015) 353–362. 10.1038/nm.3816. [DOI] [PubMed] [Google Scholar]
- [89].Han C, Nie Y, Lian H, Liu R, He F, Huang H, Hu S, Acute inflammation stimulates a regenerative response in the neonatal mouse heart, Cell Res. 25 (2015) 1137–1151. 10.1038/cr.2015.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Horckmans M, Ring L, Duchene J, Santovito D, Schloss MJ, Drechsler M, Weber C, Soehnlein O, Steffens S, Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype, Eur. Heart J 38 (2017) 187–197. 10.1093/eurheartj/ehw002. [DOI] [PubMed] [Google Scholar]
- [91].Hu T, Lu YR, BCYRN1, a c-MYC-activated long non-coding RNA, regulates cell metastasis of non-small-cell lung cancer, Cancer Cell Int. 15 (2015) 1–8. 10.1186/s12935-015-0183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Güngör N, Knaapen AM, Munnia A, Peluso M, Haenen GR, Chiu RK, Godschalk RWL, Van Schooten FJ, Genotoxic effects of neutrophils and hypochlorous acid, Mutagenesis. 25 (2010) 149–154. 10.1093/mutage/gep053. [DOI] [PubMed] [Google Scholar]
- [93].Reczek CR, Chandel NS, The two faces of reactive oxygen species in cancer, Annu. Rev. Cancer Biol 1 (2017) 79–98. 10.1146/annurev-cancerbio-041916-065808. [DOI] [Google Scholar]
- [94].Granot ZBR, Henke E, Comen EA, King TA, Norton L, Tumor entrained neutrophils inhibit seeding in the premetastatic lung, Cancer Cell. 20 (2011) 300–314. 10.1016/j.ccr.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Nogueira V, Park Y, Chen CC, Xu PZ, Chen ML, Tonic I, Unterman T, Hay N, Akt Determines Replicative Senescence and Oxidative or Oncogenic Premature Senescence and Sensitizes Cells to Oxidative Apoptosis, Cancer Cell. 14 (2008) 458–470. 10.1016/j.ccr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Lewis A, Du J, Liu J, Ritchie JM, Oberley LW, Cullen JJ, Metastatic progression of pancreatic cancer: Changes in antioxidant enzymes and cell growth, Clin. Exp. Metastasis 22 (2005) 523–532. 10.1007/s10585-005-4919-7. [DOI] [PubMed] [Google Scholar]
- [97].Gorrini C, Harris IS, Mak TW, Modulation of oxidative stress as an anticancer strategy, Nat. Rev. Drug Discov 12 (2013) 931–947. 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- [98].Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar CM, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D, Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET, Nat. Med 18 (2012) 883–891. 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Azambuja JH, Ludwig N, Yerneni SS, Braganhol E, Whiteside TL, Arginase-1+ Exosomes from Reprogrammed Macrophages Promote Glioblastoma Progression, Int. J. Mol. Sci 21 (2020) 3990. 10.3390/ijms21113990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Liang W, Ferrara N, The complex role of Neutrophils in tumor angiogenesis and metastasis, Cancer Immunol. Res 4 (2016) 83–91. 10.1158/2326-6066.CIR-15-0313. [DOI] [PubMed] [Google Scholar]
- [101].Kowanetz M, Wu X, Lee J, Tan M, Hagenbeek T, Qu X, Yu L, Ross J, Korsisaari N, Cao T, Bou-Reslan H, Kallop D, Weimer R, Ludlam MJC, Kaminker JS, Modrusan Z, Van Bruggen N, Peale FV, Carano R, Meng YG, Ferrara N, Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes, Proc. Natl. Acad. Sci. U. S. A 107 (2010) 21248–21255. 10.1073/pnas.1015855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Igor P, Franco R, Perillo A, Borges L, Menezes D, Pacheco M, Pathology - Research and Practice Tumor microenvironment components : Allies of cancer progression, Pathol. - Res. Pract (2019) 152729. 10.1016/j.prp.2019.152729. [DOI] [PubMed] [Google Scholar]
- [103].Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, Manova-todorova K, Leversha M, Hogg N, Seshan VE, Norton L, Brogi E, A CXCL1 Paracrine Network Links Cancer Chemoresistance and Metastasis, (2012) 165–178. 10.1016/j.cell.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Leal AC, Mizurini DM, Gomes T, Rochael NC, Saraiva EM, Dias MS, Werneck CC, Sielski MS, Vicente CP, Monteiro RQ, Tumor-Derived Exosomes Induce the Formation of Neutrophil Extracellular Traps: Implications For The Establishment of Cancer-Associated Thrombosis, Sci. Rep 7 (2017) 1–12. 10.1038/s41598-017-06893-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].V Orlova V, Choi EY, Xie C, Chavakis E, Bierhaus A, Ihanus E, Ballantyne CM, Gahmberg CG, Bianchi ME, Nawroth PP, Chavakis T, A novel pathway of HMGB1-mediated inflammatory cell recruitment that requires Mac-1-integrin., EMBO J. 26(4) (2007) 1129–1139. 10.1038/sj.emboj.7601552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Shi Y, Zhang J, Mao Z, Jiang H, Liu W, Shi H, Ji R, Xu W, Qian H, Zhang X, Extracellular Vesicles From Gastric Cancer Cells Induce PD-L1 Expression on Neutrophils to Suppress T-Cell Immunity, Front. Oncol 10 (2020) 1–9. 10.3389/fonc.2020.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Cheng WC, Liao TT, Lin CC, Yuan LTE, Lan HY, Lin HH, Teng HW, Chang HC, Lin CH, Yang CY, Huang SC, Jiang JK, Yang SH, Yang MH, Hwang WL, RAB27B-activated secretion of stem-like tumor exosomes delivers the biomarker microRNA-146a-5p, which promotes tumorigenesis and associates with an immunosuppressive tumor microenvironment in colorectal cancer, Int. J. Cancer 145 (2019) 2209–2224. 10.1002/ijc.32338. [DOI] [PubMed] [Google Scholar]
- [108].Li L, Cao B, Liang X, Lu S, Luo H, Wang Z, Wang S, Jiang J, Lang J, Zhu G, Microenvironmental oxygen pressure orchestrates an anti- and pro-tumoral γδ T cell equilibrium via tumor-derived exosomes, Oncogene. 38 (2019) 2830–2843. 10.1038/s41388-018-0627-z. [DOI] [PubMed] [Google Scholar]
- [109].Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, Boireau W, Rouleau A, Simon B, Lanneau D, De Thonel A, Multhoff G, Hamman A, Martin F, Chauffert B, Solary E, Zitvogel L, Garrido C, Ryffel B, Borg C, Apetoh L, Rébé C, Ghiringhelli F, Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells, J. Clin. Invest 120 (2010) 457–471. 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Costa-silva B, Aiello NM, Ocean AJ, Singh S, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J, Zhang T, Theilen T, García-santos G, Williams C, Ararso Y, Huang Y, Rodrigues G, Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver, Nat Cell Biol. 17 (2015) 816–826. 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Perego M, Tyurin VA, Tyurina YY, Yellets J, Nacarelli T, Lin C, Nefedova Y, Kossenkov A, Liu Q, Sreedhar S, Pass H, Roth J, Vogl T, Feldser D, Zhang R, Kagan VE, Gabrilovich DI, Reactivation of dormant tumor cells by modified lipids derived from stress-activated neutrophils, Sci. Transl. Med 12 (2020) 1–17. 10.1126/SCITRANSLMED.ABB5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Schuldner M, Dörsam B, Shatnyeva O, Reiners KS, Kubarenko A, Hansen HP, Finkernagel F, Roth K, Theurich S, Nist A, Stiewe T, Paschen A, Knittel G, Reinhardt HC, Müller R, Hallek M, von Strandmann EP, Exosome-dependent immune surveillance at the metastatic niche requires BAG6 and CBP/p300-dependent acetylation of p53, Theranostics. 9 (2019) 6047–6062. 10.7150/thno.36378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Tyagi A, Sharma S, Wu K, Wu SY, Xing F, Liu Y, Zhao D, Deshpande RP, D’Agostino RB, Watabe K, Nicotine promotes breast cancer metastasis by stimulating N2 neutrophils and generating pre-metastatic niche in lung, Nat. Commun 12 (2021) 1–18. 10.1038/s41467-020-20733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Chennakrishnaiah S, Meehan B, D’Asti E, Montermini L, Lee TH, Karatzas N, Buchanan M, Tawil N, Choi D, Divangahi M, Basik M, Rak J, Leukocytes as a reservoir of circulating oncogenic DNA and regulatory targets of tumor-derived extracellular vesicles, J. Thromb. Haemost 16 (2018) 1800–1813. 10.1111/jth.14222. [DOI] [PubMed] [Google Scholar]
- [115].Bobrie A, Thery C, Exosomes and communication between tumours and the immune system: Are all exosomes equal?, Biochem. Soc. Trans 41 (2013) 263–267. 10.1042/BST20120245. [DOI] [PubMed] [Google Scholar]
- [116].Shopova IA, Belyaev I, Dasari P, Jahreis S, Brakhage AA, Human Neutrophils Produce Antifungal Extracellular Vesicles against Aspergillus fumigatus, Host Microbe Biol. (2020) 1–19. 10.1128/mBio.00596-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Yinwei Zhang P, PhD, Li Liangwei, PhD, Liu Yuan, PhD, and Liu Zhi-Ren, G. Department of Biology, Georgia State University, Atlanta, PKM2 released by neutrophils at wound site facilitates early wound healing by promoting angiogenesis, Wound Repair Regen. 24 (2016) 328–336. 10.1038/s41395-018-0061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Hwang WL, Lan HY, Cheng WC, Huang SC, Yang MH, Tumor stem-like cell-derived exosomal RNAs prime neutrophils for facilitating tumorigenesis of colon cancer, J. Hematol. Oncol 12 (2019) 1–17. 10.1186/s13045-019-0699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Diao J, Yang X, Song X, Chen S, He Y, Wang Q, Chen G, Luo C, Wu X, Zhang Y, Exosomal Hsp70 mediates immunosuppressive activity of the myeloid-derived suppressor cells via phosphorylation of Stat3, Med. Oncol 32 (2015). 10.1007/s12032-014-0453-2. [DOI] [PubMed] [Google Scholar]
- [120].Gobbo J, Marcion G, Cordonnier M, Dias AMM, Pernet N, Hammann A, Richaud S, Mjahed H, Isambert N, Clausse V, Rébé C, Bertaut A, Goussot V, Lirussi F, Ghiringhelli F, De Thonel A, Fumoleau P, Seigneuric R, Garrido C, Restoring Anticancer Immune Response by Targeting Tumor-Derived Exosomes with a HSP70 Peptide Aptamer, J. Natl. Cancer Inst 108 (2016) 1–11. 10.1093/jnci/djv330. [DOI] [PubMed] [Google Scholar]


