Abstract
Tendon injuries are one of the most common musculoskeletal disorders that cause considerable morbidity and significantly compromise the patients’ quality of life. The innate limited regenerative capacity of tendon poses a substantial treating challenge for clinicians. MicroRNAs (miRNAs) are a family of small non-coding RNAs that play a vital role in orchestrating many biological processes through post-transcriptional regulation. Increasing evidence reveals that miRNA-based therapeutics may serve as an innovative strategy for the treatment of tendon pathologies. In this review, we briefly present miRNA biogenesis, the role of miRNAs in tendon cell biology and their involvement in tendon injuries, followed by a summary of current miRNA-based approaches in tendon tissue engineering with a special focus on attenuating post-injury fibrosis. Next, we discuss the advantages of miRNA-functionalized scaffolds in achieving sustained and localized miRNA administration to minimize off-target effects, and thus hoping to inspire the development of effective miRNA delivery platforms specifically for tendon tissue engineering. We envision that advancement in miRNA-based therapeutics will herald a new era of tendon tissue engineering and pave a way for clinical translation for the treatments of tendon disorders.
Keywords: MicroRNAs (miRNAs), Tendon injuries, Tendon healing, Tissue engineering, Antiadhesion, Biomaterials
1. Introduction
Tendon injuries are one of the most common disorders in the musculoskeletal system. The etiology of tendon injuries is multifactorial, including trauma, chronic overuse, aging, inflammation and genetic factors [1–4]. Compared to other musculoskeletal tissues such as bone and muscle, the innate hypocellular and hypovascular nature of tendon renders it lacking sufficient healing ability [5]. Currently, exercise-based strategies, injection therapy and surgical intervention are the standard treating modalities for tendon injuries [6]. However, they are associated with high failure rates owing to different reasons, among which significant adhesion formation substantially weakens tissue strength, affects the restoration of function and patients’ quality of life. It has been estimated that approximately one third of flexor tendon injuries lead to scarring [7, 8], and the retear rate after rotator cuff repair is up to 90% due to fibrovascular scar formation [9]. Unlike flexor tendon injuries that often result from direct lacerations, tendinopathy is the most common cause of injuries to the patellar tendon, the Achilles tendon and the rotator cuff. The prominent pathological changes of tendinopathy include disorganized collagen fibers, dysregulated extracellular matrix (ECM), and increased microvasculature with neoinnervation [10]. Normal tendons consist predominantly of collagen I with small amount of collagen III. In tendinopathic tendons, greater amount of collagen III is produced and fails to be replaced by collagen I with time, thus leading to mechanical inferiority and eventual tendon rupture since collagen III tends to form thin and random fibers [11, 12].
To improve tendon healing quality and functional outcomes, numerous novel approaches have been developed, among which tissue engineering plays a key role in provoking the regenerative potential of injured tissue through a combination of cell- and/or scaffold-based strategies, which may create a favorable microenvironment for tissue healing [13–16]. Although cells and scaffolds are two fundamental components of tissue engineering, the concept of utilizing cell-free or scaffold-free approaches has gained increasing popularity in the field of regenerative medicine over the past few years. Notably, the effect of microRNAs (miRNAs) on tissue repair and regeneration has been exploited and miRNA-based therapeutics have demonstrated a great translatable value. miRNAs are ubiquitously expressed in different species and are evolutionarily conserved in expression [17]. To date, there have been around 2,600 mature miRNAs identified in humans that participate in the regulation of more than 60% of coding genes [18]. In contrast to short interfering RNAs (siRNAs), a unique feature of miRNAs is that they are commonly binding to the target mRNA with partial complementarity which means one miRNA is capable of regulating hundreds of target genes. Therefore, the expression change of miRNAs could exert considerable influence on many biological processes involving cell proliferation, migration, differentiation or apoptosis. While sometimes work as potentiators [19, 20], miRNAs mainly act as a silencer of coding genes, the expression of miRNAs is thought to inversely related to the regenerative potential of injured tissue. Observation of the mouse fetal skin healing pattern at different developmental stages revealed the regulatory role of miRNAs in tissue regeneration. Scarless healing of the skin at an early embryonic stage is characterized by a global suppression of miRNA function compared to scar formation at a later stage [21]. Additionally, the downregulation of miRNAs resulted in cell proliferation and enhanced tissue regeneration in patients receiving liver transplantation [22]. These results suggested that if certain miRNA functions are selectively and purposely suppressed in the injured tissue, the silencing of coding genes responsible for tissue repair and regeneration could be revoked and may restore the regenerative potential akin to the in-utero state [23].
Compelling evidence have shown dysregulation of miRNA expression is correlated with diseases in the musculoskeletal, cardiovascular and nervous system [24–28]. More importantly, miRNAs play a critical role in maintaining normal tissue homeostasis and has been associated with the occurrence of tendinopathy [29–33]. Herein, we review the current knowledge of miRNA function related to tendon cell biology and miRNA profiling in tendon injuries. In addition, we summarize the application of miRNAs in tendon tissue engineering, especially in preventing strategy for tendon disorders.
2. miRNA biogenesis
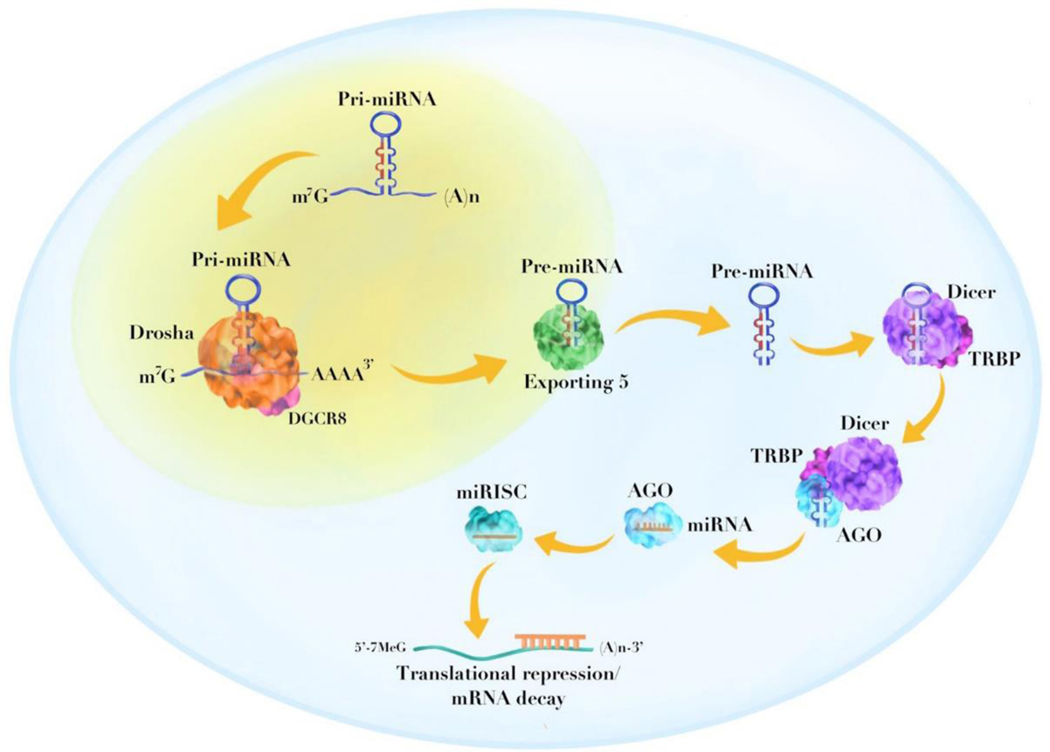
miRNAs are a class of small noncoding endogenous RNAs (normally 20–24 nucleotides long) 12 that regulate post-transcriptional gene expression. miRNAs can be categorized into two types: intracellular miRNAs and extracellular miRNAs (i.e., circulating miRNAs) [34]. The miRNA genes are first transcribed by RNA polymerase II or III into primary miRNAs (pri-miRNAs), which have a stem-loop structure [35, 36]. The pri-miRNAs are further processed by RNase III enzyme, Drosha and DGCR8 (DiGeorge Syndrome Critical Region 8) in the nucleus into precursor miRNAs (pre-miRNAs) with a hairpin structure (approximately 60–80 nucleotides in length) [37, 38]. The pre-miRNAs are then exported to the cytoplasm and subjected to processing by Dicer and TRBP (transactivation response element RNA-binding protein) to generate mature miRNA duplexes. The miRNA duplexes are subsequently unwinded to form single-stranded miRNA molecules which enter the RNA-induced silencing complex (RISC) by interacting with Argonaute (AGO) proteins. Eventually, the RISC binds to the 3ʹ untranslated region (UTR) of the target messenger RNA (mRNA) and functions by either inducing mRNA degradation or repressing gene translation [39] (Figure 1).
Figure 1.
Schematic illustration showing the biogenesis of miRNA. The pri-miRNA is first cleaved by Drosha and DGCR8 to form pre-miRNA with a hairpin structure. The pre-miRNA is exported to the cytoplasm by Exporting 5 and subjected to further processing by Dicer and TRBP. The mature miRNA duplex is unwinded and enter the RISC by interacting with AGO proteins. The RISC binds to the 3’ UTR of target mRNA to induce either translation repression or mRNA degradation. DGCR8, DiGeorge syndrome critical region 8; TRBP, transactivation response element RNA-binding protein; RISC, RNA-induced silencing complex; AGO, argonaute; UTR, untranslated region.
3. miRNAs in tendon tissue engineering
3.1. miRNA profiling and tendon injuries
Tendon injuries encompass chronic tendinopathy and acute tendon rupture. Under normal conditions, physiological loading can lead to increased ECM synthesis and tenocyte density [40]. When a tendon fails to compensate the mechanical stimuli and age-related changes, including oxidative stress, cellular senescence, calcification or fatty infiltration, tendinopathy persists causing pain and dysfunction [41]. Taking a profiling approach, previous studies have revealed differentially expressed miRNAs in tendinopathy. Millar et al. [42] collected 17 samples of supraspinatus tendon (torn tendon) and subscapularis tendon (early tendinopathy) from the same patients during shoulder surgery, respectively. Ten subscapularis tendon samples from patients without rotator cuff tears were served as controls. They found expression of miR-29 family in all tendon samples with miR-29a demonstrating a remarkable reduction in early tendinopathic tendons compared with control and torn tendons. Hall et al. [43] acquired ten paired supraspinatus (tendinopathy) and subscapularis (normal) tendon samples arthroscopically from five patients and identified 21 differentially expressed miRNAs using RNA sequencing. In particular, six miRNAs (let-7g, miR-7a, miR-22, miR-26a/b and miR-29a) were predicted to regulate genes involved in tendinopathy including bone morphogenetic protein (BMP)-2, BMP-7, interleukin (IL)-6, collagen I and III. [42]
Matrix metalloproteinases (MMPs) and tissue inhibitors of matrix metalloproteinases (TIMPs) are two major players in maintaining normal tendon homeostasis [44]. The imbalance between MMPs and TIMPs could be the reason of ECM disorganization, a hallmark of tendinopathy. One study evaluated the miRNAs expression pattern in the long head of biceps tendons obtained from patients with or without glenohumeral arthritis [33]. A total of 1001 miRNAs were identified by microarray analysis to mediate the expression of 81 genes responsible for ECM disorganization. More importantly, seven highly altered miRNAs (miR-145–5p, miR-151a-3p, miR-382–5p, miR-199a-5p, miR-21–5p, miR-125a-5p and miR-498) were projected to be associated with the tendon ECM reorganization through regulating MMPs expression [33]. Persistent inflammation is another hallmark of tendinopathy. Following the above study, Thankam et al. [32] examined the miRNA profiling and associated inflammatory pathway in patients with or without glenohumeral arthritis and rotator cuff tears by sampling the long head of biceps tendon. There were 235 miRNAs demonstrated fold changes, among which 39 miRNAs and 196 miRNAs were significantly upregulated and downregulated, respectively. The authors screened 284 genes that closely related with inflammation via 120 different pathways which subjected to the regulation by approximately 1500 miRNAs. By selecting miRNAs that have multiple target genes, 77 miRNAs were found to have regulatory control over more than 10 genes. Furthermore, the expression of miR-145–5p, miR-100–5p, miR-23b-3p, let-7d-5p, miR-146a-5p, miR-150–5p, miR-181a-5p and miR-193b-3p was decreased more than 20-fold in patients who had severe inflammation, suggesting their potent role in regulating inflammatory genes and interconnecting pathways. Additionally, Thankam et al. [45] identified 13 miRNAs related to fatty infiltration and inflammation from rotator cuff injured patients that are associated with genes involved in metabolic homeostasis and inflammation.
More recently, circulating miRNAs were detected in liquid biopsies (i.e., venous blood) from 14 patients with chronic tendinopathy or degenerative tear of the rotator cuff to identify distinct miRNA profiles [31]. The serum level of six miRNAs (miR-19b-3p, miR-192–5p, miR-25–3p, miR-19a-3p, miR-18b-5p and miR-93–5p) was only downregulated in degenerative tear samples when compared to chronic tendinopathy samples or healthy controls. Another six miRNAs (miR-30a-5p, miR-324–3p, miR-210–3p, miR-140–3p, miR-425–5p and miR-222–3p) were differentially expressed in pathological serum samples compared to healthy controls. Subsequently, nine miRNAs repressed in sera (miR-29a-3p, miR-29c-3p, miR-30a-5p, miR-140-3p, miR-192–5p, miR-210–3p, miR-222–3p, miR-324–3p and miR-425–5p) were selected to examine their expression in torn supraspinatus or intact subscapularis tendons collected from patients. Of note, except for miR-222–3p, the rest miRNAs either demonstrated a significant repression or a clear trend of repression when compared to healthy controls, which was in good agreement with the serum results. These findings provided us with a distinct signature of miRNAs in degenerative rotator cuff injuries and may direct the development of novel diagnostic tools for such disorders [31]. Major findings from clinical studies described in this section are summarized in Table 1.
Table 1.
miRNA profiling in clinical studies
| Condition/mode l | Sample type | Profiling method | Major findings | Observed expression | Ref |
|---|---|---|---|---|---|
| Rotator cuff tendinopathy | Supraspinatus tendon | RNA sequencing | Identified 6 miRNAs predicted to bind to genes involved in tendinopathy | Upregulated: miR-7a-5p Downregulated: let-7g-5p, miR-22– 3p, miR-26a/b-5p, miR-29a-3p |
[43] |
| Glenohumeral arthritis | Long head of biceps tendon | Microarray | Identified 7 miRNAs associated with genes in the pathways involved in collagen disorganization | Upregulated: miR-498 Downregulated: miR-145–5p, miR- 151a-3p, miR-382– 5p, miR-199a-5p, miR-21 −5p, miR- 125a-5p |
[33] |
| Glenohumeral arthritis and massive RCTs | Long head of biceps tendon | Microarray | Identified 8 miRNAs with over 20-fold change that may participate in regulating inflammation | Downregulated: miR-145–5p, miR-100–5p, miR-23b- 3p, let-7d-5p, miR- 146a-5p, miR-150– 5p, miR-181a-5p, miR-193b-3p | [32] |
| Rotator cuff injury with fatty infiltration and inflammation | Long head of biceps tendon | Microarray | Identified 13 miRNAs associated with genes in the pathways involved in metabolic homeostasis and inflammation | Upregulated: miR-297 Downregulated: miR-145–5p, miR- 99a-5p, miR100– 5p, miR-150–5p, miR-193b-3p, miR-103a-3p, miR-31 −5p, miR-195–5p, miR-497– 5p, miR-15a-5p, miR-16–5p, let-7b- 5p |
[45] |
| Rotator cuff tendinopathy | Subscapularis tendon | qPCR array | Identified miR29a/b differentially expressed in early tendinopathic tendons | Downregulated: miR-29a/b | [42] |
| Rotator cuff tendinopathy/degenerative RCTs | Serum/Suprasp inatus tendon | qPCR array | Identified 6 miRNAs differentially expressed in sera from patients with either RCTs or tendinopathy/RCTs, respectively. 5 out of these 12 miRNAs were significantly repressed in tendon biopsy samples | Serum from RCTs samples Downregulated: miR-19b-3p, miR-192–5p, miR-25– 3p, miR-19a-3p, miR-18b-5p, miR-93-5p Serum from tendinopathy/RCTs samples Downregulated: miR-30a-5p, miR- 324–3p, miR-210– 3p, miR-140–3p, miR-425–5p, miR- 222–3p Tendon biopsies Downregulated: miR-29a-3p, miR- 29c-3p, miR-30a–5p, miR-140–3p, miR-192–5p |
[31] |
RCTs, rotator cuff tears.
3.2. miRNAs in tendon cell biology
The physiological role of miRNAs in many biological processes have been well established [17, 30, 46, 47]. Among them, several studies have demonstrated the significance of miRNAs in mediating tenogenesis, tendon cell apoptosis or senescence [48–51] as summarized in Table 2. For example, Wang et al. [51] found decreased collagen formation during the tenogenesis of human tendon derived stem cells (TDSCs) as a result of targeted suppression of early growth response-1(EGR1) by miR-124. Similarly, miR-217 inhibited the differentiation of human TDSCs towards tenogenic pathway by decreasing EGR1 expression [52]. Chen et al. [48] demonstrated miR-135a can promote the tenogenic differentiation of rat TDSCs through upregulating the gene expression of scleraxis (Scx) and tenomodulin (Tnmd) which critically involved in tenocyte specification. In another study using mouse TDSCs, the transfection of miR-378a mimics led to significant decrease of collagen synthesis and tenogenic gene expression. The miR-378a inhibitory regulation of tenogenesis was further confirmed by targeting TGF-β2 [49].
Table 2.
Selected miRNAs and their roles in tendon cell biology
| miRNA(s) | Cell type | Target gene(s) and effect | Function | Ref |
|---|---|---|---|---|
| miR-29b-3p | Human TDSCs | TGF-β1 and COL1A1 ↓ | Inhibit tenogenic differentiation | [85] |
| miR-29b | Rat fibroblasts | TGF-β1 ↓ | Inhibit tendon fibroblasts growth | [84] |
| miR17–92 | Primary human tenocytes | bim ↓ | Suppress apoptosis | [50] |
| miR-135a | Rat TDSCs | Scx and Tnmd ↑ ROCK1 ↓ |
Promote tenogenic differentiation Suppress senescence | [48] |
| miR-124 | Human TDSCs | EGR1 ↓ | Inhibit tenogenic differentiation | [51] |
| miR-217 | Human TDSCs | EGR1 ↓ | Inhibit tenogenic differentiation | [52] |
| miR-140–5p | Human TDSCs | Pin1 ↓ | Promote senescence | [54] |
| miR-378a | Mouse TDSCs | TGF-β2 ↓ | Inhibit tenogenic differentiation | [49] |
Aging is thought to have negative effects on the composition and function of tendon. A comprehensive RNA expression analysis of young and aged human Achilles tendon identified a suppression of miR-1245a in aged tendon compared with young tendon [53]. When comparing TDSCs isolated from aged and young rats, Chen et al. [54] found remarkable downregulation and upregulation of miR-135a in aged and young TDSCs, which was accompanied by increased and decreased expression of Rho-associated coiled-coil protein kinase 1 (ROCK1), respectively. Overexpression of miR-135a in aged TDSCs resulted in decreased expression of ROCK1 and other senescence markers such as p16 and β-galactosidase compared to controls. Conversely, the suppression of miR-135a in young TDSCs aggravated senescence as evidenced by increased expression of ROCK1, p16 and β-galactosidase. Meanwhile, cell proliferation and migration capacities were improved in aged TDSCs overexpressing miR-135a whereas opposite effects were seen in young TDSCs with anti-miR-135a. Furthermore, the authors revealed the control of miR-135a over TDSCs senescence was mediated by repressing ROCK1 through direct binding to its 3’ UTR. Since ROCK1 is correlated with TDSC aging [55], the application of miR-135a may contribute to the reversal of tendon degeneration [48].
The response of tendons to mechanical stimuli has also been associated with altered miRNA expression. Mendias et al. [56] compared the expression of miRNAs in Achilles tendons between sedentary and treadmill exercised rats. They found miR-100 and miR-378, which involved in cell proliferation and ECM production, were differentially expressed under mechanical loading. Additionally, three miRNAs (miR-338, miR-381 and miR-743a) that predicted to bind to the Scx was significantly suppressed in loaded tendons. Given the important regulatory role of Scx in tendon cell biology, it is reasonable to postulate that these miRNAs may participate in the mechano-adaptation of tendons.
Although several miRNAs have been identified in in vitro studies to regulate tendon cell biology, the majority of these studies have not taken into account that there are heterogeneous populations of cells reside within the tendon, such as tenoblasts, tenocytes and TDSCs, on which different miRNAs may have specific subpopulation functions. It is intriguing to explore the potential differential effect of a single miRNA on each distinct cell population in an in vivo environment. In addition, how miRNAs guide the differentiation of TDSCs is not fully understood. Previous reports have shown TDSCs differentiated towards nontenocyte lineages when responded to excessive mechanical loading, but whether miRNA is associated with this process remains unknown [57, 58]. Future studies are warranted to unveil the role of miRNAs in determining the fate of TDSCs after tendon injury.
3.3. miRNAs alleviate adhesion formation
Due to the limited regenerative capacity, tendon often heals via the formation of scar tissue with suboptimal histological and mechanical properties [5]. In rotator cuff injuries, the tendon-to-bone interface is filled with fibrovascular scar tissue after repair that never recapitulates the functional transition zone in the native enthesis [59]. Adhesion formation is also a major complication after flexor tendon injury which impairs the digit mobility and may require reoperation [7]. Tendon healing can be divided into three stages: inflammation, proliferation and remodeling. Both extrinsic and intrinsic mechanisms are involved in each healing stage [5, 8]. Extrinsic healing is characterized by the recruitment and infiltration of surrounding fibroblasts which leads to scar formation. In contrast, intrinsic healing activates tenocytes in the endotenon and epitenon together with the recruitment of TDSCs within the tendon proper contributing to scarless healing [60]. However, in most cases, extrinsic healing prevails over intrinsic healing and damages tendon gliding properties. While various methods have been attempted to reduce the formation of adhesions, including hyaluronic acid, growth factors, lubricin and mechanical barriers, none of them have yielded satisfactory outcomes in clinical settings [61–64].
Mechanistically, the fibrosis after tendon injury can be attributed to the disruption of tissue mechanical homeostasis, which sustains the normal tissue stiffness and cell contractility. To reveal the regulatory feedback pathways mediating mechanical homeostasis, Moro et al. [29] identified a network of 122 AGO2-associated miRNA families responsible for keeping constant tissue properties through buffering 73 targeted mRNAs that encodes cytoskeletal, contractile, adhesive and extracellular matrix proteins. By quantifying tissue stiffness with atomic force microscopy in a zebrafish fin-fold model, it was shown that ago2 −/− mutants had approximately 30% higher elastic modules compared to wild type (WT) embryos. This abnormal tissue stiffness was recovered by administration of human AGO2 mRNA. In addition, the limb repair speed was comparable between WT embryos and ago2 −/− mutants which received human AGO2 mRNA. These results shed a light on the role of miRNA-dependent regulatory network in stabilizing mechanical homeostasis and promoting tissue healing.
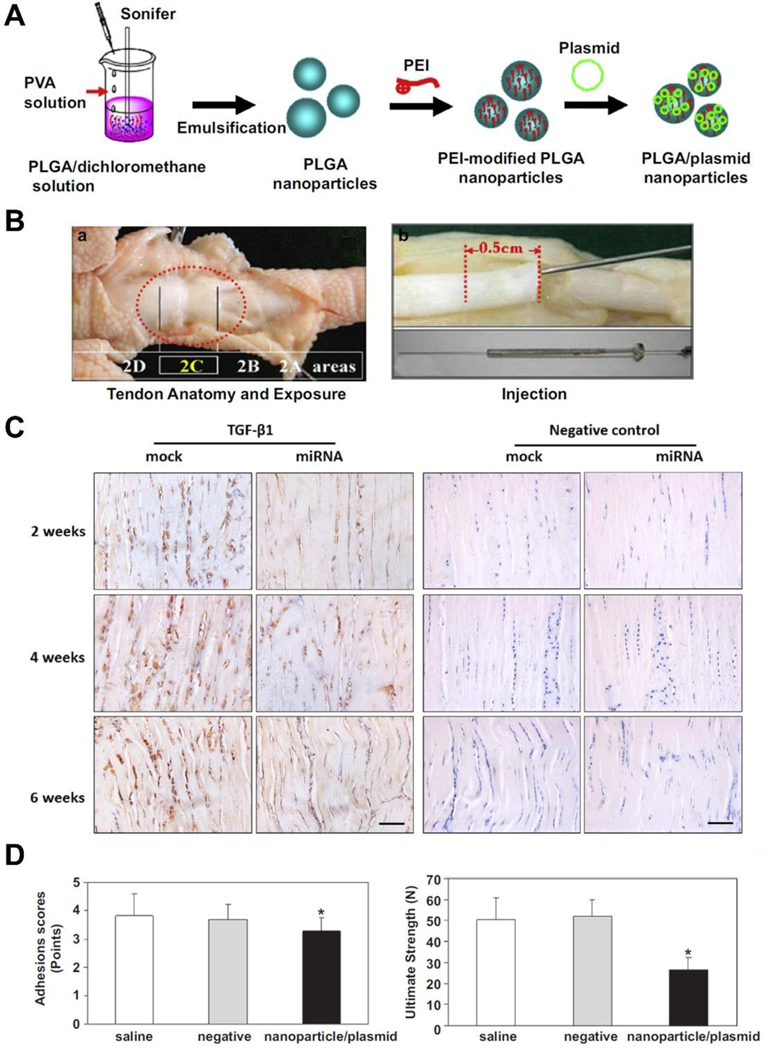
Among various growth factors that participate in tendon healing, TGF-β1 is one of the most extensively studied factors that contributes to scar tissue formation [65–67]. Engineered miRNA plasmid targeting TGF-β1 gene was delivered by polylactic-co-glycolic acid (PLGA) nanoparticles into tenocytes in vitro and to the flexor tendon laceration site in vivo to modulate TGF-β1 expression [68, 69] (Figure 2). The nanoparticle/miRNA plasmid significantly suppressed the TGF-β1 protein synthesis in tenocytes after 3 days of culture, and this effect persisted to 21 days of culture [69]. Similarly, the injection of nanoparticle/miRNA plasmid in vivo resulted in decreased TGF-β1 expression accompanied with downregulation of collagen I and III mRNA compared to nanoparticle/mock miRNA.
Figure 2.
(A) Schematic illustration demonstrating the process of preparing nanoparticle/plasmid complexes; (B) a) The area between the proximal interphalangeal and distal interphalangeal joint levels of chicken toes corresponds to zone II of human hands and the major pulley structure (2C); b) Various agents were injected to the cross-section of tendon cut at a depth of 0.5 cm using a microinjection needle; (C) Representative images of immunohistochemical staining of TGF-β1 in repaired tendons treated with nanoparticle/miRNA plasmid or nanoparticle/mock plasmid at 2, 4 and 6 weeks after surgery. Scale bar=100 μm; (D) Adhesion scores and ultimate strength were significantly lower in nanoparticle/miRNA plasmid treated tendons compared with saline and negative (nanoparticle/mock plasmid) controls at 6 weeks after surgery. *p < 0.05 compared with saline and negative controls. Data are expressed as mean ± SD. (A), (B), (D) Adapted and reprinted with permission from Ref. [68]; (C) Adapted and reprinted with permission from Ref. [69].
Of note is that although the application of nanoparticle/miRNA plasmid reduced adhesion formation and did not elicit notable inflammation after tendon repair, the ultimate repair strength was significantly lower than the nanoparticle/mock plasmid treated tendons [68, 69]. Using adeno-associated virus (AAV) as a vector, Wu et al. [70] transferred TGF-β1-miRNA to the injured flexor tendon in a chicken model. At postoperative 6 and 8 weeks, the TGF-β1 protein synthesis was decreased accompanied with improved tendon gliding property and adhesion formation. However, consistent with previous reports, the ultimate strength of repaired tendons treated with TGF-β1-miRNA was 12–24% lower than that of the control tendons. The inferior mechanical properties observed in TGF-β1-miRNA treated tendons suggested that purely inhibition of TGF-β1 function have a mixed influence on flexor tendon healing. This could be attributed to the fact that TGF-β1 does not only play a role in scar tissue formation but also other biological processes related to tendon healing and regeneration such as cell proliferation, migration or ECM production [71, 72]. Katzel et al. [73] found that TGF-β deficiency mice had lower flexor tendon repair strength and decreased expression of collagen I compared to WT controls. It has also been shown that TGF-β inhibition significantly reduced collagen I production in cultured rabbit flexor tendon cells [74]. Therefore, the downregulation of collagen I induced by TGF-β1-miRNA is likely the reason for the decreased mechanical strength in the above studies.
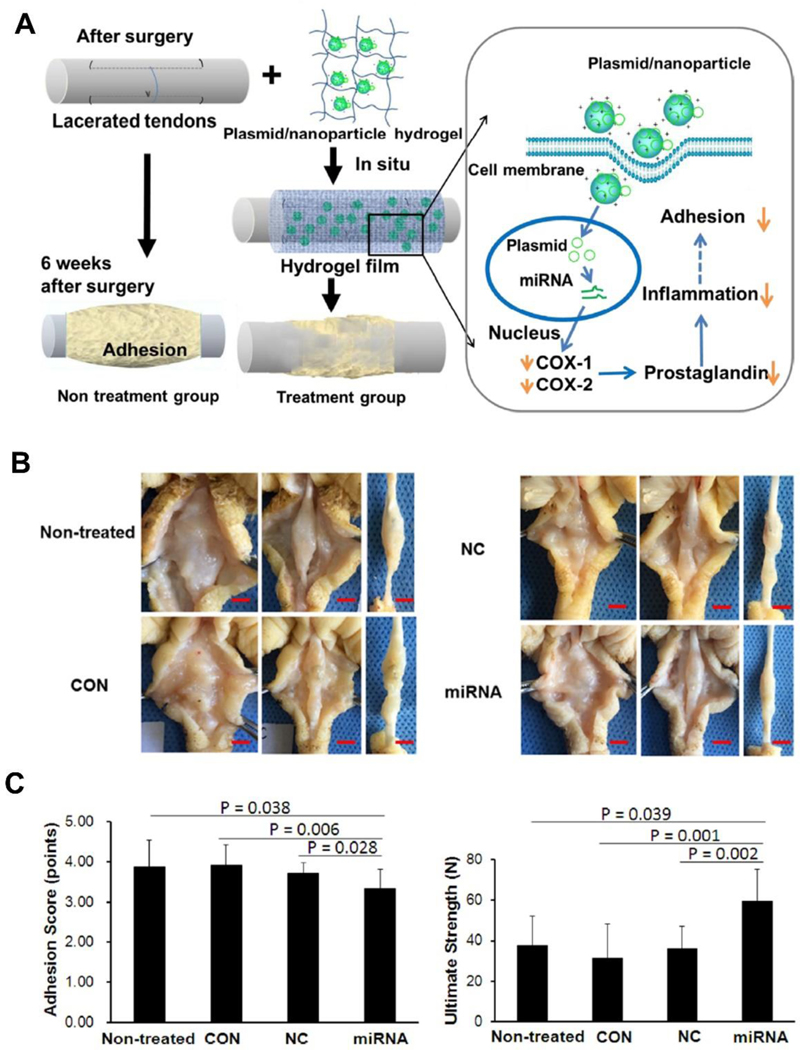
In addition to TGF-β1, cyclooxygenases (COX-1/COX-2) are important inflammation-related proteins that regulate the inflammatory response after tendon injury [75, 76]. The degree of adhesion formation of an injured tendon is closely related to the degree of inflammatory response [77]. Consequently, controlling or reducing the inflammation after tendon injury can effectively prevent the formation of adhesions. To this end, Zhou et al. [78] developed a novel system for controlled topical delivery of cyclooxygenase-engineered miRNA plasmids via PLGA nanoparticles and hyaluronic acid hydrogels. Three different miRNA plasmids were screened for their effects on COX-1/COX-2 gene silencing. It was shown that COX-1-miRNA1 and COX-2- miRNA2 had the most substantial inhibition of COX-1/COX-2 in tenocytes, respectively. These two miRNAs were subsequently used for in vivo studies which effectively downregulated the COX-1/COX-2 protein in the injured tendon and surrounding subcutaneous tissue one-week after surgery. Functionally, the tendon gliding excursions and work of flexion were both enhanced in the nanoparticle/miRNA plasmid hydrogel group compared to other groups, though the result of work of flexion did not reach statistical significance. Additionally, the gross observation and adhesion scoring were better in the nanoparticle/miRNA plasmid hydrogel group, indicating effective reduction of adhesions. More encouragingly, the mechanical strength of the nanoparticle/miRNA plasmid hydrogel treated tendons was significantly higher than that of the other groups (Figure 3). Unlike the blockade of TGF-β1, targeted silencing of COX-1/COX-2 genes with miRNA-based strategy yielded histological and mechanical satisfactory results, which may serve as a more desired approach in the modulation and improvement of tendon healing.
Figure 3.
(A) Schematic diagram of adhesion formation after tendon injury in the non-treatment group and treatment group. Tendon adhesion in the treatment group was reduced by the local delivery of nanoparticle/miRNA plasmid targeting COX1/COX2 in hydrogel through inhibiting the synthesis of prostaglandins; (B) Representative images of repaired tendons after 6 weeks of surgery. There were less adhesions in the nanoparticle/miRNA plasmid hydrogel treated group compared to non-treated, hydrogel only (CON) and nanoparticle/mock plasmid hydrogel (NC) treated groups. Scale bar=0.5 cm; (C) Adhesion score and ultimate strength of the repaired tendons were significantly improved in the nanoparticle/miRNA plasmid hydrogel group compared with other groups at 6 weeks post-surgery. Adapted and reprinted with permission from Ref. [78].
During the early inflammatory stage of tendon healing, besides the infiltration of fibroblasts, the migration of macrophages to the injury site is considered to exert a negative effect on the healing process [1, 79]. Previous reports have demonstrated that by depleting macrophages in Achilles tendon injury, the mechanical properties of the repaired tendon were significantly improved [80]. In a study by Cui et al. [81], the depletion of bone marrow-derived macrophages (BMDMs) in mice flexor tendon injury led to minor fibrosis and less inflammatory cell infiltration around the repair site as well as unimpaired joint mobility. Interestingly, the ultimate tensile strength of repaired tendons was not compromised in the macrophage-depleted group which is quite counterintuitive as accumulation of fibrous tissue could increase the repair strength. Thus, it was postulated that diminishing fibrous tissue within the tendon cutting ends allowing better collagen fiber regeneration and integration contributed to the maintained tendon healing strength. Further, the introduction of BMDMs-exosome reactivated the fibrotic process, which was found to be mediated by exosomal miR-21–5p. More importantly, it was revealed that miR-21–5p exerted its pro-fibrogenic function through suppressing Smad7 expression. Recently, Yao et al. [82] found that human umbilical cord mesenchymal stem cell (MSC)-derived exosomes (HUMSC-Exos) decreased rat fibroblast proliferation and downregulated fibrosis-related genes expression in vitro. Additionally, localized delivery of HUMSC-Exos alleviated formation of adhesions in injured Achilles tendons. Specifically, the HUMSC-Exos contained low amount of miR-21a-3p by which the activity of p65 and COX-2 was repressed, which is consistent with previous study that inhibition of RelA/p65 pathway may prevent tendon adhesions [83]. Taken together, regulating exosomal miR-21 could promote tendon healing by reducing fibrosis through Smad7 and p65 mediated pathways.
Another major fibrosis regulator is miR-29. Previous reports have shown miR-29b affected fibroblasts growth and improved Achilles tendon healing via inhibiting TGF-β1/Smad3 pathway [84]. In contrast, Lu et al. [85] demonstrated that miR-29b-3p negatively regulate tenogenesis by targeting TGF-β1 and collagen I expression. This discrepancy further substantiates the notion that inhibition of TGF-β1 alone may not achieve desired tendon healing. For rotator cuff injured patients with stiffed shoulder, a systemic (serum) and local (bursa tissue and cell) reduction in miR-29a expression is associated with the severe subacromial bursa fibrosis [86]. When inducing rotator cuff injury in miR-29a overexpression mice, the tissue fibrosis, inflammation response and gait profiles of injured limbs were significantly improved compared to WT mice. The favorable result of miR-29a in mitigating fibrotic subacromial bursa was owing to targeted silencing of collagen III gene [86].
Compiled evidence has shown miR-21 and miR-29 mediate fibrosis in many organs, such as the heart [87, 88], liver [89, 90], lung [91, 92] and kidney [93, 94]. miR-21 also serves as a long-term fibrotic memory keeper of MSCs [95]. However, their pro-fibrotic role in tendon injury has not been elucidated previously. These above studies extended our understanding of the relationship between miRNAs and tissue fibrosis, which may pave new avenues for miRNA-based approaches in improving peritendinous adhesion formation.
3.4. miRNAs facilitate tendon remodeling
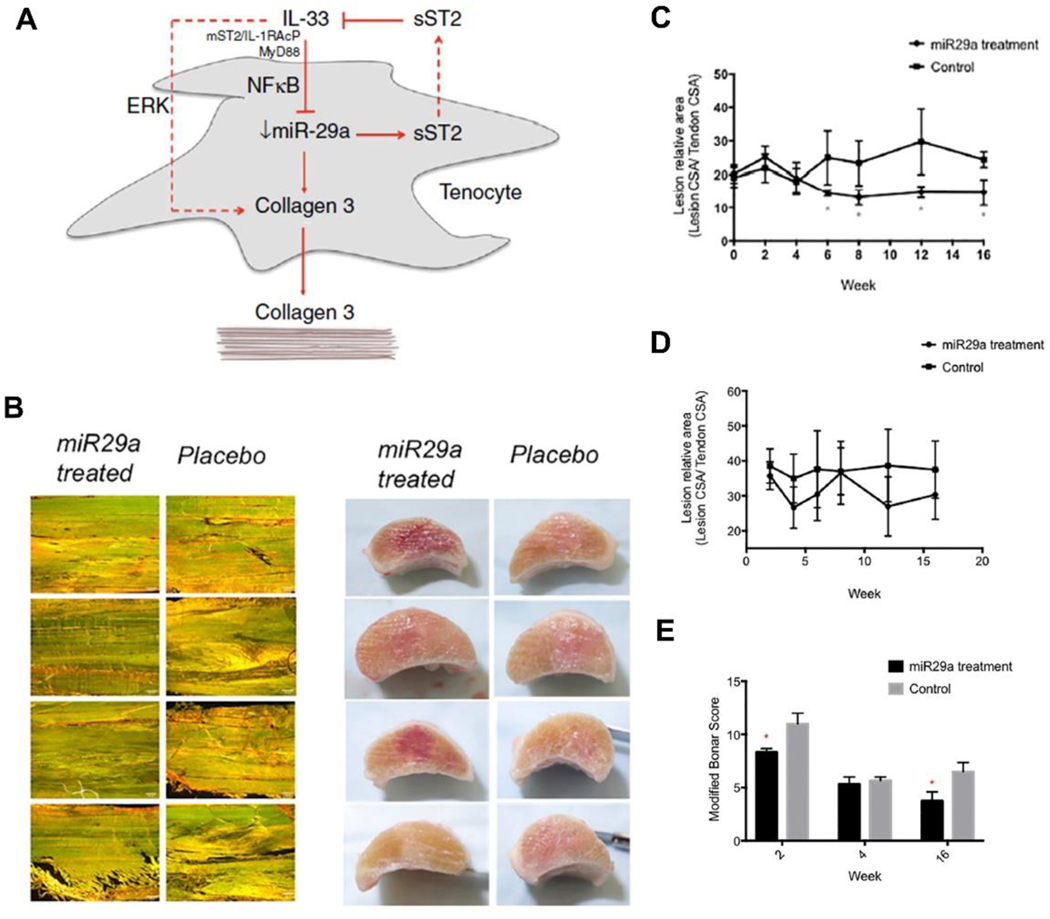
Similar to the pathophysiology of tendinopathy, the imbalance of collagen I to collagen III ratio, namely increased collagen III and decreased collagen I synthesis during the tendon remodeling phase, contributes to the inferior mechanical strength and undesired tendon healing [12, 41]. It has been suggested that IL-33 play a role in inflammation response and fibrotic disorders [96, 97], however, whether it’s implicated in tendon diseases and how it influences tendon healing remains unknown. Based on animal and human tendinopathy models, Millar et al. [42] revealed that inflammatory response and imbalance in collagen production was initiated by IL-33/ST2 pathway. In particular, the IL-33/ST2 pathway functions by increasing collagen III synthesis after tendon injury which is associated with decreased mechanical strength. Considering that miR-29 family target collagen I and III genes, the researchers predicted that miR-29 may regulate IL-33/ST2 pathway with computational algorithms, especially miR-29a which had substantial expression change in tendinopathy biopsies. Next, transfection assays in human tenocytes confirmed that miR-29a directly inhibited the expression of soluble ST2. In a mice tendon healing model, the miR-29a expression was downregulated by 22-fold and 6-fold on day 1 and day 3 post injury, respectively. The administration of exogenous IL-33 resulted in a 10-fold decrease of miR-29a expression in injured WT mice while had no effect on St2−/− mice, indicating miR-29a is mediated by IL-33/ST2 system to some extent. Finally, the delivery of miR-29a mimic to treat the injured tendon significantly reduced collagen III production without affecting collagen I synthesis.
Following the above study, Watts et al. [98] evaluated the effect of miR-29a replacement on treating tendinopathy in a randomized study using an equine model. The miR-29a mimic transfected equine tenocytes had decreased collagen III expression but unchanged collagen I expression, which corresponds well with previous reports on human tenocytes [42]. When administering miR-29a mimic or placebo to tendon lesions one-week post injury, the expression of collagen III but not collagen I was significantly decreased at two weeks after injury. In addition, miR-29a treated tendons showed improved histology in terms of lower normalized lesion cross-sectional area and modified Bonar scores (Figure 4). These results provided further evidence supporting the potential therapeutic role of miR-29a delivery in facilitating tendon remodeling and improving tendon healing after injury.
Figure 4.
(A) Schematic illustration showing the role of IL-33 and miR-29a in tendon disease; (B) Longitudinal section images of horse superficial digital flexor tendon stained with Picrosirius Red with miR-29a or placebo treatment after 16 weeks, scale bar=200 μm (Left column), and cross sectional gross images of miR-29a or placebo treated tendons after 16 weeks; Normalized lesion to tendon cross sectional area (CSA) for miR29a- and placebo-treated tendons at different time points post treatment evaluated by ultrasound (C) and MRI (D). *p < 0.05 versus control (one-tailed Student’s t test); (E) Modified Bonar score (cell density, vascularity, linear fiber, and polarized collagen) was significantly improved in miR-29a-treated tendons compared to controls at 2 and 16 weeks. *p < 0.05 versus controls (Mann-Whitney U test). (A) Adapted and reprinted with permission from Ref. [42]; (B), (C), (D), (E) Adapted and reprinted with permission from Ref. [98].
3.5. miRNAs in tendon angiogenesis
Being a hypovascular tissue, angiogenesis is a key factor in tendon healing. A variety of miRNAs have been reported to regulate the process of angiogenesis [46], among which miR-210 has been investigated in promoting tendon healing. In a rat Achilles tendon injury model, miR-210 injection resulted in significantly higher collagen fiber diameter, maximum failure load and capillary density at two weeks. The expression of vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF)-2 and collagen I were also upregulated in miR-210 treated tendons three days after injection. However, the positive effect of miR-210 on capillary density diminished at six weeks, suggesting the miR-210 mediated angiogenesis was confined to the early stage of tendon healing [99]. Recently, in a rat rotator cuff injury model, Xu et al. [100] demonstrated that MSCs overexpression VEGF contributed to significantly higher ultimate failure load at 4 and 8 weeks postoperatively compared to controls. The expression of collagen I and II at the tendon-bone interface were upregulated via MSCs-VEGF treatment. It was further confirmed that VEGF was targeted by miR-205–5p during the healing process. Through inhibiting miR-205–5p in MSCs, the VEGF expression was remarkably increased as well as the repair strength, indicating improved tendon-to-bone healing.
4. miRNA delivery for future clinical translation
miRNA-based therapies can generally be classified into miRNA inhibition therapy and miRNA replacement therapy. The miRNA inhibition therapy is conducted by using anti-miRs or miRNA inhibitors. The miRNA replacement therapy is conducted by introducing miRNA mimics into cells which act as exogenous source of additional miRNAs [101]. Since systemic administration of miRNA is subjected to rapid clearance, the success of miRNA therapy mainly lies in an effective localized and controlled delivery to desired tissues or organs. Scaffold-based system possesses the advantage of realizing site-specific miRNA delivery by which the off-target effects of miRNA therapy could be avoided and thus maximizing miRNAs’ function. In addition, the natural degradation of scaffolds can ensure a sustained release of encapsulated or immobilized miRNA. Recently, a variety of biomaterials have been developed to serve as novel delivery platforms for miRNA-based therapeutics such as hydrogels, collagen-nanohydroxyapatite scaffold, electrospun scaffold and microspheres [102–105].
Hydrogels are one of the most widely utilized materials in tendon tissue engineering [106]. The miRNA can be encapsulated into hydrogels without compromising its bioactivity. More importantly, the degradation rate of hydrogels could be fined-tuned through adjusting its physicochemical properties to enable long-term sustained delivery of miRNAs. Conde et al. [103] reported a self-assembled RNA-triple-helix hydrogel bearing miR-221 antagomir and miR-205 mimic for tumor abrogation in a triple-negative breast cancer mouse model. The RNA-triple-helix was initially conjugated to dendrimers (triplex nanoconjugates) and then mixed with dextran aldehyde to form hydrogel scaffolds that can readily adhere to the tumor tissue. The cellular uptake efficiency of the triplex nanoconjugates was close to 100% (99.8±2.5%) with the internalization began at 3h and reached peak at 48h in cancer cells in vitro. After implanting the RNA triplex hydrogel scaffolds to the tumor in vivo, neither inflammation around the surgical site nor body weight change was noticed, indicating good biocompatibility of the hydrogel scaffolds. Regarding tumor suppression, approximately 90% reduction of tumor size was obtained by the RNA triplex hydrogel scaffolds at 13 days after implantation. Furthermore, the considerable tumor shrinkage was correlated with significantly prolonged animal survival. Hence, the dextran-dendrimer hydrogel scaffold could be an effective platform for efficient local miRNA delivery.
Electrospun fibrous scaffolds are capable of replicating the complex ECM topography and providing essential biophysical cues in guiding stem cell differentiation and promoting cell proliferation [107–109]. Various electrospun fibrous scaffolds have been used in tendon tissue engineering to improve tendon repair and regeneration [110–112]. However, the reports on electrospun fibrous scaffolds for localized miRNA delivery have been elusive. Zhou et al. [104] prepared electrospun poly (ethylene glycol)-b-poly(L-lactide-co-e-caprolactone) (PELCL) and poly(e-caprolactone) (PCL) bilayer scaffolds combined with REDV peptide-modified trimethyl chitosan for vascular endothelial cell-specific delivery of miR-126. The sustained release of miR-126 from the electrospun scaffolds was observed for up to 56 days. The miR-126-loaded scaffolds not only promoted human umbilical vein endothelial cells (HUVECs) proliferation but also demonstrated excellent cellular biocompatibility as evidenced by well-preserved cell morphology and phenotype. Additionally, the target gene expression of SPRED-1 in HUVECs was downregulated by nearly 50% after seeding on miR-126-loaded scaffolds for 3 days. Postoperative evaluation of the transplanted bilayer scaffolds by immunofluorescent staining with anti-CD31 further confirmed its positive effect on endothelialization in vivo.
More recently, Milbreta et al. [113] used a hybrid scaffold consisting of poly (caprolactone-co-ethyl ethylene phosphate) (PCLEEP)-aligned fibers and collagen hydrogel for sustained delivery of miR-219 and miR-338 in guiding oligodendrocyte differentiation, maturation and myelination. The hybrid scaffolds released 10% of loaded miRs within the first 1h and gradually released 65% of miRs over a period of 20 days. The hybrid scaffolds also demonstrated a steady degradation rate with around 60% of mass lost in 30 days. In a rat spinal cord injury model, the authors found that miR-219/miR-338 activated scaffolds led to significantly higher number of oligodendroglia lineage cells compared to control group both at 2- and 4-weeks post implantation. Moreover, the myelination after injury was almost doubled in miR-219/miR-338 scaffolds implanted group compared with control group. Altogether, these results highlighted the potential of the fiber-hydrogel scaffolds acting as a non-viral, sustained delivery platform for miRNAs therapeutics.
It is noteworthy that although the above studies revealed the efficacy of miRNA-functionalized scaffolds in cell- or tissue-specific tissue engineering, none of them have been reported in improving tendon regeneration. Given the similar design concept and mature technique of loading miRNAs [114], it is not difficult to learn from the already successful miRNA-activated scaffolds delivery system and derivate platforms specifically for tendon tissue engineering. More importantly, a variety of bioscaffolds with multi-phasic structures which highly recapitulates the tendon ECM microenvironment or gradient tendon-bone interface have been developed and showed promising results [14, 115–118]. Therefore, it is optimistic to assume that the combination of biomimetic scaffolds and miRNA delivery could exert favorable synergistic effects on modulating tissue repair and regeneration after tendon injury.
5. Concluding remarks
miRNA-based therapeutics have received growing interest in the field of regenerative medicine given its profound potential in regulating a plethora of cellular functions. Promisingly, the miRNA profiling in tendinopathy and the roles of single miRNA in mediating tendon repair and healing are beginning to be exploited. miRNA-based strategies have shown a considerable beneficial effect on improving tendon healing in terms of alleviating adhesion, facilitating remodeling and promoting angiogenesis. Consequently, mimicry or antagonism of targeted miRNAs might open an exciting avenue for the treatment of tendon injury. However, successful clinical translation mainly relies on addressing the off-target effects associated with miRNA-based approaches. An effective way of circumventing the off-target effects is ensuring a localized delivery of miRNA to the desired site or coding gene. Therefore, the design and development of novel delivery platform for miRNA-based therapies, specifically miRNA-functionalized bioscaffolds holds enormous translational potential to serve as off-the-shelf product for clinical use. To achieve this, more future studies are needed to deepen the understanding of miRNAs in tendon biology, the miRNA regulatory mechanism in tendon injury, and the interplay between miRNAs and bioscaffolds. While miRNA-based therapies for tendon injury lag behind those of other musculoskeletal tissues including bone, cartilage and muscle, its endowed with great promise in the near future to realize scarless healing and obtain optimal outcomes. We believe that advancement in miRNA-based therapeutics will herald a new era of tendon tissue engineering and thereby initiating clinical trials for tendon applications.
Supplementary Material
Acknowledgment
This work was supported by National Natural Science Foundation of China (No. 81902221), Natural Science Foundation of Hunan Province (2019JJ30035) and NIH/NIAMS (AR73811).
Footnotes
Conflict of interest
None.
Credit Author Statement
Conceptualization: Qian Liu, Yunahi Peter Yang, Chunfeng Zhao
Literature Review: Qian Liu, Yaxi Zhu, Weihong Zhu, Ge Zhang, Yunzhi Peter Yang, Chunfeng Zhao
Manuscript drafting: Qian Liu, Yaxi Zhu, Weihong Zhu, Ge Zhang, Yunzhi Peter Yang, Chunfeng Zhao
Manuscript editing: Qian Liu, Yaxi Zhu, Weihong Zhu, Ge Zhang, Yunzhi Peter Yang, Chunfeng Zhao
Manuscript finalizing: Qian Liu, Yaxi Zhu, Weihong Zhu, Ge Zhang, Yunzhi Peter Yang, Chunfeng Zhao
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Dakin SG, Martinez FO, Yapp C, Wells G, Oppermann U, Dean BJ, Smith RD, Wheway K, Watkins B, Roche L, Carr AJ, Inflammation activation and resolution in human tendon disease, Sci Transl Med 7(311) (2015) 311ra173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].de Jong JP, Nguyen JT, Sonnema AJ, Nguyen EC, Amadio PC, Moran SL, The incidence of acute traumatic tendon injuries in the hand and wrist: a 10-year population-based study, Clin Orthop Surg 6(2) (2014) 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dudhia J, Scott CM, Draper ER, Heinegard D, Pitsillides AA, Smith RK, Aging 10 enhances a mechanically-induced reduction in tendon strength by an active process involving matrix metalloproteinase activity, Aging Cell 6(4) (2007) 547–56. [DOI] [PubMed] [Google Scholar]
- [4].Gwilym SE, Watkins B, Cooper CD, Harvie P, Auplish S, Pollard TC, Rees JL, Carr AJ, Genetic influences in the progression of tears of the rotator cuff, J Bone Joint Surg Br 91(7)(2009) 915–7. 647e. [DOI] [PubMed] [Google Scholar]
- [5].Titan AL, Foster DS, Chang J, Longaker MT, Flexor Tendon: Development, Healing, Adhesion Formation, and Contributing Growth Factors, Plast Reconstr Surg 144(4) (2019) 639e–647e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nourissat G, Berenbaum F, Duprez D, Tendon injury: from biology to tendon repair, Nat Rev Rheumatol 11(4) (2015) 223–33. [DOI] [PubMed] [Google Scholar]
- [7].Dy CJ, Hernandez-Soria A, Ma Y, Roberts TR, Daluiski A, Complications after flexor tendon repair: a systematic review and meta-analysis, J Hand Surg Am 37(3) (2012) 543–551 e1. [DOI] [PubMed] [Google Scholar]
- [8].Loiselle AE, Kelly M, Hammert WC, Biological Augmentation of Flexor Tendon Repair: A Challenging Cellular Landscape, J Hand Surg Am 41(1) (2016) 144–9; quiz 149. [DOI] [PubMed] [Google Scholar]
- [9].Charles MD, Christian DR, Cole BJ, The Role of Biologic Therapy in Rotator Cuff Tears and Repairs, Curr Rev Musculoskelet Med 11(1) (2018) 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Millar NL, Silbernagel KG, Thorborg K, Kirwan PD, Galatz LM, Abrams GD, Murrell GAC, McInnes IB, Rodeo SA, Tendinopathy, Nat Rev Dis Primers 7(1) (2021) 1. [DOI] [PubMed] [Google Scholar]
- [11].Maffulli N, Ewen SW, Waterston SW, Reaper J, Barrass V, Tenocytes from ruptured and tendinopathic achilles tendons produce greater quantities of type III collagen than tenocytes from normal achilles tendons. An in vitro model of human tendon healing, Am J Sports Med 28(4) (2000) 499–505. [DOI] [PubMed] [Google Scholar]
- [12].Lapiere CM, Nusgens B, Pierard GE, Interaction between collagen type I and type III in conditioning bundles organization, Connect Tissue Res 5(1) (1977) 21–9. [DOI] [PubMed] [Google Scholar]
- [13].Easley J, Puttlitz C, Hackett E, Broomfield C, Nakamura L, Hawes M, Getz C, Frankle M, St Pierre P, Tashjian R, Cummings PD, Abboud J, Harper D, McGilvray K, A prospective study comparing tendon-to-bone interface healing using an interposition bioresorbable scaffold with a vented anchor for primary rotator cuff repair in sheep, J Shoulder Elbow Surg 29(1) (2020) 157–166. [DOI] [PubMed] [Google Scholar]
- [14].Liu Q, Yu Y, Reisdorf RL, Qi J, Lu CK, Berglund LJ, Amadio PC, Moran SL, Steinmann SP, An KN, Gingery A, Zhao C, Engineered tendon-fibrocartilage-bone composite and bone marrow-derived mesenchymal stem cell sheet augmentation promotes rotator cuff healing in a non-weight-bearing canine model, Biomaterials 192 (2019) 189–198. [DOI] [PubMed] [Google Scholar]
- [15].Rossbach BP, Gulecyuz MF, Kempfert L, Pietschmann MF, Ullamann T, Ficklscherer A, Niethammer TR, Zhang A, Klar RM, Muller PE, Rotator Cuff Repair With Autologous Tenocytes and Biodegradable Collagen Scaffold: A Histological and Biomechanical Study in Sheep, Am J Sports Med 48(2) (2020) 450–459. [DOI] [PubMed] [Google Scholar]
- [16].Rothrauff BB, Smith CA, Ferrer GA, Novaretti JV, Pauyo T, Chao T, Hirsch D, Beaudry MF, Herbst E, Tuan RS, Debski RE, Musahl V, The effect of adipose-derived stem cells on enthesis healing after repair of acute and chronic massive rotator cuff tears in rats, J Shoulder Elbow Surg 28(4) (2019) 654–664. [DOI] [PubMed] [Google Scholar]
- [17].Bartel DP, MicroRNAs: genomics, biogenesis, mechanism, and function, Cell 116(2) (2004) 281–97. [DOI] [PubMed] [Google Scholar]
- [18].Friedman RC, Farh KK, Burge CB, Bartel DP, Most mammalian mRNAs are conserved targets of microRNAs, Genome Res 19(1) (2009) 92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fineberg SK, Kosik KS, Davidson BL, MicroRNAs potentiate neural development, Neuron 64(3) (2009) 303–9. [DOI] [PubMed] [Google Scholar]
- [20].Kwok GTY, Zhao JT, Glover AR, Gill AJ, Clifton-Bligh R, Robinson BG, Ip JCY, Sidhu SB, microRNA-431 as a Chemosensitizer and Potentiator of Drug Activity in Adrenocortical Carcinoma, Oncologist 24(6) (2019) e241–e250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cheng J, Yu H, Deng S, Shen G, MicroRNA profiling in mid- and late-gestational fetal skin: implication for scarless wound healing, Tohoku J Exp Med 221(3) (2010) 203–9. [DOI] [PubMed] [Google Scholar]
- [22].Salehi S, Brereton HC, Arno MJ, Darling D, Quaglia A, O’Grady J, Heaton N, Aluvihare VR, Human liver regeneration is characterized by the coordinated expression of distinct microRNA governing cell cycle fate, Am J Transplant 13(5) (2013) 1282–95. [DOI] [PubMed] [Google Scholar]
- [23].Schwartzfarb E, Kirsner RS, Understanding scarring: scarless fetal wound healing as a model, J Invest Dermatol 132(2) (2012) 260. [DOI] [PubMed] [Google Scholar]
- [24].Heller KN, Mendell JT, Mendell JR, Rodino-Klapac LR, MicroRNA-29 overexpression by adeno-associated virus suppresses fibrosis and restores muscle function in combination with micro-dystrophin, JCI Insight 2(9) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dong S, Cheng Y, Yang J, Li J, Liu X, Wang X, Wang D, Krall TJ, Delphin ES, Zhang C, MicroRNA expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction, J Biol Chem 284(43) (2009) 29514–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S, MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts, Nature 456(7224) (2008) 980–4. [DOI] [PubMed] [Google Scholar]
- [27].Hebert SS, De Strooper B, Molecular biology. miRNAs in neurodegeneration, Science 317(5842) (2007) 1179–80. [DOI] [PubMed] [Google Scholar]
- [28].Sugatani T, Vacher J, Hruska KA, A microRNA expression signature of osteoclastogenesis, Blood 117(13) (2011) 3648–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Moro A, Driscoll TP, Boraas LC, Armero W, Kasper DM, Baeyens N, Jouy C, Mallikarjun V, Swift J, Ahn SJ, Lee D, Zhang J, Gu M, Gerstein M, Schwartz M, Nicoli S, MicroRNA-dependent regulation of biomechanical genes establishes tissue stiffness homeostasis, Nat Cell Biol 21(3) (2019) 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kasper DM, Moro A, Ristori E, Narayanan A, Hill-Teran G, Fleming E, MorenoMateos M, Vejnar CE, Zhang J, Lee D, Gu M, Gerstein M, Giraldez A, Nicoli S, MicroRNAs Establish Uniform Traits during the Architecture of Vertebrate Embryos, Dev Cell 40(6) (2017) 552–565 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Plachel F, Heuberer P, Gehwolf R, Frank J, Tempfer H, Lehner C, Weissenbacher N, Wagner A, Weigl M, Moroder P, Hackl M, Traweger A, MicroRNA Profiling Reveals Distinct Signatures in Degenerative Rotator Cuff Pathologies, J Orthop Res 38(1) (2020) 202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Thankam FG, Boosani CS, Dilisio MF, Agrawal DK, MicroRNAs associated with inflammation in shoulder tendinopathy and glenohumeral arthritis, Mol Cell Biochem 437(1–2) (2018) 81–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Thankam FG, Boosani CS, Dilisio MF, Dietz NE, Agrawal DK, MicroRNAs Associated with Shoulder Tendon Matrisome Disorganization in Glenohumeral Arthritis, PLoS One 11(12) (2016) e0168077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].McAlinden A, Im GI, MicroRNAs in orthopaedic research: Disease associations, potential therapeutic applications, and perspectives, J Orthop Res 36(1) (2018) 33–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN, MicroRNA genes are transcribed by RNA polymerase II, EMBO J 23(20) (2004) 4051–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Borchert GM, Lanier W, Davidson BL, RNA polymerase III transcribes human microRNAs, Nat Struct Mol Biol 13(12) (2006) 1097–101. [DOI] [PubMed] [Google Scholar]
- [37].Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S, Kim VN, The nuclear RNase III Drosha initiates microRNA processing, Nature 425(6956) (2003) 415–9. [DOI] [PubMed] [Google Scholar]
- [38].Han J, Lee Y, Yeom KH, Kim YK, Jin H, Kim VN, The Drosha-DGCR8 complex in primary microRNA processing, Genes Dev 18(24) (2004) 3016–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Treiber T, Treiber N, Meister G, Regulation of microRNA biogenesis and its crosstalk with other cellular pathways, Nat Rev Mol Cell Biol 20(1) (2019) 5–20. [DOI] [PubMed] [Google Scholar]
- [40].Mendias CL, Gumucio JP, Bakhurin KI, Lynch EB, Brooks SV, Physiological loading of tendons induces scleraxis expression in epitenon fibroblasts, J Orthop Res 30(4) (2012) 606–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sharma P, Maffulli N, Biology of tendon injury: healing, modeling and remodeling, J Musculoskelet Neuronal Interact 6(2) (2006) 181–90. [PubMed] [Google Scholar]
- [42].Millar NL, Gilchrist DS, Akbar M, Reilly JH, Kerr SC, Campbell AL, Murrell GAC, Liew FY, Kurowska-Stolarska M, McInnes IB, MicroRNA29a regulates IL-33-mediated tissue remodelling in tendon disease, Nat Commun 6 (2015) 6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hall KE, Sarkissian EJ, Sharpe O, Robinson WH, Abrams GD, Identification of differentially expressed micro-RNA in rotator cuff tendinopathy, Muscles Ligaments Tendons J 8(1) (2018) 8–14. [Google Scholar]
- [44].Gardner K, Arnoczky SP, Caballero O, Lavagnino M, The effect of stress-deprivation and cyclic loading on the TIMP/MMP ratio in tendon cells: an in vitro experimental study, Disabil Rehabil 30(20–22) (2008) 1523–9. [DOI] [PubMed] [Google Scholar]
- [45].Thankam FG, Boosani CS, Dilisio MF, Gross RM, Agrawal DK, Genes interconnecting AMPK and TREM-1 and associated microRNAs in rotator cuff tendon injury,Mol Cell Biochem 454(1–2) (2019) 97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Landskroner-Eiger S, Moneke I, Sessa WC, miRNAs as modulators of angiogenesis, Cold Spring Harb Perspect Med 3(2) (2013) a006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Dubin JA, Greenberg DR, Iglinski-Benjamin KC, Abrams GD, Effect of micro-RNA on tenocytes and tendon-related gene expression: A systematic review, J Orthop Res 36(11) (2018) 2823–2829. [DOI] [PubMed] [Google Scholar]
- [48].Chen L, Wang GD, Liu JP, Wang HS, Liu XM, Wang Q, Cai XH, miR-135a modulates tendon stem/progenitor cell senescence via suppressing ROCK1, Bone 71 (2015) 210–6. [DOI] [PubMed] [Google Scholar]
- [49].Liu Y, Feng L, Xu J, Yang Z, Wu T, Zhang J, Shi L, Zhu D, Zhang J, Li G, MiR-378a suppresses tenogenic differentiation and tendon repair by targeting at TGF-beta2, Stem Cell Res Ther 10(1) (2019) 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Poulsen RC, Knowles HJ, Carr AJ, Hulley PA, Cell differentiation versus cell death: extracellular glucose is a key determinant of cell fate following oxidative stress exposure, Cell Death Dis 5 (2014) e1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wang B, Guo J, Feng L, Suen CW, Fu WM, Zhang JF, Li G, MiR124 suppresses collagen formation of human tendon derived stem cells through targeting egr1, Exp Cell Res 347(2) (2016) 360–6. [DOI] [PubMed] [Google Scholar]
- [52].Han W, Wang B, Liu J, Chen L, The p16/miR-217/EGR1 pathway modulates age-related tenogenic differentiation in tendon stem/progenitor cells, Acta Biochim Biophys Sin (Shanghai) 49(11) (2017) 1015–1021. [DOI] [PubMed] [Google Scholar]
- [53].Peffers MJ, Fang Y, Cheung K, Wei TK, Clegg PD, Birch HL, Transcriptome analysis of ageing in uninjured human Achilles tendon, Arthritis Res Ther 17 (2015) 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Chen L, Liu J, Tao X, Wang G, Wang Q, Liu X, The role of Pin1 protein in aging of human tendon stem/progenitor cells, Biochem Biophys Res Commun 464(2) (2015) 487–92. [DOI] [PubMed] [Google Scholar]
- [55].Kohler J, Popov C, Klotz B, Alberton P, Prall WC, Haasters F, Muller-Deubert S, Ebert R, Klein-Hitpass L, Jakob F, Schieker M, Docheva D, Uncovering the cellular and molecular changes in tendon stem/progenitor cells attributed to tendon aging and degeneration, Aging Cell 12(6) (2013) 988–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Mendias CL, Gumucio JP, Lynch EB, Mechanical loading and TGF-beta change the expression of multiple miRNAs in tendon fibroblasts, J Appl Physiol (1985) 113(1) (2012) 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zhang J, Wang JH, Mechanobiological response of tendon stem cells: implications of tendon homeostasis and pathogenesis of tendinopathy, J Orthop Res 28(5) (2010) 639–43. [DOI] [PubMed] [Google Scholar]
- [58].Rui YF, Lui PP, Ni M, Chan LS, Lee YW, Chan KM, Mechanical loading increased BMP-2 expression which promoted osteogenic differentiation of tendon-derived stem cells, J Orthop Res 29(3) (2011) 390–6. [DOI] [PubMed] [Google Scholar]
- [59].Rothrauff BB, Pauyo T, Debski RE, Rodosky MW, Tuan RS, Musahl V, The Rotator Cuff Organ: Integrating Developmental Biology, Tissue Engineering, and Surgical Considerations to Treat Chronic Massive Rotator Cuff Tears, Tissue Eng Part B Rev 23(4) (2017)318–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zhang C, Zhu J, Zhou Y, Thampatty BP, Wang JH, Tendon Stem/Progenitor Cells and 11 Their Interactions with Extracellular Matrix and Mechanical Loading, Stem Cells Int 2019 (2019) 3674647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Golash A, Kay A, Warner JG, Peck F, Watson JS, Lees VC, Efficacy of ADCON-T/N 14 after primary flexor tendon repair in Zone II: a controlled clinical trial, J Hand Surg Br 28(2) (2003) 113–5. [DOI] [PubMed] [Google Scholar]
- [62].Hagberg L, Exogenous hyaluronate as an adjunct in the prevention of adhesions after flexortendon surgery: a controlled clinical trial, J Hand Surg Am 17(1) (1992) 132–6. [DOI] [PubMed] [Google Scholar]
- [63].Zhao C, Sun YL, Kirk RL, Thoreson AR, Jay GD, Moran SL, An KN, Amadio PC, Effects of a lubricin-containing compound on the results of flexor tendon repair in a canine model in vivo, J Bone Joint Surg Am 92(6) (2010) 1453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Henn RF 3rd, Kuo CE, Kessler MW, Razzano P, Grande DP, Wolfe SW, Augmentation of zone II flexor tendon repair using growth differentiation factor 5 in a rabbit model, J Hand Surg Am 35(11) (2010) 1825–32. [DOI] [PubMed] [Google Scholar]
- [65].Chan KM, Fu SC, Wong YP, Hui WC, Cheuk YC, Wong MW, Expression of transforming growth factor beta isoforms and their roles in tendon healing, Wound Repair Regen 16(3) (2008) 399–407. [DOI] [PubMed] [Google Scholar]
- [66].Chang J, Thunder R, Most D, Longaker MT, Lineaweaver WC, Studies in flexor tendon wound healing: neutralizing antibody to TGF-beta1 increases postoperative range of motion, Plast Reconstr Surg 105(1) (2000) 148–55. [DOI] [PubMed] [Google Scholar]
- [67].Margadant C, Sonnenberg A, Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing, EMBO Rep 11(2) (2010) 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Zhou Y, Zhang L, Zhao W, Wu Y, Zhu C, Yang Y, Nanoparticle-mediated delivery of TGF-beta1 miRNA plasmid for preventing flexor tendon adhesion formation, Biomaterials 34(33) (2013) 8269–78. [DOI] [PubMed] [Google Scholar]
- [69].Zhou Y, Zhu C, Wu YF, Zhang L, Tang JB, Effective modulation of transforming growth factor-beta1 expression through engineered microRNA-based plasmid-loaded nanospheres, Cytotherapy 17(3) (2015) 320–9. [DOI] [PubMed] [Google Scholar]
- [70].Wu YF, Mao WF, Zhou YL, Wang XT, Liu PY, Tang JB, Adeno-associated virus-2-mediated TGF-beta1 microRNA transfection inhibits adhesion formation after digital flexor tendon injury, Gene Ther 23(2) (2016) 167–75. [DOI] [PubMed] [Google Scholar]
- [71].Farhat YM, Al-Maliki AA, Chen T, Juneja SC, Schwarz EM, O’Keefe RJ, Awad HA, Gene expression analysis of the pleiotropic effects of TGF-beta1 in an in vitro model of flexor tendon healing, PLoS One 7(12) (2012) e51411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Majewski M, Porter RM, Betz OB, Betz VM, Clahsen H, Fluckiger R, Evans CH, Improvement of tendon repair using muscle grafts transduced with TGF-beta1 cDNA, Eur Cell Mater 23 (2012) 94–101; discussion 101–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Katzel EB, Wolenski M, Loiselle AE, Basile P, Flick LM, Langstein HN, Hilton MJ, Awad HA, Hammert WC, O’Keefe RJ, Impact of Smad3 loss of function on scarring and adhesion formation during tendon healing, J Orthop Res 29(5) (2011) 684–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Tan V, Zhang AY, Pham H, Ho F, Teng K, Longaker MT, Chang J, Inhibition of TGF-beta-induced collagen production in rabbit flexor tendons, J Hand Surg Am 29(2) (2004) 230–5. [DOI] [PubMed] [Google Scholar]
- [75].Su B, O’Connor JP, NSAID therapy effects on healing of bone, tendon, and the enthesis, J Appl Physiol (1985) 115(6) (2013) 892–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Nourbakhsh A, Capo J, Cottrell JA, Meyenhofer M, O’Connor JP, Effects of nonsteroidal anti-inflammatory drugs on flexor tendon adhesion, J Hand Surg Am 35(6) (2010) 941–7. [DOI] [PubMed] [Google Scholar]
- [77].Manning CN, Havlioglu N, Knutsen E, Sakiyama-Elbert SE, Silva MJ, Thomopoulos S, Gelberman RH, The early inflammatory response after flexor tendon healing: a gene expression and histological analysis, J Orthop Res 32(5) (2014) 645–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Zhou YL, Yang QQ, Yan YY, Zhu C, Zhang L, Tang JB, Localized delivery of miRNAs targets cyclooxygenases and reduces flexor tendon adhesions, Acta Biomater 70 (2018) 237–248. [DOI] [PubMed] [Google Scholar]
- [79].Millar NL, Murrell GA, McInnes IB, Inflammatory mechanisms in tendinopathy - towards translation, Nat Rev Rheumatol 13(2) (2017) 110–122. [DOI] [PubMed] [Google Scholar]
- [80].de la Durantaye M, Piette AB, van Rooijen N, Frenette J, Macrophage depletion reduces cell proliferation and extracellular matrix accumulation but increases the ultimate tensile strength of injured Achilles tendons, J Orthop Res 32(2) (2014) 279–85. [DOI] [PubMed] [Google Scholar]
- [81].Cui H, He Y, Chen S, Zhang D, Yu Y, Fan C, Macrophage-Derived miRNA-Containing Exosomes Induce Peritendinous Fibrosis after Tendon Injury through the miR-21–5p/Smad7 Pathway, Mol Ther Nucleic Acids 14 (2019) 114–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Yao Z, Li J, Wang X, Peng S, Ning J, Qian Y, Fan C, MicroRNA-21–3p Engineered Umbilical Cord Stem Cell-Derived Exosomes Inhibit Tendon Adhesion, J Inflamm Res 13 (2020) 303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Chen S, Jiang S, Zheng W, Tu B, Liu S, Ruan H, Fan C, RelA/p65 inhibition prevents tendon adhesion by modulating inflammation, cell proliferation, and apoptosis, Cell Death Dis 8(3) (2017) e2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Chen Q, Lu H, Yang H, Chitosan inhibits fibroblasts growth in Achilles tendon via TGF-beta1/Smad3 pathway by miR-29b, Int J Clin Exp Pathol 7(12) (2014) 8462–70. [PMC free article] [PubMed] [Google Scholar]
- [85].Lu YF, Liu Y, Fu WM, Xu J, Wang B, Sun YX, Wu TY, Xu LL, Chan KM, Zhang JF, Li G, Long noncoding RNA H19 accelerates tenogenic differentiation and promotes tendon healing through targeting miR-29b-3p and activating TGF-beta1 signaling, Faseb J 31(3) (2017) 954–964. [DOI] [PubMed] [Google Scholar]
- [86].Ko JY, Lian WS, Tsai TC, Chen YS, Hsieh CK, Kuo CW, Wang FS, MicroRNA-29a Mitigates Subacromial Bursa Fibrosis in Rotator Cuff Lesion with Shoulder Stiffness, Int J Mol Sci 20(22) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Cavarretta E, Condorelli G, miR-21 and cardiac fibrosis: another brick in the wall?, Eur Heart J 36(32) (2015) 2139–41. [DOI] [PubMed] [Google Scholar]
- [88].van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN, Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis, Proc Natl Acad Sci U S A 105(35) (2008) 13027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Lai S, Iwakiri Y, Is miR-21 a potent target for liver fibrosis?, Hepatology 67(6) (2018) 2082–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Roderburg C, Urban GW, Bettermann K, Vucur M, Zimmermann H, Schmidt S, Janssen J, Koppe C, Knolle P, Castoldi M, Tacke F, Trautwein C, Luedde T, Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis, Hepatology 53(1) (2011) 209–18. [DOI] [PubMed] [Google Scholar]
- [91].Liu G, Friggeri A, Yang Y, Milosevic J, Ding Q, Thannickal VJ, Kaminski N, Abraham E, miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis, J Exp Med 207(8) (2010) 1589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Xiao J, Meng XM, Huang XR, Chung AC, Feng YL, Hui DS, Yu CM, Sung JJ, Lan HY, miR-29 inhibits bleomycin-induced pulmonary fibrosis in mice, Mol Ther 20(6) (2012) 1251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Chau BN, Xin C, Hartner J, Ren S, Castano AP, Linn G, Li J, Tran PT, Kaimal V, Huang X, Chang AN, Li S, Kalra A, Grafals M, Portilla D, MacKenna DA, Orkin SH, Duffield JS, MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways, Sci Transl Med 4(121) (2012) 121ra18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Wang B, Komers R, Carew R, Winbanks CE, Xu B, Herman-Edelstein M, Koh P, Thomas M, Jandeleit-Dahm K, Gregorevic P, Cooper ME, Kantharidis P, Suppression of microRNA-29 expression by TGF-beta1 promotes collagen expression and renal fibrosis, J Am Soc Nephrol 23(2) (2012) 252–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Li CX, Talele NP, Boo S, Koehler A, Knee-Walden E, Balestrini JL, Speight P, Kapus A, Hinz B, MicroRNA-21 preserves the fibrotic mechanical memory of mesenchymal stem cells, Nat Mater 16(3) (2017) 379–389. [DOI] [PubMed] [Google Scholar]
- [96].Liew FY, Pitman NI, McInnes IB, Disease-associated functions of IL-33: the new kid in the IL-1 family, Nat Rev Immunol 10(2) (2010) 103–10. [DOI] [PubMed] [Google Scholar]
- [97].Rankin AL, Mumm JB, Murphy E, Turner S, Yu N, McClanahan TK, Bourne PA, Pierce RH, Kastelein R, Pflanz S, IL-33 induces IL-13-dependent cutaneous fibrosis, J Immunol 184(3) (2010) 1526–35. [DOI] [PubMed] [Google Scholar]
- [98].Watts AE, Millar NL, Platt J, Kitson SM, Akbar M, Rech R, Griffin J, Pool R, Hughes T, McInnes IB, Gilchrist DS, MicroRNA29a Treatment Improves Early Tendon Injury, Mol Ther 25(10) (2017) 2415–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Usman MA, Nakasa T, Shoji T, Kato T, Kawanishi Y, Hamanishi M, Kamei N, Ochi M, The effect of administration of double stranded MicroRNA-210 on acceleration of Achilles tendon healing in a rat model, J Orthop Sci 20(3) (2015) 538–46. [DOI] [PubMed] [Google Scholar]
- [100].Xu Q, Sun WX, Zhang ZF, High expression of VEGFA in MSCs promotes tendon-bone healing of rotator cuff tear via microRNA-205–5p, Eur Rev Med Pharmacol Sci 23(10) (2019) 4081–4088. [DOI] [PubMed] [Google Scholar]
- [101].Rupaimoole R, Slack FJ, MicroRNA therapeutics: towards a new era for the management of cancer and other diseases, Nat Rev Drug Discov 16(3) (2017) 203–222. [DOI] [PubMed] [Google Scholar]
- [102].Mencia Castano I, Curtin CM, Shaw G, Murphy JM, Duffy GP, O’Brien FJ, A novel collagen-nanohydroxyapatite microRNA-activated scaffold for tissue engineering applications capable of efficient delivery of both miR-mimics and antagomiRs to human mesenchymal stem cells, J Control Release 200 (2015) 42–51. [DOI] [PubMed] [Google Scholar]
- [103].Conde J, Oliva N, Atilano M, Song HS, Artzi N, Self-assembled RNA-triple-helix hydrogel scaffold for microRNA modulation in the tumour microenvironment, Nat Mater 15(3) (2016) 353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Zhou F, Jia X, Yang Y, Yang Q, Gao C, Hu S, Zhao Y, Fan Y, Yuan X, Nanofibermediated microRNA-126 delivery to vascular endothelial cells for blood vessel regeneration, Acta Biomater 43 (2016) 303–313. [DOI] [PubMed] [Google Scholar]
- [105].Zhang X, Li Y, Chen YE, Chen J, Ma PX, Cell-free 3D scaffold with two-stage delivery of miRNA-26a to regenerate critical-sized bone defects, Nat Commun 7 (2016) 10376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Liu R, Zhang S, Chen X, Injectable hydrogels for tendon and ligament tissue engineering, J Tissue Eng Regen Med 14(9) (2020) 1333–1348. [DOI] [PubMed] [Google Scholar]
- [107].Kiros S, Lin S, Xing M, Mequanint K, Embryonic Mesenchymal Multipotent Cell Differentiation on Electrospun Biodegradable Poly(ester amide) Scaffolds for Model Vascular Tissue Fabrication, Ann Biomed Eng 48(3) (2020) 980–991. [DOI] [PubMed] [Google Scholar]
- [108].Sperling LE, Reis KP, Pozzobon LG, Girardi CS, Pranke P, Influence of random and oriented electrospun fibrous poly(lactic-co-glycolic acid) scaffolds on neural differentiation of mouse embryonic stem cells, J Biomed Mater Res A 105(5) (2017) 1333–1345. [DOI] [PubMed] [Google Scholar]
- [109].Birhanu G, Akbari Javar H, Seyedjafari E, Zandi-Karimi A, Dusti Telgerd M, An improved surface for enhanced stem cell proliferation and osteogenic differentiation using electrospun composite PLLA/P123 scaffold, Artif Cells Nanomed Biotechnol 46(6) (2018) 1274–1281. [DOI] [PubMed] [Google Scholar]
- [110].Rinoldi C, Kijenska E, Chlanda A, Choinska E, Khenoussi N, Tamayol A, Khademhosseini A, Swieszkowski W, Nanobead-on-string composites for tendon tissue engineering, J Mater Chem B 6(19) (2018) 3116–3127. [DOI] [PubMed] [Google Scholar]
- [111].Orr SB, Chainani A, Hippensteel KJ, Kishan A, Gilchrist C, Garrigues NW, Ruch DS, Guilak F, Little D, Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering, Acta Biomater 24 (2015) 117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Chainani A, Hippensteel KJ, Kishan A, Garrigues NW, Ruch DS, Guilak F, Little D, Multilayered electrospun scaffolds for tendon tissue engineering, Tissue Eng Part A 19(23–24) (2013) 2594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Milbreta U, Lin J, Pinese C, Ong W, Chin JS, Shirahama H, Mi R, Williams A, Bechler ME, Wang J, Ffrench-Constant C, Hoke A, Chew SY, Scaffold-Mediated Sustained, Non-viral Delivery of miR-219/miR-338 Promotes CNS Remyelination, Mol Ther 27(2) (2019) 411423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Peng B, Chen Y, Leong KW, MicroRNA delivery for regenerative medicine, Adv Drug Deliv Rev 88 (2015) 108–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Zhu C, Pongkitwitoon S, Qiu J, Thomopoulos S, Xia Y, Design and Fabrication of a Hierarchically Structured Scaffold for Tendon-to-Bone Repair, Adv Mater 30(16) (2018) e1707306. 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Liu H, Yang L, Zhang E, Zhang R, Cai D, Zhu S, Ran J, Bunpetch V, Cai Y, Heng BC, Hu Y, Dai X, Chen X, Ouyang H, Biomimetic tendon extracellular matrix composite gradient scaffold enhances ligament-to-bone junction reconstruction, Acta Biomater 56 (2017) 129–140. [DOI] [PubMed] [Google Scholar]
- [117].Ker DFE, Wang D, Behn AW, Wang ETH, Zhang X, Zhou BY, Mercado-Pagan AE, Kim S, Kleimeyer J, Gharaibeh B, Shanjani Y, Nelson D, Safran M, Cheung E, Campbell P, Yang YP, Functionally Graded, Bone- and Tendon-Like Polyurethane for Rotator Cuff Repair, Adv Funct Mater 28(20) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Wang D, Ker DFE, Ng KW, Li K, Gharaibeh B, Safran M, Cheung E, Campbell P, Weiss L, Yang YP, Combinatorial mechanical gradation and growth factor biopatterning strategy for spatially controlled bone-tendon-like cell differentiation and tissue formation, NPG Asia Materials 13(1) (2021) 26. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.