Abstract
Reducing tendon failure after repair remains a challenge due to its poor intrinsic healing ability. The purpose of this study is to investigate the effect of a novel tissue-engineered purified exosome product (PEP) patch on tendon healing in a canine ex vivo model. Lacerated flexor digitorum profundus (FDP) tendons from three canines’ paws underwent simulated repair with Tisseel patch alone or biopotentiated with PEP. For ex vivo model, FDP tendons were randomly divided into 3 groups: FDP tendon repair alone group (Control), Tisseel patch alone group, and Tisseel plus PEP (TEPEP) patch group. Following four weeks of tissue culture, the failure load, stiffness, histology and gene expression of the healing tendon were evaluated. The transmission electron microscopy (TEM) revealed that exosomes of PEP the diameters ranged from 93.70 to 124.65 nm, and the patch release test showed this TEPEP patch could stably release the extracellular vesicle over two weeks. The failure strength of tendon in the TEPEP patch group was significantly higher than that of the Control group and Tisseel alone group. The results of histology showed that the TEPEP patch group had the smallest healing gap and the largest number of fibroblasts on the surface of the injured tendon. Quantitative RT-PCR showed that TEPEP patch increased the expression of COL3, MMP2, MMP3, MMP14, and reduced the expression of TGF-beta1, IL-6. This study shows that the TEPEP patch could promote tendon repair by reducing gap formation and inflammatory response, increasing the activity of endogenous cells and the formation of type III collagen.
Keywords: Flexor digitorum profundus tendon, Tendon healing, Fibrin Sealant, Purified exosome products
1. Introduction
Flexor digitorum profundus (FDP) tendon injury accounts for a high proportion of hand trauma.1 Although recently tendon repair techniques have been improved, the frequent occurrence of repair site catastrophic rupture and adhesion formation, especially in Zone II and III, remains a clinically challenging problem.2; 3 Previous reports have shown that a lot of patients have a repaired tendon re-rupture after repair of the flexor tendon. Although there are multiple factors that may lead to repaired tendon rupture, the hypocellular and hypovascular nature of the flexor tendon has been considered as the major underlying reason for the low intrinsic healing ability of this tendon.4 Therefore, the novel regenerative strategies to enhance the flexor tendon intrinsic healing capacity are demanding to prevent repair tendon gap formation or rupture, thus improving clinical outcomes following flexor tendon repair.
Exosomes are membrane vesicles with a diameter of 30–150 nm secreted by multiple types of cells.5–7 Exosomes are distributed in various body fluids such as blood8, saliva9, urine10, cerebrospinal fluid11 and breast milk12. Due to the different cellular sources, exosomes have the capacity to carry different kinds of proteins13, lipids14, messenger RNAs15 and play a critical role in intercellular communication. Recently, the purified exosome products (PEP) derived from human plasma was identified as a positive role in regulating tenocyte biological behaviors in vitro.16 Exosomes extracted from blood have broad potential applications in medical diagnosis, health monitoring and personalized medicine due to their biocompatibility and ease of crossing various physical barriers.17
Fibrin Sealant (FS) has been widely used as an adhesive for tissue repair during surgical treatment of various diseases.18–20 It consists of two human plasma-derived components: (1) highly concentrated fibrinogen complex (FC) and (2) high potency thrombin (TH). FS has been shown to be a suitable delivery vehicle for many different kinds of cells and exogenous growth factors that could be used to accelerate bone growth and vascularization.21 In addition, fibrin sealant is an effective and well-tolerated therapeutic option for patients with Crohn’s disease and perianal fistula tracts.22 Previous studies have shown the fibrin sealant as a new type of scaffold for tendon and bone healing.23 Due to its biocompatibility and biodegradability, it can be used as an excellent carrier for tissue engineering carrying a variety of cells and growth factors.24; 25 However, studies on the tendon healing with exosomes attached to the fibrin sealant scaffold have not been reported.
The aim of this study was to develop a novel biopotentiated tissue-engineered purified exosome product (TEPEP) patch and to evaluate its effects on flexor tendon healing in an ex vivo canine tendon tissue culture model. We hypothesized that augmentation of flexor tendon repair with the TEPEP patch would improve the tendon’s intrinsic healing ability.
2. Method and materials
2.1. Study Rationale and Design
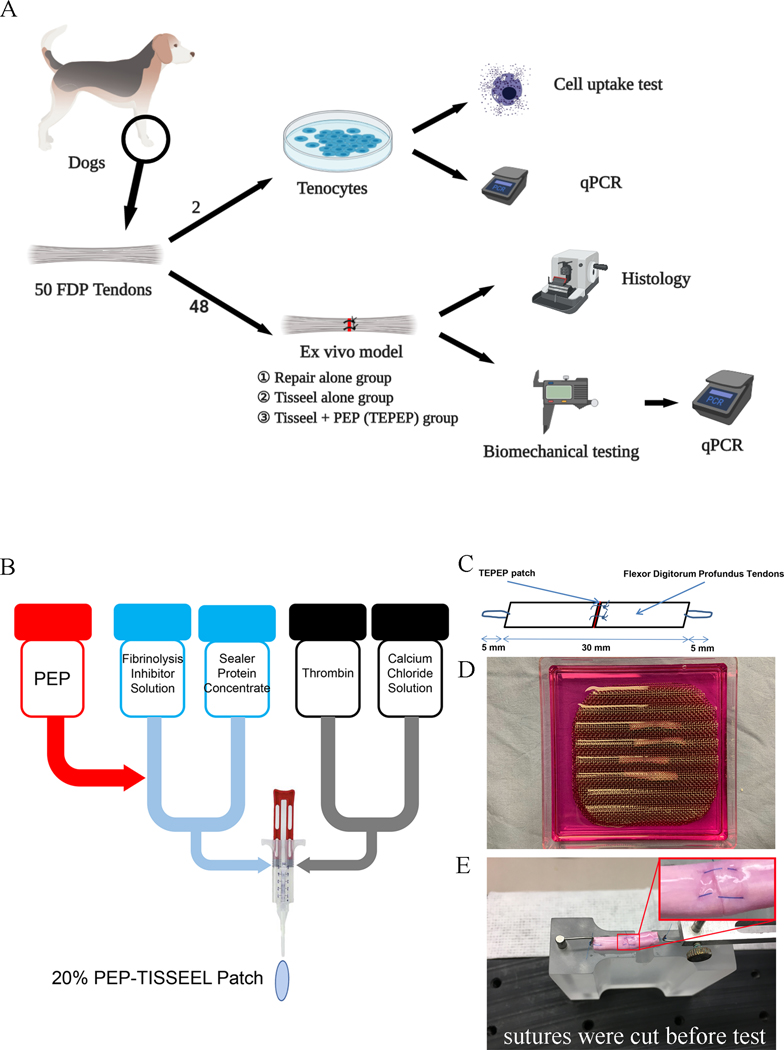
The flexor tendon ex vivo model has been widely used to investigate tendon intrinsic healing ability, as the lacerated viable tendon can heal in a culture media.26 Although the healing strength was relative low compared to in vivo healing model, ex vivo model does provide important information regarding tendon intrinsic healing at the cellular level. In this study, we used a total of 50 flexor digitorum profundus tendons from 3 dogs (8 months, weighing 23.7 ± 2.3 kg) sacrificed from other IACUC approved projects that did not involve tendon related research. PEP (Good Manufacturing Practice grade, Rion LLC Rochester, MN) was manufactured and supplied by the Advanced Product Incubator (API) of Mayo Clinic. In brief, the production process includes separating multiple expired human donor plasma, separating exosomes from the separated plasma (filtration and centrifugation), and encapsulating the exosomes according to the patent description (US Patent 20160324A1). PEP exosomes are provided in room temperature stable lyophilized form with specific exosomal properties as previously described.16 The carrier we used for PEP was commercially available Tisseel (Baxter Healthcare Corp., USA). Among these flexor digitorum profundus tendons, 2 were utilized for tenocytes isolation and cellular studies, while other 48 were randomly divided into 3 groups: FDP tendon repair alone group (Control, n=16), Tisseel patch alone group (n=16), and Tisseel plus PEP (TEPEP) (n=16). Following four weeks of tissue culture, thirty repaired tendons (ten per group) were evaluated with mechanical properties and quantitative RT-PCR, others (six per group) were evaluated with histology. (Fig. 1-A)
Figure 1:
An explant in an ex vivo model and mechanical evaluation. A. Study design of this study. B. PEP-Tisseel patches fabrication procedures. C. Each test specimen was 40 mm long, which included 5 mm of suture loop at each end and 30 mm of tendon. D. Repaired tendons were kept straight without tension between the grooves of custom-made wire meshes. E. Repaired tendon mounted on the experimental platform. (Sutures were cut before mechanical evaluation)
2.2. PEP-Tisseel Patch Preparation
TEPEP patch was prepared by rehydrating lyophilized PEP within the Tisseel solution following the protocol illustrated in Fig. 1-B. In brief, the PEP was dissolved into 1 ml PBS in order to make a PEP solution representing 5×1012 vesicles per ml (100%). 400 μl PEP mixed solution (2×1012 vesicles) and 600 μl Fibrinolysis inhibitor solution were added to Sealer Protein Concentrate to make the Fibrinogen. Then, 1000 μl Calcium Chloride Solution was added to Thrombin to make the Thrombin. Finally, both two reagents were mixed to get a TEPEP patch with 20% PEP concentration (1×1012 vesicles per ml).
2.3. Tendon Repair and Culture
All FDP tendons were harvested immediately following dog sacrifice and placed in tissue culture medium to maintain the tendon viability. Each tendon was transected in the middle of a 30 mm length centered at the proximal interphalangeal joint level. This section of the FDP tendon consisted of two collagen bundles. A simple suture of 6–0 Prolene sutures (Ethicon, USA) was placed in each bundle. Before tightening the suture loop, TEPEP patch, Tisseel patch or no patch were implanted between the tendon ends and then the sutures were knotted to close the repaired tendon (Fig. 1-C). The PEP patch was a gel like material, and after closuring the tendon end, the patch was scraped and no visible gap was observed following tendon repair. The repaired tendons were placed between 2 wire meshes with longitudinal grooves designed to maintain the tendons in a straight position without applying any tensional force on the tendon (Fig. 1-D). After 4 weeks cultured in Dulbecco’s modified Eagle’s Medium (DMEM; Gibco®, USA) containing 10% fetal bovine serum (FBS; Gibco®, USA) and 1% antibiotic–antimycotic (AA; Gibco®, USA), the tendons were assessed biomechanically and histologically. In the culturing process of the tendon model, a large number of adherent tenocytes were observed at the bottom of the square dish of different groups, all tenocytes were quantified by Image J (v1.48).
2.4. Transmission electron microscopy of PEP
According to the a our previous proposal for exosome observation,16 100% (v/v) concentration PEP solution was fixed with 1mL 2.5% glutaraldehyde in 0.1M sodium cacodylate solution for 1 hour at 4°C. After rinsed with 0.1M sodium cacodylate buffer and 2% osmium tetroxide for 1 hour, all samples dehydrated using a graded series of ethanol and stained with 2% uranyl acetate. Then, a total of 50 μL samples (5×1010 vesicles) per grid were viewed using a transmission electron microscope (TEM, JEM-1400Plus; JEOL) at an acceleration voltage of 80 kV.
2.5. Release Test and Cellular Uptake of PEP
The release ratio of TEPEP patch was tested using a NanoSight System (NS300, Malvern, UK). Briefly, 20% TEPEP patch was mixed with 3 ml PBS, following incubation in a 15 ml tube at 37 °C. After incubating in a 37 °C incubator for 1 day, the supernatant PBS was collected and moved to another 6 ml collecting tube for testing. Add the new PBS and repeat until day 14. The particle concentration and size distribution was measured by nanoparticle tracking analysis (v3.20 software).
For the cellular uptake of PEP, tenocytes were harvested from fresh FDP tendons from Mixed-breed dogs mentioned above based on well-established protocol.16 Tenocytes (P4) were seeded with 300 cells per well on a Cell Culture Slides (Corning®, USA). After 2 days cultured in DMEM containing 10% FBS and 1% AA. The PEP was labeled with 1 mM Vybrant® CM-Dil solution (Invitrogen, USA). Then, the CM-Dil labeled PEP was incubated with tenocytes in serum-free DMEM at 37 °C for 2 h. After washed with PBS and fixed in 4% paraformaldehyde solution (Santa Cruz, USA), the tenocytes were stained with F-actin Alexa Fluor™ 488 Phalloidin (Invitrogen, A12379, USA) and Hoechst 33342 (Thermo, USA) respectively. Finally, the fluorescent images were observed and captured using confocal laser scanning microscopy (LSM 780, Zeiss, Germany).
2.6. Biomechanical Testing
After the 4 weeks culture, repaired tendons (n=10 each group) were mounted on to a custom-designed microtester to characterize mechanical properties of the repaired tendon healing. The testing apparatus included a load transducer, a stepper-motor driven stage, and a potentiometer that connected to a hook. The repaired tendon ends were placed with single loop suture that connected to the testing hooks (Fig. 1-E). Before testing, tendon repair sutures were cut without disrupting the repair site, which enabled assessment of the strength of the healing tissue rather than the composite strength of suture and healing. The tendons were placed on a flat, plastic platform moistened with saline. Specimens were tested to failure at a rate of 0.1 mm/second. Failure strength and displacement were measured by the transducer and recorded at a sample rate of 20 Hz. Stiffness was defined as the slope of the linear region of the force/displacement curve.
2.7. Histology
After four weeks of culture, repaired tendons (n=6 each group) were used for histology described below. Tendon samples were embedded in optimal cutting temperature compound and cut at 7μm slices with a cryostat. Sections were stained with Hematoxylin and Eosin (Thermo, USA), Sirius Red (Polysciences, USA), and Masson staining (American Mastertech Scientific, USA) to evaluate the morphology and cellularity with light microscopy. Image J (v1.48) was used to quantify the cell number or healing gap of tendons.
2.8. Quantitative real-time RT-PCR
After biomechanical testing, the tendons tissue (n=10) were used for quantitative real-time PCR gene expression analysis. In addition, Dog’s tenocytes of three above treatments also be harvested in order to investigate whether Gene expression difference compare with tendon tissue (Supplement Figure 1). All samples were performed on C1000 Touch™ Thermal Cycler using SYBR Green PCR Master Mix (Quantabio, USA) to measure the gene expressions of Collagen type I alpha 2 chain (COL1A2), Collagen type III alpha 1 chain (COL3A1), Transforming Growth Factor beta-1 (TGF-β), Interleukin 6 (IL-6), Matrix Metallopeptidase 2 (MMP2), Matrix Metallopeptidase 3 (MMP3), and Matrix Metallopeptidase 14 (MMP14). GAPDH is as an internal control. The primers are shown in Table 1. The relative quantification of the genes of interest was calculated using the 2-ΔΔCt method.
Table 1.
Primer sequences used for quantitative RT-PCR.
| Gene | Accession No. | Forward | Reverse | Product Length (bp) |
|---|---|---|---|---|
| COL1A2 | NM_001003187 | tggaagtcgtggtgatggtg | tctttaccagcagcaccagg | 126 |
| COL3A1 | XM_845916.4 | ccgtgccaaatatgcgtctg | aggaggagatgttggaggct | 144 |
| TGF-β | NM_001003309 | tccggcagctctacattgac | ctccaaatgtaggggcaggg | 109 |
| IL-6 | NM_001003301.1 | cggcaaaatctctgcactga | actccacaagaccggtagtg | 182 |
| MMP2 | XM_014109407 | cacggccaactatgatgatg | agaatgctccagtcccattg | 112 |
| MMP3 | NM_001002967.1 | cattccctgggtctctttca | ggaggaatcagagggaggtc | 150 |
| MMP14 | AY534615.1 | tgctgctctcttctggatgc | ttttggggtactcgctgtcc | 107 |
| cGAPDH | NM_001003142 | aacatcatccctgcttccac | ggcaggtcagatccacaact | 130 |
2.9. Statistical Analysis
The outcomes of primary interest were the biomechanical parameters of ultimate failure strength and stiffness, relative gene expression, and histology outcomes of morphology and cellularity. The histology outcomes were reported descriptively only. Otherwise, outcomes comprised of continuous variables were compared between the 3 tendon repair groups using mixed-models analysis of variance, incorporating the dog ID as a random term to account for the within-animal correlation among the digits contributed by each dog. If the overall tests of the tendon repair group were statistically significant, pairwise contrasts were generated to identify which groups were different from the others. In order to guard against the increased type-I error rate associated with multiple comparisons, the p-values from the pairwise contrasts were adjusted using the Benjamini-Hochberg procedure to control the false discovery rate. All statistical tests were two-sided and p-values less than 0.05 were considered significant.
3. Results
The morphological investigation of exosomes was performed by transmission electron microscope. The TEM micrographs showed that exosomes of PEP present hollow vesicle spherical structures with hypodense in the center and hyperdense in the around. (Fig. 2-A) The diameters of exosomes were ranged from 93.70 nm to 124.65 nm as measured with Image J software. We incorporated PEP into the Tisseel as a gel patch, which could release exosomes to the surroundings in a sustained manner. The particles concentration of PEP was calculated every day and the size distribution was 50~200 nm. (Fig. 2-B, 2-C and 2-D) The representative picture of NanoSight detecting process showed that flash light spots were particles in different focus planes. (Fig. 2-E) Fig. 2-F showed the TEPEP patch could release particles stably over a two-week observation period.
Figure 2:
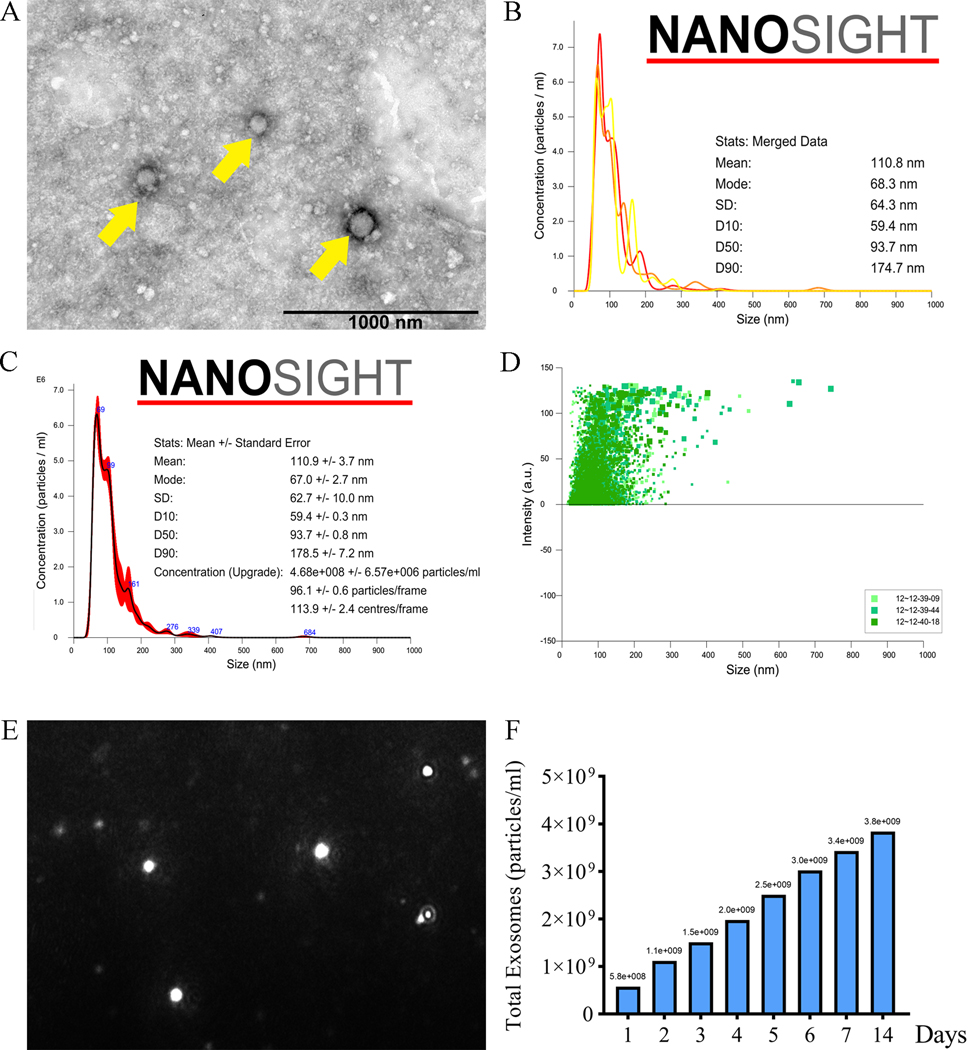
Characteristic and releasing test of the TEPEP patch. A. The structure of exosomes was observed by TEM. (Yellow arrows) B. Concentration of particles released by TEPEP patch in day 4. C. Mean size of particles released by TEPEP patch in day 4. D. Size distribution of TEPEP patch in day 4. E. Flash light spots were particles in different focus planes. F. Release test of TEPEP patch within two weeks.
All F-actin and Hoechst 33342 showed a positive fluorescence in cytoplasmic and nuclear of tenocytes respectively. The nanoparticles showed a higher fluorescence in cytoplasm of tenocytes with 20% PEP compared to tenocytes without PEP. (Fig. 3-A and 3-B) In addition, in the culturing process of the tendon model, a large number of adherent tenocytes were observed in the TEPTP patch group at day 14, versus the smaller number of tenocytes observed in both Control and Tisseel patch group (Fig. 3-C and 3-D). During mechanical testing, all specimens failed at the repair site. The failure strength in the Tisseel patch (40.0243 mN, p = 0.0025) and TEPTP patch (57.9858 mN, p < 0.0001) group were significantly higher than that of the Control group (18.5197 mN), while the TEPTP patch was found superior to the Tisseel patch alone group (p = 0.0223) (Fig. 3-E). Tensile stiffness in the Tisseel patch (16.4584 mN/mm, p = 0.0011) group and the TEPTP patch (13.6093 mN/mm, p < 0.0001) group was significantly higher than that in the Control group (5.7209 mN/mm).There was no significant difference between the TEPTP patch group and the Tisseel patch group (p = 0.06305) (Fig. 3-F).
Figure 3:
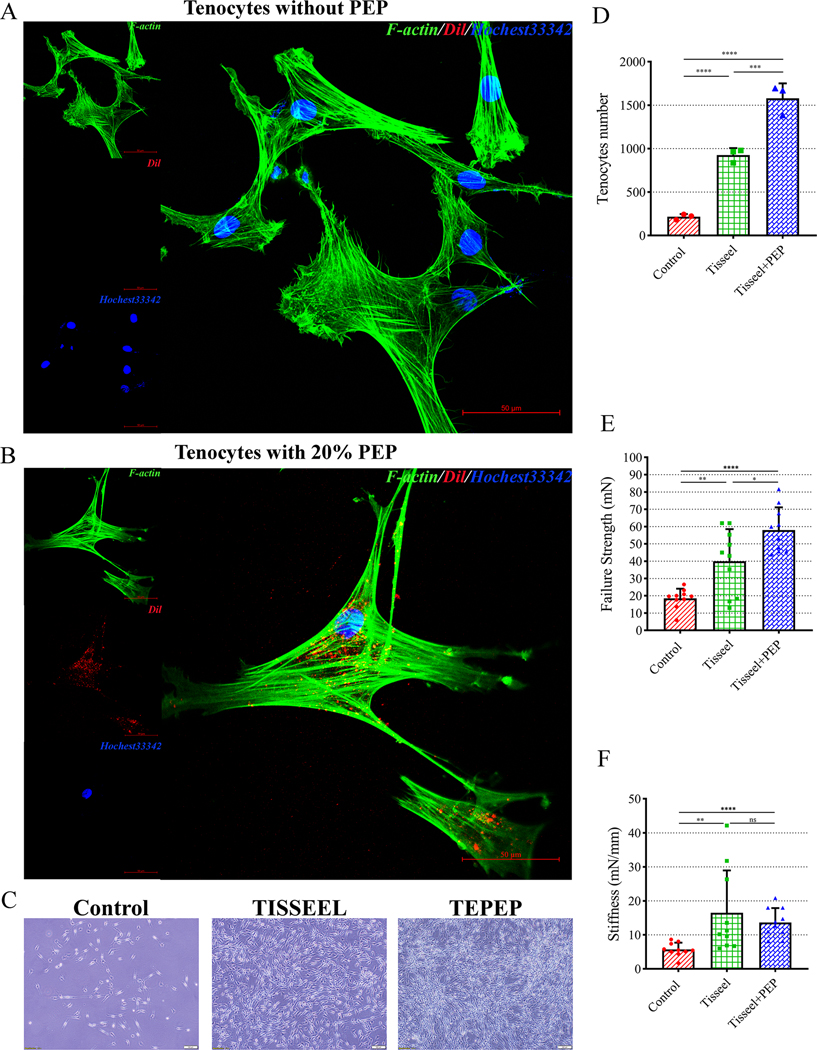
Cellular uptake and mechanical testing results. A. Cellular uptake of PEP into tenocytes without PEP. (Scale bar: 50 μm) B. Cellular uptake of PEP into tenocytes with 20% PEP. (Scale bar: 50 μm) C. Tenocytes attached on the square dish in different groups at day 14. (Scale bar: 100 μm) D. Quantification of the tenocytes number among three groups. E. Mean failure strength of the repaired tendon. F. Mean stiffness of the repaired tendon. Error bars represent standard deviation. (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001)
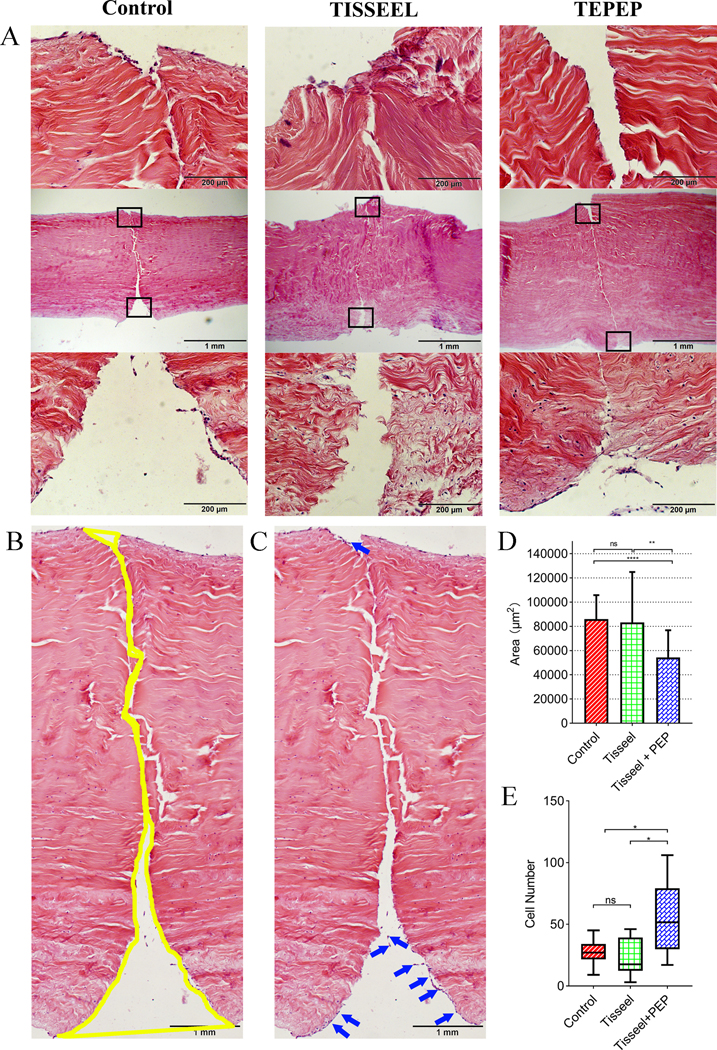
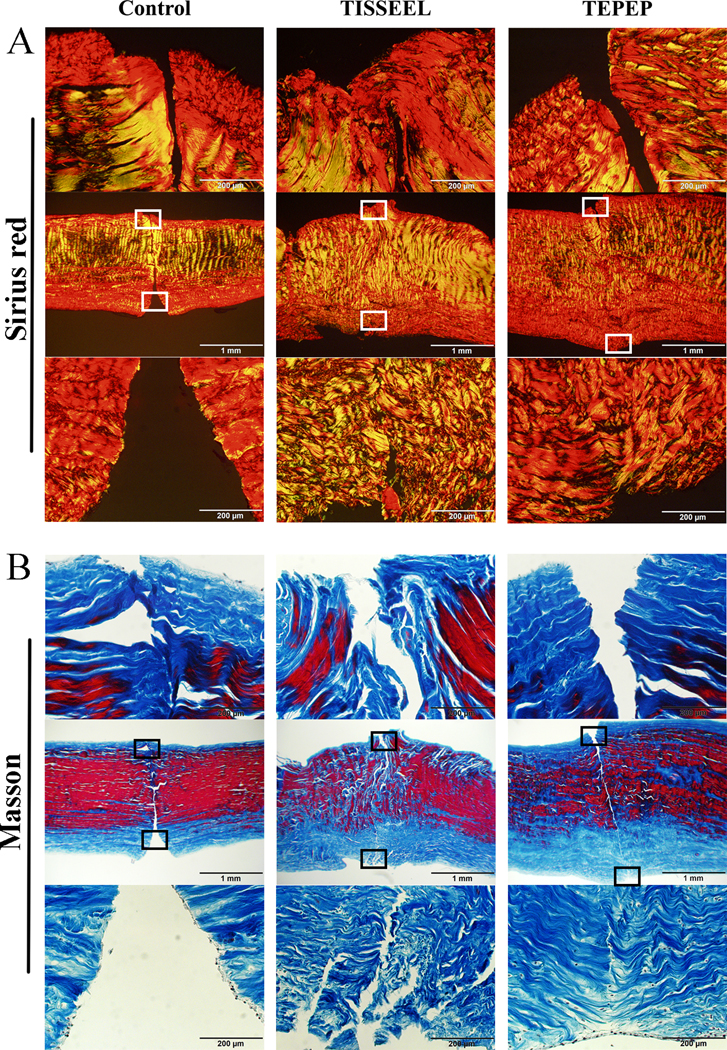
Partial healing was histologically documented in all the three groups (Fig. 4-A), with healing gap quantified among the three groups with Image J software (Fig. 4-B). The healing gap in the TEPTP patch group was significantly smaller than both the Control group (p < 0.0001) and the Tisseel patch group (p = 0.0014). There were no differences between the Control group and the Tisseel patch group (p = 0.5561) (Fig. 4-D). In addition, we also found a lot of fibroblasts migrated into the injury site of the flexor tendon in PEP-Tisseel group (Fig. 4-C). The cell number in TEPTP patch group was significantly higher than both Control group (p = 0.0241) and Tisseel (p = 0.0139) patch group (Fig. 4-E). While there were no differences between the Control group and the Tisseel patch group (p = 0.4625). Moreover, the Sirius red staining showed most of the collagen fibers belong to type I collagen and difference in collagen alignment was observed (Fig. 5-A). Masson trichrome staining showed most of the fibers are collagen fibers (Fig. 5-B).
Figure 4:
Histology results of healing tendons in treatment groups at 4 weeks. A: Representative H&E-stained images. B-C: Representative H&E-stained images from control group. Yellow area showed the healing gap of tendons. Blue arrows showed the fibroblasts migrate to the surface of injury tendon. (Scale bar: 1 mm) D. Quantification of healing among three groups. E. Quantification of the number of fibroblasts among three groups. (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001)
Figure 5:
Histology results of healing tendons in treatment groups at 4 weeks. A. Representative Sirius red staining images at 4 weeks after surgery. B. Representative Masson staining images at 4 weeks after surgery.
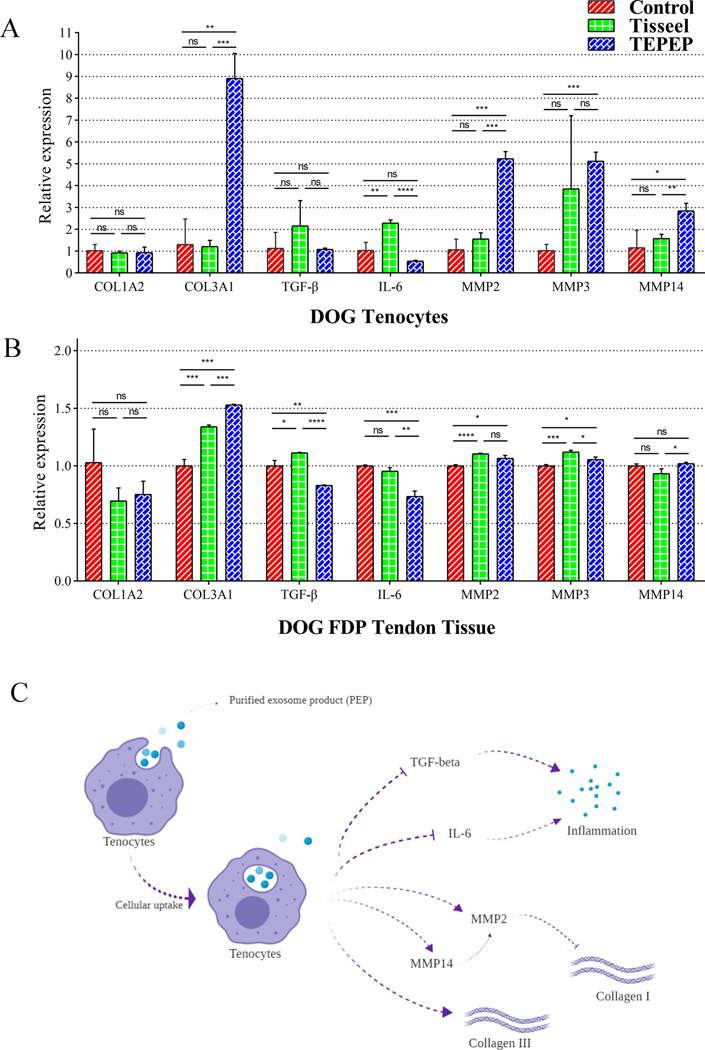
For the gene expression in the dog tenocytes, the expression of COL3A1, MMP2, MMP3, and MMP14 was significantly increased in the TEPEP patch Group compared to other groups. For the expression of COL1A2, TGF-β and IL-6, there were no significant changes between TEPEP patch and Control groups (Fig. 6-A). Besides, the gene expression of COL1A2, COL3A1, MMP2, and MMP3 in the FDP tendon tissue were consistent with above tenocytes study. The expression of TGF-β and IL-6 was significantly decreased in the TEPEP patch Group. And there were no significant changes in COL1A2 and MMP14 between TEPEP patch and Control groups (Fig. 6-B). Therefore, the role of PEP in this ex vivo tendon repair model may be to reduce inflammation and increase expression of type III collagen. (Fig. 6-C)
Figure 6A and B:
Gene expressions of tenocytes and healing tendon tissue among three groups at 4 weeks. C. The role of PEP for tendon healing in the ex vivo model.
4. Discussion
A number of studies have been published recently on the effect of exosomes on tendon healing. For tendon repair, previous studies mainly focused on surgical techniques, growth factors, cell therapy, and tissue engineering scaffolds.27–29 Despite new biological scaffolds and other treatments designed to improve tendon healing and minimize scar formation, the mechanical properties and proliferation of tenocytes remain problematic.30; 31 In this study, we proposed a novel engineered purified exosome product patch for tendon healing, our results suggested that the Tisseel could improve biomechanics properties and the PEP could accelerate fibroblast migrates to the injury site of tendon gap and reduce the inflammatory microenvironment in the injured site. Moreover, PEP is a GMP grade product, also a traceable, safe, pure and effective drug. This makes the novel ex vivo model have potential clinical transformation prospects. Therefore, this study represented an innovative method in tendon repair strategies that could be translated for flexor digitorum profundus tendon injuries in human patients.
With the development of current biological scaffolds, composite materials such as biological materials combined with cells or various factors have been deeply explored.32 However, finding a bioactive material that can be rapidly degraded and stably release certain targeting factors has become a common problem faced by scientists worldwide. In this study, the TEPEP patch, which could release exosomes to the surroundings in a sustained manner within two weeks. The size distribution of particles was 50~200 nm and most of the particles released into the surrounding area could be well uptake by tenocytes. Although we didn’t explore molecular or drug delivery capacity of PEP, there is no doubt that we could add certain mRNAs or related drugs via this way according to previous research.33 In addition, this novel tissue-engineered tendon ex vivo model, it can mimic the intrinsic repair ability of tendon tissue and avoid the ethics or time constraints of in vivo experiments. In tendon explants, cells could still transfer mechanical stimuli and biological signals in a way that mimics the in vivo complexity of cell-cell and cell-matrix communication. This also makes it possible to study the histological morphology and mechanical mechanics of tendons. Different from our previous tendon model studies (bone marrow stromal cells,34 growth and differentiation factor 5,35 or different ratios of fibrin gels36), this study first adopted PEP exosomes, a GMP grade product, as a safe and off-the-shelf biologics-based strategy to promote endogenous repair of tendon tissue.
Mechanical properties are one of the most important concerns for tendon healing. Our previous study attempted to use different concentrations of Fibrin gel (5–80 mg/mL Fibrinogen, 25–500 NIH units/mL Thrombin) to repair tendon, we found using high concentration of Fibrinogen as the patch have better mechanical properties (include the Failure Load, Failure Stress, and Tensile Stiffness) than the low one.37 The methods of mechanical testing and delivery scaffold were consistent with our research. In this study, compared with other groups, the mechanical properties of TEPEP group are better. This is most likely caused by the PEP’s stimulation of tendon cell proliferation and the migration of fibroblasts on the tendon surface to the site of injury. Use of PEP (with no dose-to-dose variability) instead of bone marrow stromal cells, greatly reduced the inter-group error and enhanced the mechanical stability of the patch itself. Moreover, failure stress is also important indicator to evaluate the mechanical properties of tendon.38 Therefore, a systematic biomechanical assessment of tendon recovery at the right time becomes particularly important.
The extracellular matrix of tendons is composed of several types of collagen molecules (e.g. Type I/III collagen), which play an important role in the repair and regeneration of injured tendon tissue. In this study, we found that the type III collagen increased in the TEPEP group compare with other groups. Due to collagen’s unique triple helical structure, it can provide stable mechanical properties, good support, and elasticity for cells. Besides, it participates in various cell signaling pathways and cell differentiation. The synthesis of collagen III involves the early stages of wound repair because of a large number of fibroblasts migrate into the surface of the tendon rupture to form the fibronectin matrix.39 This further confirms our hypothesis that PEP can promote accumulation in collagen III and accelerate the process tendon healing. On the other hand, after the tendon ruptures, its microenvironment is broken. In the healing process of tendons, a variety of cytokines and matrix metalloproteinases (e.g. MMP2, MMP3, MMP14), inflammatory factors (e.g. TGF-β, IL-6) and angiogenic factors constitute an inseparable interrelationship.40 This project found that the application of PEP can effectively reduce the expression of IL-6 and TGF-β, further decrease the inflammatory response during tendon healing and increase the stability of the extracellular matrix.41; 42 Although we have not detected much gene expression changes in inflammatory factors and matrix metalloproteinases in cellular experiments, this may be due to the short exposure time of the cells to exosomes or due to an absence of immune cells in the ex vivo context. It should be noted that some gene expressions between tenocytes and tendon tissue were different. There may be several reasons. 1) Large amount of cells in the tenocyte model compared to tissue culture; 2) The PEP in cell culture medial may have a direct impact on the culture cells; 3) PEP intake by cells may be more predominant in cell culture model than the tissue culture; 4) extracellular matrix environment is different between cell culture and tissue culture. However, several gene expressions, such as type I and III collagen and IL-6, have shown the same trend between tenocyte model and repair tendon model.
The matrix metalloproteinases, synthesized and secreted by fibroblasts, are a class of enzymes that digests collagen and other structural molecules.43 It has been reported that MMP2 plays an important role in the degradation of collagen type I.44 This may further explain why we detected large amounts MMP2 of in tendon tissue but did not find much expression of type I collagen. In previous tumor invasion studies, MMP2 activation was furthermore shown to be closely related to MMP14 expression, consistent with the results detected in our project.45 Above all, the specific role of PEP in different collagens by regulating various types of matrix metalloproteinases still requires further exploration.
The conclusions from this project is limited by the fact that the tendons utilized this study were of canine origin being treated with exosomes of human origin. Although the impact of PEP exosomes in humans would be anticipated to be stronger, the results reported herein will need further verification. Additionally, the ex vivo designs of this study provided a targeted focus on tenocyte mediated tendon healing. However, with the significant impact of microvasculature as well as the immune system on tissue regeneration, in vivo verification of the findings described is required.
In summary, application of the PEP loaded TISSEEL patch after flexor tendon repair increases the tendon intrinsic healing strength, cellularity, and tenocyte migration, while decreasing the repair gap formation and tenocyte inflammatory response in an ex vivo tendon healing model. Since our PEP is a fully off-the-shelf GMP grade biologics and TISSEEL is pharmaceutical agent, this approach has high potential for clinical translation. However, combination of PEP with a hydrogel delivery system in a pre-clinical in vivo study is required to validate the findings from the current study before clinical trial application can be pursued.
Supplementary Material
Supplement Figure 1: Process of culture dog tenocytes and harvest for quantitative RT-PCR.
Acknowledgement
This work was supported by grants from Orthopedic Research Review Committee of Mayo Clinic (94064024), NIH NIAMS AR57745, and Mayo Clinic Kelly Fellow Award. Mechanical testing was supported by the Mayo Clinic Biomechanics Core Facility. This study also thanks for the technical support from Dr. Zeling Long and Ramona. The authors Guidong Shi, Zhanwen Wang are funded by the China Scholarship Council.
Footnotes
Conflicts of interest
AB is a co-founder of Rion LLC.
References:
- 1.Polfer EM, Sabino JM, Katz RD. 2019. Zone I Flexor Digitorum Profundus Repair: A Surgical Technique. J Hand Surg Am 44:164.e161–164.e165. [DOI] [PubMed] [Google Scholar]
- 2.Lee YW, Fu SC, Mok TY, et al. 2017. Local administration of Trolox, a vitamin E analog, reduced tendon adhesion in a chicken model of flexor digitorum profundus tendon injury. Journal of orthopaedic translation 10:102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen SH, Chen CH, Fong YT, et al. 2014. Prevention of peritendinous adhesions with electrospun chitosan-grafted polycaprolactone nanofibrous membranes. Acta Biomater 10:4971–4982. [DOI] [PubMed] [Google Scholar]
- 4.Duci SB, Ahmeti HR. 2016. Partially Divided Flexor Tendon Injuries: Should They Be Repaired or Not? Surgery journal (New York, NY) 2:e89–e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qiu G, Zheng G, Ge M, et al. 2018. Mesenchymal stem cell-derived extracellular vesicles affect disease outcomes via transfer of microRNAs. Stem Cell Res Ther 9:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui H, He Y, Chen S, et al. 2019. Macrophage-Derived miRNA-Containing Exosomes Induce Peritendinous Fibrosis after Tendon Injury through the miR-21–5p/Smad7 Pathway. Molecular Therapy - Nucleic Acids 14:114–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hofer HR, Tuan RS. 2016. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res Ther 7:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davidson SM, Yellon DM. 2018. Exosomes and cardioprotection - A critical analysis. Molecular aspects of medicine 60:104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cecchettini A, Finamore F, Puxeddu I, et al. 2019. Salivary extracellular vesicles versus whole saliva: new perspectives for the identification of proteomic biomarkers in Sjogren’s syndrome. Clinical and experimental rheumatology 37 Suppl 118:240–248. [PubMed] [Google Scholar]
- 10.Street JM, Koritzinsky EH, Glispie DM, et al. 2017. Urine Exosomes: An Emerging Trove of Biomarkers. Advances in clinical chemistry 78:103–122. [DOI] [PubMed] [Google Scholar]
- 11.Kong FL, Wang XP, Li YN, et al. 2018. The role of exosomes derived from cerebrospinal fluid of spinal cord injury in neuron proliferation in vitro. Artificial cells, nanomedicine, and biotechnology 46:200–205. [DOI] [PubMed] [Google Scholar]
- 12.Qin W, Tsukasaki Y, Dasgupta S, et al. 2016. Exosomes in Human Breast Milk Promote EMT. Clinical cancer research : an official journal of the American Association for Cancer Research 22:4517–4524. [DOI] [PubMed] [Google Scholar]
- 13.Othman N, Jamal R, Abu N. 2019. Cancer-Derived Exosomes as Effectors of Key Inflammation-Related Players. Frontiers in immunology 10:2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pick H, Alves AC, Vogel H. 2018. Single-Vesicle Assays Using Liposomes and Cell-Derived Vesicles: From Modeling Complex Membrane Processes to Synthetic Biology and Biomedical Applications. Chemical reviews 118:8598–8654. [DOI] [PubMed] [Google Scholar]
- 15.Barile L, Vassalli G. 2017. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacology & therapeutics 174:63–78. [DOI] [PubMed] [Google Scholar]
- 16.Qi J, Liu Q, Reisdorf RL, et al. 2020. Characterization of a purified exosome product and its effects on canine flexor tenocyte biology. J Orthop Res. [DOI] [PubMed] [Google Scholar]
- 17.Wu M, Ouyang Y, Wang Z, et al. 2017. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proceedings of the National Academy of Sciences 114:10584–10589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanandiya ND, Lee S, Rho S, et al. 2019. Tunichrome-inspired pyrogallol functionalized chitosan for tissue adhesion and hemostasis. Carbohydr Polym 208:77–85. [DOI] [PubMed] [Google Scholar]
- 19.Spotnitz WD. 2014. Fibrin Sealant: The Only Approved Hemostat, Sealant, and Adhesive-a Laboratory and Clinical Perspective. ISRN Surg 2014:203943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu R, Wilson A, Travieso R, et al. 2017. Fibrin Tissue Sealant as an Adjunct to Cleft Palate Repair. The Journal of craniofacial surgery 28:1164–1166. [DOI] [PubMed] [Google Scholar]
- 21.Wong C, Inman E, Spaethe R, et al. 2003. Fibrin-based biomaterials to deliver human growth factors. Thrombosis and haemostasis 89:573–582. [PubMed] [Google Scholar]
- 22.Grimaud JC, Munoz-Bongrand N, Siproudhis L, et al. 2010. Fibrin glue is effective healing perianal fistulas in patients with Crohn’s disease. Gastroenterology 138:2275–2281, 2281.e2271. [DOI] [PubMed] [Google Scholar]
- 23.Solakoglu C, Mahirogullari M, Cakmak S, et al. 2010. Fibrin sealant in the treatment of acute ruptures of the Achilles tendon: long-term results. Eklem Hastalik Cerrahisi 21:124–129. [PubMed] [Google Scholar]
- 24.Soreide E, Denbeigh JM, Lewallen EA, et al. 2018. Fibrin glue mediated delivery of bone anabolic reagents to enhance healing of tendon to bone. Journal of cellular biochemistry 119:5715–5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frauz K, Teodoro LFR, Carneiro GD, et al. 2019. Transected Tendon Treated with a New Fibrin Sealant Alone or Associated with Adipose-Derived Stem Cells. Cells 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao C, Chieh H-F, Bakri K, et al. 2009. The effects of bone marrow stromal cell transplants on tendon healing in vitro. Medical Engineering & Physics 31:1271–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Georgescu AV, Matei IR, Olariu O. 2019. Zone II Flexor Tendon Repair by Modified Brunelli Pullout Technique and Very Early Active Mobilization. J Hand Surg Am 44:804.e801–804.e806. [DOI] [PubMed] [Google Scholar]
- 28.Chamberlain CS, Clements AEB, Kink JA, et al. 2019. Extracellular Vesicle-Educated Macrophages Promote Early Achilles Tendon Healing. Stem Cells 37:652–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang S, Shi X, Li X, et al. 2019. Oriented collagen fiber membranes formed through counter-rotating extrusion and their application in tendon regeneration. Biomaterials 207:61–75. [DOI] [PubMed] [Google Scholar]
- 30.Shi Z, Wang Q, Jiang D. 2019. Extracellular vesicles from bone marrow-derived multipotent mesenchymal stromal cells regulate inflammation and enhance tendon healing. Journal of translational medicine 17:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hurley CM, Reilly F, Callaghan S, et al. 2019. Negative Predictors of Outcomes of Flexor Tendon Repairs. Cureus 11:e4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wunderli SL, Blache U, Snedeker JG. 2020. Tendon explant models for physiologically relevant in vitro study of tissue biology - a perspective. Connect Tissue Res:1–16. [DOI] [PubMed] [Google Scholar]
- 33.Zhang K, Zhao X, Chen X, et al. 2018. Enhanced Therapeutic Effects of Mesenchymal Stem Cell-Derived Exosomes with an Injectable Hydrogel for Hindlimb Ischemia Treatment. ACS Appl Mater Interfaces 10:30081–30091. [DOI] [PubMed] [Google Scholar]
- 34.Ozasa Y, Gingery A, Thoreson AR, et al. 2014. A comparative study of the effects of growth and differentiation factor 5 on muscle-derived stem cells and bone marrow stromal cells in an in vitro tendon healing model. J Hand Surg Am 39:1706–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayashi M, Zhao C, An KN, et al. 2011. The effects of growth and differentiation factor 5 on bone marrow stromal cell transplants in an in vitro tendon healing model. J Hand Surg Eur Vol 36:271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirkness MW, Lehmann K, Forde NR. 2019. Mechanics and structural stability of the collagen triple helix. Curr Opin Chem Biol 53:98–105. [DOI] [PubMed] [Google Scholar]
- 37.Uehara K, Zhao C, Gingery A, et al. 2015. Effect of Fibrin Formulation on Initial Strength of Tendon Repair and Migration of Bone Marrow Stromal Cells in Vitro. J Bone Joint Surg Am 97:1792–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kew SJ, Gwynne JH, Enea D, et al. 2012. Synthetic collagen fascicles for the regeneration of tendon tissue. Acta Biomater 8:3723–3731. [DOI] [PubMed] [Google Scholar]
- 39.Snedeker JG, Foolen J. 2017. Tendon injury and repair - A perspective on the basic mechanisms of tendon disease and future clinical therapy. Acta Biomater 63:18–36. [DOI] [PubMed] [Google Scholar]
- 40.Schulze-Tanzil G, Al-Sadi O, Wiegand E, et al. 2011. The role of pro-inflammatory and immunoregulatory cytokines in tendon healing and rupture: new insights. Scand J Med Sci Sports 21:337–351. [DOI] [PubMed] [Google Scholar]
- 41.Cianciaruso C, Beltraminelli T, Duval F, et al. 2019. Molecular Profiling and Functional Analysis of Macrophage-Derived Tumor Extracellular Vesicles. Cell reports 27:3062–3080.e3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rayamajhi S, Nguyen TDT, Marasini R, et al. 2019. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater 94:482–494. [DOI] [PubMed] [Google Scholar]
- 43.Davis ME, Gumucio JP, Sugg KB, et al. 2013. MMP inhibition as a potential method to augment the healing of skeletal muscle and tendon extracellular matrix. Journal of applied physiology (Bethesda, Md : 1985) 115:884–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wertheim KY, Roose T. 2017. A Mathematical Model of Lymphangiogenesis in a Zebrafish Embryo. Bull Math Biol 79:693–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nyante SJ, Wang T, Tan X, et al. 2019. Quantitative expression of MMPs 2, 9, 14, and collagen IV in LCIS and paired normal breast tissue. Sci Rep 9:13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure 1: Process of culture dog tenocytes and harvest for quantitative RT-PCR.