Abstract
BACKGROUND
We report long-term survival and development of selected health conditions in Ontario-based referred and screened C282Y homozygotes for hemochromatosis treated by phlebotomy compared with an untreated control group known to be without HFE mutations.
METHODS
Patient characteristics and outcomes (all-cause mortality, liver cancer, diabetes, cirrhosis, hip or knee joint replacement, and osteoarthritis) were ascertained using a linked health administrative database held at ICES. Outcomes were assessed between groups without the outcome at baseline using Cox proportional hazards regression adjusted for age and sex. All C282Y homozygotes with elevated serum ferritin were treated by phlebotomy to reach serum ferritin of 50 µg/L. Our cohort included 527 C282Y homozygotes (311 men, 216 women, mean age 48 years) and 12,879 control participants (5,667 men and 7,212 women).
RESULTS
C282Y homozygotes had an increased risk of all-cause mortality (aHR 1.44 [1.19–1.75], p <0.001); hepatocellular carcinoma (aHR 8.30 [3.97–17.34], p <0.001); hip or knee joint replacement (aHR 3.06 [2.46–3.81], p <0.001); osteoarthritis (aHR 1.72 [1.47–2.01], p <0.001); and cirrhosis (aHR 3.87 [3.05–4.92], p <0.001). C282Y homozygotes did not have an increased risk for diagnosis of diabetes) (aHR 0.84 [0.67–1.07], p = 0.16) during follow-up (median 17.7 y).
CONCLUSIONS
C282Y homozygotes experience higher death and complication rates than individuals without HFE mutations, despite treatment by phlebotomy. Diabetes did not increase after phlebotomy therapy.
Keywords: hemochromatosis, iron, iron overload, phlebotomy
Though iron overload has been the defining feature of hemochromatosis, it is not clear that all the complications are caused by iron overload (1) or if iron depletion would prevent the future development of symptoms (2,3,4). In this study, the development of health conditions associated with C282Y-linked hemochromatosis and mortality after phlebotomy therapy are compared with a large untreated control population without HFE mutations.
Study design and setting
We conducted a retrospective cohort study in Ontario—Canada’s largest province—which provides universal access to health care through a single-payer system. Participant data were linked using unique encoded identifiers and analyzed at ICES. ICES is a prescribed entity under section 45 of Ontario’s Personal Health Information Protection Act, which authorizes ICES to collect personal health information without consent for the purpose of analysis or compiling statistical information with respect to the management, evaluation, or monitoring of the allocation of resources to, or planning for, all or part of the health system. The use of data in this project was approved by the Western University Health Science Research Ethics Board. This study follows RECORD reporting guidelines (5).
Participants
Our cohort was derived from two sources: patients included C282Y homozygotes referred to University Hospital in London, Ontario between 1974 and 2018, and Ontario-based patients (C282Y homozygotes and controls without HFE mutations) screened during the Hemochromatosis and Iron Overload Study (HEIRS) study between 2000 and 2005 (1). We considered matching control participants based on age and gender, but we chose to include all available control participants without HFE mutations. The large sample size of the control population provides the best estimate of the clinical outcomes and a narrow confidence interval. The ICES database does not contain data on genetic testing, so ascertainment of C282Y homozygotes was not possible. C282Y homozygotes with an elevated serum ferritin (men >300 µg/L, women >200 µg/L) were treated by weekly 500 mL by phlebotomy until the serum ferritin was 50 µg/L (6). Maintenance therapy was individualized based on the monitoring of serum ferritin over the follow-up period. Periodic voluntary blood donation was recommended every 3 months for patients with persistent or rising serum ferritin. The therapy was supervised by a single physician in 95% of patients. The ICES database does not include laboratory data such as serum ferritin, so it was not compared with referred patients in this study. Serum ferritin at entry in all control patients without HFE mutations from the HEIRS study (including our 12,879 control participants) has been previously reported (1). Referred patients were evaluated for cirrhosis and liver failure, but this information was not available for the control population, so it was not compared. We have previously reported on cirrhosis in referred patients, which was 30% in male probands, and 7% in female probands (7). Due to the much broader referral period among University Hospital patients—which includes a period during which outcome data was unavailable at ICES—individuals from this group were only included if alive and in Ontario as of a randomly assigned index date that followed the distribution of index dates (the date of inclusion into the HEIRS study) for HEIRS patients. Where multiple records for an individual were present (for example, an individual was present in both sources), the earliest index date was assigned. In referred C282Y homozygotes, cirrhosis and hepatocellular carcinoma were diagnosed by triphasic imaging, alpha-fetoprotein, and biopsy in selected cases. No patient had chronic hepatitis B or C or alcoholic liver disease. This differs from our control population assessment, in which cirrhosis was based on a validated proxy for cirrhosis and hepatocellular carcinoma diagnosis was from public databases. Statistical comparisons were restricted to clinical outcomes where only ICES data was used. We have previously reported on our referred patients with regard to survival, liver pathology, serum ferritin, hepatocellular carcinoma (7,8), and alcohol abuse (9), and this was not reported since we did not have similar data on the control population. All patients with hepatocellular carcinoma as a complication of hemochromatosis at our centre have had cirrhosis.
Outcomes
We followed participants to identify a series of outcomes previously linked to hemochromatosis (1,4,7,8,10–19): hepatocellular carcinoma, chronic liver disease, cirrhosis, knee or hip replacement, osteoarthritis, diabetes, and all-cause death. We also looked back for evidence of these outcomes in the patient’s health history. For each outcome, individuals were censored at index if the outcome was already present. During follow-up, individuals were also censored upon death or at the end of the observation window (for most outcomes, December 31, 2019; for death, March 31, 2020). Complete concept definitions are provided in Table 1.
Table 1:
Covariate concept definitions and codes
| Variable | Data source | Definition description | Standardized codes applied |
|---|---|---|---|
| Age | RPDB | Age of the individual at index, years. Categories for this variable include <30; 30–39; 40–49; 50–59; 60+ | |
| Sex | RPDB | Biological sex of the individual | |
| Congestive heart failure | CHF (20) | Presence in the database indicates the individual has a history of congestive heart failure | |
| Diabetes | ODD (21) | Presence in the database indicates the individual has a history of diabetes | |
| Rheumatoid arthritis | ORAD (22) | Presence in the database indicates the individual has a history of rheumatoid arthritis | |
| Osteoarthritis | DAD | At least one health care encounter in any of the listed data sources occurring within 5 years prior to index or during the follow-up period having a code related to osteoarthritis | ICD-9: 715 |
| NACRS | ICD-10: M15; M16; M17; M18; M19; M47 | ||
| OHIP | OHIP dx: 715 | ||
| Pneumonia | DAD | At least one health care encounter in any of the listed data sources occurring within 3 years prior to index or during follow-up having a code related to pneumonia | ICD-9: 480; 481; 482; 483; 484; 485; 486 |
| NACRS | ICD-10: J12; J13; J14; J15; J16; J17; J18 | ||
| OHIP | OHIP dx: 498 | ||
| Chronic liver disease | DAD | At least one health care encounter in any of the listed data sources occurring within 5 years prior to index having a code related to chronic liver disease | ICD-9: 070; 571; 573; 2750; 2751; 4561; 4562; 5722; 5723; 5724; 5728; 7824; 7891; 7895; V026 |
| NACRS | ICD-10: B16; B17; B18; B19; I85; R17; R18; R160; R162; B942; Z225; E831; E830; K70; K713; K714; K715; K717; K721; K729; K73; K74; K753; K754; K758; K759; K76; K77 | ||
| OHIP | OHIP dx: 571; 573; 070 | ||
| OHIP fee: Z551; Z554 | |||
| Dementia | DEMENTIA (24) | Presence in the database indicates the individual has a history of dementia | |
| Parkinson’s disease | DAD | At least one health care encounter in any of the listed data sources occurring within 5 years prior to index or during follow-up having a code related to Parkinson’s disease | ICD-9: 3320 |
| NACRS | ICD-10: G20 | ||
| OHIP | OHIP dx: 332 | ||
| Recent osteoporotic fracture | DAD | At least one health care encounter in any of the listed data sources occurring within 3 years prior to index with a code related to an osteoporotic fracture | ICD-9: 812; 8056; 8057; 808; 813; 8210; 8211; 8200; 8201; 8208; 8209; 8202; 8203 |
| NACRS | ICD-10: S422; S52; S720; S721; S722; S321; S322; S324; S323; S325; S327; S328; S723 | ||
| CCP: 9101; 9121; 9141; 9111; 9131; 9052; 9104; 9124; 9054; 9114; 9134; 9104; 9124; 9054; 9114; 9134; 935; 936 | |||
| CCI: 1TV73; 1TV74; 1TV03; 1VC73; 1VC74; 1VC03; 1VC80; 1VA73; 1VC73; 1VA74; 1VA53; 1VC74; 1VA80 | |||
| OHIP | OHIP fee: F014; F022; F023; F025; F026; F028; F030; F032; F033; F046; F024; F027; F031; Z203; F095; F096; F097; Z211 | ||
| Cirrhosis | DAD | At least one health care encounter in any of the listed data sources occurring within 3 years prior to index or during follow-up having a code related to cirrhosis | ICD-9: 4561; 5712; 5715 |
| NACRS | ICD-10: K700; K702; K73; K754; K758; K759; K760; B180; B181; B182; B188; B189 | ||
| OHIP | OHIP dx: 571 | ||
| All-cause death | RPDB | Death due to any cause | |
| Liver cancer | OCR | Diagnosis during follow-up with a liver tumour site | Topography code = C220 |
| Knee or hip joint replacement | DAD | At least one health care encounter in any of the listed data sources occurring during follow-up having a code related to knee or hip joint replacement | CCP: 9351; 9359; 9341 |
| NACRS | CCI: 1VA53; 1VG53 | ||
| OHIP | OHIP fee: R241; R439; R440; R481; R491; R553; R244; R248; R441; R442; R482; R483; R509 |
Data source: RPDB = ICES Registered Persons Database; CHF = Congestive heart failure; ODD = Ontario diabetes database; ORAD = Ontario rheumatoid arthritis database; DAD = Canadian Institute for Health Information Discharge Abstract Database; NACRS = Canadian Institute for Health Information National Ambulatory Care Reporting System; OHIP = Ontario Health Insurance Plan database
Standardized codes: ICD-9 = International Classification of Diseases Version 9; ICD-10 = International Classification of Diseases Version 10 (Canadian Version); OHIP fee = Ontario Health Insurance Plan fee codes; CCP = Canadian Classification of Diagnostic, Therapeutic and Surgical Procedures; CCI = Canadian Classification of Health Interventions; OHIP dx = Ontario Health Insurance Plan Diagnosis Codes
Covariates
We obtained covariates for the cohort at index including age, sex, health history (pneumonia in past 3 years; osteoporotic fracture in past 5 years), and comorbidities [congestive heart failure (20), diabetes (21), rheumatoid arthritis (22), osteoarthritis, chronic liver disease (23), dementia (24), Parkinson’s disease (24), and cirrhosis (23)] (Table 2). Covariate concept definitions, including codes, are provided in Table 1.
Table 2:
Description of the cohort at index
| No. (%)* | ||||||
|---|---|---|---|---|---|---|
| Total (N = 13,406) | C282Y homozygote (n = 527) | Wild type (n = 12,879) | p-value | Standardized difference† | ||
| Demographics | ||||||
| Age at index date, years | ||||||
| Mean (SD) | 49.00 (13.78) | 48.14 (15.61) | 49.04 (13.70) | 0.145 | 6% | |
| Median (IQR) | 48 (39–58) | 49 (38–58) | 48 (39–58) | 0.811 | 1% | |
| <30 | 2,734 (20.4) | 81 (15.4) | 2,653 (20.6) | <0.001 | 13% | |
| 30–39 | 3,615 (27.0) | 128 (24.3) | 3,487 (27.1) | 6% | ||
| 40–49 | 3,002 (22.4) | 128 (24.3) | 2,874 (22.3) | 5% | ||
| 50–59 | 3,100 (23.1) | 126 (24.0) | 2,974 (23.1) | 2% | ||
| ≥60 | 950 (7.1) | 62 (11.8) | 888 (6.9) | 17% | ||
| Sex = male | 5,512 (41.1) | 309 (58.6) | 5,203 (40.4) | <0.001 | ||
| Comorbidities and health history | ||||||
| Congestive heart failure | 223 (1.7) | 12 (2.3) | 211 (1.6) | 0.256 | 5% | |
| Diabetes | 1,268 (9.5) | 61 (11.6) | 1,207 (9.4) | 0.085 | 7% | |
| Rheumatoid arthritis | 164 (1.2) | 6 (1.1) | 158 (1.2) | 0.863 | 1% | |
| Osteoarthritis‡ | 1,478 (11.0) | 84 (15.9) | 1,394 (10.8) | <0.001 | 15% | |
| Recent osteoporotic fracture§ | 168 (1.3) | 15 (2.8) | 153 (1.2) | <.001 | 12% | |
| Chronic liver disease§ | 891 (6.6) | 186 (35.3) | 705 (5.5) | <0.001 | 80% | |
| Pneumonia§ | 582 (4.3) | 37 (7.0) | 545 (4.2) | 0.002 | 12% | |
| Dementia | NR | ≤5 | 25 (0.2) | 0.985 | 0% | |
| Parkinson's disease§ | NR | ≤5 | 42 (0.3) | 0.83 | 1% | |
| Cirrhosis‡ | 77 (0.6) | 20 (3.8) | 57 (0.4) | <0.001 | 23% | |
* Unless otherwise specified
† A standard difference >10% is statistically significant (25)
‡ Evidence within 5 years prior to index
§ Evidence within 3 years prior to index
IQR = Interquartile range; NR = Not recorded
Statistical Methods
We describe cohorts at index using a combination of one-way ANOVA, Kruskal–Wallis, and chi-square tests, as appropriate. We also provide standardized differences, which assess differences between group means as a percentage of the pooled standard deviation (a difference of >10% is considered significant). The standardized difference is the difference between two group means divided by the standard deviation of pooled groups. It is not as susceptible to identifying a statistically significant difference that is not clinically significant with large sample sizes as hypothesis testing is, which is why it is used in the analysis of large datasets (25).
We then report crude and event rates per 100 person-years. The hazard of receiving a given outcome for C282Y homozygotes was summarized using an adjusted hazard ratio (aHR) from a multivariate Cox proportional hazards model adjusted for age and sex. We ensured the proportional hazards assumption was met. Throughout, small cells (fewer than 6) are suppressed to protect patient privacy. We conducted all analyses using SAS statistical software, version 9.4 (SAS Software, Cary, NC, USA).
Results
We included 13,406 patients (527 C282Y homozygotes and 12,879 patients without HFE mutations) from both sources after exclusions (Figure 1). C282Y homozygotes were more likely male (58.9% versus 40.4%). They were not substantially more likely to have chronic conditions other than cirrhosis (3.8% versus 0.4%), chronic liver disease (35.4% versus 5.5%), and osteoarthritis (16% versus 10.8%). They were also more likely to have selected health history events (osteoporotic fracture, 2.9% versus 1.2%; pneumonia, 7.0% versus 4.2%) (Table 2).
Figure 1:
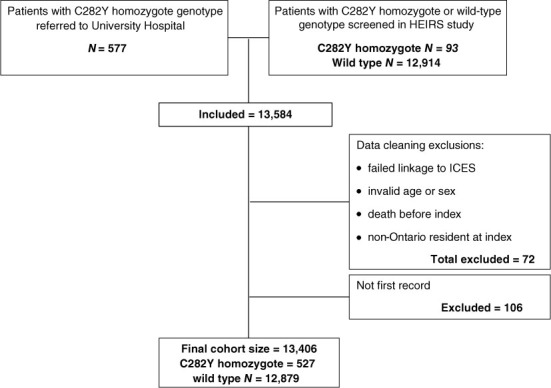
Cohort creation
HEIRS = Hemochromatosis and iron overload study
The aHRs of selected outcomes for C282Y homozygotes compared with wild-type participants are summarized in Table 3. The median follow-up time was 17.7 years. Liver cancer rates are lower than the UK Biobank study (10) and earlier studies (7), but cirrhosis rates are similar to previous studies in referred patients (11,12,13) and much higher than a population-based study from Newfoundland, Canada (4).
Table 3:
Adjusted hazard of developing selected outcomes during follow-up among C282Y homozygotes, compared with wild-type patients
| Adjusted* hazard ratio (95% CI) | ||||
|---|---|---|---|---|
| HR | Lower CL | Upper CL | p-value | |
| All-cause death | 1.436 | 1.182 | 1.745 | 0.0003 |
| Liver cancer | 8.297 | 3.97 | 17.341 | <0.001 |
| Knee or hip joint replacement | 3.063 | 2.461 | 3.811 | <0.001 |
| Diabetes | 0.844 | 0.667 | 1.067 | 0.1567 |
| Cirrhosis | 3.872 | 3.047 | 4.920 | <0.001 |
| Osteoarthritis | 1.719 | 1.472 | 2.007 | <0.001 |
* Adjusted for age and sex
After adjusting for age and sex, C282Y homozygotes had a 44% increased hazard of experiencing all-cause death (aHR 1.44 [95% CI 1.18–1.75]). They are over eight times more likely to experience hepatocellular carcinoma (aHR 8.30 [95% CI 3.97–17.34]) (Table 2). C282Y homozygotes are nearly four times more likely to experience cirrhosis during the follow-up (aHR 3.87 [95% CI 3.05–4.92]), over three times more likely to experience hip or knee replacement (aHR 3.06 [95% CI 2.46–3.81]) and are 72% more likely to develop osteoarthritis (aHR 1.72 [95% CI 1.47–2.01]). C282Y homozygotes were not more likely to develop diabetes during follow-up. Outcomes are compared between male and female C282Y homozygotes in Table 4 and between screened and referred patients in Table 5.
Table 4:
Outcomes by sex
| Male | Female | |||||
|---|---|---|---|---|---|---|
| Homozygote, No. (%) (n = 309) | Wild type, No. (%) (n = 5,203) | χ2 (p-value) | Homozygote, No. (%) (n = 218) | Wild type, No. (%) (n = 7,676) | χ2 (p-value) | |
| Death | 75 (24.27) | 992 (19.07) | 4.8 (0.03) | 33 (15.14) | 992 (12.92) | 1.03 (0.31) |
| Diabetes | 48 (15.53) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Cirrhosis | 59 (19.09) | 965 (18.55) | 1.14 (0.28) | 24 (11.01) | 1,164 (15.16) | 2.95 (0.08) |
| Osteoarthritis | 141 (45.63) | 229 (4.40) | 125.9 (<0.001) | 19 (8.72) | 291 (3.79) | 13.9 (<0.001) |
| Knee or hip joint replacement | 52 (16.83) | 1,571 (30.19) | 31.5 (<0.001) | 98 (44.95) | 2,857 (37.22) | 5.9 (0.01) |
Note: Numbers of patients with hepatocellular carcinoma are too small to perform χ2 analysis
Table 5:
Outcomes in screened and referred C282Y homozygotes
| Screened*, No. (%) (n = 135) | Referred†, No. (%) (n = 392) | χ2 (p-value) | |
|---|---|---|---|
| Death | 22 (16.29) | 86 (21.94) | 1.96 (0.16) |
| Cirrhosis | 19 (14.07) | 59 (15.05) | 0.07 (0.78) |
| Knee or hip joint replacement | 24 (17.78) | 65 (16.58) | 0.34 (0.56) |
* Screened refers to a C282Y homozygote discovered during a population screening study
† Referred is a C282Y homozygote referred to a specialty clinic by a primary care physician
Discussion
Since the discovery of the HFE gene in 1996 (26), there have been conflicting opinions about the clinical expression of the C282Y mutation of the HFE gene1 (4,12–19) and the efficacy of phlebotomy therapy in preventing long-term medical complications. Population studies using genetic testing have identified many non-expressing C282Y homozygotes, most commonly in young women (1). Earlier studies in referred patients may have introduced a referral bias and overestimated the morbidity and mortality (19). In this study, we have included both screened and referred C282Y homozygotes (Table 5) and non-HFE controls linked to health administrative data permitting identification of health outcomes over a median observation window of 17.7 years.
Liver complications, including cirrhosis and hepatocellular carcinoma, are the most serious and life-threatening and account for most disease-related mortality (4,7,8,10,11,12,27). Early treatment of hemochromatosis by phlebotomy has been demonstrated to prevent cirrhosis and hepatocellular carcinoma (28). Regression of liver fibrosis has also been reported after phlebotomy therapy (28–30), but complete reversal of cirrhosis is uncommon. In the current study, treated C282Y homozygotes had over eight times the risk of hepatocellular carcinoma compared with a large control population without HFE mutations. A larger sample of untreated C282Y homozygotes identified by genochip array analysis in the UK Biobank study had a hazard ratio of 15.5 (27). They predicted that 7.2 % of male homozygotes would develop hepatocellular carcinoma by age 75 years. The reduced hazard ratio for hepatocellular carcinoma in our study is not directly comparable, but improvement in fibrosis by phlebotomy therapy is a potential factor. It is possible in both studies that the follow-up period was not extended long enough to capture all eventual liver cancer cases. C282Y homozygotes in the present study had a nearly four times hazard of experiencing cirrhosis during the observation window, which could be a conservative estimate since liver biopsies were not done in all cases. A recent report from Newfoundland reported cirrhosis in 5.8% of C282Y homozygotes (4). Previous studies have suggested that hemochromatosis patients today may have a less severe phenotype than previously reported in earlier studies (31).
C282Y homozygotes were over three times more likely to require hip and knee joint replacements. Similar results were previously reported (32), and iron depletion has not been shown to improve arthropathy of hemochromatosis in most studies (33). C282Y homozygotes were 71% more likely to have evidence of osteoarthritis during the observation window. Both osteoarthritis and rheumatoid arthritis were more common in the UK Biobank study, with odds ratios of 2.01 and 2.23 (10). Arthropathy of hemochromatosis has often been shown to be the presenting complaint in women, and it can have a major deleterious effect on quality of life (34).
Although hemochromatosis was originally called “bronze diabetes,” we did not demonstrate an increased rate of diabetes, similar to several previous population-based studies (1,13,27). We previously demonstrated that most hemochromatosis patients with diabetes have insulin resistance, not low insulin from iron in the pancreas (35). The clinical factors associated with insulin resistance (obesity, metabolic syndrome) are also very common in the control population, which may explain why there is no increased risk of diabetes in the hemochromatosis cohort. Phlebotomy therapy was expected to possibly improve existing symptoms and prevent new symptoms from developing in C282Y homozygotes. This study demonstrates that cirrhosis, hepatocellular carcinoma, joint replacement, and osteoarthritis continue to occur after iron depletion. Hepatocellular carcinoma has rarely been reported in non-cirrhotic hemochromatosis patients (36), but an early diagnosis and treatment prior to the development of cirrhosis is likely the best strategy to prevent cirrhosis and hepatocellular carcinoma.
Limitations
We rely on health administrative data for the detection of outcomes. We used validated case definitions where possible for outcomes, but certain outcomes (in particular, osteoarthritis) have unknown operating characteristics in administrative data, which may result in misclassification. Certain individuals in the referral group have a blackout period of years or decades before starting their observation window. We do not know what happened before, whether it be treatment, or health outcomes, that might affect their behaviour. Data has been previously reported on cohorts of C282Y homozygotes that were untreated (27,37–39) but may not be directly comparable to our patient cohorts.
Acknowledgements:
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The study was completed at the ICES Western site, where core funding is provided by the Academic Medical Organization of Southwestern Ontario, the Schulich School of Medicine and Dentistry, Western University, and the Lawson Health Research Institute. Parts of this material are based on data and information compiled and provided by CIHI and Cancer Care Ontario. The analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred. We acknowledge the support of the National Heart, Lung and Blood Institute of the National Institute of Health (Bethesda, MD, USA) for the HEIRS study (NHLBI-HC-99-04), which contributed participants to this study.
Ethics Approval:
The use of data in this project was approved by the Western University Health Science Research Ethics Board. This study follows RECORD reporting guidelines (5).
Informed Consent:
N/A
Registry and registration no. of the study/trial:
N/A
Funding:
No funding was received for this work.
Disclosures:
The authors have nothing to disclose.
Peer Review:
This article has been peer reviewed.
References
- 1.Adams PC, Reboussin DM, Barton JC, et al. Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med. 2005;352(17):1769–78. 10.1056/NEJMoa041534. Medline: [DOI] [PubMed] [Google Scholar]
- 2.McDonell S, Preston B, Jewell S, et al. A survey of phlebotomy among persons with hemochromatosis. Transfusion. 1999;39(6):651–6. 10.1046/j.1537-2995.1999.39060651.x. Medline: [DOI] [PubMed] [Google Scholar]
- 3.Adams PC, Deugnier Y, Moirand R, Brissot P. The relationship between iron overload, clinical symptoms and age in 410 hemochromatosis patients. Hepatology. 1997; 25(1):162–6. 10.1002/hep.510250130. Medline: [DOI] [PubMed] [Google Scholar]
- 4.Lim DR, Vidyasankar G, Phua C, Borgaonkar M. Clinical penetrance of hereditary hemochromatosis-related endo-organ damage of C282Y homozygosity, a Newfoundland experience. Clin Transl Gastroenterol. 2020; 11(11):e00258. 10.14309/ctg.0000000000000258. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benchimol E, Smeeth L, Guttmann A, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement. PLoS Med. 2015;12(10):e1001885. 10.1371/journal.pmed.1001885. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams PC, Barton JC. How I treat hemochromatosis. Blood. 2010;116(3):317–25. 10.1182/blood-2010-01-261875. Medline: [DOI] [PubMed] [Google Scholar]
- 7.Wojcik J, Speechley M, Kertesz AE, et al. Natural history of C282Y homozygotes for hemochromatosis. Can J Gastroenterol. 2002;16(5):297–302. 10.1155/2002/161569. Medline: [DOI] [PubMed] [Google Scholar]
- 8.Adams PC. Hepatocellular carcinoma in hereditary hemochromatosis. Can J Gastroenterol. 1993;7:37–41. 10.1155/1993/537378. [DOI] [Google Scholar]
- 9.Adams PC, Agnew S. Alcoholism in hereditary hemochromatosis revisited: prevalence and clinical consequences among homozygous siblings. Hepatology. 1996;23(4):724–7. 10.1002/hep.510230411. Medline: [DOI] [PubMed] [Google Scholar]
- 10.Pilling LC, Tamosauskaite J, Jones G, et al. Common conditions associated with hereditary haemochromatosis genetic variants: cohort study in UK Biobank. BMJ. 2019;364:k5222. 10.1136/bmj.k5222. Medline:. Erratum in: BMJ. 2019;367. Corrected at: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niederau C, Fischer R, Sonnenberg A, et al. Survival and causes of death in cirrhotic and in noncirrhotic patients with primary hemochromatosis. N Engl J Med. 1985;313(20):1256–62. 10.1056/NEJM198511143132004. Medline: [DOI] [PubMed] [Google Scholar]
- 12.Ellervik C, Birgens H, Tybjærg-Hansen A, Nordestgaard B. Hemochromatosis genotypes and risk of 31 disease endpoints: meta-analyses including 66,000 cases and 226,000 controls. Hepatology. 2007;46(4):1071–80. 10.1002/hep.21885. Medline: [DOI] [PubMed] [Google Scholar]
- 13.Beutler E, Felitti V, Koziol J, et al. Penetrance of the 845G to A (C282Y) HFE hereditary haemochromatosis mutation in the USA. Lancet. 2002;359(9302):211–18. 10.1016/S0140-6736(02)07447-0. [DOI] [PubMed] [Google Scholar]
- 14.McLaren GD, McLaren CE, Adams PC, et al. Clinical manifestations of hemochromatosis in HFE C282Y homozygotes identified by screening. Can J Gastroenterol. 2008; 22(11):923–30. 10.1155/2008/907356. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asberg A, Hveem K, Kruger O, Bjerve KS.Persons with screening-detected haemochromatosis: as healthy as the general population? Scand J Gastroenterol. 2002;37(6):719–24. 10.1080/00365520212510. Medline: [DOI] [PubMed] [Google Scholar]
- 16.Allen KJ, Gurrin LC, Constantine CC, et al. Iron-overload-related disease in HFE hereditary hemochromatosis. N Engl J Med. 2008;358(3):221–30. 10.1056/NEJMoa073286. Medline: [DOI] [PubMed] [Google Scholar]
- 17.Fargion S, Mandelli C, Piperno A, et al. Survival and prognostic factors in 212 Italian patients with genetic hemochromatosis. Hepatology. 1992;15(4):655–9. 10.1002/hep.1840150417. Medline: [DOI] [PubMed] [Google Scholar]
- 18.Milman N, Pedersen P, Steig T, Byg K, Graudal N, Fenger K. Clinically overt hemochromatosis in Denmark 1948-1985: epidemiology, factors of significance for long-term survival, and causes of death in 179 patients. Ann Hematol. 2001;80(12):737–44. 10.1007/s002770100371. Medline: [DOI] [PubMed] [Google Scholar]
- 19.Bomford A, Williams R. Long term results of venesection therapy in idiopathic haemochromatosis. Q J Med. 1976;45(180):611–23. Medline: [PubMed] [Google Scholar]
- 20.Schultz SE, Rothwell DM, Chen Z, Tu K. Identifying cases of congestive heart failure from administrative data: a validation study using primary care patient records. Chronic Dis Inj Can. 2013;33(3):160–6. 10.24095/hpcdp.33.3.06. Medline: [DOI] [PubMed] [Google Scholar]
- 21.Hux JE, Ivis F, Flintoft V, Bica A. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002;25(3):512–6. 10.2337/diacare.25.3.512. Medline: [DOI] [PubMed] [Google Scholar]
- 22.Widdifield J, Bombardier C, Bernatsky S, et al . An administrative data validation study of the accuracy of algorithms for identifying rheumatoid arthritis: the influence of the reference standard on algorithm performance. BMC Musculoskeletal Disord. 2014;15:216. 10.1186/1471-2474-15-216. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lapointe-Shaw L, Georgie F, Carlone D, et al. Identifying cirrhosis, decompensated cirrhosis and hepatocellular carcinoma in health administrative data: a validation study. PLoS One. 2018;13(8):e0201120. 10.1371/journal.pone.0201120. Medline:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaakkimainen RL, Bronskill SE, Tierney M, et al . Identification of physician-diagnosed Alzheimer’s disease and related dementias in population-based administrative data: a validation study using family physicians’ electronic medical records. J Alzheimers Dis. 2016;54(1):337–49. 10.3233/JAD-160105. Medline: [DOI] [PubMed] [Google Scholar]
- 25.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(6):1228–34. 10.1080/03610910902859574. [DOI] [Google Scholar]
- 26.Feder JN, Gnirke A, Thomas W, et al. A novel MHC class I-like gene is mutated in patients with hereditary hemochromatosis. Nat Genet. 1996;13(4):399–408. 10.1038/ng0896-399. Medline: [DOI] [PubMed] [Google Scholar]
- 27.Atkins JL, Pilling L, Masoli J, et al. Primary hepatic carcinoma and mortality risks with hemochromatosis mutations in the UK Biobank community cohort. JAMA. 2020;324(20):2048–57. 10.1001/jama.2020.21566. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bardou-Jacquet E, Morandeau E, Anderson G, et al. Regression of fibrosis stage with treatment reduces long-term risk of liver cancer in patients with hemochromatosis caused by mutation in HFE. Clin Gastro Hep. 2020;18(8):1851–7. 10.1016/j.cgh.2019.10.010. Medline: [DOI] [PubMed] [Google Scholar]
- 29.Falize L, Guillygomarc’h A, Perrin M, et al. Reversibility of hepatic fibrosis in treated genetic hemochromatosis: a study of 36 cases. Hepatology. 2006;44(2):472–7. 10.1002/hep.21260. Medline: [DOI] [PubMed] [Google Scholar]
- 30.Powell LW, Dixon J, Ramm G, et al. Screening for hemochromatosis in asymptomatic subjects with or without a family history. Arch Int Med. 2006;166(3):294–301. 10.1001/archinte.166.3.294. Medline: [DOI] [PubMed] [Google Scholar]
- 31.Bardou-Jacquet E, Morcet J, Manet G, et al. Decreased cardiovascular and extrahepatic cancer-related mortality in treated patients with mild HFE hemochromatosis. J Hepatol. 2015;62(3):682–9. 10.1016/j.jhep.2014.10.025. Medline: [DOI] [PubMed] [Google Scholar]
- 32.Sahinbegovic E, Dallos T, Aigner E, et al. Hereditary hemochromatosis as a risk factor for joint replacement surgery. Am J Med. 2010;123(7):659–62. 10.1016/j.amjmed.2010.01.024. Medline: [DOI] [PubMed] [Google Scholar]
- 33.Kiely DW. Haemochromatosis arthropathy – a conundrum of the Celtic curse. J R Coll Physicians Edinb. 2018;48(3):233–8. 10.4997/JRCPE.2018.307. Medline: [DOI] [PubMed] [Google Scholar]
- 34.Adams PC, Speechley M. The effect of arthritis on the quality of life in hereditary hemochromatosis. J Rheumatol. 1996;23(4): 707–10. Medline: [PubMed] [Google Scholar]
- 35.Hramiak I, Finegood D, Adams PC. Factors affecting glucose tolerance in hereditary hemochromatosis. Clin Invest Med. 1997; 20(1):110–18. Medline: [PubMed] [Google Scholar]
- 36.Von Delius S, Lersch C, Schulte-Frohlinde E, et al. Hepatocellular carcinoma associated with hereditary hemochromatosis occurring in non-cirrhotic liver. Z Gastroenterol. 2006;44(1): 39–42. 10.1055/s-2005-858567. Medline: [DOI] [PubMed] [Google Scholar]
- 37.Olynyk J, Hagan S, Cullen D, Beiby J, Whitall D. Evolution of untreated hereditary hemochromatosis in the Busselton population: a 17-year study. Mayo Clin Proc. 2004; 79(3):309–13. 10.4065/79.3.309. Medline: [DOI] [PubMed] [Google Scholar]
- 38.Adams PC. The natural history of untreated HFE-related hemochromatosis. Acta Haematol. 2009;122(2-3):134–9. 10.1159/000243797. Medline: [DOI] [PubMed] [Google Scholar]
- 39.Andersen R, Tybjaerg-Hansen A, Appleyard M, Birgens H, Nordestgaard B. Hemochromatosis mutations in the general population: iron overload progression rate. Blood. 2004;103(8): 2914–9. 10.1182/blood-2003-10-3564. Medline: [DOI] [PubMed] [Google Scholar]


