Abstract
Background
Heartburn is one of the most common gastrointestinal symptoms in pregnant women. It can occur in all trimesters of pregnancy. The symptoms of heartburn in pregnancy may be frequent, severe and distressing, but serious complications are rare. Many interventions have been used for the treatment of heartburn in pregnancy. These interventions include advice on diet, lifestyle modification and medications. However, there has been no evidence‐based recommendation for the treatment of heartburn in pregnancy.
Objectives
To assess the effects of interventions for relieving heartburn in pregnancy.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 June 2015), ClinicalTrials.gov (2 March 2015), Asian & Oceanic Congress of Obstetrics & Gynaecology (AOCOG) conference proceedings (20‐23 October 2013, Centara Grand & Bangkok Convention Centre, Bangkok, Thailand), and reference lists of retrieved studies.
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTS of interventions for heartburn in pregnancy compared with another intervention, or placebo, or no intervention. Cluster‐RCTs would have been eligible for inclusion but none were identified. We excluded studies available as abstracts only and those using a cross‐over design.
Interventions could include advice on diet, lifestyle modification and medications (such as antacids, sucralfate, histamine 2‐receptor antagonists, promotility drugs and proton pump inhibitors (PPIs)).
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy.
Main results
We included nine RCTs involving 725 women. However, five trials did not contribute data. Four trials involving 358 women contributed data. Trials were generally at mixed risk of bias.
We only identified data for three comparisons: pharmaceutical treatment versus placebo or no treatment; acupuncture versus no treatment and pharmacological intervention versus advice on dietary and lifestyle changes.
Pharmaceutical treatment compared with placebo or no treatment
Two trials evaluated any pharmaceutical treatment compared with placebo or no treatment. One trial examined a treatment rarely used nowadays (intramuscular prostigmine 0.5 mg versus placebo). One trial evaluated the effect of magnesium and aluminium hydroxide plus simethicone liquid and tablet compared with placebo. For the primary outcome of this review (relief of heartburn), women who received pharmaceutical treatment reported complete heartburn relief more often than women receiving no treatment or placebo (risk ratio (RR) 1.85, 95% confidence interval (CI) 1.36 to 2.50 in two RCTs of 256 women, I2 = 0%, moderate‐quality evidence). Data on partial relief of heartburn were heterogenous and showed no clear difference (average RR 1.35, 95% CI 0.38 to 4.76 in two RCTs of 256 women, very low‐quality evidence). In terms of secondary outcomes, there was no clear difference in the rate of side effects between the pharmaceutical treatment group and the placebo/no treatment group (RR 0.63, 95% CI 0.21 to 1.89 in two RCTs of 256 women, very low‐quality evidence).
Pharmacological intervention versus advice on dietary and lifestyle choices
One study compared 1 g of sucralfate with advice on dietary and lifestyle choices in treating heartburn. More women in the sucralfate group experienced complete relief of heartburn compared to women who received advice on diet and lifestyle choices (RR 2.41, 95% CI 1.42 to 4.07; participants = 65; studies = one). The only secondary outcome of interest addressed by this trial was side effects. The evidence was not clear on intervention side effects rate between the two groups (RR 1.74, 95% CI 0.07 to 41.21; participants = 66; studies = one). There was only one instance of side effects in the pharmacological group.
Acupuncture compared with no treatment
One trial evaluated acupuncture compared with no treatment but did not report data relating to this review's primary outcome (relief of heartburn). In terms of secondary outcomes, there was no difference in the rate of side effects between women who had acupuncture and women who had no treatment (RR 2.43, 95% CI 0.11 to 55.89 in one RCT of 36 women). With regard to quality of life, women who had acupuncture reported improved ability to sleep (RR 2.80, 95% CI 1.14 to 6.86) and eat (RR 2.40, 95% CI 1.11 to 5.18 in one RCT of 36 women).
The following secondary outcomes were not reported upon in any of the trials included in the review: miscarriage, preterm labour, maternal satisfaction, fetal anomalies, intrauterine growth restriction, low birthweight.
Authors' conclusions
There are no large‐scale RCTs to assess heartburn relief in pregnancy. This review of nine small studies (which involved data from only four small studies) indicates that there are limited data suggesting that heartburn in pregnancy could be completely relieved by pharmaceutical treatment. Three outcomes were assessed and assigned a quality rating using the GRADE methods. Evidence from two trials for the outcome of complete relief of heartburn was assessed as of moderate quality. Evidence for the outcomes of partial heartburn relief and side effects was graded to be of very low quality. Downgrading decisions were based in part on the small size of the trials and on heterogenous and imprecise results.
There are insufficient data to assess acupuncture versus no treatment and no data to assess other comparisons (miscarriage, preterm labour, maternal satisfaction, fetal anomalies, intrauterine growth restriction, low birthweight).
Further RCTs are needed to fully evaluate the effectiveness of interventions for heartburn in pregnancy. Future research should also address other medications such as histamine 2‐receptor antagonists, promotility drugs, proton pump inhibitors, and a raft‐forming alginate reflux suppressant in treatment of heartburn in pregnancy. More research is needed on acupuncture and other complimentary therapies as treatments for heartburn in pregnancy. Future research should also evaluate any adverse outcomes, maternal satisfaction with treatment and measure pregnant women's quality of life in relation to the intervention.
Plain language summary
Interventions for heartburn in pregnancy
What is the issue?
This review aims to evaluate the effectiveness of interventions for relieving heartburn in pregnancy. Interventions include advice on diet, lifestyle modification, medications and complementary therapies.
Why is this important?
Heartburn is a sensation of burning in the upper part of the digestive tract including the throat. It is one of the most common gut symptoms in pregnant women and it can occur anytime during pregnancy. It is caused by pregnancy hormones affecting the muscle that keeps food in the stomach, and letting acid in the stomach come back up the throat. The symptoms may be frequent, severe and distressing, but serious complications are rare. Many interventions have been suggested. Lifestyle modifications are suggested for treating mild symptoms. Women are often advised to eat smaller meals, chew gum, not to eat late at night, to elevate the head of the bed and avoid foods and medications that cause heartburn. Abstinence from alcohol and tobacco are encouraged to reduce reflux symptoms and to avoid fetal exposure to these harmful substances. For more troubling reflux symptoms, medications are sometimes used. The common drugs used for the treatment of heartburn in pregnancy include antacids, drugs that stimulate the muscles of the gastrointestinal tract to prevent acids from staying in the stomach too long.
What evidence did we find?
We found four small trials that provided data on 358 women. We estimated that the risk of bias was low for women enrolled in the study and the researchers as far as knowing if they were in the treatment group or the control (or placebo) group. It was unclear if there was a risk of bias for how the decisions were made to for women to be in the treatment or control/placebo groups, for those looking at the results and if all the results were reported.
Two trials looked at medication compared with placebo or no treatment. One study examined the effect of a medication(sucralfate) in comparison to advice on dietary and lifestyle choice. One trial evaluated acupuncture versus no treatment.
Women who received medication reported complete relief from heartburn more often than women receiving no treatment or placebo, or women who received advice on diet and lifestyle choices (moderate quality of evidence). We found no difference in partial relief of heartburn nor in side effects between the treatment groups (very low quality of evidence). We also found women who received acupuncture reported improved quality of life in terms of improved ability to sleep and eat, and no difference in the rate of side effects compared to women who received no acupuncture,
What does this mean?
From the little evidence there is, medication seems to help relieve heartburn but there is not enough data to say which medication is best. Acupuncture seems to help women to eat and sleep better when troubled with heartburn.
Further research is needed to fully evaluate the effectiveness of interventions for heartburn in pregnancy. Future research should also address other medications such as histamine 2‐receptor antagonists, promotility drugs, proton pump inhibitors, and a raft‐forming alginate reflux suppressant in treatment of heartburn in pregnancy. More research is needed on acupuncture and other complimentary therapies as treatments for heartburn in pregnancy. Future research should also consider any adverse outcomes, maternal satisfaction with treatment and measure pregnant women's quality of life in relation to the intervention.
Summary of findings
Summary of findings for the main comparison. Any pharmaceutical treatment compared with placebo or no treatment for heartburn in pregnancy.
| Any pharmaceutical treatment compared with placebo or no treatment for heartburn in pregnancy | ||||||
| Patient or population: pregnant women with heartburn in pregnancy Settings: US and UK Intervention: any pharmaceutical treatment Comparison: placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no treatment | Any pharmaceutical treatment | |||||
| Complet relief of heartburn | Study population | RR 1.85 (95% CI 1.36 to 2.50) | 256 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 301 per 1000 | 557 per 1000 (409 to 752) | |||||
| Moderate | ||||||
| 291 per 1000 | 539 per 1000 (396 to 728) | |||||
| Partial relief of heartburn | Study population | RR 1.35 (95% CI 0.38 to 4.76) | 256 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1,2,3 | ||
| 252 per 1000 | 340 per 1000 (96 to 1000) | |||||
| Moderate | ||||||
| 238 per 1000 | 321 per 1000 (90 to 1000) | |||||
| Side effects | Study population | RR 0.63 (95% CI 0.21 to 1.89) | 256 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 4,5 | ||
| 57 per 1000 | 36 per 1000 (12 to 108) | |||||
| Moderate | ||||||
| 48 per 1000 | 30 per 1000 (10 to 91) | |||||
| Quality of life | Study population | not estimable | (0 studies) | |||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Maternal satisfaction | Study population | not estimable | (0 studies) | |||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Low birthweight | Study population | not estimable | (0 studies) | |||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Preterm labour | Study population | not estimable | (0 studies) | |||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. CI: confidence interval; RR: risk ratio | ||||||
1Most studies contributing data had design limitations (‐1)
2Statistical heterogeneity, I2 = 88% (‐1).
3Small sample size and wide confidence interval crossing the line of no effect (‐2).
4Most weight from a study with design limitations (‐1). Reisfield 1971 excluded 6 women for having side effects.
5Few events and wide confidence interval crossing the line of no effect (‐2).
Background
Description of the condition
Heartburn is a sensation of burning in the upper part of the digestive tract including the throat (Richter 2005). It is one of the most common gastrointestinal symptoms in pregnant women. The worldwide incidence of heartburn in pregnancy is 17% to 80% (Audu 2006; Ho 1998; Law 2010; Quartarone 2013; Richter 2003; Richter 2005) and it can occur in all trimesters of pregnancy. Most women begin their symptoms late in the first trimester or in the second trimester and these symptoms become more frequent and severe in the final months of pregnancy (Richter 2005). The symptoms of heartburn in pregnancy may be frequent, severe and distressing, but serious complications are rare (Neilson 2008).
Risk factors for heartburn in pregnancy include advancing gestational age, heartburn antecedent to the pregnancy and women who have previously had one or more babies (Richter 2005).
The pathogenesis of heartburn in pregnancy involves decreasing lower oesophageal sphincter pressure. The increased circulating progesterone during pregnancy causes lower oesophageal sphincter relaxation (Marrero 1992; VanThiel 1977). In addition, the enlarging gravid uterus causes increased intra‐abdominal pressure. The normal compensatory response of the lower oesophageal sphincter to accommodate this change is impaired during pregnancy ( VanThiel 1981). Abnormal gastric emptying or delayed small bowel transit might also contribute to heartburn in pregnancy (Richter 2005). One study also demonstrated an inappropriate response of the sphincter to injections with pentagastrin, edrophonium, and methacholine, or a protein meal (Fisher 1978).
The diagnosis of heartburn is based on clinical history. Upper endoscopy and other diagnostic tests are infrequently performed (Baron 1993; Richter 2005).
Description of the intervention
There are no Cochrane systematic reviews on the best approach for treatment of heartburn in non‐pregnant adults. There have been reports of interventions for relieving heartburn in pregnancy but the effectiveness of such interventions has not been established. Lifestyle modification, advice on diet and medications have been used for treatment of heartburn in pregnancy (Richter 2005).
There have also been some reports of complications from medications. One retrospective, case‐controlled study reported a significant increase in major and minor congenital anomalies in infants exposed to antacids during the third trimester of pregnancy (Witter 1981). An observation cohort study demonstrated two elective miscarriages in 12 pregnancies taking cisapride during the first trimester (Wilton 1998). One study on the use of proton pump inhibitors (PPIs) in the first trimester, found no difference in the prevalence of major birth defects, low birthweight and prematurity (Nielsen 1999).
The relief of heartburn may be complete or partial relief. This can be measured by the disappearance or decreasing frequency of heartburn. Not only maternal morbidities (miscarriage, preterm labour) and fetal morbidities (anomalies, intrauterine growth restriction, low birthweight), but also quality of life and satisfaction with interventions should be assessed.
Lifestyle modification is used for treating mild symptoms. Women are often advised to eat smaller meals, chew gum, not to eat late at night, to elevate the head of the bed and avoid foods and medications that cause heartburn. Abstinence from alcohol and tobacco are encouraged to reduce reflux symptoms and to avoid fetal exposure to these harmful substances (Richter 2005). Another lifestyle recommendation in the United States is avoidance of acidic foods (such as citrus, tomatoes, coffee).
For more troubling reflux symptoms, medications are sometimes used. The common drugs used for the treatment of heartburn in pregnancy include antacids, sucralfate, histamine 2‐receptor antagonists, promotility drugs (drugs that stimulate the muscles of the gastrointestinal tract to prevent acids from staying in the stomach too long), PPIs, and a raft‐forming alginate reflux suppressant (Mandel 2000; Richter 2005; Strugala 2012). Traditional Chinese Medicine such as acupuncture has been used in treatment of heartburn in pregnancy in one study (da Silva 2009)
How the intervention might work
Smaller meals, lifestyle modification and elevation of the head of the bed may help to reduce gastric secretion and gastric reflux (Richter 2005).
Chewing gum stimulates the salivary glands and can help neutralise acid. Abstinence from alcohol and tobacco are encouraged to reduce reflux symptoms (Richter 2005).
Medications work to relieve the symptoms of heartburn. Mechansims of drugs to relieve heartburn in pregnancy include: 1. acid neutralisation (antacids, sucralfate, histamine 2‐receptor antagonists); 2. Increase lower oesophageal sphincter pressure, improve oesophageal acid clearance, and promote gastric emptying (promotility drugs); 3. inhibit gastric acid secretion (PPIs); and 4. prevent reflux of acid and food into oesophagus (a alginate reflux suppressant) (Brucker 1988; Christopher 2005; Richter 2005).
Traditional Chinese Medicine such as acupuncture has an effect in the regulation of gastrointestinal motor activity and secretion through opioid and other neural pathways (Li 1992).
Why it is important to do this review
Heartburn is a common gastrointestinal symptom that occurs during pregnancy. Although the symptom is mild, it may disturb the pregnant woman. Many interventions have been used for the treatment of heartburn in pregnancy. These interventions include advice on diet, lifestyle modification and medications. However, there has been no evidence‐based recommendation for the treatment of heartburn in pregnancy. A previous Cochrane review (Neilson 2008) found that there was little information to draw conclusions on the effectiveness of interventions in the treatment of heartburn in pregnancy. Thus, identification of the most effective interventions for relieving heartburn in pregnancy in order to help pregnant women suffering from the symptoms of heartburn is important. The safety of interventions used to treat heartburn, particularly medications, is also paramount. Finally, it is important to consider maternal satisfaction with treatments and evaluate the potential impact of specific interventions on quality of life.This systematic review replaces the earlier Cochrane review, published in 2008 (Neilson 2008), which is no longer being updated.
Objectives
To assess the effects of interventions for relieving heartburn in pregnancy.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐randomised trials of interventions for heartburn in pregnancy compared with another intervention, or placebo, or no intervention. Cluster‐RCTs would have been eligible for inclusion but none were identified. We excluded studies available as abstracts only and those using a cross‐over design.
Types of participants
Pregnant women who have heartburn.
Types of interventions
One intervention for treating heartburn compared with another intervention for treating heartburn, or placebo or no treatment. The interventions for the treatment of heartburn in pregnancy include advice on diet, lifestyle modification, medications (such as antacids, sucralfate, histamine 2‐receptor antagonists, promotility drugs and PPIs), and complimentary therapies.
We planned to assess the following groups of comparisons:
intervention versus placebo or no treatment;
one intervention versus another intervention;
intervention versus combined placebo/no treatment (if study is multi‐arm).
Types of outcome measures
Primary outcomes
Relief of heartburn (complete or partial relief measured by frequency of heartburn)
Secondary outcomes
Maternal
Miscarriage
Preterm labour
Side effects of intervention (gastrointestinal, insomnia, constipation, diarrhoea, muscle cramp, allergy)
Quality of life (as defined by trial authors)
Maternal satisfaction (as defined by trial authors)
Fetal/infant
Fetal anomalies
Intrauterine growth restriction
Low birthweight
Search methods for identification of studies
The following methods section of this protocol is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (30 June 2015).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
In addition, we searched ClinicalTrials.gov (Appendix 1) (2 March 2015).
Searching other resources
We handsearched the proceedings of the Asian & Oceanic Congress of Obstetrics & Gynaecology (AOCOG), 20‐23 October 2013, Centara Grand & Bangkok Convention Centre, Bangkok, Thailand.
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
The following methods section of this protocol is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Both review authors independently assessed for inclusion all of the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third assessor.
We created a Study flow diagram to map out the number of records identified, included and excluded.
Data extraction and management
We designed a form to extract data. For eligible studies, both review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third assessor. We entered data into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details and noted this in the included studies tables.
Assessment of risk of bias in included studies
Both review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and will assess whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses undertaken.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. If in future updates there are sufficient data, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessing the quality of the body of evidence using the GRADE approach
The quality of the evidence has been assessed using the GRADE approach (Schunemann 2009).
We planned to assess the quality of the body of evidence relating to the following outcomes for the main comparisons.
Complete heartburn relief
Partial heartburn relief
Side effects
However, data were only available to allow us to compare these outcomes for pharmaceutical versus placebo/no treatment. In future updates if we have sufficient data, we would also include the following outcomes in a 'Summary of findings' table.
Quality of life
Maternal satisfaction
Low birthweight
Preterm labour
We used GRADEprofiler (GRADE 2014) to import data from Review Manager 5.3 (RevMan 2014) in order to create a ’Summary of findings’ table. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we planned to use the mean difference if outcomes were measured in the same way between trials. We would have used the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
This review did not include any cluster trials. If in future updates, relevant cluster‐randomised trials are identified and included, we will include these trials in the analyses along with individually‐randomised trials. We will adjust their sample sizes using the methods described in the Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials are not eligible for inclusion in this review. Cross‐over trials are rarely a valid study design for Pregnancy and Childbirth reviews and were therefore ineligible for inclusion in this review.
Other unit of analysis issues
Trials with multiple arms
Trials with more than two treatment groups: if in future updates we include a study that has a group with an intervention, a group with a placebo and a group with no treatment, we plan to combine the placebo and no intervention groups and compare those findings with those from the intervention group.
Multiple pregnancy
If in future updates we include any trials with multiple pregnancies, we will consider the women to be the unit of randomisation (not each fetus separately). Outcomes from the first of the multiples (e.g. twin 1, triplet 1, etc) will be used in the analysis.
Dealing with missing data
For included studies, we noted levels of attrition. If there are sufficient data in future updates, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either a T² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. Where significant heterogeneity was noted, we added potential sources of differences between trials in the text of the results section.
Assessment of reporting biases
We did not investigate publication bias due to insufficient data. If in future updates there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects, and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials.
If we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
We did not conduct subgroup analysis for this version of the review due to insufficient data. If in future updates we have sufficient data and identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
In future updates, if there are sufficient data, we plan to carry out the following subgroup analyses.
Nulliparity versus multiparity
Gestational age less than 20 weeks versus > 20 weeks
Singleton versus multiple pregnancy
Subgroup analysis will be restricted to the review's primary outcome.
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi2 statistic and P value, and the interaction test I² value.
Sensitivity analysis
With sufficient data, we plan to conduct sensitivity analyses to determine the effects of selection, performance and attrition bias on the estimates of effect, by excluding trials at high risk of bias due to these potential biases from the analyses, in order to assess whether this made any difference to the overall result. Sensitivity analysis will be restricted to the review’s primary outcome.
Results
Description of studies
Results of the search
See: Figure 1.
1.
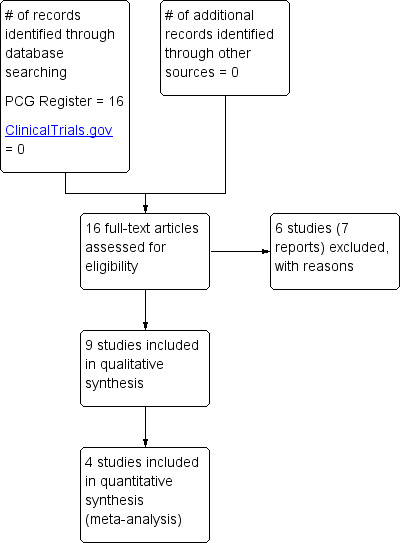
Study flow diagram.
The search identified 16 reports, related to 15 studies. Six studies have been excluded (Atlay 1978; Briggs 1972; Carne 1964; Hey 1978; Larson 1997; Marks 1997) and nine studies have been included in the review (Bower 1961; Brunclik 1988; da Silva 2009; Kovacs 1990; Lang 1989; Ranchet 1990; Rayburn 1999; Reisfield 1971; Shaw 1978).
Included studies
(1) Any pharmaceutical treatment versus placebo or no treatment
Five studies were included under this comparison. Two studies involving 256 women contributed data under this comparison
The first study (Bower 1961) involved 100 pregnant women who failed to obtain relief from antacid treatment for heartburn. It was carried out in United Kingdom more than 50 years ago and examined a treatment that is rarely used nowadays (intramuscular prostigmine 0.5 mg versus placebo). The trial by (Reisfield 1971) evaluated the effect of magnesium and aluminium hydroxide plus simethicone liquid and tablet compared with placebo on 156 pregnant women. This trial was conducted in the United States of America. The study by Kovacs 1990 included 50 pregnant women aged 20 to 40, who were more than 20 weeks pregnant and who had experienced moderately severe heartburn at least once a day in the previous week. This study was supported by Wyeth Pharmaceuticals and conducted in Australia. the intervention was compared in three ways: Mucaine with oxethazaine, Mucaine or placebo. Heartburn relief data from this study were not used in the analysis because they were presented as a mean. The Shaw 1978 study included 120 pregnant women in their third trimester. This study was conducted in the United Kingdom. The study examined the effect of syn‐ergel (containing aluminium phosphate with pectin and agar agar gel) and pectin and agar agar gel alone (active placebo).The trial reported to have 23% missing data and measuring the impact of treatment up to 60 minutes after the medication was taken. It was not feasible to obtain usable data from this study, therefore the study was not included in the analysis.
The Brunclik 1988 trial included 61 women aged 20 to 35 years, 30 to 36 weeks' gestation who had suffered at least one episode a day of heartburn in the week before the trial. This trial was conducted in Germany. This study had three arms. Women in the first group received mucaine, while participants in group two were randomised to receive mucaine with oxetacaine (mucainex) and women in the third group were assigned to take placebo mixtures. Nearly one fifth (12 of 61) of enrolled women were excluded after randomisation for not meeting study requirements. In addition, all women were told they could take a “low natrium antacid” if they did not gain relief within 15 minutes of consuming the allocated treatment. The study did not specify how many women did take the antacid; therefore, we did not include the data from this study in the analysis as we thought that including them might introduce bias in relation to the treatment effect. The publication is in German and our translation is incomplete and data were unclear. If we had included this study in the analysis, we would have combined the group of women who received mucaine with the ones who received mucainex and compared them with the placebo group.
(2) One intervention versus another intervention
a. Pharmacological intervention versus advice on dietary and lifestyle choices
Only one trial were categorised under this comparison (Ranchet 1990); 66 pregnant women were included in this trial. This trial was conducted in Italy. The study arms were not balanced (42 versus 24); women in the first group took sucralfate 1 g, three times daily and the women in the comparison group received advice on dietary and lifestyle choices.
b. Pharmacological intervention versus other pharmacological intervention
The study by Lang 1989 included 57 pregnant women, all were less than 38 weeks of gestation and had symptoms of dyspepsia during pregnancy of recent onset. This trial was conducted in the United Kingdom. Women in the first group were given algicon suspension and the women in the comparison group received magnesium trisilicate mixture. There was a high post‐randomisation attrition rate in the study (38% lost to follow‐up by two weeks). Data on heart burn relief in this study were presented in different ways (daytime heartburn relief and night‐time heartburn relief at week one and week two). Given the high attrition rate and the manner of reporting the outcomes not aligning with our prespecified outcomes, we decided not to include these data in the analysis.
(3) Acupuncture versus no treatment
There was just one study (da Silva 2009), which compared acupuncture (20 women) with no treatment (16 women). This study was conducted in Brazil. Women were aged from 15 to 39 years, at 15 to 30 weeks of pregnancy. Participants were not in a high‐risk pregnancy group and had not received any acupuncture in the preceding year.
(4) Any treatment versus combined intervention
Only one study (Rayburn 1999) compared a combined intervention of 75 mg ranitidine daily, plus antacids (15 women) with placebo plus antacids (15 women). This study was supported by a pharmaceutical company (GlaxoWellcome). We did not include this study in the data analysis because there were no usable data for prespecified outcomes.
(5) Intervention versus combined placebo no treatment (if study is multi‐arm)
We did not identify any study that could fit under this comparison.
Excluded studies
Six studies were excluded. We excluded four studies (Atlay 1978; Briggs 1972; Carne 1964; Larson 1997) because they were cross‐over trials. The Marks 1997 study was reported in abstract form only. We excluded the Hey 1978 study because the relief of heartburn was not an objective of the trial.
Risk of bias in included studies
See Figure 2; Figure 3 for a summary of ’Risk of bias’ assessments.
2.
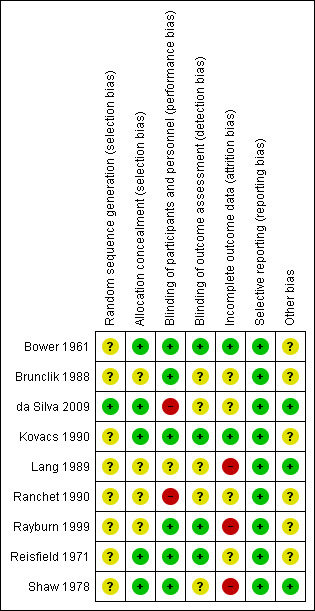
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
3.
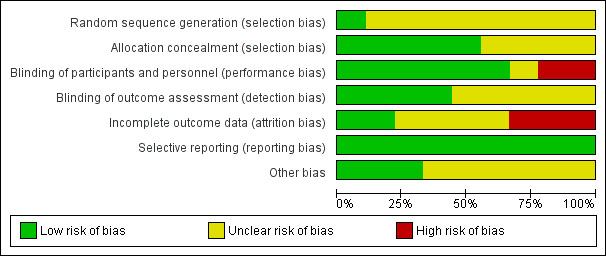
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
The method of randomisation in eight studies was not stated (Bower 1961; Brunclik 1988; Kovacs 1990; Lang 1989; Ranchet 1990; Rayburn 1999; Reisfield 1971; Shaw 1978), and these were assessed as being at unclear risk of bias. Randomisation in (da Silva 2009) was achieved by a nurse from the research team selecting from a box a closed piece of paper with a treatment order written on it; this trial was assessed a being of low risk of bias for method of randomisation because the sequence resulting would have been random.
Four of included studies did not report the method of allocation (Brunclik 1988; Lang 1989; Ranchet 1990; Rayburn 1999) these were judged of unclear risk of bias for allocation concealment. The remaining five included studies were assessed to be of low risk of bias for the method of allocation used because the pharmacy prepared identical treatment and placebo packs, the treatments were pre‐coded or the allocation slips were folded/concealed.
Blinding
Of the studies we included, five (Bower 1961; Kovacs 1990; Rayburn 1999, Reisfield 1971; Shaw 1978) used placebo in combination with other methods of heartburn relief. The study by Brunclik 1988 was described as being double‐blinded. Lang 1989 did not report whether treatment was blinded or not. The treatment was not blinded in the other trials (da Silva 2009; Ranchet 1990). Where treatment was not blinded, there is a possibility of bias in assessment of outcomes. Six studies (Bower 1961;Brunclik 1988; Kovacs 1990; Rayburn 1999; Reisfield 1971;Shaw 1978) were judged of low risk of performance bias. Two studies (da Silva 2009; Ranchet 1990) were judged of high risk of performance bias while one study (Lang 1989) was judged of unclear risk of performance bias.
Five studies (Brunclik 1988; da Silva 2009; Lang 1989; Ranchet 1990; Shaw 1978) were judged of unclear risk of detection bias. Four studies (Bower 1961; Kovacs 1990; Rayburn 1999; Reisfield 1971) were judged of low risk of detection bias.
Incomplete outcome data
In one study (da Silva 2009), six women were excluded. One woman from each treatment group moved, and four women (20%) in the control group missed two consecutive interviews. In the Ranchet 1990 study no dropouts or withdrawals were reported; however; denominators suggest missing data for the outcome of total remission of heartburn (one active, three no treatment). Lang 1989 was conducted over two weeks and there were high post‐randomisation attrition rates where 38% were lost to follow‐up by two weeks. Twelve of 61 enrolled women in Brunclik 1988 trial were excluded after randomisation for not meeting study requirements. More than one‐fifth (23%) of data were missing in Shaw 1978. There was no evidence of incomplete outcome data in the other trials. Four studies (Brunclik 1988; da Silva 2009; Ranchet 1990; Reisfield 1971) were judged of unclear risk of attrition bias. Three studies (Lang 1989; Rayburn 1999; Shaw 1978) were judged of high risk of attrition bias, while two studies (Bower 1961; Kovacs 1990) were judged of low risk of attrition bias.
Selective reporting
There are no obvious cases of selective reporting and all studies were assessed as being at a low risk of reporting bias.
Other potential sources of bias
Several studies had support from pharmaceutical companies (Kovacs 1990; Rayburn 1999; Reisfield 1971), which we judged to be of unclear risk of bias. A further three studies (Bower 1961; Brunclik 1988; Ranchet 1990) were also judged of unclear risk of other potential sources of bias. Three studies (da Silva 2009; Lang 1989; Shaw 1978) were judged of low risk of other potential sources of bias.
Effects of interventions
See: Table 1
We included nine studies (involving 725 women) in this review but only four studies (involving 358 women) contributed data to analyses (Bower 1961; da Silva 2009; Ranchet 1990; Reisfield 1971).
Any pharmaceutical treatment versus placebo or no treatment (Comparison 1)
Two trials provided data for this comparison (Bower 1961; Reisfield 1971). One study (100 women) used intramuscular prostigmine 0.5 mg versus intramuscular water, and one study (156 women) used magnesium and aluminium hydroxide plus simethicone liquid and tablet versus placebo liquid and placebo.
Primary outcome
Relief of heartburn
Women who received pharmaceutical treatment reported complete heartburn relief more often than women receiving no treatment or placebo (risk ratio (RR) 1.85, 95% confidence interval (CI) 1.36 to 2.50; participants = 256; studies = two; fixed‐effect analysis, Heterogeneity: I² = 0%); Analysis 1.1 (moderate quality of evidence)).
1.1. Analysis.
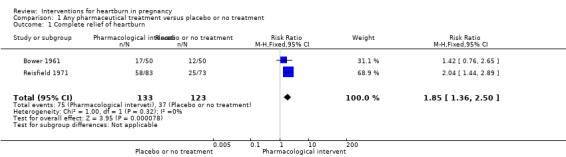
Comparison 1 Any pharmaceutical treatment versus placebo or no treatment, Outcome 1 Complete relief of heartburn.
Data on partial relief of heartburn were heterogenous and showed no difference (average RR 1.35, 95% CI 0.38 to 4.76; participants = 256; studies = two; random‐effects analysis, Heterogeneity:Tau² = 0.73; I² = 88%); Analysis 1.2. The quality of evidence of this outcome was graded as very low. The downgrading of evidence was based on presence of heterogeneity and design limitations of the contributing studies. Differences in pharmaceutical treatments and regimens are likely sources of the differences between trials and statistical heterogeneity. Sample size may also play a part, although statistical heterogeneity was not noted for the similar outcome of complete relief.
1.2. Analysis.
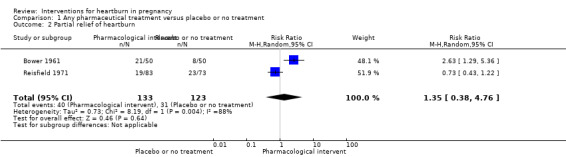
Comparison 1 Any pharmaceutical treatment versus placebo or no treatment, Outcome 2 Partial relief of heartburn.
Secondary outcomes
Side effects of intervention
Two trials contributed data on treatment side effects (Bower 1961; Reisfield 1971). There was no difference in the side‐effect rate between the treatment groups (RR 0.63, 95% CI 0.21 to 1.89; participants = 256; studies = two; fixed‐effect analysis); Analysis 1.3. The reported side effects included gastrointestinal, constipation, diarrhoea and allergy. The quality of evidence in relation to this outcome was very low. The decision to downgrade evidence was taken on basis of the presence of few events and wide CIs crossing the line of no effect and finding that most of the weight came from a study with design limitations (Reisfield 1971). Additionally, this study excluded six women who had side effects.
1.3. Analysis.
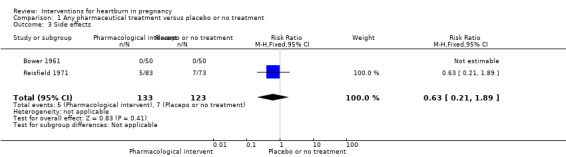
Comparison 1 Any pharmaceutical treatment versus placebo or no treatment, Outcome 3 Side effects.
Pharmacological intervention versus advice on dietary and lifestyle choices (Comparison 2)
There was one study under this comparison (Ranchet 1990) ‐ the study included 66 women between the age of 19 and 36, both primigravida and multipara, with at least one symptom of gravidic pyrosis (heartburn during pregnancy).
Primary outcome
Relief of heartburn
More women in the sucralfate experienced complete relief of heartburn in comparison to women who received advice on diet and lifestyle choices (RR 2.41, 95% CI 1.42 to 4.07; participants = 65; studies = one); Analysis 2.1.
2.1. Analysis.
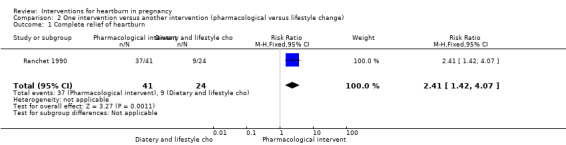
Comparison 2 One intervention versus another intervention (pharmacological versus lifestyle change), Outcome 1 Complete relief of heartburn.
Secondary outcomes
Side effects of intervention
The only secondary outcome of interest addressed by this trial was side effects. The rate of side effects did not differ between the groups (RR 1.74, 95% CI 0.07 to 41.21; participants = 66; studies = one); Analysis 2.2. There was only one instance of side effects (diarrhoea) in the pharmacological group.
2.2. Analysis.
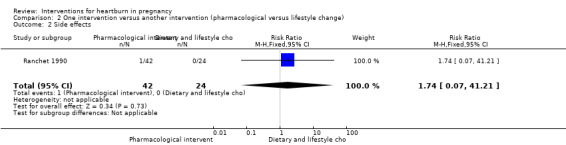
Comparison 2 One intervention versus another intervention (pharmacological versus lifestyle change), Outcome 2 Side effects.
Acupuncture versus no treatment (Comparison 3)
There was just one study (36 women at 15 to 30 weeks of pregnancy) in this comparison ‐ da Silva 2009 compared acupuncture (20 women) versus no treatment (16 women).
Primary outcome
Relief of heartburn
The results from the da Silva 2009 study included information on severity and frequency of heartburn using a numerical rating scale (NRS) zero to 10; we did not incorporate these continuous data into our analyses.
Secondary outcomes
Side effects of intervention
There was no difference in the rate of side effects between women who had acupuncture and women who received no treatment (RR 2.43, 95% CI 0.11 to 55.89; participants = 36; studies = one; Analysis 3.1.
3.1. Analysis.
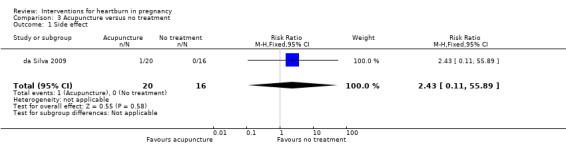
Comparison 3 Acupuncture versus no treatment, Outcome 1 Side effect.
Quality of life
With regard to quality of life, women who had acupuncture reported improved ability to sleep (RR 2.80, 95% CI 1.14 to 6.86; participants = 36; studies = one) and to eat (RR 2.40, 95% CI 1.11 to 5.18; participants = 36; studies = one; Analysis 3.2.
3.2. Analysis.
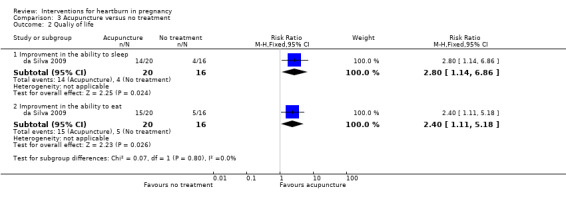
Comparison 3 Acupuncture versus no treatment, Outcome 2 Qualiy of life.
Other secondary outcomes
The following secondary outcomes were not reported upon in any of the trials included in the review: miscarriage, preterm labour, maternal satisfaction, fetal anomalies, intrauterine growth restriction, low birthweight.
Discussion
Summary of main results
Heartburn is a common gastrointestinal symptom that occurs during pregnancy. Although the symptom is often mild, it may disturb the pregnant woman. Many interventions have been used for the treatment of heartburn in pregnancy. These interventions include advice on diet, lifestyle modification, medications and complimentary therapies. Identification of the most effective interventions for relieving heartburn in pregnancy in order to help pregnant women suffering from the symptoms of heartburn is important. The safety of interventions used to treat heartburn, particularly medications, is also important. We must also consider maternal satisfaction with treatments and evaluate the potential impact of interventions on quality of life.
In this review of nine randomised controlled trials (RCTs), including 725 women, two trials (involving 256 women, with one trial reported over 50 years ago) demonstrated that women who received pharmaceutical treatment reported complete heartburn relief more often than women receiving no treatment or placebo, and side effects were comparable between the two treatment groups. One trial compared pharmacological intervention (sucralfate) to the effect of advice on diet and lifestyle choices on heartburn. The study showed that more women in the sucralfate group experienced complete relief of heartburn. The rate of side effects in this trial did not differ between the groups. One study evaluated acupuncture versus no treatment and demonstrated that there was no difference in the rate of side effects between women who had acupuncture and women who had no treatment. With regard to quality of life; women who had acupuncture reported an improved ability to sleep and to eat.
There were no data reported for the secondary outcomes of miscarriage, preterm labour, maternal satisfaction, fetal anomalies, intrauterine growth restriction, or low birthweight, so this review is unable to evaluate the effect of the interventions on those outcomes.
Overall completeness and applicability of evidence
We found four RCTs that contributed data evaluating the treatment of heartburn in pregnancy. Three studies were conducted in developed countries while one study was conducted in a developing country. The findings of the review are generalisable.
Two trials evaluated the effect of pharmaceutical treatment versus placebo or no treatment. One trial examined a treatment rarely used nowadays (intramuscular prostigmine 0.5 mg versus placebo). One evaluated the effect of magnesium and aluminium hydroxide plus simethicone liquid and tablet compared with placebo. One trial evaluated pharmaceutical treatment (sucralfate) versus advice on dietary and lifestyle choices. These small studies, albeit one being reported over 50 years ago, found that pharmaceutical treatment resulted in complete heartburn relief for pregnant women suffering heartburn.
One trial compared acupuncture versus no treatment. This trial showed improved quality of life for acupuncture compared with no treatment.
None of the trials reported on the secondary outcomes of miscarriage, preterm labour, maternal satisfaction, fetal anomalies, intrauterine growth restriction, or low birthweight. Thus, we unable to evaluate the effect of the interventions on those outcomes.
We could not perform subgroup analyses based on parity, gestational age and number of fetuses, as the data were not available. Overall, our analysis of included trials suggests that the pharmaceutical treatments that have been evaluated provide pregnant women with relief from heartburn. Further research is necessary to specify which pharmaceutical interventions are most helpful to women. The ability to make conclusions about treatments for heartburn is severely limited by the lack of evidence available on this subject.
Quality of the evidence
Three outcomes were assessed and assigned a quality rating using the GRADE methods. Evidence from two trials for the outcome of complete relief of heartburn was assessed as of moderate quality. Evidence for the outcomes of partial heartburn relief and side effects was graded to be of very low quality. Downgrading decisions were based in part on the small size of the trials and on heterogenous and imprecise results.
Potential biases in the review process
The biases in the review process were minimised. There was a systematic evaluation at all stages, including literature searching, study selection, data extraction and analysis. All relevant studies were identified and all relevant data could be obtained. Two review authors did this independently and resolved discrepancies by discussion between the review authors. All the outcomes were prespecified in the protocol.
Agreements and disagreements with other studies or reviews
Vazquez 2010 performed a systematic review of constipation, haemorrhoids and heartburn in pregnancy, including RCTs and other non‐randomised study designs. The authors found no evidence for the efficacy of specific interventions, including acid‐reducing drugs, raising the bed when sleeping, reducing caffeine an other dietary methods.
A previous Cochrane review by Neilson 2008 (that the present review replaces) demonstrated that there was little information to draw conclusions on the overall effectiveness of interventions to relieve heartburn in pregnancy. However, the review showed positive findings in favour of the intervention groups. The interventions included intramuscular prostigmine, an antacid preparation, and antacid plus ranitidine.
Authors' conclusions
Implications for practice.
Three small studies found that pharmaceutical treatment resulted in complete heartburn relief for pregnant women suffering heartburn, although we note that one of these studies, reported over 50 years ago, included women who had a pharmaceutical (antacid) that failed to relieve symptoms before they were exposed to intramuscular prostigmine 0.5mg, a pharmaceutical that is not in current use for this indication). One trial showed improved quality of life for acupuncture compared with no treatment. Overall, there is very little evidence to show that heartburn in pregnancy can be completely relieved by pharmaceutical treatment. The lack of good‐quality evidence from randomised controlled trials limits our ability to draw conclusions that would be relevant to clinical practice.
Implications for research.
There is very limited evidence to show that heartburn in pregnancy could be completely relieved by any pharmaceutical treatment. Future research should address other medications such as histamine 2‐receptor antagonists, promotility drugs, proton pump inhibitors, and a raft‐forming alginate reflux suppressant in treatment of heartburn in pregnancy. More research is needed on acupuncture and other complimentary therapies as treatments for heartburn in pregnancy. Future research should also evaluate maternal satisfaction with treatment and measure pregnant women's quality of life relation to the intervention.
Acknowledgements
We would like to thank the editors and other referees for their helpful feedback on the protocol and review.
We would like to thank Nasreen Aflaifel and Nancy Medley for their support. Nasreen Aflaifel helped to extract and enter data, set up the comparison table, drafted the results and created the 'Summary of findings' table for this review. Nancy Medley assisted with eligibility assessments and the preparation of the included studies tables. Nasreen Aflaifel's and Nancy Medley's work was financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization. The named authors alone are responsible for the views expressed in this publication.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), members of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
This project was supported by the National Institute for Health research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. ClinicalTrials.gov search terms
heartburn AND pregnancy
heartburn AND pregnant
dyspepsia AND pregnancy
dyspepsia AND pregnant
Data and analyses
Comparison 1. Any pharmaceutical treatment versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete relief of heartburn | 2 | 256 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [1.36, 2.50] |
| 2 Partial relief of heartburn | 2 | 256 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.38, 4.76] |
| 3 Side effects | 2 | 256 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.21, 1.89] |
Comparison 2. One intervention versus another intervention (pharmacological versus lifestyle change).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete relief of heartburn | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.41 [1.42, 4.07] |
| 2 Side effects | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [0.07, 41.21] |
Comparison 3. Acupuncture versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Side effect | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [0.11, 55.89] |
| 2 Qualiy of life | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Improvment in the ability to sleep | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.8 [1.14, 6.86] |
| 2.2 Improvment in the ability to eat | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.4 [1.11, 5.18] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bower 1961.
| Methods | Study described as a randomised controlled trial. Oldchurch Hospital, Essex, UK. | |
| Participants | 100 pregnant women who failed to obtain relief from antacid treatment for heartburn were recruited when attending outpatient antenatal care. Degree of heartburn of women was not stated at the study entry. | |
| Interventions | Intervention group: intramuscular prostigmine 0.5 mg (N = 50) Comparison group: intramuscular water (N = 50) |
|
| Outcomes | Relief of heartburn (complete relief, useful relief), injection useless. | |
| Notes | The authors stated that the average duration of relief obtained from the injections was 5 days. Otherwise, follow‐up is unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Low risk | Pharmacy prepared identical ampoules with treatment and placebo, so that staff would not have been aware of the next group assignment when women were recruited. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical treatment and placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Treatment allocation unknown to obstetric staff. Code broken after outcome data recorded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for all women randomised. |
| Selective reporting (reporting bias) | Low risk | Planned outcomes appear to have been reported. |
| Other bias | Unclear risk | Duration of follow‐up not stated. |
Brunclik 1988.
| Methods | Study described as a double‐blind randomised trial. Publication is in German and our translation is incomplete. | |
| Participants | 61 women aged 20‐35 years, 30‐36 weeks' gestation who had suffered at least 1 episode a day of heartburn in the week before the trial. Exclusion criteria were women who had other known gastrointestinal conditions requiring medication, or were taking other medications, or who had chronic respiratory disorders. Degree of heartburn of women was not stated at the study entry. | |
| Interventions | Group 1: 15 women received mucaine. Group 2: 15 women received mucaine with oxetacaine (mucainex). Group 3: 17 women received placebo mixtures. Study duration: 1 week. |
|
| Outcomes | Relief of heartburn, symptoms severity and side effects. | |
| Notes | 12 of 61 enrolled women were excluded after randomisation for not meeting study requirements. In addition, all women were told they could take a “low natrium antacid” if they did not gain relief within 15 minutes of consuming the allocated treatment. The study did not specify how many women did take the antacid; therefore, we did not include the data from this study in the analysis as we thought that including them might introduce bias in relation to the treatment effect. Publication is in German and our translation is incomplete and data were unclear. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised. No information on how randomisation was carried out. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study design. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Publication is in German and our translation is incomplete. |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes seem to be reported. |
| Other bias | Unclear risk | Publication is in German and our translation is incomplete. |
da Silva 2009.
| Methods | Prospective randomised controlled trial in Sao Paulo, Brazil. | |
| Participants | 40 pregnant women aged from 15 to 39 years, at 15–30 weeks of pregnancy and with dyspepsia symptoms. Participants had no underlying disease as a possible cause of the symptoms or history of similar symptoms prior to pregnancy. Participants were not in a high‐risk pregnancy group and had no acupuncture in the preceding year. Degree of heartburn of women was not stated at the study entry. Women who missed more than 2 interviews were excluded. |
|
| Interventions | Intervention group: acupuncture (N = 20) Acupuncture once per week but occasionally twice per week during 8 weeks. A minimum of 8 and a maximum of 12 sessions of traditional acupuncture “respecting the classical acupuncture points including depth of insertion. Sterilised stainless steel needles of 40 mm in length and 0.2 mm diameter were used. Neither electro‐stimulation nor ear acupuncture was used. On average 12 needles were used, always attempting to achieve the de qi sensation (sensation of soreness, numbness or distension around the point). Needles were left at place for about 25 minutes. ...The most commonly used points were: LI4 (hands); PC6 (forearms); CV12, ST21, LR13 (abdomen); ST36 (legs) and SP4, ST44 (feet)". Comparison group: no treatment (N = 16) Data collection at baseline and every 2 weeks until completion of the treatment at 8 weeks. Women were interviewed by a research assistant. |
|
| Outcomes | Severity and frequency of heartburn using a NRS 0‐10. Secondary outcomes were antacid consumption and the ability to sleep and eat (also NRS). Authors report birthweight, Apgar score. | |
| Notes | The Research Ethics Committee of the Federal University of Sao Paulo, Brazil approved this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved by a nurse from the research team selecting from a box a closed piece of paper with a treatment order written on it. This is the same as shuffling sealed cards to determine a sequence and is adequate. |
| Allocation concealment (selection bias) | Low risk | As above, subsequent treatment allocation would have been concealed from person conducting randomisation because papers drawn from box were "closed". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was an attempt made to blind the interviewers who collected the data. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 40 women randomised; 6 women excluded. 1 woman from each treatment group moved, and 4 women (20%) in the control group missed 2 consecutive interviews. Denominators stated are 20 and 16, so it appears that some data were collected before the 2 women moved. |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes have been reported. |
| Other bias | Low risk | None noted. |
Kovacs 1990.
| Methods | RCT. Women recruited from 4 private obstetric practices, May 1985–June 1987, Australia. | |
| Participants | 50 pregnant women aged 20‐40, more than 20 weeks pregnant and having experienced moderately severe heartburn at least once a day in the previous week. Women with known gastrointestinal disorders, systemic medication or chronic respiratory diseases, emphysema or recurrent pneumonia were excluded. Women had moderate and severe degree of heartburn at study entry. | |
| Interventions | Intervention groups: mucaine with oxethazaine, mucaine. Data for these women have been combined for analysis. N = 32. Comparison group: placebo. N = 18. Study duration: 1 week. Intervention and treatment were provided in identical bottles. Women were allowed to take an antacid if treatment was not successful after 15 minutes of the drug dose. Women were asked to record the severity of heartburn before the trial and after the 7 day study period, as well as the number of doses used each day and antacids taken. Side effects were also recorded at the end of the week. |
|
| Outcomes | Relief of heartburn, symptoms severity and relief, use of medication, willingness to use the medication again. All outcomes were analysed using mean and standard deviation | |
| Notes | This study was supported by Wyeth Pharmaceuticals. Heartburn relief data were not used in the analysis as it were presented as a mean. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. Study described as randomised. |
| Allocation concealment (selection bias) | Low risk | Women allocated to trial number in sequence. Identical treatments and placebo prepared, so that staff and women would not have been aware of the next allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treatment and placebo identical. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Treatment and placebo identical. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for all women recruited. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes appear to have been reported. |
| Other bias | Unclear risk | This study was supported by Wyeth Pharmaceuticals. |
Lang 1989.
| Methods | Study described as a randomised trial. | |
| Participants | 157 pregnant women, all were less than 38 weeks of gestation and had symptoms of dyspepsia during pregnancy of recent onset. Exclusion criteria were women who had signs or symptoms of pre‐eclampsia, with a history of dyspepsia or suspected peptic ulcer prior to pregnancy. Women had mild, moderate and severe degree of heartburn at study entry. | |
| Interventions | Group 1: 10 mL of algicon suspension. (N = 79) Group 2: 10 mL of magnesium trisilicate mixture. (N = 78) Study duration: 2 weeks. |
|
| Outcomes | Relief of heartburn, symptoms incidence and severity and side effects. | |
| Notes | High post‐randomisation attrition rates (38% lost to follow‐up by 2 weeks and therefore at high risk of bias, see below). Data on heartburn relief from this study were presented into different categories (daytime and night‐time ; at week 1 and week 2); therefore data were not included in the analysis. We did not attempt to contact the authors as the study was conducted several years ago (1989). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on how randomisation was carried out. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 38% lost to follow‐up by 2 weeks. |
| Selective reporting (reporting bias) | Low risk | Relevant data reported. |
| Other bias | Low risk | None noted. |
Ranchet 1990.
| Methods | RCT. Publication is in Italian and the report has been translated. Location of trial is still unclear. | |
| Participants | 66 pregnant women between the age of 19 and 36, both primipara and multipara, with at least 1 symptom of gravidic pyrosis. Degree of heartburn of women was not stated at the study entry. Exclusion criteria not stated. |
|
| Interventions | Intervention group: sucralfate 1 g, 3 times daily. N = 42. Comparison group: advice on dietary and lifestyle choices. N = 24. Follow‐up recorded at 15 and at 30 days. |
|
| Outcomes | Symptom relief of heartburn and acid regurgitations, epigastric pain, sialorrhoea, remission of symptoms, side effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on how randomisation was carried out. Study described as randomised. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study described as single‐blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropouts or withdrawals reported. Denominators suggest missing data for the outcome of total remission of heartburn (1 active, 3 no treatment). |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes seem to be reported. |
| Other bias | Unclear risk | Randomised treatment groups are unequal in size. |
Rayburn 1999.
| Methods | RCT. Outpatient obstetric patients in Oklahoma, USA. | |
| Participants | 30 pregnant women (20 weeks or beyond) who had 4 or more episodes of moderate to severe heartburn during a week of antacid therapy were eligible for randomisation. Women had moderate and severe degree of heartburn at study entry. | |
| Interventions | Intervention group: 75 mg ranitidine daily, plus antacids. N = 15. Comparison group: placebo plus antacids. N = 15. Study duration: 3 weeks. 1 week of eligibility assessment took place, with candidates taking antacids only. Women who did not experience relief from antacids were eligible for randomisation. Data collection occurred at 7 and at 14 days. |
|
| Outcomes | Heartburn intensity, number of antacids consumed. Unspecified birth outcomes were said to be favourable, women reported no side effects were reported. | |
| Notes | This study was supported by GlaxoWellcome, USA. An institutional review board at the University of Oklahoma approved the protocol for this study. Study drug consumption during the third week was discontinued in 7, 47% patients receiving the placebo‐antacids, because of inadequate relief of heartburn compared with none who received the ranitidine‐antacids (P = < 0.05). No usable data in relation to the prespecified outcomes. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on how randomisation was carried out. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study described as placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study described as placebo‐controlled. Data collected was self‐reported heartburn relief and intensity. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data available for all women randomised. Authors state that almost half of the placebo arm was discontinued during the third week of the trial due to inadequate heartburn relief. Data stated for the number of antacids consumed relates to the baseline week and to week 2 of the trial. Data for heartburn intensity is reported for the baseline week, the second week and the third week of the trial. |
| Selective reporting (reporting bias) | Low risk | Relevant data reported. |
| Other bias | Unclear risk | This study was supported by GlaxoWellcome, USA. |
Reisfield 1971.
| Methods | Double‐blind randomised trial in New Jersey, USA. | |
| Participants | 156 pregnant women suffering from heartburn. Women had mild, moderate and severe degree of heartburn at study entry. | |
| Interventions | Intervention group: magnesium and aluminium hydroxide plus simethicone liquid and tablet. N = 83. Comparison group: placebo liquid and placebo tablet. N = 73. |
|
| Outcomes | Relief of heartburn, side effects. | |
| Notes | The intervention in this trial, Mylanta liquid and tablets, were supplied by Stuart Pharmaceuticals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence not described. |
| Allocation concealment (selection bias) | Low risk | Prior coding of identical active and placebo treatment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Staff and participants blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind study design. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition not reported. 6 women discontinued use of medications due to side effects (5 of these women were taking the placebo and 1 active treatment). |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes reported. |
| Other bias | Unclear risk | Length of follow‐up is not clear. Results are stated for 10 and 28 weeks, but it is not clear if these were the only data collection time points for all participants. 19 women were taking oral iron therapy; 3 of women experienced gastrointestinal symptoms attributed to the iron. The intervention in this trial, Mylanta liquid and tablets, were supplied by Stuart Pharmaceuticals. |
Shaw 1978.
| Methods | The study was described as randomised, placebo‐controlled trial. | |
| Participants | 120 pregnant women all were in the third trimester. Women had mild, moderate and severe degree of heartburn at study entry. | |
| Interventions | Intervention group: 60 women received Syn‐ergel containing aluminium phosphate with pectin and agar agar gel. Control group: 60 women received pectin and agar agar gel alone. Study duration: 1 week. |
|
| Outcomes | Relief and severity of heartburn. | |
| Notes | This trial compared Syn‐ergel containing aluminium phosphate with pectin and agar agar gel versus pectin and agar gel alone. Data from this study have not been included in this review as there was 23% missing data and the investigators only measured the impact of treatment up to 60 minutes after the medication was taken. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised. Sequence generation not described. |
| Allocation concealment (selection bias) | Low risk | "Envelope was opened for each patient entered the trial which indicated whether she will receive preparation A or B." The code was broken only at the end of the trial. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "...the code was broken only at the end of the trial." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10 women in the intervention group and 18 in the placebo group were excluded from the analysis. |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes reported. |
| Other bias | Low risk | None noted. |
LI: large intestine, PC: pericardium, CV: conception vessel, ST: stomach, LR: liver, SP: spleen, NRS: numerical rating scale, RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Atlay 1978 | This trial is a cross‐over trial examining the effects of an alkali, an acid and a placebo treatment, with each treatment given for 1 week before crossing over to the next treatment. Cross‐over trials are not eligible for this review. |
| Briggs 1972 | This trial is a cross‐over trial examining the effects of 2 compounds for the treatment of heartburn: Alcin tablets (360 mg anhydrous sodium aluminium salicylate and basic magnesium aluminate) and aluminium hydroxide tablets. Participants were instructed to take 1 set of tablets for 1 week before switching to the alternate tablet. Cross‐over trials are not eligible for this review. |
| Carne 1964 | This trial is a cross‐over trial comparing solutions of Mucaine and an antacid gel of aluminium and magnesium hydroxide. Pregnant women were advised to take the medications on alternate days. 1 arm had only Mucaine. Cross‐over trials are not eligible for inclusion in this review. |
| Hey 1978 | This study focuses on gastric sphincter pressure. The intervention was 10 mg metoclopramide intravenously compared with placebo (water) intravenously. Relief of heartburn was not an objective of the trial. |
| Larson 1997 | This trial was a cross‐over trial, comparing 3 weekly regimens: twice‐daily doses of ranitidine 150 mg, once daily ranitidine 150 mg and placebo. The study was double‐blind. Cross‐over trials are not eligible for this systematic review. |
| Marks 1997 | This study has been reported in abstract form only. The abstract describes a randomised clinical trial of gum‐chewing versus no treatment for relief of heartburn symptoms during pregnancy. Heartburn intensity was the primary outcome. Previous review authors attempted to contact trial report author (2007). Author's contact details not found for current update. |
Differences between protocol and review
There are some differences between our published protocol (Phupong 2014) and the full review.
We planned to run a search of CINAHL, however, we had difficulty accessing this database. A search of CINAHL is now included in the broad search that makes up the Pregnancy and Childbirth Group's Trials Register so we removed our separate search.
We have added methods for using the GRADE approach to assess the quality of the body of evidence. We planned to carry out the GRADE/SoF for the main comparisons. However, data were only available to allow us to compare pharmaceutical versus placebo/no treatment in this review.
We have edited our 'adverse effects of intervention' secondary outcome to 'side effects of the intervention'.
Contributions of authors
V Phupong is guarantor of this review. V Phupong and T Hanprasertpong conceived the review; drafted the proposal, developed the search strategies for searches in addition to those developed by the Pregnancy and Childbirth Group's Trials Search Co‐ordinator and finalised the protocol for publication.
V Phupong and T Hanprasertpong conducted eligibility assessments. V Phupong and T Hanprasertpong extracted, entered data, conducted analyses and drafted the text of the review. V Phupong and T Hanprasertpong were responsible for the finalisation of the data analyses and the text of the review.
Sources of support
Internal sources
Chulalongkorn University, Thailand.
External sources
UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Declarations of interest
None known.
New
References
References to studies included in this review
Bower 1961 {published data only}
- Bower D. The use of prostigmine in the heartburn of pregnancy. Journal of Obstetrics and Gynaecology of the British Commonwealth 1961;63:846‐7. [DOI] [PubMed] [Google Scholar]
Brunclik 1988 {published data only}
- Brunclik V, Privrel T. Double‐blind comparison of an oxetacain‐antacid combination against the antacid alone in the treatment of heartburn in pregnancy. Schweizerische Rundschau fur Medizin Praxis 1988;77:587‐91. [PubMed] [Google Scholar]
da Silva 2009 {published data only}
- Silva JB, Nakamura MU, Cordeiro JA, Kulay LJ, Saidah R. Acupuncture for dyspepsia in pregnancy: a prospective, randomised, controlled study. Acupuncture in Medicine 2009;27(2):50‐3. [DOI] [PubMed] [Google Scholar]
Kovacs 1990 {published data only}
- Kovacs GT, Campbell J, Francis D, Hill D, Adena MA. Is mucaine an appropriate medication for the relief of heartburn during pregnancy?. Asia‐Oceania Journal of Obstetrics and Gynaecology 1990;16:357‐62. [DOI] [PubMed] [Google Scholar]
Lang 1989 {published data only}
- Lang GD, Dougall A. Comparative study of algicon suspension and magnesium trisilicate mixture in the treatment of reflux dyspepsia of pregnancy. British Journal of Clinical Practice 1989;66:48‐51. [PubMed] [Google Scholar]
Ranchet 1990 {published data only}
- Ranchet G, Gangemi O, Petrone M. Sucralfate in the treatment of pregnancy pyrosis [Il Sucralfato nel trattamento della pirosi gravidica]. Giornale Italiano di Ostetricia e Ginecologia 1990;12(1):91‐6. [Google Scholar]
Rayburn 1999 {published data only}
- Rayburn W, Liles E, Christensen H, Robinson M. Antacids vs antacids plus non‐prescription ranitidine for heartburn during pregnancy. International Journal of Gynecology & Obstetrics 1999;66(1):35‐7. [DOI] [PubMed] [Google Scholar]
Reisfield 1971 {published data only}
- Reisfield DR. Pyrosis and pregnancy. Current Therapeutic Research, Clinical & Experimental 1971;13(11):680‐4. [PubMed] [Google Scholar]
Shaw 1978 {published data only}
- Shaw RW. Randomized controlled trial of Syn‐Ergel and an active placebo in the treatment of heartburn of pregnancy. Journal of International Medical Research 1978;6:147‐51. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Atlay 1978 {published data only}
- Atlay RD, Weekes ARL, Entwistle GD, Parkinson DJ. Treating heartburn in pregnancy: comparison of acid and alkali mixtures. BMJ 1978;2:919‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Briggs 1972 {published data only}
- Briggs DW, Hart DM. Heartburn in pregnancy ‐ a continuation study. British Journal of Clinical Practice 1972;26(4):167‐9. [PubMed] [Google Scholar]
Carne 1964 {published data only}
- Carne S. Double‐blind trial of mucaine in heartburn of pregnancy. Journal of the College of General Practitioners 1964;8(1):135‐9. [PMC free article] [PubMed] [Google Scholar]
Hey 1978 {published data only}
- Hey VMF, Ostick DG. Metoclopramide and the gastro‐oesophageal sphincter ‐ a study in pregnant women with heartburn. Anaesthesia 1978;33:462‐5. [DOI] [PubMed] [Google Scholar]
Larson 1997 {published data only}
- Larson J, Patatanian E, Miner P, Rayburn W, Robinson M. Double‐blind, placebo controlled study of ranitidine (zantac(r)) for gastroesophageal reflux symptoms during pregnancy. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S23. [DOI] [PubMed] [Google Scholar]
- Larson JD, Patatanian E, Miner PBJ, Rayburn WF, Robinson MG. Double‐blind, placebo‐controlled study of ranitidine for gastroesophageal reflux symptoms during pregnancy. Obstetrics & Gynecology 1997;90(1):83‐7. [DOI] [PubMed] [Google Scholar]
Marks 1997 {published data only}
- Marks RD, Singh S, Owen J. Does chewing gum reduce pregnancy associated heartburn?. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S184. [Google Scholar]
Additional references
Audu 2006
- Audu BM, Mustapha SK. Prevalence of gastrointestinal symptoms in pregnancy. Nigerian Journal of Clinical Practice 2006;9(1):1‐6. [PubMed] [Google Scholar]
Baron 1993
- Baron TH, Ramirez B, Richter JE. Gastrointestinal motility disorders during pregnancy. Annals of Internal Medicine 1993;118(5):366‐75. [DOI] [PubMed] [Google Scholar]
Brucker 1988
- Brucker MC. Management of common minor discomforts in pregnancy. Part II: Managing minor pain in pregnancy. Journal of Nurse Midwifery 1988;33(1):25‐30. [DOI] [PubMed] [Google Scholar]
Christopher 2005
- Christopher L. The role of proton pump inhibitors in the treatment of heartburn during pregnancy. Journal of the American Academy of Nurse Practitioners 2005;17(1):4‐8. [DOI] [PubMed] [Google Scholar]
Fisher 1978
- Fisher RS, Roberts GS, Grabowski CJ, Cohen S. Altered lower esophageal sphincter function during early pregnancy. Gastroenterology 1978;74(6):1233‐7. [PubMed] [Google Scholar]
GRADE 2014 [Computer program]
- McMaster University. GRADEpro. [Computer program on www.gradepro.org]. Version 2015. McMaster University, 2014.
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ho 1998
- Ho KY, Kang JY, Viegas OA. Symptomatic gastro‐oesophageal reflux in pregnancy: a prospective study among Singaporean women. Journal of Gastroenterology and Hepatology 1998;13(10):1020‐6. [DOI] [PubMed] [Google Scholar]
Law 2010
- Law R, Maltepe C, Bozzo P, Einarson A. Treatment of heartburn and acid reflux associated with nausea and vomiting during pregnancy. Canadian Family Physician 2010;56(2):143‐4. [PMC free article] [PubMed] [Google Scholar]
Li 1992
- Li Y, Tougas G, Chiverton SG, Hunt RH. The effect of acupuncture on gastrointestinal function and disorders. American Journal of Gastroenterology 1992;87:1372‐81. [PubMed] [Google Scholar]
Mandel 2000
- Mandel KG, Daggy BP, Brodie DA, Jacoby HI. Review article: alginate‐raft formulations in the treatment of heartburn and acid reflux. Alimentary Pharmacology & Therapeutics 2000;14(6):669‐90. [DOI] [PubMed] [Google Scholar]
Marrero 1992
- Marrero JM, Goggin PM, Caestecker JS, Pearce JM, Maxwell JD. Determinants of pregnancy heartburn. British Journal of Obstetrics and Gynaecology 1992;99(9):731‐4. [DOI] [PubMed] [Google Scholar]
Nielsen 1999
- Nielsen GL, Sørensen HT, Thulstrup AM, Tage‐Jensen U, Olesen C, Ekbom A. The safety of proton pump inhibitors in pregnancy. Alimentary Pharmacology & Therapeutics 1999;13(8):1085‐9. [DOI] [PubMed] [Google Scholar]
Quartarone 2013
- Quartarone G. Gastroesophageal reflux in pregnancy: a systematic review on the benefit of raft forming agents. Minerva Ginecologica 2013;65(5):541‐9. [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richter 2003
- Richter JE. Gastroesophageal reflux disease during pregnancy. Gastroenterology Clinics of North America 2003;32(1):235‐61. [DOI] [PubMed] [Google Scholar]
Richter 2005
- Richter JE. Review article: the management of heartburn in pregnancy. Alimentary Pharmacology and Therapeutics 2005;22(9):749‐57. [DOI] [PubMed] [Google Scholar]
Schunemann 2009
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. [DOI] [PubMed] [Google Scholar]
Strugala 2012
- Strugala V, Bassin J, Swales VS, Lindow SW, Dettmar PW, Thomas EC. Assessment of the safety and efficacy of a raft‐dorming alginate reflux suppressant (liquid Gaviscon) for the treatment of heartburn during pregnancy. ISRN Obstetrics and Gynecology 2012;2012:481870. [DOI] [PMC free article] [PubMed] [Google Scholar]
VanThiel 1977
- Thiel DH, Gavaler JS, Joshi SN, Sara RK, Stremple J. Heartburn of pregnancy. Gastroenterology 1977;72(4 Pt 1):666‐8. [PubMed] [Google Scholar]
VanThiel 1981
- Thiel DH, Wald A. Evidence refuting a role for increased abdominal pressure in the pathogenesis of the heartburn associated with pregnancy. American Journal of Obstetrics and Gynecology 1981;140(4):420‐2. [DOI] [PubMed] [Google Scholar]
Vazquez 2010
- Vazquez JC. Constipation, haemorrhoids, and heartburn in pregnancy. Clinical Evidence 2010;8:1411. [PMC free article] [PubMed] [Google Scholar]
Wilton 1998
- Wilton LV, Pearce GL, Martin RM, Mackay FJ, Mann RD. The outcomes of pregnancy in women exposed to newly marketed drugs in general practice in England. British Journal of Obstetrics and Gynaecology 1998;105(8):882‐9. [DOI] [PubMed] [Google Scholar]
Witter 1981
- Witter FR, King TM, Blake DA. The effects of chronic gastrointestinal medication on the fetus and neonate. Obstetrics and Gynecology 1981;58(5 Suppl):79S‐84S. [PubMed] [Google Scholar]
References to other published versions of this review
Neilson 2008
- Neilson JP. Interventions for heartburn in pregnancy. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007065.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Phupong 2014
- Phupong V, Hanprasertpong T. Interventions for heartburn in pregnancy. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD011379] [DOI] [PMC free article] [PubMed] [Google Scholar]


