Abstract
Neutrophils, the most abundant type of leukocyte in human blood, play a major role in host defense against invading pathogens and in sterile injury. Neutrophil infiltration is characteristic of inflammation because of its antimicrobial and cytotoxic activities. Neutrophils also actively participate in the resolution of inflammation and subsequent tissue repair by acting as a critical mediator between the inflammation and resolution phases of tissue damage. However, neutrophils that are consistently exposed to inflammatory conditions lose their self-resolving capabilities and maintain an inflammatory phenotype, further exacerbating tissue damage. The current review describes how neutrophils interact with tissue microenvironments and acquire disease-specific phenotypes under chronic inflammatory conditions. Here, we aim to provide a better understanding of neutrophil-mediated pathogenesis of various liver diseases.
The liver is a central organ that performs numerous essential functions, including coordination of immune responses. Hepatocytes and nonparenchymal cells (including Kupffer cells, liver-resident macrophages) interact in the liver, which gives rise to liver-specific metabolic and immunologic features. The liver is continuously exposed to various gut-derived components via the portal vein, including food antigens and microbial components. In health, the liver is “tolerant” to these low-grade intrusive exposures, but excessive stimulation induces inflammation that contributes to the progression of most chronic liver diseases.
Neutrophils are bone marrow–derived granulocytes that circulate through the bloodstream for immune surveillance. On infection or liver injury, neutrophils rapidly migrate from circulation to sites of inflammation and execute various antimicrobial and cytotoxic activities. Once tissue-infiltrating neutrophils complete their task, they mostly undergo programmed cell death, although a small portion of intact neutrophils can return to the vasculature.(1) The increased neutrophils death is then compensated through emergency granulopoiesis, which is a significantly enhanced neutrophil production and subsequent release from bone marrow during excessive infection or injury.(1) As first responders, neutrophils dictate whether inflammatory conditions should be maintained via the recruitment of additional immune cells or ceased via the initiation of the resolution phase (Fig. 1). Hence, excessive or dysfunctional neutrophils could exacerbate tissue damage and lead to pathogenesis. In this review, we summarize the contribution of neutrophils on the pathogenesis of various liver diseases and highlight their beneficial roles in controlling inflammation and maintaining liver homeostasis.
FIG. 1.
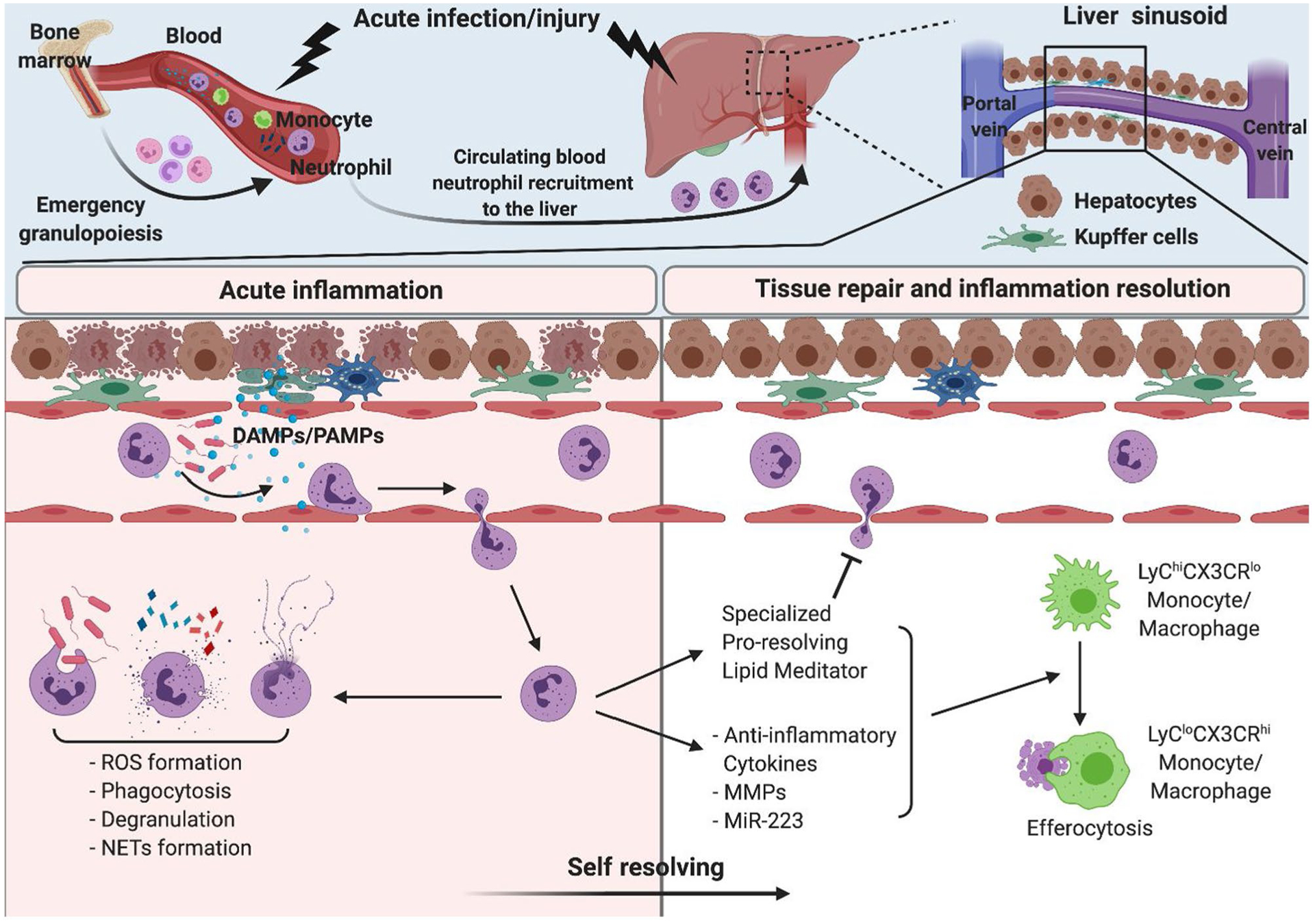
Neutrophils in acute infection/inflammation and self-resolution. Circulating blood neutrophils rapidly migrate into the site of infection/inflammation in the liver. Neutrophils exert antimicrobial and cytotoxic roles through ROS formation, phagocytosis, degranulation, and neutrophil extracellular trap (NET) formation. Increased neutrophil consumption is compensated through emergency granulopoiesis. During inflammation, neutrophils also actively participate in resolution of inflammation to prevent unwanted tissue damage and maintain homeostasis by production of anti-inflammatory cytokines and lipid mediators as well as monocyte/macrophage phenotype shift. PAMP, pathogen-associated molecular pattern.
Neutrophils in Host Defense
Neutrophil-mediated antimicrobial and cytotoxic activities occur primarily through the production of reactive oxygen species (ROS), phagocytosis, cytokine release, degranulation, and the formation of neutrophil extracellular traps (NETs). Enriched pre-made granular proteins enable neutrophils to achieve immediate, potent, neutrophil-specific microbicidal responses.(2) Several adhesion molecules are involved in circulating neutrophil rolling, adhesion, and transmigration.(2) The tethering of these adhesion molecules partially activates neutrophils, which results in multiprotein enzyme complex nicotinamide adenine dinucleotide phosphate oxidase (NOX) assembly on the plasma membrane or phagosomes and leads to the production of ROS, such as superoxide anion and hydrogen peroxide. Hydrogen peroxide is further converted to hypochlorous acid by myeloperoxidase (MPO), enhancing its bactericidal effect. Production of ROS and its derivatives inhibits bacterial growth and induces direct cytotoxicity by destroying the cellular membrane.(3) NOX assembly under toll-like receptor (TLR) 4 and TNF receptor engagement involves kinase activation, such as p38 mitogen-activated protein kinase or extracellular signal-regulated kinase 1/2.(3) Hence, ROS production is often coupled with degranulation and cytokine production. Because neutrophil granules are a reservoir of various proteases, oxidase, and antimicrobial peptides, phagosome fusion with granules following phagocytosis enhances pathogen clearance. Furthermore, neutrophil serine proteases cleave essential bacterial proteins for virulence or membrane integrity, directly killing invading microbes. Bactericidal/permeability-increasing protein, a well-known antimicrobial peptide, binds to lipopolysaccharides (LPS) and accelerates phagocytosis of bacteria.(2) In addition, neutrophils release decondensed chromatin mixed with various bactericidal granular proteins (NETs) when they encounter excessive amounts of pathogen. The NETs formation is an efficient antimicrobial strategy that traps pathogens within its web-like structure and prevents pathogen dissemination.(2) In sterile inflammation, recruited neutrophils have been observed in the necrotic area,(4) suggesting that neutrophils may be necessary to clear cellular debris and aid in the return to homeostasis.
Pathogenic Roles of Neutrophils in Liver Diseases
ALCOHOL-ASSOCIATED LIVER DISEASE
Alcohol-associated liver disease (ALD) encompasses the spectrum of alcohol-associated steatosis, alcohol-associated hepatitis (AH), and cirrhosis caused by excessive or chronic alcohol intake. An increase of neutrophils in peripheral blood and liver tissue is consistently reported in patients with ALD.(5,6) Increased neutrophil levels from liver biopsy correlate with poor clinical outcomes in AH. Increased blood neutrophil-lymphocyte ratio (NLR) is also associated with higher susceptibility to infection, acute kidney injury, and poor clinical outcome following steroid treatment in patients with AH.(7) Activated Kupffer cells and damaged hepatocytes recruit neutrophils to the liver via the release of chemokines and cytokines.(8) These infiltrating neutrophils (including immature neutrophils) contribute to alcohol-induced liver damage in various ways, although their specific role is not yet understood.
Although ROS production is a potent bactericidal process, excessive or uncontrolled ROS formation causes undesirable tissue damage. Peripheral neutrophils isolated from patients with AH/alcohol-associated cirrhosis have significantly higher resting ROS levels compared with neutrophils from healthy donors, suggesting that neutrophils in patients with ALD are activated and produce ROS even without stimulation.(5,6) Because superoxide anion and hydrogen peroxide are highly diffusible, extracellular ROS can travel across cellular membranes and activate or cause damage in nearby cells. Indeed, advanced oxidized protein products are significantly increased with alcohol exposure, further demonstrating enhanced oxidative stress in patients with severe AH. Notably, significantly increased oxidized albumin directly induces neutrophil respiratory burst and cytokine production and augments inflammatory reaction in patients with AH.(6) The mobilization of the primary granule to the plasma membrane on stimulation is significantly increased in the neutrophils from patients with ALD,(9) indicating an increased possibility of degranulation in the neutrophils of these patients. Indeed, the plasma levels of several neutrophil granular proteins, such as lactoferrin, neutrophil elastase (NE), and lipocalin 2 (LCN2), are increased in patients with ALD.(9,10) Studies using blocking antibodies to ameliorate alcohol-induced liver damage in mice also correlate with granular protein-mediated liver damage in patients with ALD.(10) Neutrophils from patients with AH also produce more TNF-α and IL-8 (or chemokine [C-X-C motif] ligand 8 [CXCL8]) on LPS stimulation, further providing evidence for hyperresponsive neutrophils in AH.(11) Finally, binge alcohol consumption induces spontaneous NETs formation in the liver and results in sustained inflammation in mice. Intriguingly, NET formation results in a proinflammatory phenotype in macrophages after efferocytosis.(12) This suggests that neutrophils can maintain inflammation through activation of neighboring cells and subsequently exacerbate alcohol-induced liver damage.
NAFLD AND NASH
NAFLD, ranging from simple steatosis to more progressive forms, including steatohepatitis (NASH) and cirrhosis, is initiated by a disruption in glucose and lipid metabolism. In patients with NAFLD, excessive fat accumulation, hepatocyte ballooning, lobular inflammation, and fibrosis characterize pathologic liver biopsies.(13) Hepatic fat accumulation renders hepatocytes susceptible to additional “hits,” including oxidative stress and mitochondrial dysfunction, which are sterile danger signals thought to promote persistent inflammation and contribute to the complex development of NAFLD.(14) Recently, clinical data showed that NLR correlates with histological features of NASH and fibrosis grade, suggesting that NLR could be a useful marker to predict NASH and fibrosis in patients with NAFLD.(15) However, despite an increase in neutrophil numbers, patients with NASH are more susceptible to infection.(16)
Neutrophil contribution to the progression of NAFLD is supported by experiments where in vivo neutrophil depletion in methionine/choline-deficient (MCD) diet and high-fat and high-cholesterol (HFHC) diet induced mouse models of NASH remarkably reduce NASH phenotypes, including macrovesicular/microvesicular steatosis and inflammation as well as liver damage in early stages of NASH. Interestingly, the protective effect was less distinct when neutrophils were removed in advanced NASH, indicating a contribution of neutrophils to the onset of NASH rather than progression.(17) Neutrophil-mediated pathogenesis occurs primarily through granular proteins in NASH. Indeed, lipidomics analysis demonstrated that increased NE in patients with NASH and a corresponding mouse model is associated with increased ceramide synthesis. This suggests that NE participates in ceramide metabolism and may induce lipid disturbance in NASH.(18) Furthermore, the absence of NE reduces steatosis and inflammatory cytokine production,(17) supporting the hypothesis that NE partially mediates steatosis and inflammation in NASH. Moreover, significantly elevated LCN2 has been associated with altered C-X-C motif chemokine receptor (CXCR) 2 expression levels in neutrophils, which leads to neutrophil recruitment and initiates a positive feedback loop in mouse models of NASH. Genetic deletion of LCN2 leads to a significant reduction of neutrophil recruitment to the liver and subsequent decreased inflammation and fewer instances of liver injury, in HFHC diet and MCD diet induced NASH in mice.(19) Patients with NASH also show significantly increased levels of neutrophil-derived MPO in liver tissue. This increased MPO accelerates production of modified ROS and induces protein modification and DNA damage.(20) Furthermore, patients with NASH also present increased NET formation in the liver. This augments inflammation through increased monocyte recruitment and cytokine production and, consequently, drives HCC development from NASH.(13)
FIBROSIS/CIRRHOSIS AND ACUTE-ON-CHRONIC LIVER FAILURE
Chronic inflammation and activation of HSCs contribute to the development and progression of fibrosis and cirrhosis regardless of etiologies, including ALD, NAFLD, and chronic viral infection. Neutrophil count is associated with disease severity in cirrhosis, and increased NLR is associated with poor prognosis and high mortality in patients with cirrhosis.(21) Neutrophil-derived IL-17A and IL-22 increase is prominent in patients and mouse models with fibrosis/cirrhosis. Inflammatory cytokine production and profibrotic gene expression are also IL-17A dependent. IL-17A directly activates IL-17A receptor–expressing HSCs, and activated HSCs subsequently produce transforming growth factor beta and collagens, resulting in developmental fibrosis.(22,23) Accordingly, neutrophil-derived IL-17A is considered to be a driving force behind fibrosis/cirrhosis development in patients with ALD and NAFLD.(23,24) Granule proteins also contribute to neutrophil-mediated fibrosis/cirrhosis development and progression. Transgenic mice expressing human neutrophil peptide (HNP)-1 show significantly enhanced fibrosis phenotypes on alcohol intake. HNP-1 directly activates HSCs and promotes alcohol-induced fibrosis.(25) MPO-induced oxidative stress also directly activates HSCs and up-regulates fibrosis-related gene expression.(26) This supports the clinical finding that a fraction of ROS-producing neutrophils is positively correlated with severity of liver cirrhosis indicated by Child-Pugh stage.(27) Interestingly, activated HSCs suppress neutrophil apoptosis by releasing granulocyte macrophage colony-stimulating factors,(23) suggesting that neutrophil-HSC interactions may form a positive feedback loop and accelerate fibrosis progression (Fig. 2).
FIG. 2.
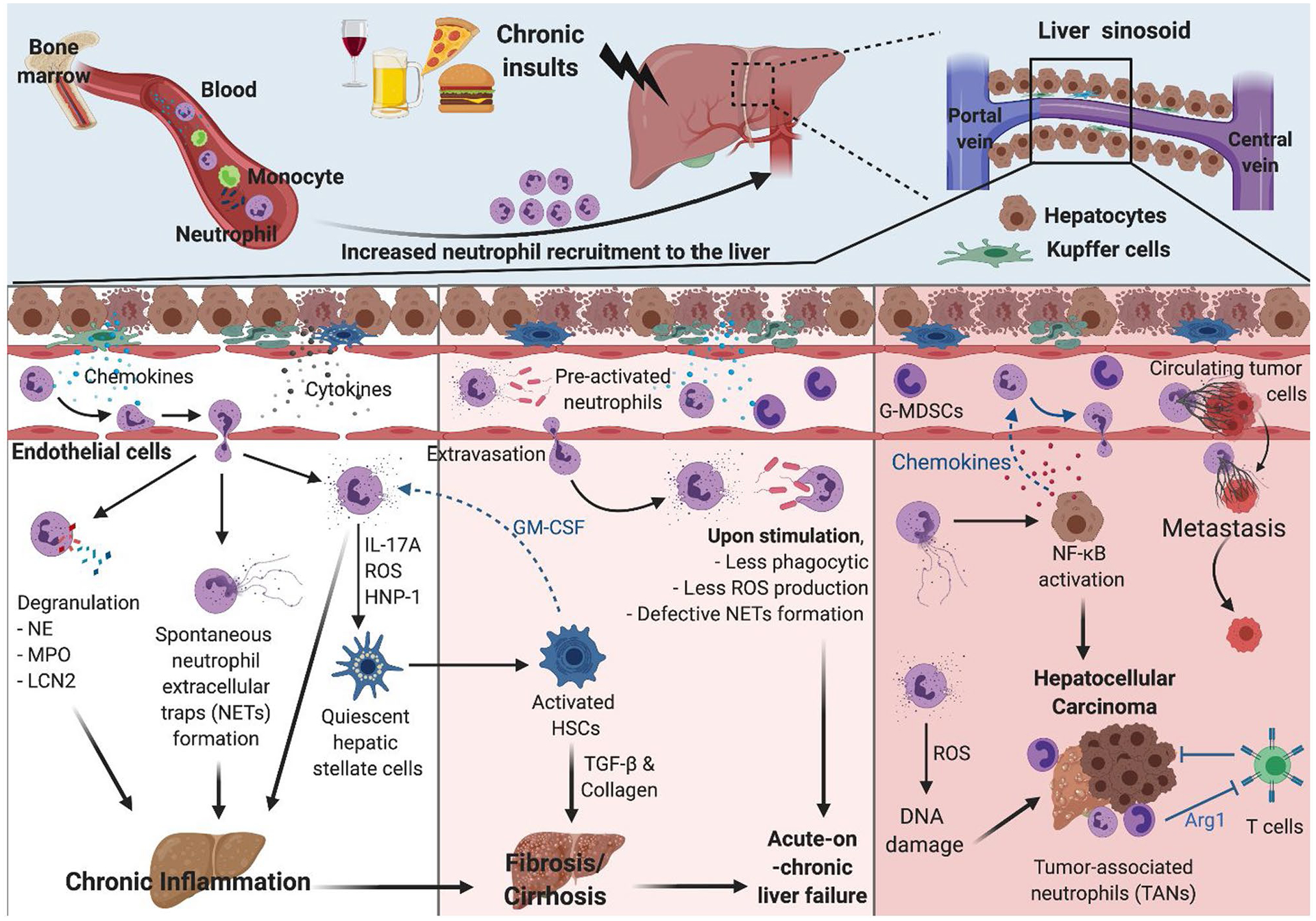
Neutrophils in chronic inflammation and advanced liver diseases. Neutrophils contribute to chronic inflammation through release of granular proteins, inflammatory cytokines, and spontaneous neutrophil extracellular traps (NETs). Particularly, neutrophil-derived IL-17A, ROS, and HNP-1 can directly activate HSCs, leading to development of fibrosis/cirrhosis. Neutrophils in patients with cirrhosis are preactivated but are less responsive to stimulation, rendering patients with cirrhosis more susceptible to infection and liver failure. Neutrophils also contribute to HCC development via continuous ROS-mediated DNA damage and up-regulation of oncogene expression. NETs activate NF-kB, which promotes hepatocyte proliferation. NETs also enhance the adherence of CTCs on vasculature and thus tumor metastasis. The TANs and G-MDSC that are observed in HCC maintain the tumor microenvironment and facilitate tumor growth by suppression of T cell–mediated antitumor activities. GM-CSF, granulocyte macrophage colony-stimulating factor.
In patients with cirrhosis, chronic inflammation leads to the continuous exposure of damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns on neutrophils, resulting in elevated expression of immune receptors. For example, neutrophils isolated from patients with cirrhosis have significantly elevated TLR2 and TLR4 expression levels compared with those from healthy controls,(16) which correlates with heightened activation of neutrophils in patients with cirrhosis. Activated phenotypes with up-regulated surface molecules have been associated with chemotaxis, including CD11b, CXCR1, and CXCR2.(28) Moreover, higher resting oxidative bursts occur in neutrophils isolated from patients with cirrhosis. However, these neutrophils produce less ROS and show reduced degranulation and impaired phagocytic capacity on ex vivo stimulation. This is partially due to defects in expression of NOX2 components gp91phox, p22phox, and p47phox,(29) which affects downstream signaling pathways in neutrophils. Indeed, activation of AKT, p38, and extracellular signal-regulated kinase 1/2, major downstream pathways following ROS generation, are impaired on N-formyl-methionyl-leucyl-phenylanine–induced stimulation in neutrophils from patients with advanced alcohol-associated cirrhosis.(30) Neutrophils from patients with cirrhosis also show significant defects on NET release on stimulation.(31) Hence, neutrophils are preactivated (but show significant defects in neutrophil antimicrobial functions in response to stimuli) and contribute to increased susceptibility to infection in patients with cirrhosis. Moreover, patients with cirrhosis have a higher chance of developing acute-on-chronic liver failure (ACLF) that is characterized by acute collapse of liver function (decompensation) and high short-term mortality. Several precipitating factors, including bacterial infection and binge alcohol intake, are also involved.(32) Of these factors, increased spontaneous microbial infection correlates with the neutrophil dysfunctions described. Despite different etiologies, neutrophils found in patients with ACLF show similar defects on their functions, such as increased chemokine receptor expressions, higher resting ROS burst, and impaired phagocytosis. Dysfunctional neutrophil count is also positively correlated with disease severity, indicated by Model for End-Stage Liver Disease and Child-Turcotte-Pugh score, and survival rate in patients with ACLF.(33)
HCC
HCC is one of the most common primary types of cancer worldwide. Risk factors for HCC development vary greatly from alcohol abuse to NASH and chronic viral hepatitis. Repetitive inflammation and resolution disrupt hepatocyte cell cycle control and apoptosis, which leads to hepatocytes with tumorigenic features.(34) Clinical data suggest a correlation between increased neutrophil levels and NLR with poor clinical outcomes in patients with HCC.(35) Recent studies have shown distinctive neutrophil subpopulations in tumor microenvironments that contribute to tumor progression and metastasis. Granulocytic myeloid-derived suppressor cells (G-MDSCs) in circulation appear enriched in patients with cancer and may facilitate tumor growth by suppressing T-cell antitumor activities. Interestingly, these G-MDSCs, or “low-density neutrophils,” are often coisolated with peripheral blood mononuclear cells. Neutrophil populations found specifically in tumor microenvironments are known as tumor-associated neutrophils (TANs).(36,37) A growing body of literature emphasizes the protumor function of TANs on HCC progression. First, increased spontaneous ROS production causes damage to proteins and DNA. Increased 8-hydroxydeoxyguanosine, a DNA adduct that is generated as a result of oxidative stress, is apparent in patients with HCC and mirrors stress-induced DNA damage.(38) Continuous DNA damage can cause up-regulation of prototypic oncogenes such as c-myc, as demonstrated by clinical studies highlighting oncogene-mediated tumor development.(39) Inflammatory cytokines and growth factors are also key players in hepatocyte proliferation. Peritumoral neutrophils are a cellular source of hepatocyte growth factor (HGF) and drive metastasis through enhanced HGF/c-Met interaction in HCC.(40) Neutrophil-derived IL-17A is also found in the peritumoral region, and high IL-17 levels are significantly associated with increased incidences of HCC recurrence.(41) Moreover, matrix metalloproteases (MMPs) within NETs degrade and modify the extracellular matrix; NET formation during extravasation greatly enhances circulating tumor cell (CTC) adherence on vasculature and consequently facilitates CTC vascular invasion and, ultimately, metastasis. In addition, NETs activate TLR4/9 and lead to NF-kB pathway activation and cyclooxygenase 2 up-regulation, which results in hepatocyte proliferation and enhanced invasion capacity.(42) NF-kB activation in HCC also results in production of key chemokines for neutrophil recruitment. CXCL1/2 and CXCL5 are increased after activation of NF-kB pathway in murine hepatocytes, initiating a positive feedback loop in neutrophil-driven HCC development in mice.(43) Furthermore, TAN-induced immunosuppressive tumor microenvironment is a critical risk factor for tumor growth and the development of resistance to therapy. Of neutrophil-derived proteases, arginase 1 (ARG1) depletes L-arginine, which is essential for T-cell proliferation and activation. TANs show enhanced ARG1 expression compared with those from healthy donors, inhibiting T cell–mediated antitumor effect.(37) TANs also produce chemokine (C-C motif) ligand (CCL) 2 and CCL17, which recruit macrophage and regulatory T cells and maintain the immunosuppressive tumor microenvironment(44) (Fig. 2).
ACUTE LIVER INJURY
Acetaminophen (APAP) overdose is the most common cause of drug-induced acute liver injury worldwide. Excessive metabolic intermediate N-acetyl-p-quinone imine initiates a cytotoxic response and results in massive centrilobular necrosis in the liver. Infiltrated neutrophils preferentially remain in the necrotic zone, which is a hepatocyte-derived DNA-rich area. Neutrophils sensitize the extracellular DNA through enhanced expression of TLR9 on their plasma membrane, which leads to NF-kB activation in neutrophils.(4) Neutrophil depletion in mice via injection of an antibody targeting either granulocyte receptor 1 or lymphocyte antigen 6 complex locus G (Ly6-G) improves survival rate and ameliorates liver damage indicated by reduced ALT levels and necrosis area in APAP injury.(45,46) Neutrophil absence is also associated with less hydrogen peroxide production, decreased inducible nitric oxide synthase (iNOS) expression, and reduced peroxynitrite-induced liver damage.(46) In addition, the neutrophils treated with the supernatant from APAP-stimulated HepG2 cells produce more ROS, suggesting that a necrotic environment activates the recruited neutrophil.(45) These data demonstrate neutrophil-mediated oxidative stress may cause additional tissue damage in APAP-induced acute liver injury. However, several studies report conflicting data and question neutrophil involvement. Neutropenia achieved via anti–Ly6-G antibody injection or genetic depletion of CD18 does not show significant protective effects on APAP-induced liver damage.(47,48)
Ischemia/reperfusion injury is another type of acute and sterile liver injury caused by restricted oxygen supply to the liver. During ischemia, exposure of hepatocytes and KCs to oxidative stress causes necrotic cell death. On reperfusion, these damaged or dying cells release various DAMPs, cytokines, and chemokines, recruiting additional immune cells and exacerbating inflammation.(49) Massive neutrophil infiltration occurs particularly in liver sinusoid and microvasculature. Neutrophil recruitment also depends on the length of ischemia, which determines the extent of inflammation and liver damage.(50) Recruited neutrophils exert cytotoxicity mainly through increased ROS formation and inflammatory cytokine production, including IL-17A,(49,51) as shown in a mouse model of ischemia/reperfusion injury following neutrophil depletion.(49)
Beneficial Role of Neutrophils in Liver Inflammation and Repair
Neutrophil recruitment and activation generally lead to potent cytotoxic and inflammatory responses at the site of injury or inflammation. However, neutrophils also control inflammation to prevent unwanted tissue damage and maintain tissue homeostasis. Consequently, neutrophils actively participate in the resolution of inflammation through several mechanisms. Neutrophils promote tissue repair by regulating a macrophage phenotype switch. Neutrophil-mediated ROS formation drives Ly6Chi CX3CR1lo proinflammatory monocytes/macrophages conversion to Ly6Clo C-X3-C motif chemokine receptor 1 (CX3CR1)high reparative macrophages in acute liver injury(52) (Fig. 1). Macrophages engulfing apoptotic neutrophils produce less cytokine and chemokine,(53) suggesting that neutrophils act as a cue to cease inflammation and initiate resolution. Furthermore, MMPs released during neutrophil extravasation suppress fibrosis progression in a carbon tetrachloride–induced fibrosis mouse model. Particularly, MMP-8 and MMP-9 reduce collagen accumulation and promote liver repair by degrading fibrotic components.(54) An MMP blockade delays liver tissue repair and function restoration measured by indocyanine green disruption ratio following APAP-induced acute liver injury. Protease inhibition shows similar effects, and this study recapitulates findings showing that neutrophils are crucial for liver repair after inflammation.(48) MicroRNA-223 (miR-223) is the most abundant microRNA in neutrophils, and neutrophil-derived miR-223 is associated with down-regulation of iNOS expression and inflammatory cytokine production in macrophage, switching classically activated macrophage to M2 phenotype. Moreover, miR-223 suppresses NLR family, pyrin domain-containing 3 (NLRP3) expression and inflammatory cytokine production in macrophages, contributing to a cessation of inflammation. Neutrophil or miR-223 absence results in impaired spontaneous resolution during early fibrosis.(55) MiR-223 also controls ROS production through IL-6–dependent suppression of p47phox. Neutrophils regulate oxidative stress via miR-223 in alcohol-induced liver damage.(56) Moreover, specialized proresolving lipid mediators (SPMs), such as lipoxins, protectins, resolvins, and maresins, shed light on the initiation of resolution phase. SPMs promote tissue repair by inhibiting neutrophil chemotaxis, suppressing cytokine production, and enhancing phagocytosis (Fig. 1). Neutrophils with platelets play an essential role in the synthesis of these lipid mediators by providing enzymes required in the multistep conversion.(57) Of the synthesized SPMs, Lipoxin A4 leads to neutrophil reverse migration and removes the remaining neutrophils at the site of inflammation.(58) Additionally, maresin 1 prevents hepatocyte apoptosis caused by lipotoxicity-induced or hypoxia-induced endoplasmic reticulum stress.(59)
Concluding Remarks
Neutrophils play a critical role in host defense against sterile injury and microbe-induced infection. Neutrophil recruitment causes inflammation but is also required for inflammation resolution and tissue repair. However, neutrophils demonstrate abnormal functions under chronic inflammation conditions, which alters the self-resolving capacity of neutrophils and leads to tissue damage. Here, we describe several mechanisms by which neutrophils may contribute to the pathogenesis of various liver diseases. Notably, neutrophil dysfunctions are often acquired from tissue microenvironments under certain disease states. Furthermore, neutrophils actively participate in inflammation resolution and the return to homeostatic conditions, which is a critical component in our understanding of neutrophil-mediated inflammation and liver damage.
Acknowledgment:
We thank Jaclyn Mallard and Olivia Potvin for assistance in preparing the manuscript.
Supported by NIH/NIAAA grants U01AA026977 and R01AA011576 (to G. S.). The funders had no role in the preparation of this review.
Potential conflict of interest:
Dr. Szabo consults for and owns stock in Glympse and Zomagen. She consults for Allergan, Alnylam, Arrow, Durect, Generon, Merck Novartis, Pandion, Quest, Surrozen, and Terra Firma. She received grants from Gilead.
Abbreviations:
- ACLF
acute-on-chronic liver failure
- AH
alcohol-associated hepatitis
- ALD
alcohol-associated liver disease
- APAP
acetaminophen
- CTC
circulating tumor cell
- CXCL
chemokine (C-X-C motif) ligand
- CXCR
C-X-C motif chemokine receptor
- G-MDSC
granulocyte-like myeloid-derived suppressor cell
- HNP
human neutrophil peptide
- LCN2
lipocalin 2
- miR-223
microRNA-223
- MMP
matrix metalloproteinase
- MPO
myeloperoxidase
- NE
neutrophil elastase
- NLR
neutrophil-lymphocyte ratio
- NOX
nicotinamide adenine dinucleotide phosphate oxidase
- ROS
reactive oxygen species
- SPM
specialized proresolving lipid mediator
- TAN
tumor-associated neutrophil
- TLR
toll-like receptor
REFERENCES
- 1).Manz MG, Boettcher S. Emergency granulopoiesis. Nat Rev Immunol 2014;14:302–314. 10.1038/nri3660. [DOI] [PubMed] [Google Scholar]
- 2).Teng TS, Ji AL, Ji XY, Li YZ. Neutrophils and immunity: From bactericidal action to being conquered. J Immunol Res 2017;2017:9671604. 10.1155/2017/9671604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Nguyen GT, Green ER, Mecsas J. Neutrophils to the ROScue: Mechanisms of NADPH oxidase activation and bacterial resistance. Front Cell Infect Microbiol 2017;7:373. 10.3389/fcimb.2017.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Marques PE, Oliveira AG, Pereira RV, David BA, Gomides LF, Saraiva AM, et al. Hepatic DNA deposition drives drug-induced liver injury and inflammation in mice. Hepatology 2015;61:348–360. 10.1002/hep.27216. [DOI] [PubMed] [Google Scholar]
- 5).Mookerjee RP, Stadlbauer V, Lidder S, Wright GA, Hodges SJ, Davies NA, et al. Neutrophil dysfunction in alcoholic hepatitis superimposed on cirrhosis is reversible and predicts the outcome. Hepatology 2007;46:831–840. 10.1002/hep.21737. [DOI] [PubMed] [Google Scholar]
- 6).Das S, Maras JS, Hussain MS, Sharma S, David P, Sukriti S, et al. Hyperoxidized albumin modulates neutrophils to induce oxidative stress and inflammation in severe alcoholic hepatitis. Hepatology 2017;65:631–646. 10.1002/hep.28897. [DOI] [PubMed] [Google Scholar]
- 7).Rachakonda V, Bataller R, Duarte-Rojo A. Recent advances in alcoholic hepatitis. F1000Res 2020;9:97. 10.12688/f1000research.20394.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Szabo G Gut–liver axis in alcoholic liver disease. Gastroenterology 2015;148:30–36. 10.1053/j.gastro.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Tranah TH, Vijay GK, Ryan JM, Abeles RD, Middleton PK, Shawcross DL. Dysfunctional neutrophil effector organelle mobilization and microbicidal protein release in alcohol-related cirrhosis. Am J Physiol Gastrointest Liver Physiol 2017;313:G203–G211. 10.1152/ajpgi.00112.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Wieser V, Tymoszuk P, Adolph TE, Grander C, Grabherr F, Enrich B, et al. Lipocalin 2 drives neutrophilic inflammation in alcoholic liver disease. J Hepatol 2016;64:872–880. 10.1016/j.jhep.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 11).Taieb J, Mathurin P, Elbim C, Cluzel P, Arce-Vicioso M, Bernard B, et al. Blood neutrophil functions and cytokine release in severe alcoholic hepatitis: effect of corticosteroids. J Hepatol 2000;32:579–586. 10.1016/s0168-8278(00)80219-6. [DOI] [PubMed] [Google Scholar]
- 12).Bukong TN, Cho Y, Iracheta-Vellve A, Saha B, Lowe P, Adejumo A, et al. Abnormal neutrophil traps and impaired efferocytosis contribute to liver injury and sepsis severity after binge alcohol use. J Hepatol. 2018;69:1145–1154. 10.1016/j.jhep.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).van der Windt DJ, Sud V, Zhang H, Varley PR, Goswami J, Yazdani HO, et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology 2018;68:1347–1360. 10.1002/hep.29914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016;65:1038–1048. 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 15).Alkhouri N, Morris-Stiff G, Campbell C, Lopez R, Tamimi TA, Yerian L, et al. Neutrophil to lymphocyte ratio: A new marker for predicting steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease. Liver Int 2012;32:297–302. 10.1111/j.1478-3231.2011.02639.x. [DOI] [PubMed] [Google Scholar]
- 16).Tritto G, Bechlis Z, Stadlbauer V, Davies N, Frances R, Shah N, et al. Evidence of neutrophil functional defect despite inflammation in stable cirrhosis. J Hepatol 2011;55:574–581. 10.1016/j.jhep.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 17).Zang S, Wang L, Ma X, Zhu G, Zhuang Z, Xun Y, et al. Neutrophils play a crucial role in the early stage of nonalcoholic steatohepatitis via neutrophil elastase in mice. Cell Biochem Biophys 2015;73:479–487. 10.1007/s12013-015-0682-9. [DOI] [PubMed] [Google Scholar]
- 18).Chen J, Liang B, Bian D, Luo Y, Yang J, Li Z, et al. Knockout of neutrophil elastase protects against western diet induced non-alcoholic steatohepatitis in mice by regulating hepatic ceramides metabolism. Biochem Biophys Res Commun 2019;518:691–697. 10.1016/j.bbrc.2019.08.111. [DOI] [PubMed] [Google Scholar]
- 19).Ye D, Yang K, Zang S, Lin Z, Chau HT, Wang Y, et al. Lipocalin-2 mediates non-alcoholic steatohepatitis by promoting neutrophil-macrophage crosstalk via the induction of CXCR2. J Hepatol 2016;65:988–997. 10.1016/j.jhep.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 20).Rensen SS, Slaats Y, Nijhuis J, Jans A, Bieghs V, Driessen A, et al. Increased hepatic myeloperoxidase activity in obese subjects with nonalcoholic steatohepatitis. Am J Pathol. 2009;175:1473–1482. 10.2353/ajpath.2009.080999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Biyik M, Ucar R, Solak Y, Gungor G, Polat I, Gaipov A, et al. Blood neutrophil-to-lymphocyte ratio independently predicts survival in patients with liver cirrhosis. Eur J Gastroenterol Hepatol 2013;25:435–441. 10.1097/MEG.0b013e32835c2af3. [DOI] [PubMed] [Google Scholar]
- 22).Fabre T, Molina MF, Soucy G, Goulet JP, Willems B, Villeneuve JP, et al. Type 3 cytokines IL-17A and IL-22 drive TGF-beta-dependent liver fibrosis. Sci Immunol 2018;3:eaar7754. 10.1126/sciimmunol.aar7754. [DOI] [PubMed] [Google Scholar]
- 23).Zhou Z, Xu MJ, Cai Y, Wang W, Jiang JX, Varga ZV, et al. Neutrophil-hepatic stellate cell interactions promote fibrosis in experimental steatohepatitis. Cell Mol Gastroenterol Hepatol 2018;5:399–413. 10.1016/j.jcmgh.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Lemmers A, Moreno C, Gustot T, Marechal R, Degre D, Demetter P, et al. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology 2009;49:646–657. 10.1002/hep.22680. [DOI] [PubMed] [Google Scholar]
- 25).Ibusuki R, Uto H, Oda K, Ohshige A, Tabu K, Mawatari S, et al. Human neutrophil peptide-1 promotes alcohol-induced hepatic fibrosis and hepatocyte apoptosis. PLoS One 2017;12:e0174913. 10.1371/journal.pone.0174913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Pulli B, Ali M, Iwamoto Y, Zeller MW, Schob S, Linnoila JJ, et al. Myeloperoxidase-hepatocyte-stellate cell cross talk promotes hepatocyte injury and fibrosis in experimental nonalcoholic steatohepatitis. Antioxid Redox Signal 2015;23:1255–1269. 10.1089/ars.2014.6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Bruns T, Peter J, Hagel S, Herrmann A, Stallmach A. The augmented neutrophil respiratory burst in response to Escherichia coli is reduced in liver cirrhosis during infection. Clin Exp Immunol 2011;164:346–356. 10.1111/j.1365-2249.2011.04373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Huang CH, Jeng WJ, Ho YP, Teng W, Hsieh YC, Chen WT, et al. Increased EMR2 expression on neutrophils correlates with disease severity and predicts overall mortality in cirrhotic patients. Sci Rep 2016;6:38250. 10.1038/srep38250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Rolas L, Boussif A, Weiss E, Letteron P, Haddad O, El-Benna J, et al. NADPH oxidase depletion in neutrophils from patients with cirrhosis and restoration via toll-like receptor 7/8 activation. Gut 2018;67:1505–1516. 10.1136/gutjnl-2016-313443. [DOI] [PubMed] [Google Scholar]
- 30).Boussif A, Rolas L, Weiss E, Bouriche H, Moreau R, Perianin A. Impaired intracellular signaling, myeloperoxidase release and bactericidal activity of neutrophils from patients with alcoholic cirrhosis. J Hepatol 2016;64:1041–1048. 10.1016/j.jhep.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 31).Agraz-Cibrian JM, Delgado-Rizo V, Segura-Ortega JE,Maldonado-Gomez HA, Zambrano-Zaragoza JF, Duran-Avelar MJ, et al. Impaired neutrophil extracellular traps and inflammatory responses in the peritoneal fluid of patients with liver cirrhosis. Scand J Immunol 2018;88:e12714. 10.1111/sji.12714. [DOI] [PubMed] [Google Scholar]
- 32).Szabo G Pathogenesis of acute-on-chronic liver failure in patients with infection. Clin Liver Dis 2019;14:103–106. 10.1002/cld.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Khanam A, Trehanpati N, Riese P, Rastogi A, Guzman CA, Sarin SK. Blockade of neutrophil’s chemokine receptors CXCR1/2 abrogate liver damage in acute-on-chronic liver failure. Front Immunol. 2017;8:464. 10.3389/fimmu.2017.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Yu LX, Ling Y, Wang HY. Role of nonresolving inflammation in hepatocellular carcinoma development and progression. NPJ Precis Oncol 2018;2:6. 10.1038/s41698-018-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Margetts J, Ogle LF, Chan SL, Chan AW, Chan KC, Jamieson D, et al. Neutrophils: Driving progression and poor prognosis in hepatocellular carcinoma? Br J Cancer 2018;118:248–257. 10.1038/bjc.2017.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Lecot P, Sarabi M, Pereira Abrantes M, Mussard J, Koenderman L, Caux C, et al. Neutrophil heterogeneity in cancer: from biology to therapies. Front Immunol 2019;10:2155. 10.3389/fimmu.2019.02155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Masucci MT, Minopoli M, Carriero MV. Tumor associated neutrophils. Their role in tumorigenesis, metastasis, prognosis and therapy. Front Oncol 2019;9:1146. 10.3389/fonc.2019.01146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Cardin R, Piciocchi M, Bortolami M, Kotsafti A, Barzon L, Lavezzo E, et al. Oxidative damage in the progression of chronic liver disease to hepatocellular carcinoma: An intricate pathway. World J Gastroenterol 2014;20:3078–3086. 10.3748/wjg.v20.i12.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Xin B, Yamamoto M, Fujii K, Ooshio T, Chen X, Okada Y, et al. Critical role of Myc activation in mouse hepatocarcinogenesis induced by the activation of AKT and RAS pathways. Oncogene 2017;36:5087–5097. 10.1038/onc.2017.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).He M, Peng A, Huang XZ, Shi DC, Wang JC, Zhao Q, et al. Peritumoral stromal neutrophils are essential for c-Met-elicited metastasis in human hepatocellular carcinoma. OncoImmunology 2016;5:e1219828. 10.1080/2162402X.2016.1219828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Motomura T, Shirabe K, Mano Y, Muto J, Toshima T, Umemoto Y, et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol 2013;58:58–64. 10.1016/j.jhep.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 42).Yang LY, Luo Q, Lu L, Zhu WW, Sun HT, Wei R, et al. Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response. J Hematol Oncol 2020;13:3. 10.1186/s13045-019-0836-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Wilson CL, Jurk D, Fullard N, Banks P, Page A, Luli S, et al. NFkappaB1 is a suppressor of neutrophil-driven hepatocellular carcinoma. Nat Commun 2015;6:6818. 10.1038/ncomms7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Zhou SL, Zhou ZJ, Hu ZQ, Huang XW, Wang Z, Chen EB, et al. Tumor-associated neutrophils recruit macrophages and T-regulatory cells to promote progression of hepatocellular carcinoma and resistance to sorafenib. Gastroenterology 2016;150:1646–1658.e17. 10.1053/j.gastro.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 45).Marques PE, Amaral SS, Pires DA, Nogueira LL, Soriani FM, Lima BH, et al. Chemokines and mitochondrial products activate neutrophils to amplify organ injury during mouse acute liver failure. Hepatology 2012;56:1971–1982. 10.1002/hep.25801. [DOI] [PubMed] [Google Scholar]
- 46).Ishida Y, Kondo T, Kimura A, Tsuneyama K, Takayasu T, Mukaida N. Opposite roles of neutrophils and macrophages in the pathogenesis of acetaminophen-induced acute liver injury. Eur J Immunol 2006;36:1028–1038. 10.1002/eji.200535261. [DOI] [PubMed] [Google Scholar]
- 47).Williams CD, Bajt ML, Farhood A, Jaeschke H. Acetaminophen-induced hepatic neutrophil accumulation and inflammatory liver injury in CD18-deficient mice. Liver Int 2010;30:1280–1292. 10.1111/j.1478-3231.2010.02284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).Alvarenga DM, Mattos MS, Lopes ME, Marchesi SC, Araujo AM, Nakagaki BN, et al. Paradoxical role of matrix metalloproteinases in liver injury and regeneration after sterile acute hepatic failure. Cells 2018;7:247. 10.3390/cells7120247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49).Sun L, Wu Q, Nie Y, Cheng N, Wang R, Wang G, et al. A role for MK2 in enhancing neutrophil-derived ROS production and aggravating liver ischemia/reperfusion injury. Front Immunol. 2018;9:2610. 10.3389/fimmu.2018.02610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50).Kotzampassi K, Kalekou CH, Paramythiotis D, Elefthrtiadis E. Portal sinusoids’ neutrophil plugging after experimental ischemia reperfusion liver injury. Ann Gastroenterol 2003;16:346–351. [Google Scholar]
- 51).Tan Z, Jiang R, Wang X, Wang Y, Lu L, Liu Q, et al. RORgammat+IL-17+ neutrophils play a critical role in hepatic ischemia-reperfusion injury. J Mol Cell Biol. 2013;5:143–146. 10.1093/jmcb/mjs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52).Yang W, Tao Y, Wu Y, Zhao X, Ye W, Zhao D, et al. Neutrophils promote the development of reparative macrophages mediated by ROS to orchestrate liver repair. Nat Commun 2019;10:1076. 10.1038/s41467-019-09046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53).Holub M, Cheng CW, Mott S, Wintermeyer P, van Rooijen N, Gregory SH. Neutrophils sequestered in the liver suppress the proinflammatory response of Kupffer cells to systemic bacterial infection. J Immunol 2009;183:3309–3316. 10.4049/jimmunol.0803041. [DOI] [PubMed] [Google Scholar]
- 54).Saijou E, Enomoto Y, Matsuda M, Yuet-Yin Kok C, Akira S, Tanaka M, et al. Neutrophils alleviate fibrosis in the CCl4-induced mouse chronic liver injury model. Hepatol Commun 2018;2:703–717. 10.1002/hep4.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55).Calvente CJ, Tameda M, Johnson CD, Del Pilar H, Lin YC, Adronikou N, et al. Neutrophils contribute to spontaneous resolution of liver inflammation and fibrosis via microRNA-223. J Clin Invest 2019;129:4091–4109. 10.1172/JCI122258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Li M, He Y, Zhou Z, Ramirez T, Gao Y, Gao Y, et al. MicroRNA-223 ameliorates alcoholic liver injury by inhibiting the IL-6-p47(phox)-oxidative stress pathway in neutrophils. Gut 2017;66:705–715. 10.1136/gutjnl-2016-311861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57).Serhan CN, Takano T, Chiang N, Gronert K, Clish CB. Formation of endogenous “anti-inflammatory” lipid mediators by transcellular biosynthesis. Lipoxins and aspirin-triggered lipoxins inhibit neutrophil recruitment and vascular permeability. Am J Respir Crit Care Med 2000;161(Pt 2):S95–S101. 10.1164/ajrccm.161.supplement_1.ltta-19. [DOI] [PubMed] [Google Scholar]
- 58).Loynes CA, Lee JA, Robertson AL, Steel MJ, Ellett F, Feng Y, et al. PGE2 production at sites of tissue injury promotes an anti-inflammatory neutrophil phenotype and determines the outcome of inflammation resolution in vivo. Sci Adv 2018;4:eaar8320. 10.1126/sciadv.aar8320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59).Rius B, Duran-Guell M, Flores-Costa R, Lopez-Vicario C, Lopategi A, Alcaraz-Quiles J, et al. The specialized proresolving lipid mediator maresin 1 protects hepatocytes from lipotoxic and hypoxia-induced endoplasmic reticulum stress. FASEB J 2017;31:5384–5398. 10.1096/fj.201700394R. [DOI] [PubMed] [Google Scholar]


