Abstract
Colorectal carcinoma (CRC) is a common malignancy with steadily declining incidence rates and mortality, secondary to improved screening and lifestyle changes (eg, decreased smoking rates). The association between pelvic inflammatory disease (PID) and CRC has been unclear in the past. However, multiple studies showed a positive association between PID and underlying malignancy (gynecologic and pelvic primarily). Several studies evaluated the relation between PID and CRC, but the results were conflicting. We describe a case of a 33-year-old female patient, with a history of PID and recurrent pelvic abscesses, who was found to have CRC. Of note, the patient’s diagnosis was based on abnormal computed tomography findings, which were further investigated (by colonoscopy and biopsy), rather than on symptoms suggestive of CRC, such as rectal bleeding, constipation, symptomatic anemia, or abdominal pain.
Keywords: gastroenterology, colorectal carcinoma, pelvic inflammatory disease, occult malignancy
Introduction
Colorectal carcinoma (CRC) is one of the most common malignancies worldwide, with high morbidity and mortality rates. Pelvic inflammatory disease (PID) is an ascending infection affecting female pelvic organs with adjacent spread. The association between CRC and PID has been a topic of discussion as PID was found to be associated with gynecologic malignancies in the past. Our case report describes a 33-year-old female patient with a history of PID, with recurrent pelvic abscesses diagnosed with sigmoid adenocarcinoma.
Case Presentation
The patient is a 33-year-old woman with a past medical history of PID, complicated by recurrent pelvic abscesses with a history of drain placement by interventional radiology 3 months prior to admission. She presented to the emergency department (ED) with worsening left lower quadrant abdominal pain. Pain is of 4 months duration, constant, non-radiating, colicky in character, rated as 5/10, without alleviating or exacerbating factors present. The patient also had associated loss of appetite and unintentional weight loss of 26 pounds over 4 months. She denied associated nausea, vomiting, melena, hematochezia, constipation, coffee ground emesis, or hematemesis. One month prior to admission, the patient had an increase in the number of bowel movements (2-3 per day) and described the stool as solid and brown in color. During her admission, she had episodes of dysuria and a “reddish” tinge to her urine. However, she denied any air sensation (pneumaturia), fecal particles, or foul odor in her urine stream. The patient did not have a history of heavy alcohol use, smoking, or illicit drug use. Family history was unremarkable for colon cancer or other malignancies. The patient had 1 sexual partner over the past year, uses barrier protection, and denied history of sexually transmitted diseases (STD) or recent vaginal discharge. She stated that she had a history of bacterial vaginosis 1 year ago, which was treated.
On physical examination, the patient was found to be afebrile, normotensive, with no heart rate or respiratory rate abnormalities. Conjunctival pallor was noted. Abdominal examination was unremarkable as the abdomen was soft, scaphoid, non-tender, no rigidity or guarding, and bowel sounds were heard in 4 quadrants. Rectal examination was unremarkable.
Labs showed evidence of hypochromic microcytic anemia (Hgb 9.0 g/dl, mean corpuscular volume [MCV] 74.3 FEMTO L, mean corpuscular hemoglobin [MCH] 23.4 picosulfate [PICO] G), with an elevated red blood cell distribution width (RDW; 15.8), low iron 35 mcg/dl (normal: 37-170 mcg/dl), low transferrin 168 mg/dl (normal: 206-381 mg/dl), low total iron-binding capacity (TIBC) 229 mcg/dl (normal: 265-497 mcg/dl), and normal ferritin 49.2 (normal: 6.24-137.0 ng/ml). The iron studies’ findings were suggestive of a mixed pattern: iron deficiency anemia and anemia of chronic disease. No evidence of leukocytosis was present. Complete metabolic panel was within normal limits. Carcinoembryonic antigen (CEA) was elevated at 10.4 ng/ml (normal: <3) and CA19-9 was within normal limits. Blood cultures revealed no growth.
The patient was started on intravenous piperacillin/tazobactam and underwent contrast enhanced computed tomography (CT) of the abdomen and pelvis. The CT showed wall thickening of the sigmoid colon bowel loop and the adjacent urinary bladder surrounding severe fat stranding. There was interval increase in the size of the preexisting multiloculated rim-enhancing pelvic collection, measuring 10.3 × 7.3 cm2 (previously 1.9 × 4.0 cm2), in addition to inflammatory changes surrounding the uterus and left adnexa, extending to the anterior abdominal wall. No foci of air was seen in the urinary bladder. However, colovesical fistula could not be fully ruled out. Given the symptoms of dysuria, recurrent pelvic abscesses, and CT findings, the surgical team was consulted and recommended CT of the abdomen and pelvis with rectal contrast to assess for colovesical fistula. The CT of the abdomen and pelvis with rectal contrast showed significant intraluminal narrowing secondary to mass-like eccentric sigmoid colon wall thickening, without evidence of colovesical fistula (Figures 1 and 2). Therefore, the gastroenterology team was consulted to evaluate the sigmoid colon thickening and a decision was made to proceed with colonoscopy.
Figure 1.
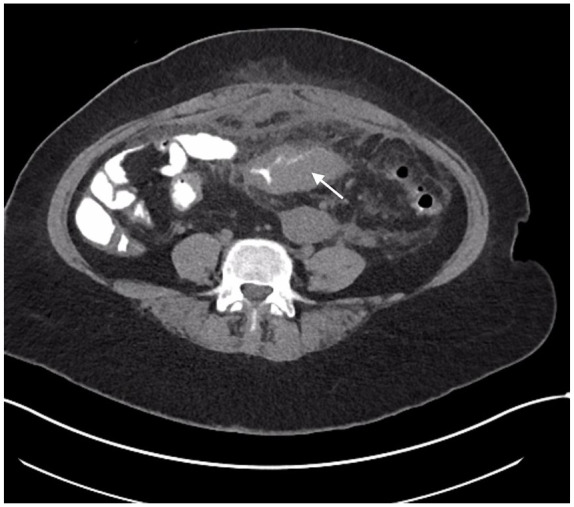
The CT of the abdomen and pelvis with oral and rectal contrast (axial view), showing circumferential sigmoid thickening (white arrow) with luminal narrowing.
Abbreviation: CT, computed tomography.
Figure 2.
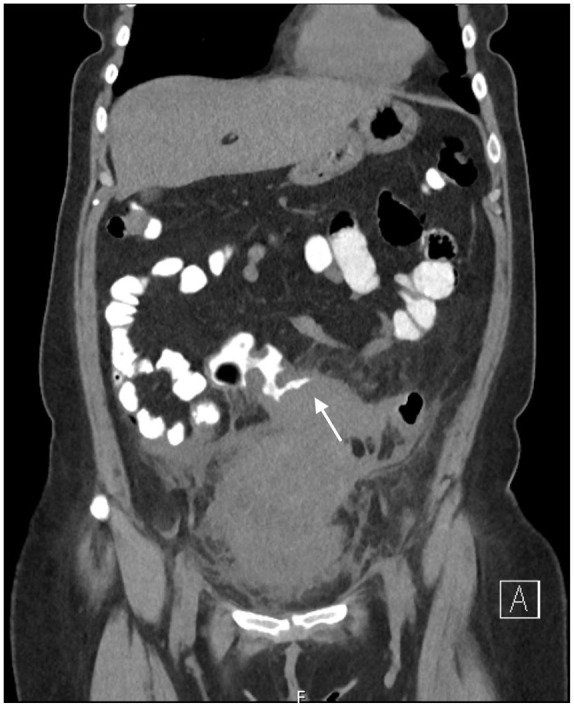
The CT of the abdomen and pelvis with oral and rectal contrast (coronal view), showing circumferential sigmoid thickening (white arrow) with luminal narrowing and large multiloculated thick-walled fluid collection inferiorly.
Abbreviation: CT, computed tomography.
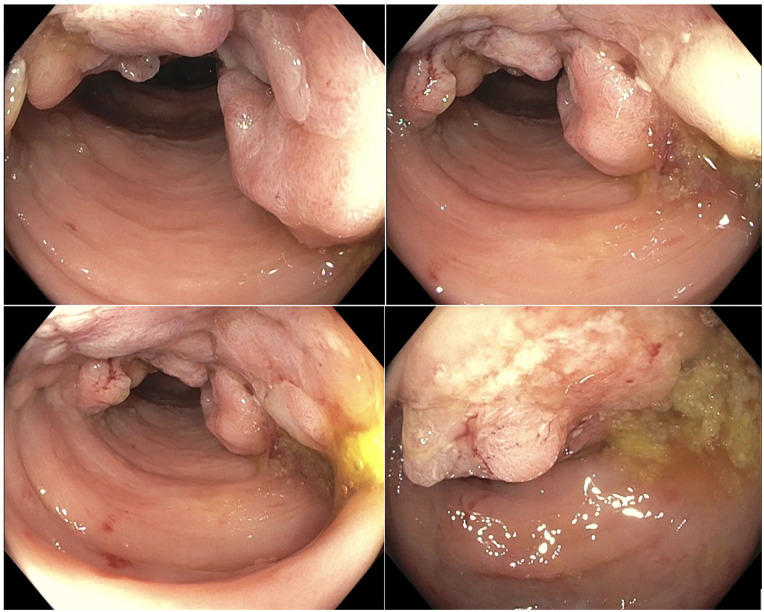
Colonoscopy showed an 8 cm infiltrative, partially obstructing large mass in the sigmoid colon, 35 cm proximal to the anal verge. The mass was partially circumferential (involving half of the lumen circumference; Figure 3). Biopsies were collected and showed invasive adenocarcinoma with normal mismatch repair (MMR) expression (no microsatellite instability) and negative Kirsten rat sarcoma virus (KRAS) mutation.
Figure 3.
Colonoscopy showing an infiltrative, partially obstructing large mass in the sigmoid colon measuring 8 cm in length with no bleeding present.
The CT of the thorax and the CT of the head were subsequently done and showed no evidence of metastatic disease. Colorectal surgery was consulted and due to the significant inflammation in the surrounding organs, and possibility of multi-organ removal, a decision was made to place a diverting colostomy to decrease inflammation, in addition to chemotherapy followed by colectomy depending on patient response. Hence, the patient underwent laparoscopic diverting loop transverse colostomy.
Hematology/Oncology was consulted and recommended FOLFOX-6 for 6 cycles followed by reassessment based on patient response and surgical assessment. The patient still follows with surgery, hematology/oncology, and gastroenterology as outpatient.
Discussion
Colorectal cancer (CRC) is the malignant growth of tissue in the colon and/or the rectum. It is the third most commonly diagnosed malignancy and has the second highest mortality risk among malignancies diagnosed in the United States. With the advancement in the gastroenterology field and the implementation of screening colonoscopies, the incidence of CRC has decreased. In 1976, the incidence of CRC was estimated to be 60.7 per 100,000, whereas the estimated incidence of CRC between 2009 and 2013 was 40.7 per 100,000 people.1,2 Moreover, the mortality rates have also declined, with a 35% decrease in mortality between 1990 and 2007. 3 The improvement in the incidence and mortality of CRC could be secondary to the application of screening colonoscopies, improvement in risk factors (smoking rates), and improved treatment modalities.
The PID is an infection involving the female upper genital tract. It can include the uterus, fallopian ducts, ovaries, and often involves adjacent organs (such as liver capsule—Fitz-Hugh Curtis syndrome). The PID presentation can be acute, subacute, or chronic, with symptoms varying, depending on the involved organ and associated complications. Presentations are variable and include severe abdominal pain with peritonitis symptoms, abnormal uterine bleeding, vaginal discharge, urinary frequency, palpable mass (in patients with abscess), infertility, fever, or weight loss. Risk factors for PID include younger age, multiple sexual partners, previous history of STD, previous history of PID, or no use of barrier contraception. 4
Our case brings up the question, “Is PID the result of underlying colorectal cancer or is there an association between PID and colorectal cancer?”
The PID and its association with underlying malignancies have been assessed in the past.
A retrospective review by Hsiao SM et al, between the years 1992 and 2000, assessed the features of tubo-ovarian abscess in premenopausal and postmenopausal patients. Despite the small sample size, postmenopausal patients were found to have a higher risk of associated malignancy (1 case of sigmoid colon cancer) compared with premenopausal women with statistical significance (P value .037). 5
Another retrospective study by Protopapas AG et al evaluated the risk of PID and associated malignancy, and the differences between premenopausal and postmenopausal patients. Postmenopausal patients were found to have a higher risk of underlying gynecologic malignancy (47% with P value <.001). This study did not find evidence of colorectal malignancy in patients with PID complicated with abscess formation. 6 However, the association between malignancy and PID with abscess was present.
Moreover, a population-based study by Lin HW et al evaluated patients with PID and risk of malignancy between the years 2000 and 2013. The study included 47 333 PID and 189 332 control group patients. Patients in the PID group differed significantly from the control group with the risk of associated malignancy. Patients with PID were found to be more likely to have associated gynecologic malignancy (ovarian, endometrial, and cervical cancer) and urinary tract malignancies, with a hazard ratio (HR) of 2.1 and 1.3, respectively (P value <.0001 and .0412, respectively). However, there was no significant association between PID and CRC in this study. Most of the gynecologic malignancies were diagnosed within 1 year of PID diagnosis. 7
Another retrospective population-based study by Hsu MI et al evaluated the risk of CRC specifically with PID. The study included 19 029 women with PID, who were matched to a control group of 76 116. The study showed that the CRC HR in the PID patient group was 2.00 (95% CI [1.30-3.08]), with an adjusted HR of 1.7 (95% CI [1.10-2.65]). The incidence of CRC per 100 000 person-years was 37.9 in the PID group versus 19.7 in the control group. The risk of CRC increased as the number of follow-up years increased. 8 This begs the question, “Is PID a risk factor for CRC?” A reanalysis of the data evaluated the association between CRC-predicted probability and the number of PID visits. The relation between CRC and PID during Visits 1 to 12 showed an odds ratio (OR) of 1.72 (95% CI [1.1-2.71], P value .019). However, the OR of CRC and PID with >12 PID visits was 2.84 (95% CI [1.04-7.8], P value .043). This shows an association between the duration of PID and risk of CRC, and suggests possible causality as the study met the Bradford Hill criteria in a biological system. 9
Furthermore, a study by Baruch G et al showed that 1.4% of premenopausal patients with PID and 42.8% of postmenopausal patients with PID had underlying pelvic malignancy (P value <.001). 10
The role of inflammation and development of CRC has been reviewed in the literature in the past. The major example showing the association between CRC and inflammation is in cases of inflammatory bowel disease (IBD). Inflammation can contribute to malignancy through 3 mechanisms: inflammation-associated tumorigenesis, tumor-elicited inflammation, and therapy-induced inflammation. Inflammation-associated tumorigenesis can be classified as infections, unregulated immune response, and external factors (smoking, alcohol use, and diet). Chronic inflammation leads to oxidative stress due to reactive oxygen species, which can in turn lead to DNA damage (nucleotide alterations, single- and double-stranded breaks, genome-wide mutations, and abasic sites), epigenetic alterations, and proliferative stimuli. 11 Moreover, chronic inflammation causes barrier defects and immune response that also lead to further oxidative stress, epigenetic alterations (silencing of tumor suppressor genes), and proliferative stimuli. Interestingly, epithelial barrier defects lead to exposure of the intestinal stem cells to external mutagens (smoking by-products and alcohol) and allows interactions between the intestinal stem cells and inflammatory cells, leading to genotoxic products. 12 Furthermore, intestinal barrier defects induce interactions between intestinal stem cells and intestinal microbiota components that possess pro-tumorigenic characteristics. 13
In IBD, inflammation can result in gut microbiome alterations, with an increase in adherent invasive E. coli and a decrease in butyrate producing bacteria, such as Ruminococcacaeae, Eubacterium, Clostridia, and Firmicutes. Adherent invasive E. coli can survive and replicate in macrophages. The decrease in butyrate producing organisms results in a lower anti-inflammatory effect through a decrease in the inhibition of histone deacetylases (HDACs)/NF-kB (in macrophages) and decreased IL-10 secretion. 14 In addition, butyrate was found to improve mucosal integrity through tight junction assembly and hence minimizes the exposure of intestinal stem cells to external mutagens and bacterial translocation. The exact relation between the microbiome and IBD remains controversial due to the differences between human and animal models. Although gut microbiome alterations exist in IBD, potentially due to the inflammatory environment, causality remains unknown. 15 It is important to note that the association between inflammation and CRC in IBD cannot be directly compared with the association between inflammation and CRC in PID as PID is not a colonic pathology.
Conclusion
The PID is an inflammatory status and hence can theoretically result in malignancy as seen in a population-based study that was related to the duration of PID. Multiple studies in the past showed an association between PID and malignancy, albeit more commonly gynecologic malignancy. The number of studies evaluating PID and CRC is scarce, however, an association was found in a population-based study. Nonetheless, the exact association remains unclear, whether PID causes CRC versus CRC increasing the risk of underlying infection due to local inflammation and possible bacterial translocation. This case report highlights the importance of exploring the possibility of malignancy in PID patients, which was found in our report.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Verbal informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iDs: M Ammar Kalas  https://orcid.org/0000-0002-6230-9172
https://orcid.org/0000-0002-6230-9172
Andrew Jonathen Ortega  https://orcid.org/0000-0002-6925-9884
https://orcid.org/0000-0002-6925-9884
References
- 1. Cheng L, Eng C, Nieman LZ, et al. Trends in colorectal cancer incidence by anatomic site and disease stage in the United States from 1976 to 2005. Am J Clin Oncol. 2011;34(6):573-580. doi: 10.1097/COC.0b013e3181fe41ed. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3. Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212-236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 4. Mitchell C, Prabhu M. Pelvic inflammatory disease: current concepts in pathogenesis, diagnosis and treatment. Infect Dis Clin North Am. 2013;27(4):793-809. doi: 10.1016/j.idc.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hsiao SM, Hsieh FJ, Lien YR. Tuboovarian abscesses in postmenopausal women. Taiwan J Obstet Gynecol. 2006;45(3):234-238. doi: 10.1016/S1028-4559(09)60231-X. [DOI] [PubMed] [Google Scholar]
- 6. Protopapas AG, Diakomanolis ES, Milingos SD, et al. Tubo-ovarian abscesses in postmenopausal women: gynecological malignancy until proven otherwise? Eur J Obstet Gynecol Reprod Biol. 2004;114(2):203-209. doi: 10.1016/j.ejogrb.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 7. Lin HW, Wang PH, Lee CY, et al. The risk of gynecologic and urinary tract cancer with pelvic inflammatory disease: a population-based cohort study. J Cancer. 2019;10(1):28-34. doi: 10.7150/jca.29278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hsu MI, Lin HW. Risk of colorectal cancer in women with pelvic inflammatory disease: a matched cohort study. BJOG. 2014;121(3):337-342. doi: 10.1111/1471-0528.12420. [DOI] [PubMed] [Google Scholar]
- 9. Hsu MI, Lin HW. Authors’ reply: risk of colorectal cancer in women with pelvic inflammatory disease: a matched cohort study. BJOG. 2014;121(11):1449-1450. doi: 10.1111/1471-0528.12815. [DOI] [PubMed] [Google Scholar]
- 10. Ben-Baruch G, Menashe Y, Leibovitz S, et al. Pelvic malignancy presenting as a pelvic inflammatory process in pre and postmenopausal women. Eur J Gynaecol Oncol. 1991;12(5): 347-349. [PubMed] [Google Scholar]
- 11. Canli Ö, Nicolas AM, Gupta J, et al. Myeloid cell-derived reactive oxygen species induce epithelial mutagenesis. Cancer Cell. 2017;32(6):869-883.e5. doi: 10.1016/j.ccell.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 12. Janney A, Powrie F, Mann EH. Host-microbiota maladaptation in colorectal cancer. Nature. 2020;585(7826):509-517. doi: 10.1038/s41586-020-2729-3. [DOI] [PubMed] [Google Scholar]
- 13. Schmitt M, Greten FR. The inflammatory pathogenesis of colorectal cancer. Nat Rev Immunol. 2021;21(10):653-667. doi: 10.1038/s41577-021-00534-x. [DOI] [PubMed] [Google Scholar]
- 14. Wang J, Chen WD, Wang YD. The relationship between gut microbiota and inflammatory diseases: the role of macrophages. Front Microbiol. 2020;11:1065. doi: 10.3389/fmicb.2020.01065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ni J, Wu GD, Albenberg L, et al. Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol. 2017;14(10):573-584. doi: 10.1038/nrgastro.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]