Abstract
Effective prevention of thromboembolism is essential for patients with mechanical prosthetic heart valves. For this group of patients, vitamin K antagonists (VKAs) remain the drug group of choice despite the widespread use of new anticoagulants in other diseases. As a consequence, warfarin resistance remains a serious challenge for physicians. The current report describes a 65-year-old male patient that had a mechanical prosthetic aortic valve implanted due to severe aortic insufficiency after infective endocarditis. Despite consistent increases in his warfarin dose, the level of international normalized ratio (INR) remained very low. The patient was considered to have warfarin resistance. Warfarin was successfully replaced by another VKA, acenocoumarol, which resulted in a stable INR observed over 1 year of follow-up. Achieving the target INR in patients with mechanical prosthetic heart valves using VKAs is the main goal of thromboprophylaxis. Although the genetic changes that cause warfarin resistance are understood, the options to overcome these pharmacogenetic issues remain limited. Based on the success with this current patient, physicians with similar patients with warfarin resistance might wish to consider replacing warfarin with acenocoumarol.
Keywords: Prevention of prosthetic valve thrombosis, warfarin resistance, acenocoumarol
Introduction
The problem of thrombosis and thromboembolism (TE) is extremely relevant today. The number of risk factors for the development of the components of the Virchow's triad is constantly increasing: (i) changes in blood flow; (ii) hypercoagulation; (iii) endothelial damage. 1 The increase in the incidence of cardiovascular diseases, cancer, the widespread introduction of various surgical procedures and the use of drugs that lead to blood clotting elevates the frequency of TE.2–4 In the last year, Covid-19 was added to the list of disease risk factors for these life-threatening complications. 5 Therefore, drugs that can prevent and effectively treat thromboembolism are being actively developed, studied and implemented.6,7 Currently, the following groups of drugs are used: fibrinolytic, direct anticoagulants, vitamin K antagonists (VKAs) and oral anticoagulants (OACs).8–10
For a long time, the main group of drugs used for the prevention of TE and stroke was the VKAs.11,12 However, these drugs are difficult to use because it is necessary to maintain the international normalized ratio (INR) within the target range of 2.0 to 3.5.8,9 This is challenging because of drug–drug interactions with a wide range of drugs of different pharmacological groups and their interactions with certain foods.13,14
Currently, there is considerable attention focused on the OACs by researchers and clinicians.9,10 This group includes rivaroxaban, dabigatran, apixaban and edoxaban. OACs have pharmacological advantages over VKAs, which include no food interactions, limited drug–drug interactions, and the ability to be administered in a fixed dosage without the need to maintain a special diet or undergo regular monitoring of blood clotting.6,7
Dabigatran, a direct thrombin inhibitor, was first approved by the FDA in 2010. The drug actively binds to the active centre of thrombin, preventing the conversion of soluble fibrinogen into insoluble fibrin. 6 In the RE-SONATE clinical trial, the drug reduced the risk of venous thromboembolic complications by more than 90% compared with placebo. 15
Rivaroxaban, apixaban and edoxaban are direct inhibitors of factor Xa. 7 These drugs competitively inhibit free and clot-bound factor Xa. 7 In addition, apixaban has no direct effects on platelet aggregation but indirectly inhibits platelet aggregation induced by thrombin.7,16 The AMPLIFY and AMPLIFY-EXT clinical trials have evaluated the efficacy of apixaban in deep vein thrombosis/pulmonary embolism, which was similar to the standard therapy of enoxaparin and warfarin, but it was safer in terms of the development of haemorrhagic complications. 16 Several clinical trials (EINSTEIN-DVT, EINSTEIN-РЕ, EINSTEIN-Extension) have investigated the effectiveness of rivaroxaban to treat and prevent deep vein thrombosis/pulmonary embolism, with the findings showing that rivaroxaban was more effective than standard therapy (i.e. enoxaparin with the transition to warfarin). 17
Mechanical prosthetic heart valves can be a site of thrombus formation, which can lead to cardioembolic stroke.8,18 The rate of these thromboembolic events is 0.7–6%. 18 The only group of anticoagulants that are approved for use after prosthetic valve implantation to prevent thromboembolic complications is the VKAs. 8 The use of OACs is not recommended (class III level B). 8 This current case report describes a patient with warfarin resistance that was treated successfully by replacing warfarin with acenocoumarol.
Case report
On 7 February 2020, a 65-year-old male patient with a 2-month history of irregular heartbeats and general weakness was admitted to Kyiv Municipal Heart Centre, Kyiv, Ukraine. His body temperature was elevated to a subfebrile level. The patient suffered from severe shortness of breath, palpitations and swelling of his legs. The electrocardiogram (ECG) revealed a sinus irregular heart rhythm, a heart rate of 85 beats/min (bpm), ventricular premature complexes and signs of left ventricular hypertrophy with systolic overload. The results of echocardiography were as follows: (i) mitral valve – moderate fibrosis, regurgitation +(+); (ii) aortic valve – severe fibrosis, regurgitation 4+, the presence of mobile and prolapsing vegetation 1.8 × 1.2 cm on the noncoronary valve, and valve abscess with perforation, aortic valve pressure gradient 14 mmHg; (iii) pulmonary artery pressure gradient 56 mmHg; (iv) end diastolic volume – 219 ml, ejection fraction – 40%. There was diffuse hypokinesis of the left ventricular myocardium. A chest X-ray demonstrated cardiomegaly, pneumosclerosis, aortic sclerosis and bilateral exudative pleural effusion.
Routine laboratory analyses demonstrated the following complete blood count: red blood cells (RBC) – 4.1 × 1012/l, white blood cells (WBC) – 9.79 × 109/l; haemoglobin (Hb) – 115 g/l; haematocrit – 35.3%, platelets – 219 × 109/l; erythrocyte sedimentation rate (ESR) – 28mm/h. Biochemical profiles were as follows: alanine transpeptidase – 26 U/l, aspartate transpeptidase – 19 U/l, creatinine – 75 µmol/l, total protein – 64 g/l, potassium – 3.8 mmol/l, sodium – 135 mmol/l, glucose – 10.8 mmol/l, C-reactive protein – 58.51 mg/ml, N-terminal prohormone of brain natriuretic peptide – 7561 pg/ml.
According to his clinical manifestations, ECG, echocardiography and laboratory findings, the patient was diagnosed as follows: infective endocarditis of native aortic valve; abscess, perforation of the non-coronary aortic valve; aortic insufficiency stage D; mitral insufficiency stage B; ventricular premature complexes Lown III; secondary pulmonary hypertension II degree; anasarca; bilateral exudative pleural effusion; ascites; heart failure with reduced ejection fraction (HFrEF). The patient also had type 2 diabetes mellitus.
The patient underwent implantation of a mechanical prosthetic aortic valve (St. Jude Medical 25 mm; St. Jude Medical, Saint Paul, MN, USA) on 25 February 2020 at the Kyiv Municipal Heart Centre. The medical management consisted of rifampicin, sulperazone, bisoprolol, amiodarone, pantoprazole, ramipril, torasemide, hypothiazide, enoxaparine, metformin and glimepiride.
On 12 March 2020, the patient was admitted to the Oleksandrivska Kyiv City Clinical Hospital, Kyiv, Ukraine for cardiac rehabilitation. On examination, his heart rate was 70 bpm and his blood pressure was 115/75 mmHg. Cardiac auscultation presented dull heart sounds. Lung examination was characterized by an absence of breathing sounds in the bottom of the left lung due to pleural effusion. The patient was noted to have ankle oedema. On admission, his clinical data were as follows: ECG revealed sinus regular heart rhythm and signs of left ventricular hypertrophy; echocardiography data showed gradient on aortic prosthetic valve – 22 mmHg, left atrium – 3.7 × 5.9 cm, end diastolic volume – 204 ml, and ejection fraction – 43%; complete blood count showed RBC – 4.15 × 1012/l, WBC – 5.4 × 109/l, Hb – 118 g/l, platelets –274 × 109/l and ESR – 34 mm/h; biochemical profiles were creatinine –106 µmol/l, total protein – 65 g/l, albumin – 37 g/l, potassium – 3.2 mmol/l, sodium – 142 mmol/l, glucose – 7.6 mmol/l and total cholesterol –5.6 mmol/l. According to his clinical manifestations, ECG, echocardiography and laboratory findings, the patient was diagnosed as follows: prosthetic aortic valve implanted (25.02.20); healed infective endocarditis; heart failure with mildly reduced ejection fraction (HFmrEF); and type 2 diabetes mellitus.
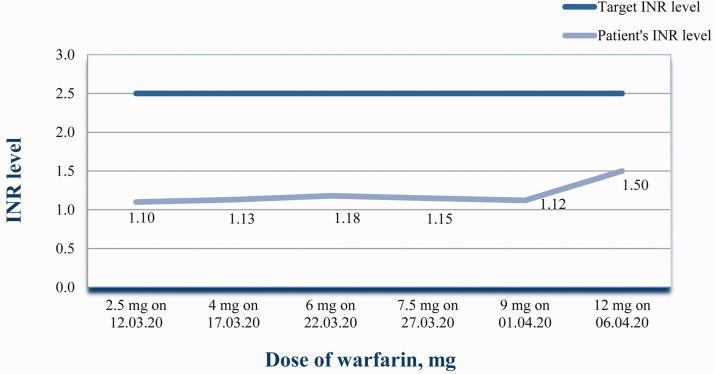
To prevent thromboembolic complications, the patient had been prescribed warfarin under control of INR at the Kyiv Municipal Heart Centre . At admission to the Oleksandrivska Kyiv City Clinical Hospital, the warfarin dose of 2.5 mg resulted in an INR –1.1. So the dose was increased to ∼4 mg (3.75 mg/day), but after 5 days the INR remained low (Figure 1). All factors that can influence warfarin metabolism were evaluated. The patient strictly followed the dietary recommendations. He had no diseases that could result in malabsorption. By this time rifampicin and sulperazone, which had been used for infective endocarditis treatment before surgery and in the early postoperative period, were replaced by 1.5 million units benzathine benzylpenicillin intramuscular, once every 3 weeks, for 1 year. The patient’s long-term management included the following: 2.5 mg bisoprolol oral once a day, 2.5 mg ramipril oral once a day, 200 mg amiodarone oral once a day, 12.5 mg hydrochlorothiazide oral once a day, 5 mg torasemide oral once a day, 0.4 ml enoxaparin subcutaneous once a day, 500 mg metformin oral twice a day and 2 mg glimepiride oral once a day. All of these drugs except enoxaparin were administered for 6 months, after which amiodarone and the diuretics were discontinued. The last enoxaparin injection was administered on 13 April 2020. Amiodarone and hydrochlorothiazide are on the list of drugs that potentiate the effects of warfarin. 13 The dose of warfarin was gradually increased and the INR checked every 5–6 days. Pharmacogenetic testing was not undertaken because it is not recommended by guidelines due to a lack of good-quality evidence. 8 The dynamics of the changes in the dose of the warfarin and the INR are presented in Figure 1. As it was impossible to achieve the appropriate INR over a long period of time, the patient was at a high risk of thromboembolism, so warfarin was replaced by acenocoumarol as described below.
Figure 1.
Dependence of the level of international normalized ratio (INR) on the dose of warfarin in a 65-year-old male patient after implantation of a mechanical prosthetic aortic valve on 25 February 2020. The colour version of this figure is available at: http://imr.sagepub.com.
Acenocoumarol is a 4-hydroxycoumarin derivative with anticoagulant activity (Figure 2). 19 The mechanism of action of acenocoumarol and other VKAs (Figure 3) results in the inhibition of vitamin K epoxide reductase, thereby inhibiting the reduction of vitamin K and the availability of vitamin K H2.13,14 This prevents gamma carboxylation of glutamic acid residues near the N-terminals of the vitamin K-dependent clotting factors, including factor II, VII, IX, and X and anticoagulant proteins C and S.13,14 This prevents their activity and thus thrombin formation. Acenocoumarol is rapidly absorbed from the gastrointestinal tract resulting in a peak concentration in 2–3 h.19,20 After oral administration, the half-life is 8–11 h and the maximum effect in terms of increasing prothrombin time is observed between 24–30 h.19,20 The cytochrome P450 family 2 subfamily C member 9 (CYP2C9) isoenzyme is the most important enzyme for the clearance of warfarin, while it plays a less important role in acenocoumarol clearance. 20 In vitro acenocoumarol has higher intrinsic anticoagulant properties. 13
Figure 2.
The molecular structure of acenocoumarol.
Figure 3.
The mechanism of action of vitamin K antagonists.
The patient was observed over 1 year. The level of INR was maintained in the target range (Figure 4). In the year after surgery, the patient had no complaints. On examination, his heart rate was 68 bpm, blood pressure was 120/80 mmHg. His heart sounds were regular with no murmur. His lungs were clear. The patient received the following: 6 mg acenocoumarol oral once a day, 5 mg bisoprolol oral once a day, 2.5 mg ramipril oral once a day, 500 mg metformin oral twice a day and 2 mg glimepiride oral once a day. The patient was advised to continue this treatment until their next follow-up appointment after 6 months. The reporting of this study conforms to CARE guidelines. 21 The authors obtained written informed consent from the patient for submission of this manuscript for publication.
Figure 4.
Dependence of the level of international normalized ratio (INR) on the dose of acenocoumarol in a 65-year-old male patient with a mechanical prosthetic heart valve in whom warfarin was replaced by acenocoumarol due to the inability to obtain the target INR level. The colour version of this figure is available at: http://imr.sagepub.com.
Discussion
Only VKAs are recommended for the long-term prevention of TE in the patients with mechanical prosthetic heart valves. 8 Warfarin is widely used in clinical practice. In the trials of OACs, their efficacy and safety were compared with warfarin.15–17 The INR is used to estimate the effect of VKA therapy because a balance must be achieved between preventing TE (if INR is <2.0) and bleeding (if INR > 3.5). However, a small proportion of patients have resistance to warfarin. Resistance to warfarin has been described as the inability to prolong the prothrombin time or raise the INR into the therapeutic range when the drugs are given at the recommended doses. 14 The highest recommended daily warfarin dose to maintain a therapeutic INR ranges from 15 mg to 20 mg.13,14,22
Warfarin resistance can be classified as acquired versus hereditary.13,14,20 Acquired resistance to warfarin may depend on poor patient compliance, high consumption of vitamin K, decreased absorption of warfarin, increased clearance and drug–drug interactions.13,14,20,22 The following drugs can increase the effect of warfarin: acetaminophen, alcohol, allopurinol, amiodarone, aspirin, atorvastatin, cefixime, co-trimoxazole, erythromycin, esomeprazole, pantoprazole, fenofibrate, fluvoxamine, gemfibrozil, phenylbutazone, ibuprofen, indomethacin, metronidazole, mefenamicacid, thiazides and urokinase.13,14 Drugs that can decrease the effect of warfarin include barbiturates, carbamazepine, corticosteroids, spironolactone, griseofulvin, rifampicin and oral contraceptives containing oestrogen.13,14
Hereditary warfarin resistance is due to genetic polymorphisms that result in either the faster metabolism of warfarin (a form of pharmacokinetic resistance) or a reduction in its activity (pharmacodynamic resistance). 14 Pharmacogenetic analyses can be undertaken to estimate warfarin resistance by determining the following gene polymorphisms: cytochrome P450 family 2 subfamily C member 9 (CYP2C9), cytochrome P450 family 4 subfamily F member 2 (CYP4F2) and vitamin K epoxide reductase complex subunit 1 (VKORC1).22,23 The VKORC1 gene encodes vitamin K epoxide reductase, which is a target for coumarin derivatives such as warfarin or phenprocoumon.22,23 Vitamin K epoxide reductase is an integral membrane protein that functions to convert K-epoxide to vitamin K-quinone, which activates the blood clotting factors II, VII, IX and X.22,24 In large trials, mutations in the CYP2C9 and VKORC1 genes have been shown to lead to warfarin resistance.22,25 A VKORC1 heterozygous mutation has been identified in warfarin-resistant individuals. 22 Patients with the G allele for VKORC1-1639G>A had a significantly higher number of TE complications per month during warfarin therapy. 25 Carriers of the VKORC1-1639G>A variant and wild-type CYP2C9*1/*1 may require a higher dose of warfarin to achieve adequate anticoagulation. 25 Mutations in the CYP4F2 gene, which is responsible for the synthesis of the enzyme that inactivates vitamin K, result in a reduction in enzyme production, so patients with CYP4F2 gene mutations require higher doses of warfarin. 23
Since 2010 in the US, the warfarin label has contained a dosing table with a range of therapeutic warfarin doses for CYP2C9 and/or VKORC1 variant carriers. 26 However, a pharmacogenetic analysis of patients prior to warfarin administration is not obligatory or routinely used. Several studies have been conducted to assess the cost-effectiveness of pharmacogenetic analyses of patients.27,28 A review of previous economic evaluations found that if genetic information was freely available, 75% would support pharmacogenetic-guided treatment and 25% would show cost-effectiveness. 26 Therefore, routine testing in more populations remains controversial.
Other than pharmacogenetic analyses, it is possible to analyse the plasma levels of warfarin and clotting factors II and X. A previous report proposed an algorithm for managing warfarin resistance. 14 If the INR is <2.0 on a warfarin (Coumadin) dose >15 mg/day, then initially the patient’s noncompliance and potential interference from other medications and diet should be investigated. 14 Malabsorption disorders (gastroenteritis, coeliac disease, chronic pancreatitis, short gut syndrome) should also be considered. 14 Then, factor II and factor X activity should be checked. According to the results of these investigations, there are two options: (i) if activity <40% of normal, this suggests therapeutic warfarin dose and unreliable INR, so consider checking the plasma warfarin level to confirm the diagnosis; (ii) if activity ≥40% of normal, the physician should suspect pharmacokinetic or pharmacodynamic resistance and should check the plasma warfarin level. A therapeutic level suggests pharmacodynamic resistance, whereas a subtherapeutic level suggests pharmacokinetic resistance or noncompliance. 14
There are several options for overcoming warfarin resistance. For example, increasing compliance to treatment and educating patients about food and drug–drug interactions should be considered.13,22,24 In addition, the dose of warfarin could be increased. A previous study demonstrated the safety of very high doses of warfarin, which were administered at a median dose of 32 mg/day (range, 22–55 mg/day). 22 Another approach is to replace warfarin with low-molecular-weight heparins but these require long-term daily injections.14,24 In some recommendations, other VKAs, such as acenocoumarol or phenprocoumon, are suggested as substitutes for warfarin.13,14 In the current case, the replacement of warfarin with acenocoumarol resulted in a stable INR observed over 1 year of follow-up.
In conclusion, although OACs have many positive properties, they cannot completely replace VKAs. There are clear medical indications for this group of drugs, including the prevention of TE in patients with mechanical prosthetic heart valves. It is vitally important to achieve a timely diagnosis of warfarin resistance in these patients so that effective treatment can be initiated to keep them protected against TE.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605221103959 for Warfarin resistance: possibilities to solve this problem. A case report by Halyna Mostbauer, Olga Nishkumay, Oksana Rokyta and Valeriia Vavryniuk in Journal of International Medical Research
Footnotes
Declaration of conflicting interest: The authors declare that there are no conflicts of interest.
Funding: This research received no specific grant from funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Oksana Rokyta https://orcid.org/0000-0002-7248-2817
References
- 1.Monie DD, DeLoughery EP. Pathogenesis of thrombosis: cellular and pharmacogenetic contributions. Cardiovasc Diagn Ther 2017; 7(Suppl 3): S291–S298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weir NU. An update on cardioembolic stroke. Postgrad Med J 2008; 84:133–142. [DOI] [PubMed] [Google Scholar]
- 3.Rogers MA, Levine DA, Blumberg N, et al. Triggers of hospitalization for venous thromboembolism. Circulation 2012; 125: 2092–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bang OY, Chung JW, Lee MJ, et al . Cancer-Related Stroke: An Emerging Subtype of Ischemic Stroke with Unique Pathomechanisms. J Stroke 2020; 22: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helms J, Tacquard C, Severac F, et al . High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020; 46: 1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hankey GJ, Eikelboom JW. Dabigatran etexilate: a new oral thrombin inhibitor. Circulation 2011; 123: 1436–1450. [DOI] [PubMed] [Google Scholar]
- 7.DeHaas K. The Direct Oral Anticoagulants Apixaban, Rivaroxaban, and Edoxaban. American Society for Clinical Laboratory Science 2017; 30: 2–6. [Google Scholar]
- 8.Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2022; 43: 561–632. [DOI] [PubMed] [Google Scholar]
- 9.Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 2020; 41: 543–603. [DOI] [PubMed] [Google Scholar]
- 10.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021; 42: 373–498. [DOI] [PubMed] [Google Scholar]
- 11.Deykin D. Warfarin therapy. 1. N Engl J Med 1970; 283: 691–694. [DOI] [PubMed] [Google Scholar]
- 12.Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(6 Suppl): 160S–198S. [DOI] [PubMed] [Google Scholar]
- 13.Lakshmi R, Anitha S, Anila KN, et al. Warfarin Resistance – Mechanisms And Management. IJPSR 2012; 13: 353–357. [Google Scholar]
- 14.Osinbowale O, Al Malki M, Schade A, et al. An algorithm for managing warfarin resistance. Cleve Clin J Med 2009; 76: 724–730. [DOI] [PubMed] [Google Scholar]
- 15. Clinical Trials.gov. Twice-daily Oral Direct Thrombin Inhibitor Dabigatran Etexilate in the Long Term Prevention of Recurrent Symptomatic VTE (RE-SONATE), https://clinicaltrials.gov/ct2/show/NCT00558259, (27 June 2014, accessed 18 May 2022).
- 16.Clinical Trials.gov. Efficacy and Safety Study of Apixaban for Extended Treatment of Deep Vein Thrombosis or Pulmonary Embolism, https://www.clinicaltrials.gov/ct2/show/NCT00633893, (25 November 2013, accessed 18 May 2022).
- 17.Wells PS, Prins MH, Levitan B, et al. Long-term Anticoagulation With Rivaroxaban for Preventing Recurrent VTE: A Benefit-Risk Analysis of EINSTEIN-Extension. Сhest 2016; 150: 1059–1068. [DOI] [PubMed] [Google Scholar]
- 18.Roudaut R, Serri K, Lafitte S. Thrombosis of prosthetic heart valves: diagnosis and therapeutic considerations. Heart 2007; 93: 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DrugBank online. Acenocoumarol. https://go.drugbank.com/drugs/DB01418, (accessed 18 May 2022).
- 20.Ufer M. Comparative pharmacokinetics of vitamin K antagonists: warfarin, phenprocoumon and acenocoumarol. Clin Pharmacokinet 2005; 44: 1227–1246. [DOI] [PubMed] [Google Scholar]
- 21.Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 22.Harrington DJ, Gorska R, Wheeler R, et al. Pharmacodynamic resistance to warfarin is associated with nucleotide substitutions in VKORC1. J Thromb Haemost 2008; 6: 1663–1670. [DOI] [PubMed] [Google Scholar]
- 23.Rusdiana T, Araki T, Nakamura T, et al. Responsiveness to low-dose warfarin associated with genetic variants of VKORC1, CYP2C9, CYP2C19, and CYP4F2 in an Indonesian population. Eur J Clin Pharmacol 2013; 69: 395–405. [DOI] [PubMed] [Google Scholar]
- 24.Ildizli M, Karaca M. Genetic warfarin resistance in a patient with mechanical prosthetic aortic valve. International Journal of the Cardiovascular Academy 2016; 2: 155–156. [Google Scholar]
- 25.Bazan NS, Sabry NA, Rizk A, et al. Factors affecting warfarin dose requirements and quality of anticoagulation in adult Egyptian patients: role of gene polymorphism. Ir J Med Sci 2014; 183: 161–172. [DOI] [PubMed] [Google Scholar]
- 26.Verbelen M, Weale ME, Lewis CM. Cost-effectiveness of pharmacogenetic-guided treatment: are we there yet? Pharmacogenomics J 2017; 17: 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hatz MH, Schremser K, Rogowski WH. Is individualized medicine more cost-effective? A systematic review. Pharmacoeconomics 2014. May; 32: 443–455. [DOI] [PubMed] [Google Scholar]
- 28.Stergiopoulos K, Brown DL. Genotype-guided vs clinical dosing of warfarin and its analogues: meta-analysis of randomized clinical trials. JAMA Intern Med 2014; 174: 1330–1338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605221103959 for Warfarin resistance: possibilities to solve this problem. A case report by Halyna Mostbauer, Olga Nishkumay, Oksana Rokyta and Valeriia Vavryniuk in Journal of International Medical Research