Abstract
Introduction
Escitalopram is commonly prescribed to patients with recurrent depressive disorder. Some of them do not show adequate response to treatment with escitalopram, while many of them experience adverse drug reactions.
Objective
The objective of our study was to evaluate the impact of −806C>T polymorphism of CYP2C19 (CYP2C19*17) on the concentration/dose ratio of escitalopram in patients with recurrent depressive disorder.
Material and methods
Our study enrolled 267 patients with recurrent depressive disorder (average age −40.2 ± 16.4 years). Treatment regimen included escitalopram in an average daily dose of 12.5 ± 5.0 mg per day. The efficacy and safety rates of treatment were evaluated using the international psychometric scales. For genotyping, we performed the real-time polymerase chain reaction. Therapeutic drug monitoring has been performed using HPLC-MS/MS.
Results
Our findings revealed the statistically significant results in terms of both treatment efficacy evaluation (HAMD scores at the end of the treatment course): (CC) 9.0 [7.0; 11.0], (CT) 4.0 [2.0; 6.0] and (TT) 2.0 [1.0; 4.0], p < 0.001; and safety profile (the UKU scores): (CC) 7.0 [7.0; 8.0], (CT) 3.0 [3.0; 4.0] and (TT) 3.0 [2.0; 3.0], p < 0.001. We revealed no statistically significant results for the concentration/dose ratio of escitalopram in patients with different genotypes: (CC) 5.762 [3.939; 9.076], (CT) 5.714 [3.485; 8.533] and (TT) 7.388 [4.618; 10.167], p = 0.268).
Conclusion
The CYP2C19*17 genetic variant significantly affected the efficacy and safety profiles of escitalopram in a group of 267 patients with recurrent depressive disorder but did not greatly affect its equilibrium plasma concentration.
Keywords: escitalopram, CYP2C19, pharmacogenetics, biotransformation, personalized medicine, depressive disorders, alcohol use disorder
Introduction
Depression is a common mental disorder that affects approximately 300.2 million people worldwide (~3.8% of world population).1 The COVID-19 pandemic has led to a drastic increase in depressive and anxiety disorders globally in 2020; the overall number of cases of mental disorders rose dramatically, with an additional 53.2 million and 76.2 million cases of anxiety and depressive disorders, respectively.2 Studies show that depressive disorders are the most common co-occurring psychiatric disorders among people with alcohol use disorders (AUD), and people with AUD are 2.3 times more likely to have a comorbid major depressive disorder in the previous year in comparison with those with no AUD.3
Currently, there are six classes of medications approved to treat depression, including selective serotonin reuptake inhibitors (SSRIs), serotonin and noradrenaline reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), monoamine oxidase inhibitors (MAOIs), norepinephrine and dopamine reuptake inhibitors (NDRIs) and non-competitive N-methyl-D-aspartate receptor antagonists.4 Selective serotonin reuptake inhibitors (SSRIs) are the most commonly prescribed medications for the treatment of depression.5 The six major SSRIs that are marketed in the USA today (fluoxetine, citalopram, escitalopram, paroxetine, sertraline, and fluvoxamine) are a group of structurally unrelated molecules that share a similar mechanism of action.6 Even though their primary mechanism of action is similar, each SSRI has unique pharmacokinetics, pharmacodynamics, efficacy, and side effect profile. Studies show that SSRIs’ efficacy is variable and incomplete: 60%–70% of the patients do not experience remission, while 30%–40% do not show a significant response.7 With regards to adverse effects, many are shared among all SSRIs to varying degrees, including sexual dysfunction, gastrointestinal distress, prolonged QT interval, and xerostomia.8
CYP2C19 genetic polymorphism can influence the metabolism of SSRIs, thereby affecting drug efficacy and safety.9 Thus, patients may be predisposed to poor therapeutic outcomes due to CYP2C19 genetic polymorphism that alter SSRI biotransformation.9 Escitalopram is an antidepressant belonging to the selective serotonin reuptake inhibitors (SSRIs) group. In humans, escitalopram is primarily metabolized by CYP2C19 (36%), CYP2D6 (30%) and CYP3A4 (34%).10 Allele variants in the CYP2C19 gene (categorized as no function, normal function or increased function) directly modulate the enzyme’s efficiency in escitalopram metabolism.11 The metabolizer status is determined by the two alleles a person carries, and is categorized into poor, intermediate, normal, rapid or ultrarapid metabolizer (UM) status.12 CYP2C19 poor metabolizers are carriers of the variant alleles *2 and *3, whereas the *17 variant is associated with increased gene transcription and enzymatic activity (rapid or ultrarapid metabolizer).
Most studies have focused on the effects of CYP2C19*2 and *3 genetic variants, finding that *2 (G681A) and *3 (G636A) variations reduce enzyme activity.13,14 Meanwhile, CYP2C19*17 (−806C>T) was found to be associated with increased expression of the CYP2C19 gene and enzymatic activity.15 Notably, phenotyping of CYP2C19 performed in 2015 in 971 Russian patients demonstrated a variable minor allele frequency: CYP2C19*2 − 0.140, CYP2C19*3 − 0.006, CYP2C19*17 − 0.274.16 A high frequency of CYP2C19*17 minor allele associated with modified response to medications suggests the preferred choice of this polymorphic marker for the development of pharmacogenetic approaches to prescribing escitalopram.
The objective of our study was to evaluate the effect of −806C>T polymorphism of the CYP2C19 gene (CYP2C19*17, rs12248560) on the concentration/dose ratio of escitalopram in patients diagnosed with recurrent depressive disorder and comorbid AUD.
Material and Methods
Clinical Characteristics of the Study Subjects
The study involved 267 male patients (average age — 40.20 ± 16.38 years). The inclusion criteria were as follows: the dual diagnosis of “Depressive episode (F32.x, according to ICD-10)” and “Mental and behavioral disorders due to use of alcohol. Dependence syndrome. Currently abstinent but in a protected environment (F.10.212)”; signed informed consent, and 8-weeks escitalopram monotherapy. Exclusion criteria were the presence of any other mental disorders; presence of severe somatic disorders (except alcoholic hepatitis and toxic encephalopathy); presence of any other psychotropic medications in treatment regimen; creatinine clearance values <50 mL/min, creatinine concentration in plasma >1.5 mg/dL (133 mmol/L), bodyweight less than 60 kg or greater than 100 kg, age of 75 years or more and presence of any contraindications for escitalopram use.
Therapy Efficacy and Safety Evaluation
In order to assess escitalopram efficacy, several international psychometric scales were used: Hospital Anxiety and Depression Scale (HADS)17 and Hamilton Depression Rating Scale (HAMD).18 The safety profile was evaluated using the UKU Side-Effect Rating Scale (UKU).19 Patients were examined on weeks 1, 4 and 8 of escitalopram therapy.
Genotyping
For genotyping, venous blood samples were collected into VACUETTE® (Greiner Bio-One, Austria) vacuum tubes on week 8 of escitalopram therapy. The single nucleotide polymorphism (SNP) rs12248560 (CYP2C19*17) was analyzed by real-time PCR using “Dtlite” DNA amplifiers (DNA Technology, Moscow, Russia) on a CFX96 Touch Real-Time System with CFX Manager software (Bio-Rad Laboratories Inc., Hercules, CA, USA) and the “SNP-screen” sets (Syntol, Moscow, Russia). In every set, two allele-specific hybridizations were used, which allowed simultaneous determination of both alleles of the respective SNP using two fluorescence channels.
Therapeutic Drug Monitoring
For therapeutic drug monitoring (TDM), venous blood samples were collected on week 8 of escitalopram therapy. Both plasma calibration standards (St) and quality control samples (QC) were made from a stock solution prepared by consistent dissolving of substantial amounts of venous blood in methanol with subsequent dilution to the relevant concentrations. Calibration curve was created using 5, 10, 20, 50, 100, 200, 500, 1000, 2000 ng/mL calibration standards along with 5 ng/mL (LLOQ), 15 ng/mL (Low QC), 1000 ng/mL (Medium QC), and 1500 ng/mL (High QC) quality control samples (QC). Diazepam (250 ng/mL in acetonitrile) was used as the internal standard.
Sample preparation. Sample preparation was carried out by precipitation of proteins. For this, 200 μL of plasma and 600 μL of acetonitrile containing internal standard were introduced into a test tube with a capacity of 1.5 mL. The sample was vortexed for 10 min, then centrifuged for 10 min at 14 500 rpm at 4°C. After centrifugation, the supernatant was transferred into a vial and placed in the autosampler of the HPLC. The study was carried out using an Agilent 1260 high performance liquid chromatography (Agilent Technologies, California, USA) and an Agilent 6460 tandem mass-selective detector (Agilent Technologies, California, USA) with a Jet Stream Electrospray Ionization source.
Conditions for chromatographic analysis. The stationary phase was an Agilent Poroshell 120 EC-C18 column (2.7 μm, 3.0 × 50 mm) with an InfinityLab Poroshell 120 EC-C18 guard column (2.7 μm, 3.0 × 5.0 mm) (Agilent Technologies, California, USA). The column oven temperature was 50° C. The mobile phase consisted of eluent A (10 mM aqueous solution of ammonium formate with 0.1% formic acid) and eluent B (methanol with 0.1% formic acid). The flow rate was 0.4 ml/min. Elution took place in a gradient mode (Table 1). Analysis time for each sample was 9.0 min. The volume of the injected sample was 2 μL. Under these conditions, the retention time was 4.75 min for escitalopram and 4.84 min for sertraline.
Table 1. Gradient of the Mobile Phase.
| Time of analysis, min | Eluent A volume ratio, % | Eluent B volume ratio, % |
| 0.00 | 95 | 5 |
| 0.50 | 95 | 5 |
| 1.00 | 50 | 50 |
| 1.50 | 5 | 95 |
| 3.00 | 5 | 95 |
| 3.01 | 95 | 5 |
| 5.00 | 95 | 5 |
Local Ethical Committee
The research was approved by the local ethical committee of the Russian Medical Academy of Continuous Professional Education of the Ministry of Health of the Russian Federation (The protocol No. 6 from 5/16/2017).
Statistical Analysis
Statistical analysis of the results was performed in Statsoft Statistica v. 10.0 (Dell Statistica, Tulsa, OK, USA). The normality of sample distribution was evaluated using the Shapiro-Wilk test and was taken into account for selecting parametric or non-parametric tests. The differences were considered statistically significant at p < 0.05 (power above 80 %). Two samples of continuous independent data were compared using the Mann-Whitney U-tests with further correction of the obtained p-value using the Benjamin-Hochberg test, due to the multiple comparison procedure. Several samples of continuous data were analyzed using the Kruskal-Wallis H-test. Correlation analysis was performed using the Spearman nonparametric test, taking into account the abnormal nature of sample distribution. Pearson’s Chi2 test for evaluation of the sampling distribution of the alleles (Hardy Weinberg equilibrium) has been used. Research data are presented in the form of the median and interquartile range (Me [Q1; Q3]), or in case of their normal distribution, as the arithmetic mean and standard deviation (Mean ± SD).
Study Results
In total, 125 out of 267 patients (46.8%) did not carry the CYP2C19*17 allele, whereas 117 patients (43.8%) were heterozygous for the respective variant. There were 25 carriers of the TT (homozygous) genotype (9.4%). Genotype distributions followed the Hardy–Weinberg equilibrium (Chi2 = 0.10, p-value = 0.75).
The results of data analysis performed for psychometric assessments (HADS, HAMD) and side-effect rating scale (UKU) on weeks 1, 4 and 8 in patients who received escitalopram are summarized in Table 2. Dynamics of changes in HAMD scale scores among the patients with different genotypes are shown in Figure 1. By week 1, there were no statistically significant differences across the compared groups: (CC) 22.0 [21.0; 23.0], (CT) 22.0 [21.0; 22.0] and (TT) 22.0 [21.0; 22.0], p = 0.330. By week 4, we revealed statistically significant differences in patients with different genotypes: (CC) 16.0 [14.0; 18.0], (CT) 15.0 [14.0; 16.0] and (TT) 12.0 [10.0; 15.0], p < 0.001. On week 8, the statistically significant differences were also observed: (CC) 9.0 [7.0; 11.0], (CT) 4.0 [2.0; 6.0] and (TT) 2.0 [1.0; 4.0], p < 0.001. For other psychometric scales, the same dynamics of changes in scores were obtained.
Table 2. Data from the Psychometric Assessments and Side-Effect Rating Scale in Patients who Received Escitalopram, on Weeks 1, 4 and 8 of the Study.
| Scale | CC | CT | TT | p-value | ||||
| Week 1 | ||||||||
| HADS | 37.0 [36.0; 38.0] | 37.0 [36.0; 37.0] | 37.0 [36.0; 38.0] | 0.335 | ||||
| HAMD | 22.0 [21.0; 23.0] | 22.0 [21.0; 22.0] | 22.0 [21.0; 22.0] | 0.330 | ||||
| UKU | 1.0 [1.0; 1.0] | 1.0 [1.0; 1.0] | 1.0 [1.0; 1.0] | 0.359 | ||||
| Week 4 | ||||||||
| HADS | 27.0 [23.0; 30.0] | 26.0 [23.0; 29.0] | 20.0 [17.0; 22.0] | < 0.001 | ||||
| HAMD | 16.0 [14.0; 18.0] | 15.0 [14.0; 16.0] | 12.0 [10.0; 15.0] | < 0.001 | ||||
| UKU | 3.0 [2.0; 3.0] | 2.0 [2.0; 3.0] | 2.0 [1.0; 2.0] | < 0.001 | ||||
| Week 8 | ||||||||
| HADS | 16.0 [12.0; 19.0] | 5.0 [2.0; 9.0] | 3.0 [3.0; 5.0] | < 0.001 | ||||
| HAMD | 9.0 [7.0; 11.0] | 4.0 [2.0; 6.0] | 2.0 [1.0; 4.0] | < 0.001 | ||||
| UKU | 7.0 [7.0; 8.0] | 3.0 [3.0; 4.0] | 3.0 [2.0; 3.0] | < 0.001 | ||||
p – p-value obtained in Benjamini-Hochberg multiple testing correction (based on the results of Mann-Whitney U test), Data are presented as Me and IQR.
Figure 1.
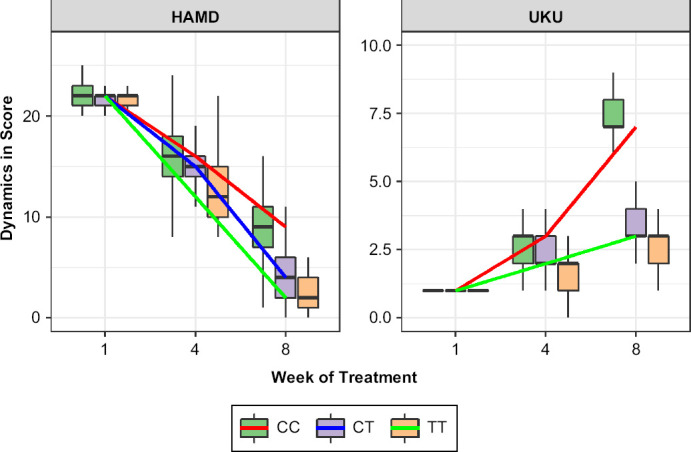
Dynamics of Changes in the HAMD and UKU Scales Scores Across Patients with Different Genotypes by the Polymorphic Marker −806C>T of CYP2C19 (rs12248560)
Data are presented as Me and IQR (colored lines connect the medians on different week of the study).
Dynamics of changes in UKU scores among the patients are presented in Figure 1. The graph shows that on week 1 the compared groups had no statistically significant differences: (CC) 1.0 [1.0; 1.0], (CT) 1.0 [1.0; 1.0] and (TT) 1.0 [1.0; 1.0], p = 0.359. By week 4, a statistically significant difference was obtained: (CC) 3.0 [2.0; 3.0], (CT) 2.0 [2.0; 3.0] and (TT) 2.0 [1.0; 2.0], p < 0.001. A statistically significant difference was also observed on week 8: (CC) 7.0 [7.0; 8.0], (CT) 3.0 [3.0; 4.0] and (TT) 3.0 [2.0; 3.0], p < 0.001. In addition, the differences were statistically significant when comparing the indicator between individual genotypes.
Table 3 summarizes the data on concentration/dose ratio (C/D) values obtained for escitalopram through pharmacokinetic studies. No statistical significance was revealed for escitalopram C/D ratio in patients with different genotypes: (CC) 5.762 [3.939; 9.076], (CT) 5.714 [3.485; 8.533] and (TT) 7.388 [4.618; 10.167], p = 0.268 (Figure 2).
Table 3. The values of Escitalopram Equilibrium Concentration in Patients with Different Genotypes by the Polymorphic Marker −806C>T of CYP2C19 (rs12248560).
| Parameter | CC | CT | TT | p-value |
| Concentration of escitalopram, ng/ml | 78.89 [38.51; 118.63] |
68.91 [39.89; 93.25] |
95.05 [44.12; 134.48] |
0.010 |
| C/D of escitalopram, u.e. | 5.76 [3.94; 9.08] |
5.71 [3.49; 8.53] |
7.39 [4.62; 10.17] |
0.268 |
p – p-value obtained in Benjamini-Hochberg multiple testing correction (based on the results of Mann-Whitney U test), Data are presented as Me and IQR.
Figure 2.
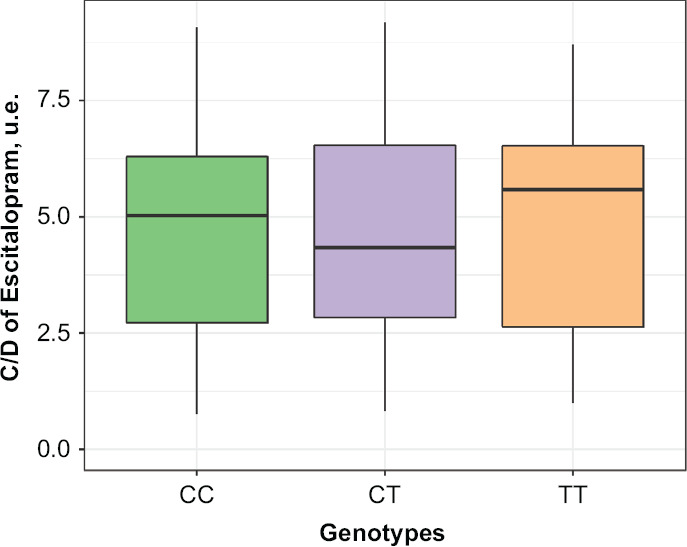
Differences in Escitalopram Concentration/Dose Ratio Values in Patients with Different Genotypes by the Polymorphic Marker –806C>T of CYP2C19 (rs12248560)
Data are presented as Me and IQR (colored lines connect the medians on different week of the study).
Discussion
This study sought to understand the association between CYP2C19*17 genetic polymorphism and escitalopram efficacy and safety in a sample of Russian patients with recurrent depressive disorder. Our findings did not reveal a statistically significant difference between the values of escitalopram equilibrium concentration in patients with different CYP2C19*17 genotypes: patients carrying the C allele have a lower level of drug equilibrium concentration than those with the T allele but no statistical significance was found (p = 0.268). As a result, carriage of the T allele does not lead to a drug accumulation in plasma and an increased risk of adverse drug reactions. Rudberg et al., showed that CYP2C19*17 homozygous phenotype may be associated with lower serum concentrations of escitalopram, which might imply an increased risk of therapeutic failure.20 In another study, despite the greater escitalopram dosage in the utrarapid group, it was demonstrated that the utrarapid metabolizers only achieved remission of depression symptoms using a pharmacological combination of escitalopram with another antidepressant.21 This result agrees with the present study where we showed that escitalopram alone does not lead to a drug accumulation in plasma and an increased risk of adverse drug reactions in different genotypes.
Statistical analysis of the data on the clinical efficacy profile of escitalopram in patients with different CYP2C19*17 genotypes revealed the statistically significant differences in the efficacy rates (p < 0.001). The analysis of escitalopram safety data also revealed the statistically significant difference (p < 0.001). The value of these parameters is statistically significantly lower for carriers of the minor allele than for carriers of the major allele. This may indicate that the carriage of this polymorphic marker may lead to a decreased risk of the adverse drug reactions of escitalopram in comparison with carriers of nonmutant alleles. It is probably due to an increased CYP2C19 activity in these patients resulting in the increased rate of escitalopram biotransformation and elimination, and decrease in drug plasma level.
Thus, based on the results obtained that the −806C>T genetic polymorphism affects the efficacy and safety rates of escitalopram in patients with recurrent depressive disorder, it is possible to assume that it is necessary to take the results of CYP2C19*17 genotyping into account before prescribing escitalopram to such patients.
This study has some limitations. The study included only one CYP2C19 gene and one polymorphic variant of this gene. Other genetic factors (CYP2C19*2 and *3) were not evaluated. The study did not assess the influence of gender as all subjects were males. Finally, the study did not assess the effect of other molecular biomarkers (serum biomarkers of liver function, micro-RNA, etc.). Regardless, this study can provide individualized assessments of escitalopram metabolism by CYP2C19*17 genetic variant; leading to more personalized medicine strategies for individuals with recurrent depressive disorder.
Conclusion
In summary, we showed that the CYP2C19*17 genetic polymorphism greatly influenced the efficacy and safety profiles of escitalopram in a group of 267 patients with recurrent depressive disorder but did not affect its equilibrium plasma concentration.
Footnotes
Funding Source
The study was supported by the grant from the President of the Russian Federation for state support of young Russian scientists - PhDs (project MK-3902.2021.3): Personalized pharmacotherapy with tranquilizers for patients with anxiety disorders comorbid with alcoholism, based on pharmacogenetic, pharmacokinetic and pharmacotranscriptomic biomarkers.
Disclosure
The authors report no conflicts of interest in this work.
Author’s Contribution
M.S. Zastrozhin – conception and design of the study, recruitment of study participants, biomaterial sampling, carrying out of the genotyping, statistical processing of the obtained data, drafting of the article. V.Yu. Skryabin, D.S. Horyaev – recruitment of study participants, biomaterial sampling, carrying out of the genotyping, statistical processing of the obtained data, drafting of the article. F. Rwere - analysis of the results of pharmacokinetics, description of methods, revision and editing of the article. A.E. Petukhov – development of a method for determining the equilibrium concentration of escitalopram, revision and editing of the article. E.P. Pankratenko, V.V. Noskov, N.V. Vinokurova – carrying out therapeutic drug monitoring, revision and editing of the article. S.A. Pozdnyakov, V.A. Ivanchenko, I.A. Zaytsev, N.V. Grishina E.A. – design of the laboratory part of the study, carrying out of the genotyping, revision and editing of the article. R.V. Vlasovskih – conception and design of the study, assistance in resolving administrative and ethical issues, revision and editing of the article. E.A. Bryun– conception and design of the study, revision and editing of the article. D.A. Sychev – the idea of the study, conception and design of the study, revision and editing of the article. All authors made a substantial contribution to the research and preparation of the research paper, and were involved in drafting of the article or its critical revision for important intellectual content and gave final approval of the revision to be published.
Funding
This work was financially supported by the project 16-15-00227 entitled “Fundamental research and exploratory research in priority areas of research” of the Russian Science Foundation.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Contributor Information
MS Zastrozhin, Zastrozhin, PhD, M.D., Postdoctoral Fellow, 1University of California, San Francisco, CA, USA; Head of laboratory of genetics and fundamental studies, Moscow Research and Practical Centre on Addictions of the Moscow Department of Healthcare, Moscow, Russia; Associate professor of addiction psychiatry department, Russian Medical Academy of Continuous Professional Education of the Ministry of Health of the Russian Federation, Moscow, Russian Federation..
VYu Skryabin, Skryabin, PhD, M.D., head of clinical department, Moscow Research and Practical Centre on Addictions of the Moscow Department of Healthcare, Moscow, Russia; Associate professor of addiction psychiatry department, Russian Medical Academy of Continuous Professional Education of the Ministry of Health of the Russian Federation, Moscow, Russian Federation..
F Rwere, Rwere, PhD, instructor of the department of Anesthesiology, perioperative and pain medicine, Stanford University School of Medicine, Stanford, CA, USA..
AE Petukhov, Petukhov, PhD, M.D., clinical laboratory diagnostician of the analytical toxicology lab of the Reference center for psychoactive substances use monitoring, Moscow Research and Practical Centre on Addictions of the Moscow Department of Healthcare, Moscow, Russia; associate professor of pharmaceutical and toxicological chemistry, I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russian Federation..
EP Pankratenko, Pankratenko, paramedic-laboratory assistant of the analytical toxicology lab of the Reference center for psychoactive substances use monitoring, Moscow Research and Practical Centre on Addictions of the Moscow Department of Healthcare, Moscow, Russia..
SA Pozdniakov, Pozdniakov, researcher of the laboratory of genetics and fundamental studies, Moscow Research and Practical Centre on Addictions of the Moscow Department of Healthcare, Moscow, Russia..
VA Ivanchenko, Ivanchenko, laboratory assistant, Moscow Research and Practical Centre on Addictions of the Moscow Department of Healthcare, Moscow, Russia..
VV Noskov, Noskov, laboratory assistant, Moscow Research and Practical Centre on Addictions of the Moscow Department of Healthcare, Moscow, Russia..
IA Zaytsev, Zaytsev, laboratory assistant, Moscow Research and Practical Centre on Addictions of the Moscow Department of Healthcare, Moscow, Russia..
NV Vinokurova, Vinokurova, laboratory assistant, Moscow Research and Practical Centre on Addictions of the Moscow Department of Healthcare, Moscow, Russia..
DS Horyaev, Horyaev, M.D., psychiatrist, addiction psychiatrist of clinical department, Moscow Research and Practical Centre on Addictions of the Moscow Department of Healthcare, Moscow, Russia..
RV Vlasovskih, Vlasovskih, PhD, M.D., vice-director, Moscow Research and Practical Centre on Addictions of the Moscow Department of Healthcare, Moscow, Russia..
EA Bryun, Bryun, PhD, M.D., professor, president, Moscow Research and Practical Centre on Addictions of the Moscow Department of Healthcare, Moscow, Russia; head of addiction psychiatry department, Russian Medical Academy of Continuous Professional Education of the Ministry of Health of the Russian Federation, Moscow, Russian Federation..
DA Sychev, Sychev, corresponding member of the Academy of Sciences of Russia, M.D., PhD, professor, rector, head of clinical pharmacology and therapy department, Russian Medical Academy of Continuous Professional Education of the Ministry of Health of the Russian Federation, Moscow, Russian Federation..
References
- 1.Liu Q, He H, Yang J, Feng X, Zhao F, Lyu J. Changes in the global burden of depression from 1990 to 2017: Findings from the Global Burden of Disease study. J Psychiatr Res . 2020;126:134–140. doi: 10.1016/j.jpsychires.2019.08.002. doi: [DOI] [PubMed] [Google Scholar]
- 2.COVID-19 Mental Disorders Collaborators. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet . 2021;398(10312):1700–1712. doi: 10.1016/S0140-6736(21)02143-7. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McHugh RK, Weiss RD. Alcohol Use Disorder and Depressive Disorders. Alcohol Res . 2019;40(1) doi: 10.35946/arcr.v40.1.01. arcr.v40.1.01. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheffler ZM, Abdijadid S. Antidepressants. 2021 In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Jan. 2021 Sep 9. [PubMed] [Google Scholar]
- 5.Darab MG, Hedayati A, Khorasani E, Bayati M, Keshavarz K. Selective serotonin reuptake inhibitors in major depression disorder treatment: an umbrella review on systematic reviews. Int J Psychiatry Clin Pract . 2020;24(4):357–370. doi: 10.1080/13651501.2020.1782433. doi: [DOI] [PubMed] [Google Scholar]
- 6.Edinoff AN, Akuly HA, Hanna TA, Ochoa CO, Patti SJ, Ghaffar YA, Kaye AD, Viswanath O, Urits I, Boyer AG, Cornett EM, Kaye AM. Selective Serotonin Reuptake Inhibitors and Adverse Effects: A Narrative Review. Neurol Int . 2021;13(3):387–401. doi: 10.3390/neurolint13030038. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan Z, Chen Z, Xue M, Zhang J, Leng L. Application of antidepressants in depression: A systematic review and meta-analysis. J Clin Neurosci . 2020;80:169–181. doi: 10.1016/j.jocn.2020.08.013. doi: [DOI] [PubMed] [Google Scholar]
- 8.David DJ, Gourion D. Antidépresseurs et tolérance: Déterminants et prise en charge des principaux effets indésirables [Antidepressant and tolerance: Determinants and management of major side effects] Encephale . 2016;42(6):553–561. doi: 10.1016/j.encep.2016.05.006. doi: [DOI] [PubMed] [Google Scholar]
- 9.Hicks JK, Bishop JR, Sangkuhl K, Müller DJ, Ji Y, Leckband SG, Leeder JS, Graham RL, Chiulli DL, LLerena A, Skaar TC, Scott SA, Stingl JC, Klein TE, Caudle KE, Gaedigk A, Clinical Pharmacogenetics Implementation Consortium Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 Genotypes and Dosing of Selective Serotonin Reuptake Inhibitors. Clin Pharmacol Ther . 2015;98(2):127–34. doi: 10.1002/cpt.147. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sangkuhl K, Klein TE, Altman RB. PharmGKB summary: citalopram pharmacokinetics pathway. Pharmacogenet Genomics . 2011;21(11):769–72. doi: 10.1097/FPC.0b013e328346063f. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aldrich SL, Poweleit EA, Prows CA, Martin LJ, Strawn JR, Ramsey LB. Influence of CYP2C19 Metabolizer Status on Escitalopram/Citalopram Tolerability and Response in Youth With Anxiety and Depressive Disorders. Front Pharmacol . 2019;10:99. doi: 10.3389/fphar.2019.00099. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caudle KE, Dunnenberger HM, Freimuth RR, Peterson JF, Burlison JD, Whirl-Carrillo M, Scott SA, Rehm HL, Williams MS, Klein TE, Relling MV, Hoffman JM. Standardizing terms for clinical pharmacogenetic test results: consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC) Genet Med . 2017;19(2):215–223. doi: 10.1038/gim.2016.87. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeong YH, Kim IS, Kwak CH, Hwang JY. AS-192: the CYP2C19*2 and CYP2C19*3 polymorphisms are associated with high posttreatment platelet reactivity in patients with acute myocardial infarction. Am J Cardiol . 2009;103:82B. doi: 10.1016/j.amjcard.2009.01.380. doi: [DOI] [Google Scholar]
- 14.Chi NF, Wang SJ. CYP2C19 loss-of-function alleles: A common but overlooked problem associated with clopidogrel resistance. Chin Med Assoc . 2019;82(10):746–747. doi: 10.1097/JCMA.0000000000000168. doi: [DOI] [PubMed] [Google Scholar]
- 15.Sim SC, Risinger C, Dahl ML, Aklillu E, Christensen M, Bertilsson L, Ingelman-Sundberg M. A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clin Pharmacol Ther . 2006;79:103–113. doi: 10.1016/j.clpt.2005.10.002. doi: [DOI] [PubMed] [Google Scholar]
- 16.Sychev DA, Denisenko NP, Sizova ZM, Grachev AV, Velikolug KA. The frequency of CYP2C19 genetic polymorphisms in Russian patients with peptic ulcers treated with proton pump inhibitors. Pharmgenomics Pers Med . 2015;8:111–4. doi: 10.2147/PGPM.S78986. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand . 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry . 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl . 1987;334:1–100. doi: 10.1111/j.1600-0447.1987.tb10566.x. [DOI] [PubMed] [Google Scholar]
- 20.Rudberg I, Mohebi B, Hermann M, Refsum H, Molden E. Impact of the ultrarapid CYP2C19*17 allele on serum concentration of escitalopram in psychiatric patients. Clin Pharmacol Ther . 2008;83(2):322–327. doi: 10.1038/sj.clpt.6100291. doi: Epub 2007 Jul 11. PMID: 17625515. [DOI] [PubMed] [Google Scholar]
- 21.Bernini de Brito R, Ghedini PC. CYP2C19 polymorphisms and outcomes of Escitalopram treatment in Brazilians with major depression. Heliyon . 2020;6(5):e04015. doi: 10.1016/j.heliyon.2020.e04015. doi: PMID: 32509985; PMCID: PMC7264488. [DOI] [PMC free article] [PubMed] [Google Scholar]


