Abstract
Objectives
The mechanism of many neuropsychiatric disorders remains unknown, but the ineffectiveness of the sodium channel blocker lidocaine has been suggested to be a biomarker for Attention Deficit Hyperactivity Disorder (ADHD) and a severe form of Premenstrual Syndrome (PMS) that is considered psychiatric. We conducted single-arm double-blind clinical trials to test whether lidocaine ineffectiveness can be used as a biomarker to identify people with these conditions and provide a clue as to the molecular mechanism and potential psychopharmacological intervention.
Experimental Design
We developed a noninvasive taste test for lidocaine ineffectiveness, validated by comparing lidocaine injections to pain testing in 12 subjects, and assessed it in individuals with ADHD and PMS.
Principal Observations
Lidocaine ineffectiveness had a strong association in women with ADHD + PMS in a sample of 53 subjects and controls (p < 0.001).
Conclusions
These results suggest the possibility of the biological understanding of the combination of ADHD and PMS that is characteristic of the psychiatric disorder Premenstrual Dysphoric Disorder (PMDD). These results and comparison to family pedigrees of a neuromuscular channelopathy with overlapping symptoms suggest the possibility that the clinical phenotype in PMDD is produced by sensory overstimulation, and amenable to molecular understanding and treatment.
Keywords: attention deficit hyperactivity disorder (ADHD), premenstrual dysphoric disorder (PMDD), lidocaine, hypokalemic periodic paralysis, channelopathies
Introduction
We have described families in which affected individuals have ineffectiveness of the local anesthetic lidocaine and Attention Deficit Hyperactivity Disorder (ADHD) and severe Premenstrual Syndrome (PMS; Segal, Jurkat-Rott and Lehmann-Horn, unpublished).1,2 Here we systematize such observations and report on single-arm double-blind clinical trials using a validated questionnaire for ADHD and PMS-related questions. We also discuss the relationship to Premenstrual Dysphoric Disorder (PMDD), a possible molecular mechanism, and novel treatments.
Materials and Methods
Taste Test for Lidocaine Ineffectiveness
To assess lidocaine ineffectiveness we developed a non-invasive test based on whether subjects can taste after the tongue is treated with topical lidocaine. The rationale was to have a test that avoids injecting lidocaine and avoids a painful assessment. In the lidocaine taste test, the subject had tastant solutions spread on the front central half of the top of the tongue using a swab. The solutions were sodium chloride (25 g added to 75 ml water) or sucrose (60 grams sucrose added to 40 ml water). Before applying lidocaine, the type of taste was disclosed, and the subject was asked to rate the intensity on a 10-point scale. Lidocaine gel (0.75 g premeasured dose of Septodont 5% oral gel NDC 0362-0221-10) was then spread over the front half of the top and edges of the tongue using a swab to attempt to achieve surface anesthesia. After 2 minutes, the subject was challenged in a double-blinded manner with application in random sequence of 4 solutions: 2 of sodium chloride and 2 of sucrose. Subjects responded by pointing to a card with depictions of tastes and by pointing to taste intensity scores. Lidocaine effectiveness was quantitated as the reduction or elimination of taste by lidocaine compared to taste scores reported before lidocaine. For each of the 4 before/after pairs, the change in taste intensity was calculated, and a weighted average score was computed. Incorrectly identified tastes were excluded. Scores with a reduction in taste ≥50% were considered to be lidocaine effective.
Comparison of Taste Test to Lidocaine Injection
The taste test was assessed in 12 subjects by comparing its results on 4 separate days (2 using lidocaine, 2 using polyethylene glycol (PEG) placebo) to those of injections of lidocaine in the maxillary mucobuccal fold (1 ml of 1% lidocaine without epinephrine) on a fifth day. The effectiveness of the injected lidocaine was assessed 10 minutes after injection using a dental explorer probe pressed against the soft tissue and measuring the area with full anesthesia. Results were quantitated using the Wong-Baker FACES Pain Rating Scale.3 If the subject reported no numbness, a second dose was administered. Personnel assessing the injections and taste tests were blinded to the result of the other test type.
Recruitment and Human Subjects Approval
For the injection versus taste test validation, employees at Jacobi Medical Center (Bronx NY) ages 19–59 (mean 37, SD 7.6) were recruited with no eligibility conditions about ADHD or lidocaine response history.
For assessment of lidocaine ineffectiveness in adults, subjects ages 18 to 49 (mean 31, SD 7.5) were recruited at Boston Clinical Trials (Boston MA) and Jacobi Medical Center according to self-reporting of an ADHD diagnosis or no history of an ADHD diagnosis, in equal numbers.
For assessment of lidocaine ineffectiveness in treatment-resistant ADHD subjects ages 7 to 49 (mean 16, SD 7.3) were recruited at the behavioral-neurology focused NeurAbilities Healthcare (Voorhees, NJ).
Consent to participate was obtained from all study participants or their parents/guardians.
The ADHD status of adult subjects was then checked using the ADHD-RS questionnaire with adult prompts, and for children the ADHD-RS-5 with child prompts (4, authorized under a license from NYU-MGH). A score of ≥18 was considered as ADHD.
For women, PMS was also assessed using a custom scale of 9 questions with scores 0–3 to score symptoms during the relevant period during the month, (agitation, headaches including migraine, cramps in hands and legs, cramps in stomach, backache, edema, overwhelmed, trouble sleeping, difficulty concentrating). A score of ≥12 was considered as PMS.
Exclusion criteria were inability to taste salt or sugar before the application of lidocaine, use of medications known to affect serum potassium, major neurological diagnoses (epilepsy, IQ < 80, severe head trauma, severe autism), major psychiatric disorders (major depression, schizophrenia, bipolar disorder, general anxiety disorder) and active mouth sores.
The clinical trial was stopped prior to completion of recruitment due to the coronavirus pandemic, but the number of enrolled subjects was close to goals and the results were analyzed.
Statistical Analysis
Fisher’s exact test was used for comparing proportions and the kappa coefficient for measuring chance-corrected level of agreement in assessing lidocaine ineffectiveness between taste test and injection. Logistic regression was used to determine the odds of lidocaine ineffectiveness for individuals with PMS, those with ADHD, and those having ADHS and PMS versus controls with 95% confidence intervals around the estimated odds ratio (OR). Statistical analysis was performed using Stata software release 16.1 (StataCorp LLC, College Station, Texas) with two-tailed p < 0.05 considered statistically significant.
Results
The lidocaine taste anesthesia test was compared to lidocaine injections in 12 subjects as described in Methods. Eight had lidocaine effectiveness using both techniques, and 2 had lidocaine ineffectiveness using both techniques, one showed ineffectiveness only by taste test and one only by injection. The chance-corrected kappa coefficient was 0.56 (standard error = 0.27; p = 0.046). Because of the non-invasiveness of the taste-based testing, this was used in the studies described below to look for neurobehavioral correlates of lidocaine ineffectiveness.
We studied a group of 90 adults (53 female, 37 male; Table 1) to assess the correlation between lidocaine ineffectiveness and the symptoms of PMS and ADHD observed in preliminary studies.1,2 The subjects were recruited as either having ADHD or being controls without ADHD. PMS was also assessed in the females.
Table 1. Count of Subjects at the Sites that Included Controls at the Same Site, by Group.
| Male | Female | Total | |||||
| ADHD | No | Yes | No | No | Yes | Yes | |
| PMS | – | – | No | Yes | No | Yes | |
| Lidocaine ineffective | 12 | 5 | 6 | 0 | 0 | 8 | 31 |
| Lidocaine works | 11 | 9 | 16 | 5 | 15 | 3 | 59 |
| TOTAL | 23 | 14 | 22 | 5 | 15 | 11 | 90 |
| Avg. age, years | 32.7 | 26.5 | 32.8 | 32.6 | 27.1 | 29.8 | 30.4 |
| Age std. deviation | 7.6 | 7.1 | 6.4 | 10.0 | 6.1 | 8.1 | 7.5 |
The correlation of lidocaine ineffectiveness with PMS was significant (p = 0.017; Table 2). This correlation was even stronger in women with both PMS and ADHD (p < 0.001; Table 3), the combination of symptoms found in our preliminary studies.1,2 All subjects with PMS and lidocaine ineffectiveness also had ADHD, and all female subjects with ADHD and lidocaine ineffectiveness also had PMS (Table 1).
Table 2. Lidocaine Ineffectiveness (Assessed by Taste Test) in PMS.
| PMS | Control | Total | |
| Lidocaine ineffective | 8 | 6 | 14 |
| Lidocaine works | 8 | 31 | 39 |
| TOTAL | 16 | 37 | 53 |
| % Lidocaine Ineffective | 50% | 16% | 26% |
Odds Ratio 5.17 (95% CI 1.40–19.21; p = 0.017).
Table 3. Lidocaine Ineffectiveness (Assessed by Taste Test) in PMS with ADHD.
| PMS with ADHD | Rest of female subjects | Total | |
| Lidocaine ineffective | 8 | 6 | 14 |
| Lidocaine works | 3 | 36 | 39 |
| TOTAL | 11 | 42 | 53 |
| % Lidocaine Ineffective | 73% | 14% | 26% |
Odds Ratio 16.0 (95% CI 3.28–77.95; p = 0.001).
We characterized sensory overstimulation in these individuals by sensory modality and effects of overstimulation such as cramping (Figure 1). Women having the combination of both ADHD and PMS reported a higher frequency of sensory overstimulation on various modalities than women with only ADHD, PMS or neither.
Figure 1.
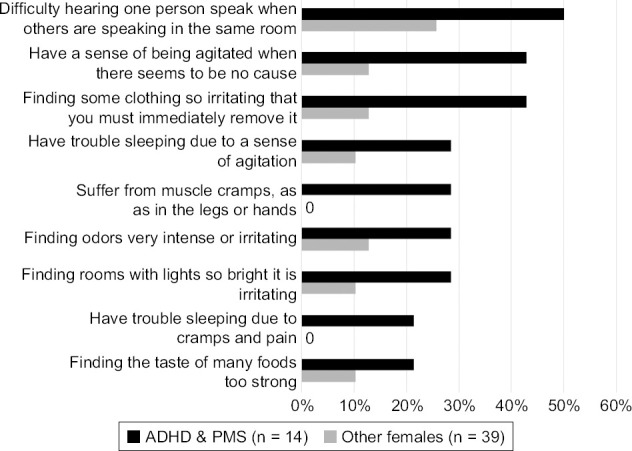
Sensory Overstimulation Reporting by Females with Both ADHD and PMS (n = 11) vs. Other Females (n = 42)
Electrolyte levels in these subjects were not assessed. However, because of observations suggesting that potassium treatment might be effective for such disorders1,5 we did queries about potassium levels in PMDD (which has features of both ADHD and PMS) versus normals in the National Institute of Health “All of Us” database.6 No differences in these random serum potassium levels were found.
There was no significant correlation between lidocaine ineffectiveness and ADHD alone in this adult sample in which all subjects were either untreated for ADHD or well treated by ADHD medications (Table 4). However, we also studied a primarily pediatric sample (mean age 16) with difficult-to-treat ADHD at NeurAbilities Healthcare. Subjects were recruited using the criteria of a prior diagnosis of ADHD and having poor response to standard treatment, with the intention of conducting follow-on studies of potassium treatments that appeared to be effective in preliminary PMS and ADHD studies.1,2,5 Of these subjects, 27 of 28 (96%) showed ineffectiveness of lidocaine using the taste anesthesia test. We had planned to assess lidocaine ineffectiveness in control subjects in the pediatric age group at other sites because the patient population at NeurAbilities Healthcare consisted of individuals primarily with neurobehavioral/neuropsychiatric disorders. However, due to an Institutional Review Board (IRB) ruling at the other sites, subjects at those sites were restricted initially to adults, so we can only compare this primarily-pediatric subset with difficult to treat ADHD to low rates of lidocaine ineffectiveness by injection in the literature (12%)7 and adults in our study by taste test without ADHD (18 of 49; 37%; Table 1).
Table 4. Lidocaine Ineffectiveness (Assessed by Taste Test) in ADHD for Males and Females.
| ADHD | Control | Total | |
| Lidocaine ineffective | 13 | 18 | 31 |
| Lidocaine works | 27 | 32 | 59 |
| TOTAL | 40 | 50 | 90 |
| % Lidocaine Ineffective | 33% | 36% | 34% |
Odds Ratio 0.86 with p = 0.8.
Discussion
These studies demonstrate that women with ADHD + PMS were far more likely to have lidocaine ineffectiveness than other women (Table 3; p < 0.001), validating previous anecdotal reports.1,2
The combination of ADHD + PMS is biologically interesting because, as shown in Table 5, it maps onto the clinical entity of PMDD. However, much work remains to be done to establish the robustness, breadth, and significance of the association of lidocaine ineffectiveness with PMDD. Since the assessment was designed to take only a single visit, no formal PMDD assessment was done, which would have required a 2-month collection of contemporaneous data.8
Table 5. Findings in PMDD versus PMS + ADHD.
| PMDD (DRSP) | PMS (PMTS-OR) | ADHD (ADHD-RS) |
| Less interest in activities | Decreased interest in usual activities | |
| Interfered with hobbies or social activities | ||
| Depressed, hopeless, worthless | Depressed mood | |
| More sensitive to rejection | ||
| Mood swings | Affective Lability (Emotional, mood swings) | |
| Angry/irritable/conflicts | Irritability/Hostility | |
| Fatigued | Marked Lack of Energy | |
| Increased appetite | Eating habits | |
| Cravings for specific foods | ||
| Overwhelmed | Overwhelmed | |
| Slept more | Sleeping Habits | |
| Trouble getting or staying asleep | ||
| Anxious | Anxiety/Tension | |
| Headache | Physical symptoms | |
| Joint or muscle pain | ||
| Breast tenderness | ||
| Edema | ||
| Difficulty concentrating | Concentration difficulties | Sustaining attention, easily distractible, Forgetful in daily activities, loses items, carelessness, can’t organize |
| Out of control | Runs/climbs excessively, can’t play/work quietly, hyperactive: squirms & fidgets, can’t stay seated, on the go, “driven by a motor | |
| Interfered with relationships | Intrudes/interrupts others, can’t wait for turn, blurts out answers, talks excessively | |
| Loss of productivity | No follow through, avoids sustained mental effort |
PMDD is considered to be a psychiatric condition because of behavioral symptoms that go far beyond the PMS symptoms. In PMDD, the mood disturbance is intense enough to be very problematic in social and work situations: there are 3.8 years of cumulative disability, and a rate of suicidal thoughts and attempts twice that of the general population.9–11
PMDD was added as an independent diagnostic entity in the DSM 5 in 2013.12 This decision was controversial, with some people contending that PMDD is merely a disparaging label used to stigmatize women.13 Finding a biomarker of ineffectiveness of lidocaine is PMDD would provide support for the decision to include PMDD in the DSM.
The pathophysiology of PMDD remains poorly understood. There are strong indications that it is heritable14 and there is a consensus that it is not the result of hormonal abnormalities.15,16 The results presented here provide preliminary evidence for a biological model for understanding the pathophysiology of PMDD. The large families we had begun to characterize before the death of our collaborator Frank Lehmann-Horn are particularly illuminating for understand a molecular mechanism. In these families, there is an autosomal dominant pattern of ADHD and lidocaine ineffectiveness in affected individuals, PMS in affected females, and the affected individuals also have hypokalemic periodic paralysis (HypoPP), a combination suggestive of a form of PMDD with severe muscular symptoms. In most other families with HypoPP, the disease results from a variant in the sodium channel gene SCN4A or in the calcium channel gene CACNA1A.17 However, in the large families we studied, neither of those genes had variants, suggesting that there is a third gene for HypoPP. Since the mechanism of action of lidocaine is blocking of sodium channels, a variant in such a third gene may also result in a channelopathy and be important for understanding the molecular mechanism of both HypoPP and PMDD.
Therapeutically, patients with HypoPP18 and those with PMS and ADHD described here and in small studies1,5 can be treated by raising serum potassium. Accordingly, there is a prospect that these common conditions could be treated in ways that avoid the problematic effects of other treatments for ADHD and PMDD.19–22
It is difficult to draw any firm conclusion about the results for ADHD alone. The data presented here are consistent with the conclusion that treatment-resistant ADHD is highly associated with lidocaine ineffectiveness. The lack of association of generic ADHD with lidocaine ineffectiveness could indicate an important stratification among different groups of patients meeting ADHD criteria. However, without controls done at the same site one can’t exclude the possibility that there was some systematic difference among lidocaine testing procedures at different sites. The results here also suggest the importance of testing the hypothesis that girls with treatment-resistant ADHD will become women with PMDD.
Such testing for lidocaine ineffectiveness could also result in coordination on diagnosis of these neuropsychiatric conditions with dentists, who are interested in whether patients have lidocaine ineffectiveness in order to switch to using a local anesthesia such as articaine that is effective in people with lidocaine ineffectiveness.1 Such information would also be important for surgeons, who can use bupivacaine effectively, as reported to us by individuals with lidocaine ineffectiveness.
Women with PMDD are often dismissed as having a supra-tentorial problem, subjecting them to stigma and discrimination.20,23 It would be an advance to demonstrate a molecular mechanism and simple treatment. It would be particularly illuminating to show a similar phenomenon to that in ADHD, which primarily affects males, thereby demonstrating a similar phenomenon in both females and males, with different manifestations but similar molecular mechanisms.
Conclusion
Much remains to be done to further define PMDD and ADHD and find the underlying genetic variants, but the evidence presented here suggests a testable molecular hypothesis for the pathogenesis of these conditions, and the possibility of treatments based on the molecular mechanism.
Acknowledgements
Funding
Research reported in this publication was supported by the National Institute of Child Health and Development (NICHD) of the National Institutes of Health under award number 1R43HD094628 to Michael Segal at PhenoSolve. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Ethics Approval
The taste tests of adults at Boston Clinical Trials and Jacobi Medical Center were approved by Advarra Pro00039879 protocol 2019-01A. The taste-based test validation relative to lidocaine injections and taste tests of adults at Jacobi Medical Center were approved by the Albert Einstein College of Medicine IRB #054329. The taste tests of children with hard-to-treat ADHD at NeurAbilities Healthcare were approved by the Advarra IRB, Pro00036315, protocol 2017-01A.
Competing Interests
Authors LKF and MMS are employees of PhenoSolve, which does not have any intellectual property relating to the work described here.
ClinicalTrials.gov registration numbers NCT03680885 (Test validation), NCT03676725 (Lidocaine ineffectiveness in adults), and NCT04167189 (Hard-to-treat ADHD).
Contributor Information
Mark Mintz, Mintz M, MD, NeurAbilities Healthcare, NJ..
Victor Badner, Badner, DMD MPH, Department of Dentistry, Jacobi Medical Center, NY..
Lynn K Feldman, Feldman, PhenoSolve Inc., MA..
Pnina Mintz, Mintz P, PhD., NeurAbilities Healthcare, NJ..
Mana Saraghi, Saraghi, DMD, Department of Dentistry, Jacobi Medical Center, NY..
Jonathan Diaz, Diaz, Department of Dentistry, Jacobi Medical Center, NY..
Irina Mezhebovsky, Mezhebovsky, MD, Boston Clinical Trials, MA..
Irene Axelrod, Axelrod, RN, Boston Clinical Trials, MA..
Joseph Gleeson, Gleeson, MD, Departments of Neurosciences and Pediatrics, University of California San Diego, CA..
Chang Liu, Liu, Departments of Neurosciences and Pediatrics, University of California San Diego, CA..
Cathy Smith, Smith, NeurAbilities Healthcare, NJ..
Helen Chow, Chow, Boston Clinical Trials, MA..
David Zurakowski, Zurakowski, PhD, Departments of Anesthesiology and Surgery, Boston Childrens Hospital, Harvard Medical School, Boston, MA..
Michael M Segal, Segal, MD PhD, PhenoSolve Inc., MA..
References
- 1.Segal MM, Rogers GF, Needleman HL, Chapman CA. Hypokalemic Sensory Overstimulation. J Child Neurol . 2007;22:1408–1410. doi: 10.1177/0883073807307095. [DOI] [PubMed] [Google Scholar]
- 2.Segal MM. We Cannot Say Whether Attention Deficit Hyperactivity Disorder Exists, but We Can Find Its Molecular Mechanisms. Pediatric Neurology . 2014;51:15–16. doi: 10.1016/j.pediatrneurol.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Wong DL, Baker CM. Pain in Children: Comparison of Assessment Scales. Pediatric Nursing . 1998;14(1):9–17. [PubMed] [Google Scholar]
- 4.Zhang S, Faries DE, Vowles M, Michelson D. ADHD Rating Scale IV: Psychometric Properties from a Multinational Study as a Clinician-Administered Instrument. Int J Methods Psychiatr Res . 2005;14:186–201. doi: 10.1002/mpr.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takacs BE. Potassium: A New Treatment for Premenstrual Syndrome. J Orthomol Med . 1998 4th Quarter13 [Google Scholar]
- 6.NIH. [14 March 2022];2022 All of Us. https://allofus.nih.gov/ Accessed. [Google Scholar]
- 7.Nakai Y, Milgrom P, Mancl L et al. Effectiveness of Local Anesthesia in Pediatric Dental Practice. J Am Dent Assoc . 2000;131:1699–1705. doi: 10.14219/jada.archive.2000.0115. [DOI] [PubMed] [Google Scholar]
- 8.Endicott J, Nee J, Harrison W. Daily Record of Severity of Problems (DRSP): Reliability and Validity. Arch Womens Ment Health . 2006;9:41–49. doi: 10.1007/s00737-005-0103-y. [DOI] [PubMed] [Google Scholar]
- 9.Halbreich U, Borenstein J, Pearlstein T, Kahn LS. The Prevalence, Impairment, Impact, and Burden of Premenstrual Dysphoric Disorder (PMS/PMDD) Psychoneuroendocrinology . 2003;28(Suppl 3):1–23. doi: 10.1016/s0306-4530(03)00098-2. [DOI] [PubMed] [Google Scholar]
- 10.Pilver CE, Libby DJ, Hoff RA. Premenstrual Dysphoric Disorder as a Correlate of Suicidal Ideation, Plans, and Attempts Among a Nationally Representative Sample. Soc Psychiatry Psychiatr Epidemiol . 2013;48:437–446. doi: 10.1007/s00127-012-0548-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofmeister S, Bodden S. Premenstrual Syndrome and Premenstrual Dysphoric Disorder. Am Fam Physician . 2016;94:236–240. [PubMed] [Google Scholar]
- 12.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 2013 Washington DC. [Google Scholar]
- 13.Chrisler JC, Caplan P. The Strange Case of Dr. Jekyll and Ms. Hyde: How PMS became a Cultural Phenomenon and a Psychiatric Disorder. Annu Rev Sex Res . 2002;13:274–306. [PubMed] [Google Scholar]
- 14.Kendler KS, Karkowski LM, Corey LA, Neale MC. Longitudinal Population-based Twin Study of Retrospectively Reported Premenstrual Symptoms and Lifetime Major Depression. Am J Psychiatry . 1998;155:1234–1240. doi: 10.1176/ajp.155.9.1234. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt PJ, Nieman LK, Danaceau MA et al. Differential Behavioral Effects of Gonadal Steroids in Women with and in Those without Premenstrual Syndrome. N Engl J Med . 1998;338:209–216. doi: 10.1056/NEJM199801223380401. [DOI] [PubMed] [Google Scholar]
- 16.Wyatt KM, Dimmock PW, Ismail KM et al. The Effectiveness of GnRHa With and Without ‘add-back’ Therapy in Treating Premenstrual Syndrome: A Meta Analysis. BJOG . 2004;111:585–593. doi: 10.1111/j.1471-0528.2004.00135.x. [DOI] [PubMed] [Google Scholar]
- 17.Weber F, Lehmann-Horn F. Hypokalemic Periodic Paralysis. [14 March 2022];2018 GeneReviews. https://www.ncbi.nlm.nih.gov/books/NBK1338/ Accessed. [PubMed] [Google Scholar]
- 18.Levitt JO. Practical Aspects in the Management of Hypokalemic Periodic Paralysis. J Translational Medicine . 2008;6:18. doi: 10.1186/1479-5876-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennedy SH, Eisfeld BS, Dickens SE et al. Antidepressant-induced Sexual Dysfunction During Treatment with Moclobemide, Paroxetine, Sertraline, and Venlafaxine. J Clin Psychiatry . 2000;1:276–281. doi: 10.4088/jcp.v61n0406. [DOI] [PubMed] [Google Scholar]
- 20.Pearlstein T, Steiner M. Premenstrual Dysphoric Disorder: Burden of Illness and Treatment Update. J Psychiatry Neurosci . 2008;33:291–301. [PMC free article] [PubMed] [Google Scholar]
- 21.Graf WD, Miller G, Nagel SK. Addressing the Problem of ADHD Medication as Neuroenhancements. Expert Rev Neurother . 2014;14:569–581. doi: 10.1586/14737175.2014.908707. [DOI] [PubMed] [Google Scholar]
- 22.Clemow DB. Misuse of Methylphenidate. Curr Top Behav Neurosci . 2017;34:99–124. doi: 10.1007/7854_2015_426. [DOI] [PubMed] [Google Scholar]
- 23.Hartlage SA, Breaux CA, Yonkers KA. Addressing Concerns About the Inclusion of Premenstrual Dysphoric Disorder in DSM-5. J Clin Psychiatry . 2014;75:70–76. doi: 10.4088/JCP.13cs08368. [DOI] [PubMed] [Google Scholar]
- 24.Steiner M, Peer M, Macdougall M, Haskett R. The Premenstrual Tension Syndrome Rating Scales: An Updated Version. J Affect Disord . 2011;135:82–88. doi: 10.1016/j.jad.2011.06.058. [DOI] [PubMed] [Google Scholar]


