Abstract
A diverse array of ixodid and argasid ticks infest dogs and cats in North America, resulting in skin lesions, blood loss, and disease. The ticks most commonly found on pets in this region are hard ticks of the genera Amblyomma, Dermacentor, Ixodes, and Rhipicephalus, as well as the more recently established Haemaphysalis longicornis. Soft tick genera, especially Otobius and Ornithodoros, are also reported from pets in some regions. In this review, we provide a summary of the complex and diverse life histories, distinct morphologies, and questing and feeding behaviors of the more common ticks of dogs and cats in North America with a focus on recent changes in geographic distribution. We also review pathogens of dogs and cats associated with the different tick species, some of which can cause serious, potentially fatal disease, and describe the zoonotic risk posed by ticks of pets. Understanding the natural history of ticks and the maintenance cycles responsible for providing an ongoing source of tick-borne infections is critical to effectively combatting the challenges ticks pose to the health of pets and people.
Keywords: Amblyomma, Cat, Dermacentor, Dog, Ixodes, Haemaphysalis, Ornithodoros, Otobius, Rhipicephalus, Tick
1. Introduction
Ticks are common hematophagous ectoparasites of both medical and veterinary importance that readily infest a variety of vertebrate hosts including dogs, cats, and humans. In contrast to many other arthropod vectors, where only specific instars imbibe blood, all motile tick stages feed on blood (Nicholson et al., 2019). In addition to direct effects from attachment and blood feeding, such as trauma to the skin and anemia, ticks vector a variety of pathogens, many of which are zoonotic (Childs and Paddock, 2003; Sonenshine and Roe, 2013; Eisen et al., 2017; Centers for Disease Control and Prevention, 2018). Several tick-borne pathogens are readily transmitted to dogs and cats (Groves et al., 1975; Little et al., 2010; Nicholson et al., 2010; Little et al., 2018). Clinical disease due to these agents is well-characterized in dogs (Shaw et al., 2001a), and occurs, although is less well understood, in cats (Shaw et al., 2001b; Magnarelli et al., 2005, 2007).
In North America, vaccines are only available to limit disease due to Borrelia burgdorferi infection in dogs, thus routine use of effective tick control products and avoiding tick infestations are crucial to protecting pets. However, the complex biology and diverse array of ticks active in this region make control and prevention inherently difficult. Ticks are relatively long-lived and prolific parasites with varying life histories, both among species and within species in differing geographic locations (Yunik et al., 2015; Ogden et al., 2018). Further complicating control efforts, most tick species spend a majority of their life span in the environment and dogs and cats are continually re-exposed to questing ticks in nature.
Over the past several decades, tick populations have increased and their geographic ranges have expanded (Paddock et al., 2016; Sonenshine, 2018). These changes are the result of a variety of factors, many of which are anthropogenic, including climate change, habitat fragmentation, and host availability (Paddock and Yabsley, 2007; Diuk-Wasser et al., 2010; Paddock and Goddard, 2015). Tick ranges and populations are dynamic, as are the infestation and disease risk faced by dogs and cats. In addition to the increases in tick abundance and geographic range, the establishment of a novel tick species in North America (Beard et al., 2018) provides further evidence that the tick risk facing dogs and cats has shifted in the last few decades. The objectives of the current review are to (1) describe the species of ticks that commonly infest dogs and cats in North America and (2) review the biology and current geographic range of each species and the veterinary and zoonotic pathogens they transmit.
1.1. Ixodid (hard) ticks
Ticks infesting dogs and cats are from two families: Ixodidae (hard ticks) and Argasidae (soft ticks). Ixodid ticks are characterized by the presence of a distinct hard sclerotized plate on the dorsum called a scutum, with or without ornamentation, and a capitulum (mouthparts) that extends anterior to the idiosoma (body). The life cycle of ixodid ticks consists of four stages: egg, larva, nymph, and adult, of which the latter three are motile. In female, nymphal, and larval ticks, the scutum extends from the capitulum covering the anterior one-third to half of the dorsal body surface; in males the scutum covers the entire dorsal surface of the idiosoma (Fig. 1). The incomplete scutum of females, nymphs, and larvae allows the posterior portion of the body to greatly expand as the tick imbibes blood, whereas the complete scutum of males limits the degree of expansion achieved while feeding (Nicholson et al., 2019).
Fig. 1.

Dorsal and lateral view of non-fed and fed female and male Amblyomma americanum.
All common species of hard ticks found on dogs and cats in North America have a three-host life cycle, with each new motile stage feeding on a different host. Eggs laid by adult females hatch and give rise to six legged larvae, commonly referred to as seed ticks. For sylvatic species, large numbers of unfed larvae seek hosts in low lying vegetation often utilizing small rodents, lizards, or birds. Larvae will drop from the host after feeding to repletion and molt to eight-legged nymphs. The unfed nymphs then quest for a suitable host, feed, drop off of the host, and molt to eight-legged adults. The unfed adults then seek a third, final host. Although feeding behavior varies between species, both males and females of the common ixodid species in North America attach and imbibe blood. Adult females will feed and mate, becoming dramatically engorged before detaching from the host to lay eggs and die. Females in populations of some species are able to reproduce parthenogenetically. Female ixodid ticks have only a single gonotrophic cycle, meaning a single clutch containing thousands of eggs are laid at one time before she dies (Apanaskevich and Oliver, 2013).
The majority of an ixodid tick’s life cycle is spent off-host in the environment (Nicholson et al., 2019). The time to life cycle completion varies greatly depending on climate, particularly temperature and humidity. In laboratory studies some ixodid species exhibited generation times ranging from 3 to 4 months while others had longer generation times of up to 8 months (Loomis, 1961; Troughton and Levin, 2007). Generally speaking ticks in tropical regions develop more quickly and may complete multiple generations within a single year. However, in areas with both dry and rainy seasons, completion takes longer as ticks stop host seeking when humidity is low (Nicholson et al., 2019). Ticks inhabiting areas with colder temperatures also develop more slowly, and can undergo diapause when it is coldest, extending the time to life cycle completion to two years or more (Nicholson et al., 2019).
Host-seeking strategies vary among tick species but can largely be categorized as either nidicolous (nest or burrow dwelling) or non-nidicolous (Waladde and Rice, 1982; Carroll et al., 2002). Within the non-nidicolous ticks there are two major “questing” strategies for locating hosts: ambush and hunting (Nicholson et al., 2019). Ambush ticks climb vegetation and passively wait for hosts with their first pair of legs extended to grasp the fur, feathers, or clothing of passing hosts (Nicholson et al., 2019). This first pair of legs contains the Haller’s organ, structures with setae that sense humidity, temperature, and carbon dioxide to assist with locating a host (Sonenshine and Roe, 2013; Carr et al., 2017). In contrast, ticks that exhibit hunting behavior actively pursue their host and are located on or very close to the ground, also utilizing the Haller’s organ for chemoreception, particularly of carbon dioxide (Sonenshine, 2018; Nicholson et al., 2019). Ticks that ambush hosts include Ixodes scapularis and Dermacentor variabilis (Carroll et al., 2002), while ticks such as Amblyomma americanum are considered hunters (Carroll et al., 2002; Sonenshine, 2018). Once a potential host has been acquired, the ixodid tick determines if the host is appropriate to attach and feed. If the correct host-recognition cues (e.g. odor, heat, and carbon dioxide) are not found, the tick may drop off and resume host seeking (Nicholson et al., 2019). Even after the suitability of the host has been recognized, ixodid ticks may spend several minutes to hours locating a feeding site (Nicholson et al., 2019). Preferred feeding sites for host attachment have been demonstrated for numerous tick species and stages on several different hosts including dogs, cats, cattle, birds, and humans (Koch, 1982; Barnard et al., 1989; Felz et al., 1996; Heylen et al., 2014; Duscher et al., 2013; Beck et al., 2014; Little et al., 2018; Saleh et al., 2019).
After reaching the feeding site the tick uses chelicerae to lacerate the epidermis and insert the chelicerae and hypostome into the dermis (Moorhouse, 1969; Kemp et al., 1982), initiating the attachment process. The first one to two days an adult female tick is attached, the tick prepares the feeding lesion by secreting cement from the salivary glands (Moorhouse, 1969; Sonenshine and Anderson, 2013). The amount of cement produced varies among tick species. Genera with shorter mouthparts (Dermacentor, Haemaphysalis, and Rhipicephalus) that only penetrate as far as the dermal-epidermal junction secrete more cement, and tick genera with longer mouthparts (Amblyomma and Ixodes) extending into the dermis produce less cement (Moorhouse, 1969). The cement secretion hardens around the chelicerae and helps secure the feeding position, ensuring the tick is firmly affixed (Sonenshine and Anderson, 2013). Once secure, the tick alternates salivating and imbibing, injecting anticoagulants and immunomodulatory compounds as it salivates, thus enlarging the wound (Ribeiro, 1989; Steen et al., 2006; Wikel, 2013; Nicholson et al., 2019) and enabling the tick to feed slowly from the pooling blood for several days (Suppan et al., 2018). As feeding progresses the tick must produce fresh cuticle to allow for the expansion of the body as it engorges (Lees, 1952; Flynn and Kaufman, 2011). Duration of attachment and time to engorgement range from as little as 2–3 days for larval ticks and from 2 weeks or longer for adult females (United States Department of Agriculture, 1976; Apanaskevich and Oliver, 2013). To allow for prolonged attachment to the host, ticks mute the host immune response by the secretion of numerous pharmacologically active compounds in the tick saliva, including anti-inflammatory and immunosuppressive proteins (Ribeiro, 1989; Steen et al., 2006; Wikel, 2013).
1.2. Argasid (soft) ticks
Unlike Ixodidae, Argasidae lack a scutum and as such are commonly referred to as soft ticks. Most species have a rounded body margin, and as adults the capitulum and mouthparts are not visible protruding from the body when the tick is viewed dorsally (Nicholson et al., 2019). In contrast to the life cycles exhibited by ixodid ticks, argasids are more variable with the majority feeding multiple times on multiple hosts. Argasid ticks also develop through the same basic life stages (egg, larva, nymph, and adult) but have several nymphal instars rather than the single nymphal instar of ixodid ticks. The number of argasid nymphal instars varies among species and is influenced by temperature, engorgement status, and number of previous instars (Apanaskevich and Oliver, 2013). Some species of argasid ticks do not take blood meals as larvae or as stage I nymphs, instead directly molting to the next stage using nutrients stored from their previous stage while others do not feed as adults (Apanaskevich and Oliver, 2013).
Most argasids are long-lived nidicolous ticks, living in caves, burrows, or nests of their hosts and often undergoing several month-long intervals between feedings when nests are vacant (Nicholson et al., 2019). Like ixodid ticks, argasid ticks possess and utilize the specialized sensory Haller’s organ to locate their host (Gray et al., 2013). Argasid ticks are relatively rapid feeders, with some active stages feeding in as little as fifteen to thirty minutes (Apanaskevich and Oliver, 2013). With a few exceptions, the majority of soft ticks do not secrete cement. No new cuticle is secreted during feeding, and soft tick engorgement is limited by how far the existing cuticle can stretch (Suppan et al., 2018; Nicholson et al., 2019). Females are able to complete multiple gonotrophic cycles, and usually lay several small batches of a few hundred eggs each after taking a small blood meal, repeating the process sometimes months later. Other species are autogenous and lay eggs without taking a blood meal in the adult stage (Nicholson et al., 2019).
2. Ticks infesting dogs and cats in North America
Ixodid ticks of the most importance to dogs and cats in North America include: Amblyomma americanum (lone star tick), Amblyomma maculatum (Gulf Coast tick), Dermacentor variabilis (American dog tick), Haemaphysalis longicornis (longhorned or bush tick), Ixodes pacificus (western black-legged tick), Ixodes scapularis (eastern black-legged or deer tick), and Rhipicephalus spp. (brown dog ticks) (Koch, 1982; Dryden and Payne, 2004; Burroughs et al., 2016; Thomas et al., 2016; Shannon et al., 2017; Little et al., 2018; Saleh et al., 2019). Other less common ixodid tick species may also occasionally be found on dogs and cats in this region (Bishopp and Trembley, 1945; Koch, 1982; Wells et al., 2004; Little et al., 2018; Saleh et al., 2019; Ghosh et al., 2021; Duncan et al., 2020, 2021). Additionally, two genera of argasid ticks, Otobius spp. and Ornithodoros spp., infest dogs and cats in North America (Cooley and Kohls, 1944a, 1944b; Bishopp and Trembley, 1945; Breitschwerdt et al., 1994; Kelly et al., 2014; Esteve-Gasent et al., 2017; Saleh et al., 2019).
2.1. Amblyomma americanum (lone star tick)
2.1.1. Environment
Amblyomma americanum, the lone star tick, is a three-host ixodid tick commonly found in the eastern United States. Historically this tick was primarily restricted to the southeastern and southcentral United States (Bishopp and Trembley, 1945). More recently, the range of A. americanum has expanded considerably (Brown et al., 2011; Cortinas and Spomer, 2013; Barrett et al., 2015; Dahlgren et al., 2016) and can now be found in parts of most northeastern and Midwestern states (Springer et al., 2014; Monzon et al., 2016). Additionally, A. americanum is found with increasing frequency in southern Ontario (Nelder et al., 2019). Application of bioclimatic modelling and climate change forecasts to predict future geographical distributions of A. americanum suggest continued expansion in the upper Midwest, contraction in areas of coastal Florida, Alabama, Mississippi, Louisiana, and eastern Texas, and no westward expansion beyond the current limit of the 100th meridian (Springer et al., 2015). Others examining the current and future potential distribution of A. americanum with ecological niche modelling support continued expansion of the lone star tick northward (Raghavan et al., 2019). Additionally, broad areas in northern California as well as the west coast of Oregon, Washington, and British Colombia are thought to be climactically suitable for A. americanum although there is no evidence to suggest populations of lone star ticks have established in these areas (Raghavan et al., 2019).
Amblyomma americanum can be found in a variety of habitats ranging from open grasslands and prairies to mature, climax forest. The greatest number of lone star ticks are found in wooded habitats with dense underbrush or trees < 6 m in height and low-lying branches (Hair and Howell, 1970; Semtner et al., 1971; Sonenshine et al., 1966). Within its preferred habitat, lone star ticks are not uniformly distributed and are found most along ecotones (Semtner et al., 1971), within forest openings, and areas with abundant animal hosts (Hair and Howell, 1970). Amblyomma americanum overwinters as adults and nymphs. Activity of both stages of lone star ticks in the spring are thought largely to be stimulated by mean and maximum daily temperature, however, temperature and humidity within the tick’s micro-habitat are key for their development and survival (Hair and Howell, 1970). Data collected in New Jersey noted that A. americanum adults did not quest at temperatures below 4.4 °C (Schulze et al., 2001).
Across the core range of A. americanum in the United States, each feeding stage of the tick fluctuates seasonally in levels of activity (Sonenshine et al., 1966; Hair and Howell, 1970; Jackson et al., 1996; Lavender and Oliver, 1996; Kollars et al., 2000; Goddard, 2007). Adults and nymphal A. americanum emerge in early spring and peak in activity in May. Adult A. americanum will remain active through June and July but their activity begins to subside by August. Despite the clear seasonality of A. americanum, adult lone star ticks can be found on preferred hosts, white- tailed deer (Odocoileus virginianus) anytime of the year suggesting they become active during warm weather breaks in colder months (Hair and Howell, 1970). Nymphal activity also subsides through summer but increases for a second time in September and into October. Activity of A. americanum larvae increases in June and continues through August and September, but declines rapidly thereafter.
Amblyomma americanum is euryxenous and will feed on a wide range of vertebrate animals. When active, lone star ticks aggressively seek hosts and are relatively non-specific when selecting a host. Nevertheless, larvae, nymphs, and adults of A. americanum most commonly infest medium- and large-sized wild and domestic mammals (Bishopp and Trembley, 1945; Hair and Howell, 1970; Childs and Paddock, 2003). White-tailed deer are principal hosts to all feeding stages of A. americanum (Koch, 1988) and are critical to maintain populations of lone star ticks in natural environments. Ground-inhabiting birds, such as wild turkey (Meleagris gallopavo) and bobwhite quail (Colinus virginianus) are also readily infested with A. americanum larvae and nymphs (Bishopp and Trembley, 1945; Hair and Howell, 1970). Small mammals are rarely to occasionally infested with A. americanum larvae and nymphs (Bishopp and Trembley, 1945; Hair and Howell, 1970; Zimmerman et al., 1987; Kollars et al., 2000).
2.1.2. Morphology
Amblyomma americanum are medium-sized ticks with females being a little larger than males (Fig. 2). Lone star ticks are reddish brown, ornate, have long mouth parts, eyes, a rectangular basis capitulum, and festoons. Female A. americanum are easily identified by the conspicuous iridescent white dot on the posterior margin of the scutum. Its designation as the lone star tick is attributed to the resemblance of the conspicuous dot to the prominent single white star on the Texas state flag, known as the “Lone Star” flag. Male A. americanum are readily identified by having white markings on the margin of the scutum and flecked on the festoons. Nymphs and larvae of A. americanum, like most other immature ixodids, are more difficult to distinguish from other species of ticks. Nymphal A. americanum are light brown to reddish brown in color and have long mouth parts. Nymphs resemble adult A. americanum but are inornate and approximately half the size. Larval A. americanum are commonly called “seed ticks” because they are about the size of a poppy seed. Larval A. americanum are pale in color and difficult to see on an animal due to their small size. Seed ticks often occur in the 100s or 1000s on hosts as they migrate very little from where a clutch of eggs was deposited by a female. Keys are available to aid the identification of adult and immature A. americanum (Keirans and Litwak, 1989; Keirans and Durden, 1998; Coley, 2015).
Fig. 2.
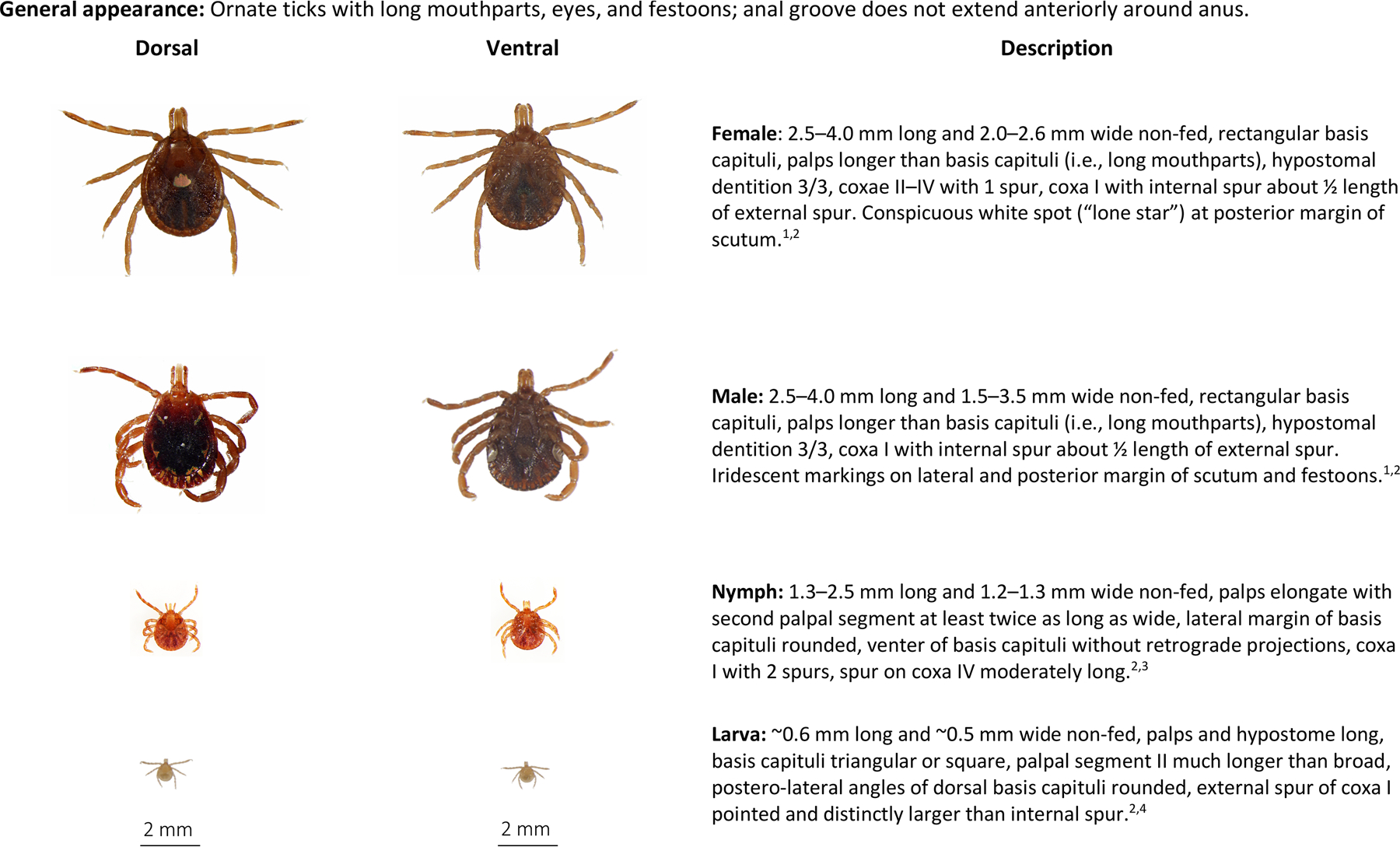
Amblyomma americanum, lone star tick, dorsal and ventral view of each stage and description of key morphologic features. From top to bottom: Female, male, nymph, larva. Descriptions adapted from:
1Keirans, J.E., and T.R. Litwak. 1989. Pictorial key to the adults of hard ticks, family Ixodidae (Ixodida: Ixodoidea), East of the Mississippi River. Journal of Medical Entomology 26: 435–448.
2Cooley, R.A., G.M. Kohls. 1944. The genus Amblyomma (Ixodidae) in the United States. Journal of Parasitology. 30: 77–111.
3Keirans, J.E., and L.A. Durden. 1998. Illustrated key to the nymphs of the tick genus Amblyomma (Acari: Ixodidae) found in the United States. Journal of Medical Entomology 35: 489–495.
4Coley, K. 2015. Identification Guide to Larval Stages of Ticks of Medical Importance in the USA. University Honors Program Thesis, paper 110. Georgia Southern University.
2.1.3. Feeding and disease transmission
Dogs and cats are both readily infested by larval, nymphal, and adult A. americanum. In a national survey of ticks on dogs and cats recovered by veterinarians in the United States (Saleh et al., 2019), A. americanum was present on 23.1% of dogs (345 of 1494) with lone star ticks collected every month except February and November. In the same survey, A. americanum was collected from 29.5% of cats (99 of 336) with lone star ticks collected every month except January, February, and December. Proportions of dogs and cats infested with lone star ticks will vary depending on geographic region with infestations more common in the southcentral and southeastern United States. Examination of A. americanum infested dogs from southeastern Oklahoma and northwestern Arkansas, noted the fewest number of adult lone star ticks on the dorsum with an even distribution over the rest of the body (Koch, 1982). Nymphs were most abundant on the chest and front legs whereas larvae were mostly attached to dogs’ ears. Saleh et al. (2019) noted adult A. americanum preferred to attach to the abdomen, axillary, and inguinal regions of dogs. On cats, however, significantly more adult A. americanum were attached to the tail and perianal region (Saleh et al., 2019).
The bite of lone star ticks is painful and irritating due to their long mouthparts. Despite relatively high tick burdens, levels of A. americanum infestation are rarely severe enough on domestic animals to result in debilitating clinical disease from the tick bite alone. However, in white-tailed deer fawns, severe infestations of A. americanum may result in death due to anemia, tissue destruction, and secondary bacterial infection (Bolte et al., 1970; Hair et al., 1992). Lone star ticks are aggressive and annoying. Grossly, the bite of a lone star tick appears superficial and relatively innocuous in contrast to histopathological changes that take place in a tick bite lesion. Compared to pre-infestation levels, the number of immune cells in the bite lesion of an A. americanum female increased over the duration of attachment (Thomas et al., 2020). Significantly more neutrophils, eosinophils, lymphocytes, and macrophages were noted 8 days post-infestation in dogs whereas significantly more eosinophils, lymphocytes, and macrophages were noted in cats. Pruritus due to infestation with A. americanum on dogs and cats may result in trauma and secondary bacterial infection; intradermal infestations with A. americanum described in red foxes (Vulpes vulpes) have not yet been reported in pets in the region (Smith et al., 1986).
Amblyomma americanum are primary vectors for a variety of pathogens to dogs and cats. These pathogens include: Cytauxzoon felis, Ehrlichia chaffeensis, Ehrlichia ewingii, and Francisella tularensis (Table 1). Although experimental and limited field evidence suggests A. americanum may be a secondary vector of Rickettsia rickettsii (Breitschwerdt et al., 2011; Levin et al., 2017), more research is needed to explore the relative importance of this tick in transmitting the true Rocky Mountain spotted fever (RMSF) agent. Amblyomma americanum is a vector for other spotted fever group Rickettsia spp. including Rickettsia amblyommatis (Barrett et al., 2014).
Table 1.
Tick species, commonly reported hosts, known canine and feline pathogens, and current distribution of ticks commonly found parasitizing dogs and cats in North America.
| Species | Primary hosts (stages) | Disease agents of dogs and cats | Current distribution in North America |
|---|---|---|---|
|
| |||
| Amblyomma americanum | White-tailed deer (L, N, A), large and medium-sized mammals (L, N, A), birds (L, N) |
Cytauxzoon felis
Ehrlichia ewingii Ehrlichia chaffeensis Francisella tularensis |
Eastern half of North America from Gulf of Mexico to northern United States |
| Amblyomma maculatum | Cattle and other large mammals (A), birds and small mammals (L, N) | Hepatozoon americanum | Found 402 km inland in states along the Gulf Coast and in states bordering the Atlantic Coast as far northeast as Delaware; also found in several land-locked Midwestern and southern states |
| Dermacentor variabilis | Dogs, coyotes, cattle, horses, and raccoons (A), small mammals (L, N) |
Rickettsia rickettsii
Cytauxzoon felis |
Eastern half of North America from Gulf of Mexico to southern Canada; isolated populations along the Pacific Coast in the United States, and extending eastward into Idaho |
| Haemaphysalis longicornis | White-tailed deer, cattle, raccoons, opossums (L, N, A) | Not known to be a primary vector of any canine or feline disease agents in North America at this time | Reported in 15 eastern states and in Arkansas, but documented distribution continues to spread |
| Ixodes pacificus | Black-tailed deer (A), lizards, small rodents (L, N) |
Anaplasma phagocytophilum
Borrelia burgdorferi |
California, western Oregon, western Washington and northward to British Columbia; also, in Utah and Nevada |
| Ixodes scapularis | White-tailed deer (A), small mammals (L, N in northern North America), lizards (L, N in the southern United States) |
Anaplasma phagocytophilum
Borrelia burgdorferi Ehrlichia muris |
Eastern half of United States from Florida to central Texas and northward to eastern North Dakota and Maine; also common in southern Atlantic, Central, and some Western provinces in Canada |
| Rhipicephalus spp. | Dogs (L, N, A) |
Anaplasma platys
*
Babesia vogeli Babesia gibsoni * Cercopithifilaria sp. * Ehrlichia canis Hepatozoon canis * Rickettsia rickettsii |
Considered ubiquitous wherever there are dogs, with populations more intense in the southern United States, Hawaii, Mexico, and in the Caribbean |
| Otobius megnini | Cattle, goats, horses, sheep, wild ungulates (L, N) | Not a primary vector of any known disease agent | Southwestern and southcentral states, in states bordering the Pacific Coast into British Columbia, and in some southeastern states |
Abbreviations: L larva; N nymph; A adult.
Transmission by ticks has not been confirmed in North America.
2.1.4. Pathogens
Amblyomma americanum has been demonstrated as a vector of C. felis to domestic cats (Reichard et al., 2009, 2010). In addition to adult A. americanum transmitting C. felis, nymphal lone star ticks have been shown to transmit the piroplasm to domestic cats (Allen et al., 2019). Dermacentor variabilis has also been demonstrated as a competent vector for C. felis to domestic cats (Blouin et al., 1984) and bobcats (Blouin et al., 1987). Cytauxzoonosis in domestic cats occurs throughout the southcentral and southeastern United States (Sherrill and Cohn, 2015), in an area that overlaps the distribution of both A. americanum and D. variabilis. In a study conducted in Oklahoma by Reichard et al. (2008), the highest number of cytauxzoonosis cases occurred in May and September when activities of A. americanum adults and nymphs were expected to be highest. Cytauxzoon felis has been documented in bobcats from two states, North Dakota (Shock et al., 2011) and Pennsylvania (Birkenheuer et al., 2008), in which no known cases of cytauxzoonosis have been reported from domestic cats. The prevalence of C. felis in bobcats is higher in states with established populations of A. americanum (Shock et al., 2011). Cytauxzoon felis DNA was present in A. americanum nymphs collected from 3 cats with cytauxzoonosis in Missouri (Bondy et al., 2005). In wild-collected, unfed A. americanum and D. variabilis from Oklahoma, the minimum infection of C. felis was 0.5% in male (1 of 153), 0.8% in nymphal (3 of 393), and 1.9% in female (3 of 161) lone star ticks, and 0.0% in female (n = 74) and 0.0% in male (n = 86) American dog ticks (Reichard et al., 2010). Shock et al., 2014 examined A. americanum and D. variabilis from Kentucky, Tennessee, Georgia, and Texas and found C. felis DNA only in American dog ticks from Tennessee (1.8%; 8 of 442) and Georgia (0.8%; 1 of 125).
Amblyomma americanum is a primary vector for E. chaffeensis (Ewing et al., 1995; Varela-Stokes, 2007) and E. ewingii (Anziania et al., 1990). Examination of 8662 dogs from the southcentral and southeastern United States, where A. americanum populations are most abundant, showed a higher percentage of dogs are exposed to E. ewingii (5.1%) than E. chaffeensis (2.8%) (Beall et al., 2012). Despite dogs being naturally exposed to and infected by E. chaffeensis, attempts to infect dogs experimentally by transmission feeding infected A. americanum have failed (Ewing et al., 1995). Transovarial transmission of E. chaffeensis in A. americanum does not appear to take place (Long et al., 2003). Numerous publications exist reporting the presence of E. chaffeensis and E. ewingii DNA in A. americanum throughout the geographic range of the tick, and prevalence generally ranges from <1.0% to approximately 10% (Anderson et al., 1993; Lockhart et al., 1997; Yu et al., 1997; Burket et al., 1998; Murphy et al., 1998; Roland et al., 1998; Steiner et al., 1999; Ijdo et al., 2000; Irving et al., 2000; Whitlock et al., 2000; Wolf et al., 2000; Stromdahl et al., 2001; Steiert and Gilfoy, 2002; Goddard et al., 2003; DeShields et al., 2004; Long et al., 2004; Mixson et al., 2004; Varela et al., 2004a; Schulze et al., 2005; Mixson et al., 2006; Castellaw et al., 2010; Cohen et al., 2010; Yabsley, 2010; Schulze et al., 2011; Fritzen et al., 2011; Fitak et al., 2014; Gaines et al., 2014; Maegli et al., 2016; Sayler et al., 2016; Simpson et al., 2019) depending on experimental design, detection methodology, and sample size.
Francisella tularensis (subspecies tularensis, or type A), causative agent of tularemia, can be vectored by several arthropods including ticks. Transmission of F. tularensis through ticks is considered biological whereas transmission through other arthropods is mechanical. It is believed that D. variabilis is the primary vector of F. tularensis and maintains infections among wild animals, whereas A. americanum acts as a bridge vector for human infection (Eisen, 2007; Mani et al., 2015). Information on infection of F. tularensis in A. americanum is limited to several reports of either natural infections from lone star ticks collected off hosts in Arkansas (Calhoun, 1954), experimental infection and demonstration of transstadial transmission (Hopla, 1953; Hopla and Downs, 1953), and quantification, colonization, and duration of infection (Mani et al., 2015).
2.1.5. Zoonotic concerns
Amblyomma americanum has emerged as a serious parasite and vector of pathogens to humans in the United States (Childs and Paddock, 2003; Paddock and Yabsley, 2007). Larvae, nymphs, and adults of A. americanum all can infest and feed on humans. Amblyomma americanum is the most commonly recovered tick from humans in the southeastern United States (Merten and Durden, 2000). In surveys, 83% of ticks recovered from humans in Georgia and South Carolina were A. americanum (Felz et al., 1996); 63% of ticks collected off humans in Mississippi were A. americanum (Goddard, 2002). Along with D. variabilis (34.0%), A. americanum (34.3%) was the most commonly recovered tick species from United States Air Force personnel (Campbell and Bowles, 1994). Amblyomma americanum is either a known or suspected vector for several established or potential pathogens of humans including: Ehrlichia chaffeensis (Childs and Paddock, 2003; Paddock and Yabsley, 2007), E. ewingii (Childs and Paddock, 2003; Paddock and Yabsley, 2007), Panola Mountain Ehrlichia (PME) (Loftis et al., 2008; Reeves et al., 2008), Rickettsia amblyommatis (Billeter et al., 2007a, b; Jiang et al., 2010; Karpathy et al., 2016), Bourbon virus (Lambert et al., 2015; Savage et al., 2017; Jackson et al., 2019), Heartland virus (Brault et al., 2018), and Borrelia lonestari (James et al., 2001; Varela et al., 2004b). The bite of A. americanum has been associated with development of red meat allergy in humans. This delayed anaphylaxis is believed to be initiated by feeding of A. americanum and the production of antibodies against galactose-alpha-1,3-galactose (alpha-gal), a carbohydrate produced in all mammals besides humans, great apes, and new world monkeys (Commins et al., 2011; Steinke et al., 2015).
2.2. Amblyomma maculatum (Gulf Coast tick)
2.2.1. Environment
Amblyomma maculatum is an aggressive, three-host species commonly known as the Gulf Coast tick (Paddock and Goddard, 2015; Nadolny and Gaff, 2018). Historically found only as far as 161–257 km (100–160 miles) inland in states bordering the Gulf Coast and southern Atlantic Coast, this tick is now established 402 km (250 miles) inland in coastal states and is endemic in states along the Atlantic Coast into Delaware and in several land-locked Midwestern and southern states (Paddock and Goddard, 2015; Portugal and Goddard, 2016; Lockwood et al., 2018; Nadolny and Gaff, 2018; Maestas et al., 2020; Phillips et al., 2020). Immature Gulf Coast ticks feed on a variety of birds and rodents, while adult A. maculatum infest larger mammals including cattle, coyotes (Canis latrans), feral swine (Sus scrofa), horses, sheep, and white-tailed deer. However, Gulf Coast ticks occasionally feed on domestic dogs and cats (Dryden and Payne, 2004; Paddock and Goddard, 2015; Little et al., 2018; Nadolny and Gaff, 2018; Saleh et al., 2019).
Gulf Coast ticks are able to tolerate exposed, arid and hot environments. Common habitats include mesquite areas, oak savannah, prairie, and scrublands, especially when adjacent to wetland areas (Paddock and Goddard, 2015; Nadolny and Gaff, 2018; Maestas et al., 2020). The catholic feeding behavior of A. maculatum, their ability to thrive in both dry and humid environments, expansion of white-tailed deer and feral swine populations, frequent movement of livestock, and migration patterns of avian hosts have all likely contributed to the broadening geographic distribution of this tick species over the past 50–70 years. The establishment and spread of coastal A. maculatum populations are also facilitated by anthropogenic disturbances, and inland populations may contract or bloom according to precipitation patterns (Paddock and Goddard, 2015; Nadolny and Gaff, 2018).
Host-seeking adult Gulf Coast ticks are found on vegetation in open, exposed areas, and are able to tolerate harsh summertime conditions in which other common sympatric tick species would languish. Seasonal peak activity of immature A. maculatum has not fully been elucidated, and field survey data investigating questing behaviors vary depending on region (Goddard, 2007; Paddock and Goddard, 2015; Portugal and Goddard, 2016; Nadolny and Gaff, 2018). Experimentally, nymphal Gulf Coast ticks quest low on vegetation and migrate horizontally at a slow speed. This questing behavior may be the reason why immature A. maculatum are commonly found on ground-dwelling birds and other small animals. Adults of A. maculatum are active during March through September, but as with immatures, data differ somewhat with the geographic region surveyed and months of activity (Portugal and Goddard, 2016; Nadolny and Gaff, 2018).
2.2.2. Morphology
Amblyomma maculatum is a member of the Amblyomma maculatum group, which also includes A. neumanni, A. parvitarsum, A. tigrinum, and A. triste. Within this group, A. maculatum, A. tigrinum, and A. triste are exceptionally difficult to distinguish by morphology during all instars, and therefore misidentifications have likely occurred since their original descriptions (Estrada-Peña et al., 2005; Mertins et al., 2010; Lado et al., 2018). Phylogenetic comparisons have led some researchers to hypothesize that A. maculatum and A. triste are indeed the same species. However, further studies are needed to support or refute this hypothesis (Lado et al., 2018), and therefore A. maculatum is regarded as distinct from A. triste in the current review.
Keys are available to aid in species identification of immature and adult Amblyomma spp. in the United States (Cooley and Kohls, 1944a, 1944b; Keirans and Durden, 1998). Like other Amblyomma species, A. maculatum have characteristically elongate mouthparts (Fig. 3) (Cooley and Kohls, 1944a, 1944b). Because morphologies are quite similar among immature Amblyomma spp., consultation with a diagnostic lab having specific entomologic expertise may be advisable. Subtle morphologic differences between immature Amblyomma spp. as observed under magnification include characteristic margins of the basis capitulum with presence/absence of lateral projections, presence/absence of projections on the ventral basis capitulum, shape and dentition of hypostomes, and presence/absence of spurs on Coxae I–III (Cooley and Kohls, 1944a, [Cooley and Kohls, 1944b] 1944b; Keirans and Durden, 1998).
Fig. 3.
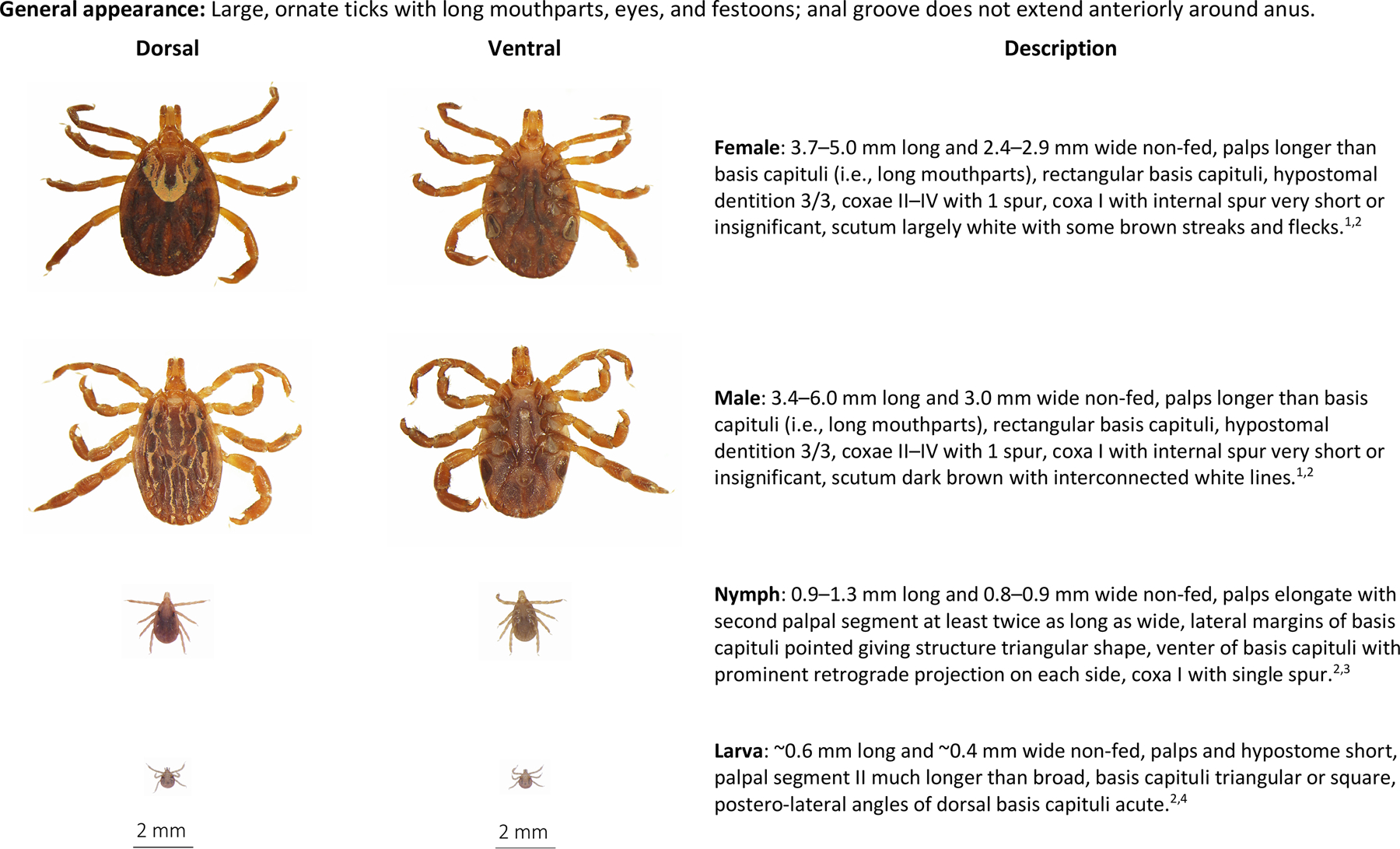
Amblyomma maculatum, Gulf Coast tick, dorsal and ventral view of each stage and description of key morphologic features. From top to bottom: Female, male, nymph, larva. Descriptions adapted from:
1Keirans, J.E., and T.R. Litwak. 1989. Pictorial key to the adults of hard ticks, family Ixodidae (Ixodida: Ixodoidea), East of the Mississippi River. Journal of Medical Entomology 26: 435–448.
2Cooley, R.A., G.M. Kohls. 1944. The genus Amblyomma (Ixodidae) in the United States. Journal of Parasitology. 30: 77–111.
3Keirans, J.E., and L.A. Durden. 1998. Illustrated key to the nymphs of the tick genus Amblyomma (Acari: Ixodidae) found in the United States. Journal of Medical Entomology 26: 489–495.
4Coley, K. 2015. Identification Guide to Larval Stages of Ticks of Medical Importance in the USA. University Honors Program Thesis, paper 110. Georgia Southern University.
Amblyomma spp. adults are more easily differentiated. Among other characteristics, female A. maculatum have an ornate scutum with pale markings in an extensive pattern and a very short spur on the first pair of coxae. Male A. maculatum have an ornate scutum with a filigree pattern in white and brown, and one spur on the second, third, and fourth pair of coxae (Cooley and Kohls, 1944a, [Cooley and Kohls, 1944b] 1944b; Lado et al., 2018). Amblyomma maculatum and D. variabilis males and females are sometimes confused based on their large sizes and ornate patterns (Paddock and Goddard, 2015), but careful examination of mouthpart length and basis capitulum shape can lead to successful species identification (Cooley and Kohls, 1944a, 1944b).
2.2.3. Feeding and disease transmission
Little is known about the direct effects of feeding A. maculatum on cats and dogs, and attachment site preferences have not been reported. On wildlife hosts, immature A. maculatum most often attach near or on the head region. On cattle, immature A. maculatum most often attach to dorsal body areas (withers, midline, and tail-head) (Ketchum et al., 2005). Adult A. maculatum preferentially feed on inner and outer surfaces of the external ear of livestock (especially cattle), and over time may cause the inflammatory, destructive condition dubbed “gotch ear” (Edwards, 2011; Paddock and Goddard, 2015). Other direct effects of feeding Gulf Coast ticks on cattle are well-documented and include abscesses, anemia, edema, and predisposition to myiasis and secondary infection (Nadolny and Gaff, 2018). Similar consequences from A. maculatum infestations in dogs and cats may occur but reports are lacking and warrant further consideration.
Amblyomma maculatum is primarily of concern to canine medicine because it is the vector of Hepatozoon americanum, the apicomplexan parasite causing American canine hepatozoonosis (ACH; Table 1). Immature A. maculatum acquire H. americanum via feeding on infected canids; the parasite subsequently develops within ticks during ecdysis, resulting in numerous sporulated oocysts in emerged instars. Dogs become infected with H. americanum via ingestion of Gulf Coast ticks harboring the oocysts. Ingestion of ticks may occur through grooming of self or other dogs, or possibly by the incidental ingestion of infected ticks parasitizing prey species (Vincent-Johnson et al., 1997; Mathew et al., 1998; Johnson et al., 2009; Allen et al., 2011). Rarely, the feeding of Gulf Coast ticks has been associated with paralysis in dogs (Gothe et al., 1979; Espinoza-Gomez et al., 2011; Paddock and Goddard, 2015).
2.2.4. Pathogens
Experimentally, oocysts of H. americanum are found free in the body cavity of A. maculatum, and may be abundant. Each oocyst contains numerous sporocysts that are comprised of hundreds of infective sporozoites (Mathew et al., 1998). In enzootic areas, prevalence of H. americanum infection in Gulf Coast tick populations is not known but seems low (KE Allen, unpublished data). Coyotes may serve as reservoirs of H. americanum to feeding A. maculatum in Oklahoma and Texas (Kocan et al., 2000; Garrett et al., 2005; Starkey et al., 2013). Dogs with ACH are typically diagnosed in regions where Gulf Coast ticks are established, but infections have been documented in other states due to displacement or travel history (Allen et al., 2008; Li et al., 2008).
DNA of Anaplasma platys, E. chaffeensis, and E. ewingii has been documented within field- collected A. maculatum. These zoonotic canine pathogens transmit via tick feeding and are thought to primarily use other tick vectors in nature (Williamson et al., 2010; Maegli et al., 2016; Breitschwerdt et al., 2014; Mays et al., 2016; Allerdice et al., 2017; Maestas et al., 2020). Gulf Coast ticks have also been experimentally shown to vector Panola Mountain Ehrlichia (PME), which naturally cycles between A. americanum and likely white-tailed deer reservoirs in the United States (Paddock and Goddard, 2015; Loftis et al., 2016); DNA of PME was detected in 0.5–4.3% of A. maculatum tested from several Gulf Coast states (Loftis et al., 2016). However, the potential role of A. maculatum as a natural alternative vector for any of these bacterial organisms is not clear (Williamson et al., 2010; Jiang et al., 2012; Loftis et al., 2016; Allerdice et al., 2017; Maestas et al., 2020).
2.2.5. Zoonotic concerns
Amblyomma maculatum is chiefly recognized as a risk to human health because it is the vector of Rickettsia parkeri, an emerging spotted fever rickettsia. Cases have been documented in mid-Atlantic, southeastern, and southern states, and in some regions of Arizona (Hardstone Yoshimizu and Billeter, 2018). DNA of R. parkeri has been detected in the blood of domestic dogs by PCR; dogs are not known to develop apparent clinical signs with infection, and may serve as potential reservoirs to feeding ticks (Grasperge et al., 2012; Hardstone Yoshimizu and Billeter, 2018). Field- collected Gulf Coast ticks have also been shown to harbor DNA of Rickettsia felis, an emerging flea-borne infection in humans that is found world-wide. Although the pathogen has been molecularly detected in dogs in Africa, to date it has not been documented in dogs in North America (Moonga et al., 2019).
Gulf Coast ticks may also transmit more poorly understood spotted fever rickettsia to humans including R. amblyommatis (formerly Rickettsia amblyommii and Candidatus Rickettsia amblyommii) and Rickettsia montanensis (Nadolny et al., 2014; Karpathy et al., 2016; Lee et al., 2017; Harris et al., 2017; Hardstone Yoshimizu and Billeter, 2018; Maestas et al., 2020). Molecular evidence suggests that both of these organisms can infect dogs (Barrett et al., 2014; Hardstone Yoshimizu and Billeter, 2018), and therefore possibly Gulf Coast ticks feeding on dogs. Also, as in canine medicine, the role of A. maculatum as a vector of Ehrlichia spp. to humans is not clear (Williamson et al., 2010; Jiang et al., 2012; Breitschwerdt et al., 2014; Paddock and Goddard, 2015; Loftis et al., 2016; Mays et al., 2016; Allerdice et al., 2017; Maestas et al., 2020). Feeding Gulf Coast ticks have also been associated with tick paralysis (Gothe et al., 1979; Espinoza-Gomez et al., 2011; Paddock and Goddard, 2015).
2.3. Dermacentor variabilis (American dog tick)
A variety of Dermacentor spp. occur in North America and infest dogs and cats, including D. albipictus, D. andersoni, D. occidentalis, and D. variabilis. However, D. variabilis, the American dog tick, is the most common species recovered from dogs and cats throughout the United States (Little et al., 2018; Saleh et al., 2019). Dermacentor albipictus, the winter tick, is found across the United States and Canada and commonly infests large ungulates but has been reported from a few dogs and cats in the Midwestern and western United States and from Alberta and British Columbia in Canada (Lindquist et al., 2016; Saleh et al., 2019; Duncan et al., 2020). Dermacentor andersoni is found in Rocky Mountain states and western Canada. The Pacific Coast tick, D. occidentalis, is found from central Oregon to California in the United States; this tick commonly infests humans and has occasionally been reported from dogs (Hooker et al., 1912; Merten and Durden, 2000; Vigil, 2013). In Mexico D. occidentalis is found in Baja California and Baja California Sur (Guzmán-Cornejo et al., 2016). The most common Dermacentor sp. infesting dogs and cats in North America is D. variabilis and will be the focus of the current review.
2.3.1. Environment
Dermacentor variabilis is one of the most widespread ixodid ticks in North America. Its distribution ranges from Florida to southern Canada in the eastern half of the United States, and extends to the Gulf of Mexico, with isolated populations also occurring along the Pacific Coast in California, Oregon, and Washington, and extending eastward into Idaho (Bishopp and Trembley, 1945; Wilkinson, 1967; Stout et al., 1971; Rotramel et al., 1976; Easton et al., 1977). In Canada, D. variabilis is present in 8 of 10 provinces and 2 of 3 territories, with the greatest abundance in parts of Nova Scotia as well as southern Manitoba and Ontario. The species has also been documented in Alaska (Dergousoff et al., 2013; Durden et al., 2016; Lindquist et al., 2016). In Mexico, D. variabilis has been documented in 18 of 32 states throughout the country (Guzmán-Cornejo et al., 2016). Traditionally, D. variabilis has been considered absent from the Rocky Mountain region of North America, with a related species, D. andersoni, aptly called the Rocky Mountain wood tick, present in this area. However, as far back as 1939, there have been reports that these two Dermacentor spp. overlapped in distribution in eastern California, southern Oregon, in addition to localities in Montana, North and South Dakota, and Nebraska in the United States, and in south-central Saskatchewan in Canada (Gibbons, 1939; Bishopp and Trembley, 1945; Dergousoff et al., 2013). Generally, D. andersoni is found in areas with hot and dry summers, while D. variabilis is in areas with summers that are more humid (Wilkinson, 1967).
Dermacentor variabilis is commonly found questing in low elevation grasslands and along the forest edge in addition to the boundaries of trails and roadways (Easton et al., 1977; Sonenshine, 1979; Dergousoff et al., 2013). Typical host species for D. variabilis range from small mammals such as voles (Microtus spp.) and chipmunks (Tamias spp.) for immature ticks to medium and larger-sized mammals such as opossums (Didelphis virginianus), raccoons (Procyon lotor), dogs, white-tailed deer, and humans for adult ticks (Kollars et al., 2000). Populations of American dog ticks in Canada have expanded greatly northward in recent years and it is considered established in portions of Ontario, Saskatchewan, Manitoba, and Nova Scotia (Dergousoff et al., 2013; Yunik et al., 2015; Wood et al., 2016). Recent work predicting the future range of D. variabilis in response to climate change indicated that areas of suitable habitat could increase by as much as 50% over the next 50 years (Minigan et al., 2018), allowing for even further northward expansion of the American dog tick into northern parts of Canada. Additionally, suitable alternate hosts for immature stages of D. variabilis (Sonenshine, 2018) are present, supporting the recent northward expansion.
2.3.2. Morphology
Dermacentor variabilis is an ornate reddish-brown tick; both males and females have a distinct lacy silvery-white pattern covering the scutum, a rectangular basis capitulum with the width exceeding the length, and palps that are relatively broad and short (Fig. 4). All life stages of Dermacentor spp. have festoons and eyes, but only adults possess ornate scuta (Brinton et al., 1965). Several species of Dermacentor have similar appearances making morphologic identification difficult (Goddard et al., 2020). Geography has traditionally been used to inform specific identification, but recent work has demonstrated that in areas that were historically considered D. andersoni range, D. variabilis may be the more abundant tick (Dergousoff et al., 2013; Duncan et al., 2021). Keys are available to differentiate adult and immature Dermacentor ticks, but in cases where there is uncertainty molecular techniques to speciate ticks should be utilized.
Fig. 4.
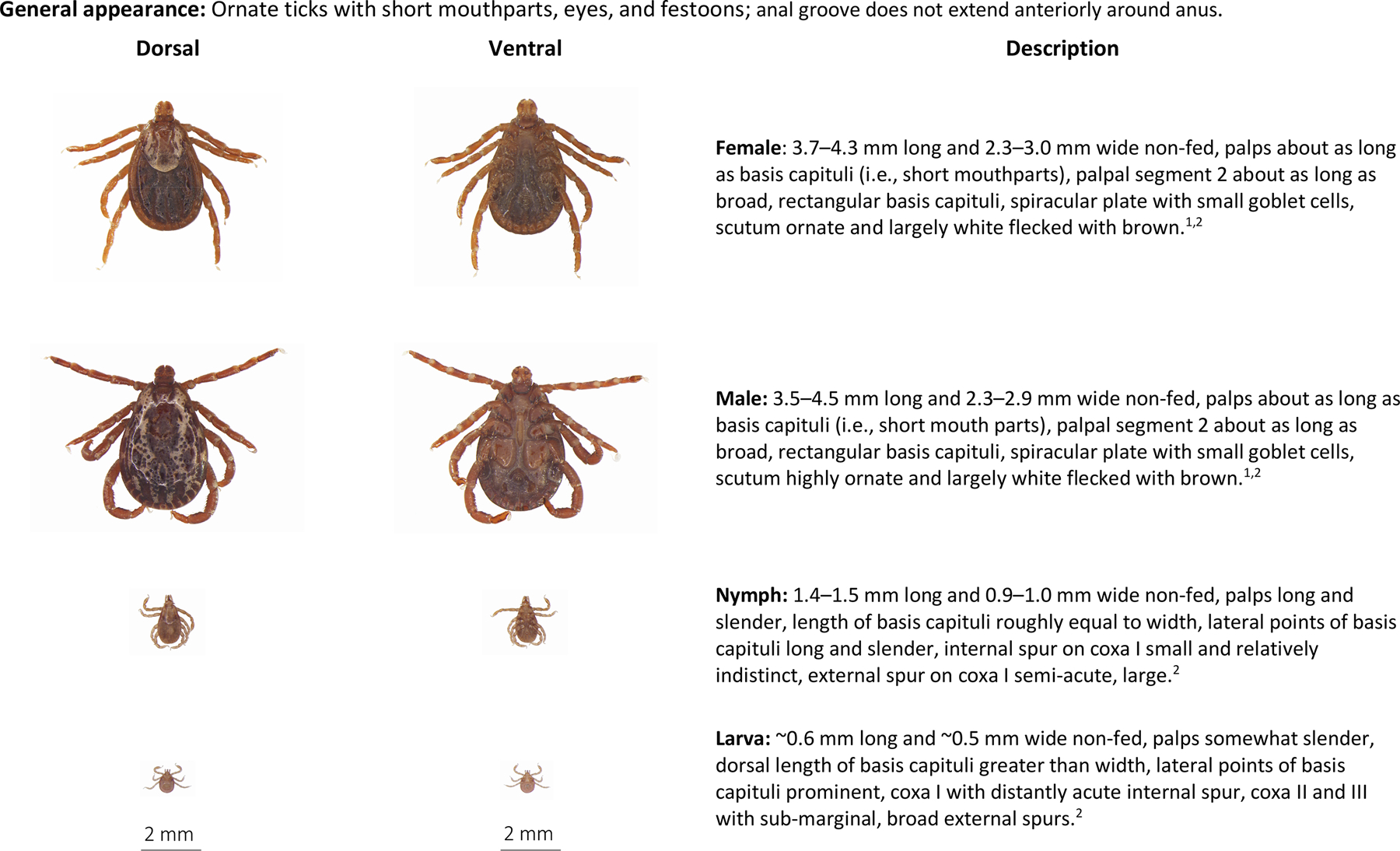
Dermacentor variabilis, American dog tick, dorsal and ventral view of each stage and description of key morphologic features. From top to bottom: Female, male, nymph, larva. Descriptions adapted from:
1Keirans, J.E., and T.R. Litwak. 1989. Pictorial key to the adults of hard ticks, family Ixodidae (Ixodida: Ixodoidea), East of the Mississippi River. Journal of Medical Entomology 26: 435–448.
2Brinton, E. P.; D.E. Beck, and D.M. Allred. 1965. Identification of the adults, nymphs and larvae of ticks of the genus Dermacentor Koch (Ixodidae) in the western United States. Brigham Young University Science Bulletin, Biological Series: Vol. 5 : No. 4, Article 1.
2.3.3. Feeding and disease transmission
American dog ticks commonly attach and feed on both dogs and cats throughout their geographic range. While ticks can be found anywhere on the host, a significant dorsal preference is seen for attachment, with most ticks being found on the head, neck, ears, and back for both dogs and cats (Koch, 1982; Little et al., 2018; Saleh et al., 2019). An engorged female American dog tick consumes more than 1.4 mL of blood from a canine host (Koch and Sauer, 1984). Compared to other ixodid ticks when feeding the mouthparts of D. variabilis are restricted to the superficial epidermis, but a large amount of cement is secreted resulting in marked hypertrophy of the epidermis at the site of attachment (Moorhouse, 1969).
Dermacentor variabilis transmits a variety of pathogens to dogs and cats via saliva during tick feeding (Table 1). The most important are R. rickettsii and other spotted fever group Rickettsia spp., as well as F. tularensis (Philip and Jellison., 1934; Allison and Little, 2013). However, American dog ticks are considered a possible secondary or minor vector for a number of other pathogens including Cytauxzoon felis, E. chaffeensis, and E. ewingii, which are more commonly transmitted by the lone star tick (Reichard et al., 2010; Little, 2010).
2.3.4. Pathogens
In North America D. variabilis is one of the most important vectors of R. rickettsii, especially in the southeastern and central United States (McDade and Newhouse, 1986; Hecht et al., 2019). Rickettsia rickettsii is maintained in D. variabilis populations transovarially and transstadially, making the tick both the vector and reservoir of the pathogen (McDade and Newhouse, 1986). Canine serosurveys identified R. rickettsii antibodies in 10.4% of dogs in the United States (Yancey et al., 2014). However, PCR surveys of D. variabilis often do not detect R. rickettsii DNA or detect it at a very low prevalence, less than 1% (Stromdahl et al., 2010; Hecht et al., 2019), but often detect other spotted fever group Rickettsii spp., such as R. montanensis (Fritzen et al., 2011; Little et al., 2018).
The proportion of D. variabilis infected with a pathogen varies depending on the specific pathogen and the geographic location. Cytauxzoon felis is present in the eastern and southern regions of the United States. The bobcat is considered the historic reservoir, but in recent years domestic cats with persistent infections have been considered potentially important reservoirs of C. felis (Birkenheuer et al., 2008; Reichard et al., 2010). Reported prevalences of C. felis detected via PCR from healthy asymptomatic cats in the southern United States range from less than 1% up to 30.3% (Haber et al., 2007; Brown et al., 2010; Rizzi et al., 2015; Nagamori, 2016). DNA of C. felis was detected in 0–15.8% of D. variabilis tested from the central and southern United States (Bondy et al., 2005; Shock et al., 2014; Zieman et al., 2017; Little et al., 2018). However, A. americanum is now considered an important vector of C. felis and covers a large portion of the geographic region where cytauxzoonosis occurs most commonly (Reichard et al., 2010; Mueller et al., 2013).
While D. variabilis has been shown to transmit Ehrlichia canis experimentally, this pathogen has only been described from field-caught D. variabilis in a few reports (Johnson et al., 1998; Sosa-Gutierrez et al., 2016). Ehrlichia chaffeensis has been documented in 0–6.7% of field-collected D. variabilis in Virginia, Kentucky, and Missouri, while the prevalence of E. ewingii in these ticks was slightly lower ranging from 0 to 3.3% (Steiert and Gilfoy, 2002; Fritzen et al., 2011; Wright et al., 2014). DNA of E. ewingii has also been recovered from D. variabilis removed from an infected dog in Oklahoma (Murphy et al., 1998).
2.3.5. Zoonotic concerns
Humans and dogs are both susceptible to infection with R. rickettsii from D. variabilis (Nicholson et al., 2010). Infection with R. rickettsii in cats is considered uncommon (Allison and Little, 2013). Concurrent infections with R. rickettsii and Ehrlichia spp. have been documented in humans and their pet dogs in the United States (Buller et al., 1999; Paddock et al., 2002; Elchos and Goddard, 2003). Dermacentor variabilis is a principal vector of R. rickettsii to both dogs and humans in the eastern and central United States, while in the West, D. andersoni is considered the more important vector of R. rickettsii. The majority of Rocky Mountain spotted fever cases reported in humans in the United States originate from states extending from Oklahoma and Missouri east to North Carolina and Virginia (Drexler et al., 2017; Centers for Disease Control and Prevention, 2018). Dermacentor variabilis is also an important vector of F. tularensis, and is considered a key source of human infection (Klock et al., 1973; Petersen et al., 2009). Dogs and cats are typically infected with F. tularensis after contact with or ingestion of infected tissues from rabbits or other wild mammals; clinical disease is most commonly seen in cats and is considered rare in dogs (Feldman et al., 2003). Additionally, the absorption of toxins from D. variabilis saliva can sometimes result in tick paralysis (Lane et al., 1984; Gothe and Neitz, 1991; Diaz, 2010).
2.4. Haemaphysalis longicornis (longhorned tick)
2.4.1. Environment
Historically Haemaphysalis spp. in North America have been considered ticks of wildlife with limited medical and veterinary importance, rarely infesting domestic animals and people (Keirans and Litwak, 1989; Egizi et al., 2019). The rabbit tick, H. leporispalustris, has been infrequently found on dogs in North America, while H. chordeilis and H. juxtakochi primarily infest birds and ungulates, respectively (Bishopp and Trembley, 1945; Kohls, 1960; Lindquist et al., 2016). However, in 2017, natural infestations with the longhorned tick, H. longicornis, were identified for the first time in North America from a sheep in New Jersey, United States (Rainey et al., 2018; United States Department of Agriculture, 2020). Haemaphysalis longicornis is native to East Asia and is established in Australia and New Zealand (Hoogstraal et al., 1968), where it is considered a significant pest of people, livestock, and companion animals (Shimada et al., 2003; Heath, 2013; Heath, 2016; Greay et al., 2016). Since the initial report in 2017, H. longicornis has been reported in 15 different states in the eastern and south-central United States and on numerous hosts including humans, dogs, and cats in addition to domestic livestock (cattle, sheep) and wildlife (white-tailed deer, coyotes, raccoons, opossums), with its range continuing to increase (Beard et al., 2018; Saleh et al., 2019; United States Department of Agriculture, 2020). Subsequent reexamination of archived samples demonstrated that H. longicornis has been infesting North American wildlife since at least 2010 (Beard et al., 2018). Introduced populations of the longhorned tick are parthenogenic (Oliver et al., 1973; Heath, 2013; Rainey et al., 2018).
Haemaphysalis longicornis thrive in humid, warm-temperate conditions but can tolerate temperatures ranging from −2 °C to 40 °C and occupy a variety of climates and latitudes (Hoogstraal et al., 1968; Sutherst and Moorhouse, 1972; Heath, 2013, 2016). Populations are often found among long grass and rushes (Heath, 2016), with unfed nymphs surviving longer in areas with long as opposed to short grass (Sutherst and Bourne, 1991). In the first recognized infestations in New Jersey, United States, all feeding stages of longhorned ticks were recovered from the sheep, and larvae were found on vegetation within the sheep paddock, but not outside the paddock where the grass was mowed (Rainey et al., 2018).
As a recently established species, the seasonality of North American populations of H. longicornis is still being determined, but early work in the northeastern United States found that activity of adult longhorned ticks peaked in late July, with nymphs peaking from June to July, and larvae having the highest activity in August (Tufts et al., 2019). This pattern is similar to that seen in populations of H. longicornis in South Korea and China (Zheng et al., 2012; Johnson et al., 2017). The full extent of the longhorned tick’s geographic distribution in North America is currently unknown. Habitat and climate preferences of H. longicornis in other regions have been used to model the potential range of this species in North America. Although predictions vary, potential ranges include the southeastern United States, the Pacific Coast extending into Canada, as well as Mexico, and areas of the Great Lakes region in the United States and Canada (Raghavan et al., 2019; Rochlin, 2019). To date, both models confirm extensive suitable habitat for H. longicornis in the southeastern and central Midwestern United States (Raghavan et al., 2019; Rochlin, 2019).
2.4.2. Morphology
Adult H. longicornis are inornate, reddish-brown ticks with short mouthparts and a rectangular dorsal basis capitulum with straight lateral margins (Fig. 5). The lateral projection of the second palpal segment is characteristic of the genus; eyes are absent, but festoons are present (Cooley, 1946). Haemaphysalis longicornis adults are differentiated from its cogeners in North America (H. chordeilis, H. juxtakochi, and H. leporispalustris) by the presence of a dorsal spur on the third palpal segment, and keys are available to aid in the identification of adults and immature stages (Cooley, 1946; Egizi et al., 2019).
Fig. 5.
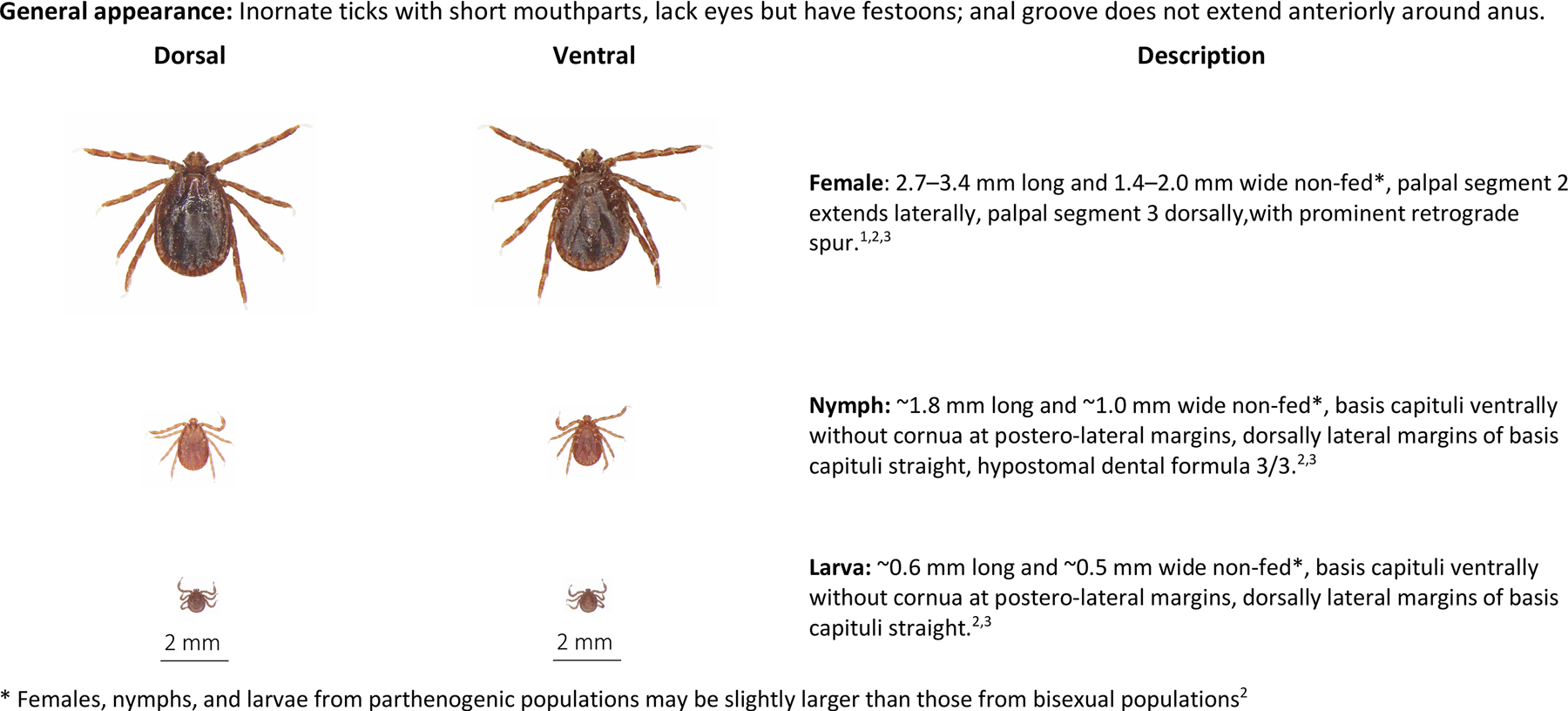
Haemaphysalis longicornis, longhorned tick, bush tick, cattle tick, dorsal and ventral view of female (top), nymph (middle), larva (bottom) and description of key morphologic features. Descriptions adapted from:
1Keirans, J.E., and T.R. Litwak. 1989. Pictorial key to the adults of hard ticks, family Ixodidae (Ixodida: Ixodoidea), East of the Mississippi River. Journal of Medical Entomology 26: 435–448.
2Hoogstraal, H., F.H.S. Roberts, G.M. Kohls, V.J. Tipton. 1968. Review of Haemaphysalis (Kaiseriana) longicornis Neumann (resurrected) of Australia, New Zealand, New Caledonia, Fiji, Japan, Korea, and Northeastern China and USSR, and its parthenogenic and bisexual populations (Ixodoidea, Ixodidae). Journal of Parasitology 54: 1197–1213.
3Egizi, A.M., R.G. Robbins, L. Beati, S. Nava, C.R. Evans, J.L. Occi, D.M. Fonseca. 2019. A pictorial key to differentiate the recently detected exotic Haemaphysalis longicornis Neumann, 1901 (Acari, Ixodidae) from native congeners in North America. ZooKeys 818: 117–128.
2.4.3. Feeding and disease transmission
In parts of the world where H. longicornis is established, longhorned ticks readily infest dogs and cats and are commonly removed from pets (Iwakami et al., 2014; Greay et al., 2016; Zhang et al., 2017). On livestock and wildlife hosts, H. longicornis was found to infest the head and ears, including the periocular region more commonly than elsewhere on the host (Heath et al., 1987; Zheng et al., 2011, 2012). This attachment preference was also noted in the recent North American introduction in New Jersey where ticks were concentrated on the face and ears of the infested sheep (Rainey et al., 2018). Attachment site preferences for dogs and cats have not been reported.
2.4.4. Pathogens
Haemaphysalis longicornis is capable of transmitting several pathogens to pets, livestock, and people (Beard et al., 2018). To date, there have been no reports of pathogens recovered from longhorned ticks removed from dogs and cats in North America, and the only pathogen reported from the United States has been Theileria orientalis Ikeda genotype in host seeking H. longicornis from Virginia (Beard et al., 2018; Thompson et al., 2020). Under experimental conditions, H. longicornis failed to transmit B. burgdorferi sensu stricto (s.s.), the causative agent of Lyme disease, but it was able to acquire and transmit R. rickettsii at a very low frequency between tick generations (Breuner et al., 2020; Stanley et al., 2020). The importance of H. longicornis as an experimentally competent vector of R. rickettsii in North America is unknown at this time, but highlights the need for additional studies to determine the potential role of longhorned ticks in pathogen transmission in North America.
2.4.5. Zoonotic concerns
In its historic range H. longicornis transmits several different pathogens including severe fever with thrombocytopenia syndrome virus (SFTSV), which causes a hemorrhagic fever in people (Luo et al., 2015). However, while H. longicornis has been shown to attach and feed on people in North America, to date no human pathogens have been recovered from these ticks (Beard et al., 2018; Thompson et al., 2020; Tufts et al., 2019). The apparent rapid spread of H. longicornis in North America, specifically in the eastern United States, has been partially attributed to its ability to reproduce parthenogenetically (Stanley et al., 2020). As the longhorned tick becomes further established and more widespread in North America, its involvement in transmission of zoonotic pathogens warrants further consideration.
2.5. Ixodes scapularis (black-legged tick, deer tick) and Ixodes pacificus (western black-legged tick)
Ixodes spp. are ticks of wildlife that readily infest domestic animals and people. Approximately 40 Ixodes spp. have been described in North America, and several have been reported from dogs and cats in this region, including I. affinis, I. angustus, I. banksi, I. cookei, I. kingi, I. marxi, I. muris, and I. texanus (Rand et al., 2007; Durden et al., 2016; Nadolny and Gaff, 2018; Little et al., 2018; Saleh et al., 2019; Ghosh et al., 2021). However, by far the most common species found on domestic animals, including pets, are I. scapularis in the eastern United States and I. pacificus in the West; these two species will be the focus of the current review.
2.5.1. Environment
Just a few decades ago, I. scapularis populations were considered to be somewhat concentrated in fairly focal areas of the upper Midwestern and northeastern United States, with additional populations present in the South (Dennis et al., 1998). Apparent changes in habitat, host, and climate in recent years have allowed this tick to re-establish throughout most of eastern North America, with populations of I. scapularis now found from the Atlantic Coast across to central Texas and up into eastern North Dakota and extending as far north as the southern regions of the Western, Central, and Atlantic provinces of Canada (Eisen et al., 2016a; Clow et al., 2017). In contrast, the geographic range of I. pacificus in western North America has remained fairly stable and extends from the foothills of the Sierra Nevada in California northward into British Columbia (Eisen et al., 2016a).
Ixodes scapularis and I. pacificus populations are found in wooded habitats. A dense understory and accumulation of leaf litter provides favorable microclimates for development and survival of immature and adult ticks in the environment (Lindsay et al., 1999). Seasonal activity of the different stages varies by geographic region. In northern North America, adult I. scapularis are usually most active in the fall and winter with peak activity in October and November when temperatures are between 5–15 °C; a second, smaller peak of adult activity occurs with the onset of spring weather in March and April. In contrast, in Florida and South Carolina, adult I. scapularis activity is highest January through March (Ogden et al., 2004, 2018). In northern regions of North America, immature I. scapularis are most commonly found questing in late spring and summer. Nymphs become active at 15–25 °C and often emerge a few weeks before the larvae of the next generation (Ogden et al., 2004). The pattern of activity for immature stages in the southern United States is less well defined; immature stages in this region quest below the leaf litter, usually feed on lizards rather than small mammals, and are rarely found on people or pets (Oliver, 1996; Arsnoe et al., 2015; Little et al., 2018; Saleh et al., 2019). In northwestern California, adult I. pacificus quest from late October through May with activity peaking in January, nymphs are active January through October, and larvae are found April through June. At the southern extent of the I. pacificus range, seasonal activity, particularly of the immature stages, is confined to a much shorter period of time and all three stages are primarily active in the winter (Salkeld et al., 2014; MacDonald and Briggs, 2016).
Recent increases in the distribution of I. scapularis in the northern and mid-Atlantic United States have been attributed to re-establishment of the historical range of this tick, continuing a trend seen for the past several decades (Spielman, 1994; Eisen et al., 2016a). This range expansion of I. scapularis is considered to be a result of the combined influences of habitat change, increase in white-tailed deer populations, and climate change (Eisen et al., 2016b; Ogden et al., 2018). In the past two decades, the previously distinct foci of tick populations in the northeastern and upper Midwestern United States have largely converged (Eisen et al., 2016a). Ixodes scapularis populations have also spread across western New York, western Pennsylvania, and northern Maine, as well as into higher elevations of Appalachia (Simmons et al., 2015; Herrin et al., 2014; Eddens et al., 2019; Dewage et al., 2019), putting more people and pets at risk of both I. scapularis infestation and infection with associated pathogens (Little et al., 2021).
2.5.2. Morphology
Adult Ixodes spp. are inornate, reddish brown to black in color, and have mouthparts longer than the basis capitulum (Fig. 6). Both eyes and festoons are absent. All stages (larva, nymph, adult) of Ixodes spp. bear a distinct anal groove that arches anterior to the anus. Confirming identification to the species level, even for adult ticks, may require consultation with a diagnostic laboratory with specific expertise; for less common species, both morphologic and molecular identification may be preferred. The presence and number of spurs on the coxae, shape of the scutum, and pattern of dentition on the hypostome are all helpful in confirming the identity of a given Ixodes sp. specimen. Adult I. scapularis have prominent internal spurs on the first pair of coxae. Keys are available to aid in identification of adult and immature Ixodes spp. (Keirans and Litwak, 1989; Durden and Keirans, 1996).
Fig. 6.

Ixodes scapularis, black-legged tick or deer tick, dorsal and ventral view of each stage and description of key morphologic features. From top to bottom: Female, male, nymph, larva. Descriptions adapted from:
1Keirans, J.E., and T.R. Litwak. 1989. Pictorial key to the adults of hard ticks, family Ixodidae (Ixodida: Ixodoidea), East of the Mississippi River. Journal of Medical Entomology 26: 435–448.
2Durden, L.A., and J.E. Keirans. 1996. Nymphs of the Genus Ixodes (Acari: Ixodidae) of the United States: Taxonomy, Identification Key, Distribution, Hosts, and Medical Importance. Thomas Say Publications in Entomology: Monographs. Lanham, Maryland. 76 pp.
3Coley, K. 2015. Identification Guide to Larval Stages of Ticks of Medical Importance in the USA. University Honors Program Thesis, paper 110. Georgia Southern University.
2.5.3. Feeding and disease transmission
Ixodes scapularis and I. pacificus have broad host ranges. Larvae, nymphs, and adult I. scapularis infest and feed on a diverse array of wild and domestic mammals, birds, and reptiles (Yuval and Spielman, 1990). However, differences are observed in host preference according to geographic location and tick species (Bishopp and Trembley, 1945; Cooley and Kohls, 1945; Keirans et al., 1996; Castro and Wright, 2007). Surveying naturally infested hosts throughout the range of I. scapularis demonstrated that immature black-legged ticks in northern areas feed on small mammals (e.g., white-footed mice (Peromyscus leucopus), shrews (Soricidae), chipmunks, etc.) followed by ground-dwelling birds (Mather et al., 1989). In southern areas, immature I. scapularis prefer to feed on lizards (Oliver et al., 1993a) over small mammals. In comparison to surveys of naturally infested animals, host- selection experiments conducted in the lab (James and Oliver, 1990), demonstrated that when given a choice, immature I. scapularis, regardless of whether ticks originated from northern or southern populations, preferentially feed on mice over other hosts. It is likely that natural feeding patterns of I. scapularis result from a multitude of variables (e.g., host availability, questing duration, questing height, climate, etc.), many of which have yet to be elucidated in detail (James and Oliver, 1990). Regardless of geographic location, both northern and southern adult I. scapularis feed (Oliver et al., 1993b) and rely (Wilson et al., 1990) on white-tailed deer to maintain tick populations. In addition to white-tailed deer, other medium and large-sized mammals are commonly infested with I. scapularis adults and nymphs.
In a national survey of ticks on canine and feline companion animals recovered by veterinarians in the United States, I. scapularis was present on 27.4% (409 of 1494) of infested dogs and 46.4% (156 of 336) of cats (Saleh et al., 2019). Dogs were mostly infested with adult I. scapularis but a few nymphs were also recorded. Cats were also mostly infested with adult I. scapularis but a larger proportion of nymphs and larvae were recovered from cats. Proportions of dogs and cats with I. scapularis vary depending on geographic region with differences in tick activity based on local climate and weather (Eisen et al., 2016b). Examination of tick infested dogs from southeastern Oklahoma and northwestern Arkansas (Koch, 1982) noted adult I. scapularis attached to the head and neck. Saleh et al. (2019) also demonstrated that I. scapularis adults preferentially attached to the head, ears, neck and dorsum of infested dogs. Similarly, I. scapularis were commonly recovered from the head, ears, neck, and dorsum of infested cats (Little et al., 2018; Saleh et al., 2019).
In the western United States, I. pacificus also infests a diverse array of wild and domestic mammals, birds, and lizards (Padgett and Lane, 2001; Castro and Wright, 2007). Western fence lizards (Sceloporus occidentalis) are considered preferred hosts for immature I. pacificus which are found more in greater abundance and prevalence on reptiles compared to wild deer mice (Peromyscus spp.) and pinyon mice (P. truei) (Lane and Loye, 1989). Columbian black-tailed deer (O. hemionus columbianus) along with other medium and large-sized mammals are hosts for adult I. pacificus (Padgett and Lane, 2001).
In comparison to humans, I. scapularis and I. pacificus transmit relatively few pathogens to dogs and cats, namely Anaplasma phagocytophilum and B. burgdorferi (Table 1). Ixodes scapularis also transmits Ehrlichia muris eauclairensis in the upper Midwest. From a public health perspective, I. scapularis presents a serious public health concern as this tick vectors seven known pathogens to humans: Anaplasma phagocytophilum, B. burgdorferi, B. mayonii, B. miyamotoi, E. muris eauclairensis, Babesia microti, and Powassan virus (Eisen and Eisen, 2018). Nymphal I. scapularis, in particular, are an important stage for pathogen transmission in the Northeast and Upper Midwest of the United States (Mather et al., 1996; Diuk-Wasser et al., 2010). On the West Coast, I. pacificus vectors A. phagocytophilum and B. burgdorferi, and is a presumed vector of B. miyamotoi to humans (Eisen and Paddock, 2020). Dogs and cats are not primary hosts for any of these pathogens and humans become infected from spill-over events from wild animal reservoirs.
2.5.4. Pathogens
Anaplasma phagocytophilum was shown to be transmitted by I. scapularis in 1996 (Telford et al., 1996), and I. pacificus in 2006 (Teglas and Foley, 2006). Ticks become infected by feeding on A. phagocytophilum infected wild rodents such as the white-footed mouse and eastern chipmunk in the eastern and Midwestern United States, and dusky-footed woodrats (Neotoma fuscipes), gray squirrels (Sciurus griseus), and chipmunks in western states (Carrade et al., 2009). Seroprevalence of A. phagocytophilum in dogs has been decreasing in mid-Atlantic states (e.g., Connecticut, Maryland, New Jersey, Rhode Island) as well as in Virginia, Minnesota, and Wisconsin (Dewage et al., 2019). However, seroprevalence of A. phagocytophilum has continued to increase in Massachusetts, Maine, New Hampshire, and Vermont (Dewage et al., 2019). Cats living in A. phagocytophilum endemic areas can be seropositive but clinical disease appears uncommon (Billeter et al., 2007a, b; Lappin et al., 2020).
Prevalence of A. phagocytophilum in I. scapularis ranges from 0.1–20% in adults and 2.7–13% in nymphs (reviewed by Little and Molaei, 2020). One study suggested that I. scapularis were 98% more likely to be infected with A. phagocytophilum if infected with B. burgdorferi compared to ticks not infected (Little and Molaei, 2020). Prevalence of A. phagocytophilum in I. pacificus ranges from 0.4–4.3% in adults to 0.0–0.2% in nymphs (Eisen and Paddock, 2020).
Borrelia burgdorferi was first identified as the etiological agent of Lyme disease in 1982 (Burgdorfer et al., 1982). Experimental infections of B. burgdorferi in laboratory-reared dogs corroborated observations of naturally infected dogs in that Lyme disease was a multisystemic, polyarthritic condition in canines (Appel et al., 1993). Estimates of B. burgdorferi antibodies in dogs across the United States suggests a decrease in seroprevalence in several states along the mid-Atlantic coast and in Wisconsin (Dewage et al., 2019). These numbers are in stark contrast to an increase in the seroprevalence of B. burgdorferi antibodies in dogs from other northeastern and Midwestern states (e.g., Iowa, Michigan, Maine, New York, Pennsylvania) where Lyme borreliosis is endemic or emerging, as well as in a few states in the upper South (e.g., North Carolina, South Carolina, West Virginia) (Dewage et al., 2019; Little et al., 2021). Cats are susceptible to infection with B. burgdorferi but little is known about the clinical course of the disease (Hoyt et al., 2018; Lappin et al., 2015; Magnarelli et al., 2005).
In New England and the upper Midwestern United States, the prevalence of B. burgdorferi in I. scapularis is relatively high compared to other tick-borne pathogens, typically ranging from 24–64% in adults and 10–23% in nymphs (Tilly et al., 2008; Hamer et al., 2010; Turtinen et al., 2015; Little and Molaei, 2020), depending on experimental methodology, sampling strategy, and endemicity of sampling location. In other regions of the United States, the prevalence of B. burgdorferi is much lower to non-detectable (Maggi et al., 2019). Although, recent data suggests that prevalence of B. burgdorferi is increasing in I. scapularis in areas where the tick is expanding in range (Hickling et al., 2018). Immature ticks become infected when they ingest the spirochete while feeding on B. burgdorferi infected wild rodents (Mather et al., 1989). Nymphal I. scapularis are considered the most important stage for transmitting B. burgdorferi to humans and animals (Mather et al., 1996; Diuk-Wasser et al., 2010). Questing nymphs of I. scapularis are rarely collected by dragging techniques or found infesting people in the southeastern United States (Arsnoe et al., 2015). The prevalence of B. burgdorferi in I. pacificus is considerably lower than that of I. scapularis, with estimates usually less than 5% (Burgdorfer et al., 1985; MacDonald et al., 2017). Analysis of I. pacificus and other ticks in California for infection with B. burgdorferi suggest the highest risk of Lyme disease is in north-central and Sierra Nevada foothill regions of the state with little to no risk in southern regions (Rose et al., 2019).
Ehrlichia muris eauclairensis was first identified in human patients in the upper Midwest with most cases occurring in Minnesota and Wisconsin (Pritt et al., 2011; Johnson et al., 2015; Pritt et al., 2017). Ixodes scapularis larvae were able to acquire and nymphs were able to transmit E. muris eauclairensis to C57BL/6 J mice (Karpathy et al., 2016). Prevalence of E. muris eauclairensis was 2.9% (22 of 757) in I. scapularis adults and 0.9% (10 of 1150) in nymphs (Murphy et al., 2017). Electron microscopy demonstrated that E. muris eauclairensis exhibits an affinity for epithelial cells, neuronal cells of the synganglion, salivary glands, and male accessory glands of I. scapularis (Lynn et al., 2015). Infection of E. muris eauclairensis in a dog resulted in decreased appetite, lethargy, recurrent bouts of vomiting, and fever (Hegarty et al., 2012). Dogs seroconvert to infection with E. muris eauclairensis and antibodies to the pathogen are thought to contribute to the rise in seroprevalence to Ehrlichia spp. in the upper Midwest (Little et al., 2014).
2.5.5. Zoonotic concerns
Both I. scapularis and I. pacificus bite and feed on humans. In addition to the risk of pathogen transmission (Eisen and Eisen, 2018), tick bites are painful and annoy humans (Merten and Durden, 2000; Nelder et al., 2014; Lindquist et al., 2016). Borrelia burgdorferi that causes Lyme disease in humans is the most commonly reported vector-borne pathogen in the United States (Rosenberg et al., 2018). All stages of I. scapularis are commonly reported from people in the eastern and northern United States (Merten and Durden, 2000) and eastern Ontario (Nelder et al., 2014). Ixodes pacificus are commonly recovered from humans in far western states (Merten and Durden, 2000). Despite a basic understanding of the epidemiology of I. scapularis and Lyme disease, infestations of black-legged ticks on humans (Eisen and Eisen, 2018) and companion animals continue to commonly occur (Saleh et al., 2019). Furthermore, it appears I. scapularis is expanding its range in the upper Midwest, northeast and mid-Atlantic regions, while remaining stable in the southeastern United States (Eisen et al., 2016a).
Risk of infestation with I. scapularis is highest for humans in woodland, mixed grassy, brushy, or ecotones of natural areas located around domestic and peridomestic communities or recreation areas (Carroll et al., 1992; Stafford and Connecticut Agricultural Experiment, S., 2007; Hahn et al., 2018). Outdoor recreational activities (Salkeld et al., 2019) and contact with wood (e.g., sitting on logs, gathering wood, sitting against trees, walking, stirring and sitting on leaf litter, just sitting on leaf litter) increase the risk of I. pacificus infestation (Lane et al., 2004). Engorgement indices quantifying specific tick morphological measurements at known time intervals have been developed to estimate duration of attachment as an indirect indicator for risk of pathogen transmission (Yeh et al., 1995). Over 60% of infested people removed I. scapularis adults by 36 h of attachment, but only 10% found and removed nymphal blacklegged ticks within the first 24 h of feeding (Yeh et al., 1995).
2.6. Rhipicephalus spp. (brown dog ticks)
2.6.1. Environment
Brown dog ticks (Rhipicephalus spp.) have the widest geographic distribution of any tick in the world and are now recognized to be a species group comprised of as many as five different species, or operational taxonomic units (OTUs), including R. sanguineus sensu lato (=tropical) and R. sp. I–IV (Dantas-Torres et al., 2013). Because the taxonomy is still under debate, this review will refer to these ticks as Rhipicephalus spp. or brown dog ticks. Two main populations of brown dog ticks have been identified in North America to date. Commonly referred to as “tropical” and “temperate,” these populations do not mate and produce offspring, and can be distinguished from one another on the basis of mitochondrial sequences (16S rDNA, 12S rDNA, and cox1), morphology, and through cross-breeding experiments (Levin et al., 2012; Dantas-Torres et al., 2013; Sanches et al., 2016; Zemtsova et al., 2016; Jones et al., 2017). Intense brown dog tick infestations are more common in warmer areas in the region including Mexico, the Caribbean, and the southern United States. However, due to the propensity of these ticks to establish and flourish indoors, populations of brown dog ticks can be found anywhere there are dogs (Dryden and Payne, 2004).
Brown dog ticks have a strong host preference for dogs and are distinctly endophilic. Accordingly, infestations are commonly associated with kennels or homes where dogs are present, and these ticks are often found crawling on walls, emerging from gaps in the structures, under upholstery cushions, and in carpeted areas where the dogs spend the majority of their time. In warmer areas, these ticks can also survive outdoors so long as cracks and crevices in walls, between rocks, or in the ground are present to provide safe havens for egg deposition by females (Walker et al., 2000; Dantas-Torres, 2010). Although translocation events occasionally occur, tropical brown dog ticks are generally found in areas with annual mean temperatures of >20 °C and temperate brown dog ticks are present where annual mean temperatures are less than 20 °C (Zemtsova et al., 2016; Jones et al., 2017). This disparate species distribution is thought to be related, in part, to poor survival of fed immature stages of tropical brown dog ticks under more severe winter conditions (Labruna et al., 2017).
Survey of dogs has shown infestations with adult brown dog ticks in Oklahoma are most common in July, August, and September, with a second, smaller peak of adult activity in March, April, and May. Larvae were only found on dogs June through September, and nymphs were present in July and again in September and October (Koch, 1982). In Mexico, dogs were found infested with all stages throughout the year although prevalence of infestation was highest spring through fall and lowest in the winter months (Cruz-Vazquez and Garcia-Vazquez, 1999). Brown dog ticks exhibit a strong host preference for domestic dogs in all stages (larvae, nymphs, adults) although specimens are occasionally reported from cats and people (Demma et al., 2005; Thomas et al., 2016; Little et al., 2018; Saleh et al., 2019). In recent years, apparent rapid northward expansion of tropical brown dog ticks has been identified in California and western Arizona (Villarreal et al., 2018).
2.6.2. Morphology
Adult Rhipicephalus spp. are inornate, reddish brown, and have relatively short mouthparts and a hexagonal basis capitulum (Fig. 7). Both festoons and eyes are present. The different taxonomic groups are considered morphologically similar although morphology varies, including in the appearance of festoons, Haller’s organ, accessory shield, adanal shield, and spiracular plates (Dantas-Torres et al., 2013; Caetano et al., 2017). Short mouthparts that are approximately the length of the hexagonal basis capitulum are also evident on larvae and nymphs (Dantas-Torres, 2010).
Fig. 7.

Rhipicephalus sp., brown dog tick, dorsal and ventral view of each stage and description of key morphologic features. From top to bottom: Female, male, nymph, larva. Descriptions adpated from:
1Keirans, J.E., and T.R. Litwak. 1989. Pictorial key to the adults of hard ticks, family Ixodidae (Ixodida: Ixodoidea), East of the Mississippi River. Journal of Medical Entomology 26: 435–448.
2Walker, J.B., J.E. Keiran, and I.G. Horak. 2000. The genus Rhipicephalus (Acari, Ixodidae): A guide to the brown ticks of the world. Cambridge University Press, Cambridge, New York. 643 pp.
3Coley, K. 2015. Identification Guide to Larval Stages of Ticks of Medical Importance in the USA. University Honors Program Thesis, paper 110. Georgia Southern University.
2.6.3. Feeding and disease transmission
Brown dog ticks readily attach to and feed on dogs, and the presence of dogs is critical to long-term establishment of a population of these ticks in a given area. Rhipicephalus spp. ticks may attach and feed anywhere and on any dog, and adults are documented to move between infested dogs resulting in interrupted feeding (Little et al., 2007). However, both feeding site preferences and breed preferences of brown dog ticks have been described. A slight but significant dorsal preference is seen for attachment, and most ticks are found on the head, especially the ears, and the back, but brown dog ticks also readily attach between the toes and in the inguinal and axillary regions (Dantas-Torres, 2010; Saleh et al., 2019). A comparison study using co-housed dogs of different breeds revealed Cocker Spaniel dogs harbored many more ticks than Beagles in the same environment, suggesting ticks were less attracted to Beagles (Louly et al., 2010).
Dogs with brown dog tick infestations often present with very high numbers of ticks, and blood loss anemia — at times severe and life threatening — can occur when infestations are intense (Dantas-Torres, 2010; Herndon and Little, 2015). Bite site reactions may become inflamed and secondary infections occasionally develop, especially with high intensity infestations, but due in part to shorter mouthparts, the trauma caused by brown dog tick feeding is often less severe than that of other tick species. However, brown dog ticks transmit a wide array of pathogens to dogs (Table 1), including Babesia vogeli, E. canis, R. rickettsii, and other spotted fever group Rickettsia spp. (Dantas-Torres, 2010). Most of these agents are transmitted through saliva during tick feeding, but for Hepatozoon canis dogs ingest the tick to become infected (Baneth et al., 2007). While suspected, tick transmission has not been confirmed for some Rhipicephalus sp.-associated pathogens in North America including A. platys, Cercopithifilaria sp., H. canis, and Babesia gibsoni (Allen et al., 2008; Dantas-Torres, 2010; Lineberry et al., 2020). The latter agent is considered more likely to move between dogs in this region via fighting and bite wounds (Yeagley et al., 2009).
2.6.4. Pathogens
Disease caused by brown dog tick-transmitted pathogens is much more common in the southern United States, Mexico, and the Caribbean where this tick commonly occurs than in other areas of North America. Even in regions where disease associated with brown dog ticks is well documented, the prevalence of a given bacterial or protozoal pathogen in ticks is usually low (not detected or <1%) in the absence of active, ongoing transmission among dogs on a given premise. However, when ticks are removed from infected dogs, and especially when engorged ticks that contain concentrated blood from several days of feeding are tested, prevalence of infection in ticks may be much higher. For example, in Yucatan, Mexico, 36% of fed brown dog ticks removed from dogs and 45% of those from an animal shelter with ongoing E. canis transmission were PCR positive for E. canis (Pat-Nah et al., 2015). In contrast, PCR for Anaplasma spp. and Ehrlichia spp. failed to confirm any infections in 519 engorged adult brown dog ticks removed from 100 dogs in northern Mexico despite the fact that A. platys was detected in 3 dogs and E. canis was found in 4 dogs (Almazán et al., 2016). In another study in southern California, United States, E. canis was not detected in unfed, questing Rhipicephalus sp. ticks although R. rickettsii was detected by PCR in one tick (Wikswo et al., 2007).
As the strongly preferred host, dogs serve as the reservoir of pathogens transmitted by brown dog ticks, and in the absence of tick control, infection of dogs is very likely. Canine serosurveys and PCR surveys in the Caribbean, where brown dog ticks are common, have documented E. canis antibodies or DNA in 25–50% of dogs and A. platys antibodies or DNA in 10–25% (Yabsley et al., 2008; Qurollo et al., 2014; Starkey et al., 2016). In the southwestern United States, Mexico, and Central America, antibodies reactive to spotted fever group Rickettsia spp. are detected in approximately 10% of dogs tested although during an outbreak canine seroprevalence as high as 70% has been reported (Demma et al., 2006; Wikswo et al., 2007; Moreira-Soto et al., 2016; Pieracci et al., 2019).
2.6.5. Zoonotic concerns
Brown dog ticks are an important vector of R. rickettsii in the southwestern United States, Mexico, and Central America. Large outbreaks of human disease have been associated with dog overpopulation, dramatic increases in Rhipicephalus sp. numbers, and spillover leading to human infection (Demma et al., 2005, 2006; Nicholson et al., 2006; Eremeeva et al., 2011). Human cases are more commonly seen in areas where canine seroprevalence is high, and dogs can be used as sentinels to predict risk in humans (Demma et al., 2005; McQuiston et al., 2011). Dogs can also serve as an amplifying host for R. rickettsii, with infection detected in approximately 10% of nymphs and 36% of adults that fed as larvae and nymphs, respectively, on infected dogs (Piranda et al., 2011). Rhipicephalus sp. females also pass R. rickettsii infection transovarially, but the route appears inefficient, suggesting horizontal transmission would be necessary to maintain a source of infected ticks in a given area (Piranda et al., 2011). Rickettsia massiliae has been described in brown dog ticks in North America although the role of this agent as a human pathogen in the region is unclear (Eremeeva et al., 2006; Beeler et al., 2011; Fornadel et al., 2013). Zoonotic infection with E. canis has also been described (Perez et al., 2006).
2.7. Otobius megnini (spinose ear tick)
Otobius spp. are soft ticks which parasitize a single host during their life cycle, and only the immature stages are parasitic. Two species present in North America are known to infest dogs and cats — O. megnini and O. lagophilus. Otobius megnini prefers the arid climate of southwestern states, but has extended beyond these regions and infests a variety of animals across the United States including dogs and cats (Soulsby, 1968; Sonenshine and Roe, 2013; Levin, 2016; Little et al., 2018; Saleh et al., 2019); thus, the species is of primary concern in veterinary medicine. Otobius lagophilus, predominantly found in the southwestern United States, infests wild rabbits (Sylvilagus spp. and Lepus spp.) and rarely cats (Cooley and Kohls, 1944a, 1944b; Bacha, 1957).
2.7.1. Environment
Otobius megnini, commonly called the spinose ear tick, was first described in 1883 under the scientific name Argas megnini, was later transferred to the genus Ornithodoros, and finally the genus Otobius was erected in 1912 (Keirans and Pound, 2003; Niebuhr et al., 2013). The tick species prefers dry, hot environments and was historically limited to the southwestern United States and Mexico. Immature stages of O. megnini feed within the external ear canals of a variety of mammals including cattle, goats, horses, sheep, wild ungulates, and less frequently dogs and cats (Soulsby, 1968; Sonenshine and Roe, 2013; Levin, 2016; Little et al., 2018; Saleh et al., 2019). Infestation occurs more often in winter and spring months, but O. megnini can be found on animals year-round (Levin, 2016). Seasonal abundance may vary according to geographic region and annual climactic pattern (Nava et al., 2009; Niebuhr et al., 2013).
Time to life cycle completion of O. megnini depends upon humidity and temperature, which influence time of egg incubation, feeding and molting of immatures, oviposition by females, and larval hatch rate (Vial, 2009; Diyes and Rajakaruna, 2017; Rajakaruna and Diyes, 2019). Unfed larvae are quite active; after finding and climbing onto a suitable host, larval spinose ear ticks will migrate to the ears and locate far within the external acoustic meatus to feed and undergo ecdysis. Although O. megnini is generally accepted to have at least two nymphal instars, consensus among all researchers has yet to be reached. Replete nymphs will drop from host ear canals into the environment, seek shelter in protected microenvironments, and molt to adults (Loomis, 1961; Hoogstraal, 1985; Wanchinga and Barker, 1986; Nava et al., 2009; Niebuhr et al., 2013; Diyes and Rajakaruna, 2017; Njaa, 2017; Rajakaruna and Diyes, 2019).
Adult O. megnini are free-living and thrive in semi-arid climates. They are often found within cracks and crevices of tree bark, log fences, barns, in pasture shelters under accumulated debris, and under logs and fence posts. Unmated females can survive an average of nearly 5 months and a maximum of 18 months under laboratory conditions. Males can live an average of 100 days (Niebuhr et al., 2013; Rajakaruna and Diyes, 2019). Adults mate in the environment, and within approximately two weeks gravid females will produce 4–10 egg clutches comprised of often hundreds of eggs; oviposition occurs during a single, protracted gonotrophic cycle. Larvae may emerge within two-week’s time, but this short of a hatch period appears to occur when environmental temperatures are 25–33 °C. Emerged O. megnini larvae may survive in favorable environmental conditions off-host without a blood meal for nearly three months (Wanchinga and Barker, 1986; Niebuhr et al., 2013; Diyes and Rajakaruna, 2017).
The covert anatomic location that spinose ticks parasitize likely resulted in the inadvertent translocation of infested livestock. Now, in addition to southwestern states previously recognized, the tick species is documented in states bordering the Pacific Coast into British Columbia, in southcentral states, and in several southeastern states. Also, spinose ear ticks are now found in Australia, Africa, Asia, Central and South America, and Europe (Niebuhr et al., 2013; Levin, 2016; Rajakaruna and Diyes, 2019).
A related species, O. lagophilus has been rarely reported infesting cats (Cooley and Kohls, 1944a, [Cooley and Kohls, 1944b] 1944b; Bacha, 1957). Otobius lagophilus is primarily found in the southwestern United States, although isolated reports have occurred in other geographic regions including Canada. Otobius lagophilus has a similar biology to O. megnini, but the parasitic phase of O. lagophilus most commonly attaches around the face and ears of hosts, instead of within the ear canals. Adults of O. lagophilus are found free in the environment but can be found within rodent burrows, suggesting rodents may also serve as natural wildlife hosts. Nymphs of O. megnini and O. lagophilus are morphologically similar, but can be differentiated based on integument spine size, hypostome dentition, and spiracular shape. Otobius lagophilus was once suggested as a vector of Colorado tick fever and a poorly characterized spotted fever group rickettsial agent in the 1950s; however, the tick species is not currently known to vector any pathogens (Bacha, 1957).
2.7.2. Morphology
Adults of O. megnini are non- parasitic and therefore of little clinical significance. Male and female O. megnini possess vestigial mouthparts which are not visible from the dorsal aspect, and their cuticles are somewhat granular. Males and females are morphologically similar except that females are typically larger and have a slightly smaller genital aperture. Adults are greyish to dark brown in color (Diyes and Rajakaruna, 2017). Capitula of nymphs and larvae extend anteriorly when viewed from the dorsal aspect (Fig. 8). Only the nymphal instars have backward-projecting spines that cover the integument; characteristic spines and preferred anatomic location on hosts earned the tick species its common name. Typically, nymphs are the stages observed infesting animals.
Fig. 8.

Otobius megnini, spinose ear tick, dorsal and ventral view of nymph (top) and larva (bottom) and description of key morphologic features. Descriptions adapted from:
1Cooley, R.A., and G.M. Kohls. 1944. The Argasidae of North America, Central America, and Cuba. American Midland Naturalist Monograph No. 1.
Engorged nymphs appear greyish-blue in color, and have a midline constriction that confers a violin-shaped body. Unfed nymphs are less than half the size of engorged nymphs and may appear ivory or reddish brown in color. Larvae are usually not observed infesting hosts due to their small size and location deep within the external ear canal. Engorged larvae may appear greyish, pinkish, or ivory in color. Unfed larvae are minute especially unlikely to be detected within host ear canals or in the environment (Sonenshine and Roe, 2013; Soulsby, 1982; Diyes and Rajakaruna, 2017; Njaa, 2017; Saari, 2019).
2.7.3. Feeding and disease transmission
Otobius megnini larvae feed for up to two weeks (Niebuhr et al., 2013; Diyes and Rajakaruna, 2017). After this feeding period, they molt to nymphs in host ear canals within two-week’s time (Loomis, 1961; Niebuhr et al., 2013; Diyes and Rajakaruna, 2017). Nymphs feed intermittently for approximately 2–4 months, remaining in the ear of the same host throughout (Loomis, 1961; Vial, 2009; Niebuhr et al., 2013; Diyes and Rajakaruna, 2017). However, the collective parasitic phase of O. megnini has been documented to exceed 200 days (Nava et al., 2009). As nymphs mature and grow, they can be observed feeding in more shallow portions of the ear canal or attached to the auricular dermis (Njaa, 2017).
Consequences of infestation with O. megnini are related to the trauma caused by blood-feeding larvae and nymphs within the ear canal and associated discomfort. Severe irritation leads to otitis externa with a waxy exudate. Local reactions at attachment sites include perivascular to interstitial dermatitis containing abundant eosinophils and neutrophils (Duron et al., 2015; Njaa, 2017). Large numbers of spinose ear ticks can produce ulcerative lesions. Animals may exhibit vigorous head-shaking. Ear scratching and rubbing of the head may lead to excoriation of the ear pinnae (Levin, 2016; Njaa, 2017; Saari, 2019).
2.7.4. Pathogens
Infections of O. megnini with Coxiella burnetii (Q fever), Colorado tick fever virus, F. tularensis, and R. rickettsii have been reported, but the tick’s vector competence for these agents is unknown (Bowman, 2013; Levin, 2016). Investigations into the vector capacity of O. megnini for B. burgdorferi and E. canis have yielded no evidence supporting competence. In general, O. megnini is not considered an important vector of any known pathogenic agent in North America (Ewing et al., 1990; Rajakaruna and Diyes, 2019). The detriment of spinose ear tick infestations seems mainly due to annoyance and direct injury caused by immature instars within the external ear canal (Sonenshine and Roe, 2013; Njaa, 2017; Diyes and Rajakaruna, 2017; Levin, 2016).
2.7.5. Zoonotic concerns
Sparse reports of O. megnini infesting humans include immature stages found attached to the conjunctiva of the eye and within the ear canal (Jellison et al., 1948; Naudé et al., 2001; Diyes and Rajakaruna, 2017). Paralysis as a possible result of O. megnini infestation has been documented (Peacock, 1958; Nava et al., 2009). The spinose ear tick was once suspected as a potential vector of C. burnetii and F. tularensis to humans (Jellison et al., 1948; Nava et al., 2009). However, as humans are rarely ever parasitized by O. megnini, the risk of pathogen transmission to humans by this tick species is extraordinarily low.
2.8. Ornithodoros spp.
2.8.1. Environment
Ornithodoros species are soft ticks that are found worldwide (Mullen and Durden, 2019; Horton, 2015; Elelu, 2018). The two species of concern in companion animal medicine in North America are O. hermsi and O. turicata. Ornithodoros hermsi is found in coniferous forests at elevations of 1500–8000 feet in Rocky Mountain States, in states west of the Rocky Mountains, and in southern British Columbia. Ornithodoros turicata prefers drier habitats at lower elevations; the species is found in southcentral and southwestern states and into Mexico (Dworkin et al., 2008). The subspecies O. turicata americanus, only present in Florida, was proposed based on the marked geographic distance from western populations and possible difference in biology (Beck et al., 1986; Lopez et al., 2016; Krishnavahjala et al., 2018).
Ornithodoros hermsi preferentially feeds on deer and diurnal rodents including chipmunks (Tamias spp.) and tree squirrels (Tamiasciurus spp.), and tends to infest nests or tree cracks and crevices near nests (Fritz et al., 2013; Sage et al., 2017; Talagrand-Reboul et al., 2018). Ornithodoros turicata has been collected in association with numerous mammals including cattle, ground squirrels (Spermophilus spp.), pigs, and prairie dogs (Cynomys spp.) (Donaldson et al., 2016). Coyotes may serve as a natural host of O. turicata in Texas. Ornithodoros turicata is often found in underground burrows and cavern floors. In Florida, O. turicata americanus is consistently found in dens of gopher tortoises (Gopherus polyphemus), suggesting they serve as natural hosts of the tick subspecies. Occasionally, Ornithodoros spp. infesting shelter-seeking wildlife may be translocated to vacation cabins or rustic residences and there subsist on other hosts present including dogs and humans (Dworkin et al., 2008; Donaldson et al., 2016; Lopez et al., 2016; Krishnavahjala et al., 2018; Talagrand-Reboul et al., 2018).
2.8.2. Morphology
Ornithodoros species possess hypostomes that are well developed and very similar between males, females, and nymphal instars. The integument is comprised of both discs and mammilla which create various patterns that are continuous from dorsal to ventral surfaces. The bodies typically have very flattened appearances except after feeding, in which case the dorsa are markedly convex. As adults, species may be distinguishable by the presence or absence of eyes and tarsal features. Ornithodoros hermsi is significantly smaller than O. turicata. The integument of O. turicata is made up of characteristically conical mammillae, and the tarsi on the first pair of legs have dorsal projections. Nymphal stages can be differentiated from adults by characteristic protuberances on legs, which become more prominent with successive molts (United States Department of Agriculture, 1976; Dworkin et al., 2008).
2.8.3. Feeding and disease transmission
The life cycles of O. hermsi, O. turicata, and O. turicata americanus are very similar, despite preferring different natural habitats and associated hosts (Beck et al., 1986; Dworkin et al., 2008). Like other Ornithodoros spp., they are endophilic, nidicolous, nocturnal, rapid feeders (≤ 1.5 h until replete), and directly leave hosts after blood meal acquisition. Motile stages include a single larval instar and several successive nymphal instars preceding the molt to females or males. Larvae and younger nymphs require a single blood meal before ecdysis, while larger nymphs feed more than once. Adults feed numerous times during their lifespans, and females oviposit after each blood meal. In the laboratory, nymphs of O. turicata can survive prolonged periods without a blood meal. Adults can survive without a blood meal for 5 years, but can live for over 10 years with regular feedings. Based on field studies, natural Ornithodoros spp. populations may be similarly hardy and long-lived (Beck et al., 1986; Donaldson et al., 2016; Talagrand-Reboul et al., 2018).
Ornithodoros hermsi and O. turicata transmit Borrelia hermsii and Borrelia turicatae, respectively, which are causative agents of tick-borne relapsing fever (TBRF) (Dworkin et al., 2008; Elelu, 2018; Talagrand-Reboul et al., 2018). Geographically, the Borrelia sp. causing TBRF generally corresponds to the distribution of its Ornithodoros sp. vector; in Rocky Mountain and western states, TBRF is predominantly caused by B. hermsii, while in arid regions of the southern United States, TBRF is predominantly caused by B. turicatae (Horton, 2015; Krishnavahjala et al., 2018). Borrelia hermsii and B. turicatae have been isolated from dogs in Washington and Texas, respectively (Kelly et al., 2014). Additionally, B. turicatae was identified in a dog in Florida (Schwan et al., 2009). Ornithodoros turicata americanus is present in Florida, but the natural transmission of B. turicatae by the tick subspecies is not well understood (Krishnavahjala et al., 2018). Rodents and other small mammals are regarded as natural wildlife hosts for TBRF spirochetes (Talagrand-Reboul et al., 2018).
2.8.4. Pathogens
Although Ornithodoros species tend to stay in protected habitats near or where wildlife hosts reside to support their frequent need for blood meals, they are generalists and will feed on other animals in close proximity. Dogs may become infested with Ornithodoros spp. by sleeping in infested cabins or by exploring natural habitats (for example, caves or underground burrows) (Kelly et al., 2014; Elelu, 2018; Talagrand-Reboul et al., 2018). The quick transfer time of Borrelia spp. by Ornithodoros spp., and also that spirochetes may be secreted in both saliva and coxal fluid, facilitates transmission. Borrelia spp. are maintained transstadially in their respective vectors and may transmit transovarially. Despite multiple factors contributing to efficiency of Borrelia spp. transmission by Ornithodoros spp., the diagnosis of TBRF in dogs is rare. As Ornithodoros spp. ticks are fast-feeders, nocturnal, and typically flee hosts after repletion, they are unlikely to be observed by owners. However, TBRF is recognized as an emerging tick-borne disease that is under-diagnosed in veterinary medicine (Dworkin et al., 2008; Elelu, 2018; Talagrand-Reboul et al., 2018).
2.8.5. Zoonotic concerns
Ornithodoros spp. occasionally feed on humans. Cases of human TBRF caused by B. hermsii are often seasonal and typically traced back to events in which humans were sleeping in rustic cabins or within sleeping bags in caves in areas of the western United States (Dworkin et al., 2008; Talagrand-Reboul et al., 2018). Human TBRF cases caused by B. turicatae have occurred predominantly in Texas, with older reports in Kansas and Oklahoma. Human cases of B. turicatae in Florida (potentially transmitted by O. turicata americanus) have not been reported (Dworkin et al., 2008). In California, Borrelia parkeri was documented as the causative agent of TBRF in a single human patient in 1939, and has since been detected in Ornithodoros parkeri collected within the state; the true medical importance of this spirochete remains unclear (Dworkin et al., 2008; Barbour and Miller, 2014).
3. Conclusions
Tick species in North America are diverse in ecology, host-seeking behavior, and pathogens they transmit. Ticks are also dynamic and adaptable, as demonstrated by their changing geographic distributions and establishment in new areas. Factors promoting this expansion include anthropogenic disturbances of terrain, climate change, movement of livestock, translocation via migratory bird hosts, and increases in wildlife populations, particularly white-tailed deer. Here we summarized the current geographic distribution, feeding habits and preferences, and known or novel pathogen transmission patterns for important tick species feeding on dogs and cats in North America. The wildlife-associated ixodid ticks of North America, including A. americanum (lone star tick), A. maculatum (Gulf Coast tick), D. variabilis (American dog tick), I. scapularis (black-legged tick, deer tick), I. pacificus (western black-legged tick), and now H. longicornis (longhorned tick), continue to extend beyond their historic geographic ranges. The domestic dog dependent Rhipicephalus spp. (brown dog ticks) are now documented to occasionally feed on cats and humans. The argasid O. megnini (spinose ear tick), once limited to the southwestern United States, is now found internationally due to movement of infested livestock. Ornithodoros spp. vector relapsing fever in humans, a zoonotic infection now considered emerging in dogs.
As humans continue to encroach into natural tick habitats, modify landscape, and transport livestock and companion animals long distances, tick species will adapt accordingly and extend into new areas. It is critical to continually reassess where tick species occur and evaluate associated pathogen endemicity. Regular surveillance and geospatial predictive mapping efforts investigating changing tick populations in North America are important to protect the health of pets and owners alike. Understanding the basic biology, life history, distribution, and pathogen transmission systems of the common ticks of dogs and cats in this region is vitally important to successfully address the health challenges ticks create.
Acknowledgements
We are grateful to the Department of Veterinary Pathobiology, College of Veterinary Medicine, Oklahoma State University, for supporting the page charges for this review publication, and the National Tick Research and Education Resource at Oklahoma State University for specimens used in images.
Funding
This work was supported in part by the National Center for Veterinary Parasitology, the Krull-Ewing Endowment at Oklahoma State University, and grants from the National Science Foundation (IIA-1920946) and the National Institutes of Health (AI149638).
Footnotes
Declaration of Competing Interest
In the past five years, MNS, KEA, MWL, SEL, and MVR have received honoraria or research support from multiple veterinary pharmaceutical companies directly or through their involvement with the National Center for Veterinary Parasitology. These activities were unrelated to the current review and the authors declare no potential conflicts of interest with respect to the research, authorship, or publication of this article.
CRediT authorship contribution statement
Meriam N. Saleh: Conceptualization, Writing - original draft, Writing - review & editing, Project administration. Kelly E. Allen: Conceptualization, Writing - original draft, Writing - review & editing. Megan W. Lineberry: Visualization, Resources. Susan E. Little: Conceptualization, Writing - original draft, Writing - review & editing. Mason V. Reichard: Conceptualization, Writing - original draft, Writing - review & editing.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
References
- Allen KE, Li Y, Kaltenboeck B, Johnson EM, Reichard MV, Panciera RJ, Little SE, 2008. Diversity of Hepatozoon species in naturally infected dogs in the southern United States. Vet. Parasitol. 154, 220–225. [DOI] [PubMed] [Google Scholar]
- Allen KE, Johnson EM, Little SE, 2011. Hepatozoon spp. Infections in the United States. Vet. Clin. North Am. Small Anim. Pract. 41, 1221–1238. [DOI] [PubMed] [Google Scholar]
- Allen KE, Thomas JE, Wohltjen ML, Reichard MV, 2019. Transmission of Cytauxzoon felis to domestic cats by Amblyomma americanum nymphs. Parasit. Vectors 12, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allerdice ME, Hecht JA, Karpathy SE, Paddock CD, 2017. Evaluation of Gulf Coast ticks (Acari: Ixodidae) for Ehrlichia and Anaplasma species. J. Med. Entomol. 54, 481–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison RW, Little SE, 2013. Diagnosis of rickettsial diseases in dogs and cats. Vet. Clin. Pathol. 42, 127–144. [DOI] [PubMed] [Google Scholar]
- Almazán C, González-Álvarez VH, Fernández de Mera IG, Cabezas-Cruz A, Rodríguez-Martínez R, de la Fuente J, 2016. Molecular identification and characterization of Anaplasma platys and Ehrlichia canis in dogs in Mexico. Ticks Tick Borne Dis. 7, 276–283. [DOI] [PubMed] [Google Scholar]
- Anderson BE, Sims KG, Olson JG, Childs JE, Piesman JF, Happ CM, Maupin GO, Johnson BJ, 1993. Amblyomma americanum: a potential vector of human ehrlichiosis. Am. J. Trop. Med. Hyg. 49, 239–244. [DOI] [PubMed] [Google Scholar]
- Anziania O, Ewing S, Barker R, 1990. Experimental transmission of a granulocytic form of the tribe ehrlichieae by Dermacentor variabilis and Amblyomma americanum to dogs. Am. J. Vet. Res. 51, 929–931. [PubMed] [Google Scholar]
- Apanaskevich AA, Oliver JH Jr., 2013. Chapter 3 – Life cycles and natural history of ticks. In: Sonenshine DE, Roe MR (Eds.), Biology of Ticks, 2nd edition, Vol. 1. Oxford University Press, New York, NY, pp. 3–16. [Google Scholar]
- Appel MJ, Allan S, Jacobson RH, Lauderdale TL, Chang YF, Shin SJ, Thomford JW, Todhunter RJ, Summers BA, 1993. Experimental Lyme disease in dogs produces arthritis and persistent infection. J. Infect. Dis. 167, 651–654. [DOI] [PubMed] [Google Scholar]
- Arsnoe IM, Hickling GJ, Ginsberg HS, McElreath R, 2015. Different populations of blacklegged tick nymphs exhibit differences in questing behavior that have implications for human Lyme disease risk. PLoS One. 10, e0127450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacha WJ Jr, 1957. The life history of Otobius lagophilus. J. Parasitol. 43, 560–565. [PubMed] [Google Scholar]
- Baneth G, Samish M, Shkap V, 2007. Life cycle of Hepatozoon canis (Apicomplexa: Adeleorina: Hepatozoidae) in the tick Rhipicephalus sanguineus and domestic dog (Canis familiaris). J. Parasitol. 93, 283–299. [DOI] [PubMed] [Google Scholar]
- Barbour AG, Miller SC, 2014. Genome sequence of Borrelia parkeri, an agent of enzootic relapsing fever in Western North America. Genome Announc. 2 10.1128/genomeA.00018-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard DR, Morrison RD, Ervin RT, 1989. Sites of attachment and density assessment in Amblyomma americanum (Acari: Ixodidae) on nursing beef calves. Exp. Appl. Acarol. 6, 245–252. [DOI] [PubMed] [Google Scholar]
- Barrett A, Little SE, Shaw E, 2014. “Rickettsia amblyommii” and R. montenensis infection in dogs following natural exposure to ticks. Vector Borne Zoonotic Dis. 14, 20–25. [DOI] [PubMed] [Google Scholar]
- Barrett AW, Noden BH, Gruntmeir JM, Holland T, Mitcham JR, Martin JE, Johnson EM, Little SE, 2015. County scale distribution of Amblyomma americanum (Ixodida: Ixodidae) in Oklahoma: addressing local deficits in tick maps based on passive reporting. J. Med. Entomol. 52, 269–273. [DOI] [PubMed] [Google Scholar]
- Beall MJ, Alleman AR, Breitschwerdt EB, Cohn LA, Couto CG, Dryden MW, Guptill LC, Iazbik C, Kania SA, Lathan P, 2012. Seroprevalence of Ehrlichia canis, Ehrlichia chaffeensis and Ehrlichia ewingii in dogs in North America. Parasit. Vectors 5, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard CB, Occi J, Bonilla DL, Egizi AM, Fonseca DM, Mertins JW, Backenson BP, Bajwa WI, Barbarin AM, Bertone MA, Brown J, Connally NP, Connell ND, Eisen RJ, Falco RC, James AM, Krell RK, Lahmers K, Lewis N, Little SE, Neault M, Pérez de León AA, Randall AR, Ruder MG, Saleh MN, Schappach BL, Schroeder BA, Seraphin LL, Wehtje M, Wormser GP, Yabsley MJ, Halperin W, 2018. Multistate infestation with the exotic disease-vector tick Haemaphysalis longicornis - United States, August 2017-September 2018. Morb. Mortal. Wkly. Rep. 67, 1310–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AF, Holscher KH, Butler JF, 1986. Life cycle of Ornithodoros turicata americanum (Acari: Argasidae) in the laboratory. J. Med. Entomol. 23, 313–319. [DOI] [PubMed] [Google Scholar]
- Beck S, Schreiber C, Schein E, Krücken J, Baldermann C, Pachnicke S, von Samson-Himmelstjerna G, Kohn B, 2014. Tick infestation and prophylaxis of dogs in northeastern Germany: a prospective study. Ticks Tick. Borne. Dis. 5, 336–342. [DOI] [PubMed] [Google Scholar]
- Beeler E, Abramowicz KF, Zambrano ML, Sturgeon MM, Khalaf N, Hu R, Dasch GA, Eremeeva ME, 2011. A focus of dogs and Rickettsia massiliae-infected Rhipicephalus sanguineus in California. Am. J. Trop. Med. Hyg. 84, 244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter SA, Blanton HL, Little SE, Levy MG, Breitschwerdt EB, 2007a. Detection of “Rickettsia amblyommii” in association with a tick bite rash. J. Med. Entomol 7, 607–610. [DOI] [PubMed] [Google Scholar]
- Billeter SA, Spencer JA, Griffin B, Dykstra CC, Blagburn BL, 2007b. Prevalence of Anaplasma phagocytophilum in domestic felines in the United States. Vet. Parasitol. 147, 194–198. [DOI] [PubMed] [Google Scholar]
- Birkenheuer AJ, Marr HS, Warren C, Acton AE, Mucker EM, Humphreys JG, Tucker MD, 2008. Cytauxzoon felis infections are present in bobcats (Lynx rufus) in a region where cytauxzoonosis is not recognized in domestic cats. Vet. Parasitol. 153, 126–130. [DOI] [PubMed] [Google Scholar]
- Bishopp FC, Trembley HL, 1945. Distribution and hosts of certain North American ticks. J. Parasitol. 81, 1–54. [Google Scholar]
- Blouin EF, Kocan AA, Glenn BL, Kocan KM, Hair JA, 1984. Transmission of Cytauxzoon felis Kier, 1979 from bobcats, Felis rufus (Schreber), to domestic cats by Dermacentor variabilis (Say). J. Wildl. Dis. 20, 241–242. [DOI] [PubMed] [Google Scholar]
- Blouin EF, Kocan AA, Kocan KM, Hair J, 1987. Evidence of a limitied schizogonous cycle for Cytauxzoon felis in bobcats following exposure to infected ticks. J. Wildl. Dis. 23, 499–501. [DOI] [PubMed] [Google Scholar]
- Bolte JR, Hair JA, Fletcher J, 1970. White-tailed deer mortality following tissue destruction induced by lone star ticks. J. Wildl. Manage. 34, 546–552. [Google Scholar]
- Bondy PJ, Cohn LA, Tyler JW, Marsh AE, 2005. Polymerase chain reaction detection of Cytauxzoon felis from field-collected ticks and sequence analysis of the small subunit and internal transcribed spacer 1 region of the ribosomal RNA gene. J. Parasitol. 91, 458–461. [DOI] [PubMed] [Google Scholar]
- Bowman DD, 2013. Arthropods. In: Bowman DD (Ed.), Georgi’s Parasitology for Veterinarians, 10th edition. Elsevier Saunders, St. Louis, MS, pp. 11–85. [Google Scholar]
- Brault AC, Savage HM, Duggal NK, Eisen RJ, Staples JE, 2018. Heartland virus epidemiology, vector association, and disease potential. Viruses 10, 498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitschwerdt EB, Nicholson WL, Kiehl AR, Steers C, Meuten DJ, Levine JF, 1994. Natural infections with Borrelia spirochetes in two dogs from Florida. J. Clin. Microbiol. 32, 352–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitschwerdt EB, Hegarty BC, Maggi RG, Lantos PM, Aslett DM, Bradley JM, 2011. Rickettsia rickettsii transmission by a lone star tick, North Carolina. Emerg. Infect. Dis. 17, 873–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitschwerdt EB, Hegarty BC, Qurollo BA, Saito TB, Maggi RG, Blanton LS, Bouyer DH, 2014. Intravascular persistence of Anaplasma platys, Ehrlichia chaffeensis, and Ehrlichia ewingii DNA in the blood of a dog and two family members. Parasit. Vectors. 7, 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuner NE, Ford SL, Hojgaard A, Osikowicz LM, Parise CM, Rosales Rizzo MF, Bai Y, Levin ML, Eisen RJ, Eisen L, 2020. Failure of the Asian longhorned tick, Haemaphysalis longicornis, to serve as an experimental vector of the lyme disease spirochete, Borrelia burgdorferi sensu stricto. Ticks Tick Borne Dis. 11, 101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton EP, Beck DE, Allred DM, 1965. Identification of the adults, nymphs and larvae of ticks of the genus Dermacentor Koch (Ixodidae) in the western United States. Brigham Young university science bulletin. Biological Series. 4, 1. [Google Scholar]
- Brown HM, Lockhart JM, Latimer KS, Peterson DS, 2010. Identification and genetic characterization of Cytauxzoon felis in asymptomatic domestic cats and bobcats. Vet. Parasitol. 172, 311–316. [DOI] [PubMed] [Google Scholar]
- Brown HE, Yates KF, Dietrich G, MacMillan K, Graham CB, Reese SM, Helterbrand WS, Nicholson WL, Blount K, Mead PS, 2011. An acarologic survey and Amblyomma americanum distribution map with implications for tularemia risk in Missouri. Am. J. Trop. Med. Hyg. 84, 411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller RS, Arens M, Hmiel SP, Paddock CD, Sumner JW, Rikihisa Y, Unver A, Gaudreault- Keener M, Manian FA, Liddell AM, Schmulewitz N, Storch GA, 1999. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. New England J Med. 341, 148–155. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP, 1982. Lyme disease-a tick-borne spirochetosis? Science. 216, 1317–1319. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W, Lane RS, Barbour AG, Gresbrink RA, Anderson JR, 1985. The western black-legged tick, Ixodes pacificus: a vector of Borrelia burgdorferi. Am. J. Trop. Med Hyg. 34, 925–930. [DOI] [PubMed] [Google Scholar]
- Burket CT, Vann CN, Pinger RR, Chatot CL, Steiner FE, 1998. Minimum infection rate of Ambylomma americanum (Acari: Ixodidae) by Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) in Southern Indiana. J. Med. Entomol. 35, 653–659. [DOI] [PubMed] [Google Scholar]
- Burroughs JE, Thomasson JA, Marsella R, Greiner EC, Allan SA, 2016. Ticks associated with domestic dogs and cats in Florida. USA. Exp. Appl. Acaraol. 69, 87–95. [DOI] [PubMed] [Google Scholar]
- Caetano RL, Vizzoni VF, Bitencourth K, Carriço C, Sato TP, Pinto ZT, De Oliveira SV, Amorim M, Voloch CM, Gazeta GS, 2017. Ultrastructural morphology and molecular analyses of tropical and temperate “species” of Rhipicephalus sanguineus sensu lato (Acari: Ixodidae) in Brazil. J. Med. Entomol. 54, 1201–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun EL, 1954. Natural occurrence of tularemia in the lone star tick, Amblyomma americanum (Linn.), and in dogs in Arkansas. Am. J. Trop. Med. Hyg. 3, 360–366. [DOI] [PubMed] [Google Scholar]
- Campbell BS, Bowles DE, 1994. Human tick bite records in a United States air force population, 1989–1992: implications for tick-borne disease risk. J. Wilderness Med. 5, 405–412. [Google Scholar]
- Carr AL, Mitchell III RD, Dhammi A, Bissinger BW, Sonenshine DE, Roe RM, 2017. Tick haller’s organ, a new paradigm for arthropod olfaction: How ticks differ from insects. Int. J. Mol. Sci. 18, 1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrade D, Foley JE, Borjesson DL, Sykes JE, 2009. Canine granulocytic anaplasmosis: a review. J. Vet. Intern. Med. 23, 1129–1141. [DOI] [PubMed] [Google Scholar]
- Carroll MC, Ginsberg HS, Hyland KE, Hu R, 1992. Distribution of Ixodes dammini (Acari: Ixodidae) in residential lawns on prudence Island, Rhode Island. J. Med. Entomol. 29, 1052–1055. [DOI] [PubMed] [Google Scholar]
- Carroll JF, Allen PC, Hill DE, Pound JM, Miller JA, George JE, 2002. Control of Ixodes scapularis and Amblyomma americanum through use of the’ 4-poster’ treatment device on deer in Maryland. Exp. Appl. Acarol. 28, 289–296. [DOI] [PubMed] [Google Scholar]
- Castellaw A, Showers J, Goddard J, Chenney E, Varela-Stokes A, 2010. Detection of vector-borne agents in lone star ticks, Amblyomma americanum (Acari: Ixodidae), from Mississippi. J. Med. Entomol. 47, 473–476. [DOI] [PubMed] [Google Scholar]
- Castro MB, Wright SA, 2007. Vertebrate hosts of Ixodes pacificus (Acari: Ixodidae) in California. J. Vector. Ecol. 32, 140–149. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2018. Tickborne Diseases of the United States, 5th edition. Accessed 27 Oct 2020. https://www.cdc.gov/ticks/tickbornediseases/TickborneDiseases-P.pdf.
- Childs JE, Paddock CD, 2003. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu. Rev. Entomol. 48, 307–337. [DOI] [PubMed] [Google Scholar]
- Clow KM, Leighton PA, Ogden NH, Lindsay LR, Michel P, Pearl DL, Jardine CM, 2017. Northward range expansion of Ixodes scapularis evident over a short timescale in Ontario, Canada. PLoS One. 12, e0189393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SB, Yabsley MJ, Freye JD, Dunlap BG, Rowland ME, Huang J, Dunn JR, Jones TF, Moncayo AC, 2010. Prevalence of Ehrlichia chaffeensis and Ehrlichia ewingii in ticks from Tennessee. Vector Borne Zoonotic Dis. 10, 435–440. [DOI] [PubMed] [Google Scholar]
- Coley K, 2015. Identification Guide to Larval Stages of Ticks of Medical Importance in the USA. Honors Thesis. Georgia Southern University; https://digitalcommons.georgiasouthern.edu/honors-theses/110. [Google Scholar]
- Commins SP, James HR, Kelly LA, Pochan SL, Workman LJ, Perzanowski MS, Kocan KM, Fahy JV, Nganga LW, Ronmark E, Cooper PJ, Platts-Mills TAE, 2011. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-alpha-1,3-galactose. J. Allergy Clin. Immunol. 127, 1286–1293 e1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley RA, 1946. The genera Boophilus, Rhipicephalus, and Haemaphysalis (Ixodidae) of the New world. NIH Bulletin No. 187, 54. [Google Scholar]
- Cooley RA, Kohls GM, 1944a. The genus Amblyomma (Ixodidae) in the United States. J. Parastol. 30, 77–111. [Google Scholar]
- Cooley RA, Kohls GM, 1945. The Genus Ixodes in North America. US Government Printing Office. [Google Scholar]
- Cooley RA, Kohls GM, 1944b. The Argasidae of North America, Central America, and Cuba. American Midland Naturalist Monograph. No. 1. [Google Scholar]
- Cortinas R, Spomer S, 2013. Lone star tick (Acari: Ixodidae) occurrence in Nebraska: historical and current perspectives. J. Med. Entomol. 50, 244–251. [DOI] [PubMed] [Google Scholar]
- Cruz-Vazquez C, Garcia-Vazquez Z, 1999. Seasonal distribution of Rhipicephalus sanguineus ticks (Acari: Ixodidae) on dogs in an urban area of morelos, Mexico. Exper. Appl. Acarol. 23, 277–280. [DOI] [PubMed] [Google Scholar]
- Dahlgren FS, Paddock CD, Springer YP, Eisen RJ, Behravesh CB, 2016. Expanding range of Amblyomma americanum and simultaneous changes in the epidemiology of spotted fever group rickettsiosis in the United States. Am. J. Trop. Med. Hyg. 94, 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantas- Torres F, 2010. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit. Vector. 3, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantas- Torres F, Latrofa MS, Annoscia G, Giannelli A, Parisi A, Otranto D, 2013. Morphological and genetic diversity of Rhipicephalus sanguineus sensu lato from the New and Old worlds. Parasit. Vector. 6, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demma LJ, Traeger MS, Nicholson WL, Paddock CD, Blau DM, Eremeeva ME, Dasch GA, Levin ML, Singleton J Jr, Zaki SR, Cheek JE, Swerdlow DL, McQuiston JH, 2005. Rocky Mountain spotted fever from an unexpected tick vector in Arizona. New. Eng. J. Med. 353, 587–594. [DOI] [PubMed] [Google Scholar]
- Demma LJ, Traeger M, Blau D, Gordon R, Johnson B, Dickson J, Ethelbah R, Piontkowski S, Levy C, Nicholson WL, Duncan C, Heath K, Cheek J, Swerdlow DL, McQuiston JH, 2006. Serologic evidence for exposure to Rickettsia rickettsii in eastern Arizona and recent emergence of Rocky Mountain spotted fever in this region. Vector Borne Zoonotic Dis. 6, 423–429. [DOI] [PubMed] [Google Scholar]
- Dennis DT, Nekomoto TS, Victor JC, Paul WS, 1998. Reported distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the United States. J. Med. Entomol. 35, 629–638. [DOI] [PubMed] [Google Scholar]
- Dergousoff SJ, Galloway TD, Lindsay LR, Curry PS, Chilton NB, 2013. Range expansion of Dermacentor variabilis and Dermacentor andersoni (Acari: Ixodidae) near their northern distributional limits. J. Med. Entomol. 50, 510–520. [DOI] [PubMed] [Google Scholar]
- DeShields A, Borman-Shoap E, Peters JE, Gaudreault-Keener M, Arens MQ, Storch GA, 2004. Detection of pathogenic Ehrlichia in ticks collected at acquisition sites of human ehrlichiosis in Missouri. Mo. Med. 101, 132. [PubMed] [Google Scholar]
- Dewage BG, Little S, Payton M, Beall M, Braff J, Szlosek D, Buch J, Knupp A, 2019. Trends in canine seroprevalence to Borrelia burgdorferi and Anaplasma spp. In the eastern USA, 2010–2017. Parasit. Vectors. 12, 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz JH, 2010. A 60-year meta-analysis of tick paralysis in the United States: a predictable, preventable, and often misdiagnosed poisoning. J. Med. Toxicol. 6, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diuk- Wasser MA, Vourc’h G, Cislo P, Hoen AG, Melton F, Hamer SA, Rowland M, Cortinas R, Hickling GJ, Tsao JI, Barbour AG, Kitron U, Piesman J, Fish D, 2010. Field and climate- based model for predicting the density of host-seeking nymphal Ixodes scapularis, an important vector of tick-borne disease agents in the eastern United States. Glo. Ecol. Biogeogr. 19, 504–514. [Google Scholar]
- Diyes GCP, Rajakaruna RS, 2017. Life cycle of spinose ear ticks, Otobius megnini (Acari: Argasidae) infesting the race horses in nuwara eliya, Sri Lanka. Acta Trop. 166, 164–176. [DOI] [PubMed] [Google Scholar]
- Donaldson TG, Pèrez de León AA, Castro–Arellano I, Wozniak E, Boyle WK, Hargrove R, Wilder HK, Kim HJ, Teel PD, Lopez JE, 2016. Assessment of the geographic distribution of Ornithodoros turicata (Argasidae): climate variation and host diversity. PLoS Negl. Trop. Dis 10. 10.1371/journal.pntd.0004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler NA, Yaglom H, Casal M, Fierro M, Kriner P, Murphy B, Kjemtrup A, Paddock CD, 2017. Fatal Rocky Mountain spotted fever along the United States-Mexico border, 2013–2016. Emerg. Infect. Dis. 23, 1621–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryden MW, Payne PA, 2004. Biology and control of ticks infesting dogs and cats in North America. Vet Ther. 5, 139–154. [PubMed] [Google Scholar]
- Duncan KT, Clow KM, Sundstrom KD, Saleh MN, Reichard MV, Little SE, 2020. Recent reports of winter tick, Dermacentor albipictus, from dogs and cats in North America. Vet. Parasitol. Reg. Stud. Reports. 22, 100490. [DOI] [PubMed] [Google Scholar]
- Duncan KT, Saleh MN, Sundstrom KD, Little SE, 2021. Dermacentor variabilis is the predominant Dermacentor spp. (Acari: Ixodidae) feeding on dogs and cats throughout the United States. J. Med. Entomol. Tjab007 (online ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durden LA, Keirans JE, 1996. Nymphs of the genus Ixodes of the United States. Lanham, Maryland: entomol. Soc. Am. [Google Scholar]
- Durden LA, Beckmen KB, Gerlach RF, 2016. New records of ticks (Acari: Ixodidae) from dogs, cats, humans, and some wild vertebrates in Alaska: invasion potential. J. Med. Entomol. 53, 1391–1395. [DOI] [PubMed] [Google Scholar]
- Duron O, Sidi–Boumedine K, Rousset E, Moutailler S, Jourdain E, 2015. The importance of ticks in Q fever transmission: what has (and has not) been demonstrated? Trends Parasitol. 31, 536–552. [DOI] [PubMed] [Google Scholar]
- Duscher GG, Feiler A, Leschnik M, Joachim A, 2013. Seasonal and spatial distribution of ixodid tick species feeding on naturally infested dogs from eastern Austria and the influence of acaricides/repellents on these parameters. Parasit. Vectors. 6, 76–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin MS, Schwan TG, Anderson DE Jr., Borchardt SM, 2008. Tick–borne relapsing fever. Infect. Dis. Clin. North Am. 22, 449–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton ER, Keirans JE, Gresbrink RA, Clifford CM, 1977. The distribution in oregon of Ixodes pacificus, Dermacentor andersoni, and Dermacentor occidentalis with a note on Dermacentor variabilis (Acarina: Ixodidae). J. Med. Entomol. 13, 501–506. [DOI] [PubMed] [Google Scholar]
- Eddens T, Kaplan DJ, Anderson AJM, Nowalk AJ, Campfield BT, 2019. Insights from the geographic spread of the Lyme disease epidemic. Clin. Infect. Dis. 68, 426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards KT, 2011. Gotch ear: a poorly described, local, pathologic condition of livestock associated primarily with the Gulf Coast tick. Vet. Parasitol. 183, 1–7. [DOI] [PubMed] [Google Scholar]
- Egizi AM, Robbins RG, Beati L, Nava S, Vans CR, Occi JL, Fonseca DM, 2019. A pictorial key to differentiate the recently detected exotic Haemaphysalis longicornis neumann, 1901 (Acari: Ixodidae) from native congeners in North America. ZooKeys. 818, 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen L, 2007. A call for renewed research on tick-borne Francisella tularensis in the Arkansas-Missouri primary national focus of tularemia in humans. J. Med. Entomol. 44, 389–397. [DOI] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, 2018. The blacklegged tick, Ixodes scapularis: an increasing public health concern. Trends. Parasitol. 34, 295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Paddock CD, 2020. Tick and tickborne pathogen surveillance as a public health tool in the United States. J. Med. Entomol. 10.1093/jme/tjaa087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, Beard CB, 2016a. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the continental United States. J. Med. Entomol. 53, 349–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, Ogden NH, Beard CB, 2016b. Linkages of weather and climate with Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae), enzootic transmission of Borrelia burgdorferi, and Lyme disease in North America. J. Med. Entomol. 53, 250–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Kugeler KJ, Eisen L, Beard CB, Paddock CD, 2017. Tick-Borne zoonoses in the United States: persistent and emerging threats to human health. ILAR. J. 58, 319–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elchos BN, Goddard J, 2003. Implications of presumptive fatal Rocky Mountain spotted fever in two dogs and their owner. J. Am. Vet. Med. Assoc. 223, 1450–1433. [DOI] [PubMed] [Google Scholar]
- Elelu N, 2018. Tick-borne relapsing fever as a potential veterinary medical problem. Vet. Med. Sci. 4, 271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eremeeva ME, Bosserman EA, Demma LJ, Zambrano ML, Blau DM, Dasch GA, 2006. Isolation and identification of Rickettsia massiliae from Rhipicephalus sanguineus ticks collected in Arizona. Appl. Environ. Microbiol. 72, 5569–5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eremeeva ME, Zambrano ML, Anaya L, Beati L, Karpathy SE, Santos- Silva MM, Salceda B, MacBeth D, Olguin H, Dasch GA, Aranda CA, 2011. Rickettsia rickettsii in Rhipicephalus ticks, Mexicali, Mexico. J. Med. Entomol. 48, 418–421. [DOI] [PubMed] [Google Scholar]
- Espinoza-Gomez F, Newton-Sanchez O, Flores- Cazares G, De la Cruz-Ruiz M, Melnikov V, Austria- Tejeda J, Rojas-Larios F, 2011. Tick paralysis caused by Amblyomma maculatum on the Mexican pacific Coast. Vector Borne Zoonotic Dis. 11, 945–946. [DOI] [PubMed] [Google Scholar]
- Esteve-Gasent MD, Snell CB, Adetunji SA, Piccione J, 2017. Serological detection of tick-borne relapsing fever in Texan domestic dogs. PLOS ONE 12, e0189786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Peña A, Venzal JM, Mangold AJ, Cafrune MM, Guglielmone AA, 2005. The Amblyomma maculatum Koch, 1844 (Acari: Ixodidae: Amblyomminae) tick group: diagnostic characters, description of the larva of A. parvitarsum Neumann, 1901, 16s rDNA sequences, distribution and hosts. Syst. Parasitol. 60, 99–112. [DOI] [PubMed] [Google Scholar]
- Ewing SA, Harkess JR, Kocan KM, Barker RW, Fox JC, Tyler RD, Cowell RL, Morton RB, 1990. Failure to transmit Ehrlichia canis (Rickettsiales: Ehrlichieae) with Otobius megnini (Acari: Argasidae). J. Med. Entomol. 27, 803–806. [DOI] [PubMed] [Google Scholar]
- Ewing S, Dawson J, Kocan A, Barker R, Warner C, Panciera R, Fox J, Kocan K, Blouin E, 1995. Experimental transmission of Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) among white-tailed deer by Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 32, 368–374. [DOI] [PubMed] [Google Scholar]
- Feldman KA, Stiles-Enos D, Julian K, Matyas BT, Telford SR 3rd, Chu MC, Petersen LR, Hayes EB, 2003. Tularemia on Martha’s Vineyard: seroprevalence and occupational risk. Emerg. Infect. Dis. 9, 350–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felz MW, Durden LA, Oliver JH Jr, 1996. Ticks parasitizing humans in Georgia and South Carolina. J. Parasitol. 82, 505–508. [PubMed] [Google Scholar]
- Fitak RR, Kelly DJ, Daniels MK, Jiang J, Richards AL, Fuerst PA, 2014. The prevalence of rickettsial and ehrlichial organisms in Amblyomma americanum ticks collected from Ohio and surrounding areas between 2000 and 2010. Ticks Tick Borne Dis. 5, 797–800. [DOI] [PubMed] [Google Scholar]
- Flynn PC, Kaufman WR, 2011. Female ixodid ticks grow endocuticle during the rapid phase of engorgement. Exp. Appl. Acarol. 53, 167–178. [DOI] [PubMed] [Google Scholar]
- Fornadel CM, Smith JD, Zawada SE, Arias JR, Norris DE, 2013. Detection of Rickettsia massiliae in Rhipicephalus sanguineus from the eastern United States. Vector Borne Zoonotic Dis. 13, 67–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz CL, Payne JR, Schwan TG, 2013. Serologic evidence for Borrelia hermsii infection in rodents on federally owned recreational areas in California. Vector Borne Zoonotic Dis. 13, 376–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzen CM, Huang J, Westby K, Freye JD, Dunlap B, Yabsley MJ, Schardein M, Dunn JR, Jones TF, Moncayo AC, 2011. Infection prevalences of common tick-borne pathogens in adult lone star ticks (Amblyomma americanum) and American dog ticks (Dermacentor variabilis) in Kentucky. Am. J. Trop. Med. Hyg. 85, 718–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaines DN, Operario DJ, Stroup S, Stromdahl E, Wright C, Gaff H, Broyhill J, Smith J, Norris DE, Henning T, 2014. Ehrlichia and spotted fever group rickettsiae surveillance in Amblyomma americanum in Virginia through use of a novel six-plex real-time PCR assay. Vector Borne Zoonotic Dis. 14, 307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett JJ, Kocan AA, Reichard MV, Panciera RJ, Bahr RJ, Ewing SA, 2005. Experimental infection of adult and juvenile coyotes with domestic dog and wild coyote isolates of Hepatozoon americanum (Apicomplexa: Adeleorina). J. Wildl. Dis. 41, 588–592. [DOI] [PubMed] [Google Scholar]
- Ghosh P, Saleh MN, Sundstrom KD, Ientile M, Little SE, 2021. Ixodes spp. from dogs and cats in the United States: diversity, seasonality, and prevalence of Borrelia burgdorferi and Anaplasma phagocytophilum. Vector Borne Zoon Dis. 21, 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons RJ, 1939. Survey of Rocky Mountain spotted fever and sylvatic plague in western Canada during 1938. Can. J. Public Health 30, 184–187. [Google Scholar]
- Goddard J, 2002. A ten-year study of tick biting in Mississippi: implications for human disease transmission. J. Agromedicine 8, 25–32. [DOI] [PubMed] [Google Scholar]
- Goddard J, 2007. Seasonal activity of Amblyomma spp. in Mississippi. J. Vector Ecol. 32, 157–158. [DOI] [PubMed] [Google Scholar]
- Goddard J, Sumner JW, Nicholson WL, Paddock CD, Shen J, Piesman J, 2003. Survey of ticks collected in Mississippi for Rickettsia, Ehrlichia, and Borrelia species. J. Vector Ecol. 28, 184–189. [PubMed] [Google Scholar]
- Goddard J, Allerdice M, Portugal JS, Moraru GM, Paddock CD, King J, 2020. What Is going on with the genus Dermacentor? Hybridizations, introgressions, Oh My! J. Med. Entomol. 57 (May 3), 653–656. [DOI] [PubMed] [Google Scholar]
- Gothe R, Neitz AWH, 1991. Tick paralysis: pathogeneses and etiology. In: Harris KF (Ed.), Advances in Disease Vector Research, Vol. 8. Springer, New York, NY, pp. 177–204. [Google Scholar]
- Gothe R, Kunze K, Hoogstraal H, 1979. The mechanisms of pathogenicity in the tick paralyses. J. Med. Entomol. 16, 357–369. [DOI] [PubMed] [Google Scholar]
- Grasperge BJ, Wolfson W, Macaluso KR, 2012. Rickettsia parkeri infection in domestic dogs, southern Louisiana, USA. Emerg. Infect. Dis. 18, 995–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JS, Estrada-Peña A, Vial L, 2013. Chapter 2 – Biology of nidocolous ticks. In: Sonenshine DE, Roe MR (Eds.), Biology of Ticks, 2nd edition, Vol. 1. Oxford University Press, New York, NY, pp. 39–60. [Google Scholar]
- Greay TL, Oskam CL, Gofton AW, Rees RL, Ryan UM, Irwin PJ, 2016. A survey of ticks (Acari: Ixodidae) of companion animals in Australia. Parasit. Vectors. 9, 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves MG, Dennis GL, Amyx HL, Huxsoll DL, 1975. Transmission of Ehrlichia canis to dogs by ticks (Rhipicephalus sanguineus). Am. J. Vet. Res. 36, 937–940. [PubMed] [Google Scholar]
- Guzmán-Cornejo C, Robbins RG, Guglielmone AA, Montiel-Parra G, Rivas G, Pérez TM, 2016. The Dermacentor (Acari, Ixodida, Ixodidae) of Mexico: hosts, geographical distribution and new records. ZooKeys. 569, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber MD, Tucker MD, Marr HS, Levy JK, Burgess J, Lappin MR, Birkenheuer AJ, 2007. The detection of Cytauxzoon felis in apparently healthy free-roaming cats in the USA. Vet. Parasitol. 146, 316–320. [DOI] [PubMed] [Google Scholar]
- Hahn MB, Bjork JK, Neitzel DF, Dorr FM, Whitemarsh T, Boegler KA, Graham CB, Johnson TL, Maes SE, Eisen RJ, 2018. Evaluating acarological risk for exposure to Ixodes scapularis and Ixodes scapularis-borne pathogens in recreational and residential settings in Washington County, Minnesota. Ticks Tick. Borne. Dis. 9, 340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hair JA, Howell DE, 1970. Lone star ticks; their biology and control in Ozark recreation areas. Oklahoma Agric. Exper. Station Bullet. B–679. [Google Scholar]
- Hair JA, Hoch A, Buckner R, Barker R, 1992. Fawn hematology and survival following tick infestation and theileriasis. J. Agric. Entomol. 9, 301–319. [Google Scholar]
- Hamer SA, Tsao JI, Walker ED, Hickling GJ, 2010. Invasion of the Lyme disease vector Ixodes scapularis: implications for Borrelia burgdorferi endemicity. EcoHealth. 7, 47–63. [DOI] [PubMed] [Google Scholar]
- Hardstone Yoshimizu M, Billeter SA, 2018. Suspected and confirmed vector-borne rickettsioses of North America associated with human diseases. Trop. Med. Infect. Dis. 3, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris EK, Verhoeve VI, Banajee KH, Macaluso JA, Azad AF, Macaluso KR, 2017. Comparative vertical transmission of Rickettsia by Dermacentor variabilis and Amblyomma maculatum. Ticks Tick Borne Dis. 8, 598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath ACG, 2013. Implications for New Zealand of potentially invasive ticks sympatric with Haemaphysalis longicornis Neumann, 1901 (Acari: Ixodidae). Syst Appl. Acaraol. 18, 1–26. [Google Scholar]
- Heath ACG, 2016. Biology, ecology and distribution of the tick, Haemaphysalis longicornis Neumann (Acari: Ixodidae) in New Aealand. New Zealand Vet. J 64, 10–20. [DOI] [PubMed] [Google Scholar]
- Heath ACG, Tenquist JD, Bishop DM, 1987. Goats, hares, and rabbits as hosts for the New Zealand cattle tick, Haemaphysalis longicornis. New Zealand J. Zool. 14, 549–555. [Google Scholar]
- Hecht JA, Allerdice MEJ, Dykstra EA, Mastel L, Eisen RJ, Johnson TL, Gaff HD, Varela-Stokes AS, Goddard J, Pagac BB, Paddock CD, Karpathy SE, 2019. Multistate survey of American dog ticks (Dermacentor variabilis) for Rickettsia species. Vector Borne Zoonotic Dis. 19, 652–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty B, Maggi R, Koskinen P, Beall M, Eberts M, Chandrashekar R, Breitschwerdt E, 2012. Ehrlichia muris infection in a dog from Minnesota. J. Vet. Intern. Med. 26, 1217–1220. [DOI] [PubMed] [Google Scholar]
- Herndon A, Little S, 2015. Complications of severe tick infestation. Clin. Brief. 4, 19–21. [Google Scholar]
- Herrin BH, Zajac AM, Little SE, 2014. Confirmation of Borrelia burgdorferi sensu stricto and Anaplasma phagocytophilum in Ixodes scapularis, southwestern Virginia. Vector Borne Zoonotic Dis. 14, 821–823. [DOI] [PubMed] [Google Scholar]
- Heylen D, De Coninck E, Jansen F, Madder M, 2014. Differential diagnosis of three common Ixodes spp. Ticks infesting songbirds of western Europe: Ixodes arboricola, I. frontalis and I. ricinus. Ticks Tick Borne Dis. 5, 693–700. [DOI] [PubMed] [Google Scholar]
- Hickling GJ, Kelly JR, Auckland LD, Hamer SA, 2018. Increasing prevalence of Borrelia burgdorferi sensu stricto–Infected blacklegged ticks in Tennessee Valley, Tennessee, USA. Emerg. Infect. Dis. 24, 1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogstraal H, 1985. Argasid and nuttalliellid ticks as parasites and vectors. Adv. Parasitol. 24, 135–238. [DOI] [PubMed] [Google Scholar]
- Hoogstraal H, Roberts FHS, Kohls G, Tipton V, 1968. Review of Haemaphysalis (Kaiseriana) longicornis Neumann (resurrected) of Australia, New Zealand, New Caledonia, Fiji, Japan, Korea, and Northeastern China and USSR, and its parthenogenetic and bisexual populations (Ixodoidea, Ixodidae). J. Parasitol. 54, 1197–1213. [PubMed] [Google Scholar]
- Hooker WA, Bishopp FC, Wood HP, 1912. The life history and bionomics of some North American ticks. Bull. Bur. Entomol. U. S. Dept. Agric. 106, 239 pp. [Google Scholar]
- Hopla CE, 1953. Experimental studies on tick transmission of tularemia organisms. Am. J. Hyg. 58, 101–118. [DOI] [PubMed] [Google Scholar]
- Hopla CE, Downs CM, 1953. The isolation of Bacterium tularense from the tick, Amblyomma americanum. J. Kans. Entomol. Soc. 26, 72–73. [Google Scholar]
- Horton JM, 2015. Relapsing fever caused by Borrelia species (chapter 242). In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 8th edition, Vol 2. Elsevier Saunders, Philadelphia, PA, pp. 2721–2724. [Google Scholar]
- Hoyt K, Chandrashekar R, Beall M, Leutenegger C, Lappin MR, 2018. Evidence for clinical anaplasmosis and borreliosis in cats in Maine. Top. Companion Anim. Med. 33, 40–44. [DOI] [PubMed] [Google Scholar]
- Ijdo JW, Wu C, Magnarelli LA, Stafford KC, Anderson JF, Fikrig E, 2000. Detection of Ehrlichia chaffeensis DNA in Amblyomma americanum ticks in Connecticut and Rhode Island. J. Clin. Microbiol. 38, 4655–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving R, Pinger R, Vann C, Olesen J, Steiner F, 2000. Distribution of Ehrlichia chaffeensis (Rickettsiales: Rickettsiaeceae) in Amblyomma americanum in southern Indiana and prevalence of E. chaffeensis- reactive antibodies in white-tailed deer in Indiana and Ohio in 1998. J. Med. Entomol. 37, 595–600. [DOI] [PubMed] [Google Scholar]
- Iwakami S, Ichikawa Y, Inokuma H, 2014. A nationwide survey of ixodid tick species recovered from domestic dogs and cats in Japan in 2011. Ticks Tick Borne Dis. 5, 771–779. [DOI] [PubMed] [Google Scholar]
- Jackson LK, Gaydon DM, Goddard J, 1996. Seasonal activity and relative abundance of Amblyomma americanum in Mississippi. J. Med. Entomol. 33, 128–131. [DOI] [PubMed] [Google Scholar]
- Jackson KC, Gidlewski T, Root JJ, Bosco-Lauth AM, Lash RR, Harmon JR, Brault AC, Panella NA, Nicholson WL, Komar N, 2019. Bourbon virus in wild and domestic animals, Missouri, USA, 2012–2013. Emerg. Inf. Dis. 25, 1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AM, Oliver JH Jr., 1990. Feeding and host preference of immature Ixodes dammini, I. scapularis, and I. pacificus (Acari: Ixodidae). J. Med. Entomol. 27, 324–330. [DOI] [PubMed] [Google Scholar]
- James AM, Liveris D, Wormser GP, Schwartz I, Montecalvo MA, Johnson BJ, 2001. Borrelia lonestari infection after a bite by an Amblyomma americanum tick. J. Infect. Dis. 183, 1810–1814. [DOI] [PubMed] [Google Scholar]
- Jellison WL, Bell EJ, Hurbner RJ, Parker RR, Welsh HH, 1948. Q fever studies in southern California; Occurrence of Coxiella burnetii. Public Health Rep. 63, 1483–1489. [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Yarina T, Miller MK, Stromdahl EY, Richards AL, 2010. Molecular detection of Rickettsia amblyommii in Amblyomma americanum parasitizing humans. Vector Borne Zoonotic Dis. 10, 329–340. [DOI] [PubMed] [Google Scholar]
- Jiang J, Stromdahl EY, Richards AL, 2012. Detection of Rickettsia parkeri and Candidatus Rickettsia andeanae in Amblyomma maculatum Gulf Coast ticks collected from the United States. Vector Borne Zoonotic Dis. 12, 175–182. [DOI] [PubMed] [Google Scholar]
- Johnson EM, Ewing SA, Barker RW, Fox JC, Crow DW, Kocan KM, 1998. Experimental transmission of Ehrlichia canis (Rickettsiales: Ehrlichieae) by Dermacentor variabilis (Acari: Ixodidae). Vet. Parasitol. 74, 277–288. [DOI] [PubMed] [Google Scholar]
- Johnson EM, Allen KE, Panciera RJ, Ewing SA, Little SE, 2009. Experimental transmission of Hepatozoon americanum to New Zealand while rabbits (Oryctolagus cuniculus) and infectivity of cystozoites for a dog. Vet. Parasitol. 164, 162–166. [DOI] [PubMed] [Google Scholar]
- Johnson DKH, Schiffman EK, Davis JP, Neitzel DF, Sloan LM, Nicholson WL, Fritsche TR, Steward CR, Ray JA, Miller TK, 2015. Human infection with Ehrlichia muris–like pathogen, United States, 2007–2013. Emerg. Infect. Dis. 21, 1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JL, Kim H. c., Coburn JM, Chong S. t., Chang NW, Robbins RG, Klein TA, 2017. Tick surveillance in two southeastern provinces, including three metropolitan areas, of the Republic of Korea during 2014. Systemic Appl. Acarol. 22, 271–288. [Google Scholar]
- Jones EO, Gruntmeir JM, Hamer SA, Little SE, 2017. Temperate and tropical lineages of brown dog ticks in North America. Vet. Parasitol. 7, 58–61. [DOI] [PubMed] [Google Scholar]
- Karpathy SE, Allerdice ME, Sheth M, Dasch GA, Levin ML, 2016. Co-feeding transmission of the Ehrlichia muris–like agent to mice (Mus musculus). Vector Borne Zoonotic Dis. 16, 145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirans JE, Durden LA, 1998. Illustrated key to nymphs of the tick genus Amblyomma (Acari: Ixodidae) found in the United States. J. Med. Entomol. 35, 489–495. [DOI] [PubMed] [Google Scholar]
- Keirans JE, Litwak TR, 1989. Pictorial key to the adults of hard ticks, family ixodidae (Ixodida: Ixodoidea), east of the Mississippi River. J Med Entomol. 26, 435–448. [DOI] [PubMed] [Google Scholar]
- Keirans JE, Pound JM, 2003. An annotated bibliography of the spinose ear tick, Otobius megnini (Dugès, 1883) (Acari: Ixodida: Argasidae) 1883–2000. Systemic Appl. Acaraol. 13, 1–68. [Google Scholar]
- Keirans JE, Hutcheson HJ, Durden LA, Klompen J, 1996. Ixodes (Ixodes) scapularis (Acari: Ixodidae): redescription of all active stages, distribution, hosts, geographical variation, and medical and veterinary importance. J. Med. Entomol. 33, 297–318. [DOI] [PubMed] [Google Scholar]
- Kelly AL, Raffel SJ, Fischer RJ, Bellinghausen M, Stevenson C, Schwan TG, 2014. First isolation of the relapsing fever spirochete, Borrelia hermsii, from a domestic dog. Ticks Tick Borne Dis. 5 (2), 95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp DH, Stone BF, Binnington KC, 1982. Chapter 4 - Tick attachment and feeding: role of the mouthparts, feeding apparatus, salivary gland secretions and the host response. In: Obenchain FD, Galun R (Eds.), Physiology of Ticks. Pergamon, pp. 119–168. [Google Scholar]
- Ketchum HR, Teel PD, Strey OF, Longnecker MT, 2005. Feeding predilection of Gulf Coast tick, Amblyomma maculatum Koch, nymphs on cattle. Vet. Parasitol. 133, 349–356. [DOI] [PubMed] [Google Scholar]
- Klock LE, Olsen PF, Fukushima T, 1973. Tularemia epidemic associated with the deerfly. J Am. Med. Assoc. 226, 149–152. [PubMed] [Google Scholar]
- Kocan AA, Cummings CA, Panciera RJ, Mathew JS, Ewing SA, Barker RW, 2000. Naturally occurring and experimentally transmitted Hepatozoon americanum in coyotes from Oklahoma. J. Wild. Dis. 36, 149–153. [DOI] [PubMed] [Google Scholar]
- Koch HG, 1982. Seasonal incidence and attachment sites of tick (Acari: Ixodidae) on domestic dogs in southeatern Oklahoma and northwestern Arkansas, USA. J. Med. Entomol. 19, 293–298. [DOI] [PubMed] [Google Scholar]
- Koch HG, 1988. Suitability of white-tailed deer, cattle, and goats as hosts for the lone star tick, Amblyomma americanum (Acari: Ixodidae). J. Kans. Entomol. Soc. 61, 251–257. [Google Scholar]
- Koch HG, Sauer JR, 1984. Quantity of blood ingested by four species of hard ticks (Acari: Ixodidae) fed on domestic dogs. Annals Entomol. Soc. Am 77, 142–146. [Google Scholar]
- Kohls G, 1960. Records and new synonymy of New world Haemaphysalis ticks, with descriptions of the nymph and larva of H. juxtakochi Cooley. J. Parasitol. 46, 355–361. [PubMed] [Google Scholar]
- Kollars TM Jr., Oliver JH Jr., Durden LA, Kollars PG, 2000. Host associations and seasonal activity of Amblyomma americanum (Acari: Ixodidae) in Missouri. J. Parasitol. 86, 1156–1159. [DOI] [PubMed] [Google Scholar]
- Krishnavahjala A, Armstrong BA, Lopez JE, 2018. Vector competence of geographical populations of Ornithodoros turicata for the tick-borne relapsing fever spirochete Borrelia turicatae. Appl. Environ. Microbiol 84, e01505–18 https://doi:1128/AEM.01505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labruna MB, Gerardi M, Krawczak FS, Moraes-Filho J, 2017. Comparative biology of the tropical and temperate species of Rhipicephalus sanguineus sensu lato (Acari: Ixodidae) under different laboratory conditions. Ticks Tick Borne Dis 8, 146–156. [DOI] [PubMed] [Google Scholar]
- Lado P, Nava S, Mendoza-Uribe L, Caceres AG, Delgado-de la Mora J, Licona-Enriquez J, Delgado-de la Mora, Labruna MB, Durden LA, Allerdice MEJ, Paddock CD, Szabó MPJ, Guglielmone AA, Beati L, 2018. The Amblyomma maculatum Koch, 1844 (Acari: Ixodidae) group of ticks: phenotypic plasticity or incipient speciation? Parasit. Vectors 11, 610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J, Velez JO, Brault AC, Calvert AE, Bell-Sakyi L, Bosco- Lauth AM, Staples JE, Kosoya OI, 2015. Molecular, serological and in vitro culture-based characterization of Bourbon virus, a newly described human pathogen of the genus thogotovirus. J. Clin. Virol. 73, 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RS, Loye JE, 1989. Lyme disease in California: interrelationship of Ixodes pacificus (Acari: Ixodidae), the western fence lizard (Sceloporus occidentalis), and Borrelia burgdorferi. J. Med. Entomol. 26, 272–278. [DOI] [PubMed] [Google Scholar]
- Lane RS, Peek J, Donaghey PJ, 1984. Tick (Acari: Ixodidae) paralysis in dogs from northern California: acarological and clinical findings. J. Med. Entomol. 21, 321–326. [DOI] [PubMed] [Google Scholar]
- Lane RS, Steinlein DB, Mun J, 2004. Human behaviors elevating exposure to Ixodes pacificus (Acari: Ixodidae) nymphs and their associated bacterial zoonotic agents in a hardwood forest. J. Med. Entomol. 41, 239–248. [DOI] [PubMed] [Google Scholar]
- Lappin MR, Chandrashekar R, Stillman B, Liu JY, Mather TN, 2015. Evidence of Anaplasma phagocytophilum and Borrelia burgdorferi infection in cats after exposure to wild-caught adult Ixodes scapularis. J. Vet. Diagn. Invest. 27, 522–525. [DOI] [PubMed] [Google Scholar]
- Lappin MR, Tasker S, Roura X, 2020. Role of vector-borne pathogens in the development of fever in cats: 2. Tick-and sandfly-associated diseases. J. Fel. Med. Surg 22, 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavender DR, Oliver JH Jr., 1996. Ticks (Acari: Ixodidae) in bulloch County, Georgia. J. Med. Entomol. 33, 224–231. [DOI] [PubMed] [Google Scholar]
- Lee JK, Moraru GM, Stokes JV, Wills RW, Mitchell E, Unz E, Moore-Henderson B, Harber AB, Varela-Stokes AS, 2017. Rickettsia parkeri and “Candidatus Rickettsia andeanae” in questing Amblyomma maculatum (Acari: Ixodidae) from Mississippi. J. Med Entomol. 54, 476–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees AD, 1952. The role of cuticle growth in the feeding process of ticks. Proc. Zool. Soc. Lond. 121, 759–772. [Google Scholar]
- Levin M, 2016. Ticks. Merck Veterinary Manual, 11th ed, pp. 925–942. [Google Scholar]
- Levin ML, Studer E, Killmaster L, Zemtsova G, Mumcuoglu KY, 2012. Crossbreeding between different geographical populations of the brown dog tick, Rhipicephalus sanguineus (Acari: Ixodidae). Exper. Appl. Acarol. 58, 51–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ML, Zemtsova GE, Killmaster LF, Snellgrove A, Schumacher LBM, 2017. Vector competence of Amblyomma americanum (Acari: Ixodidae) for Rickettsia rickettsii. Ticks Tick Borne Dis. 8, 615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang C, Allen KE, Little SE, Ahluwalia SK, Gao D, Macintire DK, Blagburn BL, Kaltenboeck B, 2008. Diagnosis of canine Hepatozoon spp. infection by quantitative pcr. Vet. Parasitol 157, 50–58. [DOI] [PubMed] [Google Scholar]
- Lindquist EE, Wu KW, Flahey B, 2016. A handbook to the ticks of Canada (Ixodida: Ixodidae, Argasidae). Biolo. Survey Canada Ottawa. [Google Scholar]
- Lindsay LR, Mathison SW, Barker IK, McEwen SA, Gillespie TJ, Surgeoner GA, 1999. Microclimate and habitat in relation to Ixodes scapularis (Acari: Ixodidae) populations on Long Point, Ontario, Canada. J. Med. Entomol. 36, 255–262. [DOI] [PubMed] [Google Scholar]
- Lineberry MW, Sundstrom KD, Little SE, Stayton EM, Allen KE, 2020. Detection of Cercopithifilaria bainae infection in shelter dogs and ticks in Oklahoma, USA. Parasit. Vector. 13, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little SE, 2010. Ehrlichiosis and anaplasmosis in dogs and cats. Vet. Clin. North Am: Small Animal Practice. 40, 1121–1140. [DOI] [PubMed] [Google Scholar]
- Little EA, Molaei G, 2020. Passive tick surveillance: exploring spatiotemporal associations of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae), Babesia microti (Piroplasmida: Babesiidae), and Anaplasma phagocytophilum (Rickettsiales: Anaplasmataceae) infection in Ixodes scapularis (Acari: Ixodidae). Vector Borne Zoonotic Dis. 20, 177–186. [DOI] [PubMed] [Google Scholar]
- Little SE, Hostetler J, Kocan KM, 2007. Movement of Rhipicephalus sanguineus adults between co-housed dogs during active feeding. Vet. Parasitol. 150, 139–145. [DOI] [PubMed] [Google Scholar]
- Little SE, Heise SR, Blagburn BL, Callister SM, Mead PS, 2010. Lyme borreliosis in dogs and humans in the USA. Trends. Parasitol. 26, 213–218. [DOI] [PubMed] [Google Scholar]
- Little SE, Beall MJ, Bowman DD, Chandrashekar R, Stamaris J, 2014. Canine infection with Dirofilaria immitis, Borrelia burgdorferi, Anaplasma spp., and Ehrlichia spp. in the United States, 2010–2012. Parasit Vectors. 7, 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little SE, Barrett AW, Nagamori Y, Herrin BH, Normile D, Heaney K, Armstrong R, 2018. Ticks from cats in the United States: patterns of infestation and infection with pathogens. Vet. Parasitol. 257, 15–20. [DOI] [PubMed] [Google Scholar]
- Little S, Braff J, Place J, Buch J, Dewage BG, Knupp A, Beall M, 2021. Canine infection with Dirofilaria immitis, Borrelia burgdorferi, Anaplasma spp., and Ehrlichia spp. in the United States, 2013–2019. Parasit. Vectors 14, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart JM, Davidson WR, Stallknecht DE, Dawson JE, Little SE, 1997. Natural history of Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) in the piedmont physiographic province of Georgia. J. Parasitol. 83, 887–894. [PubMed] [Google Scholar]
- Lockwood BH, Stasiak I, Pfaff MA, Cleveland CA, Yabsley MJ, 2018. Widespread distribution of ticks and selected tick-borne pathogens in Kentucky (USA). Ticks Tick Borne Dis. 9, 738–741. [DOI] [PubMed] [Google Scholar]
- Loftis AD, Mixson TR, Stromdahl EY, Yabsley MJ, Garrison LE, Williamson PC, Fitak RR, Fuerst PA, Kelly DJ, Blount KW, 2008. Geographic distribution and genetic diversity of the Ehrlichia sp. From panola Mountain in Amblyomma americanum. MC Infect. Dis. 8, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftis AD, Kelly PJ, Paddock CD, Blount K, Johnson JW, Gleim ER, Yabsley MJ, Levin ML, Beati L, 2016. Panola Mountain Ehrlichia in Amblyomma maculatum from the United States and Amblyomma variegatum (Acari: Ixodidae) from the Caribbean and Africa. J. Med. Entomol. 53, 696–698. [DOI] [PubMed] [Google Scholar]
- Long SW, Zhang X, Zhang J, Ruble RP, Teel P, Yu X-J, 2003. Evaluation of transovarial transmission and transmissibility of Ehrlichia chaffeensis (Rickettsiales: Anaplasmataceae) in Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 40, 1000–1004. [DOI] [PubMed] [Google Scholar]
- Long SW, Pound JM, Yu XJ, 2004. Ehrlichia prevalence in Amblyomma americanum, central Texas. Emerg. Infect. Dis. 10, 1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis EC, 1961. Life histories of ticks under laboratory conditions (Acarina: Ixodidae and Argasidae). J. Parasitol. 47, 91–99. [PubMed] [Google Scholar]
- Lopez JE, Krishnavahjala A, Garcia MN, Bermudez S, 2016. Tick-borne relapsing fever spirochetes in the Americas. Vet. Sci. 10.3390/vetsci2020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louly CC, Soares SF, da Nóbrega Silveira D, Guimarães MS, Borges LM, 2010. Differences in the behavior of Rhipicephalus sanguineus tested against resistant and susceptible dogs. Exper. Appl. Acarol. 51, 353–362. [DOI] [PubMed] [Google Scholar]
- Luo LM, Zhao L, Wen HL, Zhang ZT, Liu JW, Fang LZ, Xue ZF, Ma DQ, Zhang XS, Ding SJ, Lei XY, Yu XJ, 2015. Haemaphysalis longicornis ticks as reservoir andv of severe fever with thrombocytopenia syndrome virus in China. Emerg. Infect. Dis. 21, 1770–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn GE, Oliver JD, Nelson CM, Felsheim RF, Kurtti TJ, Munderloh UG, 2015. Tissue distribution of the Ehrlichia muris-like agent in a tick vector. PLoS One 10, e0122007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AJ, Briggs CJ, 2016. Truncated seasonal activity patterns of the western blacklegged tick (Ixodes pacificus) in central and southern California. Ticks Tick Borne Dis. 7, 234–242. [DOI] [PubMed] [Google Scholar]
- MacDonald AJ, Hyon DW, Brewington JB, O’Connor KE, Swei A, Briggs CJ, 2017. Lyme disease risk in southern California: abiotic and environmental drivers of Ixodes pacificus (Acari: Ixodidae) density and infection prevalence with Borrelia burgdorferi. Parasit. Vectors. 10, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maegli A, Loy JD, Cortinas R, 2016. Note on Ehrlichia chaffeensis, Ehrlichia ewingii, and “Borrelia lonestari” infection in lone star ticks (Acari: Ixodidae), Nebraska, USA. Ticks and Tick Borne Dis. 7, 154–158. [DOI] [PubMed] [Google Scholar]
- Maestas LP, Reeser SR, McGay PJ, Buoni MH, 2020. Surveillance for Amblyomma maculatum (Acari: Ixodidae) and Rickettsia parkeri (Rickettsiales: Rickettsiaceae) in the state of Delaware, and their public health implications. J. Med. Entomol. 57, 979–983. [DOI] [PubMed] [Google Scholar]
- Maggi RG, Toliver M, Richardson T, Mather T, Breitschwerdt EB, 2019. Regional prevalences of Borrelia burgdorferi, Borrelia bissettiae, and Bartonella henselae in Ixodes affinis, Ixodes pacificus and Ixodes scapularis in the USA. Ticks Tick Borne Dis. 10, 360–364. [DOI] [PubMed] [Google Scholar]
- Magnarelli LA, Bushmich SL, Ijdo JW, Fikrig E, 2005. Seroprevalence of antibodies against Borrelia burgdorferi and Anaplasma phagocytophilum in cats. Am. J. Vet. Res. 66, 1895–1899. [DOI] [PubMed] [Google Scholar]
- Magnarelli L, Levy S, Koski R, 2007. Detection of antibodies to Francisella tularensis in cats. Res. Vet. Sci. 82, 22–26. [DOI] [PubMed] [Google Scholar]
- Mani RJ, Metcalf JA, Clinkenbeard KD, 2015. Amblyomma americanum as a bridging vector for human infection with Francisella tularensis. PLoS One. 10, e0130513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather TN, Wilson ML, Moore SI, Ribeiro JMC, Spielman A, 1989. Comparing the relative potential of rodents as reservoirs of the Lyme disease spirochete (Borrelia burgdorferi). Am. J. Epidemiol. 130, 143–150. [DOI] [PubMed] [Google Scholar]
- Mather TN, Nicholson MC, Donnelly EF, Matyas BT, 1996. Entomologic index for human risk of Lyme disease. Am. J. Epidemiol. 144, 1066–1069. [DOI] [PubMed] [Google Scholar]
- Mathew JS, Ewing SA, Panciera RJ, Woods JP, 1998. Experimental transmission of Hepatozoon americanum Vincent-Johnson et al., 1997 to dogs by the Gulf Coast tick, Amblyomma maculatum Koch. Vet. Parasitol 80, 1–14. [DOI] [PubMed] [Google Scholar]
- Mays SE, Houston AE, Trout Fryxell RT, 2016. Specifying pathogen associations of Amblyomma maculatum (Acari: Ixodidae) in western Tennessee. J. Med. Entomol 53, 435–440. [DOI] [PubMed] [Google Scholar]
- McDade JE, Newhouse VF, 1986. Natural history of Rickettsia rickettsii. Ann. Rev. Microbiol. 40, 287–309. [DOI] [PubMed] [Google Scholar]
- McQuiston JH, Guerra MA, Watts MR, Lawaczeck E, Levy C, Nicholson WL, Adjemian J, Swerdlow DL, 2011. Evidence of exposure to spotted fever group rickettsiae among Arizona dogs outside a previously documented outbreak area. Zoonoses Public Health. 58, 85–92. [DOI] [PubMed] [Google Scholar]
- Merten HA, Durden LA, 2000. A state-by-state survey of ticks recorded from humans in the United States. J. Vector Ecol. 25, 102–113. [PubMed] [Google Scholar]
- Mertins JW, Moorhouse AS, Alfred JT, Hutcheson HJ, 2010. Amblyomma triste (Acari: Ixodidae): new North American collection records, including the first from the United States. J. Med. Entomol. 47 (4), 536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minigan JN, Hager HA, Peregrine AS, Newman JA, 2018. Current and potential future distribution of the American dog tick (Dermacentor variabilis, Say) in North America. Ticks Tick Borne Dis. 9, 354–362. [DOI] [PubMed] [Google Scholar]
- Mixson TR, Ginsberg HS, Campbell SR, Sumner JW, Paddock CD, 2004. Detection of Ehrlichia chaffeensis in adult and nymphal Amblyomma americanum (Acari: Ixodidae) ticks from Long Island, New York. J. Med. Entomol. 41, 1104–1110. [DOI] [PubMed] [Google Scholar]
- Mixson TR, Campbell SR, Gill JS, Ginsberg HS, Reichard MV, Schulze TL, Dasch GA, 2006. Prevalence of Ehrlichia, Borrelia, and Rickettsial agents in Amblyomma americanum (Acari: Ixodidae) collected from nine states. J. Med. Entomol. 43, 1261–1268. [DOI] [PubMed] [Google Scholar]
- Monzon JD, Atkinson EG, Henn BM, Benach JL, 2016. Population and evolutionary genomics of Amblyomma americanum, an expanding arthropod disease vector. Genome Biol. Evol. 8, 1351–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonga LC, Hayashida K, Nakao R, Lisulo M, Kaneko C, Nakamura I, Eshita Y, Mweene AS, Namangala B, Sugimoto C, Yamagishi J, 2019. Molecular detection of Rickettsia felis in dogs, rodents and cat fleas in Zambia. Parasit. Vectors. 12, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorhouse DE, 1969. The attachment of some ixodid ticks to their natural hosts. In: Evans GO (Ed.), Proceedings of the 2nd International Congress of Acarology, Sutton Bonington, England. Akadémiai Kiadó, Budapest, pp. 319–327. [Google Scholar]
- Moreira-Soto A, Carranza MV, Taylor L, Calderón-Arguedas O, Hun L, Troyo A, 2016. Exposure of dogs to spotted fever group rickettsiae in urban sites associated with human rickettsioses in Costa Rica. Ticks Tick Borne Dis. 7, 748–753. [DOI] [PubMed] [Google Scholar]
- Mueller EK, Baum KA, Papes M, Cohn LA, Cowell AK, Reichard MV, 2013. Potential ecological distribution of Cytauxzoon felis in domestic cats in Oklahoma, Missouri, and Arkansas. Vet. Parasitol. 192, 104–110. [DOI] [PubMed] [Google Scholar]
- Mullen GM, Durden LA, 2019. Medical and veterinary entomology. Ticks (Ixodidae), 3rd ed. Academic Press, London, UK, pp. 603–663. Chapter 27). [Google Scholar]
- Murphy GL, Ewing S, Whitworth LC, Fox JC, Kocan AA, 1998. A molecular and serologic survey of Ehrlichia canis, E. chaffeensis, and E. ewingii in dogs and ticks from Oklahoma. Vet. Parasitol. 79, 325–339. [DOI] [PubMed] [Google Scholar]
- Murphy DS, Lee X, Larson SR, Johnson DKH, Loo T, Paskewitz SM, 2017. Prevalence and distribution of human and tick infections with the Ehrlichia muris-like agent and Anaplasma phagocytophilum in Wisconsin, 2009–2015. Vector Borne Zoonotic Dis. 17, 229–236. [DOI] [PubMed] [Google Scholar]
- Nadolny RM, Gaff HD, 2018. Natural history of Ixodes affinis in Virginia. Ticks Tick Borne Dis. 9, 109–119. [DOI] [PubMed] [Google Scholar]
- Nadolny RM, Wright CL, Sonenshine DE, Hynes WL, Gaff HD, 2014. Ticks and spotted fever group rickettsiae of southeastern Virginia. Ticks Tick Borne Dis. 5, 53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamori Y, 2016. Transmission of Cytauxzoon felis by Amblyomma americanum: Engorgement Weight of Nymphs and Attachment Time of Adults for Transmission to Domestic Cats. Oklahoma State University, Stillwater, OK USA. [Google Scholar]
- Naudé TW, Heyne H, van der Merwe IR, Benic MJ, 2001. Spinose ear tick Otobius megnini (Dugès, 1884) as the cause of an incident of painful otitis externa in humans. J. S. Afr. Vet. Assoc. 72, 118–119. [DOI] [PubMed] [Google Scholar]
- Nava S, Mangold AJ, Guglielmone AA, 2009. Field and laboratory studies in a neotropical population of the spinose ear tick, Otobius megnini. Med. Vet. Entomol. 23, 1–5. [DOI] [PubMed] [Google Scholar]
- Nelder MP, Russell C, Lindsay LR, Dhar B, Patel SN, Johnson S, Moore S, Kristjanson E, Li Y, Ralevski F, 2014. Population-based passive tick surveillance and detection of expanding foci of blacklegged ticks Ixodes scapularis and the Lyme disease agent Borrelia burgdorferi in Ontario, Canada. PLoS One 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelder MP, Russell CB, Clow KM, Johnson S, Weese JS, Cronin K, Ralevski F, Jardine CM, Patel SN, 2019. Occurrence and distribution of Ambylomma americanum as determined by passive surveillance in Ontario, Canada (1999–2016). Ticks Tick Borne Dis. 10, 146–155. [DOI] [PubMed] [Google Scholar]
- Nicholson WL, Paddock CD, Demma L, Traeger M, Johnson B, Dickson J, McQuiston J, Swerdlow D, 2006. Rocky Mountain spotted fever in Arizona: documentation of heavy environmental infestations of Rhipicephalus sanguineus at an endemic site. Ann. NY Acad. Sci. 1078, 338–341. [DOI] [PubMed] [Google Scholar]
- Nicholson WL, Allen KE, McQuiston JH, Breitschwerdt EB, Little SE, 2010. The increasing recognition of rickettsial pathogens in dogs and people. Trends Parasitol. 26, 205–212. [DOI] [PubMed] [Google Scholar]
- Nicholson WL, Sonenshine DE, Noden BH, Brown RN, 2019. Chapter 27 - Ticks (ixodida). In: Mullen GR, Durden LA (Eds.), Medical and Veterinary Entomology, 3rd edition. Academic Press, pp. 603–672. [Google Scholar]
- Niebuhr CN, Breeden JB, Lambert BD, Eyres AI, Haefele HJ, Kattes DH, 2013. Off-host collection methods of the Otobius megnini (Acari: Argasidae). J. Med. Entomol. 50, 994–998. [DOI] [PubMed] [Google Scholar]
- Njaa BL, 2017. The ear (Chapter 20). In: Zachary JF (Ed.), Pathologic Basis of Veterinary Disease, 6th ed. Elsevier, St. Louis, Missouri, pp. 1223–1262. [Google Scholar]
- Ogden NH, Lindsay LR, Beauchamp G, Charron D, Maarouf A, O’Callaghan CJ, Waltner-Toews D, Barker IK, 2004. Investigation of relationships between temperature and developmental rates of tick Ixodes scapularis (Acari: Ixodidae) in the laboratory and field. J. Med. Entomol. 41, 622–633. [DOI] [PubMed] [Google Scholar]
- Ogden NH, Pang G, Ginsberg HS, Hickling GJ, Burke RL, Beati L, Tsao JI, 2018. Evidence for geographic variation in life-cycle processes affecting phenology of the Lyme disease vector Ixodes scapularis (Acari: Ixodidae) in the United States. J. Med. Entomol. 55, 1386–1401. [DOI] [PubMed] [Google Scholar]
- Oliver JH Jr, 1996. Lyme borreliosis in the southern United States: a review. J. Parasitol. 82, 926–935. [PubMed] [Google Scholar]
- Oliver JH Jr., Tanaka K, Sawada M, 1973. Cytogenetics of ticks (Acari: Ixodoidea). 12. Chromosome and hybridization studies of bisexual and parthenogenetic Haemaphysalis longicornis races from Japan and Korea. Chromosoma 42, 269–288. [DOI] [PubMed] [Google Scholar]
- Oliver JH Jr., Cummins GA, Joiner MS, 1993a. Immature Ixodes scapularis (Acari: Ixodidae) parasitizing lizards from the southeastern USA. J. Parasitol. 79, 684–689. [PubMed] [Google Scholar]
- Oliver JH Jr., Owsley MR, Hutcheson HJ, James AM, Chen C, Irby WS, Dotson EM, Mclain DK, 1993b. Conspecificity of the ticks Ixodes scapularis and I. Dammini (Acari: Ixodidae). J. Med. Entomol. 30, 54–63. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Goddard J, 2015. The evolving medical and veterinary importance of the Gulf Coast tick (Acari: Ixodidae). J. Med. Entomol. 52 (2), 230–252. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Yabsley MJ, 2007. Ecological havoc, the rise of white-tailed deer, and the emergence of Amblyomma americanum-associated zoonoses in the United States. In: Childs JE, Mackenzie JS, Richt JA (Eds.), Wildlife and Emerging Zoonotic Diseases: The Biology, Circumstances and Consequences of Cross-Species Transmission. Springer Berlin Heidelberg, Berlin, Heidelberg, pp. 289–324. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Brenner O, Vaid C, Boyd DB, Berg JM, Joseph RJ, Zaki SR, Childs JE, 2002. Short report: concurrent Rocky Mountain spotted fever in a dog and its owner. Am. J. Trop. Med. Hyg. 66, 197–199. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Lane RS, Staples JE, Labruna MB, 2016. Changing paradigms for tick-borne diseases in the Americas. In: Mack A (Ed.), Global Health Impacts of Vector-Borne Diseases: Workshop Summary. National Academies Press, Washington DC, pp. 221–257. [Google Scholar]
- Padgett KA, Lane RS, 2001. Life cycle of Ixodes pacificus (Acari: Ixodidae): timing of developmental processes under field and laboratory conditions. J. Med. Entomol. 38, 684–693. [DOI] [PubMed] [Google Scholar]
- Pat-Nah H, Rodriguez-Vivas RI, Bolio-Gonzalez ME, Villegas-Perez SL, Reyes-Novelo E, 2015. Molecular diagnosis of Ehrlichia canis in dogs and ticks Rhipicephalus sanguineus (Acari: Ixodidae) in Yucatan, Mexico. J. Med. Entomol. 52, 101–104. [DOI] [PubMed] [Google Scholar]
- Peacock PB, 1958. Tick paralysis or poliomyelitis. S. Afr. Med. J. 32, 201–202. [PubMed] [Google Scholar]
- Perez M, Bodor M, Zhang C, Xiong Q, Rikihisa Y, 2006. Human infection with Ehrlichia canis accompanied by clinical signs in Venezuela. Ann. NY Acad. Sci. 1078, 110–117. [DOI] [PubMed] [Google Scholar]
- Petersen JM, Mead PS, Schriefer ME, 2009. Francisella tularensis: an arthropod-borne pathogen. Vet. Res. 40, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip C, Jellison W, 1934. The American dog tick, Dermacenter variabilis, as a host of bacterium tularense. Pub Health Rep. 49, 386–392. [Google Scholar]
- Phillips VC, Zieman EA, Kim CH, Stone CM, Tuten HC, Jiménez FA, 2020. Documentation of the expansion of the Gulf Coast tick (Amblyomma maculatum) and Rickettsia parkeri: first report in Illinois. J. Parasitol. 106, 9–13. [PubMed] [Google Scholar]
- Pieracci EG, De La Rosa J, Rubio DL, Perales M, Contreras MV, Drexler NA, Nicholson WL, De La Rosa J, Chung IH, Kato C, Barton Behravesh C, Enríquez M, Roldan J, Villarino ME, 2019. Seroprevalence of spotted fever group rickettsiae in canines along the United States-Mexico border. Zoonoses Public Health. 66, 918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piranda EM, Faccini JL, Pinter A, Pacheco RC, Cançado PH, Labruna MB, 2011. Experimental infection of Rhipicephalus sanguineus ticks with the bacterium Rickettsia rickettsii, using experimentally infected dogs. Vector Borne Zoonotic Dis. 11, 29–36. [DOI] [PubMed] [Google Scholar]
- Portugal JS, Goddard J, 2016. Field observations of questing and dispersal by colonized nymphal Amblyomma maculatum Koch (Acari: Ixodidae). J. Parasitol 102, 481–483. [DOI] [PubMed] [Google Scholar]
- Pritt BS, Sloan LM, Johnson DKH, Munderloh UG, PIaskewitz SM, McElroy KM, McFadden JD, Binnicker MJ, Neitzel DF, Liu G, 2011. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, 2009. New Eng. J. Med. 365, 422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritt BS, Allerdice ME, Sloan LM, Paddock CD, Munderloh UG, Rikihisa Y, Tajima T, Paskewitz SM, Neitzel DF, Johnson DKH, 2017. Proposal to reclassify Ehrlichia muris as Ehrlichia muris subsp. muris subsp. nov. and description of Ehrlichia muris subsp. eauclairensis subsp. Nov., a newly recognized tick-borne pathogen of humans. Int. J. Syst. Evol. Microbiol. 67, 2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qurollo BA, Chandrashekar R, Hegarty BC, Beall MJ, Stillman BA, Liu J, Thatcher B, Pultorak E, Cerrito B, Walsh M, Breitschwerdt EB, 2014. A serological survey of tick-borne pathogens in dogs in North America and the Caribbean as assessed by Anaplasma phagocytophilum, A. platys, Ehrlichia canis, E. chaffeensis, E. ewingii, and Borrelia burgdorferi species-specific peptides. Infect. Ecol. Epidemiol. 4 10.3402/iee.v4.24699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan RK, Peterson AT, Cobos ME, Ganta R, Foley D, 2019. Current and future distribution of the lone star tick, Amblyomma americanum (L.) (Acari: Ixodidae) in North America. PLoS One. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey T, Occi JL, Robbins RG, Egizi A, 2018. Discovery of Haemaphysalis longicornis (Ixodida: Ixodidae) parasitizing a sheep in New Jersey, United States. J. Med. Entomol. 55, 757–759. [DOI] [PubMed] [Google Scholar]
- Rajakaruna RS, Diyes CP, 2019. Spinose ear tick Otobius megnini infestations in race horses. In: Bakar M, Perera PK (Eds.), Ticks and Tick-Borne Pathogens. Abudoi. 10.5772/intechopen.80784. [DOI] [Google Scholar]
- Rand PW, Lacombe EH, Dearborn R, Cahill B, Elias S, Lubelczyk CB, Beckett GA, Smith RP Jr, 2007. Passive surveillance in Maine, an area emergent for tick-borne diseases. J. Med. Entomol. 44, 1118–1129. [DOI] [PubMed] [Google Scholar]
- Reeves WK, Loftis AD, Nicholson WL, Czarkowski AG, 2008. The first report of human illness associated with the panola Mountain Ehrlichia species: a case report. J. Med. Case Rep. 2, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard MV, Baum KA, Cadenhead SC, Snider TA, 2008. Temporal occurrence and environmental risk factors associated with cytauxzoonosis in domestic cats. Vet. Parasitol. 152, 314–320. [DOI] [PubMed] [Google Scholar]
- Reichard MV, Meinkoth JH, Edwards AC, Snider TA, Kocan KM, Blouin EF, Little SE, 2009. Transmission of Cytauxzoon felis to a domestic cat by Amblyomma americanum. Vet. Parasitol. 161, 110–115. [DOI] [PubMed] [Google Scholar]
- Reichard MV, Edwards AC, Meinkoth JH, Snider TA, Meinkoth KR, Heinz RE, Little SE, 2010. Confirmation of Amblyomma americanum (Acari: Ixodidae) as a vector for Cytauxzoon felis (Piroplasmorida: Theileriidae) to domestic cats. J. Med. Entomol. 47, 890–896. [DOI] [PubMed] [Google Scholar]
- Ribeiro JMC, 1989. Role of saliva in tick/host interactions. Exp. Appl. Acar. 7, 15–20. [DOI] [PubMed] [Google Scholar]
- Rizzi TE, Reichard MV, Cohn LA, Birkenheuer AJ, Taylor JD, Meinkoth JH, 2015. Prevalence of Cytauxzoon felis infection in healthy cats from enzootic areas in Arkansas, Missouri, and Oklahoma. Parasite. Vectors 8 (13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochlin I, 2019. Modeling the Asian longhorned tick (Acari: Ixodidae) suitable habitat in North America. J. Med. Entomol. 56, 384–391. [DOI] [PubMed] [Google Scholar]
- Roland WE, Everett ED, Cyr TL, Hasan SZ, Dommaraju CB, McDonald GA, 1998. Ehrlichia chaffeensis in Missouri ticks. Am. J. Trop. Med. Hyg. 59, 641–643. [DOI] [PubMed] [Google Scholar]
- Rose I, Yoshimizu MH, Bonilla DL, Fedorova N, Lane RS, Padgett KA, 2019. Phylogeography of Borrelia spirochetes in Ixodes pacificus and Ixodes spinipalpis ticks highlights differential acarological risk of tick-borne disease transmission in northern versus southern California. PLoS One 14, e0214726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R, Lindsey NP, Fischer M, Gregory CJ, Hinckley AF, Mead PS, Paz-Bailey G, Waterman SH, Drexler NA, Kersh GJ, 2018. Vital signs: trends in reported vectorborne disease cases—United States and Territories, 2004–2016. Morb. Mortal. Wkly. Rep. 67, 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotramel GL, Schwan TG, Doty RE, 1976. Distribution of suspected tick vectors and reported cases of Rocky Mountain spotted fever in California. Am. J. Epidemiol. 104, 287–293. [PubMed] [Google Scholar]
- Saari S, 2019. Canine Parasites and Parasitic Diseases; Arachnida (Chapter 9 ). Academic Press, London, UK, pp. 187–228. [Google Scholar]
- Sage KM, Johnson TL, Teglas MB, Nieto NC, Schwan TG, 2017. Ecological niche modeling and distribution of Ornithodoros hermsi associated with tick-borne relapsing fever in western North America. PLoS Negl Trop Dis. 11 (10), e0006047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh MN, Sundstrom KD, Duncan KT, Ientile MM, Jordy J, Ghosh P, Little SE, 2019. Show us your ticks: a survey of ticks infesting dogs and cats across the USA. Parasit. Vectors. 12, 595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkeld DJ, Castro MB, Bonilla D, Kjemtrup A, Kramer VL, Lane RS, Padgett KA, 2014. Seasonal activity patterns of the western black-legged tick, Ixodes pacificus, in relation to onset of human Lyme disease in northwestern California. Ticks Tick Borne Dis. 5, 790–796. [DOI] [PubMed] [Google Scholar]
- Salkeld DJ, Porter WT, Loh SM, Nieto NC, 2019. Time of year and outdoor recreation affect human exposure to ticks in California, United States. Ticks Tick. Borne. Dis. 10, 1113–1117. [DOI] [PubMed] [Google Scholar]
- Sanches GS, Évora PM, Mangold AJ, Jittapalapong S, Rodriguez- Mallon A, Guzmán PE, Bechara GH, Camargo- Mathias MI, 2016. Molecular, biological, and morphometric comparisons between different geographical populations of Rhipicephalus sanguineus sensu lato (Acari: Ixodidae). Vet. Parasitol. 215, 78–87. [DOI] [PubMed] [Google Scholar]
- Savage HM, Burkhalter KL, Godsey MS Jr, Panella NA, Ashley DC, Nicholson WL, Lambert AJ, 2017. Bourbon virus in field-collected ticks, Missouri, USA. Emerg. Inf. Dis. 23, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayler KA, Loftis AD, Beatty SK, Boyce CL, Garrison E, Clemons B, Cunningham M, Alleman AR, Barbet AF, 2016. Prevalence of tick-borne pathogens in host-seeking Amblyomma americanum (Acari: Ixodidae) and Odocoileus virginianus (Artiodactyla: Cervidae) in Florida. J. Med. Entomol. 53, 949–956. [DOI] [PubMed] [Google Scholar]
- Schulze TL, Jordan RA, Hung RW, 2001. Effects of selected meteorological factors on diurnal questing of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 38, 318–324. [DOI] [PubMed] [Google Scholar]
- Schulze TL, Jordan RA, Schulze CJ, Mixson T, Papero M, 2005. Relative encounter frequencies and prevalence of selected Borrelia, Ehrlichia, and Anaplasma infections in Amblyomma americanum and Ixodes scapularis (Acari: Ixodidae) ticks from central New Jersey. J. Med. Entomol. 42, 450–456. [DOI] [PubMed] [Google Scholar]
- Schulze TL, Jordan RA, White JC, Roegner VE, Healy SP, 2011. Geographical distribution and prevalence of selected Borrelia, Ehrlichia, and Rickettsia infections in Amblyomma americanum (Acari: Ixodidae) in New Jersey. J. Am. Mosq. Control Assoc. 27, 236–244. [DOI] [PubMed] [Google Scholar]
- Schwan TG, Raffel SJ, Schrumpf ME, Gill JS, Piesman J, 2009. Characterization of a novel relapsing fever spirochete in the midgut, coxal fluid, and salivary glands of the bat tick Carios kelleyi. Vector Borne Zoon. Dis. 9, 643–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semtner PJ, Howell D, Hair JA, 1971. The ecology and behavior of the lone star tick (Acarina: Ixodidae) I. The relationship between vegetative habitat type and tick abundance and distribution in Cherokee Co., Oklahoma. J. Med. Entomol. 8, 329–335. [DOI] [PubMed] [Google Scholar]
- Shannon AB, Rucinsky R, Gaff HD, Brinkerhoff RJ, 2017. Borrelia miyamotoi, other vector-borne agents in cat blood and ticks in eastern Maryland. EcoHealth. 14, 816–820. [DOI] [PubMed] [Google Scholar]
- Shaw SE, Day MJ, Birtles RJ, Breitschwerdt EB, 2001a. Tick-borne infectious diseases of dogs. Trends. Parasitol. 17, 74–80. [DOI] [PubMed] [Google Scholar]
- Shaw SE, Birtles RJ, Day MJ, 2001b. Arthropod-transmitted infectious diseases of cats. J. Feline. Med. Surgy. 3, 193–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrill MK, Cohn LA, 2015. Cytauxzoonosis: diagnosis and treatment of an emerging disease. J. Feline Med. Surg. 17, 940–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada Y, Beppu T, Inokuma H, Okuda M, Onishi T, 2003. Ixodid tick species recovered from domestic dogs in Japan. Med. Vet. Entomol. 17, 38–45. [DOI] [PubMed] [Google Scholar]
- Shock BC, Murphy SM, Patton LL, Shock PM, Olfenbuttel C, Beringer J, Prange S, Grove DM, Peek M, Butfiloski JW, 2011. Distribution and prevalence of Cytauxzoon felis in bobcats (Lynx rufus), the natural reservoir, and other wild felids in thirteen states. Vet. Parasitol. 175, 325–330. [DOI] [PubMed] [Google Scholar]
- Shock BC, Moncayo A, Cohen S, Mitchell EA, Williamson PC, Lopez G, Garrison LE, Yabsley MJ, 2014. Diversity of piroplasms detected in blood-fed and questing ticks from several states in the United States. Ticks Tick Borne Dis. 5, 373–380. [DOI] [PubMed] [Google Scholar]
- Simmons TW, Shea J, Myers- Claypole MA, Kruise R, Hutchinson ML, 2015. Seasonal activity, density, and collection efficiency of the blacklegged tick (Ixodes scapularis) (Acari: Ixodidae) in mid-western Pennsylvania. J. Med. Entomol. 52, 1260–1269. [DOI] [PubMed] [Google Scholar]
- Simpson DT, Teague MS, Weeks JK, Kaup BZ, Kerscher O, Leu M, 2019. Habitat amount, quality, and fragmentation associated with prevalence of the tick-borne pathogen Ehrlichia chaffeensis and occupancy dynamics of its vector, Amblyomma americanum. Landsc. Ecol. 34, 2435–2449. [Google Scholar]
- Smith DD, Frenkel JK, Smith EI, 1986. Intradermal infestation of a red fox (Vulpes vulpes) by the lone star tick (Amblyomma americanum). J. Wild. Dis. 22, 122–124. [DOI] [PubMed] [Google Scholar]
- Sonenshine DE, 1979. Zoogeography of the American dog tick, Dermacentor variabilis. In: Rodriguez J (Ed.), Recent Advances in Acarology, Vol. 2. Academic Press, New York, pp. 123–134. [Google Scholar]
- Sonenshine DE, 2018. Range expansion of tick disease vectors in north america: implications for spread of tick-borne disease. Int. J. Environ. Res. Public Health. 15, 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshine DE, Anderson JM, 2013. Chapter 6 – Mouthparts and digestive system. In: Sonenshine DE, Roe MR (Eds.), Biology of Ticks, 2nd edition, Vol. 1. Oxford University Press, New York, NY, pp. 3–16. [Google Scholar]
- Sonenshine DE, Roe MR, 2013. Chapter 1 – Overview: ticks, people, and animals. In: Sonenshine DE, Roe MR (Eds.), Biology of Ticks, 2nd edition, Vol. 1. Oxford University Press, New York, NY, pp. 122–162. [Google Scholar]
- Sonenshine DE, Atwood EL, Lamb JT Jr, 1966. The ecology of ticks transmitting Rocky Mountain spotted fever in a study area in Virginia. Ann. Entomol. Soc. Am. 59, 1234–1262. [DOI] [PubMed] [Google Scholar]
- Sosa- Gutierrez CG, Vargas-Sandoval M, Torres J, Gordillo- Pérez G, 2016. Tick-borne rickettsial pathogens in questing ticks, removed from humans and animals in Mexico. J. Vet. Sci. 17, 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulsby EJL, 1968. Ballèiere, tindall, and cassel ltd, London, WC. Helminths, Arthropods and Protozoa of Domesticated Animals (Mönnig), 6th ed, p. 468. [Google Scholar]
- Soulsby EJL, 1982. Helminths, Arthropods and Protozoa of Domesticated Animals, seventh edition. Lea and Febiger, Philadelphia, Bailliere Tindall, London, pp. 456–458. [Google Scholar]
- Spielman A, 1994. The emergence of Lyme disease and human babesiosis in a changing environment. Ann N Y Acad Sci. 740, 146–156. [DOI] [PubMed] [Google Scholar]
- Springer YP, Eisen L, Beati L, James AM, Eisen RJ, 2014. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae). J. Med. Entomol. 51, 342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer YP, Jarnevich CS, Barnett DT, Monaghan AJ, Eisen RJ, 2015. Modeling the present and future geographic distribution of the lone star tick, Amblyomma americanum (Ixodida: Ixodidae), in the continental United States. Am. J. Trop. Med. Hyg. 93, 875–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford KC, Connecticut Agricultural Experiment S, 2007. Tick Management Handbook : An Integrated Guide for Homeowners, Pest Control Operators, and Public Health Officials for the Prevention of Tick- Associated Disease. Connecticut Agricultural Experiment Station, New Haven, Conn. [Google Scholar]
- Stanley HM, Ford SL, Snellgrove AN, Hartzer K, Smith EB, Krapiunaya I, Levin ML, 2020. The ability of the invasive Asian longhorned tick Haemaphysalis longicornis (Acari: Ixodidae) to acquire and transmit Rickettsia rickettsii (Rickettsiales: Rickettsiaceae), the agent of Rocky Mountain spotted fever, under laboratory conditions. J. Med. Entomol. 57, 1635–1639. [DOI] [PubMed] [Google Scholar]
- Starkey LA, Panciera RJ, Paras K, Allen KE, Reiskind MH, Reichard MV, Johnson EM, Little SE, 2013. Genetic diversity of Hepatozoon spp. In coyotes from the south-central United States. J. Parasitol. 99, 375–378. [DOI] [PubMed] [Google Scholar]
- Starkey LA, Newton K, Brunker J, Crowdis K, Edourad E, Meneus P, Little SE, 2016. Prevalence of vector-borne pathogens in dogs from Haiti. Vet. Parasitol. 224, 7–12. [DOI] [PubMed] [Google Scholar]
- Steen NA, Barker SC, Alewood PF, 2006. Proteins in the saliva of the ixodida (ticks): pharmacological features and biological significance. Toxicon 47, 1–20. [DOI] [PubMed] [Google Scholar]
- Steiert JG, Gilfoy F, 2002. Infection rates of Amblyomma americanum and Dermacentor variabilis by Ehrlichia chaffeensis and Ehrlichia ewingii in southwest Missouri. Vector Borne Zoonotic Dis. 2, 53–60. [DOI] [PubMed] [Google Scholar]
- Steiner FE, Pinger RR, Vann CN, 1999. Infection rates of Amblyomma americanum (Acari: Ixodidae) by Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) and prevalence of E. chaffeensis-reactive antibodies in white-tailed deer in southern Indiana, 1997. J. Med. Entomol. 36, 715–719. [DOI] [PubMed] [Google Scholar]
- Steinke JW, Platts-Mills TAE, Commins SP, 2015. The alpha-gal story: lessons learned from connecting the dots. J. Allergy Clin. Immunol. 135, 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout IJ, Clifford CM, Keirans JE, Portman RW, 1971. Dermacentor variabilis (Say) (Acarina: Ixodidae) established in southeastern Washington and northern Idaho. J. Med. Entomol. 8, 143–147. [DOI] [PubMed] [Google Scholar]
- Stromdahl E, Evans S, O’brien J, Gutierrez A, 2001. Prevalence of infection in ticks submitted to the human tick test kit program of the US Army Center for health promotion and preventive medicine. J. Med. Entomol. 38, 67–74. [DOI] [PubMed] [Google Scholar]
- Stromdahl EY, Jiang J, Vince M, Richards AL, 2010. Infrequency of Rickettsia rickettsii in Dermacentor variabilis removed from humans, with comments on the role of other human-biting ticks associated with spotted fever group rickettsiae in the United States. Vector Borne Zoonotic Dis. 11, 969–977. [DOI] [PubMed] [Google Scholar]
- Suppan J, Engel B, Marchetti-Deschmann M, Nürnberger S, 2018. Tick attachment cement - reviewing the mysteries of a biological skin plug system. Biol. Rev. Camb. Philos. Soc. 93, 1056–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherst RW, Bourne AS, 1991. Development, survival, fecundity and behaviour of Haemaphysalis (Kaiseriana) longicornis (Ixodidae) at two locations in southeast Queensland. Int. J. Parasitol. 21, 661–672. [DOI] [PubMed] [Google Scholar]
- Sutherst RW, Moorhouse DE, 1972. The seasonal incidence of ixodid ticks on cattle in an elevated area of South-Eastern Queensland. Aust. J. Agric. Res. 23, 195–204. [Google Scholar]
- Talagrand-Reboul E, Boyer PH, Bergstöm S, Vial L, Boulanger N, 2018. Relapsing fevers: neglected tick-borne diseases. Front. Cell Infect. Microbiol. 8, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teglas MB, Foley J, 2006. Differences in the transmissibility of two Anaplasma phagocytophilum strains by the North American tick vector species, Ixodes pacificus and Ixodes scapularis (Acari: Ixodidae). Exp. Appl. Acarol. 38, 47–58. [DOI] [PubMed] [Google Scholar]
- Telford SR, Dawson JE, Katavolos P, Warner CK, Kolbert CP, Persing DH, 1996. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc. Natl. Acad. Sci. 93, 6209–6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JE, Staubus L, Goolsby JL, Reichard MV, 2016. Ectoparasites of free-roaming domestic cats in the central United States. Vet. Parasitol. 228, 17–22. [DOI] [PubMed] [Google Scholar]
- Thomas JE, Scimeca RC, Schunack B, Ohmes CM, Duncan KT, Lineberry MW, Beam RA, Whitley D, Reichard MV, 2020. Sequential histologic comparisons of naïve and subsequent Amblyomma americanum bite lesions from induced infestations on dogs and cats. In: 65th Annual Meeting of the American Association of Veterinary Parasitologists. Virtual Conference June 20–23, 2020. [Google Scholar]
- Thompson AT, White S, Shaw D, Egizi A, Lahmers K, Ruder MG, Yabsley MJ, 2020. Theileria orientalis Ikeda in host-seeking Haemaphysalis longicornis in Virginia, U.S.A. Ticks Tick Borne Dis. 11, 101450. [DOI] [PubMed] [Google Scholar]
- Tilly K, Rosa PA, Stewart PE, 2008. Biology of infection with Borrelia burgdorferi. Infect. Dis Clin. North Am. 22, 217–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troughton DR, Levin ML, 2007. Life cycles of seven ixodid tick species (Acari: Ixodidae) under standardized laboratory conditions. J. Med. Entomol. 44, 732–740. [DOI] [PubMed] [Google Scholar]
- Tufts DM, VanAcker MC, Fernandez MP, DeNicola A, Egizi A, Diuk- Wasser MA, 2019. Distribution, host-seeking phenology, and host and habitat associations of Haemaphysalis longicornis ticks, Staten Island, New York, USA. Emerg. Infect. Dis. 25, 792–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turtinen LW, Kruger AN, Hacker MM, 2015. Prevalence of Borrelia burgdorferi in adult female ticks (Ixodes scapularis), Wisconsin 2010–2013. J. Vector Ecol. 40, 195–197. [DOI] [PubMed] [Google Scholar]
- United States Department of Agriculture, 1976. Ticks of veterinary importance. Agriculture Handbook No. 485. Accessed 1 Oct 2020. https://naldc.nal.usda.gov/download/CAT87208761/PDF.
- United States Department of Agriculture, 2020. National Haemaphysalis longicornis (Asian Longhorned Tick) Situation Report. https://www.aphis.usda.gov/animal_health/animal_diseases/tick/downloads/longhorned-tick-sitrep.pdf.
- Varela A, Moore V, Little S, 2004a. Disease agents in Amblyomma americanum from northeastern Georgia. J. Med. Entomol. 41, 753–759. [DOI] [PubMed] [Google Scholar]
- Varela AS, Luttrell MP, Howerth EW, Moore VA, Davidson WR, Stallknecht DE, Little SE, 2004b. First culture isolation of Borrelia lonestari, putative agent of southern tick- associated rash illness. J. Clin. Microbiol. 42, 1163–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela- Stokes A, 2007. Transmission of Ehrlichia chaffeensis from lone star ticks (Amblyomma americanum) to white- tailed deer (Odocoileus virginianus). J. Wildl. Dis. 43, 376–381. [DOI] [PubMed] [Google Scholar]
- Vial L, 2009. Biological and ecological characteristics of soft ticks (Ixodida: Argasidae) and their impact for predicting tick and associated disease distribution. Parasite. 16, 191–202. [DOI] [PubMed] [Google Scholar]
- Vigil KJ, 2013. Molecular Investigations of Two Spotted Fever Group Rickettsia Phylotypes in Ixodes pacificus, Dermacentor occidentalis and Domestic Dogs. Thesis (M.S.). Humboldt State University, Biology. http://humboldt-dspace.calstate.edu/handle/2148/1477. [Google Scholar]
- Villarreal Z, Stephenson N, Foley J, 2018. Possible northward introgression of a tropical lineage of Rhipicephalus sanguineus ticks at a site of emerging Rocky Mountain spotted fever. J. Parasitol. 104, 240–245. [DOI] [PubMed] [Google Scholar]
- Vincent-Johnson NA, Macintire DK, Lindsay DS, Lenz SD, Baneth G, Shkap V, Blagburn BL, 1997. A new Hepatozoon species from dogs: description of the causative agent of canine hepatozoonosis in North America. J. Parasitol. 83, 1165–1172. [PubMed] [Google Scholar]
- Waladde S, Rice M, 1982. The sensory basis of tick feeding behaviour. In: Obenchain FD, Galum R (Eds.), Physiology of Ticks. Pergamon Press, Oxford, UK, pp. 71–118. [Google Scholar]
- Walker JB, Keiran JE, Horak IG, 2000. The Genus Rhipicephalus (Acari, Ixodidae): A Guide to the Brown Ticks of the World. Cambridge University Press, Cambridge, New York, 643 pp. [Google Scholar]
- Wanchinga DM, Barker RW, 1986. Colonization and laboratory development of Otobius megnini (Acari: Argasidae). J. Econ. Entomol. 79, 999–1002. [DOI] [PubMed] [Google Scholar]
- Wells AB, Durden LA, Smoyer JH, 2004. Ticks (Acari: Ixodidae) parasitizing domestic dogs in southeastern Georgia. J. Entomol. Sci. 39, 426–432. [Google Scholar]
- Whitlock J, Fang Q, Durden L, Oliver J, 2000. Prevalence of Ehrlichia chaffeensis (Rickettsiales: Rickettsiaceae) in Amblyomma americanum (Acari: Ixodidae) from the Georgia coast and barrier islands. J. Med. Entomol. 37, 276–280. [DOI] [PubMed] [Google Scholar]
- Wikel S, 2013. Ticks and tick-borne pathogens at the cutaneous interface: host defenses, tick countermeasures, and a suitable environment for pathogen establishment. Front. Microbiol. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikswo ME, Hu R, Metzger ME, Eremeeva ME, 2007. Detection of Rickettsia rickettsii and Bartonella henselae in Rhipicephalus sanguineus ticks from California. J. Med. Entomol. 44, 158–162. [DOI] [PubMed] [Google Scholar]
- Wilkinson PR, 1967. The distribution of Dermacentor ticks in Canada in relation to bioclimatic zones. Can. J. Zool. 45, 517–537. [DOI] [PubMed] [Google Scholar]
- Williamson PC, Billingsley PM, Teltow GJ, Seals JP, Turnbough M, 2010. Borrelia, Ehrlichia, and Rickettsia spp. In ticks removed from persons, Texas, USA. Emerg. Infect. Dis. 16, 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ML, Ducey AM, Litwin TS, Gavin TA, Spielman A, 1990. Microgeographic distribution of immature Ixodes dammini ticks correlated with that of deer. Med. Vet. Entomol. 4, 151–159. [DOI] [PubMed] [Google Scholar]
- Wolf L, McPherson T, Harrison B, Engber B, Anderson A, Whitt P, 2000. Prevalence of Ehrlichia ewingii in Amblyomma americanum in North Carolina. J. Clin. Microbiol. 38, 2795–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood H, Dillon L, Patel SN, Ralevski F, 2016. Prevalence of Rickettsia species in Dermacentor variabilis ticks from Ontario, Canada. Ticks Tick Borne Dis. 7, 1044–1046. [DOI] [PubMed] [Google Scholar]
- Wright CL, Gaff HD, Hynes WL, 2014. Prevalence of Ehrlichia chaffeensis and Ehrlichia ewingii in Amblyomma americanum and Dermacentor variabilis collected from southeastern Virginia, 2010–2011. Ticks Tick Borne Dis. 5, 978–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabsley MJ, 2010. Natural history of Ehrlichia chaffeensis: vertebrate hosts and tick vectors from the United States and evidence for endemic transmission in other countries. Vet. Parasitol. 167, 136–148. [DOI] [PubMed] [Google Scholar]
- Yabsley MJ, McKibben J, Macpherson CN, Cattan PF, Cherry NA, Hegarty BC, Breitschwerdt EB, O’Connor T, Chandrashekar R, Paterson T, Perea ML, Ball G, Friesen S, Goedde J, Henderson B, Sylvester W, 2008. Prevalence of Ehrlichia canis, Anaplasma platys, Babesia canis vogeli, Hepatozoon canis, Bartonella vinsonii berkhoffii, and Rickettsia spp. In dogs from Grenada. Vet. Parasitol. 151, 279–285. [DOI] [PubMed] [Google Scholar]
- Yancey CB, Hegarty BC, Qurollo BA, Levy MG, Birkenheuer AJ, Weber DJ, Diniz PPVP, Breitschwerdt EB, 2014. Regional seroreactivity and vector-borne disease co- exposures in dogs in the United States from 2004–2010: utility of canine surveillance. Vector Borne Zoonotic Dis. 14, 724–732. [DOI] [PubMed] [Google Scholar]
- Yeagley TJ, Reichard MV, Hempstead JE, Allen KE, Parsons LM, White MA, Little SE, Meinkoth JH, 2009. Detection of Babesia gibsoni and the canine small Babesia’ Spanish isolate’ in blood samples obtained from dogs confiscated from dogfighting operations. J. Am. Vet. Med. Assoc. 235, 535–539. [DOI] [PubMed] [Google Scholar]
- Yeh MT, Bak JM, Hu R, Nicholson MC, Kelly C, Mather TN, 1995. Determining the duration of Ixodes scapularis (Acari: Ixodidae) attachment to tick-bite victims. J. Med Entomol. 32, 853–858. [DOI] [PubMed] [Google Scholar]
- Yu X, Piesman JF, Olson JG, Walker DH, 1997. Geographic distribution of different genetic types of Ehrlichia chaffeensis. Am. J. Trop. Med. Hyg. 56, 679–680. [DOI] [PubMed] [Google Scholar]
- Yunik MEM, Galloway TD, Lindsay LR, 2015. Assessment of prevalence and distribution of spotted fever group rickettsiae in Manitoba, Canada, in the American dog tick, Dermacentor variabilis (Acari: Ixodidae). Vector Borne. Zoonotic. Dis. 15, 103–108. [DOI] [PubMed] [Google Scholar]
- Yuval B, Spielman A, 1990. Duration and regulation of the developmental cycle of Ixodes dammini (Acari: Ixodidae). J. Med Entomol. 27, 196–201. [DOI] [PubMed] [Google Scholar]
- Zemtsova GE, Apanaskevich DA, Reeves WK, Hahn M, Snellgrove A, Levin ML, 2016. Phylogeography of Rhipicephalus sanguineus sensu lato and its relationships with climatic factors. Exper. Appl. Acarol. 69, 191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Liu Q, Wang D, Li W, Beugnet F, Zhou J, 2017. Epidemiological survey of ticks and tick-borne pathogens in pet dogs in southeastern China. Parasite 24, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Yu Z, Chen Z, Zhou L, Zheng B, Ma H, Liu J, 2011. Development and biological characteristics of Haemaphysalis longicornis (Acari: Ixodidae) under field conditions. Exp. Appl. Acarol. 53, 377–388. [DOI] [PubMed] [Google Scholar]
- Zheng H, Yu Z, Zhou L, Yang X, Liu J, 2012. Seasonal abundance and activity of the hard tick Haemaphysalis longicornis (Acari: Ixodidae) in north China. Exp. Appl. Acarol. 56, 133–141. [DOI] [PubMed] [Google Scholar]
- Zieman EA, Jiménez FA, Nielsen CK, 2017. Concurrent examination of bobcats and ticks reveals high prevalence of Cytauxzoon felis in southern Illinois. J. Parasitol. 103, 343–348. [DOI] [PubMed] [Google Scholar]
- Zimmerman RH, McWherter GR, Bloemer SR, 1987. Role of small mammals in population dynamics and dissemination of Amblyomma americanum and Dermacentor variabilis (Acari: Ixodidae) at Land between the Lakes, Tennessee. J. Med. Entomol. 24, 370–375. [DOI] [PubMed] [Google Scholar]


