Abstract
Melittin, the main venom component of the European Honeybee, is a cationic linear peptide-amide of 26 amino acid residues with the sequence: GIGAVLKVLTTGLPALISWIKRKRQQ-NH2. Melittin binds to lipid bilayer membranes, folds into amphipathic α-helical secondary structure and disrupts the permeability barrier properties of membranes. Since melittin was first described, a remarkable array of activities and potential applications in biology and medicine have been described. Melittin is also a favorite model system for biophysicists to study the structure, folding and function of peptides and proteins in membranes. Melittin has also been used as a template for the evolution of new activities in membranes. Here we overview the rich history of scientific research of the activities of melittin and outline exciting future applications.
INTRODUCTION
A brief biological history of Honeybee venom and melittin
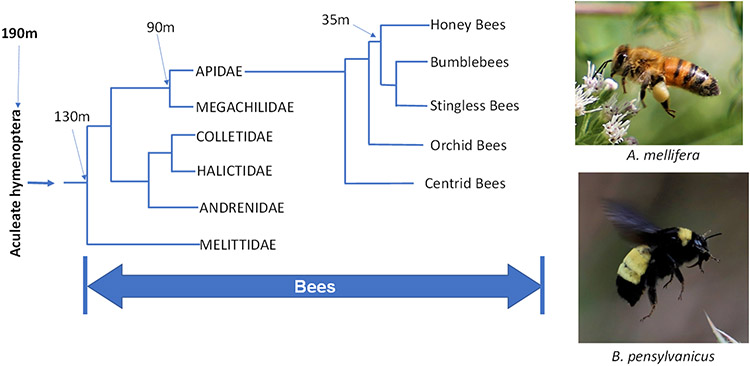
Roughly 190 million years ago, the extremely successful aculeate hymenopteran lineage of stinging ants, wasps, and bees arose when female ovipositors and sexual accessory glands were co-opted by natural selection to create a unique venom delivery system; the sting (1-4). This system is used by tens of thousands of extant insect species to deliver peptides, proteins, bioactive amines, and other components to prey, predator, or kleptoparasite species, including humans. Bees diverged from various wasp lineages about 130 million years ago, Fig. 1. About 90 million years ago, a bee lineage diverged that would lead to the Honeybees and their close relatives, the Bumblebees, the Orchid bees, and others. Honeybees and Bumblebees diverged from each other about 30 million years ago, leading to the ~10 extant species of Honeybees, including the globally important agricultural pollinator and honey producer, the Western Honeybee, Apis melifera.
Figure 1.
Evolution of the melittin-producing Honey Bees. Marked nodes include the origin of the sting (190 m years), the divergence of bees (130 m years), the divergence of the Honey bees and relatives (90 m years) and the divergence of the honey bees (35 m years). Photographs show the Western Honey Bee (A. melifera) and the related bumblebee (B. pensylvanicus). Photographs by WC Wimley, used with permission.
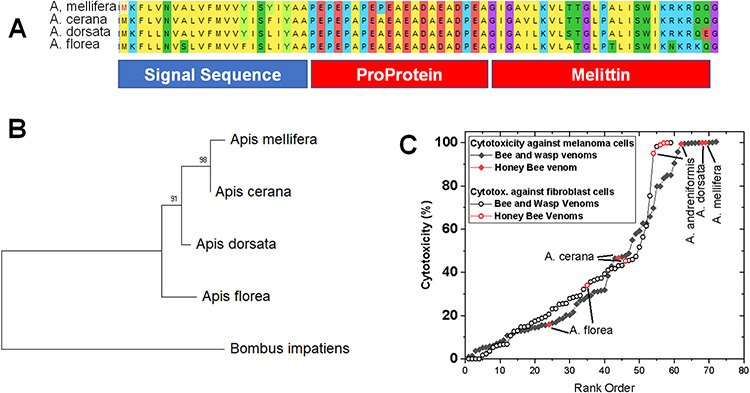
The 26-residue cytolytic peptide melittin is the main component, by weight, of the venom of all members of the genus apis, the Honeybees. Related cytolytic peptides, called bombolittins are found in Bumblebee (Genus Bombus) venom. These and other venom peptides are produced by secretory cells associated with the venom glands. Like the peptide hormones from which it likely evolved, melittin is synthesized as a preproprotein. The 70-residue preproprotein has an N-terminal signal sequence to direct its synthesis to the secretory pathways of the endoplasmic reticulum. After cleavage of the signal peptide, the remaining proprotein has an N-terminal domain composed of multiple anionic residues, interspersed with proline and alanine residues at every other residue, Fig. 2A. The anionic residues may neutralize the action of the C-terminal melittin sequence through electrostatic interactions with the five basic residues of melittin. The Pro and Ala residues in the proprotein enable the mature melittin peptide to be released from the proprotein through the sequential action of dipeptidyl peptidase IV (DPP IV) on the N-terminal residues. For this purpose, DPP IV is also secreted into the venom gland (5). Finally, the C-terminal glutamine-glycine dipeptide is enzymatically converted to a terminal glutamine-amide to give the native 26-residue peptide amide. In Fig. 2A, the sequences of melittins from Apis species are shown, and a partial phylogenetic tree is shown for Honeybees in Fig. 2B. One species of Bombus is included to validate the root of the tree. Among melittin sequences, a few conservative substations occur, but they are otherwise nearly identical. As we discuss below, melittin is highly cytolytic and comprises up to 50% of the dry weight of Apis venom. Therefore, Honeybee venom is among the most cytotoxic bee/wasp venoms known (Fig 2C).
Figure 2.
Production and properties of Honey Bee venom. A. Prepromelittin sequences from four Apis species show the architecture that is used the production of peptide toxins. A signal sequence directs the protein to secretory pathways and the proprotein stabilizes the peptide until it is released in the venom gland. B. Apis phylogeny inferred form the prepromelittin sequences. C. Relative cytotoxicity of bee and wasp venoms (1).
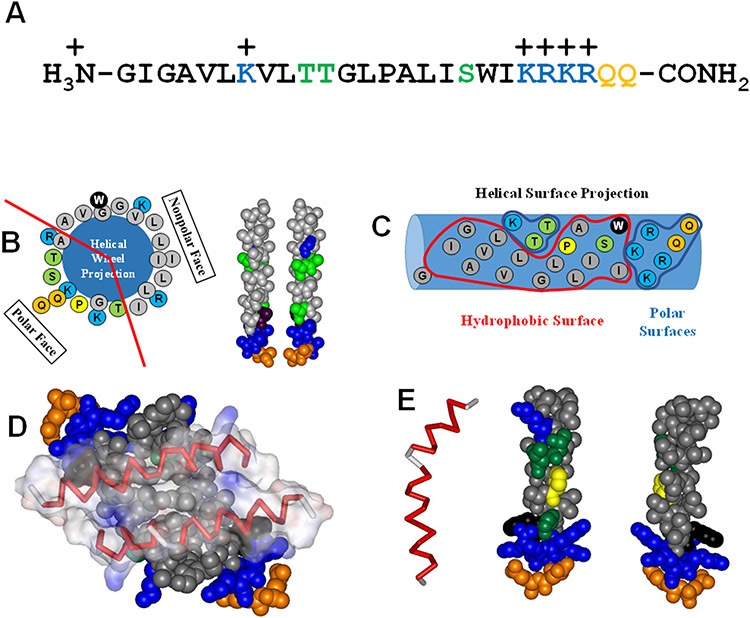
The crystal structure of melittin was determined nearly 40 years ago (6) and its structure in solution has also been elucidated by NMR (7, 8). Melittin partitions into membranes because of its hydrophobicity (6). It has amphipathicity in two-dimensions (Fig. 3A-C). First, it is amphipathic in primary sequence; its N-terminal half is much more hydrophobic than its C-terminal half, which is highly polar and cationic (Fig. 3A,C). Second, melittin is amphipathic in secondary structure as it folds into an α-helix with a continuous hydrophobic face on one surface and a distinct polar face on the opposite surface (Fig. 3B). In solution, melittin α-helices fold into tetramers (Fig. 3D) in which the hydrophobic surfaces of the amphipathic helices are buried, and the polar surfaces are exposed (6). This structure enables melittin to have an extremely high solubility in water.
Figure 3.
Sequence and structure of melittin. Hydrophoboic residues are black. Moderately polar residues (S,T) are green. More polar glutamines (Q) are orange. Basic lysines (K) are blue. A. The amino acid sequence of melittin from A. melifera. B. The helical wheel diagram and idealized helical structures show that when melittin folds into an α-helix the structure is highly amphipathic, with hydrophobic and hydrophilic residues clustered on opposite faces. C. This helical surface projection shows that the amphipathicity of the melittin helix is two-dimensional, both on the face of the helix, and along the helix length where the C-terminal portion on the right is highly polar. D. In solution, the amphipathicity of melittin drives the formation of tetramers with the hydrophobic surfaces forming the interior of the structure. E. The Gly-Leu-Pro sequence at positions 12-14 break the melittin structure into two helical segments. This helix break is critical for the biological activity of melittin.
The secondary structure of melittin is a bent rod or helix-hinge-helix motif which is common to many membrane active peptides (9-11) and transmembrane helices (12). Residues 3 to 10 form an α-helix followed by a hinge region (residues 12-14), which connects to a longer α-helix consisting of residues 15 to 24 (6, 7), Fig. 3E. The central glycine-X-proline motif at residues 12-14 is responsible for creating the bend by disrupting the conformation and hydrogen bonding of the helical structure. The hinge region serves an important function by separating the more hydrophobic N-helix from the more hydrophilic C-helix, which gives melittin an amphiphilic arrangement along the length of the sequence (13, 14). A recent 2D-NMR study utilized isotope-labeled (15N and 13C) melittin to determine the structures of cis and trans Pro-14 melittin in aqueous solution and 30% trifluoroethanol, a membrane-mimicking solvent (8, 15). In accordance with previous NMR studies, most of the melittin molecules were determined to contain the trans Leu13–Pro14 bond; however, about 25% of the melittin helices contained the cis-Pro configuration. The cis Leu13-Pro14 bond alters the secondary structure of the longer C-terminal α-helix, resulting in a lower helicity. This is supported by the crystal structure and other evidence that trans-Pro-melittin is exclusively involved in tetramer formation in solution (16).
Melittin is the archetypal membrane-active peptide. It is hydrophobic and amphipathic, which drives strong partitioning into membranes. Membrane binding is coupled to folding into α-helical secondary structure. Though the mechanism is still debated (17-19), and may be variable in some instances, the insertion of amphipathic melittin into a bilayer dramatically reduces the permeability barrier by disrupting the strict segregation of polar, and non-polar moieties across the bilayer. Under some rare conditions, melittin may assemble into stable transmembrane bundles (20) to form pores but this is generally not the case. Instead, in synthetic bilayers under most conditions melittin causes transient permeabilization of membranes with a burst of leakage that occurs in the minutes after melittin is first added. After the initial burst, leakage slows or stops as we have discussed in detail elsewhere (17).
When melittin permeabilizes a biological membrane, water, ions, metabolites and other molecules pass freely through the membrane and the cell is rapidly killed. Natural selection evolved Honeybee venom to protect the hive from predators that include insects, reptiles, mammals and birds. Accordingly, melittin permeabilizes many different types of membranes indiscriminately. At a locally high concentration after a sting, the physiological activity of melittin is straightforward; it lyses cell membranes and disrupts tissue to cause immediate pain and discomfort in the predator or parasite. At a high enough concentration, the toxicity of melittin becomes systemic and can be fatal.
Beyond these limited natural scenarios, an extremely wide array of interesting and potentially useful biological activities have been reported for melittin. These many studies are mostly carried out under conditions where melittin is not uniformly toxic to all cells or to the host animal. These conditions include melittin at low concentrations, conjugated to proteins to other molecules, or formulated in nanoparticles, liposomes, or formulations that reduce its undesirable effects. Its activity has been characterized in a broad array of systems, in vitro and in vivo. It has been tested against host and pathogen cells from all branches of life, against infectious diseases and disease pathogens, and against cancer, and many other diseases from many different tissues. Next, we describe the many biological activities that have been observed for melittin.
THE BIOLOGICAL ACTIVITIES OF MELITTIN
Bacteria
The anti-bacterial activity of bee venom was first reported in 1941 by Schmidt and Lange (21). Melittin was recognized as the bactericidal constituent of bee venom, over two decades later, by an experiment that demonstrated whole bee venom and a sub-fraction, melittin, have similar anti-bacterial effects against penicillin resistant Staphylococcus aureus (22). In the same study, melittin was also shown to have activity against clinical isolates of gram-negative Pseudomonas aeruginosa, Stenotrophomonas maltophilia, Acinetobacter lwoffii, Klebsiella aerogenes (previously known as Enterobacter aerogenes), Enterobacter cloacae, and Salmonella enterica as well as isolates of gram-positive Enterococcus faecalis, Streptococcus liquefaciens, Corynebacterium sp., and Staphylococcus aureus 3A, 53, and 80 (22, 23). The mechanism by which melittin sterilized bacteria was not known then and still is not completely understood. However, experimental and computational studies have provided evidence that melittin insertion into bacterial cytoplasmic membranes is a prerequisite to membrane permeabilization that leads to cell death by membrane disruption (24-26).
In 1971, the activity of melittin was demonstrated against acid-fast Mycobacterium phlei (27). More recently, melittin was shown to be active against clinical isolates of Mycobacterium tuberculosis and Mycobacterium avium (28). Melittin also has activity against mollicutes, a class of parasitic bacteria distinguished phenotypically by the absence of a cell wall (29). Melittin exhibits excellent activity against many genera of mollicutes including, Acholeplasma laidlawii A-PGS, Mycoplasma gallisepticum S6, Mycoplasma genitalium G37, Mycoplasma mycoides subsp. mycoides KH3J, Spiroplasma citri R8A2, Spiroplasma melliferum BC3, and Mycoplasma hominis strain H34 (29-31). Melittin has also been reported to possess anti-bacterial activity against Borrelia burgdorferi, the etiologic agent for Lyme disease (32, 33). Borrelia spp., although a spirochetal bacterium, can exist as round bodies, stationary phase persisters, and biofilm forms. Different states of Borrelia have varying susceptibility to conventional antibiotics, with persisters and biofilms being most resistant (34, 35). Due to the limited activity of conventional antibiotics against Borrelia spp., melittin-like membrane permeabilizing peptides may be a promising therapeutic alternative for Lyme disease. Melittin is also reported to inhibit Chlamydia trachomatis infection of cultured human cells(30). It is proven to be effective against different strains of Listeria as well (36). Melittin shows inhibitory effects against bacteria that infect aquatic creatures such as Vibrio parahaemolyticus and Edwardsiella tarda (23). Melittin also shows potency against plant associated bacteria including Xanthomonas oryzae that causes rice blight disease (23, 37). Additionally, melittin has been used synergistically with other peptides such as cecropin, nisin,and with conventional antibiotics. These combination formulations have higher bactericidal activity than individual peptides or antibiotics. Some combinations with antibiotics also have antibacterial activity against previously resistant strains (38-43). During the last two decades, melittin has also been tested for its activity against biofilms made by Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli, Acinetobacter baumannii and Klebsiella pneumoniae. Melittin has demonstrated the ability to reduce biofilm formation by destroying biofilms, lysing biofilm-embedded bacteria, and preventing bacterial surface attachment (38, 42, 44). One such study demonstrated that melittin can even inhibit the formation of polymicrobial biofilms made of combinations of various resistant bacteria (45).
In addition to in vitro studies, several animal models have also been used to study the activity of melittin against bacteria in vivo. These studies show that melittin has protective effects against Chlamydia Trachomatis, Cutibacterium acnes (previously known as Propionibactierium acnes), Mycoplasma gallispticum, Methicillin resistant Staphylococcus aureus (MRSA), and extensively drug resistant (XDR) Acinetobacter baumannii infections in animal models (46-49). A plasmid vector was delivered to chickens in which the expression of melittin was under the control of an inducible tetracycline-dependent human cytomegalovirus (CMV) promoter. The plasmid also contained the gene that codes for the trans-activation protein rtTA (47). The delivery of the plasmid vector was accomplished through aerosol administration to chickens. The chickens were infected with Mycoplasma gallisepticum, which is a bacterium which causes severe disease in several types of birds (45, 47). The chickens which were given the melittin plasmid vector were able to inhibit development of infection and bacterialdissemination. Later, a similar melittin plasmid vector was delivered by transfection intravaginally to BALB/C mice (46). The melittin-expressing mice were able to inhibit vaginal infection of two types of bacteria, Mycoplasma hominis and Chlamydia trachomatis, and many of the animals were completely pathogen free within 3-4 weeks (46). These data suggest that antimicrobial plasmid vectors may be a viable antibacterial option in several contexts.
Melittin exhibits antibacterial activity against two human bacterial pathogens which can cause severe infections. One of these studies used intraperitoneal (IP) infection of CD1 mice with Methicillin-resistant Staphylococcus aureus (MRSA) and then followed that with another IP injection of melittin after 1 hour (48) . Interestingly, melittin was able to increase the survival rate of the mice infected with MRSA; this is likely the first instance in which melittin demonstrated potential in controlling in vivo infection by MRSA. Another study used SKH-1 mice in a wound model in which the wound was infected with a biofilm of a bioluminescent derivative of Pseudomonas aeruginosa (PAO1) (50). The melittin in this study was reconstituted in agarose hydrogels to serve as a delivery system and applied to the wound. The melittin hydrogel, especially when used in combination with tobramycin, resulted in a several-fold reduction in the bioluminescence of the PAO1. This study demonstrates the applicability of melittin to novel combination antibacterial treatments, and is the first record of utilizing hydrogels as a delivery system to an animal model of biofilm bacterial infection.
Melittin has also been the basis of many novel AMP discoveries. For example, pioneering work done by Bruce Merrifield and collaborators showed that a hybrid peptide made by combining parts of melittin with the insect antimicrobial peptide cecropin was 100 times more potent than cecropin A alone against S. aureus. The hybrid also had broad-spectrum activity against bacteria and against Plasmodium, the parasite that causes malaria (51). The work of Shai and colleagues showed that melittin diastereomer, containing several helix-disrupting D-amino acids retained antibacterial activity while becoming 100-fold less cytotoxic (52)
Interestingly, there are a few species of bacteria that are resistant to melittin and other antimicrobial peptides. For example, Burkholderia thailandensis, a close relative of the biothreat organism Burkholderia pseudomallei is completely resistant to melittin and other AMPs up to concentrations as high as 50 μM (S. Guha, W.C. Wimley, unpublished). This is probably due to the inability of the peptide to cross the Burkholderia outer membrane lipopolysaccharide and access the inner membrane.
Viruses
Melittin has potent broad-spectrum inhibitory activity against both enveloped and non-enveloped viruses, as discussed recently (53). For example, multiple studies have been conducted on the Herpesviridae family of viruses, which include Herpes Simplex Virus (HSV-1, HSV-2), and Bovine Herpes Virus (BoHV-1). One of these studies showed that melittin can disrupt HSV-1 cell fusion in certain glycoprotein mutants of the virus (53, 54). Specifically, melittin limited the ability of HSV-1 to enter eukaryotic Vero cells (53, 54). Two other studies confirmed that melittin has antiviral activity against HSV-1, and also demonstrated antiviral activity against HSV-2 in Vero cells (53, 55, 56). Specifically, melittin completely inactivated the viruses, thereby disrupting their potential for infectivity. Melittin also exerts potent antiviral activity against BoHV-1 and can work in a temperature-independent manner (53).
There is limited data demonstrating the antiviral activity of melittin against viral members of the Orthomyxoviridae family. One such study tested melittin against A/PR/8 Influenza A virus (53, 57), and demonstrated that melittin decreased viral titers and reduced the number of (infected MDCK cells. There is a large body of literature demonstrating efficacy of melittin against the Retroviridae family, which contains the human immunodeficiency virus (HIV) virus. The exposure of the murine leukemia virus (MuLV) to melittin resulted in the disintegration of the viral membrane (53, 58). Melittin is also able to permeabilize the viral membrane of rous-associated virus (RAV-2), resulting in virolysis (53, 59). In 1990, melittin was demonstrated to permeabilize HIV-1 membranes with direct virolysis (53, 60). Melittin reduced infectivity of HIV-1 in the supernatants of KE37/1 T lymphoma cells (53, 61). In a dose-dependent manner, melittin inhibited virus production in T lymphoma cells and in fibroblast cells via gene expression modulation (53, 61). Melittin-loaded nanoparticles were able to capture HIV-1 and reduce infectivity (53, 62).
The activity of melittin has also been tested against non-enveloped viruses. Treatment with melittin reduced the infectivity of enterovirus (EV-71), which can cause epidemics of neurological disease and is the causative virus of hand, foot, and mouth disease. Melittin decreased the cytopathic effects induced by the virus against HeLa cells (53, 57). Melittin was also tested against coxsackievirus (H3), a member of the Picornaviridae family. This virus causes a wide array of disease symptoms (53, 57). Using a GFP-tagged H3 virus, the authors found that melittin reduced viral titers as well as the number of GFP-positive infected cells.
The antiviral activity of melittin has also been studied using animal models. One study used C57BL/6 mice to intranasally deliver both H1N1 Influenza A virus and melittin after a preincubation of melittin with a minimum lethal dose (MLD50) of the virus (57). The mice that were given melittin instead of the vehicle control were able to maintain body weight during the 8 days duration of the study, had a 100% survival rate (n=6), and virus titers orders of magnitude lower than the vehicle control group at the end point (57). These data suggest a potential for melittin or melittin-like peptides to be used as an antiviral agent either therapeutically or prophylactically. There are many more examples demonstrating the specific antiviral activity of melittin against Arenaviridae, Flaviviridae, Pneumoviridae, and Virgaviridae families. The hypothesized antiviral mechanisms of melittin have recently been described (53).
Fungi
Melittin has inhibitory and antifungal effects against Candida albicans, one of the main pathogens responsible for human fungal infections, at concentration levels that are lower than the ones used for Amphotericin B, a traditional antifungal drug. Melittin likely triggers fungal apoptosis through the reactive oxygen species-mediated mitochondria/caspase-dependent pathway (63-65). Melittin has been used to inhibit the production of ECM materials from nasal passage fibroblasts, which were then exposed to Alterneria and Aspergillus (66). Interestingly, the inhibition of the production of collagen,and other extracellular matrix (ECM) components by melittin reduced the number of fungi that attached to the ECM, suggesting a potential use in fungi-induced airway inflammatory diseases. Melittin may also be useful within an agricultural setting. One study found that melittin inhibits Rhizoctonia solani, a type of fungus that causes rice sheath blight and aerial blight of soy beans in the United States (67). Additionally, melittin has displayed antifungal activity against a fungus, Penicillium digitatum, which affects citrus fruit postharvest decay, thus potentially acting as an agricultural fungicide (68, 69).
Parasites
Melittin has direct antiparasitic effects against the sporogonic and gametocyte stages of the Plasmodium life cycle (70) (71). Melittin has been tested against Leishmania parasites, the causative agent of leishmaniasis. The peptides had inhibitory activity against the promastigote life stage of the parasite and can even inhibit the intracellular amastigote form of Leishmania (72, 73). A melittin-cecropin hybrid peptide, previously described and named CM11, has also shown antiparasitic activity against the amastigote form of Leishmania (74). Leishmaniasis is a deadly disease caused by the Leishmania genus of parasites, namely Leishmania major, and is responsible for millions of new infections and several thousand deaths yearly (75).
In recent years, a select few antimicrobial peptides have been tested against Trypanosoma cruzi, that causes Chagas disease which affects millions of people worldwide on a yearly basis (76, 77). Melittin was one of the very few compounds that yielded positive results. Melittin exerted antiparasitic activity on all developmental stages of Trypanosoma cruzi, including the intracellular amastigote forms. Likely, this killing was mediated through various programmed cell death pathways based on the life stage of the parasite (78). Melittin also displays synergistic killing of Trypanosoma cruzi when combined with other potent antimicrobial peptides (79). Melittin demonstrated antiparasitic activity against Toxoplasma gondii when monitoring the release of β-galactosidase, a marker for cell death, further indicating antiparasitic activity (80). Toxoplasmosis is a disease caused by the parasite Toxoplasma gondii and may affect up to one third of the entire world population (81). A CM11 melittin-cecropin hybrid peptide has high toxicity to the parasite Entamoeba histolytica, which causes Amebiasis, estimated to affect millions of people every year, especially those without access to clean water (82).
Eukaryotic cells
Melittin acts as a non-specific lytic peptide with a broad-spectrum activity against eukaryotic cells. The lytic effect of melittin has been shown to arise from its ability to disrupt plasma membrane by insertion into phospholipid bilayers. The characteristic action of melittin is hemolysis as erythrocytes membranes are an abundant cell type in target animals (83-85). In a monomeric form, melittin binds to the cell membranes of erythrocytes and induces rapid partial release of hemoglobin even at submicromolar concentrations (86, 87). This process is coupled with penetration of melittin into the membrane, followed by a second slower release of cytoplasmic contents, proposed to be caused by the self-assembly of membrane bound melittin (87). Besides erythrocytes, melittin also exhibits cytotoxicity in other hematopoietic cells, lymphocytes, and thymocytes. In human peripheral lymphocytes, melittin induces morphological changes in the cell membrane, and causes granulation leading to cell lysis. Even at non-cytotoxic concentrations, melittin causes DNA damage in the leukocytes indicated by increased oxidative stress, elevated formation of micronuclei and nuclear buds, and decreased lymphocytes proliferation (88, 89). In studies performed on rat thymocytes, melittin has varying concentration-dependent effects. At low concentrations, melittin stimulates thymocyte proliferation while in high concentrations, it is cytotoxic to the cells (90).
Melittin interacts with mammalian cells to cause plasma membrane disruption. For example, in vitro treatment of gastrointestinal epithelial cell lines with melittin results in loss of plasma membrane integrity even in low concentrations. The peptide is highly toxic to the epithelial cells, demonstrated by pronounced changes in cellular morphology, depletion of microvilli, increase in cell debris, decrease in tight junction and loss of cell-cell adhesion (91, 92). On account of this paracellular permeability of melittin, its utility in the delivery of poorly bioavailable hydrophilic drugs, through mucosal and absorption limited gastrointestinal tract, has been widely studied (93-95). Another study with endothelial cells also demonstrated that melittin causes large scale damage to the plasma membrane, leading to clear morphological signs of necrosis as well as disintegration and rapid decline in cell viability (96). Melittin also perturbs a broad range of metabolic functions in eukaryotic cells. Melittin inhibits the proliferation of vascular smooth muscle cells (VSMC) via activation of the AKT pathway and enhancement in the expression of apoptotic proteins (97). Additionally, in vitro and in vivo studies have demonstrated that melittin also exerts cytotoxic effects and inhibits the proliferation of hepatic cells by inducing apoptotic cell death (98). Conversely, optimal concentrations of melittin have protective effects against TGF-β1-mediated and TNF-α-induced hepatic damage. Under these conditions, melittin can inhibit the apoptosis initiated by TGF-β1 and TNF-α (99, 100).
Cancer
In cell and animal models, melittin has been shown to affect cell proliferation, apoptosis, and cancer cell metastasis, all of which indicate a possible use of melittin or melittin-derived peptides for cancer treatment (101, 102). The IC50 values against a variety of cancer cells (e.g. lung, breast, ovarian) (103-105) have largely been in the 1-10 μg/mL range.
Melittin induces apoptosis in human prostate cancer cells in vitro and, as a major component of bee venom, inhibits tumor growth in nude mice implanted with prostate cancer cells (106). Recently, prostate cancer cells have been killed by targeting their upregulation of MMP-2. Branched poly-ethylenimine was used to create nanoparticles that encompassed the melittin gene and were externally crosslinked to chlorotoxin, which targets MMP-2. The nanoparticles selectively bound to prostate cancer cells and delivered melittin DNA for transcription and translocation, which caused cell death (107).
Melittin has also been found to inhibit cell growth in multiple ovarian cancer cell lines (108). Melittin can be targeted to kill ovarian cells after being fused to urokinase-type plasminogen activator 1 (109). The anti-ovarian cancer cell activity of fused melittin was later enhanced by conjugation to the amino-terminal fragment of urokinase-type plasminogen activator 1 (110). Separately, the synergistic effect of melittin with cisplatin in ovarian cancer cells reduced metabolites in the CAC, ETC, purine, pyrimidine, arginine, and proline pathways and has therapeutic implications for drug tolerability and resistance and disease relapse (111).
Melittin causes a calcium influx and induces apoptosis in osteosarcoma cells (112). Furthermore, in a nude mouse osteosarcoma model, melittin directly decreased the viability of osteosarcoma cells, as well as the adhesion, migration and tube formation of endothelial progenitor cells (113). In terms of gastric cancer, melittin was found to inhibit cell proliferation and induce cell death in human gastric adenocarcinoma cells (114). Melittin has been confirmed to kill gastric cancer cells by the induction of apoptosis in additional in vitro studies (115). Interestingly, melittin killed gastric cancer cells more effectively as a monomer, rather than as a dimer, at lower concentrations (116).
Melittin kills human hepatocellular carcinoma cells when its gene is delivered by recombinant adenovirus (117). Building on previous findings, melittin was tested in additional hepatocellular carcinoma cell lines and was found to be cytotoxic. In nude mouse models, melittin was able to suppress hepatocellular carcinoma tumor growth and metastasis (118). Following the success of melittin in killing human hepatocellular carcinoma cells in vitro and in vivo, the mechanism of action for melittin in this system was explored (110, 119-122). Hepatocellular carcinoma cells with high VEGFR expression have been targeted through the creation of VEGF-melittin fusion proteins. Melittin analogs with hydrocarbon staples have also been designed that are useful against human hepatocellular carcinoma cells (123).
Melittin has long been known to inhibit the growth of human promyelocytic leukemic granulocytes by inhibiting calmodulin (124). Later studies also supported the activity of melittin against leukemia by demonstrating that melittin can induce cytolysis in human monocytic leukemia cells (125). The killing capacity of melittin in human monocytic leukemia cells was replicated, and the mechanism was further investigated (126). Most recently, melittin was shown to induce apoptosis in cell lines related to different origin acute lymphoblastic leukemia and chronic myelogenous leukemia (127).
Melittin inhibits human breast cancer cell motility and migration that is typically induced by EGF (128). Melittin suppresses PMA-induced cell differentiation (128). With the assistance of carbon nanoparticles, melittin induces death in brain cancer cell lines (129). The cytolytic effect of melittin in additional breast cancer cell lines has been investigated, as has its associated pathways (130). To improve the delivery of melittin to estrogen responsive breast cancer cells, micelles containing melittin and coated in estrone were developed (131). Melittin suppresses PMA-induced (phorbol 12-myristate 13-acetate, a potent tumor promoter) invasion and migration in renal carcinoma cells (132).
Melittin may halt the progression of human cervical cancer by inhibiting angiogenesis (133). More so, melittin directly induces apoptosis in human cervical cancer cells (134). Recently, nanocomposites containing melittin were developed and tested in additional human cervical cancer cell lines that allow for safe delivery and extended release (135). Pretreatment with melittin sensitizes esophageal squamous cell carcinoma to radiation, yielding increased apoptosis (136). In bladder cancer cells, melittin induces apoptosis by regulating key cancer related signaling pathways (137). In vitro, melittin inhibits the proliferation, migration and invasion of bladder cancer cells by inhibiting gene expression at the mRNA, protein and phosphorylation levels (138). Melittin selectively induces apoptosis in colorectal carcinoma cells over normal colorectal cells and suppresses the expression of genes involved in biotransformation (139). In both colorectal and gastric cancer cells, melittin is extremely fast acting and its membrane effects can be easily observed (140).
Melittin can be used to control the metastasis of cancer within an animal model. One study describes the effect of melittin on hepatocellular carcinoma (HCC) in which nude mice were subcutaneously injected with cells to produce implanted tumors (118). Once the tumors reached a certain size and volume, they were resected and transplanted into the liver parcel of other nude mice. Melittin and vehicle control were then administered to the mouse via tail vein injections daily. Melittin drastically inhibited cell motility of this aggressive HCC and prevented metastasis via suppression of the Rac-1-dependent pathway. In a rat model for chronic prostatitis and chronic prostatic pain, melittin was found to act as an anti-inflammatory and analgesic treatment (141). Melittin significantly reduces tumor growth of a highly metastatic lung cancer model in mice (142). Interestingly, in a similar Lewis lung carcinoma mouse model, melittin was found to reduce M2 tumor-associated macrophages, which reduces angiogenesis and immunosuppression (143). Melittin appears to selectively target human bronchogenic carcinoma cells over human lung fibroblast cells (144). The ability of melittin to inhibit cell proliferation, suppress tumor growth and decrease angiogenesis has also been reported in vitro and in vivo in non-small cell lung cancer cells (145). The mechanism for these findings has been elucidated (146).
Lung cancer has been targeted indirectly using melittin, as melittin acts on tumor-associated macrophages (TAM), which are major components of the tumor stroma and facilitate tumor cell progression (143, 147). In this work, a Lewis lung carcinoma (LLC) mouse model was used, in which C57BL/6 mice were subcutaneously injected with LLC cells into the right flank in order to produce a tumor. Intraperitoneal (IP) melittin suppressed the tumor growth and improved the survival of the tumor-affected mice by reducing the number of macrophages in the tumor microenvironment, specifically the M2-like macrophages (148). In addition, an orthotopic tumor model was used, in which the left lobe of the lung within the mice was injected with the LLC cells (147). The authors found that the IP-delivered melittin targeted M2-like macrophages, by inducing mitochondrial death and inhibiting orthotopic tumor growth. These studies indicate the potential of melittin as a potent anticancer agent in targeting TAMs. The effects of melittin on a tumor formed by the non-small cell lung cancer line, A549, were also studied (145). The A549 cells were subcutaneously injected into the right flank of BALB/C mice to produce tumors, and then melittin was administered by the same route at two different concentrations. The mice that were treated with melittin exhibited lower tumor volume and weight at both concentrations of peptide. These data indicate that melittin may be a viable template for designing treatments for lung cancer.
Melittin prevents proliferation and induces apoptosis in human melanoma cells (149). Additionally, melittin inhibits the migration, invasion and production of melanin in melanoma cells. Most recently, melittin has been applied to kill melanoma and breast cancer cells at reduced concentrations due to synergism with plasma treated phosphate buffered saline solution (150). Melittin promotes apoptosis in pancreatic ductal adenocarcinoma cells and suppresses tumor growth in a xenograft mouse model in combination with gemcitabine (151). Although not melittin itself, a concatenated melittin mutant has been used to target human thyroid cancer cells and to induce apoptosis both in vitro and in a xenograft nude mouse model (152). Melittin inhibits astrocytoma cells at nanomolar concentrations (153). Melittin has since been shown to induce apoptosis in grade IV human glioma cells in vitro and to cause disintegration of cell membranes (129). Interestingly, melittin can successfully be delivered at lower concentrations and with greater killing efficacy than previously reported with the help of carbon nanoparticles (129). Melittin was also recently incorporated into a self-assembling peptide nanofiber hydrogel to kill C6 glioma cells in vitro and was additionally found to reduce tumor size and recurrence rate in nude mice bearing tumors (154). Similarly, melittin was packaged with paclitaxel into lipodiscs for synergistic glioma killing both in vitro and in vivo (155). To target breast cancer brain metastases with melittin, poly(lactone-co-β-amino ester) nanoparticles coated in AMD3100 (plerixafor), which antagonizes upregulated CXCR4, were used to deliver the gene promelittin, which was then transcribed and translated to melittin. This strategy was successful in killing glioma cells and prolonging the survival time of tumor bearing mice (156). Melittin likewise inhibits head and neck squamous cell carcinoma cell growth and induces apoptosis in vitro, as well as reduces tumor growth in a xenograft mouse model (157).
Blood brain barrier
Recently, sub-toxic concentrations of melittin were shown to transiently open the paracellular tight junctions of the blood-brain barrier (BBB)(158). This phenomenon was first studied in cell culture and then in a tissue engineered model of the human BBB to optimize dosing and determine mechanism of action. Lastly, a 150 μL dose containing 3 μM of melittin at caused a dramatic increase in BBB permeability without causing obvious toxicity or neurological effects.
Immune modulation
Melittin may also have value as an adjuvant to cancer radiotherapy (136) as demonstrated by a study focused on esophageal squamous cell carcinoma (ESCC) within the context of a tumor xenograft model using BALB/C mice. In this experiment, mice were subcutaneously injected with ECA109 cells on their right leg. Once the tumor grew to a certain size, they were split into four treatment groups (vehicle, melittin, irradiation, and melittin + irradiation). The authors found that melittin increased the radiosensitivity of the ESCC within the context of the right leg subcutaneous tumors and enhanced the apoptosis of the irradiated cancer cells.
Melittin may play a useful role in immunotherapy. The application of a mutant IL2-melittin fusion protein enhanced immune cell killing of multiple cancer cell types and their respective tumors in vivo (147). Melittin was also effectively fused to dKLA to induce cell death in M2-like tumor-associated macrophages, which reduced tumor growth, weight and angiogenesis in vivo (148). Lastly, self-assembling melittin lipid nanoparticles have been found to accumulate in lymph nodes, to activate antigen presenting cells, to increase T-cell responses, and to inhibit primary and distant tumor growth in a melanoma mouse model (159).
Analgesic/anti-inflammatory effects
Honeybee venom has long been used as an anti-inflammatory agent (160). In one study that specifically focuses on the anti-inflammatory action of melittin, a dose of D-galactosamine (GalN), along with LSP (endotoxin), was administered to C57BL/6 mice, causing endotoxic shock and acute hepatic failure similar to what is seen in the clinic (161). The administration of melittin intraperitoneally prevented GalN/LPS-induced liver failure in mice by suppressing apoptosis and the inflammatory response in the liver (161). These data suggest that melittin may potentially be useful as a therapeutic agent against acute hepatic injury or failure (161). Another study with C57BL/6 mice used an established method of inducing acute pancreatitis (AP) by intraperitoneal administration of supramaximal concentrations of a CCK analogue called cerulein (162). Melittin was delivered subcutaneously (at a gradient of concentrations) to the AP and control mice at different time points either before or after the administration of the cerulein to induce AP (162). Melittin inhibited the activation of the c-Jun NH2- terminal protein kinase (JNK) in the pancreas, which reduced cell death and the production of pro-inflammatory cytokines. These data suggest that melittin may be able to attenuate AP and AP-induced injury outside of the pancreas.
Melittin may also be beneficial for inflammatory conditions caused by infections. One study used a mouse model to observe the induction of skin inflammation by the bacteria, Propionibacterium acnes (163). In this model, ICR mice were given intradermal injections of P. acnes into the ears to induce inflammation (163). After the onset of inflammation in the ears, the melittin-treated mice were given melittin admixed with Vaseline as a topical treatment (163). The mice that received the melittin showed markedly reduced swelling and granulomatous responses compared to the untreated mice; these data demonstrate the potential of melittin as a therapeutic agent against inflammatory skin disease caused by P. acnes. Melittin may also act as a therapeutic agent for neuropathy, which shares common features with inflammatory pain (164). Subcutaneous injections of melittin alleviated the pain response in rats, which were given the chemotherapeutic drug oxaliplatin, known to induce peripheral neuropathy (164). Noteworthy, peripheral neuropathy, manifested as allodynia (pain from stimuli that are normally not painful), is a common side effect of oxaliplatin treatment. A possible mechanism for this neuropathic pain alleviation by melittin may require the activation of the spinal α1- and α2-adrenegic receptors.
Melittin not only perturbs cell membranes but also affects cellular pathways which can lead to reduced inflammation. For instance, acne is caused by P. acnes and the resulting inflammation (102). Lee et al. showed that melittin decreased P. acne induced inflammatory cytokine production by blocking NFkB and p38 pathways in HaCaT cells. They also showed reduced inflammation in P. acnes injected ears in animal models (163). Anti-inflammatory properties were also seen in the BV2 microglial cell line, where it suppresses NO and iNOS expression, indicating that melittin can be used to combat neurodegenerative disease (165).
In an atherosclerosis study, mice treatment with 0.1 mg/kg of IP injected melittin resulted in a marked reduction in atherosclerosis lesions in the aorta and heart. Reduced levels of cholesterol, triglycerides, pro-inflammatory cytokines, adhesion molecules, and proatherogenic proteins were found as well (166).
Dermatological effects
There are only a few studies that specifically focus on the dermatological effects of melittin in vivo (160). Atopic Dermatitis (AD) is a common and chronic multifactorial skin disease, which manifests with pruritus and skin lesions (167). This disease has a mouse model using six-week old BALB/C mice, with induced AD through the application of 1-chloro-2,4-dinitrobenzene (DNCB) to the skin of the mouse (168). The application of melittin to the dorsal skin after AD induction resulted in decreased skin lesions and reduced pro-inflammatory cytokines. This demonstrates the ability of melittin to work in an extracutaneous manner. The same research group also published results regarding an AD mouse model in which an ovalbumin (OVA) patch was used to induce AD in six-week-old BALB/C mice (169). They were able to demonstrate that melittin is able to decrease OVA-induced skin thickening and inflammatory infiltration. However, in this study, they administered melittin intraperitoneally. This exemplifies the ability of melittin to act as a dermatological agent against differently induced AD, and demonstrates that multiple routes of administration can also be applied to treat AD. Mechanistically, the amelioration of AD via melittin appears to work through the upregulation of the Decay-Accelerating Factor (DAF/CD55) as described by another research group (170). This group used the previously described model of DNCB-induced AD in seven-week-old BALB/C mice, and demonstrated that subcutaneous injections of melittin alter the onset of AD symptoms through modulation of the complement system and the ERK signaling pathway. Overall, it appears that melittin may be a dynamic and effective therapeutic agent against AD.
General tissue response
Much of the in vitro data, as well as data from single cells show that melittin can be toxic and can cause cellular dysregulation or even death at high enough concentrations. One such study conducted in 1997 showed that intramuscular injection of melittin caused necrosis of murine skeletal muscle within 30 minutes of administration (171). Melittin also caused hyper contraction of the muscle tissue. Yet, 24 hours after administration, the affected cells formed an amorphous mass, demonstrating the potent myotoxin activity of melittin when the concentration is high enough. Another study from 1999 applied melittin to the cerebrospinal fluid (CSF) of rats to probe the effect of melittin on the cerebral cortex (172). The authors of this study found that administration of melittin resulted in the release of amino acids, neurotransmitters, and fatty acids from the cerebral cortex, potentially through the activation of cerebral cortical phospholipases. These results are comparable to cerebral cortical ischemia induced by phospholipase A2 (PLA2) and demonstrate the disruptive activity of melittin. In another study, melittin was injected intracerebroventricularly into normotensive rats to observe the effects of melittin on mean arterial pressure (MAP) and heart rate (HR) (173). The authors found that melittin increased MAP and decreased HR in conscious rats in a dose-dependent manner, likely through the activation of PLA2 .
Melittin has been shown to reduce tissue injury in an animal model, where lung tissue injury was induced by paraquat, a commonly used herbicide (174). Paraquat is highly toxic to humans and currently, no treatment options exist for paraquat poisoning. In the study, mice were singularly administered paraquat intraperitoneally (IP), and this injection was followed by multiple IP injections of melittin for four weeks. The authors found that the mice which were given melittin had greatly reduced lung injury, as a result of anti-apoptotic effects and modification of oxidative stress response pathways. Melittin has also been used as an adjuvant in vaccines. Bramwell et al. showed that 4 μg of melittin given with a tetanus toxoid showed marked increases in antibody titers over the free antigen only group.
Effects on plants
There is very little available research regarding the direct effects of melittin on plants and plant cells, however, these limited studies provide interesting insights. In an older study, increasing concentrations of melittin, when incubated with chloroplasts harvested from peas, silver beets, maize, and wheat, decreased the ability of the chloroplasts to conduct photochemical reactions (175). Melittin also appears to increase the production of inositol triphosphate when directly applied to plant cells, indicating that signal transduction pathways of plants may be dysregulated by melittin treatment (176). From an ecological standpoint, a small study has also demonstrated that melittin exerted toxic and genotoxic effects against a microalgae species, Pseudokirchneriella subcapitata (45). These data indicated that melittin is generally toxic to plant cells and would likely dysregulate normal plant cell function.
The greater potential value of melittin regarding plants resides in its plant protection from pathogens. One study demonstrated that the direct application of melittin to whole rice plants did not cause deleterious effects to the plant, while conferring resistance to the bacterial phytopathogen specific to rice plants, Xanthomonas oryzae pv. Oryzae (37). A study of the effect of melittin against a species of corn earworm, Heliothis zea, found that melittin can induce antifeeding behavior in these insects, yielding protection to the corn (177). Since then, higher level scientific technologies have been used to observe the plant protection effects of melittin and its analogues. One study described the transgenic engineering of two potato cultivars of Solanum tuberosum to constitutively produce a melittin-cecropin hybrid peptide; these transgenic plants demonstrated broad-spectrum resistance to bacterial and fungal pathogens (178). Furthermore, these transgenic tubers retained resistance for up to a year, exemplifying stability in the transgenic model. Also, they did not negatively affect rodents, which were given the transgenic potatoes to eat. Another research group engineered a transgenic tobacco plant, Nicotiana tabacum, that also expresses a melittin-cecropin hybrid peptide (179). They utilized a promoter that is specifically activated in response to plant pathogens, to trigger the production of the hybrid melittin-cecropin in response to fungal attack. Recently, a study described the phytopathogenic bacteria, Xylella fastidiosa, and how it is transmitted to plants via an arthropod carrier, the Glassy-Winged Sharpshooter [GWSS] (Homalodisca vitripennis) (180). The researchers developed an innovative paratransgenic strategy where a bacterial symbiont of GWSS, Pantoea agglomerans, underwent a transgenic modification where they expressed melittin. This study demonstrated antimicrobial activity against X. fastidiosa, but not against P. agglomerans, and was thereby able to reduce the transmission of X. fastidiosa to grape plants by GWSS. To that end, melittin may be a valuable antimicrobial agent especially in the context of agricultural settings.
Effects on invertebrate animals
The earliest study of the effects of melittin on insects was conducted in 1971, when melittin was administered to Drosophila melanogaster (fruit fly) larvae (181). The in vivo administration of melittin was highly toxic to the larvae and likely inhibited acetylcholinesterase through global cellular disruption. This was one of the first attempts to determine the potential of melittin as an insecticide. Another study described the administration of melittin to insect cells derived from a cabbage moth (Mamestra brassicae) and found that melittin increases cyclic AMP (cAMP) levels at lower concentrations and is able to induce insect cell death at several concentrations (182). More recently, a study of the administration of melittin to a beetle (Tenebrio molitor) reported cardiotropic and immunotropic effects (183). Once injected into these beetles, melittin increased the number of apoptotic cells in the hemolymph in a dose-dependent manner, but this did not affect the number of phagocytic hemocytes. Additionally, melittin exhibited cardiotropic effects by increasing the heart rates of the beetles in a dose-dependent manner.
Melittin can affect insect biology in an indirect manner. One relevant study focuses on an invasive urban Formosan subterranean termite species called Coptotermes formosanus Shikari (184). In this study, the researchers took advantage of a symbiotic termite protozoa which resides in the gut of the termites and delivered a genetically engineered melittin-producing yeast to the gut of the termite. This killed the protozoa and weakened the gut lining of the termites themselves, leading to termite death. Finally, there are two examples of non-insect invertebrate effects of melittin. In the first example, melittin exhibited antinematode activity against the commonly used Caenorhabditis elegans, as it decreased the hatching rates and the motility in a dose-dependent manner (185). This is likely the first time melittin was described to have antinematodal effects. The second example involves an aquatic microcrustacean (Daphnia magna) as part of an ecological study on the effect of melittin (186). In this study, melittin was found to have toxic effects against the microcrustacean leading to decreased and delayed reproduction. At sublethal concentrations, melittin appeared to cause DNA damage to the microcrustaceans. Overall, it appears that melittin can be quite toxic to invertebrate life forms and may have potential utility as an insecticide.
Synthetic lipid bilayer vesicles – Mechanism of action studies.
Many of the activities described above are attributed to the interaction of melittin with the membrane. Melittin is believed to insert in biological membranes and destabilize them. Decades of studies with synthetic vesicles have revealed many of the important principles of melittin activity, although some questions still remain (17, 18). The detailed understanding of melittin, in turn, has provided insights that are relevant to thousands of other membrane active peptides. Early studies highlighted the effect of vesicle composition on changes in melittin intrinsic fluorescence in the presence of vesicles (187-189). Different lipid headgroups such as phosphatidylcholine, phosphatidylserine, phosphatidylglycerol were explored and membrane activity was found to be a function of vesicle composition, especially membrane charge and fluidity. Further studies explored the effects of vesicle size- small unilamellar vesicles versus giant unilamellar vesicles (190). Model membranes were further used to investigate changes in the secondary structure of melittin through NMR and CD spectroscopy (191-195). These studies revealed that the activity of melittin against vesicles relied on binding, followed by the formation of secondary, alpha helical structure which is required for permeabilizing activity. Ultimately, model membrane studies verify that binding and insertion are the critical mechanistic steps. Variations that increase melittin binding, such as the addition of anionic lipids, increase melittin activity, while variations that reduce lipid disorder, such as formation of gel phases or addition of cholesterol, reduce melittin activity.
The permeabilizing activity of melittin in membranes relies on the association of multiple melittin monomers to form a larger structure. The early literature describes melittin as a “fusion inducing” peptide capable of causing the fusion of multiple vesicles or vesicle-like structures (196, 197). Over time this concept evolved into an understanding of melittin as a peptide that can induce leakage of fluorescent reporters from vesicles (198-200). The key quantitative measurement for comparison in these assays is the peptide to lipid (peptide:lipid, or P:L) ratio . Melittin is reported to cause total leakage of calcein reporters from POPC vesicles at peptide:lipid ratios between 1:20 and 1:100 (199, 201, 202). This effect is modulated by adding charged lipids or other membrane components such as cholesterol, which often requires a P:L ratio of more than 1:100 to induce leakage (200). Extensive research has shown that at some P:L ratios, as high as 1:20, melittin can form stable pores in some bilayers, but it forms transient pores when fewer peptides are present (17, 18, 201-205).
Many sequence-structure-function studies have been conducted to investigate the significance of individual amino acids for the function of melittin. The substitution of the first 20 amino acids by another helical sequence was found not to alter the hemolytic activity of melittin (86), conversely, the deletion of any of the residues in helical regions Gly-1—Leu-9 and Leu-13—Ile-20 resulted in a decrease in hemolytic activity (206). A leucine zipper motif has also been discovered within the structure of melittin: Leu-6, Leu-13 and Ile-20, which are each about 7 residues apart (207). Tryptophan (Trp-19) is the only aromatic residue in melittin and has been found to be critical for its hemolytic activity (206, 208). A considerable number of studies have focused on the structural importance of the central proline residue (Pro-14). Pro→Ala mutations resulted in increased peptide binding, and decreased leakage in model membranes (13). Pro→Cys mutations yielded increased leakage for model membranes and decreased antimicrobial activity (209). It was also observed that changing the stereochemistry of Pro-14 eliminates both hemolytic and cytotoxic activity (210).
The mechanism by which melittin permeabilizes cell membranes has long been debated, as there has been evidence for a variety of models. This diversity can be understood if the differences in experimental conditions are taken into account. These experimental conditions include: the use of different phospholipids and membrane components, different peptide to lipid ratios, and different experimental designs for determining permeabilization. At high P:L ratios (1:15), melittin forms disk-shaped fragments in concordance with a detergent-like model of membrane solubilization.22,23 Oriented circular dichroism and neutron diffraction studies have also provided evidence that melittin forms toroidal pores under some conditions (20). The stability of these pores is also dependent on experimental conditions. Electrochemical impedance spectroscopy (211) and leakage assays (201, 202) in PC bilayers have demonstrated that melittin forms transient pores at P:L ratios of 1:200 or less, with maximum leakage only in the minutes immediately after peptide addition, slowing or stopping thereafter. This is further supported by a study utilizing single-cell fluorescence microscopy of live E. coli cells (19). Green fluorescent protein (GFP) and SYTOX Orange were used to visualize real time leakage events in the presence of 10 μM melittin. The authors described seven distinct membrane-related events: 1) the outer membrane (OM) is permeabilized to GFP, 2) periplasmic GFP bubbles begin to form, 3) the cytoplasmic membrane (CM) is permeabilized to SYTOX Orange, 4) the CM is permeabilized to GFP, 5) the OM is re-sealed to GFP, 6) the CM is re-sealed to GFP, and 7) CM and OM are both re-permeabilized to GFP. Since the mechanism of melittin is highly dependent on experimental conditions, it is perhaps more helpful to discuss its activity in terms of mechanistic landscapes rather than competing mechanistic theories (205).
Synthetic molecular evolution of new functions in melittin
As we have detailed above, the natural sequence of melittin has many interesting and potentially useful bioactivities alone, or when conjugated to other biomolecules. However, many of these bioactivities are incidental, often not directly related to the cytolytic and cytotoxic activity of melittin derived from natural selection. To translate these activities into more useful ones, we should optimize the sequence of melittin to have more desirable properties, such as better membrane selectivity, specific permeabilization properties, controllable activation. It would obviously be highly beneficial to retain the useful activities while reducing incidental toxicity. Yet, there is a major impediment to the optimization of membrane active peptides in general - there are just a few useful sequence structure-function relationship rules. Therefore, rational engineering of membrane active peptides is very difficult. Examination of the literature shows that variants are usually created by small scale trial and error.
Here we will discuss a much more efficient approach to finding optimized variants of melittin or other membrane active peptides: synthetic molecular evolution (SME). The SME approach, which was developed for understanding and optimizing membrane-active peptides (202, 212-222), is the iterative design of small peptide libraries subjected to orthogonal screening to find gain-of-function variants. This entails using knowledge of the basic thermodynamic and structural principles of peptide-membrane interactions to design iterative peptide libraries. These libraries enable exploration of the sequence space around a fundamental concept or a template sequence with known activity. These iterative libraries are screened using complex parallel screens that enable selection of peptides with a desired combination of properties. Combinatorial libraries have been employed for decades in the study of peptides owing to the synthetic simplicity of solid phase peptide synthesis (223-226). Our small library synthesis approach (218, 220, 227, 228) provides μg amounts of each member, enough for complex parallel screens in multi-well plates. This is fundamentally different from most high throughput approaches. In each generation of screening, we up-select in parallel for multiple desirable activities (see below for examples), and down-select for multiple negative activities (such as insolubility and toxicity). Several peptide libraries have undergone multiple generations of evolutionary processes (229-231).
First generation of melittin derivatives – Evolution of equilibrium pore formers.
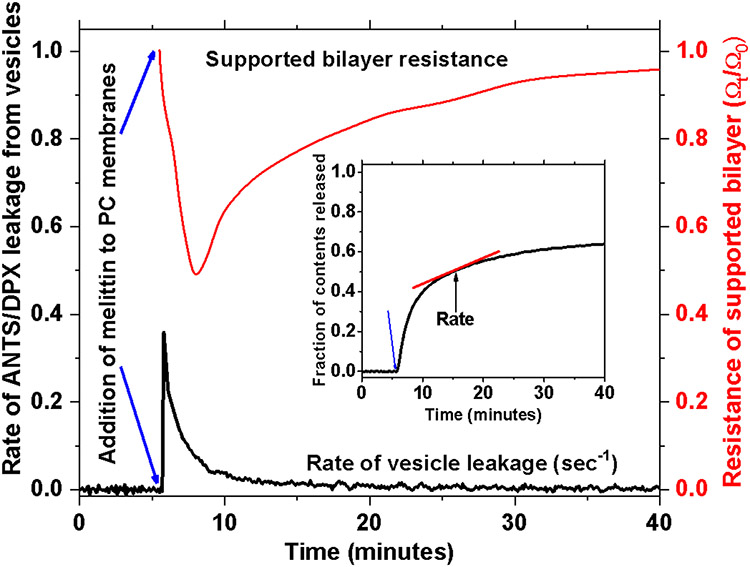
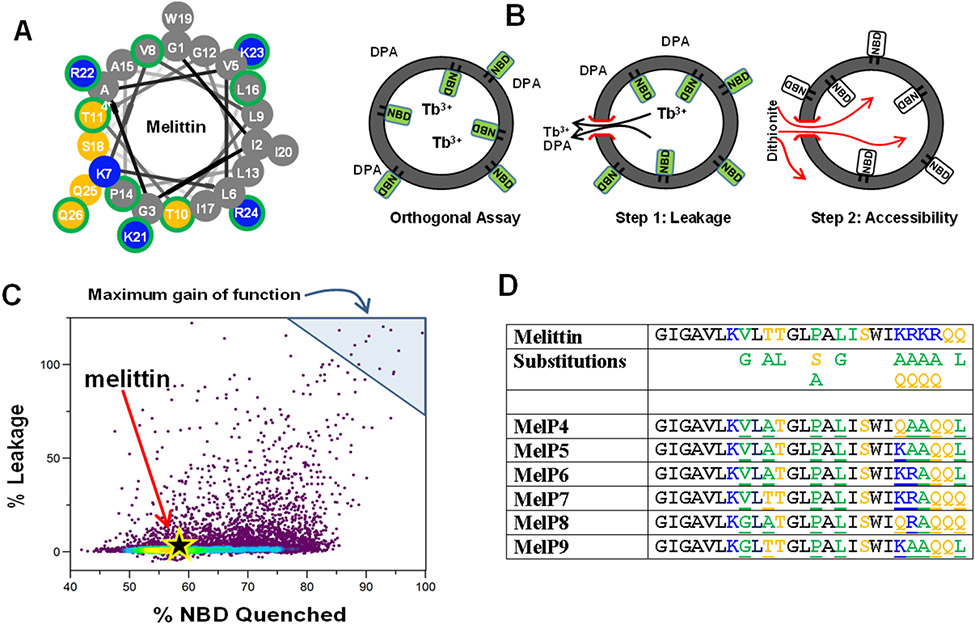
Melittin does not form explicit pores in membranes under most conditions. Instead, it forms transient pores which enable leakage from membranes only for a limited time after the peptide is added to bilayers (17, 18, 201, 211). See Fig. 4. After the transient phase, leakage slows or stops completely despite the continued presence of melittin bound to the bilayers. In the first generation of synthetic molecular evolution of melittin, Krauson and Wimley sought to identity melittin variants that formed potent equilibrium, not transient, pores in phosphatidylcholine membranes (202).
Figure 4.
Transient permeabilization of PC bilayers caused by melittin. In vesicle leakage and electrochemical impedance, when P:L is less than 1:200 melittin causes a rapid burst of permeabilization immediately after addition to the bilayers. But almost immediately, leakage dramatically slows with a halftime of 5-10 minutes. In the vesicle studies above, leakage stops before all the contents have been released from the vesicles.
The design of the library, Fig. 5A, simultaneously tested several structural hypotheses (202). First, they hypothesized that helical propensity would be important, so they varied the helix breaking proline at position 14. Second, they hypothesized that improving the two-dimensional amphipathicity of the helix would be important. When folded into a helix, the N-terminal region of melittin has a polar and nonpolar face. This amphiphilicity is disrupted by cationic residues at the C-terminus. Thus, they allowed for variation in the four cationic residues of the highly polar C-terminus to polar or nonpolar residues. They also allowed for variation in Thr10, Thr11 which bound the polar face of the helix in the middle of the sequence. Third, they hypothesized that intermolecular helix-helix interactions could be improved by introducing potential GXXXG helix dimerization motifs (232, 233). So they allowed Val8 and Leu16 to be glycine, along with the native Gly12. This motif, known as the glycine zipper, is a feature common to α-helical transmembrane proteins and channels and is involved in their dimerization or oligomerization. Altogether, 10 critical residues out of 26 were varied, with each varied position containing the native residue and one or two others. These variations gave a 7,776 member iterative combinatorial peptide library, with all members having at least 62% identity to melittin.
Figure 5.
Synthetic molecular evolution of potent equilibrium pore formers from melittin. A. The amphipathic helix of melittin was used to rationally vary specific residues in the melittin sequence. Residues maked with green were varied. B. The members of this library were screened using an assay that tests for equilibrium pore formation, rather than the transient pore formation that melittin causes. In this assay leakage of entrapped probes is measured as usual in Step 1. Later, access of a polar quencher to the vesicle interior at equilibrium is measured in Step 2. Only equilibrium pore formers enable access and ≥90% quenching. C. 10,000 library members were screened using this assay at a low peptide concentration. Most were inactive, as was melittin. D. A few potent equilibrium pore-formers were identified. Sequences of the best -studied examples are shown.
An orthogonal, two-step assay was designed to assess the potency of the peptides and the continued existence of pores at equilibrium (Fig. 5B). First, unilamellar PC vesicles were prepared with encapsulated terbium and a trace of phospholipids labeled with the fluorescent dye NBD. The vesicles were mixed with dipicolinic acid (DPA), an aromatic chelator that remains outside intact vesicles. If pores formed upon the addition of a peptide library member, the diffusion of terbium and DPA across the permeabilized membrane would allow them to interact and form a luminescent complex which can be quantitated. This is the Y-axis of Fig. 5C. Second, the existence of pores that persisted upon reaching equilibrium was tested hours later by adding membrane-impermeant dithionite to quench the fluorescence of NBD lipids. In the absence of equilibrium pores, only the NBD on the outer leaflet (55%) is quenched. The presence of equilibrium pores enables access of dithionite to the vesicle interior, leading to ≥90% NBD quenching.
About 10,000 library members were assayed at a very stringent peptide to lipid ratio of 1:1000, where native melittin is inactive. Most library members show little to no activity in either assay. A few exceptional library members were found. Fourteen gain-of-function variants were identified in the screen and six were carefully characterized (Fig. 5D). These potent peptides are able to induce leakage at P:L ≤ 1:1000, a concentration at which melittin is not active, and they form equilibrium pores. Their sequences suggest that potent equilibrium pore formation is dependent upon three factors: the conservation of proline 14, the formation of a more ideal amphipathic helix with polar and nonpolar residues segregated along the folded peptide, and the extension of helix into the formerly cationic C-terminal tail. Specifically, two changes that improve helicity and amphipathicity dominate the gain of function variants: Thr11 to Alanine and Lys23 to Alanine. Curiously, there is little difference in the membrane binding affinity of melittin compared to the gain-of-function variants. However, oriented circular dichroism indicates that the variants are significantly more likely to be in a transmembrane state. This suggests that the sequence motifs of the gain-of-function variants drive an increase in potency by promoting the folding of the peptide into an ideal amphipathic helix, over more of the peptide length, that adopts a transmembrane configuration.
The most active melittin variant, MelP5 is a very potent equilibrium pore forming peptide (202). Based on data suggesting the formation of very large pores that release macromolecules (234) we developed an assay for macromolecule leakage. We labelled a dextran (10 kDa in the assay described here and 40 kDa in the assay described in the next section) with the dye TAMRA and with biotin. TAMRA acts as a FRET quencher, when complexed with Alexafluor488-labelled streptavidin via the biotin moiety. In the assay, TAMRA-biotin dextran (TBD) is entrapped inside lipid vesicles and streptavidin (65 kDa) added to the external space. If a peptide enables macromolecular leakage, the TBD-streptavidin complex forms and quenches the AF488 fluorescence, which we can measure. Using this assay and others, we showed that MelP5 releases macromolecules from PC vesicles at low concentration (234). MelP5 allows the escape of 10 kDa dextrans from vesicles at P:L as low as 1:5000, and creates pores that allow the passage of dextrans as large as 40 kDa at P:L ≤ 1:500. This is a uniquely useful property found in very few pore-forming peptides.
Second generation of melittin variants - pH triggered macromolecular poration
Equilibrium pore formation, especially macromolecular poration, can be useful in a variety of contexts. It could be much more useful if it is activated upon a physiologically relevant trigger. One potentially useful trigger is acidic pH, which is a marker of the tumor microenvironment and is also found in endosomes and lysosomes of endocytotic pathways. For example, a peptide that can be triggered to form macromolecule-sized pores only in tumors could induce the selective apoptosis of malignant cells. Alternatively, macromolecule-sized pore formed in the endosome could allow the cytosolic delivery of co-encapsulated cargoes such as peptides and proteins. For these reasons we sought to modify MelP5 to retain macromolecular poration, but to only be active at acidic pH.
The advantage of SME was highlighted by a partially successful attempt to rationally redesign MelP5. Wiedman et al. attempted to encode pH sensitivity by taking inspiration from the peptides GALA and pHLIP (235). GALA, derived from a viral fusion peptide, forms small pores at acidic pH (236, 237). pHLIP, a peptide derived from bacteriorhodopsin, is not a pore-forming peptide, but inserts into membranes at acidic pH (238, 239). Upon examination of the sequences of GALA and pHLIP, we identified that acidic residues could modulate the activity of membrane-associating peptides. The rationally designed variants of MelP5, named MelP5_Δ4 and MelP5_Δ6, had 4 or 6 acidic residues. This attempt at rational design was only partially successful. The peptides bound to membranes and folded into alpha helices in a pH-sensitive manner, but lost their ability to form large pores, indicating that the properties of pore-formation and pH-sensitivity and stable pores are not fully additive (235). This also underscored the limitations in our understanding of peptide-lipid structure-function relationships that is fundamental to successful rational design.
Consequently, we used a synthetic molecular evolution approach (228). We designed a library that used MelP5 as a template. The design of the library tested several hypotheses (Fig. 6A). pH-sensitivity was encoded by incorporating six potential acidic residues, aspartate or glutamate, along the polar face of the helix. This placement would allow the peptide to maintain its amphiphilicity upon folding into a helix. However, at neutral pH, the negative charges of the adjacent residues with helical spacings were expected to oppose folding of the peptide into the active helix. Several other residues were modified to allow for an increase in amphipathicity, including Ile17 which was allowed to be Gln. Further, the two basic lysines of MelP5 were allowed to be histidine, having a positive charge only at acidic pH. In total, we varied 9 of 26 resides, which resulted in a library of 18,432 members.
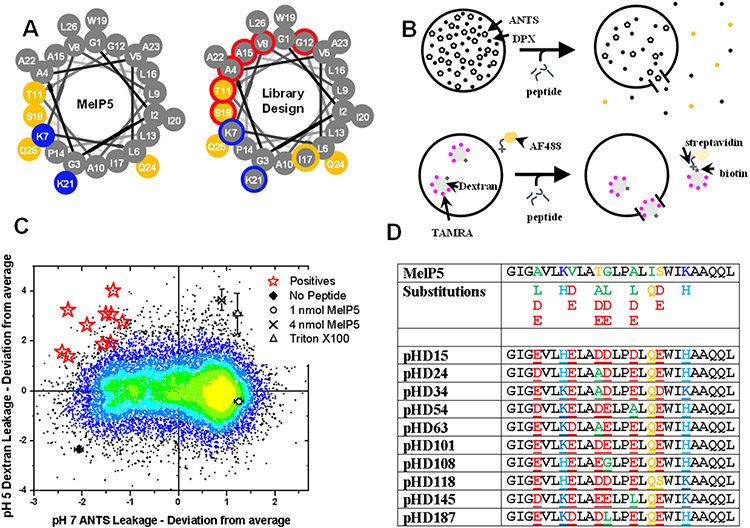
Figure 6.
Synthetic evolution of pH-triggered formers of macromolecule-sized pores. A. The best equilibrium pore-forming variant, MelP5, was shown to form macromolecule-sized pores. In the generation of evolution described in this figure, we allowed the library to have acidic residues at six positions marked by red borders. B. We screened for lack of release of small molecules at pH 7 and high P:L, and for high release of macromolecules at pH 5 and low P:L. C. Screening data show the results of the two assays. Outliers in the upper left cause pH triggered macromolecular poration. D. The red stars in panel C were sequenced to give the pHD peptides, which mostly have five out of six possible acidic residues.
The goal in this screen was to identify library members that formed macromolecule-sized pores at acidic pH, but had no activity at neutral pH. We used lipid vesicles made from phosphatidylcholine and performed two sequential assays (Fig. 6B). First, we measured leakage of small molecules at pH 7 and P:L = 1:200. Small molecule leakage and high P:L constitutes very permissive conditions for detecting membrane permeabilization. Next, we measured leakage of TBD (40 kDa) at pH 5 and P:L = 1:800. These conditions were achieved by adding TBD-containing vesicles at pH 4 to the wells already used for small molecule leakage. These are much more stringent conditions due to lower P:L and much larger probe molecules. The resulting distribution of activities is shown in Fig. 6C. Many library members have MelP5 like activity; high leakage of small molecule at pH 7 and some leakage of macromolecules. The screen enabled us to identify peptides with the properties we sought; low leakage of small molecules at pH 7 and high leakage of macromolecules at pH 5.
Ten gain-of-function variants, named the pH-dependent, or pHD peptides, were identified and sequenced (Fig. 6D). They shared a common motif in which five or six acidic residues were selected. Peptides with five acidic residues were more potent and more common than those with six acidic residues. All variants had the original isoleucine at position 17 modified into glutamine, which may allow hydrogen bonding between adjacent peptides, enabling lateral association and aggregation.
Compared to the thousands of known membrane permeabilizing peptides, the pHD peptides have completely unprecedented properties. They are inactive at pH 7 but have sharply pH-dependent activity. Their apparent pKa values (which we call pH50) is between 5 and 6 (228, 240, 241) The pHD peptides induce release of macromolecules from PC vesicles at very low peptide concentrations. In fact, the pHD peptides are significantly more potent than MelP5 at releasing 40 kDa dextran from vesicles.
The pHD peptides work cooperatively to induce leakage, as is evident in the sigmoidal dependence of binding, folding, and leakage on pH (240). The transition from 0 to 100% typically occurs over one pH unit, suggesting that the protonation and deprotonation occur synergistically. The binding and folding are coupled. These peptides are some of the most potent pore-formers known, as they are capable of inducing 50% leakage of 50% dextran at 1 bound peptide per 800 lipids. pH functions to regulate the number of peptides bound per vesicle. It was proposed that these peptides work by transitioning from a charged, random coil in solution at neutral pH to a membrane-bound, actively pore-forming helical peptide that inserts across the bilayer to stabilize the exposed bilayer edges of a large pore at acidic pH. The peptides are dynamic, exchanging between vesicles, and forming pores that form, fuse, and dissipate over time.
Second generation of melittin variants – potent macromolecular poration at neutral pH
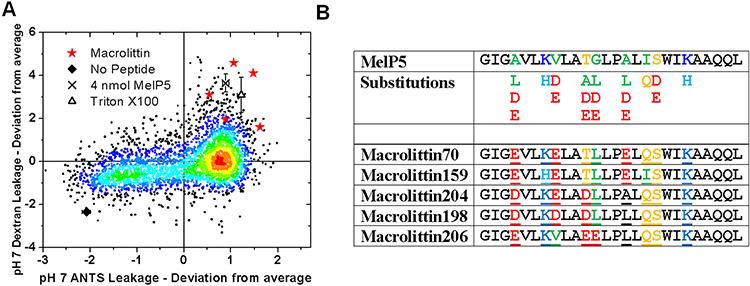
Most membrane active peptides permeabilize membranes transiently and release only small molecules (17, 201, 205). Furthermore, their activity mostly occurs at high concentration (205). Thus, in addition to the pHD peptides just described, we also sought to identify peptides that could form macromolecule-sized pores at very low peptide to lipid ratios without a pH trigger. To discover such peptides, we used the same screen described above, but we selected for peptides that had exceptionally high leakage of small molecules at pH 7 and high leakage of TBD at pH 5.
The screen data are shown in Fig. 7A. Five gain-of-function variants, named the macrolittins, were identified (Fig. 7B). They share a common motif of three or four acidic residues, and a strong preference for lysine over histidine at locations where basic residues were varied. The macrolittins and the pHD peptides share important features in common, including the fact that residues 4 and 8 are almost always acidic (29/30 opportunities) in the selected peptides, suggesting an important role in macromolecular poration. At the four other positions that could contain acidic residues, most macrolittins have one acidic residue, and most pHD peptides have 3 acidic residues. We interpret this to mean that acidic residues 4 and 8 and important for macromolecular poration due to their contribution to pore structure, and acidic residues 11, 12 15 and 18 are responsible for pH triggering. In the pHD peptides, several of these residues must be protonated for activity, while in the macrolittins the native sequence, with one acidic group is active at pH 7.
Figure 7.
Synthetic evolution of pH-insensitive formers of macromolecule-sized pores. A. The same library and assay described in Figure 6 were used to measure release of small and large molecules, except that both assays were done at pH 7. Highly potent macromolecular poration gives the outliers in the upper right. B. The macrolittins are similar to the pHD peptides except that they have three acidic residues out of six possible.
The macrolittins are highly potent pore-formers, inducing 50% leakage of a 40 kDa dextran at a P:L ≤ 1:800. This is unprecedented activity. Melittin itself has little macromolecular poration activity until P:L >> 1:50, the concentration at which it begins to have detergent-like activity. Even MelP5 is less potent than the macrolittins when the probe molecule is 40 kDa, inducing 50% leakage at P:L = 1:50. The macrolittins fold into an α-helix that lies in a transmembrane orientation, which may explain its potency. We have suggested that the macrolittins and pHD peptides form large pores by existing in a transmembrane orientation with the hydrophobic surface of the helix in contact with lipids, stabilizing the exposed edge of the lipids, and the hydrophilic surface in contact with the interior of the water-filled pore.
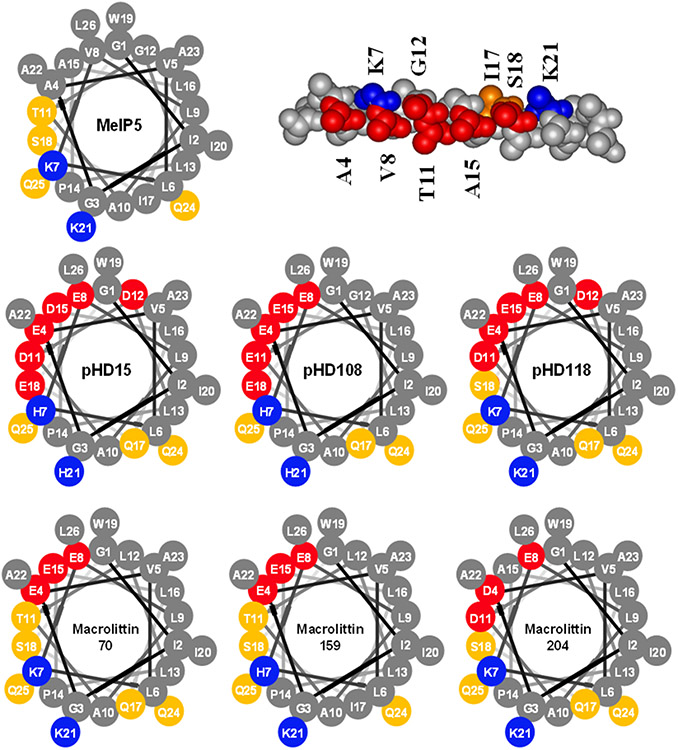
In Fig. 8 we show helical wheel diagrams of MelP5, three pHD peptides, and three macrolittins. The pHD peptides have a larger polar face that covers roughly half of the helix circumference but some of those acidic residues must be protonated for the peptide to function. If we consider the macrolittins to mimic a protonated pHD peptide, then we hypothesize that the acidic residues at the edges of the polar face, such positions 12 and 18 constitute the pH sensor while the highly conserved acidic residues at positions 4 and 8 comprise the polar surface of the pore and are ionized when the pore is functional. These comparisons show that relatively simple changes in the structure of melittin can dramatically change the functions of the peptide.
Figure 8.
Helical wheel diagrams of evolved gain of function analogs of melittin In the upper right, an idealized MelP5 helix shows the location of the residues that allowed to contain acidic and basic residues. Helical wheel diagram of MelP5 (upper right) shows a narrow polar face, while the pHD peptides (middle) have a polar face that is about 180° wide. Helical wheel diagrams of the macrolittins (bottom) show their intermediate polar face angle.
Towards future optimization of melittin and its variants
As we have detailed above, the bee venom peptide, melittin has many different effects on cells and tissues. Although the medical significance is unknown, bee venom has long been used in traditional Chinese medicine for the treatment of a variety of ailments, including arthritis, chronic pain and inflammation, skin diseases, and cancer. Melittin-induced cytotoxic and apoptotic effects continue to be studied for their therapeutic benefits because melittin has the ability to inhibit cancer cell proliferation, arrest cell cycle, induce apoptosis, and cease cell metastasis, motility and migration through various mechanisms against many different types of cancers. To be effective in translational applications, however, further optimization is needed to create melittin analogs that are not toxic to bystander cells, that are selective for target cells and tissues, and that possess useful pharmacodynamic and pharmacokinetic properties. Rational optimization and synthetic molecular evolution can be used to achieve the needed changes. Since the pH of the tumor microenvironment is 6.5-6.8, the pHD peptides could be optimized for activity at this specific pH range. Conjugating the peptides with a toxic payload might make them effective against tumors.
With rising antimicrobial resistance, untreatable viral diseases and the emergence of novel viruses, such as the SARS-CoV2 pandemic coronavirus, novel chemotypes for infectious disease treatment are needed. As we have detailed above, melittin and melittin-like peptides have shown significant promise as anti-infectives. Indeed, melittin is highly potent against broad spectrum of bacteria, including drug-resistant and biofilm forming bacteria. It can also be used in combination with antibiotics and other peptides against pan resistant bacterial infections. Furthermore, melittin and other membrane active peptides are naturally active against many enveloped viruses. There is hope that melittin or melittin-based peptides can become an alternative to conventional antibiotics or antivirals.
The potential drug delivery applications of melittin are also promising. Melittin analogs may be used to facilitate the cellular entry of molecules which do not obey the Lipinski’s rules of five, i.e. molecules that are neither small nor hydrophobic. For instance, the pHD peptides, described above, can be optimized for such applications because of their endosomolytic properties. Further, melittin and melittin-like peptides can help deliver oligonucleotides to cells because they are cationic and amphipathic like most transfection reagents.
Table 1.
Studies of melittin effects in cancer cells.
| Cancer type |
Model/organism(s) | Form of melittin | Effect | Ref. |
|---|---|---|---|---|
| Lung | A549 and NCI-H460 cells | Bee venom (BV) containing 45-50% melittin | Inhibited cell growth, induced apoptotic cell death | 103 |
| Breast | MCa cells and transplanted tumor in CBA mice | Purified BV, intravenous (IV) and subcutaneous (SC) | IV injection reduced metastatic nodes in the lung | 104 |
| Ovary | A2780cp cells | BV + cisplatin | Apoptotic cell killing, greater in combination than individual treatment | 105 |
| Prostate | LNCaP, DU145, PC-3 cells and BALB/c athymic nude mice | Intraperitoneal (IP) BV, melittin peptide | Inhibition of cell growth, inhibition of tumor growth and apoptotic cell death | 106 |
| Prostate | PC-3 cells | Polyethylenimine-chlorotoxin nanoparticles containing melittin DNA | Decreased cell viability | 107 |
| Ovary | SKOV-3 and PA-1 cells | BV, melittin peptide | Induction of apoptotic cell death | 108 |
| Ovary | SKOV-3 cells | Urokinase-type plasminogen activator amino acids 1-43 conjugated to melittin | Inhibit cell growth, arrest cell cycle, induce apoptosis | 109 |
| Liver | HepG2 cells | Melittin peptide | Inhibition of cell growth | 110 |
| Ovary | A2780 cells | Melittin peptide | Reduced metabolic pathways in synergism with cisplatin | 111 |
| Bone | MG63 cells | Melittin peptide | Induction of apoptotic cell death | 112 |
| Bone | BALB/c nu/nu mice and UMR-106 cells and endothelial progenitor cells (EPCs) | Intratumor multi-point local injections of melittin peptide | Decreased EPC functions, reduced tumor size | 113 |
| Stomach | AGS cells | Melittin peptide | Inhibited proliferation and induced cell death | 114 |
| Stomach | SGC-7901 cells | Melittin peptide | Induction of apoptosis | 115 |
| Stomach | NUGC-3, MKN-7, and MKN-74 cells | Monomeric and dimeric melittin peptide | Monomeric peptide more cytotoxic at low concentrations, cell membrane disruption, nuclear localization | 116 |
| Liver | BEL-7402 cells | Adenovirus containing the α-fetoprotein gene and the melittin gene (Ad-rAFP-Mel) | Inhibition of cell proliferation, induction of apoptosis, upregulation of Fas expression | 117 |
| Liver | MHCC97L and MHCC97H cells, Transplanted liver tumor in nude mice | Tail vein injection of melittin peptide | Inhibits cancer cell viability and motility, suppresses Rac1 activity | 118 |
| Liver | Hep3B, HepG2, SMMC-7721 and BEL-7402 cells | Melittin peptide in combination with TNF-related apoptosis-inducing ligand (TRAIL) | Induction of apoptosis in synergism with TRAIL | 119 |
| Liver | SMMC-7721 cells | Melittin peptide | Inhibits cell proliferation, delays cell cycle progression | 120 |
| Liver | MHCC97-H, Bel-7402, L02, HepG2, SMMC7721, Hep3B and Huh7 cells | Melittin peptide | Inhibition of cellular proliferation, invasion and angiogenesis | 121 |
| Liver | HepG-2, MHCC97-H and AsPC-1 cells, BALB/c and BALB/c athymic nude mice | IV injection of VEGF165-melittin fusion protein | Inhibition of cells highly expressing VEGFR-2 and inhibition of tumor xenograft growth | 122 |
| Liver | SMCC-7221 and HepG2 cells | Hydrocarbon stapled variants of melittin | Increased stability in α-helix and against proteolytic degradation, enhanced anti-cancer cell activity of melittin | 123 |
| Bone marrow | L1210, L5178Y and HL-60 cells | Melittin peptide | Inhibition of cell growth and clonogenicity | 124 |
| Bone marrow | U937 cells | Melittin peptide | Membrane disruption, cell hypertrophy, aggregation and cytolysis | 125 |
| Bone marrow | U937 cells overexpressing Bcl-2 | Melittin peptide | Induction of apoptosis through regulation of Akt signaling pathway | 126 |
| Bone marrow | CCRF-CEM and K-562 cells | Melittin peptide | Reduced cell viability, induced apoptosis | 127 |
| Breast | MDA-MB-231 and MCF-7 cells | Melittin peptide | Inhibition of cellular invasion and migration | 128 |
| Brain | U87 cells | Nanoparticles composed of pristine graphene, nanographene oxide, graphite, nanodiamond and hierarchical nanoporous carbons containing melittin peptide | Increased cell death relative to melittin alone | 129 |
| Breast | 4T1 cells | Melittin peptide | Greater cytotoxicity and hemolysis than cisplatin and doxorubicin, induced apoptosis, upregulation of Drp1 and Mfn1 mRNA | 130 |
| Breast | MCF-7 and MDA-MB-231 cells | Estrone conjugated polyion complex micelle containing melittin peptide | Micelles increased cytotoxicity and cellular uptake of melittin into cells | 131 |
| Kidney | Caki-1 cells | Melittin peptide | Inhibition of invasion and migration, suppression of MMP-9 expression | 132 |
| Cervix | CaSki cells | Melittin peptide | Suppression of EGF-induced migration and angiogenesis, inhibited expression of HIF-1α | 133 |
| Cervix | HeLa cells | Melittin peptide | Induction of apoptosis | 134 |
| Cervix | HeLa cells | Graphene oxide based magnetic nanocomposites containing melittin peptide | Pore formation in membranes and cell lysis, less degradation and continuous release of melittin | 135 |
| Esophagus | ECA109 and TE13 cells, subcutaneous injection into BALB/c nude mice | IP melittin peptide, radiation | Enhanced sensitization to radiation followed by increased apoptosis, delayed growth of tumor | 136 |
| Bladder | T24 and 5637 cells | Melittin peptide | Suppression of cell proliferation and migration | 137 |
| Bladder | UM-UC-3 and 5637 cells | Melittin peptide | Inhibition of proliferation, migration and invasion | 138 |
| Colorectal | HCT-116 and SW-480 cells | Melittin peptide | Reduced cell viability, induction of apoptosis and necrosis, increased expression of Fas receptors and caspase 9 and mRNA involved in apoptosis and biotransformation | 139 |
| Colorectal and gastric | AGS, COLO205 and HCT-15 cells | Melittin peptide | Induction of cell death, changes in membrane morphology and membrane damage | 140 |
| Prostate | Sprague-Dawley rats with complete Freund’s adjuvant induced prostatitis | Melittin peptide | Raised the pain threshold, decreased inflammation and demonstrated analgesic effects | 141 |
| Lung | Highly metastatic mouse Lewis lung carcinoma cells (hm LLC) in vitro and SC injection into C57BL/6 mice, VEGF transfected HUVEC cells | Melittin peptide | Inhibits tumor growth and angiogenesis in mice, decreases VEGFR inflammation and targets VEGFR and COX-2 signaling pathway | 142 |
| Lung | LLC cells SC injected into C57BL/6 cells and in vitro LLC, MLE12, A549, and H441 cells | Rhodamine conjugated melittin peptide | Reduced tumor growth and angiogenesis, increased survival, decreased macrophage in tumor environment, preferentially bound M2 macrophages, increased M1/M2 ratio | 143 |
| Lung | ChaGo-K1 and THP-1 cells | Melittin peptide | Induction of apoptosis and G1 cell cycle arrest, inhibition of differentiation into TAMs, reduced colony formation | 144 |
| Lung | A549 cells SC injected into BALB/C nude mice | Melittin peptide | Inhibited invasion and migration, suppressed tumor growth, decreased expression of VEGF and HIF-1α | 145 |
| Lung | NCI-H441, A549 and BEAS-2B cells and NCI-H441 cells SC injected into BALB/C nu/nu mice | Melittin peptide | Inhibition of cell growth, migration, invasion and miR-183 expression, inhibition of tumor growth, induction of apoptosis | 146 |
| Lung, liver and ovary | CTLL-2 cells and SMMC-7721, A549 and SKOV3 cells were SC injected into BALB/c mice | Mutant IL-2 fused to melittin peptide | Induced proliferation of lymphocytes, promoted production of IFN-γ, inhibited cancer cell proliferation, inhibited tumor growth, prevented metastases | 147 |
| Lung | RAW264.7 cells and LLC cells SC injected into C57BL/6 cells | Melittin fused to dKLA peptide | Induction of M2 macrophage death, membrane disruption leading to apoptosis, reduction of tumor growth and angiogenesis, targeting of M2-like TAMs | 148 |
| Skin | A2058 cells | Melittin peptide and BV | Melittin contributes in large part to BV induced apoptosis and elevation of calcium | 149 |
| Skin and breast | MCF7 and A375 cells | Melittin peptide | Synergistic effect of melittin targeting with plasma induced oxidation | 150 |
| Pancreas | SW1990, Capan1, AsPC-1 and BxPC-3 cells, SW1990 cells SC injected into nude mice | Melittin peptide | Inhibited cell growth, induced apoptosis and cell cycle arrest, downregulated biosynthetic pathways, induced gemcitabine sensitization in vitro and in vivo | 151 |
| Thyroid | TT cells, TT cells injected intradermally into nude mice | Melittin derivative, TT1 peptide | Suppressed tumor growth, induced cell death and apoptosis, upregulated Bax, downregulated Bcl-2, activated caspases 3 and 9 | 152 |
| Brain | C6 rat astrocytoma cells | Melittin peptide | Inhibition of calmodulin and cell growth | 153 |
| Brain | Rat C6 glioma cells in vitro and injected SC into BALB/c nude mice | (RADA)8-GG-melittin hydrogel | Reduced tumor size and tumor recurrence rate | 154 |
| Brain | U87 and bEnd.3 cells, intracranial U87 xenogrsfts in ICR and BALB/c nude mice | Lipodisk loaded with paclitaxel and melittin | Paclitaxel and melittin showed synergism in vitro, loaded disks prolonged survival time in mice | 155 |
| Breast | NHA and 231BR cells, intracardiac injections of 231BR cells into NCr nu/nu mice | Nanoparticles with surface AMD3100, containing a modified promelittin gene | Increased accumulation of melittin in tumor cells, inhibited tumor progression, enhanced survival of tumor bearing mice | 156 |
| Head and neck | CNE-2 and KB cells, SC injection of CNE-2 cells into BALB/C nude mice | Melittin peptide | Inhibition of cell growth, induction of apoptosis, reduction in HIF-1α and VEGF expression, reduction in tumor growth | 157 |
References
- 1.Baumann K, University of Queensland, (2018). [Google Scholar]
- 2.Bossert S, Murray EA, Almeida EAB, Brady SG, Blaimer BB, Danforth BN, Combining transcriptomes and ultraconserved elements to illuminate the phylogeny of Apidae. Mol Phylogenet Evol 130, 121–131 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Sann M, Niehuis O, Peters RS, Mayer C, Kozlov A, Podsiadlowski L, Bank S, Meusemann K, Misof B, Bleidorn C, Ohl M, Phylogenomic analysis of Apoidea sheds new light on the sister group of bees. BMC Evol Biol 18, 71 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hedtke SM, Patiny S, Danforth BN, The bee tree of life: a supermatrix approach to apoid phylogeny and biogeography. BMC Evol Biol 13, 138 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kreil G, Haiml L, Suchanek G, Stepwise cleavage of the pro part of promelittin by dipeptidylpeptidase IV. Evidence for a new type of precursor--product conversion. Eur J Biochem 111, 49–58 (1980). [DOI] [PubMed] [Google Scholar]
- 6.Terwilliger TC, Eisenberg D, The structure of melittin. II. Interpretation of the structure. J. Biol. Chem 257, 6016–6022 (1982). [PubMed] [Google Scholar]
- 7.Bazzo R, Tappin MJ, Pastore A, Harvey TS, Carver JA, Campbell ID, The structure of melittin. A 1H-NMR study in methanol. European Journal of Biochemistry 173, 139–146 (1988). [DOI] [PubMed] [Google Scholar]
- 8.Ramirez LS, Pande J, Shekhtman A, Helical Structure of Recombinant Melittin. J Phys Chem B 123, 356–368 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park CB, Yi KS, Matsuzaki K, Kim MS, Kim SC, Structure-activity analysis of buforin II, a histone H2A-derived antimicrobial peptide: the proline hinge is responsible for the cell-penetrating ability of buforin II. Proc Natl Acad Sci U S A 97, 8245–8250 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sani MA, Lee TH, Aguilar MI, Separovic F, Proline-15 creates an amphipathic wedge in maculatin 1.1 peptides that drives lipid membrane disruption. Biochim Biophys Acta 1848, 2277–2289 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Lee JK, Gopal R, Park SC, Ko HS, Kim Y, Hahm KS, Park Y, A proline-hinge alters the characteristics of the amphipathic alpha-helical AMPs. PLoS One 8, e67597 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cordes FS, Bright JN, Sansom MS, Proline-induced distortions of transmembrane helices. J Mol Biol 323, 951–960 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Rex S, A Pro --> Ala substitution in melittin affects self-association, membrane binding and pore-formation kinetics due to changes in structural and electrostatic properties. Biophys. Chem 85, 209–228 (2000). [DOI] [PubMed] [Google Scholar]
- 14.Dempsey CE, Bazzo R, Harvey TS, Syperek I, Boheim G, Campbell ID, Contribution of proline-14 to the structure and actions of melittin. FEBS Lett 281, 240–244 (1991). [DOI] [PubMed] [Google Scholar]
- 15.Ramirez L, Shekhtman A, Pande J, Nuclear Magnetic Resonance-Based Structural Characterization and Backbone Dynamics of Recombinant Bee Venom Melittin. Biochemistry 57, 2775–2785 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miura Y, NMR studies on the monomer-tetramer transition of melittin in an aqueous solution at high and low temperatures. Eur Biophys J 41, 629–636 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Wimley WC, Hristova K, The Mechanism of Membrane Permeabilization by Peptides: Still an Enigma. Aust J Chem 73, 96–103 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wimley WC, How Does Melittin Permeabilize Membranes? Biophys J 114, 251–253 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Z, Choi H, Weisshaar JC, Melittin-induced Permeabilization, Re-sealing, and Re-permeabilization of E. coli Membranes. Biophys J 114, 368–379 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang L, Harroun TA, Weiss TM, Ding L, Huang HW, Barrel-stave model or toroidal model? A case study on melittin pores. Biophys. J 81, 1475–1485 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt-Lange W, [The germicidal effects of bee venom]. Hedizinische Wochenschrift (Munchener) 88, 935 (1941). [Google Scholar]
- 22.Fennell JF, Shipman WH, Cole LJ, Antibacterial action of a bee venom fraction (melittin) against a penicillin-resistant staphylococcus and other microorganisms. USNRDL-TR-67-101. Res Dev Tech Rep, 1–13 (1967). [DOI] [PubMed] [Google Scholar]
- 23.Memariani H, Memariani M, Shahidi-Dadras M, Nasiri S, Akhavan MM, Moravvej H, Melittin: from honeybees to superbugs. Appl Microbiol Biotechnol 103, 3265–3276 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Huang HW, Action of antimicrobial peptides: two-state model. Biochemistry 39, 8347–8352 (2000). [DOI] [PubMed] [Google Scholar]
- 25.Lee MT, Sun TL, Hung WC, Huang HW, Process of inducing pores in membranes by melittin. Proc Natl Acad Sci U S A 110, 14243–14248 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong J, Lu X, Deng Z, Xiao S, Yuan B, Yang K, How Melittin Inserts into Cell Membrane: Conformational Changes, Inter-Peptide Cooperation, and Disturbance on the Membrane. Molecules 24, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dorman LC, Markley LD, Solid phase synthesis and antibacterial activity of N-terminal sequences of melittin. J Med Chem 14, 5–9 (1971). [DOI] [PubMed] [Google Scholar]
- 28.Portell-Buj E, Vergara A, Alejo I, Lopez-Gavin A, Monte MR, San Nicolas L, Gonzalez-Martin J, Tudo G, In vitro activity of 12 antimicrobial peptides against Mycobacterium tuberculosis and Mycobacterium avium clinical isolates. J Med Microbiol 68, 211–215 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Beven L, Wroblewski H, Effect of natural amphipathic peptides on viability, membrane potential, cell shape and motility of mollicutes. Res Microbiol 148, 163–175 (1997). [DOI] [PubMed] [Google Scholar]
- 30.Lazarev VN, Parfenova TM, Gularyan SK, Misyurina OY, Akopian TA, Govorun VM, Induced expression of melittin, an antimicrobial peptide, inhibits infection by Chlamydia trachomatis and Mycoplasma hominis in a HeLa cell line. Int J Antimicrob Agents 19, 133–137 (2002). [DOI] [PubMed] [Google Scholar]
- 31.Aleksandrova NM, Bevova MR, Govorun VM, [Cytotoxic activity of melittin expressed by recombinant vectors in Acholeplasma laidlawii and Mycoplasma hominis cells]. Genetika 37, 46–53 (2001). [PubMed] [Google Scholar]
- 32.Lubke LL, Garon CF, The antimicrobial agent melittin exhibits powerful in vitro inhibitory effects on the Lyme disease spirochete. Clin Infect Dis 25 Suppl 1, S48–51 (1997). [DOI] [PubMed] [Google Scholar]
- 33.Socarras KM, Theophilus PAS, Torres JP, Gupta K, Sapi E, Antimicrobial Activity of Bee Venom and Melittin against Borrelia burgdorferi. Antibiotics (Basel) 6, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sapi E, Kaur N, Anyanwu S, Luecke DF, Datar A, Patel S, Rossi M, Stricker RB, Evaluation of in-vitro antibiotic susceptibility of different morphological forms of Borrelia burgdorferi. Infect Drug Resist 4, 97–113 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barbour AG, Hayes SF, Biology of Borrelia species. Microbiol Rev 50, 381–400 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu X, Singh AK, Wu X, Lyu Y, Bhunia AK, Narsimhan G, Characterization of antimicrobial activity against Listeria and cytotoxicity of native melittin and its mutant variants. Colloids Surf B Biointerfaces 143, 194–205 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Shi W, Li C, Li M, Zong X, Han D, Chen Y, Antimicrobial peptide melittin against Xanthomonas oryzae pv. oryzae, the bacterial leaf blight pathogen in rice. Appl Microbiol Biotechnol 100, 5059–5067 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bardbari AM, Arabestani MR, Karami M, Keramat F, Aghazadeh H, Alikhani MY, Bagheri KP, Highly synergistic activity of melittin with imipenem and colistin in biofilm inhibition against multidrug-resistant strong biofilm producer strains of Acinetobacter baumannii. Eur J Clin Microbiol Infect Dis 37, 443–454 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Dosler S, Gerceker AA, In vitro activities of antimicrobial cationic peptides; melittin and nisin, alone or in combination with antibiotics against Gram-positive bacteria. J Chemother 24, 137–143 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Boman HG, Wade D, Boman IA, Wahlin B, Merrifield RB, Antibacterial and antimalarial properties of peptides that are cecropin-melittin hybrids. FEBS Lett 259, 103–106 (1989). [DOI] [PubMed] [Google Scholar]
- 41.Cao Y, Yu RQ, Liu Y, Zhou HX, Song LL, Cao Y, Qiao DR, Design, recombinant expression, and antibacterial activity of the cecropins-melittin hybrid antimicrobial peptides. Curr Microbiol 61, 169–175 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Dosler S, Karaaslan E, Alev Gerceker A, Antibacterial and anti-biofilm activities of melittin and colistin, alone and in combination with antibiotics against Gram-negative bacteria. J Chemother 28, 95–103 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Giacometti A, Cirioni O, Kamysz W, D'Amato G, Silvestri C, Del Prete MS, Lukasiak J, Scalise G, Comparative activities of cecropin A, melittin, and cecropin A-melittin peptide CA(1-7)M(2-9)NH2 against multidrug-resistant nosocomial isolates of Acinetobacter baumannii. Peptides 24, 1315–1318 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Picoli T, Peter CM, Zani JL, Waller SB, Lopes MG, Boesche KN, Vargas GDA, Hubner SO, Fischer G, Melittin and its potential in the destruction and inhibition of the biofilm formation by Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa isolated from bovine milk. Microb Pathog 112, 57–62 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Galdiero E, Siciliano A, Gesuele R, Di Onofrio V, Falanga A, Maione A, Liguori R, Libralato G, Guida M, Melittin Inhibition and Eradication Activity for Resistant Polymicrobial Biofilm Isolated from a Dairy Industry after Disinfection. Int J Microbiol 2019, 4012394 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lazarev VN, Shkarupeta MM, Titova GA, Kostrjukova ES, Akopian TA, Govorun VM, Effect of induced expression of an antimicrobial peptide melittin on Chlamydia trachomatis and Mycoplasma hominis infections in vivo. Biochem Biophys Res Commun 338, 946–950 (2005). [DOI] [PubMed] [Google Scholar]
- 47.Lazarev VN, Stipkovits L, Biro J, Miklodi D, Shkarupeta MM, Titova GA, Akopian TA, Govorun VM, Induced expression of the antimicrobial peptide melittin inhibits experimental infection by Mycoplasma gallisepticum in chickens. Microbes Infect 6, 536–541 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Choi JH, Jang AY, Lin S, Lim S, Kim D, Park K, Han SM, Yeo JH, Seo HS, Melittin, a honeybee venomderived antimicrobial peptide, may target methicillinresistant Staphylococcus aureus. Mol Med Rep 12, 6483–6490 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pashaei F, Bevalian P, Akbari R, Pooshang Bagheri K, Single dose eradication of extensively drug resistant Acinetobacter spp. In a mouse model of burn infection by melittin antimicrobial peptide. Microb Pathog 127, 60–69 (2019). [DOI] [PubMed] [Google Scholar]
- 50.Yeo CK, Vikhe YS, Li P, Guo Z, Greenberg P, Duan H, Tan NS, Chan-Park MB, Hydrogel Effects Rapid Biofilm Debridement with ex situ Contact-Kill to Eliminate Multidrug Resistant Bacteria in vivo. ACS Appl Mater Interfaces 10, 20356–20367 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Boman HG, Wade D, Boman IA, Wahlin B, Merrifield RB, Antibacterial and antimalarial properties of peptides that are cecropin-melittin hybrids. FEBS Lett 259, 103–106 (1989). [DOI] [PubMed] [Google Scholar]
- 52.Oren Z, Shai Y, Selective lysis of bacteria but not mammalian cells by diastereomers of melittin: Structure-function study. Biochemistry 36, 1826–1835 (1997). [DOI] [PubMed] [Google Scholar]
- 53.Memariani H, Memariani M, Moravvej H, Shahidi-Dadras M, Melittin: a venom-derived peptide with promising anti-viral properties. Eur J Clin Microbiol Infect Dis 39, 5–17 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baghian A, Kousoulas KG, Role of the Na+,K+ pump in herpes simplex type 1-induced cell fusion: melittin causes specific reversion of syncytial mutants with the syn1 mutation to Syn+ (wild-type) phenotype. Virology 196, 548–556 (1993). [DOI] [PubMed] [Google Scholar]
- 55.Yasin B, Pang M, Turner JS, Cho Y, Dinh NN, Waring AJ, Lehrer RI, Wagar EA, Evaluation of the inactivation of infectious Herpes simplex virus by host-defense peptides. Eur J Clin Microbiol Infect Dis 19, 187–194 (2000). [DOI] [PubMed] [Google Scholar]
- 56.Albiol Matanic VC, Castilla V, Antiviral activity of antimicrobial cationic peptides against Junin virus and herpes simplex virus. Int J Antimicrob Agents 23, 382–389 (2004). [DOI] [PubMed] [Google Scholar]
- 57.Uddin MB, Lee BH, Nikapitiya C, Kim JH, Kim TH, Lee HC, Kim CG, Lee JS, Kim CJ, Inhibitory effects of bee venom and its components against viruses in vitro and in vivo. J Microbiol 54, 853–866 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Esser AF, Bartholomew RM, Jensen FC, Muller-Eberhard HJ, Disassembly of viral membranes by complement independent of channel formation. Proc Natl Acad Sci U S A 76, 5843–5847 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boone LR, Skalka A, Two species of full-length cDNA are synthesized in high yield by melittin-treated avian retrovirus particles. Proc Natl Acad Sci U S A 77, 847–851 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yong WH, Wyman S, Levy JA, Optimal conditions for synthesizing complementary DNA in the HIV-1 endogenous reverse transcriptase reaction. AIDS 4, 199–206 (1990). [DOI] [PubMed] [Google Scholar]
- 61.Wachinger M, Saermark T, Erfle V, Influence of amphipathic peptides on the HIV-1 production in persistently infected T lymphoma cells. FEBS Lett 309, 235–241 (1992). [DOI] [PubMed] [Google Scholar]
- 62.Hood JL, Jallouk AP, Campbell N, Ratner L, Wickline SA, Cytolytic nanoparticles attenuate HIV-1 infectivity. Antivir. Ther 18, 95–103 (2013). [DOI] [PubMed] [Google Scholar]
- 63.Do N, Weindl G, Grohmann L, Salwiczek M, Koksch B, Korting HC, Schafer-Korting M, Cationic membrane-active peptides - anticancer and antifungal activity as well as penetration into human skin. Exp Dermatol 23, 326–331 (2014). [DOI] [PubMed] [Google Scholar]
- 64.Lee J, Lee DG, Melittin triggers apoptosis in Candida albicans through the reactive oxygen species-mediated mitochondria/caspase-dependent pathway. FEMS Microbiol Lett 355, 36–42 (2014). [DOI] [PubMed] [Google Scholar]
- 65.Park C, Lee DG, Melittin induces apoptotic features in Candida albicans. Biochem Biophys Res Commun 394, 170–172 (2010). [DOI] [PubMed] [Google Scholar]
- 66.Shin SH, Ye MK, Choi SY, Park KK, The Effects of Melittin and Apamin on Airborne Fungi-Induced Chemical Mediator and Extracellular Matrix Production from Nasal Polyp Fibroblasts. Toxins (Basel) 9, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oard S, Rush MC, Oard JH, Characterization of antimicrobial peptides against a US strain of the rice pathogen Rhizoctonia solani. J Appl Microbiol 97, 169–180 (2004). [DOI] [PubMed] [Google Scholar]
- 68.Munoz A, Lopez-Garcia B, Marcos JF, Comparative study of antimicrobial peptides to control citrus postharvest decay caused by Penicillium digitatum. J Agric Food Chem 55, 8170–8176 (2007). [DOI] [PubMed] [Google Scholar]
- 69.Munoz A, Lopez-Garcia B, Marcos JF, Studies on the mode of action of the antifungal hexapeptide PAF26. Antimicrob Agents Chemother 50, 3847–3855 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carter V, Underhill A, Baber I, Sylla L, Baby M, Larget-Thiery I, Zettor A, Bourgouin C, Langel U, Faye I, Otvos L, Wade JD, Coulibaly MB, Traore SF, Tripet F, Eggleston P, Hurd H, Killer bee molecules: antimicrobial peptides as effector molecules to target sporogonic stages of Plasmodium. PLoS Pathog 9, e1003790 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Habtewold T, Tapanelli S, Masters EKG, Hoermann A, Windbichler N, Christophides GK, Streamlined SMFA and mosquito dark-feeding regime significantly improve malaria transmission-blocking assay robustness and sensitivity. Malar J 18, 24 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Perez-Cordero JJ, Lozano JM, Cortes J, Delgado G, Leishmanicidal activity of synthetic antimicrobial peptides in an infection model with human dendritic cells. Peptides 32, 683–690 (2011). [DOI] [PubMed] [Google Scholar]
- 73.Pereira AV, de Barros G, Pinto EG, Tempone AG, Orsi Rde O, Dos Santos LD, Calvi S, Ferreira RS Jr., Pimenta DC, Barraviera B, Melittin induces in vitro death of Leishmania (Leishmania) infantum by triggering the cellular innate immune response. J Venom Anim Toxins Incl Trop Dis 22, 1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khalili S, Mohebali M, Ebrahimzadeh E, Shayan E, Mohammadi-Yeganeh S, Moosazadeh Moghaddam M, Elikaee S, Akhoundi B, Sharifi-Yazdi MK, Antimicrobial activity of an antimicrobial peptide against amastigote forms of Leishmania major. Vet Res Forum 9, 323–328 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Torres-Guerrero E, Quintanilla-Cedillo MR, Ruiz-Esmenjaud J, Arenas R, Leishmaniasis: a review. F1000Res 6, 750 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pereira PC, Navarro EC, Challenges and perspectives of Chagas disease: a review. J Venom Anim Toxins Incl Trop Dis 19, 34 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Teixeira AR, Nitz N, Guimaro MC, Gomes C, Santos-Buch CA, Chagas disease. Postgrad Med J 82, 788–798 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Adade CM, Oliveira IR, Pais JA, Souto-Padron T, Melittin peptide kills Trypanosoma cruzi parasites by inducing different cell death pathways. Toxicon 69, 227–239 (2013). [DOI] [PubMed] [Google Scholar]
- 79.Fieck A, Hurwitz I, Kang AS, Durvasula R, Trypanosoma cruzi: synergistic cytotoxicity of multiple amphipathic anti-microbial peptides to T. cruzi and potential bacterial hosts. Exp Parasitol 125, 342–347 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Seeber F, An enzyme-release assay for the assessment of the lytic activities of complement or antimicrobial peptides on extracellular Toxoplasma gondii. J Microbiol Methods 39, 189–196 (2000). [DOI] [PubMed] [Google Scholar]
- 81.Saadatnia G, Golkar M, A review on human toxoplasmosis. Scand J Infect Dis 44, 805–814 (2012). [DOI] [PubMed] [Google Scholar]
- 82.Shirley DT, Farr L, Watanabe K, Moonah S, A Review of the Global Burden, New Diagnostics, and Current Therapeutics for Amebiasis. Open Forum Infect Dis 5, ofy161 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hider RC, Khader F, Tatham AS, Lytic activity of monomeric and oligomeric melittin. Biochim Biophys Acta 728, 206–214 (1983). [DOI] [PubMed] [Google Scholar]
- 84.Tosteson MT, Holmes SJ, Razin M, Tosteson DC, Melittin lysis of red cells. J Membr Biol 87, 35–44 (1985). [DOI] [PubMed] [Google Scholar]
- 85.Saberwal G, Nagaraj R, Cell-lytic and antibacterial peptides that act by perturbing the barrier function of membranes: facets of their conformational features, structure-function correlations and membrane-perturbing abilities. Biochim Biophys Acta 1197, 109–131 (1994). [DOI] [PubMed] [Google Scholar]
- 86.DeGrado WF, Kézdy FJ, Kaiser ET, Design, synthesis, and characterization of cytotoxic peptide with melittin-like activity. J. Am. Chem. Soc 103, 679–681 (1981). [Google Scholar]
- 87.DeGrado WF, Musso GF, Lieber M, Kaiser ET, Kezdy FJ, Kinetics and mechanism of hemolysis induced by melittin and by a synthetic melittin analogue. Biophys J 37, 329–338 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gajski G, Domijan AM, Zegura B, Stern A, Geric M, Novak Jovanovic I, Vrhovac I, Madunic J, Breljak D, Filipic M, Garaj-Vrhovac V, Melittin induced cytogenetic damage, oxidative stress and changes in gene expression in human peripheral blood lymphocytes. Toxicon 110, 56–67 (2016). [DOI] [PubMed] [Google Scholar]
- 89.Garaj-Vrhovac V, Gajski G, Evaluation of the cytogenetic status of human lymphocytes after exposure to a high concentration of bee venom in vitro. Arh Hig Rada Toksikol 60, 27–34 (2009). [DOI] [PubMed] [Google Scholar]
- 90.Shaposhnikova VV, Egorova MV, Kudryavtsev AA, Levitman M, Korystov Yu N, The effect of melittin on proliferation and death of thymocytes. FEBS Lett 410, 285–288 (1997). [DOI] [PubMed] [Google Scholar]
- 91.Maher S, McClean S, Investigation of the cytotoxicity of eukaryotic and prokaryotic antimicrobial peptides in intestinal epithelial cells in vitro. Biochem Pharmacol 71, 1289–1298 (2006). [DOI] [PubMed] [Google Scholar]
- 92.Maher S, McClean S, Melittin exhibits necrotic cytotoxicity in gastrointestinal cells which is attenuated by cholesterol. Biochem Pharmacol 75, 1104–1114 (2008). [DOI] [PubMed] [Google Scholar]
- 93.Maher S, Feighery L, Brayden DJ, McClean S, Melittin as a permeability enhancer II: in vitro investigations in human mucus secreting intestinal monolayers and rat colonic mucosae. Pharm Res 24, 1346–1356 (2007). [DOI] [PubMed] [Google Scholar]
- 94.Maher S, Feighery L, Brayden DJ, McClean S, Melittin as an epithelial permeability enhancer I: investigation of its mechanism of action in Caco-2 monolayers. Pharm Res 24, 1336–1345 (2007). [DOI] [PubMed] [Google Scholar]
- 95.Maher S, Kennelly R, Bzik VA, Baird AW, Wang X, Winter D, Brayden DJ, Evaluation of intestinal absorption enhancement and local mucosal toxicity of two promoters. I. Studies in isolated rat and human colonic mucosae. Eur J Pharm Sci 38, 291–300 (2009). [DOI] [PubMed] [Google Scholar]
- 96.Cerne K, Erman A, Veranic P, Analysis of cytotoxicity of melittin on adherent culture of human endothelial cells reveals advantage of fluorescence microscopy over flow cytometry and haemocytometer assay. Protoplasma 250, 1131–1137 (2013). [DOI] [PubMed] [Google Scholar]
- 97.Son DJ, Ha SJ, Song HS, Lim Y, Yun YP, Lee JW, Moon DC, Park YH, Park BS, Song MJ, Hong JT, Melittin inhibits vascular smooth muscle cell proliferation through induction of apoptosis via suppression of nuclear factor-kappaB and Akt activation and enhancement of apoptotic protein expression. J Pharmacol Exp Ther 317, 627–634 (2006). [DOI] [PubMed] [Google Scholar]
- 98.Hu H, Chen D, Li Y, Zhang X, Effect of polypeptides in bee venom on growth inhibition and apoptosis induction of the human hepatoma cell line SMMC-7721 in-vitro and Balb/c nude mice in-vivo. J Pharm Pharmacol 58, 83–89 (2006). [DOI] [PubMed] [Google Scholar]
- 99.Lee WR, Park JH, Kim KH, Park YY, Han SM, Park KK, Protective effects of melittin on transforming growth factor-beta1 injury to hepatocytes via anti-apoptotic mechanism. Toxicol Appl Pharmacol 256, 209–215 (2011). [DOI] [PubMed] [Google Scholar]
- 100.Park JH, Lee WR, Kim HS, Han SM, Chang YC, Park KK, Protective effects of melittin on tumor necrosis factor-alpha induced hepatic damage through suppression of apoptotic pathway and nuclear factor-kappa B activation. Exp Biol Med (Maywood) 239, 1705–1714 (2014). [DOI] [PubMed] [Google Scholar]
- 101.Moreno M, Giralt E, Three valuable peptides from bee and wasp venoms for therapeutic and biotechnological use: melittin, apamin and mastoparan. Toxins (Basel) 7, 1126–1150 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee G, Bae H, Anti-Inflammatory Applications of Melittin, a Major Component of Bee Venom: Detailed Mechanism of Action and Adverse Effects. Molecules 21, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Choi KE, Hwang CJ, Gu SM, Park MH, Kim JH, Park JH, Ahn YJ, Kim JY, Song MJ, Song HS, Han SB, Hong JT, Cancer cell growth inhibitory effect of bee venom via increase of death receptor 3 expression and inactivation of NF-kappa B in NSCLC cells. Toxins (Basel) 6, 2210–2228 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Orsolic N, Sver L, Verstovsek S, Terzic S, Basic I, Inhibition of mammary carcinoma cell proliferation in vitro and tumor growth in vivo by bee venom. Toxicon 41, 861–870 (2003). [DOI] [PubMed] [Google Scholar]
- 105.Alizadehnohi M, Nabiuni M, Nazari Z, Safaeinejad Z, Irian S, The synergistic cytotoxic effect of cisplatin and honey bee venom on human ovarian cancer cell line A2780cp. J Venom Res 3, 22–27 (2012). [PMC free article] [PubMed] [Google Scholar]
- 106.Park MH, Choi MS, Kwak DH, Oh KW, Yoon DY, Han SB, Song HS, Song MJ, Hong JT, Anti-cancer effect of bee venom in prostate cancer cells through activation of caspase pathway via inactivation of NF-kappaB. Prostate 71, 801–812 (2011). [DOI] [PubMed] [Google Scholar]
- 107.Tarokh Z, Naderi-Manesh H, Nazari M, Towards prostate cancer gene therapy: Development of a chlorotoxin-targeted nanovector for toxic (melittin) gene delivery. Eur J Pharm Sci 99, 209–218 (2017). [DOI] [PubMed] [Google Scholar]
- 108.Jo M, Park MH, Kollipara PS, An BJ, Song HS, Han SB, Kim JH, Song MJ, Hong JT, Anti-cancer effect of bee venom toxin and melittin in ovarian cancer cells through induction of death receptors and inhibition of JAK2/STAT3 pathway. Toxicol Appl Pharmacol 258, 72–81 (2012). [DOI] [PubMed] [Google Scholar]
- 109.Su M, Chang W, Cui M, Lin Y, Wu S, Xu T, Expression and anticancer activity analysis of recombinant human uPA143-melittin. Int J Oncol 46, 619–626 (2015). [DOI] [PubMed] [Google Scholar]
- 110.Zhang H, Zhao B, Huang C, Meng XM, Bian EB, Li J, Melittin restores PTEN expression by downregulating HDAC2 in human hepatocelluar carcinoma HepG2 cells. PLoS One 9, e95520 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alonezi S, Tusiimire J, Wallace J, Dufton MJ, Parkinson JA, Young LC, Clements CJ, Park JK, Jeon JW, Ferro VA, Watson DG, Metabolomic Profiling of the Synergistic Effects of Melittin in Combination with Cisplatin on Ovarian Cancer Cells. Metabolites 7, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chu ST, Cheng HH, Huang CJ, Chang HC, Chi CC, Su HH, Hsu SS, Wang JL, Chen IS, Liu SI, Lu YC, Huang JK, Ho CM, Jan CR, Phospholipase A2-independent Ca2+ entry and subsequent apoptosis induced by melittin in human MG63 osteosarcoma cells. Life Sci 80, 364–369 (2007). [DOI] [PubMed] [Google Scholar]
- 113.Qin G, Chen Y, Li H, Xu S, Li Y, Sun J, Rao W, Chen C, Du M, He K, Ye Y, Melittin inhibits tumor angiogenesis modulated by endothelial progenitor cells associated with the SDF-1alpha/CXCR4 signaling pathway in a UMR-106 osteosarcoma xenograft mouse model. Mol Med Rep 14, 57–68 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mahmoodzadeh A, Zarrinnahad H, Bagheri KP, Moradia A, Shahbazzadeh D, First report on the isolation of melittin from Iranian honey bee venom and evaluation of its toxicity on gastric cancer AGS cells. J Chin Med Assoc 78, 574–583 (2015). [DOI] [PubMed] [Google Scholar]
- 115.Kong GM, Tao WH, Diao YL, Fang PH, Wang JJ, Bo P, Qian F, Melittin induces human gastric cancer cell apoptosis via activation of mitochondrial pathway. World J Gastroenterol 22, 3186–3195 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jamasbi E, Lucky SS, Li W, Hossain MA, Gopalakrishnakone P, Separovic F, Effect of dimerized melittin on gastric cancer cells and antibacterial activity. Amino Acids 50, 1101–1110 (2018). [DOI] [PubMed] [Google Scholar]
- 117.Li B, Gu W, Zhang C, Huang XQ, Han KQ, Ling CQ, Growth arrest and apoptosis of the human hepatocellular carcinoma cell line BEL-7402 induced by melittin. Onkologie 29, 367–371 (2006). [DOI] [PubMed] [Google Scholar]
- 118.Liu S, Yu M, He Y, Xiao L, Wang F, Song C, Sun S, Ling C, Xu Z, Melittin prevents liver cancer cell metastasis through inhibition of the Rac1-dependent pathway. Hepatology 47, 1964–1973 (2008). [DOI] [PubMed] [Google Scholar]
- 119.Wang C, Chen T, Zhang N, Yang M, Li B, Lu X, Cao X, Ling C, Melittin, a major component of bee venom, sensitizes human hepatocellular carcinoma cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by activating CaMKII-TAK1-JNK/p38 and inhibiting IkappaBalpha kinase-NFkappaB. J Biol Chem 284, 3804–3813 (2009). [DOI] [PubMed] [Google Scholar]
- 120.Wu X, Zhao B, Cheng Y, Yang Y, Huang C, Meng X, Wu B, Zhang L, Lv X, Li J, Melittin induces PTCH1 expression by down-regulating MeCP2 in human hepatocellular carcinoma SMMC-7721 cells. Toxicol Appl Pharmacol 288, 74–83 (2015). [DOI] [PubMed] [Google Scholar]
- 121.Zhang Z, Zhang H, Peng T, Li D, Xu J, Melittin suppresses cathepsin S-induced invasion and angiogenesis via blocking of the VEGF-A/VEGFR-2/MEK1/ERK1/2 pathway in human hepatocellular carcinoma. Oncol Lett 11, 610–618 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang W, Tong CY, Liu B, Liu XY, Li T, Preparation and functional characterization of tumor-targeted folic acid-chitosan conjugate nanoparticles loaded with mitoxantrone. J Control Release 213, e110–111 (2015). [DOI] [PubMed] [Google Scholar]
- 123.Wu YH, M-F.; Liu C; Liu T-X; Zou Y,; Li B; Liao H-L , Design, synthesis, and antiproliferative activities of stapled melittin peptides. RSC Advances 7, 17514–17518 (2017). [Google Scholar]
- 124.Hait WN, Grais L, Benz C, Cadman EC, Inhibition of growth of leukemic cells by inhibitors of calmodulin: phenothiazines and melittin. Cancer Chemother Pharmacol 14, 202–205 (1985). [DOI] [PubMed] [Google Scholar]
- 125.Saini SS, Chopra AK, Peterson JW, Melittin activates endogenous phospholipase D during cytolysis of human monocytic leukemia cells. Toxicon 37, 1605–1619 (1999). [DOI] [PubMed] [Google Scholar]
- 126.Moon DO, Park SY, Choi YH, Kim ND, Lee C, Kim GY, Melittin induces Bcl-2 and caspase-3-dependent apoptosis through downregulation of Akt phosphorylation in human leukemic U937 cells. Toxicon 51, 112–120 (2008). [DOI] [PubMed] [Google Scholar]
- 127.Ceremuga M, Stela M, Janik E, Gorniak L, Synowiec E, Sliwinski T, Sitarek P, Saluk-Bijak J, Bijak M, Melittin-A Natural Peptide from Bee Venom Which Induces Apoptosis in Human Leukaemia Cells. Biomolecules 10, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jeong YJ, Choi Y, Shin JM, Cho HJ, Kang JH, Park KK, Choe JY, Bae YS, Han SM, Kim CH, Chang HW, Chang YC, Melittin suppresses EGF-induced cell motility and invasion by inhibiting PI3K/Akt/mTOR signaling pathway in breast cancer cells. Food Chem Toxicol 68, 218–225 (2014). [DOI] [PubMed] [Google Scholar]
- 129.Biniecka P, Jaworski S, Bugajska Z, Daniluk K, Carbon nanoparticles as transporters of melittin to glioma grade IV U87 cells in in vitro model. Annals of Warsaw University of Life Sciences – SGGW Animal Science 56, 23–32 (2017). [Google Scholar]
- 130.Moghaddam FD, Mortazavi P, Hamedi S, Nabiuni M, Roodbari NH, Apoptotic Effects of Melittin on 4T1 Breast Cancer Cell Line is associated with Up Regulation of Mfn1 and Drp1 mRNA Expression. Anticancer Agents Med Chem 20, 790–799 (2020). [DOI] [PubMed] [Google Scholar]
- 131.Raveendran R, Chen F, Kent B, Stenzel MH, Estrone-Decorated Polyion Complex Micelles for Targeted Melittin Delivery to Hormone-Responsive Breast Cancer Cells. Biomacromolecules 21, 1222–1233 (2020). [DOI] [PubMed] [Google Scholar]
- 132.Park JH, Jeong YJ, Park KK, Cho HJ, Chung IK, Min KS, Kim M, Lee KG, Yeo JH, Park KK, Chang YC, Melittin suppresses PMA-induced tumor cell invasion by inhibiting NF-kappaB and AP-1-dependent MMP-9 expression. Mol Cells 29, 209–215 (2010). [DOI] [PubMed] [Google Scholar]
- 133.Shin JM, Jeong YJ, Cho HJ, Park KK, Chung IK, Lee IK, Kwak JY, Chang HW, Kim CH, Moon SK, Kim WJ, Choi YH, Chang YC, Melittin suppresses HIF-1alpha/VEGF expression through inhibition of ERK and mTOR/p70S6K pathway in human cervical carcinoma cells. PLoS One 8, e69380 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zarrinnahad H, Mahmoodzadeh A, Hamidi MP, Mahdavi M, Moradi A, Bagheri KP, Shahbazzadeh D, Apoptotic Effect of Melittin Purified from Iranian Honey Bee Venom on Human Cervical Cancer HeLa Cell Line. Int J Pept Res Ther 24, 563–570 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Qi J, Chen Y, Xue T, Lin Y, Huang S, Cao S, Wang X, Su Y, Lin Z, Graphene oxide-based magnetic nanocomposites for the delivery of melittin to cervical cancer HeLa cells. Nanotechnology 31, 065102 (2020). [DOI] [PubMed] [Google Scholar]
- 136.Zhu H, Yang X, Liu J, Ge Y, Qin Q, Lu J, Zhan L, Liu Z, Zhang H, Chen X, Zhang C, Xu L, Cheng H, Sun X, Melittin radiosensitizes esophageal squamous cell carcinoma with induction of apoptosis in vitro and in vivo. Tumour Biol 35, 8699–8705 (2014). [DOI] [PubMed] [Google Scholar]
- 137.Jin Z, Yao J, Xie N, Cai L, Qi S, Zhang Z, Li B, Melittin Constrains the Expression of Identified Key Genes Associated with Bladder Cancer. J Immunol Res 2018, 5038172 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yao J, Zhang Z, Li S, Li B, Wang XH, Melittin inhibits proliferation, migration and invasion of bladder cancer cells by regulating key genes based on bioinformatics and experimental assays. J Cell Mol Med 24, 655–670 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nikodijević DD, Milutinović MG, Cvetković DM, Ćupurdija MĐ, Jovanović MM, Mrkić IV, Jankulović-Gavrović MĐ, Marković SD, Impact of bee venom and melittin on apoptosis and biotransformation in colorectal carcinoma cell lines. Toxin Reviews, 1–8 (2019). [Google Scholar]
- 140.Soliman C, Eastwood S, Truong VK, Ramsland PA, Elbourne A, The membrane effects of melittin on gastric and colorectal cancer. PLoS One 14, e0224028 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lin L, Zhu BP, Cai L, Therapeutic effect of melittin on a rat model of chronic prostatitis induced by Complete Freund's Adjuvant. Biomed Pharmacother 90, 921–927 (2017). [DOI] [PubMed] [Google Scholar]
- 142.Huh JE, Kang JW, Nam D, Baek YH, Choi DY, Park DS, Lee JD, Melittin suppresses VEGF-A-induced tumor growth by blocking VEGFR-2 and the COX-2-mediated MAPK signaling pathway. J Nat Prod 75, 1922–1929 (2012). [DOI] [PubMed] [Google Scholar]
- 143.Lee C, Bae SS, Joo H, Bae H, Melittin suppresses tumor progression by regulating tumor-associated macrophages in a Lewis lung carcinoma mouse model. Oncotarget 8, 54951–54965 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tipgomut C, Wongprommoon A, Takeo E, Ittiudomrak T, Puthong S, Chanchao C, Melittin Induced G1 Cell Cycle Arrest and Apoptosis in Chago-K1 Human Bronchogenic Carcinoma Cells and Inhibited the Differentiation of THP-1 Cells into Tumour- Associated Macrophages. Asian Pac J Cancer Prev 19, 3427–3434 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang SF, Chen Z, Melittin exerts an antitumor effect on nonsmall cell lung cancer cells. Mol Med Rep 16, 3581–3586 (2017). [DOI] [PubMed] [Google Scholar]
- 146.Gao D, Zhang J, Bai L, Li F, Dong Y, Li Q, Melittin induces NSCLC apoptosis via inhibition of miR-183. Onco Targets Ther 11, 4511–4523 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu M, Wang H, Liu L, Wang B, Sun G, Melittin-MIL-2 fusion protein as a candidate for cancer immunotherapy. J Transl Med 14, 155 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee C, Jeong H, Bae Y, Shin K, Kang S, Kim H, Oh J, Bae H, Targeting of M2-like tumor-associated macrophages with a melittin-based pro-apoptotic peptide. J Immunother Cancer 7, 147 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tu WC, Wu CC, Hsieh HL, Chen CY, Hsu SL, Honeybee venom induces calcium-dependent but caspase-independent apoptotic cell death in human melanoma A2058 cells. Toxicon 52, 318–329 (2008). [DOI] [PubMed] [Google Scholar]
- 150.Shaw P, Kumar N, Hammerschmid D, Privat-Maldonado A, Dewilde S, Bogaerts A, Synergistic Effects of Melittin and Plasma Treatment: A Promising Approach for Cancer Therapy. Cancers (Basel) 11, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang X, Xie J, Lu X, Li H, Wen C, Huo Z, Xie J, Shi M, Tang X, Chen H, Peng C, Fang Y, Deng X, Shen B, Melittin inhibits tumor growth and decreases resistance to gemcitabine by downregulating cholesterol pathway gene CLU in pancreatic ductal adenocarcinoma. Cancer Lett 399, 1–9 (2017). [DOI] [PubMed] [Google Scholar]
- 152.Wan L, Zhang D, Zhang J, Ren L, TT-1, an analog of melittin, triggers apoptosis in human thyroid cancer TT cells via regulating caspase, Bcl-2 and Bax. Oncol Lett 15, 1271–1278 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lee GL, Hait WN, Inhibition of growth of C6 astrocytoma cells by inhibitors of calmodulin. Life Sci 36, 347–354 (1985). [DOI] [PubMed] [Google Scholar]
- 154.Jin H, Zhao G, Hu J, Ren Q, Yang K, Wan C, Huang A, Li P, Feng JP, Chen J, Zou Z, Melittin-Containing Hybrid Peptide Hydrogels for Enhanced Photothermal Therapy of Glioblastoma. ACS Appl Mater Interfaces 9, 25755–25766 (2017). [DOI] [PubMed] [Google Scholar]
- 155.Wang H, Wang S, Wang R, Wang X, Jiang K, Xie C, Zhan C, Wang H, Lu W, Co-delivery of paclitaxel and melittin by glycopeptide-modified lipodisks for synergistic anti-glioma therapy. Nanoscale 11, 13069–13077 (2019). [DOI] [PubMed] [Google Scholar]
- 156.Zhou Y, Zhang S, Chen Z, Bao Y, Chen AT, Sheu WC, Liu F, Jiang Z, Zhou J, Targeted Delivery of Secretory Promelittin via Novel Poly(lactone-co-beta-amino ester) Nanoparticles for Treatment of Breast Cancer Brain Metastases. Adv Sci (Weinh) 7, 1901866 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yang X, Zhu H, Ge Y, Liu J, Cai J, Qin Q, Zhan L, Zhang C, Xu L, Liu Z, Yang Y, Yang Y, Ma J, Cheng H, Sun X, Melittin enhances radiosensitivity of hypoxic head and neck squamous cell carcinoma by suppressing HIF-1alpha. Tumour Biol 35, 10443–10448 (2014). [DOI] [PubMed] [Google Scholar]
- 158.Linville RM, Komin A, Lan X, DeStefano JG, Chu C, Liu G, Walczak P, Hristova K, Searson PC, Reversible blood-brain barrier opening utilizing the membrane active peptide melittin in vitro and in vivo. Biomaterials 275, 120942 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yu X, Dai Y, Zhao Y, Qi S, Liu L, Lu L, Luo Q, Zhang Z, Melittin-lipid nanoparticles target to lymph nodes and elicit a systemic anti-tumor immune response. Nat Commun 11, 1110 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cornara L, Biagi M, Xiao J, Burlando B, Therapeutic Properties of Bioactive Compounds from Different Honeybee Products. Front Pharmacol 8, 412 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Park JH, Kim KH, Lee WR, Han SM, Park KK, Protective effect of melittin on inflammation and apoptosis in acute liver failure. Apoptosis 17, 61–69 (2012). [DOI] [PubMed] [Google Scholar]
- 162.Yun SW, Bae GS, Kim MS, Park KC, Koo BS, Kim BJ, Kim TH, Seo SW, Shin YK, Lee SH, Song HJ, Park SJ, Melittin inhibits cerulein-induced acute pancreatitis via inhibition of the JNK pathway. Int Immunopharmacol 11, 2062–2072 (2011). [DOI] [PubMed] [Google Scholar]
- 163.Lee WR, Kim KH, An HJ, Kim JY, Chang YC, Chung H, Park YY, Lee ML, Park KK, The protective effects of melittin on Propionibacterium acnes-induced inflammatory responses in vitro and in vivo. J Invest Dermatol 134, 1922–1930 (2014). [DOI] [PubMed] [Google Scholar]
- 164.Xu Q, Yaksh TL, A brief comparison of the pathophysiology of inflammatory versus neuropathic pain. Curr Opin Anaesthesiol 24, 400–407 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Moon DO, Park SY, Lee KJ, Heo MS, Kim KC, Kim MO, Lee JD, Choi YH, Kim GY, Bee venom and melittin reduce proinflammatory mediators in lipopolysaccharide-stimulated BV2 microglia. Int Immunopharmacol 7, 1092–1101 (2007). [DOI] [PubMed] [Google Scholar]
- 166.Kim SJ, Park JH, Kim KH, Lee WR, Kim KS, Park KK, Melittin inhibits atherosclerosis in LPS/high-fat treated mice through atheroprotective actions. J Atheroscler Thromb 18, 1117–1126 (2011). [DOI] [PubMed] [Google Scholar]
- 167.Kapur S, Watson W, Carr S, Atopic dermatitis. Allergy Asthma Clin Immunol 14, 52 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.An HJ, Kim JY, Kim WH, Gwon MG, Gu HM, Jeon MJ, Han SM, Pak SC, Lee CK, Park IS, Park KK, Therapeutic effects of bee venom and its major component, melittin, on atopic dermatitis in vivo and in vitro. Br J Pharmacol 175, 4310–4324 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kim WH, An HJ, Kim JY, Gwon MG, Gu H, Jeon M, Sung WJ, Han SM, Pak SC, Kim MK, Park KK, Beneficial effects of melittin on ovalbumin-induced atopic dermatitis in mouse. Sci Rep 7, 17679 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kim Y, Lee YW, Kim H, Chung DK, Bee Venom Alleviates Atopic Dermatitis Symptoms through the Upregulation of Decay-Accelerating Factor (DAF/CD55). Toxins (Basel) 11, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ownby CL, Powell JR, Jiang MS, Fletcher JE, Melittin and phospholipase A2 from bee (Apis mellifera) venom cause necrosis of murine skeletal muscle in vivo. Toxicon 35, 67–80 (1997). [DOI] [PubMed] [Google Scholar]
- 172.Phillis JW, Song D, O'Regan MH, Melittin enhances amino acid and free fatty acid release from the in vivo cerebral cortex. Brain Res 847, 270–275 (1999). [DOI] [PubMed] [Google Scholar]
- 173.Yalcin M, Erturk M, The involvement of the central cholinergic system in the pressor and bradycardic effects of centrally administrated melittin in normotensive conscious rats. Neuropeptides 41, 103–110 (2007). [DOI] [PubMed] [Google Scholar]
- 174.El-Aarag B, Magdy M, AlAjmi MF, Khalifa SAM, El-Seedi HR, Melittin Exerts Beneficial Effects on Paraquat-Induced Lung Injuries In Mice by Modifying Oxidative Stress and Apoptosis. Molecules 24, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bishop DG, Kenrick JR, Melittin : an inhibitor of chloroplast photochemical reactions. Biochem Biophys Res Commun 97, 1082–1090 (1980). [DOI] [PubMed] [Google Scholar]
- 176.Drobak BK, Watkins PA, Inositol(1,4,5)trisphosphate production in plant cells: stimulation by the venom peptides, melittin and mastoparan. Biochem Biophys Res Commun 205, 739–745 (1994). [DOI] [PubMed] [Google Scholar]
- 177.Ross DC, Crim JW, Brown MR, Herzog GA, Lea AO, Toxic and antifeeding actions of melittin in the corn earworm, Heliothis zea (Boddie): comparisons to bee venom and the insecticides chlorpyriphos and cyromazine. Toxicon 25, 307–313 (1987). [DOI] [PubMed] [Google Scholar]
- 178.Osusky M, Zhou G, Osuska L, Hancock RE, Kay WW, Misra S, Transgenic plants expressing cationic peptide chimeras exhibit broad-spectrum resistance to phytopathogens. Nat. Biotechnol 18, 1162–1166 (2000). [DOI] [PubMed] [Google Scholar]
- 179.Yevtushenko DP, Romero R, Forward BS, Hancock RE, Kay WW, Misra S, Pathogen-induced expression of a cecropin A-melittin antimicrobial peptide gene confers antifungal resistance in transgenic tobacco. J Exp Bot 56, 1685–1695 (2005). [DOI] [PubMed] [Google Scholar]
- 180.Arora AK, Pesko KN, Quintero-Hernandez V, Possani LD, Miller TA, Durvasula RV, A paratransgenic strategy to block transmission of Xylella fastidiosa from the glassy-winged sharpshooter Homalodisca vitripennis. BMC Biotechnol 18, 50 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mitchell HK, Lowy PH, Sarmiento L, Dickson L, Melittin: toxicity to Drosophila and inhibition of acetylcholinesterase. Arch Biochem Biophys 145, 344–348 (1971). [DOI] [PubMed] [Google Scholar]
- 182.Knowles BH, Farndale RW, Activation of insect cell adenylate cyclase by Bacillus thuringiensis delta-endotoxins and melittin. Toxicity is independent of cyclic AMP. Biochem J 253, 235–241 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lubawy J, Urbanski A, Mrowczynska L, Matuszewska E, Swiatly-Blaszkiewicz A, Matysiak J, Rosinski G, The Influence of Bee Venom Melittin on the Functioning of the Immune System and the Contractile Activity of the Insect Heart-A Preliminary Study. Toxins (Basel) 11, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Husseneder C, Donaldson JR, Foil LD, Genetically Engineered Yeast Expressing a Lytic Peptide from Bee Venom (Melittin) Kills Symbiotic Protozoa in the Gut of Formosan Subterranean Termites. PLoS One 11, e0151675 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Park Y, Jang SH, Lee DG, Hahm KS, Antinematodal effect of antimicrobial peptide, PMAP-23, isolated from porcine myeloid against Caenorhabditis elegans. J Pept Sci 10, 304–311 (2004). [DOI] [PubMed] [Google Scholar]
- 186.Galdiero E, Maselli V, Falanga A, Gesuele R, Galdiero S, Fulgione D, Guida M, Integrated analysis of the ecotoxicological and genotoxic effects of the antimicrobial peptide melittin on Daphnia magna and Pseudokirchneriella subcapitata. Environ Pollut 203, 145–152 (2015). [DOI] [PubMed] [Google Scholar]
- 187.Dufourcq J, Faucon JF, Intrinsic fluorescence study of lipid-protein interactions in membrane models. Binding of melittin, an amphipathic peptide, to phospholipid vesicles. Biochim. Biophys. Acta 467, 1–11 (1977). [DOI] [PubMed] [Google Scholar]
- 188.Ladokhin AS, Holloway PW, Fluorescence quenching study of melittin-membrane interactions. Ukrainian Biochemical Journal 67, 34–40 (1995). [PubMed] [Google Scholar]
- 189.Vaz WL, Kaufmann K, Nicksch A, Use of energy transfer to assay the association of proteins with lipid membranes. Anal Biochem 83, 385–393 (1977). [DOI] [PubMed] [Google Scholar]
- 190.Ladokhin AS, Jayasinghe S, White SH, How to measure and analyze tryptophan fluorescence in membranes properly, and why bother? Anal. Biochem 285, 235–245 (2000). [DOI] [PubMed] [Google Scholar]
- 191.Vogel H, Incorporation of melittin into phosphatidylcholine bilayers: Study of binding and conformational changes. FEBS Lett 134, 37–42 (1981). [DOI] [PubMed] [Google Scholar]
- 192.Vogel H, Jähnig F, The structure of melittin in membranes. Biophys. J 50, 573–582 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Vogel H, Comparison of the conformation and orientation of alamethicin and melittin in lipid membranes. Biochemistry 26, 4562–4572 (1987). [DOI] [PubMed] [Google Scholar]
- 194.Smith R, Separovic F, Milne TJ, Whittaker A, Bennett FM, Cornell BA, Makriyannis A, Structure and orientation of the pore-forming peptide, melittin, in lipid bilayers. J. Mol. Biol 241, 456–466 (1994). [DOI] [PubMed] [Google Scholar]
- 195.Podo F, Strom R, Crifo C, Zulauf M, Dependence of melittin structure on its interaction with multivalent anions and with model membrane systems. Int J Pept Protein Res 19, 514–527 (1982). [DOI] [PubMed] [Google Scholar]
- 196.Dufourcq J, Faucon J-F, Fourche G, Dasseux J-L, Le Marie M, Gulik-Krzywicki T, Morphological changes of phosphatidylcholine bilayers induced by melittin: vesicularization, fusion, discoidal particles. Biochim. Biophys. Acta 859, 33–48 (1986). [DOI] [PubMed] [Google Scholar]
- 197.Murata M, Nagayama K, Ohnishi S, Membrane fusion activity of succinylated melittin is triggered by protonation of its carboxyl groups. Biochemistry 26, 4056–4062 (1987). [DOI] [PubMed] [Google Scholar]
- 198.Subbarao NK, MacDonald RC, Lipid unsaturation influences melittin-induced leakage of vesicles. Biochim. Biophys. Acta 1189, 101–107 (1994). [DOI] [PubMed] [Google Scholar]
- 199.Benachir T, Lafleur M, Study of vesicle leakage induced by melittin. Biochim. Biophys. Acta 1235, 452–460 (1995). [DOI] [PubMed] [Google Scholar]
- 200.Benachir T, Monette M, Grenier J, Lafleur M, Melittin-induced leakage from phosphatidylcholine vesicles is modulated by cholesterol: A property used for membrane targeting. Eur. Biophys. J 25, 201–210 (1997). [Google Scholar]
- 201.Krauson AJ, He J, Wimley WC, Determining the mechanism of membrane permeabilizing peptides: Identification of potent, equilibrium pore-formers. Biochim. Biophys. Acta 1818, 1625–1632 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Krauson AJ, He J, Wimley WC, Gain-of-Function Analogues of the Pore-Forming Peptide Melittin Selected by Orthogonal High-Throughput Screening. J. Am. Chem. Soc 134, 12732–12741 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Andersson A, Danielsson J, Graslund A, Maler L, Kinetic models for peptide-induced leakage from vesicles and cells. Eur. Biophys J 36, 621–635 (2007). [DOI] [PubMed] [Google Scholar]
- 204.Lee MT, Sun TL, Hung WC, Huang HW, Process of inducing pores in membranes by melittin. Proc. Natl. Acad. Sci. U. S. A 110, 14243–14248 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Guha S, Ghimire J, Wu E, Wimley WC, Mechanistic Landscape of Membrane-Permeabilizing Peptides. Chem. Rev, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Blondelle SE, Houghten RA, Hemolytic and antimicrobial activities of the twenty-four individual omission analogues of melittin. Biochemistry 30, 4671–4678 (1991). [DOI] [PubMed] [Google Scholar]
- 207.Asthana N, Yadav SP, Ghosh JK, Dissection of antibacterial and toxic activity of melittin - A leucine zipper motif plays a crucial role in determining its hemolytic activity but not antibacterial activity. Journal of Biological Chemistry 279, 55042–55050 (2004). [DOI] [PubMed] [Google Scholar]
- 208.Zhu WL, Park Y, Park IS, Park YS, Kim Y, Hahm KS, Shin SY, Improvement of bacterial cell selectivity of melittin by a single Trp mutation with a peptoid residue. Protein Pept Lett 13, 719–725 (2006). [DOI] [PubMed] [Google Scholar]
- 209.Jamasbi E, Batinovic S, Sharples RA, Sani MA, Robins-Browne RM, Wade JD, Separovic F, Hossain MA, Melittin peptides exhibit different activity on different cells and model membranes. Amino Acids 46, 2759–2766 (2014). [DOI] [PubMed] [Google Scholar]
- 210.Hewish DR, Barnham KJ, Werkmeister JA, Kirkpatrick A, Bartone N, Liu ST, Norton RS, Curtain C, Rivetta DE, Structure and activity of D-Pro14 melittin. J Protein Chem 21, 243–253 (2002). [DOI] [PubMed] [Google Scholar]
- 211.Wiedman G, Herman K, Searson P, Wimley WC, Hristova K, The electrical response of bilayers to the bee venom toxin melittin: evidence for transient bilayer permeabilization. Biochim. Biophys Acta 1828, 1357–1364 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rausch JM, Marks JR, Wimley WC, Rational combinatorial design of pore-forming beta-sheet peptides. Proc. Natl. Acad. Sci. U. S. A 102, 10511–10515 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Rathinakumar R, Wimley WC, High-throughput discovery of broad-spectrum peptide antibiotics. FASEB J 24, 3232–3238 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Rathinakumar R, Walkenhorst WF, Wimley WC, Broad-spectrum antimicrobial peptides by rational combinatorial design and high-throughput screening: The importance of interfacial activity. J. Am. Chem. Soc 131, 7609–7617 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Rathinakumar R, Wimley WC, Biomolecular engineering by combinatorial design and high-throughput screening: small, soluble peptides that permeabilize membranes. J. Am. Chem. Soc 130, 9849–9858 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Marks JR, Placone J, Hristova K, Wimley WC, Spontaneous membrane-translocating peptides by orthogonal high-throughput screening. J. Am. Chem. Soc 133, 8995–9004 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Krauson AJ, Hall OM, Fuselier T, Starr CG, Kauffman WB, Wimley WC, Conformational Fine-Tuning of Pore-Forming Peptide Potency and Selectivity. J. Am. Chem. Soc 137, 16144–16152 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Krauson AJ, He J, Hoffmann AR, Wimley AW, Wimley WC, Synthetic molecular evolution of pore-forming peptides by Iterative combinatorial library screening. ACS Chem. Biol 8, 823–831 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.He L, Hoffmann AR, Serrano C, Hristova K, Wimley WC, High-throughput selection of transmembrane sequences that enhance receptor tyrosine kinase activation. J. Mol. Biol 412, 43–54 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kauffman WB, Guha S, Wimley WC, Synthetic molecular evolution of hybrid cell penetrating peptides. Nat. Commun 9, 2568 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Starr CG, Ghimire J, Guha S, Hoffmann JP, Wang Y, Sun L, Landreneau BN, Kolansky ZD, Kilanowski-Doroh IM, Sammarco MC, Morici LA, Wimley WC, Synthetic molecular evolution of host cell-compatible, antimicrobial peptides effective against drug-resistant, biofilm-forming bacteria. Proc Natl Acad Sci U S A 117, 8437–8448 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Li S, Kim SY, Pittman AE, King GM, Wimley WC, Hristova K, Potent Macromolecule-Sized Poration of Lipid Bilayers by the Macrolittins, A Synthetically Evolved Family of Pore-Forming Peptides. J Am. Chem. Soc 140, 6441–6447 (2018). [DOI] [PubMed] [Google Scholar]
- 223.Houghten RA, Pinilla C, Blondelle SE, Appel JR, Dooley CT, Cuervo JH, Generation and use of synthetic peptide combinatorial libraries for basic research and drug discovery. Nature 354, 84–86 (1991). [DOI] [PubMed] [Google Scholar]
- 224.Pinilla C, Appel J, Blondelle S, Dooley C, Dorner B, Eichler J, Ostresh J, Houghten RA, A review of the utility of soluble peptide combinatorial libraries. Biopolymers 37, 221–240 (1995). [DOI] [PubMed] [Google Scholar]
- 225.Fauchere JL, Henlin JM, Boutin JA, Peptide and nonpeptide lead discovery using robotically synthesized soluble libraries. Can J Physiol Pharmacol 75, 683–689 (1997). [PubMed] [Google Scholar]
- 226.Fleeman R, LaVoi TM, Santos RG, Morales A, Nefzi A, Welmaker GS, Medina-Franco JL, Giulianotti MA, Houghten RA, Shaw LN, Combinatorial Libraries As a Tool for the Discovery of Novel, Broad-Spectrum Antibacterial Agents Targeting the ESKAPE Pathogens. J Med Chem 58, 3340–3355 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wimley WC, Application of Synthetic Molecular Evolution to the Discovery of Antimicrobial Peptides. Adv Exp Med Biol 1117, 241–255 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wiedman G, Kim SY, Zapata-Mercado E, Wimley WC, Hristova K, PH-Triggered, Macromolecule-Sized Poration of Lipid Bilayers by Synthetically Evolved Peptides. J. Am. Chem. Soc 139, 937–945 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lee KH, Development of short antimicrobial peptides derived from host defense peptides or by combinatorial libraries. Curr Pharm Des 8, 795–813 (2002). [DOI] [PubMed] [Google Scholar]
- 230.Jones RM, Bulaj G, Conotoxins - new vistas for peptide therapeutics. Curr Pharm Des 6, 1249–1285 (2000). [DOI] [PubMed] [Google Scholar]
- 231.Han TS, Teichert RW, Olivera BM, Bulaj G, Conus venoms - a rich source of peptide-based therapeutics. Curr Pharm Des 14, 2462–2479 (2008). [DOI] [PubMed] [Google Scholar]
- 232.Lemmon MA, Treutlein HR, Adams PD, Brünger AT, Engelman DM, A dimerization motif for transmembrane alpha-helices. Nature Struct. Biol 1, 157–163 (1994). [DOI] [PubMed] [Google Scholar]
- 233.MacKenzie KR, Prestegard JH, Engelman DM, A transmembrane helix dimer: Structure and implications. Science 276, 131–133 (1997). [DOI] [PubMed] [Google Scholar]
- 234.Wiedman G, Fuselier T, He J, Searson PC, Hristova K, Wimley WC, Highly efficient macromolecule-sized poration of lipid bilayers by a synthetically evolved peptide. J Am. Chem. Soc 136, 4724–4731 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wiedman G, Wimley WC, Hristova K, Testing the limits of rational design by engineering pH sensitivity into membrane-active peptides. Biochim Biophys Acta 1848, 951–957 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Parente RA, Nir S, Szoka F, Mechanism of leakage of phospholipid vesicle contents induced by the peptide GALA. Biochemistry 29, 8720–8728 (1990). [DOI] [PubMed] [Google Scholar]
- 237.Li W, Nicol F. o., Szoka FC, GALA: a designed synthetic pH-responsive amphipathic peptide with applications in drug and gene delivery. Advanced Drug Delivery Reviews 56, 967–985 (2004). [DOI] [PubMed] [Google Scholar]
- 238.Zoonens M, Reshetnyak YK, Engelman DM, Bilayer interactions of pHLIP, a peptide that can deliver drugs and target tumors. Biophys. J 95, 225–235 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Thevenin D, An M, Engelman DM, pHLIP-mediated translocation of membrane-impermeable molecules into cells. Chem Biol 16, 754–762 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kim SY, Pittman AE, Zapata-Mercado E, King GM, Wimley WC, Hristova K, Mechanism of Action of Peptides That Cause the pH-Triggered Macromolecular Poration of Lipid Bilayers. J Am Chem Soc 141, 6706–6718 (2019). [DOI] [PubMed] [Google Scholar]
- 241.Kim SY, Bondar AN, Wimley WC, Hristova K, pH-triggered pore-forming peptides with strong composition-dependent membrane selectivity. Biophys J 120, 618–630 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]