Abstract
Uterine leiomyoma is the most common tumor of the female reproductive system and originates from a single transformed myometrial smooth muscle cell. Despite the immense medical, psychosocial, and financial impact, the exact underlying mechanisms of leiomyoma pathobiology are poorly understood. Alterations of signaling pathways are thought to be instrumental in leiomyoma biology. Wnt/β-catenin pathway appears to be involved in several aspects of the genesis of leiomyomas. For example, Wnt5b is overexpressed in leiomyoma, and the Wnt/β-catenin pathway appears to mediate the role of MED12 mutations, the most common mutations in leiomyoma, in tumorigenesis. Moreover, Wnt/β-catenin pathway plays a paracrine role where estrogen/progesterone treatment of mature myometrial or leiomyoma cells leads to increased expression of Wnt11 and Wnt16, which induces proliferation of leiomyoma stem cells and tumor growth. Constitutive activation of β-catenin leads to myometrial hyperplasia and leiomyoma-like lesions in animal models. Wnt/β-catenin signaling is also closely involved in mechanotransduction and extracellular matrix regulation and relevant alterations in leiomyoma, and crosstalk is noted between Wnt/β-catenin signaling and other pathways are known to regulate leiomyoma development and growth such as estrogen, progesterone, TGFβ, PI3K/Akt/mTOR, Ras/Raf/MEK/ERK, IGF, Hippo, and Notch signaling. Finally, evidence suggests that inhibition of the canonical Wnt pathway using β-catenin inhibitors inhibits leiomyoma cell proliferation. Understanding the molecular mechanisms of leiomyoma development is essential for effective treatment. The specific Wnt/β-catenin pathway molecules discussed in this review constitute compelling candidates for therapeutic targeting.
Keywords: uterine fibroid, leiomyoma, pathobiology, signaling pathway, wnt/β-catenin pathway
Introduction
Uterine leiomyoma/fibroid (UL) is the most common benign tumor of the female reproductive system with a global incidence of up to 77% [1]. UL is characterized by myometrium smooth muscle cell proliferation and accumulation of extracellular matrix. UL occur throughout women’s reproductive life, and the most commonly reported symptom is excessive menstrual bleeding resulting in anemia due to iron deficiency. Other possible symptoms also occur including pelvic pain, subfertility, miscarriages, and preterm labor, all of which ultimately affect women’s quality of life [1, 2]. UL is a global burden as its annual estimated total cost is up to $34 billion in the US alone [3]. Despite this immense societal impact, the pathobiology of UL and their exact underlying mechanisms are still unclear. While new and emerging treatments are currently available [4], most only offer short term benefit, and satisfactory medical treatment is still urgently needed to mitigate the economic, social, and psychological burden. Targeting UL signaling pathways represents a compelling opportunity to develop an effective precise treatment.
UL is made of dysregulated smooth muscle cells and fibroblasts. An increasing body of evidence has supported the hypothesis that UL originates from myometrial stem cells that transform into tumor-initiating cells. Myometrial and leiomyoma stem cells have previously been isolated, and several differences between these two cells have been established, giving insight into the genetic and epigenetic changes that are necessary for UL development [5–11]. UL stem cells interact with mature myometrial cells through paracrine pathways resulting in proliferation and extracellular matrix accumulation (ECM) [7]. Resident uterine fibroblasts produce large amounts of ECM proteins such as fibronectin, collagen type I and III, vimentin, and versican, resulting in the stiff nature of UL [12, 13]. This dysregulation is reported to be induced by environmental stimuli and intracellular signaling pathways. Several intracellular pathways are involved including Wnt/β-catenin, TGF-β (transforming growth factor-β)/SMAD, MAPK (mitogen-activated protein kinase)/p38, Hippo/YAP (yes-associated protein)/TAZ (transcriptional coactivator with PDZ-binding motif), Notch, PI3K (phosphotidyl-inositol 3-kinase/protein kinase B)/AKT/mTOR (mammalian target of rapamycin), and JAK (Janus kinase)/STAT (signal transducer and activator of transcription) pathways [14–20]. Previous studies suggest that targeting Wnt/β-catenin signaling can be a promising therapeutic approach for UL due to their aberrant activation compared to the myometrial cells [21, 22]. Thus, understanding the complicated relationship between Wnt/β-catenin signaling and UL development is crucial for precise therapeutic targeting.
In this review, we discuss the critical role of Wnt/β-catenin signaling in UL pathobiology and their possible association with other intracellular signaling pathways. We also review the clinical relevance of Wnt/β-catenin and the drugs targeting this pathway as potential UL treatments.
Search strategy and selection criteria
We performed a PubMed and Scopus literature search until September 2020 using keywords: uterine fibroid, leiomyoma, Wnt/β-catenin signaling, signaling crosstalk, treatment and therapeutics. In this review, we included English language articles focused on Wnt/β-catenin signaling-related leiomyoma pathobiology, its interconnections to other pathways, its therapeutic potential, and anticipated clinical applications.
Overview of the Wnt signaling pathway
The Wingless-type MMTV integration site family (Wnt) signaling pathway is one of the evolutionarily conserved signal pathways that regulate several cellular functions, including cell proliferation, apoptosis, cell fate determination, polarity, migration during development, and stem cell maintenance in adults [23–26]. Dysregulation of the Wnt pathway traditionally represents a critical factor for developing human tumors [27, 28].
There are several Wnt genes, with the number differing in different species, and humans encode a total of nineteen [29]. The Wnt proteins are secreted as lipid-modified glycoproteins, typically 350–400 amino acids in length [30]. On the cell surface, Wnt proteins function as ligands and bind to specific receptors, including Frizzled 1–10 (FZD1–10), seven-pass transmembrane proteins, and their co-receptors, low-density lipoprotein receptor-related protein 5/6 (LRP5/6) before activating intracellular signaling pathways [26]. When Wnt binds to its receptor, disheveled (Dsh or Dvl) is activated and modulates the multiple intracellular downstream Wnt signaling pathways [31, 32]. These pathways are broadly classified into canonical (β-catenin dependent) and non-canonical (β-catenin independent) Wnt pathways [29] (figure 1). In the canonical Wnt pathway, a stable β-catenin detaches from a multimeric destruction complex that contains adenomatous polyposis coli (APC), scaffolding proteins (Axin), casein kinase 1 (CK1), and glycogen synthase kinase 3 (GSK-3) [33–36]. β-catenin then is free to translocate to the nucleus to alter gene transcription by acting as a transcriptional co-activator. Several β-catenin targets have been identified, the most important of which is the LEF (lymphoid enhancer factor)/TCF (T-cell factor) binding transcription factors. The β-catenin-LEF/TCF complex binds to the promoter of various target genes resulting in their activation. However, in the absence of Wnt activation, the unstable β-catenin (phospho-β-catenin), phosphorylated by CK1 and GSK-3, binds to the destruction protein complex and is consequently degraded through ubiquitination [37–39].
Figure 1. Schematic diagram of canonical and noncanonical Wnt signaling pathways.
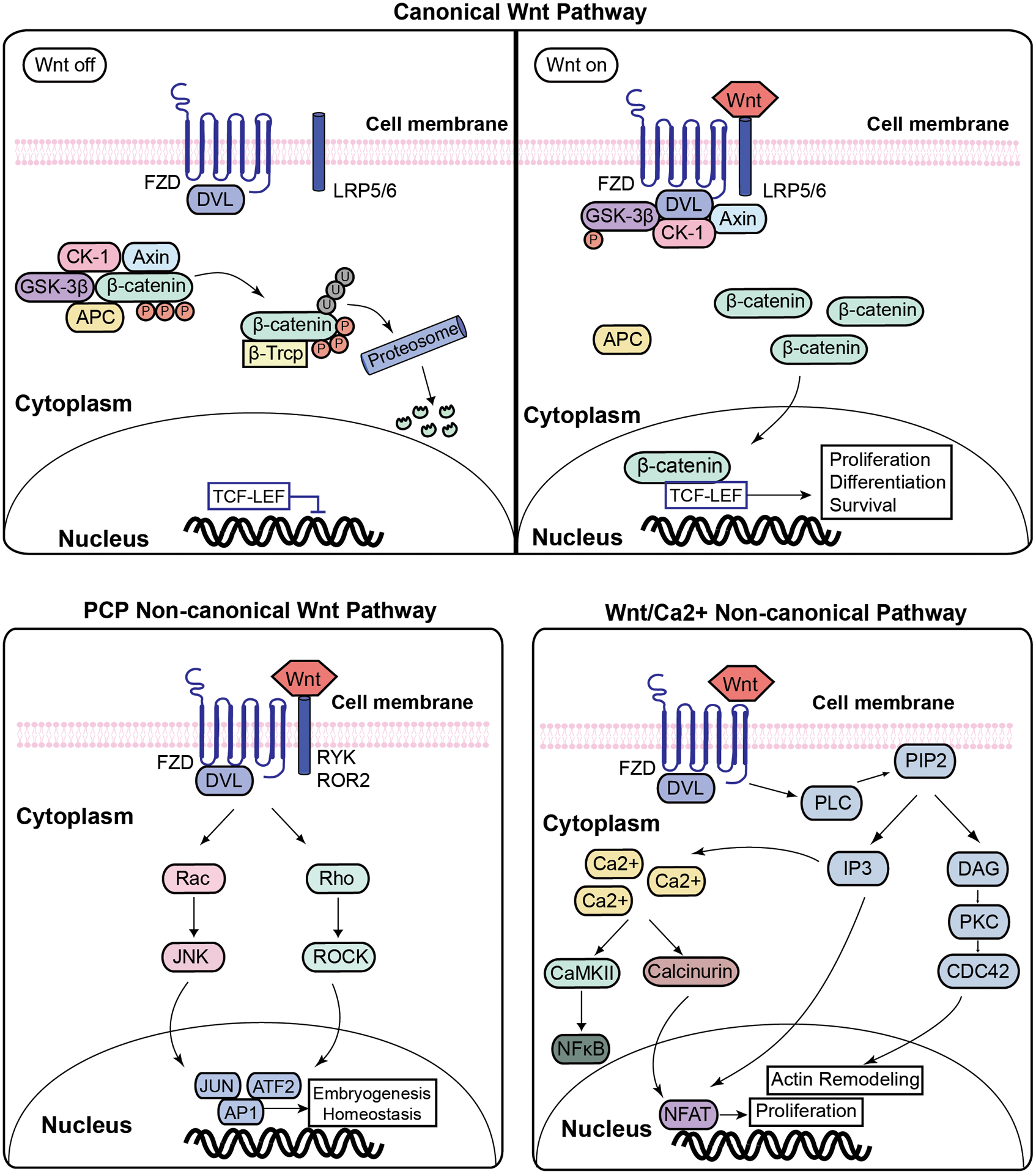
In the absence of Wnt ligand, β-catenin binds to the destruction complex formed by APC, axin, CK1 and GSK3β, resulting in the phosphorylation of β-catenin, its ubiquitination and proteosomal degradation, thus inhibiting its entry to the nucleus. In the presence of Wnt ligand, its receptor gets activated, resulting in the phosphorylation of GSK3β. This inhibits the formation of the degradation complex, freeing the β-catenin to accumulate in the cytoplasm and translocate to the nucleus, where it associates with the TCF/LEF complex, altering gene transcription related to proliferation, differentiation and survival. Wnt can also alter other cellular processes, including cell adhesion, tissue polarity and tumorigenesis, through the non-canonical pathways shown here. The two most studied non-canonical pathways are the planar cell polarity (PCP) and Wnt/Ca2+ pathways. PCP pathway is activated with Wnt binding to FZD and co-receptors such as Ryk, ROR2, or NRH which recruits Dvl. This activates Rho family GTPases and or c-Jun-N-terminal kinase (JNK). In the Wnt/Ca2+ pathway, the binding of Wnt receptor to FZD results in a temporary increase in the concentration of Ca2+ through the activation of phospholipase C (PLC) which results in the formation of inositol 1,4,5-triphosphate (IP3). IP3 increase results in the release of Ca2+ and the activation of calcium-calmodulin-dependent protein kinase II (CaMKII). Ca2+ and PLC pathway activate several regulatory proteins, including NFxB, CREB, and NFAT.
Abbreviation: Wnt- Wingless-Type MMTV Integration Site Family; FZD- frizzled; LRP- low-density lipoprotein receptor-related protein; Dvl- disheveled; GSK3- glycogen synthase kinase 3; CK1- casein kinase 1; TCF/LEF- T-cell factor/lymphoid enhancer factor; RYK- Receptor Like Tyrosine Kinase; ROR- receptor tyrosine kinase-like orphan receptor; RAC- Rho family of GTPases; JNK- c-Jun N-terminal kinases; AP1- Activator protein 1; ATF2- Activating Transcription Factor 2; ROCK- Rho-associated protein kinase; RhoA- Ras homolog family member A; CaMKII- Calcium/calmodulin-dependent protein kinase type II alpha chain; PLC- Phospholipase C; PIP2- Phosphatidylinositol 4,5-bisphosphate; IP3- inositol 1,4,5-trisphosphate; NFAT- nuclear factor of activated T-cells; NF-κB- nuclear factor-κB, DAG- diacylglycerol; PKC- Protein kinase C; CDC42- Cell division control protein 42.
There are several non-canonical Wnt pathways (figure 1). The non-canonical planar cell polarity (PCP) pathway has been shown to be implicated in tissue organization in embryogenesis and in adult tissue homeostasis. It does not involve β-catenin or the coreceptor LRP5. This pathway is activated with Wnt binding to FZD and co-receptors such as Ryk, ROR2, or NRH and results in the recruitment of Dvl which activates Rho family GTPases and or c-Jun-N-terminal kinase (JNK) [40, 41]. Another pathway is the non-canonical Wnt/Ca2+ pathway, which also does not involve β-catenin. The binding of Wnt receptor to FZD results in a temporary increase in the concentration of Ca2+ through the activation of phospholipase C (PLC) which results in the formation of inositol 1,4,5-triphosphate (IP3). IP3 interacts with calcium channels on the endoplasmic reticulum, resulting in the increase of Ca2+ and the activation of calcium-calmodulin-dependent protein kinase II (CaMKII) [42]. Ca2+ and PLC pathway activate several regulatory proteins, including NFxB, CREB, and NFAT [42]. YAP-TAZ and FYN-STAT have been reported to be associated with the non-canonical Wnt pathway [43–45], but more studies are needed to confirm their involvement. Moreover, there are several proteins, including the Wnt inhibitory factors (WIFs), Dkk family, and secreted Fzd-related proteins (sFRP), that play an antagonistic role in the Wnt pathway and ultimately alter the Wnt pathway-mediated development and tumorigenesis [46]. For instance, a study showed that the expression of WIF1, dikkopf WNT signaling pathway inhibitor 1 (DKK1), and secreted frizzled-related protein 4 (SFRP4) was altered in UL compared to the myometrium [47], while their exact involvement in UL development and tumorigenesis remains elusive. The common canonical and non-canonical Wnt/β-catenin pathways are summarized in Figure 1.
Wnt/β-catenin pathway has been shown to be highly activated in human cancers. One of the most studied cancers implicated in this pathway is colorectal cancer (CRC), with up to 70% of the tumors displaying an APC mutation [48]. APC mutation is also the cause of hereditary colon cancer syndrome (familial adenomatous polyposis) [49]. This mutation is considered an early step in CRC adenoma development, and additional genetic alterations are required for malignant transformation to CRC [50]. APC not only plays a role in β-catenin degradation, but it also controls interactions between E-cadherin and β-catenin, which influences migration and chromosomal stability [51–53]. AXIN1 has also been implicated in several cancers, and mutations have been found throughout the whole coding sequence of the AXIN1 gene [54]. The CTNNB1 gene that encodes β-catenin was shown to be mutated in several cancers such as hepatocellular carcinoma and endometrial cancer [55, 56]. Moreover, the phosphorylation sites necessary for β-catenin degradation are mutational hotspots; these mutations allows β-catenin to accumulate and translocate to the nucleus, allowing it to alter gene expression [57].
Role of Wnt signaling in leiomyoma biology: what is currently known?
As described above, Wnt signaling is one of the complex and multi-regulatory pathways that mediate several cellular processes. It has been reported to be upregulated in leiomyoma cells and involved in leiomyomatogenesis.
UL are hypothesized to be monoclonal tumors, meaning that each tumor arises from a single adult myometrial stem cell [58, 59]. The involvement of the Wnt/β-catenin pathway in UL has been increasingly described in recent years [47, 60, 21, 61]. However, the expression of β-catenin in UL varies among different studies. β-catenin overexpression in UL compared with myometrium was shown using human primary UL cells [62, 63], while a report showed no notable difference when comparing human UL tissue to myometrial tissue [64]. Several Wnt ligands are overexpressed in UL and consequently activate the Wnt/β-catenin pathway to promote UL formation and growth. For example, Wnt4 and Wnt5A were found to be overexpressed in human primary UL, and Wnt11 and Wnt16 were overexpressed in leiomyoma stem cells [65, 66, 21]. Interestingly, besides Wnt-responsive genes, Wnt inhibitory genes, such as WIF1, FBXW11 (F-box and WD repeat domain containing 11), NKD1 (NKD inhibitor of WNT signaling pathway 1), SFRP1, and SFRP4, were shown to be significantly upregulated in human UL tissue relative to normal adjacent myometrium while other Wnt inhibitors such as APC, DKK1, and DKK3, were significantly decreased [47]. Fukuhara et al. also showed an increase in sFRP1 mRNA and protein in UL tissue compared to adjacent myometrium, and the inhibition of sFRP1 in a leiomyosarcoma cell line resulted in induction of apoptosis [67], suggesting a dual role of Wnt pathway in UL that necessitates further investigation.
Wnt signaling and leiomyoma stem cell biology
An increasing body of evidence has shown that UL originates from stem cells in the myometrium [68]. Several groups have identified side population (SP) stem cells from UL that express stemness markers such as octamer-binding transcription factor 4 (OCT4), Nanog homeobox (NANOG), DNA (cystosine-5-)-methyltransferase 3 beta (DNMT3B), and growth differentiation factor-3 (GDF-3) [8]. UL stem cells express genetic and epigenetic aberrations compared to myometrial stem and differentiated cells and have altered signaling pathways [10, 69, 70, 7], and this includes the Wnt/β-catenin pathway [71].
The involvement of the Wnt/β-catenin pathway in UL development is supported by embryologic studies. Constitutive overexpression of active β-catenin during embryonic development of mice resulted in myometrial hyperplasia that developed into tumors that histologically resembled UL [72]. Moreover, a marked reduction in uterine size was observed in uterine mesenchyme during embryonic development of female mice containing a selectively deleted form of β-catenin with the cells being replaced by adipocytes [73], suggesting a possible role of Wnt/β-catenin pathway in UL development.
Leiomyoma SP cells are considered tumor initiating cells, and despite their dependence on estrogen and progesterone for growth, these cells have a remarkably low expression of estrogen and progesterone receptors. Ono et al. showed that leiomyoma SP cells interact with mature myometrial or leiomyoma cells through the Wnt/β-catenin which enables the growth of these cells [21]. In leiomyoma SP cells cocultured with mature myometrial cells, estrogen and progesterone promoted nuclear translocation of β-catenin, modifying the transcriptional activity of its heterodimeric partner TCF and its target gene, axis inhibition protein 2 (AXIN2), and ultimately resulting in the proliferation of these SP cells [21]. This effect was not seen in the absence of myometrial mature cells, supporting the necessary role of paracrine signaling between mature myometrial cells and leiomyoma SP cells. Moreover, ectopic expression of β-catenin inhibitor in these cells blocked the hormone dependent growth of human tumors in vivo, highlighting the importance of the β-catenin pathway in UL development [21]. A recent study on UL stem cells showed that UL stem cells primarily expressed Wnt receptor FZD6 compared to the more differentiated cells, and WNT4 was overexpressed in intermediately less differentiated cells [71]. The same group used primary UL cells to show that WNT4 induced proliferation through Akt-dependent β-catenin activation and the resulting activation of pro-proliferative genes such as c-Myc and cyclin D1 [71]. TGFβ3 also seems to contribute to the Wnt/β-catenin pathway in UL stem cells since fucoidan, an anti-fibrotic polysaccharide, was shown to inhibit TGFβ3-induced cell growth and reduced β-catenin translocation into the nucleus in the Eker rat-derived uterine leiomyoma cells (ELT-3 cells) [74].
Wnt signaling in cell differentiation and proliferation
The Wnt/β-catenin pathway has several transcriptional outputs that influence cell proliferation and differentiation. Due to their tight association with cancer, one of the most studied targets of the Wnt/β-catenin pathway are c-MYC, a protooncogene, and cyclin-D1, a regulatory subunit necessary for cell cycle progression [75, 76], and an aberrant Wnt/β-catenin pathway has been linked to desregulation of these pro-proliferation genes in several cancers [77, 78]. Wnt/β-catenin pathway has also been implicated in the PI3K/Akt/mTOR pathway, which is a key signaling pathway in cell differentiation and proliferation. This pathway will be discussed in more detail in subsection 5.4 [79]. Moreover, the Wnt/β-catenin pathway was shown to be involved in promoting self-renewal of human embryonic stem cells, and the inhibition of either Wnt or β-catenin resulted in reduced cell proliferation of the naïve cells [80]. In uterine leiomyoma, the addition of three different Wnt/β-catenin inhibitors (ICAT, niclosamide, and XAV939) resulted in suppressed growth and proliferation in human primary leiomyoma cells, highlighting the role of this pathway in leiomyoma cell proliferation [63]. Ono et al. also showed that the inhibition of β-catenin and TCF4, through the tranfection with adenoviral vectors expressing inhibitors, reduced the growth of leiomyoma-like tumors in immunodeficient mice [21]. They also showed that secreted frizzled-related protein 1, sFRP, a natural Wnt inhibitor, disrupted growth of leiomyoma SP cells in co-culture with mature myometrial cells [21]. These studies highlight the involvement of this pathway in UL cell proliferation.
Wnt signaling and Med12 mutations
Genetic mutations play a prominent role in UL development, and approximately 70% of patients with UL present specific mutations in the mediator complex subunit 12, MED12, gene [81]. MED12 encodes a subunit of Mediator complex, which regulates transcription initiation and elongation by joining regulatory elements in promotors to the RNA polymerase initiation complex. The mutations of the MED12 in UL predominantly occur in exons 1 and 2 of the MED12 gene, and most of them are deletion, insertion, and missense mutations [82]. The role of these mutations is not fully understood, but evidence suggests that MED12 mutations are implicated in the regulation of the Wnt/β-catenin pathway. It was shown that β-catenin targeted the MED12 subunit in Mediator to activate transcription, and, mediator was recruited to Wnt-responsive genes in a β-catenin dependent manner in HeLa cells and 293Top cells [83]. The same group showed that the inhibition of the β-catenin/MED12 interaction suppressed β-catenin activation in response to Wnt signaling [83]. Since MED12 was shown to be essential for canonical Wnt signaling and MED12 limits β-catenin-dependent growth during mouse embryonic development [84], it could be postulated that MED12 mutations resulting in absent or defective MED12 can lead to a β-catenin pathway dependent growth. Indeed, fibroids with MED12 mutations are associated with increased the expression of the Wnt ligand, Wnt4, in UL cells when compared fibroids without the MED12 mutation [65]. Similarly, using an immortalized human uterine myometrial smooth muscle cell line, El Andaloussi et al. showed that the overexpression of mutant MED12 resulted in increased protein expression of Wnt4 and β-catenin when compared to the cells with the overexpression of wild type MED12 [85]. Cells with mutant MED12 also expressed higher levels of mTOR protein and cyclin D, which are implicated in proliferation as will be discussed in subsection 4.5. A more recent study also showed that the expression of Wnt/β-catenin genes was significantly higher in primary fibroid cells with MED12 mutations, and vitamin D treatment significantly reduced the expression of WNT4 expression in MED12 mutated samples [86]. Interestingly, Mehine and colleagues demonstrated that Wnt antagonists, such as WIFI1 and SFRP1, were significantly upregulated in leiomyomas with MED12 mutations [87], demonstrating the dysregulation of both Wnt agonists and antagonists in leiomyoma development.
Wnt signaling and mechanotransduction
Mechanotransduction is reported to have a significant role in both the normal development and tumorigenesis by regulating signaling pathways and gene expression [88–93]. Several mechanical factors, including osmotic pressure, shear stress, spring forces, surface tension, and tensional forces, are eventually converted to cellular biochemical signals [94, 95]. Several studies reported that the Wnt/β-catenin pathway directly interacts with mechanotransduction in Drosophila, zebrafish, and vertebrates [95–97]. It was also proposed that mechanical forces can induce the Wnt target genes in stem cells, chondrocytes, epithelium, osteoblasts, as well as vascular and lymphatic endothelium [97].
Several studies suggested that mechanosignalling is present in the quiescent epithelium and within the tumor microenvironment through the regulation of β-catenin. For instance, in epithelial cells, increased β-catenin stabilization is observed with augmented matrix stiffness or tension [98, 99]. The mechanical strain can stimulate the β-catenin stabilization in the dormant epithelium, affecting cell cycle re-entry through an E-cadherin-dependent mechanism (Figure 2) [100]. Tissue strain results in the unbinding of β-catenin from E-cadherin through an increase of accessibility of phosphorylation site, resulting in the release of β-catenin [101]. In tumor-adjacent cells in a mouse colon tumor model, the expression of β-catenin and its target genes was increased due to the mechanical stress that consequently drives proliferation, resulting in augmented tumor growth and fibrosis [102]. In UL, Ko and colleagues showed that primary fibroid cells expressed higher levels of β-catenin when cultured on stiffer surfaces, highlighting the biomechanical cues influencing β-catenin expression [47]. Similar results were reported in bone marrow mesenchymal cells and primary chondrocytes, with stiff ECM increasing the expression of several members of the Wnt/β-catenin pathway, and the increased expression was linked to activation of the integrin/focal adhesion kinase (FAK)/Akt pathway (Figure 2) which regulates GSK3β [103]. This integrin-FAK pathway is known to be hyperactive in stiffer UL [104]. While mechanootransduction has been suggested as an important signaling pathway in uterine fibroids [13], more studies are needed to understand the interaction between mechanotransduction and Wnt/β-catenin signaling pathways in UL.
Figure 2. Role of Wnt signaling in mechanotransduction and extracellular matrix (ECM) alterations in uterine fibrosis.
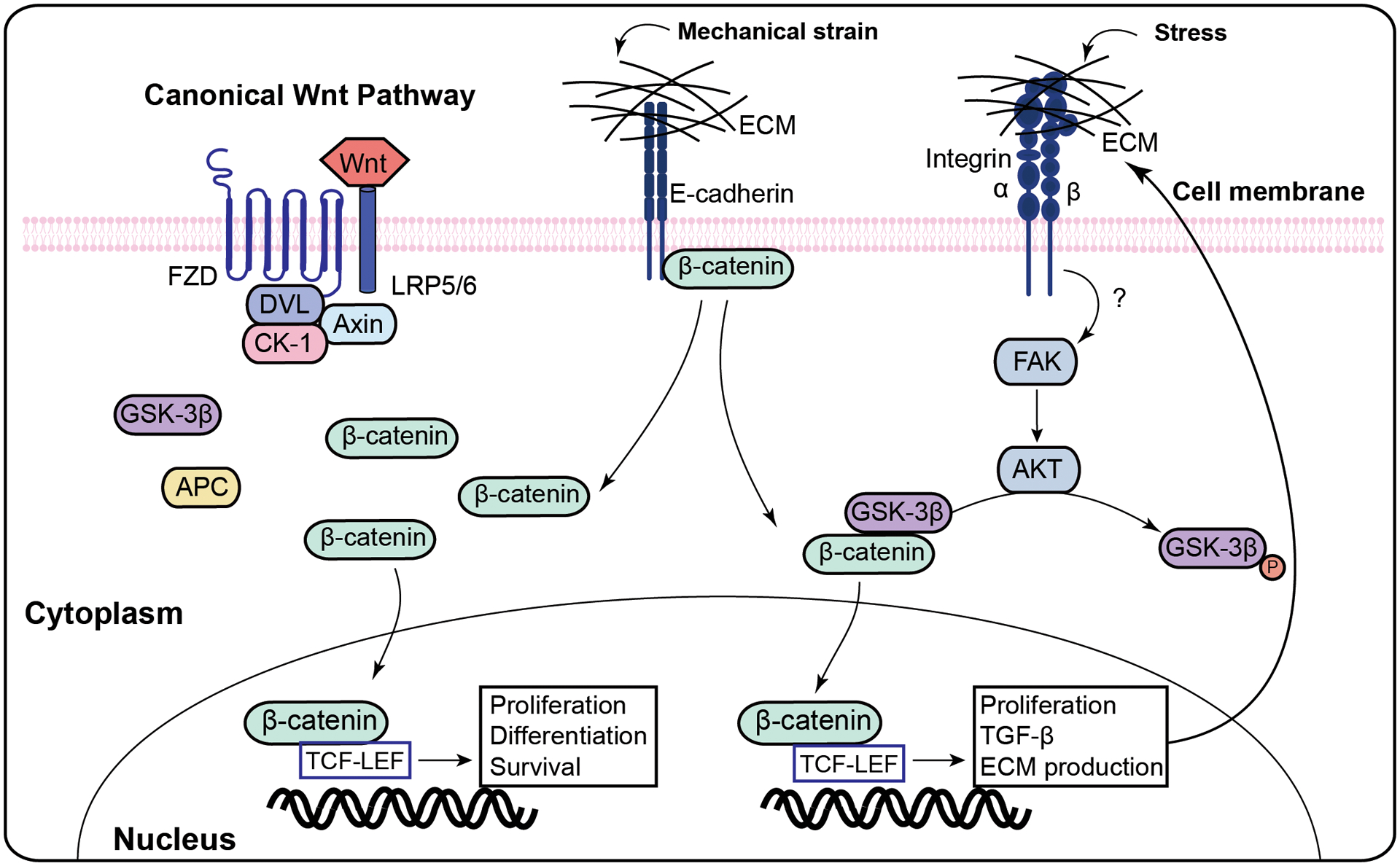
Mechanical strain alters E-cadherin and integrinαvβ3, resulting in the release of β-catenin, and the activation of transcription of genes related to proliferation and ECM formation.
Abbreviation: FAK- Focal adhesion kinase; ECM- extracellular matrix; TGFβ3- Transforming growth factor beta-3.
Wnt signaling and extracellular matrix
Excessive ECM accumulation is considered critical for UL development and appears to play a crucial role in the formation of the bulk structure of these tumors and their associated symptoms [12]. There is evidence that ECM accumulation is due to an imbalance between synthesis and dissolution, similar to disordered wound healing observed in keloid formation [105]. Other factors that might be involved in this complicated process include growth factors such as TGFβ, activin-A, and PDGF, steroid hormones such as estrogen and progesterone, and cytokines such as TNF-α [12]. Several intermediate mediators such as caveolae, cytoskeleton, integrins, ion channels, and surface receptors can receive communication from ECM and transmit mechanical signals to intracellular signaling pathways and subsequently produce more ECM through a feedback loop [91]. It was reported that the Wnt/β-catenin pathway could play an essential role in TGFβ1-mediated ECM production in airway smooth muscle cells [106]. The involvement of Wnt/β-catenin in this complex process in UL development and its interaction with the mediators of ECM formation in UL are not clearly elucidated. It was reported that mutant mice with constitutive activation of β-catenin developed dysplastic lesions in the myometrium and an extracellular matrix that resembled the one found in human uterine leiomyoma [72]. Analysis of the uteri of the mutant mice showed an augmented expression of TGFβ3, compared to the surrounding myometrium [72]. TGFB is known to be over-expressed in UL and is a key modulator of ECM formation [107, 108]. These findings suggest that mechanotransduction and ECM formation are critical factors for UL growth that might be partially mediated via the intracellular Wnt/β-catenin pathway.
Wnt crosstalks with other signaling pathways in ULs
As shown above, the selective activation of the Wnt pathway plays an essential role in UL development. There are several other pathways also involved in UL formation. Intracellular pathways could interact with the Wnt pathway, although our understanding of the direct crosstalk between Wnt and other pathways in UL is less clear. The proposed crosstalks between Wnt and other pathways are summarized in Figure 3.
Figure 3. Schematic representation of the crosstalk between Wnt and other major signaling pathways in uterine fibrosis.
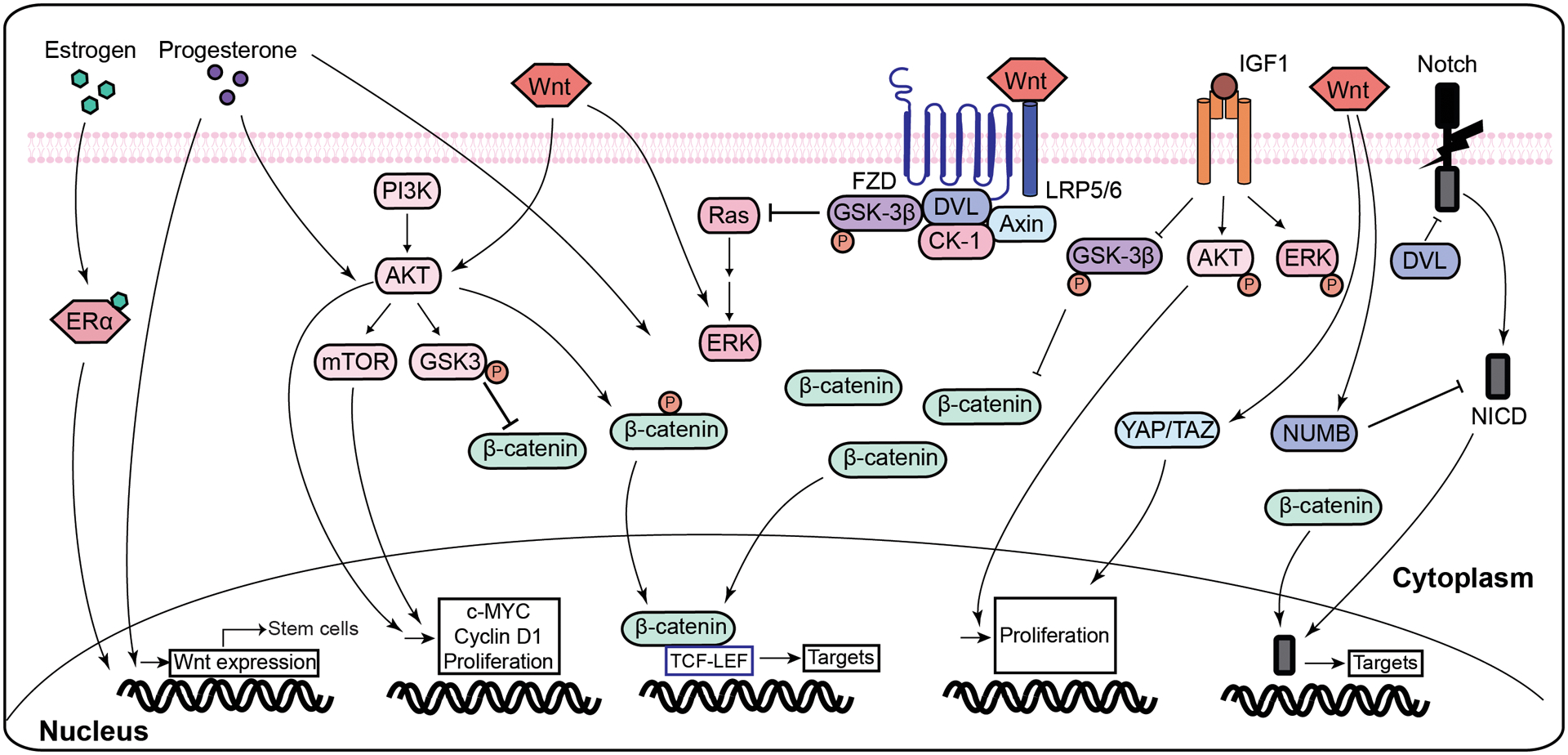
Several signaling pathways have been implicated in Wnt/β-catenin signaling. Estrogen and progesterone induce Wnt expression in mature myometrial cells, which exerts a pro-proliferative effect on stem cells. Wnt, progesterone, and IGF were shown to activate Akt which has phosphorylated β-catenin at Ser552, activating it and allowing it to induce the expression of its target genes. Akt activation also increases expression of downstream signaling targets of Wnt/β-catenin pathway including c-MYC and cyclin D1. Progesterone and IGF were shown to induce the expression of ERK, and Ras, an upstream activator of ERK, was shown to be degraded by the degradation complex in a similar manner to β-catenin. Hippo pathway exerts its effect through YAP/TAZ, which is activated with Wnt. Notch pathway is both activated and inhibited by Wnt/β-catenin pathway, as Wnt induces the expression of its inhibitor, NUMB, while β-catenin allows for the expression of its downstream signaling targets.
Abbreviation: Wnt- Wingless-Type MMTV Integration Site Family; FZD- frizzled; LRP- low-density lipoprotein receptor-related protein; Dvl- disheveled; GSK3- glycogen synthase kinase 3; CK1- casein kinase 1; TCF/LEF- T-cell factor/lymphoid enhancer factor; Ras- ERK- extracellular signal-regulated kinases; ERα- estrogen receptor α; IGF- Insulin-like growth factor; NICD-notch intracellular domain; PI3K- phosphatidylinositol 3-kinase; AKT- protein kinase B; mTOR-mammalian target of rapamycin.
Crosstalk with estrogen signaling
Estrogen receptor (ER) signaling is activated by binding its ligand, estrogen, triggering both genomic and non-genomic downstream signaling [109]. Estrogen and its receptors, ERα and ERβ, are overexpressed in UL and play a crucial role in its development and growth [110]. Estrogen signaling is also altered due to the epigenetic modification of ERα by histone deacetylases (HDACs) in UL [111]. Experiments on UL suggest that ERα could regulate the canonical Wnt pathway, both directly and indirectly, via HDACs modification [112, 22]. It was shown that UL tissue expressed higher protein levels of class I HDAC enzymes compared to myometrium tissue. The addition of an HDAC inhibitor resulted in a time and dose dependent growth inhibitory effect which was linked to the Wnt/β-catenin pathway since there was a decrease in nuclear translocation of β-catenin and the decreased expression of β-catenin responsive markers such as cyclin D1 and C-Myc, quantified by immunofluorescence staining, in immortalized human UF (HuLM) cells [22]. In the same study, Ali et al. also reported that 17β-estradiol (E2) increased the RNA expression of CCND1 and MYC in immortalized human uterine smooth muscle (UTSM) cells, and using an ER antagonist abrogated this stimulatory effect of estrogen [22]. The interaction between E2 and Wnt/β-catenin is reciprocal since the addition of a β-catenin inhibitor resulted in a reduction in ER mRNA expression by almost 5-fold in HuLM cells [22]. Zhang et al. confirmed that the induction of β-catenin expression with E2 treatment in primary endometrial stromal cells might result from the selective activation of the β-catenin promoter [113]. They found that E2 can stimulate ER binding to the β-catenin promoter at the estrogen response element (ERE) site [113]. As mentioned earlier, the paracrine activation of the Wnt/β-catenin pathway could induce UL growth in response to estrogen and progesterone by transferring mitogenic signals from mature myometrium or UL cells to neighboring stem cells [21]. The above findings can explain, at least partially, how estrogen signaling cross-talks with the Wnt/β-catenin pathway in UL cells since estrogen can regulate Wnt/β-catenin in other ways. However, substantial molecular research is essential to adequately explore the exact mechanism underlying estrogen and Wnt/β-catenin signaling regulation.
Crosstalk with progesterone signaling
Progesterone is essential for the development and growth of UL [114]. Like estrogen, progesterone binds to progesterone receptors (PRs), followed by activation of genomic and non-genomic intracellular signaling. Genomic signaling involves activating progesterone response elements (PRE) while nongenomic signaling activates several protein kinases, including MEK and MAPK [115]. The proline-rich motif of PRs could directly bind and activate c-Src tyrosine kinases and subsequently induce ERK signaling [116]. Wnt/β-catenin has been linked to progesterone in several studies. One study showed that PR could co-localize with Wnt4 in the luminal compartment of the ductal epithelium [117]. Wnt4 expression was also upregulated in mammary epithelial cells upon progesterone treatment during pregnancy [117]. Rider et al. showed that progesterone increases Wnt5A mRNA expression through increasing Wnt5A mRNA stability in uterine stromal cells isolated from rat uteri, and they showed that Wnt6A induced stromal cell proliferation [118]. Progesterone was also shown to regulate Dkk-1, Wnt inhibitor, through increasing its mRNA and protein expression in endometrial stromal cells isolate from human tissue [119]. This effect was not seen with the addition of estrogen. Wnt signaling has been shown to mediate a regulatory function in uterine development associated with progesterone signaling as the ablation of Wnt4 resulted in defects in responsiveness to progesterone signaling in female mice [120]. As previously mentioned under section 4.1 on Wnt signaling and leiomyoma stem cell biology, estrogen and progesterone-induced nuclear translocation of β-catenin and the transcriptional activity of its target gene, and the ectopic expression of β-catenin inhibitor blocked estrogen and progesterone dependent growth in leiomyoma stem cells [21]. This study highlighted the interplay of both estrogen and progesterone with the Wnt pathway, reasserting the need for further investigation to properly understand the underlying mechanisms.
Crosstalk with TGFβ signaling
TGF-β is a family of ubiquitously expressed, pleiotropic small polypeptides responsible for modulating several autocrine and paracrine factors of various cellular processes, including differentiation, proliferation, survival, cytoskeletal reorganization, and ECM production [121]. Besides these roles in the development and tissue homeostasis, TGF-β is reported to represent a vital regulator of fibrosis, an essential process in UL growth [121]. The human genome has 33 functional genes encoding TGF-β family polypeptides. The three isoforms TGF-β1, -β2, and -β3, are translated from distinct genes but bind to the same receptors. The binding of TGF-β results in heterotetrameric complex formation and phosphorylation, resulting in the activation of the Smad family of intracellular mediators and non-SMAD pathways [122]. Lee et al. demonstrated that the expression of TGF-β is higher in UL compared with the normal myometrium, and the UL cells are refractory to its usual antiproliferative effects [123]. Moreover, the addition of exogenous TGF-β3 induced a similar molecular phenotype in immortalized myometrial cells, compared to leiomyoma cells, with increased expression of collagen1A1, fibronectin 1, and connective tissue growth factor [124]. While TGF-β and Wnt ligands have distinct intracellular pathways, several differentiation and patterning events require the involvement of both [125]. It was shown that constitutive activation of β-catenin in mice uteri resulted in higher levels of TGF-β3 expression in the endothelium, endometrial stroma, and in central areas of leiomyoma-like lesions when compared to the myometrium [72]. Moreover, as discussed previously in section 4.1 on Wnt signaling and leiomyoma stem cell biology, fucoidan, an anti-fibrotic polysaccharide, inhibited TGFβ3-induced cell growth and reduced β-catenin translocation into the nucleus, establishing a link between TGFβ3’s influence on growth and the Wnt/ β-catenin pathway [74].
Crosstalk with PI3K/Akt/mTOR Pathway
PI3K/Akt/mTOR signaling is involved in several cellular processes governing nutrient uptake, anabolic reactions, cell growth, tumorigenesis and survival [126]. Activation of PI3K results in phosphorylation of Akt, localizing it in the plasma membrane and allowing it to activate several downstreams signals, including the mTOR. There is compelling evidence of its involvement in the development of UL. Hoekstra et al. showed that AKT is activated in primary leiomyoma cells, which is increased in response to the addition of a progesterone receptor agonist (R5020), and the inhibition of AKT was associated with reduced cellular proliferation [127]. Protein expression of phosphorylated GSK3 and cyclin D2, downstream signaling components in the Akt pathway, was higher in primary leiomyoma cells compared with the paired primary myometrial tissue [128]. The involvement of the Akt pathway in UL development was further confirmed using MK-2206, an allosteric Akt inhibitor, was shown to induce caspase-independent cell death of cultured primary leiomyoma tissue and reduce the growth of primary UL cell grafts in a mouse xenograft model [129]. The same inhibitor increased levels of reactive oxygen species in primary UL cells, which was linked to stress-induced premature senescence and an increase in the expression of miR-182 and miR-200a/c, which are implicated in DNA damage response, cellular senescence and proliferation [130]. Silencing of AKT using siRNA against AKT1, AKT2, and AKT3 in primary UL cells resulted in reduced the anti-apoptotic Bcl-2 protein expression, which could be one of the mechanisms in which AKT promotes UL survival and proliferation [131]. Moreover, HMGA2 overexpression, which is seen in 10–15% of UL, is associated with higher levels of Akt signaling, demonstrated by strong immunoreactivity of pAKT compared to without this mutation [132]. Another study reported an augmented mTOR pathway in UL in humans and in the Eker rat animal model [133]. Inhibition of the mTOR pathway in female Eker rate with rapamycin analogue WAY-129327 resulted in decreased cell proliferation after 2 weeks and decreased tumor incidence and size after 4 months. [133]. Previous studies have reported the interaction of Akt and the Wnt/β-catenin pathway. In an epidermoid carcinoma cell line, Akt was shown to phosphorylate β-catenin at Ser552, which stabilized β-catenin, increased its translocation to the nucleus and its transcriptional activity, and induced tumor invasion [134]. Moreover, WNT1 was shown to induce Akt phosphorylation and activation in PC12 cell line, and this phorphorylation was inhibited with the addition of the sFRP, a natural Wnt antagonist [135]. The same group showed that, in the presence of Dvl, Akt modulates β-catenin levels and its target gene expression through increasing phosphorylated GSK3β in the the Axin complex [135]. Focusing on UL, Liu et al. showed that WNT4 treatment increased Akt phosphorylation in primary UL cells, and the addition of an Akt activator (SC79) resulted in β-catenin phosphorylation (at Ser552) and increased levels of active β-catenin (non-phosphorylated at Ser45) [71]. The addition of the Akt inhibitor MK-2206 inhibited WNT4-induced β-catenin phosphorylation at Ser552 and inhibited the WNT4-induced primary UL cell proliferation [71]. The same inhibitor inhibited WNT4-induced mRNA expression of pro-proliferative genes, c-Myc and cyclin D1, in leiomyoma stem cells [71]. Al-Hendy et al. reported an interaction between Wnt/β-catenin and mTOR pathways in response to vitamin D3 in immortalized HuLM and human primary UL cells. In their study, the administration of vitamin D3 reduced the levels of Wnt4, β-catenin as well as mTOR signaling, and the silencing of vitamin D receptor induced increased expression of Wnt/β-catenin, with a subsequent increase in cell division and ECM deposition [136]. The mTOR and Wnt/β-catenin signaling have been previously reported to converge and regulate the progression of cell cycle and cancer metabolism [79], which prompts further research on the crosstalk between Wnt/β-catenin and PI3K/Akt/mTOR signaling in leiomyoma pathobiology.
Crosstalk with Ras/Raf/MEK/ERK Pathway
Ras/Raf/MEK/ERK pathway is another vital signaling pathway regulating various cellular properties, including proliferation, survival, and differentiation [137–139]. MAPK cascades dysfunction have been described in cancer and pathologic disorders [140]. Studies suggest that the Ras/Ref/MEK/ERK pathway is also involved in UL development as some of the proteins involved in this pathway, including Grb2, Shc, and ERK, as well as 15 distinct RTKs are overexpressed compared to healthy myometrium [141]. A study showed that the expression of phospho-ERK appeared to be upregulated upon estrogen treatment in immortalized UL cells, but not in immortalized myometrial cells [142]. Ras/Raf/MEK/ERK and Wnt/β-catenin were shown to interact in several cancers, particularly in colon cancer. ERK pathway was shown to be activated by Wnt/β-catenin through multiple mechanisms in an immortalized mouse cell line (NIH3T3), including a β-catenin-independent Wnt3a activation as well as a β-catenin/Tcf-4-dependent post gene transcriptional activation [143]. Moreover, similar to β-catenin, Ras phosphorylation by GSK3β kinase results in its degradation, and using an immortalized cell line (HEK 293), an aberrant Wnt/β-catenin pathway was reported to stabilize RAS through inhibition of the GSK3β kinase [144]. As expected, phosphorylation mediated Ras degradation inhibits ERK pathway and decreases proliferation [144]. The crosstalk between Wnt/β-catenin and the Ras/Raf/MEK/ERK pathway has not been extensively studied in UL. Silencing of the MED12 gene decreased both ERK and Wnt/β-catenin signaling in UL cells, suggesting interconnectedness of both pathways in UL [60]. More studied are needed to elucidate the interaction of these two pathways in UL.
Crosstalk with IGFs signaling
Insulin-like growth factor (IGF) ligand binding to its receptor results in activation of receptor tyrosine kinase activity which phosphorylates various downstream targets including insulin receptor substrates (IRSs) and Src homology collagen (SHC). IGF signaling is implicated in cell differentiation and survival, and IGFs play a permissive role that is implicated in several signaling factors discussed in this review. IGF signaling appears to be dysregulated in one-third of the UL cells [145]. One study suggested increased IGFs (IGF1 and IGF2) protein expression levels, increased IGF2 mRNA transcript levels, with no significant changes in IGF1 mRNA level in 33 randomly selected fibroids in 33 premenopausal women when compared to its matched myometrium [145]. The same study found that IGF1 levels correlate with AKT activation and with increased tumor size [145]. The addition of IGF1 to primary UL cells resulted in a dose dependent increase in proliferating cell nuclear antigen (PCNA) labeling index and protein expression and an increase in apoptosis-inhibiting protein Bcl-2 compared with control cultures, allowing authors to conclude that IGF-1 plays a role in UL growth by promoting proliferation and imhibiting apoptosis [146]. It was also shown that IGF2 mutation is commonly present in UL of the mutation HMGA2 subtype [87]. Other studies demonstrated that IGF1 interacts with several other pathways including the PI3K and the Ras/Raf/MAPK cascades [147]. IGF2 treatment in primary UL cells resulted in the activation of the MAPK/ERK pathway with increased ERK phosphorylation, and blocking the ERK pathway resulted in inhibition of IGF2-induced proliferation [148]. There is also evidence suggesting IGF and Wnt signaling interplay. Axin2, a Wnt signaling responsive gene, was shown to represent a target of IGF signaling, providing negative feedback to the Wnt/β-catenin pathway in extra-embryonic endoderm with IGF increasing the stability and transcription of Axin 1 [149]. Desbois-Mouthon et al. showed that IGF-1 induced the transcription of Lef/Tcf reporter gene through activating PI3K/Akt and Ras pathways and inhibiting GSK3β in hepatoma cells [150]. The convergence of these pathways suggests an interplay between IGF and Wnt signaling. Further studies are needed to clarify the crosstalk of IGF and Wnt signaling in leiomyoma pathobiology.
Crosstalk with Hippo signaling
Hippo signaling, a highly conserved pathway composed of kinases, plays a significant role in organ development, tissue regeneration, self-renewal, and cancer [151, 152]. The Hippo signaling transcriptional co-activator, YAP/TAZ, is reported to be upregulated in UL, and its inhibition could decrease the fibroid growth by decreasing ECM production [17, 153]. Purdy and coworkers showed that this pathway is regulated by mechanotransduction since decreasing substrate stiffness resulted in reduced YAP/TAZ nuclear localization in both primary UL and matched myometrial cells [17]. Inhibiting YAP/TAZ through siRNA targeting resulted in reduced mRNA expression of connective tissue growth factor, and the treatment with verteporfin, a YAP inhibitor, resulted in decreased cell survival and fibronectin deposition [17]. YAP/TAZ transcriptional co-activator was shown to interact with other signaling pathways and processes involving cell proliferation, survival, and tumorigenesis [154, 155, 43, 156], and this includes Wnt signaling pathway [157]. In a human breast cell line, Varelas et al. demonstrated that TAZ could inhibit the phosphorylation of Dvl which results in the inhibition of the Wnt/β-catenin pathway, and the inhibition of Hippo signaling resulted in increased nuclear translocation of β-catenin and the expression of its target genes [157]. Moreover, the level and nuclear translocation of YAP/TAZ seem to be regulated by Wnt signaling, as TAZ was shown to be sequestered in the β-catenin destruction complex in the absence of Wnt in HEK293 cells [158, 159]. In the presence of Wnt, however, the stable β-catenin escapes the destruction complex and protects TAZ from degradation allowing its accumulation and the expression of Hippo pathway target genes [159, 158]. These findings suggest there might be an interaction between Hippo and Wnt signaling in UL pathobiology, which is yet to be elucidated.
Crosstalk with Notch Signaling
Notch proteins are single-pass transmembrane receptors that function as a membrane-bound transcription factors. Upon ligand binding and activation, Notch undergoes cleavage, releasing the intracellular domain of Notch (NICD), which translocates to the nucleus, binds to RBPj transcription factor, and induces the expression of its target genes in the Hes and Hey family [160]. Notch signaling pathway is involved in organ development, tissue regeneration, self-renewal, and cancer [161–163]. It was also shown to regulate fibrosis by inducing the proliferation of fibroblasts [164]. Gonzalez-Foruria et al. reported hyperactivation of Notch signaling in stromal cells isolated from ectopic endometriosis lesions, and this was associated with oxidative stress and fibrosis [165]. There are many studies suggesting that Notch and Wnt signaling pathways interact with each other and affect the output of both pathways. It was reported that Dvl inhibits Notch through physical interaction with the receptors in vivo and in yeast studies [166]. Dvl also directly binds and inhibits the transcription factor downstream of Notch receptors, regulating cell-fate specification in vivo during Xenopus development [167]. In stem and colon cancer cells, Notch was shown to reduce expression of active β-catenin, and this regulation did not depend on Notch ligand binding [168]. Liu et al. showed that Wnt3a increases the mRNA and protein expression of Numb, an inhibitor of the Notch pathway, through β-catenin being recruited to the proximal promotor of the Numb gene in C2C12 myoblasts [169]. Uterine leiomyomas were shown to have reduced expression of the Notch signal transduction pathway inhibitor Numb and increased expression of the Notch 2 receptor when compared to the surrounding myometrium [170]. An interaction between Wnt and Notch pathways has not yet been elucidated in UL, which deserves further studies.
Therapeutic targeting of the Wnt/β-catenin pathway in UL
Available drugs for the management of UL are limited, and these have several side effects, with a majority of symptomatic women undergoing surgery to alleviate symptoms. This requires the need for alternative oral agents to prevent and treat UL [171]. Given that the Wnt/β-catenin pathway is involved in UL pathobiology and interacts with various signaling pathways, inhibiting the Wnt/β-catenin pathway directly or indirectly via inhibiting other signaling pathways represent a compelling strategy to inhibit UL development and growth. However, caution needs to be considered when inhibiting Wnt/β-catenin pathway since its signaling is an essential pathway for several cellular processes, which can be hampered with its inhibition. Since this pathway regulates organ development, its inhibition can affect pregnant women, a situation that can be clinically relevant as some patients seeking UL treatment can be attempting to conceive.
Preclinical relevance
Several FDA-approved nonspecific Wnt/β-catenin inhibitors exert a potential preclinical inhibitory effect on Wnt/β-catenin pathway. Table 1 includes drugs which can potentially be used as inhibitors for this pathway. For example, non-steroidal anti-inflammatory drugs (NSAID), including celecoxib and 2,5‐dimethylcelecoxib, well-known COX inhibitors, were shown to inhibit the Wnt pathway by augmenting β-catenin degradation through phosphorylation in colon cancer and by targeting TCF/LEF in intestinal cancer [172, 173]. Another NSAID drug, diclofenac, was shown to inhibit Wnt/β-catenin/TCF pathway in glioblastoma cells [174]. Also, studies reported that vitamin D could target the Wnt/β-catenin/TCF pathway and consequently decrease the proliferation and increase apoptosis in colon cancer [175] and UL cells [136]. Pendás-Franco et al. also reported that vitamin D treatment could induce DKK1 expression, which results in the inhibition of the Wnt/β-catenin pathway [175]. A natural flavonoid compound called isoquercitrin had a suppressing effect on colon cancer development by inhibiting Wnt/ β-catenin pathway [176]. As these drugs are FDA-approved, their pharmacokinetics and safety profile are well-known, which makes them compelling options for prevention or treatment of UL.
Table 1.
Available drugs which can potentially inhibits Wnt/β-catenin pathway.
| Name of drug | Direct target | Indirect target | IC50 conc. (nM) | Cancer type | Reference |
|---|---|---|---|---|---|
| Niclosamide | STAT3 | Axin, Wnt/β-catenin-mediated transcription | - | UF | [63] |
| XAV-939 | tankyrase1/2 | Axin, Wnt/β-catenin-mediated transcription | 11/4 | UF, lymphoma | [63, 177, 182] |
| Fucoidan | - | canonical Wnt pathway | - | UF | [74] |
| Vitamin D | - | canonical Wnt pathway | - | CC, UF | [136, 175] |
| Isoquercitrin | Wnt/β-catenin | canonical Wnt pathway | - | CC | [176] |
| Celecoxib | COX | canonical Wnt pathway | - | CC | [172] |
| 2,5‐dimethylcelecoxib | COX | canonical Wnt pathway | - | CC | [172, 173] |
| Diclofenac | - | canonical Wnt pathway | - | GBM | [174] |
| WIKI4 | tankyrase1/2 | Wnt/beta-catenin signaling | 15 | CC, OTS | [179] |
| JW55 | PARP domain of tankyrase1/2 | canonical Wnt pathway | - | CC | [180] |
| GNF-6231 | PORCN | canonical Wnt pathway | 0.8 | CAC | [182] |
| Wnt-C59 (C59) | PORCN | Wnt3A, TCF-mediated transcription | 0.074 | Any cancer | [183] |
| ETC-159 | PORCN | β-catenin | 2.9 | CC | [183] |
| IWP-2 | PORCN | Wnt palmitoylation | 27 | PC | [185] |
| LGK-974 | PORCN | Wnt signaling | 0.4 | HNC, solid tumor | [187] |
| IWP-O1 | PORCN | Wnt, Dvl2/3 | 0.08 | CRC | [188] |
| IWP4 | PORCN | Wnt/β-catenin | 25 | CC | [189] |
| ICG-001 | CREB-binding protein (CBP) | Wnt/β-catenin/TCF-mediated transcription | 3000 | CC | [190] |
| NLS-StAx-h | - | Wnt/β-catenin | 1400 | CC | [222] |
| iCRT14 | β-catenin/Tcf | canonical Wnt pathway | 54000 | CC | [223] |
| iCRT3 | - | Wnt/β-catenin | 8.2 | CC | [223] |
| PNU-75654 | disrupts β-catenin and TCF interaction | canonical Wnt pathway | 450 | ACT | [224] |
| LF3 | disrupts β-catenin and TCF interaction | canonical Wnt pathway | 2000 | CC, HNC | [225] |
| FH535 | PPARγ and PPARδ | Wnt/β-catenin | - | LC | [226] |
| NCB-0846 | TNIK (TRAF2 and NCK-Interacting Kinase) | Wnt signaling | 21 | CC | [227] |
| JW67 | GSK-3β/AXIN/APC | Wnt/β-catenin | 1170 | CC | [191] |
| JW74 | GSK-3β/AXIN/APC | Wnt/β-catenin | 1170 | CC | [191] |
| KYA1797K | GSK-4β/AXIN/APC | Wnt/β-catenin | 750 | CC | [192] |
| IWR-1-endo | - | Wnt3A, Axin2, β-catenin | 180 | LC | [228] |
| CCT 031374 hydrobromide | GSK-4β/AXIN/APC | β-catenin/TCF-dependent transcription | - | CC, LC | [228] |
Other drugs can target Wnt/β-catenin pathway-dependent tumor progression. For instance, XAV-939, a selective inhibitor of tankyrase1/2, can significantly inhibit the Wnt/β-catenin pathway by regulating Axin levels in cancer cells [177, 178]. Other tankyrase inhibitors (JW55 and WIKI4) can also negatively affect the Wnt/β-catenin pathway [179, 180]. Another study showed that XAV939 and niclosamide could specifically inhibit the canonical Wnt/β-catenin pathway and thereby decrease the expression of Wnt-responsive downstream genes in human primary leiomyoma cells, resulting in inhibition of cell growth and proliferation [63]. Chen et al. demonstrated that a natural compound, fucoidan, inhibits UL cell proliferation and growth by inhibiting TGF-β3-induced β-catenin expression [74].
Another possible target is the porcupine (PORCN), a membrane-bound O-acyltransferase, which is necessary for Wnt palmitoylation, secretion, and biologic activity. PORCN is required for proper activation of all human Wnt pathways, suggesting that blocking PORCN could be an alternative treatment option for Wnt/β-catenin pathway-dependent tumors. ETC-159, Wnt-C59 (C59), LGK-974, IWP-2, IWP4, IWP-O1, and GNF-6231 are potent PORCN inhibitors which can also inhibit the Wnt/β-catenin pathway and thereby prevent tumorigenesis with moderate IC50 dose [181–189].
Targeting the cAMP response element-binding protein (CBP)/β-catenin interaction or multimeric destruction protein complex (GSK-3β/AXIN/APC/CK1) could represent another potential approach to inhibit the Wnt/β-catenin pathway in tumor cells. For example, ICG-001, a CBP/β-catenin binding antagonist, is reported to inhibit cancer cells by regulating the expression of Wnt target genes, including Survivin and cyclin D1 [190]. On the other hand, small molecules, such as JW67, JW74, and KYA1797K were reported to reduce active β-catenin and downregulate its target genes by enhancing the β-catenin destruction complex, resulting in β-catenin degradation in colorectal cells [191, 192]. The structure of the small molecules mentioned in the review are shown in figure 4. These studies suggest a possibility to redeploy Wnt-dependent cancer therapy to target UL pathogenesis.
Figure 4. The structure of the small molecule inhibitors.
The structures of SM08502 and CWP232291 are not publicly available.
ULs are extensively associated with cholesterol, as cholesterol is the substrate for sex steroid hormones, such as progesterone and estrogens [193]. Evidence indicates that cholesterol accumulation in the cell plasma membrane activates the interaction between FZD receptors and LRP5/6 by recruiting Dvl resulting in Wnt binding to the FZDs/LRP5/6 complex and the activation of the canonical Wnt/β-catenin pathway [194–196]. Another study reported that the expression of Wnts, particularly Wnt4, could be regulated by estrogen through the ERα-dependent pathway [197]. Therefore, it can be hypothesized that targeting cholesterol biosynthesis and accumulation in plasma membrane could represent a potential approach for targeting Wnt/β-catenin mediated ULs pathogenesis. Our previous study demonstrated that simvastatin, an FDA-approved anti-hypercholesterolemia drug, has a potential inhibitory effect on UL cell proliferation and growth and ECM production in vitro and in vivo via various signaling pathways [198–202]. More studies are needed to see if simvastatin can alter the Wnt/β-catenin pathway.
Clinical relevance
Besides preclinical studies, several drugs have been tested or are currently in clinical trials for Wnt/β-catenin pathway-directed therapy, which are summarized in table 2. Their site of action is elucidated in figure 5. It is important to note that most of these trials are in the early phases to test toxicity and response rate of these inhibitors. These drugs primarily target specific Wnt pathway molecules, including Wnt ligand, FZDs, CBP/β-catenin, β-catenin targets, and Wnt/β-catenin pathway responsive enzymes such as tankyrases and PORCN [57, 178, 203, 204]. Several PORCN inhibitors are currently used in clinical trials. For example, WNT974 (LGK974; clinicaltrials.gov trials: NCT02278133, NCT01351103, and NCT02649530), CGX1321 (NCT02675946 and NCT03507998), RXC004 (NCT03447470), and ETC-1922159 (ETC-159; NCT02521844) are selective PORCN inhibitors which commonly interact with PORCN in the endoplasmic reticulum, inhibiting the secretion of Wnt ligand by altering its post-translational modification and consequently blocking the Wnt/β-catenin pathway [205, 181, 206, 207]. On the other hand, there are several FZD antagonists/antibodies currently approved for clinical trials. For instance, vantictumab (OMP-18R5; NIH clinical trial numbers: NCT01345201, NCT01973309, NCT02005315, and NCT01957007), ipafricept (OMP-54F28; NIH clinical trial numbers: NCT01608867, NCT02050178, NCT02069145, and NCT02092363), and OTSA101-DTPA-90Y (NIH clinical trial number NCT01469975) are specific antibodies which target several FZDs (FZD1, 2, 5, 7, 8, and 10) and consequently inhibit the binding of Wnt ligand to FZD and LRP receptors by disrupting FZD and LRP receptors interaction [208–211].
Table 2:
Wnt/β-catenin signaling inhibitors in current and past clinical trials.
| Therapeutics | Mechanism of action | Interventions | Cancer type | Development phase | Number of patients | Trial identifier |
|---|---|---|---|---|---|---|
| Vitamin D | β-catenin | Cholecalciferol | Stage III Colorectal Cancer | N/A | 70 | NCT02603757 |
| β-catenin | XELOX/mFOLFOX | Stage I-III Colon Cancer or Resectable Colon Cancer Liver Metastases | I | 80 | NCT02172651 | |
| Curcumin | Tcf/β-catenin | 5-fluorouracil | Colon Cancer | I | 13 | NCT02724202 |
| Tcf/β-catenin | Irinotecan | Solid Tumors | I | 23 | NCT01859858 | |
| Tcf/β-catenin | Celecoxib | Metastatic Colon Cancer | III | 100 | NCT00295035 | |
| Tcf/β-catenin | preoperative neoadjuvant | Rectal Cancer | II | 45 | NCT00745134 | |
| Tcf/β-catenin | Placebo/Mirtoselect | Colorectal Adenoma | N/A | 100 | NCT01948661 | |
| Genistein | GSK3β | mFOLFOX/ mFOLFOX+Avastin | Metastatic Colorectal Cancer | I/II | 13 | NCT01985763 |
| Resveratrol | PDE4 | SRT501 | Colorectal Cancer and Hepatic Metastases | I | 9 | NCT00920803 |
| PDE4 | grapes | Colon Cancer | I | 30 | NCT01564797 | |
| Aspirin | β-catenin | Placebo | Colorectal Cancer | III | 3000 | NCT02607072 |
| LGK974 / WNT974 | PORCN | PDR001 | Pancreatic cancer, BRAF mutant CRC, Melanoma, TNBC, H&N, Squamous cell cancer (cervical, esophageal, lung) | I | 184 | NCT01351103 |
| BBI608 | β-catenin/Stat3 | Placebo | Advanced Colorectal Carcinoma | III | 282 | NCT01830621 |
| β-catenin/Stat3 | Panitumumab+Cet | Advanced Colorectal Carcinoma | II | 203 | NCT01776307 | |
| β-catenin/Stat3 | Pembrolizumab | Metastatic Colorectal Cancer | II | 94 | NCT02851004 | |
| β-catenin/Stat3 | Nivolumab | Refractory Colorectal Cancer | II | 90 | NCT03647839 | |
| PRI-724 | CBP/β-catenin | - | Advanced pancreatic cancer, Metastatic pancreatic cancer, Pancreatic adenocarcinoma | I | 20 | NCT01764477 |
| PRI-724 | CBP/β-catenin | mFOLFOX6 | Advanced solid tumors | I | 23 | NCT01302405 |
| PRI-724 | CBP/β-catenin | - | Acute myeloid leukemia, Chronic myeloid leukemia | II | 49 | NCT01606579 |
| WNT974 (with LGX818 and Cetuximab) | PORCN | LGX818 and Cetuximab | BRAF-mutant Metastatic Colorectal Cancer | I | 20 | NCT02278133 |
| ETC-1922159, WNT974 | PORCN, PORCN | Pembrolizumab- | Solid tumor, Metastatic Head and Neck Squamous Cell Carcinoma | III | 83 | NCT02521844 |
| RXC004, ETC-1922159 | PORCN, PORCN | -Pembrolizumab | Solid tumor, Solid tumor | II | 59 | NCT03447470 |
| CGX1321, RXC004 | PORCN, PORCN | -- | Advanced Gastrointestinal Tumors, Solid tumor | II | 39 | NCT03507998 |
| CGX1321 (with Pembrolizumab)CGX1321 | PORCN, PORCN | Pembrolizumab- | Solid tumors, GI cancer, Advanced Gastrointestinal Tumors | II | 72 | NCT02675946 |
| OTSA101-DTPA-90Y, CGX1321 (with Pembrolizumab) | FZD10, PORCN | -Pembrolizumab | Sarcoma, Synovial, Solid tumors, GI cancer | II | 20 | NCT01469975 |
| OMP-18R5 (with Docetaxel), OTSA101-DTPA-90Y | FZD10, FZD | Docetaxel- | Solid tumors, Sarcoma, Synovial | II | 34 | NCT01957007 |
| OMP-18R5, OMP-18R5 (with Docetaxel) | FZDs | paclitaxelDocetaxel | Metastatic breast cancer, Solid tumors | II | 37 | NCT01973309 |
| OMP-18R5 | FZDs | -paclitaxel | Solid tumors, Metastatic breast cancer | II | 35 | NCT01345201 |
| OMP-18R5 (with Nab-Paclitaxel and Gemcitabine), OMP-18R5 | FZDs | Nab-Paclitaxel and Gemcitabine- | Pancreatic cancer, Stage IV pancreatic cancer, Solid tumors | II | 30 | NCT02005315 |
| OMP-54F28 (with Sorafenib), OMP-18R5 (with Nab-Paclitaxel and Gemcitabine) | FZD8 | SorafenibNab-Paclitaxel and Gemcitabine | Hepatocellular cancer, Pancreatic cancer, Stage IV pancreatic cancer | II | 10 | NCT02069145 |
| OMP-54F28 (with Paclitaxel & Carboplatin), OMP-54F28 (with Sorafenib) | FZD8 | Paclitaxel and CarboplatinSorafenib | Ovarian cancer, Hepatocellular cancer | II | 37 | NCT02092363 |
| OMP-54F28 (with Nab-Paclitaxel and Gemcitabine), OMP-54F28 (with Paclitaxel & Carboplatin) | FZD8 | Nab-Paclitaxel and GemcitabinePaclitaxel and Carboplatin | Pancreatic cancer, Stage IV pancreatic cancer, Ovarian cancer | II | 26 | NCT02050178 |
| OMP-54F28, OMP-54F28 (with Nab-Paclitaxel and Gemcitabine) | FZD8 | -Nab-Paclitaxel and Gemcitabine | Solid tumors, Pancreatic cancer, Stage IV pancreatic cancer | II | 26 | NCT01608867 |
| SM08502, Niclosamide | β-catenin-controlled gene expression inhibitor, FZDs | -- | Solid tumors, Colon Cancer | II | 42 | NCT03355066 |
| CWP232291, SM08502 | β-catenin-controlled gene expression inhibitor | ara-C - | Acute myeloid leukemia, Solid tumors | I/III | 45 | NCT03055286 |
| CWP232291 | β-catenin-controlled gene expression inhibitor | Lenalidomide, Dexamethasoneara-C | Refractory Myeloma, Acute myeloid leukemia | Ia/IbI/II | 25 | NCT02426723 |
| CWP232291 | β-catenin-controlled gene expression inhibitor | -Lenalidomide, Dexamethasone | Acute Myeloid Leukemia, Chronic Myelomonocytic Leukemia, Myelodysplastic Syndrome, Myelofibrosis, Refractory Myeloma | IIa/Ib | 69 | NCT01398462 |
| DKN-01, CWP232291 | β-catenin-controlled gene expression inhibitor | Paclitaxel- | Epithelial Endometrial Cancer, Epithelial Ovarian Cancer, or Carcinosarcoma, Acute Myeloid Leukemia, Chronic Myelomonocytic Leukemia, Myelodysplastic Syndrome, Myelofibrosis | III | 124 | NCT03395080 |
| DKN-01 | β-catenin-controlled gene expression inhibitor | SorafenibPaclitaxel | Advanced Liver Cancer, Epithelial Endometrial Cancer, Epithelial Ovarian Cancer, or Carcinosarcoma | I/IIII | 70 | NCT03645980 |
| Daunorubicin, DKN-01 | β-catenin-controlled gene expression inhibitor | -Sorafenib | Acute Leukemia, Advanced Liver Cancer | II/II | 18 | NCT02914977 |
| Foxy-5, Daunorubicin | Wnt5aβ-catenin-controlled gene expression inhibitor | FOLFOX regimen- | Colon Cancer, Acute Leukemia | III | 100 | NCT03883802 |
Figure 5. Schematic diagram of the site of action of the Wnt signaling pathway inhibitors that are in current or were in past clinical trials.
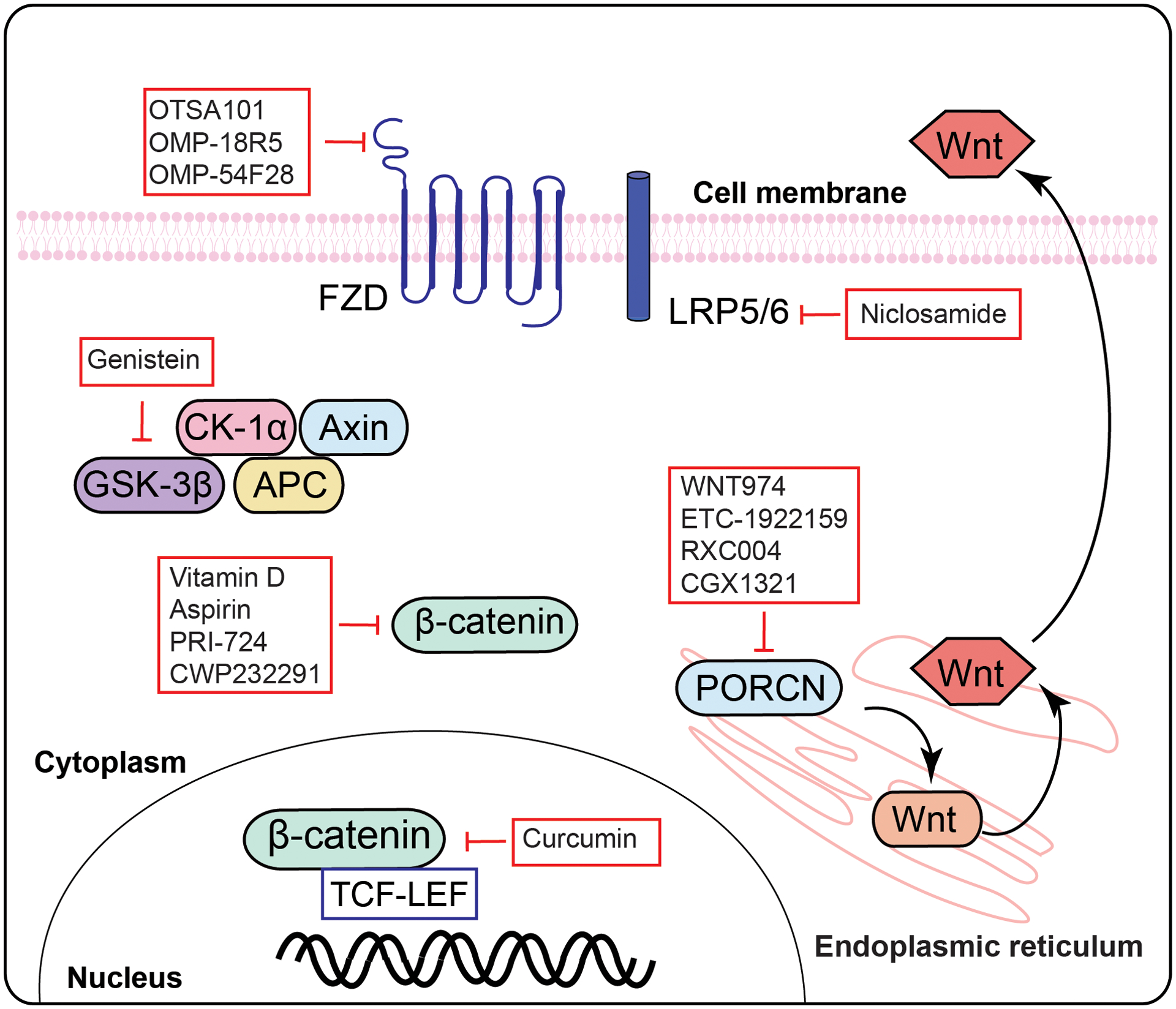
Abbreviation: Wnt- Wingless-Type MMTV Integration Site Family; FZD- frizzled; LRP- low-density lipoprotein receptor-related protein; Dvl- disheveled; GSK3- glycogen synthase kinase 3; CK1- casein kinase 1; TCF/LEF- T-cell factor/lymphoid enhancer factor; PORCN-Porcupine O-acyltransferase.
PRI-724 is a β-catenin/CBP antagonist which inhibits the binding of β-catenin/CBP to WRE (Wnt-responsive element; 5′-CTTTGA/TA/T-3′). This antagonist is currently in clinical trials (NCT01302405, NCT01606579, NCT01764477, and NCT02413853) for Wnt/β-catenin pathway-dependent cancer therapy [212]. Inhibiting target genes of the Wnt/β-catenin pathway could remain another possible option to inhibit Wnt pathway-mediated pathogenesis, and there are several small molecules approved for use in clinical trials. For example, SM08502 (NCT03355066) and CWP232291 (NCT03055286, NCT01564797, and NCT01398462) can potentially inhibit the Wnt pathway target genes and subsequently inhibit the Wnt pathway-dependent myeloma development [213–215].
Several natural compounds such as vitamin D, curcumin, genistein, and resveratrol are potential inhibitors of the Wnt/β-catenin pathway. Vitamin D has been shown to inhibit this pathway by several mechanisms in human cancers, and there are ongoing clinical on Vitamin D (NCT02603757 and NCT02172651) to test its effect [216, 217]. Curcumin (NCT02724202, NCT01859858, and NCT00295035) is a potent inhibitor of TCF/β-catenin [218, 219]. Genistein (NCT01985763) and resveratrol (NCT00920803 and NCT00578396) can also inhibit the Wnt/β-catenin pathway-dependent tumorigenesis [220, 221].
Concluding remarks
Fibrotic progression remains a dynamic pathobiological phenomenon in UL (hence given the common name, fibroids). Exploring molecular mechanisms of this progression could offer solutions to this common condition. Mechanisms of uterine fibrosis are complex and involve multiple signaling mediators imposing therapeutic challenges. The pieces of evidence in this review show that the Wnt/β-catenin pathway plays a critical role in the pathogenesis of UL including mechanotransduction and ECM production. Extensive research has been performed to develop therapeutic drugs for targeting Wnt/β-catenin signaling in tumor development; however, the blockade of Wnt signaling requires further investigation due to the complexity of the involved cellular signaling pathways. Several Wnt signaling regulators are expressed in tumors, which might be targetable with improvised drugs. These drugs can be potentially deployed in leiomyoma treatment and can open new horizons in reproductive medicine.
Funding:
This work was supported, in part, by NIH grant 1R01HD094380 to Mostafa Borahay
Footnotes
Conflicts of interest/competing interests: The authors declare no conflict of interest.
Availability of data and material:
No new data were generated or analysed in support of this research.
REFERENCES
- 1.Fortin C, Flyckt R, Falcone T. Alternatives to hysterectomy: The burden of fibroids and the quality of life. Best Pract Res Clin Obstet Gynaecol. 2018;46:31–42. doi: 10.1016/j.bpobgyn.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Gupta S, Jose J, Manyonda I. Clinical presentation of fibroids. Best practice & research Clinical obstetrics & gynaecology. 2008;22(4):615–26. [DOI] [PubMed] [Google Scholar]
- 3.Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol. 2012;206(3):211 e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fritton K, Borahay MA. New and Emerging Therapies for Uterine Fibroids. Semin Reprod Med. 2017;35(6):549–59. doi: 10.1055/s-0037-1606303. [DOI] [PubMed] [Google Scholar]
- 5.Ono M, Maruyama T, Masuda H, Kajitani T, Nagashima T, Arase T et al. Side population in human uterine myometrium displays phenotypic and functional characteristics of myometrial stem cells. Proc Natl Acad Sci U S A. 2007;104(47):18700–5. doi: 10.1073/pnas.0704472104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang HL, Senaratne TN, Zhang L, Szotek PP, Stewart E, Dombkowski D et al. Uterine leiomyomas exhibit fewer stem/progenitor cell characteristics when compared with corresponding normal myometrium. Reprod Sci. 2010;17(2):158–67. doi: 10.1177/1933719109348924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ono M, Qiang W, Serna VA, Yin P, Coon JSt, Navarro A et al. Role of stem cells in human uterine leiomyoma growth. PLoS One. 2012;7(5):e36935. doi: 10.1371/journal.pone.0036935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mas A, Cervello I, Gil-Sanchis C, Faus A, Ferro J, Pellicer A et al. Identification and characterization of the human leiomyoma side population as putative tumor-initiating cells. Fertil Steril. 2012;98(3):741–51 e6. doi: 10.1016/j.fertnstert.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 9.Mas A, Nair S, Laknaur A, Simon C, Diamond MP, Al-Hendy A. Stro-1/CD44 as putative human myometrial and fibroid stem cell markers. Fertil Steril. 2015;104(1):225–34 e3. doi: 10.1016/j.fertnstert.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin P, Ono M, Moravek MB, Coon JSt, Navarro A, Monsivais D et al. Human uterine leiomyoma stem/progenitor cells expressing CD34 and CD49b initiate tumors in vivo. J Clin Endocrinol Metab. 2015;100(4):E601–6. doi: 10.1210/jc.2014-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patterson AL, George JW, Chatterjee A, Carpenter TJ, Wolfrum E, Chesla DW et al. Putative human myometrial and fibroid stem-like cells have mesenchymal stem cell and endometrial stromal cell properties. Hum Reprod. 2020;35(1):44–57. doi: 10.1093/humrep/dez247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Islam MS, Ciavattini A, Petraglia F, Castellucci M, Ciarmela P. Extracellular matrix in uterine leiomyoma pathogenesis: a potential target for future therapeutics. Hum Reprod Update. 2018;24(1):59–85. doi: 10.1093/humupd/dmx032. [DOI] [PubMed] [Google Scholar]
- 13.Leppert PC, Jayes FL, Segars JH. The extracellular matrix contributes to mechanotransduction in uterine fibroids. Obstet Gynecol Int. 2014;2014:783289. doi: 10.1155/2014/783289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corachán A, Ferrero H, Aguilar A, Garcia N, Monleon J, Faus A et al. Inhibition of tumor cell proliferation in human uterine leiomyomas by vitamin D via Wnt/β-catenin pathway. Fertility and sterility. 2019;111(2):397–407. [DOI] [PubMed] [Google Scholar]
- 15.Shen Y, Lu Q, Zhang P, Wu Y, Ren M. The effect of TGF-β signaling on regulating proliferation of uterine leiomyoma cell via ERα signaling activated by bisphenol A, octylphenol and nonylphenol in vitro. Journal of cancer research and therapeutics. 2018;14(9):276. [DOI] [PubMed] [Google Scholar]
- 16.Zhu Y, Xu J, Li Z, Xie S, Zhou J, Guo X et al. Ginsenoside Rh2 suppresses growth of uterine leiomyoma in vitro and in vivo and may regulate ERα/c-Src/p38 MAPK activity. Journal of functional foods. 2015;18:73–82. [Google Scholar]
- 17.Purdy MP, Ducharme M, Haak AJ, Ravix J, Tan Q, Sicard D et al. YAP/TAZ are Activated by Mechanical and Hormonal Stimuli in Myometrium and Exhibit Increased Baseline Activation in Uterine Fibroids. Reprod Sci. 2020;27(4):1074–85. doi: 10.1007/s43032-019-00106-4. [DOI] [PubMed] [Google Scholar]
- 18.Makker A, Goel MM, Mahdi AA, Bhatia V, Das V, Agarwal A et al. PI3K/Akt/mTOR signaling & its regulator tumour suppressor genes PTEN & LKB1 in human uterine leiomyomas. Indian J Med Res. 2016;143(Supplement):S112–S9. doi: 10.4103/0971-5916.191808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malik M, Britten JL, Catherino W. IL6 and STAT-3 pathway highlight the differences in molecular responses in myometrium and uterine fibroids. Fertility and sterility. 2019;112(3):e350. [Google Scholar]
- 20.Borahay MA, Al-Hendy A, Kilic GS, Boehning D. Signaling Pathways in Leiomyoma: Understanding Pathobiology and Implications for Therapy. Molecular medicine. 2015. doi: 10.2119/molmed.2014.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ono M, Yin P, Navarro A, Moravek MB, Coon JSt, Druschitz SA et al. Paracrine activation of WNT/beta-catenin pathway in uterine leiomyoma stem cells promotes tumor growth. Proc Natl Acad Sci U S A. 2013;110(42):17053–8. doi: 10.1073/pnas.1313650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ali M, Shahin SM, Sabri NA, Al-Hendy A, Yang Q. Activation of β-Catenin Signaling and its Crosstalk With Estrogen and Histone Deacetylases in Human Uterine Fibroids. The Journal of Clinical Endocrinology & Metabolism. 2020;105(4):dgz227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gruber J, Yee Z, Tolwinski NS. Developmental Drift and the Role of Wnt Signaling in Aging. Cancers (Basel). 2016;8(8). doi: 10.3390/cancers8080073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaur P, Jin HJ, Lusk JB, Tolwinski NS. Modeling the Role of Wnt Signaling in Human and Drosophila Stem Cells. Genes (Basel). 2018;9(2). doi: 10.3390/genes9020101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng LF, Kaur P, Bunnag N, Suresh J, Sung ICH, Tan QH et al. WNT signaling in disease. Cells. 2019;8(8):826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169(6):985–99. [DOI] [PubMed] [Google Scholar]
- 27.Martin-Orozco E, Sanchez-Fernandez A, Ortiz-Parra I, Ayala-San Nicolas M. WNT Signaling in Tumors: The Way to Evade Drugs and Immunity. Front Immunol. 2019;10:2854. doi: 10.3389/fimmu.2019.02854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2017;36(11):1461–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4(2):68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11(24):3286–305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 31.Pfister AS, Kühl M. Of Wnts and ribosomes. Progress in molecular biology and translational science. Elsevier; 2018. p. 131–55. [DOI] [PubMed] [Google Scholar]
- 32.Habas R, Dawid IB. Dishevelled and Wnt signaling: is the nucleus the final frontier? Journal of biology. 2005;4(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minde DP, Anvarian Z, Rüdiger SG, Maurice MM. Messing up disorder: how do missense mutations in the tumor suppressor protein APC lead to cancer? Molecular cancer. 2011;10(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Iii WLP et al. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90(1):181–92. [DOI] [PubMed] [Google Scholar]
- 35.Cruciat C-M. Casein kinase 1 and Wnt/β-catenin signaling. Current opinion in cell biology. 2014;31:46–55. [DOI] [PubMed] [Google Scholar]
- 36.Wu D, Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci. 2010;35(3):161–8. doi: 10.1016/j.tibs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108(6):837–47. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 39.Rao TP, Kuhl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ Res. 2010;106(12):1798–806. doi: 10.1161/CIRCRESAHA.110.219840. [DOI] [PubMed] [Google Scholar]
- 40.van Amerongen R Alternative Wnt pathways and receptors. Cold Spring Harb Perspect Biol. 2012;4(10). doi: 10.1101/cshperspect.a007914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.VanderVorst K, Dreyer CA, Konopelski SE, Lee H, Ho HH, Carraway KL 3rd. Wnt/PCP Signaling Contribution to Carcinoma Collective Cell Migration and Metastasis. Cancer Res. 2019;79(8):1719–29. doi: 10.1158/0008-5472.CAN-18-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De A Wnt/Ca2+ signaling pathway: a brief overview. Acta Biochim Biophys Sin (Shanghai). 2011;43(10):745–56. doi: 10.1093/abbs/gmr079. [DOI] [PubMed] [Google Scholar]
- 43.Park HW, Kim YC, Yu B, Moroishi T, Mo JS, Plouffe SW et al. Alternative Wnt Signaling Activates YAP/TAZ. Cell. 2015;162(4):780–94. doi: 10.1016/j.cell.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gujral TS, Chan M, Peshkin L, Sorger PK, Kirschner MW, MacBeath G. A noncanonical Frizzled2 pathway regulates epithelial-mesenchymal transition and metastasis. Cell. 2014;159(4):844–56. doi: 10.1016/j.cell.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Green J, Nusse R, van Amerongen R. The role of Ryk and Ror receptor tyrosine kinases in Wnt signal transduction. Cold Spring Harb Perspect Biol. 2014;6(2). doi: 10.1101/cshperspect.a009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Semënov MV, He X. Secreted antagonists/modulators of Wnt signaling. Madame Curie Bioscience Database [Internet]. Landes Bioscience; 2013. [Google Scholar]
- 47.Ko Y-A, Jamaluddin MFB, Adebayo M, Bajwa P, Scott RJ, Dharmarajan AM et al. Extracellular matrix (ECM) activates β-catenin signaling in uterine fibroids. Reproduction. 2018;155(1):61–71. [DOI] [PubMed] [Google Scholar]
- 48.Kwong LN, Dove WF. APC and its modifiers in colon cancer. Adv Exp Med Biol. 2009;656:85–106. doi: 10.1007/978-1-4419-1145-2_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66(3):589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 50.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61(5):759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 51.Caldwell CM, Kaplan KB. The role of APC in mitosis and in chromosome instability. Adv Exp Med Biol. 2009;656:51–64. doi: 10.1007/978-1-4419-1145-2_5. [DOI] [PubMed] [Google Scholar]
- 52.Hulsken J, Birchmeier W, Behrens J. E-cadherin and APC compete for the interaction with beta-catenin and the cytoskeleton. J Cell Biol. 1994;127(6 Pt 2):2061–9. doi: 10.1083/jcb.127.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Juanes MA, Bouguenina H, Eskin JA, Jaiswal R, Badache A, Goode BL. Adenomatous polyposis coli nucleates actin assembly to drive cell migration and microtubule-induced focal adhesion turnover. J Cell Biol. 2017;216(9):2859–75. doi: 10.1083/jcb.201702007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salahshor S, Woodgett JR. The links between axin and carcinogenesis. J Clin Pathol. 2005;58(3):225–36. doi: 10.1136/jcp.2003.009506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Machin P, Catasus L, Pons C, Munoz J, Matias-Guiu X, Prat J. CTNNB1 mutations and beta-catenin expression in endometrial carcinomas. Hum Pathol. 2002;33(2):206–12. doi: 10.1053/hupa.2002.30723. [DOI] [PubMed] [Google Scholar]
- 56.Cieply B, Zeng G, Proverbs-Singh T, Geller DA, Monga SP. Unique phenotype of hepatocellular cancers with exon-3 mutations in beta-catenin gene. Hepatology. 2009;49(3):821–31. doi: 10.1002/hep.22695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jung Y-S, Park J-I. Wnt signaling in cancer: therapeutic targeting of Wnt signaling beyond β-catenin and the destruction complex. Experimental & Molecular Medicine. 2020:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lobel MK, Somasundaram P, Morton CC. The genetic heterogeneity of uterine leiomyomata. Obstet Gynecol Clin North Am. 2006;33(1):13–39. doi: 10.1016/j.ogc.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 59.Linder D, Gartler SM. Glucose-6-phosphate dehydrogenase mosaicism: utilization as a cell marker in the study of leiomyomas. Science. 1965;150(3692):67–9. [DOI] [PubMed] [Google Scholar]
- 60.Al-Hendy A, Laknaur A, Diamond MP, Ismail N, Boyer TG, Halder SK. Silencing Med12 gene reduces proliferation of human leiomyoma cells mediated via Wnt/β-catenin signaling pathway. Endocrinology. 2017;158(3):592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bulun SE. Uterine fibroids. New England Journal of Medicine. 2013;369(14):1344–55. [DOI] [PubMed] [Google Scholar]
- 62.Zaitseva M, Holdsworth-Carson SJ, Waldrip L, Nevzorova J, Martelotto L, Vollenhoven BJ et al. Aberrant expression and regulation of NR2F2 and CTNNB1 in uterine fibroids. Reproduction. 2013;146(2):91–102. doi: 10.1530/REP-13-0087. [DOI] [PubMed] [Google Scholar]
- 63.Ono M, Yin P, Navarro A, Moravek MB, Coon VJ, Druschitz SA et al. Inhibition of canonical WNT signaling attenuates human leiomyoma cell growth. Fertil Steril. 2014;101(5):1441–9. doi: 10.1016/j.fertnstert.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tai CT, Lin WC, Chang WC, Chiu TH, Chen GT. Classical cadherin and catenin expression in normal myometrial tissues and uterine leiomyomas. Mol Reprod Dev. 2003;64(2):172–8. doi: 10.1002/mrd.10248. [DOI] [PubMed] [Google Scholar]
- 65.Markowski DN, Bartnitzke S, Loning T, Drieschner N, Helmke BM, Bullerdiek J. MED12 mutations in uterine fibroids--their relationship to cytogenetic subgroups. Int J Cancer. 2012;131(7):1528–36. doi: 10.1002/ijc.27424. [DOI] [PubMed] [Google Scholar]
- 66.Mangioni S, Viganò P, Lattuada D, Abbiati A, Vignali M, Di Blasio AM. Overexpression of the Wnt5b gene in leiomyoma cells: implications for a role of the Wnt signaling pathway in the uterine benign tumor. The Journal of Clinical Endocrinology & Metabolism. 2005;90(9):5349–55. [DOI] [PubMed] [Google Scholar]
- 67.Fukuhara K, Kariya M, Kita M, Shime H, Kanamori T, Kosaka C et al. Secreted frizzled related protein 1 is overexpressed in uterine leiomyomas, associated with a high estrogenic environment and unrelated to proliferative activity. The Journal of Clinical Endocrinology & Metabolism. 2002;87(4):1729–36. [DOI] [PubMed] [Google Scholar]
- 68.El Sabeh M, Afrin S, Singh B, Miyashita-Ishiwata M, Borahay M. Uterine Stem Cells and Benign Gynecological Disorders: Role in Pathobiology and Therapeutic Implications. Stem Cell Rev Rep. 2020. doi: 10.1007/s12015-020-10075-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mas A, Cervello I, Fernandez-Alvarez A, Faus A, Diaz A, Burgues O et al. Overexpression of the truncated form of High Mobility Group A proteins (HMGA2) in human myometrial cells induces leiomyoma-like tissue formation. Mol Hum Reprod. 2015;21(4):330–8. doi: 10.1093/molehr/gau114. [DOI] [PubMed] [Google Scholar]
- 70.Orciani M, Caffarini M, Biagini A, Lucarini G, Delli Carpini G, Berretta A et al. Chronic Inflammation May Enhance Leiomyoma Development by the Involvement of Progenitor Cells. Stem Cells Int. 2018;2018:1716246. doi: 10.1155/2018/1716246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu S, Yin P, Dotts AJ, Kujawa SA, Coon VJ, Wei JJ et al. Activation of protein kinase B by WNT4 as a regulator of uterine leiomyoma stem cell function. Fertil Steril. 2020. doi: 10.1016/j.fertnstert.2020.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tanwar PS, Lee H-J, Zhang L, Zukerberg LR, Taketo MM, Rueda BR et al. Constitutive activation of Beta-catenin in uterine stroma and smooth muscle leads to the development of mesenchymal tumors in mice. Biology of reproduction. 2009;81(3):545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arango NA, Szotek PP, Manganaro TF, Oliva E, Donahoe PK, Teixeira J. Conditional deletion of beta-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev Biol. 2005;288(1):276–83. doi: 10.1016/j.ydbio.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 74.Chen HY, Huang TC, Lin LC, Shieh TM, Wu CH, Wang KL et al. Fucoidan Inhibits the Proliferation of Leiomyoma Cells and Decreases Extracellular Matrix-Associated Protein Expression. Cell Physiol Biochem. 2018;49(5):1970–86. doi: 10.1159/000493660. [DOI] [PubMed] [Google Scholar]
- 75.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D’Amico M, Pestell R et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999;96(10):5522–7. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yochum GS, Sherrick CM, Macpartlin M, Goodman RH. A beta-catenin/TCF-coordinated chromatin loop at MYC integrates 5’ and 3’ Wnt responsive enhancers. Proc Natl Acad Sci U S A. 2010;107(1):145–50. doi: 10.1073/pnas.0912294107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guo L, Yilamu D, Sun L, Liu S, Ma F. Association among the expression of beta-catenin, cyclin D1 and estrogen receptor-beta in human breast cancer. Exp Ther Med. 2015;10(4):1423–8. doi: 10.3892/etm.2015.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rennoll S, Yochum G. Regulation of MYC gene expression by aberrant Wnt/beta-catenin signaling in colorectal cancer. World J Biol Chem. 2015;6(4):290–300. doi: 10.4331/wjbc.v6.i4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vadlakonda L, Pasupuleti M, Pallu R. Role of PI3K-AKT-mTOR and Wnt Signaling Pathways in Transition of G1-S Phase of Cell Cycle in Cancer Cells. Front Oncol. 2013;3:85. doi: 10.3389/fonc.2013.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu Z, Robitaille AM, Berndt JD, Davidson KC, Fischer KA, Mathieu J et al. Wnt/beta-catenin signaling promotes self-renewal and inhibits the primed state transition in naive human embryonic stem cells. Proc Natl Acad Sci U S A. 2016;113(42):E6382–E90. doi: 10.1073/pnas.1613849113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mäkinen N, Mehine M, Tolvanen J, Kaasinen E, Li Y, Lehtonen HJ et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science. 2011;334(6053):252–5. [DOI] [PubMed] [Google Scholar]
- 82.Kampjarvi K, Park MJ, Mehine M, Kim NH, Clark AD, Butzow R et al. Mutations in Exon 1 highlight the role of MED12 in uterine leiomyomas. Hum Mutat. 2014;35(9):1136–41. doi: 10.1002/humu.22612. [DOI] [PubMed] [Google Scholar]
- 83.Kim S, Xu X, Hecht A, Boyer TG. Mediator is a transducer of Wnt/β-catenin signaling. Journal of Biological Chemistry. 2006;281(20):14066–75. [DOI] [PubMed] [Google Scholar]
- 84.Rocha PP, Scholze M, Bleiß W, Schrewe H. Med12 is essential for early mouse development and for canonical Wnt and Wnt/PCP signaling. Development. 2010;137(16):2723–31. [DOI] [PubMed] [Google Scholar]
- 85.El Andaloussi A, Al-Hendy A, Ismail N, Boyer TG, Halder SK. Introduction of Somatic Mutation in MED12 Induces Wnt4/beta-Catenin and Disrupts Autophagy in Human Uterine Myometrial Cell. Reprod Sci. 2020;27(3):823–32. doi: 10.1007/s43032-019-00084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Corachan A, Trejo MG, Carbajo-Garcia MC, Monleon J, Escrig J, Faus A et al. Vitamin D as an effective treatment in human uterine leiomyomas independent of mediator complex subunit 12 mutation. Fertil Steril. 2020. doi: 10.1016/j.fertnstert.2020.07.049. [DOI] [PubMed] [Google Scholar]
- 87.Mehine M, Kaasinen E, Heinonen HR, Makinen N, Kampjarvi K, Sarvilinna N et al. Integrated data analysis reveals uterine leiomyoma subtypes with distinct driver pathways and biomarkers. Proc Natl Acad Sci U S A. 2016;113(5):1315–20. doi: 10.1073/pnas.1518752113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol. 2009;10(1):34–43. doi: 10.1038/nrm2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hoffman BD, Grashoff C, Schwartz MA. Dynamic molecular processes mediate cellular mechanotransduction. Nature. 2011;475(7356):316–23. doi: 10.1038/nature10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gillespie PG, Walker RG. Molecular basis of mechanosensory transduction. Nature. 2001;413(6852):194–202. doi: 10.1038/35093011. [DOI] [PubMed] [Google Scholar]
- 91.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260(5111):1124–7. [DOI] [PubMed] [Google Scholar]
- 92.Broders-Bondon F, Nguyen Ho-Bouldoires TH, Fernandez-Sanchez ME, Farge E. Mechanotransduction in tumor progression: The dark side of the force. J Cell Biol. 2018;217(5):1571–87. doi: 10.1083/jcb.201701039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang J, Zhou Y, Tang PMK, Cheng ASL, Yu J, To KF et al. Mechanotransduction and Cytoskeleton Remodeling Shaping YAP1 in Gastric Tumorigenesis. Int J Mol Sci. 2019;20(7). doi: 10.3390/ijms20071576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mammoto T, Ingber DE. Mechanical control of tissue and organ development. Development. 2010;137(9):1407–20. doi: 10.1242/dev.024166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pukhlyakova E, Aman AJ, Elsayad K, Technau U. β-Catenin–dependent mechanotransduction dates back to the common ancestor of Cnidaria and Bilateria. Proceedings of the National Academy of Sciences. 2018;115(24):6231–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brunet T, Bouclet A, Ahmadi P, Mitrossilis D, Driquez B, Brunet AC et al. Evolutionary conservation of early mesoderm specification by mechanotransduction in Bilateria. Nat Commun. 2013;4:2821. doi: 10.1038/ncomms3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Warboys CM. Mechanoactivation of Wnt/β-catenin pathways in health and disease. Emerging Topics in Life Sciences. 2018;2(5):701–12. [DOI] [PubMed] [Google Scholar]
- 98.Samuel MS, Lopez JI, McGhee EJ, Croft DR, Strachan D, Timpson P et al. Actomyosin-mediated cellular tension drives increased tissue stiffness and β-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer cell. 2011;19(6):776–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mouw JK, Yui Y, Damiano L, Bainer RO, Lakins JN, Acerbi I et al. Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. Nat Med. 2014;20(4):360–7. doi: 10.1038/nm.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Benham-Pyle BW, Pruitt BL, Nelson WJ. Mechanical strain induces E-cadherin–dependent Yap1 and β-catenin activation to drive cell cycle entry. Science. 2015;348(6238):1024–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Röper J-C, Mitrossilis D, Stirnemann G, Waharte F, Brito I, Fernandez-Sanchez M-E et al. The major β-catenin/E-cadherin junctional binding site is a primary molecular mechano-transductor of differentiation in vivo. Elife. 2018;7:e33381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fernandez-Sanchez ME, Barbier S, Whitehead J, Béalle G, Michel A, Latorre-Ossa H et al. Mechanical induction of the tumorigenic β-catenin pathway by tumour growth pressure. Nature. 2015;523(7558):92–5. [DOI] [PubMed] [Google Scholar]
- 103.Du J, Zu Y, Li J, Du S, Xu Y, Zhang L et al. Extracellular matrix stiffness dictates Wnt expression through integrin pathway. Scientific reports. 2016;6:20395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Malik M, Segars J, Catherino WH. Integrin beta1 regulates leiomyoma cytoskeletal integrity and growth. Matrix Biol. 2012;31(7–8):389–97. doi: 10.1016/j.matbio.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Leppert PC, Catherino WH, Segars JH. A new hypothesis about the origin of uterine fibroids based on gene expression profiling with microarrays. Am J Obstet Gynecol. 2006;195(2):415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Baarsma HA, Menzen MH, Halayko AJ, Meurs H, Kerstjens HA, Gosens R. β-Catenin signaling is required for TGF-β1-induced extracellular matrix production by airway smooth muscle cells. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2011;301(6):L956–L65. [DOI] [PubMed] [Google Scholar]
- 107.Arici A, Sozen I. Transforming growth factor-beta3 is expressed at high levels in leiomyoma where it stimulates fibronectin expression and cell proliferation. Fertil Steril. 2000;73(5):1006–11. doi: 10.1016/s0015-0282(00)00418-0. [DOI] [PubMed] [Google Scholar]
- 108.Catherino WH, Leppert PC, Stenmark MH, Payson M, Potlog‐Nahari C, Nieman LK et al. Reduced dermatopontin expression is a molecular link between uterine leiomyomas and keloids. Genes, Chromosomes and Cancer. 2004;40(3):204–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fuentes N, Silveyra P. Estrogen receptor signaling mechanisms. Advances in protein chemistry and structural biology. 2019;116:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Borahay MA, Asoglu MR, Mas A, Adam S, Kilic GS, Al-Hendy A. Estrogen Receptors and Signaling in Fibroids: Role in Pathobiology and Therapeutic Implications. Reprod Sci. 2017;24(9):1235–44. doi: 10.1177/1933719116678686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sant’Anna GDS, Brum IS, Branchini G, Pizzolato LS, Capp E, Corleta HVE. Ovarian steroid hormones modulate the expression of progesterone receptors and histone acetylation patterns in uterine leiomyoma cells. Gynecol Endocrinol. 2017;33(8):629–33. doi: 10.1080/09513590.2017.1301924. [DOI] [PubMed] [Google Scholar]
- 112.Hou X, Tan Y, Li M, Dey SK, Das SK. Canonical Wnt signaling is critical to estrogen-mediated uterine growth. Molecular endocrinology. 2004;18(12):3035–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang L, Xiong W, Xiong Y, Liu H, Liu Y. 17 β-Estradiol promotes vascular endothelial growth factor expression via the Wnt/β-catenin pathway during the pathogenesis of endometriosis. Mhr: Basic science of reproductive medicine. 2016;22(7):526–35. [DOI] [PubMed] [Google Scholar]
- 114.Ishikawa H, Ishi K, Serna VA, Kakazu R, Bulun SE, Kurita T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology. 2010;151(6):2433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lange CA. Integration of progesterone receptor action with rapid signaling events in breast cancer models. J Steroid Biochem Mol Biol. 2008;108(3–5):203–12. doi: 10.1016/j.jsbmb.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL et al. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Molecular cell. 2001;8(2):269–80. [DOI] [PubMed] [Google Scholar]
- 117.Brisken C, Heineman A, Chavarria T, Elenbaas B, Tan J, Dey SK et al. Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev. 2000;14(6):650–4. [PMC free article] [PubMed] [Google Scholar]
- 118.Rider V, Talbott A, Bhusri A, Krumsick Z, Foster S, Wormington J et al. WINGLESS (WNT) signaling is a progesterone target for rat uterine stromal cell proliferation. J Endocrinol. 2016;229(2):197–207. doi: 10.1530/JOE-15-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tulac S, Overgaard MT, Hamilton AE, Jumbe NL, Suchanek E, Giudice LC. Dickkopf-1, an inhibitor of Wnt signaling, is regulated by progesterone in human endometrial stromal cells. J Clin Endocrinol Metab. 2006;91(4):1453–61. doi: 10.1210/jc.2005-0769. [DOI] [PubMed] [Google Scholar]
- 120.Franco HL, Dai D, Lee KY, Rubel CA, Roop D, Boerboom D et al. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. The FASEB Journal. 2011;25(4):1176–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Massague J TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13(10):616–30. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Derynck R, Budi EH. Specificity, versatility, and control of TGF-beta family signaling. Sci Signal. 2019;12(570). doi: 10.1126/scisignal.aav5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee BS, Nowak RA. Human leiomyoma smooth muscle cells show increased expression of transforming growth factor-beta 3 (TGF beta 3) and altered responses to the antiproliferative effects of TGF beta. J Clin Endocrinol Metab. 2001;86(2):913–20. [DOI] [PubMed] [Google Scholar]
- 124.Joseph DS, Malik M, Nurudeen S, Catherino WH. Myometrial cells undergo fibrotic transformation under the influence of transforming growth factor beta-3. Fertil Steril. 2010;93(5):1500–8. doi: 10.1016/j.fertnstert.2009.01.081. [DOI] [PubMed] [Google Scholar]
- 125.Attisano L, Labbe E. TGFbeta and Wnt pathway cross-talk. Cancer Metastasis Rev. 2004;23(1–2):53–61. doi: 10.1023/a:1025811012690. [DOI] [PubMed] [Google Scholar]
- 126.Yu JS, Cui W. Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development. 2016;143(17):3050–60. doi: 10.1242/dev.137075. [DOI] [PubMed] [Google Scholar]
- 127.Hoekstra AV, Sefton EC, Berry E, Lu Z, Hardt J, Marsh E et al. Progestins activate the AKT pathway in leiomyoma cells and promote survival. J Clin Endocrinol Metab. 2009;94(5):1768–74. doi: 10.1210/jc.2008-2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Karra L, Shushan A, Ben-Meir A, Rojansky N, Klein BY, Shveiky D et al. Changes related to phosphatidylinositol 3-kinase/Akt signaling in leiomyomas: possible involvement of glycogen synthase kinase 3α and cyclin D2 in the pathophysiology. Fertility and sterility. 2010;93(8):2646–51. [DOI] [PubMed] [Google Scholar]
- 129.Sefton EC, Qiang W, Serna V, Kurita T, Wei JJ, Chakravarti D et al. MK-2206, an AKT inhibitor, promotes caspase-independent cell death and inhibits leiomyoma growth. Endocrinology. 2013;154(11):4046–57. doi: 10.1210/en.2013-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xu X, Lu Z, Qiang W, Vidimar V, Kong B, Kim JJ et al. Inactivation of AKT induces cellular senescence in uterine leiomyoma. Endocrinology. 2014;155(4):1510–9. doi: 10.1210/en.2013-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vidimar V, Chakravarti D, Bulun SE, Yin P, Nowak R, Wei JJ et al. The AKT/BCL-2 Axis Mediates Survival of Uterine Leiomyoma in a Novel 3D Spheroid Model. Endocrinology. 2018;159(3):1453–62. doi: 10.1210/en.2017-03191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xie J, Ubango J, Ban Y, Chakravarti D, Kim JJ, Wei JJ. Comparative analysis of AKT and the related biomarkers in uterine leiomyomas with MED12, HMGA2, and FH mutations. Genes Chromosomes Cancer. 2018;57(10):485–94. doi: 10.1002/gcc.22643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Crabtree JS, Jelinsky SA, Harris HA, Choe SE, Cotreau MM, Kimberland ML et al. Comparison of human and rat uterine leiomyomata: identification of a dysregulated mammalian target of rapamycin pathway. Cancer Res. 2009;69(15):6171–8. [DOI] [PubMed] [Google Scholar]
- 134.Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H et al. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282(15):11221–9. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fukumoto S, Hsieh CM, Maemura K, Layne MD, Yet SF, Lee KH et al. Akt participation in the Wnt signaling pathway through Dishevelled. J Biol Chem. 2001;276(20):17479–83. doi: 10.1074/jbc.C000880200. [DOI] [PubMed] [Google Scholar]
- 136.Al-Hendy A, Diamond MP, Boyer TG, Halder SK. Vitamin D3 inhibits Wnt/β-catenin and mTOR signaling pathways in human uterine fibroid cells. The Journal of Clinical Endocrinology & Metabolism. 2016;101(4):1542–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bashanfer SAAS, Saleem M, Heidenreich O, Moses EJ, Yusoff NM. Disruption of MAPK1 expression in the ERK signalling pathway and the RUNX1‑RUNX1T1 fusion gene attenuate the differentiation and proliferation and induces the growth arrest in t (8; 21) leukaemia cells. Oncology reports. 2019;41(3):2027–40. [DOI] [PubMed] [Google Scholar]
- 138.Kurtzeborn K, Kwon HN, Kuure S. MAPK/ERK Signaling in Regulation of Renal Differentiation. Int J Mol Sci. 2019;20(7). doi: 10.3390/ijms20071779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kolch W Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351 Pt 2:289–305. [PMC free article] [PubMed] [Google Scholar]
- 140.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26(22):3291–310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 141.Yu L, Saile K, Swartz CD, He H, Zheng X, Kissling GE et al. Differential expression of receptor tyrosine kinases (RTKs) and IGF-I pathway activation in human uterine leiomyomas. Molecular medicine. 2008;14(5–6):264–75. doi: 10.2119/2007-00101.Yu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nierth-Simpson EN, Martin MM, Chiang T-C, Melnik LI, Rhodes LV, Muir SE et al. Human uterine smooth muscle and leiomyoma cells differ in their rapid 17β-estradiol signaling: implications for proliferation. Endocrinology. 2009;150(5):2436–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yun MS, Kim SE, Jeon SH, Lee JS, Choi KY. Both ERK and Wnt/beta-catenin pathways are involved in Wnt3a-induced proliferation. J Cell Sci. 2005;118(Pt 2):313–22. doi: 10.1242/jcs.01601. [DOI] [PubMed] [Google Scholar]
- 144.Jeong WJ, Yoon J, Park JC, Lee SH, Lee SH, Kaduwal S et al. Ras stabilization through aberrant activation of Wnt/beta-catenin signaling promotes intestinal tumorigenesis. Sci Signal. 2012;5(219):ra30. doi: 10.1126/scisignal.2002242. [DOI] [PubMed] [Google Scholar]
- 145.Peng L, Wen Y, Han Y, Wei A, Shi G, Mizuguchi M et al. Expression of insulin-like growth factors (IGFs) and IGF signaling: molecular complexity in uterine leiomyomas. Fertil Steril. 2009;91(6):2664–75. [DOI] [PubMed] [Google Scholar]
- 146.Gao Z, Matsuo H, Wang Y, Nakago S, Maruo T. Up-regulation by IGF-I of proliferating cell nuclear antigen and Bcl-2 protein expression in human uterine leiomyoma cells. J Clin Endocrinol Metab. 2001;86(11):5593–9. doi: 10.1210/jcem.86.11.8008. [DOI] [PubMed] [Google Scholar]
- 147.Gkioka E, Msaouel P, Philippou A, Vlaghogiannis NI, Vogkou CT, Margiolis A et al. Review: The Role of Insulin-like Growth Factor-1 Signaling Pathways in Uterine Leiomyoma. In Vivo. 2015;29(6):637–49. [PubMed] [Google Scholar]
- 148.Moravek MB, Yin P, Coon JSt, Ono M, Druschitz SA, Malpani SS et al. Paracrine Pathways in Uterine Leiomyoma Stem Cells Involve Insulinlike Growth Factor 2 and Insulin Receptor A. J Clin Endocrinol Metab. 2017;102(5):1588–95. doi: 10.1210/jc.2016-3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schlupf J, Steinbeisser H. IGF antagonizes the Wnt/β-Catenin pathway and promotes differentiation of extra-embryonic endoderm. Differentiation. 2014;87(5):209–19. [DOI] [PubMed] [Google Scholar]
- 150.Desbois-Mouthon C, Cadoret A, Blivet-Van Eggelpoel MJ, Bertrand F, Cherqui G, Perret C et al. Insulin and IGF-1 stimulate the beta-catenin pathway through two signalling cascades involving GSK-3beta inhibition and Ras activation. Oncogene. 2001;20(2):252–9. doi: 10.1038/sj.onc.1204064. [DOI] [PubMed] [Google Scholar]
- 151.Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13(8):877–83. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pan D The hippo signaling pathway in development and cancer. Dev Cell. 2010;19(4):491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Islam MS, Maher JY, Afrin S, Su S-C, Segars J. Verteporfin inhibits fibrosis, inflammation and angiogenesis related genes in uterine fibroid cells. Fertility and sterility. 2019;112(3):e349. [Google Scholar]
- 154.Piersma B, Bank RA, Boersema M. Signaling in fibrosis: TGF-β, WNT, and YAP/TAZ converge. Frontiers in medicine. 2015;2:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Luo K Signaling cross talk between TGF-β/Smad and other signaling pathways. Cold Spring Harb Perspect Biol. 2017;9(1):a022137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kim M, Jho E-h. Cross-talk between Wnt/β-catenin and Hippo signaling pathways: a brief review. BMB reports. 2014;47(10):540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Varelas X, Miller BW, Sopko R, Song S, Gregorieff A, Fellouse FA et al. The Hippo pathway regulates Wnt/beta-catenin signaling. Dev Cell. 2010;18(4):579–91. doi: 10.1016/j.devcel.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 158.Azzolin L, Zanconato F, Bresolin S, Forcato M, Basso G, Bicciato S et al. Role of TAZ as mediator of Wnt signaling. Cell. 2012;151(7):1443–56. [DOI] [PubMed] [Google Scholar]
- 159.Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S et al. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158(1):157–70. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 160.Egan SE, St-Pierre B, Leow CC. Notch receptors, partners and regulators: from conserved domains to powerful functions. Curr Top Microbiol Immunol. 1998;228:273–324. doi: 10.1007/978-3-642-80481-6_11. [DOI] [PubMed] [Google Scholar]
- 161.Zlobin A, Bloodworth JC, Baker AT, Osipo C. Notch Signaling Pathway in Carcinogenesis. In: Badve S, Kumar GL, editors. Predictive Biomarkers in Oncology: Applications in Precision Medicine. Cham: Springer International Publishing; 2019. p. 223–30. [Google Scholar]
- 162.McIntyre B, Asahara T, Alev C. Overview of Basic Mechanisms of Notch Signaling in Development and Disease. In: Reichrath J, Reichrath S, editors. Notch Signaling in Embryology and Cancer: Molecular Biology of Notch Signaling. Cham: Springer International Publishing; 2020. p. 9–27. [DOI] [PubMed] [Google Scholar]
- 163.Kiyokawa H, Morimoto M. Notch signaling in the mammalian respiratory system, specifically the trachea and lungs, in development, homeostasis, regeneration, and disease. Dev Growth Differ. 2020;62(1):67–79. doi: 10.1111/dgd.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kavian N, Servettaz A, Weill B, Batteux F. Suppl 1: New Insights into the Mechanism of Notch Signalling in Fibrosis. The open rheumatology journal. 2012;6:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gonzalez-Foruria I, Santulli P, Chouzenoux S, Carmona F, Chapron C, Batteux F. Dysregulation of the ADAM17/Notch signalling pathways in endometriosis: from oxidative stress to fibrosis. Mol Hum Reprod. 2017;23(7):488–99. doi: 10.1093/molehr/gax028. [DOI] [PubMed] [Google Scholar]
- 166.Collu GM, Hidalgo-Sastre A, Brennan K. Wnt-Notch signalling crosstalk in development and disease. Cell Mol Life Sci. 2014;71(18):3553–67. doi: 10.1007/s00018-014-1644-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Collu GM, Hidalgo-Sastre A, Acar A, Bayston L, Gildea C, Leverentz MK et al. Dishevelled limits Notch signalling through inhibition of CSL. Development. 2012;139(23):4405–15. doi: 10.1242/dev.081885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kwon C, Cheng P, King IN, Andersen P, Shenje L, Nigam V et al. Notch post-translationally regulates beta-catenin protein in stem and progenitor cells. Nat Cell Biol. 2011;13(10):1244–51. doi: 10.1038/ncb2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liu XH, Wu Y, Yao S, Levine AC, Kirschenbaum A, Collier L et al. Androgens up-regulate transcription of the Notch inhibitor Numb in C2C12 myoblasts via Wnt/beta-catenin signaling to T cell factor elements in the Numb promoter. J Biol Chem. 2013;288(25):17990–8. doi: 10.1074/jbc.M113.478487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Christman GM T H, Ahmad I, Stribley JM, editor. Differential expression of the Notch signal transduction pathway: ligands, receptors and Numb in uterine leiomyomas vs. myometrium. The Society For Reproductive Endocrinology and Infertility; 2007 2007. [Google Scholar]
- 171.Ciebiera M, Lukaszuk K, Meczekalski B, Ciebiera M, Wojtyla C, Slabuszewska-Jozwiak A et al. Alternative Oral Agents in Prophylaxis and Therapy of Uterine Fibroids-An Up-to-Date Review. Int J Mol Sci. 2017;18(12). doi: 10.3390/ijms18122586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Maier T Jr, Janssen A, Schmidt R, Geisslinger G, Grösch S. Targeting the beta-catenin/APC pathway: a novel mechanism to explain the cyclooxygenase-2-independent anticarcinogenic effects of celecoxib in human colon carcinoma cells. The FASEB Journal. 2005;19(10):1353–5. [DOI] [PubMed] [Google Scholar]
- 173.Egashira I, Takahashi‐Yanaga F, Nishida R, Arioka M, Igawa K, Tomooka K et al. Celecoxib and 2, 5‐dimethylcelecoxib inhibit intestinal cancer growth by suppressing the Wnt/β‐catenin signaling pathway. Cancer science. 2017;108(1):108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sareddy GR, Kesanakurti D, Kirti PB, Babu PP. Nonsteroidal anti-inflammatory drugs diclofenac and celecoxib attenuates Wnt/β-catenin/Tcf signaling pathway in human glioblastoma cells. Neurochemical research. 2013;38(11):2313–22. [DOI] [PubMed] [Google Scholar]
- 175.Pendas-Franco N, Aguilera O, Pereira F, González-Sancho JM, Munoz A. Vitamin D and Wnt/β-catenin pathway in colon cancer: role and regulation of DICKKOPF genes. Anticancer Research. 2008;28(5A):2613–23. [PubMed] [Google Scholar]
- 176.Amado NG, Predes D, Fonseca BF, Cerqueira DM, Reis AH, Dudenhoeffer AC et al. Isoquercitrin suppresses colon cancer cell growth in vitro by targeting the Wnt/β-catenin signaling pathway. J Biol Chem. 2014;289(51):35456–67. doi: 10.1074/jbc.M114.621599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lazarian G, Friedrich C, Quinquenel A, Tran J, Ouriemmi S, Dondi E et al. Stabilization of β-catenin upon B-cell receptor signaling promotes NF-kB target genes transcription in mantle cell lymphoma. Oncogene. 2020;39(14):2934–47. [DOI] [PubMed] [Google Scholar]
- 178.Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461(7264):614–20. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 179.James RG, Davidson KC, Bosch KA, Biechele TL, Robin NC, Taylor RJ et al. WIKI4, a novel inhibitor of tankyrase and Wnt/ß-catenin signaling. PloS one. 2012;7(12):e50457–e. doi: 10.1371/journal.pone.0050457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Waaler J, Machon O, Tumova L, Dinh H, Korinek V, Wilson SR et al. A Novel Tankyrase Inhibitor Decreases Canonical Wnt Signaling in Colon Carcinoma Cells and Reduces Tumor Growth in Conditional APC Mutant Mice. Cancer Res. 2012;72(11):2822–32. doi: 10.1158/0008-5472.can-11-3336. [DOI] [PubMed] [Google Scholar]
- 181.Madan B, Ke Z, Harmston N, Ho SY, Frois AO, Alam J et al. Wnt addiction of genetically defined cancers reversed by PORCN inhibition. Oncogene. 2016;35(17):2197–207. doi: 10.1038/onc.2015.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cheng D, Liu J, Han D, Zhang G, Gao W, Hsieh MH et al. Discovery of pyridinyl acetamide derivatives as potent, selective, and orally bioavailable porcupine inhibitors. ACS medicinal chemistry letters. 2016;7(7):676–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kothari V, Goodwin JF, Zhao SG, Drake JM, Yin Y, Chang SL et al. DNA-dependent protein kinase drives prostate cancer progression through transcriptional regulation of the wnt signaling pathway. Clinical Cancer Research. 2019;25(18):5608–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wellenstein MD, Coffelt SB, Duits DEM, van Miltenburg MH, Slagter M, de Rink I et al. Loss of p53 triggers WNT-dependent systemic inflammation to drive breast cancer metastasis. Nature. 2019;572(7770):538–42. doi: 10.1038/s41586-019-1450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Proffitt KD, Madan B, Ke Z, Pendharkar V, Ding L, Lee MA et al. Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT-driven mammary cancer. Cancer Res. 2013;73(2):502–7. doi: 10.1158/0008-5472.CAN-12-2258. [DOI] [PubMed] [Google Scholar]
- 186.Jiang X, Hao HX, Growney JD, Woolfenden S, Bottiglio C, Ng N et al. Inactivating mutations of RNF43 confer Wnt dependency in pancreatic ductal adenocarcinoma. Proc Natl Acad Sci U S A. 2013;110(31):12649–54. doi: 10.1073/pnas.1307218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Liu J, Pan S, Hsieh MH, Ng N, Sun F, Wang T et al. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc Natl Acad Sci U S A. 2013;110(50):20224–9. doi: 10.1073/pnas.1314239110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.You L, Zhang C, Yarravarapu N, Morlock L, Wang X, Zhang L et al. Development of a triazole class of highly potent Porcn inhibitors. Bioorganic & medicinal chemistry letters. 2016;26(24):5891–5. doi: 10.1016/j.bmcl.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan C-W et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nature chemical biology. 2009;5(2):100–7. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M et al. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected]. Proc Natl Acad Sci U S A. 2004;101(34):12682–7. doi: 10.1073/pnas.0404875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Waaler J, Machon O, von Kries JP, Wilson SR, Lundenes E, Wedlich D et al. Novel Synthetic Antagonists of Canonical Wnt Signaling Inhibit Colorectal Cancer Cell Growth. Cancer Res. 2011;71(1):197–205. doi: 10.1158/0008-5472.can-10-1282. [DOI] [PubMed] [Google Scholar]
- 192.Cha P-H, Cho Y-H, Lee S-K, Lee J, Jeong W-J, Moon B-S et al. Small-molecule binding of the axin RGS domain promotes β-catenin and Ras degradation. Nature chemical biology. 2016;12(8):593–600. doi: 10.1038/nchembio.2103. [DOI] [PubMed] [Google Scholar]
- 193.Cook J, Walker C. The Eker rat: establishing a genetic paradigm linking renal cell carcinoma and uterine leiomyoma. Current molecular medicine. 2004;4(8):813–24. [DOI] [PubMed] [Google Scholar]
- 194.Sheng R, Kim H, Lee H, Xin Y, Chen Y, Tian W et al. Cholesterol selectively activates canonical Wnt signalling over non-canonical Wnt signalling. Nat Commun. 2014;5:4393. doi: 10.1038/ncomms5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wong HC, Mao J, Nguyen JT, Srinivas S, Zhang W, Liu B et al. Structural basis of the recognition of the dishevelled DEP domain in the Wnt signaling pathway. Nat Struct Biol. 2000;7(12):1178–84. doi: 10.1038/82047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tauriello DV, Jordens I, Kirchner K, Slootstra JW, Kruitwagen T, Bouwman BA et al. Wnt/beta-catenin signaling requires interaction of the Dishevelled DEP domain and C terminus with a discontinuous motif in Frizzled. Proc Natl Acad Sci U S A. 2012;109(14):E812–20. doi: 10.1073/pnas.1114802109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Miyakoshi T, Kajiya H, Miyajima K, Takei M, Tobita M, Takekoshi S et al. The Expression of Wnt4 Is Regulated by Estrogen via an Estrogen Receptor Alpha-dependent Pathway in Rat Pituitary Growth Hormone-producing Cells. ACTA HISTOCHEMICA ET CYTOCHEMICA. 2009;42(6):205–13. doi: 10.1267/ahc.09033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Malik M, Britten J, Borahay M, Segars J, Catherino WH. Simvastatin, at clinically relevant concentrations, affects human uterine leiomyoma growth and extracellular matrix production. Fertility and sterility. 2018;110(7):1398–407. e1. [DOI] [PubMed] [Google Scholar]
- 199.Malik M, Catherino W, Laknaur A, Ali M, Al-Hendy A, Segars J et al. Synergistic effects of simvastatin and ulipristal acetate on uterine leiomyoma. Fertility and sterility. 2017;108(3):e65. [Google Scholar]
- 200.Borahay MA, Vincent K, Motamedi M, Sbrana E, Kilic GS, Al-Hendy A et al. Novel effects of simvastatin on uterine fibroid tumors: in vitro and patient-derived xenograft mouse model study. Am J Obstet Gynecol. 2015;213(2):196 e1–8. doi: 10.1016/j.ajog.2015.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Borahay MA, Kilic GS, Yallampalli C, Snyder RR, Hankins GD, Al-Hendy A et al. Simvastatin potently induces calcium-dependent apoptosis of human leiomyoma cells. J Biol Chem. 2014;289(51):35075–86. doi: 10.1074/jbc.M114.583575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Afrin S, Islam MS, Patzkowsky K, Malik M, Catherino WH, Segars JH et al. Simvastatin ameliorates altered mechanotransduction in uterine leiomyoma cells. Am J Obstet Gynecol. 2020. doi: 10.1016/j.ajog.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kadowaki T, Wilder E, Klingensmith J, Zachary K, Perrimon N. The segment polarity gene porcupine encodes a putative multitransmembrane protein involved in Wingless processing. Genes Dev. 1996;10(24):3116–28. doi: 10.1101/gad.10.24.3116. [DOI] [PubMed] [Google Scholar]
- 204.Kim YM, Kahn M. The role of the Wnt signaling pathway in cancer stem cells: prospects for drug development. Res Rep Biochem. 2014;4:1–12. doi: 10.2147/RRBC.S53823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Liu J, Pan S, Hsieh MH, Ng N, Sun F, Wang T et al. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proceedings of the National Academy of Sciences. 2013;110(50):20224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mullighan CG, Zhang J, Kasper LH, Lerach S, Payne-Turner D, Phillips LA et al. CREBBP mutations in relapsed acute lymphoblastic leukaemia. Nature. 2011;471(7337):235–9. doi: 10.1038/nature09727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wang X, Moon J, Dodge ME, Pan X, Zhang L, Hanson JM et al. The development of highly potent inhibitors for porcupine. J Med Chem. 2013;56(6):2700–4. doi: 10.1021/jm400159c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Fischer MM, Cancilla B, Yeung VP, Cattaruzza F, Chartier C, Murriel CL et al. WNT antagonists exhibit unique combinatorial antitumor activity with taxanes by potentiating mitotic cell death. Sci Adv. 2017;3(6):e1700090. doi: 10.1126/sciadv.1700090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Jimeno A, Gordon M, Chugh R, Messersmith W, Mendelson D, Dupont J et al. A First-in-Human Phase I Study of the Anticancer Stem Cell Agent Ipafricept (OMP-54F28), a Decoy Receptor for Wnt Ligands, in Patients with Advanced Solid Tumors. Clin Cancer Res. 2017;23(24):7490–7. doi: 10.1158/1078-0432.CCR-17-2157. [DOI] [PubMed] [Google Scholar]
- 210.Moore KN, Gunderson CC, Sabbatini P, McMeekin DS, Mantia-Smaldone G, Burger RA et al. A phase 1b dose escalation study of ipafricept (OMP54F28) in combination with paclitaxel and carboplatin in patients with recurrent platinum-sensitive ovarian cancer. Gynecol Oncol. 2019;154(2):294–301. doi: 10.1016/j.ygyno.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Giraudet AL, Cassier PA, Iwao-Fukukawa C, Garin G, Badel JN, Kryza D et al. A first-in-human study investigating biodistribution, safety and recommended dose of a new radiolabeled MAb targeting FZD10 in metastatic synovial sarcoma patients. BMC Cancer. 2018;18(1):646. doi: 10.1186/s12885-018-4544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Takemaru KI, Moon RT. The transcriptional coactivator CBP interacts with beta-catenin to activate gene expression. J Cell Biol. 2000;149(2):249–54. doi: 10.1083/jcb.149.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Cortes JE, Faderl S, Pagel J, Jung CW, Yoon S-S, Koh Y et al. Phase 1 study of CWP232291 in relapsed/refractory acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). American Society of Clinical Oncology; 2015. [Google Scholar]
- 214.Manasanch EE, Yoon S-S, Min C-K, Kim JS, Shain KH, Hauptschein R et al. Interim results from the phase 1a/1b dose-finding study of CWP232291 (CWP291) in relapsed or refractory myeloma (RRMM) alone or in combination with Lenalidomide and dexamethasone. Blood. 2017;130(Supplement 1):3091-. [Google Scholar]
- 215.Yoon S-S, Min C-K, Kim JS, Manasanch EE, Hauptschein R, Choi J et al. Ongoing phase 1a/1b dose-finding study of CWP232291 (CWP291) in relapsed or refractory multiple myeloma (MM). American Society of Hematology Washington, DC; 2016. [Google Scholar]
- 216.Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159(1):80–93. doi: 10.1016/j.cell.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Larriba MJ, Gonzalez-Sancho JM, Barbachano A, Niell N, Ferrer-Mayorga G, Munoz A. Vitamin D Is a Multilevel Repressor of Wnt/b-Catenin Signaling in Cancer Cells. Cancers (Basel). 2013;5(4):1242–60. doi: 10.3390/cancers5041242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Patel BB, Sengupta R, Qazi S, Vachhani H, Yu Y, Rishi AK et al. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int J Cancer. 2008;122(2):267–73. doi: 10.1002/ijc.23097. [DOI] [PubMed] [Google Scholar]
- 219.Lev-Ari S, Strier L, Kazanov D, Madar-Shapiro L, Dvory-Sobol H, Pinchuk I et al. Celecoxib and curcumin synergistically inhibit the growth of colorectal cancer cells. Clin Cancer Res. 2005;11(18):6738–44. doi: 10.1158/1078-0432.CCR-05-0171. [DOI] [PubMed] [Google Scholar]
- 220.Patel KR, Brown VA, Jones DJ, Britton RG, Hemingway D, Miller AS et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010;70(19):7392–9. doi: 10.1158/0008-5472.CAN-10-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Holcombe RF, Martinez M, Planutis K, Planutiene M. Effects of a grape-supplemented diet on proliferation and Wnt signaling in the colonic mucosa are greatest for those over age 50 and with high arginine consumption. Nutrition journal. 2015;14(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Dietrich L, Rathmer B, Ewan K, Bange T, Heinrichs S, Dale TC et al. Cell Permeable Stapled Peptide Inhibitor of Wnt Signaling that Targets β-Catenin Protein-Protein Interactions. Cell Chemical Biology. 2017;24(8):958–68.e5. doi: 10.1016/j.chembiol.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 223.Gonsalves FC, Klein K, Carson BB, Katz S, Ekas LA, Evans S et al. An RNAi-based chemical genetic screen identifies three small-molecule inhibitors of the Wnt/wingless signaling pathway. Proc Natl Acad Sci U S A. 2011;108(15):5954–63. doi: 10.1073/pnas.1017496108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Leal LF, Bueno AC, Gomes DC, Abduch R, de Castro M, Antonini SR. Inhibition of the Tcf/beta-catenin complex increases apoptosis and impairs adrenocortical tumor cell proliferation and adrenal steroidogenesis. Oncotarget. 2015;6(40):43016–32. doi: 10.18632/oncotarget.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Fang L, Zhu Q, Neuenschwander M, Specker E, Wulf-Goldenberg A, Weis WI et al. A Small-Molecule Antagonist of the β-Catenin/TCF4 Interaction Blocks the Self-Renewal of Cancer Stem Cells and Suppresses Tumorigenesis. Cancer Res. 2016;76(4):891–901. doi: 10.1158/0008-5472.can-15-1519. [DOI] [PubMed] [Google Scholar]
- 226.Gedaly R, Galuppo R, Daily MF, Shah M, Maynard E, Chen C et al. Targeting the Wnt/β-catenin signaling pathway in liver cancer stem cells and hepatocellular carcinoma cell lines with FH535. PloS one. 2014;9(6):e99272–e. doi: 10.1371/journal.pone.0099272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Masuda M, Uno Y, Ohbayashi N, Ohata H, Mimata A, Kukimoto-Niino M et al. TNIK inhibition abrogates colorectal cancer stemness. Nat Commun. 2016;7(1):12586. doi: 10.1038/ncomms12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ewan K, Pajak B, Stubbs M, Todd H, Barbeau O, Quevedo C et al. A useful approach to identify novel small-molecule inhibitors of Wnt-dependent transcription. Cancer Res. 2010;70(14):5963–73. doi: 10.1158/0008-5472.can-10-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.


