Abstract
Alcohol-associated liver disease (ALD) is emerging worldwide as the leading cause of liver-related morbidity, mortality, and indication for liver transplantation. The ALD Special Interest Group and the Clinical Research Committee at the digital American Association for the Study of Liver Diseases meeting in November 2020 held the scientific sessions to identify clinical unmet needs in ALD, and addressing these needs using clinical research methodologies. Of several research methodologies, the sessions were focused on (a) studying disease burden of ALD using large administrative databases, (b) developing biomarkers for noninvasive diagnosis of alcohol-associated hepatitis (AH) and estimation of disease prognosis, (c) identifying therapeutic targets for ALD and AH, (d) deriving accurate models to predict prognosis or posttransplant alcohol relapse as a basis for developing treatment algorithm and a uniform protocol on patient-selection criteria for liver transplantation, and (e) examining qualitative research methodologies in studying the barriers to implementation of multidisciplinary integrated care model by hepatology and addiction teams for the management of dual pathology of liver disease and of alcohol use disorder. Prospective multicenter studies are required to address many of these clinical unmet needs. Further, multidisciplinary care models are needed to improve long-term outcomes in patients with ALD.
INTRODUCTION
Alcohol-associated liver disease (ALD) is one of the most common liver diseases worldwide, with 2.2 million people in the US affected by alcohol-associated cirrhosis in 2017.[1] Although the research efforts and funding for ALD have increased substantially over the last decade, there still remain several unmet clinical needs.[2] Recently, the ALD Special Interest Group and the Clinical Research Committee of the American Association for Study of Liver Diseases (AASLD) held individual sessions at the digital AASLD meeting in November 2020 to discuss clinical research methodologies to address clinical unmet needs. Although there is a need for animal models mimicking the human phenotype of ALD,[3] this manuscript focuses on clinical research methodologies that can help in addressing unmet needs in patients with ALD.
EPIDEMIOLOGY AND HEALTH CARE BURDEN OF EARLY ALD
ALD is the leading cause of liver-related morbidity and mortality.[1,4] Worldwide, approximately 2.5 billion people consume alcohol, with 300 million having alcohol use disorder (AUD). Approximately 25 million people worldwide have compensated cirrhosis due to alcohol, with 10% having decompensated cirrhosis, including HCC, resulting in 750,000 deaths, which accounts for 1% of all annual deaths.[5] Furthermore, increasing mortality due to ALD is occurring in women and younger people,[6] especially among individuals 25–34 years of age, with annual increase of 10.5% over the past decade.[7] Proportion of liver transplants (LT) performed for ALD, including those with alcohol-associated hepatitis (AH), is also increasing, and ALD is now the leading indication for LT worldwide.[8]
Compared with other liver diseases, ALD often presents at an advanced stage of cirrhosis or its complications. In a study of 3000 patients worldwide with chronic liver disease, only 3.8% of patients with ALD were seen at an early stage (without complications from portal hypertension) compared with 17%–30% for those with NAFLD or viral hepatitis.[9] Furthermore, patients at an early unrecognized subclinical stage of ALD are commonly seen in drug or alcohol addiction clinics, and often not referred to specialists.[9] Thus, strategies for population-level awareness and detection of early-stage ALD are needed.[5] It will be necessary to improve screening for AUD and early ALD using validated accurate tools such as the Alcohol Use Disorder Identification Test on self-reported alcohol use.
Several tools are available to assess the burden of ALD such as observational cohorts derived from single-center and multicenter registries, clinical trials, and large administrative databases. Large databases are advantageous due to their sample size, and less concerns about statistical power (Table 1). Because the administrative databases collect important clinical data including demographics, diagnoses, procedures, service use, and billing on a large scale, they can be leveraged to study epidemiologic trends, disparities, costs, and outcomes on a population level.[10,11] Analysis of data from the US Census Bureau compiled by the Center for Disease Control and Prevention’s Wide-ranging Online Data for Epidemiologic Research revealed an increase in cirrhosis-related mortality driven by ALD. National Health and Nutrition Examination Survey (NHANES), Nationwide Inpatient Sample, and United Network for Organ Sharing (UNOS) registries have been used to show an increasing severity of ALD in the US.[7,12]
TABLE 1.
Large databases that can be leveraged for the study of ALD
| Description | Strengths | Weaknesses | |
|---|---|---|---|
| Organ Procurement and Transplantation Network | US national transplant registry | Longitudinal; includes wait-list and transplant outcomes; low cost | Lacks data on several comorbidities |
| National Health and Nutrition Examination Survey | Survey of US residents | Accurate; includes alcohol use, laboratory, and imaging data (e.g., FIB-4, steatosis) | Selection bias; cross-sectional design |
| Medicare | Health care claims for inpatient, outpatient, and pharmacy services | Beneficiary-level and provider-level data; comprehensive | Expensive; no laboratory data; predominantly older adults age ≥ 65 |
| Optum Clinformatics DataMart | Commercial claims for inpatient, outpatient, and pharmacy services | Longitudinal; clinical use and expenditures; some laboratory data | Expensive; only privately insured; cannot cross over geographic, socioeconomic, and mortality files |
| Truven Marketscan | Claims from commercial and employer health plans, Medicare, and Medicaid | Longitudinal; person-specific clinical use and expenditures | Expensive; claims cannot be aggregated at provider level |
| Veterans Health Administration | Health system | Longitudinal and granular data; annual AUDIT-C | Not representative of US population; can be resource-intensive to access data |
| Nationwide Inpatient Sample | All-payer inpatient claims database; survey of participating hospitals | Low cost; easily accessible | Unable to track patients longitudinally; no laboratory data; no data from veterans |
Abbreviations: AUDIT-C, Alcohol Use Disorders Identification Test–Concise; and FIB-4, Fibrosis-4 Index.
It is challenging to study ALD due to inconsistency in documenting alcohol-use patterns, including type and amount consumed. Socioeconomic factors, education, comorbid psychiatric illness, and co-existing nonalcoholic fatty liver may not be available in large administrative databases. Merging with other databases may provide more detailed information on alcohol use, such as the “National Epidemiologic Survey on Alcohol and Related Conditions.” Stages of fibrosis in ALD may be particularly difficult to capture in databases, compared with clinical trials or single or multicenter prospective studies. Nonetheless, several studies have leveraged large databases to study fibrosis stage as estimated by Fibrosis-4 using aspartate aminotransferase (AST), alanine aminotransferase (ALT), platelet count, and age in the NHANES and describe population level trends on advanced fibrosis among individuals with ALD.[13] Association of AUD treatments with reduced risk of hepatic decompensation and patient mortality has been demonstrated in studies using the Veterans Health Administration and commercial claims database of privately insured individuals, respectively.[14,15]
Data in large administrative databases are retrospective, prone to missing data, and inaccurate on some important variables of interest. For example, only 35% of patients with AH included in the ACCELERATE consortium were coded as “AH” in the UNOS registry.[16] Data from large database studies must also be interpreted in the context of clinical relevance. Use of validated diagnostic coding algorithms reduces misclassification bias in these claims-based databases.[17]
These limitations can be partly addressed with adequate domain knowledge and using well-characterized cohorts. Statistical knowledge and collaborative relationship with a biostatistician are needed to ensure appropriate selection of analytic procedures, handling the missing data, correction for multiple testing, and interpretation of results. Propensity score or instrumental variable analysis may help to overcome confounding and selection bias. Machine learning methods such as shrinkage, random forest, and neural networks can handle many variables and account for complex and nonlinear interactions.
NONINVASIVE BIOMARKERS FOR THE MANAGEMENT OF ALD
Although large databases are powerful tools to study epidemiology, there remains a need for accurate biomarkers for disease diagnosis, stratification, and prognosis. Over 50% of patients with AUD have elevations in serum AST and ALT.[18] AUD patients with mild liver enzyme elevation compared to those with normal liver enzymes have greater evidence of gut barrier dysfunction, inflammation, and nutritional changes, potentially leading to progression of ALD.[18] Thus, screening for ALD using aminotransferases combined with novel noninvasive biomarkers for different stages of ALD should be implemented.
The progression to symptomatic AH with jaundice and acute-on-chronic liver failure negatively impacts short-term patient survival.[19] Liver biopsy is the gold standard to diagnose AH, but is not routinely performed in clinical practice.[20,21] In 2016, the National Institute on Alcohol Abuse and Alcoholism (NIAAA)–funded consortia developed standard definitions for AH.[22] Patients are considered to have severe AH if they had a Maddrey discriminant function (DF) score ≥ 32 or a Model for End-Stage Liver Disease (MELD) score > 20. Although MELD and DF can predict short-term mortality,[23] they cannot differentiate AH from decompensated cirrhosis. Moreover, serum AST and ALT levels do not predict severity of liver injury in AH. In a recent study, patients with AUD enrolled in a treatment program with normal serum bilirubin had AST/ALT levels higher than hospitalized patients with severe AH.[24] Clearly, improved biomarkers for AH and ALD are needed.
Keratin 18 (K18) is released into the bloodstream with epithelial cell death.[24–26] During hepatocyte apoptosis, activated caspases cleave K18, and the cleaved K18 (M30) fragment can be detected in plasma by the M30 ELISA, whereas the M65 ELISA detects both caspase-cleaved and uncleaved K18. Thus, K18 ELISAs can quantify hepatocyte death and differentiate necrosis versus apoptosis.[24–26] In patients undergoing liver biopsy for suspected AH,[25] K18 (M65) was found to be the most useful biomarker evaluated, with levels > 2000 IU/L being highly diagnostic for severe AH. Using the same cutoff, another study demonstrated that K18 reflected severity of liver disease and identified patients who died within 90 days with greater accuracy than MELD and DF.[24] All healthy controls had values below the upper limit of normal (500 IU/L) (Figure 1). Some patients with AUD and some patients with moderate AH had levels > 2000 IU/L, suggesting greater liver injury than indicated by liver tests. Finally, a third of patients classified as “severe AH” had levels < 2000 IU/L, suggesting they may have limited ongoing liver injury and inflammation, and may not be optimal candidates for prednisolone. Atkinson et al. also reported that K18 is a diagnostic, prognostic, and theragnostic marker to predict who will benefit from prednisolone therapy.[26]
FIGURE 1.
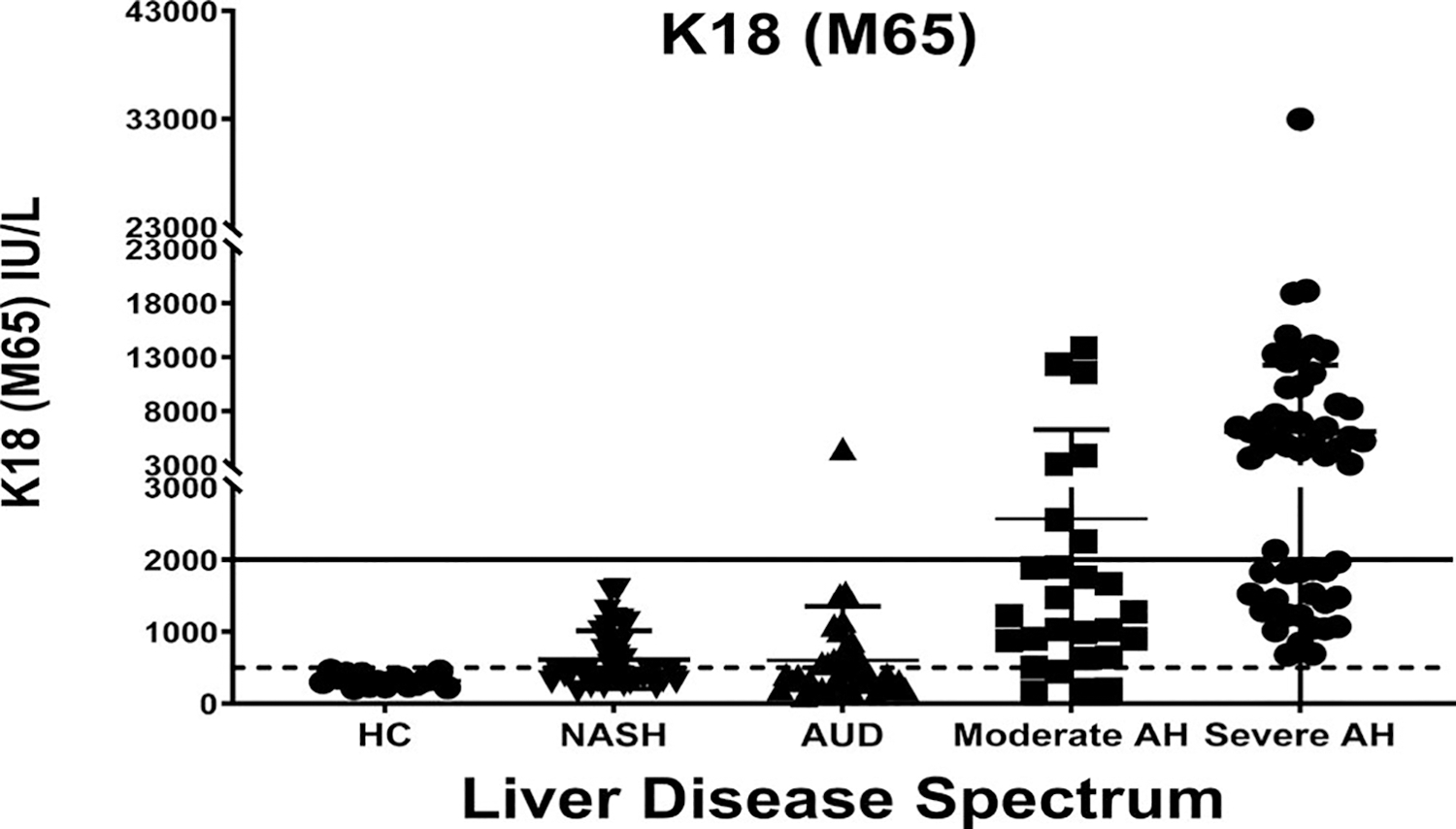
Keratin 18 (K18 or M65) was measured in healthy controls (HC) and in patients with liver disease. Patients with NASH all had liver biopsies with a NAFLD Activity Score ≥ 4; patients with alcohol use disorder (AUD) were in alcohol treatment programs (all had normal serum bilirubin); patients with moderate alcohol-associated hepatitis (AH) were hospitalized with MELD ≤ 20; patients with severe AH were hospitalized with MELD > 20. Solid line (> 2000) represents lower limit for diagnosis of AH, and dotted line (500) represents the upper limit of normal value
Bile acids play an important role in the development of ALD.[27] In a recent study, several urinary bile acids levels were shown to be elevated in patients with AH compared to healthy controls, with area under the receiver operating characteristic curve (AUROC) of > 0.7 for most bile acids and 0.94 for taurochenodeoxycholate. Furthermore, serum bile acids showed a progressive increase from asymptomatic liver disease to increasing Child-Turcotte-Pugh stage of cirrhosis.[28] Biomarkers are also needed to optimize selection of therapeutics based on individual metabolism, and to identify patients at risk for development of infections or acute kidney injury, common causes of patient mortality in patients with ALD and/or AH.[29]
Alcohol abstinence is the most important factor to improve long-term survival.[30] Biomarkers of alcohol consumption are also needed. Breath and blood levels of alcohol or its metabolites such as ethyl glucuronide or ethyl sulphate are accurate for recent alcohol ingestion in the last few days.[31] Phosphatidylethanol, another metabolite of alcohol, can identify alcohol use over the last few weeks.[32] Prospective multicenter collaborative studies are needed to test and validate biomarkers and examine cost-effectiveness to maximize their usability in the paradigm of clinical care of patients with ALD.
EMERGING THERAPEUTIC TARGETS FOR MANAGEMENT OF PATIENTS WITH ALD
Currently, corticosteroid is the only available pharmacological treatment for severe AH, with a modest benefit at 28 days, and no long-term benefit on patient survival.[33] Better understanding of the disease mechanisms has translated into identifying newer therapeutic targets for the treatment of ALD.[34]
Interactions between gut and liver is the major mechanism mediating development of ALD (Figure 2). The metabolism of alcohol in the liver results in oxidative stress due to changes in mitochondrial electron transport,[35] resulting in hepatocyte apoptosis and production of extracellular vesicles.[36] MicroRNAs (miRs) contained in these vesicles mediate steatosis, fibrosis, and neutrophil dysfunction.[37] The dying hepatocytes release damage-associated molecular patterns (DAMPs) such as uric acid, ATP, and HMGB1 (high mobility group box protein 1).[38]
FIGURE 2.
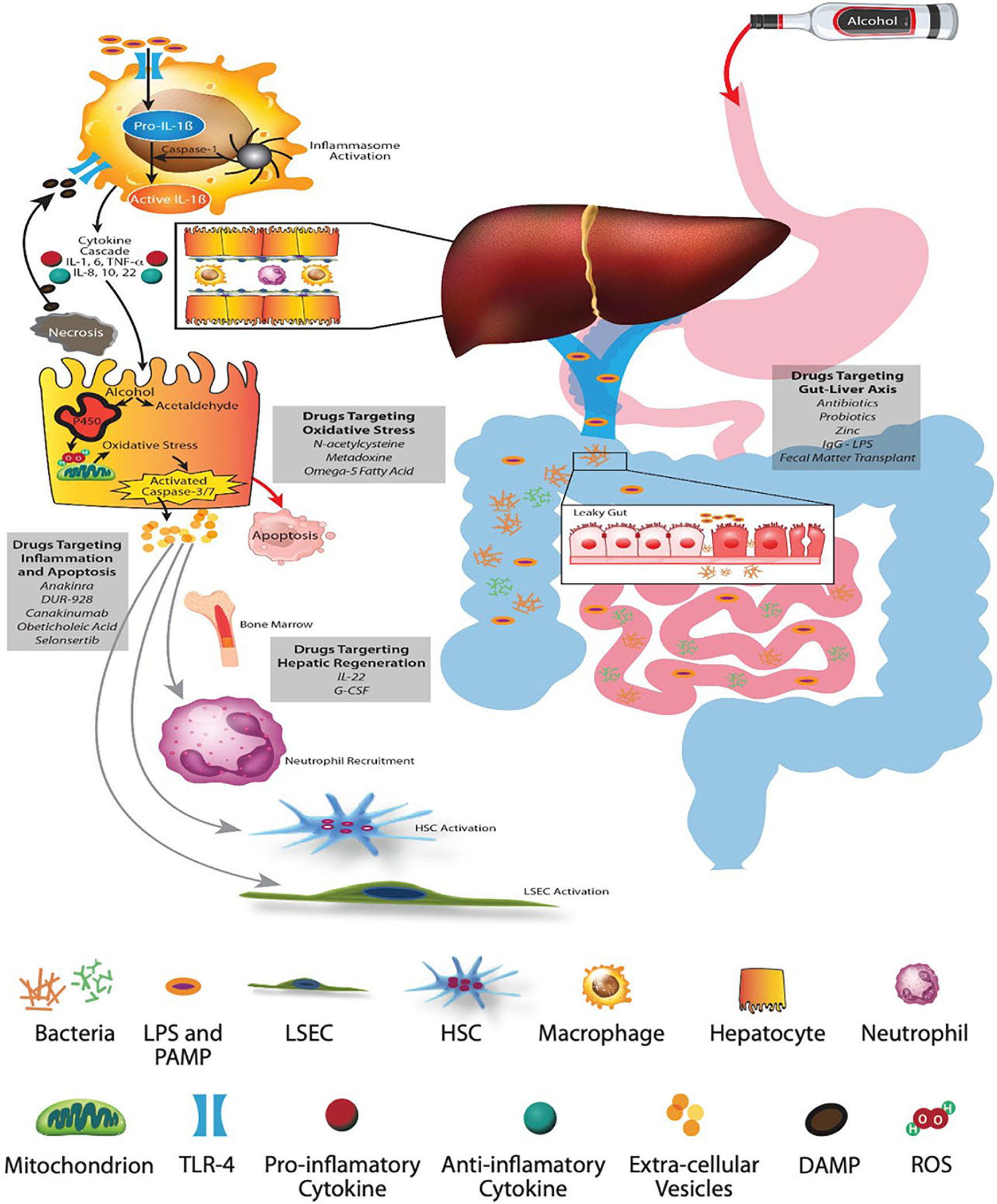
Pathophysiology of alcohol-associated liver disease and AH. (A) Alcohol-mediated increased gut permeability with leaky gut results in translocation of pathogen-associated molecular patterns and bacterial lipopolysaccharides (LPS) through the portal vein. (B) Schematic representation of the hepatic lobule with hepatocytes and sinusoids lined with liver sinusoid endothelial cells (LSECs) containing macrophages and neutrophils, and space of Disse containing HSCs. (C) LPS binds to membrane and cytosolic receptors of hepatocyte immune cells, macrophages, HSCs, and sinusoidal endothelial cells. LPS and its binding protein complex activates toll-like receptor-4 on the surface of hepatic macrophages, leading to an inflammatory cascade and signaling of chemokines and cytokines. The inflammasome complex (pro-IL-1β and caspase-1) activates pro-caspase-1 to generate IL-1β. (D) The metabolism of alcohol to acetaldehyde causes direct hepatocyte injury with generation of reactive oxygen species, leading to mitochondrial dysfunction, oxidative stress, and hepatocyte apoptosis. Damage associated molecular patterns released from the injured hepatocytes, especially HMGB-1 (high mobility group box protein 1) and microRNA-122, perpetuate ongoing hepatocyte injury. (E) Extracellular vesicles released from injured hepatocytes along with chemokines and cytokines recruit neutrophils from the bone marrow to the hepatic circulation and sinusoids. The inflammatory cascade cross talks with sinusoidal cells, resulting in activation of LSECs and HSCs, leading to portal hypertension and laying down of collagen with development of fibrosis
Chronic excessive alcohol use compromises the gut epithelial barrier function, resulting in enhanced entrance of pathogen-associated molecular patterns like lipopolysaccharide and bacterial DNA into the portal circulation.[39] Alcohol also modifies the gut microbiome, with reduction in Akkermansia, Lactobacilli and Furmicutes, and increased proportion of Bacteriodes.[40] The fungal microbiome is also affected, and is associated with severity of ALD.[41] Initial pilot studies with fecal microbiota transplant showed improvement in liver disease, and a positive impact on AUD with reduced cravings and maintenance of abstinence.[42,43] Obeticholic acid targeting the nuclear farnesoid X receptor provides beneficial effects on gut integrity and HSC activity.
DAMPs are recognized by toll-like receptors and other pattern recognition receptors on several hepatic cells such as macrophages, hepatocytes and stellate cells, resulting in NF-kB-mediated pro-inflammatory cytokine and chemokine production. Activation of intracellular inflammasome complex consisting of caspase-1 and IL-1β amplifies inflammation, promotes fibrosis, and impairs liver regeneration.[44] In a preclinical model of ALD, IL-1 receptor antagonist, anakinra, attenuated steatosis, inflammation, and fibrosis.[45] Anakinra in conjunction with zinc and pentoxifylline in a phase 2 clinical trial tended to improve 6 months’ survival in patient with severe AH as compared to those treated with prednisolone. Currently, this drug is being tested in a larger clinical trial.
Liver regeneration is impaired in ALD due to reduced levels of hepatocyte nuclear factor-4 and miR-122, and abnormalities in hedgehog signaling.[46,47] IL-22, a peotropic cytokine produced during acute phase response is anti-apoptotic, hepatoprotective, and promotes cell proliferation and regeneration.[48] F-652, an IL-22 fusion protein in a dose-ranging study in patients with moderate AH (MELD 11–20) provided a superior response (measured by Lille score) compared whit propensity-matched historical controls, paving the way for testing this molecule in larger studies.[49] Granulocyte-colony stimulating factor mobilizes myeloid precursors from the bone marrow, with improved immune function and hepatic regeneration.[50] Clinical trials using this agent have shown mixed results with encouraging data from Asia, but not from Europe.[51] Studies are ongoing in the United States to substantiate the role of this molecule in the treatment of ALD.
DUR-928 is an epigenetic regulator with a net effect of reducing the inflammation and improving regeneration.[52] In a pilot clinical trial, this drug improved response rate (as measured by Lille score) in patients with AH as compared with historical steroid treated controls.[53] Larger trials of this agent are ongoing.
ESTIMATING PROGNOSIS OF AND SELECTION CRITERIA FOR LT IN PATIENTS WITH ALD
Several models are available to predict patient survival and response to corticosteroids (Table 2). For example, MELD at baseline and the Lille score after 1 week of medical treatment identifies sickest patients with AH and at high risk of short-term mortality.[54]
TABLE 2.
Clinical scores in the management of ALD and AH
| Score (reference) | Elements | Clinical outcome | Clinical application and thresholds |
|---|---|---|---|
| DF[70] | Serum bilirubin and prothrombin time | Survival at 30 days | ≥32 high risk of mortality and candidacy for corticosteroids |
| MELD[71] | Serum bilirubin, INR, serum creatinine | Survival at 30 and 90 days | ≥21 high risk of mortality and candidacy for corticosteroids |
| ABIC[72] | Serum bilirubin, INR, serum creatinine, age of the patient | Survival at 90 days and 1 year | ≥9 high risk of mortality ≥6.71 intermediate risk |
| GAHS[73] | Serum bilirubin, prothrombin time, serum creatinine, patient’s age, WBC count | Survival at 28 and 84 days | ≥9 high risk of mortality and candidacy for corticosteroids |
| Lille[19] | Serum bilirubin at days 0 and 7, prothrombin time, serum creatinine, patient’s age, serum albumin | 6-month survival with corticosteroid treatment | ≥0.45 at day 7; nonresponse to corticosteroids |
| SALT[60] | > 10 drinks per day at initial hospitalization, multiple prior rehabilitation attempts, prior alcohol-related legal issues, and prior illicit substance abuse | Sustained alcohol use after LT | ≤5 low risk for alcohol relapse after LT |
Abbreviations: ABIC, age, bilirubin, INR, creatinine; DF, discriminant function; GAHS, Glasgow alcohol hepatitis score; INR, international normalized ratio; MELD, Model for End-Stage Liver Disease; SALT, sustained alcohol use after liver transplant; and WBC, white blood cell.
LT is considered for patients who continue to deteriorate despite optimal medical treatment. However, patient selection for LT in ALD poses difficult dilemmas due to lack of uniform protocol across transplant centers worldwide, shortage of organ donors, and variable views of the public on alcohol use.[55]
LT is indicated in patients with decompensated alcohol-associated cirrhosis who do not improve their liver disease despite at least 3 months of abstinence. However, patients with the most severe form of ALD ] with AH and acute-on-chronic liver failure and not responding to medical treatment have a mortality risk of up to 80% at 6 months, and cannot afford to wait that long.[56]
The 6-month rule was introduced to allow recovery of liver disease in response to abstinence and not as a predictor for alcohol relapse after LT.[57] Applying this 6-month rule to select patients for LT is not adapted, because alcohol use is a complex situation to be judged and cannot be summarized by a simple time period. The main goal is to identify patients with greatest survival benefit and an acceptable risk of posttransplant alcohol relapse. Younger age, lack of adequate social support, history of psychiatric disorders and multiple rehabilitation attempts, and poor patient insight for their disease are more important predictors of posttransplant alcohol relapse.[58]
Developing a prediction model requires selection of candidate predictors, the type of model, criteria for predictor selection, evaluation of model performance, and validation (Table 3). A model simple for use by clinicians is favored over a complex one. Typically at least 10 events for each predictor variable are needed for models with binary or time-to-event outcomes, but this rule has been supplanted by a more tailored approach focusing on minimizing model overfitting and maximizing the estimate precision.[59]
TABLE 3.
Considerations in developing prediction models for patients with AH
| Step | Element | Lille score[19] | SALT score[60] |
|---|---|---|---|
| 1 | Select clinically relevant outcome | Survival with corticosteroid therapy | Relapse of harmful alcohol use after liver transplant |
| 2 | Select candidate explanatory variables (predictors) • Evaluate data quality • Address missing values |
Serum bilirubin at days 0 and 7, prothrombin time, serum creatinine, patient’s age, serum albumin | Pre-transplant history of Non-THC illicit substance abuse, ≥2 prior rehab attempts, any legal issues, and ≥10 drinks/day at presentation |
| 3 | Select model type guided by type of outcome variable (binary, continuous, time to event) and number/collinearity of explanatory variables | Logistic regression: binary outcome = survival at 6 months | Logistic, cox, and Lasso regression as well as classification and regression tree analysis |
| 4 | Choose strategy for selecting variables for final model (forward, backward, or bidirectional) and criterion for inclusion/exclusion of variables | Forward selection | Forward and backward |
| Criterion: p value | Criterion: p value | ||
| 5 | Create the prediction risk score | Complex: using coefficients from model | Simple: assign integers based on coefficients |
| Range 0–1 | Range 0–11 | ||
| 6 | Measure how model performs and/or accuracy of prediction • Discrimination • Calibration • PPV, NPV, specificity, sensitivity |
AUROC, c statistic | AUROC, c statistic |
| Sensitivity and specificity | PPV, NPV, specificity, sensitivity | ||
| 7 | Validate model Internal: bootstrapping; data splitting External: another patient cohort representative of the target population |
External: another cohort and prior RCTs of patients with AH treated with corticosteroids | Internal: Random splitting of cohort into 10 groups; leave out each group in turn and estimate from other 9 |
Abbreviations: AUROC, area under the receiver operating characteristic curve; Lasso, least absolute shrinkage selection operator regression; NPV, negative predictive value; PPV, positive predictive value; RCT, randomized control trial; and THC, tetrahydrocannabinol.
Key aspects of prognostic modeling are highlighted in Table 2 using two examples: the Lille score[19] and the sustained alcohol use after LT (SALT) score.[60] Lille score was developed using a logistic regression model to estimate survival at 30 and at 90 days, with a forward-selection approach on static and dynamic clinical and laboratory variables as candidate predictors. The final model included a set of five baseline variables combined with change in serum bilirubin at 1 week of corticosteroid treatment. The SALT score was developed using least absolute shrinkage selection operator regression (Lasso) and bidirectional selection approach on demographic and psychosocial variables as predictors.
To derive the final model, a given variable can be removed from (backward selection) or added to (forward selection) the model. Although 5% significance level is most commonly used, there remains a risk of overfitting, and the model will be too closely adapted to the data.[61] Akaike or Bayesian information criterion are methods of assessing the model fit that includes a penalty for models with a larger number of predictors, thus reducing the risk of overfitting. It should be noted that differences in the assumptions potentially underlying the selection criterion used may influence the predictors selected, so using more than one selection criterion may help achieve a model less at risk for overfitting or underfitting.[62] Lasso regression is ideal for multivariate analysis in which a rare outcome is anticipated to be predicted by a small number of variables or in models with a high level of multicollinearity.
Once the final model has been derived, presenting the “score” can be in a complex or simplified form. The Lille score used the complex approach, in which the weight of the multivariable model coefficients produces the risk score. As this is not an easy calculation, authors typically provide a “link” to an online calculation. The SALT score used the simplified method that assigns integer points to each risk factor based on the relative weight of their coefficients. The benefit of the simple approach is that it can be applied at the bedside and clarifies which of the predictors carries the most “weight” in prediction.
AUROC or the concordance (“c”) statistic are methods to measure discrimination on developing the event. Based on the performance characteristics, a cutoff value may be used, such as 0.45 for Lille score, with a sensitivity of 81% and a specificity of 76% in identifying patients who are likely to die within 6 months. The SALT score, at a threshold of ≤ 5, had a negative predictive value of 95% and a positive predictive value of only 25%, highlighting its limitation in identifying patients at high risk of alcohol relapse.
Finally, the model needs validation, which can be internal (on the same data set used for developing the model) such as SALT score,[63] or external (using another patient population with similar characteristics as the one used to develop the model) such as Lille score. Ideally, both internal and external validation should be included.
The performance of prognostic models may wane over time due to changes in patient characteristics and management strategies. Existing models can be improved by adding novel predictors[64] or new biomarkers[26] as a basis to change the current treatment paradigm of ALD. Additionally, there is a need for prognostic models in (a) patients with moderate AH, (b) those awaiting LT, and (c) LT recipients.
CHALLENGES TO IMPLEMENT INTEGRATED MULTIDISCIPLINARY MANAGEMENT OF AUD IN PATIENTS WITH ALD
Apart from accessible biological parameters (laboratory, imaging, clinical exam findings), there are abstract and complex psychosocial variables (psychology, relationships, lifestyle factors) that are involved in the management of patients with ALD. Additionally, many patients are affected by polysubstance use; pain foci treated by opioids, marijuana, or neuropathic agents; and poor coping. Although many clinical unmet needs, as mentioned earlier, can be addressed using a quantitative approach, research on management of AUD involves qualitative or a mixed quantitative-qualitative approach. As ALD is too psychiatrically complex for hepatology and too medically complex for psychiatry, a single professional discipline cannot adequately treat or study this breadth of phenomena. As hepatology and psychiatry do not often collaborate clinically or academically within a health system, this disconnect between specialties has significant consequences for clinical care and research.
Hepatology and psychiatry each have their own challenges when it comes to taking care of patients with ALD. Rigorous hepatology training with large clinical load leaves little room for adding additional training on management of AUD and other substance use disorders. Similarly, lack of training on liver disease during psychiatry residency training is a significant barrier for psychiatrists in prescribing psychopharmacology in patients with ALD, particularly among those with severe forms of the disease with liver and/or kidney failure. Apart from the concerns among mental health providers for polypharmacy, toxicity, and worsening medical pathology, presence of hepatic encephalopathy in patients with ALD may impact their ability for meaningful engagement of patients for AUD treatment.
These interprofessional challenges are also mirrored in the research environment. Traditional AUD and ALD research outcomes tend to be alcohol abstinence and no heavy drinking days. While these are important and meaningful parameters, the effects of novel interventions may be difficult to assess without a broader array of study outcomes reflecting the medical and psychiatric nature of ALD. Quantifying drinking often depends on the recall of the research subject, which may often be imprecise, especially in those with decompensated disease with HE. Like many other health behaviors, patients with AUD tend to not reveal the accurate information and conceal the true nature of their alcohol use.[65] The principal clinical goal and primary research endpoint should be full abstinence from alcohol, given the mortality and decompensation risks in ALD with any drinking.[66] This should not preclude the study of harm-reduction efforts, as these efforts often precede abstinence in the real-world cycle of motivation and change. Table 4 contains a list of multimodal research outcomes that should be considered while studying patients with ALD. The research methods to study these outcomes should include mixed methods and qualitative research that can address fundamental questions about ALD treatment, and disease course including granular data about why patients continue to drink, why they do not attend AUD treatment, and how they perceive their illness.
TABLE 4.
Multimodal research outcomes in ALD
| Medical | Psychiatric | Other |
|---|---|---|
| 1. Improved liver disease (MELD score) 2. Reduced decompensation and cirrhosis complications from portal hypertension 3. Reduced rates of hospitalization and visits to emergency room 4. Improved overall and LT-free survival |
1. Amount of alcohol and other substance use as assessed by the TLFB and biomarkers of alcohol use (BAL, uEtG, PEth) and urine drug screen for other substance use 2. Rates of discordance between patient self-report and biomarkers 3. Reduction in alcohol cravings 4. Rates of regained sobriety 5. Treatment engagement and retention rates for AUD 6. Improvement in tracked psychometric scores (e.g., anxiety, depression, sleep) 7. Nature of and changes in understanding and insight on AUD |
1. Quality of life 2. ALD team reimbursement and revenue generation 3. Cost savings from reduction in hospitalization and resource use 4. Value-based population management metrics 5. Implementation metrics: clinic cancellation and no-show rates, referral rates 6. Geographic areas served 7. Validated metrics to evaluate ALD clinician teamwork |
Abbreviations: BAL, blood alcohol level; PEth, phosphatidylethanol; TLFB, timeline follow-back; and uEtG, urinary ethyl glucuronide.
The multidisciplinary nature of ALD demands improved interprofessional care and research. Although such multidisciplinary integrated care models have shown benefits among patients listed for or recipients of LT,[67,68] data are emerging on these models on effective treatment of the dual pathology of liver disease and of AUD in the management of patients with ALD.[69] Co-located hepatology and addiction teams cultivate strong personal and professional relationships. They seek the buy-in of institutional leadership ahead of establishing a clinic, making it clear to stakeholders as to how the liver clinic will provide a return on investment in clinical care, research, and education. These integrated teams flatten traditional medical hierarchies, resulting in a team culture of respect for all roles, reciprocal interprofessional training and support, constructive dissent, lateral and multidisciplinary clinic leadership, and openness to course correction and creative solutions.
Patients with ALD can be challenging to care for hepatologists and psychiatrists, clinician wellness, and conflict resolution should be prioritized. ALD care entails building long-term relationships with patients, as their insight, motivation, mental health, and medical disease fluctuate over time. This requires an efficient clinical communication and data management through the use of updated data reviewed during regular team meetings led by ALD case managers. In addition to medical care, ALD treatment plans should be personalized in terms of prescribed psychotherapy and psychopharmacology. Patients should be aware that toxicology will be used regularly, and that they will be informed as to how the team will use these data. Between clinic visits, ALD case managers should reach out via phone calls and patient portal messages to gather data, update tracked psychometric instruments, provide encouragement, and support treatment adherence. ALD teams pursue networking and outreach activities with intramural and extramural colleagues in hepatology and psychiatry. ALD clinics function well adjacent to the transplant centers, as all of these efforts will be invaluable to the patient and the team, should the need for transplant arise. In spite of the obvious benefits of such integrated care models, these are not routinely used in clinical practice. Clearly, studies are needed to overcome challenges in more widespread implementation of the integrated multidisciplinary care models for patients with ALD.
In summary, the burden of ALD continues to rise, especially in the young. Identification of at-risk populations allows diagnosis of early-stage ALD and targets interventions to prevent progression to advanced disease. Understanding the mechanisms of liver injury in ALD has opened avenues to therapeutic targets such as gut microbiome, inflammatory mediators, and liver regeneration, with a potential to translate into developing effective pharmacological interventions. With LT evolving as an effective salvage therapy for selected individuals with ALD, continued efforts are needed to derive prediction models as a basis for homogenizing criteria for patient selection for this therapy. Federal efforts with the ongoing NIAAA-funded consortia (Alcoholic Hepatitis Network project) and the upcoming National Institute of Diabetes and Digestive and Kidney Disease–funded consortia (Liver Cirrhosis Network) would be of immense value in addressing many of the research strategies to address clinical unmet needs in ALD (Figure 3). Finally, multidisciplinary research models will be required to gain greater insight to address AUD and improve the long-term outcomes of patients with ALD.
FIGURE 3.
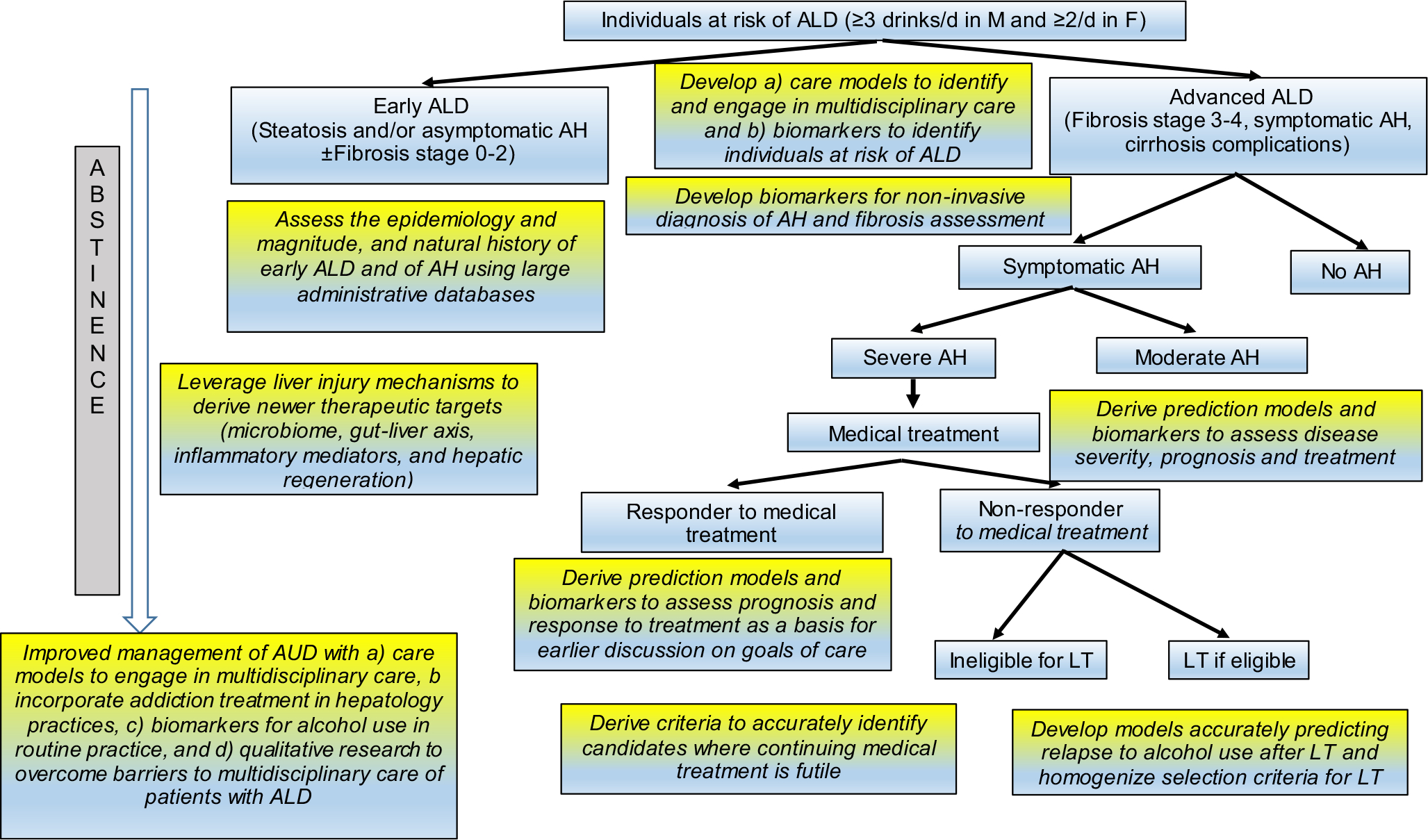
Research strategies to address clinical unmet needs in the background of the current algorithm in the management of ALD. Boxes highlighted in gray-yellow depict the clinical unmet needs and research methodologies to address the corresponding unmet need. LT, liver transplantation
Abbreviations:
- AH
alcohol-associated hepatitis
- ALD
alcohol-associated liver disease
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AUD
alcohol use disorder
- AUROC
area under the receiver operating characteristic curve
- DF
discriminant function
- K-18
keratin 18
- Lasso
least absolute shrinkage selection operator regression
- LT
liver transplantation
- MELD
Model for End-Stage Liver Disease
- NHANES
National Health and Nutrition Examination Survey
- SALT
sustained alcohol use after liver transplant
Footnotes
CONFLICT OF INTEREST
Dr. Singal consults for CSL Behring. He advises Gilead and Arrowhead. Dr. Kwo owns stock in Durect. Dr. Szabo consults and owns stock in Glympse. She consults for Durect, Evive, Novartis, Pfizer, Zomagen, Quest, Surrozen, and Pandion. She advises Terra Firma. Dr. Terrault consults for Enyo, PPD Pharma, and Entourage. She received grants from Gilead, Genentech, Roche, and EXIGO.
REFERENCES
- 1.GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:245–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah VH. Alcoholic liver disease: the buzz may be gone, but the hangover remains. Hepatology. 2010;51:1483–4. [DOI] [PubMed] [Google Scholar]
- 3.Szabo G, Kamath PS, Shah VH, Thursz M, Mathurin P, Addolorato G, et al. Alcohol-related liver disease: areas of consensus, unmet needs and opportunities for further study. Hepatology. 2019;69:2271–83. [DOI] [PubMed] [Google Scholar]
- 4.Axley PD, Richardson CT, Singal AK. Epidemiology of alcohol consumption and societal burden of alcoholism and alcoholic liver disease. Clin Liver Dis. 2019;23:39–50. [DOI] [PubMed] [Google Scholar]
- 5.Asrani SK, Mellinger J, Arab JP, Shah VH. Reducing the global burden of alcohol-associated liver disease: a blueprint for action. Hepatology. 2021;73:2039–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singal AK, Arora S, Wong RJ, Satapathy SK, Shah VH, Kuo YF, et al. Increasing burden of acute-on-chronic liver failure among alcohol-associated liver disease in the young population in the United States. Am J Gastroenterol. 2020;115:88–95. [DOI] [PubMed] [Google Scholar]
- 7.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: observational study. BMJ 2018;362:k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asrani SK, Trotter J, Lake J, Ahmed A, Bonagura A, Cameron A, et al. Meeting Report: The Dallas Consensus Conference on liver transplantation for alcohol associated hepatitis. Liver Transpl. 2020;26:127–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah ND, Ventura-Cots M, Abraldes JG, Alboraie M, Alfadhli A, Argemi J, et al. Alcohol-related liver disease is rarely detected at early stages compared with liver diseases of other etiologies worldwide. Clin Gastroenterol Hepatol. 2019;17:2320–9.e2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mellinger JL, Shedden K, Winder GS, Tapper E, Adams M, Fontana RJ, et al. The high burden of alcoholic cirrhosis in privately insured persons in the United States. Hepatology. 2018;68:872–82. [DOI] [PubMed] [Google Scholar]
- 11.Guirguis J, Chhatwal J, Dasarathy J, Rivas J, McMichael D, Nagy LE, et al. Clinical impact of alcohol-related cirrhosis in the next decade: estimates based on current epidemiological trends in the United States. Alcohol Clin Exp Res. 2015;39:2085–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dang K, Hirode G, Singal AK, Sundaram V, Wong RJ. Alcoholic liver disease epidemiology in the United States: a retrospective analysis of 3 US databases. Am J Gastroenterol. 2020;115:96–104. [DOI] [PubMed] [Google Scholar]
- 13.Wong T, Dang K, Ladhani S, Singal AK, Wong RJ. Prevalence of alcoholic fatty liver disease among adults in the United States, 2001–2016. JAMA. 2019;321:1723–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mellinger JL, Fernandez A, Shedden K, Winder GS, Fontana RJ, Volk ML, et al. Gender disparities in alcohol use disorder treatment among privately insured patients with alcohol-associated cirrhosis. Alcohol Clin Exp Res. 2019;43:334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogal S, Youk A, Zhang H, Gellad WF, Fine MJ, Good CB, et al. Impact of alcohol use disorder treatment on clinical outcomes among patients with cirrhosis. Hepatology. 2020;71:2080–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee BP, Im GY, Rice JP, Weinberg E, Hsu C, Fix OK, et al. Underestimation of liver transplantation for alcoholic hepatitis in the national transplant database. Liver Transpl. 2019;25:706–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tapper EB, Korovaichuk S, Baki J, Williams S, Nikirk S, Waljee AK, et al. Identifying patients with hepatic encephalopathy using administrative data in the ICD-10 era. Clin Gastroenterol Hepatol. 2021;19:604–6.e601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirpich IA, McClain CJ, Vatsalya V, Schwandt M, Phillips M, Falkner KC, et al. Liver injury and endotoxemia in male and female alcohol-dependent individuals admitted to an alcohol treatment program. Alcohol Clin Exp Res. 2017;41:747–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louvet A, Naveau S, Abdelnour M, Ramond M-J, Diaz E, Fartoux L, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology. 2007;45:1348–54. [DOI] [PubMed] [Google Scholar]
- 20.Singal AK, Salameh H, Singal A, Jampana SC, Freeman DH, Anderson KE, et al. Management practices of hepatitis C virus infected alcoholic hepatitis patients: a survey of physicians. World J Gastrointest Pharmacol Ther. 2013;4:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thursz MR, Richardson P, Allison M, Austin A, Bowers M, Day CP, et al. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med. 2015;372:1619–28. [DOI] [PubMed] [Google Scholar]
- 22.Crabb DW, Bataller R, Chalasani NP, Kamath PS, Lucey M, Mathurin P, et al. Standard definitions and common data elements for clinical trials in patients with alcoholic hepatitis: recommendation from the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology. 2016;150:785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liangpunsakul S, Puri P, Shah VH, Kamath P, Sanyal A, Urban T, et al. Effects of age, sex, body weight, and quantity of alcohol consumption on occurrence and severity of alcoholic hepatitis. Clin Gastroenterol Hepatol. 2016;14:1831–8.e1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vatsalya V, Cave MC, Kong M, Gobejishvili L, Falkner KC, Craycroft J, et al. Keratin 18 is a diagnostic and prognostic factor for acute alcoholic hepatitis. Clin Gastroenterol Hepatol. 2020;18:2046–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bissonnette J, Altamirano J, Devue C, Roux O, Payancé A, Lebrec D, et al. A prospective study of the utility of plasma biomarkers to diagnose alcoholic hepatitis. Hepatology. 2017;66:555–63. [DOI] [PubMed] [Google Scholar]
- 26.Atkinson SR, Grove JI, Liebig S, Astbury S, Vergis N, Goldin R, et al. In severe alcoholic hepatitis, serum keratin-18 fragments are diagnostic, prognostic, and theragnostic biomarkers. Am J Gastroenterol. 2020;115:1857–68. [DOI] [PubMed] [Google Scholar]
- 27.He L, Vatsalya V, Ma X, Zhang J, Yin X, Kim S, et al. Metabolic profiling of bile acids in the urine of patients with alcohol-associated liver disease. Hepatol Commun. 2021;5:798–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Z, Kusumanchi P, Ross RA, Heathers L, Chandler K, Oshodi A, et al. Serum metabolomic profiling identifies key metabolic signatures associated with pathogenesis of alcoholic liver disease in humans. Hepatol Commun. 2019;3:542–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byrnes SA, Weigl BH. Selecting analytical biomarkers for diagnostic applications: a first principles approach. Expert Rev Mol Diagn. 2018;18:19–26. [DOI] [PubMed] [Google Scholar]
- 30.Peeraphatdit TB, Kamath PS, Karpyak VM, Davis B, Desai V, Liangpunsakul S, et al. Alcohol rehabilitation within 30 days of hospital discharge is associated with reduced readmission, relapse, and death in patients with alcoholic hepatitis. Clin Gastroenterol Hepatol. 2020;18:477–85.e475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halter CC, Dresen S, Auwaerter V, Wurst FM, Weinmann W. Kinetics in serum and urinary excretion of ethyl sulfate and ethyl glucuronide after medium dose ethanol intake. Int J Legal Med. 2008;122:123–8. [DOI] [PubMed] [Google Scholar]
- 32.Helander A, Peter O, Zheng Y. Monitoring of the alcohol biomarkers PEth, CDT and EtG/EtS in an outpatient treatment setting. Alcohol Alcohol. 2012;47:552–7. [DOI] [PubMed] [Google Scholar]
- 33.Singh S, Murad MH, Chandar AK, Bongiorno CM, Singal AK, Atkinson SR, et al. Comparative effectiveness of pharmacological interventions for severe alcoholic hepatitis: a systematic review and network meta-analysis. Gastroenterology. 2015;149:958–70.e912. [DOI] [PubMed] [Google Scholar]
- 34.Mathurin P, Thursz M. Endpoints and patient stratification in clinical trials for alcoholic hepatitis. J Hepatol. 2019;70:314–8. [DOI] [PubMed] [Google Scholar]
- 35.Seitz HK, Bataller R, Cortez-Pinto H, Gao B, Gual A, Lackner C, et al. Alcoholic liver disease. Nat Rev Dis Primers. 2018;4:1–22. [DOI] [PubMed] [Google Scholar]
- 36.Szabo G, Momen-Heravi F. Extracellular vesicles in liver disease and potential as biomarkers and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2017;14:455–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szabo G, Bala S. MicroRNAs in liver disease. Nat Rev Gastroenterol Hepatol. 2013;10:542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petrasek J, Iracheta-Vellve A, Saha B, Satishchandran A, Kodys K, Fitzgerald KA, et al. Metabolic danger signals, uric acid and ATP, mediate inflammatory cross-talk between hepatocytes and immune cells in alcoholic liver disease. J Leukoc Biol. 2015;98:249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szabo G Gut–liver axis in alcoholic liver disease. Gastroenterology. 2015;148:30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grander C, Adolph TE, Wieser V, Lowe P, Wrzosek L, Gyongyosi B, et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut. 2018;67:891–901. [DOI] [PubMed] [Google Scholar]
- 41.Lang S, Duan YI, Liu J, Torralba MG, Kuelbs C, Ventura-Cots M, et al. Intestinal fungal dysbiosis and systemic immune response to fungi in patients with alcoholic hepatitis. Hepatology. 2020;71:522–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Philips CA, Pande A, Shasthry SM, Jamwal KD, Khillan V, Chandel SS, et al. Healthy donor fecal microbiota transplantation in steroid-ineligible severe alcoholic hepatitis: a pilot study. Clin Gastroenterol Hepatol. 2017;15:600–2. [DOI] [PubMed] [Google Scholar]
- 43.Bajaj JS, Gavis EA, Fagan A, Wade JB, Thacker LR, Fuchs M, et al. A randomized clinical trial of fecal microbiota transplant for alcohol use disorder. Hepatology. 2021;73:1688–700. [DOI] [PubMed] [Google Scholar]
- 44.Tilg H, Moschen AR, Szabo G. Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2016;64:955–65. [DOI] [PubMed] [Google Scholar]
- 45.Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Investig. 2012;122:3476–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Florentino RM, Fraunhoffer NA, Morita K, Takeishi K, Ostrowska A, Achreja A, et al. Cellular location of HNF4α is linked with terminal liver failure in humans Hepatol Commun. 2020;4:859–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Machado MV, Diehl AM. Hedgehog signalling in liver pathophysiology. J Hepatol. 2018;68:550–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sabat R, Ouyang W, Wolk K. Therapeutic opportunities of the IL-22-IL-22R1 system. Nat Rev Drug Discov. 2014;13:21–38. [DOI] [PubMed] [Google Scholar]
- 49.Arab JP, Sehrawat TS, Simonetto DA, Verma VK, Feng D, Tang T, et al. An open-label, dose-escalation study to assess the safety and efficacy of IL-22 agonist F-652 in patients with alcohol-associated hepatitis. Hepatology. 2020;72:441–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spahr L, Lambert JF, Rubbia-Brandt L, Chalandon Y, Frossard JL, Giostra E, et al. Granulocyte-colony stimulating factor induces proliferation of hepatic progenitors in alcoholic steatohepatitis: a randomized trial. Hepatology. 2008;48:221–9. [DOI] [PubMed] [Google Scholar]
- 51.Marot A, Singal AK, Moreno C, Deltenre P. Granulocyte colonystimulating factor for alcoholic hepatitis: a systematic review and meta-analysis of randomised controlled trials. JHEP Rep. 2020;2:100139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Lin W, Brown JE, Chen L. Pandak WM, Hylemon PB, et al. 25-Hydroxycholesterol 3-sulfate is an endogenous ligand of DNA methyltransferases in hepatocytes. J Lipid Res. 2021;62:10G063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McClain CJ, Vatsalya V, Rex R, Hassanein Tl, Stein LL, Flamm SL, et al. DUR-928 therapy for acute alcoholic hepatitis: a pilot study. Hepatology. 2019;70:1483–1484 A. [Google Scholar]
- 54.Louvet A, Labreuche J, Artru F, Boursier J, Kim DJ, O’Grady J, et al. Combining data from liver disease scoring systems better predicts outcomes of patients with alcoholic hepatitis. Gastroenterology. 2015;149:398–406.e398; quiz e316–397. [DOI] [PubMed] [Google Scholar]
- 55.Volk ML, Biggins SW, Huang MA, Argo CK, Fontana RJ, Anspach RR. Decision making in livertransplant selection committees: a multicenter study. Ann Intern Med. 2011;155:503–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mathurin P, Moreno C, Samuel D, Dumortier J, Salleron J, Durand F, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med. 2011;365:1790–800. [DOI] [PubMed] [Google Scholar]
- 57.Foster PF, Fabrega F, Karademir S, Sankary HN, Mital D, Williams JW. Prediction of abstinence from ethanol in alcoholic recipients following liver transplantation. Hepatology. 1997;25:1469–77. [DOI] [PubMed] [Google Scholar]
- 58.Addolorato G, Mirijello A, Barrio P, Gual A. Treatment of alcohol use disorders in patients with alcoholic liver disease. J Hepatol. 2016;65:618–30. [DOI] [PubMed] [Google Scholar]
- 59.Riley RD, Ensor J, Snell KIE, Harrell FE, Martin GP, Reitsma JB, et al. Calculating the sample size required for developing a clinical prediction model. BMJ. 2020;368:m441. [DOI] [PubMed] [Google Scholar]
- 60.Lee BP, Vittinghoff E, Hsu C, Han H, Therapondos G, Fix OK, et al. Predicting low risk for sustained alcohol use after early liver transplant for acute alcoholic hepatitis: the sustained alcohol use post-liver transplant score. Hepatology. 2019;69:1477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harrell FJ. Regression modeling strategies with applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. [Google Scholar]
- 62.Royston P, Moons KG, Altman DG, Vergouwe Y. Prognosis and prognostic research: developing a prognostic model. BMJ 2009;338:b604. [DOI] [PubMed] [Google Scholar]
- 63.Altman DG, Royston P. What do we mean by validating a prognostic model? Stat Med. 2000;19:453–73. [DOI] [PubMed] [Google Scholar]
- 64.Forrest EH, Storey N, Sinha R, Atkinson SR, Vergis N, Richardson P, et al. Baseline neutrophil-to-lymphocyte ratio predicts response to corticosteroids and is associated with infection and renal dysfunction in alcoholic hepatitis. Aliment Pharmacol Ther. 2019;50:442–53. [DOI] [PubMed] [Google Scholar]
- 65.Zuckoff A “Why won’t my patients do whaťs good for them?” Motivational interviewing and treatment adherence. Surg Obes Relat Dis. 2012;8:514–21. [DOI] [PubMed] [Google Scholar]
- 66.Mellinger J, Winder GS, Fernandez ACJH. Measuring the alcohol in alcohol-related liver disease: choices and challenges for clinical research. Hepatology. 2021;73:1207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Addolorato G, Mirijello A, Barrio P, Gual A. Treatment of alcohol use disorders in patients with alcoholic liver disease. J Hepatol. 2016;65:618–30. [DOI] [PubMed] [Google Scholar]
- 68.Asrani SK, Trotter J, Lake J, Ahmed A, Bonagura A, Cameron A, et al. Meeting Report: The Dallas Consensus Conference on liver transplantation for alcohol associated hepatitis. Liver Transpl 2020;26:127–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Winder GS, Fernandez AC, Klevering K, Mellinger JL. Confronting the crisis of comorbid alcohol use disorder and alcohol-related liver disease with a novel multidisciplinary clinic. Psychosomatics. 2020;61:238–53. [DOI] [PubMed] [Google Scholar]
- 70.Maddrey WC, Boitnott JK, Bedine MS, Weber FL, Mezey E, White Rl Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978;75:193–9. [PubMed] [Google Scholar]
- 71.Dunn W, Jamil LH, Brown LS, Wiesner RH, Kim WR, Menon KVN, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. 2005;41:353–8. [DOI] [PubMed] [Google Scholar]
- 72.Dominguez M, Rincon D, Abraldes JG, Miquel R, Colmenero J, Bellot P, et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol. 2008;103:2747–56. [DOI] [PubMed] [Google Scholar]
- 73.Forrest EH, Evans CD, Stewart S, Phillips M, Oo YH, McAvoy NC, et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut. 2005;54:1174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


