Abstract
Purpose:
We evaluated 5-year oncologic and functional outcomes of hemigland cryoablation of localized prostate cancer.
Materials and Methods:
We reviewed the records of 160 consecutive men who underwent hemigland cryoablation of localized prostate cancer. Recurrent and/or residual clinically significant prostate cancer was defined as Grade Group 2 or greater on followup biopsy. A prostate specific antigen nadir plus 2 ng/ml according to the Phoenix criteria was used to define biochemical failure. Radical treatment was defined as any whole gland therapy. Treatment failure was defined as any radical and/or whole gland treatment, systemic therapy initiation, metastasis or prostate cancer specific mortality. The study primary end point was treatment failure-free survival. The secondary end points were survival free of biochemical failure, clinically significant prostate cancer and radical treatment. Followup biopsy and functional outcomes were also evaluated. Statistical analysis included the Kaplan-Meier method, and univariate and multivariable Cox and logistic regression with significance considered at p <0.05.
Results:
Median patient age was 67 years, baseline prostate specific antigen was 6.3 ng/ml and followup was 40 months. A total of 131 patients (82%) had D’Amico intermediate (66%) or high risk (16%) prostate cancer. At 5 years the treatment failure-free survival rate was 85%, the biochemical failure-free survival rate was 62% and the survival rate free of clinically significant prostate cancer was 89%. Higher baseline prostate specific antigen independently predicted treatment failure (p <0.001), biochemical failure (p = 0.048), recurrence and radical treatment (p <0.01). Grade Group 3 or greater independently predicted treatment failure (p = 0.04). The metastasis-free survival rate was 100% at 5 years. Pad-free continence and potency (erections sufficient for intercourse) were retained in 97% and 73% of patients, respectively. There was no rectal fistula or mortality.
Conclusions:
Hemigland cryoablation of localized prostate cancer provides effective midterm oncologic out-comes with good continence and potency. Patients with higher baseline prostate specific antigen are at increased risk for biochemical failure, recurrent cancer and treatment failure.
Keywords: prostatic neoplasms, cryosurgery, prostate specific antigen, treatment failure, risk
Cryoablation is an alternative treatment option for localized PCa.1 In fact, a randomized controlled trial showed similar long-term oncologic outcomes for whole gland cryoablation and radiation therapy of localized PCa.2 Recently primary focal cryoablation has gained attention because of encouraging oncologic outcomes and a low side effect profile.3–6 However, these reports mainly focused on low to intermediate risk PCa as such data on focal cryoablation of high risk PCa are sparse.
We previously reported functional and oncologic outcomes in 73 men with low to intermediate risk PCa who underwent focal cryoablation as primary treatment.3 In this study we updated our series of 160 men and present the outcomes of hemigland cryoablation in men with high risk PCa. Additionally, we analyzed predictors of BF, recurrent and/or residual CSPCa on followup biopsy, conversion to radical treatment and TF after hemigland cryoablation of localized PCa.
MATERIALS AND METHODS
Using an Institutional Review Board approved database (IRB No. HS-17–00749) we retrospectively reviewed the records of 160 consecutive men who underwent hemigland cryoablation as treatment of localized low, intermediate or high risk PCa from 2002 to 2014. Hemigland cryoablation was defined as hemigland ablation of the prostate lobe harboring the dominant, biopsy proven PCa. The study inclusion criterion for focal cryoablation was unilateral PCa or select men with bilateral PCa in whom there was low volume disease in the contralateral lobe (GG 2 or less, less than 10% of the core involved by cancer and 2 or fewer positive cores). Patients treated with whole gland or salvage cryoablation were excluded from analysis.
Hemigland cryoablation was performed by 1 of 3 experts (DKB, OU or ALA) at a total of 2 facilities. Deidentified data were retrospectively merged after receiving IRB approval (HS-17–00749). Hemigland (1 lobe) cryoablation was performed as 2 freeze-thaw cycles using a freehand technique under TRUS guidance as previously described (supplementary material, https://www.jurology.com).3,7 Demographics, clinical characteristics, and perioperative and followup data were obtained retrospectively from the patient records and by contacting patients and physicians. Patients were categorized into a low, intermediate or high risk PCa cohort according to the D’Amico criteria.8 Patients with high risk PCa underwent metastatic workup, including a whole body bone scan and computerized tomography of the abdomen and pelvis. Any neoadjuvant ADT was discontinued before cryoablation. No patient received adjuvant ADT.
All study procedures in human participants were done in accordance with the ethical standards of the institutional and/or national research committee and according to the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants.
At study entry gray scale and Doppler multiparametric TRUS (Hitachi, Santa Clara, California) was performed, followed by image targeting of suspicious lesions and systematic sextant prostate biopsy. This cohort dates back to 2002, when the availability of MRI of PCa was limited. Nevertheless, detailed cancer location mapping was created in 3-dimensional schematic drawings and multiparametric TRUS images or multiparametric MRI in recent years were electronically stored for surgical planning and followup as previously described.3,7,9
Followup was typically scheduled every 3 months in year 1, every 6 months in years 2 to 5 and annually thereafter to assess digital rectal examination, PSA and multiparametric imaging (TRUS or MRI).3,7 Followup biopsy, consisting of systematic sextant biopsy and image targeted biopsy of suspicious areas, was strongly recommended in all patients at 12 months, or at any time if clinically indicated, as guided by BF, rising PSA or suspicion of PCa recurrence on digital rectal examination or multiparametric imaging.3,7
The Phoenix criterion of the PSA nadir plus 2 ng/ml was used to define BF.10 PCa was reported according to the ISUP (International Society of Urological Pathology) standards.11 CSPCa was defined as GG 2 or greater (Gleason score 3 + 4 or greater) on followup biopsies.11 RT was defined as any radical and/or whole gland treatment, including whole gland ablation, radiation or surgery.6,10,12,13 TF was defined as any whole gland treatment (RT criteria), ADT initiation (systemic therapy), metastasis and PCa specific mortality.6,12
Continence, potency and the I-PSS or the AUA-SS (American Urological Association Symptom Score) were evaluated using the best score on validated questionnaires within 2 years after focal cryoablation.3 Continence was strictly defined as no pad use.3 Potency was defined as a patient reported score of 3 or higher on the IIEF-5 (International Index of Erectile Function-5) question 2.3,14 The study primary end point was TF-free survival.6,12 The secondary end points were survival free of BF, CSPCa and RT. Followup biopsy outcomes were evaluated according to the GG at baseline and followup biopsy status. Urinary symptoms, continence and erectile function were evaluated. We assessed baseline predictors of the primary and secondary end points.
Statistical analysis was done with EZR, version 1.35 (Saitama Medical Center, Kawagoe, Japan). The Mann-Whitney and chi-square tests or the Fisher exact test were used for continuous and categorical variables, respectively. We constructed Kaplan-Meier plots to estimate survival probability and used the log rank test to test differences among the risk groups. Multivariable Cox regression analysis was performed for clinically important and statistically significant parameters on exploratory comprehensive univariate Cox regression analysis. Multivariable logistic regression analysis was done for functional outcomes. Statistical significance was considered at p <0.05.
RESULTS
Demographic data included median age 67 years (IQR 60–74), median PSA 6.3 ng/ml (IQR 4.2–9.0), median prostate volume 40 cc (IQR 31–50) and median PSAD 0.16 ng/ml/cc (IQR 0.09–0.24) (table 1). Median followup was 40 months (IQR 20–59) and followup was longer than 1 year in 141 men (88%). A total of 131 patients (82%) had D’Amico intermediate (66%) or high risk (16%) prostate cancer. At study entry the contralateral untreated lobe harbored PCa in 9 patients, which was GG 1 in 8 and GG 2 in less than 5% of 1 core in an 84-year-old patient (table 1).
Table 1.
Baseline data on PCa hemigland cryoablation
| No. pts | 160 | |
| Median age (IQR) | 67 | (60–74) |
| Median ng/ml PSA (IQR) | 6.3 | (4.2–9.0) |
| Median cc prostate vol (IQR) | 40 | (31–50) |
| Median ng/ml/cc PSA density (IQR) | 0.16 | (0.09–0.24) |
| No. clinical stage (%): | ||
| T1c | 98 | (61) |
| T2a | 49 | (31) |
| T2b | 13 | (8) |
| No. Gleason Grade Group (%): | ||
| 1 | 39 | (24) |
| 2 | 55 | (34) |
| 3 | 45 | (28) |
| 4 | 17 | (11) |
| 5 | 4 | (3) |
| No. D’Amico risk group (%): | ||
| Low | 29 | (18) |
| Intermediate | 106 | (66) |
| High | 25 | (16) |
| No. pos biopsy side (%): | ||
| Unilat | 151 | (94) |
| Bilat | 9 | (6) |
| No. neoadjuvant ADT (%) | 29 | (18) |
| Median study entry biopsy results (IQR): | ||
| No. pos biopsy cores | 2 | (2–3) |
| Max Ca core length/core (mm) | 6 | (2.5–9) |
| Max % Ca/core | 40 | (20–65) |
During followup 104 men (65%) underwent a total of 176 biopsies. The median PSA nadir was 0.9 ng/ml (IQR 0.5–2.3) and median time to the PSA nadir was 4 months (IQR 3–8). The median PSA decrease from preoperatively to the postoperative nadir was 83% (IQR 63–92) (table 2). Figure 1 shows PSA kinetics.
Table 2.
Oncologic outcomes after hemigland cryoablation by D’Amico criteria PCa risk group
| PCa Risk Group |
||||||||
|---|---|---|---|---|---|---|---|---|
| Overall | Low | Intermediate | High | |||||
| No. pts (%) | 160 | 29 | (18) | 106 | (66) | 25 | (16) | |
| Median mos followup (IQR) | 40 | (20–59) | 45 | (22–74) | 34 | (19–56) | 36 | (19–57) |
| Median ng/ml PSA nadir (IQR) | 0.9 | (0.5–2.3) | 1.2 | (0.75–2.3) | 0.86 | (0.49–2.1) | 0.8 | (0.24–3.1) |
| Median mos to PSA nadir (IQR) | 4 | (3–8) | 6 | (3–11) | 4 | (3–8) | 4 | (3–8) |
| Median % PSA decrease (IQR) | 83 | (63–92) | 76 | (51–86) | 84 | (63–92) | 85 | (66–94) |
| No. greater than 70% PSA decrease (%)* | 107 | (67) | 18 | (62) | 71 | (67) | 18 | (72) |
| % 5-Yr survival: | ||||||||
| Phoenix criteria biochemical failure-free | 62 | 78 | 57 | 67 | ||||
| Clinically significant PCa-free on followup biopsy | 70 | 87 | 64 | 74 | ||||
| Radical treatment-free† | 89 | 95 | 86 | 96 | ||||
| ADT-free | 95 | 100 | 96 | 81 | ||||
| Treatment failure-free‡ | 85 | 95 | 83 | 78 | ||||
| 5-Yr repeat focal cryoablation-free | 86 | 82 | 87 | 88 | ||||
Five-year treatment failure-free survival, metastasis-free survival, cancer specific survival and overall survival were 100%.
Percent of PSA decreased at nadir = (PSA at entry – PSA nadir)/(PSA at entry × 100).
Radical treatment defined as any whole gland treatment.
Treatment failure defined as any whole gland treatment, ADT initiation, metastasis and PCa specific mortality.
Figure 1.
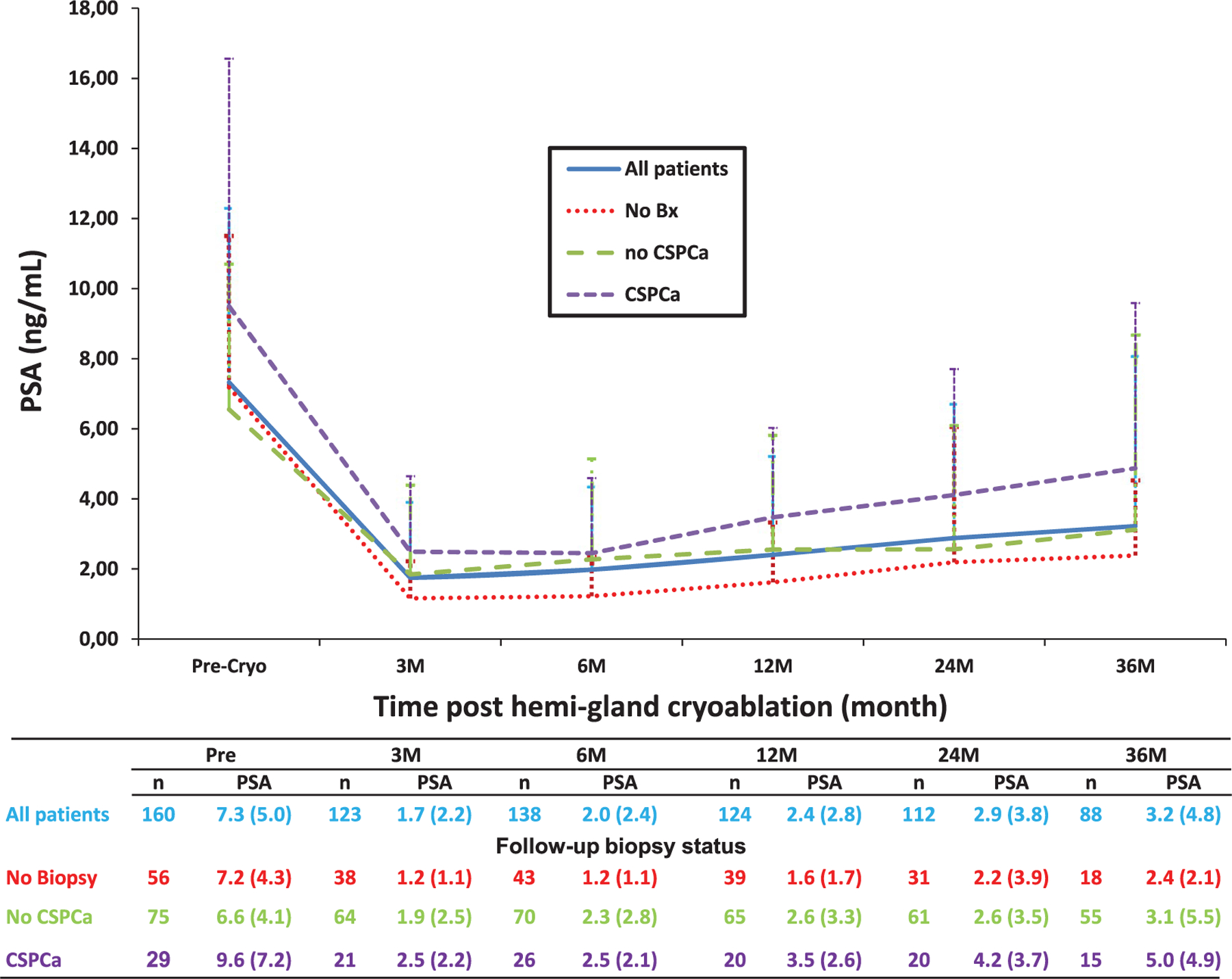
Mean ± SD followup PSA kinetics after hemigland cryoablation by followup prostate biopsy status. No Bx, no prostate biopsy. CSPCa, CSPCa on followup biopsy. No CSPCa, no CSPCa on followup biopsy. Cryo, cryoablation. M, month. n, number of patients.
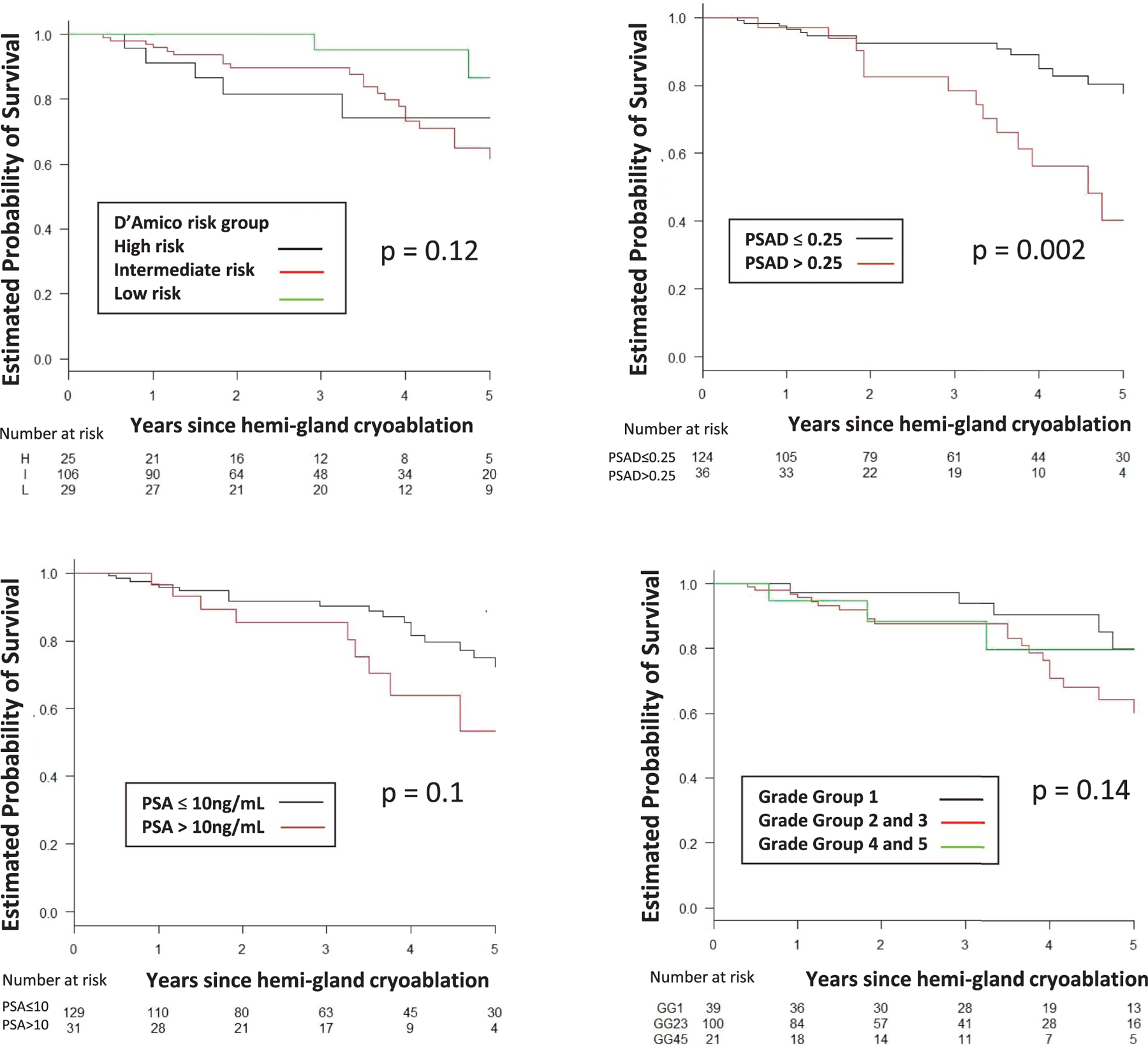
The 5-year estimated rate of survival free of TF, BF, CSPCa and RT was 85%, 62%, 70% and 89%, respectively (table 2). Figures 2 to 5 show estimated survival by PCa risk, PSA, PSAD and GG. Table 3 lists followup biopsy outcomes. Bone metastasis developed in 1 patient and in another lymph node metastasis was discovered at salvage prostatectomy. The rate of metastasis-free survival was 100% at 5 years and 89% at 7 years. No patient died. Five-year cancer specific and overall survival was 100% and 100%, respectively. Higher baseline PSA independently predicted TF (p <0.001), BF (p = 0.048), recurrent CSPCa (p <0.01) and RT (p <0.01). GG 3 or greater independently predicted TF (p = 0.04, supplementary table 1, https://www.jurology.com).
Figure 2.
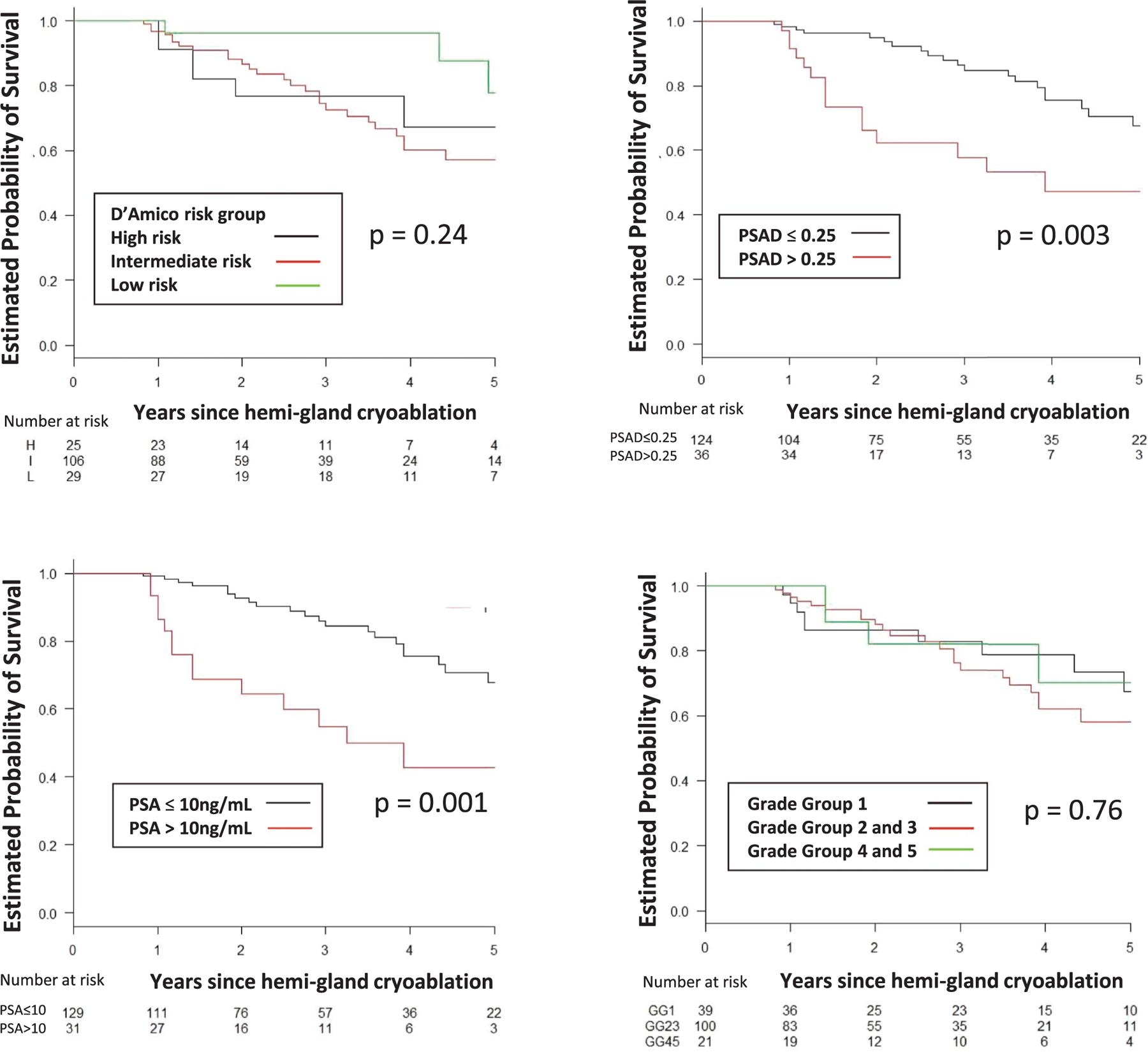
BF-free survival by D’Amico risk group, PSA, PSAD in ng/ml/cc and GG. H, high. I, intermediate. L, low.
Figure 5.
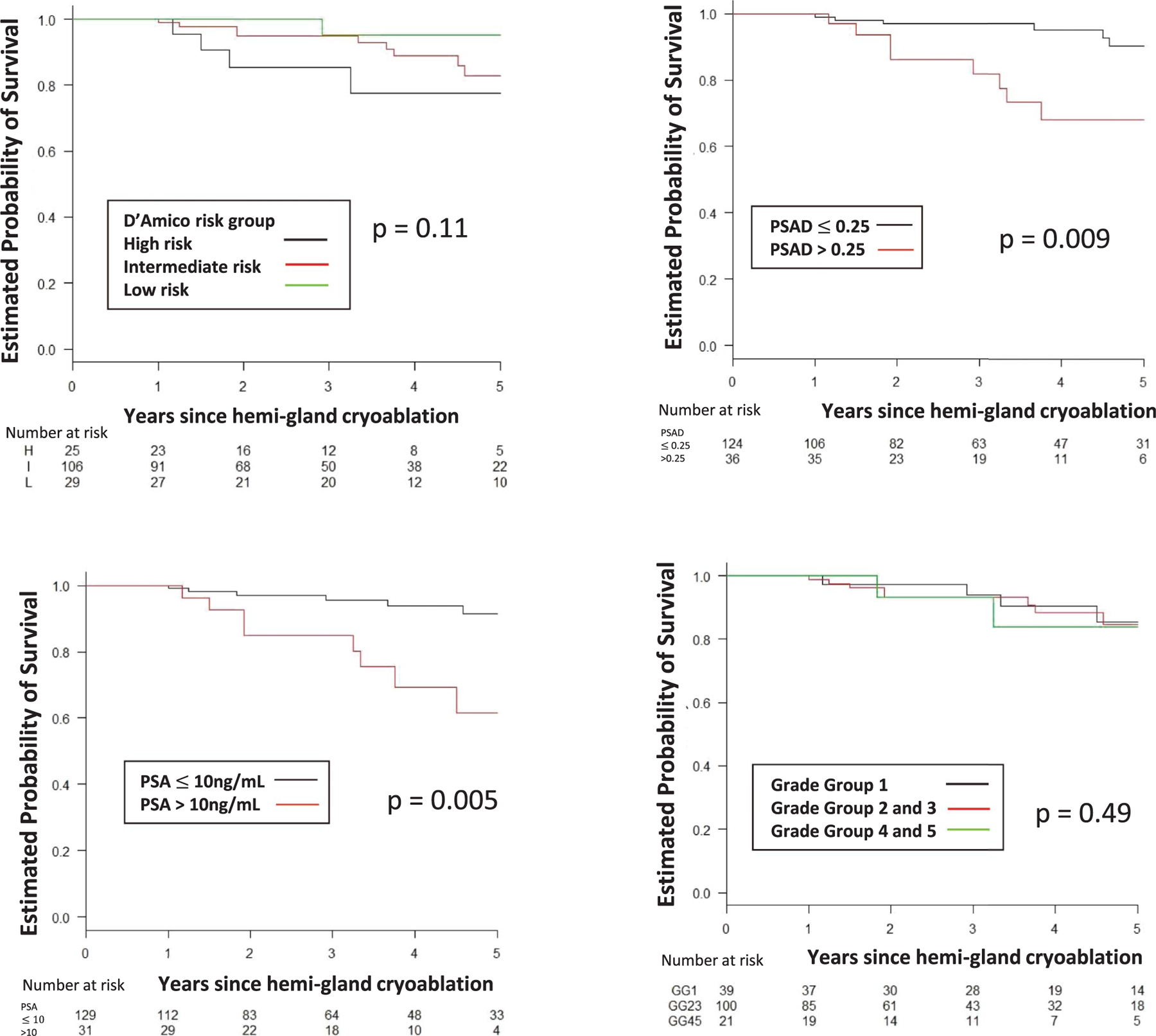
Treatment failure-free survival by D’Amico risk group, PSA, PSAD in ng/ml/cc and GG. H, high. I, intermediate. L, low.
Table 3.
Followup biopsy outcomes after hemigland cryoablation by baseline PCa Grade Group and followup biopsy status
| % 3/5-Yr Followup Biopsy Survival Outcome* |
|||
|---|---|---|---|
| GG | No. Pts | GG 1–5 PCa-Free | Clinically Significant PCa-Free |
| Any at baseline: | |||
| All pts | 160 | 79/58 | 89/70 |
| Followup biopsy† | 104 | 70/47 | 85/63 |
| 1 at Baseline: | |||
| All pts | 39 | 70/62 | 94/80 |
| Followup biopsy† | 34 | 67/57 | 93/77 |
| 2 or Greater at baseline: | |||
| All pts | 121 | 81/58 | 88/64 |
| Followup biopsy† | 70 | 72/43 | 81/60 |
Considering any Grade Group (1–5) cancer on followup prostate biopsy.
Considering only the patients who underwent followup prostate biopsy.
Overall 29 patients (18%) received neoadjuvant ADT prior to focal cryoablation for a median of 12 months, including 1 at low risk, 17 at intermediate risk and 11 at high risk. Neoadjuvant ADT did not predict the oncologic end points (supplementary tables 1 and 2, https://www.jurology.com).
Continence and potency were retained in 97% and 73% of patients, respectively. In 100 patients (63%) with an available I-PSS score before and after focal cryoablation the median I-PSS decreased by 2 points (p <0.001, table 4, and figs. 6 and 7). No rectal fistula was reported.
Table 4.
Functional outcomes after PCa hemigl and cryoablation
| No. potent before hemigland cryoablation (%) | 91 | (57) |
| No. potent before hemigland cryoablation by IIEF-5 question 2 score (range 1–5): | ||
| 3 | 8 | |
| 4 | 29 | |
| 5 | 54 | |
| No. potent after hemigland cryoablation by IIEF-5 question 2 score (range 1–5): | ||
| 1–2 | 25 | |
| 3 | 18 | |
| 4 | 29 | |
| 5 | 19 | |
| No. potency maintained (%): | ||
| After hemigland cryoablation | 66 | (73) |
| Using phosphodiesterase 5-inhibitor | 21 | (30) |
| No. continence (%): | ||
| Before hemigland cryoablation | 157 | (98) |
| Maintained after hemigland cryoablation | 153 | (97) |
| Median AUA-SS (IQR): | ||
| Before hemigland cryoablation | 7 | (3–12) |
| After hemigland cryoablation | 5 | (3–8) |
| Median I-PSS difference before vs after hemigland ablation (IQR) | 2 | (−1–5)* |
Continence was defined as no pad use and functional outcomes were assessed within 2 years of followup by best score on patient self-reported questionnaire and definition of potency considered patient report of score of 3 or more on IIEF-5 question 2, “When you had erections with sexual stimulation, how often were your erections hard enough for penetration (entering your partner)?” 1 Point—almost never or never, 2 points—few times (much less than half time), 3 points— -sometimes (about half time), 4 points—most times (much more than half time) and 5 points—almost always or always.
In 100 patients with available I-PSS (p<0.001).
Figure 6.
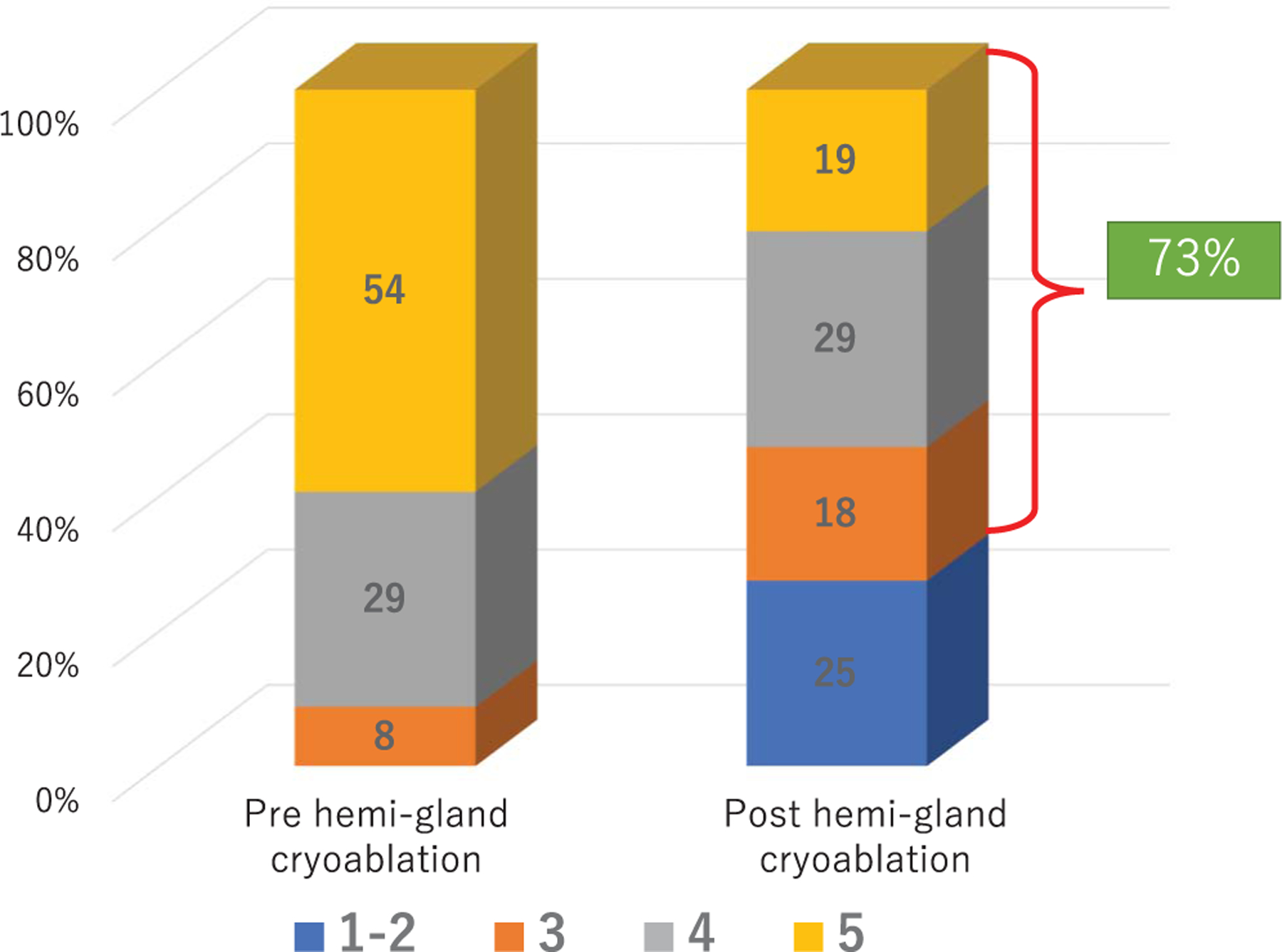
Intercourse score on IIEF-5 question 2 before and after PCa hemigland cryoablation in 91 preoperatively potent men.
Figure 7.
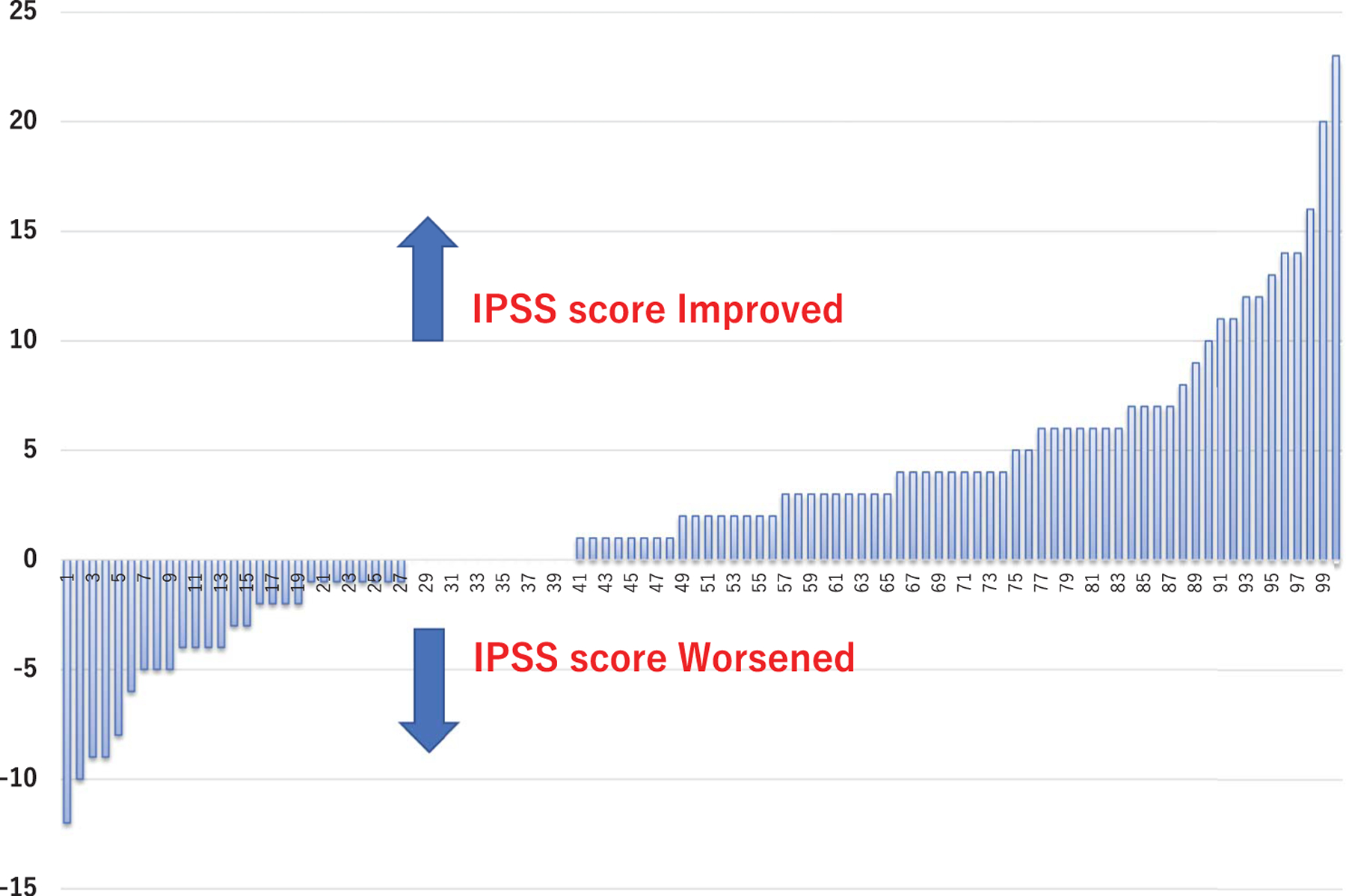
I-PSS difference before to after PCa hemigland cryoablation
DISCUSSION
Delivering cancer control while avoiding radical treatment and maintaining quality of life are the central goals of focal therapy. We report TF-free survival to reflect patients who underwent RT or ADT initiation, in whom metastasis developed or who died of PCa, similar to other recent studies on focal therapy of PCa, including cryoablation and HIFU.6,12 Our cohort comprised 160 patients treated with hemigland cryoablation of localized PCa with a median 40 months of followup, including 106 with intermediate and 25 with high risk PCa.
Five-year TF-free survival, which was the main end point of our study, was 85% in the entire cohort, and 95%, 83% and 78% in men with low, intermediate and high risk PCa, respectively. A recent multi-institutional study described early term to midterm outcomes (median followup 27.8 months) in 122 patients treated with focal cryoablation, including 87 with intermediate and 35 with high risk PCa. Using a definition similar to ours as the main end point, the authors reported a 3-year TF-free survival rate of 90.5% in all patients, and 93.3% and 84.7% in those with intermediate and high risk PCa, respectively.6 Comparably, using the same definition as in this report, 5-year TF-free survival after focal HIFU was 88% in all PCa cases, 96% in low risk cases, 88% in intermediate risk cases and 84% in high risk cases.12
We report 86% 5-year freedom from repeat hemigland cryoablation. Additionally, we report freedom from RT with 89% survival free of radical treatment in 5 years. Recently 3 multi-institutional studies described the short-term to midterm outcomes of partial prostate gland ablation using HIFU in men with low to high risk PCa.12,15,16 For focal HIFU the rate of freedom from RT at 2 years was 81% to 89%15,16 and at 5 years it was 96%, 88% and 84% for low, intermediate and high risk PCa, respectively.12
There is a paucity of studies on predictors of PCa recurrence or radical and/or salvage treatment after focal cryoablation. Kongnyuy et al reported that the number of positive biopsy cores before focal cryoablation and a higher PSA nadir after focal cryoablation predicted BF.5 Others reported that baseline PSA and stage T3 disease correlated with TF after focal HIFU.12 We found that higher PSA at study entry was an independent predictor of TF, BF, recurrent CSPCa and the need for RT after hemigland cryoablation. Additionally, we found that GG greater than 3 before hemigland cryoablation prostate biopsy was a predictor of TF (supplementary table 1, https://www.jurology.com).
In our study followup biopsy was not performed in all patients. In fact, 104 men (65%) underwent a total of 176 biopsies. Followup biopsy may be affected by indication (for cause vs mandatory), followup duration and patient compliance. Patients with low and stable PSA without clinical suspicion for PCa recurrence may defer followup biopsy.15 Figure 1 shows that PSA kinetics were similar in patients with negative biopsy after hemigland cryoablation and those with no followup biopsy. Recent retrospective studies described the performance of followup biopsy in 36% to 52% of men who underwent focal HIFU ablation.12,15 A focal cryoablation prospective registry reported that 29 of 122 patients underwent followup biopsy for cause due to rising PSA and suspicious findings on MRI.6 A prospective study in 62 men with low to intermediate risk PCa who underwent focal cryoablation showed that mandatory 1-year followup biopsy was negative in 50 (81%).4 The 12 men with positive followup biopsy findings had Gleason score 6 disease with 1 or 2 positive cores.
We report 70% 5-year CSPCa-free survival in the entire cohort and rates of 87%, 74% and 64% for low, intermediate and high risk PCa, respectively. Table 3 shows followup biopsy outcomes after hemigland cryoablation according to the PCa GG at baseline and followup biopsy status. The supplementary figure (https://www.jurology.com) shows the secondary and tertiary treatments after CSPCa was detected on followup biopsy after hemigland cryoablation.
There is a lack of consensus on the definition of BF after focal therapy. In our study we used the Phoenix criteria because of broad acceptance and it can be applied in men with localized PCa who undergo neoadjuvant ADT.3,5,10,13 The role of PSA in followup monitoring after focal therapy is debatable.17 We noted a median 83% PSA reduction from before to after cryoablation. As reported by others, our data indicate that if the PSA reduction after focal cryoablation is more than 50% of preoperative PSA, followup biopsies are likely to be negative for CSPCa.18 After hemigland cryoablation patients with biopsies positive for CSPCa had PSA greater than 4 ng/ml and/or a PSA reduction less than 50% after 2 years of followup (fig. 1). A recent study showed that an 80% PSA reduction was associated with no BF after focal cryoablation, indicating appropriate ablation of the index cancer.3,19–21
Whole gland ablative therapies, including cryoablation and HIFU, have been applied to treat select men with high risk PCa.13,22 We recently reported outcomes of whole gland cryoablation with 5-year 69% clinical recurrence-free survival in men with high risk PCa and 95% overall metastasis-free survival.13 Along this continuum, carefully selected, well informed, motivated men with high risk, unilateral, low volume, clinically localized disease might be potential candidates for focal therapy. Encouraging outcomes of focal HIFU and focal cryoablation in men with high risk PCa were recently reported. In conjunction with our study, they represent the largest series with longer followup of patients with high risk PCa who underwent focal therapy.6,12,23 It is interesting that different operators applying different ablative energy achieved similar outcomes, demonstrating the reproducibility of focal therapy of PCa.
To our knowledge from a mechanistic and pathophysiological standpoint there are no data to indicate that GG 4 or greater disease would be more resistant to cryotherapy than GG 1 to 3 disease. Double freeze-thaw cycles, which rapidly generate a temperature of –40C, induce irreversible cell death.24,25 In our study patients who underwent hemigland cryoablation of GG 5 PCa were likely to be older and would not accept adverse quality of life outcomes. In fact, 3 of the 4 patients with GG 5 PCa in our cohort, who were 74, 78 and 82 years old, had no biopsy evidence of PCa recurrence at a followup of 25, 35 and 18 months, respectively. In the remaining patient, who was 59 years old, followup biopsy revealed GG 1 PCa in the untreated lobe after focal cryoablation. In this patient PSA remained stable at 1 to 2 ng/ml during 9 years of AS.
Currently select patients with low risk PCa are candidates for AS. In our practice patients with low risk PCa were offered AS. However, our cohort began in 2002, when AS was still early in its adoption curve. Nonetheless, a critique of our study is that the patients at low risk would be candidates for AS. In this regard recent Level 1 randomized data showed benefits of focal ablation compared to AS in men with low risk PCa.26 Men with low risk PCa had lower rates of conversion to radical treatment, cancer progression and CSPCa on followup biopsy in the ablation group compared to the AS group.26
Functional preservation is the main rationale to consider organ sparing treatment. In our study the urinary continence rate, stringently defined as no pad use, was 97%, similar to that in other reports.3,4,22 Urinary symptoms were evaluated using validated AUA-SS initially with transition to the I-PSS more recently. In the 100 men with available I-PSS data the median I-PSS decreased 2 points from before to after hemigland cryoablation.4 The potency rate varies considerably after cryoablation according to the definition used. Potency was preserved in 73% of patients, of whom 30% used pharmaceuticals, similar to other reports.3,4,22
Our study is limited by its retrospective nature. However, it represents real-world clinical outcomes with some of the longest reported hemigland cryoablation followup and a higher rate of followup biopsy (65%) compared to other reports.6,12,23 Our cohort started in 2002 when prostate multiparametric MRI and MRI/TRUS fusion biopsy were not widely performed. Therefore, it is possible that followup MRI may identify more patients with local recurrence.27 However, multiparametric MRI may also provide better patient selection at entry, thus improving the outcomes. Additionally, patients did not undergo reconfirming biopsy prior to hemigland cryoablation, which may have led to biopsy misattribution at study entry. Of the patients 18% had received neoadjuvant ADT. Because no patient received adjuvant ADT, the Phoenix criteria could still be applied, similar to patients who undergo radiation therapy.2,3,5,7,10,13 Nevertheless, neo-adjuvant ADT did not predict any oncologic end point (supplementary table 1, https://www.jurology.com).
Given the limitations of this study, hemigland cryoablation should be performed with caution and in carefully selected patients, especially in those with high risk PCa. Additional data from prospective trials with large and multicenter cohorts are necessary to validate our reported findings.
CONCLUSIONS
As primary treatment of localized PCa hemigland cryoablation provides effective midterm oncologic outcomes with a minimal impact on continence and potency. Patients with higher baseline PSA are at increased risk for BF, recurrent cancer and TF.
Supplementary Material
Figure 3.
CSPCa-free survival by D’Amico risk group, PSA, PSAD in ng/ml/cc and GG. H, high. I, intermediate. L, low.
Figure 4.
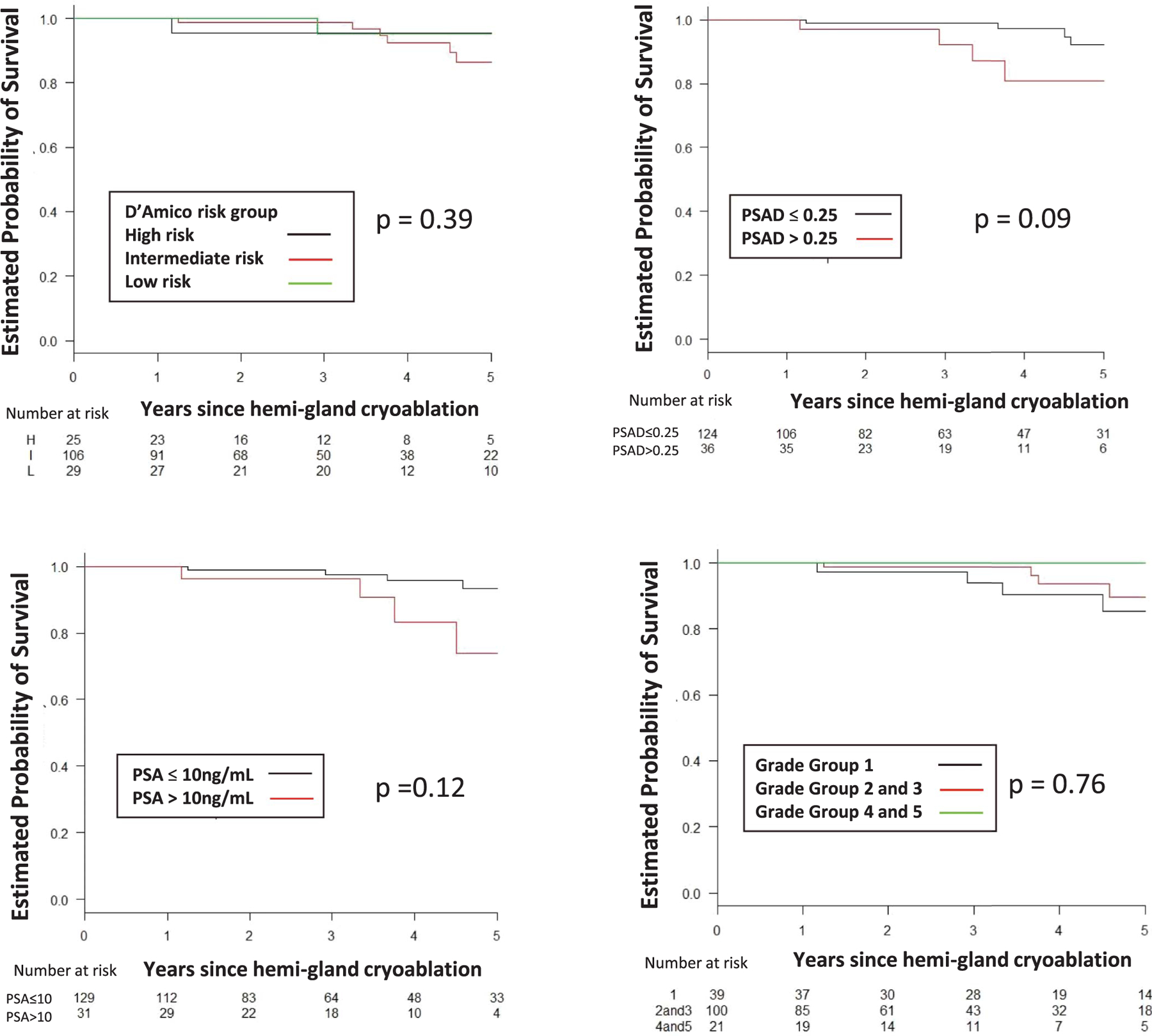
RT-free survival by D’Amico risk group, PSA, PSAD in ng/ml/cc and GG. H, high. I, intermediate. L, low.
Abbreviations and Acronyms
- ADT
androgen deprivation therapy
- AS
active surveillance
- BF
biochemical failure
- CSPCa
clinically significant PCa
- GG
Grade Group
- HIFU
high intensity focused ultrasound
- I-PSS
International Prostate Symptom Score
- MRI
magnetic resonance imaging
- PCa
prostate cancer
- PSA
prostate specific antigen
- PSAD
PSA density
- RT
radical treatment
- TF
treatment failure
- TRUS
transrectal ultrasound
Footnotes
The corresponding author certifies that, when applicable, a statement(s) has been included in the manuscript documenting institutional review board, ethics committee or ethical review board study approval; principles of Helsinki Declaration were followed in lieu of formal ethics committee approval; institutional animal care and use committee approval; all human subjects provided written informed consent with guarantees of confidentiality; IRB approved protocol number; animal approved project number.
No direct or indirect commercial, personal, academic, political, religious or ethical incentive is associated with publishing this article.
Contributor Information
Masakatsu Oishi, USC Institute of Urology and Catherine and Joseph Aresty Department of Urology, Ventura, California; Departments of Urology, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto, Japan.
Inderbir S. Gill, USC Institute of Urology and Catherine and Joseph Aresty Department of Urology, Ventura, California
Alessandro Tafuri, USC Institute of Urology and Catherine and Joseph Aresty Department of Urology, Ventura, California; Kyoto, Japan, and University of Verona, Azienda Ospedaliera Universitaria Integrata Verona, Verona, Italy.
Aliasger Shakir, USC Institute of Urology and Catherine and Joseph Aresty Department of Urology, Ventura, California.
Giovanni E. Cacciamani, USC Institute of Urology and Catherine and Joseph Aresty Department of Urology, Ventura, California
Tsuyoshi Iwata, USC Institute of Urology and Catherine and Joseph Aresty Department of Urology, Ventura, California.
Atsuko Iwata, USC Institute of Urology and Catherine and Joseph Aresty Department of Urology, Ventura, California.
Akbar Ashrafi, USC Institute of Urology and Catherine and Joseph Aresty Department of Urology, Ventura, California.
Daniel Park, USC Institute of Urology and Catherine and Joseph Aresty Department of Urology, Ventura, California.
Jie Cai, USC Institute of Urology and Catherine and Joseph Aresty Department of Urology, Ventura, California.
Mihir Desai, USC Institute of Urology and Catherine and Joseph Aresty Department of Urology, Ventura, California.
Osamu Ukimura, USC Institute of Urology and Catherine and Joseph Aresty Department of Urology, Ventura, California; Departments of Urology, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto, Japan.
Duke K. Bahn, USC Institute of Urology and Catherine and Joseph Aresty Department of Urology, Ventura, California; Keck School of Medicine, University of Southern California, Los Angeles and Prostate Institute of America, Community Memorial Hospital, Ventura, California
Andre Luis Abreu, USC Institute of Urology and Catherine and Joseph Aresty Department of Urology, Ventura, California.
REFERENCES
- 1.Babaian RJ, Donnelly B, Bahn D et al. : Best practice statement on cryosurgery for the treatment of localized prostate cancer. J Urol 2008; 180: 1993. [DOI] [PubMed] [Google Scholar]
- 2.Donnelly BJ, Saliken JC, Brasher PM et al. : A randomized trial of external beam radiotherapy versus cryoablation in patients with localized prostate cancer. Cancer 2010; 116: 323. [DOI] [PubMed] [Google Scholar]
- 3.Bahn D, de Castro Abreu AL, Gill IS et al. : Focal cryotherapy for clinically unilateral, low-intermediate risk prostate cancer in 73 men with a median follow-up of 3.7 years. Eur Urol 2012; 62: 55. [DOI] [PubMed] [Google Scholar]
- 4.Barqawi AB, Stoimenova D, Krughoff K et al. : Targeted focal therapy for the management of organ confined prostate cancer. J Urol 2014; 192: 749. [DOI] [PubMed] [Google Scholar]
- 5.Kongnyuy M, Lipsky MJ, Islam S et al. : Predictors of biochemical recurrence after primary focal cryosurgery (hemiablation) for localized prostate cancer: a multi-institutional analytic comparison of Phoenix and Stuttgart criteria. Urol Oncol 2017; 35: 530.e15. [DOI] [PubMed] [Google Scholar]
- 6.Shah TT, Peters M, Eldred-Evans D et al. : Early-medium-term outcomes of primary focal cryotherapy to treat nonmetastatic clinically significant prostate cancer from a prospective multicentre registry. Eur Urol 2019; 76: 98. [DOI] [PubMed] [Google Scholar]
- 7.de Castro Abreu AL, Bahn D, Chopra S et al. : Real-time transrectal ultrasonography-guided hands-free technique for focal cryoablation of the prostate. BJU Int 2014; 114: 784. [DOI] [PubMed] [Google Scholar]
- 8.D’Amico AV, Moul J, Carroll PR et al. : Cancer-specific mortality after surgery or radiation for patients with clinically localized prostate cancer managed during the prostate-specific antigen era. J Clin Oncol 2003; 21: 2163. [DOI] [PubMed] [Google Scholar]
- 9.Ukimura O, de Castro Abreu AL, Gill IS et al. : Image visibility of cancer to enhance targeting precision and spatial mapping biopsy for focal therapy of prostate cancer. BJU Int 2013; 111: E354. [DOI] [PubMed] [Google Scholar]
- 10.Roach M III, Hanks G, Thames H Jr et al. : Defining biochemical failure following radio-therapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix consensus conference. Int J Radiat Oncol Biol Phys 2006; 65: 965. [DOI] [PubMed] [Google Scholar]
- 11.Epstein JI, Egevad L, Amin MB et al. : The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol 2016; 40: 244. [DOI] [PubMed] [Google Scholar]
- 12.Guillaumier S, Peters M, Arya M et al. : A multicentre study of 5-year outcomes following focal therapy in treating clinically significant non-metastatic prostate cancer. Eur Urol 2018; 74: 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oishi M, Gill IS, Ashrafi AN et al. : Primary whole-gland cryoablation for prostate cancer: biochemical failure and clinical recurrence at 5.6 years of follow-up. Eur Urol 2019; 75: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosen RC, Riley A, Wagner G et al. : The Inter-national Index of Erectile Function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997; 49: 822. [DOI] [PubMed] [Google Scholar]
- 15.Bass R, Fleshner N, Finelli A et al. : Oncologic and functional outcome of partial gland ablation with high intensity focused ultrasound for localized prostate cancer. J Urol 2019; 201: 113. [DOI] [PubMed] [Google Scholar]
- 16.Rischmann P, Gelet A, Riche B et al. : Focal high intensity focused ultrasound of unilateral localized prostate cancer: a prospective multicentric hemiablation study of 111 patients. Eur Urol 2017; 71: 267. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed HU, Freeman A, Kirkham A et al. : Focal therapy for localized prostate cancer: a phase I/II trial. J Urol 2011; 185: 1246. [DOI] [PubMed] [Google Scholar]
- 18.Inoue Y, Ushijima S, Shiraishi T et al. : Biochemical and magnetic resonance image response in targeted focal cryotherapy to ablate targeted biopsy-proven index lesion of prostate cancer. Int J Urol 2019; 26: 317. [DOI] [PubMed] [Google Scholar]
- 19.Kongnyuy M, Islam S, Mbah AK et al. : PSA kinetics following primary focal cryotherapy (hemiablation) in organ-confined prostate cancer patients. World J Urol 2018; 36: 209. [DOI] [PubMed] [Google Scholar]
- 20.Babaian RJ, Troncoso P, Steelhammer LC et al. : Tumor volume and prostate specific antigen: implications for early detection and defining a window of curability. J Urol 1995; 154: 1808. [DOI] [PubMed] [Google Scholar]
- 21.Ohori M, Eastham JA, Koh H et al. : Is focal therapy reasonable in patients with early stage prostate cancer (CAP)—an analysis of radical prostatectomy (RP) specimens (abstract 1574). J Urol, suppl, 2006; 175: 507. [Google Scholar]
- 22.Valerio M, Cerantola Y, Eggener SE et al. : New and established technology in focal ablation of the prostate: a systematic review. Eur Urol 2017; 71: 17. [DOI] [PubMed] [Google Scholar]
- 23.Stabile A, Orczyk C, Hosking-Jervis F et al. : Medium term oncological outcomes in a large cohort of men treated with either focal or hemiablation using HIFU for primary localized prostate cancer. BJU Int 2019; 124: 431. [DOI] [PubMed] [Google Scholar]
- 24.Baust J, Gage A, Johansen TB et al. : Mechanisms of cryoablation: clinical consequences on malignant tumors. Cryobiology 2014; 68: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bahn DK, Silverman P, Lee F Sr et al. : In treating localized prostate cancer the efficacy of cryoablation is independent of DNA ploidy type. Technol Cancer Res Treat 2004; 3: 253. [DOI] [PubMed] [Google Scholar]
- 26.Gill IS, Azzouzi A-R, Emberton M et al. : Randomized trial of partial gland ablation with vascular targeted phototherapy versus active surveillance for low risk prostate cancer: extended followup and analyses of effectiveness. J Urol 2018; 200: 786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller B, Van Den Bos W, Brausi M et al. : Follow-up modalities in focal therapy for prostate cancer: results from a delphi consensus project. World J Urol 2015; 33: 1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


