Abstract
Purpose:
The clinical significance of a positive surgical margin after partial nephrectomy remains controversial. The association between positive margin and risk of disease recurrence in patients with clinically localized renal neoplasms undergoing partial nephrectomy was evaluated.
Materials and Methods:
A retrospective multi-institutional review of 1,240 patients undergoing partial nephrectomy for clinically localized renal cell carcinoma between 2006 and 2013 was performed. Recurrence-free survival was estimated using the Kaplan-Meier method and evaluated as a function of positive surgical margin with the log rank test and Cox models adjusting for tumor size, grade, histology, pathological stage, focality and laterality. The relationship between positive margin and risk of relapse was evaluated independently for pathological high risk (pT2-3a or Fuhrman grades III-IV) and low risk (pT1 and Fuhrman grades I-II) groups.
Results:
A positive surgical margin was encountered in 97 (7.8%) patients. Recurrence developed in 69 (5.6%) patients during a median followup of 33 months, including 37 (10.3%) with high risk disease (eg pT2-pT3a or Fuhrman grade III-IV). A positive margin was associated with an increased risk of relapse on multivariable analysis (HR 2.08, 95% CI 1.09–3.97, p=0.03) but not with site of recurrence. In a stratified analysis based on pathological features, a positive surgical margin was significantly associated with a higher risk of recurrence in cases considered high risk (HR 7.48, 95% CI 2.75–20.34, p <0.001) but not low risk (HR 0.62, 95% CI 0.08–4.75, p=0.647).
Conclusions:
Positive surgical margins after partial nephrectomy increase the risk of disease recurrence, primarily in patients with adverse pathological features.
Keywords: nephrectomy, laparoscopy, carcinoma, renal cell, kidney neoplasms
Partial nephrectomy has emerged as the treatment of choice for clinically localized renal masses.1 Although oncologic outcomes comparable to those of radical nephrectomy have been demonstrated, a realistic concern is violation of the tumor during resection, leaving residual disease in the nephrectomy bed.2 A positive surgical margin has been shown to increase the recurrence risk for many solid organ malignancies.3–9 However, several studies evaluating outcomes after PN for renal cell carcinoma have failed to demonstrate the prognostic significance of a PSM.10,11
Lack of consensus surrounding the clinical relevance of a PSM may result from broad interstudy variability in the pathological characteristics of the populations studied. Similar to prostate cancer, our appreciation for the heterogeneous behavior of RCC has matured in recent years.12–14 Lesions low in Fuhrman grade and stage follow a relatively indolent course, whereas tumors of advanced pathological phenotype exhibit a higher proclivity for growth and systemic spread, warranting early intervention.15–17 It seems plausible, then, that residual tumor in the context of PSM mimics the primary lesion rather than universally signifying disease meant to progress.
The low incidence of positive surgical margins and the relative infrequency of pathologically aggressive lesions treated with NSS limits the high risk patients evaluated in many series.10,11 Contemporary studies often lack the statistical power to discern differences between high risk patients with and without PSM as well as between high and low risk patients with PSM. We evaluated the impact of PSM on recurrence-free survival after NSS, using a multi-institutional cohort comprised of greater numbers of pathological high risk cases. Oncologic outcomes stratified by margin status and patient risk group were also analyzed.
METHODS
After institutional review board approval, data from patients (age 18 years or older) undergoing PN for clinically localized renal masses (clinical stage T1 or T2) between 2006 and 2013 at 4 high volume centers (University of California Irvine, North Shore LIJ, University of Southern California, University of Chicago) were collected. Patient demographics, surgical approach (minimally invasive vs open), tumor pathology (laterality, histology, Fuhrman grade, focality, pathological stage, size and margin status), duration and disease status at followup, and time and site of recurrence were evaluated. Patients with clinical stage T3 or greater disease, solitary kidney, benign pathology, familial RCC or RCC treated before 2006 were excluded from analysis. Institutional databases were prospectively collected, with de-identified data merged and analyzed.
Laparoscopic, robotic and open PN techniques have been previously described.18–20 Based on surgeon preference, extirpation was completed with tumor enucleation or sharp excision, and intraoperative biopsy of the resection bed was evaluated using frozen section. Specimens were sent for pathological evaluation, where margins were stained before manipulation. Malignant cells at the stained margin were reported as PSM. A negative surgical margin was defined as the absence of malignancy at the stained margin.
All patients had more than 1 year of postoperative surveillance, consisting of an initial visit between 6 and 12 months, followed by semiannual or annual visits. History, examination, laboratory testing, and imaging of the abdomen, pelvis and chest were performed at each visit. Imaging included the exclusive use of cross-sectional imaging or baseline CT followed by alternating use of ultrasound and CT based on surgeon preference. X-ray was used to screen the chest, except for pT3a disease, for which CT may have been obtained. Recurrence was considered if imaging demonstrated new lesions, with definitive diagnosis assigned only after tissue confirmation of histological congruence with the original tumor. Lesions in tissue adjacent to the resection site were classified as local recurrence, whereas metachronous lesions in the ipsilateral kidney away from the nephrectomy bed or in the contralateral kidney were not considered recurrence. Lesions in distant organs were considered metastatic. Patients with a PSM received no adjuvant intervention.
Patients were divided into 2 groups based on the presence of cancer at the surgical margin. Univariable comparisons of baseline characteristics between margin groups were performed using the Fisher exact test and the Student t-test. Time to recurrence was estimated using the Kaplan-Meier method. The primary objective was to compare the relapse risk after NSS between patients with and those without a PSM. The association of margin status with time to recurrence was evaluated with the log rank test on univariable analysis and the Cox proportional hazards model on multivariable analysis, adjusting for age, gender, tumor size, pathological stage (pT1, pT2, pT3a), histology (clear cell, papillary, chromophobe, other), Fuhrman grade (I-IV) and laterality.
Patients were divided into 2 composite subgroups based on recurrence risk observed for individual pathological metrics in relation to PSM, those of low risk (pT1 and Fuhrman grade I-II) and high risk (pT2-3 and/or Fuhrman grade III-IV). The relationship between margin status and relapse risk was evaluated independently for these pathological risk groups. Subgroup analysis was performed with unadjusted Cox proportional hazard models and the log rank test. The association of PSM with location of recurrence (local or metastatic) was analyzed with Fisher’s exact test. All statistical analyses were 2-tailed, and performed using Stata® 12.0 and R 3.2.0 (R Foundation for Statistical Computing, Vienna, Austria), with p <0.05 considered statistically significant.
RESULTS
Between 2006 and 2013, 1,240 patients underwent PN for clinical stage T1-T2N0M0 RCC, with 1,232 demonstrating cT1N0M0 disease and 8 cT2N0M0 disease. Minimally invasive and open PN were performed in 1,095 (88.3%) and 145 (11.7%) cases, respectively. Clinicopathological features are presented in table 1. Lesions up staged to pathological T3a revealed perirenal fat invasion and none demonstrated venous involvement. A PSM was observed in 97 (7.8%) cases, with 69 (71.1%) low risk and 28 (28.9%) high risk. PSM was unrelated to tumor size, histology, focality, Fuhrman grade, pathological stage or laterality (all p >0.05).
Table 1.
Baseline patient and disease characteristics
| Overall | PSM | NSM | p Value | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| No. pts (%) | 1,240 | (100) | 97 | (8) | 1,143 | (92) | – |
| Mean pt age (SD) | 59.1 (11.9) | 59.7 (11.5) | 59.0 (11.9) | 0.57 | |||
| No. gender (%): | 0.82 | ||||||
| M | 832 | (67) | 64 | (66) | 768 | (67) | |
| F | 408 | (33) | 33 | (34) | 375 | (33) | |
| Mean cm tumor size (SD) | 3.2 (1.7) | 3.3 (1.8) | 3.2 (1.6) | 0.64 | |||
| No. Fuhrman grade (%): | 0.29 | ||||||
| I | 184 | (15) | 12 | (12) | 172 | (15) | |
| II | 743 | (60) | 60 | (62) | 683 | (60) | |
| III | 290 | (23) | 21 | (22) | 269 | (24) | |
| IV | 23 | (2) | 4 | (4) | 19 | (2) | |
| No. tumor histology (%): | 0.56 | ||||||
| Clear cell | 851 | (69) | 69 | (71) | 782 | (69) | |
| Papillary | 321 | (26) | 21 | (22) | 300 | (26) | |
| Chromophobe | 50 | (4) | 5 | (5) | 45 | (4) | |
| Other | 18 | (1) | 2 | (2) | 16 | (1) | |
| No. tumor stage (%): | 0.16 | ||||||
| pT1 | 1,145 | (92) | 86 | (89) | 1,059 | (93) | |
| pT2 | 32 | (3) | 2 | (2) | 30 | (3) | |
| pT3a | 63 | (5) | 9 | (9) | 54 | (5) | |
| No. tumor focality (%): | 0.61 | ||||||
| Unifocal | 1,219 | (98) | 95 | (98) | 1,124 | (98) | |
| Multifocal | 21 | (2) | 2 | (2) | 19 | (2) | |
| No. tumor side (%): | 0.95 | ||||||
| Rt | 635 | (51) | 50 | (52) | 585 | (51) | |
| Lt | 580 | (47) | 46 | (47) | 534 | (47) | |
| Bilat | 25 | (2) | 1 | (1) | 24 | (2) | |
There were 69 (5.6%) recurrences during a median followup of 33 months (IQR 15–57) after PN. Median time to recurrence was 19 months (IQR 12–35). Local recurrence was appreciated in 42 (60.9%) patients and metastasis in 27 (39.1%) (table 2). Characteristics associated with increased recurrence risk on multivariable analysis included increasing tumor size (p <0.001), higher grade (grades III and IV, p=0.04 and 0.02, respectively), higher stage (pT3a, p <0.001) and clear cell histology (p=0.05).
Table 2.
Sites of disease recurrence
| No. PSMs | No. NSMs | |
|---|---|---|
|
| ||
| Local | 8 | 34 |
| Distant: | ||
| Lungs | 2 | 9 |
| Retroperitoneal lymph nodes | 3 | 5 |
| Bones | 1 | 3 |
| Adrenal | 1 | 2 |
| Liver | 1 | 2 |
| Omentum | 0 | 1 |
| Peritoneum | 1 | 1 |
| Gallbladder | 0 | 1 |
PSM was associated with increased recurrence risk after PN on univariable (fig. 1, p=0.003) and multivariable analysis (table 3, HR 2.08, 95% CI 1.09–3.97, p=0.03). PSM was not predictive of recurrence site.
Figure 1.
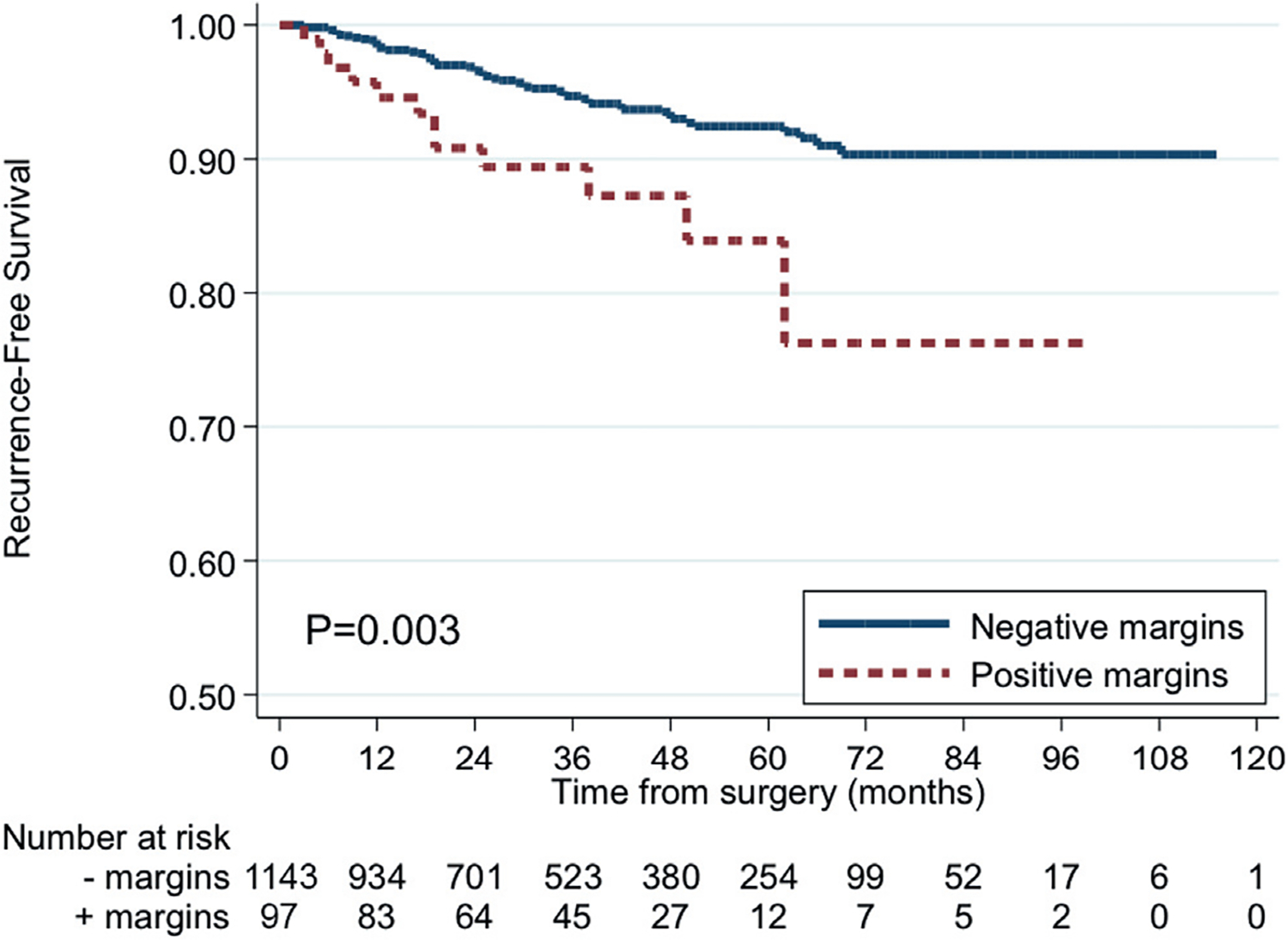
RFS by margin status
Table 3.
Multivariable analysis of time to recurrence
| HR | 95% CI | p Value | |
|---|---|---|---|
|
| |||
| PSM | 2.08 | 1.09–3.97 | 0.03 |
| Age | 1.00 | 0.98–1.02 | 0.66 |
| Tumor size (cm) | 1.37 | 1.18–1.60 | <0.001 |
| Fuhrman grade: | |||
| I | Reference | – | – |
| II | 1.40 | 0.54–3.66 | 0.49 |
| III | 2.78 | 1.04–7.39 | 0.04 |
| IV | 5.16 | 1.32–20.18 | 0.02 |
| Tumor stage: | |||
| pT1 | Reference | – | – |
| pT2 | 0.34 | 0.08–1.45 | 0.15 |
| pT3a | 4.08 | 2.15–7.74 | <0.001 |
| Tumor histology: | |||
| Clear cell | Reference | – | – |
| Papillary | 0.54 | 0.29–1.00 | 0.05 |
| Chromophobe | Not applicable | – | – |
| Other | 0.64 | 0.09–4.78 | 0.66 |
| Tumor side: | |||
| Rt | Reference | – | – |
| Lt | 1.18 | 0.72–1.93 | 0.51 |
| Bilat | 1.75 | 0.41–7.41 | 0.45 |
| Gender: | |||
| M | Reference | – | – |
| F | 1.41 | 0.79–2.54 | 0.25 |
Subgroups in which PSM was a predictor of recurrence were identified. We performed subgroup analysis stratified by stage (pT1 vs pT2-3a), histology (clear cell vs nonclear cell) and Fuhrman grade (I-II vs III-IV), the results of which are presented in figure 2. PSM was associated with a higher risk of recurrence among patients with higher stage disease, higher Fuhrman grade and clear cell histology. Thus, we divided patients into the 2 composite subgroups of low risk (pT1 and Fuhrman grade I-II) and high risk (pT2-3 and/or Fuhrman grade III-IV). Stratification of tumors into these categories resulted in 870 (70.2%) low risk and 370 (29.8%) high risk cases. We further divided low risk and high risk groups based on margin status and plotted survivals (fig. 3). Patients with a low risk PSM had RFS similar to those with a NSM. High risk patients with a PSM had a higher risk of recurrence compared to the other 3 groups (nearly 45% in 5 years, p <0.001). Multivariable analysis of PSM and time to metastasis stratified by risk group showed PSM to be significantly associated with a higher risk of recurrence among cases considered high risk (HR 7.48, 95% CI 2.75–20.34, p <0.001) but not low risk (HR 0.62, 95% CI 0.08–4.75, p=0.647).
Figure 2.
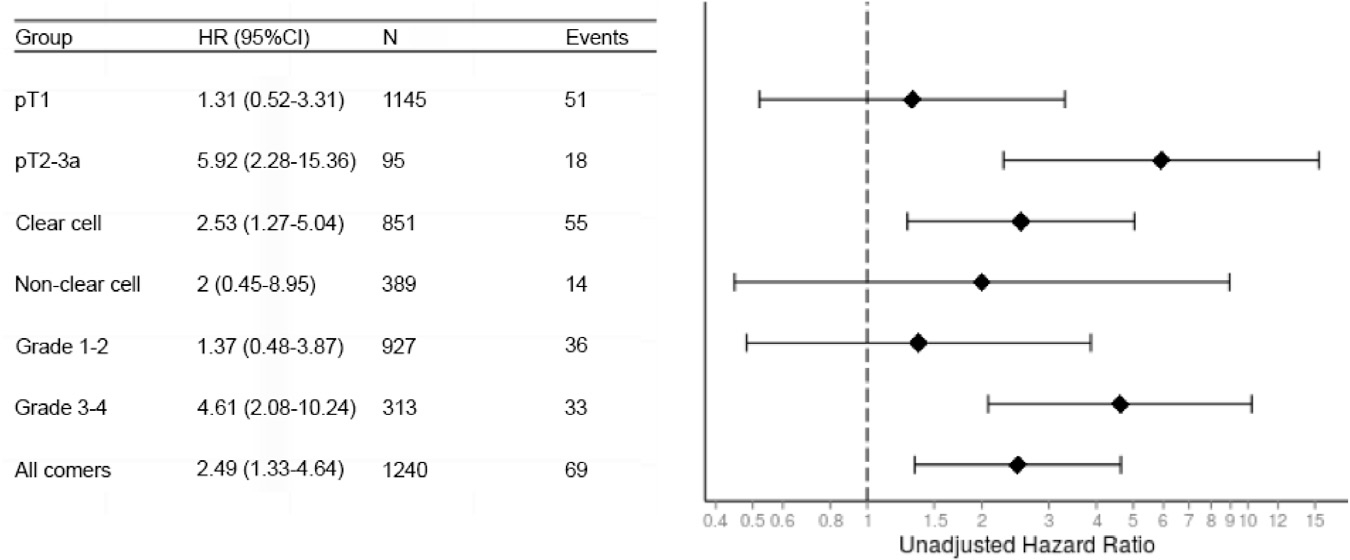
Association of PSM with recurrence by subgroups
Figure 3.
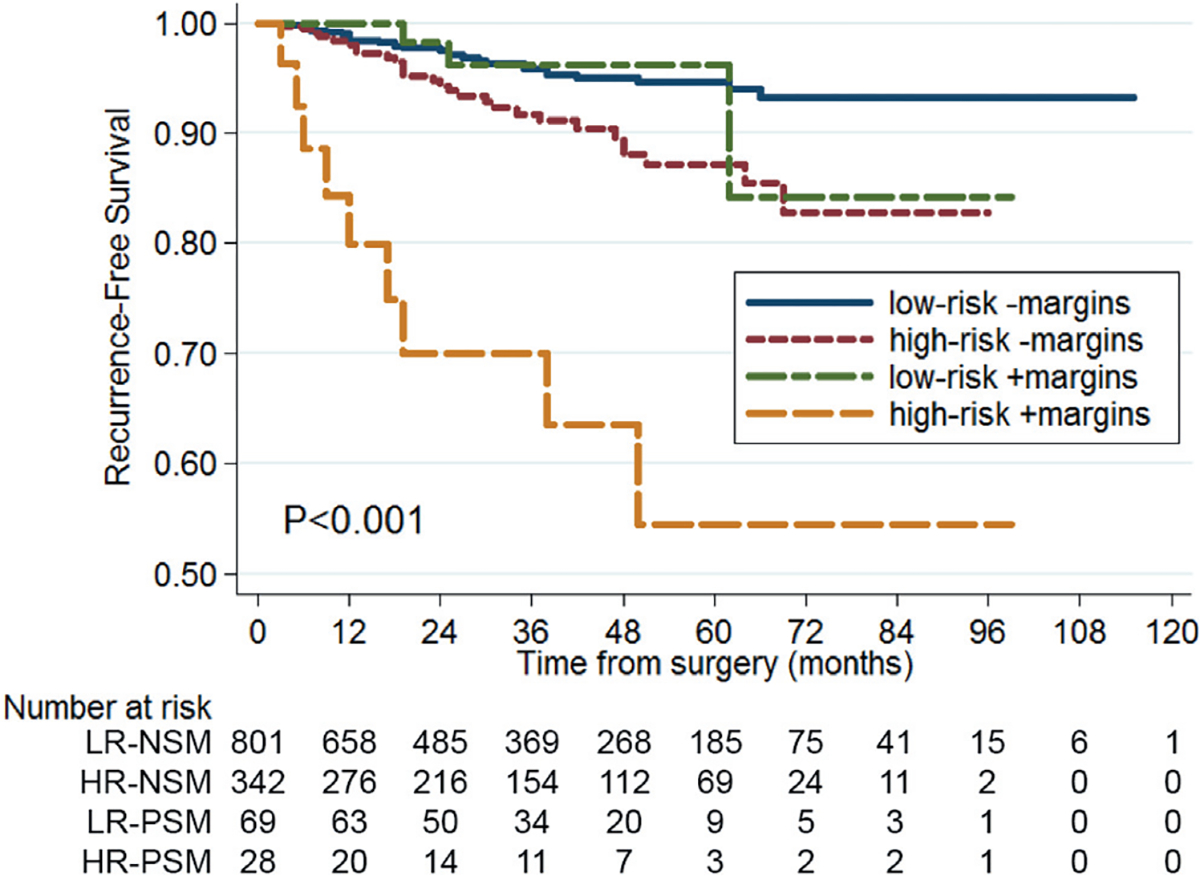
RFS by margin status and risk group. Low risk (LR)—pT1 and Fuhrman grade I-II. High risk (HR)—pT2-3 or Fuhrman grade III-IV.
DISCUSSION
Complete tumor resection is a cardinal principle in the surgical management of neoplastic disease as PSM is linked to inferior oncologic outcomes for many malignancies, including prostate and bladder cancer.3,4 Although the pursuit of a NSM during PN for renal cell carcinoma seems only logical, the significance of PSM has been questioned, with several studies unable to demonstrate oncologic detriment.8,10,11 In this study we evaluated the association between PSM and disease recurrence in patients undergoing PN for localized RCC in a multi-institutional cohort.
PSM was independently associated with local and metastatic tumor recurrence after PN. Interestingly this relationship was observed specifically for tumors with higher pathological stage (pT2-3a), higher Fuhrman grade (III-IV) or clear cell histology. Stratification into low risk (pT1 and Fuhrman grades I-II) and high risk (pT2-3 and/or Fuhrman grades III-IV) groups revealed high risk patients with a PSM to be at highest risk for recurrence compared to low and high risk patients with a NSM as well as low risk patients with a PSM. RFS was similar among low risk patients regardless of margin status.
This study’s results may serve to reconcile the dichotomy regarding the significance of margin status. PSM may not be an absolute prognosticator, but rather an adverse feature acting in concert with the histopathological properties of the primary lesion to influence recurrence patterns. The presence of a PSM must be interpreted in the context of tumor grade and stage. For high risk tumors our results suggest that PSM represents residual disease with high intrinsic malignant potential and, thus, the propensity for recurrence exceeding that of completely extirpated lesions. Conversely, with the relative indolence of low risk RCC already borne out through the followup of patients on active surveillance for incidentally detected masses, a PSM in low risk patients is expected to be of minimal prognostic relevance, as any residual tumor in the nephrectomy bed would obey slower growth kinetics and possess a low predilection for systemic spread.12
Several studies highlight the impact of PSM on RFS. A review of 777 patients undergoing PN revealed an increased relapse risk only among those with PSM and high risk pathology, with no recurrences observed for low grade or low stage tumors with PSM.7 We corroborate these findings in a larger cohort of high risk patients, enabling more meaningful analysis of PSM significance. Although their study linked PSM with local recurrence, we observed an increased risk of local as well as metastatic relapse. Higher rates of metastasis with PSM were also reported by Khalifeh et al after a review of 943 patients undergoing robotic PN.9 However, their study did not delineate groups most vulnerable to relapse.
Despite the clear association with disease recurrence shown in several reports, studies failing to assign prognostic relevance to PSM cannot be ignored. Permpongkosol et al observed no recurrences after NSS among patients with PSM, concluding PSM does not indicate residual disease.10 These results are not necessarily in contest with the current study conclusions and should be interpreted in context of the patients evaluated. Review of tumor characteristics in patients with a PSM reveals all sporadic lesions to have been low risk, pT1 disease. These results reaffirm our study finding that PSM is of minimal oncologic relevance in the setting of indolent histopathological features. In a separate study Bensalah et al did not associate PSM with decreased RFS on multivariable analysis.8 However, a matched cohort analysis revealed a significantly increased risk of relapse when controlling for prognostically relevant histopathological features. These findings are fundamentally in agreement with our conclusion that although not all positive margins progress, they do indeed increase the risk of recurrence.
We evaluate significance of PSM in the context of RFS, although CSS and overall survival analyses may be necessary to fully understand the clinical relevance of PSM. Recurrence remains a sentinel event shown to impact patient longevity.21–23 Understanding predictors of recurrence helps optimize surveillance protocols, enabling early detection when disease is most amenable to treatment.23–27 Studies that do not find PSM to compromise CSS often omit the discussion of management strategies for cases of recurrence, heterogeneity in which can influence oncologic outcomes and confound the true effect of PSM on CSS.8,28
The incidence of PSM in our study was 7.8%. Although reports cite rates between 0% and 7%, this is largely in the context of low stage lesions.2 Higher tumor stage and perinephric fat invasion have been shown to be independent predictors of PSM after NSS.28 The higher PSM rate in our series may be attributed to greater numbers of higher stage lesions, particularly those with perirenal fat involvement. As the use of PN extends beyond cT1a tumors, encompassing more anatomically complex lesions, the prevalence of PSM may increase, as evidenced in a study by Ani et al demonstrating an incidence of 10.7%.28
The optimal management of PSM after NSS remains uncertain. Considering that we found prognostic significance to depend on primary lesion pathology, it seems reasonable to tailor intervention to patient risk. Patients with high risk disease features (pT2-pT3a and/or Fuhrman grades III-IV) are at greatest risk for relapse when margins demonstrate tumor. However, the usefulness of repeat resection or completion nephrectomy in this cohort is qualified by several studies indicating that PSM does not necessarily signify residual disease. Bensalah et al observed residual disease in only 39% of patients with PSM who underwent repeat surgery, raising questions regarding the accuracy of PSM.8 Additionally, the oncologic benefit of preemptive nephrectomy remains dubious, as no study to our knowledge has shown this to forestall relapse among these patients. Until such studies materialize, the potential morbidity and risk of renal dysfunction with repeat surgery may not be justified.
Preservation of renal function has fueled the impetus to expand indications for NSS beyond cT1a disease to include cT1b and cT2 lesions. Our study indicates clinically localized lesions greater than 7 cm pose a higher recurrence risk in the setting of PSM. For anatomically complex cT2 lesions where concern exists over the feasibility of complete resection, our study findings support the use of radical nephrectomy to preserve oncologic integrity, particularly in view of recent reports failing to demonstrate the overall survival benefit of PN.29
This study has several limitations in addition to its retrospective nature. The study cohort was derived from multiple institutions. Any resultant heterogeneity in surgical technique and patient selection may have influenced outcomes. Information on intraoperative biopsy of the resection bed, including management of positive biopsies, was unavailable for all institutions. As such, the current study focuses on positive margin identified on final histology. Similarly, data on nephrometry scores were not available at all institutions. The magnitude of PSM may have been influenced by tumor complexity, with residual disease burden greater for lesions of higher nephrometry score due to limitations in the feasibility of resection. Considering the demonstration of a direct relationship between nephrometry score and high risk pathological features, tumor anatomy may partly explain the observed relationship between higher Fuhrman grade and increased recurrence risk with PSM.30 Surveillance algorithms were not standardized among surgeons, which may have influenced recurrence detection patterns. Although the majority of patients underwent imaging at 6 to 12-month intervals, a small proportion had imaging at intervals exceeding 12 months, all of whom were disease-free on final imaging. Margins were considered positive if malignant cells were identified at the specimen resection edge. Depth and magnitude of circumferential involvement were not evaluated, which may correlate with residual disease burden and influence propensity for recurrence. Lastly, although the association between PSM and recurrence site was not observed, the relatively limited number of local and distant recurrences precluded a detailed risk assessment of recurrence location among patients with PSM.
CONCLUSIONS
PSM resulting from NSS increases the risk of disease recurrence, primarily in patients with adverse pathological features. As such, disease at the surgical margin is not uniformly prognostic. Instead, its clinical relevance is influenced by the intrinsic malignant potential of the primary lesion. Surveillance schemes emphasizing rigorous followup for high risk patients with PSM may facilitate early detection and more effective treatment of recurrence. Future studies are necessary to discern potential oncologic benefit from early completion nephrectomy in this cohort. Conversely, PSM is of minimal prognostic relevance with low stage, low grade tumors and may not necessitate aggressive surveillance or adjuvant intervention. Given the current limitations in definitively differentiating between low and high risk RCC preoperatively, it remains prudent to strive for negative margins during NSS.
Abbreviations and Acronyms
- CSS
cancer specific survival
- CT
computerized tomography
- NSM
negative surgical margin
- NSS
nephron sparing surgery
- PN
partial nephrectomy
- PSM
positive surgical margin
- RCC
renal cell carcinoma
- RFS
recurrence-free survival
Footnotes
No direct or indirect commercial incentive associated with publishing this article.
The corresponding author certifies that, when applicable, a statement(s) has been included in the manuscript documenting institutional review board, ethics committee or ethical review board study approval; principles of Helsinki Declaration were followed in lieu of formal ethics committee approval; institutional animal care and use committee approval; all human subjects provided written informed consent with guarantees of confidentiality; IRB approved protocol number; animal approved project number.
Contributor Information
Paras H. Shah, Department of Urology, Smith Institute for Urology, North Shore LIJ, New Hyde Park, New York.
Daniel M. Moreira, Department of Urology, Mayo Clinic, Rochester, Minnesota
Zhamshid Okhunov, Department of Urology, University of California, Irvine.
Vinay R. Patel, Department of Urology, Smith Institute for Urology, North Shore LIJ, New Hyde Park, New York
Sameer Chopra, USC Institute of Urology, University of Southern California, Los Angeles, California.
Aria A. Razmaria, Department of Urology, University of Chicago, Chicago, Illinois
Manaf Alom, Department of Urology, Smith Institute for Urology, North Shore LIJ, New Hyde Park, New York.
Arvin K. George, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Oksana Yaskiv, Department of Urology, Smith Institute for Urology, North Shore LIJ, New Hyde Park, New York.
Michael J. Schwartz, Department of Urology, Smith Institute for Urology, North Shore LIJ, New Hyde Park, New York
Mihir Desai, USC Institute of Urology, University of Southern California, Los Angeles, California.
Manish A. Vira, Department of Urology, Smith Institute for Urology, North Shore LIJ, New Hyde Park, New York
Lee Richstone, Department of Urology, Smith Institute for Urology, North Shore LIJ, New Hyde Park, New York.
Jaime Landman, Department of Urology, University of California, Irvine.
Arieh L. Shalhav, Department of Urology, University of Chicago, Chicago, Illinois
Inderbir Gill, USC Institute of Urology, University of Southern California, Los Angeles, California.
Louis R. Kavoussi, Department of Urology, Smith Institute for Urology, North Shore LIJ, New Hyde Park, New York
REFERENCES
- 1.Campbell SC, Novick AC, Belldegrun A et al. : Guideline for management of the clinical T1 renal mass. J Urol 2009; 182: 1271. [DOI] [PubMed] [Google Scholar]
- 2.Borghesi M, Brunocilla E, Schiavina R et al. : Positive surgical margins after nephron-sparing surgery for renal cell carcinoma: incidence, clinical impact, and management. Clin Genitourin Cancer 2013; 11: 5. [DOI] [PubMed] [Google Scholar]
- 3.Epstein JI, Partin AW, Sauvageot J et al. : Prediction of progression following radical prostatectomy. A multivariate analysis of 721 men with long-term follow-up. Am J Surg Pathol 1996; 20: 286. [DOI] [PubMed] [Google Scholar]
- 4.Mitra AP, Quinn DI, Dorff TB et al. : Factors influencing post-recurrence survival in bladder cancer following radical cystectomy. BJU Int 2012; 109: 846. [DOI] [PubMed] [Google Scholar]
- 5.Bickenbach KA, Gonen M, Strong V et al. : Association of positive transection margins with gastric cancer survival and local recurrence. Ann Surg Oncol 2013; 20: 2663. [DOI] [PubMed] [Google Scholar]
- 6.Houssami N, Macaskill P, Marinovich ML et al. : The association of surgical margins and local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy: a meta-analysis. Ann Surg Oncol 2014; 21: 717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwon EO, Carver BS, Snyder ME et al. : Impact of positive surgical margins in patients undergoing partial nephrectomy for renal cortical tumours. BJU Int 2007; 99: 286. [DOI] [PubMed] [Google Scholar]
- 8.Bensalah K, Pantuck AJ, Rioux-Leclercq N et al. : Positive surgical margin appears to have negligible impact on survival of renal cell carcinomas treated by nephron-sparing surgery. Eur Urol 2010; 57: 466. [DOI] [PubMed] [Google Scholar]
- 9.Khalifeh A, Kaouk JH, Bhayani S et al. : Positive surgical margins in robot-assisted partial nephrectomy: a multi-institutional analysis of oncologic outcomes (leave no tumor behind). J Urol 2013; 190: 1674. [DOI] [PubMed] [Google Scholar]
- 10.Permpongkosol S, Colombo JR Jr, Gill IS et al. : Positive surgical parenchymal margin after laparoscopic partial nephrectomy for renal cell carcinoma: oncological outcomes. J Urol 2006; 176: 2401. [DOI] [PubMed] [Google Scholar]
- 11.Yossepowitch O, Thompson RH, Leibovich BC et al. : Positive surgical margins at partial nephrectomy: predictors and oncological outcomes. J Urol 2008; 179: 2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gill IS, Aron M, Gervais DA et al. : Clinical practice. Small renal mass. N Engl J Med 2010; 362: 624. [DOI] [PubMed] [Google Scholar]
- 13.Newcomb LF, Thompson IM Jr, Boyer HD et al. : Outcomes of active surveillance for the management of clinically localized prostate cancer in the prospective, multi-institutional Canary PASS cohort. J Urol 2016; 195: 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tosoian JJ, Mamawala M, Epstein JI et al. : Intermediate and longer-term outcomes from a prospective active-surveillance program for favorable-risk prostate cancer. J Clin Oncol 2015; 33: 3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pierorazio PM, Johnson MH, Ball MW et al. : Five-year analysis of a multi-institutional prospective clinical trial of delayed intervention and surveillance for small renal masses: the DISSRM Registry. Eur Urol 2015; 68: 408. [DOI] [PubMed] [Google Scholar]
- 16.Lane BR and Kattan MW: Prognostic models and algorithms in renal cell carcinoma. Urol Clin North Am 2008; 35: 613. [DOI] [PubMed] [Google Scholar]
- 17.Lohse CM and Cheville JC: A review of prognostic pathologic features and algorithms for patients treated surgically for renal cell carcinoma. Clin Lab Med 2005; 25: 433. [DOI] [PubMed] [Google Scholar]
- 18.Uzzo RG and Novick AC: Nephron sparing surgery for renal tumors: indications, techniques and outcomes. J Urol 2001; 166: 6. [PubMed] [Google Scholar]
- 19.Gill IS, Desai MM, Kaouk JH et al. : Laparoscopic partial nephrectomy for renal tumor: duplicating open surgical techniques. J Urol 2002; 167: 469. [DOI] [PubMed] [Google Scholar]
- 20.Patel MN, Bhandari M, Menon M et al. : Robotic-assisted partial nephrectomy. BJU Int 2009; 103: 1296. [DOI] [PubMed] [Google Scholar]
- 21.Maldazys JD and deKernion JB: Prognostic factors in metastatic renal carcinoma. J Urol 1986; 136: 376. [DOI] [PubMed] [Google Scholar]
- 22.Eggener SE, Yossepowitch O, Pettus JA et al. : Renal cell carcinoma recurrence after nephrectomy for localized disease: predicting survival from time of recurrence. J Clin Oncol 2006; 24: 3101. [DOI] [PubMed] [Google Scholar]
- 23.Thomas AZ, Adibi M, Borregales LD et al. : Surgical management of local retroperitoneal recurrence of renal cell carcinoma after radical nephrectomy. J Urol 2015; 194: 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dabestani S, Marconi L, Hofmann F et al. : Local treatments for metastases of renal cell carcinoma: a systematic review. Lancet Oncol 2014; 15: e549. [DOI] [PubMed] [Google Scholar]
- 25.Motzer RJ, Hutson TE, Tomczak P et al. : Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009; 27: 3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alt AL, Boorjian SA, Lohse CM et al. : Survival after complete surgical resection of multiple metastases from renal cell carcinoma. Cancer 2011; 117: 2873. [DOI] [PubMed] [Google Scholar]
- 27.Escudier B, Michaelson MD, Motzer RJ et al. : Axitinib versus sorafenib in advanced renal cell carcinoma: subanalyses by prior therapy from a randomised phase III trial. Br J Cancer 2014; 110: 2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ani I, Finelli A, Alibhai SM et al. : Prevalence and impact on survival of positive surgical margins in partial nephrectomy for renal cell carcinoma: a population-based study. BJU Int 2013; 111: E300. [DOI] [PubMed] [Google Scholar]
- 29.Van Poppel H, Da Pozzo L, Albrecht W et al. : A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol 2011; 59: 543. [DOI] [PubMed] [Google Scholar]
- 30.Kutikov A, Smaldone MC, Egleston BL et al. : Anatomic features of enhancing renal masses predict malignant and high-grade pathology: a preoperative nomogram using the RENAL Nephrometry score. Eur Urol 2011; 60: 241. [DOI] [PMC free article] [PubMed] [Google Scholar]


