Abstract
Mismatch negativity (MMN) amplitude is reliably reduced in psychotic disorders. While several studies have examined this effect in first-degree relatives of individuals with schizophrenia, few have sought to quantify deficits in relatives of individuals with other psychotic disorders. While some conclude that, compared to healthy subjects, first-degree relatives of schizophrenia show reduced MMN, others contradict this finding. Furthermore, though MMN is often shown to be associated with cognitive impairments and clinical symptoms in psychotic disorders, to our knowledge no studies have sought to fully examine these relationships in studies of first-degree relatives. The present study sought to clarify the extent of MMN amplitude reductions in a large sample of siblings of individuals with diverse psychotic disorders (n=67), compared to probands with psychosis (n=221) and never psychotic comparison subjects (n=251). We further examined associations of MMN amplitude with cognition and schizotypal symptoms across these groups. We found that MMN amplitude was intact in siblings compared to probands. MMN amplitude was associated with cognition and schizotypal symptoms dimensionally across levels of familial risk. The present results imply that MMN reductions do not reflect genetic risk for psychotic disorders per se, and instead emerge as a result of, or in conjunction with, clinical features associated with psychosis. Such findings carry important implications for the utility of MMN amplitude as an indicator of inherited risk, and suggest that this component may be best conceptualized as an endophenotype for clinical symptoms and cognitive impairments, rather than risk for psychosis per se.
Keywords: Mismatch Negativity (MMN), Psychosis, Schizophrenia, Genetic Risk, Schizotypy
1. Introduction
The mismatch negativity (MMN) is an event-related potential (ERP) commonly elicited by the presentation of an unexpected stimulus. In auditory MMN paradigms, expectations are set through the frequent presentation of a standard stimulus, and the MMN arises when expectations are violated by the presentation of a deviant stimulus (Näätänen, 1995). Reduced MMN amplitude is one of the most well-replicated psychophysiological impairments in schizophrenia (Erickson, Ruffle, & Gold, 2016; Umbricht & Krljes, 2005); evidence suggests reductions are also present across other psychotic disorders (Donaldson et al., 2020; Erickson et al., 2016).
Several studies have examined MMN in samples at genetic high risk for schizophrenia (Earls, Curran, & Mittal, 2016; Erickson et al., 2016). Some conclude that, compared to healthy subjects, first-degree relatives of individuals with schizophrenia show reduced MMN amplitude (Jessen et al., 2001; Michie, Innes-Brown, Todd, & Jablensky, 2002; Şevik et al., 2011). Conversely, some studies demonstrate MMN reductions only in patients, not relatives (Ahveninen et al., 2006; Bramon et al., 2004; Hall et al., 2006; Hong, Moran, Du, O’Donnell, & Summerfelt, 2012; Magno et al., 2008). This discrepancy extends to two recent meta-analyses. One compared patients with schizophrenia, relatives, and comparison subjects across eight studies and found no differences between relatives and controls (Erickson et al., 2016). However, though the second replicated this finding when scoring the MMN broadly, a significant difference emerged between relatives and controls when restricting analyses to electrode Fz, where the component is often maximal (Earls et al., 2016). Similarly, studies report heritability estimates of MMN amplitude of around 50–60% (Hall et al., 2006; Hall et al., 2009), suggesting genetic effects may be contributory, but not wholly deterministic of MMN amplitude. Thus, while this literature is lacking in consensus, the preponderance of findings among studies thus far suggests intact MMN amplitude in unaffected relatives. This may be because MMN reductions emerge as a result of psychotic illness (such as is suggested by some prior studies of patients, such as Salisbury et al., 2007), or because MMN amplitude represents an endophenotype specific to certain symptom domains associated with psychotic disorders. Studies of MMN in relatives of individuals with schizophrenia have been few and limited in sample size, which may have contributed to the lack of clarity on this topic thus far.
Fewer studies still have examined MMN in relatives of individuals with psychotic disorders other than schizophrenia, despite considerable overlap in symptoms (Reininghaus et al., 2016). Studies of MMN in siblings of individuals with diverse psychotic disorders include one sample of patients with bipolar disorder and their siblings (Hall et al., 2009) and one mixed psychosis sample comprised predominantly of schizophrenia-spectrum disorders (N= 21; 88% of the sample; Ranlund et al., 2016). However, diagnostic instability, particularly early in illness, is high. Many probands initially diagnosed as other psychosis eventually are diagnosed with schizophrenia-spectrum disorders (Bromet et al., 2011; Heslin et al., 2015; Ruggero, Carlson, Kotov, & Bromet, 2010; Ruggero et al., 2011), complicating comparisons between sibling groups. Thus, further study of MMN amplitude in first-degree relatives of individuals with a variety of stable, well-defined psychotic disorder diagnoses, including those other than schizophrenia, is needed in order to examine this putatively genetically transmitted neural marker of psychosis in samples at familial risk for developing a psychosis-spectrum illness. If MMN amplitude reductions are indeed endophenotypic for particular symptom domains, associations between reduced MMN and such symptoms would likely emerge in siblings of individuals across diagnoses in which symptoms are present.
Consistent with this interpretation, reduction in MMN amplitude is often associated with poor cognitive functioning in schizophrenia (Baldeweg, Klugman, Gruzelier, & Hirsch, 2004; Näätänen, Kujala, Kreegipuu, Carlson, Escera, Baldeweg, & Ponton, 2011; Näätänen et al., 2014) and other psychotic disorders (Donaldson et al., 2020; Hermens et al., 2010; Kaur et al., 2011; Thompson et al., 2005). Only one study to our knowledge has sought to extend this relationship to first-degree relatives. This study shows associations between MMN and cognitive functioning across a sample of patients with schizophrenia, relatives, and controls (Hong et al., 2012). This relationship did not reach significance when relatives were examined separately. To our knowledge, these effects have not been replicated, nor extended to relatives of individuals with other psychotic disorders. Examination of the relationship between MMN and cognition across levels of familial risk for psychosis represents an important next step in elucidating the degree to which this may represent an at-risk state for psychosis.
Additionally, reduced MMN amplitude is associated with psychotic symptoms in studies with large patient samples or targeted recruitment of symptoms (Donaldson et al., 2020; Fisher et al., 2011; Fisher et al., 2008, 2012; Light et al., 2015). While one recent-meta analysis did not find evidence of this relationship (Erickson et al., 2017), this may be due to effects collapsed across both symptom dimensions and deviant types (Donaldson et al., 2020). To our knowledge, the relationship between psychotic symptoms and MMN in relatives of individuals with psychotic disorders has not been examined. Of course, establishing the degree to which MMN represents an endophenotype for psychotic symptoms in this way is complicated by the lower rates of and variability in full-threshold psychotic symptoms in non-clinical samples. However, first-degree relatives of individuals with schizophrenia report sub-threshold, psychotic-like experiences at a higher-than-average rate (Johnstone et al., 2000; Kendler, 1985; Tarbox & Pogue-Geile, 2011) and are at increased risk for both schizophrenia and schizotypal personality disorder (SPD or “schizotypy”; Asarnow et al., 2001; Erlenmeyer-Kimling et al., 1995). Indeed, MMN is reduced in individuals with SPD (Niznikiewicz et al., 2009). Schizotypy, like schizophrenia, presents with both positive and negative symptoms; however, the relationship between positive and negative symptoms of schizotypy and MMN in relatives of individuals with psychosis has yet to be evaluated.
The aim of the present study is to clarify the extent of MMN amplitude reductions in siblings, and thereby the extent to which reduced MMN amplitude in probands may reflect genetic and shared environmental vulnerability for psychosis. This examination follows a previous study with this sample demonstrating associations between reduced MMN amplitude, auditory hallucinations, and cognitive functioning in psychotic disorders (Donaldson et al., 2020). Here, we seek to extend these findings to familial liability for psychosis in order to examine the utility of MMN as an endophenotype for particular symptom domains associated with psychotic illness. To accomplish this aim, we examined the relationships among MMN amplitude, cognitive functioning, and both positive and negative schizotypal traits across three groups (people with psychotic disorders, unaffected biological siblings, and never-psychotic comparison subjects). In line with findings in patient samples, we hypothesize that reduced MMN amplitude will be associated with worse cognitive functioning and greater positive schizotypy, and that these relationships will not differ between groups. As an exploratory inquiry, we also examined relationships between MMN and negative schizotypy across groups. This study aims to provide insight into whether reduced MMN amplitude reflects risk for psychosis, which may be present but attenuated in siblings, or factors associated with the illness itself. Utilizing a sample of individuals with diverse psychotic disorders and their biological siblings allows us to extend relationships that exist transdiagnostically amongst individuals with psychotic disorders to their first-degree relatives.
2. Method
2.1. Participants
Study participants consisted of individuals with mixed psychotic disorders (N=221, including schizophrenia-spectrum disorders, mood disorders with psychosis, and other psychotic disorders), biological siblings not diagnosed with a psychotic disorder (N=67; 35 siblings of individuals diagnosed with schizophrenia-spectrum disorders (SZ), 32 siblings of individuals diagnosed with other psychotic disorders (OP)), and 251 never-psychotic zip-code- and demographically-matched comparison subjects (NP). All study participants were drawn from the 20-year follow up of the Suffolk County Mental Health Project (Bromet et al., 2011; Kotov et al., 2017), an epidemiologic study of first admission psychosis. Probands were recruited from psychiatric inpatient units in Suffolk County, New York, between 1990 and 1995. Inclusion criteria included first admission within 6 months, clinical evidence of psychosis not due to a medical condition, ages 15–60, IQ > 70, proficient in English, and a Suffolk County resident. Siblings and NP subjects were recruited at the 20-year follow-up, from which the present data is drawn. Additional inclusion criteria for sibling and NP subjects included the absence of psychotic symptoms (Kotov et al., 2017); these subjects were not excluded for the presence of a non-psychotic Axis I disorder. Lifetime prevalence of such disorders in these subjects, as measured by the Structured Clinical Interview for DSM-IV (SCID; First, Spitzer, Gibbon, & Williams, 2001), is presented in Supplementary Table 1. Sample characteristics are presented in Table 1. MMN amplitude in probands and never-psychotic comparison subjects in a partially overlapping sample has previously been reported (Donaldson et al., 2020). MMN amplitude was reduced in probands, an effect shown not to differ based on psychotic disorder diagnosis, and correlated with cognitive functioning, disorganization, and auditory hallucinations. We sought to examine whether similar relationships extend to siblings of this patient group.
Table 1.
Sample Characteristics
| Probands | Siblings | NP | Probands v. Siblings v. NP | |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Gender | — | — | — | X2=10.85** |
| Female | 91 (42.3) | 40 (65.6) | 110 (44.4) | — |
| Male | 124 (57.7) | 21 (34.4) | 138 (55.6) | — |
| Race1 | — | — | — | X2=17.51* |
| Asian | 5 (2.3) | 1 (1.5) | 2 (.8) | — |
| African American | 26 (11.8) | 9 (13.4) | 14 (5.6) | — |
| White | 169 (76.5) | 55 (82.1) | 223 (89.2) | — |
| More than one race | 9 (4.1) | 0 (0) | 4 (1.6) | — |
| Other/Unknown | 12 (5.4) | 2 (3.0) | 7 (2.8) | — |
| Ethnicity | — | — | — | X2=4.1 |
| Hispanic/Latino | 30 (13.9) | 6 (9.8) | 20 (8.1) | — |
| Education | X2=43.09*** | |||
| Not a HS graduate | 23 (1.4) | 5 (7.5) | 7 (2.8) | — |
| Highschool/GED | 77 (34.7) | 15 (22.4) | 59 (23.5) | — |
| Some college | 51 (23.0) | 15 (22.4) | 60 (23.9) | — |
| Associate’s degree | 21 (9.5) | 4 (6.0) | 36 (14.3) | — |
| Bachelor’s degree | 30 (13.5) | 9 (13.4) | 45 (17.9) | — |
| Some graduate classes | 3 (1.4) | 4 (6.0) | 3 (1.2) | — |
| MA or equivalent | 13 (5.9) | 12 (17.9) | 35 (13.9) | — |
| MD/PhD/JD | 1 (.5) | 2 (3.0) | 5 (2.0) | — |
| Antipsychotic Medication | 126 (58.3) | 1 (1.7) | 4 (1.6) | X2=217.16*** |
| Mean (SD) | Mean (SD) | Mean (SD) | Probands v. Siblings v. NP | |
| Age (years) | 47.9 (8.6) | 49.1 (9.0) | 50.5 (8.8) | F=5.04** |
Due to the small number of individuals identifying as a race other than Caucasian, group differences were examined as white vs other.
Indicates a significant difference (p<.05) between groups;
p<.01;
p<.001.
NP = Never Psychotic, SD = standard deviation, HS = high-school, GED = General Education Diploma, MA = Master’s Degree, MD = Medical Doctor, PhD = Doctor of Philosophy; JD = Juris Doctor.
2.2. Measures
Clinical and neuropsychological assessments were completed by each participant at study visits concurrent with the MMN task. Diagnosis was determined using the SCID, information from which was used by study psychiatrists to generate consensus diagnoses. Psychotic symptoms were absent in sibling and NP groups by design; thus, dimensional measures of schizotypy were used. Schizotypal symptoms were assessed using the Schedule for Nonadaptive and Adaptive Personality (SNAP; Clark, Simms, Wu, & Casillas, 1993). The SNAP yields three subscales in its assessment of schizotypal traits– eccentric perceptions and mistrust, which assess positive schizotypy, and detachment assessing negative schizotypy– which were used independently in the present analyses (Cicero, Jonas, Li, Perlman, & Kotov, 2019).
Cognitive functioning was assessed during a comprehensive neuropsychological battery, including the Controlled Oral Word Association Test (Ruff, Light, Parker, & Levin, 1996), Verbal Paired Associates and Visual Reconstruction from the Wechsler Memory Scale-Revised (both immediate and delay trials; Wechsler, 2012), Symbol-Digit Modalities, Letter-Number Sequencing, and Vocabulary from the Wechsler Adult Intelligence Scale-R (Wechsler, 1981), Trails A and B (Reitan, 1955), and the Stroop Test (Trenerry, Crosson, DeBoe, & Leber, 1989). A single composite score reflecting performance across these tasks was computed using an exploratory factory analysis, described in further detail in previous studies (Jonas et al., 2019; Martin et al., 2021).
2.3. Task
The auditory MMN task was administered in the background of a visual picture/word matching task (Donaldson et al., 2020; Mathalon, Roach, & Ford, 2010). 2458 tones were presented at an interval of 500 ms, at 78 dB and 633 Hz, with 10ms rise/fall. 80% of tones were considered ‘standards’ (50ms, 633Hz), while 10% deviated in duration, eliciting the duration MMN (MMN-D, 100ms at 633Hz) and 10% deviated in frequency, eliciting the frequency MMN (MMN-F, 50ms at 1000 Hz).
2.4. Psychophysiological Data Acquisition and Preprocessing
EEG data were acquired using an ActiveTwo BioSemi system (BioSemi, Amsterdam, Netherlands). Scalp electrodes were placed according to an extension of the international 10/20 system for 34 electrode sites. Signal was digitized at 24-bit resolution at a sampling rate of 1024 Hz and referenced to a common mode sense active electrode forming a monopolar channel. Electrodes were placed above and below and at the outer canthi of each eye in order to record horizontal and vertical electrooculogram.
Offline data analyses were completed using Brain Vision Analyzer (Brain Products, Munich, Germany). Linked mastoids served as the reference, and data were band-pass filtered from .1 to 30 Hz. Gratton-Coles ocular correction algorithm was used to remove eye movements (Gratton, Coles, & Donchin, 1983), and a trial-wise artifact rejection was applied based on a 50+ mV step between trials or 75 mV difference within trials. Baseline correction was applied with reference to a 200-ms pre-stimulus window. ERPs were stimulus-locked to onset of standard and deviant tones, and difference waves (duration deviant minus standard, MMN-D; frequency deviant minus standard, MMN-F) were computed for each subject. Semi-automatic peak detection was performed from the difference wave at electrode Fz, in line with where significant effects in siblings have emerged in previous studies (Earles et al., 2016) and where it is often maximal (Duncan et al., 2009; Sinkkonen & Tervaniemi, 2000). Inspection of and adjustments to the automated peak detection were completed by the first author, who was blind to participant group status during data processing. MMN-D and MMN-F were computed as the 50ms area under these peaks.
2.5. Statistical Analyses
IBM SPSS Statistics (Version 26.0, IBM, Armonk, NY) was used for statistical analyses, save for paired t-tests which were computed in the R programming environment (R Core Team, 2020) with data organization supported by the dplyr package (Wickham, François, Henry, & Muller, 2020). Differences between groups in demographic factors were assessed using one-way analyses of variance (ANOVAs) and chi-square goodness of fit tests.
2.5.1. Group Differences.
MMN-D and MMN-F were used as separate outcome variables in all models. Differences in MMN amplitude, schizotypy, and cognition between probands, siblings, and never-psychotic participants were each examined using an ANOVA, with group as the between-subjects factor and MMN amplitude, clinical symptoms, or cognition as the dependent variable. Differences between siblings of SZ and siblings of OP were further parsed using an ANOVA with this grouping variable (case vs sibling of SZ vs sibling of OP vs NP) as the between-subjects factor and MMN amplitude, clinical symptoms, or cognition as the dependent variable. In each model, Tukey post-hoc analyses were used to query hypothesized differences between each group. Given three post-hoc comparisons for each statistic of interest (probands vs siblings, probands vs NP, siblings vs NP), a Bonferroni-adjusted p value of <.017 was considered significant for post-hoc analyses. Finally, in order to evaluate whether significant effects were robust to the comparatively small N of the sibling group, paired samples t-tests were performed in which MMN amplitude in probands was compared to MMN amplitude in their matched biological sibling, using a subset of the present sample restricted by the size of the sibling group. Previous work with this sample has demonstrated a lack of difference in MMN-D and MMN-F amplitude amongst probands based on psychotic disorder diagnosis (Donaldson et al., 2020); thus, probands were examined as a single group in the present study.
2.5.2. Clinical Relationships.
Associations of MMN with cognition were assessed using bivariate Pearson correlations. A hierarchical regression model was then employed to examine group differences in this relationship. MMN amplitude was the dependent variable, with cognition, dummy-coded group (probands, siblings, or NP), and their interaction entered sequentially into the model. A non-significant cognition X group interaction term indicated the relationship between MMN and cognitive functioning did not differ systematically by group (Donaldson et al., 2020; Sheffield et al., 2017). The same analysis plan was carried out for each of the three schizotypy subscales, which were centered prior to forming interaction terms.
3. Results
3.1. Group Differences
3.1.1. MMN Amplitude.
A significant main effect of group on MMN-D (F(2, 537)=11.54, p<.001) emerged; Tukey HSD post-hoc analyses revealed that MMN-D in probands was significantly reduced compared to NP (Hedges’ g =.41, p = .000; as described in a similar sample in Donaldson et al., 2020). MMN-D was diminished in probands compared to siblings (Hedges’ g =.46, p=.002), and siblings did not differ from NP (Hedges’ g =.06, p=.87). A second model confirmed that siblings of probands with SZ and OP did not differ from each other (Hedges’ g =.16, p=.73). Group differences in MMN-D amplitude are displayed in Figure 1. Paired t-tests confirm that MMN-D amplitude differs significantly between probands and their paired siblings (t(45)=3.67; p=.001). Differences remained controlling for age, gender, and race (p<.001). Conversely, MMN-F amplitude was unable to distinguish between the three study groups in the omnibus model (F(2, 537)=2.84, p=.059; Figure 1). Due to this lack of overall group difference in MMN-F, further analyses focused on the MMN-D component.
Figure 1.
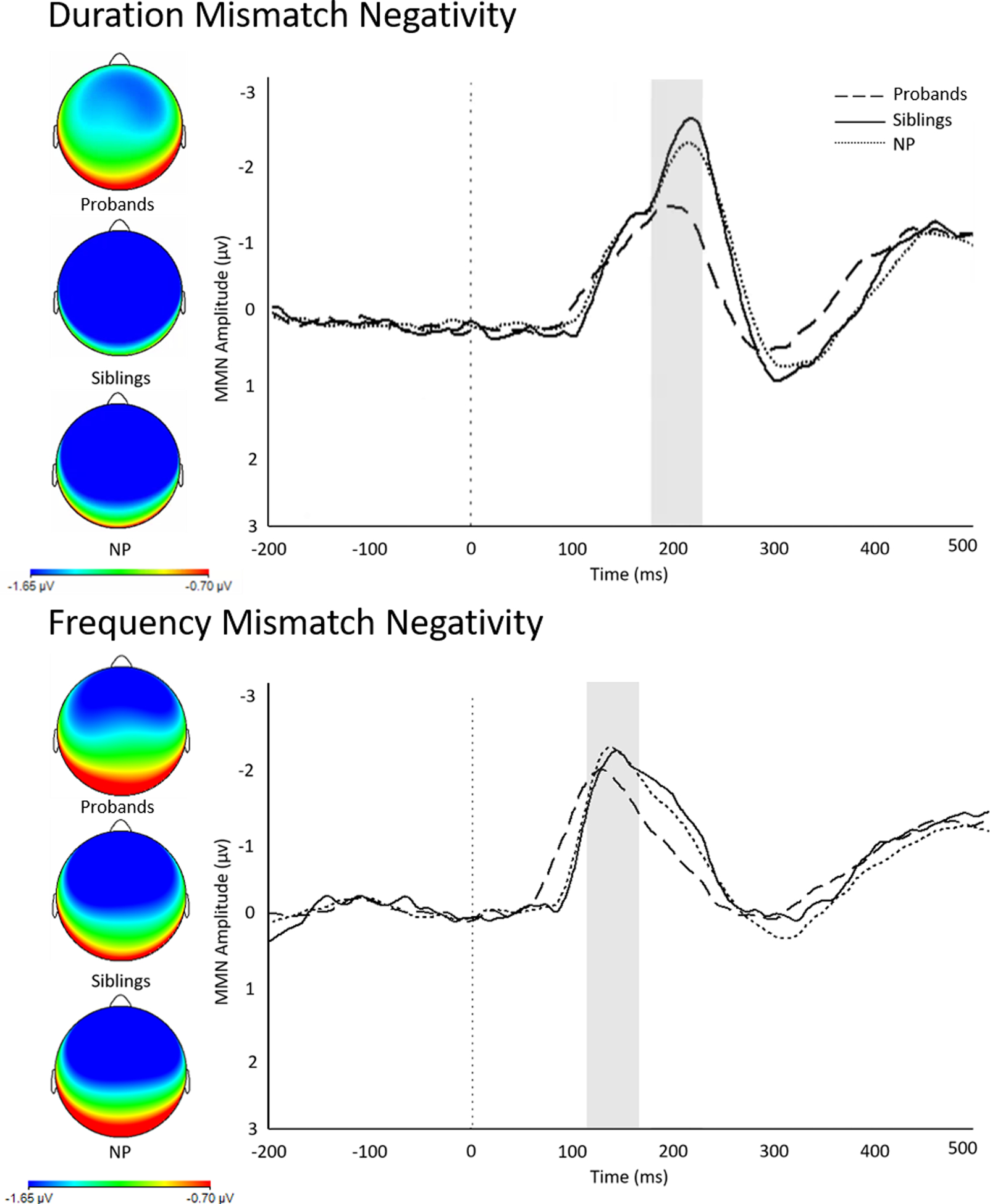
ERP Waveforms and Scalp Distributions
Note. Duration mismatch negativity (MMN-D, top) and frequency mismatch negativity (MMN-F, bottom) at electrode Fz in probands with psychosis, their siblings, and never psychotic comparison subjects. MMN amplitude scored as difference wave at electrode Fz. Highlighted area represents amplitude displayed in scalp topography map (left). NP = never psychotic; ms = milliseconds; μV = microvolts.
3.1.2. Clinical and Cognitive Measures.
Significant differences emerged in cognition (F(2,487)=54.33, p<.001). Tukey HSD follow-up analyses revealed that cognition in probands differed from NP (Hedges’ g = −.97, p<.001) and siblings (Hedges’ g = −.77, p<.001), while siblings did not differ from NP (Hedges’ g = −.23, p=.37). Group differences in cognitive functioning are presented in Table 2. Group comparisons in schizotypal traits revealed significant group effects on dimensions of mistrust (F(2,464)=40.85, p<.001), detachment (F(2,470)=34.13, p<.001), and eccentric perceptions (F(2,477)=29.64, p<.001). Tukey HSD follow-up analyses revealed that schizotypal traits were elevated in probands compared to siblings (mistrust, Hedges’ g =.64; detachment, Hedges’ g =.86; eccentric perceptions, Hedges’ g = .71; all p<.001) and NP (mistrust, Hedges’ g =.85; detachment, Hedges’ g =.76; eccentric perceptions, Hedges’ g = .65; all p<.001), while siblings did not differ significantly from NP (mistrust, Hedges’ g =.23; detachment, Hedges’ g = −.11; eccentric perceptions, Hedges’ g = −.03; Table 2). In no models did siblings of SZ and siblings of OP differ from one another (cognition, Hedges’ g =.48, p=.41; mistrust, Hedges’ g =.03, p=.98; detachment, Hedges’ g =.01, p=.98; eccentric perceptions, Hedges’ g =.11, p=.99). Overall group differences in cognition and schizotypy remained when controlling for age, gender, and race (all p<.001). Given overall group differences in cognitive functioning, a hierarchical regression model was conducted examining whether the group effect on MMN-D is robust to differences in vocabulary, used here as a proxy for pre-morbid IQ (step 1: vocabulary, step 2: group, step 3: vocabulary X group interaction; Supplementary Table 3). Group remained a significant predictor of MMN-D over and above vocabulary, and this effect did not differ based on vocabulary ability.
Table 2.
Cognition & Schizotypy Across Groups
| Probands | Siblings | NP | Group Comparison | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Cognition1 | −.43(1.0) | .24(.71) | .40(.68) | F=54.33*** |
| Case v NP | — | — | — | p=.000** |
| Case v Sibling | — | — | — | p=.000** |
| Sibling v NP | — | — | — | p=.368 |
| Mistrust | 6.32(5.2) | 3.41(3.8) | 2.60(3.3) | F=40.85*** |
| Case v NP | — | — | — | p=.000** |
| Case v Sibling | — | — | — | p=.000** |
| Sibling v NP | — | — | — | p=.418 |
| Detachment | 6.70(4.2) | 3.38(3.5) | 3.77(3.5) | F=35.13*** |
| Case v NP | — | — | — | p=.000** |
| Case v Sibling | — | — | — | p=.000** |
| Sibling v NP | — | — | — | p=.779 |
| Eccentric Perceptions | 3.21(3.4) | 1.40(1.2) | 1.45(1.7) | F=29.54*** |
| Case v NP | — | — | — | p=.000** |
| Case v Sibling | — | — | — | p=.000** |
| Sibling v NP | — | — | — | p=.980 |
Overall cognition score derived factor analytically from scores on a large neuropsychological functioning battery.
NP = never psychotic;
p<.05;
p<.01;
p<.001.
3.2. Relationships of MMN with cognitive and clinical measures.
Bivariate correlations revealed significant associations between reduced MMN-D amplitude and worse cognitive functioning (r=−.26, p=.000), greater mistrust (r=.15, p=.001), greater detachment (r=.12, p=.009), and greater eccentric perceptions (r=.12, p=.011). Results are displayed in Figure 2. Follow-up hierarchical regression analyses show that these relationships did not differ based on group status (case vs sibling vs NP; Table 3). As siblings of SZ and siblings OP did not differ from one another on any measure of interest, they were entered as a single group in regression models. Correlations in each group are presented in Supplementary Table 2.
Figure 2.
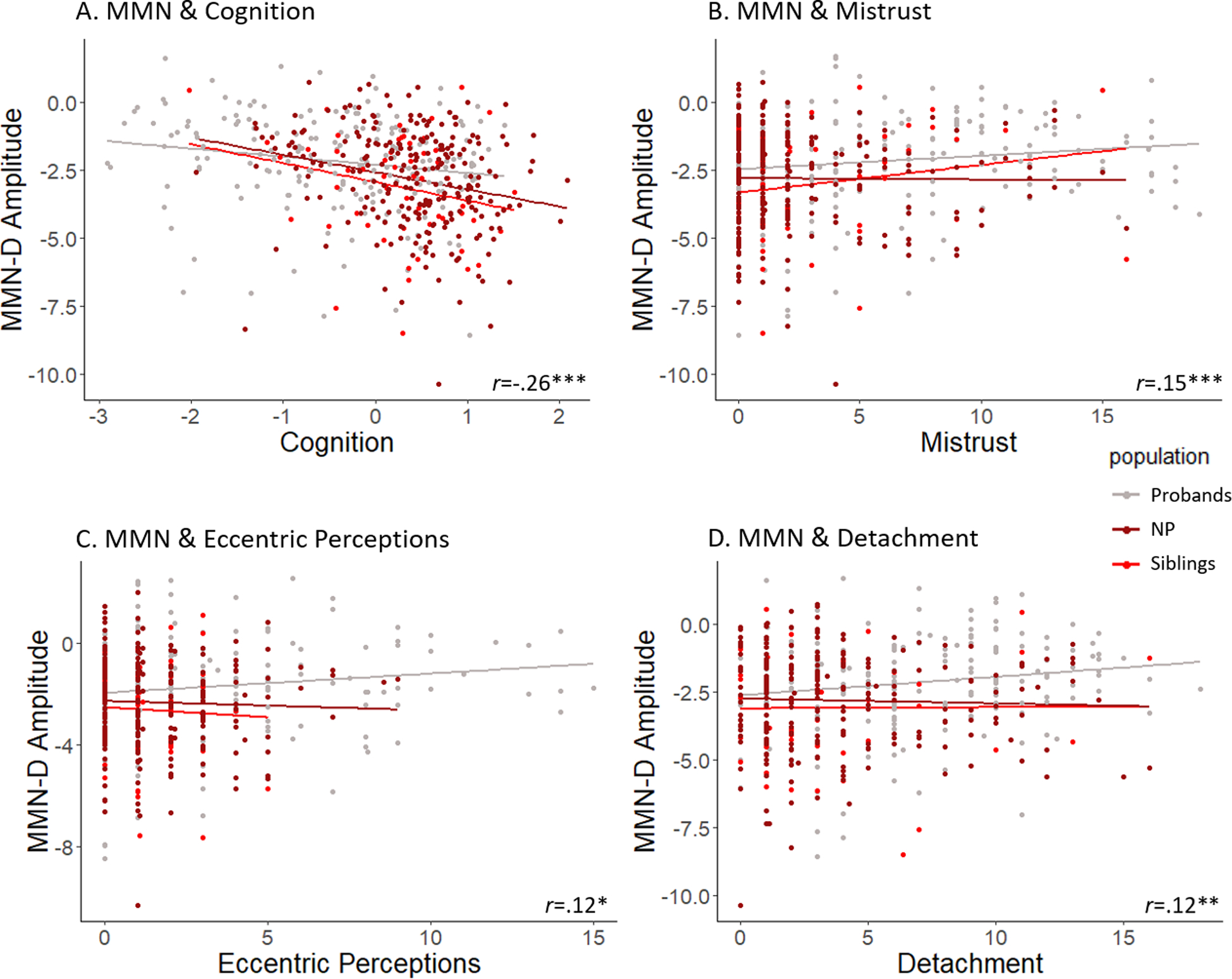
Relationships of MMN Amplitude with Cognition and Schizotypy
Note. Scatterplots illustrating associations between duration mismatch negativity (MMN-D) and measures of overall cognition score (A) and schizotypal symptoms, including mistrust (B), eccentric perceptions (C), and detachment (D). Effects are illustrated across probands, siblings, and never-psychotic comparison subjects. *Indicates p<.05; **p<.01; ***p<.001.
Table 3.
Hierarchical Regression
| Model | Predictors | R 2 | ΔR2 | p value |
|---|---|---|---|---|
| Cognition | Cognition | .07 | .07 | .000** |
| Group1 | .07 | .01 | .066 | |
| Interaction | .08 | .01 | .217 | |
| Mistrust | Mistrust | .02 | .02 | .001** |
| Group | .05 | .02 | .008** | |
| Interaction | .05 | .01 | .272 | |
| Detachment | Detachment | .01 | .01 | .009** |
| Group | .04 | .03 | .002** | |
| Interaction | .0 | .01 | .158 | |
| Eccentric Perceptions | Eccentric Perceptions | .01 | .01 | .011* |
| Group | .04 | .03 | .001** | |
| Interaction | .05 | .00 | .593 |
Probands vs Siblings vs Never Psychotic;
p<.05,
p<.01
4. Discussion
This study demonstrates the specificity of the deficit in MMN-D amplitude to individuals affected by psychosis, revealing intact MMN-D amplitude in siblings of both individuals with schizophrenia spectrum and other psychotic disorders. This finding replicates some previous studies (Ahveninen et al., 2006; Bramon et al., 2004; Hong, Moran, Du, O’Donnell, & Summerfelt, 2012; Magno et al., 2008), including one recent meta-analysis (Erickson et al., 2016), while contradicting others (Jessen et al., 2001; Michie, Innes-Brown, Todd, & Jablensky, 2002; Şevik et al., 2011). Our inclusion of siblings of individuals with a diverse array of psychotic disorders, as well as our demonstration that sibling groups do not differ from each other, extends these previous null findings to a broader transdiagnostic sample. These results are consistent with earlier work in bipolar disorder (Hall et al., 2009) and in one small mixed psychosis sample (Ranlund et al., 2016). We found that MMN-F amplitude was not able to distinguish overall differences between probands, siblings, and comparison subjects, suggesting specificity in effects discussed here to MMN generated in response to duration deviants. This is in line with prior work suggesting that reductions in MMN-D are most relevant to prediction of psychosis (Lepock et al., 2018; Randeniya, Oestreich, & Garrido, 2018), and are more reliably reported in first episode psychosis (Erickson et al., 2016; Haigh et al., 2017); as well as work suggesting that MMN-D and MMN-F are supported by different neural substrates (Lee et al., 2017). Taken together, these results do not support the notion that MMN amplitude offers a genetically-transmitted marker for psychotic disorders broadly defined. However, relationships emerged across groups between MMN-D and clinical features of psychosis providing support for the notion that MMN-D amplitude may represent an endophenotype for particular symptom domains.
Cognitive functioning was significantly associated with MMN-D amplitude, a relationship which did not differ based on group status. While this finding echoes previous work revealing relationships between MMN and cognition in psychosis samples (Baldeweg et al., 2004; Näätänen, Kujala, Kreegipuu, Carlson, Escera, Baldeweg, & Ponton, 2011; Näätänen et al., 2014; Hermens et al., 2010; Kaur et al., 2011; Thompson et al., 2005), the extension of this relationship to unaffected siblings of diverse psychotic disorders is novel. This suggests that associations between MMN and cognition span dimensionally across psychosis risk. Indeed, studies have demonstrated associations between MMN and intellectual functioning in first episode psychosis (Salisbury et al., 2017), supporting this notion. Furthermore, we demonstrated no difference between siblings of individuals with SZ and OP in MMN-D amplitude or cognitive functioning. These analyses were based on 539 observations, but may be underpowered due to the relatively small size of the sibling group (n=67). The present results would benefit from replication with a larger group of first-degree relatives of individuals with diverse psychotic disorders.
Similarly, to our knowledge no prior studies have characterized relationships between MMN and schizotypal traits. The present study not only provides first evidence that MMN-D is associated with symptoms of schizotypy, in line with prior work demonstrating reduced MMN amplitude in SPD (Niznikiewicz et al., 2009), but also shows these relationships are consistent across probands, siblings of individuals with SZ and OP, and comparison subjects. Furthermore, results demonstrate relationships with MMN-D amplitude across domains of schizotypal traits. Mistrust and eccentric perception scales assess positive schizotypy and are correlated with measures of paranoia and psychoticism, respectively (Cicero et al., 2019). The present findings echo previous associations between MMN and positive symptoms in psychotic disorders (Fisher et al., 2011; Fisher et al., 2008, 2012; Light et al., 2015; Donaldson et al., 2020). Conversely, the detachment subscale correlates with measures of social anhedonia and assess negative schizotypy (Cicero et al., 2019), replicating some less consistent associations between MMN and negative symptoms (e.g., Baldeweg et al., 2004; Javitt, Shelley, & Ritter, 2000) including in one sample of individuals at clinical high risk for psychosis (Sehatpour et al., 2021). These findings provide support for the notion that reduced MMN amplitude may represent an endophenotype for these psychotic symptom domains. However, it is important to note that it is possible that the relationships between clinical symptoms and MMN amplitude may be driven, at least in part, by relatively elevated scores on measures of schizotypy in the patient group.
These results provide preliminary evidence that MMN-D amplitude tracks clinical and cognitive functioning across levels of vulnerability to psychosis. Indeed, it is possible that prior findings of decreased MMN in siblings did not reflect genetic vulnerability per se, but attenuated symptoms and/or cognitive impairment in siblings in these samples. Some prior studies suggest that first-degree relatives of individuals with psychotic disorders present with mild decrements in cognitive ability relative to healthy controls (Arts, Jabben, Krabbendam, & Van Os, 2008; Bromet et al., 1996; Hill et al., 2013; Russo et al., 2017; Sitskoorn, Aleman, Ebisch, Appels, & Kahn, 2004; Snitz, MacDonald III, & Carter, 2006; Toulopoulou et al., 2005; Zalla et al., 2004). It may therefore be the case that while the MMN does not carry sufficient power as a candidate biomarker for genetic or familial risk, reduction in MMN amplitude may reflect attenuated symptoms, or clinical high risk for psychosis, a notion supported by studies suggesting that MMN reductions may emerge as a function of psychotic illness (Salisbury et al., 2007). Indeed, schizotypal symptoms themselves represent a clinical high-risk state, supporting this notion. In fact, there is emerging evidence that MMN is reduced in individuals at clinical high risk for psychosis (Perez et al., 2014; Erickson, Ruffle, & Gold, 2016; Atkinson, Michie, & Schall, 2011) and may predict conversion to psychotic disorders (Perez et al., 2014; Bodatsch et al., 2011; Shaikh et al., 2012; Naatanen et al., 2015), further supporting this idea. However, individuals at genetic risk for psychosis are also at an elevated risk for SPD (Asarnow et al., 2001; Barrantes-Vidal, Grant, & Kwapil, 2015; Erlenmeyer-Kimling et al., 1995; Kwapil & Varrantes-Bidal, 2015). Thus, while the absence of diagnosable SPD in our non-psychotic samples supports the notion of associations between dimensional, clinical risk and MMN impairments, we cannot rule out underlying genetic effects as impacting the expression of such symptoms. Future work is needed in this area to tease apart relationships of MMN amplitude with clinical, cognitive, and genetic risk factors in order to fully evaluate its effectiveness as a candidate marker of genetic risk for psychotic illness.
A strength of the present study is our large transdiagnostic sample of individuals with psychotic disorders and our inclusion of siblings of both individuals with SZ and OP. However, it may also be of note that effects in the present sample are small; though, this is not unusual in brain-behavior relationships, and is in fact in line with literature demonstrating that significant variability in neuroimaging measures accounts for only a small portion of variance in clinical phenotypes (Patrick et al., 2013; Paulus & Thompson, 2019). Though small effects such as these may not be useful in single-subject predictions, effects may be informative longer-term and with regard to the etiology of psychosis (Funder & Ozer, 2019). It is possible that our large sample size enabled detection of such effects, and as such these findings will benefit from replication. Finally, the overall lack of cognitive impairment in our sibling group is contrary to several prior studies (Arts, Jabben, Krabbendam, & Van Os, 2008; Bromet et al., 1996; Hill et al., 2013; Russo et al., 2017; Sitskoorn, Aleman, Ebisch, Appels, & Kahn, 2004; Snitz, MacDonald III, & Carter, 2006; Toulopoulou et al., 2005; Zalla et al., 2004) which may have impacted the present findings. There is greater evidence of cognitive impairments in siblings of individuals with SZ than OP; thus, cognitive impairments in siblings may have been occluded by the use of a mixed sibling group. However, as neither MMN amplitude, nor clinical and cognitive impairments, in the sibling group differed based on the corresponding case’s diagnosis, we believe this is unlikely. Future studies recruiting larger sibling samples, or utilizing approaches such as twin designs and molecular genetic analyses, will be an important step in yielding deeper knowledge regarding these relationships. In addition, it is important to note that the probands and never-psychotic subjects were not matched in the present study on premorbid IQ. It is possible that differences in cognitive functioning may account for some variance in group differences in MMN amplitude; future studies recruiting comparison subjects matched for premorbid IQ may help to shed more light on these effects. However, the group effect on MMN amplitude did not differ based on premorbid IQ, suggesting that results may be robust to these differences.
4.1. Conclusions
In sum, the present study demonstrates no significant reduction in MMN-D or MMN-F amplitude in siblings of individuals with psychosis. Results extend previous examinations restricted primarily to siblings of individuals with schizophrenia-spectrum disorder to risk for psychotic disorders broadly defined. While no significant reductions of MMN amplitude were observed in siblings in the present sample, associations of MMN-D with both positive and negative schizotypy and cognitive functioning emerged across study groups. This pattern of effects may imply that MMN-D amplitude reductions emerge as a result of, or in conjunction with, clinical features associated with psychosis. Such findings carry important implications for the utility of MMN-D amplitude as an indicator of inherited risk, and suggest that this component may be best conceptualized as an endophenotypic marker for transdiagnostic psychotic symptoms, negative symptoms, and cognitive dysfunction, rather than risk for psychosis per se.
Supplementary Material
Acknowledgements:
We thank Evelyn Bromet, cohort founder. The authors gratefully acknowledge the support of the participants and mental health community of Suffolk County for contributing their time and energy to this project. They are also indebted to dedicated efforts of study coordinators, interviewers for their careful assessments, and to the psychiatrists who derived the consensus diagnoses.
Funding Statement:
This work was supported by an NIH grant from the National Institute of Mental Health to Roman Kotov (MH110434) and 2014 NARSAD Young Investigator Award to Greg Perlman.
Footnotes
Conflicts of Interest: None
References
- Ahveninen J, Jääskeläinen IP, Osipova D, Huttunen MO, Ilmoniemi RJ, Kaprio J, … Therman S (2006). Inherited auditory-cortical dysfunction in twin pairs discordant for schizophrenia. Biological psychiatry, 60(6), 612–620. [DOI] [PubMed] [Google Scholar]
- Arts B, Jabben N, Krabbendam L, & Van Os J (2008). Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychological medicine, 38(6), 771. [DOI] [PubMed] [Google Scholar]
- Asarnow RF, Nuechterlein KH, Fogelson D, Subotnik KL, Payne DA, Russell AT, … Kendler KS (2001). Schizophrenia and schizophrenia-spectrum personality disorders in the first-degree relatives of children with schizophrenia: the UCLA family study. Archives of General Psychiatry, 58(6), 581–588. [DOI] [PubMed] [Google Scholar]
- Bache SM, and Wickham H (2014). magrittr: A Forward-Pipe Operator for R. R package version 1.5. https://CRAN.R-project.org/package=magrittr
- Baldeweg T, Klugman A, Gruzelier J, & Hirsch SR (2004). Mismatch negativity potentials and cognitive impairment in schizophrenia. Schizophrenia research, 69(2), 203–217. [DOI] [PubMed] [Google Scholar]
- Barrantes-Vidal N, Grant P, & Kwapil TR (2015). The role of schizotypy in the study of the etiology of schizophrenia spectrum disorders. Schizophrenia bulletin, 41(suppl_2), S408–S416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramon E, Croft RJ, McDonald C, Virdi GK, Gruzelier JG, Baldeweg T, … Murray RM (2004). Mismatch negativity in schizophrenia: a family study. Schizophrenia research, 67(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Bromet EJ, Jandorf L, Fennig S, Lavelle J, Kovasznay B, Ram R, … Craig T (1996). The Suffolk County Mental Health Project: demographic, pre-morbid and clinical correlates of 6-month outcome. Psychological medicine, 26(5), 953–962. [DOI] [PubMed] [Google Scholar]
- Bromet EJ, Kotov R, Fochtmann LJ, Carlson GA, Tanenberg-Karant M, Ruggero C, & Chang S. w. (2011). Diagnostic shifts during the decade following first admission for psychosis. American Journal of Psychiatry, 168(11), 1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero DC, Jonas KG, Li K, Perlman G, & Kotov R (2019). Common taxonomy of traits and symptoms: linking schizophrenia symptoms, schizotypy, and normal personality. Schizophrenia Bulletin, 45(6), 1336–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Simms L, Wu K, & Casillas A (1993). Schedule for nonadaptive and adaptive personality (SNAP): Minneapolis, MN: University of Minnesota Press. [Google Scholar]
- Donaldson KR, Novak Keisha D., Foti D, Marder M, Perlman G Kotov R, Mohanty A (2020). Associations of Mismatch Negativity With Psychotic Symptoms and Functioning Transdiagnostically Across Psychotic Disorders. Journal of abnormal psychology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan CC, Barry RJ, Connolly JF, Fischer C, Michie PT, Näätänen R, … & Van Petten C (2009). Event-related potentials in clinical research: guidelines for eliciting, recording, and quantifying mismatch negativity, P300, and N400. Clinical Neurophysiology, 120(11), 1883–1908. [DOI] [PubMed] [Google Scholar]
- Earls HA, Curran T, & Mittal V (2016). A meta-analytic review of auditory event-related potential components as endophenotypes for schizophrenia: perspectives from first-degree relatives. Schizophrenia Bulletin, 42(6), 1504–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson MA, Albrecht M, Ruffle A, Fleming L, Corlett P, & Gold J (2017). No association between symptom severity and MMN impairment in schizophrenia: A meta-analytic approach. Schizophrenia research: cognition, 9, 13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson MA, Ruffle A, & Gold JM (2016). A meta-analysis of mismatch negativity in schizophrenia: from clinical risk to disease specificity and progression. Biological psychiatry, 79(12), 980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlenmeyer-Kimling L, Squires-Wheeler E, Adamo UH, Bassett AS, Cornblatt BA, Kestenbaum CJ, … Gottesman II (1995). The New York High-Risk Project: psychoses and cluster A personality disorders in offspring of schizophrenic parents at 23 years of follow-up. Archives of General Psychiatry, 52(10), 857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanous A, Gardner C, Walsh D, & Kendler KS (2001). Relationship between positive and negative symptoms of schizophrenia and schizotypal symptoms in nonpsychotic relatives. Archives of General Psychiatry, 58(7), 669–673. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, & Williams J (2001). Structured clinical interview for DSM-IV-TR disorders—Non-patient edition (SCID—NP). New York: New York State Psychiatric Institute. [Google Scholar]
- Fisher DJ, Grant B, Smith DM, Borracci G, Labelle A, & Knott VJ (2011). Effects of auditory hallucinations on the mismatch negativity (MMN) in schizophrenia as measured by a modified ‘optimal’multi-feature paradigm. International Journal of Psychophysiology, 81(3), 245–251. [DOI] [PubMed] [Google Scholar]
- Fisher DJ, Labelle A, & Knott VJ (2008). The right profile: mismatch negativity in schizophrenia with and without auditory hallucinations as measured by a multi-feature paradigm. Clinical neurophysiology, 119(4), 909–921. [DOI] [PubMed] [Google Scholar]
- Fisher DJ, Labelle A, & Knott VJ (2012). Alterations of mismatch negativity (MMN) in schizophrenia patients with auditory hallucinations experiencing acute exacerbation of illness. Schizophrenia research, 139(1–3), 237–245. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, & Donchin E (1983). A new method for off-line removal of ocular artifact. Electroencephalography and clinical neurophysiology, 55(4), 468–484. [DOI] [PubMed] [Google Scholar]
- Hall MH, Schulze K, Rijsdijk F, Picchioni M, Ettinger U, Bramon E, … & Sham P (2006). Heritability and reliability of P300, P50 and duration mismatch negativity. Behavior genetics, 36(6), 845–857. [DOI] [PubMed] [Google Scholar]
- Hall M, Schulze K, Rijsdijk F, Kalidindi S, McDonald C, Bramon E, … Sham P (2009). Are auditory P300 and duration MMN heritable and putative endophenotypes of psychotic bipolar disorder? A Maudsley Bipolar Twin and Family Study. Psychological medicine, 39(8), 1277. [DOI] [PubMed] [Google Scholar]
- Hermens DF, Ward PB, Hodge MAR, Kaur M, Naismith SL, & Hickie IB (2010). Impaired MMN/P3a complex in first-episode psychosis: cognitive and psychosocial associations. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 34(6), 822–829. [DOI] [PubMed] [Google Scholar]
- Heslin M, Lomas B, Lappin J, Donoghue K, Reininghaus U, Onyejiaka A, … Fearon P (2015). Diagnostic change 10 years after a first episode of psychosis. Psychological medicine, 45(13), 2757–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SK, Reilly JL, Keefe RS, Gold JM, Bishop JR, Gershon ES, … Sweeney JA (2013). Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. American Journal of Psychiatry, 170(11), 1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Moran LV, Du X, O’Donnell P, & Summerfelt A (2012). Mismatch negativity and low frequency oscillations in schizophrenia families. Clinical neurophysiology, 123(10), 1980–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Doneshka P, Grochowski S, & Ritter W (1995). Impaired mismatch negativity generation reflects widespread dysfunction of working memory in schizophrenia. Archives of General Psychiatry, 52(7), 550–558. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Shelley A-M, & Ritter W (2000). Associated deficits in mismatch negativity generation and tone matching in schizophrenia. Clinical neurophysiology, 111(10), 1733–1737. [DOI] [PubMed] [Google Scholar]
- Jessen F, Fries T, Kucharski C, Nishimura T, Hoenig K, Maier W, … Heun R (2001). Amplitude reduction of the mismatch negativity in first-degree relatives of patients with schizophrenia. Neuroscience letters, 309(3), 185–188. [DOI] [PubMed] [Google Scholar]
- Johnstone EC, Abukmeil SS, Byrne M, Clafferty R, Grant E, Hodges A, … Owens DG (2000). Edinburgh high risk study—findings after four years: demographic, attainment and psychopathological issues. Schizophrenia research, 46(1), 1–15. [DOI] [PubMed] [Google Scholar]
- Kaur M, Battisti RA, Ward PB, Ahmed A, Hickie IB, & Hermens DF (2011). MMN/P3a deficits in first episode psychosis: comparing schizophrenia-spectrum and affective-spectrum subgroups. Schizophrenia research, 130(1–3), 203–209. [DOI] [PubMed] [Google Scholar]
- Kendler KS (1985). Diagnostic approaches to schizotypal personality disorder: A historical perspective. Schizophrenia Bulletin, 11(4), 538–553. [DOI] [PubMed] [Google Scholar]
- Kotov R, Fochtmann L, Li K, Tanenberg-Karant M, Constantino EA, Rubinstein J, … Carlson G (2017). Declining clinical course of psychotic disorders over the two decades following first hospitalization: evidence from the Suffolk County Mental Health Project. American Journal of Psychiatry, 174(11), 1064–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapil TR, & Barrantes-Vidal N (2015). Schizotypy: looking back and moving forward. Schizophrenia bulletin, 41(suppl_2), S366–S373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Sehatpour P, Hoptman MJ, Lakatos P, Dias EC, Kantrowitz JT, … Javitt DC (2017). Neural mechanisms of mismatch negativity dysfunction in schizophrenia. Molecular Psychiatry, 22, 1585–1593. 10.1038/mp.2017.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepock JR, Mizrahi R, Korostil M, Bagby RM, Pang EW, & Kiang M (2018). Event-related potentials in the clinical high-risk (CHR) state for psychosis: a systematic review. Clinical EEG and neuroscience, 49(4), 215–225. [DOI] [PubMed] [Google Scholar]
- Light GA, Swerdlow NR, Thomas ML, Calkins ME, Green MF, Greenwood TA, … Nuechterlein KH (2015). Validation of mismatch negativity and P3a for use in multi-site studies of schizophrenia: characterization of demographic, clinical, cognitive, and functional correlates in COGS-2. Schizophrenia research, 163(1–3), 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magno E, Yeap S, Thakore JH, Garavan H, De Sanctis P, & Foxe JJ (2008). Are auditory-evoked frequency and duration mismatch negativity deficits endophenotypic for schizophrenia? High-density electrical mapping in clinically unaffected first-degree relatives and first-episode and chronic schizophrenia. Biological psychiatry, 64(5), 385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EA, Jonas KG, Lian W, Foti D, Donaldson KR, Bromet EJ, & Kotov R (2021). Predicting Long-Term Outcomes in First-Admission Psychosis: Does the Hierarchical Taxonomy of Psychopathology Aid DSM in Prognostication?. Schizophrenia Bulletin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon DH, Roach BJ, & Ford JM (2010). Automatic semantic priming abnormalities in schizophrenia. International Journal of Psychophysiology, 75(2), 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie PT, Innes-Brown H, Todd J, & Jablensky AV (2002). Duration mismatch negativity in biological relatives of patients with schizophrenia spectrum disorders. Biological psychiatry, 52(7), 749–758. [DOI] [PubMed] [Google Scholar]
- Näätänen R (1995). The mismatch negativity: a powerful tool for cognitive neuroscience. Ear and hearing, 16(1), 6–18. [PubMed] [Google Scholar]
- Näätänen R, Kujala T, Kreegipuu K, Carlson S, Escera C, Baldeweg T and Ponton C (2011). The mismatch negativity: an index of cognitive decline in neuropsychiatric and neurological diseases and in ageing. Brain topography, 14312. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Sussman ES, Salisbury D, & Shafer VL (2014). Mismatch negativity (MMN) as an index of cognitive dysfunction. Brain topography, 27(4), 451–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niznikiewicz MA, Spencer KM, Dickey C, Voglmaier M, Seidman LJ, Shenton ME, & McCarley RW (2009). Abnormal pitch mismatch negativity in individuals with schizotypal personality disorder. Schizophrenia research, 110(1–3), 188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Venables NC, Yancey JR, Hicks BM, Nelson LD, & Kramer MD (2013). A construct-network approach to bridging diagnostic and physiological domains: Application to assessment of externalizing psychopathology. Journal of Abnormal Psychology, 122, 902–916. 10.1037/a0032807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, & Thompson WK (2019). The challenges and opportunities of small effects: The new normal in academic psychiatry. Journal of the American Medical Association Psychiatry, 76, 353–354. 10.1001/jamapsychiatry.2018.4540 [DOI] [PubMed] [Google Scholar]
- Randeniya R, Oestreich L, & Garrido M (2018). Sensory prediction errors in the continuum of psychosis. Schizophrenia research, 191, 109–122. [DOI] [PubMed] [Google Scholar]
- Ranlund S, Adams RA, Díez Á, Constante M, Dutt A, Hall MH, … Schulze K (2016). Impaired prefrontal synaptic gain in people with psychosis and their relatives during the mismatch negativity. Human Brain Mapping, 37(1), 351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. [Google Scholar]
- Reininghaus U, Böhnke JR, Hosang G, Farmer A, Burns T, McGuffin P, & Bentall RP (2016). Evaluation of the validity and utility of a transdiagnostic psychosis dimension encompassing schizophrenia and bipolar disorder. The British Journal of Psychiatry, 209(2), 107–113. [DOI] [PubMed] [Google Scholar]
- Reitan RM (1955). The relation of the trail making test to organic brain damage. Journal of consulting psychology, 19(5), 393. [DOI] [PubMed] [Google Scholar]
- Ruff R, Light R, Parker S, & Levin H (1996). Benton controlled oral word association test: Reliability and updated norms. Archives of Clinical Neuropsychology, 11(4), 329–338. [PubMed] [Google Scholar]
- Ruggero CJ, Carlson GA, Kotov R, & Bromet EJ (2010). Ten-year diagnostic consistency of bipolar disorder in a first-admission sample. Bipolar disorders, 12(1), 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero CJ, Kotov R, Carlson GA, Tanenberg-Karant M, González DA, & Bromet EJ (2011). Consistency of the diagnosis of major depression with psychosis across 10 years. The Journal of clinical psychiatry, 72(9), 1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo M, Van Rheenen T, Shanahan M, Mahon K, Perez-Rodriguez M, Cuesta-Diaz A, … Burdick K (2017). Neurocognitive subtypes in patients with bipolar disorder and their unaffected siblings. Psychological medicine, 47(16), 2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Şevik AE, Yağcıoğlu AEA, Yağcıoğlu S, Karahan S, Gürses N, & Yıldız M (2011). Neuropsychological performance and auditory event related potentials in schizophrenia patients and their siblings: a family study. Schizophrenia research, 130(1–3), 195–202. [DOI] [PubMed] [Google Scholar]
- Sheffield JM, Kandala S, Tamminga CA, Pearlson GD, Keshavan MS, Sweeney JA, … Barch DM (2017). Transdiagnostic associations between functional brain network integrity and cognition. JAMA psychiatry, 74(6), 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkkonen J, & Tervaniemi M (2000). Towards optimal recording and analysis of the mismatch negativity. Audiology and Neurotology, 5(3–4), 235–246. [DOI] [PubMed] [Google Scholar]
- Sitskoorn MM, Aleman A, Ebisch SJ, Appels MC, & Kahn RS (2004). Cognitive deficits in relatives of patients with schizophrenia: a meta-analysis. Schizophrenia research, 71(2–3), 285–295. [DOI] [PubMed] [Google Scholar]
- Snitz BE, MacDonald III AW, & Carter CS (2006). Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. [DOI] [PMC free article] [PubMed]
- Tarbox SI, & Pogue-Geile MF (2011). A multivariate perspective on schizotypy and familial association with schizophrenia: a review. Clinical psychology review, 31(7), 1169–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JM, Gallagher P, Hughes JH, Watson S, Gray JM, Ferrier IN, & Young AH (2005). Neurocognitive impairment in euthymic patients with bipolar affective disorder. The British Journal of Psychiatry, 186(1), 32–40. [DOI] [PubMed] [Google Scholar]
- Toulopoulou T, Mapua-Filbey F, Quraishi S, Kravariti E, Morris RG, McDonald C, … Murray RM (2005). Cognitive performance in presumed obligate carriers for psychosis. The British Journal of Psychiatry, 187(3), 284–285. [DOI] [PubMed] [Google Scholar]
- Trenerry MR, Crosson B, DeBoe J, & Leber W (1989). Stroop neuropsychological screening test. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Umbricht D, & Krljes S (2005). Mismatch negativity in schizophrenia: a meta-analysis. Schizophrenia research, 76(1), 1–23. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1981). Wechsler adult intelligence scale-revised (WAIS-R). Psychological Corporation. [Google Scholar]
- Wechsler D (2012). Wechsler preschool and primary scale of intelligence—fourth edition. The Psychological Corporation; San Antonio, TX. [Google Scholar]
- Wickham H, François R, Henry L, and Müller K (2020). dplyr: A Grammar of Data Manipulation. R package version 1.0.2. https://CRAN.Rproject.org/package=dplyr
- Zalla T, Joyce C, Szöke A, Schürhoff F, Pillon B, Komano O, … Dubois B (2004). Executive dysfunctions as potential markers of familial vulnerability to bipolar disorder and schizophrenia. Psychiatry research, 121(3), 207–217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


