Abstract
Background:
Life events (LEs) are a risk factor for first onset and relapse of psychotic disorders. However, the impact of LEs on specific symptoms—namely reality distortion, disorganization, negative symptoms, depression, and mania—remains unclear. Moreover, the differential effects of negative versus positive LEs are poorly understood.
Methods:
The present study utilizes an epidemiologic cohort of patients (N=428) ascertained at first-admission for psychosis and followed for a decade thereafter. Symptoms were assessed at 6-, 24-, 48- and 120-month follow-ups.
Results:
We examined symptom change within-person and found that negative events in the previous 6 months predicted an increase in reality distortion (β= .07), disorganized (β= .07), manic (β= .08), and depressive symptoms (β= .06), and a decrease in negative symptoms (β= −.08). Conversely, positive LEs predicted fewer reality distortion (β= −.04), disorganized (β= −.04), and negative (β= −.13) symptoms, and were unrelated to mood symptoms. A between-person approach to the same hypotheses confirmed that negative LEs predicted change in all symptoms, while positive LEs predicted change only in negative symptoms. In contrast, symptoms rarely predicted future LEs.
Conclusions:
These findings confirm that LEs have an effect on symptoms, and thus contribute to the burden of psychotic disorders. That LEs increase positive symptoms and decrease negative symptoms suggests at least two different mechanisms underlying the relationship between LEs and symptoms. Our findings underscore the need for increased symptom monitoring following negative LEs, as symptoms may worsen during that time.
Keywords: Life Stress, Psychosis, Schizophrenia, LEs, Cohort Study
Stress has long been associated with psychosis (Bebbington et al., 1993). Often, stress is presumed to play a contributory role in the onset and relapse of psychotic disorders. This is consistent with the diathesis-stress and vulnerability models of psychosis, both of which posit that life events (LEs) ‘activate’ a clinical or genetic vulnerability (Lukoff, Snyder, Ventura, & Nuechterlein, 1984; Nuechterlein & Dawson, 1984; Nuechterlein et al., 1994; Walker & Diforio, 1997). This model has been supported by research showing that LEs often occur shortly before the onset of psychosis (Beards et al., 2013; Bebbington et al., 1993; Day et al., 1987; Ira et al., 2014; March-Llanes, Marqués-Feixa, Mezquita, Fañanás, & Moya-Higueras, 2017; Trotman et al., 2014), even when limited to fateful events unrelated to a person’s symptoms and behavior (Bebbington et al., 1993; Malla, Cortese, Shaw, & Ginsberg, 1990; Raune, Kuipers, & Bebbington, 2009). There are several hypotheses regarding the mechanism of this relationship. The stress-generation model holds that the presence of symptoms increases the likelihood of experiencing LE’s (Hammen, 1991, 2006), described by Zubin & Spring (1977) as a “stress-prone pattern of living”. This pattern is supported by research showing LEs can be predicted by earlier symptoms and illness characteristics (Brown & Birley, 1968; Lukoff et al., 1984; March-Llanes et al., 2017; Shakoor et al., 2016).
While the relationship between LEs and illness onset is well-established, fewer studies have tracked LEs and symptom relapse or exacerbation. Brown & Birley’s (1968) seminal work demonstrated a link between retrospectively assessed LEs and relapse in schizophrenia. Multiple prospective studies of psychotic disorders have confirmed that LEs increase prior to relapse (Hirsch et al., 1996; Malla et al., 1990; Nuechterlein et al., 1994; Ventura, Nuechterlein, Lukoff, & Hardesty, 1989), where relapse is defined as an episode requiring hospitalization (Castine, Meador-Woodruff, & Dalack, 1998), or as a significant exacerbation in psychotic symptoms (Doering et al., 1998). One particularly large prospective study, however, failed to replicate the association between LEs and relapse (Hirsch et al., 1996), and in a review of the literature, Fallon (2008) concluded that the association between LEs and relapse was unresolved.
These inconsistent findings could be explained by unique effects of LEs on specific symptom domains, which are obscured when the outcome is relapse. Relapse implies positive symptoms are present, but could involve multiple symptom domains. Furthermore, utilizing a categorical (i.e., relapse or no relapse) rather than dimensional outcome measure may have obscured relationships present across the spectrum of symptom severity. Psychotic disorders manifest in diverse symptoms, including reality distortion (hallucinations and delusions), disorganization, negative symptoms, depression, and mania (Reininghaus et al., 2019; Reininghaus et al., 2016; Stochl et al., 2014). However, few studies have investigated the effect of LEs on these domains. One of the first, a retrospective study of patients with schizophrenia, found that those whose psychosis was rated as being “reactive to life events” had fewer negative symptoms than other patients (Fenton & McGlashan, 1991). More recently, a prospective study of individuals with schizophrenia spectrum disorders showed that LEs and emotional reactivity together predicted increases in positive symptoms, but not negative or mood symptoms (Docherty, St-Hilaire, Aakre, & Seghers, 2008). Similarly, a longitudinal study of adolescents with schizotypal personality disorder showed that LEs predicted reality distortion but not negative symptoms (Tessner, Mittal, & Walker, 2009), and a study of community adolescents found that LEs were positively correlated with psychotic experiences, but negatively correlated with anhedonia (Shakoor et al., 2016). The apparent inverse relationship between LEs and negative symptoms appears contrary to the well-established literature linking LEs and depression (Kessler, 1997; Stroud, Davila, & Moyer, 2008; Tennant, 2002), which is often confounded with negative symptoms. In a retrospective analysis of patients with schizophrenia, LEs were more strongly associated with depression than psychotic symptoms, and uncorrelated with negative symptoms (Schwartz & Myers, 1977). Negative LEs have also been associated with the onset or relapse of bipolar disorder (Ellicott, Hammen, Gitlin, Brown, & Jamison, 1990; Hammen & Gitlin, 1997; Hunt, Bruce-Jones, & Silverstone, 1992; Johnson & Roberts, 1995). Several studies have shown negative LEs precede manic episodes, but others fail to replicate this effect (for a review, see Johnson, 2005). Furthermore, several longitudinal studies suggest that negative LEs are not predictive of increased manic symptoms (Johnson, 2005; Mcpherson, Herbison, & Romans, 1993; Reilly-Harrington, Alloy, Fresco, & Whitehouse, 1999; Reilly-Harrington, Fresco, Whitehouse, & Zechmeister). Overall, evidence suggests that LEs predict exacerbation of reality distortion and depression symptoms in psychotic disorders, but the role of stress in disorganization, negative symptoms, and mania in the context of psychotic disorders is uncertain.
Finally, the research on LEs has generally focused on negative LEs, either ignoring positive LEs or treating them as equivalent, potentially conflating two opposite effects. For instance, Beards and colleagues’ 2013 meta-analysis identified only one study which distinguished positive and negative LEs. That study found that negative, but not positive LEs, predict onset of acute or transient psychosis (Chakraborty, Chatterjee, Choudhary, Singh, & Chakraborty, 2007). Outside of the psychosis literature, several studies have shown that positive events may reduce psychopathology (Cohen, McGowan, Fooskas, & Rose, 1984; S. Cohen & Hoberman, 1983; Dixon & Reid, 2000), or at least buffer against the effect of negative LEs (Davidson, Shahar, Lawless, Sells, & Tondora, 2006), but these findings must be interpreted carefully, as individuals with psychotic disorders may respond differently to LEs than healthy individuals. Studies of bipolar disorder in particular have suggested increased manic symptoms following goal-attainment, but not other positive events (Johnson et al., 2000), suggesting that in some instances positive LEs may precede worsening of symptoms. Overall, compared to negative LEs, the research on positive LEs is sparse, and the effects of positive LEs on symptoms in psychotic disorders is unclear.
The present study seeks to address these gaps by examining the impact of LEs on change in specific symptom domains of psychotic disorders, using both within- and between-person analyses. We further examined the directionality of these effects; whether LEs predict symptoms or symptoms predict LEs. Specifically, we utilized a cohort of individuals with psychotic disorders who were followed for ten years after first psychiatric hospitalization. We examined the impact of positive and negative LEs on reality distortion, disorganization, negative symptoms, depression, and mania. We hypothesized that negative LEs would exacerbate symptoms of psychosis, disorganization, depression, and mania. We also examined the impact of positive LEs, an exploratory aim given the limited knowledge in this area. Finally, there is mixed support for a stress generation model positing that symptoms can elicit LEs, and thus we further sought to examine whether symptoms lead to increased LEs at subsequent timepoints.
Methods
Participants
Participants are from the Suffolk County Mental Health Project (SCMHP; Bromet et al., 1992), an epidemiological study of first-admission psychosis. This study follows a cohort of individuals with schizophrenia, schizoaffective disorder, bipolar disorder with psychosis, major depressive disorder with psychosis, drug-induced psychosis, and psychotic disorders not otherwise specified. Data utilized in the present analyses were collected at 6-month, 24-month, 48-month, and 120-month follow-up. Recruitment and inclusion criteria are described in further detail by Bromet and colleagues (1992). All participants were consenting adults or assenting minors for whom parental consent was obtained; full participant demographics are presented in Table 1. This study was approved by the Stony Brook University Institutional Review Board (IRB). The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Table 1.
Sample Characteristics
| N | % | Mean | SD | |
|---|---|---|---|---|
| Baseline Gender | — | — | — | — |
| Female | 176 | 41.1 | — | — |
| Male | 252 | 58.9 | — | — |
| Diagnosis1 | — | — | — | — |
| Schizophrenia Spectrum | 207 | 48.4 | — | — |
| Other Psychosis | 221 | 51.6 | — | — |
| Race | — | — | — | — |
| White | 325 | 75.9 | — | — |
| Other Race | 103 | 24.1 | — | — |
| Baseline Age | — | — | 29.06 | 9.4 |
| Baseline SES (blue collar or less2) | 193 | 45.1 | — | — |
| Symptoms3 | — | — | — | — |
| Reality Distortion | — | — | 3.20 | 6.44 |
| Disorganization | — | — | 2.44 | 4.10 |
| Negative Symptoms | — | — | 14.64 | 13.42 |
| Depression | — | — | 12.22 | 4.24 |
| Mania | — | — | 1.29 | .761 |
| Number LEs Endorsed4 | — | — | — | — |
| Positive | — | — | .84 | 1.0 |
| Negative | — | — | .57 | .75 |
| Participation Rates | — | — | — | — |
| 6-month follow-up | 428 | 100 | — | — |
| 24-month follow-up | 375 | 87.6 | — | — |
| 48-month follow-up | 314 | 73.3 | — | — |
| 120-month follow-up | 354 | 82.7 | — | — |
Diagnosis assessed at most recent study wave. Schizophrenia spectrum includes schizophrenia and schizoaffective disorder.
SES of household primary breadwinner.
Distribution across 4 study intervals.
Measures
Life events.
Both positive and negative LEs were assessed using the Life Chart (adapted from McGonagle & Kessler, 1990), a checklist-based approach to assessing LEs. Interviewers rated LEs based on information obtained from participants during interviews, interviews with significant others, and review of medical records. Negative LEs from 13 categories and positive events from 9 categories were evaluated. Specific categories of events are included in Supplemental Table 1. Composites were scored at each follow-up by counting the number of LEs in each category (positive and negative) endorsed by the participant as occurring during the previous six months.
Symptoms.
Symptoms were assessed using the Scales for the Assessment of Positive Symptoms (SAPS; Andreasen, 1984) and the Scales for the Assessment of Negative Symptoms (SANS; Andreasen, 1989). Prior factor analysis of the SAPS and SANS in this sample indicated that the SANS may best be scored as a single index of negative symptoms, whereas the SAPS is best broken down into reality distortion and disorganization scales (Kotov, Guey, Bromet, & Schwartz, 2010). Mania was assessed using the excitement rating of the Brief Psychiatric Rating Scale (BPRS; Huber et al., 2012; Overall, 1974) and depression using the sum of nine symptoms of current depression from the Structured Clinical Interview for DSM-IV (SCID-IV, First, Spitzer, Gibbon, & Williams, 2002) as described previously (Kotov et al., 2017). All clinical variables were rated for the month preceding interview.
Analyses
Multilevel models.
In order to evaluate dynamic relationships within people over time, multilevel models were fit to the data using SAS (Version 9.4). The five symptom domains were the outcomes, predicted by either the positive or negative LE composite, for a total of 10 models. Random intercepts and slopes were included, estimating individual trajectories for each participant. Time-point of data collection was entered as a categorical covariate to control for any differences between follow-ups. Residual autocorrelations were estimated using an unstructured covariance matrix. All symptom and LE variables were grand mean standardized to facilitate interpretability.
Structural equation models.
Structural equation modeling was used to determine the direction of effects. These analyses employed a cross-lagged model, in which symptoms at each timepoint were predicted by both LEs preceding that timepoint and symptoms at the previous timepoint; likewise, LEs were predicted by previous LEs and symptoms (Figure 1). The model therefore tested the impact of LEs on future symptoms above and beyond previous symptoms, and the impact of symptoms on future LEs above and beyond previous LEs. For both positive and negative LEs, paths were constrained to be equal from symptoms to LEs and LEs to symptoms at each wave, save for between 48- and 120-months—due to the substantial difference in time between these study waves no constraints were placed on these paths. Disturbances on symptoms at non-adjacent waves were allowed to correlate, as symptom stability did not follow a simple autoregressive model. The addition of these correlations improved fit of all models. In evaluating the model, we considered Comparative Fit Index (CFI) > .90 and Root Mean Square Error of Approximation (RMSEA) < .08 to indicate good fit; and CFI > .95 and RMSEA < .05 to indicate excellent fit (Hu & Bentler, 1999; Marsh, Hau, & Wen, 2004). Structural equation models were estimated using SAS version 9.4. Fit statistics are presented in Table 2.
Figure 1. Model of Between-Subjects Effects.
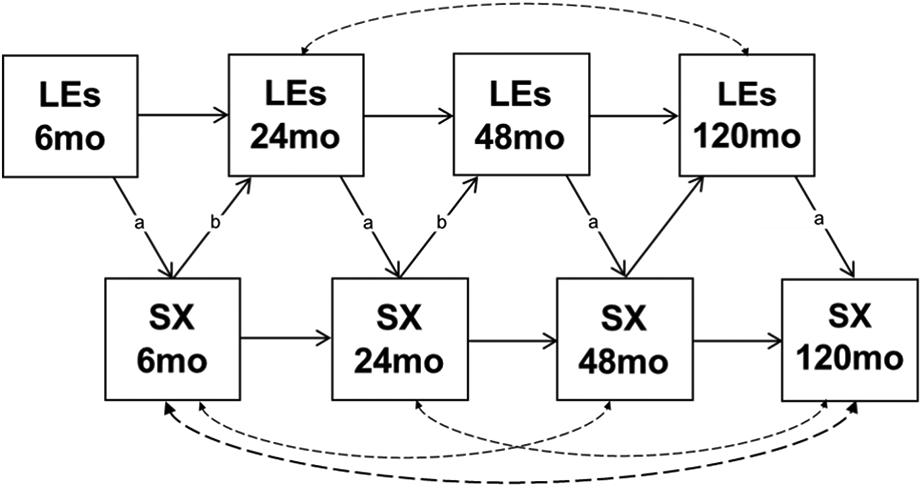
Cross-lagged structural equation models of the interplay between LEs and symptoms between-subjects. Paths with the same label were constrained to be equal. The same model was fit for all SX/LE combinations reported in Tables. LE = life events; SX = symptoms. Dashed lines indicate correlations included to improve model fit and not described in tables.
Table 2.
Fit Indexes Across Structural Equation Models
| Model | CFI1 | RMSEA2 |
|---|---|---|
| Reality Distortion | ||
| Positive LE | .91 | .05 |
| Negative LE | .95 | .04 |
| Disorganization | ||
| Positive LE | .92 | .05 |
| Negative LE | .98 | .03 |
| Negative Symptoms | ||
| Positive LE | .98 | .04 |
| Negative LE | .98 | .04 |
| Mania | ||
| Negative LE | .97 | .03 |
| Depression | ||
| Negative LE | .98 | .02 |
Comparative Fit Index
Root Mean Square Error of Approximation
Data were 86% complete across waves, and 1,471 observations were available for analyses. Analyses used Full Information Maximum Likelihood, which allowed us to retain all available observations.
Results
Sample characteristics
Participants were predominantly male, and 29 years of age at study entry on average. About half had a schizophrenia spectrum disorder (Table 1). Participants experienced on average fewer than one LE at each timepoint (mean negative = .57; mean positive = .84).
Within-person effects of LEs on symptoms
Multilevel models were fit to the data in order to examine effects of LEs on symptoms over time. Results are presented in Table 3. We observed significant within-person effect of negative LEs on increases in reality distortion, disorganization, mania, and depression. This indicates that when a person experienced more LEs relative to their own average, reality distortion, disorganization, mania, and depression were elevated, compared to intervals with fewer LEs. Negative LEs also predicted a decrease in negative symptoms. Effects of adverse LEs on symptoms remained consistent after controlling for demographic characteristics (Supplemental Table 2). The impact of demographic factors on slopes of the adverse LE – symptom relationships are presented in Supplemental Table 3 and Supplemental Figures 1 & 2.
Table 3.
Within-Person Effects of LEs Across Multi-Level Models
| Life Events | β | SE | df | p |
|---|---|---|---|---|
| Reality Distortion | ||||
| Positive | −0.04 | 0.02 | 358 | 0.025* |
| Negative | 0.07 | 0.03 | 375 | 0.006* |
| Disorganization | ||||
| Positive | −0.04 | 0.02 | 349 | 0.045* |
| Negative | 0.07 | 0.02 | 365 | 0.002* |
| Negative Symptoms | ||||
| Positive | −0.13 | 0.02 | 344 | <.0001* |
| Negative | −0.08 | 0.02 | 364 | <.0001* |
| Mania | ||||
| Positive | 0.05 | 0.03 | 358 | 0.064 |
| Negative | 0.08 | 0.03 | 375 | 0.002* |
| Depression | ||||
| Positive | −0.04 | 0.02 | 356 | 0.1334 |
| Negative | 0.06 | 0.02 | 374 | 0.019* |
Indicates significance p<.05.
Positive life LEs predicted decreases in reality distortion, disorganization, and negative symptoms. After a person experienced more positive LEs relative to their own average, levels of these three symptom dimensions decreased compared when they had fewer positive LEs than their own average. Positive LEs were not significantly associated with symptoms of depression or mania.
Direction of effects
Structural equation models were used to test the directions of the LE-symptom associations observed in multilevel models. Path effects are presented in Table 4. Structural equation models are illustrated in Figure 1. Across models, all paths from negative LEs to later symptoms were significant, with negative LEs predicting exacerbation in all domains save for negative symptoms, in which LEs predicted a reduction in symptom severity. Symptoms did not consistently predict occurrence of negative LEs, except for negative symptoms which predicted fewer LEs in the first 48 months of the study.
Table 4.
Path Effects in Structural Equation Models of LEs
| Negative LEs | |||||
|---|---|---|---|---|---|
| Effect |
Reality distortion (β) 1 |
Disorgani
zation (β) |
Negative
SX (β) |
Mania
(β) |
Depression
(β) |
| Negative LE 6M on SX 6M 2 | .10 | .11 | −.07 | .11 | .06 |
| Negative LE 24M on SX 24M | .10 | .11 | −.08 | .10 | .06 |
| Negative LE 48M on SX 48M | .11 | .10 | −.08 | .11 | .06 |
| Negative LE 120M on SX 120M | .05 | .05 | −.05 | .06 | .04 |
| SX 6M on Negative LE 24M | -- | -- | −.11 | -- | -- |
| SX 24M on Negative LE 48M | -- | -- | −.11 | -- | -- |
| SX 48M on Negative LE 120M | -- | −.10 | -- | −.13 | .17 |
| Negative LE 6M on Negative LE 24M | .20 | .20 | .20 | .20 | .20 |
| Negative LE 24M on Negative LE 48M | .30 | .30 | .28 | .29 | .29 |
| Negative LE 48M on Negative LE 120M | -- | -- | -- | -- | -- |
| SX 6M on SX 24M | .44 | .41 | .67 | .42 | .32 |
| SX 24M on SX 48M | .51 | .42 | .63 | .22 | .33 |
| SX 48M on SX 120M | .27 | .38 | .40 | .24 | .27 |
| Positive LEs | |||||
|
Reality distortion
(β) |
Disorganization
(β) |
Negative SX (β) | |||
| Positive LE 6M on SX 6M | -- | -- | −.16 | ||
| Positive LE 24M on SX 24M | -- | -- | −.15 | ||
| Positive LE 48M on SX 48M | -- | -- | −.13 | ||
| Positive LE 120M on SX 120M | -- | -- | −.11 | ||
| SX 6M on Positive LE 24M | -- | -- | −.09 | ||
| SX 24M on Positive LE 48M | -- | -- | −.10 | ||
| SX 48M on Positive LE 120M | -- | -- | -- | ||
| Positive LE 6M on Positive LE 24M | .14 | .14 | .12 | ||
| Positive LE 24M on Positive LE 48M | .27 | .28 | .26 | ||
| Positive LE 48M on Positive LE 120M | .17 | .17 | .16 | ||
| SX 6M on SX 24M | .44 | .40 | .66 | ||
| SX 24M on SX 48M | .51 | .43 | .59 | ||
| SX 48M on SX 120M | .28 | .38 | .41 | ||
Effect sizes reported for all p<.05 significant paths; correlations among disturbances on symptoms at non-adjacent waves are not shown.
LE = Life Events; SX = Symptoms.
Positive LEs showed few significant effects on symptoms (Table 4). The only consistent effect was for greater positive LEs predicting a reduction in negative symptoms. This effect was largely bidirectional, with reduced negative symptoms predicting increased positive LEs at most timepoints. Given the potentially confounding effects of depressive symptoms and antipsychotic medication on negative symptoms, two additional models were fit controlling for these effects. In both negative and positive LE models, results remained robust to the impacts of depression and antipsychotic medication (Supplemental Table 4).
Discussion
The present study advances understanding of the relationship between LEs and psychosis, discriminating between positive and negative LEs, examining effects on specific symptom domains, and tracking this interplay over ten years. By utilizing both between (SEM) and within (MLM) subjects analyses, we demonstrate paths from LEs to symptoms, and illustrate how this dynamic unfolds for a typical patient. We found that across illness course, negative LEs were associated with subsequent increases in reality distortion, disorganization, mania, and depression. The direction of these effects is from negative LEs to symptoms, supporting the vulnerability model rather than the stress-generation model. In fact, only with negative symptoms was the inverse relationship present, in which more severe negative symptoms predicted fewer LEs, both negative and positive. Outside of this counterintuitive effect, positive LEs were largely unrelated to symptoms.
The observed effect of negative LEs on positive symptoms echoes previous findings that LEs trigger relapse of psychosis (Brown & Birley, 1968; Doering et al., 1998; Hirsch et al., 1996; Malla et al., 1990; Nuechterlein et al., 1994; Ventura et al., 1989) and is consistent with prior literature examining relationships between stress and psychotic symptoms in the general population (Shakoor et al., 2016) and in schizotypy (Tessner et al., 2009). Present findings are also consistent with research detailing the impact of expressed emotion (interpersonal stress in the family environment) and relapse in psychotic disorders (Butzlaff & Hooley, 1998; Stirling et al., 1991). Our findings further suggest that these symptoms have little effect on risk for LEs, consistent with a diathesis-stress, rather than stress-generation, model of psychotic symptoms. Previous literature has provided mixed support for a stress-generation in psychosis (Horan et al., 2005; Lukoff et al., 1984); the present study provides clarity, supporting this model only for negative symptoms.
Similar results were observed for depression and mania. Negative LEs predicted escalation of depression, consistent with prior research on the onset of depression (Kessler, 1997; Tennant, 2002; Ventura, Nuechterlein, Subotnik, Hardesty, & Mintz, 2000), as well as depression in psychotic disorders (Schwartz & Myers, 1977). Negative LEs also predicted worsening of manic symptoms. This has been previously implied by studies of recurrence in bipolar disorder, in which LEs are shown to precede episodes which could be either manic or depressive (Ellicott et al., 1990; Hunt et al., 1992; Johnson & Roberts, 1995). Here, we show that this is in fact the case, and extend this finding to mania in the context of a psychotic disorder. We found no evidence of depressive or manic symptoms eliciting LEs, contrary to the literature on depression, in which the stress-generation model has been supported (for a review, see Liu & Alloy, 2010). The relatively long intervals between follow-ups may have prevented us from capturing this effect, as well as the relatively mild nature of depressive symptoms in our sample. Future studies should examine these relationships in studies of psychotic disorders with more frequent follow-up assessments.
Negative symptoms were the exception to the rule, bearing an inverse relationship to LEs, such that both positive and negative LEs were associated with a reduction in negative symptoms. The converse relationship also held: greater negative symptoms were associated with fewer LEs. These effects are opposite of those implied by both the vulnerability and stress-generation models. While this is inconsistent with some previous studies (Docherty et al., 2008; Tessner et al., 2009), other research has alluded to an inverse association with negative symptoms (Fenton & McGlashan, 1991; Shakoor et al., 2016). It is possible that severe negative symptoms prevent individuals from the kinds of activities that would precipitate either positive or negative LEs. For instance, one is less likely to experience the beginning or end of a romantic relationship if one is sexually and socially disinterested.
Thus far, no studies to our knowledge have examined the relationship between positive LEs and specific symptoms in psychosis (Seeman, 2017). In the present analyses, the effects of positive LEs were weak and inconsistent, except for their inverse association with negative symptoms. Interventions such as social skills training may impact negative symptoms via increasing positive LEs, although these data suggest that such a change in negative symptoms would be accompanied by a further increase in both positive and negative LEs.
A strength of the present study is our large first-episode sample of psychotic disorders, with a wide range of clinical presentations, and course observed over 10 years. Furthermore, we tested both within- and between-subject effects, and provided support for a relationship in the between LEs and symptoms across both. A limitation of this study is that assessment points were spaced years apart. Further, symptoms measured burden in the past month rather than a discrete episode onset; while conversely, LEs were rated for the preceding six months. This likely enhanced the effects of LEs on symptoms compared to effects of symptoms on LEs. Consequently, we cannot be certain that symptom change followed LEs rather than preceded them. Furthermore, data on dependence of LE on an individual’s behavior were systematically coded only at year 10, and we were unable to test the temporal associations of dependent and independent LE separately, although they may have different patterns of interplay with symptoms. Nevertheless, lack of evidence for stress generation in these data suggest that this confounding is likely to be small. Another limitation is that this study utilized an interview-based structured life events assessment, rather than a semi-structured life stress interview allowing for the weighing of context for each event. Finally, since the start of this study several concerns have been raised regarding the SANS, including that some items may reflect secondary symptoms, emerging perhaps as a result of depression or antipsychotic medication (Kirkpatrick, Fenton, Carpenter, & Marder 2006; Whiteford, Riney, & Csernansky, 1987). However, present results are demonstrated to be robust to such confounding effects. Similarly, some items in this scale are influenced by the frequency of interactions; thus, there may be some degree of overlap between certain negative symptom items and particular life events. Future work may choose to more specifically parse negative symptoms assessed to investigate these potential confounds. Despite these limitations, present findings are consistent with results of short-term studies that had more frequent and detailed assessments (Schwartz & Myers, 1977; Ventura et al., 1989), and extends them to a 10-year time span, supporting the impact of LEs on the long-term course of psychotic disorders.
In sum, negative LEs appear to contribute to exacerbations of reality distortion, disorganization, depression, and mania. Positive LEs show weaker effects but may offer some protection against negative symptoms. Contrary to what is hypothesized by the stress-generation model, symptoms did not generally predict future life events, save for relationships between more severe negative symptoms and fewer future LEs, both positive and negative. This pattern helps to inform models of illness course in psychotic disorders, and highlights that patients who were recently exposed to a negative LE could benefit from additional monitoring and services.
Supplementary Material
Acknowledgements:
The authors gratefully acknowledge the support of the participants and mental health community of Suffolk County for contributing their time and energy to this project. They are also indebted to dedicated efforts of study coordinators, interviewers for their careful assessments, and to the psychiatrists who derived the consensus diagnoses. Special thanks to Janet Lavelle for her many contributions to the study.
Funding Statement:
This work was supported by the National Institutes of Health (grant number MH44801 to E. J. B.; grant numbers MH094398 and MH110434 to R.K.)
Footnotes
Conflicts of Interest: None
References
- Alloy LB, Reilly-Harrington NA, Fresco DM, Whitehouse WG, & Zechmeister JS (1999). Cognitive Styles and Life Events in Subsyndromal Unipolar and Bipolar Disorders: Stability and Prospective Prediction of Depressive and Hypomanic Mood Swings. Journal of Cognitive Psychotherapy, 13(1), 21. [Google Scholar]
- Andreasen NC (1984). Scale for the assessment of positive symptoms (SAPS). Iowa City: University of Iowa. [Google Scholar]
- Andreasen NC (1989). The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. The British Journal of Psychiatry, 155(S7), 49–52. [PubMed] [Google Scholar]
- Beards S, Gayer-Anderson C, Borges S, Dewey ME, Fisher HL, & Morgan C (2013). Life events and psychosis: a review and meta-analysis. Schizophrenia bulletin, 39(4), 740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebbington P, Wilkins S, Jones P, Foerster A, Murray R, Toone B, & Lewis S (1993). Life events and psychosis: Initial results from the Camberwell Collaborative Psychosis Study. The British Journal of Psychiatry, 162(1), 72–79. [DOI] [PubMed] [Google Scholar]
- Bromet EJ, Schwartz JE, Fennig S, Geller L, Jandorf L, Kovasznay B, … & Rich C (1992). The epidemiology of psychosis: The Suffolk County mental health project. Schizophrenia bulletin, 18(2), 243–255. [DOI] [PubMed] [Google Scholar]
- Brown GW, & Birley JL (1968). Crises and life changes and the onset of schizophrenia. Journal of health and social behavior, 9(3), 203–214. [PubMed] [Google Scholar]
- Butzlaff RL, & Hooley JM (1998). Expressed emotion and psychiatric relapse: a meta-analysis. Archives of general psychiatry, 55(6), 547–552. [DOI] [PubMed] [Google Scholar]
- Castine M, Meador-Woodruff J, & Dalack G (1998). The role of life events in onset and recurrent episodes of schizophrenia and schizoaffective disorder. Journal of Psychiatric Research, 32(5), 283–288. [DOI] [PubMed] [Google Scholar]
- Chakraborty R, Chatterjee A, Choudhary S, Singh AR, & Chakraborty P (2007). Life events in acute and transient psychosis—a comparison with mania. Ger J Psychiatr, 10, 36–40. [Google Scholar]
- Cohen LH, McGowan J, Fooskas S, & Rose S (1984). Positive life events and social support and the relationship between life stress and psychological disorder. American Journal of Community Psychology, 12(5), 567–587. [DOI] [PubMed] [Google Scholar]
- Cohen S, & Hoberman HM (1983). Positive events and social supports as buffers of life change stress 1. Journal of applied social psychology, 13(2), 99–125. [Google Scholar]
- Davidson L, Shahar G, Lawless MS, Sells D, & Tondora J (2006). Play, pleasure, and other positive life events:“Non-specific” factors in recovery from mental illness? Psychiatry: Interpersonal and Biological Processes, 69(2), 151–163. [DOI] [PubMed] [Google Scholar]
- Day R, Nielsen J, Korten A, Ernberg G, Dube K, Gebhart J, … Olatawura M (1987). Stressful life events preceding the acute onset of schizophrenia: a cross-national study from the World Health Organization. Culture, Medicine and Psychiatry, 11(2), 123–205. [DOI] [PubMed] [Google Scholar]
- Dixon WA, & Reid JK (2000). Positive life events as a moderator of stress-related depressive symptoms. Journal of Counseling & Development, 78(3), 343–347. [Google Scholar]
- Docherty NM, St-Hilaire A, Aakre JM, & Seghers JP (2008). Life events and high-trait reactivity together predict psychotic symptom increases in schizophrenia. Schizophrenia bulletin, 35(3), 638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering S, Müller E, Köpcke W, Pietzcher A, Gaebel W, Linden M, … Schüssler G (1998). Predictors of relapse and rehospitalization in schizophrenia and schizoaffective disorder. Schizophrenia bulletin, 24(1), 87–98. [DOI] [PubMed] [Google Scholar]
- Ellicott A, Hammen C, Gitlin M, Brown G, & Jamison K (1990). Life events and the course of bipolar disorder. The American journal of psychiatry, 147(9), 1194–1198. [DOI] [PubMed] [Google Scholar]
- Fallon P (2008). Life events; their role in onset and relapse in psychosis, research utilizing semi-structured interview methods: a literature review. Journal of psychiatric and mental health nursing, 15(5), 386–392. [DOI] [PubMed] [Google Scholar]
- Fenton WS, & McGlashan TH (1991). Natural history of schizophrenia subtypes: II Positive and negative symptoms and long-term course. Archives of general psychiatry, 48(11), 978–986. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JB (2002). Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition. Retrieved from [Google Scholar]
- Hammen C (1991). Generation of stress in the course of unipolar depression. Journal of Abnormal psychology, 100(4), 555. [DOI] [PubMed] [Google Scholar]
- Hammen C (2006). Stress generation in depression: Reflections on origins, research, and future directions. Journal of clinical psychology, 62(9), 1065–1082. [DOI] [PubMed] [Google Scholar]
- Hammen C, & Gitlin M (1997). Stress reactivity in bipolar patients and its relation to prior history of disorder. The American journal of psychiatry, 154(6), 856–857. [DOI] [PubMed] [Google Scholar]
- Hirsch S, Bowen J, Emami J, Cramer P, Jolley A, Haw C, & Dickinson M (1996). A one year prospective study of the effect of life events and medication in the aetiology of schizophrenic relapse. The British Journal of Psychiatry, 168(1), 49–56. [DOI] [PubMed] [Google Scholar]
- Horan WP, Ventura J, Nuechterlein KH, Subotnik KL, Hwang SS, & Mintz J (2005). Stressful life events in recent-onset schizophrenia: reduced frequencies and altered subjective appraisals. Schizophrenia research, 75(2-3), 363–374. [DOI] [PubMed] [Google Scholar]
- Hu L. t., & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural equation modeling: a multidisciplinary journal, 6(1), 1–55. [Google Scholar]
- Huber CG, Schöttle D, Lambert M, Hottenrott B, Agorastos A, Naber D, & Schroeder K (2012). Brief Psychiatric Rating Scale—Excited Component (BPRS-EC) and neuropsychological dysfunction predict aggression, suicidality, and involuntary treatment in first-episode psychosis. Schizophrenia research, 134(2-3), 273–278. [DOI] [PubMed] [Google Scholar]
- Hunt N, Bruce-Jones W, & Silverstone T (1992). Life events and relapse in bipolar affective disorder. Journal of affective disorders, 25(1), 13–20. [DOI] [PubMed] [Google Scholar]
- Ira E, De Santi K, Lasalvia A, Bonetto C, Zanatta G, Cristofalo D, … Gardellin F (2014). Positive symptoms in first-episode psychosis patients experiencing low maternal care and stressful life events: a pilot study to explore the role of the COMT gene. Stress, 17(5), 410–415. [DOI] [PubMed] [Google Scholar]
- Johnson SL (2005). Life events in bipolar disorder: towards more specific models. Clinical psychology review, 25(8), 1008–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, & Roberts JE (1995). Life events and bipolar disorder: implications from biological theories. Psychological bulletin, 117(3), 434. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Sandrow D, Meyer B, Winters R, Miller I, Solomon D, & Keitner G (2000). Increases in manic symptoms after life events involving goal attainment. Journal of Abnormal psychology, 109(4), 721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC (1997). The effects of stressful life events on depression. Annual review of psychology, 48(1), 191–214. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Fenton WS, Carpenter WT, & Marder SR (2006). The NIMH-MATRICS consensus statement on negative symptoms. Schizophrenia bulletin, 32(2), 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Fochtmann L, Li K, Tanenberg-Karant M, Constantino EA, Rubinstein J, … Carlson G (2017). Declining clinical course of psychotic disorders over the two decades following first hospitalization: evidence from the Suffolk County Mental Health Project. American Journal of Psychiatry, 174(11), 1064–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Guey LT, Bromet EJ, & Schwartz JE (2010). Smoking in schizophrenia: diagnostic specificity, symptom correlates, and illness severity. Schizophrenia bulletin, 36(1), 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RT, & Alloy LB (2010). Stress generation in depression: A systematic review of the empirical literature and recommendations for future study. Clinical psychology review, 30(5), 582–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukoff D, Snyder K, Ventura J, & Nuechterlein KH (1984). Life events, familial stress, and coping in the developmental course of schizophrenia. Schizophrenia bulletin, 10(2), 258–292. [DOI] [PubMed] [Google Scholar]
- Malla A, Cortese L, Shaw T, & Ginsberg B (1990). Life events and relapse in schizophrenia. Social Psychiatry and Psychiatric Epidemiology, 25(4), 221–224. [DOI] [PubMed] [Google Scholar]
- March-Llanes J, Marqués-Feixa L, Mezquita L, Fañanás L, & Moya-Higueras J (2017). Stressful life events during adolescence and risk for externalizing and internalizing psychopathology: a meta-analysis. European child & adolescent psychiatry, 26(12), 1409–1422. [DOI] [PubMed] [Google Scholar]
- Marsh HW, Hau K-T, & Wen Z (2004). In search of golden rules: Comment on hypothesis-testing approaches to setting cutoff values for fit indexes and dangers in overgeneralizing Hu and Bentler's (1999) findings. Structural equation modeling, 11(3), 320–341. [Google Scholar]
- McGonagle KA, & Kessler RC (1990). Chronic stress, acute stress, and depressive symptoms. American Journal of Community Psychology, 18(5), 681–706. [DOI] [PubMed] [Google Scholar]
- Mcpherson H, Herbison P, & Romans S (1993). Life events and relapse in established bipolar affective disorder. The British Journal of Psychiatry, 163(3), 381–385. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, & Dawson ME (1984). A heuristic vulnerability/stress model of schizophrenic episodes. Schizophrenia bulletin, 10(2), 300. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Dawson ME, Ventura J, Gitlin M, Subotnik KL, Snyder KS, … Bartzokis G (1994). The vulnerability/stress model of schizophrenic relapse: a longitudinal study. Acta Psychiatrica Scandinavica, 89, 58–64. [DOI] [PubMed] [Google Scholar]
- Overall JE (1974). The Brief Psychiatric Rating Scale in psychopharmacology research. [Google Scholar]
- Raune D, Kuipers E, & Bebbington P (2009). Stressful and intrusive life events preceding first episode psychosis. Epidemiology and Psychiatric Sciences, 18(3), 221–228. [PubMed] [Google Scholar]
- Reilly-Harrington NA, Alloy LB, Fresco DM, & Whitehouse WG (1999). Cognitive styles and life events interact to predict bipolar and unipolar symptomatology. Journal of Abnormal psychology, 108(4), 567. [DOI] [PubMed] [Google Scholar]
- Reininghaus U, Böhnke JR, Chavez-Baldini U, Gibbons R, Ivleva E, Clementz BA, … Tamminga CA (2019). Transdiagnostic dimensions of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). World Psychiatry, 18(1), 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reininghaus U, Böhnke JR, Hosang G, Farmer A, Burns T, McGuffin P, & Bentall RP (2016). Evaluation of the validity and utility of a transdiagnostic psychosis dimension encompassing schizophrenia and bipolar disorder. The British Journal of Psychiatry, 209(2), 107–113. [DOI] [PubMed] [Google Scholar]
- Schwartz CC, & Myers JK (1977). Life events and schizophrenia: II. Impact of life events on symptom configuration. Archives of general psychiatry, 34(10), 1242–1245. [PubMed] [Google Scholar]
- Seeman MV (2017). Schizophrenia: Reaction to Positive Life Events. Psychiatric Quarterly, 88(3), 561–570. [DOI] [PubMed] [Google Scholar]
- Shakoor S, Zavos HM, Haworth CM, McGuire P, Cardno AG, Freeman D, & Ronald A (2016). Association between stressful life events and psychotic experiences in adolescence: evidence for gene–environment correlations. The British Journal of Psychiatry, 208(6), 532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling J, Tantam D, Thomas P, Newby D, Montague L, Ring N, & Rowe S (1991). Expressed emotion and early onset schizophrenia: a one year follow-up. Psychological Medicine, 21(3), 675–685. [DOI] [PubMed] [Google Scholar]
- Stochl J, Jones PB, Plaistow J, Reininghaus U, Priebe S, Perez J, & Croudace TJ (2014). Multilevel ordinal factor analysis of the Positive and Negative Syndrome Scale (PANSS). International journal of methods in psychiatric research, 23(1), 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud CB, Davila J, & Moyer A (2008). The relationship between stress and depression in first onsets versus recurrences: A meta-analytic review. Journal of Abnormal psychology, 117(1), 206. [DOI] [PubMed] [Google Scholar]
- Tennant C (2002). Life events, stress and depression: a review of recent findings. Australian & New Zealand Journal of Psychiatry, 36(2), 173–182. [DOI] [PubMed] [Google Scholar]
- Tessner KD, Mittal V, & Walker EF (2009). Longitudinal study of stressful life events and daily stressors among adolescents at high risk for psychotic disorders. Schizophrenia bulletin, 37(2), 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotman HD, Holtzman CW, Walker EF, Addington JM, Bearden CE, Cadenhead KS, … Mathalon DH (2014). Stress exposure and sensitivity in the clinical high-risk syndrome: initial findings from the North American Prodrome Longitudinal Study (NAPLS). Schizophrenia research, 160(1-3), 104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura J, Nuechterlein KH, Lukoff D, & Hardesty JP (1989). A prospective study of stressful life events and schizophrenic relapse. Journal of Abnormal psychology, 98(4), 407. [DOI] [PubMed] [Google Scholar]
- Ventura J, Nuechterlein KH, Subotnik KL, Hardesty JP, & Mintz J (2000). Life events can trigger depressive exacerbation in the early course of schizophrenia. Journal of Abnormal psychology, 109(1), 139. [PubMed] [Google Scholar]
- Walker EF, & Diforio D (1997). Schizophrenia: a neural diathesis-stress model. Psychological review, 104(4), 667. [DOI] [PubMed] [Google Scholar]
- Whiteford HA, Riney SJ, & Csernansky JG (1987). Distinguishing depressive and negative symptoms in chronic schizophrenia. Psychopathology, 20(5-6), 234–236. [DOI] [PubMed] [Google Scholar]
- Zubin J, & Spring B (1977). Vulnerability: a new view of schizophrenia. Journal of Abnormal psychology, 86(2), 103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


