SUMMARY.
Wild waterfowl in the order Anseriformes are recognized reservoirs for influenza A viruses (IAVs); however, prevalence of infection can vary greatly by species. Few isolates of IAVs have been reported from snow geese (Chen caerulescens), and generally they have not been regarded as an important component of this reservoir. In February 2013, 151 combined cloacal and oropharangeal swabs and 147 serum samples were collected from snow geese wintering on the Gulf coast of Texas. None of the swab samples tested positive by virus isolation, but antibodies to IAVs were detected in 87 (59%) birds tested by competitive blocking ELISA (bELISA). To further characterize these detected antibodies, positive samples were tested by virus microneutralization (MN) for antibodies to viruses representing 14 hemagglutinin subtypes (HA1–HA12, H14, and H15). By MN, antibodies to H1 (n = 41; 47%), H5 (n = 32; 37%), H6 (n = 49; 56%), H9 (n = 50; 57%), and H12 (n = 24; 28%) were detected. Snow goose populations have increased in North America since the 1960s, and their association with agricultural lands provides a potential indirect source of IAV infection for domestic poultry. This potential, as well as the detection of antibodies to HA subtypes H5, H9, and H12 that are not well represented in other waterfowl species, suggests that further snow geese surveillance is indicated.
Keywords: antibodies, Chen caerulescens, influenza A virus, snow goose, virus isolation
RESUMEN.
Anticuerpos contra el virus de la influenza A virus en gansos blancos (Chen caerulescens) durante el invierno en Texas. Las aves acuáticas silvestres del orden Anseriformes son reservorios reconocidos del virus de la influenza A (IAVs); Sin embargo, la prevalencia de la infección puede variar mucho según la especie. Se han registrado pocos aislamientos de virus de este tipo en los gansos blancos (Chen caerulescens), y por lo general no han sido considerados como reservorios importantes. En Febrero de 2013, se recolectaron 151 hisopos cloacales y orofaríngeos combinados y 147 muestras de suero de gansos blancos de la costa del Golfo de Texas durante el invierno. Ninguna de las muestras de hisopos dio positivo para el aislamiento del virus, pero se detectaron anticuerpos contra virus de este tipo en 87 (59%) las aves analizadas por ELISA por bloqueo competitivo (bELISA). Para caracterizar mejor estos anticuerpos detectados, las muestras positivas fueron analizadass por microneutralización viral (MN) para detectar anticuerpos contra los virus que representan 14 subtipos de hemaglutinina (HA1–HA12, H14 y H15). Mediante la microneutralización, se detectaron anticuerpos contra H1 (n = 41; 47%), H5 (n = 32; 37%), H6 (n = 49; 56%), H9 (n = 50; 57%) y H12 (n = 24; 28%). Las poblaciones de gansos blancos han aumentado en América del Norte desde la década de los 1960s, y su asociación con las tierras agrícolas proporciona una fuente indirecta de infección por el virus de influenza aviar para las aves comerciales. Este potencial, así como la detección de anticuerpos contra los subtipos de hemaglutinina HA H5, H9 y H12 que no están bien representados en otras especies de aves acuáticas, sugiere que una vigilancia mayor de los gansos blancos es indicada.
Wild waterfowl in the order Anseriformes are natural reservoirs for influenza type A viruses (IAVs) (8); however, prevalence of infection varies by species (17). Reported IAV isolation rates from wild geese, including snow geese (Chen caerulescens), historically have been low (12,13), and consequently they have not been regarded as an important component of this reservoir. Greater white-fronted geese (Anser albifrons albifrons) in Europe are a possible exception to this with IAV prevalence as detected by PCR reported as high as 10.7% (11). The low prevalence of IAV reported from geese may partly represent an artifact of seasonal sampling; unlike ducks, transmission and viral shedding appears to be associated with wintering or spring migration staging rather than fall migration (4,9,11,15). In contrast to low IAV isolation rates from geese, a high prevalence ofIAV antibodies have been reported from pink-footed geese (Anser brachyrhynchus) in Europe (9), and greater white-fronted geese (4), Canada geese (Branta canadensis) (10), snow geese (15), cackling geese (Branta hutchinsii), black brant (Branta bernicla), and emperor geese (Chen canagica) (4) in North America. In North America, IAV antibody prevalence estimates from more than 3000 snow geese ranged from 32.4% to 75.5%, indicating that this species in commonly infected (15). Snow geese, due to an extended range, also may be involved with long-distance IAV transport and may be locally important with regard to IAV transmission to domestic poultry on wintering areas and migratory corridors. Snow geese range extends from breeding areas in the Arctic to wintering areas in the Gulf of Mexico, and populations have recently expanded due to their utilization of food sources associated with agricultural landscapes (1). Snow geese population in the mid-continental United States increased from the late 1960s to 2005 as a result of increased survival related to abundant agricultural food supplies; in 2005 it was estimated that this population consisted of over 5 million birds (1).
The objectives of this study were 1) to determine the prevalence of IAV infection and nucleoprotein (NP) antibody prevalence in a wintering population of snow geese on the Texas Gulf coast and 2) to identify hemagglutinin (HA)-specific antibodies in this population as a means of identifying predominant subtypes infecting this population. In relation to this second objective, hemagglutination inhibition (HI) is most commonly used to detect for IAV HA-specific antibodies in avian species, but the duration of detectable HI antibodies in ducks and other wild birds is not well defined and may be short lived. In mallards experimentally infected with a low pathogenic H5N2 IAV, only 58% of mallards infected with IAV retained a detectable HI antibody response by 4 wk postexposure (5). For this reason, we elected to attempt to measure subtype specific antibodies using virus microneutralization (MN).
MATERIALS AND METHODS
In February 2013, 151 combined cloacal and oropharangeal swabs and 147 serum samples were collected from snow geese wintering on the Gulf coast of Texas. Cloacal and oropharyngeal swabs were tested for IAV by virus isolation in specific-pathogen-free chicken eggs as described (16).
Serum samples were tested by commercial blocking enzyme-linked immunosorbent assay (bELISA, IDEXX Laboratories, Westbrook, ME) for IAV antibodies as described by the manufacturer. Samples testing positive by bELISA were also tested by MN. Antigens for MN were prepared in Madin–Darby canine kidney (MDCK) cells (American Type Culture Collection, Manassas VA, USA). During virus propagation, and in all MN test procedures, cells were maintained in minimal essential media (MEM; Sigma-Aldrich, St. Louis MO) containing L-1-tosylamido2-phenylethyl chloromethyl ketone-trypsin (final concentration of 1 μg/ml; Worthington Biochemical Corporation, Lakewood, NJ) and antibiotics (final concentration of 100 units penicillin, 0.1 mg streptomycin, and 0.25 μg amphotericin B/ml; Sigma-Aldrich). Antigen was stored at −80 C until used. For antibody testing, sera were diluted 1:10 in MEM and heat inactivated at 57 C for 30 min. Serum samples were screened at a 1:20 dilution against all antigens. For the screen, 25 μL of the diluted serum (1:10) were placed in a single well of a 96-well v-bottom plate corresponding to each antigen. An additional well for each serum sample served as a serum control to determine potential toxicity. A positive control well using chicken antisera to each antigen (provided by the National Veterinary Services Laboratory, APHIS, USDA) and a negative control well using MEM were also included. Each antigen (25 μL containing 100 median tissue culture infective doses [102.0 TCID50]) was added to each well, not including the serum control wells, which received 25 μL MEM. Plates were incubated for 2 hr at room temperature after which 25 μL from each well was transferred to a second 96-well tissue culture plate with a confluent monolayer of MDCK cells. Prior to transfer, the tissue culture plate containing the MDCK cells was washed two times with Dulbecco’s phosphate buffered saline (Sigma-Aldrich), and 150 μL of trypsin supplemented MEM was added to each well. The inoculated tissue culture plate was incubated at 5% CO2 at 37 C and was visually read at 48–72 hr. For the test result to be considered valid, all controls (serum, positive, and negative) had to meet their expected negative or positive status. In addition, based on back titration in MDCK cells (four replicates per dilution), the viral titer of the antigen had to fall within 101.5 and 102.5 TCID50/25 μL. Sera were considered positive on the screen if no cytopathic effect (CPE) was observed. All positive serum samples were titrated. Each positive serum sample was diluted twofold in MEM on a 96 well v-bottom plate (final volume of 25 μL well at dilutions 1:20 to 1:640) and tested as described above. If CPE was observed at the minimum 1:20 dilution, the sample was classified as negative; if not, the positive titer was recorded as the highest dilution at which no CPE was observed. Viruses used as antigens in the MN assays included A/mallard/NJ/AI10–4263/2010 (H1N1), A/mallard/MN/AI08–2755/2008 (H2N3), A/mallard/MN/AI10–2593/2010 (H3N8), A/mallard/MN/AI10–3208/2010 (H4N6), A/mallard/MN/AI11–3933/2011 (H5N1), A/mallard/MN/AI08–2721/2008 (H6N1), A/mallard/MN/AI08–3770/2009 (H7N9), A/mallard/MN/SG-01048/2008 (H8N4), A/RUTU/NJ/AI07–293/2007 (H9N1), A/RUTU/DE/AI11–809/2011 (H9N2), A/mallard/MN/SG-00999/2008 (H10N7), A/mallard/MN/SG-00930/2008 (H11N9), A/mallard/MN/SG-3285/2007 (H12N5), A/blue-winged teal/TX/AI13–1028/2013 (H14N5), and A/wedge-tailed shearwater/Western Australia/2327/1983 (H15N6).
RESULTS
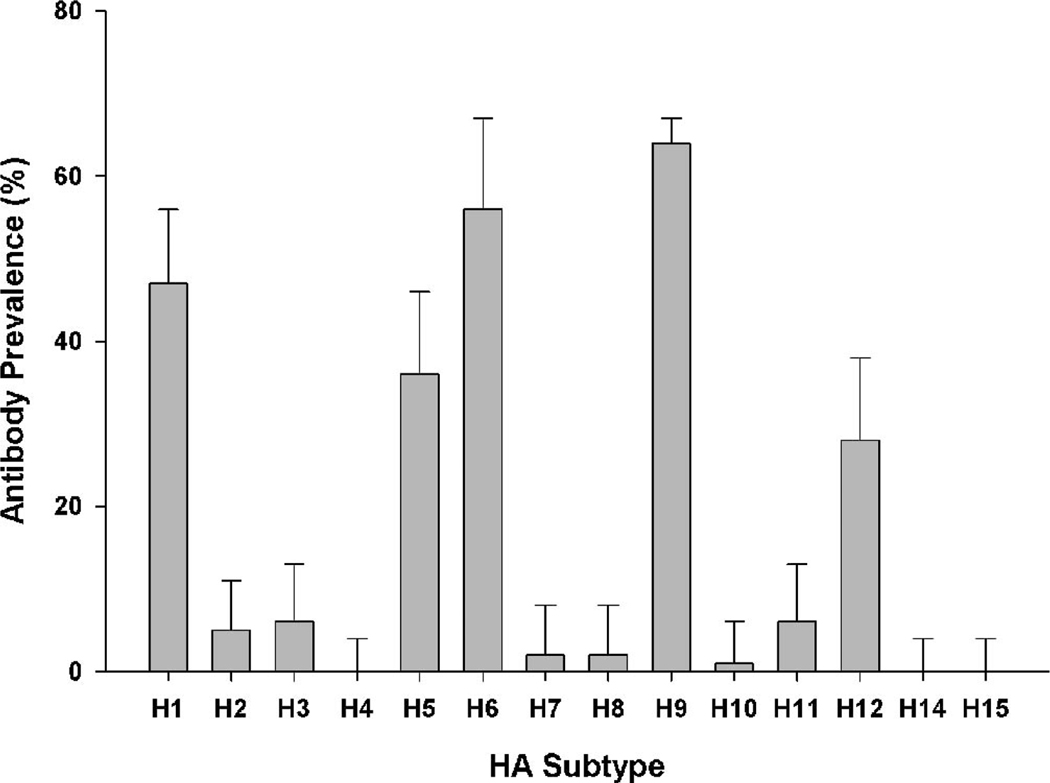
All swab samples tested negative by virus isolation. Antibodies were detected by bELISA in 87 (59%) of 147 birds. Of the 87 bELISA-positive birds tested by MN, 75 (86%) tested antibody positive to one or more HA antigens (Table 1). Subtype prevalence estimates based on MN test results are shown in Figure 1. Antibodies were detected against 11 of the 14 HA antigens, but high antibody prevalence (>20%) were detected only against H1, H5, H6, H9, and H12. The highest antibody prevalence (64%) was detected against the H9N1 antigen. To verify the H9 results, 68 samples including 56 and 12 sera that tested positive and negative to H9N1, respectively, were tested against the H9N2 antigen (Table 2). Antibody prevalence estimates for this sample subset were 82% and 90% for the H9N1 and H9N2, respectively.
Table 1.
Proportion of bELISA positive snow goose serum samples testing positive to zero, one, or multiple HA subtypes.
| Number tested | Number of HA subtypesA |
||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6+ | |
| 87 | 12 (13.8) | 12 (13.8) | 18 (20.7) | 19 (21.8) | 15 (17.2) | 6 (6.9) | 5 (5.7) |
Number of samples (%) testing positive.
Fig. 1.
Antibody prevalence to hemagglutinin subtypes 1–12, 14, and 15 in snow goose sera as determined by virus microneutralization tests. All tested sera were positive for antibodies to nucleoprotein as determined by bELISA (bars show 95% upper confidence limits on prevalence estimate).
Table 2.
Serologic test results for 68 snow goose sera using two H9 antigens: A/RUTU/NJ/AI07–283/2007 (H9N1) and A/RUTU/DE/AI11–909/2011 (H9N2).
| H9N1 | H9N2 |
|
|---|---|---|
| Positive | Negative | |
| Positive | 52 (76.5%) | 4 (5.9%) |
| Negative | 9 (13.2%) | 3 (4.4%) |
DISCUSSION
Our failure to isolate virus from this species and the high observed IAV antibody prevalence is consistent with reported isolation and serologic results from numerous goose species worldwide (4,9,10,13,15). This pattern is difficult to interpret and may reflect the combined effects of seasonal or localized infection that is difficult to capture during routine surveillance and a possible short duration of shedding associated with previous infections, as described in ducks (3). Our high observed IAV NP antibody prevalence and positive MN results for numerous IAV subtypes suggest a high level of population immunity that could greatly reduce isolation rates.
Our MN results indicate exposure to numerous IAV subtypes (Table 1), and based on virus isolation data this is a normal occurrence in waterfowl (17). Previous studies have consistently reported H3, H4, and H6 as the predominant IAV subtypes present in North American Anseriformes (8,17). In this study, antibodies to H1, H5, H6, H9, and H12 predominated. The high prevalence estimates for H9 and H12 are unique, as these subtypes are not commonly reported from ducks. However, as the duration of the detectable IAV antibody response is not well defined for waterfowl, and has never been evaluated in snow geese, it is difficult to know if these antibodies reflect recent infections or in part resulted from cross-reactivity with related HA or neuraminidase. Because of these unknowns, we did three evaluations to validate the specificity of our results. First, H14 and H15 viruses were included in our tests. The H14 viruses are rare and only recently (2010) reported in North America (7); H15 viruses are not present. No antibodies were detected against either subtype. Second, we evaluated two H9 antigens with different neuraminidase, and prevalence results for the tested subset were similar with both H9N1 and H9N2 (Table 2). Finally, to determine the potential for cross reactivity between genetically related HAs based on their relative position on the HA phylogenic tree (6), we evaluated prevalence estimates for subtypes within the H1 (H1, H2, H5, and H6) and H9 (H8, H9, and H12) clades. Within the H1 clade, monospecific results (testing positive to only one HA subtype within the clade) were detected for H1, H5, and H6, and there was no evidence of nonspecific results to H2. Within the H9 clade, monospecific results were detected only for H9; all H12 positive samples also tested positive for H9, and there was no evidence of nonspecific results for H8. Although cross-reactions cannot be ruled out by these results, they suggest that the detected high antibody prevalence estimates for H1, H5, H6, and H9 did not result from HA cross-reactivity.
Currently, there are no long-term data related to infection of wild avian species with multiple IAV subtypes to assist with the interpretation of MN or other subtype-specific serologic tests such as HI. A comparison of HI and MN results is needed to fully understand the relative performance of these tests as applied to free-ranging wild bird populations; however, due to the unknown infection histories of snow geese tested in our study, such a comparison would not be valid.
Our data are consistent with previous infections with multiple subtypes (Table 1), but these results are potentially confounded by the possibility of cross-reactions; this is especially true with the H12 data. Similar interpretive issues related to cross-reactive antibody responses exist with IAV serological data from humans (14). Although imperfect, serologic approaches do provide an additional tool to evaluate the potential reservoir status of wild bird populations (2), and based on our results, it is possible to obtain more defined serologic data related to specific HA subtypes.
Snow geese are an abundant waterfowl species in North America, and their utilization of agricultural lands provides a potential source of direct and indirect IAV infection for domestic poultry. This potential, as well as the detection of antibodies to HA subtypes H5 and H9 that are not well represented in other waterfowl species, highlights the importance of further snow geese surveillance and research related to the interpretation of serologic data.
ACKNOWLEDGMENTS
We thank Patrick Walther and personnel from Anahuac National Wildlife Refuge for assistance with snow goose capture. This work was funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract HHSN272201400006C. The funding agencies were not involved in the design, implementation, or publishing of this study, and the research presented herein represents the opinions of the authors, but not necessarily the opinions of the funding agencies.
Abbreviations:
- bELISA
blocking enzyme-linked immunosorbent assay
- CPE
cytopathic effect
- HA
hemagglutinin
- HI
hemagglutination inhibition
- IAV
influenza A virus
- MDCK
Madin–Darby canine kidney
- MEM
minimal essential media
- MN
microneutralization
- NP
nucleoprotein
- TCID50
median tissue culture infective doses
REFERENCES
- 1.Abraham KF, Jefferies RL, and Alisauskas RT The dynamics of landscape change and snow geese in mid-continental North America. Global Change Biol. 11:841–855. 2005. [Google Scholar]
- 2.Brown JD, Stallknecht DE, Berghaus RD, Beck JR, Leathers V, Kistler W, Costa T, Luttrell MP, Yabsley M, and Swayne DE Evaluation of a commercial blocking ELISA as a serologic assay for avian influenza virus in experimentally infected wild avian species. Clin. Vac. Immunol 16:824–829. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costa TP, Brown JD, Howerth EW, and Stallknecht DE Effect of a prior exposure to a low pathogenic avian influenza virus in the outcome of a heterosubtypic low pathogenic avian influenza infection in mallards (Anas platyrhynchos). Av. Dis 54:1286–1291. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ely CR, Hall JS, Schmutz JA, Pierce JM, Terenzi J, Sedinger JS, and Ip HS Evidence that life history characteristics of birds influence infection and exposure to influenza A viruses. PLoS ONE 8(3): e57614. doi: 10.1371/journal.pone.0057614. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fereidouni SR, Grund C, Häuslaigner R, Lange E, Wilking H,Harder T, Beer M, and Starick E. Dynamics of specific antibody responses induced in mallards after infection by or immunization with low pathogenicity avian influenza viruses. Av. Dis 54:79–85. 2010. [DOI] [PubMed] [Google Scholar]
- 6.Fouchier RAM, Munster V, Wallensten A, Bestebroer TM, Herfst S, Smith D, Rimmelzwaan GF, Olson B, and Osterhaus ADME Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J. Virol 79:2814–2822. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fries AC, Nolting JM, Danner A, Webster RG, Bowman AS,Krauss S, and Slemons RD Evidence for the circulation and interhemispheric movement of the H14 subtype influenza A virus. PLoS ONE 8:e59216. doi: 10.1371/journal.pone.0059216. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hinshaw VS, Webster RG, and Turner B. The perpetuation of orthomyxoviruses and paramyxoviruses in Canadian waterfowl. Can. J. Microbiol 26:622–629. 1980. [DOI] [PubMed] [Google Scholar]
- 9.Hoye BJ, Munster VJ, Nishiura H, Fouchiier RAM, Madsen J, and Klaassen M. Reconstructing an annual cycle of interaction: natural infection and antibody dynamics to avian influenza along a migratory flyway. Oikos 120:748–755. 2011. [Google Scholar]
- 10.Kistler WM, Stallknecht DE, Deliberto TJ, Swafford S, van Why K, Wolf PC, Hill JA, Bruning DL, Cumbee JC, Mickley RM, Betsill CE, Berghaus RD, and Yabsley MJ Evaluation of Canada geese (Branta canadensis) as sentinels for detection of transmission of avian influenza viruses. J. Wildl. Dis 48:999–1009. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleijn D, Munster VJ, Ebbinge BS, Jonkers DA, Müskens GJDM, Van Randen Y, and Fouchier RAM Dynamics and ecological consequences of avian influenza infection in greater white-fronted geese in their winter staging areas. Proc. R. Soc. B 277:2041–2048. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munster VJ, Baas C, Lexmond P, Waldenström J, Wallensten A, Fransson T, Rimmelzwann G, Beyer WEP, Schutten M, Olsen B, Osterhaus ADME, and Fouchier RAM Spatial, temporal, and species variation in prevalence of influenza A viruses in wild migratory birds. PLoS Pathog. 3(5):e61. doi: 10.1371/journal.ppat.0030061. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsen B, Munster VJ, Wallensten A, Waldenstrom J, Osterhaus ADME, and Fouchier RAM Global patterns of influenza A virus in wild birds. Science 312:384–388. 2006. [DOI] [PubMed] [Google Scholar]
- 14.Oshansky CM, Wong S-S Jeevan T, Smallwood HS, Webby RJ, Shafir SC, and Thomas PG Seasonal influenza vaccination is the strongest correlate of cross-reactive antibody responses in migratory bird handlers. mBIO 5(6):e02107–14. doi: 10.1128/mBio.02107-14. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samuel MD, Hall JS, Brown JD, Goldberg DR, Ip H, and Baranyuk V. The dynamics of avian influenza in western Arctic snow geese: implications for annual and migratory infection patterns. Ecol. Appl 25:1851–1859. [DOI] [PubMed] [Google Scholar]
- 16.Stallknecht DE, Shane SM, Zwank PJ, Senne DA, and Kearney MT Avian influenza viruses from migratory and resident ducks of coastal Louisiana. Av. Dis 34:398–405. 1990. [PubMed] [Google Scholar]
- 17.Wilcox BR, Knutsen GA, Berdeen J, Goekjian V, Poulson R, Goyal S, Sreevatsan S, Cardona C, Berghaus R, Swayne D, Yabsley M, and Stallknecht DE Influenza-A viruses in ducks in northwestern Minnesota: fine scale spatial and temporal variation in prevalence and subtype diversity. PLoS ONE 6:e24010. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]