Abstract
The important roles of human milk oligosaccharides (HMOS), the third major component of human milk, in the health of breast-fed infants have been increasingly recognized. Structures of more than 100 different HMOS have now been elucidated. Despite the recognition of the various functions of HMOS as prebiotics, antiadhesive antimicrobials, and immunomodulators, the roles and the applications of individual HMOS species are less clear. This is mainly due to the limited accessibility to large amounts of individual HMOS in their pure forms. Current advance on the development of enzymatic, chemoenzymatic, whole cell, and living cell systems allows for the production of a growing numbers of HMOS in increasing amounts. This effort will greatly facilitate the elucidation of the important roles and exploring the applications of HMOS as individual compounds and as a mixture of defined structures with desired functions. The structures, functions, and enzyme-catalyzed synthesis of HMOS are briefly surveyed to provide a general picture about the current progress on these aspects. Future efforts should be devoted to elucidating the structures of more complex HMOS, synthesizing more complex HMOS including those with branched structures, and to develop HMOS-based or HMOS-inspired prebiotics, additives, and therapeutics.
Keywords: human milk oligosaccharides (HMOS), prebiotic, antiadhesive, antimicrobial, immunomodulator, structure, function, synthesis
1. Introduction
Carbohydrates in human milk are presented in diverse forms including monosaccharides such as glucose and galactose, lactose (a disaccharide), oligosaccharides, glycoproteins, glycopeptides, and glycolipids.1 Human milk oligosaccharides (HMOS) containing a diverse array of oligosaccharides with three or more monosaccharide units are the subject of investigation here.
HMOS are the third major component of human milk after lactose (55–70 g/L) and lipids (16–39 g/L).2–4 Historically, purified HMOS were used to synthesize glycan antigens to obtain antibodies5, 6 which was later used as important bioreagents to identify novel glycans and detect glycoconjugates.7 The amounts of HMOS vary in lactation stages with 12–14 g/L in mature milk and 20–24 g/L in colostrum.8–10 HMOS were found to be presented in higher concentrations in preterm human milk than those in term human milk.11 The presence and the quantity of HMOS also vary among individuals and are related to the secretor status and the Lewis group type of the nursing mothers.10–13 Four human milk groups have been classified based on the HMOS profiles controlled by the Secretor (Se) status and the Lewis (Le) blood type of the nursing mother.11 Individuals (Se+/Le+) with both α1–2-fucosyltransferase FUT2 encoded by the Secretor (Se) gene and α1–3/4-fucosyltransferase FUT3 encoded by the Lewis (Le) gene represent about 70% of the European population and contain all types of fucosylated HMOS with α1–2/3/4-fucosyl linkages. Those (Se−/Le+) with no FUT2 but with FUT3 represent 20% of the population and do not have α1–2-fucosylated HMOS. Those (Se+/Le−) with FUT2 but no FUT3 represent 9% of the general population and do not have α1–4-fucosyl oligosaccharides. Finally, those (Se−/Le−) without FUT2 nor FUT3 represent 1% of the general population contains α1–3-fucosylated HMOS but not other fucosylated HMOS due to the expression of a Lewis-independent α1–3-fucosyltransferase.11, 14
Due to its structure complexity and the lack of efficient analytic methods, the presence and the functions of HMOS were unaware of in early time. For example, lactose was first isolated from milk in 1633.15 In comparison, three centuries years later in the early 1930, Polonowski and Lespagnol found and named nitrogen-containing “gynolactose”16, 17 which was confirmed two decades later to be a mixture of more than ten oligosaccharides by two-dimensional paper chromatography separation.18 In 1954, György, Kuhn, and et al. reported β-linked N-acetylglucosamine (GlcNAc)-containing oligosaccharides and polysaccharides in human milk as “bifidus factors”19 that promote the growth of Lactobacillus bifidus var. Penn (now Bifidobacterium bifidum).20–23 This ignited the efforts on elucidating the structures of HMOS. Several papers published in 1956 reported the structures of lacto-N-tetraose (LNT), 2’-fucosyllactose (2’FL), lacto-N-fucopentaose I (LNFP I), and 3-fucosyllactose (3FL).24–27 By 1965, 14 HMOS structures have been reported, mainly by the groups of Kuhn and Montreuil.28 Additional structures were soon elucidated by the efforts of Ginsburg, Kobata, and others. The introduction of mass spectrometry to the identification of HMOS29 further speeds up the progress. Modern advance on the separation and analysis method development allows fast profiling of HMOS and the structure identification of additional HMOS. More than 200 HMOS species have now been observed30, 31 and more than 100 HMOS structures have been elucidated.28, 32–34
Unlike lactose, the primary component and the principle carbohydrate of human milk which is digestible by infants and provides them nutritional needs,35 HMOS are not digestible by the infant.1, 36, 37 Therefore, the direct physiological roles of HMOS are not clear. Accumulating evidence has shown that HMOS can survive the obstacles encountered upon suckling and reach the infant gut where they regulate the microbiota population which in turn can affect the health of breastfed infants.36–39 HMOS are believed to contribute significantly to the health of breast-fed infants in lowering their risk of diarrheal disease, respiratory infections, allergy, and other infectious diseases including otitis media.15, 40–42 The prebiotic (stimulating the growth and colonization of beneficial bacteria, mainly bifidobacteria, in the gut), antiadhesive antimicrobial (acting as decoys to inhibit specific pathogenic bacteria, viruses, or parasites binding to epithelial surface and translocation), immunomodulating, and brain development nutritional functions of HMOS have also been reported.1, 15, 38, 43–46 The enrichment of bifidobacteria in the gut also leads to the increased production of lactate and short-chain fatty acids thus the decrease of pH, worsening the environment for the growth and colonization of some pathogens.1 Additional mechanism of pathogen inhibition may include the release of other anti-microbial substances by bifidobacteria.47
The functions of individual structures, however, are less clear. Only a handful of HMOS have known specific roles, and only a limited number of HMOS have been synthesized. The current knowledge about the structures, functions, and production of HMOS by enzyme-catalyzed processes is presented here.
2. Structures Of HMOS
2.1. HMOS monosaccharide building blocks, core structures, and glycosidic linkages.
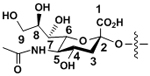
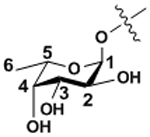
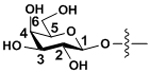
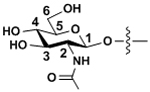
Human milk is unique in containing a large number of oligosaccharides compared to the milk of other mammals.48 Five monosaccharides have been found to be major building blocks for HMOS which include D-glucose (Glc), D-galactose (Gal), N-acetyl-D-glucosamine (GlcNAc), L-fucose (Fuc), and N-acetylneuraminic acid (Neu5Ac). These monosaccharide building blocks in HMOS are presented in the six-membered ring pyranose (for Glc) or pyranoside (for Gal, GlcNAc, Fuc, and Neu5Ac) structures. HMOS are extended from lactose (Galβ1–4Glc with Glc at the reducing end) by N-acetylglucosaminylation and/or galactosylation with or without fucosylation and/or sialylation. Among the five major HMOS monosaccharide building blocks (TABLE I), the glucose (Glc) is at the reducing end with a mix of α- and β- configuration at the anomeric carbon. While Gal and GlcNAc are always presented with β-glycosidic linkages, Fuc and Neu5Ac are always presented with α-glycosidic linkages.
TABLE I.
Major monosaccharide building blocks for HMOS.
| HMOS monosaccharide building blocks | Abbreviations | Symbols | Structures | Glycosidic Linkages in HMOS |
|---|---|---|---|---|
| N-Acetylneuraminic acid | Neu5Ac |

|
|
α-linkages |
| Fucose | Fuc |

|
|
α-linkages |
| Galactose | Gal |

|
|
β-linkages |
| N-Acetylglucosamine | GlcNAc |

|
|
β-linkages |
| Glucose | Glc |

|
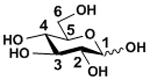
|
None (at the reducing end, a mix of α and β configurations at the anomeric carbon) |
Other than lactose (which itself is not considered an HMOS), at least fifteen neutral oligosaccharides (TABLE II), including linear and branched structures, have been identified to be able to serve as the core structures of other HMOS.32–34 It is interesting to observe that these structures rarely have GlcNAc as the terminal unit at the non-reducing end, indicating the high efficiency of galactosyltransferases (either β1–3- or β1–4-galactosyltransferase) in capping the GlcNAc residues in these HMOS. In addition, unlike N-acetyllactosamine (LacNAc, Galβ1–4GlcNAc) which can serve as both internal and non-reducing-end terminal disaccharide units, lacto-N-biose (LNB, Galβ1–3GlcNAc) can only serve as the non-reducing-end terminal disaccharide. Linear structures only contain β1–3-linked GlcNAc residue, while any β1–6-linked GlcNAc generates branching.38
TABLE II.
Lactose and neutral non-fucosylated HMOS that can serve as the core structures for other HMOS.28, 32–34
| Core # | Lactose and HMOS core structures | Abbreviations | Symbols | Ref. |
|---|---|---|---|---|
| I | Lactose (not considered an HMOS itself) | Lac |
|
49 |
| II | Lacto-N-tetraose | LNT |
|
24 |
| III | Lacto-N-neotetraose | LNnT |
|
50 |
| IV | Lacto-N-hexaose | LNH |
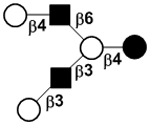
|
51 |
| V | Lacto-N-neohexaose | LNnH |
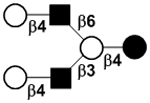
|
52 |
| VI | para-Lacto-N-hexaose | pLNH |
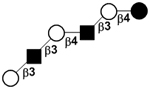
|
53 |
| VII | para-Lacto-N-neohexaose | pLNnH |
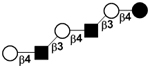
|
53 |
| VIII | Lacto-N-octaose | LNO |
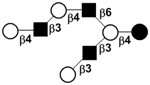
|
54 |
| IX | Lacto-N-neooctaose | LNnO |
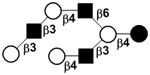
|
54 |
| X | iso-Lacto-N-octaose | iLNO |
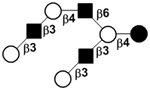
|
55 |
| XI | para-Lacto-N-octaose | pLNO |
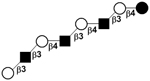
|
56 |
| XII | para-Lacto-N-neooctaose | pLNnO |
|
57 |
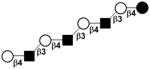
| XIII | Lacto-N-decaose | LND |
|
58 |
| XIV | Lacto-N-neodecaose | LNnD |
|
58 |
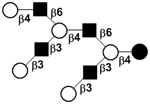
| XV |
|
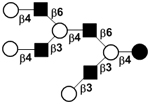
33 | ||
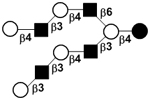
| XVI |
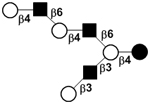
|
59 |
Symbols and abbreviations:  galactose (Gal),
galactose (Gal),  N-acetylglucosamine (GlcNAc),
N-acetylglucosamine (GlcNAc),  glucose (Glc).
glucose (Glc).
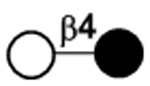
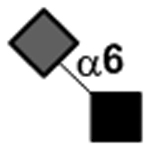
Other than the exceptions mentioned above, twelve glycosidic linkages constitute the structures of HMOS and the list is shown in TABLE III which include three types of galactosidic, two types of N-acetyl-glucosaminidic, four types of fucosidic, and three types of sialidic linkages.
TABLE III.
| Glycosidic linkages | Abbreviations | Symbol |
|---|---|---|
| Galactosidic bonds | Galβ1–4Glc |
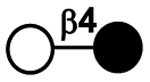
|
| Galβ1–3GlcNAc |
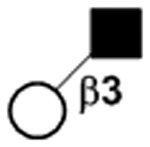
|
|
| Galβ1–4GlcNAc |
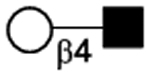
|
|
| N-acetyl-glucosaminidic bond | GlcNAcβ1–3Gal |
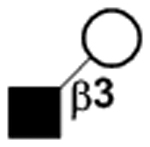
|
| GlcNAcβ1–6Gal |
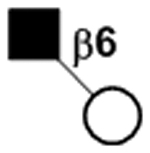
|
|
| Fucosidic bond | Fucα1–2Gal |
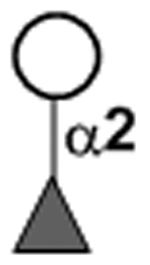
|
| Fucα1–3Glc |
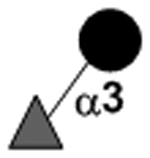
|
|
| Fucα1–3GlcNAc |
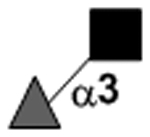
|
|
| Fucα1–4GlcNAc |
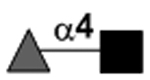
|
|
| Sialidic bond | Neu5Acα2–3Gal |
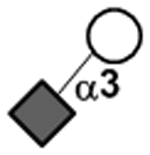
|
| Neu5Acα2–6Gal |
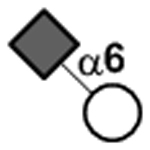
|
|
| Neu5Acα2–6GlcNAc |
|
Symbols and abbreviations:  N-acetylneuraminic acid (Neu5Ac),
N-acetylneuraminic acid (Neu5Ac),  fucose (Fuc),
fucose (Fuc),  galactose (Gal),
galactose (Gal),  N-acetylglucosamine (GlcNAc),
N-acetylglucosamine (GlcNAc),  glucose (Glc).
glucose (Glc).
2.2. HMOS structures
Over 200 individual HMOS molecular species have been found30, 31 and the structures of more than 100 HMOS have been successfully elucidated (see TABLE IV).28, 32–34 These were achieved using chromatography separation, tritium labeling, derivatization with a chromophore or fluorophore, methylation analysis, glycosidase digestion, 1H and 13C nuclear magnetic resonance spectroscopy (NMR) characterization, high performance liquid chromatography (HPLC), high-pH anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD), capillary electrophoresis, and various mass spectrometry (MS) techniques.8, 11, 30, 58, 60, 61 Diversity of HMOS comes from five different monosaccharide building blocks (TABLE I), the length, the size, the sequence (TABLE II), and twelve glycosidic linkages (TABLE III) of the glycans.15 TABLE IV lists 144 structures (lactose is not counted) in the I–XVI categories based on the differences of core structures and 12 structures in the XVII category of deviant structures.
TABLE IV.
| Core # | Lactose and HMOS | Abbreviations | Symbols | Ref. |
|---|---|---|---|---|
| I | Lactose (not considered as HMOS itself) | Lac |
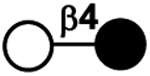
|
49 |
| LNTri II |
|
76 | ||
| a2’-Fucosyllactose | 2’FL |
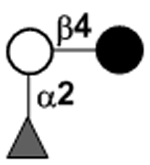
|
25 | |
| 3-Fucosyllactose | 3FL |
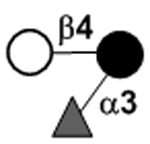
|
27 | |
| aLactodifucotetraose | LDFT |
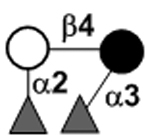
|
77 | |
| 3’-Sialyllactose | 3’SL |
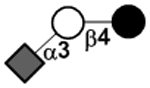
|
78 | |
| 6’-Sulfo-3’-sialyllactose | 6’-Sulfo-3’SL |
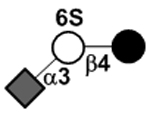
|
64 | |
| 6’-Sialyllactose | 6’SL |
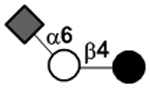
|
79 | |
| 3’-Sialyl-3-fucosyllactose | 3’S3FL |
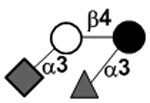
|
80 | |
| II | Lacto-N-tetraose | LNT |
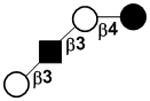
|
24 |
| aLacto-N-fucopentaose I | LNFP I |
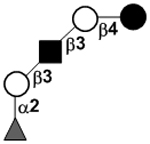
|
26 | |
| bLacto-N-fucopentaose II | LNFP II |
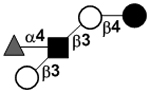
|
81 | |
| Lacto-N-fucopentaose V | LNFP V |
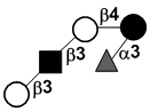
|
82 | |
| a, bLacto-N-difuco-hexaose I | LNDFH I |
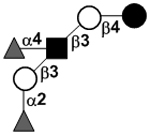
|
83 | |
| bLacto-N-difuco-hexaose II | LNDFH II |
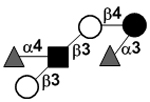
|
84 | |
| Sialyllacto-N-tetraose a | LSTa |
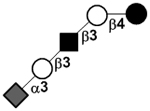
|
66 | |
| Sialyllacto-N-tetraose b | LSTb |
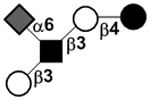
|
66 | |
| Disialyllacto-N-tetraose | DSLNT |
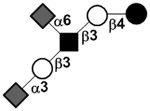
|
85 | |
| Sialylfucosyllacto-N-tetraose | S-LNF II or F-LSTa |
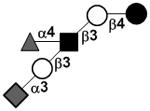
|
86 | |
| Fucosylsialyllacto-N-tetraose | S-LNF I or F-LSTb |
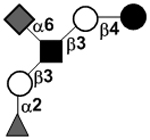
|
86 | |
| Fucosyldisialyllacto-N-tetraose | DS-LNF II or FDS-LNT I |
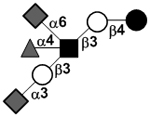
|
87 | |
| Disialylfucosyllacto-N-tetraose | DS-LNF V or FDS-LNT II |
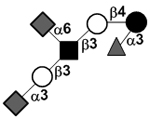
|
87 | |
| III | Lacto-N-neotetraose | LNnT |
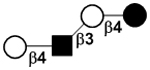
|
50 |
| Lacto-N-fucopentaose III | LNFP III |
|
88 | |
| Lacto-N-neofucopentaose V | LNnFP V |
|
89 | |
| Lacto-N-neodifucohexaose II | LNnDFH II (LNDFH III) |
|
67 | |
| Sialyllacto-N-neotetraose c | LSTc |
|
90 | |
| Fucosylsialyllacto-N-neotetroase | F-LSTc |
|
91 | |
| IV | Lacto-N-hexaose | LNH |
|
51 |
| Fucosyllacto-N-hexaose I | FLNH I |
|
92 | |
| Fucosyllacto-N-hexaose II | FLNH II |
|
93 | |
| c4120a |
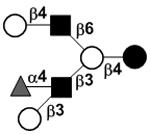
|
33 | ||
| Difucosyllacto-N-hexaose a | DF-LNH a |
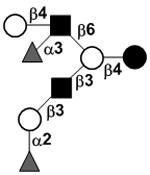
|
92 | |
| Difucosyllacto-N-hexaose b | DF-LNH b |
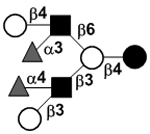
|
51 | |
| Difucosyllacto-N-hexaose c | DF-LNH c |
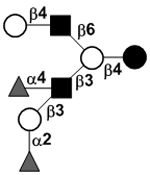
|
33 | |
| Trifucosyllacto-N-hexaose | TF-LNH |
|
55 | |
| Sialyllacto-N-hexaose | S-LNH |
|
51 | |
| c4021a |
|
34 | ||
| Disialyllacto-N-hexaose I | DS-LNH I |
|
94 | |
| Disialyllacto-N-hexaose II | DS-LNH II |
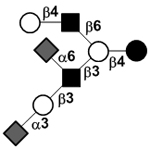
|
94 | |
| Trisialyllacto-N-hexaose | TS-LNH |
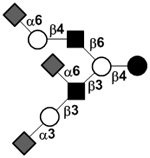
|
95 | |
| Fucosylsialyllacto-N-hexaose | FS-LNH |
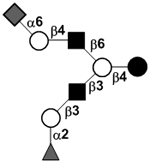
|
92 | |
| Fucosylsialyllacto-N-hexaose I | FS-LNH I |
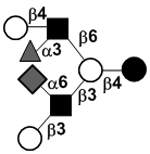
|
96 | |
| Fucosylsialyllacto-N-hexaose II | FS-LNH II |
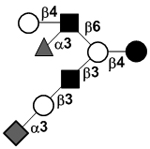
|
96 | |
| Fucosylsialyllacto-N-hexaose III | FS-LNH III |
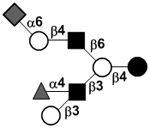
|
96 | |
| Fucosylsialyllacto-N-hexaose IV | FS-LNH IV |
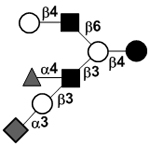
|
97 | |
| Difucosylsialyllacto-N-hexaose I | DFS-LNH I |
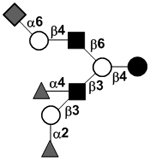
|
96 | |
| Difucosylsialyllacto-N-hexaose II | DFS-LNH II |
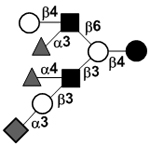
|
97 | |
| Fucosyldisialyllacto-N-hexaose I | FDS-LNH I |
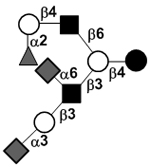
|
98 | |
| Fucosyldisialyllacto-N-hexaose II | FDS-LNH II |
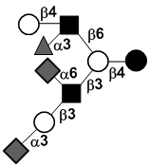
|
98 | |
| Fucosyldisialyllacto-N-hexaose III | FDS-LNH III |
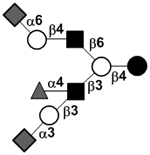
|
97 | |
| V | Lacto-N-neohexaose | LNnH |
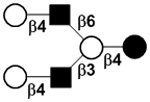
|
52 |
| Fucosyllacto-N-neohexaose | F-LNnH |
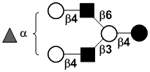
|
52 | |
| Difucosyllacto-N-neohexaose | DF-LNnH |
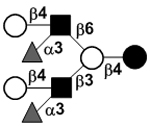
|
56 | |
| Sialyllacto-N-neohexaose I | S-LNnH I |
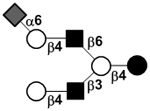
|
51 | |
| Sialyllacto-N-neohexaose II | S-LNnH II |
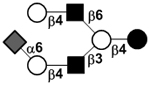
|
80 | |
| Disialyllacto-N-neohexaose | DS-LNnH |
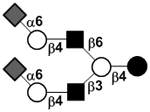
|
87 | |
| Fucosylsialyllacto-N-neohexaose I | FS-LNnH I |
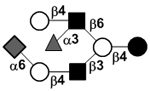
|
80 | |
| Fucosylsialyllacto-N-neohexaose II | FS-LNnH II |
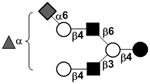
|
52 | |
| Difucosylsialyllacto-N-neohexaose | DFS-LNnH |
|
80 | |
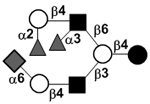
| Fucosyldisialyllacto-N-neohexaose | FDS-LNnH |
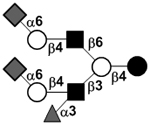 or or 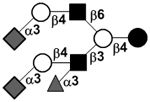
|
98 | |
| VI | para-Lacto-N-hexaose | pLNH |
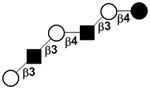
|
53 |
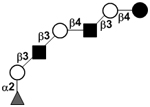
| Fucosyl-para-lacto-N-hexaose I | F-pLNH I |
|
99 | |
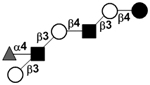
| Fucosyl-para-lacto-N-hexaose II | F-pLNH II |
|
100 | |
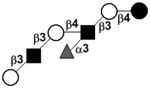
| Fucosyl-para-lacto-N-hexaose IV | F-pLNH IV |
|
100 | |
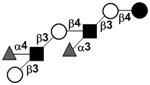
| Difucosyl-para-lacto-N-hexaose | DF-pLNH |
|
53 | |
| Trifucosyl-para-lacto-N-hexaose I | TF-pLNH I |
|
101 | |
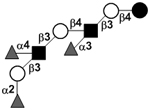
| Trifucosyl-para-lacto-N-hexaose II | TF-pLNH II |
|
100 | |
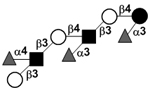
| Difucosyl-para-lacto-N-hexaose sulfate I | DF-pLNH sulfate I |
|
65 | |
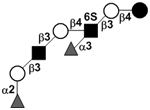
| Difucosyl-para-lacto-N-hexaose sulfate II | DF-pLNH sulfate II |
|
65 | |
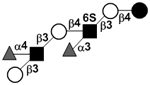
| Difucosyl-para-lacto-N-hexaose sulfate III | DF-pLNH sulfate III |
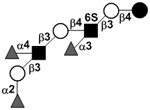
|
65 | |
| VII | para-Lacto-N-neohexaose | pLNnH |
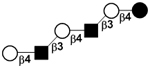
|
53 |
| Fucosyl-para-lacto-N-neohexaose | F-pLNnH or IFLNH III |
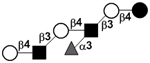
|
99 | |
| Difucosyl-para-lacto-N-neohexaose | DF-pLNnH |
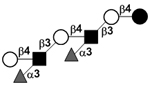
|
53 | |
| Trifucosyl-para-lacto-N-neohexaose | TF-pLNnH |
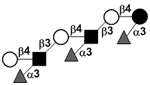
|
100 | |
| c4021b |
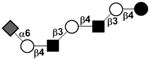
|
34 | ||
| c4121a |
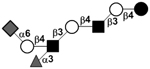
|
34 | ||
| c4121b |
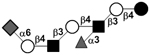
|
34 | ||
| VIII | Lacto-N-octaose | LNO |
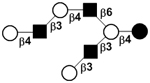
|
54 |
| c5130c |
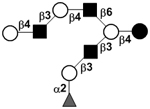
|
33 | ||
| c5130b |
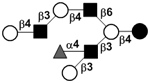
|
33 | ||
| Fucosyllacto-N-octaose | F-LNO |
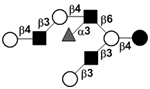
|
102 | |
| Difucosyllacto-N-octaose I | DF-LNO I |
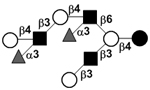
|
54 | |
| Difucosyllacto-N-octaose II | DF-LNO II |
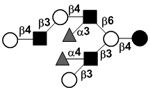
|
54 | |
| Trifucosyllacto-N-octaose | TF-LNO |
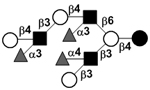
|
54 | |
| c5031a |
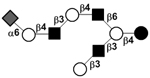
|
34 | ||
| Fucosylsialyllacto-N-octaose | FS-LNO |
|
103 | |
| c5131a |
|
34 | ||
| c5231a |
|
34 | ||
| c5231b |
|
34 | ||
| Difucosylsialyllacto-N-octaose | DFS-LNO |
|
104 | |
| c5331a |
|
34 | ||
| IX | Lacto-N-neooctaose | LNnO |
|
54 |
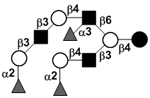
| Fucosyllacto-N-neooctaose | F-LNnO |
|
54 | |
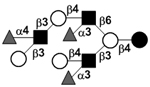
| Difucosyllacto-N-neooctaose I | DF-LNnO I |
|
54 | |
| Difucosyllacto-N-neooctaose II | DF-LNnO II |
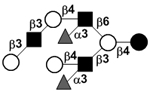
|
54 | |
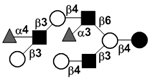
| Trifucosyllacto-N-neooctaose I | TF-LNnO I |
|
54, 55 | |
| Trifucosyllacto-N-neooctaose II | TF-LNnO II |
|
55 | |
| X | iso-Lacto-N-octaose | iLNO |
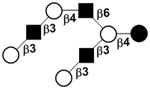
|
55 |
| c5130a Fucosyl-iso-Lacto-N-octaose | F-iLNO |
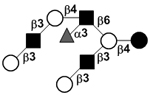
|
33, 105 | |
| Difucosyl-iso-lacto-N-octaose I | DF-iLNO I |
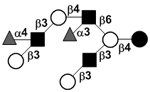
|
106 | |
| Difucosyl-iso-lacto-N-octaose II | DF-iLNO II |
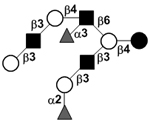
|
106 | |
| c5230a |
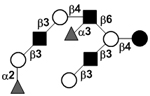
|
33 | ||
| Trifucosyl-iso-lacto-N-octaose I | TF-iLNO I |
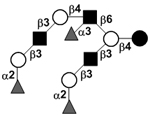
|
55 | |
| Trifucosyl-iso-lacto-N-octaose II | TF-iLNO II |
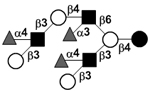
|
56, 105 | |
| c5330a |
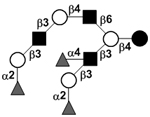
|
33 | ||
| Tetrafucosyl-iso-lacto-N-octaose | TetraF-iLNO |
|
56 | |
| pentafucosyl-iso-lacto-N-octaose | PentaF-iLNO |
|
56 | |
| Fucosylsialyl-iso-lacto-N-octaose | FS-iLNO |
|
104 | |
| Difucosylsialyl-iso-lacto-N-octaose I | DFS-iLNO I |
|
104 | |
| Difucosylsialyl-iso-lacto-N-octaose II | DFS-iLNO II |
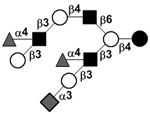
|
104 | |
| Trifucosylsialyl-iso-lacto-N-octaose | TFS-iLNO |
|
94 | |
| XI | para-Lacto-N-octaose | pLNO |
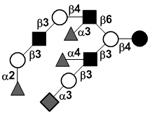
|
56 |
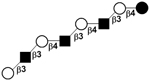
| Tetrafucosyl-para-lacto-N-octaose | TetraF-pLNO |
|
56 | |
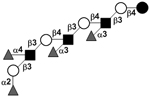
| XII | para-Lacto-N-neooctaose | pLNnO |
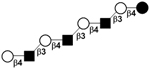
|
57 |
| XIII | Lacto-N-decaose | LND |
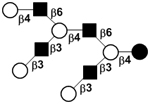
|
58, 107 |
| Fucosyl-lacto-N-decaose I | F-LND I |
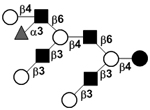
|
107 | |
| Difucosyl-lacto-N-decaose I | DF-LND I |
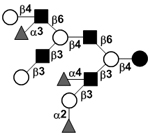
|
58 | |
| Difucosyl-lacto-N-decaose II | DF-LND II |
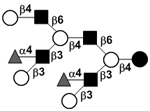
|
58 | |
| Difucosyl-lacto-N-decaose III | DF-LND III |
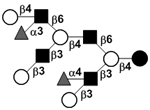
|
58 | |
| Difucosyl-lacto-N-decaose IV | DF-LND IV |
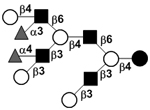
|
58 | |
| Difucosyl-lacto-N-decaose V | DF-LND V |
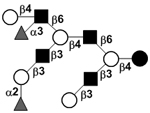
|
58 | |
| Difucosyl-lacto-N-decaose VI | DF-LND VI |
|
58 | |
|
58 | |||
|
58 | |||
| Trifucosyl-lacto-N-decaose I | TriF-LND I |
|
58 | |
| Trifucosyl-lacto-N-decaose II | TriF-LND II |
|
58 | |
| Trifucosyl-lacto-N-decaose III | TriF-LND III |
|
58 | |
| Trifucosyl-lacto-N-decaose IV | TriF-LND IV |
|
58 | |
|
58 | |||
|
58 | |||
| c6340a |
|
33 | ||
| Tetrafucosyl-lacto-N-decaose I | TetraF-LND I |
|
58 | |
| Tetrafucosyl-lacto-N-decaose II | TetraF-LND II |
|
58 | |
| Tetrafucosyl-lacto-N-decaose III | TetraF-LND III |
|
58 | |
|
58 | |||
| XIV | Lacto-N-neodecaose | LNnD |
|
58 |
| Fucosyllacto-N-neodecaose I | F-LNnD I |
|
58 | |
| Fucosyllacto-N-neodecaose II | F-LNnD II |
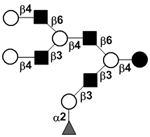
|
58 | |
| Difucosyllacto-N-neodecaose | DF-LNnD |
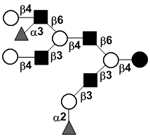
|
58 | |
| c6041a |
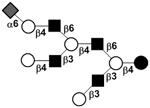
|
34 | ||
| XV |
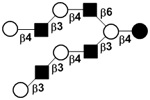
|
33 | ||
| XVI |
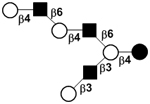
|
59 | ||
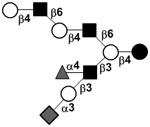
|
59 | |||
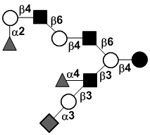
|
59 | |||
| XVII Deviant structures | A antigen-tetrasaccharide | A-Tri |
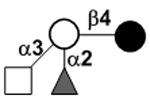
|
62, 108 |
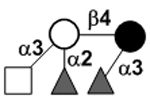
| A antigen-pentasaccharide | A-Penta |
|
62, 108–110 | |
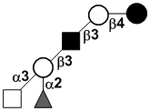
| A antigen-hexasaccharide | A-Hexa |
|
62 | |
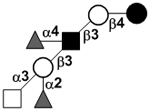
| A antigen-heptasaccharide | A-Hepta |
|
62, 108, 109 | |
|
111 | |||
| 3’-Sialyl Lewis a | 3’SLea |
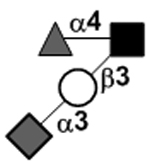
|
111 | |
| 3’-Sialyl-N-acetyllactosamine | 3’SLN |
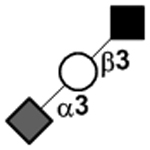
|
34 | |
| 6’-Sialyl-N-acetyllactosamine | 6’SLN |
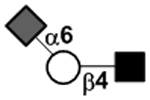
|
66 | |
| Fucosylsialyl-novo-lacto-N-pentaose I | FS-novo-LNP I |
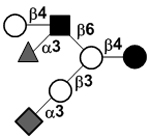
|
96 | |
| 3’-Galactosyllactose | β3’GL |
|
67 | |
| 4’-Galactosyllactose | β4’GL |
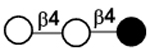
|
68 | |
| 6’-Galactosyllactose | β6’GL |
|
69 |
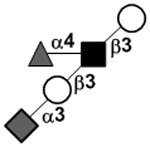
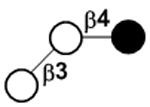
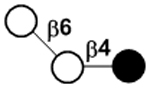
Several exceptions have been found to the general structure featured described above for HMOS. For example, a few HMOS containing a terminal N-acetylgalactosamine (GalNAc) such as A antigen-tetrasaccharide, pentasaccharide, hexasaccharide,62 and heptasaccharide63 were isolated from urine or feces of blood group A breast-fed infants. In addition, several HMOS containing 6-O-sulfated monosaccharides have been identified.64, 65 Several HMOS missing the glucose59 or lactose34, 59, 66 at the reducing end have been identified. In addition, an unusually Galβ1–3Gal,59, 67 Galβ1–4Gal,68 or Galβ1–6Gal69 component has been found in several HMOS (see XVII Deviant structures in TABLE IV).
The presence of some HMOS is related to the secretor status and the Lewis blood type of the mother.70 The milk produced by Lea+b+ secretors, presenting in 70% of the general population, has the highest diversity of HMOS.14 Fucα1–2Gal-containing HMOS such as 2’-fucosyllactose (2’FL),71, 72 lactodifucotetraose (LDFT), lacto-N-fucopentaose I (LNFP I), and lacto-N-difuco-hexoase I (LNDFH I) are missing in the milk of Lea+b− non-secretors.73 Fucα1–4GlcNAc-containing HMOS including LNFP II, LNDFH I, and LNDFH II are missing in the milk of Lewis negative (Lea−b−) individuals74 (TABLE IV). It has been shown that in the absence of blood samples, the ratios of 2’FL versus 3’FL; LNFP I, LDFT, and LNDFH I versus LNT; and 6’SL versus 3’SL in human milk can be used as specific and sensitive markers for determining the secretor status of individuals.75
High molecular weight HMOS (Mr 2242–8000),112 complex neutral HMOS with up to 10 fucose residues on a core structure containing 7 LacNAc units,60 HMOS with up to 32 monosaccharide units,113 neutral HMOS with up to 35 monosaccharides and HMOS with more than 50 monosaccharide units114 have been observed. Nevertheless, the identified of HMOS containing more than 14 monosaccharide units have not been elucidated and are not presented in TABLE IV.
Overall, about 70% of HMOS in pooled milk are fucosylated and about 20% are sialylated.30 The major components of HMOS are lacto-N-tetraose (LNT), lacto-N-neotetraose (LNnT), as well as monofucosylated, monosialylated, difucosylated, and disialylated lactose, LNT, and LNnT (TABLE IV).57 Oligosaccharides with both sialic acid and fucose are also presented in HMOS. The top 10 most abundant HMOS species are responsible for about 46% of the HMOS mass. The top 50 most abundant HMOS species in the pooled human milk sample account for 83% of the total intensity and the least abundant half of the total constitute only 8% of the entire intensities.30 Among HMOS, 20–25 of them are considered to be the major components.11 Most of them contain 3–9 monosaccharide units.8 2’FL, LNFP I, LNDFH I, and LNT were shown to be the most abundant HMOS in the colostra of Japanese Women (85% are secretors) and in mature human milk.10, 61, 115–117 The most abundant acidic HMOS were LSTc, DSLNT, 6’SL, 3’SL and LSTa.61, 115
Compared to the milk oligosaccharides (MOS) characterized for some domestic animals118–120 and other primates,48, 121 HMOS are higher in quantities and complexity with more diversity and longer structures. In general, HMOS are high in fucosylation which is rare in the MOS of cows and pigs. In comparison, sialylation is more abundant in the MOS of cows and pigs.118–120 Furthermore, N-glycolylneuraminic acid (Neu5Gc)-containing MOS found in the milk of cows, pigs, and primates48, 118–121 and 4-O-acetyl-N-acetylneuraminic acid (Neu4,5Ac2)-containing MOS found in the milk of monotremes including echidna122, 123 and platypus124–126 have not been observed in human milk. Except for 3’-galactosyllactose (3’GL), other oligo-β1–3-galactoside structures abundant in the milk of metatheria (marsupials)127–129 such as common brushtail possum, tammar wallaby, red kangaroo, and koala, have not been found in HMOS.
3. Biosynthesis Of HMOS
Our understanding on the biosynthesis of HMOS is limited. Most, if not all, HMOS are extended from lactose at the non-reducing ends and are believed to be catalyzed by glycosyltransferases in the mammary gland.130–133 Glucose and galactose can be de novo synthesized in the mammary gland by a process named hexoneogenesis although plasma glucose is the major carbon source of milk lactose.134, 135 Lactose and other MOS are most likely accumulated in the secretory vesicle and secreted by exocytotic fusion with the apical plasma membrane.136 Lactose itself is produced in the mammary gland by β1–4-galactosyltransferase 1 (β1–4GalT1) bound to α-lactalbumin in a lactose synthase complex.137–139 However, most of the specific glycosyltransferases that are responsible for the formation of HMOS structures with specific glycosidic linkages have not been identified. The best understood examples are human α1–2-fucosyltransferase FUT2 encoded by the secretor (Se) gene71, 140 and α1–3/4-fucosyltransferase FUT3 encoded by the Lewis (Le) gene74 that are responsible for the formation of α1–2- and α1–3/4-linked fucosides, respectively, in human mammary glands.130, 141 Lewis (Le) gene-independent α1–3-fucosyltransferase presented in all women has also been described.11, 14 Transgenic introduction of a human α1–2-fucosyltransferase gene to mice was shown to allow the mice to express large quantities of 2’-fucosyllactose142 which is a good indication of the ability of the mammary gland in producing corresponding oligosaccharides in the presence of suitable glycosyltransferases. Similar success was achieved for the transgenic manipulation of mice, but not rabbit, using other glycosyltransferases including a homologous galactosyltransferase and different fucosyltransferases.143
The presence of several glycosyltransferases in human milk has been confirmed. For example, β1–4GalT1,144, 145 α1–3- and α1–4-fucosyltransferases,146 as well as an α1–3/4-fucosyltransferase147–149 have been purified from human milk. The activity of β1–3-N-acetylglucosaminyltransferase was identified in human colostrums but not in bovine (Holstein and Jersey cow) colostrums studied.150 In addition to the presence of fucosyltransferase activity in human milk, α-fucosidase activity has also been identified.151
Enzymes purified from human milk have been used for the synthesis. For example, partially purified α1–3/4-fucosyltransferase from human milk was used for synthesizing sialyl Lewis a,152 Lewis a and Lewis x153 including their deoxy analogs,154 sulfated Lewis x,155 and multivalent tyrosinamide-tagged Lewis x structures.156, 157 Purified human milk fucosyltransferase preparation was also used for the synthesis of tumor-associated trimeric Lewis x158 and its sialylated structures,159 sialyl Lewis a and sialyl Lewis x tetrasaccharide structures modified at the C-2 position of the glucose residue at the reducing end.160 Fucosylation of lacto-N-neohexaose (LNnH) by a partially purified human milk α1–3-fucosyltransferase was found to add fucose at the LacNAc units of LNnH in the non-reducing end.161, 162 Human milk α1–3FucTs were also shown to fucosylate chitin oligosaccharides containing 2–4 GlcNAc units.163 Purified human milk β1–4GalT was used together with a partially purified rat liver β1–3GalT, a recombinant core 2 β1–6GlcNAcT, and a recombinant human α1–3FucT in synthesizing a sialyl Lewis x hexasaccharide.164 Carbon 13-labeled linear N-acetylpolylactosamines (LacNAc)n were enzymatically synthesized at 10–100 μmol scale using the partially purified and immobilized bovine milk β1–4GalT and human serum β1–3GlcNAcT.165 N-Acetylglucosaminyltransferase I (GnT-I) purified from human milk was shown to be able to catalyze the transfer of deoxy derivatives of GlcNAc.166, 167 These synthetic applications of human milk enzymes provide important information about their properties. Nevertheless, the syntheses were limited to small scales and were mostly used for HMOS derivatives instead of natural HMOS structures with a free reducing end.
4. Functions Of HMOS
The functional studies of HMOS were usually carried out using mixtures of HMOS that were isolated from human milk pools. The benefits of breast-feeding was observed as early as the end of the 19th century.38 Increasing evidence has now shown that HMOS contribute significantly to the health of breast-fed infants via several mechanisms by serving as listed in the following:1, 2, 15, 35, 38, 44, 45, 57, 133, 168–177
Prebiotics: HMOS are carbon and energy sources preferably used by beneficial bacteria such as probiotic bifidobacteria, thus promoting their growth, which in turn produce lactic acid and short chain fatty acid to decrease the pH of the gut, making it less desirable for the growth of pathogens. The predominant growth and colonization of bifidobacteria allow them to compete well pathogens for the limited nutrient available in the gut. Bifidobacteria also occupy the epithelia binding sites and make them less available for the binding of pathogens. Some antimicrobial substances released by bifidobacteria also generate an unfavorable environment for pathogens.47
Antiadhesive antimicrobials: HMOS mimic the glycan structures presented on the surface of gut epithelium and serve as soluble decoy receptors to pathogenic bacteria to decrease their binding to infant gut surface for colonization, thus lowering the risk for viral, bacterial and protozoan parasite infections. HMOS can also serve as inhibitors for toxins released by pathogenic bacteria.
Immunomodulators: Evidence has shown that HMOS can modulate epithelial and immune cell responses. Some HMOS can directly influence the gut epithelium functions,178 reduce excessive mucosal leukocyte infiltration and activation which can lower the risk for necrotizing enterocolitis (NEC), one of the most common and fatal intestinal disorders in preterm infants. Bifidobacterium infantis grown on HMOS can also change the functions of intestinal cells.43
Nutrient providers for brain: Some HMOS, mainly sialylated ones, may also be providers of sialic acid for the synthesis of sialic acid-containing glycolipids (gangliosides) and glycoproteins important for the development of brain and cognition of infants.
These functions have been discussed quite thoroughly in several excellent reviews published recently.1, 35, 38, 44, 45, 61, 133, 168–172 The functional roles of individual HMOS species, however, are less clear. This is mainly due to the unavailability of sufficient amounts of pure HMOS for detailed functional studies. Only a handful examples have been shown. These are discussed briefly in the sections below as three categories.
4.1. Neutral non-fucosylated HMOS
Neutral non-fucosylated HMOS constitute the core structures or the backbones of all HMOS. Despite earlier studies on identifying β-GlcNAc-containing oligosaccharides and polysaccharides in human milk as “bifidus factors”,19, 179 their identities have not been elucidated conclusively. The discovery of a novel galactose operon responsible for the assembly of GNB/LNB pathway in Bifidobacterium longum JCM1217 for galacto-N-biose (GNB) and lacto-N-biose (LNB) consumption 10 years ago pointed to lacto-N-biose (LNB, Galβ1–3GlcNAc) presented at the non-reducing end of many neutral non-fucosylated HMOS as a potential “bifidus factor”.9 This was further supported by the property of LNB in selective stimulating the growth of bifidobacteria, but not Clostridia, Enterococci, and Lactobacillus.180–182 The extracellular lacto-N-biosidase, α1–2-fucosidase, α1–3/4-fucosidase, and sialidase of B. bifidum183–185 can de-cap fucosylated and/or sialylated HMOS to release their core structures which can then be used by its extracellular lacto-N-biosidase to produce LNB.186 LNB can be transported into the bacterium by the GNB/LNB transporter in the GNB/LNB pathway and be metabolized by other enzymes involved in the GNB/LNB pathway.187 On the other hand, LacNAc-terminated core HMOS can be broken down by extracellular β-galactosidase and β-N-acetylhexosaminidase of B. bifidum.188
Among bifidobacteria species commonly found in breast-fed infant such as B. longum subsp. longum, B. longum subsp. infantis, B. bifidum, and B. breve175 B. longum subsp. infantis (e.g. JCM1222) and B. bifidum (e.g. JCM1254) were both found to consume both type I and type II HMOS core structures equally well. The other two species tested had preference towards LNT, but not LNnT. The B. longum subsp. infantis strain tested also consume mono- and di-fucosylated LNT/LNnT, disaccharides and monosaccharides monitored in the experiment quite well.182 LNnT was further confirmed to provide advantages for B. infantis versus B. thetaiotaomicron in both in vitro growth studies and germ-free mice studies.189
Different from B. bifidum which express extracellular glycosidases, B. longum subsp. infantis express internal glycosidases190–192 and rely on their glycan ABC transporters193 for internalization of the corresponding HMOS.175 The extracellular glycosidases on some bacteria such as B. bifidum could be used as a mechanism to release components from HMOS which can be readily transported into B. longum subsp. infantis or other bacteria for consumption. The symbiotic sharing of HMOS and components could be one of the mechanisms used to shape the gut microbiota. The enrichment of bifidobacteria in infant gut could be the result of coevolution of the bacteria and milk ingredients including HMOS.175, 182
LNT is a carbon source that can be used by most bifidobacteria.182 LNT in HMOS, and may be other HMOS with Gal at the non-reducing end, was shown to reduce Entamoeba histolytica (a protozoan parasite infecting ~ 50 million people and causing ~100,000 deaths per year194) attachment and its cytotoxicity towards human intestinal epithelia HT-29 cells in a dose-dependent manner.194 Further in vivo studies are needed to show the prebiotic and antimicrobial potentials of LNT.
In comparison, LNnT was shown to be a selective carbon source for certain bifidobacteria such as B. longum subsp. infantis and B. bifidum. LNnT was also shown to have immunosuppressive functions195 and can inhibit the binding of Streptococcus pneumoniae to ciliated chinchilla tracheal epithelium.196 Higher concentrations of LNnT in the milk of HIV-infected women was found to be associated with reduced postnatal transmission via breastfeeding.197 Therefore, LNnT is a potential candidate for developing prebiotics and therapeutics against infectious disease.198
4.2. Fucosylated HMOS
Fucosylated HMOS are the most abundant HMOS species.33 Their prebiotic, antiadhesive antimicrobial, and immunomodulation activities have been shown.
2’FL, 3FL, and LDFT were shown to selectively promotes the growth of bifidobacteria.199 Fucosylated HMOS including 2’FL, 3FL, LDFT, LNFP I/II/III, LNDFH I, and LNDFHII showed preferred consumption by B. longum subsp. infantis and B. bifidum compared to B. longum subsp. longum or B. breve.182 In fact, five fucosidases have been identified from B. longum subsp. infantis strain ATCC 15697 and characterized. Their ability in using fucosylated HMOS was confirmed.190
Several examples have been shown for the antiadhesive antimicrobial functions of fucosylated HMOS including antibacterial, antiyeast, and antiviral activities. Fucosylated HMOS were also found to bind norovirus200 and inhibit the adhesion of an enteropathogenic Escherichia coli (EPEC) to HEp-2 cells.201 A minor neutral fucosylated HMOS component was shown to protect suckling mice from the diarrheagenic effects caused by heat-stable enterotoxin of E. coli.202 α1–2-Fucosylated HMOS were shown to inhibit the adherence of Std fimbriated Salmonella enterica serotype Typhimurium to Caco-2 cells.203 They also inhibit the binding of Campylobacter jejuni to intestinal H(O) antigen and lower the chance of infection204 and potentially protect infants against diarrhea caused by Campylobacter or calicivirus.205 More specifically, high levels of 2’FL in mother’s milk corresponded to lower occurrences of Campylobacter diarrhea of the infants. LDFH I was also shown to correlated to lower incidences of calicivirus diarrhea.205 In addition, α1–2-fucosylated HMOS, but not those of Lewis blood group-type, were found to inhibit the binding of Candida albicans yeasts to human buccal epithelia cells.206 On the other hand, Lewis blood group antigen-containing HMOS bind well to dendritic cell-specific ICAM3-grabbing non-integrin (DC-SIGN), competing against human immunodeficiency virus (HIV) surface glycoprotein gp120 binding to DC-SIGN in vitro.207 Indeed, breastfeeding with human milk with high concentrations of α1–2-fucosylated HMOS and α1–3-fucosylated was found to be protective against mortality for HIV-exposed uninfected (HEU) children during breastfeeding.208 Lewis b (Leb) antigens including Leb-terminated LNDFH I that was synthesized enzymatically were shown to bind to Helicobacter pylori.209, 210
The immunomodulating function of fucosylated HMOS was represented by Lewis x-type LNFP III which was shown to have immunosuppressive functions.195 It was able to activate macrophages in vitro which can further activate natural killer (NK) cells.211 HMOS containing Lewis blood group antigens were also shown to reduce selectin-mediated cell-cell interactions.176, 212 2’FL and 3FL were shown to decrease colon motor contractions in a dose-dependent fashion with a better activity observed for 3FL than for 2’FL.178
The understanding of the important roles of α1–2- and α1–3/4-fucosylated HMOS for infant health is greatly facilitated by the presence of nursing mothers with differences on the secretor status (determined by α1–2-fucosyltransferase FUT2) and Lewis blood type (determined by α1–3/4-fucosyltransferase FUT3). Bifidobacteria were shown to be established earlier and more often in infants fed by secretor mothers.213 Mother’s milk with a higher ratio of α1–2-fucosylated versus non-α1–2-fucosylated HMOS was shown to provide protection of breast-fed infants against diarrhea.214
The secretor status of premature infants was also shown to be a predictor for the outcome of infants on their survival or susceptibility to diseases. Low or non-secretor status was associated with a higher death rate, higher incident of necrotizing enterocolitis (NEC) and Gram-negative sepsis.215
4.3. Sialylated HMOS
Sialylated HMOS are charged species and represent about 20% of HMOS.30 Their prebiotic, antiadhesive antimicrobial, and immunomodulating activities as well as their nutritional value for infant brain development have been shown.
A sialylated HMOS fraction was shown to inhibit the adhesion of Escherichia coli serotype O119, Vibrio cholerae, and Salmonella fyris to differentiated Caco-2 cells.216 As hemagglutinins on the surface of influenza viruses bind to sialylated glycans on host cell surface, it is not a surprise that sialylated HMOS bind to influenza virus or inhibit the viral hemagglutinin binding to its ligand.217
Sialylated HMOS have been shown to influence lymphocyte maturation218 and have anti-infective and immunomodulating effects.38 Sialylated HMOS, but not non-sialylated HMOS, reduce leukocyte rolling and adhesion in a dose-dependent manner.176 In fact, sialylated HMOS fraction in a physiological range (12.5–125 μg/mL) was shown to be even better than soluble sialyl Lewis x in inhibiting leukocyte rolling and adhesion. 3’SL and 3’S-3FL were further identified to be the key ingredients and were suggested to contribute to the lower incidence of inflammatory diseases in breast-fed infants.176 Similarly, sialylated HMOS reduce platelet-neutrophil complex formation and subsequent neutrophil activation in an ex vivo model with whole human blood.212
Sialylated HMOS may also be used as source of sialic acid for the synthesis of sialic acid-containing glycolipids (gangliosides) and glycoproteins important for the development of brain and cognition of infants.45
The simplest and the most well studied sialylated HMOS are sialyllactose including 6’SL and 3’SL. Sialyllactose inhibited cholera toxin induced fluid accumulation in a rabbit intestinal loop model. These effects are believed to be responsible for the activity of human milk and its low molecular weight fraction in inhibiting cholera toxin B subunit binding to monosialoganglioside (GM1).219 Sialyllactose was also shown to inhibit the binding of Aspergillus fumigatus conidia to laminin extracted from mouse sarcoma tumor220 and the binding of Pseudomonas aeruginosa 8830 to immobilized asialo GM1 in a microtiter plate assay221 although the mechanism for the latter is unknown. Sialyllactoses were also shown to induce differentiation in transformed human intestinal cells HT-29 and human intestinal epithelial cells HIEC.222 6’SL alone or with 3’SL, but not 3’SL alone or oligofructose alone, was shown to enhance the adhesion of B. longum subsp. infantis strain ATCC15697 to HT-29 human intestinal cells.223 3’SL was shown to bind to polyomarvirus.224 It inhibited the binding of S fimbriated E. coli to endothelial and epithelial.225, 226 It also inhibited the adhesion of Helicobacter pylori binding to human epithelial cells in vitro and was shown to decrease Helicobacter pylori colonization in a rhesus monkey antiadhesive therapy model.227 3’SL was shown to inhibit the binding of some sialyl oligosaccharides to Helicobacter pylori,228 E. coli S-fimbriate,229 and influenza viruses.173
The 3’SL level in human milk, however, can also be an indicator of HIV infection. Higher relative abundances of 3’SL were shown in the milk of HIV-infected mothers230 and in the milk of mothers who transmit HIV to their babies via breastfeeding.197
Another exiting example about the potential use of sialylated HMOS is their application in treating necrotizing enterocolitis, one of the most common and fatal intestinal disorders in preterm infants231, 232 that does not currently have an ideal therapeutic outcome.233–235 A single sialylated HMOS, disialyllacto-N-tetraose (DSLNT), but not its non-sialylated or mono-sialylated analog, was identified as a specific HMOS component that is effective for preventing necrotizing enterocolitis (NEC) in a neonatal rat model.236 Low concentrations of DSLNT in mother’s milk are corresponding to an increased risk of NEC in the preterm very-low-birth weight infants.230
5. Production Of HMOS By Enzyme-Catalyzed Processes
Chemical synthesis of more than 15 different HMOS and derivative (2’FL,237 3FL,237 LDFT,237 LNT,238–240 LNnT,238, 241 LNFP I,239, 242 LNFP III242–245 and its protected form,246 LSTa and LSTd (Neu5Acα2–3LNnT, not found in human milk) with an aglycon,247, 248 LNDFH I with a β-linked aglycone,249 pLNnH,241, 250 LNnH251 and its protected forms,252, 253 iLNO,254 pLNnO241 and its protected form,255 trifucosylated pLNnO in its protected form,255 DF-LNH II,256, 257 and DF-LNnH256, 257) with 3–11 monosaccharide units have been reported including recent successes in the synthesis of LNFP I and its α1–2-fucosylated LNnT analog using one-pot glycosylation approaches.258 These chemical synthetic efforts are out of the scope of this review. The focus of this section will be a survey on enzyme-catalyzed processes for the production of HMOS.
The production of only a handful of HMOS has been reported using enzyme-catalyzed processes259 and the synthesized HMOS are limited to those with relatively simple structures. Despite the success on the characterization of mammalian enzymes and purification of several glycosyltransferases from human milk, their application in synthesis has been limited due to the difficulties in obtaining them in large amounts and in an economically efficient manner. On the other hand, bacteria express a wide array of glycosyltransferases which are responsible for the construction of diverse lipopolysaccharides (LPS) and capsular polysaccharide structures. Some of these glycan structures mimic those found on human cell surfaces and those in HMOS.260, 261 Therefore, bacteria are a rich source of glycosyltransferases that can be used for the synthesis of HMOS as well as the glycans and glycoconjugates presented on human surface.262–265 Recombinant bacterial glycosyltransferases have been increasingly used for the synthesis of several HMOS structures in enzymatic, chemoenzymatic, whole cell, and living cell approaches.
Early enzymatic methods used expensive sugar nucleotides as donor substrates for glycosyltransferases for the production of HMOS. Glycosyltransferase-catalyzed reactions with in situ donor regeneration cycles that applied for preparative-scale synthesis of oligosaccharides266–268 can also be used for the synthesis of HMOS. Recently, highly efficient one-pot multienzyme (OPME) systems have been established for the synthesis of HMOS.264, 269–271 These systems use inexpensive, free monosaccharides as starting materials, which are enzymatically converted to sugar nucleotides with or without the formation of sugar-1-phosphate intermediates. The activated sugars in the forms of sugar nucleotides are supplied to the corresponding glycosyltransferases in one-pot for the formation of the corresponding oligosaccharides. Multiple OPME systems can be used in sequential to build up more complex oligosaccharides.269, 272 The high efficiency of the systems is facilitated by the elucidation of novel salvage pathways of sugar nucleotide biosynthesis as well as the identification and characterization of new bacterial glycosyltransferases and mutants with high expression levels in E. coli, good solubility and stability, and high activity.
Much progress has been made recently in identifying glycosyltransferase mutants with improved functions and many of these successes are based on protein crystal structure-based rational design and some are from directed evolution coupled with high-throughput screening methods.262, 273 These are effective approaches for obtaining additional or better catalysts that are not readily available from nature.
If not all glycosyltransferases that are responsible for formation of desired HMOS are available, enzymatically synthesized oligosaccharide derivatives can be as building blocks (or synthons) for chemical synthesis of more complex HMOS and derivatives. Such chemoenzymatic methods have been explored for the synthesis of sialyl galactosides,274, 275 sialyl Lewis x tetrasaccharides,276, 277 protected sialyllacto-N-tetraose a (LSTa, Neu5Acα2–3LNT) and LSTd (Neu5Acα2–3LNnT,126, 263 has not been found in human milk),278 and an LNT derivative279. The obtained LNT derivative was further used as a glycosyltransferase acceptor for the production of LSTa derivatives by OPME enzymatic sialylation.279
A limited number of HMOS have also been synthesized by whole cell synthesis and engineered E. coli living cell fermentation approaches. Both approaches take a good use of microorganisms’ own metabolic machinery for the production of some components (such as nucleotides, monosaccharides, and/or or sugar nucleotides) from less expensive materials (simple carbon and energy source such as glycerol or glucose). One of the limitations of the living cell system is the restriction of the oligosaccharide transporter systems for transfer acceptors from external sources into the cells for the product formation.262
Alternative enzymatic synthetic strategies using glycosidases, trans-glycosidases, and glycosidase mutants designed for synthesizing carbohydrates (e.g. glycosynthase280) have also been developed for obtaining HMOS. These methods require the use of glycosylated donors which may not be readily available. The synthetic donors used have to be chemically synthesized and may not be stable.281, 282 Low yields and poor regioselectivity are also common problems for glycosidase-catalyzed reactions. Strategies to improve the trans-glycosylation reactions of glycosidases including controlling acceptor/donor ratio and reaction time, removing product continuously, enzyme immobilization and recycling, using cosolvents, and enzyme engineering have been reviewed recently.283
Examples of HMOS that have been synthesized as their natural oligosaccharide forms with a free reducing end using enzymatic, whole cell, and living cell approaches are shown in the following sections.
5.1. 2’FL
Enzymatic production of 2’FL (18 mg, 65% yield) from lactose was achieved using a reaction catalyzed by Helicobacter pylori NCTC 364 α1–2-fucosyltransferase (glutathione S-transferase or GST fusion was shown to improve the expression of soluble protein)284 using GDP-L-fucose (78 mg, 78% yield) produced from GDP-D-mannose by enzymatic reactions catalyzed by E. coli K-12 GDP-D-mannose 4,6-dehydratase and GDP-4-keto-6-deoxy-D-mannose 3,5-epimerase-4-reductase.285
Living cell biosynthesis of 2’FL (1.23 g/L, 20% yield) from lactose (14.5 g/L) in batch fermentation was achieved using E. coli JM109(DE3) cells engineered to overexpress Helicobacter pylori 26695 strain (ATCC 700392) α1–2-fucosyltransferase286 and overproduce GDP-fucose.287 The production of 2’FL (6.4 g/L) was further improved using an engineered Helicobacter pylori α1–2-fucosyltransferase by adding three aspartate residues at its N-terminus in an alternative expression host obtained by engineering E. coli BL21star(DE) strain to delete its endogenous lactose operon and to introduce a lacZΔM15-containing modified lactose operon from E. coli K-12.288
An improved large-scale production of 2’FL (20 g/L) from lactose and glycerol was achieved using an antibiotic-free fed batch fermentation (13 L) of engineered E. coli JM109 (lacY+, lacZ−) cells. The cells were engineered by chromosome incorporation of genes involved in the de novo GDP-L-fucose biosynthetic pathway, two copies of Helicobacter pylori α1–2-fucosyltransferase futC gene, and Bacteroides fragilis bifunctional fucokinase and GDP-fucose pyrophosphorylase fkp gene involved in the salvage pathway of GDP-fucose formation to the chromosome.289
2’FL production was also achieved in the milk of transgenic mice by introducing to mice a fusion gene containing a human α1–2-fucosyltransferase gene downstream of a murine whey acidic protein promoter and upstream of a polyadenylation signal.142 The same transgenic manipulation on rabbits seemed to interfere with their lactation process.290 The presence of glycoconjugates containing Fucα1–2Gal epitope reduces the rate and duration of pathogen colonization in pups inoculated with pathogenic strains of Campylobacter jejuni.204
Several α1–2-fucosynthases were obtained from Bifidobacterium bifidum α1–2-fuocisdase (AfcA), an inverting glycosidase, by mutating the amino acid residues involved in catalysis (N421G, N423G, or D766G).183, 291 The D766G mutant was found to be the most effective enzyme in catalyzing the synthesis of 2’FL from β-L-fucosyl fluoride (10 mM) and lactose (30 mM). A 6% yield was obtained based on the β-L-fucosyl fluoride donor substrate used.292
5.2. 3’SL and 3’SLN
Neu5Acα2–3Lac (3’SL) and Neu5Acα2–3LacNAc (3’SLN) were synthesized using a one-pot three-enzyme (OP3E) system containing an E. coli sialic acid aldolase (EcNanA),262, 293 Neisseria meningitidis CMP-sialic acid synthetase (NmCSS),293 and a multifunctional Pasteurella multocida α2–3-sialyltransferase 1 (PmST1).294 The amount of the enzyme used and the reaction time needed to be controlled to allow the optimal production of the product due to the multi-functionality of PmST1. The synthesis can be improved by replacing the wild-type PmST1 with a PmST1 E271F/R313Y double mutant which has retained α2–3-sialyltransferase activity while with >6000-fold decreased α2–3-sialidase activity.295 PmST1 M144D mutant with decreased donor hydrolysis and lowered α2–3-sialidase activities296 can also be used for high efficient synthesis of 3’SL and 3’SLN. The sialosides can also be synthesized from Neu5Ac and a suitable acceptor using a one-pot two-enzyme system containing NmCSS and a sialyltransferase.
Production of 3’SL and 3’SLN has also been reported from CMP-Neu5Ac and lactose by catalyzed a Pasteurella multocida α2–3-sialyltransferase297 or Pasteurella dagmatis α2–3-sialyltransferase.298, 299
The α2–3-trans-sialidase activity of Pasteurella multocida α2–3-sialyltransferase (GenBank accession number AAK02272) (PmST) which differs from PmST1 protein sequence by three amino acid residues (N105D, Q135R, and E295G) and has α2–3- and α2–6- dual trans-sialidase activities was used for the synthesis of 3’SL from lactose and casein glycomacropeptide (whey protein). The product 3’SL was accumulated up to 2.75 mM from lactose (100 mM) and 5% (w/v) casein glycomacropeptide (containing 9 mM bound sialic acid) under an optimal condition at pH 6.4 and 40 °C for 6 hours.300
The trans-sialidase activities of Bacteroides fragilis sialidase,301 Arthrobacter ureafaciens or Bifidobacterium infantis sialidase,302 and Trypanosoma cruzi α2–3-trans-sialidase have also been explored for the synthesis of 3’SL.303, 304 Low or moderate yields were achieved.
A fusion protein of NmCSS and Neisseria meningitidis α2–3-sialyltransferase (Nmα2–3ST) was used in a sugar nucleotide regeneration reaction for the synthesis of 3’SL (68 g in a partial purified solid form, 68% yield) at the 100 gram scale from lactose, Neu5Ac, phosphoenolpyruvate, and catalytic amounts of ATP and CMP.305
Large-scale production of 3’SL was also achieved using a whole cell approach.306 In this process, Corynebacterium ammoniagenes DN510 cells (for the production of UTP from inexpensive orotic acid and converting CMP to CDP) and three recombinant E. coli strains (containing E. coli K12 CTP synthetase, E. coli K1 CMP-Neu5Ac synthetase, and Neisseria gonorrhoeae α2–3-sialyltransferase respectively) were permeabilized by treating cell pellets with polyoxyethylene octadecylamine (Nymeen S-215) and dimethylbenzenes (xylene). Multiple grams of 3’SL (0.99 g, 36% yield and 72 g, 44% yield) were synthesized from lactose, Neu5Ac, and orotic acid at 32 °C for 11 h.306
3’SL (2.6 g/L, 49% yield) has also been produced from Neu5Ac and lactose fed to living E. coli (lacY+, lacZ−, nanT+, nanA−) cells engineered to express N. meningitidis CMP-Neu5Ac synthetase (NmCSS) and an N. meningitidis L3 strain MC58 α2–3-sialyltransferase (Nm2–3ST). The knockout of lacZ− and nanA− genes was to ensure that the lactose and Neu5Ac fed to the cells were not broken down by the β-galactosidase and sialic acid aldolase, respectively. Neu5Ac was transported into the cells by Neu5Ac permease NanT and β-galactoside permease LacY endogenous to the E. coli host cells were responsible for transporting exogenous Neu5Ac and lactose, respectively, into E. coli cells for the production of 3’SL.307
To decrease the cost for 3’SL production, the engineered 3’SL biosynthetic E. coli K12 cells were modified further by deleting ManNAc kinase nanK gene and incorporating plasmids for the expression of Campylobacter jejuni strain ATCC43438 neuABC genes encoding GlcNAc-6-phosphate 2-epimerse, sialic acid synthase, and CMP-Neu5Ac synthetase to produce CMP-Neu5Ac from endogenous UDP-GlcNAc and avoid the need of exogenous Neu5Ac. Using this improved engineered bacterial strain, a higher concentration (25 g/L) of 3’SL was obtained.308
5.3. 6’SL and 6’SLN
Neu5Acα2–6LacNAc (6’SLN) was synthesized using a similarly OP3E sialylation system as described above for the synthesis of 3’SL and 3’SLN except for replacing the PmST1 by Photobacterium damselae α2–6-sialyltransferase (Pd2,6ST).309 Neu5Acα2–6Lac (6’SL) can also be synthesized similarly using the same OP3E system as shown for the synthesis of 6’SL derivatives.
Both 6’SL and 6’SLN have been synthesized from CMP-Neu5Ac and lactose using Pasteurella dagmatis α2–3-sialyltransferase P7H/M117A double mutant which was completely switched to an α2–6-sialyltransferase.298
More recently, 6’SL (3.33 mM) was synthesized from lactose (100 mM) and casein glycomacropeptide (containing 9 mM bound sialic acid) at pH 5.4 and 40 °C for 8 hours using the α2–6-trans-sialidase activity of PmST (GenBank accession number AAK02272) which has the dual α2–3- and α2–6-trans-sialidase activities.300 PmST1 P34H mutant with α2–6-trans-sialidase activity was used to further improve the regio-selective production of 6’SL versus 3’SL.
6’SL was also produced together with its disialylated derivative, 6,6’-disialyllactose, using a living cell system engineered to overexpress Photobacterium sp. JT-ISH-224 α2–6-sialyltransferase (Psp2,6ST)304, 310 with Campylobacter jejuni strain ATCC43438 neuABC genes encoding GlcNAc-6-phosphate 2-epimerse, sialic acid synthase, and CMP-Neu5Ac synthetase.311 A 6’SL derivative Kdoα2–6Lac was also able to be produced using a similar system with Psp2,6ST gene under the control of a strong Ptrc promoter and neuABC genes under the control of a weaker Plac promoter.311
5.4. LNT2, LNnT, LNnH, LNnO, LNnD, LSTd, and disialyl oligosaccharides
Recently, two β-N-acetylhexosaminidases HEX1 and HEX2 identified from soil-derived metagenomic library screening were found to be able to catalyze trans-glycosylation reactions using chitin oligosaccharides as donor substrates and lactose as the acceptor for the formation of lacto-N-triose II (LNT2, GlcNAcβ1–3Lac),312 the precursor for the synthesis of LNT and LNnT. Although the yields are low (2% and 8% respectively), they have the potential for improvement by mutagenesis.
LNT2 (106.3 mg) was also synthesized from lactose and UDP-GlcNAc catalyzed by bovine serum β1–3-N-acetylglucosaminyltransferase. LNnT (12 mg) was subsequently produced from LNT2 and ortho-nitrophenyl β-galactoside by a commercially available Bacillus circulans β-D-galactosidase.313
Large-scale production of LNT2 trisaccharide and LNnT in several hundred grams in a 100 L reactor has been reported. LNT2 trisaccharide (250 grams) was synthesized from lactose and UDP-GlcNAc using E. coli cells expressing β1–3-N-acetylglucosaminyltransferase (LgtA). LNnT (300 grams, >85% yield) was subsequently synthesized from LNT2 and UDP-galactose using E. coli cells expressing β1–4GalT (LgtB). Sialyllacto-N-tetraose d (LSTd, Neu5Acα2–3LNnT, has not been identified in human milk) was further produced in 50 grams with a 90% yield from LNnT and Neu5Acα2–3Lac using a recombinant Trypanosoma cruzi α2–3-trans-sialidase expressed in E. coli.263
LNT2 and LNnT were reported to be produced in kilograms in a fermentation-based system to allow the conduct of clinical trials.290, 314 At the tested concentration, LNnT was proven to be stable and safe to use as a component of infant formula although it did not reduce oropharyngeal colonization of Streptococcus pneumoniae in children of 6 months or older.315
LNnT was also synthesized from 1-thio-β-LNT2 conjugated to a polyethylene glycol (PEG)-based dendrimeric support and UDP-Glc using reactions catalyzed by UDP-Gal 4-epimerase and bovine milk β1–4GalT. In this system, the UDP-Gal 4-epimerase was responsible for the formation of UDP-Gal from less expensive UDP-Glc, thus providing donor substrate for the bovine milk β1–4GalT for the formation of LNnT. The thio-linked PEG-support was readily cleaved off using mercuric (II) trifluoroacetate (CF3CO2)2Hg (2 equivalents) in acetic acid (0.05 M) at room temperature to release free LNnT (18 mg).316
Large-scale production of LNT2 (6 g/L, 73% yield) and LNnT (> 5 g/L), and lower level formation of lacto-N-neohexaose (LNnH), lacto-N-neooctaose (LNnO), and even lacto-N-neodecaose (LNnD) were reported using living E. coli JM109 cells (lacY+ lacZ−) engineered to overexpress Neisseria meningitidis β1–3-N-acetylglucosaminyltransferase (NmLgtA) and Neisseria meningitidis β1–4GalT (NmLgtB).307
Enzymatic synthesis of LNT2 (1.36 g, 95% yield), LNnT (1.19 g, 92% yield) and disialyl glycans was successfully achieved using sequential one-pot multienzyme (OPME) systems as shown in FIG. 1 and FIG. 2.269 In these systems, free monosaccharides were added one-by-one at each one-pot systems containing multiple enzymes responsible for catalyzing monosaccharide activating followed by transfer processes. Multiple OMPE systems were used sequentially for building up complex HMOS structures. The combination of several OPME systems were used for the synthesis of disialyl oligosaccharides milk including DSLNnT (236 mg, 99% yield), GD3 tetraose (239 mg, 82% yield), DSLac (112 mg, 93% yield), and DS’LNT (268 mg, 98% yield) which are analogs of disialyl lacto-N-tetraose (DSLNT), a hexaose commonly found in human. A monosialylpentaose LSTd (or 3”’-sLNnT) (138 mg, 98% yield) was synthesized similarly using sequential OPME systems.269 Similar to DSLNT and HMOS pool,236 both synthetic DSLNnT and DS’LNT were shown to protect neonatal rats from necrotizing enterocolitis.269
FIG. 1.
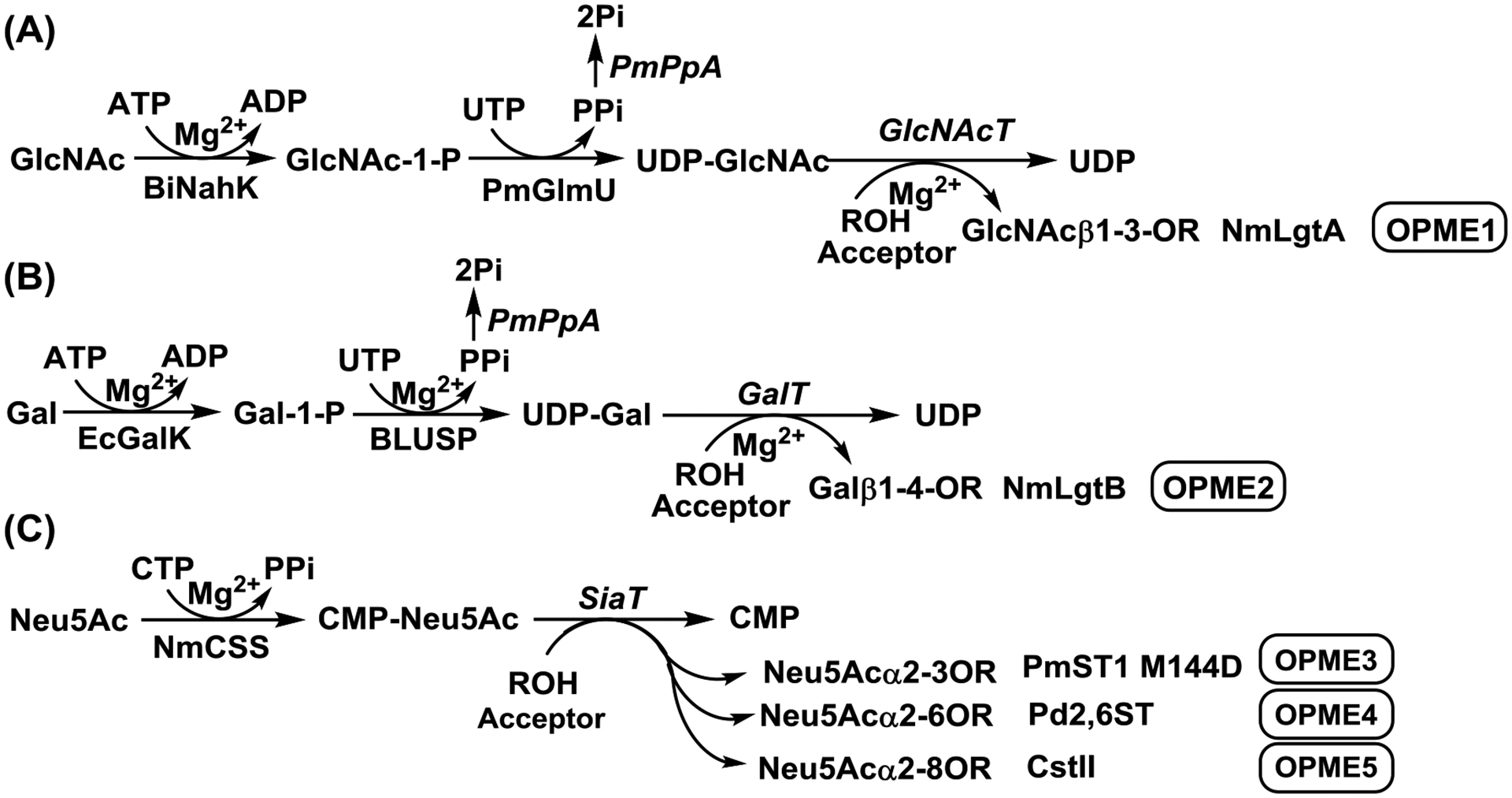
One-pot multienzyme (OPME) GlcNAc (A), Gal (B), and Neu5Ac (C) activation and transfer systems.269, 270 Enzyme abbreviations: BiNahK, B. infantis N-acetylhexosamine-1-kinase;317 PmGlmU, P. multocida N-acetylglucosamine 1-phosphate uridylyltransferase;318 PmPpA, P. multocida inorganic pyrophosphatase;318 GlcNAcT, N-acetylglucosaminyltransferase; NmLgtA, Neisseria meningitidis β1–3-N-acetylglucosaminyltransferase;319 EcGalK, E. coli K-12 galactose kinase;320 BLUSP: B. longum UDP-sugar synthase;320 GalT, galactosyltransferase; NmLgtB, Neisseria meningitidis β1–4-galactosyltransferase;321 NmCSS, Neisseria meningitidis CMP-sialic acid synthetase;293 SiaT, sialyltransferases; PmST1 M144D, Pasteurella multocida α2–3-sialyltransferase 1 M144D mutant;296 Pd2,6ST, Photobacterium damselae α2–6-sialyltransferase;309, 322 CstII, Campylobacter jejuni α2–8-sialyltransferase II.323, 324
FIG. 2.
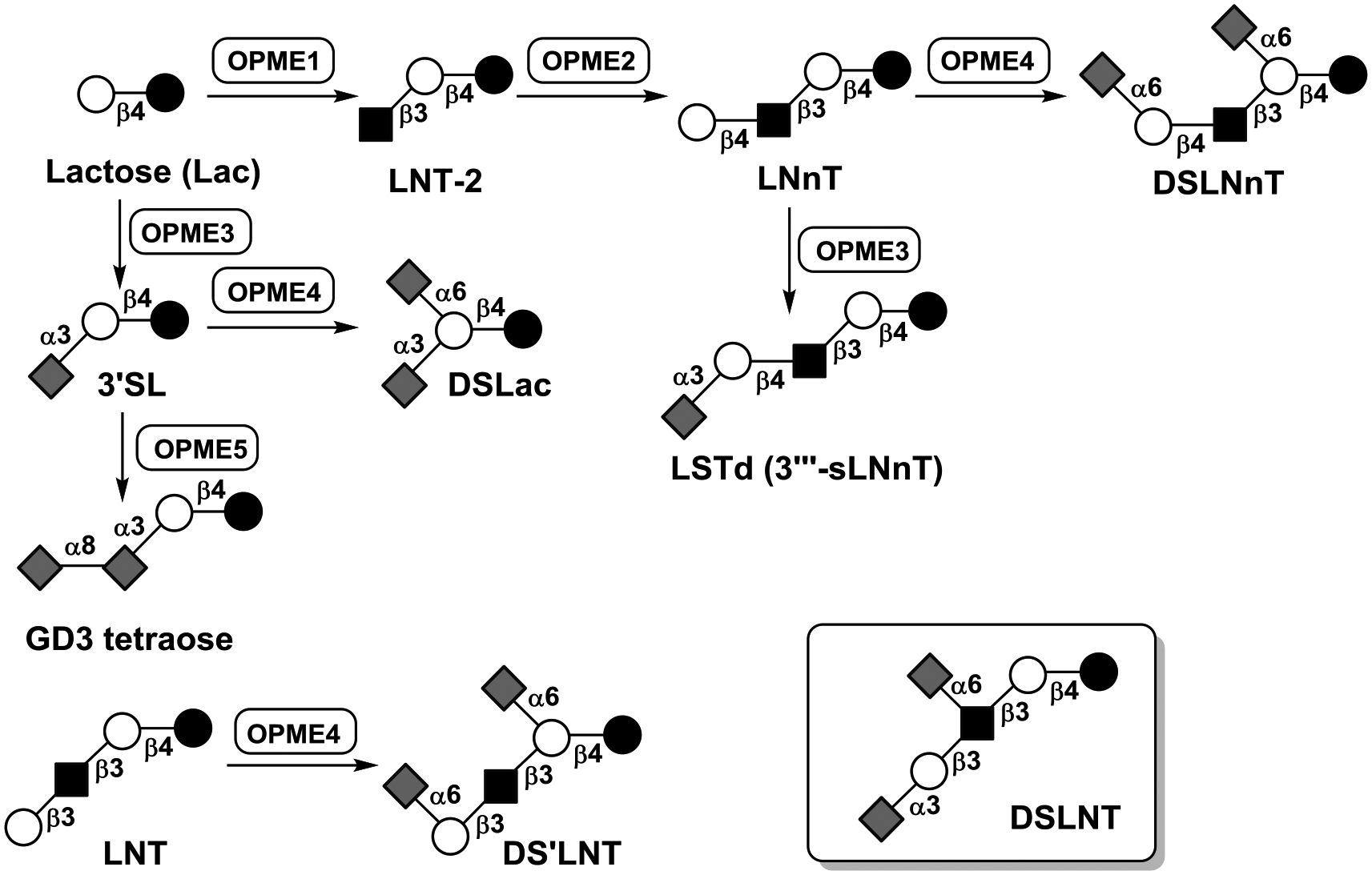
OPME and sequential OPME systems for the synthesis of disialyl oligosaccharides including DSLNnT, GD3 tetraose, DSLac, DS’LNT, and a monosialylpentaose LSTd (3”’-sLNnT).269 The structure of DSLNT found in human milk is shown for comparison purpose.
5.5. Fucα1–2LNnT
Fucα1–2LNnT, a monofucosylated pentaose that has not been identified from human milk, was produced together with 2’FL using E. coli living cells engineered to overproduce GDP-fucose325 and LNnT307 with an additional introduction of a modified H. pylori strain 26695 α1–2-fucosyltransferase.326
5.6. LNFP III, LNnFP V, and LNnDFH
Lacto-N-neofucopentaose (LNnFP V), lacto-N-neodifucohexaose (LNnDFH), and a lacto-N-neodifucooctaose [Galβ1–4GlcNAcβ1–3Galβ1–4(Fucα1–3)GlcNAcβ1–3Galβ1–4(Fucα1–3)Glc] have been synthesized from lactose using living E. coli cells engineered to inactivate genomic wcaJ gene involved in colanic acid synthesis and to express NmLgtA, NmLgtB, Helicobacter pylori strain 26695 α1–3-fucosyltransferase FutA (encoded by HP0379 gene), and RcsA (a positive regulator of the colanic acid operon). Glucose was used as a carbon source.325 The construct was further modified to improve the yield for the synthesis of LNnDFH (1.7 g/L). In addition, the living cell system containing another Helicobacter pylori strain 26695 α1–3-fucosyltransferase futB gene (HP0651) was shown to produce both lacto-N-neofucopentaose III (LNFP III) (260 mg/L) and LNnFP V (280 mg/L).327
5.7. LNT
LNT was enzymatically synthesized from LNT2 and ortho-nitrophenyl β-D-galactoside using a Bacillus circulans ATCC31382 β-galactosidase-catalyzed transglycosylation reaction. Alternatively, LNT (7.1 mg) was able to be synthesized from lactose and Galβ1–3GlcNAcβpNP using Aureobacterium sp. L-101 lacto-N-biosidase-catalyzed transglycosylation reaction.313 Inherent low yields (19–26%) were observed for typical glycosidase-catalyzed trans-glycosylation reactions.
An LNT benzyl glycoside was efficiently produced from LNT2 benzyl glycoside (synthesized by NmLgtA-catalyzed glycosylation reaction from lactose benzyl glycoside and UDP-GlcNAc) and UDP-Gal using a GST-tagged Escherichia coli O55:H7 β1–3-N-acetylglucosaminyltransferase WbgO fusion protein.313
Large-scale production of LNT was not achieved until recently using E. coli strain LJ110 (with intact LacY but with lacZ knockout) chromosomally integrated with Neisseria meningitidis β1–3-N-acetylglucosaminyltransferase lgtA and Escherichia coli O55:H7 β1–3-N-acetylglucosaminyltransferase wbgO genes.328 Nevertheless, when glucose was used as the carbon source, LNT2 was the major product and only about 5% of the lactose was converted to LNT (219 mg/L).328 By substituting the glucose with galactose, the yield of LNT production (811 mg/L) was improved by 3.6-fold. Fed-batch cultivation with galactose further improved the efficiency and produced LNT in 173 grams (12.72 g/L).329
5.8. 3FL, LDFT, LNFP II, Lea tetrasaccharide, and Lex tetrasaccharide
Several α1–3/4-fucosynthases were obtained from Bifidobacterium bifidum α1–3/4-fuocisdase (BbAfcB), a retaining glycosidase, by mutating the amino acid residue that was predicted to serve as a nucleophile (D703). Among the D703A, D703C, D703G, and D703S four mutants, D703S mutant was found to be the best α1–3/4-fucosynthase and was used for the production of several fucosylated HMOS and derivatives using β-L-fucosyl fluoride (40 mM) and a suitable acceptor (100 mM) such as Lac, 2’FL, LNT, LNB, and LacNAc. HMOS and derivatives 3FL, LDFT, LNFP II, Lea tetrasaccharide, and Lex tetrasaccharide were obtained in 13%, 5.5%, 41%, 47%, and 55% yields, respectively, based on the β-L-fucosyl fluoride donor substrate used. Increasing the LNB concentration to 200 mM was able to improve the yield to 56%.330
5.9. LNFP I and LNDFH I
LNFP I (7% yield) and LNDFH I (6% yield) was synthesized from lactose using several glycosyltransferase-catalyzed reactions with the corresponding sugar nucleotides and a galactosidase-catalyzed reaction with a corresponding synthetic donor. LNT2 (44% yield) was initially synthesized from lactose and UDP-GlcNAc catalyzed by a β1–3GlcNAcT that was partially purified from bovine blood. LNT (22% yield) was then produced from LNT2 and ortho-nitrophenyl-β-galactoside (GalβoNP) using a recombinant Bacillus circulans β1–3-galactosidase. The production of LNFP I (71% yield) was achieved from LNT and GDP-Fuc using a recombinant human α1–2-fucosyltransferase 1 (FUT1) expressed in a baculovirus system. Finally, LNDFH I (85% yield) was produced from LNFP I and GDP-Fuc by a FUT3-catalyzed reaction using a commercial enzyme.210
5.10. Other oligosaccharides
Gram-scale production of globotriose (Gb3) and globotetraose (Gb4) oligosaccharides was achieved using bacterial glycosyltransferases and sugar-nucleotides. Gb3 trisaccharide (5 grams, 75% yield) was synthesized from lactose and UDP-galactose using Neisseria gonorrhoeae α1–4-galactosyltransferase (NgLgtC). Gb4 tetrasaccharide (1.5 grams, 60% yield) was synthesized from Gb3 trisaccharide and UDP-GalNAc using Neisseria gonorrhoeae β1–3-N-acetylgalactosaminyltransferase (NgLgtD).263
UDP-galactose (44 g/L) and globotriose Galα1–4Lac (188 g/L) were also produced using permeabilized Corynebacterium ammoniagenes cells (for the production of UTP from orotic acid) and E. coli cells engineered to overexpress UDP-Gal biosynthetic genes with or without Neisseria gonorrhoeae α1–4-galactosyltransferase.331 A whole cell approach using permeabilized Corynebacterium ammoniagenes cells (for the production of GTP from GMP) and E. coli cells engineered to overexpress de novo GDP-Fuc biosynthetic enzymes, phosphoglucomutase, phosphofructokinase, and Helicobacter pylori α1–3-fucosyltransferase was also developed for the production of Lewis x trisaccharide (21 g/L in a 30 mL scale, purification yield 32%) from GMP, mannose, and N-acetyllactosamine (LacNAc).332
The engineered E. coli living cell strategy can be used for the synthesis of other HMOS by introducing plasmids for the expression of necessary glycosyltransferases and sugar nucleotide biosynthetic enzymes. For example, gram-scale synthesis of Lewis x tetrasaccharides Galβ1–4(Fucα1–3)GlcNAcβ1–4GlcNAc (0.62 g) and Galβ1–4(Fucα1–3)GlcNAcβ1–3Gal (1.84 g) was achieved by placing four de novo GDP-Fuc biosynthetic gene under Plac promoter without knocking out wcaJ involved in colanic acid synthesis or introducing RcsA (a positive regulator of the colanic acid operon).333 The engineered living cells strategy was also used to produce the oligosaccharide components of gangliosides GM2 and GM1, GalNAcβ1–4(Neu5Acα2–3)Lac and Galβ1–3GalNAcβ1–4(Neu5Acα2–3)Lac,334 has been achieved.
6. Perspectives
Future efforts on HMOS analysis should be focused on elucidating the structures of more complex and longer chain HMOS including those with more than 14 monosaccharide residues that are not currently presented in TABLE IV. These more complex structures are unlikely the byproducts of biosynthesis of HMOS. Although less abundant, they could have significant biological functions. Profiling HMOS in a high-throughput format will also help to find correlation of disease states and the roles of certain populations of HMOS. Various enzyme-catalyzed synthetic methods that have been successfully used in production of relatively simple HMOS include glycosyltransferase-catalyzed reactions with or without co-factor recycling, sialidase and trans-glycosidase-catalyzed reactions, one-pot multienzyme (OPME) and sequential OPME systems, whole cell approaches, and living cell strategies. Such efforts, however, have not been applied for the production of more complex structures, especially the ones with branches. With the limited access to all enzymes needed, especially essential glycosyltransferases, the combination of chemical and enzymatic methods can be used. Such methods have been developed but have not been broadly used for the synthesis of HMOS. The strategy of chemoenzymatic synthesis of asymmetrically branched N-glycan structures335, 336 can be readily applicable for the synthesis of branched HMOS structures. Efficient purification methods for large scale production of HMOS either by chemical, enzymatic, or cell-based systems are also in a great demand. The availability of structurally defined more complex individual HMOS species will greatly facilitate their function studies and to explore their prebiotic and therapeutic potentials. Other than functional studies using pooled HMOS and individual pure synthetic HMOS, the synergistic effect of a mixture of two or more structurally defined HMOS should also be investigated as, most likely, a single compound will not provide the desired protection against multiple pathogens.15
Acknowledgements
X.C. expresses her sincerest appreciation for the invitation to write on the subject from the late Prof. Derek Horton, who provided much needed instructions, but unfortunately could not see the submission of this work. X.C. is also grateful for the editing provided by Prof. David C. Baker. Research activities on milk oligosaccharide projects in the Chen group were supported, in part, by the National Institutes of Health (NIH) grant R01HD065122.
Abbreviations
- ATP
adenine 5’-triphoaphate
- B. breve
Bifidobacterium breve
- B. bifidum
Bifidobacterium bifidum
- B. infantis
Bifidobacterium longum subsp. infantis
- BiNahK
B. infantis N-acetylhexosamine-1-kinase
- B. longum
Bifidobacterium longum subsp. longum
- BLUSP
B. longum UDP-sugar synthase
- CDP
cytidine 5’-diphoaphate
- CMP
cytidine 5’-monophoaphate
- CMP-Neu5Ac
cytidine 5’-monophoaphate-N-acetylneuraminic acid
- CstII
Campylobacter jejuni α2–8-sialyltransferase II
- CTP
cytidine 5’-triphoaphate
- DSLNT
disialyllacto-N-tetraose
- EcGalK
E. coli K-12 galactose kinase
- EcNanA
E. coli sialic acid aldolase
- NmCSS
Neisseria meningitidis CMP-sialic acid synthetase
- PmGlmU
P. multocida N-acetylglucosamine 1-phosphate uridylyltransferase
- PmPpA
P. multocida inorganic pyrophosphatase
- PmST
Pasteurella multocida α2–3-sialyltransferase (GenBank accession number AAK02272)
- PmST1
multifunctional Pasteurella multocida α2–3-sialyltransferase 1
- 2’FL
2’-fucosyllactose
- 3FL
3-fucosyllactose
- Fuc
fucose
- FucT
fucosyltransferase
- FUT2
human α1–2-fucosyltransferase encoded by the Secretor (Se) gene
- FUT3
human α1–3/4-fucosyltransferase encoded by the Lewis (Le) gene
- FutA
Helicobacter pylori strain 26695 α1–3-fucosyltransferase
- Gal
galactose
- GalT
galactosyltransferase
- GalNAc
N-acetylgalactosamine
- GDP
guanidine 5’-diphosphate
- GMP
guanidine 5’-monophosphate
- GTP
guanidine 5’-triphosphate
- Glc
glucose
- GlcNAc
N-acetylglucosamine
- GlcNAcT
N-acetylglucosaminyltransferase
- GNB
galacto-N-biose
- GnT-I
N-acetylglucosaminyltransferase I
- GST
glutathione S-transferase
- E. coli
Escherichia coli
- HMOS
human milk oligosaccharides
- HPAEC-PAD
high-pH anion-exchange chromatography with pulsed amperometric detection
- HPLC
high performance liquid chromatography
- Lac
lactose
- LacNAc
N-acetyllactosamine
- LacY
β-galactoside permease
- LDFT
lactodifucotetraose
- Le
Lewis
- LNB
lacto-N-biose
- LNDFH
lacto-N-difuco-hexoase
- LNFP
lacto-N-fucopentaose
- LNnD
lacto-N-neodecaose
- LNnDFH
lacto-N-neodifucohexaose
- LNnFP
lacto-N-neofucopentaose
- LNnH
lacto-N-neohexaose
- LNnT
lacto-N-neotetraose
- LNT
lacto-N-tetraose
- LNT2
lacto-N-triose II
- LPS
lipopolysaccharides
- LST
sialyllacto-N-tetraose
- MOS
milk oligosaccharides
- MS
mass spectrometry
- NanT
Neu5Ac permease
- NEC
necrotizing enterocolitis
- Neu5Ac
N-acetylneuraminic acid, the most abundant sialic acid form in nature
- Neu4,5Ac2
4-O-acetyl-N-acetylneuraminic acid
- NK
natural killer
- Nmα2–3ST
Neisseria meningitidis α2–3-sialyltransferase
- NmLgtA
Neisseria meningitidis β1–3-N-acetylglucosaminyltransferase
- NmLgtB
Neisseria meningitidis β1–4-galactosyltransferase
- NMR
nuclear magnetic resonance spectroscopy
- OPME
one-pot multienzyme
- Pd2,6ST
Photobacterium damselae α2–6-sialyltransferase
- Psp2,6ST
Photobacterium sp. JT-ISH-224 α2–6-sialyltransferase
- UTP
uridine 5’-triphosphate
- Se
Secretor
- 3’S-3FL
3’-sialyl-3-fucosyllactose
- SiaT
sialyltransferase
- 3’SL
3’-sialyllactose
- 6’SL
6’-sialyllactose
- 3’SLN
3’-sialyl-N-acetyllactosamine
- 6’SLN
6’-sialyl-N-acetyllactosamine
References
- 1.Smilowitz JT; Lebrilla CB; Mills DA; German JB; Freeman SL Breast milk oligosaccharides: structure-function relationships in the neonate. Annu Rev Nutr 2014, 34, 143–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newburg DS Oligosaccharides and glycoconjugates in human milk: their role in host defense. J Mammary Gland Biol Neoplasia 1996, 1, 271–283. [DOI] [PubMed] [Google Scholar]
- 3.Jenness R The composition of human milk. Semin Perinatol 1979, 3, 225–239. [PubMed] [Google Scholar]
- 4.Jensen RG; Blanc B; Patton S The structure of milk: Implications for sampling and storage. B. Particulate constituents in human and bovine milks. in Handbook of Milk Composition. Jensen Rorbert G. Ed. Academic Press, Inc. San Diego, California, USA: 1995, 50–62. [Google Scholar]
- 5.Zopf DA; Smith DF; Drzeniek Z; Tsai CM; Ginburg V Affinity purification of antibodies using oligosaccharide-phenethylamine derivaties coupled to Sepharose. Methods Enzymol 1978, 50, 171–175. [DOI] [PubMed] [Google Scholar]
- 6.Smith DF; Prieto PA; Torres BV Rabbit antibodies against the human milk sialyloligosaccharide alditol of LS-tetrasaccharide a (NeuAc alpha 2–3Gal beta 1–3GlcNAc beta 1–3Gal beta 1–4GlcOH). Arch Biochem Biophys 1985, 241, 298–303. [DOI] [PubMed] [Google Scholar]
- 7.Prieto PA; Smith DF A new ganglioside in human meconium detected with antiserum against human milk sialyltetrasaccharide a. Arch Biochem Biophys 1986, 249, 243–253. [DOI] [PubMed] [Google Scholar]
- 8.Coppa GV; Pierani P; Zampini L; Carloni I; Carlucci A; Gabrielli O Oligosaccharides in human milk during different phases of lactation. Acta Paediatr Suppl 1999, 88, 89–94. [DOI] [PubMed] [Google Scholar]
- 9.Montreuil J; Mullet S Etude des variations des constituants glucidiques du lait de femme au cours de la lactation. Bulletin de la Societe de Chimie Biologique 1960, 42, 365–377. [PubMed] [Google Scholar]
- 10.Chaturvedi P; Warren CD; Altaye M; Morrow AL; Ruiz-Palacios G; Pickering LK; Newburg DS Fucosylated human milk oligosaccharides vary between individuals and over the course of lactation. Glycobiology 2001, 11, 365–372. [DOI] [PubMed] [Google Scholar]
- 11.Gabrielli O; Zampini L; Galeazzi T; Padella L; Santoro L; Peila C; Giuliani F; Bertino E; Fabris C; Coppa GV Preterm milk oligosaccharides during the first month of lactation. Pediatrics 2011, 128, e1520–31. [DOI] [PubMed] [Google Scholar]
- 12.Viverge D; Grimmonprez L; Cassanas G; Bardet L; Bonnet H; Solere M Variations of lactose and oligosaccharides in milk from women of blood types secretor A or H, secretor Lewis, and secretor H/nonsecretor Lewis during the course of lactation. Ann Nutr Metab 1985, 29, 1–11. [DOI] [PubMed] [Google Scholar]
- 13.Kobata A; Yamashita K; Tachibana Y Oligosaccharides from human milk. Methods Enzymol 1978, 50, 216–220. [DOI] [PubMed] [Google Scholar]
- 14.Thurl S; Henker J; Siegel M; Tovar K; Sawatzki G Detection of four human milk groups with respect to Lewis blood group dependent oligosaccharides. Glycoconj J 1997, 14, 795–799. [DOI] [PubMed] [Google Scholar]
- 15.Newburg DS; Ruiz-Palacios GM; Morrow AL Human milk glycans protect infants against enteric pathogens. Annu Rev Nutr 2005, 25, 37–58. [DOI] [PubMed] [Google Scholar]
- 16.Polonowski M; Lespagnol A Nouvelles acquisitions sur les composes glucidiques du lai de femme. Bull Soc Chim Biol (Paris) 1933, 15, 320–349. [Google Scholar]
- 17.Polonowski M; Lespagnol A Sur la nature glucidique de la substance levogyre du lait de femme. Bull Soc Biol 1929, 101, 61–63. [Google Scholar]
- 18.Polonowski M; Montreuil J Etude chromatographique des polyosides du lai de femme. C. R. Acad. Sci 1954, 238, 2263–2264. [PubMed] [Google Scholar]
- 19.Schönfeld H Über die Beziehungen der einzelnen Bestandteile der Frauenmilch zur Bifidusflora. Jahrb. Kinderheilkd 1929, 113, 19–69. [Google Scholar]
- 20.Rose CS; Kuhn R; Zilliken F; Gyorgy P Bifidus factor. V. The activity of alpha- and-beta-methyl-N-acetyl-D-glucosaminides. Arch Biochem Biophys 1954, 49, 123–129. [DOI] [PubMed] [Google Scholar]
- 21.Gyorgy P; Norris RF; Rose CS Bifidus factor. I. A variant of Lactobacillus bifidus requiring a special growth factor. Arch Biochem Biophys 1954, 48, 193–201. [DOI] [PubMed] [Google Scholar]
- 22.Gyorgy P; Kuhn R; Rose CS; Zilliken F Bifidus factor. II. Its occurrence in milk from different species and in other natural products. Arch Biochem Biophys 1954, 48, 202–208. [DOI] [PubMed] [Google Scholar]
- 23.Gauhe A; Gyorgy P; Hoover JR; Kuhn R; Rose CS; Ruelius HW; Zilliken F Bifidus factor. IV. Preparations obtained from human milk. Arch Biochem Biophys 1954, 48, 214–224. [DOI] [PubMed] [Google Scholar]
- 24.Kuhn R; Baer HH Die Konstitution der lacto-N-tetraose. Chem. Ber 1956, 89, 504–511. [Google Scholar]
- 25.Kuhn R; Baer HH; Gauhe A Kristallisierte fucosido-lactose. Chem. Ber 1956, 89, 2513. [Google Scholar]
- 26.Kuhn R; Baer HH; Gauhe A Kristallisation und Konstituionsermittlung der lacto-N-fucopentaose I. Chem. Ber 1956, 89, 2514–2523. [Google Scholar]
- 27.Montreuil J Structure de deux triholosides isoles du lai de femme. C. R. Hebd. Seances Acad. Sci 1956, 242, 192–193. [PubMed] [Google Scholar]
- 28.Kobata A Structures and application of oligosaccharides in human milk. Proc Jpn Acad Ser B Phys Biol Sci 2010, 86, 731–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egge H; Dell A; Von Nicolai H Fucose containing oligosaccharides from human milk. I. Separation and identification of new constituents. Arch Biochem Biophys 1983, 224, 235–253. [DOI] [PubMed] [Google Scholar]
- 30.Ninonuevo MR; Park Y; Yin H; Zhang J; Ward RE; Clowers BH; German JB; Freeman SL; Killeen K; Grimm R; Lebrilla CB A strategy for annotating the human milk glycome. J Agric Food Chem 2006, 54, 7471–7480. [DOI] [PubMed] [Google Scholar]
- 31.Dai D; Nanthkumar NN; Newburg DS; Walker WA Role of oligosaccharides and glycoconjugates in intestinal host defense. J Pediatr Gastroenterol Nutr 2000, 30 Suppl 2, S23–S33. [PubMed] [Google Scholar]
- 32.Newburg DS; Neubauer SH Carbohydrates in milks: Analysis, quantities, and significance. In: Handbooks of Milk Composition. Jensen Robert G. Ed., 1995, Academic Press, Inc. San Diego, California, USA: 1995, 273–349. [Google Scholar]
- 33.Wu S; Tao N; German JB; Grimm R; Lebrilla CB Development of an annotated library of neutral human milk oligosaccharides. J Proteome Res 2010, 9, 4138–4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu S; Grimm R; German JB; Lebrilla CB Annotation and structural analysis of sialylated human milk oligosaccharides. J Proteome Res 2011, 10, 856–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newburg DS Glycobiology of human milk. Biochemistry (Mosc) 2013, 78, 771–785. [DOI] [PubMed] [Google Scholar]
- 36.Engfer MB; Stahl B; Finke B; Sawatzki G; Daniel H Human milk oligosaccharides are resistant to enzymatic hydrolysis in the upper gastrointestinal tract. Am J Clin Nutr 2000, 71, 1589–1596. [DOI] [PubMed] [Google Scholar]
- 37.Gnoth MJ; Kunz C; Kinne-Saffran E; Rudloff S Human milk oligosaccharides are minimally digested in vitro. J Nutr 2000, 130, 3014–3020. [DOI] [PubMed] [Google Scholar]
- 38.Bode L Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hinde K; Lewis ZT MICROBIOTA. Mother’s littlest helpers. Science 2015, 348, 1427–1428. [DOI] [PubMed] [Google Scholar]
- 40.Grulee C; Sanford H; Schwartz H Breast and artificially fed infants; study of the age incidence in morbidity and mortality in 20,000 cases. JAMA 1935, 104, 1986–1988. [Google Scholar]
- 41.Morrow AL; Ruiz-Palacios GM; Jiang X; Newburg DS Human-milk glycans that inhibit pathogen binding protect breast-feeding infants against infectious diarrhea. J Nutr 2005, 135, 1304–1307. [DOI] [PubMed] [Google Scholar]
- 42.Stepans MB; Wilhelm SL; Hertzog M; Rodehorst TK; Blaney S; Clemens B; Polak JJ; Newburg DS Early consumption of human milk oligosaccharides is inversely related to subsequent risk of respiratory and enteric disease in infants. Breastfeed Med 2006, 1, 207–215. [DOI] [PubMed] [Google Scholar]
- 43.Chichlowski M; De Lartigue G; German JB; Raybould HE; Mills DA Bifidobacteria isolated from infants and cultured on human milk oligosaccharides affect intestinal epithelial function. J Pediatr Gastroenterol Nutr 2012, 55, 321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Etzold S; Bode L Glycan-dependent viral infection in infants and the role of human milk oligosaccharides. Curr Opin Virol 2014, 7, 101–107. [DOI] [PubMed] [Google Scholar]
- 45.Wang B Molecular mechanism underlying sialic acid as an essential nutrient for brain development and cognition. Adv Nutr 2012, 3, 465S–472S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yatsunenko T; Rey FE; Manary MJ; Trehan I; Dominguez-Bello MG; Contreras M; Magris M; Hidalgo G; Baldassano RN; Anokhin AP; Heath AC; Warner B; Reeder J; Kuczynski J; Caporaso JG; Lozupone CA; Lauber C; Clemente JC; Knights D; Knight R; Gordon JI Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gibson GR; Wang X Regulatory effects of bifidobacteria on the growth of other colonic bacteria. J Appl Bacteriol 1994, 77, 412–20. [DOI] [PubMed] [Google Scholar]
- 48.Tao N; Wu S; Kim J; An HJ; Hinde K; Power ML; Gagneux P; German JB; Lebrilla CB Evolutionary glycomics: characterization of milk oligosaccharides in primates. J Proteome Res 2011, 10, 1548–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levene PA; Sobotka H Lactone formation of lacto- and maltobionic acids and its bearing on the structure of lactose and maltose. J. Biol. Chem 1927, 71, 471–475. [Google Scholar]
- 50.Kuhn R; Gauhe A Die Konstitution der lacto-N-neotetraose. Chem. Ber 1962, 95, 518–522. [Google Scholar]
- 51.Kobata A; Ginsburg V Oligosaccharides of human milk. 3. Isolation and characterization of a new hexasaccharide, lacto-N-hexaose. J Biol Chem 1972, 247, 1525–1529. [PubMed] [Google Scholar]
- 52.Kobata A; Ginsburg V Oligosaccharides of human milk. IV. Isolation and characterization of a new hexasaccharide, lacto-N-neohexaose. Arch Biochem Biophys 1972, 150, 273–281. [DOI] [PubMed] [Google Scholar]
- 53.Yamashita K; Tachibana Y; Kobata A Oligosaccharides of human milk. Structural studies of two new octasaccharides, difucosyl derivatives of para-lacto-N-hexaose and para-lacto-N-neohexaose. J Biol Chem 1977, 252, 5408–5411. [PubMed] [Google Scholar]
- 54.Tachibana Y; Yamashita K; Kobata A Oligosaccharides of human milk: structural studies of di-and trifucosyl derivatives of lacto-N-octaose and lacto-N-neoctaose. Arch Biochem Biophys 1978, 188, 83–89. [DOI] [PubMed] [Google Scholar]
- 55.Strecker G; Fievre S; Wieruszeski JM; Michalski JC; Montreuil J Primary structure of four human milk octa-, nona-, and undeca-saccharides established by 1H- and 13C-nuclear magnetic resonance spectroscopy. Carbohydr Res 1992, 226, 1–14. [DOI] [PubMed] [Google Scholar]
- 56.Haeuw-Fievre S; Wieruszeski JM; Plancke Y; Michalski JC; Montreuil J; Strecker G Primary structure of human milk octa-, dodeca- and tridecasaccharides determined by a combination of 1H-NMR spectroscopy and fast-atom-bombardment mass spectrometry. Evidence for a new core structure, the para-lacto-N-octaose. Eur J Biochem 1993, 215, 361–371. [DOI] [PubMed] [Google Scholar]
- 57.Kunz C; Rudloff S; Baier W; Klein N; Strobel S Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu Rev Nutr 2000, 20, 699–722. [DOI] [PubMed] [Google Scholar]
- 58.Amano J; Osanai M; Orita T; Sugahara D; Osumi K Structural determination by negative-ion MALDI-QIT-TOFMSn after pyrene derivatization of variously fucosylated oligosaccharides with branched decaose cores from human milk. Glycobiology 2009, 19, 601–614. [DOI] [PubMed] [Google Scholar]
- 59.Kobata A Possible application of milk oligosaccharides for drug development. Chang Gung Med. J 2003, 26, 620–636. [PubMed] [Google Scholar]
- 60.Pfenninger A; Chan SY; Karas M; Finke B; Stahl B; Costello CE Mass spectrometric detection of multiple extended series of neutral highly fucosylated N-acetyllactosamine oligosaccharides in human milk. Int J Mass Spectrom 2008, 278, 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Urashima T; Kitaoka M; Terabayashi T; Fukuda K; Ohnishi M; Kobata A Milk oligosaccharides. In Oligosaccharides: Sources, Properties, and Applications. Gordon NG Ed. Nova Science Publishers, New York: 2011, 1–58. [Google Scholar]
- 62.Sabharwal H; Nilsson B; Chester MA; Sjoblad S; Lundblad A Blood group specific oligosaccharides from faeces of a blood group A breast-fed infant. Mol Immunol 1984, 21, 1105–1112. [DOI] [PubMed] [Google Scholar]
- 63.Strecker G; Trentesaux-Chauvet C; Riazi-Farzad T; Fournet B; Bouquelet S; Montreuil J [Demonstration of oligosaccharidosuria associated with various meliturias, and determination of the structure of the oligosaccharides excreted. Hypothesis concerning the origins of oligosaccharides in biological fluids]. C R Acad Sci Hebd Seances Acad Sci D 1973, 277, 1569–1572. [PubMed] [Google Scholar]
- 64.Sturman JA; Lin YY; Higuchi T; Fellman JH N-Acetylneuramin lactose sulfate: a newly identified nutrient in milk. Pediatr Res 1985, 19, 216–219. [DOI] [PubMed] [Google Scholar]
- 65.Guerardel Y; Morelle W; Plancke Y; Lemoine J; Strecker G Structural analysis of three sulfated oligosaccharides isolated from human milk. Carbohydr Res 1999, 320, 230–238. [DOI] [PubMed] [Google Scholar]
- 66.Kuhn R; Gauhe A Bestimmung der bindungsstelle von sialinsaureresten in oligosaccharides mit hilfe von periodat. Chem. Ber 1965, 98. [Google Scholar]
- 67.Donald AS; Feeney J Separation of human milk oligosaccharides by recycling chromatography. First isolation of lacto-N-neo-difucohexaose II and 3’-galactosyllactose from this source. Carbohydr Res 1988, 178, 79–91. [DOI] [PubMed] [Google Scholar]
- 68.Sugawara M; Idota T A new oligosaccharide 4’-galactosyllactose in human milk. Proceedings of the Annual Meeting of Japan Society for Bioscience, Biotechnology, and Agrochemistry. Sappro, Japan, p. 132. 1995. [Google Scholar]
- 69.Yamashita K; Kobata A Oligosaccharides of human milk V. Isolation and characterization of a new trisaccharide, 6’-galactosyllactose. Arch Biochem Biophys 1974, 161, 164–170. [Google Scholar]
- 70.Erney R; Hilty M; Pickering L; Ruiz-Palacios G; Prieto P Human milk oligosaccharides: a novel method provides insight into human genetics. Adv Exp Med Biol 2001, 501, 285–297. [PubMed] [Google Scholar]
- 71.Shen L; Grollman EF; Ginsburg V An enzymatic basis for secretor status and blood group substance specificity in humans. Proc Natl Acad Sci U S A 1968, 59, 224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grollman EF; Ginsburg V Correlation between secretor status and the occurrence of 2’-fucosyllactose in human milk. Biochem Biophys Res Commun 1967, 28, 50–53. [DOI] [PubMed] [Google Scholar]
- 73.Kobata A; Ginsburg V; Tsuda M Oligosaccharides of human milk. I. Isolation and characterization. Arch Biochem Biophys 1969, 130, 509–513. [DOI] [PubMed] [Google Scholar]
- 74.Grollman EF; Kobata A; Ginsburg V An enzymatic basis for Lewis blood types in man. J Clin Invest 1969, 48, 1489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Totten SM; Zivkovic AM; Wu S; Ngyuen U; Freeman SL; Ruhaak LR; Darboe MK; German JB; Prentice AM; Lebrilla CB Comprehensive profiles of human milk oligosaccharides yield highly sensitive and specific markers for determining secretor status in lactating mothers. J Proteome Res 2012, 11, 6124–6133. [DOI] [PubMed] [Google Scholar]
- 76.Dabrowski U; Egge H; Dabrowski J Proton-nuclear magnetic resonance study of peracetylated derivatives of ten oligosaccharides isolated from human milk. Arch Biochem Biophys 1983, 224, 254–260. [DOI] [PubMed] [Google Scholar]
- 77.Kuhn R; Gauhe A Uber die lacto-difuco-tetraose der Frauenmilch. Justus Liebigs Ann. Chem 1958, 611, 249–252. [Google Scholar]
- 78.Kuhn R; Brossmer R Uber das durch Viren der influenza-gruppe spaltbare trisaccharid der milch. Chem. Ber 1959, 92. [Google Scholar]
- 79.Kuhn R Biochemie der rezeptoren und resistenzfaktoren. Von der widerstandsfahigkeit der lebewesen gegen einwirkungen der umwelt. Naturwissenschaften 1959, 46, 43–50. [Google Scholar]
- 80.Gronberg G; Lipniunas P; Lundgren T; Erlansson K; Lindh F; Nilsson B Isolation of monosialyated oligosaccharides from human milk and structural analysis of three new compounds. Carbohydr Res 1989, 191, 261–278. [DOI] [PubMed] [Google Scholar]
- 81.Kuhn R; Baer HH; Gauhe A Die Konstitution der lacto-N-fucopentaose II. Chem. Ber 1958, 91, 364. [Google Scholar]
- 82.Ginsburg V; Zopf DA; Yamashita K; Kobata A Oligosaccharides of human milk. Isolation of a new pentasaccharide, lacto-N-fucopentaose V. Arch Biochem Biophys 1976, 175, 565–568. [DOI] [PubMed] [Google Scholar]
- 83.Kuhn R; Baer HH; Gauhe A 2-alpha-L-Fucopyranosyl-D-galaktose und 2-alpha-L-fucopyranosyl-D-talose. . Justus Liebigs Ann. Chem 1958, 611, 242–249. [Google Scholar]
- 84.Kuhn R; Gauhe A Uber ein kristallisiertes, Lea-aktives hexasaccharid aus frauenmilch. Chem. Ber 1960, 93, 647–651. [Google Scholar]
- 85.Grimmonprez L; Montreuil J [Physico-chemical study of 6 new oligosides isolated from human milk]. Bull Soc Chim Biol (Paris) 1968, 50, 843–855. [PubMed] [Google Scholar]
- 86.Wieruszeski JM; Chekkor A; Bouquelet S; Montreuil J; Strecker G; Peter-Katalinic J; Egge H Structure of two new oligosaccharides isolated from human milk: sialylated lacto-N-fucopentaoses I and II. Carbohydr Res 1985, 137, 127–138. [DOI] [PubMed] [Google Scholar]
- 87.Gronberg G; Lipniunas P; Lundgren T; Lindh F; Nilsson B Isolation and structural analysis of three new disialylated oligosaccharides from human milk. Arch Biochem Biophys 1990, 278, 297–311. [DOI] [PubMed] [Google Scholar]
- 88.Kobata A; Ginsburg V Oligosaccharides of human milk. II. Isolation and characterization of a new pentasaccharide, lacto-N-fucopentaose 3. J Biol Chem 1969, 244, 5496–5502. [PubMed] [Google Scholar]
- 89.Perret S; Sabin C; Dumon C; Pokorna M; Gautier C; Galanina O; Ilia S; Bovin N; Nicaise M; Desmadril M; Gilboa-Garber N; Wimmerova M; Mitchell EP; Imberty A Structural basis for the interaction between human milk oligosaccharides and the bacterial lectin PA-IIL of Pseudomonas aeruginosa. Biochem J 2005, 389, 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kuhn R; Gauhe A Uber drei saure pentasaccharide aus frauenmilch. Chem. Ber 1962, 95, 513–517. [Google Scholar]
- 91.Smith DF; Prieto PA; McCrumb DK; Wang WC A novel sialylfucopentaose in human milk. Presence of this oligosaccharide is not dependent on expression of the secretor or Lewis fucosyltransferases. J Biol Chem 1987, 262, 12040–12047. [PubMed] [Google Scholar]
- 92.Yamashita K; Tachibana Y; Kobata A Oligosaccharides of human milk: structures of three lacto-N-hexaose derivatives with H-haptenic structure. Arch Biochem Biophys 1977, 182, 546–555. [DOI] [PubMed] [Google Scholar]
- 93.Dua VK; Goso K; Dube VE; Bush CA Characterization of lacto-N-hexaose and two fucosylated derivatives from human milk by high-performance liquid chromatography and proton NMR spectroscopy. J Chromatogr 1985, 328, 259–269. [DOI] [PubMed] [Google Scholar]
- 94.Kitagawa H; Takaoka M; Nakada H; Fukui S; Funakoshi I; Kawasaki T; Tate S; Inagaki F; Yamashina I Isolation and structural studies of human milk oligosaccharides that are reactive with a monoclonal antibody MSW 113. J Biochem 1991, 110, 598–604. [DOI] [PubMed] [Google Scholar]
- 95.Fievre S; Wieruszeski JM; Michalski JC; Lemoine J; Montreuil J; Strecker G Primary structure of a trisialylated oligosaccharide from human milk. Biochem Biophys Res Commun 1991, 177, 720–725. [DOI] [PubMed] [Google Scholar]
- 96.Gronberg G; Lipniunas P; Lundgren T; Lindh F; Nilsson B Structural analysis of five new monosialylated oligosaccharides from human milk. Arch Biochem Biophys 1992, 296, 597–610. [DOI] [PubMed] [Google Scholar]
- 97.Kitagawa H; Nakada H; Kurosaka A; Hiraiwa N; Numata Y; Fukui S; Funakoshi I; Kawasaki T; Yamashina I; Shimada I; et al. Three novel oligosaccharides with the sialyl-Lea structure in human milk: isolation by immunoaffinity chromatography. Biochemistry 1989, 28, 8891–8897. [DOI] [PubMed] [Google Scholar]
- 98.Yamashita K; Tachibana Y; Kobata A Oligosaccharides of human milk. Isolation and characterization of three new disialyfucosyl hexasaccharides. Arch Biochem Biophys 1976, 174, 582–591. [DOI] [PubMed] [Google Scholar]
- 99.Pfenninger A; Karas M; Finke B; Stahl B Structural analysis of underivatized neutral human milk oligosaccharides in the negative ion mode by nano-electrospray MS(n) (part 2: application to isomeric mixtures). J Am Soc Mass Spectrom 2002, 13, 1341–1348. [DOI] [PubMed] [Google Scholar]
- 100.Bruntz R; Dabrowski U; Dabrowski J; Ebersold A; Peter-Katalinic J; Egge H Fucose-containing oligosaccharides from human milk from a donor of blood group 0 Le(a) nonsecretor. Biol Chem Hoppe Seyler 1988, 369, 257–273. [PubMed] [Google Scholar]
- 101.Strecker G; Wieruszeski JM; Michalski JC; Montreuil J Structure of a new nonasaccharide isolated form human milk: VI2-Fuc, V4Fuc, III3Fuc-p-lacto-N-hexaose. . Glycoconj. J 1988, 5, 385–396. [Google Scholar]
- 102.Yamashita K; Tachibana Y; Kobata A Oligosaccharides of human milk: isolation and characterization of two new nonasaccharides, monofucosyllacto-N-octaose and monofucosyllacto-N-neooctaose. Biochemistry 1976, 15, 3950–3955. [DOI] [PubMed] [Google Scholar]
- 103.Kitagawa H; Nakada H; Fukui S; Funakoshi I; Kawasaki T; Yamashina I; Tate S; Inagaki F Novel oligosaccharides with the sialyl-Le(a) structure in human milk. J Biochem 1993, 114, 504–508. [DOI] [PubMed] [Google Scholar]
- 104.Kitagawa H; Nakada H; Fukui S; Funakoshi I; Kawasaki T; Yamashina I; Tate S; Inagaki F Novel oligosaccharides with the sialyl-Lea structure in human milk. Biochemistry 1991, 30, 2869–2876. [DOI] [PubMed] [Google Scholar]
- 105.Kogelberg H; Piskarev VE; Zhang Y; Lawson AM; Chai W Determination by electrospray mass spectrometry and 1H-NMR spectroscopy of primary structures of variously fucosylated neutral oligosaccharides based on the iso-lacto-N-octaose core. Eur J Biochem 2004, 271, 1172–1186. [DOI] [PubMed] [Google Scholar]
- 106.Strecker G; Wieruszeski JM; Michalski JC; Montreuil J Primary structure of human milk nona- and decasaccharides determined by a combination of fast atom bombardment mass spectrometry and 1H-/13C-nuclear magnetic resonance spectroscopy. Evidence for a new core structure, iso-lacto-N-octaose. Glycoconj J 1989, 6, 169–182. [DOI] [PubMed] [Google Scholar]
- 107.Chai W; Piskarev VE; Zhang Y; Lawson AM; Kogelberg H Structural determination of novel lacto-N-decaose and its monofucosylated analogue from human milk by electrospray tandem mass spectrometry and 1H NMR spectroscopy. Arch Biochem Biophys 2005, 434, 116–127. [DOI] [PubMed] [Google Scholar]
- 108.Lundblad A; Hallgren P; Rudmark A; Svensson S Structures and serological activities of three oligosaccharides isolated from urines of nonstarved secretors and from secretors on lactose diet. Biochemistry 1973, 12, 3341–3345. [DOI] [PubMed] [Google Scholar]
- 109.Strecker G; Montruil J [Isolation and structural study of 16 oligosaccharides isolated from human urine]. C R Acad Sci Hebd Seances Acad Sci D 1973, 277, 1393–1396. [PubMed] [Google Scholar]
- 110.Lundblad A; Svensson S Letters: The structure of a urinary difucosyl pentasaccharide, characteristic of secretors with the blood-group A gene. Carbohydr Res 1973, 30, 187–189. [DOI] [PubMed] [Google Scholar]
- 111.Kitagawa H; Nakada H; Numata Y; Kurosaka A; Fukui S; Funakoshi I; Kawasaki T; Shimada I; Inagaki F; Yamashina I Occurrence of tetra- and pentasaccharides with the sialyl-Le(a) structure in human milk. J Biol Chem 1990, 265, 4859–4862. [PubMed] [Google Scholar]
- 112.Stahl B; Thurl S; Zeng J; Karas M; Hillenkamp F; Steup M; Sawatzki G Oligosaccharides from human milk as revealed by matrix-assisted laser desorption/ionization mass spectrometry. Anal Biochem 1994, 223, 218–226. [DOI] [PubMed] [Google Scholar]
- 113.Ballard O; Morrow AL Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am 2013, 60, 49–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Boehm G; Stahl B Functional dairy products. Mattila-Sandholm T, Ed. Cambridge, UK. Woodhead Publishers, pp 203. 2003. [Google Scholar]
- 115.Thurl S; Muller-Werner B; Sawatzki G Quantification of individual oligosaccharide compounds from human milk using high-pH anion-exchange chromatography. Anal Biochem 1996, 235, 202–206. [DOI] [PubMed] [Google Scholar]
- 116.Asakuma S; Urashima T; Akahori M; Obayashi H; Nakamura T; Kimura K; Watanabe Y; Arai I; Sanai Y Variation of major neutral oligosaccharides levels in human colostrum. Eur J Clin Nutr 2008, 62, 488–494. [DOI] [PubMed] [Google Scholar]
- 117.Asakuma S; Akahori M; Kimura K; Watanabe Y; Nakamura T; Tsunemi M; Arai I; Sanai Y; Urashima T Sialyl oligosaccharides of human colostrum: changes in concentration during the first three days of lactation. Biosci Biotechnol Biochem 2007, 71, 1447–1451. [DOI] [PubMed] [Google Scholar]
- 118.Tao N; DePeters EJ; Freeman S; German JB; Grimm R; Lebrilla CB Bovine milk glycome. J Dairy Sci 2008, 91, 3768–3778. [DOI] [PubMed] [Google Scholar]
- 119.Tao N; DePeters EJ; German JB; Grimm R; Lebrilla CB Variations in bovine milk oligosaccharides during early and middle lactation stages analyzed by high-performance liquid chromatography-chip/mass spectrometry. J Dairy Sci 2009, 92, 2991–3001. [DOI] [PubMed] [Google Scholar]
- 120.Tao N; Ochonicky KL; German JB; Donovan SM; Lebrilla CB Structural determination and daily variations of porcine milk oligosaccharides. J Agric Food Chem 2010, 58, 4653–4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Urashima T; Kawai Y; Nakamura T; Arai I; Saito T; Namiki M; Yamaoka K; Kawahawa K; Messer M Chemical characterisation of six oligosaccharides in a sample of colostrum of the brown capuchin, Cebus apella (Cebidae: primates). Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 1999, 124, 295–300. [DOI] [PubMed] [Google Scholar]
- 122.Oftedal OT; Nicol SC; Davies NW; Sekii N; Taufik E; Fukuda K; Saito T; Urashima T Can an ancestral condition for milk oligosaccharides be determined? Evidence from the Tasmanian echidna (Tachyglossus aculeatus setosus). Glycobiology 2014, 24, 826–839. [DOI] [PubMed] [Google Scholar]
- 123.Messer M; Kerry KR Milk carbohydrates of the echidna and the platypus. Science 1973, 180, 201–203. [DOI] [PubMed] [Google Scholar]
- 124.Messer M; Gadiel PA; Ralston GB; Griffiths M Carbohydrates of the milk of the platypus. Aust J Biol Sci 1983, 36, 129–137. [DOI] [PubMed] [Google Scholar]
- 125.Amano J; Messer M; Kobata A Structures of the oligosaccharides isolated from milk of the platypus. Glycoconj. J 1985, 2, 121–135. [Google Scholar]
- 126.Urashima T; Inamori H; Fukuda K; Saito T; Messer M; Oftedal OT 4-O-Acetyl-sialic acid (Neu4,5Ac2) in acidic milk oligosaccharides of the platypus (Ornithorhynchus anatinus) and its evolutionary significance. Glycobiology 2015, 25, 683–697. [DOI] [PubMed] [Google Scholar]
- 127.Urashima T; Fujita S; Fukuda K; Nakamura T; Saito T; Cowan P; Messer M Chemical characterization of milk oligosaccharides of the common brushtail possum (Trichosurus vulpecula). Glycoconj J 2014, 31, 387–399. [DOI] [PubMed] [Google Scholar]
- 128.Anraku T; Fukuda K; Saito T; Messer M; Urashima T Chemical characterization of acidic oligosaccharides in milk of the red kangaroo (Macropus rufus). Glycoconj J 2012, 29, 147–156. [DOI] [PubMed] [Google Scholar]
- 129.Urashima T; Taufik E; Fukuda R; Nakamura T; Fukuda K; Saito T; Messer M Chemical characterization of milk oligosaccharides of the koala (Phascolarctos cinereus). Glycoconj J 2013, 30, 801–811. [DOI] [PubMed] [Google Scholar]
- 130.Grollman EF; Kobata A; Ginsburg V Enzymatic basis of blood types in man. Ann N Y Acad Sci 1970, 169, 153–160. [DOI] [PubMed] [Google Scholar]
- 131.Grimmonprez L; Montreuil J [Isolation and physico-chemical properties of oligosaccharides of human milk]. Biochimie 1975, 57, 695–671. [DOI] [PubMed] [Google Scholar]
- 132.Newburg DS Human milk glycoconjugates that inhibit pathogens. Curr Med Chem 1999, 6, 117–127. [PubMed] [Google Scholar]
- 133.Bertino E; Peila C; Giuliani F; Martano C; Cresi F; Di Nicola P; Occhi L; Sabatino G; Fabris C Metabolism and biological functions of human milk oligosaccharides. J Biol Regul Homeost Agents 2012, 26, 35–38. [PubMed] [Google Scholar]
- 134.Sunehag AL; Louie K; Bier JL; Tigas S; Haymond MW Hexoneogenesis in the human breast during lactation. J Clin Endocrinol Metab 2002, 87, 297–301. [DOI] [PubMed] [Google Scholar]
- 135.Mohammad MA; Maningat P; Sunehag AL; Haymond MW Precursors of hexoneogenesis within the human mammary gland. Am J Physiol Endocrinol Metab 2015, 308, E680–E687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sasaki M; Eigel WN; Keenan TW Lactose and major milk proteins are present in secretory vesicle-rich fractions from lactating mammary gland. Proc Natl Acad Sci U S A 1978, 75, 5020–5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ramakrishnan B; Qasba PK Crystal structure of lactose synthase reveals a large conformational change in its catalytic component, the beta1,4-galactosyltransferase-I. J Mol Biol 2001, 310, 205–218. [DOI] [PubMed] [Google Scholar]
- 138.Brodbeck U; Denton WL; Tanahashi N; Ebner KE The isolation and identification of the B protein of lactose synthetase as alpha-lactalbumin. J Biol Chem 1967, 242, 1391–1397. [PubMed] [Google Scholar]
- 139.Brew K; Hill RL Lactose biosynthesis. Rev Physiol Biochem Pharmacol 1975, 72, 105–158. [DOI] [PubMed] [Google Scholar]
- 140.Kumazaki T; Yoshida A Biochemical evidence that secretor gene, Se, is a structural gene encoding a specific fucosyltransferase. Proc Natl Acad Sci U S A 1984, 81, 4193–4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ceppellini R On the genetics of secretor and Lewis characters: A family study. Proc. Fifth Congr. Intern. Soc. Blood Transfusion, Paris, 1954. 1955, 207–211. [Google Scholar]
- 142.Prieto PA; Mukerji P; Kelder B; Erney R; Gonzalez D; Yun JS; Smith DF; Moremen KW; Nardelli C; Pierce M; et al. Remodeling of mouse milk glycoconjugates by transgenic expression of a human glycosyltransferase. J Biol Chem 1995, 270, 29515–29519. [DOI] [PubMed] [Google Scholar]
- 143.Kelder B; Erney R; Kopchick J; Cummings R; Prieto P Glycoconjugates in human and transgenic animal milk. Adv Exp Med Biol 2001, 501, 269–278. [DOI] [PubMed] [Google Scholar]
- 144.Appert HE; Rutherford TJ; Tarr GE; Thomford NR; McCorquodale DJ Isolation of galactosyltransferase from human milk and the determination of its N-terminal amino acid sequence. Biochem Biophys Res Commun 1986, 138, 224–229. [DOI] [PubMed] [Google Scholar]
- 145.Endo T; Amano J; Berger EG; Kobata A Structure identification of the complex-type, asparagine-linked sugar chains of beta-D-galactosyl-transferase purified from human milk. Carbohydr Res 1986, 150, 241–263. [DOI] [PubMed] [Google Scholar]
- 146.Prieels JP; Monnom D; Dolmans M; Beyer TA; Hill RL Co-purification of the Lewis blood group N-acetylglucosaminide alpha 1 goes to 4 fucosyltransferase and an N-acetylglucosaminide alpha 1 goes to 3 fucosyltransferase from human milk. J Biol Chem 1981, 256, 10456–10463. [PubMed] [Google Scholar]
- 147.Eppenberger-Castori S; Lotscher H; Finne J Purification of the N-acetylglucosaminide alpha(1–3/4)fucosyltransferase of human milk. Glycoconj J 1989, 6, 101–114. [DOI] [PubMed] [Google Scholar]
- 148.Johnson PH; Watkins WM Purification of the Lewis blood-group gene associated alpha-3/4-fucosyltransferase from human milk: an enzyme transferring fucose primarily to type 1 and lactose-based oligosaccharide chains. Glycoconj J 1992, 9, 241–249. [DOI] [PubMed] [Google Scholar]
- 149.Johnson PH; Donald AS; Feeney J; Watkins WM Reassessment of the acceptor specificity and general properties of the Lewis blood-group gene associated alpha-3/4-fucosyltransferase purified from human milk. Glycoconj J 1992, 9, 251–264. [DOI] [PubMed] [Google Scholar]
- 150.Hosomi O; Takeya A The relationship between the (beta 1–3) N-acetylglucosaminyltransferase and the presence of oligosaccharides containing lacto-N-triose II structure in bovine and human milk. Nihon Juigaku Zasshi 1989, 51, 1–6. [DOI] [PubMed] [Google Scholar]
- 151.Wiederschain GY; Newburg DS Compartmentalization of fucosyltransferase and alpha-L-fucosidase in human milk. Biochem Mol Med 1996, 58, 211–220. [DOI] [PubMed] [Google Scholar]
- 152.Palcic MM; Venot AP; Ratcliffe RM; Hindsgaul O Enzymic synthesis of oligosaccharides terminating in the tumor-associated sialyl-Lewis-a determinant. Carbohydr Res 1989, 190, 1–11. [DOI] [PubMed] [Google Scholar]
- 153.Stangier K; Palcic MM; Bundle DR; Hindsgaul O; Thiem J Fucosyltransferase-catalyzed formation of L-galactosylated Lewis structures. Carbohydr Res 1997, 305, 511–515. [DOI] [PubMed] [Google Scholar]
- 154.Du M; Hindsgaul O Recognition of beta-D-Gal p-(1-->3)-beta-D-Glc pNAc-OR acceptor analogues by the Lewis alpha-(1-->3/4)-fucosyltransferase from human milk. Carbohydr Res 1996, 286, 87–105. [DOI] [PubMed] [Google Scholar]
- 155.Lubineau A; Auge C; Le Goff N; Le Narvor C Chemoenzymatic synthesis of a 3IV,6III-disulfated Lewis(x) pentasaccharide, a candidate ligand for human L-selectin. Carbohydr Res 1997, 305, 501–509. [DOI] [PubMed] [Google Scholar]
- 156.Chiu MH; Thomas VH; Stubbs HJ; Rice KG Tissue targeting of multivalent Le(x)-terminated N-linked oligosaccharides in mice. J Biol Chem 1995, 270, 24024–24031. [DOI] [PubMed] [Google Scholar]
- 157.Thomas VH; Elhalabi J; Rice KG Enzymatic synthesis of N-linked oligosaccharides terminating in multiple sialyl-Lewis(x) and GalNAc-Lewis(x) determinants: clustered glycosides for studying selectin interactions. Carbohydr Res 1998, 306, 387–400. [DOI] [PubMed] [Google Scholar]
- 158.de Vries T; Norberg T; Lonn H; Van den Eijnden DH The use of human milk fucosyltransferase in the synthesis of tumor-associated trimeric X determinants. Eur J Biochem 1993, 216, 769–777. [DOI] [PubMed] [Google Scholar]
- 159.de Vries T; van den Eijnden DH Biosynthesis of sialyl-oligomeric-Lewisx and VIM-2 epitopes: site specificity of human milk fucosyltransferase. Biochemistry 1994, 33, 9937–9944. [DOI] [PubMed] [Google Scholar]
- 160.Nikrad PV; Kashem MA; Wlasichuk KB; Alton G; Venot AP Use of human-milk fucosyltransferase in the chemoenzymic synthesis of analogues of the sialyl Lewis(a) and sialyl Lewis(x) tetrasaccharides modified at the C-2 position of the reducing unit. Carbohydr Res 1993, 250, 145–160. [DOI] [PubMed] [Google Scholar]
- 161.Natunen J; Niemela R; Penttila L; Seppo A; Ruohtula T; Renkonen O Enzymatic synthesis of two lacto-N-neohexaose-related Lewis x heptasaccharides and their separation by chromatography on immobilized wheat germ agglutinin. Glycobiology 1994, 4, 577–583. [DOI] [PubMed] [Google Scholar]
- 162.Niemela R; Natunen J; Brotherus E; Saarikangas A; Renkonen O alpha 1,3-Fucosylation of branched blood group I-type oligo-(N-acetyllactosamino)glycans by human milk transferases is restricted to distal N-acetyllactosamine units: the resulting isomers are separated by WGA-agarose chromatography. Glycoconj J 1995, 12, 36–44. [DOI] [PubMed] [Google Scholar]
- 163.Natunen J; Aitio O; Helin J; Maaheimo H; Niemela R; Heikkinen S; Renkonen O Human alpha3-fucosyltransferases convert chitin oligosaccharides to products containing a GlcNAcbeta1–4(Fucalpha1–3)GlcNAcbeta1–4R determinant at the nonreducing terminus. Glycobiology 2001, 11, 209–216. [DOI] [PubMed] [Google Scholar]
- 164.Zeng S; Gallego RG; Dinter A; Malissard M; Kamerling JP; Vliegenthart JF; Berger EG Complete enzymic synthesis of the mucin-type sialyl Lewis x epitope, involved in the interaction between PSGL-1 and P-selectin. Glycoconj J 1999, 16, 487–497. [DOI] [PubMed] [Google Scholar]
- 165.Di Virgilio S; Glushka J; Moremen K; Pierce M Enzymatic synthesis of natural and 13C enriched linear poly-N-acetyllactosamines as ligands for galectin-1. Glycobiology 1999, 9, 353–364. [DOI] [PubMed] [Google Scholar]
- 166.Srivastava G; Alton G; Hindsgaul O Combined chemical-enzymic synthesis of deoxygenated oligosaccharide analogs: transfer of deoxygenated D-GlcpNAc residues from their UDP-GlcpNAc derivatives using N-acetylglucosaminyltransferase I. Carbohydr Res 1990, 207, 259–276. [DOI] [PubMed] [Google Scholar]
- 167.Alton G; Srivastava G; Kaur KJ; Hindsgaul O Use of N-acetylglucosaminyltransferases I and II in the synthesis of a dideoxypentasaccharide. Bioorg Med Chem 1994, 2, 675–680. [DOI] [PubMed] [Google Scholar]
- 168.Bode L; Jantscher-Krenn E Structure-function relationships of human milk oligosaccharides. Adv Nutr 2012, 3, 383S–391S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jantscher-Krenn E; Bode L Human milk oligosaccharides and their potential benefits for the breast-fed neonate. Minerva Pediatr 2012, 64, 83–99. [PubMed] [Google Scholar]
- 170.Newburg DS; Morelli L Human milk and infant intestinal mucosal glycans guide succession of the neonatal intestinal microbiota. Pediatr Res 2015, 77, 115–120. [DOI] [PubMed] [Google Scholar]
- 171.Rudloff S; Kunz C Milk oligosaccharides and metabolism in infants. Adv Nutr 2012, 3, 398S–405S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kunz C; Rudloff S Potential anti-inflammatory and anti-infectious effects of human milk oligosaccharides. Adv Exp Med Biol 2008, 606, 455–465. [DOI] [PubMed] [Google Scholar]
- 173.Zopf D; Roth S Oligosaccharide anti-infective agents. Lancet 1996, 347, 1017–1021. [DOI] [PubMed] [Google Scholar]
- 174.Harmsen HJ; Wildeboer-Veloo AC; Raangs GC; Wagendorp AA; Klijn N; Bindels JG; Welling GW Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr 2000, 30, 61–67. [DOI] [PubMed] [Google Scholar]
- 175.Garrido D; Barile D; Mills DA A molecular basis for bifidobacterial enrichment in the infant gastrointestinal tract. Adv Nutr 2012, 3, 415S–421S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bode L; Kunz C; Muhly-Reinholz M; Mayer K; Seeger W; Rudloff S Inhibition of monocyte, lymphocyte, and neutrophil adhesion to endothelial cells by human milk oligosaccharides. Thromb Haemost 2004, 92, 1402–1410. [DOI] [PubMed] [Google Scholar]
- 177.Chichlowski M; German JB; Lebrilla CB; Mills DA The influence of milk oligosaccharides on microbiota of infants: opportunities for formulas. Annu Rev Food Sci Technol 2011, 2, 331–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bienenstock J; Buck RH; Linke H; Forsythe P; Stanisz AM; Kunze WA Fucosylated but not sialylated milk oligosaccharides diminish colon motor contractions. PLoS One 2013, 8, e76236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gyorgy P; Jeanloz RW; von Nicolai H; Zilliken F Undialyzable growth factors for Lactobacillus bifidus var. pennsylvanicus. Protective effect of sialic acid bound to glycoproteins and oligosaccharides against bacterial degradation. Eur J Biochem 1974, 43, 29–33. [DOI] [PubMed] [Google Scholar]
- 180.Xiao JZ; Takahashi S; Nishimoto M; Odamaki T; Yaeshima T; Iwatsuki K; Kitaoka M Distribution of in vitro fermentation ability of lacto-N-biose I, a major building block of human milk oligosaccharides, in bifidobacterial strains. Appl Environ Microbiol 2010, 76, 54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kiyohara M; Tachizawa A; Nishimoto M; Kitaoka M; Ashida H; Yamamoto K Prebiotic effect of lacto-N-biose I on bifidobacterial growth. Biosci Biotechnol Biochem 2009, 73, 1175–1179. [DOI] [PubMed] [Google Scholar]
- 182.Asakuma S; Hatakeyama E; Urashima T; Yoshida E; Katayama T; Yamamoto K; Kumagai H; Ashida H; Hirose J; Kitaoka M Physiology of consumption of human milk oligosaccharides by infant gut-associated bifidobacteria. J Biol Chem 2011, 286, 34583–34592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Katayama T; Sakuma A; Kimura T; Makimura Y; Hiratake J; Sakata K; Yamanoi T; Kumagai H; Yamamoto K Molecular cloning and characterization of Bifidobacterium bifidum 1,2-alpha-L-fucosidase (AfcA), a novel inverting glycosidase (glycoside hydrolase family 95). J Bacteriol 2004, 186, 4885–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ashida H; Miyake A; Kiyohara M; Wada J; Yoshida E; Kumagai H; Katayama T; Yamamoto K Two distinct alpha-L-fucosidases from Bifidobacterium bifidum are essential for the utilization of fucosylated milk oligosaccharides and glycoconjugates. Glycobiology 2009, 19, 1010–1017. [DOI] [PubMed] [Google Scholar]
- 185.Kiyohara M; Tanigawa K; Chaiwangsri T; Katayama T; Ashida H; Yamamoto K An exo-alpha-sialidase from bifidobacteria involved in the degradation of sialyloligosaccharides in human milk and intestinal glycoconjugates. Glycobiology 2011, 21, 437–447. [DOI] [PubMed] [Google Scholar]
- 186.Wada J; Ando T; Kiyohara M; Ashida H; Kitaoka M; Yamaguchi M; Kumagai H; Katayama T; Yamamoto K Bifidobacterium bifidum lacto-N-biosidase, a critical enzyme for the degradation of human milk oligosaccharides with a type 1 structure. Appl Environ Microbiol 2008, 74, 3996–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Suzuki R; Wada J; Katayama T; Fushinobu S; Wakagi T; Shoun H; Sugimoto H; Tanaka A; Kumagai H; Ashida H; Kitaoka M; Yamamoto K Structural and thermodynamic analyses of solute-binding Protein from Bifidobacterium longum specific for core 1 disaccharide and lacto-N-biose I. J Biol Chem 2008, 283, 13165–13173. [DOI] [PubMed] [Google Scholar]
- 188.Miwa M; Horimoto T; Kiyohara M; Katayama T; Kitaoka M; Ashida H; Yamamoto K Cooperation of beta-galactosidase and beta-N-acetylhexosaminidase from bifidobacteria in assimilation of human milk oligosaccharides with type 2 structure. Glycobiology 2010, 20, 1402–1409. [DOI] [PubMed] [Google Scholar]
- 189.Marcobal A; Barboza M; Sonnenburg ED; Pudlo N; Martens EC; Desai P; Lebrilla CB; Weimer BC; Mills DA; German JB; Sonnenburg JL Bacteroides in the infant gut consume milk oligosaccharides via mucus-utilization pathways. Cell Host Microbe 2011, 10, 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sela DA; Garrido D; Lerno L; Wu S; Tan K; Eom HJ; Joachimiak A; Lebrilla CB; Mills DA Bifidobacterium longum subsp. infantis ATCC 15697 alpha-fucosidases are active on fucosylated human milk oligosaccharides. Appl Environ Microbiol 2012, 78, 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Garrido D; Ruiz-Moyano S; Mills DA Release and utilization of N-acetyl-D-glucosamine from human milk oligosaccharides by Bifidobacterium longum subsp. infantis. Anaerobe 2012, 18, 430–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sela DA; Li Y; Lerno L; Wu S; Marcobal AM; German JB; Chen X; Lebrilla CB; Mills DA An infant-associated bacterial commensal utilizes breast milk sialyloligosaccharides. J Biol Chem 2011, 286, 11909–11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Garrido D; Kim JH; German JB; Raybould HE; Mills DA Oligosaccharide binding proteins from Bifidobacterium longum subsp. infantis reveal a preference for host glycans. PLoS One 2011, 6, e17315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jantscher-Krenn E; Lauwaet T; Bliss LA; Reed SL; Gillin FD; Bode L Human milk oligosaccharides reduce Entamoeba histolytica attachment and cytotoxicity in vitro. Br J Nutr 2012, 108, 1839–1846. [DOI] [PubMed] [Google Scholar]
- 195.Terrazas LI; Walsh KL; Piskorska D; McGuire E; Harn DA Jr. The schistosome oligosaccharide lacto-N-neotetraose expands Gr1(+) cells that secrete anti-inflammatory cytokines and inhibit proliferation of naive CD4(+) cells: a potential mechanism for immune polarization in helminth infections. J Immunol 2001, 167, 5294–303. [DOI] [PubMed] [Google Scholar]
- 196.Tong HH; McIver MA; Fisher LM; DeMaria TF Effect of lacto-N-neotetraose, asialoganglioside-GM1 and neuraminidase on adherence of otitis media-associated serotypes of Streptococcus pneumoniae to chinchilla tracheal epithelium. Microb Pathog 1999, 26, 111–119. [DOI] [PubMed] [Google Scholar]
- 197.Bode L; Kuhn L; Kim HY; Hsiao L; Nissan C; Sinkala M; Kankasa C; Mwiya M; Thea DM; Aldrovandi GM Human milk oligosaccharide concentration and risk of postnatal transmission of HIV through breastfeeding. Am J Clin Nutr 2012, 96, 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Idanpaan-Heikkila I; Simon PM; Zopf D; Vullo T; Cahill P; Sokol K; Tuomanen E Oligosaccharides interfere with the establishment and progression of experimental pneumococcal pneumonia. J Infect Dis 1997, 176, 704–712. [DOI] [PubMed] [Google Scholar]
- 199.Yu ZT; Chen C; Kling DE; Liu B; McCoy JM; Merighi M; Heidtman M; Newburg DS The principal fucosylated oligosaccharides of human milk exhibit prebiotic properties on cultured infant microbiota. Glycobiology 2013, 23, 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Huang P; Farkas T; Marionneau S; Zhong W; Ruvoen-Clouet N; Morrow AL; Altaye M; Pickering LK; Newburg DS; LePendu J; Jiang X Noroviruses bind to human ABO, Lewis, and secretor histo-blood group antigens: identification of 4 distinct strain-specific patterns. J Infect Dis 2003, 188, 19–31. [DOI] [PubMed] [Google Scholar]
- 201.Cravioto A; Tello A; Villafan H; Ruiz J; del Vedovo S; Neeser JR Inhibition of localized adhesion of enteropathogenic Escherichia coli to HEp-2 cells by immunoglobulin and oligosaccharide fractions of human colostrum and breast milk. J Infect Dis 1991, 163, 1247–1255. [DOI] [PubMed] [Google Scholar]
- 202.Newburg DS; Pickering LK; McCluer RH; Cleary TG Fucosylated oligosaccharides of human milk protect suckling mice from heat-stabile enterotoxin of Escherichia coli. J Infect Dis 1990, 162, 1075–80. [DOI] [PubMed] [Google Scholar]
- 203.Chessa D; Winter MG; Jakomin M; Baumler AJ Salmonella enterica serotype Typhimurium Std fimbriae bind terminal alpha(1,2)fucose residues in the cecal mucosa. Mol Microbiol 2009, 71, 864–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ruiz-Palacios GM; Cervantes LE; Ramos P; Chavez-Munguia B; Newburg DS Campylobacter jejuni binds intestinal H(O) antigen (Fuc alpha 1, 2Gal beta 1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J Biol Chem 2003, 278, 14112–14120. [DOI] [PubMed] [Google Scholar]
- 205.Morrow AL; Ruiz-Palacios GM; Altaye M; Jiang X; Guerrero ML; Meinzen-Derr JK; Farkas T; Chaturvedi P; Pickering LK; Newburg DS Human milk oligosaccharides are associated with protection against diarrhea in breast-fed infants. J Pediatr 2004, 145, 297–303. [DOI] [PubMed] [Google Scholar]
- 206.Brassart D; Woltz A; Golliard M; Neeser JR In vitro inhibition of adhesion of Candida albicans clinical isolates to human buccal epithelial cells by Fuc alpha 1–2Gal beta-bearing complex carbohydrates. Infect Immun 1991, 59, 1605–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hong P; Ninonuevo MR; Lee B; Lebrilla C; Bode L Human milk oligosaccharides reduce HIV-1-gp120 binding to dendritic cell-specific ICAM3-grabbing non-integrin (DC-SIGN). Br J Nutr 2009, 101, 482–486. [DOI] [PubMed] [Google Scholar]
- 208.Kuhn L; Kim HY; Hsiao L; Nissan C; Kankasa C; Mwiya M; Thea DM; Aldrovandi GM; Bode L Oligosaccharide composition of breast milk influences survival of uninfected children born to HIV-infected mothers in Lusaka, Zambia. J Nutr 2015, 145, 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Xu HT; Zhao YF; Lian ZX; Fan BL; Zhao ZH; Yu SY; Dai YP; Wang LL; Niu HL; Li N; Hammarstrom L; Boren T; Sjostrom R Effects of fucosylated milk of goat and mouse on Helicobacter pylori binding to Lewis b antigen. World J Gastroenterol 2004, 10, 2063–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Miyazaki T; Sato T; Furukawa K; Ajisaka K Enzymatic synthesis of lacto-N-difucohexaose I which binds to Helicobacter pylori. Methods Enzymol 2010, 480, 511–524. [DOI] [PubMed] [Google Scholar]
- 211.Atochina O; Harn D LNFPIII/LeX-stimulated macrophages activate natural killer cells via CD40-CD40L interaction. Clin Diagn Lab Immunol 2005, 12, 1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Bode L; Rudloff S; Kunz C; Strobel S; Klein N Human milk oligosaccharides reduce platelet-neutrophil complex formation leading to a decrease in neutrophil beta 2 integrin expression. J Leukoc Biol 2004, 76, 820–826. [DOI] [PubMed] [Google Scholar]
- 213.Lewis ZT; Totten SM; Smilowitz JT; Popovic M; Parker E; Lemay DG; Van Tassell ML; Miller MJ; Jin YS; German JB; Lebrilla CB; Mills DA Maternal fucosyltransferase 2 status affects the gut bifidobacterial communities of breastfed infants. Microbiome 2015, 3, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Newburg DS; Ruiz-Palacios GM; Altaye M; Chaturvedi P; Meinzen-Derr J; Guerrero Mde L; Morrow AL Innate protection conferred by fucosylated oligosaccharides of human milk against diarrhea in breastfed infants. Glycobiology 2004, 14, 253–263. [DOI] [PubMed] [Google Scholar]
- 215.Morrow AL; Meinzen-Derr J; Huang P; Schibler KR; Cahill T; Keddache M; Kallapur SG; Newburg DS; Tabangin M; Warner BB; Jiang X Fucosyltransferase 2 non-secretor and low secretor status predicts severe outcomes in premature infants. J Pediatr 2011, 158, 745–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Coppa GV; Zampini L; Galeazzi T; Facinelli B; Ferrante L; Capretti R; Orazio G Human milk oligosaccharides inhibit the adhesion to Caco-2 cells of diarrheal pathogens: Escherichia coli, Vibrio cholerae, and Salmonella fyris. Pediatr Res 2006, 59, 377–382. [DOI] [PubMed] [Google Scholar]
- 217.Matrosovich MN; Gambaryan AS; Tuzikov AB; Byramova NE; Mochalova LV; Golbraikh AA; Shenderovich MD; Finne J; Bovin NV Probing of the receptor-binding sites of the H1 and H3 influenza A and influenza B virus hemagglutinins by synthetic and natural sialosides. Virology 1993, 196, 111–121. [DOI] [PubMed] [Google Scholar]
- 218.Eiwegger T; Stahl B; Schmitt J; Boehm G; Gerstmayr M; Pichler J; Dehlink E; Loibichler C; Urbanek R; Szepfalusi Z Human milk--derived oligosaccharides and plant-derived oligosaccharides stimulate cytokine production of cord blood T-cells in vitro. Pediatr Res 2004, 56, 536–540. [DOI] [PubMed] [Google Scholar]
- 219.Idota T; Kawakami H; Murakami Y; Sugawara M Inhibition of cholera toxin by human milk fractions and sialyllactose. Biosci Biotechnol Biochem 1995, 59, 417–419. [DOI] [PubMed] [Google Scholar]
- 220.Bouchara JP; Sanchez M; Chevailler A; Marot-Leblond A; Lissitzky JC; Tronchin G; Chabasse D Sialic acid-dependent recognition of laminin and fibrinogen by Aspergillus fumigatus conidia. Infect Immun 1997, 65, 2717–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Devaraj N; Sheykhnazari M; Warren WS; Bhavanandan VP Differential binding of Pseudomonas aeruginosa to normal and cystic fibrosis tracheobronchial mucins. Glycobiology 1994, 4, 307–316. [DOI] [PubMed] [Google Scholar]
- 222.Kuntz S; Rudloff S; Kunz C Oligosaccharides from human milk influence growth-related characteristics of intestinally transformed and non-transformed intestinal cells. Br J Nutr 2008, 99, 462–471. [DOI] [PubMed] [Google Scholar]
- 223.Kavanaugh DW; O’Callaghan J; Butto LF; Slattery H; Lane J; Clyne M; Kane M; Joshi L; Hickey RM Exposure of subsp. to Milk Oligosaccharides Increases Adhesion to Epithelial Cells and Induces a Substantial Transcriptional Response. PLoS One 2013, 8, e67224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Stehle T; Yan Y; Benjamin TL; Harrison SC Structure of murine polyomavirus complexed with an oligosaccharide receptor fragment. Nature 1994, 369, 160–163. [DOI] [PubMed] [Google Scholar]
- 225.Stins MF; Prasadarao NV; Ibric L; Wass CA; Luckett P; Kim KS Binding characteristics of S fimbriated Escherichia coli to isolated brain microvascular endothelial cells. Am J Pathol 1994, 145, 1228–1236. [PMC free article] [PubMed] [Google Scholar]
- 226.Virkola R; Parkkinen J; Hacker J; Korhonen TK Sialyloligosaccharide chains of laminin as an extracellular matrix target for S fimbriae of Escherichia coli. Infect Immun 1993, 61, 4480–4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Mysore JV; Wigginton T; Simon PM; Zopf D; Heman-Ackah LM; Dubois A Treatment of Helicobacter pylori infection in rhesus monkeys using a novel antiadhesion compound. Gastroenterology 1999, 117, 1316–1325. [DOI] [PubMed] [Google Scholar]
- 228.Evans DG; Evans DJ Jr.; Moulds JJ; Graham DY N-Acetylneuraminyllactose-binding fibrillar hemagglutinin of Campylobacter pylori: a putative colonization factor antigen. Infect Immun 1988, 56, 2896–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Korhonen TK; Valtonen MV; Parkkinen J; Vaisanen-Rhen V; Finne J; Orskov F; Orskov I; Svenson SB; Makela PH Serotypes, hemolysin production, and receptor recognition of Escherichia coli strains associated with neonatal sepsis and meningitis. Infect Immun 1985, 48, 486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Van Niekerk E; Autran CA; Nel DG; Kirsten GF; Blaauw R; Bode L Human milk oligosaccharides differ between HIV-infected and HIV-uninfected mothers and are related to necrotizing enterocolitis incidence in their preterm very-low-birth-weight infants. J Nutr 2014, 144, 1227–1233. [DOI] [PubMed] [Google Scholar]
- 231.Neu J; Walker WA Necrotizing enterocolitis. N Engl J Med 2011, 364, 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Holman RC; Stoll BJ; Clarke MJ; Glass RI The epidemiology of necrotizing enterocolitis infant mortality in the United States. Am J Public Health 1997, 87, 2026–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Dicken BJ; Sergi C; Rescorla FJ; Breckler F; Sigalet D Medical management of motility disorders in patients with intestinal failure: a focus on necrotizing enterocolitis, gastroschisis, and intestinal atresia. J Pediatr Surg 2011, 46, 1618–1630. [DOI] [PubMed] [Google Scholar]
- 234.Clark RH; Gordon P; Walker WM; Laughon M; Smith PB; Spitzer AR Characteristics of patients who die of necrotizing enterocolitis. J Perinatol 2012, 32, 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Blakely ML; Lally KP; McDonald S; Brown RL; Barnhart DC; Ricketts RR; Thompson WR; Scherer LR; Klein MD; Letton RW; Chwals WJ; Touloukian RJ; Kurkchubasche AG; Skinner MA; Moss RL; Hilfiker ML; Network N. E. C. S. o. t. N. N. R. Postoperative outcomes of extremely low birth-weight infants with necrotizing enterocolitis or isolated intestinal perforation: a prospective cohort study by the NICHD Neonatal Research Network. Ann Surg 2005, 241, 984–989; discussion 989–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Jantscher-Krenn E; Zherebtsov M; Nissan C; Goth K; Guner YS; Naidu N; Choudhury B; Grishin AV; Ford HR; Bode L The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut 2012, 61, 1417–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Fernandez-Mayoralas A; Martin-Lomas M Synthesis of 3- and 2’-fucosyl-lactose and 3,2’-difucosyl-lactose from partially benzylated lactose derivatives. Carbohydr Res 1986, 154, 93–101. [Google Scholar]
- 238.Aly MR; Ibrahim el SI; Ashry el SH; Schmidt RR Synthesis of lacto-N-neotetraose and lacto-N-tetraose using the dimethylmaleoyl group as amino protective group. Carbohydr Res 1999, 316, 121–132. [DOI] [PubMed] [Google Scholar]
- 239.Hsu Y; Lu XA; Zulueta MM; Tsai CM; Lin KI; Hung SC; Wong CH Acyl and silyl group effects in reactivity-based one-pot glycosylation: synthesis of embryonic stem cell surface carbohydrates Lc4 and IV(2)Fuc-Lc4. J Am Chem Soc 2012, 134, 4549–4552. [DOI] [PubMed] [Google Scholar]
- 240.Takamura T; Chiba T; Ishihara H; Tejima S Chemical modification of lactose. XIII. Synthesis of lacto-N-tetraose. Chem. Pharm. Bull 1980, 28, 1804–1809. [Google Scholar]
- 241.Aly MRE; Ibrahim E-SI; El-Ashry E-SHE; Schmidt RR Synthesis of lacto-N-neohexaose and lacto-N-neooctaose using the dimethylmaleoyl moiety as an amino protective group. Eur J Org Chem 2000, 2000, 319–326. [Google Scholar]
- 242.Manzoni L; Lay L; Schmidt RR Synthesis of Lewis a and Lewis x pentasaccharides based on N-trichloroethoxycarbonyl protection. J. Carbohydr. Chem 1998, 17, 739–758. [DOI] [PubMed] [Google Scholar]
- 243.Love KR; Seeberger PH Solution syntheses of protected type II Lewis blood group oligosaccharides: study for automated synthesis. J Org Chem 2005, 70, 3168–3177. [DOI] [PubMed] [Google Scholar]
- 244.Zhang Y-M; Esnault J; Mallet J-M; Sinay P Synthesis of the beta-methyl glycoside of lacto-N-fucopentaose III. J. Carbohydr. Chem 1999, 18, 419–427. [Google Scholar]
- 245.Lay L; Manzoni L; Schmidt RR Synthesis of N-acetylglucosamine containing Lewis A and Lewis X building blocks based on N-tetrachlorophthaloyl protection--synthesis of Lewis X pentasaccharide. Carbohydr Res 1998, 310, 157–171. [DOI] [PubMed] [Google Scholar]
- 246.Cao S; Gan Z; Roy R Active-latent glycosylation strategy toward Lewis X pentasaccharide in a form suitable for neoglycoconjugate syntheses. Carbohydr Res 1999, 318, 75–81. [DOI] [PubMed] [Google Scholar]
- 247.Sherman AA; Yudina ON; Mironov YV; Sukhova EV; Shashkov AS; Menshov VM; Nifantiev NE Study of glycosylation with N-trichloroacetyl-D-glucosamine derivatives in the syntheses of the spacer-armed pentasaccharides sialyl lacto-N-neotetraose and sialyl lacto-N-tetraose, their fragments, and analogues. Carbohydr Res 2001, 336, 13–46. [DOI] [PubMed] [Google Scholar]
- 248.Mandal PK; Misra AK Concise synthesis of two pentasaccharides corresponding to the alpha-chain oligosaccharides of Neisseria gonorrhoeae and Neisseria meningitidis. Tetrahedron 2008, 64, 8685–8691. [Google Scholar]
- 249.Chernyak A; Oscarson S; Turek D Synthesis of the Lewis b hexasaccharide and squarate acid-HSA conjugates thereof with various saccharide loadings. Carbohydr Res 2000, 329, 309–316. [DOI] [PubMed] [Google Scholar]
- 250.Shimizu H; Ito Y; Kanie O; Ogawa T Solid phase synthesif of polylactosamine oligosaccharide. Bioorg Med Chem Lett 1996, 6, 2841–2846. [Google Scholar]
- 251.Takamura T; Chiba T; Tejima S Chemical modification of lactose. XVI. Synthesis of lacto-N-neohexaose. Chem. Pharm. Bull 1981, 29, 2270–2276. [Google Scholar]
- 252.Roussel F; Takhi M; Schmidt RR Solid-phase synthesis of a branched hexasaccharide using a highly efficient synthetic strategy. J Org Chem 2001, 66, 8540–8548. [DOI] [PubMed] [Google Scholar]
- 253.Maranduba A; Veyrieres A Glycosylation of lactose: synthesis of branched oligosaccharides involved in the biosynthesis of glycolipids having blood-group I activity. Carbohydr Res 1986, 151, 105–119. [DOI] [PubMed] [Google Scholar]
- 254.Knuhr P; Castro-Palomino J; Grathwohl M; Schmidt RR Complex structures of antennary human milk oligosaccharides - Synthesis of a branched octasaccharide. Eur J Org Chem 2001, 2001, 4239–4246. [Google Scholar]
- 255.Broder W; Kunz H Glycosyl azides as building blocks in convergent syntheses of oligomeric lactosamine and Lewis(x) saccharides. Bioorg Med Chem 1997, 5, 1–19. [DOI] [PubMed] [Google Scholar]
- 256.Lee JC; Wu CY; Apon JV; Siuzdak G; Wong CH Reactivity-based one-pot synthesis of the tumor-associated antigen N3 minor octasaccharide for the development of a photocleavable DIOS-MS sugar array. Angew Chem Int Ed Engl 2006, 45, 2753–2757. [DOI] [PubMed] [Google Scholar]
- 257.Kim HM; Kim IJ; Danishefsky SJ Total syntheses of tumor-related antigens N3: probing the feasibility limits of the glycal assembly method. J Am Chem Soc 2001, 123, 35–48. [DOI] [PubMed] [Google Scholar]
- 258.Jennum CA; Fenger TH; Bruun LM; Madsen R One-pot glycosylations in the synthesis of human milk oligosaccharides. Eur. J. Org. Chem 2014, 2014, 3232–3241. [Google Scholar]
- 259.Han NS; Kim TJ; Park YC; Kim J; Seo JH Biotechnological production of human milk oligosaccharides. Biotechnol Adv 2012, 30, 1268–1278. [DOI] [PubMed] [Google Scholar]
- 260.Monteiro MA; Chan KH; Rasko DA; Taylor DE; Zheng PY; Appelmelk BJ; Wirth HP; Yang M; Blaser MJ; Hynes SO; Moran AP; Perry MB Simultaneous expression of type 1 and type 2 Lewis blood group antigens by Helicobacter pylori lipopolysaccharides. Molecular mimicry between H. pylori lipopolysaccharides and human gastric epithelial cell surface glycoforms. J Biol Chem 1998, 273, 11533–11543. [DOI] [PubMed] [Google Scholar]
- 261.Smith H; Parsons NJ; Cole JA Sialylation of neisserial lipopolysaccharide: a major influence on pathogenicity. Microb Pathog 1995, 19, 365–377. [DOI] [PubMed] [Google Scholar]
- 262.Chen X; Varki A Advances in the biology and chemistry of sialic acids. ACS Chem Biol 2010, 5, 163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Johnson KF Synthesis of oligosaccharides by bacterial enzymes. Glycoconj J 1999, 16, 141–146. [DOI] [PubMed] [Google Scholar]
- 264.Chen X Fermenting next generation glycosylated therapeutics. ACS Chem Biol 2011, 6, 14–17. [DOI] [PubMed] [Google Scholar]
- 265.Chen X; Kowal P; Wang PG Large-scale enzymatic synthesis of oligosaccharides. Curr Opin Drug Discov Devel 2000, 3, 756–763. [PubMed] [Google Scholar]
- 266.Ichikawa Y; Look GC; Wong CH Enzyme-catalyzed oligosaccharide synthesis. Anal Biochem 1992, 202, 215–238. [DOI] [PubMed] [Google Scholar]
- 267.Ichikawa Y; Wang R; Wong CH Regeneration of sugar nucleotide for enzymatic oligosaccharide synthesis. Methods Enzymol 1994, 247, 107–127. [DOI] [PubMed] [Google Scholar]
- 268.Tsai TI; Lee HY; Chang SH; Wang CH; Tu YC; Lin YC; Hwang DR; Wu CY; Wong CH Effective sugar nucleotide regeneration for the large-scale enzymatic synthesis of Globo H and SSEA4. J Am Chem Soc 2013, 135, 14831–14839. [DOI] [PubMed] [Google Scholar]
- 269.Yu H; Lau K; Thon V; Autran CA; Jantscher-Krenn E; Xue M; Li Y; Sugiarto G; Qu J; Mu S; Ding L; Bode L; Chen X Synthetic disialyl hexasaccharides protect neonatal rats from necrotizing enterocolitis. Angew Chem Int Ed Engl 2014, 53, 6687–6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Yu H; Chokhawala HA; Huang S; Chen X One-pot three-enzyme chemoenzymatic approach to the synthesis of sialosides containing natural and non-natural functionalities. Nat Protoc 2006, 1, 2485–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Yu H; Lau K; Li Y; Sugiarto G; Chen X One-pot multienzyme synthesis of Lewis x and sialyl Lewis x antigens. Curr Protoc Chem Biol 2012, 4, 233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Lau K; Yu H; Thon V; Khedri Z; Leon ME; Tran BK; Chen X Sequential two-step multienzyme synthesis of tumor-associated sialyl T-antigens and derivatives. Org Biomol Chem 2011, 9, 2784–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Li Y; Chen X Sialic acid metabolism and sialyltransferases: natural functions and applications. Appl Microbiol Biotechnol 2012, 94, 887–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Mehta S; Gilbert M; Wakarchuk WW; Whitfield DM Ready access to sialylated oligosaccharide donors. Org Lett 2000, 2, 751–753. [DOI] [PubMed] [Google Scholar]
- 275.Yan F; Mehta S; Eichler E; Wakarchuk WW; Gilbert M; Schur MJ; Whitfield DM Simplifying oligosaccharide synthesis: efficient synthesis of lactosamine and siaylated lactosamine oligosaccharide donors. J Org Chem 2003, 68, 2426–2431. [DOI] [PubMed] [Google Scholar]
- 276.Hayashi M; Tanaka M; Itoh M; Miyauchi H A convenient and efficient synthesis of SLeX analogs. J Org Chem 1996, 61, 2938–2945. [DOI] [PubMed] [Google Scholar]
- 277.Cao H; Huang S; Cheng J; Li Y; Muthana S; Son B; Chen X Chemical preparation of sialyl Lewis x using an enzymatically synthesized sialoside building block. Carbohydr Res 2008, 343, 2863–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Schmidt D; Thiem J Chemical synthesis using enzymatically generated building units for construction of the human milk pentasaccharides sialyllacto-N-tetraose and sialyllacto-N-neotetraose epimer. Beilstein J Org Chem 2010, 6, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Yao W; Yan J; Chen X; Wang F; Cao H Chemoenzymatic synthesis of lacto-N-tetrasaccharide and sialyl lacto-N-tetrasaccharides. Carbohydr Res 2015, 401, 5–10. [DOI] [PubMed] [Google Scholar]
- 280.Shaikh FA; Withers SG Teaching old enzymes new tricks: engineering and evolution of glycosidases and glycosyl transferases for improved glycoside synthesis. Biochem Cell Biol 2008, 86, 169–177. [DOI] [PubMed] [Google Scholar]
- 281.Williams SJ; Withers SG Glycosyl fluorides in enzymatic reactions. Carbohydr Res 2000, 327, 27–46. [DOI] [PubMed] [Google Scholar]
- 282.Albert M; Repetschnigg W; Ortner J; Gomes J; Paul BJ; Illaszewicz C; Weber H; Steiner W; Dax K Simultaneous detection of different glycosidase activities by 19F NMR spectroscopy. Carbohydr Res 2000, 327, 395–400. [DOI] [PubMed] [Google Scholar]
- 283.Zeuner B; Jers C; Mikkelsen JD; Meyer AS Methods for improving enzymatic trans-glycosylation for synthesis of human milk oligosaccharide biomimetics. J Agric Food Chem 2014, 62, 9615–9631. [DOI] [PubMed] [Google Scholar]
- 284.Albermann C; Piepersberg W; Wehmeier UF Synthesis of the milk oligosaccharide 2’-fucosyllactose using recombinant bacterial enzymes. Carbohydr Res 2001, 334, 97–103. [DOI] [PubMed] [Google Scholar]
- 285.Albermann C; Distler J; Piepersberg W Preparative synthesis of GDP-beta-L-fucose by recombinant enzymes from enterobacterial sources. Glycobiology 2000, 10, 875–881. [DOI] [PubMed] [Google Scholar]
- 286.Wang G; Boulton PG; Chan NW; Palcic MM; Taylor DE Novel Helicobacter pylori alpha1,2-fucosyltransferase, a key enzyme in the synthesis of Lewis antigens. Microbiology 1999, 145 (Pt 11), 3245–3253. [DOI] [PubMed] [Google Scholar]
- 287.Lee WH; Pathanibul P; Quarterman J; Jo JH; Han NS; Miller MJ; Jin YS; Seo JH Whole cell biosynthesis of a functional oligosaccharide, 2’-fucosyllactose, using engineered Escherichia coli. Microb Cell Fact 2012, 11, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Chin YW; Kim JY; Lee WH; Seo JH Enhanced production of 2’-fucosyllactose in engineered Escherichia coli BL21star(DE3) by modulation of lactose metabolism and fucosyltransferase. J Biotechnol 2015, 210, 107–115. [DOI] [PubMed] [Google Scholar]
- 289.Baumgartner F; Seitz L; Sprenger GA; Albermann C Construction of Escherichia coli strains with chromosomally integrated expression cassettes for the synthesis of 2’-fucosyllactose. Microb Cell Fact 2013, 12, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Prieto PA Profiles of human milk oligosaccharides and production of some human milk oligosaccharides in transgenic animals. Adv Nutr 2012, 3, 456S–464S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Nagae M; Tsuchiya A; Katayama T; Yamamoto K; Wakatsuki S; Kato R Structural basis of the catalytic reaction mechanism of novel 1,2-alpha-L-fucosidase from Bifidobacterium bifidum. J Biol Chem 2007, 282, 18497–18509. [DOI] [PubMed] [Google Scholar]
- 292.Wada J; Honda Y; Nagae M; Kato R; Wakatsuki S; Katayama T; Taniguchi H; Kumagai H; Kitaoka M; Yamamoto K 1,2-alpha-l-Fucosynthase: a glycosynthase derived from an inverting alpha-glycosidase with an unusual reaction mechanism. FEBS Lett 2008, 582, 3739–3743. [DOI] [PubMed] [Google Scholar]
- 293.Yu H; Yu H; Karpel R; Chen X Chemoenzymatic synthesis of CMP-sialic acid derivatives by a one-pot two-enzyme system: comparison of substrate flexibility of three microbial CMP-sialic acid synthetases. Bioorg Med Chem 2004, 12, 6427–6435. [DOI] [PubMed] [Google Scholar]
- 294.Yu H; Chokhawala H; Karpel R; Yu H; Wu B; Zhang J; Zhang Y; Jia Q; Chen X A multifunctional Pasteurella multocida sialyltransferase: a powerful tool for the synthesis of sialoside libraries. J Am Chem Soc 2005, 127, 17618–17619. [DOI] [PubMed] [Google Scholar]
- 295.Sugiarto G; Lau K; Li Y; Khedri Z; Yu H; Le DT; Chen X Decreasing the sialidase activity of multifunctional Pasteurella multocida alpha2–3-sialyltransferase 1 (PmST1) by site-directed mutagenesis. Mol Biosyst 2011, 7, 3021–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Sugiarto G; Lau K; Qu J; Li Y; Lim S; Mu S; Ames JB; Fisher AJ; Chen X A sialyltransferase mutant with decreased donor hydrolysis and reduced sialidase activities for directly sialylating LewisX. ACS Chem Biol 2012, 7, 1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Endo T; Koizumi S Process for producing alpha2,3/alpha2,8-sialyltransferase and sialic acid-containign complex sugar. Patent WO 2003027297 A1, April 3. 2004.
- 298.Schmolzer K; Czabany T; Luley-Goedl C; Pavkov-Keller T; Ribitsch D; Schwab H; Gruber K; Weber H; Nidetzky B Complete switch from alpha-2,3- to alpha-2,6-regioselectivity in Pasteurella dagmatis beta-D-galactoside sialyltransferase by active-site redesign. Chem Commun 2015, 51, 3083–3086. [DOI] [PubMed] [Google Scholar]
- 299.Schmolzer K; Ribitsch D; Czabany T; Luley-Goedl C; Kokot D; Lyskowski A; Zitzenbacher S; Schwab H; Nidetzky B Characterization of a multifunctional alpha2,3-sialyltransferase from Pasteurella dagmatis. Glycobiology 2013, 23, 1293–1304. [DOI] [PubMed] [Google Scholar]
- 300.Guo Y; Jers C; Meyer AS; Arnous A; Li H; Kirpekar F; Mikkelsen JD A Pasteurella multocida sialyltransferase displaying dual trans-sialidase activities for production of 3’-sialyl and 6’-sialyl glycans. J Biotechnol 2014, 170, 60–67. [DOI] [PubMed] [Google Scholar]
- 301.Tanaka H; Ito F; Iwasaki T A system for sialic acid transfer by colominic acid and a sialidase that preferentially hydrolyzes sialyl alpha-2,8 linkages. Biosci Biotechnol Biochem 1995, 59, 638–643. [DOI] [PubMed] [Google Scholar]
- 302.Mcjarrow P; Garman J; Harvey S; Van Amelsfort A Diary process and product. Patent WO 2003049547 A2, June 19. 2003.
- 303.Pelletier M; Barker WA; Hakes DJ; Zopf DA Methods for producing sialyloligosaccharides in a dairy source. Patent US 6706492 B2, March 16. 2004.
- 304.Sallomons E; Wilbrink MH; Sanders P; Kamerling JP; Van Vuure CA; Hage JA Methods for providing sialylated oligosaccharides. Patent WO 2013085384 A1, June 13. 2013.
- 305.Gilbert M; Bayer R; Cunningham AM; DeFrees S; Gao Y; Watson DC; Young NM; Wakarchuk WW The synthesis of sialylated oligosaccharides using a CMP-Neu5Ac synthetase/sialyltransferase fusion. Nat Biotechnol 1998, 16, 769–772. [DOI] [PubMed] [Google Scholar]
- 306.Endo T; Koizumi S; Tabata K; Ozaki A Large-scale production of CMP-NeuAc and sialylated oligosaccharides through bacterial coupling. Appl Microbiol Biotechnol 2000, 53, 257–261. [DOI] [PubMed] [Google Scholar]
- 307.Priem B; Gilbert M; Wakarchuk WW; Heyraud A; Samain E A new fermentation process allows large-scale production of human milk oligosaccharides by metabolically engineered bacteria. Glycobiology 2002, 12, 235–240. [DOI] [PubMed] [Google Scholar]
- 308.Fierfort N; Samain E Genetic engineering of Escherichia coli for the economical production of sialylated oligosaccharides. J Biotechnol 2008, 134, 261–265. [DOI] [PubMed] [Google Scholar]
- 309.Yu H; Huang S; Chokhawala H; Sun M; Zheng H; Chen X Highly efficient chemoenzymatic synthesis of naturally occurring and non-natural alpha-2,6-linked sialosides: a P. damsela alpha-2,6-sialyltransferase with extremely flexible donor-substrate specificity. Angew Chem Int Ed Engl 2006, 45, 3938–3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Tsukamoto H; Takakura Y; Mine T; Yamamoto T Photobacterium sp. JT-ISH-224 produces two sialyltransferases, alpha-/beta-galactoside alpha2,3-sialyltransferase and beta-galactoside alpha2,6-sialyltransferase. J Biochem 2008, 143, 187–197. [DOI] [PubMed] [Google Scholar]
- 311.Drouillard S; Mine T; Kajiwara H; Yamamoto T; Samain E Efficient synthesis of 6’-sialyllactose, 6,6’-disialyllactose, and 6’-KDO-lactose by metabolically engineered E. coli expressing a multifunctional sialyltransferase from the Photobacterium sp. JT-ISH-224. Carbohydr Res 2010, 345, 1394–1399. [DOI] [PubMed] [Google Scholar]
- 312.Nyffenegger C; Nordvang RT; Zeuner B; Lezyk M; Difilippo E; Logtenberg MJ; Schols HA; Meyer AS; Mikkelsen JD Backbone structures in human milk oligosaccharides: trans-glycosylation by metagenomic beta-N-acetylhexosaminidases. Appl Microbiol Biotechnol 2015, 99, 7997–8009. [DOI] [PubMed] [Google Scholar]
- 313.Murata T; Inukai T; Suzuki M; Yamagishi M; Usui AT Facile enzymatic conversion of lactose into lacto-N-tetraose and lacto-N-neotetraose. Glycoconj J 1999, 16, 189–195. [DOI] [PubMed] [Google Scholar]
- 314.Prieto PA; Kleman-Leyer KM; inventors; Abbott Laboratories, a. Process for synthesizing oligosaccharides. United States 5,945,314. 1999, August 31. [Google Scholar]
- 315.Prieto PA In vitro and clinical experiences with a human milk oligosaccharide, lacto-N-neotetraose, and fructooligosaccharides. Foods Food Ingredients J Jpn 2005, 210, 1018–1030. [Google Scholar]
- 316.Renaudie L; Daniellou R; Auge C; Le Narvor C Enzymatic supported synthesis of lacto-N-neotetraose using dendrimeric polyethylene glycol. Carbohydr Res 2004, 339, 693–698. [DOI] [PubMed] [Google Scholar]
- 317.Li Y; Yu H; Chen Y; Lau K; Cai L; Cao H; Tiwari VK; Qu J; Thon V; Wang PG; Chen X Substrate promiscuity of N-acetylhexosamine 1-kinases. Molecules 2011, 16, 6396–6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Chen Y; Thon V; Li Y; Yu H; Ding L; Lau K; Qu J; Hie L; Chen X One-pot three-enzyme synthesis of UDP-GlcNAc derivatives. Chem Commun 2011, 47, 10815–10817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Guan W; Ban L; Cai L; Li L; Chen W; Liu X; Mrksich M; Wang PG Combining carbochips and mass spectrometry to study the donor specificity for the Neisseria meningitidis beta1,3-N-acetylglucosaminyltransferase LgtA. Bioorg Med Chem Lett 2011, 21, 5025–5028. [DOI] [PubMed] [Google Scholar]
- 320.Muthana MM; Qu J; Li Y; Zhang L; Yu H; Ding L; Malekan H; Chen X Efficient one-pot multienzyme synthesis of UDP-sugars using a promiscuous UDP-sugar pyrophosphorylase from Bifidobacterium longum (BLUSP). Chem Commun 2012, 48, 2728–2730. [DOI] [PubMed] [Google Scholar]
- 321.Lau K; Thon V; Yu H; Ding L; Chen Y; Muthana MM; Wong D; Huang R; Chen X Highly efficient chemoenzymatic synthesis of beta1–4-linked galactosides with promiscuous bacterial beta1–4-galactosyltransferases. Chem Commun 2010, 46, 6066–6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Sun M; Li Y; Chokhawala HA; Henning R; Chen X N-Terminal 112 amino acid residues are not required for the sialyltransferase activity of Photobacterium damsela alpha2,6-sialyltransferase. Biotechnol Lett 2008, 30, 671–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Cheng J; Yu H; Lau K; Huang S; Chokhawala HA; Li Y; Tiwari VK; Chen X Multifunctionality of Campylobacter jejuni sialyltransferase CstII: characterization of GD3/GT3 oligosaccharide synthase, GD3 oligosaccharide sialidase, and trans-sialidase activities. Glycobiology 2008, 18, 686–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Yu H; Cheng J; Ding L; Khedri Z; Chen Y; Chin S; Lau K; Tiwari VK; Chen X Chemoenzymatic synthesis of GD3 oligosaccharides and other disialyl glycans containing natural and non-natural sialic acids. J Am Chem Soc 2009, 131, 18467–18477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Dumon C; Priem B; Martin SL; Heyraud A; Bosso C; Samain E In vivo fucosylation of lacto-N-neotetraose and lacto-N-neohexaose by heterologous expression of Helicobacter pylori alpha1,3-fucosyltransferase in engineered Escherichia coli. Glycoconjugate J. 2001, 18, 465–474. [DOI] [PubMed] [Google Scholar]
- 326.Drouillard S; Driguez H; Samain E Large-scale synthesis of H-antigen oligosaccharides by expressing Helicobacter pylori alpha1,2-fucosyltransferase in metabolically engineered Escherichia coli cells. Angew Chem Int Ed Engl 2006, 45, 1778–1780. [DOI] [PubMed] [Google Scholar]
- 327.Dumon C; Samain E; Priem B Assessment of the two Helicobacter pylori alpha-1,3-fucosyltransferase ortholog genes for the large-scale synthesis of LewisX human milk oligosaccharides by metabolically engineered Escherichia coli. Biotechnol Prog 2004, 20, 412–419. [DOI] [PubMed] [Google Scholar]
- 328.Baumgartner F; Conrad J; Sprenger GA; Albermann C Synthesis of the human milk oligosaccharide lacto-N-tetraose in metabolically engineered, plasmid-free E. coli. Chembiochem 2014, 15, 1896–1900. [DOI] [PubMed] [Google Scholar]
- 329.Baumgartner F; Sprenger GA; Albermann C Galactose-limited fed-batch cultivation of Escherichia coli for the production of lacto-N-tetraose. Enzyme Microb Technol 2015, 75–76, 37–43. [DOI] [PubMed] [Google Scholar]
- 330.Sakurama H; Fushinobu S; Hidaka M; Yoshida E; Honda Y; Ashida H; Kitaoka M; Kumagai H; Yamamoto K; Katayama T 1,3–1,4-alpha-L-fucosynthase that specifically introduces Lewis a/x antigens into type-1/2 chains. J Biol Chem 2012, 287, 16709–16719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Koizumi S; Endo T; Tabata K; Ozaki A Large-scale production of UDP-galactose and globotriose by coupling metabolically engineered bacteria. Nat Biotechnol 1998, 16, 847–850. [DOI] [PubMed] [Google Scholar]
- 332.Koizumi S; Endo T; Tabata K; Nagano H; Ohnishi J; Ozaki A Large-scale production of GDP-fucose and Lewis x by bacterial coupling. J. Ind. Microbiol. Biotechnol 2000, 25, 213–217. [Google Scholar]
- 333.Dumon C; Bosso C; Utille JP; Heyraud A; Samain E Production of Lewis x tetrasaccharides by metabolically engineered Escherichia coli. Chembiochem 2006, 7, 359–365. [DOI] [PubMed] [Google Scholar]
- 334.Antoine T; Priem B; Heyraud A; Greffe L; Gilbert M; Wakarchuk WW; Lam JS; Samain E Large-scale in vivo synthesis of the carbohydrate moieties of gangliosides GM1 and GM2 by metabolically engineered Escherichia coli. Chembiochem 2003, 4, 406–12. [DOI] [PubMed] [Google Scholar]
- 335.Wang Z; Chinoy ZS; Ambre SG; Peng W; McBride R; de Vries RP; Glushka J; Paulson JC; Boons GJ A general strategy for the chemoenzymatic synthesis of asymmetrically branched N-glycans. Science 2013, 341, 379–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Li L; Liu Y; Ma C; Qu J; Calderon AD; Wu B; Wei N; Wang X; Guo Y; Xiao Z; Song J; Sugiarto G; Li Y; Yu H; Chen X; Wang PG Efficient chemoenzymatic synthesis of an N-glycan isoer library. Chem. Sci 2015, 6, 5652–5661. [DOI] [PMC free article] [PubMed] [Google Scholar]



 N-acetylneuraminic acid (Neu5Ac),
N-acetylneuraminic acid (Neu5Ac),  fucose (Fuc),
fucose (Fuc),  galactose (Gal),
galactose (Gal),  N-acetylglucosamine (GlcNAc),
N-acetylglucosamine (GlcNAc),  glucose (Glc),
glucose (Glc),  N-acetylgalactosamine (GalNAc).
N-acetylgalactosamine (GalNAc).