Abstract
Two recent studies addressed the functional properties and clinical significance of tumour antigen-specific effector T cells in human melanomas and lung carcinomas using single-cell strategies. Herein, we discuss their findings, which expand our understanding of T cell alterations in the tumour microenvironment and demonstrate that CD8+ T cell exhaustion is mediated by exposure to tumour cell-specific antigens and is associated with a tissue-resident memory phenotype.
Despite prominent clinical success of immune-checkpoint inhibitors (ICIs) targeting the PD-1–PD-L1 axis in multiple cancer types, questions remain regarding their mechanism of action, biomarkers to guide treatment, determinants of sensitivity and resistance, and treatment combinations to enhance clinical responses. Solid tumours develop and progress in the context of a complex tumour microenvironment (TME). High levels of tumour-infiltrating lymphocytes (TILs) are generally associated with favourable outcomes1; however, effector T (Teff) cells can be exposed to immunoregulatory signals from the TME and to continuous antigen stimulation that can affect transcriptional and epigenetic programmes. This exposure can limit their activity and result in a form of dysfunction referred to as T cell exhaustion2.
The Teff cell dysfunction programme can encompass multiple T cell profiles characterized by altered differentiation and cytolytic properties, deregulated proliferation and/or death programmes, and increased expression of multiple immune-inhibitory signals (such as PD-1, LAG3, TIM3, TIGIT and CD39)2,3. Exhausted Teff cells have been described in chronic viral infections and cancer, although their biological properties, disease context dependency and clinical relevance remain poorly understood. Developments in multimodal high-throughput single-cell analysis strategies coupled to computational models have enabled detailed and unsupervised functional studies of immune populations in tumour specimens.
Oliveira et al.4 evaluated single-cell transcriptomic profiles, T cell receptor (TCR) repertoires and selected surface proteins in tumour antigen-specific CD8+ Teff TILs from four patients with surgically resected melanoma. Using previously defined signatures, CD8+ TILs were classified into 13 subpopulations, which were ultimately grouped into two functional categories on the basis of the distribution of dominant Teff clonotypes: exhausted cells and non-exhausted memory cells. CD8+ Teff cells of the most-expanded TCR clonotypes predominantly had an exhausted profile and expressed transcripts associated with tissue-resident memory (TRM) cells (such as ITGAE (encoding CD103) and ZNF683). Furthermore, when transducted into T cells from individuals without cancer, the majority (83%) of TCR clones from exhausted CD8+ Teff cells but only a small fraction (10%) of TCRs from non-exhausted cells had autologous reactivity against cancer cells. In addition, the specificity of TCRs isolated from TILs was established through co-culturing with non-transformed cells pulsed with a variety of melanoma and viral antigens; most of them recognized one or more tumour-specific antigens with shared HLA restriction. Antigenic peptide-specific stimulation of Teff cells with both patient-specific mutant neopeptides or shared melanoma antigens was associated with an exhausted transcriptional profile, which was not seen in CD8+ Teff cells containing viral TCR clonotypes. Finally, in a cohort of 14 patients with melanoma, exhausted tumour antigen-specific CD8+ Teff cells were rare in peripheral blood samples, but higher numbers were detected in patients with progression after treatment with ICIs. Of note, these exhausted cells characteristically expressed high levels of PD-1 and CD39, as previously reported5.
Together, their results demonstrate that tumour antigen-specific Teff cells have exhausted functional profiles and that acquisition of this state is driven by continuous exposure to tumour antigens. These results are consistent with previous studies showing a strong association between tumour antigen specificity and Teff cell exhaustion in TILs from patients with other malignancies5,6, although these studies reported a high abundance and, in some cases, predominance of non-exhausted viral-specific bystander cells that did not seem to contribute to antitumour responses.
Caushi et al.7 analysed the single-cell transcriptomic profiles and TCR sequences of in vitro-expanded tumour antigen-specific CD8+ Teff cells from 20 patients with resectable non-small-cell lung cancer treated with neoadjuvant nivolumab. Specificity was assessed using MANAFEST, a peptide stimulation-based assay that included mutant neoantigenic peptides and MHC class I-restricted viral antigens. Based on transcriptomic profiles, 15 T cell clusters were identified, 6 of which had expression programmes consistent with TRM cells. Most neoantigen-specific CD8+ Teff cell clonotypes were allocated to discrete TRM cell clusters characterized by an incomplete effector programme, increased immune inhibitory signals, upregulation of TRM markers and expression of transcription factors associated with T cell exhaustion (PRDM1 and TOX2). These features were not seen in viral-specific bystander T cells. Together, these results are consistent with the findings from Oliveira et al.4 and with previous studies indicating that tumour antigen-specific cells display a TRM phenotype and show exhausted and/or dysfunctional profiles5,6. Of note, peptide-stimulation dose–response curves showed higher TCR–peptide avidity in CD8+ Teff cells from patients with major pathological responses after anti-PD-1 treatment, suggesting a role in treatment sensitivity. A preliminary comparative analysis of single-cell transcriptomic profiles between CD8+ Teff cells from patients with versus without major pathological responses showed upregulation of numerous exhaustion markers in the latter group, supporting a potential biomarker role. Neoantigen-specific CD8+ Teff cells also had reduced expression of IL-7R and sensitivity to IL-7 stimulation compared to influenza-specific T cells.
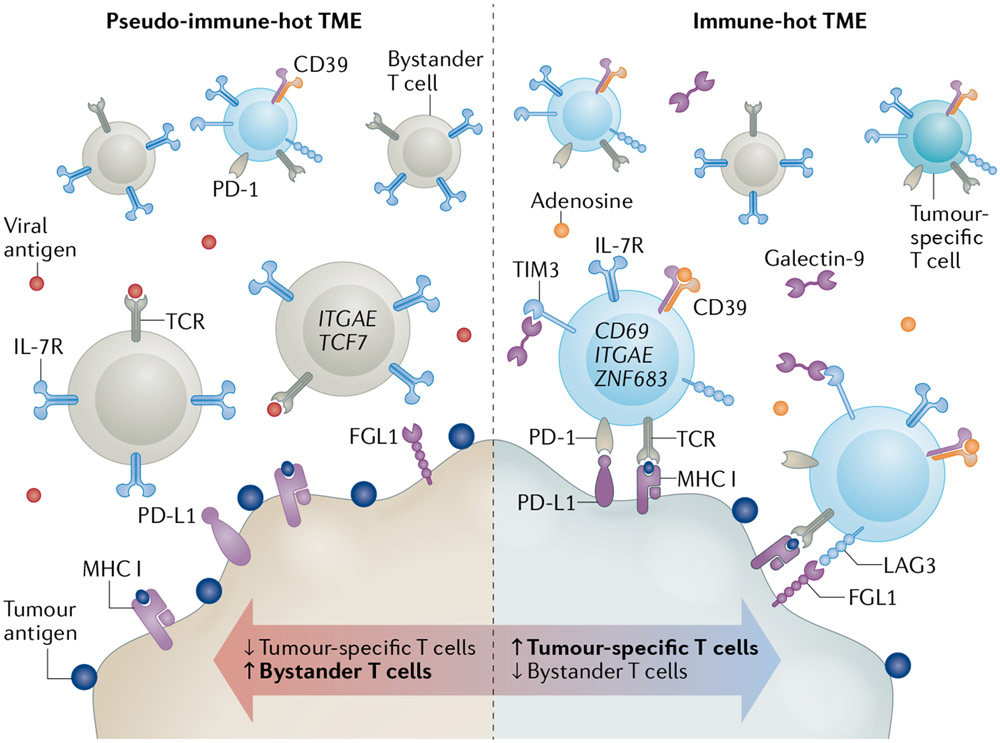
Together, both studies expand our understanding of T cell alterations in immune-‘hot’ human tumours containing MHC-presented tumour antigens and TILs. They unambiguously demonstrate that CD8+ Teff cell exhaustion is mediated by exposure to tumour-cell antigens and is prominently associated with a TRM phenotype. These studies also indicate a role for T cell exhaustion in resistance to anti-PD-1/PD-L1 antibodies and evidence the presence of variable levels of non-exhausted bystander cells with limited tumour reactivity and uncertain biological function. The presence of large numbers of these bystander cells might create ‘pseudo-immune-hot’ tumours with reduced sensitivity to ICIs (FIG. 1).
Fig. 1 ∣. Functional profile of tumour-infiltrating CD8+ Teff cells in human solid tumours.
Tumours can contain variable levels of bystander effector T (Teff) cells targeting non-tumour epitopes, which lack features of exhaustion and are not expected to mediate cancer cell elimination. Tumours with a high number of these cells could be considered as‘pseudo-hot’ (left) and are likely to be insensitive to immune-checkpoint inhibitors (ICIs). By contrast, immune-hot tumours preferentially contain tumour antigen-specific Teff cells (right). Continuous antigen stimulation and regulatory signals in the tumour microenvironment (TME) can favour an exhausted T cell phenotype and acquisition of a tissue-resident memory profile.Their accumulation in the TME is associated with reduced sensitivity to ICIs. Therapeutic interventions aiming to achieve and/or maintain a TME with a predominance of tumour antigen-specific non-exhausted Teff cells would lead to improved clinical responses to ICIs. TCR,T cell receptor. Created with BioRender.com.
These results also suggest a potential role for CD8+ Teff cell dysfunction and particularly of CD39 expression as biomarkers to identify exhausted cells and thus potentially spare treatment in patients who would derive limited benefit from anti-PD-1/PD-L1 ICIs. This role is also supported by previous studies from our group showing association between baseline TIL activation and proliferation, or expression of immune checkpoints with resistance to anti-PD-1/PD-L1 ICIs in patients with non-small-cell lung cancer3,8,9.
Possible therapeutic options to reduce or control the negative impact of T cell dysfunction in the TME include the use of combination therapies simultaneously targeting multiple co-inhibitory signals or receptors, adoptive cell therapies with genetically modified (for example, exhaustion resistant) tumour antigen-specific T cells, or modulation of the IL-7 pathway. Studies including larger patient cohorts and additional experimental approaches are required to confirm the clinical implications of these findings, expand understanding of the role of TRM cells in response to ICIs and reveal the specific molecular mechanisms by which tumour-specific T cells become dysfunctional. In this regard, the possible role of ligands for key immune checkpoints expressed in the TME, such as PD-L1, galectin-9, MHC class II and FGL1 in the acquisition or progression of the T cell exhaustion phenotype will need to be determined10. Finally, additional studies addressing dominant mechanisms of immune evasion and candidate therapeutic options in immune-cold tumours, such as those with low T cell infiltration and/or defective antigen presentation, are needed.
Acknowledgements
The authors receive funding from the Mark Foundation (EXTOL project 19-029-MIA), the NIH (grants R37CA245154, R01CA262377 and R03CA219603 to K.A.S.), Stand Up To Cancer–American Cancer Society Lung Cancer Dream Team Translational Research (SU2C-AACR-DT1715 and SU2C-AACR-DT22-17) and the Yale SPORE in Lung Cancer (grant P50CA196530).
Footnotes
Competing interests
K.A.S. has received research funding from AstraZeneca, Bristol Myers Squibb, Eli Lilly, Merck Sharpe & Dohme, Moderna, Navigate Biopharma, Pierre Fabre, Ribon Therapeutics, Surface Oncology, Takeda–Millenium Pharmaceuticals and Tesaro–GSK; and honoraria for consultancy, advisory or speaker roles from Agenus, Bristol Myers Squibb, Clinica Alemana de Santiago, EMD Serono, Fluidigm, Genmab, Merck Sharpe & Dohme, PeerView, Pierre Fabre, Shattuck Labs, Takeda–Millenium Pharmaceuticals and Torque Therapeutics. M.L.d.R. declares no competing interests.
References
- 1.Fridman WH, Zitvogel L, Sautes-Fridman C & Kroemer C The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol 14, 717–734 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Blank CU et al. Defining ‘T cell exhaustion’. Nat. Rev. Immunol 19, 665–674 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanmamed MF et al. A burned-out CD8+ T-cell subset expands in the tumor microenvironment and curbs cancer immunotherapy. Cancer Discov. 11, 1700–1715 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliveira C et al. Phenotype, specificity and avidity of antitumour CD8+ T cells in melanoma. Nature 596, 119–125 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simoni Y et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 557, 575–579 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Scheper W et al. Low and variable tumor reactivity of the intratumoral TCR repertoire in human cancers. Nat. Med 25, 89–94 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Caushi JX et al. Transcriptional programs of neoantigen-specific TIL in anti-PD-1-treated lung cancers. Nature 596, 126–132 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gettinger SN et al. A dormant TIL phenotype defines non-small cell lung carcinomas sensitive to immune checkpoint blockers. Nat. Commun 9, 3196 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Datar I et al. Expression analysis and significance of PD-1, LAG-3, and TIM-3 in human non-small cell lung cancer using spatially resolved and multiparametric single-cell analysis. Clin. Cancer Res 25, 4663–4673 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanmamed MF, Eguren-Santamaria I & Schalper KA Overview of lung cancer immunotherapy. Cancer J. 26, 473–484 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]