Abstract
Rhizoplane-rhizosphere nitrogen-fixing microorganisms (diazotrophs) are thought to provide a major source of biologically available nitrogen in salt marshes dominated by Spartina alterniflora. Compositional and functional stability has been demonstrated for this important functional group; however, the quantitative responses of specific diazotroph populations to environmental variability have not been assessed. Changes in the relative abundances of selected rhizoplane diazotrophs in response to long-term fertilization were monitored quantitatively by reverse sample genome probing. Fertilization stimulated Spartina, with plant height nearly tripling after 1 year. Fertilization also resulted in significant changes in interstitial porewater parameters. Diazotrophic activity (acetylene reduction assay) was sensitive to the fertilization treatments and was inhibited in some plots on several sampling dates. However, inhibition was never consistent across all of the replicates within a treatment and activity always recovered. The rhizoplane diazotrophs were quite responsive to environmental variability and to experimental treatments, but none were displaced by either environmental variability or experimental treatments. All strains were detected consistently throughout this study, and extensive spatial heterogeneity in the distribution patterns of these organisms was observed. The physiological traits that differentiate the diazotroph populations presumably support competitiveness and niche specialization, resulting in the observed resilience of the diazotroph populations in the rhizosphere.
Microbial communities are often considered to be quite volatile, changing readily in species richness. Such plasticity has been demonstrated in bioreacter systems (25, 64) and in natural bacterioplankton communities (38, 40, 55). This instability presumably reflects either manipulated or natural environmental variability in these systems, which clearly has substantial impacts on community composition. In contrast, microbial communities in highly structured environments (cyanobacterial mats, rhizosphere soils, and sediments) display a much higher degree of compositional stability (24, 26, 27, 56, 57, 58), suggesting that the impact of environmental variability is somewhat reduced or limited. This buffering effect seemingly helps to stabilize species composition against environmental variability, but the activity levels (19, 20) and relative abundances (60) of microorganisms in situ would be expected to be more responsive to environmental changes.
The rhizospheres of salt marsh grasses provide interesting systems in which to test ideas about microbial community plasticity and environmental buffering effects. Salt marsh systems are highly dynamic, and pronounced variability in primary productivity and edaphic environmental conditions is well known (47, 56, 57). The responsiveness of the rhizosphere microbiota within the major vegetated zones of salt marshes to environmental variability is of particular interest due to the importance of these organisms to marsh productivity.
Salt marshes along the Atlantic and northern Gulf coasts of temperate North America are dominated by the smooth cordgrass Spartina alterniflora (referred to as Spartina hereafter in this report), which grows in a virtual monoculture at low elevations in the marsh (67). High rates of primary production (18, 79) and microbially mediated nutrient cycling (13, 65) make Spartina marshes among the most productive ecosystems known. Numerous studies of salt marsh productivity (e.g., see references 17, 35, 68, and 70) and decomposition (45, 69) suggest that nitrogen is the key nutrient limiting these processes.
Nitrogen fixation has been shown to be a significant source of new nitrogen in salt marshes (33, 53, 78) and is carried out exclusively by certain species of Bacteria and Archaea (59). Nitrogen-fixing (diazotrophic) microorganisms in vegetated salt marsh sediments are largely distributed along root surfaces (rhizoplane) and within surrounding sediments directly affected by host plant activities (rhizosphere) (12, 46, 66). High rates of nitrogen fixation have been demonstrated in these root microenvironments (33, 51, 52, 66, 77). However, we know relatively little about the diazotrophs involved or their responses to key environmental variables.
Extensive physiological and phylogenetic diversity has been described for the Spartina rhizoplane-rhizosphere diazotroph assemblage by classical cultivation (3) and molecular biological approaches (44, 58). This diversity is expected to reflect the microenvironmental heterogeneity of the rhizoplane-rhizosphere (7, 12) and implies a large number of different niches occupied by diazotrophs in situ (2, 7). Phylogenetically related rhizoplane diazotrophs can differ substantially in the ability to utilize different ecologically relevant organic carbon substrates, suggesting that diazotrophs may be differentially adapted to defined physiological niches in situ (2). Physiological niche specialization would provide a competitive advantage to diazotrophs, while the sum of all diazotroph physiological traits would allow for assemblage responsiveness to and resilience (structural and functional) following environmental changes (i.e., functional redundancy). Structural and functional stability has been demonstrated for the diazotroph assemblage associated with Spartina over a seasonal cycle of edaphic variability and host plant ontogeny (58) and in response to short-term interstitial nutrient (56) and host plant manipulations (57). These studies provide preliminary evidence for high levels of assemblage stability; however, the techniques used (PCR and denaturing gradient gel electrophoresis) do not allow for quantification or detailed physiological characterization of the various species detected. In addition, manipulations were only executed for a maximum of 8 weeks and encompassed only a fraction of the Spartina ontogenetic cycle. These are important considerations for understanding the connection between assemblage structural and functional stability and environmental variability.
In this study, selected diazotrophs from short- and tall-form Spartina and Juncus roemerianus (the black needle rush, referred to as Juncus hereafter) rhizoplanes were monitored quantitatively in the rhizosphere of short-form Spartina. Nitrogen-and-phosphorus (N + P) fertilization was used to stimulate Spartina growth and productivity over a period of 13 months. We hypothesized that this treatment would result in changes in the representation of some diazotroph species through alterations in key environmental variables, such as oxygen introduction and carbon supply to the rhizosphere. In addition, nitrogen additions should ultimately alleviate nitrogen limitation within the fertilized plots and, due to the high energetic cost of nitrogen fixation, might select against diazotrophy. By eliminating the presumed selective advantage of diazotrophy, the physiological competitiveness of diazotrophs via niche specialization or other mechanisms can be assessed. Monitoring was also continued after fertilization ceased to evaluate the posttreatment responses of diazotroph populations.
MATERIALS AND METHODS
Sampling site and experimental design.
This study was conducted in the Bly Creek basin in the North Inlet estuary, near Georgetown, S.C. (79°12′ W, 33°20′ N). Spartina is the dominant macrophyte in this basin and grows largely as a monoculture stand. Two distinct growth forms of Spartina can be easily recognized at the study site. Bands of tall (≥1 m in height) Spartina are found along the banks of tidal creeks, while Spartina growth is stunted at higher elevations, resulting in short-form (≤30 cm in height) plants. Other common marsh plants (Juncus roemerianus, Spartina patens, and Salicornia virginica) are localized along the terrestrial fringe at elevations above the short-form Spartina zone.
In June 1998, a six-by-three-block design was established in the short-form Spartina stand. Garden stakes were used to designate 18 4-m2 plots, each separated by 1 m in all directions from neighboring plots. Previous studies in this system have shown that intrusion of roots from plants outside an experimental plot does not significantly impact the results of treatments within the plot (56, 57). In addition, fertilization within the plots did not have an impact on plants outside the boundaries of experimental plots. A sipper for porewater collection (10, 36) was inserted into the center of each plot to a depth of 10 cm and allowed to equilibrate for 1 week prior to the first fertilization treatment.
Plots were randomly assigned to control and experimental treatments. Each fertilized plot received 144 g of phosphorus/month (as P2O5) and 130 g of nitrogen/month (as NH4NO3). We applied phosphate by hand spreading and nitrogen either by ground application of a solution or foliar spraying using a garden sprayer. Control plots received no nutrient additions. All three treatments (control, ground, and foliar) included six replicates each, and all 12 fertilized plots were treated once per month for 1 year. Over the winter period of plant senescence (November to March), foliar spraying was expected to be problematic; consequently, these plots were ground treated to maintain elevated interstitial nutrient levels. Foliar applications were resumed after the emergence of new growth in April 1999. Treatments were stopped following the June 1999 application, resulting in a total of 13 months of N + P additions.
Field sampling.
All plots were sampled 1 week after the monthly N + P addition from June to August 1998 (active growing season), September 1998 (seed head development and flowering), January 1999 (winter die-back), August 1999 (2 months following termination of N + P additions; only porewater parameters, acetylene reduction potentials, and plant shoot heights and leaf numbers were measured), and October 1999 (4 months following termination of N + P additions). Interstitial porewater and sediment cores were collected from all of the plots on each sampling date for the measurements described below.
(i) Porewater chemistry.
Interstitial porewater was collected for analysis of salinity, pH, soluble sulfide, and ammonium concentrations. Salinity and pH were measured using a hand-held refractometer (Leica Inc., Buffalo, N.Y.) and a field-portable pH meter (Cole-Parmer Instrument Co., Chicago, Ill.), respectively. Soluble sulfide was fixed immediately upon collection with an equal volume of 2 N zinc acetate and assayed colorimetrically (29). Ammonium was fixed immediately upon collection by adding 1 drop of concentrated HCl per 2 ml of porewater. Prior to analysis, porewater was filtered (0.2-μm pore size) and assayed using a Technicon autoanalyzer (31). Porewater parameters were statistically analyzed by multiple analysis of variance (MANOVA) with Bonferroni's correction for type I error in SAS (SAS Institute, Cary, N.C.) for treatment effect, for date effect, and to account for parameter interactions. The utility of the MANOVA procedure for analysis of porewater parameters has been discussed previously (56).
(ii) Acetylene reduction assay for nitrogen fixation potential.
A small sediment core (approximately 12 g fresh weight) was collected from each plot using a steel coring device (8 by 1.5 cm) to measure nitrogen fixation potential using an acetylene reduction technique described previously (80). Upon collection, cores were immediately packed on ice and transported back to the Belle W. Baruch Institute field lab for processing. Sediments were transferred to sterile 40-ml serum vials, each containing 10 ml of filter-sterilized seawater substitute (pH 7.8) (80). The vials were crimp sealed, and 10% of the headspace gas was replaced with acetylene. All of the samples were vigorously shaken (1 to 2 s) and incubated at room temperature for 24 h. Acetylene and ethylene were analyzed by injecting 50 μl of headspace gas into a Varian 3700 gas chromatograph equipped with a flame ionization detector and a Carbosphere 80/100 column (the detection limit for ethylene is on the order of 10−10 mol; Alltech, Deerfield, Ill.). Acetylene reduction potentials were analyzed for significant differences by t test and by MANOVA for assessment of covariance with porewater parameters.
(iii) DNA extraction from sediments.
A sediment core (approximately 30 g) was collected from each plot using a steel coring device (7.5 by 2.5 cm) and held on ice for processing at the University of South Carolina main campus (Columbia). DNA extractions were started within 24 h of collection and done as previously described (43, 58). DNA quantity and quality were assessed by fluorometry and agarose gel electrophoresis, respectively, and all purified DNA extracts were stored at −20°C as alcohol precipitates until used.
(iv) Host plant measurements.
Plant shoot heights and leaf numbers were measured in a randomly selected 0.1-m2 quadrant within each plot in August 1999. Shoot heights were taken as the longest leaf, measured from the sediment surface to the leaf tip, and only live leaves were included in the leaf counts. Shoot heights and leaf numbers were statistically analyzed by t test, and covariance between shoot height and leaf number was assessed by regression analysis.
RSGP.
The reverse sample genome probing (RSGP) technique has been described in detail elsewhere (62, 72–74). Briefly, RSGP utilizes whole-community DNA extracted from environmental samples to probe against dot-blotted DNAs from different reference cultures. Under stringent hybridization conditions and using an appropriate internal standard, the quantity of probe signal detected in each dot reflects the abundance of each reference culture organism in the environmental sample. This technique allows the quantification of many different diazotrophic organisms simultaneously.
(i) Diazotroph master blot.
Isolation and characterization of rhizoplane diazotrophs from Spartina and Juncus has been described previously (3). Physiologically different diazotroph strains were evaluated by quantitative whole-genome DNA-DNA hybridization to eliminate redundancy within the strain collection. The details of these experiments are presented elsewhere (2). The master blot used in the RSGP experiments consisted of 27 physiologically and genetically different rhizoplane diazotroph strains (Table 1) and 15 additional organisms from other sources. The latter consisted primarily of diazotrophic and nondiazotrophic oxygen-utilizing and anaerobic strains that are known to associate with grasses or are considered common in intertidal salt marsh sediments (i.e., Azotobacteriaceae, Enterobacteriaceae, Vibrio sp., sulfate reducers, and Clostridium spp.) but were not represented among the diazotrophs isolated from Spartina or Juncus. This collection is not intended to be inclusive of all Spartina-associated diazotrophs but does represent several significant diazotroph groups.
TABLE 1.
Key physiological features and preliminary taxonomic affiliations of selected tall- and short-form Spartina and Juncus rhizoplane diazotrophs used on the master blota
| Strain | Key physiological features | Closest taxonomic group |
|---|---|---|
| S-M1-1 | M, ON, L | Unknown |
| S-C1-6 | F, OP, L | Unknown |
| S-G2-4 | M, OP, C | Unknown |
| S-M1-8 | F, OP, C | Unknown |
| S-C2-8 | F, ON, C, AA | Enterobacteriaceae |
| S-C2-6 | F, OP, C, AA | Vibrionaceae |
| S-G2-2 | M, ON, C | Pseudomonadaceae |
| S-G2-1 | M, ON, C | Azotobacteriaceae |
| S-C2-7 | M, ON, B | Vibrionaceae |
| S-M2-12 | M, OP, C | Unknown |
| T-M2-1 | F, ON, L | Enterobacteriaceae |
| T-M2-14 | M, ON, L | Unknown |
| T-G2-7 | F, ON, L | Enterobacteriaceae |
| T-G2-5 | F, ON, C | Enterobacteriaceae |
| T-S2-12 | A, OP, C, AA | Spirillaceae |
| T-C2-11 | F, OP, B | Vibrionaceae |
| T-G2-3 | F, ON, C | Unknown |
| J-C1-18 | M, OP, L | Spirillaceae |
| J-G1-8 | M, ON, L | Pseudomonadaceae |
| J-S2-16 | M, ON, CA, AA | Rhizobiaceae |
| J-C1-10 | M, OP, CA, AA | Unknown |
| J-C1-1A | F, OP, C | Vibrionaceae |
| J-G1-1 | M, ON, C | Unknown |
| J-S1-10 | M, ON, C | Unknown |
| J-M2-1 | F, OP, C, AA | Vibrionaceae |
| J-S2-2 | F, ON, C | Enterobacteriaceae |
| J-M2-11 | M, ON, L | Unknown |
Strains are designated by the host plant origin (S, short-form Spartina; T, tall-form Spartina; J, Juncus), the carbon source used for isolation (C, citrate; M, malate; S, sucrose; G, glucose), the pH of the isolation medium (1, pH 7.0; 2, pH 7.5); and the strain number. All of the strains are gram-negative motile rods. Physiological characteristics include oxygen requirements (A, obligate aerobe; M, microaerophile; F, facultative anaerobe), cytochrome oxidase production (OP, oxidase positive; ON, oxidase negative), and preferred substrate classes as determined by BIOLOG testing (C, carbohydrates; CA, carboxylic acids; AA, amino acids; L, low utilization of all substrate classes; B, broad substrate range).
(ii) Master blot preparation.
DNA (30 ng) from each reference strain and from bacteriophage lambda (5-, 15-, 20-, 25-, and 30-ng standards) was chemically denatured in a 96-well microtiter plate (39, 42) and loaded onto Duralose-UV membranes (Stratagene, La Jolla, Calif.) using a dot blot apparatus. Prior to blotting of the denatured DNA, each dot on the membrane was marked with 5 μl of India ink solution (1:150 dilution in distilled H2O). Membranes were air dried and baked at 80°C under a vacuum for 2 h and then stored in a desiccator at 4°C until used. All dot blots were used within 2 to 3 weeks of DNA loading.
(iii) Probe preparation.
Field DNA (1 μg) spiked with 0.1 ng of lambda phage DNA (internal standard) was digested with Sau3A1 (New England BioLabs Inc., Beverly, Mass.) for 30 min at 37°C, and the reaction was stopped by heating at 65°C for 10 min. The digests were radiolabeled using a random priming procedure (23) with [α-35S]dATP (ICN Biomedicals, Inc., Costa Mesa, Calif.), and the labeled products were purified using Sephadex G-50 spun columns (Boehringer Mannheim, Indianapolis, Ind.). The specific activity of the radiolabeled DNA was typically 108 cpm μg of DNA−1.
(iv) Master blot hybridization.
Membranes were prehybridized at 40°C for 6 h in 30 ml of prehybridization solution (10× NET, 0.1% sodium dodecyl sulfate [SDS], 3× Denhardt's solution [1× NET is 150 mM NaCl, 15 mM Tris at pH 7.5, and 1 mM EDTA, and 1× Denhardt's solution is 0.02% bovine serum albumin, 0.02% Ficoll, and 0.02% polyvinylpyrrolidone]) containing denatured salmon sperm DNA (50 μg/m) and hybridized at 40°C overnight in 30 ml of hybridization solution (50% formamide, 5× SSC [1× SSC is 150 mM NaCl plus 15 mM sodium citrate], 25 mM potassium phosphate buffer at pH 7.4, 5× Denhardt's solution), denatured salmon sperm DNA (50 μg/ml), and denatured probe (approximately 108 cpm). Membranes were washed twice in 2× SSC–0.1% SDS at room temperature for 5 min each time and twice in 0.5× SSC–0.1% SDS at 45°C for 20 min each time. Dots were punched from the membrane using a hole punch, and the radioactivity in each dot was quantified using a Packard 1500 TR liquid scintillation analyzer (Packard Instrument Co., Downers Grove, Ill.). Quench correction was done by the internal-standard method. Background radioactivity from control (no target DNA) dots was subtracted from that of experimental dots, and the quantity of DNA from each strain was calculated from regression analysis of the internal standard. RSGP profiles were generated using samples from three randomly selected treatment plots for each sampling date. Profiles were compared by PROC MIXED in SAS for a robust assessment of profile differences by treatment and sample date. This analysis procedure averages across individual populations within a profile and determines a representative profile description value for cross comparisons. RSGP profiles were analyzed in greater detail by principal-components analysis (PCA) to assess the contributions of each population to profile variability.
RESULTS
Porewater chemistry.
The means and standard deviations of the porewater parameters across all sampling dates are provided in Table 2. Fertilization had a significant effect on the porewater parameters measured (MANOVA, Wilk's lambda, P < 0.05). Interstitial concentrations of ammonium and sulfide and salinity were the major parameters contributing to the treatment effect. Soluble sulfide was significantly lower in the treatment plots than in the controls (P < 0.05) on the September 1998 and January 1999 sampling dates, while salinity was significantly higher (P < 0.05) in September 1998, January 1999, and October 1999. As expected, monthly nutrient additions maintained significantly higher ammonium concentrations in the treatment plots relative to the controls for the entire year of fertilization (P < 0.05 for the June 1998 to the January 1999 sampling dates). All porewater parameters contributed significant seasonal variability (MANOVA, Wilk's lambda, P < 0.05). However, sulfide and salinity accounted for the majority of this variability, particularly in the final months of the growing season, when both parameters reach seasonal high values (Table 2). Ammonium concentrations returned to background levels, taken as the concentrations measured in the control plots, within 2 months following the termination of N + P application (June 1999). In October 1999, a significant (P < 0.05) pulse of ammonium was measured in all of the plots relative to the previous sampling date (August 1999); however, ammonium did not differ significantly among treatments.
TABLE 2.
Porewater parameters measured at a 10-cm depth in each sample plota
| Parameter and treatment | 1998
|
1999
|
|||||
|---|---|---|---|---|---|---|---|
| June | July | August | September | January | August | October | |
| pH | |||||||
| C | 7.5 ± 0.1 | 7.5 ± 0.3 | 7.3 ± 0.3 | 7.2 ± 0.2 | 7.3 ± 0.1 | 7.7 ± 0.2 | 7.4 ± 0.1 |
| G | 7.4 ± 0.2 | 7.6 ± 0.3 | 7.3 ± 0.4 | 7.3 ± 0.5 | 6.9 ± 0.4 | 7.5 ± 0.2 | 7.3 ± 0.2 |
| F | 7.4 ± 0.1 | 7.4 ± 0.1 | 7.3 ± 0.1 | 7.1 ± 0.1 | 6.9 ± 0.2 | 7.5 ± 0.2 | 7.5 ± 0.4 |
| Salinity (ppt) | |||||||
| C | 33.5 ± 2.8 | 34.8 ± 3.3 | 36.2 ± 1.5 | 35.8 ± 1.7 | 33.2 ± 1.0 | 39.3 ± 1.0 | 31.3 ± 1.5 |
| G | 33.2 ± 3.1 | 35.0 ± 2.4 | 38.8 ± 2.7 | 38.8 ± 2.0 | 36.2 ± 1.8 | 47.7 ± 2.9 | 32.0 ± 4.4 |
| F | 31.3 ± 1.6 | 36.3 ± 1.2 | 37.2 ± 1.8 | 37.8 ± 1.0 | 36.0 ± 1.3 | 46.8 ± 2.1 | 33.0 ± 3.7 |
| H2S (mM) | |||||||
| C | 3.9 ± 0.6 | 3.6 ± 0.5 | 3.1 ± 1.1 | 4.8 ± 1.2 | 1.0 ± 0.7 | 4.9 ± 0.4 | 4.7 ± 0.7 |
| G | 3.0 ± 0.9 | 2.4 ± 0.8 | 2.2 ± 1.4 | 2.3 ± 1.8 | 0.1 ± 0.1 | 4.3 ± 0.8 | 4.2 ± 0.3 |
| F | 4.2 ± 0.6 | 3.2 ± 0.9 | 3.1 ± 1.0 | 3.2 ± 1.0 | 0.0 ± 0.0 | 4.9 ± 0.3 | 4.7 ± 0.5 |
| NH4 (μM) | |||||||
| C | 5.9 ± 3.3 | 6.1 ± 4.7 | 7.8 ± 5.7 | 9.3 ± 3.2 | 4.9 ± 3.8 | 9.1 ± 2.3 | 21.8 ± 17.3 |
| G | 24.9 ± 0.0 | 15.5 ± 8.8 | 43.4 ± 24.7 | 19.6 ± 17.8 | 29.7 ± 13.2 | 9.9 ± 5.3 | 41.3 ± 47.1 |
| F | 24.9 ± 0.0 | 21.4 ± 4.2 | 42.6 ± 16.2 | 33.8 ± 21.9 | 27.8 ± 13.3 | 10.9 ± 4.9 | 27.9 ± 11.1 |
C, control; G, ground treatment; F, foliar spray treatment. Values are the means of six results ± the standard deviations.
Host plant response.
Spartina was very responsive to monthly N + P additions. By the end of the first growing season (September 1998), all treatment plots were noticeably greener and taller than the control plots (personal observation). Fertilized plots remained green for the duration of this study, while winter die-back was observed in the control plots. The appearance and life cycle of Spartina in the control plots were typical of this site in the Bly Creek basin.
Plant shoot heights and live-leaf numbers were measured in August 1999 (2 months after the final N + P addition) for all plots. Spartina was significantly taller in the ground-treated and foliar spray-treated plots relative to the controls (P < 0.05). Shoots in fertilized plots more than doubled in height after 1 year of fertilization, with shoot heights averaging 65.5 ± 21.2, 70.5 ± 13.7, and 28.7 ± 12.0 cm for ground-treated, foliar spray-treated, and control plots, respectively. Shoot heights did not differ significantly between fertilization treatments. Spartina in the ground-treated and foliar spray-treated plots also had significantly more leaves per shoot compared to the controls (P < 0.05). Leaf numbers did not differ significantly between the two fertilization treatments. Shoot height and leaf numbers were only weakly correlated by regression: R2 = 0.39 for ground-treated plots, R2 = 0.20 for foliar spray-treated plots, and R2 = 0.42 for control plots.
Diazotrophic activity.
Acetylene reduction measurements were scored as detectable activity or no detectable activity (1 or 0, respectively) to make the trends in activity more apparent. Acetylene reduction activities (ARA), reported as the fraction of replicate plots with detectable ethylene production, by treatment and sampling date are provided in Table 3. The handling of the data in this manner, as opposed to production rates or product yields, had no effect on the outcome of the statistical analyses performed (data not shown) and was preferred since slurry incubations can yield rate measurements that are substantially different from in situ rates (76). ARA contributed significantly (MANOVA, Wilk's lambda, P < 0.05) to the variability between treatments and across sample dates. However, a significant difference (P < 0.05) in ARA between fertilization treatments was found only in January 1999. At that time, all of the control plots had detectable activity while only one out of six replicate ground-treated and foliar spray-treated plots had detectable activity. Seasonally, there was significantly (P < 0.05) higher ARA in August 1999 compared to August 1998 and October 1999; all other sampling dates were not significantly different.
TABLE 3.
ARA of each sample plota
| Treatment | 1998
|
1999
|
|||||
|---|---|---|---|---|---|---|---|
| June | July | August | September | January | August | October | |
| C | 4/6 | 5/6 | 2/6 | 5/6 | 6/6 | 5/6 | 4/6 |
| G | 4/6 | 4/6 | 1/6 | 4/6 | 1/6 | 4/6 | 1/6 |
| F | 1/6 | 4/6 | 2/6 | 4/6 | 1/6 | 5/6 | 2/6 |
C, control; G, ground treatment; F, foliar spray treatment. ARA results are presented by treatment as number of plots with detectable ethylene production (i.e., nitrogenase activity)/total number of replicate plots examined.
RSGP.
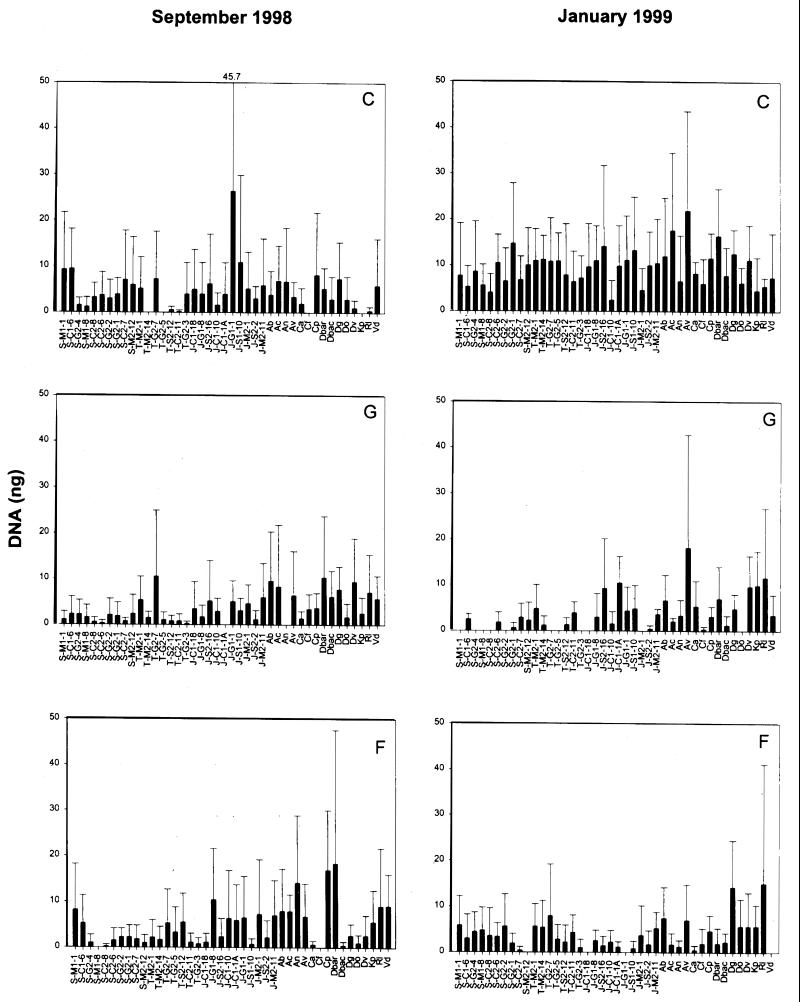
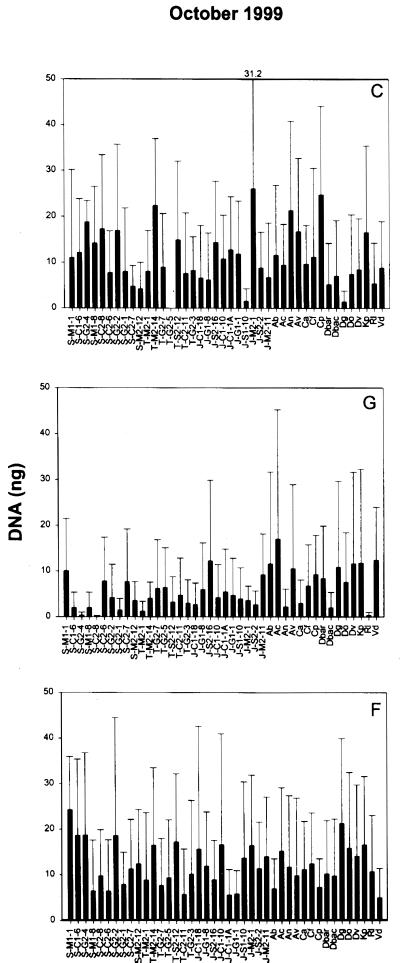
The reverse sample genome probing profiles for all treatments and sample dates are provided in Fig. 1. On average, 108 ng of labeled probe DNA was used in each hybridization experiment. Based on the RSGP profiles (Fig. 1), it is clear that the rhizoplane diazotrophs and other reference strains on the master blot did not bind a large fraction of the total probe. Pervasive treatment effects were not supported by these profiles, primarily due to variability among replicates for each treatment and date. However, a general trend toward decreasing diazotroph abundance in the ground-treated and foliar spray-treated plots in June 1998 and July 1998 presumably reflects the impact of fertilization. This impact is clearly delayed by ground treatment compared to the foliar spray treatment, which had an immediate negative impact on the majority of the populations. A similar trend was also noted in January 1999, when overall abundances were lower in the treatment plots relative to the controls. Seasonal effects were statistically (P < 0.05) supported for all treatments. Control plot profiles differed significantly (P < 0.05) among June 1998 to July 1998, July 1998 to September 1998, January 1999, and October 1999. The ground-treated plot profiles differed significantly (P < 0.05) among June 1998 to September 1998 and January 1999, July 1998 to September 1998, September 1998 to October 1999, and January 1999 to October 1999. In the foliar spray treatment plot profiles, significant differences (P < 0.05) occurred among sample dates June 1998 to October 1999, July 1998 to October 1999, September 1998 to October 1999, and January 1999 to October 1999.
FIG. 1.
RSGP profiles for each sample date by treatment (n = 3). In July, n = 2 for the foliar spray treatment profile. Values are mean nanograms of DNA ± the standard deviation for each rhizoplane diazotroph and reference strain. Standard deviations exceeding the y-axis scale are provided across the top of each panel. Strains are designated by the host plant origin (S, short-form Spartina; T, tall-form Spartina; J, Juncus), the carbon source used for isolation (C, citrate; M, malate; S, sucrose; G, glucose), the pH of the isolation medium (1, pH 7.0; 2, pH 7.5), and the strain number. Reference strains are abbreviated as follows: Ab, Azospirillum brasilense; Ac, Azotobacter chroococcum; An, Arcobacter nitrofigilis; Av, Azotobacter vinelandii; Ca, Clostridium acidiurici; Cf, Clostridium formicoaceticum; Cp, Clostridium perfringens; Dbar, Desulfovibrio baarsii; Dbac, Desulfovibrio bacculatus; Dg, Desulfovibrio gigas; Do, Desulfotomaculum orientis; Dv, Desulfovibrio vulgaris; Kp, Klebsiella pneumoniae; Rl, Rhizobium leguminosarum; Vd, Vibrio diazotrophicus. C, control; G, ground treatment; F, foliar spray treatment.
All strains accounted for roughly the same low percentages of the variability among RSGP profiles as assessed by PCA (data not shown). Up to 10 PC values were necessary to explain at least 50% of the variability among profiles when they were analyzed in toto by treatment or sample date.
DISCUSSION
Previous studies employing PCR-based methods and the acetylene reduction assay have demonstrated substantial stability in species composition and ecological function (diazotrophy) of the Spartina rhizoplane-rhizosphere diazotroph assemblage (56, 57, 58). In this study, selected rhizoplane diazotrophs were monitored to examine this stability at the level of population representation by quantitative RSGP. Under the hybridization conditions described, we estimated our lower limit for quantification of a given diazotroph to be 5 ng of DNA, approximately 5 × 106 cells (assuming 5 fg of DNA/cell). As expected, this technique is less sensitive than PCR-based methods (approximately 0.01 ng of DNA or 104 cells when using nifH primers; Y. M. Piceno and C. R. Lovell, unpublished data) but avoids amplification biases, allowing selected species (or ecotypes) to be quantified accurately. In addition, quantification of physiologically characterized diazotroph species can provide new insights into how populations of these microorganisms interact with and are controlled by their environment.
Obviously, the utility of the RSGP technique is contingent on the ability to isolate reference organisms into pure culture, the biases of which are well known and documented (1, 11, 63), or to select environmentally relevant type cultures for the master blot. All of the rhizoplane diazotrophs and other reference organisms included on the master blot (or at least their highly homologous close relatives) were detected at all dates during this study. These organisms also responded to environmental variability (natural and experimental) by increasing or decreasing in abundance. Consequently, it is expected that the diazotrophs selected for these experiments provided a suitable though clearly not inclusive group for examining responses of Spartina rhizoplane diazotrophs to environmental variability. Reference strains from non-Spartina sources were included to provide some representation of groups of anaerobes known to occur in vegetated salt marsh sediments (sulfate reducers and Clostridium spp.) (21, 22, 28, 34, 37) and additional diazotrophs that have been shown previously to be associated with Spartina or other grasses (3, 21). However, the average population sizes of these strains were not significantly different from those of Spartina rhizoplane diazotrophs (data not shown). The lack of numerical dominance of any of these diazotrophic strains may reflect the great extent of diazotroph diversity in this system (44).
The fertilization treatments clearly had a substantial impact on Spartina production and above-ground biomass. Increased evapotranspiration and oxygenation of the root zone microenvironment, likely resulting from this stimulation, also had an effect on interstitial porewater chemistry in these plots. Nitrogen additions and increased host plant production have been shown in other fertilization studies to result in elevated interstitial salinities (15, 16). Foliar spraying appears to provide a more direct route to the rhizoplane-rhizosphere microenvironment (4, 5, 75) compared to ground treatment, but both methods were effective in increasing interstitial nitrogen levels. The rhizoplane diazotrophs and other reference strains were clearly physiologically responsive (as assessed by the amount of variability in population size) to these rather dramatic changes in the abiotic and biotic environment; however, relative population size was particularly stable. A tight coupling between rhizoplane-rhizosphere diazotrophic activity and Spartina production has been demonstrated previously (9, 80), presumably reflecting a dependence of diazotrophs on host plant-derived carbon-energy resources (2, 9, 32, 33, 41). The uncoupling of this stimulatory effect on diazotrophic function (Table 3) or species richness (Fig. 1) of the strains examined suggests that other factors are limiting to growth and activity in the root microenvironments. However, it is clear that while environmental variability can be extreme, these organisms are not readily displaced by such variability.
Changes in edaphic variables are expected to have lesser impacts on rhizoplane diazotrophs relative to that of the host plant. Several oxygen-utilizing rhizoplane diazotrophs have been shown to be exceptionally tolerant of soluble sulfide exposure despite differing optimal sulfide concentration ranges for nitrogen fixation (J. R. LaRocque and C. R. Lovell, unpublished data). Presumably, similar tolerance for broad ranges of other important edaphic variables (pH, salinity, redox potential, etc.) may also occur. These findings suggest that edaphic factors are important regulators of diazotrophic activity but have relatively little impact on diazotroph abundance. However, our ability to interpret these experiments is somewhat limited by the fact that Spartina exudation (6, 14, 30, 71) and interstitial porewater parameters can change rapidly and simultaneously in intertidal sediments (14, 49, 54, 56–58). Consequently, a combination of host plant and edaphic variables may be more realistic determinants of diazotroph abundance and activity in situ than either in isolation.
High variability among replicate profiles likely reflects the extent of microenvironmental heterogeneity in these sediments (48) and along the Spartina root surfaces (8). Physiological specialization of rhizoplane diazotrophs implies niche separation (2), and presumably the distribution patterns of diazotrophs and the niches they occupy can vary over relatively small spatial scales. Spatial variability of sulfate reducers over such small scales has been demonstrated in salt marsh sediments (22). In addition, microscopic examinations of sediment particles (8, 48) and root surfaces (8, 50, 61) have shown microorganisms distributed as discontinuous aggregates with a major portion of the available surface area uncolonized. Extensive spatial heterogeneity would clearly make population level comparisons intrinsically difficult to interpret. Valid trends in RSGP profiles may be masked by variability between samples; consequently, it may be more realistic to treat each profile independently. However, there were no gross differences in averaged RSGP profiles in response to the treatments used.
All of the strains on the master blot were detected throughout this study, suggesting that even under highly enriched conditions (both combined nitrogen and host plant-derived carbon), competition between populations may be either low or simply insufficient to displace any species. Diazotroph niche specialization should prevent any strain or ecotype from numerically dominating a highly heterogeneous habitat like the rhizoplane-rhizosphere. This hypothesis is supported by the findings of Lovell et al. (44), who reported little overlap of diazotroph nifH sequences recovered from different wetland plants. If these findings are consistent for diazotrophs associated with Spartina and other wetland grasses, then it could be hypothesized that r species (fast-growing, opportunistic species) would not be successful competitors in these assemblages unless the assemblages were drastically disturbed in some way. Consequently, all of the diazotrophs we examined might behave as k species (slower-growing, resilient species). Alternatively, physiological niche adaptation may allow diazotrophs to function as both r- and k-selected species. This capability would permit responsiveness, by rapid growth and/or increased levels of activity, to favorable conditions, while slow growth or even dormancy could be maintained under limiting conditions. Minimal competition between populations and high levels of environmental heterogeneity would promote compositional stability, while differences in the physiological responses of each population would allow the maintenance of environmental function (i.e., functional redundancy) across a wide range of environmental conditions. The physiological adaptations that differentiate these rhizoplane diazotrophs clearly provide competitive advantages for diazotrophs in their specific niches.
The maintenance of diazotrophic activity in the majority of the control plots throughout this study clearly demonstrates that some fraction of the diazotroph assemblage is consistently fixing nitrogen, and is presumably abundant, despite host plant ontogeny and changes in edaphic factors. Nitrogen fixation was inhibited in many of the fertilized plots on several dates, likely due to the high interstitial concentrations of nitrogen in these plots. Despite the elevated interstitial nitrogen levels maintained in the treatment plots, inhibition of diazotrophy was never consistent across all of the replicate plots for either fertilization treatment and activity always recovered to levels comparable to that of the controls by the following sample date. Similar results showing substantial short-term resilience of diazotrophic activity have been presented previously (21, 80). In contrast, Hanson (32) found that nitrogen fixation rates were stimulated by nitrogen additions but substantially less nitrogen was added in that study relative to this and other studies. Clearly, the quantity of nitrogen added to the experimental plots in this study exceeded the requirements of the host plant; however, microenvironments suitable for nitrogen fixation were maintained despite this excess.
Previous nonquantitative studies demonstrated remarkable stability of the Spartina rhizoplane-rhizosphere diazotroph assemblage in response to natural and experimentally manipulated (short-term) environmental variability (56–58). Here, both selected rhizoplane diazotrophs and diazotrophic activity were persistent and resilient despite dramatic shifts in the abiotic and biotic environments resulting from long-term fertilization. We credit these features of the diazotroph assemblage to the physiological adaptations of diazotrophs to defined niches in situ and to the heterogeneity of the rhizosphere microenvironment. These physiological and environmental features seemingly support the maintenance of diazotroph assemblage structure and environmental function over a wide range of environmental conditions (functional redundancy) (56–58).
ACKNOWLEDGMENTS
We acknowledge Peter Bergholz for his assistance in the field and with porewater analysis, Holmes Finch for assistance with the statistical analyses performed, and the Belle W. Baruch Institute for Marine Biology and Coastal Research for access to field sites. We also thank Yvette Piceno for providing helpful comments on the manuscript.
This research was supported by NSF awards DEB-9407596 and DEB-9903623 to C.R.L. and a Slocum Lunz Foundation fellowship awarded to C.E.B.
REFERENCES
- 1.Amann R I, Ludwig W, Schleifer K H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagwell C E, Lovell C R. Microdiversity of culturable diazotrophs from the rhizoplanes of the salt marsh grasses Spartina alterniflora and Juncus roemerianus. Microb Ecol. 2000;39:128–136. doi: 10.1007/s002480000017. [DOI] [PubMed] [Google Scholar]
- 3.Bagwell C E, Piceno Y M, Ashburne-Lucas A, Lovell C R. Physiological diversity of the rhizosphere diazotroph assemblages of selected salt marsh grasses. Appl Environ Microbiol. 1998;64:4276–4282. doi: 10.1128/aem.64.11.4276-4282.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balasubramanian A, Rangaswami G. Studies on the influence of foliar nutrient sprays on the root exudation pattern in four crop plants. Plant Soil. 1969;30:210–220. [Google Scholar]
- 5.Balasubramanian A, Rangaswami G. Influence of foliar application of chemicals on the root exudations and rhizosphere microflora of Sorghum vulgare and Crotalaria juncea. Folia Microbiol. 1973;18:492–498. doi: 10.1007/BF02876796. [DOI] [PubMed] [Google Scholar]
- 6.Barber D A, Lynch J M. Microbial growth in the rhizosphere. Soil Biol Biochem. 1977;9:305–308. [Google Scholar]
- 7.Bowen G D. Misconceptions, concepts and approaches in rhizosphere biology. In: Ellwood D C, Latham M J, Hedger J N, Lynch J M, Slater J H, editors. Contemporary microbial ecology. New York, N.Y: Academic Press, Inc.; 1980. pp. 283–304. [Google Scholar]
- 8.Bowen G D, Rovira A D. Microbial colonization of plant roots. Annu Rev Phytopathol. 1976;14:121–144. [Google Scholar]
- 9.Boyle C D, Patriquin D G. Carbon metabolism of Spartina alterniflora Loisel in relation to that of associated nitrogen-fixing bacteria. New Phytol. 1981;89:275–288. [Google Scholar]
- 10.Bradley P M, Morris J T. The influence of salinity on the kinetics of NH4+ uptake in Spartina alterniflora. Oecologia. 1991;85:375–380. doi: 10.1007/BF00320613. [DOI] [PubMed] [Google Scholar]
- 11.Brock T D. The study of microorganisms in situ: progress and problems. In: Fletcher M, Gray T R G, Jones J G, editors. Ecology of Microbial Communities, 41st Symposium of the Society for General Microbiology. New York, N.Y: Cambridge University Press; 1987. pp. 1–17. [Google Scholar]
- 12.Campbell R, Greaves M P. Anatomy and community structure of the rhizosphere. In: Lynch J M, editor. The rhizosphere. New York, N.Y: John Wiley & Sons, Inc.; 1990. pp. 11–34. [Google Scholar]
- 13.Capone D G, Carpenter E J. Nitrogen fixation in the marine environment. Science. 1982;217:1140–1142. doi: 10.1126/science.217.4565.1140. [DOI] [PubMed] [Google Scholar]
- 14.Capone D G, Budin J M. Nitrogen fixation associated with rinsed roots and rhizomes of the eelgrass Zostera marina. Plant Physiol. 1982;70:1601–1604. doi: 10.1104/pp.70.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chalmers A G. The effects of fertilization on nitrogen distribution in a Spartina alterniflora salt marsh. Estuarine Coastal Mar Sci. 1979;8:327–337. [Google Scholar]
- 16.Christian R R, Bancroft K, Wiebe W J. Resistance of the microbial community within salt marsh soils to selected perturbations. Ecology. 1978;59:1200–1210. [Google Scholar]
- 17.Dai T, Wiegert R G. A field study of photosynthetic capacity and its response to nitrogen fertilization in Spartina alterniflora. Estuarine Coastal Shelf Sci. 1997;45:273–283. [Google Scholar]
- 18.Dame R F, Kenny P D. Variability of Spartina alterniflora primary production in the euhaline North Inlet estuary. Mar Ecol Prog Ser. 1986;32:71–80. [Google Scholar]
- 19.Degens B P. Decreases in microbial functional diversity do not result in corresponding changes in decomposition under different moisture conditions. Soil Biol Biochem. 1998;30:1989–2000. [Google Scholar]
- 20.Degens B P. Microbial functional diversity can be influenced by the addition of simple organic substrates to soil. Soil Biol Biochem. 1998;30:1981–1988. [Google Scholar]
- 21.Dicker H J, Smith D W. Enumeration and relative importance of acetylene-reducing (nitrogen-fixing) bacteria in a Delaware salt marsh. Appl Environ Microbiol. 1980;39:1019–1025. doi: 10.1128/aem.39.5.1019-1025.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edgcomb V P, McDonald J H, Devereux R, Smith D W. Estimation of bacterial cell numbers in humic acid-rich salt marsh sediments with probes directed to 16S ribosomal DNA. Appl Environ Microbiol. 1999;65:1516–1523. doi: 10.1128/aem.65.4.1516-1523.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feinberg A P, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 24.Felske A, Akkermans A D L. Spatial homogeneity of abundant bacterial 16S rRNA molecules in grassland soils. Microb Ecol. 1998;36:31–36. doi: 10.1007/s002489900090. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez A, Huang S, Seston S, Xing J, Hickey R, Criddle C, Tiedje J. How stable is stable? Function versus community composition. Appl Environ Microbiol. 1999;65:3697–3704. doi: 10.1128/aem.65.8.3697-3704.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferris M J, Ward D M. Seasonal distributions of dominant 16S rRNA-defined populations in a hot spring microbial mat examined by denaturing gradient gel electrophoresis. Appl Environ Microbiol. 1997;63:1375–1381. doi: 10.1128/aem.63.4.1375-1381.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferris M J, Muyzer G, Ward D M. Denaturing gradient gel electrophoresis profiles of 16S rRNA-defined populations inhabiting a hot spring microbial mat community. Appl Environ Microbiol. 1996;62:340–346. doi: 10.1128/aem.62.2.340-346.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gandy E L, Yoch D C. Relationship between nitrogen-fixing sulfate reducers and fermenters in salt marsh sediments and roots of Spartina alterniflora. Appl Environ Microbiol. 1988;54:2031–2036. doi: 10.1128/aem.54.8.2031-2036.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenberg A E, Connors J J, Jenkins D. Standard methods for the examination of water and wastewater. 15th ed. Washington, D.C.: American Public Health Association; 1980. [Google Scholar]
- 30.Hale M G, Moore L D, Griffin G J. Root exudates and exudation. In: Dommergues Y R, Krupa S V, editors. Interactions between non-pathogenic soil microorganisms and plants. New York, N.Y: Elsevier Scientific Publishing Co.; 1978. pp. 163–203. [Google Scholar]
- 31.Hansen H P, Grasshoff K. Automated chemical analysis. In: Grasshoff K, Ehrhardt M, Kremling K, editors. Methods of seawater analysis. 2nd ed. Deerfield Beach, Fla: Verlag Chemie; 1983. pp. 347–395. [Google Scholar]
- 32.Hanson R B. Nitrogen fixation (acetylene reduction) in a salt marsh amended with sewage sludge and organic carbon and nitrogen compounds. Appl Environ Microbiol. 1977;33:846–852. doi: 10.1128/aem.33.4.846-852.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanson R B. Nitrogen fixation activity (acetylene reduction) in the rhizosphere of salt marsh angiosperms, Georgia, U.S.A. Bot Mar. 1983;26:49–59. [Google Scholar]
- 34.Hines M E, Evans R S, Genthner B R S, Willis S G, Friedman S, Rooney-Varga J N, Devereux R. Molecular phylogenetic and biogeochemical studies of sulfate-reducing bacteria in the rhizosphere of Spartina alterniflora. Appl Environ Microbiol. 1999;65:2209–2216. doi: 10.1128/aem.65.5.2209-2216.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hopkinson C S, Schubauer J P. Static and dynamic aspects of nitrogen cycling in the salt marsh graminoid Spartina alterniflora. Ecology. 1984;65:961–969. [Google Scholar]
- 36.Howes B L, Dacey J W, Wakeham S G. Effects of sampling technique on measurements of porewater constituents in salt marsh sediments. Limnol Oceanogr. 1985;30:221–227. [Google Scholar]
- 37.Jones K. Nitrogen fixation in a salt marsh. J Ecol. 1974;62:553–565. [Google Scholar]
- 38.Jurgens K, Pernthaler J, Schalla S, Amann R. Morphological and compositional changes in a planktonic bacterial community in response to enhanced protozoan grazing. Appl Environ Microbiol. 1999;65:1241–1250. doi: 10.1128/aem.65.3.1241-1250.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kafatos F C, Jones C W, Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979;7:1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindstrom E S. Bacterioplankton community composition in a boreal forest lake. FEMS Microbiol Ecol. 1998;27:163–174. [Google Scholar]
- 41.Livingstone D C, Patriquin D G. Nitrogenase activity in relation to season, carbohydrates and organic acids in a temperate zone root association. Soil Biol Biochem. 1980;12:543–546. [Google Scholar]
- 42.Lovell C R, Hui Y. Design and testing of a functional group-specific DNA probe for the study of natural populations of acetogenic bacteria. Appl Environ Microbiol. 1991;57:2602–2609. doi: 10.1128/aem.57.9.2602-2609.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lovell C R, Piceno Y M. Purification of DNA from estuarine sediments. J Microbiol Methods. 1994;20:161–174. [Google Scholar]
- 44.Lovell C R, Piceno Y M, Quattro J M, Bagwell C E. Molecular analysis of diazotroph diversity in the rhizosphere of the smooth cordgrass, Spartina alterniflora. Appl Environ Microbiol. 2000;66:3814–3822. doi: 10.1128/aem.66.9.3814-3822.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marinucci A C, Hobbie J E, Helfrich J V K. Effect of litter nitrogen on decomposition and microbial biomass in Spartina alterniflora. Microb Ecol. 1983;9:27–40. doi: 10.1007/BF02011578. [DOI] [PubMed] [Google Scholar]
- 46.McClung C R, van Berkum P, Davis R E, Sloger C. Enumeration and localization of N2-fixing bacteria associated with roots of Spartina alterniflora Loisel. Appl Environ Microbiol. 1983;45:1914–1920. doi: 10.1128/aem.45.6.1914-1920.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morris J T, Haskins B. A 5-yr record of aerial primary production and stand characteristics of Spartina alterniflora. Ecology. 1990;71:2209–2217. [Google Scholar]
- 48.Nedwell D B, Gray T R G. Soils and sediments as matrices for microbial growth. In: Fletcher M, Gray T R G, Jones J G, editors. Ecology of Microbial Communities, 41st Symposium of the Society for General Microbiology. New York, N.Y: Cambridge University Press; 1987. pp. 21–54. [Google Scholar]
- 49.Newell S Y, Hopkinson C S, Scott L A. Patterns of nitrogenase activity (acetylene reduction) associated with standing, decaying shoots of Spartina alterniflora. Estuarine Coastal Shelf Sci. 1992;35:127–140. [Google Scholar]
- 50.Newman E I, Bowen H J. Patterns of distribution of bacteria on root surfaces. Soil Biol Biochem. 1974;6:205–209. [Google Scholar]
- 51.O'Donohue M J, Moriarty D J W, MacRae I C. A comparison of methods for determining rates of acetylene reduction (nitrogen fixation) by heterotrophic bacteria in seagrass sediment. J Microbiol Methods. 1991;13:171–183. [Google Scholar]
- 52.Patriquin D G. Factors affecting nitrogenase activity (acetylene reducing activity) associated with excised roots of the emergent halophyte Spartina alterniflora Loisel. Aquat Bot. 1978;4:193–210. [Google Scholar]
- 53.Patriquin D G, McClung C R. Nitrogen accretion, and the nature and possible significance of N2 fixation (acetylene reduction) in a Nova Scotian Spartina alterniflora stand. Mar Biol. 1978;47:227–242. [Google Scholar]
- 54.Patriquin D, Knowles R. Nitrogen fixation in the rhizosphere of marine angiosperms. Mar Biol. 1972;16:49–58. [Google Scholar]
- 55.Pernthaler J, Glockner F O, Unterholzner S, Alfreider A, Psenner R, Amann R. Seasonal community and population dynamics of pelagic bacteria and archaea in a high mountain lake. Appl Environ Microbiol. 1998;64:4299–4306. doi: 10.1128/aem.64.11.4299-4306.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Piceno Y M, Lovell C R. Stability in natural bacterial communities. I. Nutrient addition effects on rhizosphere diazotroph assemblage composition. Microb Ecol. 2000;39:32–40. doi: 10.1007/s002489900192. [DOI] [PubMed] [Google Scholar]
- 57.Piceno Y M, Lovell C R. Stability in natural bacterial communities. II. Plant resource allocation effects on rhizosphere diazotroph assemblage composition. Microb Ecol. 2000;39:41–48. doi: 10.1007/s002489900191. [DOI] [PubMed] [Google Scholar]
- 58.Piceno Y M, Noble P A, Lovell C R. Spatial and temporal assessment of diazotroph assemblage composition in vegetated salt marsh sediments using denaturing gradient gel electrophoresis analysis. Microb Ecol. 1999;38:157–167. doi: 10.1007/s002489900164. [DOI] [PubMed] [Google Scholar]
- 59.Postgate J. Nitrogen fixation. 3rd ed. Cambridge, United Kingdom: Cambridge University Press; 1998. [Google Scholar]
- 60.Rooney-Varga J N, Devereux R, Evans R S, Hines M E. Seasonal changes in the relative abundance of uncultivated sulfate-reducing bacteria in a salt marsh sediment and in the rhizosphere of Spartina alterniflora. Appl Environ Microbiol. 1997;63:3895–3901. doi: 10.1128/aem.63.10.3895-3901.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rovira A D, Newman E I, Bowen H J, Campbell R. Quantitative assessment of the rhizoplane microflora by direct microscopy. Soil Biol Biochem. 1974;6:211–216. [Google Scholar]
- 62.Shen Y, Stehmeier L G, Voordouw G. Identification of hydrocarbon-degrading bacteria in soil by reverse sample genome probing. Appl Environ Microbiol. 1998;64:637–645. doi: 10.1128/aem.64.2.637-645.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Staley J T, Konopka A. Measurement of in situ activities of nonphotosynthetic microorganisms in aquatic and terrestrial habitats. Annu Rev Microbiol. 1985;39:321–346. doi: 10.1146/annurev.mi.39.100185.001541. [DOI] [PubMed] [Google Scholar]
- 64.Stoffels M, Amann R, Ludwig W, Hekmat D, Schleifer K H. Bacterial community dynamics during start-up of a trickle-bed bioreactor degrading aromatic compounds. Appl Environ Microbiol. 1998;64:930–939. doi: 10.1128/aem.64.3.930-939.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Teal J M, Howes B L. Interannual variability of a salt-marsh ecosystem. Limnol Oceanogr. 1996;41:802–809. [Google Scholar]
- 66.Teal J M, Valiela I, Berlo D. Nitrogen fixation by rhizosphere and free-living bacteria in salt marsh sediments. Limnol Oceanogr. 1979;24:126–132. [Google Scholar]
- 67.Turner R E. Geographic variations in salt marsh macrophyte production: a review. Contrib Mar Sci. 1976;20:47–68. [Google Scholar]
- 68.Valiela I, Teal J M. Nutrient limitation in salt marsh vegetation. In: Riemold R J, Queen W H, editors. Ecology of halophytes. New York, N.Y: Academic Press, Inc.; 1974. pp. 547–563. [Google Scholar]
- 69.Valiela I, Teal J M, Allen S D, Van Etten R, Goehringer D, Volkmann S. Decomposition in salt marsh ecosystems: the phases and major factors affecting disappearance of above-ground organic matter. J Exp Mar Biol Ecol. 1985;89:29–54. [Google Scholar]
- 70.Valiela I, Teal J M, Sass W J. Production and dynamics of salt marsh vegetation and the effects of experimental treatment with sewage sludge. Biomass, production, and species composition. J Appl Ecol. 1975;12:973–981. [Google Scholar]
- 71.Vancura V, Prikryl Z, Kalachova L, Wurst M. Some quantitative aspects of root exudation. Ecol Bull. 1977;25:381–386. [Google Scholar]
- 72.Voordouw G, Voordouw J K, Karkhoff-Schweizer R R, Fedorak P M, Westlake D W S. Reverse sample genome probing, a new technique for identification of bacteria in environmental samples by DNA hybridization, and its application to the identification of sulfate-reducing bacteria in oil field samples. Appl Environ Microbiol. 1991;57:3070–3078. doi: 10.1128/aem.57.11.3070-3078.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Voordouw G, Voordouw J K, Jack T R, Fought J, Fedorak P M, Westlake D W S. Identification of distinct communities of sulfate-reducing bacteria in oil fields by reverse sample genome probing. Appl Environ Microbiol. 1992;58:3542–3552. doi: 10.1128/aem.58.11.3542-3552.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Voordouw G, Shen Y, Harrington C S, Telang A J, Jack T R, Westlake D W S. Quantitative reverse sample genome probing of microbial communities and its application to oil field production waters. Appl Environ Microbiol. 1993;59:4101–4114. doi: 10.1128/aem.59.12.4101-4114.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vrany J, Vancura V, Macura J. The effect of foliar application of some readily metabolized substances, growth regulators, and antibiotics on rhizosphere microflora. Folia Microbiol. 1962;7:61–70. [Google Scholar]
- 76.Welsh D T, Bourgues S, de Wit R, Herbert R A. Seasonal variations in nitrogen-fixation (acetylene reduction) and sulphate-reduction rates in the rhizosphere of Zostera noltii: nitrogen fixation by sulphate-reducing bacteria. Mar Biol. 1996;125:619–628. [Google Scholar]
- 77.Whiting G J, Gandy E L, Yoch D C. Tight coupling of root-associated nitrogen fixation and plant photosynthesis in the salt marsh grass Spartina alterniflora and carbon dioxide enhancement of nitrogenase activity. Appl Environ Microbiol. 1986;52:108–113. doi: 10.1128/aem.52.1.108-113.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Whiting G J, Morris J T. Nitrogen fixation (C2H2 reduction) in a salt marsh: its relationship to temperature and an evaluation of an in situ chamber technique. Soil Biol Biochem. 1986;18:515–521. [Google Scholar]
- 79.Whittaker R H. Communities and ecosystems. 2nd ed. New York, N.Y: MacMillan Publishing Co., Inc.; 1975. Production; pp. 192–235. [Google Scholar]
- 80.Yoch D C, Whiting G J. Evidence for NH4+ switch-off regulation of nitrogenase activity by bacteria in salt marsh sediments and roots of the grass Spartina alterniflora. Appl Environ Microbiol. 1986;51:143–149. doi: 10.1128/aem.51.1.143-149.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]