Abstract
Venous thromboembolism (VTE) is a common complication in patients with cancer. Warfarin has largely been replaced by low-molecular-weight heparin (LMWHs) and direct oral anticoagulants (DOACs) as the standard of care in cancer-associated VTE. The survival benefit of these anticoagulants over warfarin in the cancer population was not demonstrated in clinical trials, possibly due to insufficient sample size and limited follow-up duration. There are emerging population-based studies suggesting that warfarin may be associated with improved overall survival in cancers and may have a protective effect against certain types of cancers. Warfarin may exert its anti-neoplastic properties through both coagulation pathway -dependent and -independent mechanisms, the latter of which are mediated by inhibition of the Gas6-AXL signaling pathway. Further research should emphasize on identifying clinical and laboratory predictors of beneficial effects of warfarin. In this review article, we summarize and update the current evidence regarding the potential impact of warfarin on the overall survival of cancer patients and incidence of cancer, as well as review the potential mechanism of such effect and future perspectives.
Keywords: cancer-associated thrombosis, Oral anticoagulants, warfarin, low-molecular weight heparin
1. Introduction
Venous thromboembolism (VTE) is a common complication in patients with cancer, resulting in increased morbidity, mortality, and healthcare cost [1]. For half a century, warfarin and other vitamin K antagonists were the only options for the anticoagulant treatment of VTE in patients with cancers. Since the early 2000s, the use of warfarin for this indication has gradually declined from 90% to less than 30% over the course of two decades, accompanied by a corresponding rise in the use of low-molecular-weight heparin (LMWH), and more recently, direct oral anticoagulants (DOACs) [2]. This transformation in clinical practice was largely influenced by clinical trial data that showed superiority of LMWHs over warfarin in recurrent VTE reduction without increased risk of major bleeding [3–5]. More recently, DOACs (apixaban, edoxaban, and rivaroxaban) have gained rapidly evolving popularity owing to their similar therapeutic efficacy to LMWH [6–8] and ease of administration [9, 10]. Currently, LMWH and DOACs are the standard of care for VTE in cancer patients endorsed by major clinical practice guidelines [11–13]. Warfarin use in the context of cancers has become restricted to certain populations such as those with severe renal impairment, prosthetic heart valves, extreme body weights, concomitant high-risk antiphospholipid syndrome, and barriers to cost and daily injection.
Caring for cancer patients is complex. Recurrent VTE and major bleeding, the focused outcomes in clinical trials, formed the basis of guideline recommendations. However, deciding on anticoagulant strategies for cancer-associated VTE in real life is multidimensional. Of equal importance are other factors such as drug interaction with cancer therapy, patient preference, quality of life, cost, and adherence. Even more importantly, overall survival (OS) is considered the most relevant measure of clinical benefit of cancer intervention. The differential impact of LMWH or DOACs versus warfarin on survival has not been shown in previous clinical trials [14]. Whether the impact on reduction of recurrent VTE translates to longer survival remains largely unknown.
In the 1980’s evidence emerged that warfarin might prolong survival in patients with small-cell lung cancer [15]. This intriguing result has since stimulated subsequent investigation to evaluate the anti-tumor effect of warfarin and its impact on cancer progression and overall survival in various cancer patients. Today, forty years and a few dozen studies later, the conclusion to definitively confirm or rule out a clinically meaningful anti-neoplastic property of warfarin is not conclusive. Although, bodies of evidence continue to emerge with the advancing landscape in basic, clinical, and epidemiological research approaches. In this review article, we summarize and update the current evidence regarding the impact of warfarin on the overall survival of cancer patients and incidence of new cancer diagnosis, as well as discuss the potential mechanism of such effect and future perspectives.
2. Mortality data from the clinical trials in VTE
All-cause mortality is often included as one of the efficacy outcomes in phase 3 randomized control trials (RCTs) of cancer-associated VTE. According to the 5 RCTs that compared LMWH with warfarin for the long-term treatment of VTE in cancer patients, no significant difference in all-cause mortality was observed, except for the post-hoc analysis favoring LMWH in the subgroup of 150 patients without known metastases in the CLOT trial [3–5, 16–18]. There were no RCTs that directly compared DOACs with warfarin in patients with VTE and cancer. However, post-hoc analyses of cancer subgroups in 4 RCTs evaluating DOACs versus warfarin for acute VTE similarly showed the absence of mortality benefit of DOACs over warfarin (Table 1) [19–22]. In a meta-analysis summarizing these RCTs, the pooled relative risks (RRs) for all-cause mortality were 1.00 (95%CI 0.88 – 1.13, LMWH vs. warfarin, N=1747) and 0.93 (95%CI 0.71-1.21, DOAC vs. warfarin, N=1031) [14]. It was concluded that the beneficial or harmful effects of LMWH or DOACs over warfarin could not be ruled out, largely due to the lack of power.
Table 1.
Mortality outcomes from randomized control trials of anticoagulation in cancer associated VTE.
| Study | N/Intervention | Metastatic cancer (%) | Treatment duration | Follow up duration | Mortality rate (%) | P value |
|---|---|---|---|---|---|---|
| Warfarin vs. LMWH | ||||||
| CANTHANOX 2002 [16] | 67 Enoxaparin 71 Warfarin |
53.5 52.0 |
3 months | 6 months | 31.0 38.7 |
.25 |
| CLOT 2003 [3] | 336 Dalteparin 336 Warfarin/acenocoumarol |
66.4 69.0 |
6 months | 6 months | 39 41 |
.53 |
| CLOT 2003 (post-hoc analysis at 12 months) [18] | With metastases 221 Dalteparin 231 Warfarin/acenocoumarol Without metastases 75 Dalteparin 75 Warfarin/acenocoumarol |
100 100 0 0 |
6 months |
12 months |
72 69 15 26 |
.03 .46 |
| ONCENOX 2006 [17] | 29 Enoxaparin 1 mg/kg/day 32 Enoxaparin 1.5 mg/kg/day 30 Warfarin |
54.8 66.7 52.9 |
6 months | 7 months | 22.6 41.7 32.4 |
Not reported |
| LITE 2006 [5] | 100 Tinzaparin 100 warfarin |
47.0 36.0 |
3 months | 12 months | 47 47 |
Not reported |
| CATCH 2015 [4] | 449 Tinzaparin 451 Warfarin |
55.0 54.3 |
6 months | 6 months | 33.4 30.6 |
.54 |
| Warfarin vs. DOACs (Post-hoc analysis of patients with active cancers) | ||||||
| EINSTEIN-DVT and EINSTEIN-PE 2014 [19] | 354 Rivaroxaban 301 Warfarin/acenocoumarol |
19 26 |
3-12 months | 3-12 months | 58 53 |
.70 |
| AMPLIFY 2015 [20] | 88 Apixaban 81 Warfarin |
Not reported | 6 months | 6 months | 6.0 7.7 |
Not reported |
| RE-COVER I and RECOVER-II | 173 Dabigatran 162 Warfarin |
Not reported | 6 months | 6 months | 15.0 14.2 |
Not reported |
| Hokusai VTE 2016 [21] | 85 Edoxaban 77 Warfarin |
28 29 |
6 months | 12 months | 31 31 |
Not reported |
In general, prospective well-conducted randomized controlled clinical trials are considered the highest level of primary evidence evaluating the effect of pharmacologic interventions. However, when it comes to assessing mortality in cancer-associated VTE, RCTs may not be the most feasible study design for several reasons. It is impractical to recruit a sample size with adequate power required to detect subtle differences in mortality, especially in the background of the overwhelming risk of death from the progression of cancer. For example, in previous randomized studies, the overall rate of fatal pulmonary embolism was 3.7% among the 886 patients who received vitamin K antagonists [3, 5, 18]. If a 26% reduction in fatal pulmonary embolism with LMWH compared with warfarin is assumed, target enrollment would be at least 10,120 patients (two-sided α=0.05, power=0.8) with a 1:1 cohort allocation. In previous clinical trials, the number of participants in each treatment group ranged from 30 to 450. Duration of follow-up in clinical trials may not be long enough to observe the effect; most VTE studies followed participants for 3-6 months, with only a few that followed for 12 months. Moreover, heterogeneity in terms of cancer sites and stages also contributed to the dilution of mortality benefit that may be selective of certain subgroups. Hence, information regarding the mortality benefit of one anticoagulant over another could not be conclusively drawn from the clinical trial settings.
3. Warfarin and overall survival in cancer patients: from single cohorts to population-based studies
Starting in the early 1990’s, a series of RCTs were conducted to investigate the survival benefit of warfarin in patients with cancer who did not have other indications for anticoagulation (Table 3) [15, 23–27]. The U.S. Veterans Administrative Cooperative Study found that survival time doubled in the subgroup of small-cell lung cancer who received warfarin (median OS 49.5 vs. 23.0 weeks, P=.018), although such difference was not observed in other cancer types [23]. Subsequent studies focusing on small-cell lung cancer, colorectal, and metastatic breast cancer did not demonstrate any significant effect of warfarin on overall survival [24–27], although one study reported that warfarin during chemotherapy for breast cancer reduced the risk of VTE (0.6 vs. 4%, 85% relative risk reduction, P=.031). A meta-analysis of these 5 trials (1,604 patients) concluded that there was no significant reduction in overall mortality at 1-year (risk ratio 0.94, 95%CI 0.87 – 1.03, P=.20, I2 =0%) and 5-year (risk ratio 0.91, 95%CI 0.83 – 1.01, P=.08, I2 =0%) in the warfarin group; increased risk of major bleeding was also observed (risk ratio 4.24, 95%CI 1.85 – 9.68, P<.001, I2 =28%) [28]. Of note, the doses of warfarin used in these studies were lower (targeting to double the prothrombin time or INR of 1.5-2) than the therapeutic dose that would be prescribed for VTE. The possible survival benefit of warfarin could not be definitively dismissed since these early studies suffered from limited sample size and inclusion of mainly advanced stage diseases, thus, not adequately powered to characterize the effect in patients with specific cancer types and stages. It is possible that a relatively modest anti-neoplastic activity with warfarin is ineffectual for later stage disease.
Table 3.
Population-based studies evaluating survival with warfarin compared with other anticoagulants in patients with cancers.
| Data sources | Study period | Population | Cancer types | N | Adjusted hazard ratio for cancer-related or overall mortality (95%CI) | |
|---|---|---|---|---|---|---|
| Kinnunen 2017[32] | Finnish Cancer Registry and national reimbursement database |
1995 - 2015 | Men aged 55-67 | Prostate | 1074 Warfarin 978 Other AC |
Warfarin users vs. non-warfarin AC users: 1.01 (0.71-1.44) |
| Kinnunen 2019[34] | Finnish Cancer Registry and national reimbursement database | 1995 - 2016 | Men aged 55-67 | Lung, gastric, colorectal, CNS, NHL, hepatic, pancreatic, renal, bladder | 17826 Warfarin 12326 LMWH 8595 Other AC |
Warfarin users vs. non-warfarin AC users: 0.45 (0.41 – 0.50)* |
| Kinnunen 2020 [35] | Finnish Cancer Registry and national reimbursement database | 1995 - 2015 | Women median age 59 – 72 | Breast | 914 Warfarin 906 Other AC |
Warfarin users vs. non-warfarin AC users: 0.88 (0.80 – 0.98)* |
| Chiasakul 2021 [36] | SEER-Medicare | 2007 - 2016 | Aged ≥66 with VTE | Gastric, colorectal, pancreatic, lung, ovarian, or brain | 4853 LMWH 4853 Warfarin |
Warfarin vs. LMWH: 0.86 (0.83 – 0.90)* |
RR, rate ratio; HR, hazard ratio; AC, anticoagulant; VTE, venous thromboembolism
denotes statistically significant results
In the past decade, the research approach to address the effect of warfarin on cancer survival has shifted toward the utilization of cancer registries and administrative databases (originally intended for billing or quality assurance purposes). These epidemiological data provide powerful alternative resources that allow for the evaluation of outcomes in large-scale population-based cohorts, overcoming barriers in cost, location, personnel, and time required by conventional clinical trials. Also of particular relevance in this era, randomizing patients to receive warfarin in a clinical trial, when LMWH and DOACs have become the standard for cancer-associated VTE, would be ethically challenging. One limitation of database studies is the misclassification of outcomes by inaccurate coding. However, when the outcomes of interest are objective, such as overall mortality, it is less likely to be an issue.
In a recent U.S. commercial insurance claims databases study of 14,086 cancer patients with VTE who were diagnosed in 2014 to 2018, apixaban was found to be associated with lower recurrent VTE and major bleeding than warfarin, while LMWH and warfarin had comparable risks of recurrent VTE and major bleeding [29]. The findings were consistent both when the follow-up was censored at 6 months and when the entire follow-up period (up to 3 years) was used, albeit mortality was not evaluated due to incomplete information.
Recent observational population-based studies have explored the survival effect of warfarin in cancers [30–36]. These studies collected data in the late 1990s to 2010s, when the treatment of cancer-associated VTE started to transition from warfarin to LMWH and later DOACs. The numbers of participants ranged from 4000 – 70000 (compared to 100 – 400 typically enrolled by clinical trials). Study periods spanned more than 10 years in most studies. Patients who received warfarin were included regardless of the indication for which it was given, except for one study where it was limited to the treatment of cancer-associated VTE [36].
Comparative results of warfarin and other anticoagulants are evaluated in several studies (Table 3 and Figure 1) [32, 34–36]. Interestingly, in one study whereby any anticoagulant use was associated with an increased risk of cancer-related death, warfarin was associated with better survival than non-warfarin anticoagulants (mainly LMWH) (HR 0.45, 95%CI 0.41-0.50), the effect that was consistently observed in all cancer types included (lung, gastric, colorectal, central nervous system, non-Hodgkin lymphoma, hepatic, pancreatic, renal, bladder) [34]. Such difference was not seen in the prior study of the same database that included only prostate cancer[32]. In an analysis of 73,170 women with breast cancer, post-diagnosis uses of any anticoagulants increased the risk of cancer death, but the negative effect was stronger in LMWH than warfarin, with HRs of 2.62 (95%CI 2.42 – 2.83) and 1.10 (95%CI1.02 – 1.19), respectively [35]. A sensitivity analysis excluding metastatic and unknown disease at diagnosis showed an improved survival with warfarin compared to other anticoagulants (HR 0.88, 95%CI 0.80 – 0.98). Our group recently reported a SEER-Medicare analysis of patients with cancer and VTE, warfarin was associated with increased overall survival compared to LMWH with a HR of 0.86 (95%CI 0.83 – 0.90) [36]. The observed differences in survival were consistent across subgroups of cancer stages and types (except ovarian cancer), but most pronounced in gastric and pancreatic cancers and the early-stage diseases. These findings suggest that not all anticoagulants are equal with respect to their interaction with cancer survival.
Figure 1. Survival with warfarin compared with other anticoagulants in patients with cancers.
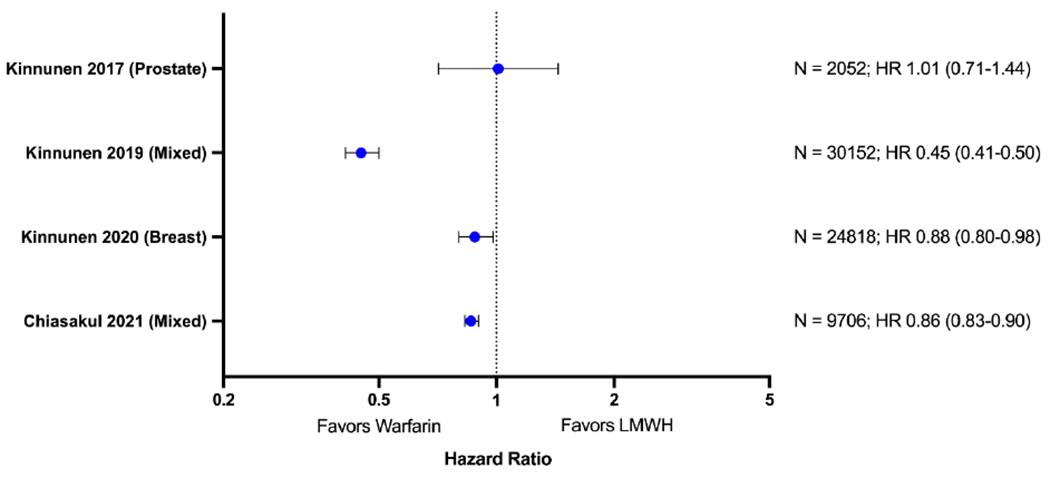
Forest plot for cancer-specific or overall mortality with warfarin compared to other anticoagulants (low-molecular-weight heparin).
Although enlightening, these reports had limitations and the results are to be interpreted with cautions. Without randomization, bias introduced by the imbalances in baseline characteristics are inevitable. For example, warfarin may be favored in healthier individuals who were able to tolerate oral medications and travel for frequent blood tests, whereas LMWH were more likely to be prescribed in patients with malabsorption or concerns for warfarin interaction with anti-cancer therapy. To minimize this, some forms of matching were performed in the analysis of these studies (such as propensity-score matching), however, it was impossible to eliminate the influence of confounders that were unmeasured or unaccounted for. Populations included were mainly the elderly (median age of more than 60) in North America or Europe, restricting the applicability to the younger population and other geographical regions. Moreover, relevant data components that could affect survival were not reported in some of these studies, such as certain comorbidities (e.g., smoking, obesity, atrial fibrillation), cancer treatments, indications of anticoagulation, duration or switching of anticoagulant therapy. Despite the availability of many published population-based studies to date, summarizing the effect across these studies would be imprudent because of their heterogeneity in terms of populations, outcome definitions (all-cause mortality vs. cancer-specific mortality), case/treatment group ascertainment, cancer types studied, and duration of follow-up. Each database also had its own strength and limitation, resulting in variation in the data completeness and accuracy.
4. Warfarin and prevention of cancers
Although controversial, the potential benefit of warfarin on the survival of cancer patients was fascinating enough to spur research interest in further exploring if warfarin has any preventative effect on cancer development. A number of studies compared the incidence of newly diagnosed cancers among warfarin users and non-users (Table 4). The first association was found in a post-hoc analysis of an RCT whereby patients who received warfarin for the treatment of VTE for 6-week had a higher risk of subsequent cancers, specifically urogenital, than the 6-month group over the follow-up period of 10 years [37, 38]. Further studies comparing warfarin users with non-users were contradictory, with some that reported positive [39–42], neutral [43–49], and negative [50] associations of warfarin and cancer incidences. The protective effect was found predominantly in prostate cancer. Interestingly in the most recent study from Sweden, which was the only one that evaluated DOACs, the incidence of prostate cancer significantly decreased among warfarin users, but not among DOACs users [42]. Notable caveats for interpreting these results include the detection bias, the immortal time bias, the inclusion of single or very short-term use of warfarin that was unlikely to exert meaningful effects, and the possibility of co-interventions with other anticoagulants in the non-warfarin group.
Table 4.
Studies evaluating warfarin and cancer risks.
| Study | Country | Study design | Population | N | Follow-up time | Cancer types | Comparison | Results (95%CI) |
|---|---|---|---|---|---|---|---|---|
| Schulman 2000 [37, 38] | Sweden | Post-hoc analysis of RCT | First episode of VTE | 419 Warfarin for 6 weeks 435 Warfarin for 6 months |
8 years | Any | Warfarin 6 weeks vs. 6 months | OR 1.6 (1.1 – 2.4) * Difference mainly due to urogenital cancers: OR 2.5 (1.3-5.0) |
| Taliani 2003 [47, 49] | Italy | Post-hoc analysis of RCT | First episode of VTE | 210 Warfarin for 3 months 219 Warfarin for 12 months |
3.6 years | Any | Warfarin 3 months vs. 12 months | RR 0.71 (0.36 – 1.41) |
| Blumentals 2004 [48] | USA | Retrospective case-control (single cohort) | White male patients seen at hospital | 330 Cases 1293 Controls |
NR | Bladder | Warfarin vs. no warfarin | OR 1.27 (0.85 – 1.89) |
| Tagalakis 2007 [39] | Canada | Retrospective case-control (Administrative database) | Men age ≥50 | 19412 Cases 116470 Controls |
NR | Urogenital | Warfarin (4-year use) vs. no warfarin | IRR 0.80 (0.65 – 0.99) * Only significant in prostate cancer: IRR 0.67 (0.53 – 0.86) * |
| Pengo 2011 [40] | Italy | Retrospective cohort (Administrative database) | Age 65-90 without prior cancer or VTE | 3231 Warfarin 72777 No warfarin |
8.2 years | Any | Warfarin vs. no warfarin | HR 0.88 (0.80 – 0.98) * Only significant in prostate cancer: HR 0.69 (0.50 – 0.97) * |
| Ahern 2011 [43] | USA | Retrospective cohort (Administrative database) | Danish population | 8724 Heart valve replacement 87240 No heart valve replacement |
NR | Any | Warfarin vs. no warfarin (heart valve replacement was used a proxy for warfarin) | No significant difference in all cancers |
| Pottegard 2013 [44] | Denmark | Retrospective case-control (Administrative database) | Danish population | 238196 Cases 1713176 Controls |
NR | Any | Warfarin for ≥ 3 years vs. no warfarin | OR 1.11 (1.07 – 1.15) Only significant in prostate cancer: OR 0.94 (0.76 – 1.17) * |
| Blanclapierre [45] | Canada | Retrospective case-control (single cohort) | Men age ≤75 | 1588 Cases 1618 Controls |
NR | Prostate | Warfarin vs. no warfarin | OR 0.76 (0.50 – 1.16) |
| Kinnunen 2016 [50] | Finland | Retrospective cohort (Administrative database) | Men age 55 – 67 | 12747 Warfarin 55674 No anticoagulants |
NR | Prostate | Warfarin vs. no anticoagulants | HR 1.11 (1.01 – 1.22) * (risk only elevated in low-dose short-term use) |
| Haaland 2017 [41] | Norway | Retrospective cohort (Administrative database) | Age >50 | 92942 Warfarin 1163783 No warfarin |
NR | Any | Warfarin for ≥ 2 years vs. no warfarin | IRR 0.84 (0.2-0.86) * Significant in many cancers including lung, breast, and prostate. |
| Kristensen 2019 [46] | Denmark | Retrospective cohort (Administrative database) | Men age 40 – 85 | 38832 Cases 388320 Controls |
NR | Prostate | VKAs for ≥ 3 years vs. no VKA | OR 1.03 (0.97 – 1.10) |
| Parker 2020 [42] | Sweden | Retrospective case-control (Administrative database) | Men | 31591 Cases 156802 Controls |
NR | Prostate | Warfarin vs. no warfarin DOACs vs. no DOACs | OR 0.92 (0.88 – 0.96)* OR 0.97 (0.90 – 1.06) |
5. The biological explanation for the anti-neoplastic effects of warfarin
Warfarin, as a vitamin K antagonist, may possess anti-neoplastic properties through several mechanisms: (1) Prevention of fatal pulmonary embolism, (2) inhibition of coagulation factors that play essential roles in tumor survival and growth, and (3) inhibition of other vitamin K-dependent proteins that are not parts of the coagulation pathway, but necessary for tumor growth (off-target effect).
In the studies where warfarin’s survival or protective benefits were observed, such association extended remarkably well beyond the period of active warfarin exposure (starting at 2 and up to 10 years) [36, 38, 51], making it unlikely that these effects were solely based on the reduction in fatal pulmonary embolism or other fatal thrombotic events. Coagulation proteins are known to be critical for tumor microenvironment [52]. Thrombin and tissue factor/FVIIa complex activates protease-activated receptors (PARs), which trigger signaling pathways that promote angiogenesis, tumor cell proliferation, and metastasis[53]. Fibrin clot formation facilitates the evasion of cancer cells from immune surveillance by natural killer cells [54] and recruits a subsets monocyte/macrophages that promote metastasis [55]. Interference with these processes by anticoagulants at the early stages may modify the natural history of the cancers. Warfarin can reduce FVII-mediated procoagulant activity in an animal model [56], and plasma from patients who take warfarin showed lower thrombin generation than rivaroxaban [57].
There exist pre-clinical models suggesting a unique anti-tumor mechanism independent of its anticoagulant activity, specifically the inhibition of Growth arrest-specific gene 6 (GAS6)-AXL pathway. GAS6, the ligand of AXL tyrosine kinase receptor, is a vitamin K-dependent protein whose function requires γ-carboxylation of its Gla domain. GAS6-AXL signaling mediates cell migration and survival, facilitates tumor-stromal cellular interaction, and is associated with metastasis, resistance to therapy, and worse outcomes in cancers [58]. AXL deficiency enhanced immune microenvironment and prolonged survival in mice models with pancreatic cancer [59]. Warfarin, at lower closes than required for anticoagulation, can inhibit GAS6-AXL signaling, resulting in reduced tumor growth, metastasis, and potentiated therapeutic effect of gemcitabine in pancreatic ductal adenocarcinoma [60]. Low-dose warfarin also promotes AXL-mediated anti-metastatic activity of natural killer cells [61]. Therapeutic agents specifically targeting GAS6 are being investigated for their clinical efficacy in ovarian cancer, renal cell carcinoma, and pancreatic cancer [58].
6. Conclusion and Future directions
The debate over warfarin’s potential as an anti-cancer agent has been going on for over 40 years. Clinical, epidemiological, and basic science investigators have harmoniously sought to find a consistent answer to this question. With re-emerging epidemiologic data suggesting as survival benefit of warfarin over LMWH, hopefully additional studies will be conducted shedding light on which cancer subgroups appear to benefit most from warfarin therapy. These may include populations with certain comorbidities (atrial fibrillation or VTE), cancer types (pancreatic, gastric, or prostate cancer), cancer stages (early disease), and possible biomarkers (D-dimer, thrombin generation, or AXL expression). Warfarin’s niche in the clinic is increasingly limited but possibly a second life will eventually emerge as an anticancer adjuvant therapy.
Table 2.
Randomized control trials evaluating survival with warfarin in patients with cancers.
| Study | N/Intervention | Cancer types | Overall Survival (OS) |
|---|---|---|---|
| Zacharski 1981 [15] | 25 Warfarin 25 Control |
SCLC | Median OS 50 vs. 24 weeks (P=.026)* |
| Zacharski 1984 [23] | 215 Warfarin 216 Control |
SCLC, NSCLC, colorectal, prostate, head and neck | Median OS 21.4 vs. 24.6 weeks (P=.42) Subgroup of SCLC (N=25/25): Median OS 49.5 vs. 23.0 weeks (P=.018)* |
| Chahinian 1989 [24] | 103 MACC + warfarin 86 MACC 105 MEPH/MACC |
SCLC (extensive) | Median OS 9.3 vs. 7.9 vs. 7.9 months (P=.098) |
| Daly 1991[25] | 158 Warfarin 181 Control |
Colorectal | 4-year OS 72.2% vs. 69.5% (P=.15) |
| Levine 1994 [26] | 152 Warfarin 159 Control |
Breast cancer (stage 4) | Mortality 57% vs 63% (P=.55) |
| Maurer 1997 [27] | 178 Warfarin 169 Control |
SCLC (limited) | Median OS 21.4 months vs. 18.6 months (P=.12) |
SCLC, small-cell lung cancer; NSCLC, non-small-cell lung cancer
denotes statistically significant results
Highlight.
Emerging population-based evidence suggests an association of warfarin with improved overall survival in cancer patients and possible preventive effect against certain types of cancers.
Anti-neoplastic mechanisms of warfarin include inhibition of thrombin and Gas6 signaling.
Implications for future research include identifying subgroups with improved outcomes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Lyman GH, Culakova E, Poniewierski MS, Kuderer NM, Morbidity, mortality and costs associated with venous thromboembolism in hospitalized patients with cancer, Thromb Res 164 Suppl 1 (2018) S112–S118. [DOI] [PubMed] [Google Scholar]
- [2].Delate T, Charlu M, Zhu S, Pai A, Clark NP, Witt DM, King JM, King JB, Temporal trends in first-line outpatient anticoagulation treatment for cancer-associated venous thromboembolism, Thromb Res 196 (2020) 367–370. [DOI] [PubMed] [Google Scholar]
- [3].Lee AY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, Rickles FR, Julian JA, Haley S, Kovacs MJ, Gent M, I. Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer, Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer, N Engl J Med 349(2) (2003) 146–53. [DOI] [PubMed] [Google Scholar]
- [4].Lee AYY, Kamphuisen PW, Meyer G, Bauersachs R, Janas MS, Jarner MF, Khorana AA, Investigators C, Tinzaparin vs Warfarin for Treatment of Acute Venous Thromboembolism in Patients With Active Cancer: A Randomized Clinical Trial, JAMA 314(7) (2015) 677–686. [DOI] [PubMed] [Google Scholar]
- [5].Hull RD, Pineo GF, Brant RF, Mah AF, Burke N, Dear R, Wong T, Cook R, Solymoss S, Poon MC, Raskob G, Investigators LT, Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer, Am J Med 119(12) (2006) 1062–72. [DOI] [PubMed] [Google Scholar]
- [6].Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, Grosso MA, Kakkar AK, Kovacs MJ, Mercuri MF, Meyer G, Segers A, Shi M, Wang TF, Yeo E, Zhang G, Zwicker JI, Weitz JI, Buller HR, Hokusai VTECI, Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism, N Engl J Med 378(7) (2018) 615–624. [DOI] [PubMed] [Google Scholar]
- [7].Young AM, Marshall A, Thirlwall J, Chapman O, Lokare A, Hill C, Hale D, Dunn JA, Lyman GH, Hutchinson C, MacCallum P, Kakkar A, Hobbs FDR, Petrou S, Dale J, Poole CJ, Maraveyas A, Levine M, Comparison of an Oral Factor Xa Inhibitor With Low Molecular Weight Heparin in Patients With Cancer With Venous Thromboembolism: Results of a Randomized Trial (SELECT-D), J Clin Oncol 36(20) (2018) 2017–2023. [DOI] [PubMed] [Google Scholar]
- [8].Agnelli G, Becattini C, Meyer G, Munoz A, Huisman MV, Connors JM, Cohen A, Bauersachs R, Brenner B, Torbicki A, Sueiro MR, Lambert C, Gussoni G, Campanini M, Fontanella A, Vescovo G, Verso M, Caravaggio I, Apixaban for the Treatment of Venous Thromboembolism Associated with Cancer, N Engl J Med 382(17) (2020) 1599–1607. [DOI] [PubMed] [Google Scholar]
- [9].Guo JD, Hlavacek P, Poretta T, Wygant G, Lane D, Gorritz M, Wang X, Chen CC, Wade RL, Pan X, Rajpura J, Stwalley B, Rosenblatt L, Inpatient and outpatient treatment patterns of cancer-associated thrombosis in the United States, J Thromb Thrombolysis 50(2) (2020) 386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schaefer JK, Li M, Wu Z, Basu T, Dorsch MP, Barnes GD, Carrier M, Griggs JJ, Sood SL, Anticoagulant medication adherence for cancer-associated thrombosis: A comparison of LMWH to DOACs, J Thromb Haemost 19(1) (2021) 212–220. [DOI] [PubMed] [Google Scholar]
- [11].Lyman GH, Carrier M, Ay C, Di Nisio M, Hicks LK, Khorana AA, Leavitt AD, Lee AYY, Macbeth F, Morgan RL, Noble S, Sexton EA, Stenehjem D, Wiercioch W, Kahale LA, Alonso-Coello P, American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer, Blood Adv 5(4) (2021) 927–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Key NS, Khorana AA, Kuderer NM, Bohlke K, Lee AYY, Arcelus JI, Wong SL, Balaban EP, Flowers CR, Francis CW, Gates LE, Kakkar AK, Levine MN, Liebman HA, Tempero MA, Lyman GH, Falanga A, Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Clinical Practice Guideline Update, J Clin Oncol 38(5) (2020) 496–520. [DOI] [PubMed] [Google Scholar]
- [13].National Comprehensive Cancer Network, NCCN guideline on cancer-associated venousthromboembolic disease. Version 2. 2021. 2021). [Google Scholar]
- [14].Kahale LA, Hakoum MB, Tsolakian IG, Matar CF, Terrenato I, Sperati F, Barba M, Yosuico VE, Schunemann H, Akl EA, Anticoagulation for the long-term treatment of venous thromboembolism in people with cancer, Cochrane Database Syst Rev 6 (2018) CD006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zacharski LR, Henderson WG, Rickles FR, Forman WB, Cornell CJ Jr., Forcier RJ, Edwards R, Headley E, Kim SH, O’Donnell JR, O’Dell R, Tornyos K, Kwaan HC, Effect of warfarin on survival in small cell carcinoma of the lung. Veterans Administration Study No. 75, JAMA 245(8) (1981) 831–5. [PubMed] [Google Scholar]
- [16].Meyer G, Marjanovic Z, Valcke J, Lorcerie B, Gruel Y, Solal-Celigny P, Le Maignan C, Extra JM, Cottu P, Farge D, Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study, Arch Intern Med 162(15) (2002) 1729–35. [DOI] [PubMed] [Google Scholar]
- [17].Deitcher SR, Kessler CM, Merli G, Rigas JR, Lyons RM, Fareed J, Investigators O, Secondary prevention of venous thromboembolic events in patients with active cancer: enoxaparin alone versus initial enoxaparin followed by warfarin for a 180-day period, Clin Appl Thromb Hemost 12(4) (2006) 389–96. [DOI] [PubMed] [Google Scholar]
- [18].Lee AY, Rickles FR, Julian JA, Gent M, Baker RI, Bowden C, Kakkar AK, Prins M, Levine MN, Randomized comparison of low molecular weight heparin and coumarin derivatives on the survival of patients with cancer and venous thromboembolism, J Clin Oncol 23(10) (2005) 2123–9. [DOI] [PubMed] [Google Scholar]
- [19].Prins MH, Lensing AWA, Brighton TA, Lyons RM, Rehm J, Trajanovic M, Davidson BL, Beyer-Westendorf J, Pap ÁF, Berkowitz SD, Cohen AT, Kovacs MJ, Wells PS, Prandoni P, Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PE): a pooled subgroup analysis of two randomised controlled trials, The Lancet Haematology 1(1) (2014) e37–e46. [DOI] [PubMed] [Google Scholar]
- [20].Agnelli G, Buller HR, Cohen A, Gallus AS, Lee TC, Pak R, Raskob GE, Weitz JI, Yamabe T, Oral apixaban for the treatment of venous thromboembolism in cancer patients: results from the AMPLIFY trial, J Thromb Haemost 13(12) (2015) 2187–91. [DOI] [PubMed] [Google Scholar]
- [21].Raskob GE, van Es N, Segers A, Angchaisuksiri P, Oh D, Boda Z, Lyons RM, Meijer K, Gudz I, Weitz JI, Zhang G, Lanz H, Mercuri MF, BÁller HR, Edoxaban for venous thromboembolism in patients with cancer: results from a non-inferiority subgroup analysis of the Hokusai-VTE randomised, double-blind, double-dummy trial, The Lancet Haematology 3(8) (2016) e379–e387. [DOI] [PubMed] [Google Scholar]
- [22].Schulman S, Goldhaber SZ, Kearon C, Kakkar AK, Schellong S, Eriksson H, Hantel S, Feuring M, Kreuzer J, Treatment with dabigatran or warfarin in patients with venous thromboembolism and cancer, Thromb Haemost 114(1) (2015) 150–7. [DOI] [PubMed] [Google Scholar]
- [23].Zacharski LR, Henderson WG, Rickles FR, Forman WB, Cornell CJ Jr., Forcier RJ, Edwards RL, Headley E, Kim SH, O’Donnell JF, et al. , Effect of warfarin anticoagulation on survival in carcinoma of the lung, colon, head and neck, and prostate. Final report of VA Cooperative Study #75, Cancer 53(10) (1984) 2046–52. [DOI] [PubMed] [Google Scholar]
- [24].Chahinian AP, Propert KJ, Ware JH, Zimmer B, Perry MC, Hirsh V, Skarin A, Kopel S, Holland JF, Comis RL, et al. , A randomized trial of anticoagulation with warfarin and of alternating chemotherapy in extensive small-cell lung cancer by the Cancer and Leukemia Group B, J Clin Oncol 7(8) (1989) 993–1002. [DOI] [PubMed] [Google Scholar]
- [25].Daly L, The first international urokinase/warfarin trial in colorectal cancer, Clin Exp Metastasis 9(1) (1991) 3–11. [DOI] [PubMed] [Google Scholar]
- [26].Levine M, Hirsh J, Gent M, Arnold A, Warr D, Falanga A, Samosh M, Bramwell V, Pritchard KI, Stewart D, et al. , Double-blind randomised trial of a very-low-dose warfarin for prevention of thromboembolism in stage IV breast cancer, Lancet 343(8902) (1994) 886–9. [DOI] [PubMed] [Google Scholar]
- [27].Maurer LH, Herndon JE 2nd, Hollis DR, Aisner J, Carey RW, Skarin AT, Perry MC, Eaton WL, Zacharski LL, Hammond S, Green MR, Randomized trial of chemotherapy and radiation therapy with or without warfarin for limited-stage small-cell lung cancer: a Cancer and Leukemia Group B study, J Clin Oncol 15(11) (1997) 3378–87. [DOI] [PubMed] [Google Scholar]
- [28].Akl EA, Kamath G, Kim SY, Yosuico V, Barba M, Terrenato I, Sperati F, Schunemann HJ, Oral anticoagulation for prolonging survival in patients with cancer, Cochrane Database Syst Rev (2) (2007) CD006466. [DOI] [PubMed] [Google Scholar]
- [29].Cohen A, Keshishian A, Lee T, Wygant G, Rosenblatt L, Hlavacek P, Mardekian J, Wiederkehr D, Sah J, Luo X, Effectiveness and Safety of Apixaban, Low-Molecular-Weight Heparin, and Warfarin among Venous Thromboembolism Patients with Active Cancer: A U.S. Claims Data Analysis, Thromb Haemost 121(3) (2021) 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tagalakis V, Tamim H, Blostein M, Hanley JA, Kahn SR, Risk of prostate cancer death in long-term users of warfarin: a population-based case-control study, Cancer Causes Control 24(6) (2013) 1079–85. [DOI] [PubMed] [Google Scholar]
- [31].O’Rorke MA, Murray LJ, Hughes CM, Cantwell MM, Cardwell CR, The effect of warfarin therapy on breast, colorectal, lung, and prostate cancer survival: a population-based cohort study using the Clinical Practice Research Datalink, Cancer Causes Control 26(3) (2015) 355–66. [DOI] [PubMed] [Google Scholar]
- [32].Kinnunen PTT, Murtola TJ, Talala K, Taari K, Tammela TLJ, Auvinen A, Prostate cancer-specific survival among warfarin users in the Finnish Randomized Study of Screening for Prostate Cancer, BMC Cancer 17(1) (2017) 585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Beg MS, Gupta A, Sher D, Ali S, Khan S, Gao A, Stewart T, Ahn C, Berry J, Mortensen EM, Impact of Concurrent Medication Use on Pancreatic Cancer Survival-SEER-Medicare Analysis, Am J Clin Oncol 41(8) (2018) 766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kinnunen PTT, Murtola TJ, Talala K, Taari K, Tammela TLJ, Auvinen A, Anticoagulants and cancer mortality in the Finnish randomized study of screening for prostate cancer, Cancer Causes Control 30(8) (2019) 877–888. [DOI] [PubMed] [Google Scholar]
- [35].Kinnunen PT, Murto MO, Artama M, Pukkala E, Visvanathan K, Murtola TJ, Anticoagulants and Breast Cancer Survival: A Nationwide Cohort Study, Cancer Epidemiol Biomarkers Prev 29(1) (2020) 208–215. [DOI] [PubMed] [Google Scholar]
- [36].Chiasakul T, Redd R, Patell R, Khan AM, McCarthy EP, Neuberg D, Zwicker JI, Overall survival with warfarin vs. low-molecular-weight heparin in cancer-associated thrombosis, J Thromb Haemost (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Schulman S, Lindmarker P, Incidence of cancer after prophylaxis with warfarin against recurrent venous thromboembolism. Duration of Anticoagulation Trial, N Engl J Med 342(26) (2000) 1953–8. [DOI] [PubMed] [Google Scholar]
- [38].Schulman S, More on: vitamin K antagonists and cancer, Journal of Thrombosis and Haemostasis 6(8) (2008) 1442–1443. [DOI] [PubMed] [Google Scholar]
- [39].Tagalakis V, Tamim H, Blostein M, Collet J-P, Hanley JA, Kahn SR, Use of warfarin and risk of urogenital cancer: a population-based, nested case-control study, The Lancet Oncology 8(5) (2007) 395–402. [DOI] [PubMed] [Google Scholar]
- [40].Pengo V, Noventa F, Denas G, Pengo MF, Gallo U, Grion AM, Iliceto S, Prandoni P, Long-term use of vitamin K antagonists and incidence of cancer: a population-based study, Blood 117(5) (2011) 1707–9. [DOI] [PubMed] [Google Scholar]
- [41].Haaland GS, Falk RS, Straume O, Lorens JB, Association of Warfarin Use With Lower Overall Cancer Incidence Among Patients Older Than 50 Years, JAMA Intern Med 177(12) (2017) 1774–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Parker J, Crawley D, Garmo H, Lindahl B, Styrke J, Adolfsson J, Lambe M, Stattin P, Van Hemelrijck M, Beckmann K, Use of Warfarin or Direct Oral Anticoagulants and Risk of Prostate Cancer in PCBaSe: A Nationwide Case-Control Study, Front Oncol 10 (2020) 571838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ahern TP, Pedersen L, Sværke C, Rothman KJ, Sørensen HT, Lash TL, The association between vitamin K antagonist therapy and site-specific cancer incidence estimated by using heart valve replacement as an instrumental variable, Am J Epidemiol 174(12) (2011) 1382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pottegard A, Friis S, Hallas J, Cancer risk in long-term users of vitamin K antagonists: a population-based case-control study, Int J Cancer 132(11) (2013) 2606–12. [DOI] [PubMed] [Google Scholar]
- [45].Blanc-Lapierre A, Weiss D, Parent M, Use of oral anticoagulants and risk of prostate cancer: a population-based case-control study in Montreal, Canada, Cancer Causes Control 25(9) (2014) 1159–66. [DOI] [PubMed] [Google Scholar]
- [46].Kristensen KB, Jensen PH, Skriver C, Friis S, Pottegard A, Use of vitamin K antagonists and risk of prostate cancer: Meta-analysis and nationwide case-control study, Int J Cancer 144(7) (2019) 1522–1529. [DOI] [PubMed] [Google Scholar]
- [47].Taliani MR, Agnelli G, Prandoni P, Becattini C, Moia M, Bazzan M, Ageno W, Tomasi C, Guazzaloca G, Ambrosio GB, Bertoldi A, Salvi R, Poggio R, Silingardi M, I. Warfarin Optimal Duration Italian Trial, Incidence of cancer after a first episode of idiopathic venous thromboembolism treated with 3 months or 1 year of oral anticoagulation, J Thromb Haemost 1(8) (2003) 1730–3. [DOI] [PubMed] [Google Scholar]
- [48].Blumentals WA, Foulis PR, Schwartz SW, Mason TJ, Does warfarin therapy influence the risk of bladder cancer?, Thromb Haemost 91(4) (2004) 801–5. [DOI] [PubMed] [Google Scholar]
- [49].Agnelli G, Taliani MR, Prandoni P, Investigators W, More on: vitamin K antagonists and cancer, J Thromb Haemost 6(8) (2008) 1441–2; author reply 1442-3. [DOI] [PubMed] [Google Scholar]
- [50].Kinnunen PT, Murtola TJ, Talala K, Taari K, Tammela TL, Auvinen A, Warfarin use and prostate cancer risk in the Finnish Randomized Study of Screening for Prostate Cancer, Scand J Urol 50(6) (2016) 413–419. [DOI] [PubMed] [Google Scholar]
- [51].Schulman S, Lindmarker P, Vitamin K antagonists and cancer: rebuttal, J Thromb Haemost 2(2) (2004) 377–8; author reply 378-9. [DOI] [PubMed] [Google Scholar]
- [52].Gil-Bernabe AM, Lucotti S, Muschel RJ, Coagulation and metastasis: what does the experimental literature tell us?, Br J Haematol 162(4) (2013) 433–41. [DOI] [PubMed] [Google Scholar]
- [53].Gay LJ, Felding-Habermann B, Contribution of platelets to tumour metastasis, Nat Rev Cancer 11(2) (2011) 123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bobek V, Boubelik M, Fiserova A, L’Uptovcova M, Vannucci L, Kacprzak G, Kolodzej J, Majewski AM, Hoffman RM, Anticoagulant drugs increase natural killer cell activity in lung cancer, Lung Cancer 47(2) (2005) 215–23. [DOI] [PubMed] [Google Scholar]
- [55].Gil-Bernabe AM, Ferjancic S, Tlalka M, Zhao L, Allen PD, Im JH, Watson K, Hill SA, Amirkhosravi A, Francis JL, Pollard JW, Ruf W, Muschel RJ, Recruitment of monocytes/macrophages by tissue factor-mediated coagulation is essential for metastatic cell survival and premetastatic niche establishment in mice, Blood 119(13) (2012) 3164–75. [DOI] [PubMed] [Google Scholar]
- [56].Francis JL, Carty N, Amirkhosravi M, Loizidou M, Cooper A, Taylor I, The effect of Warfarin and factor VII on tissue procoagulant activity and pulmonary seeding, Br J Cancer 65(3) (1992) 329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Cohen H, Hunt BJ, Efthymiou M, Arachchillage DRJ, Mackie IJ, Clawson S, Sylvestre Y, Machin SJ, Bertolaccini ML, Ruiz-Castellano M, Muirhead N, Doré CJ, Khamashta M, Isenberg DA, Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): a randomised, controlled, open-label, phase 2/3, non-inferiority trial, The Lancet Haematology 3(9) (2016) e426–e436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Tanaka M, Siemann DW, Therapeutic Targeting of the Gas6/Axl Signaling Pathway in Cancer, Int J Mol Sci 22(18) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Du W, Phinney NZ, Huang H, Wang Z, Westcott J, Toombs JE, Zhang Y, Beg MS, Wilkie TM, Lorens JB, Brekken RA, AXL Is a Key Factor for Cell Plasticity and Promotes Metastasis in Pancreatic Cancer, Mol Cancer Res 19(8) (2021) 1412–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kirane A, Ludwig KF, Sorrelle N, Haaland G, Sandal T, Ranaweera R, Toombs JE, Wang M, Dineen SP, Micklem D, Dellinger MT, Lorens JB, Brekken RA, Warfarin Blocks Gas6-Mediated Axl Activation Required for Pancreatic Cancer Epithelial Plasticity and Metastasis, Cancer Res 75(18) (2015) 3699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Paolino M, Choidas A, Wallner S, Pranjic B, Uribesalgo I, Loeser S, Jamieson AM, Langdon WY, Ikeda F, Fededa JP, Cronin SJ, Nitsch R, Schultz-Fademrecht C, Eickhoff J, Menninger S, Unger A, Torka R, Gruber T, Hinterleitner R, Baier G, Wolf D, Ullrich A, Klebl BM, Penninger JM, The E3 ligase Cbl-b and TAM receptors regulate cancer metastasis via natural killer cells, Nature 507(7493) (2014) 508–12. [DOI] [PMC free article] [PubMed] [Google Scholar]


