Abstract
Formation of a bipolar spindle is required for the faithful segregation of chromosomes during cell division. Twenty-five years ago, a transformative insight into how bipolarity is achieved was provided by Rebecca Heald, Eric Karsenti, and colleagues in their landmark publication characterizing a chromatin-mediated spindle assembly pathway in which centrosomes and kinetochores were dispensable. The discovery revealed that bipolar spindle assembly is a self-organizing process where microtubules, which possess an intrinsic polarity, polymerize around chromatin and become sorted by mitotic motors into a bipolar structure. On the 25th anniversary of this seminal paper, we discuss what was known before, what we have learned since, and what may lie ahead in understanding the bipolar spindle.
INTRODUCTION
The existence of a fusiform spindle apparatus that is wider in the middle and tapering at each end and its importance for accurate chromosome segregation had been hypothesized since cytologists such as Walther Flemming and Theodor Boveri peered through their microscopes in the late 19th century. In the 1940s, Shinya Inoué published polarized light microscopy videos of bipolar spindles in living plant and animal cells and demonstrated that the bipolar structure was composed of filaments that were in dynamic equilibrium with a soluble pool of subunits (Inoué, 1953, 1964). For decades thereafter, the question of how bipolar spindle morphology was achieved was an open one, until the “search and capture” hypothesis burst onto the scene in the 1980s (Kirschner and Mitchison, 1986). The model, which logically flowed from a rapid succession of landmark in vitro and cell-based studies, posited that spindle morphology was defined by duplicated centrosomes that nucleate microtubules (MTs) to search three-dimensional space until they capture a kinetochore and become stabilized (Mitchison and Kirschner, 1984a,b; 1985a,b; Mitchison et al., 1986). The hypothesis offered a simple and elegant molecular mechanism for the phenomenon of spindle bipolarity, in that its spatial cues came from two centrosomes searching for paired sister kinetochores. Twenty-five years ago, Heald et al. (1996) flipped the script by assembling bipolar spindles without centrosomes (search) or kinetochores (capture).
THE BEGINNING OF SPINDLE ASSEMBLY: SEARCH AND CAPTURE
Major advances in our understanding of the molecular composition of the spindle and the biochemistry of its constituents were made in the decades following Inoué’s polarized light microscopy–based characterization of spindle assembly. In the 1960s, Gary Borisy, as a student in Ed Taylor’s lab at the University of Chicago, used the drug colchicine, which had previously been shown by Inoué to disassemble bipolar spindles (Inoué, 1952), to biochemically isolate the colchicine-binding protein tubulin, the building block of the spindle filaments (Borisy and Taylor, 1967). It was subsequently shown that the subunit of the MT is a constitutive heterodimer of two tubulins called α and β tubulin (Bryan and Wilson, 1971; Ludueńa et al., 1977). The MT possesses intrinsic structural polarity, since heterodimers assemble in a head-to-tail arrangement as protofilaments (typically 13 in vivo) that assemble via lateral interactions into a hollow tube (Nogales et al., 1998; Nogales et al., 1999). It was first shown in vitro and later confirmed in cells that one end of the MT (the plus-end) is more dynamic (faster-growing and faster-shortening) than the other end (the minus-end) and that the filament exhibits a striking steady state behavior called dynamic instability where its ends stochastically transition between growing and shortening (Mitchison and Kirschner, 1984a; Cassimeris et al., 1988; Walker et al., 1988).
Kinetochores are macromolecular machines that assemble at sister centromeres during cell division. The kinetochore has two essential functions: 1) mediating load-bearing attachments between dynamic spindle MTs and centromeres to move chromosomes, and 2) coordinating a biochemical pathway called the spindle assembly checkpoint (SAC) to delay anaphase onset until all the chromosomes are correctly attached to the spindle MTs (Musacchio and Desai, 2017). When Heald and Karsenti published their work in 1996, both functions were largely understood at the phenomenological level and characterization of the molecular mechanisms of attachment and SAC regulation was in its early stages. It had been convincingly established that unattached kinetochores were the source of the wait-anaphase signal (Rieder et al., 1994; Rieder et al., 1995). The core checkpoint proteins had been identified in genetic screens in budding yeast (Hoyt et al., 1991; Li and Murray, 1991), but the observation that one of these checkpoint proteins (Mad2) localized to unattached vertebrate kinetochores would be published several months after the description of chromatin-mediated spindle assembly by Heald et al. (1996; Chen et al., 1996; Li and Benezra, 1996). While the identity of centromere proteins (CENP-A, B, C) were known (Earnshaw and Rothfield, 1985), the attachment factors of the outer kinetochore had not yet been characterized although it had been established in vitro and in cells that kinetochores could capture, stabilize, and even move on MTs (Mitchison and Kirschner, 1985b; Mitchison et al., 1986; Hyman and Mitchison, 1991).
Research on the molecular basis of attachment and chromosome movement at that time focused on MT-based motors, since dynein, CENP-E, and MCAK had all been shown to localize to mammalian kinetochores by immunofluorescence (Pfarr et al., 1990; Steuer et al., 1990; Yen et al., 1991, 1992; Wordeman and Mitchison, 1995). The kinetochore localization of CENP-E and dynein was consistent with observations of poleward kinetochore-based motility along MTs in cells and bidirectional movement of kinetochores on MTs in vitro, although the plus-end directionality of CENP-E was not established until 1997—the year after Heald et al. was published (Merdes and De Mey, 1990; Rieder and Alexander, 1990; Hyman and Mitchison, 1991; Wood et al., 1997). Beyond the kinetochore, a greater appreciation for the roles of mitotic motors in organizing the spindle was emerging in 1996 (Hyman and Karsenti, 1996). The initial study localizing dynein to vertebrate kinetochores also reported that the motor localized to centrosomes and spindle poles. Chromosome-associated kinesins (chromokinesins) had just been discovered (Afshar et al., 1995; Vernos et al., 1995; Wang and Adler, 1995); the minus-end directed motor Ncd (Walker et al., 1990) had been found two years before to be required for spindle pole organization in Drosophila melanogaster (Endow et al., 1994). Importantly, it was discovered 6 months before Heald et al. (1996) that disruption of the dynactin (dynein regulatory) complex caused severe spindle assembly defects in mammalian cells, leading the authors to hypothesize that dynein focused microtubule minus ends into the spindle poles (Echeverri et al., 1996).
While it was beginning to become evident that mitotic motors were contributing to spindle morphology, in 1996 centrosomes were still thought to provide the dominant spatial cues for achieving spindle bipolarity. In addition to their description of MT dynamic instability in 1984, an accompanying paper by Mitchison and Kirchner showed that centrosomes nucleated MTs in vitro (Mitchison and Kirschner, 1984b). A year later they reconstituted the capture of MT ends by kinetochores in vitro (Mitchison and Kirschner, 1985b) and then demonstrated that kinetochores stabilized MTs in cells (Mitchison et al., 1986). The culmination of this body of work was the articulation of the search and capture model of spindle morphogenesis whereby astral MTs nucleated from centrosomes search for kinetochores in three-dimensional space via dynamic instability and become stabilized once they are captured by kinetochores (Kirschner and Mitchison, 1986). The search and capture hypothesis was further bolstered when Rieder and colleagues visualized astral MT capture by kinetochores in living cells, although the kinetochores bound to the sides rather than to the ends of the MTs (Hayden et al., 1990; Rieder and Alexander, 1990).
BIPOLAR SPINDLE ASSEMBLY WITHOUT SEARCH AND CAPTURE: THE DISCOVERY OF CHROMATIN-MEDIATED ASSEMBLY
It is noteworthy that some of the first videos of bipolar spindles in living cells were taken of pollen cells from the Easter lily (Inoué, 1953, 1964) because plants do not have centrosomes. Similarly, female meiotic cells in most animals lack centrosomes, yet assemble bipolar structures (Dumont and Desai, 2012). Thus, while search and capture applied nicely to spindles in animal somatic cells, it was not meant to explain spindle bipolarity universally. In the 1980s, Karsenti and colleagues showed that injection of high–molecular weight DNA (lacking any centromeric sequences) into metaphase-arrested Xenopus laevis eggs promoted MT assembly (Karsenti et al., 1984). A decade later, Rebecca Heald began coupling linearized and biotinylated “Bluescript plasmid containing a 5-kb insert of non-coding Drosophila DNA” to streptavidin-coated magnetic beads to build “artificial chromosomes” lacking kinetochores in X. laevis egg extracts (Heald et al., 1996). Mitotic chromatin could be assembled on the beads by cycling them, via addition of Ca2+ to the extract, through interphase and then adding back fresh metaphase-arrested egg extract. During interphase the DNA was replicated and the chromatin beads assembled functional nuclei with a double membrane and nuclear lamina that supported nuclear transport (Heald et al., 1996). As Heald describes it, after using the mitotic chromatin-coated beads for some “horrible” radioactive phosphatase activity assays, she decided to spike some fluorescently labeled tubulin into the extract and “look at them instead” under the microscope (Rebecca Heald, personal communication). The first glimpse through the eyepiece must have been a “Eureka!” moment, for the chromatin-coated beads assembled beautiful bipolar spindles that were morphologically indistinguishable from spindles assembled in the extract using replicated sperm chromosomes that possessed kinetochores and centrosomes (Heald et al., 1996). Because the DNA beads did not contain centromeric sequences and the Xenopus egg extract did not have centrosomes, the major driving force for bipolar spindle assembly in this assay was the chromatin itself (Heald et al., 1996). Today, this pathway is referred to as chromatin-mediated spindle assembly (Supplemental Video 1).
Video S1.
Sperm and bead spindle assembly in Xenopus laevis egg extracts. Xenopus egg extracts supplemented with fluorescently labeled tubulin to visualize MTs and either sperm nuclei (top panels) or DNA-coated beads (bottom panels) were imaged by time-lapse fluorescence microcopy to visualize spindle assembly in the presence (sperm) and absence (beads) of centrosomes and kinetochores. The upper left panel shows spindle assembly around a sperm nucleus with evident centrosomes. Note that one of the centrosomes becomes fully detached (arrow) from the spindle pole without affecting spindle morphology. The upper right panel shows assembly of a bipolar spindle around a sperm nucleus lacking centrosomes. Kinetochores are present in both types of sperm spindle assembly reactions. The bottom two panels show examples of chromatin-mediated bipolar spindle assembly around DNA-coated beads that lack centrosomes and kinetochores.
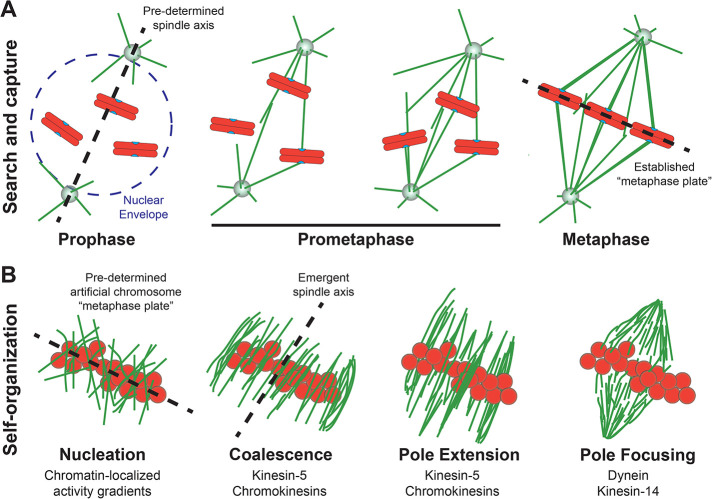
A more detailed investigation into the stages of chromatin-mediated spindle assembly revealed that the process depends on self-organization of MTs into a bipolar array (Figure 1). Randomly oriented MTs were nucleated in the vicinity of the beads in the first 15–30 min, after which they began to “coalesce” into antiparallel bundles before becoming focused into spindle poles that extended away from the beads into a bipolar structure after 60–90 min. Interestingly, polarity-marked MT seeds within the bead spindles moved poleward, with their minus-ends leading in a dynein-dependent manner, and dynein activity was shown to be required for the establishment and maintenance of focused bead spindle poles (Heald et al., 1996). Heald and colleagues concluded the paper with a new model for spindle assembly in which they proposed that microtubules are self-organized into a bipolar structure by the combined activities of plus- and minus-end directed motors acting upon the randomly oriented array that is nucleated in the vicinity of the mitotic chromatin. More specifically, they envisioned that dynein clusters MT minus-ends to focus them into poles, while microtubule-associated proteins and plus-end directed motors bundle MTs into arrays that define the bipolar axis of the spindle and extend the focused poles away from the beads.
FIGURE 1:
A comparison of two models of spindle assembly: search and capture versus self-organization. (A) In the classic search and capture model, the spindle axis is predetermined by centrosomes, which are positioned on either side of the nucleus during prophase. After the nuclear envelope breaks down in prometaphase, centrosome-nucleated MTs (green) search the three-dimensional volume of the cell via dynamic instability until they physically contact a kinetochore (blue) and become captured and stabilized. The attachment of MTs from opposite centrosomes to the two sister kinetochores on a chromosome (orange) coupled with a balance of forces leads to its central positioning between the two centrosomes—culminating with the alignment of every pair of sister chromatids at a metaphase plate. (B) In the self-organization model (shown here around chromatin beads [orange]), randomly oriented MTs (green) assemble in the vicinity of mitotic chromatin due to localized activity gradients. The spindle axis is defined when the MTs are sorted (often into antiparallel arrays) and coalesced into bundles by the actions of plus-end directed motor proteins, including the tetrameric kinesin-5 and chromokinesins, which also drive extension of the MTs away from the DNA. The minus-ends of the MTs are clustered and focused into spindle poles by the minus-end directed motors dynein or kinesin-14. During bead spindle assembly these “steps” are occurring concomitantly and, once assembled, they are required to continuously maintain the fusiform structure, as inhibiting molecular mediators of nucleation (e.g., Ran pathway), coalescence and pole extension (e.g., kinesin-5), or pole focusing (e.g., dynein) affects the morphology of an assembled bipolar spindle.
WHAT WE’VE LEARNED SINCE
The model that emerged from experiments on spindle assembly around DNA-coated beads defined immediate next steps to be taken in the field—specifically, in identifying mitotic motors that contributed to self-organization. As with many important contributions, there were skeptics who argued that the chromatin-mediated pathway was limited to female meiotic cells and plants and would not contribute significantly to spindle assembly in animal somatic cells. Heald’s observation that MTs polymerized locally around DNA beads immediately segued into new research aimed at understanding the molecular nature of chromatin-based spatial signals that promote MT nucleation and growth.
Characterization of motors that contribute to self-organization
Researchers from the Karsenti and Mitchison groups collaborated on a follow-up study to Heald et al. (1996) in which chromatin-mediated spindle assembly was assayed following inhibition of various motor proteins in the Xenopus egg extract system (Walczak et al., 1998). They concluded that the plus-end directed tetrameric kinesin Eg5 (kinesin-5) was critical for bundling and sorting antiparallel MTs, the plus-end directed chromokinesin Xklp1 (kinesin-4) contributed to MT-chromatin interactions and extending the spindle poles away from the beads, and the minus-end directed motors dynein and XCTK2 (kinesin-14) contributed to pole focusing. Thus, multiple motors contribute to the self-organization phenomenon and, in many cases, these motor functions are conserved in somatic cell spindle assembly.
Chromatin-meditated pathways function in somatic animal cells
The skeptics’ argument that the chromatin-mediated pathway was limited to plant and female meiotic cells was silenced by a series of studies over the next decade. First, it was shown that functional bipolar spindles assembled normally following laser ablation of one or both centrosomes in vertebrate somatic tissue culture cells (Khodjakov et al., 2000). It was later shown that centrosomes were dispensable for spindle bipolarity in various mutants of D. melanogaster that lacked centrosomes (Bonaccorsi et al., 2000; Giansanti et al., 2001; Megraw et al., 2001; Basto et al., 2006). The hypothesis in Heald et al. that “the real function” of centrosomes was to regulate spindle orientation/positioning by linking the spindle poles to the cell cortex has been supported by years of subsequent research.
Chromatin-based signals for MT assembly
At the conclusion of their work, Heald and colleagues noted that they did not know how chromatin induced MT assembly, but they favored the explanation that the mitotic chromatin locally altered the state of the cytoplasm to promote nucleation and stabilization. This hypothesis fueled much research in the field over the subsequent 25 years, which led to our present-day understanding that spatial gradients around mitotic chromatin promote MT polymerization. A gradient of RanGTP, which is generated by its chromatin-associated GEF RCC1, triggers the local release of spindle assembly factors (SAFs) from import receptors around mitotic chromatin (Carazo-Salas et al., 1999; Kalab et al., 1999; Ohba et al., 1999; Wilde and Zheng, 1999; Zhang et al., 1999; Carazo-Salas et al., 2001; Gruss et al., 2001; Nachury et al., 2001; Wiese et al., 2001; Kalab et al., 2002; Kaláb et al., 2006; Kalab and Heald, 2008; Halpin et al., 2011). There are many SAFs targeted by the RanGTP gradient, but, at present, the SAF with the most direct link to MT nucleation is TPX2, since it binds to and activates Aurora A kinase, which in turn phosphorylates NEDD1 to promote γ-TuRC-mediated MT nucleation (Scrofani et al., 2015). Interestingly, the chromosomal passenger complex (CPC) and its constituent Aurora B kinase are also an important regulator of MT assembly around chromosomes through local inhibition of the catastrophe factors MCAK and Stathmin/Op18 (Andersen et al., 1997; Andrews et al., 2004; Sampath et al., 2004; Gadea and Ruderman, 2006; Kelly et al., 2007; Maresca et al., 2009). In this case, the activity gradient promotes MT stability, since it reduces the catastrophe frequency of polymerizing MTs in the vicinity of mitotic chromatin. Thus, regarding their hypothesis about the nature of the MT assembly signal around chromatin, Heald and colleagues were prescient in the 1996 paper.
WHAT LIES AHEAD
Self-organizational processes are prevalent and function on multiple scales during spindle assembly. The significance of assembling bead spindles in the absence of kinetochores was rightly emphasized by Heald and colleagues in 1996. However, like micrometer-sized DNA-coated beads, nanometer-scale kinetochores also nucleate and organize MTs (Telzer et al., 1975; Mitchison and Kirschner, 1985a; Khodjakov et al., 2003; Maiato et al., 2004). In fact, kinetochores nucleate randomly oriented MTs that are coalesced into a bundle and extended away from the chromosome with MT minus-ends oriented away from the kinetochore—a phenomenon that is remarkably similar to Heald’s description of the steps of chromatin-mediated spindle assembly. The kinetochore-mediated process also utilizes molecules that are central to chromatin-mediated spindle assembly: import receptors, TPX2, dynein, and the CPC (Tulu et al., 2006). Furthermore, sorting of randomly oriented MTs around kinetochores during coalescence is mediated by the plus-end directed kinetochore-associated motor protein CENP-E (Sikirzhytski et al., 2018).
Like chromatin-mediated spindle assembly, the kinetochore-mediated pathway appears to be as far from the classic search and capture model as one could imagine—at least in its early stages. Mathematical modeling revealed that search and capture that relies on dynamic MTs nucleated from centrosomes could not work in a human cell with physiologically relevant timing unless it was spatially biased (Wollman et al., 2005). It is now known that there is a noncentrosomal MT nucleation pathway that structurally biases the direction of MT growth. Branching MT nucleation is mediated by recruitment of the γ-TuRC to a MT by the augmin complex (Goshima et al., 2007; Uehara et al., 2009; Petry et al., 2013; Verma and Maresca, 2019; Alfaro-Aco et al., 2020; Tariq et al., 2020). Importantly, daughter MTs are nucleated so that their growing plus-ends are oriented in the same direction as the mother MT.
Most spindle MTs do not persist long enough to support branching nucleation, since the multistep process takes ∼30 s (Verma and Maresca, 2019). However, during kinetochore-mediated assembly, CENP-E sorts MTs and converts them from lateral interactions into end-on attachments where the MT plus-ends are inserted into the kinetochore and stabilized, at which point they will live long enough to support branching. At this stage, daughter MTs nucleated via branching will 1) be closer to the kinetochore than if they were nucleated by the centrosome, and 2) grow with their plus-ends oriented in the direction of the kinetochore. Thus, while self-organization mechanisms initially organize kinetochore MTs, the mature kinetochore fiber is likely assembled with a biased search and capture mechanism that relies on branching (rather than centrosomal) MT nucleation (Goshima et al., 2008; Uehara et al., 2009; Kamasaki et al., 2013; David et al., 2019; Verma and Maresca, 2019; Almeida et al., 2021). As more is learned about how functional bipolar spindles assemble, we should not be surprised to discover that both biased search and capture and self-organization mechanisms contribute to building this incredibly complex machine—it may just take slightly altering your perspective to observe when and where they are at work.
Acknowledgments
The spindle assembly movies were taken by T.J.M. at the Marine Biological Laboratory in Woods Hole, MA as part of the Cell Division Group collaboration between the Salmon and Mitchison labs. The time-lapse movies were made late at night using epic X. laevis egg extracts produced alongside Aaron Groen and Jay Gatlin—thanks guys. Thank you also to Rebecca Heald for publishing the work that inspired this perspective and for sharing recollections about that exciting time of discovery. This work was supported by an NIH grant (GM107026) to T.J.M.
Abbreviations used:
- GEF
guanine nucleotide exchange factor
- GTP
guanosine triphosphate
- Mad2
mitotic arrest deficient 2
- MCAK
mitotic centromere-associated kinesin
- Ncd
nonclaret disjunctional
- NEDD1
neural precursor cell expressed developmentally down-regulated gene 1
- Op 18
oncoprotein 18
- Ran
ras-related nuclear protein
- RCC1
regulator of chromosome condensation
- TPX2
targeting protein for Xklp2
- XCTK2
xenopus COOH-terminal kinesin 2
- Xklp1
xenopus kinesin-like protein 1.
Footnotes
REFERENCES
- Afshar K, Barton NR, Hawley RS, Goldstein LS (1995). DNA binding and meiotic chromosomal localization of the Drosophila nod kinesin-like protein. Cell 81, 129–138. [DOI] [PubMed] [Google Scholar]
- Alfaro-Aco R, Thawani A, Petry S (2020). Biochemical reconstitution of branching microtubule nucleation. eLife 9, e49797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida AC, Oliveira J, Drpic D, Cheeseman LP, Damas J, Lewin HA, Larkin DM, Aguiar P, Pereira AJ, Maiato H (2021). Kinetochore-mediated microtubule assembly and Augmin-dependent amplification drive k-fiber maturation in mammals. bioRxiv, 2021.2008.2018.456780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SS, Ashford AJ, Tournebize R, Gavet O, Sobel A, Hyman AA, Karsenti E (1997). Mitotic chromatin regulates phosphorylation of Stathmin/Op18. Nature 389, 640–643. [DOI] [PubMed] [Google Scholar]
- Andrews PD, Ovechkina Y, Morrice N, Wagenbach M, Duncan K, Wordeman L, Swedlow JR (2004). Aurora B regulates MCAK at the mitotic centromere. Dev Cell 6, 253–268. [DOI] [PubMed] [Google Scholar]
- Basto R, Lau J, Vinogradova T, Gardiol A, Woods CG, Khodjakov A, Raff JW (2006). Flies without centrioles. Cell 125, 1375–1386. [DOI] [PubMed] [Google Scholar]
- Bonaccorsi S, Giansanti MG, Gatti M (2000). Spindle assembly in Drosophila neuroblasts and ganglion mother cells. Nat Cell Biol 2, 54–56. [DOI] [PubMed] [Google Scholar]
- Borisy GG, Taylor EW (1967). The mechanism of action of colchicine. Colchicine binding to sea urchin eggs and the mitotic apparatus. J Cell Biol 34, 535–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan J, Wilson L (1971). Are cytoplasmic microtubules heteropolymers? Proc Natl Acad Sci USA 68, 1762–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carazo-Salas RE, Gruss OJ, Mattaj IW, Karsenti E (2001). Ran-GTP coordinates regulation of microtubule nucleation and dynamics during mitotic-spindle assembly. Nat Cell Biol 3, 228–234. [DOI] [PubMed] [Google Scholar]
- Carazo-Salas RE, Guarguaglini G, Gruss OJ, Segref A, Karsenti E, Mattaj IW (1999). Generation of GTP-bound Ran by RCC1 is required for chromatin-induced mitotic spindle formation. Nature 400, 178–181. [DOI] [PubMed] [Google Scholar]
- Cassimeris L, Pryer NK, Salmon ED (1988). Real-time observations of microtubule dynamic instability in living cells. J Cell Biol 107, 2223–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RH, Waters JC, Salmon ED, Murray AW (1996). Association of spindle assembly checkpoint component XMAD2 with unattached kinetochores. Science 274, 242–246. [DOI] [PubMed] [Google Scholar]
- David AF, Roudot P, Legant WR, Betzig E, Danuser G, Gerlich DW (2019). Augmin accumulation on long-lived microtubules drives amplification and kinetochore-directed growth. J Cell Biol 218, 2150–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont J, Desai A (2012). Acentrosomal spindle assembly and chromosome segregation during oocyte meiosis. Trends Cell Biol 22, 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw WC, Rothfield N (1985). Identification of a family of human centromere proteins using autoimmune sera from patients with scleroderma. Chromosoma 91, 313–321. [DOI] [PubMed] [Google Scholar]
- Echeverri CJ, Paschal BM, Vaughan KT, Vallee RB (1996). Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J Cell Biol 132, 617–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endow SA, Chandra R, Komma DJ, Yamamoto AH, Salmon ED (1994). Mutants of the Drosophila ncd microtubule motor protein cause centrosomal and spindle pole defects in mitosis. J Cell Sci 107(Pt 4), 859–867. [DOI] [PubMed] [Google Scholar]
- Gadea BB, Ruderman JV (2006). Aurora B is required for mitotic chromatin-induced phosphorylation of Op18/Stathmin. Proc Natl Acad Sci USA 103, 4493–4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giansanti MG, Gatti M, Bonaccorsi S (2001). The role of centrosomes and astral microtubules during asymmetric division of Drosophila neuroblasts. Development 128, 1137–1145. [DOI] [PubMed] [Google Scholar]
- Goshima G, Mayer M, Zhang N, Stuurman N, Vale RD (2008). Augmin: a protein complex required for centrosome-independent microtubule generation within the spindle. J Cell Biol 181, 421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima G, Wollman R, Goodwin SS, Zhang N, Scholey JM, Vale RD, Stuurman N (2007). Genes required for mitotic spindle assembly in Drosophila S2 cells. Science 316, 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss OJ, Carazo-Salas RE, Schatz CA, Guarguaglini G, Kast J, Wilm M, Le Bot N, Vernos I, Karsenti E, Mattaj IW (2001). Ran induces spindle assembly by reversing the inhibitory effect of importin alpha on TPX2 activity. Cell 104, 83–93. [DOI] [PubMed] [Google Scholar]
- Halpin D, Kalab P, Wang J, Weis K, Heald R (2011). Mitotic spindle assembly around RCC1-coated beads in Xenopus egg extracts. PLoS Biol 9, e1001225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden JH, Bowser SS, Rieder CL (1990). Kinetochores capture astral microtubules during chromosome attachment to the mitotic spindle: direct visualization in live newt lung cells. J Cell Biol 111, 1039–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R, Tournebize R, Blank T, Sandaltzopoulos R, Becker P, Hyman A, Karsenti E (1996). Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature 382, 420–425. [DOI] [PubMed] [Google Scholar]
- Hoyt MA, Totis L, Roberts BT (1991). S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell 66, 507–517. [DOI] [PubMed] [Google Scholar]
- Hyman AA, Karsenti E (1996). Morphogenetic properties of microtubules and mitotic spindle assembly. Cell 84, 401–410. [DOI] [PubMed] [Google Scholar]
- Hyman AA, Mitchison TJ (1991). Two different microtubule-based motor activities with opposite polarities in kinetochores. Nature 351, 206–211. [DOI] [PubMed] [Google Scholar]
- Inoué S (1952). The effect of colchicine on the microscopic and submicroscopic structure of the mitotic spindle. Exp Cell Res 2, 305–318. [Google Scholar]
- Inoué S (1953). Polarization optical studies of the mitotic spindle. I. The demonstration of spindle fibers in living cells. Chromosoma 5, 487–500. [DOI] [PubMed] [Google Scholar]
- Inoué S (1964). Organization and function of the mitotic spindle. In: Primitive Motile Systems in Cell Biology: Academic Press; New York, NY, 549–598. [Google Scholar]
- Kalab P, Heald R (2008). The RanGTP gradient—a GPS for the mitotic spindle. J Cell Sci 121, 1577–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaláb P, Pralle A, Isacoff EY, Heald R, Weis K (2006). Analysis of a RanGTP-regulated gradient in mitotic somatic cells. Nature 440, 697–701. [DOI] [PubMed] [Google Scholar]
- Kalab P, Pu RT, Dasso M (1999). The ran GTPase regulates mitotic spindle assembly. Curr Biol 9, 481–484. [DOI] [PubMed] [Google Scholar]
- Kalab P, Weis K, Heald R (2002). Visualization of a Ran–GTP gradient in interphase and mitotic Xenopus egg extracts. Science 295, 2452–2456. [DOI] [PubMed] [Google Scholar]
- Kamasaki T, O’Toole E, Kita S, Osumi M, Usukura J, McIntosh JR, Goshima G (2013). Augmin-dependent microtubule nucleation at microtubule walls in the spindle. J Cell Biol 202, 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenti E, Newport J, Kirschner M (1984). Respective roles of centrosomes and chromatin in the conversion of microtubule arrays from interphase to metaphase. J Cell Biol 99, 47s–54s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AE, Sampath SC, Maniar TA, Woo EM, Chait BT, Funabiki H (2007). Chromosomal enrichment and activation of the aurora B pathway are coupled to spatially regulate spindle assembly. Dev Cell 12, 31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov A, Cole RW, Oakley BR, Rieder CL (2000). Centrosome-independent mitotic spindle formation in vertebrates. Curr Biol 10, 59–67. [DOI] [PubMed] [Google Scholar]
- Khodjakov A, Copenagle L, Gordon MB, Compton DA, Kapoor TM (2003). Minus-end capture of preformed kinetochore fibers contributes to spindle morphogenesis. J Cell Biol 160, 671–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner M, Mitchison T (1986). Beyond self-assembly: from microtubules to morphogenesis. Cell 45, 329–342. [DOI] [PubMed] [Google Scholar]
- Li R, Murray AW (1991). Feedback control of mitosis in budding yeast. Cell 66, 519–531. [DOI] [PubMed] [Google Scholar]
- Li Y, Benezra R (1996). Identification of a human mitotic checkpoint gene: hsMAD2. Science 274, 246–248. [DOI] [PubMed] [Google Scholar]
- Ludueńa RF, Shooter EM, Wilson L (1977). Structure of the tubulin dimer. J Biol Chem 252, 7006–7014. [PubMed] [Google Scholar]
- Maiato H, Rieder CL, Khodjakov A (2004). Kinetochore-driven formation of kinetochore fibers contributes to spindle assembly during animal mitosis. J Cell Biol 167, 831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresca TJ, Groen AC, Gatlin JC, Ohi R, Mitchison TJ, Salmon ED (2009). Spindle assembly in the absence of a RanGTP gradient requires localized CPC activity. Curr Biol 19, 1210–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megraw TL, Kao LR, Kaufman TC (2001). Zygotic development without functional mitotic centrosomes. Curr Biol 11, 116–120. [DOI] [PubMed] [Google Scholar]
- Merdes A, De Mey J (1990). The mechanism of kinetochore–spindle attachment and polewards movement analyzed in PtK2 cells at the prophase–prometaphase transition. Eur J Cell Biol 53, 313–325. [PubMed] [Google Scholar]
- Mitchison T, Kirschner M (1984a). Dynamic instability of microtubule growth. Nature 312, 237–242. [DOI] [PubMed] [Google Scholar]
- Mitchison T, Kirschner M (1984b). Microtubule assembly nucleated by isolated centrosomes. Nature 312, 232–237. [DOI] [PubMed] [Google Scholar]
- Mitchison TJ, Kirschner MW (1985a). Properties of the kinetochore in vitro. I. Microtubule nucleation and tubulin binding. J Cell Biol 101, 755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison TJ, Kirschner MW (1985b). Properties of the kinetochore in vitro. II. Microtubule capture and ATP-dependent translocation. J Cell Biol 101, 766–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T, Evans L, Schulze E, Kirschner M (1986). Sites of microtubule assembly and disassembly in the mitotic spindle. Cell 45, 515–527. [DOI] [PubMed] [Google Scholar]
- Musacchio A, Desai A (2017). A molecular view of kinetochore assembly and function. Biology (Basel) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury MV, Maresca TJ, Salmon WC, Waterman-Storer CM, Heald R, Weis K (2001). Importin beta is a mitotic target of the small GTPase Ran in spindle assembly. Cell 104, 95–106. [DOI] [PubMed] [Google Scholar]
- Nogales E, Whittaker M, Milligan RA, Downing KH (1999). High-resolution model of the microtubule. Cell 96, 79–88. [DOI] [PubMed] [Google Scholar]
- Nogales E, Wolf SG, Downing KH (1998). Structure of the alpha beta tubulin dimer by electron crystallography. Nature 391, 199–203. [DOI] [PubMed] [Google Scholar]
- Ohba T, Nakamura M, Nishitani H, Nishimoto T (1999). Self-organization of microtubule asters induced in Xenopus egg extracts by GTP-bound Ran. Science 284, 1356–1358. [DOI] [PubMed] [Google Scholar]
- Petry S, Groen AC, Ishihara K, Mitchison TJ, Vale RD (2013). Branching microtubule nucleation in Xenopus egg extracts mediated by augmin and TPX2. Cell 152, 768–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfarr CM, Coue M, Grissom PM, Hays TS, Porter ME, McIntosh JR (1990). Cytoplasmic dynein is localized to kinetochores during mitosis. Nature 345, 263–265. [DOI] [PubMed] [Google Scholar]
- Rieder CL, Alexander SP (1990). Kinetochores are transported poleward along a single astral microtubule during chromosome attachment to the spindle in newt lung cells. J Cell Biol 110, 81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder CL, Cole RW, Khodjakov A, Sluder G (1995). The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J Cell Biol 130, 941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder CL, Schultz A, Cole R, Sluder G (1994). Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. J Cell Biol 127, 1301–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath SC, Ohi R, Leismann O, Salic A, Pozniakovski A, Funabiki H (2004). The chromosomal passenger complex is required for chromatin-induced microtubule stabilization and spindle assembly. Cell 118, 187–202. [DOI] [PubMed] [Google Scholar]
- Scrofani J, Sardon T, Meunier S, Vernos I (2015). Microtubule nucleation in mitosis by a RanGTP-dependent protein complex. Curr Biol 25, 131–140. [DOI] [PubMed] [Google Scholar]
- Sikirzhytski V, Renda F, Tikhonenko I, Magidson V, McEwen BF, Khodjakov A (2018). Microtubules assemble near most kinetochores during early prometaphase in human cells. J Cell Biol 217, 2647–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuer ER, Wordeman L, Schroer TA, Sheetz MP (1990). Localization of cytoplasmic dynein to mitotic spindles and kinetochores. Nature 345, 266–268. [DOI] [PubMed] [Google Scholar]
- Tariq A, Green L, Jeynes JCG, Soeller C, Wakefield JG (2020). In vitro reconstitution of branching microtubule nucleation. eLife 9, e49769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer BR, Moses MJ, Rosenbaum JL (1975). Assembly of microtubules onto kinetochores of isolated mitotic chromosomes of HeLa cells. Proc Natl Acad Sci USA 72, 4023–4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulu US, Fagerstrom C, Ferenz NP, Wadsworth P (2006). Molecular requirements for kinetochore-associated microtubule formation in mammalian cells. Curr Biol 16, 536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara R, Nozawa RS, Tomioka A, Petry S, Vale RD, Obuse C, Goshima G (2009). The augmin complex plays a critical role in spindle microtubule generation for mitotic progression and cytokinesis in human cells. Proc Natl Acad Sci USA 106, 6998–7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma V, Maresca TJ (2019). Direct observation of branching MT nucleation in living animal cells. J Cell Biol 218, 2829–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernos I, Raats J, Hirano T, Heasman J, Karsenti E, Wylie C (1995). Xklp1, a chromosomal Xenopus kinesin-like protein essential for spindle organization and chromosome positioning. Cell 81, 117–127. [DOI] [PubMed] [Google Scholar]
- Walczak CE, Vernos I, Mitchison TJ, Karsenti E, Heald R (1998). A model for the proposed roles of different microtubule-based motor proteins in establishing spindle bipolarity. Curr Biol 8, 903–913. [DOI] [PubMed] [Google Scholar]
- Walker RA, O’Brien ET, Pryer NK, Soboeiro MF, Voter WA, Erickson HP, Salmon ED (1988). Dynamic instability of individual microtubules analyzed by video light microscopy: rate constants and transition frequencies. J Cell Biol 107, 1437–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RA, Salmon ED, Endow SA (1990). The Drosophila claret segregation protein is a minus-end directed motor molecule. Nature 347, 780–782. [DOI] [PubMed] [Google Scholar]
- Wang SZ, Adler R (1995). Chromokinesin: a DNA-binding, kinesin-like nuclear protein. J Cell Biol 128, 761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese C, Wilde A, Moore MS, Adam SA, Merdes A, Zheng Y (2001). Role of importin-beta in coupling Ran to downstream targets in microtubule assembly. Science 291, 653–656. [DOI] [PubMed] [Google Scholar]
- Wilde A, Zheng Y (1999). Stimulation of microtubule aster formation and spindle assembly by the small GTPase Ran. Science 284, 1359–1362. [DOI] [PubMed] [Google Scholar]
- Wollman R, Cytrynbaum EN, Jones JT, Meyer T, Scholey JM, Mogilner A (2005). Efficient chromosome capture requires a bias in the “search-and-capture” process during mitotic-spindle assembly. Curr Biol 15, 828–832. [DOI] [PubMed] [Google Scholar]
- Wood KW, Sakowicz R, Goldstein LS, Cleveland DW (1997). CENP-E is a plus end-directed kinetochore motor required for metaphase chromosome alignment. Cell 91, 357–366. [DOI] [PubMed] [Google Scholar]
- Wordeman L, Mitchison TJ (1995). Identification and partial characterization of mitotic centromere-associated kinesin, a kinesin-related protein that associates with centromeres during mitosis. J Cell Biol 128, 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen TJ, Compton DA, Wise D, Zinkowski RP, Brinkley BR, Earnshaw WC, Cleveland DW (1991). CENP-E, a novel human centromere-associated protein required for progression from metaphase to anaphase. EMBO J 10, 1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen TJ, Li G, Schaar BT, Szilak I, Cleveland DW (1992). CENP-E is a putative kinetochore motor that accumulates just before mitosis. Nature 359, 536–539. [DOI] [PubMed] [Google Scholar]
- Zhang C, Hughes M, Clarke PR (1999). Ran-GTP stabilises microtubule asters and inhibits nuclear assembly in Xenopus egg extracts. J Cell Sci 112(Pt 14), 2453–2461. [DOI] [PubMed] [Google Scholar]