Abstract
BACKGROUND
Negative-pressure hydrocephalus (NePH) is a rare clinical entity that presents on the background of ventriculomegaly with atypical symptoms. Its diagnosis is difficult, and some patients experience several shunt revisions until the proper solution is found.
OBSERVATIONS
The authors present a patient who developed acute deterioration due to iatrogenic NePH after surgery for a vertebral artery thrombosed giant aneurysm. The deterioration occurred after the insertion of a lumbar drain by which the authors intended to reduce a postoperative subcutaneous cerebrospinal fluid (CSF) collection. The drainage created an unexpected negative-pressure gradient in the CSF spaces, which resulted in NePH. Interventions, such as extraventricular drainage and blood patch, corrected the negative transmantle pressure and stabilized the patient’s condition.
LESSONS
Because the pathophysiology of NePH is theoretically considered to be caused by negative transmantle pressure, the intervention should be performed in order to deal with the coexistence of obstruction in the CSF pathways and a CSF leak. A blood patch would be an effective option in treating the CSF leak when the site of leakage is certain. This is the first case in which a blood patch was effectively applied in the treatment for NePH with a favorable outcome without any permanent CSF diversion.
Keywords: negative-pressure hydrocephalus, low-pressure hydrocephalus, blood patch
ABBREVIATIONS : CSAS = cortical subarachnoid space, CSF = cerebrospinal fluid, CT = computerized tomography, ETV = endoscopic third ventriculostomy, EVD = external ventricular drainage, GCS = Glasgow Coma Scale, MRI = magnetic resonance imaging, NePH = negative-pressure hydrocephalus, POD = postoperative day, VA = vertebral artery
Negative-pressure hydrocephalus (NePH) or low-pressure hydrocephalus are rare clinical entities that present on the background of an increase in ventricular size and symptoms such as headache, nausea, cranial neuropathies, breathing distress, and worsening mental status. Because of the atypical symptoms and images, it is a difficult entity for diagnosis, and some patients experience several shunt revisions until a proper treatment solution is found. Etiology includes subarachnoid hemorrhage, posterior fossa tumor cases, or conditions after lumbar puncture, among others.1–4 Negative-pressure external ventricular drainage (EVD),1,3 endoscopic third ventriculostomy (ETV),5,6 neck wrapping (or a cervical tourniquet),1,7 intermittent shunt valve pressing, and enforced recumbency were applied to treat patients with poor outcomes.4,7–9
This report describes an adult patient with iatrogenic NePH whose management was successful and whose symptoms disappeared without requiring permanent cerebrospinal fluid (CSF) diversion.
Illustrative Case
Clinical Course Before Surgery
A 48-year-old man with a vertebral artery (VA) thrombosed giant aneurysm developed right hemiparesis and hiccups with the presentation of slight enlargement of the lesion and brainstem hemorrhage on magnetic resonance imaging (MRI). We completed the treatment in three separate stages: 1) occlusion of the VA proximal to the aneurysm (proximal VA), 2) occlusion of the VA distal to the aneurysm (distal VA), and 3) debulking and vasa vasorum obliteration. We successfully finished trapping the VA with partial aneurysmectomy because of the attachment to the brainstem.10
Postoperative Course
Postoperatively, the right hemiparesis, hiccups, and nausea completely disappeared. The next day after surgery, we placed a lumbar drain to treat accumulating subcutaneous CSF. The drainage pressure was set initially at 8 cmH2O and was gradually reduced because of the small amount of drained CSF. Within 7 hours of this reduction, the patient’s consciousness was impaired (Glasgow Coma Scale [GCS] score 6) with respiratory distress. Then, the drainage pressure setting was at −4 cmH2O but only 10 ml of CSF was drained. The patient underwent emergency computerized tomography (CT) with his bed flat and drainage clamped. The CT scan showed bilateral ventriculomegaly, which had not been shown previously (Fig. 1A–C). While returning to his room, the patient’s level of consciousness recovered up to GCS score 14 (Fig. 2A). The drain was removed on postoperative day (POD) 2, and the patient was kept in the supine position with the bed flat. Though the ventriculomegaly remained, the GCS score was 14 in the supine position. We gradually lifted his head up to 30° on POD 4. His consciousness was impaired again, and the CT scan showed bilateral ventriculomegaly (Fig. 2B). We decided to perform emergency EVD.
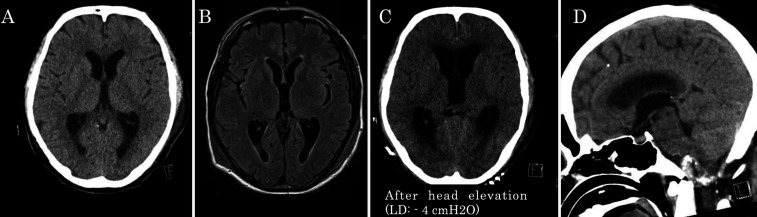
Fig. 1.
A and B: CT scan before the operation and the MRI on POD 1 showed no change in the ventricular size. C: After starting lumbar drainage (LD) to treat the accumulating subcutaneous CSF, the patient’s consciousness became impaired (GCS score 6) and the CT scan showed prominent bilateral ventriculomegaly. D: Sagittal image of the same CT scan at the time of deterioration. Clear downward shift of the posterior fossa contents cannot be confirmed because of the presence of residual aneurysm portions and postoperative artifacts in the foramen magnum area. Significant posterior bony decompression of the craniovertebral junction is visible. Magendie foramen patency cannot be confirmed.
Fig. 2.
A: POD 2. Though the ventriculomegaly remained, the GCS score was 14 in the supine position. B: POD 4. After head elevation, the patient’s consciousness became impaired again (GCS score 6) and the CT scan showed further enlarged ventricles. C: POD 4. Following EVD, the scan revealed obvious pneumocephalus with the ventricular size reduced. The patient’s mental status recovered to slight disorientation (GCS score 14). D: POD 6. After a blood patch, the CT scan demonstrated normal- and stable-sized ventricles.
Surgical Findings
After dural incision, the cortical surface was found to be gradually sinking with little CSF outflow. The opening ventricular drainage pressure was −2 cmH2O and the condition was considered to be NePH. We kept the drainage at 20 cmH2O with the bed flat. The postoperative CT showed normal-sized ventricles with a large amount of air in the subdural space (Fig. 2C), and at the same time, his level of consciousness recovered with only slight disorientation (GCS score 14). The CSF leakage from the lumbar puncture site seemed to contribute to the second deterioration; therefore, on POD 5, we performed a blood patch, which resulted in an increase of the CSF drainage pressure from 7 (pre–blood patch) to 16 cmH2O (post–blood patch). The patient became alert, and the CT scan demonstrated normal- and stable-sized ventricles (Fig. 2D).
Discussion
Pathophysiological Basis of Low-Pressure Hydrocephalus
The syndrome of NePH was discussed first by Pang and Altschuler.3 NePH has been suspected especially with repeated shunt failures and the presence of a CSF leak and loss of communication between the ventricles and the cortical subarachnoid space (CSAS).7
NePH is often explained with the effects of brain turgor and the transmantle pressure. Brain parenchyma has viscoelastic properties and possesses the ability to resist or permit distortion due to changes in brain turgor. The shift of cerebral venous blood and extracellular fluid, caused by the negative pressure, and the ventriculomegaly result in decreased brain turgor. Brain turgor is a target for treatment in some reported cases, and not only the influence of EVD but also the application of a cervical tourniquet was attempted with successful results.7
Transmantle pressure is defined as the differential pressure equal to the ventricular pressure minus the CSAS pressure and reflects the pressure transmitted through the brain parenchyma.11 Filippidis et al. theorized that two mechanisms are necessary to establish this pressure gradient: (1) the blockage of CSF pathways between the ventricles and the CSAS, and (2) the presence of a CSF leak that communicates between the subarachnoid space compartment and atmospheric pressure.1 Ventriculomegaly can occur when the ventricular pressure is higher than the pressure of the CSAS, as when there is a blockage of CSF outflow pathways, regardless of its absolute value, but only with the existence of such a gradient. The importance in treating the CSF leak has been implied; however, EVD, despite the negative pressure, has been the main choice, and eventually most cases are designated for permanent CSF shunt diversion.1,6,12
In the literature, this phenomenon has been given several names, including “(acute) low-pressure hydrocephalus,” “NePH,” and “syndrome of inappropriately low-pressure hydrocephalus.” The term for this condition should be integrated; even “low-pressure ventriculomegaly” can appropriately express the current status of knowledge. Because similar reported conditions were named “negative-pressure hydrocephalus,” we used the term for this report. These conditions seem to be created by a common situation.
Treatment Approach Analysis
In our case, NePH occurred on the background of lack of communication between the ventricles and the subarachnoid spaces due to ventral compression of the brainstem by the thrombosed aneurysm residue, combined with excessive CSF drainage from the placed lumbar drain. After the lumbar drainage, lifting the patient’s head triggered the deteriorations. The postural changes enhanced the leakage from the lumbar puncture site, which resulted in a decreased CSF pressure and subsequent NePH. These episodes were similar to those in the cases reported by Dias et al.4 The recovery from coma after the EVD was due to the regaining of pressure equilibrium between subarachnoid spaces and ventricles (transmantle) by the air entering in the subdural space. After the diagnosis, because the CSF leak was obviously caused from the site of lumbar puncture, to correct the continued subarachnoid pressure decrease, we could logically perform a blood patch at the drainage site. ETV has also been reported as an alternative treatment because it restores the communication between intra- and extraventricular spaces.5–7 However, because we understood that the main point of the treatment of NePH is correcting the negative transmantle pressure, we selected a blood patch to stop the CSF leak intervening on the pressure outside ventricles.
Historically, cases were commonly treated with negative-pressure EVD, and according to a recent systematic review of 195 patients, the mortality rate was 11% in both pediatric and adult patients. More than 20% of pediatric and only 1% of adult patients recovered without requiring permanent CSF diversion (shunt or ETV).6 This is the first case in which a blood patch has been effective as a definitive treatment for NePH with a favorable outcome without any CSF diversion, and we could accurately perform this treatment because the site of the leakage was certain. We considered that we could achieve a good outcome by using the proper treatment against the factors of negative transmantle pressure (CSF blockage and CSF leak).
Observations
This is the first case in which a blood patch was effectively applied in the treatment of NePH with a favorable outcome without any permanent CSF diversion.
Lessons
Because the pathophysiology of NePH is theoretically considered to be caused by negative transmantle pressure, the intervention should be performed in order to deal with the coexistence of obstruction in CSF pathways and CSF leak. A blood patch would be an effective option in treating the CSF leak when the site of leakage is certain.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Suzuki, Murayama. Acquisition of data: Suzuki. Analysis and interpretation of data: Suzuki, Karagiozov, Murayama. Drafting the article: Suzuki, Karagiozov, Murayama. Critically revising the article: Suzuki, Karagiozov, Murayama. Reviewed submitted version of manuscript: Suzuki, Karagiozov, Murayama. Approved the final version of the manuscript on behalf of all authors: Suzuki. Study supervision: Kaku, Murayama.
References
- 1. Filippidis AS, Kalani MY, Nakaji P, et al. Negative-pressure and low-pressure hydrocephalus: the role of cerebrospinal fluid leaks resulting from surgical approaches to the cranial base. J Neurosurg. 2011;115(5):1031–1037. doi: 10.3171/2011.6.JNS101504. [DOI] [PubMed] [Google Scholar]
- 2. Strand A, Balise S, Leung LJ, et al. Low-pressure hydrocephalus: a case report and review of the literature. World Neurosurg. 2018;109:e131–e135. doi: 10.1016/j.wneu.2017.09.120. [DOI] [PubMed] [Google Scholar]
- 3. Pang D, Altschuler E. Low-pressure hydrocephalic state and viscoelastic alterations in the brain. Neurosurgery. 1994;35(4):643–656. doi: 10.1227/00006123-199410000-00010. [DOI] [PubMed] [Google Scholar]
- 4. Dias MS, Li V, Pollina J. Low-pressure shunt ‘malfunction’ following lumbar puncture in children with shunted obstructive hydrocephalus. Pediatr Neurosurg. 1999;30(3):146–150. doi: 10.1159/000028783. [DOI] [PubMed] [Google Scholar]
- 5. Foster KA, Deibert CP, Choi PA, et al. Endoscopic third ventriculostomy as adjunctive therapy in the treatment of low-pressure hydrocephalus in adults. Surg Neurol Int. 2016;7:26. doi: 10.4103/2152-7806.178522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Keough MB, Isaacs AM, Urbaneja G, et al. Acute low-pressure hydrocephalus: a case series and systematic review of 195 patients J Neurosurg 2020[in press]. Article in press. [DOI] [PubMed] [Google Scholar]
- 7. Rekate HL, Nadkarni TD, Wallace D. The importance of the cortical subarachnoid space in understanding hydrocephalus. J Neurosurg Pediatr. 2008;2(1):1–11. doi: 10.3171/PED/2008/2/7/001. [DOI] [PubMed] [Google Scholar]
- 8. Wu X, Zang D, Wu X, et al. Diagnosis and management for secondary low- or negative-pressure hydrocephalus and a new hydrocephalus classification based on ventricular pressure. World Neurosurg. 2019;124:e510–e516. doi: 10.1016/j.wneu.2018.12.123. [DOI] [PubMed] [Google Scholar]
- 9. Smalley ZS, Venable GT, Einhaus S. Low-pressure hydrocephalus in children : a case series and review of the literature. 2017;80(3):439–447. doi: 10.1093/neuros/nyw046. [DOI] [PubMed] [Google Scholar]
- 10. Suzuki T, Kaku S, Nishimura K, et al. Multistage “hybrid” (open and endovascular) surgical treatment of vertebral artery-thrombosed giant aneurysm by trapping and thrombectomy. World Neurosurg. 2018;114:144–150. doi: 10.1016/j.wneu.2018.03.055. [DOI] [PubMed] [Google Scholar]
- 11. Miller JD. Volume and pressure in the craniospinal axis. Clin Neurosurg. 1975;22:76–105. doi: 10.1093/neurosurgery/22.cn_suppl_1.76. [DOI] [PubMed] [Google Scholar]
- 12. Diaz-Romero Paz R, Avendaño Altimira P, Coloma Valverde G, et al. A rare case of negative-pressure hydrocephalus: a plausible explanation and the role of transmantle theory. World Neurosurg. 2019;125:6–9. doi: 10.1016/j.wneu.2019.01.117. [DOI] [PubMed] [Google Scholar]