Abstract
Skeletal muscle immune cells, such as macrophages, are necessary for proper regrowth following muscle disuse. We suggest that the important role of macrophages concerning muscle regrowth following disuse is divergent compared to young mice (i.e. dysregulated) during the recovery period. Modulation of macrophages may be a promising future therapeutic target to enhance the impaired muscle growth during recovery from disuse in older adults.
Keywords: immune cells, polarization, physical inactivity, injury, rehabilitation, aging
Summary:
In this review, the role of macrophages in the recovery of muscle after disuse or injury in aging are discussed.
Introduction
Aging coincides with a higher frequency of muscle disuse and recovery events (due to illness, surgery, pain) that are likely to lead to rapid loss of muscle mass and strength. Full recovery of muscle mass and function following these disuse / recovery periods may not occur for many of these older adults while others may find recovery much slower than optimal (1). Indeed, several aging studies have reported impaired skeletal muscle recovery in rats (2–4), mice (5, 6) and humans (7–9) following disuse. The concern is that the impaired recovery following muscle disuse events may lead to an acceleration of sarcopenia (10) thereby aiding the development of muscle and functional decline. Several theories as to why aged skeletal muscle recovery from disuse is impaired (2, 5) are just beginning to be investigated. Since macrophages have been demonstrated to have a pivotal role in muscle regrowth following disuse in young rodents (11–15) our hypothesis is that dysregulated macrophages (a response divergent from the young adult mouse) in aged skeletal muscle are also a contributing factor to the age-related impairment in muscle recovery following disuse.
Role of macrophages in skeletal muscle injury repair
Research involving rodents describes a complex and well-orchestrated series of events involving a host of different cell types (e.g., immune, satellite cells, endothelial) enable complete skeletal muscle regeneration from robust muscle injury (16, 17). Macrophages are immune cells found in skeletal muscle and they are essential for complete muscle regeneration (18, 19). This critical role has been demonstrated in great detail following macrophage depletion studies (20–22) and experiments blocking macrophage recruitment/migration (23–25) and activity (22–24, 26, 27). Macrophages exist under a spectrum of polarized states but, in general, can be characterized as either pro- (M1-like) or anti-inflammatory (M2-like). In response to injury, granulocytes rapidly migrate to the tissue within 48h. This is thought to be followed by a flux of monocytes/macrophages (Ly6c+/CCL2+/CX3CR1low) and resident tissue macrophages (1–5 days), which function in a pro-inflammatory state commonly thought to remove debris, induce vascularization and stimulate proliferation and fusion of muscle satellite cells. Resident-tissue macrophages are not able to completely handle pronounced skeletal muscle damage and therefore, the circulatory monocytes/macrophages are recruited to muscle where they are differentiated to pro-inflammatory macrophages and potentially re-polarized to a different state later on during the recovery process (20). The pro-inflammatory response is resolved in the 3–7 days following injury by a polarization to macrophages with a predominantly anti-inflammatory phenotype (Ly6c−/CCL2−/CX3CR1high) such that the majority of immune cells residing in muscle throughout the remainder of the recovery is anti-inflammatory, as the total number of macrophages begins to decline. These anti-inflammatory macrophages serve to dampen the initial inflammatory response and stimulate angiogenesis, collagen deposition, satellite cell differentiation and myofiber regrowth (28). A key function of monocytes and macrophages is to release cytokines and growth factors that work in a paracrine fashion to influence muscle regeneration and regrowth (13, 29). For a more comprehensive review of the role of macrophages during muscle injury and regeneration see (28, 30–32).
Muscle disuse vs muscle injury/regeneration models
When examining the role of these immune cells in skeletal muscle of rodents the important distinctions between research models used should be briefly highlighted. Extreme injury and regeneration models (i.e., myotoxin, crush, laceration, freeze, ischemia/reperfusion, eccentric exercise) are not equivalent (33) and some models are more damaging than others (i.e. ischemia/reperfusion is more damaging than myotoxic injury (34)). It is important to note that the damage response caused by ambulatory recovery from muscle disuse is several orders of magnitude less than extreme injury and regeneration models. The injury/regeneration models employ an insult that can ablate whole sections of muscle requiring formation of new myofibers followed by their regeneration. With disuse (e.g., hindlimb unloading, hindlimb immobilization), the myofibers remain intact, but experience myofiber atrophy, weakness and increased susceptibility to injury, especially with aging (35). For example, 2d of reloading after hindlimb unloading in young adult rodents resulted in ~2% injured/necrotic myofibers, which receded to none being detectable at 4 days following reloading when only 1–2% of centrally-located regenerating myofibers are observed (11). Whereas following 1–4d post-toxin injury, ~10–75% of myofibers are injured/necrotic (20, 24) and typically >50% are myofibers classified as regenerative in the following few days (20, 24, 36). These responses are even more exaggerated following the ischemia/reperfusion injury model because it is more damaging than myotoxic injury (34). As a result, there are also drastic differences in the abundance of macrophages during reloading versus the injury models mentioned above as determined by flow cytometry (Figure 1). The monocyte/macrophage abundance in uninjured mouse tibialis anterior muscle was ~200 cells/mg muscle (20), at 4d post-toxin injury this amount increased in the same muscle to ~15,000 cells/mg muscle (20) and 3d post-ischemia-reperfusion increased to ~34,000 cells/mg muscle in the gastrocnemius (37). We demonstrate that the fast-twitch gastrocnemius or plantaris in young adult mice contained ~50 cells/mg muscle whereas the slow-twitch soleus had ~200 cells/mg in “uninjured” control muscle (38). After 4d of ambulatory recovery following 14d of hindlimb unloading, we observed a relatively minor increase of ~120 cells/mg muscle in the gastrocnemius and ~600 cells/mg muscle in the soleus (Figure 1). Although, the elevated macrophage number during recovery from disuse is far less that during regeneration, they still play an important role during the muscle regrowth process (see below).
Figure 1.
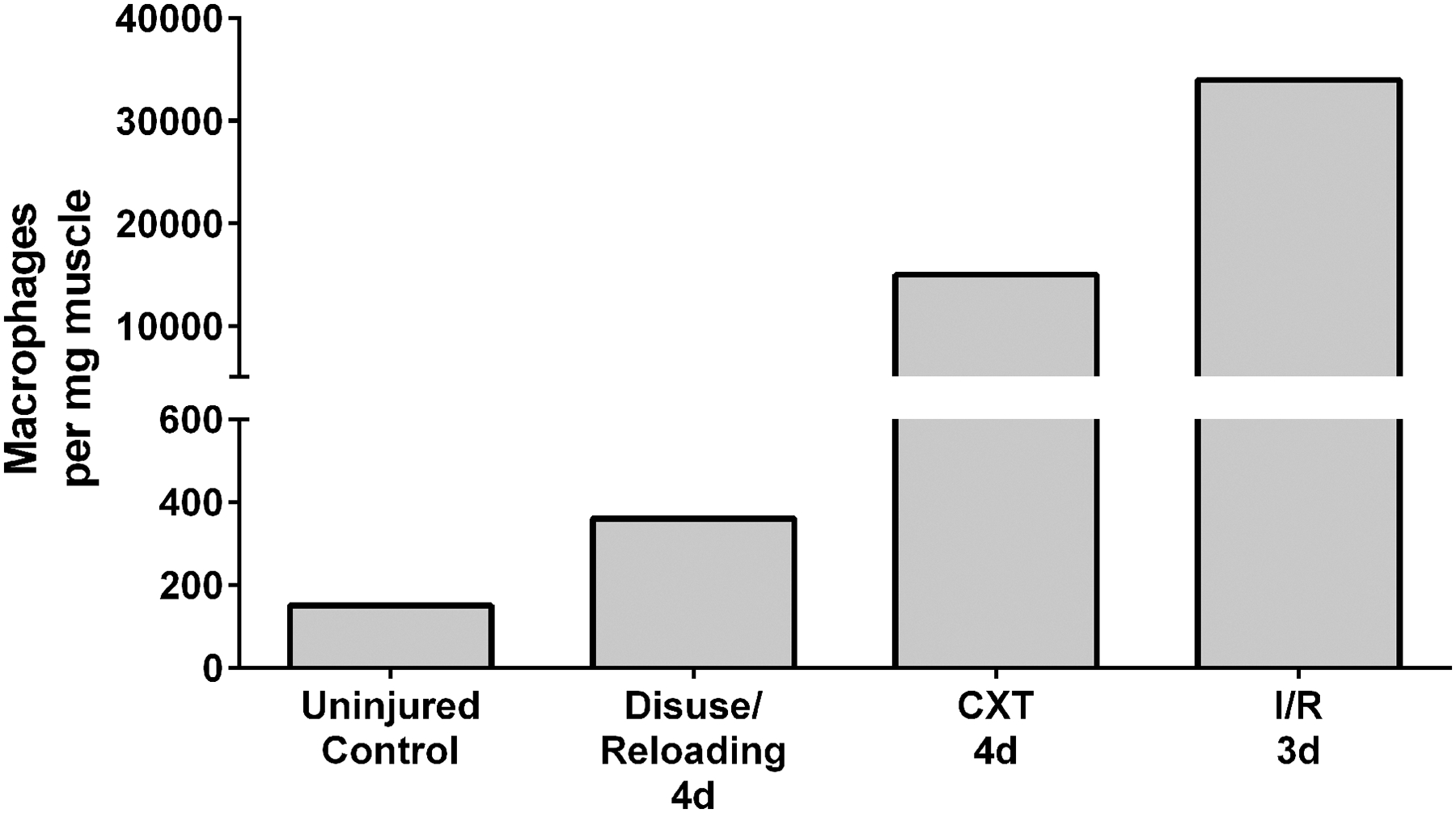
Representation of differences in mouse macrophage abundance (using flow cytometry) in skeletal muscle from “uninjured” controls, following 4 days reloaded from disuse (38), 4 days post-CXT (cardiotoxin) (20) injection, and 3 days post-I/R (ischemia/reperfusion) (37). Uninjured control was an average of 200 cells/ per mg from the tibialis anterior (20) and our work demonstrating 50 and 200 cells/ per mg from the gastrocnemius and soleus, respectively (38). The muscle disuse/reloading 4d timepoint (representing 14d hindlimb unloading followed by 4d of recovery) was an average from our data (38) demonstrating 120 and 600 cells/ per mg from the gastrocnemius and soleus, respectively.
Macrophages and recovery from muscle disuse
Myeloid cell responses during ambulatory recovery from muscle disuse (i.e., hindlimb unloading, hindlimb immobilization) have been well examined in young adult rodents (11, 12, 16, 39–41). Like the robust injury models, macrophages undergo a similar sequence of events following recovery from muscle disuse (16, 17, 40). Early studies using pharmacological approaches or genetic models (11–13, 15) have demonstrated the requirement of macrophages or their activity to maximize regrowth of myofibers during the recovery process from disuse in rodents. Indeed, depletion of circulating monocytes (13), all macrophages (11) or cytokines regulating macrophage function (12, 15) impair muscle recovery following disuse in young adult mice. Additionally, we have recently completed preliminary experiments demonstrating that CCL2 KO young adult mice have impaired muscle CSA recovery following disuse (Reidy PT and Drummond MJ, 2019 – unpublished). This further adds credence to the concept that macrophages recruitment are critical for maximum regrowth of muscle following disuse. Although, we have a more comprehensive understanding of the role of muscle macrophages in young adult rodents during recovery from disuse very little is understood in the context of aging skeletal muscle. In particular, growth factors produced by macrophages (e.g. IGF-1) are a key component of muscle regeneration (29) and regrowth (13). Since IGF-1 transcripts are attenuated in aging skeletal muscle following recovery from disuse (42), this could be a likely site of targeted immunotherapies.
Impact of aging on macrophages and muscle injury repair
Evidence suggests that aged tissues have a defective or delayed immune cell response under a variety of conditions (43), such as muscle regeneration following injury (43–46). In general, uninjured aging skeletal muscles have a predominance of macrophages with a M2-like phenotype skewed toward the development of fibrosis (47, 48). Specific macrophage phenotype polarization (M1 vs M2-like) and time course following injury has not been thoroughly examined in aged skeletal muscle following injury with appropriate methodologies (i.e., flow cytometry and cell sorting), but it is clear that human and rodent aged skeletal muscle has a dysregulated immune cell response in comparison to young muscle. Qualitative histopathological assessments have suggested a delayed (49, 50) or heightened (50, 51) accumulation of pro-inflammatory cells in aged skeletal muscle following injury (49). Other reports indicate elevated and prolonged mRNA expression of pro-inflammatory mediators (in whole muscle) (52), excessive cytokine (53), neutrophil (44, 54, 55) and macrophage abundance (54, 55) in aged skeletal muscle following injury. For example, Patsalos et al. demonstrated a prolonged greater proportion of inflammatory Ly6Chigh macrophages and a reduced proportions of repair Ly6Clow macrophages during recovery from acute sterile injury in old mice compared to young mice (56). The timing of these events from the studies listed above is unclear in aging and confounded by various injury models. Yet, taken together, these reports support a desynchronization of the immune cell response in aging skeletal muscle following injury compared to young in both human and rodent research.
Macrophages and the recovery of muscle tissue following disuse with aging
There are far fewer studies (two to date) describing muscle macrophage responses during the course of muscle disuse and recovery in aged animals. White et al. used a single M2-like macrophage marker, CD163+ cells (via IHC), and demonstrated a trend (non-significant) for young rat soleus muscle to increase the level of M2-like macrophages above both control and late (14d) recovery conditions following 14d of hindlimb unloading (4). Alternately, the old rat muscle showed greater CD163+ cells (vs young) at all time points. CD163+ cells decreased in the old with hindlimb unloading and only returned to baseline after 14d recovery while muscle regrowth remained impaired in aged animals.
Recently, we showed that macrophage recruitment and a shift in M1-like and M2-like macrophage proportions were dysregulated in aging mouse muscle during recovery from disuse in a muscle-specific manner (38). Using flow cytometry, the old gastrocnemius muscle demonstrated a higher level of pro-inflammatory monocyte (Ly6c+ cells) infiltration that started during disuse and was maintained throughout the recovery phase. These data were accompanied by a greater C-C Motif Chemokine Ligand 2 (CCL2) mRNA and attenuated interferon regulatory factor 7 (IRF7) mRNA expression during recovery; the coded protein that is responsible to differentiate monocytes to macrophages (57). These data suggest that the old muscle may have an impaired ability to differentiate monocytes to macrophages. When examining the soleus muscle, even greater age-related impairments on macrophages and muscle regrowth were observed compared to the gastrocnemius. We found that the soleus at 4d recovery not only had impaired recruitment of monocytes and macrophages, but also lower abundance of M1-like macrophages. This finding was further confirmed with specific cytokine and growth factor gene expression on sorted CD45+CD11b+ monocytes/macrophage cells which suggested that the macrophages in the old were possibly not shifting their macrophage phenotype similar to young during this M1-like to M2-like transitional time point. Together, these data suggest an altered immune cell response between old and young rodents during the disuse / recovery paradigm. More intensive studies are needed to confirm the role of muscle macrophages in aging following disuse, specifically in humans, while also examining immunotherapies to assist in muscle regrowth with aging.
Potential therapies to enhance muscle regrowth following muscle disuse in aging
Immunotherapy has more recently been sought as a route to optimize tissue regeneration (26) and regrowth. In brief, the most prominent immunotherapy techniques include: 1) delivery of ex-vivo activated macrophages or 2) delivery of molecules to alter macrophage abundance and/or phenotype. Intramuscular injection of macrophages into injured muscle has been shown to accelerate the regeneration/regrowth process in young adult animals (37, 58, 59). Macrophage immunotherapy has been successful in improving muscle recovery following ischemia-reperfusion (37, 58) and laceration (59) injury. Two studies to date have shown that injection of M1-like macrophages during the early inflammatory phase (58) and M2-like macrophages (37) at the peak of inflammation following injury improved muscle size and function. However, these studies were conducted in young rodents and in a model that does not mimic recovery from a disuse event. Interestingly, injection of macrophage colony-stimulating factor (M-CSF), a cytokine to promote recruitment, proliferation, and maturation of macrophages, into soleus muscle of young mice during disuse, resulted in faster muscle recovery (14). Indeed, our preliminary data (Reidy PT and Drummond MJ, 2019- unpublished) suggests that intramuscular injection of M-CSF (versus PBS) promoted greater regrowth of the soleus muscle of old mice after 2 weeks of reloading. One recent investigation has shown that immunotherapy via supplementation of a macrophage‐derived cytokine, growth differentiation factor 3 (GDF3) could improve regeneration in old, but not young, mice following cardiotoxin injury (56). Therefore, these models of immunomodulation may have potential application to improve the recovery of aged skeletal muscle following disuse atrophy.
Immunomodulation may also be applied through cyclic compressive loading (CCL) in skeletal muscle such as massage (60) or even certain types of muscle contraction (61, 62). Massage improves recovery following eccentric exercise in rabbits by reducing edema and macrophage infiltration (63). We showed that the application of CCL to normal muscle facilitated a shift in the expression of genes in the immunity pathways, an increase in muscle macrophages, and a shift in the muscle macrophage pool towards the M2 phenotype, which is supportive of muscle growth (64). Cyclic compressive loading given during 8d recovery from 14d of hindlimb unloading improved recovery in skeletal muscle of young rats and observed a clear trend for elevated M2-like macrophage marker CD163 (65).
Certain types of exercise may also provide immunomodulation. Aerobic exercise in the form of voluntary wheel running (66), can enhance recovery following muscle disuse in young rodents. It is unknown if the improved muscle recovery following disuse with aerobic exercise is mediated via the effects of macrophages, but it is known that a single bout of aerobic exercise is capable of increasing M2-like macrophages in muscle (62). Moreover, an accumulation of muscle macrophages with aerobic exercise training has been associated with muscle growth (61). Future investigations should examine the potential immunomodulatory impact of aerobic exercise on recovery from disuse atrophy in young and old skeletal muscle.
Conclusions
The reasons for the impaired muscle recovery from disuse with aging are still being explored and macrophages in skeletal muscle are one potential avenue of inquiry. It is clear that macrophage and immune cell responses in aged skeletal muscle are dyssynchronous when compared to young muscles following robust injury, although this is an area in need of further exploration. Using the more generalizable disuse and recovery/regrowth model, a dysregulated skeletal muscle macrophage response is also observed with aging (Figure 2). However, care should be taken when comparing the robust injury models (freeze, ischemia/reperfusion, cardiotoxin) to the disuse/reloading events as the former has a much greater amount of damage, immune cell infiltration, a longer immune response and likely a greater reliance on circulating immune cells than the latter. Future investigations need to utilize the tools of flow cytometry to better characterize the time course, phenotype and role of macrophages in regeneration and regrowth after disuse, and to determine the effectiveness of possible immunotherapies to enhance recovery of aged skeletal muscle.
Figure 2.

Hypothetical macrophage immune cell response following muscle disuse or injury in young and old skeletal muscle. During the first few days during ambulatory recovery (“early recovery”) following muscle disuse or injury in young adult rodents, the number of pro-inflammatory M1-like macrophages increase within the muscle tissue in order to remove debris and to facilitate the proliferation and fusion of muscle satellite cells. During the latter days of muscle recovery (“late recovery”), M1-like macrophages polarize into M2-like macrophages, thereby increasing the population of these anti-inflammatory macrophages (M2-like). M2-like macrophages serve to induce angiogenesis, collagen deposition, differentiation of satellite cells and myofiber regrowth. During recovery from disuse or injury, aged skeletal muscle is characterized by a dyssynchronous macrophage infiltration and polarization. It is hypothesized that evidence-based immunomodulatory therapies such as injection of activated macrophages or molecules that alter the activate or abundance of macrophages or application of massage or aerobic exercise may be effective to correct the timing and polarization of macrophages and ultimately aide in the restoration of muscle tissue from injury or disuse in aged muscle. An important distinction between the disuse/regrowth and injury/regeneration models is the amount of damage, inflammatory cells, and changes in muscle size. Regarding muscle size, the disuse/regrowth model undergoes atrophy and then regrowth while the injury model does not experience fiber atrophy, but growth of regenerated myofibers. Even with these differences both models require much further investigation using flow cytometry and cell sorting.
Key Point Summary:
Muscle recovery (size) following muscle injury or a disuse period is impaired or delayed in rodents and humans.
Macrophage timing and polarization are essential to the recovery process during regrowth
In aged skeletal muscle, the timing of pro- and anti-inflammatory macrophages is dysregulated, during recovery from injury or disuse.
Potential therapies such as macrophage replacement, immunomodulation, or exercise may optimize macrophage polarization patterns and possibly enhance muscle recovery in aged muscle
Funding:
This work was supported by the National Institute of Aging (R01AG050781, R21AG042699), the National Center for Complementary and Integrative Health (R01AT009268) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (F32AR072481).
Footnotes
Authors declare no conflict of interest
References
- 1.Gill TM, Gahbauer EA, Han L, Allore HG. The relationship between intervening hospitalizations and transitions between frailty states. The journals of gerontology. Series A, Biological sciences and medical sciences. 2011;66(11):1238–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baehr LM, West DW, Marcotte G et al. Age-related deficits in skeletal muscle recovery following disuse are associated with neuromuscular junction instability and ER stress, not impaired protein synthesis. Aging (Albany NY). 2016;8(1):127–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallegly JC, Turesky NA, Strotman BA, Gurley CM, Peterson CA, Dupont-Versteegden EE. Satellite cell regulation of muscle mass is altered at old age. Journal of applied physiology (Bethesda, Md. : 1985). 2004;97(3):1082–90. [DOI] [PubMed] [Google Scholar]
- 4.White JR, Confides AL, Moore-Reed S, Hoch JM, Dupont-Versteegden EE. Regrowth after skeletal muscle atrophy is impaired in aged rats, despite similar responses in signaling pathways. Exp Gerontol. 2015;64:17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X, Trevino MB, Wang M et al. Impaired Mitochondrial Energetics Characterize Poor Early Recovery of Muscle Mass Following Hind Limb Unloading in Old Mice. J Gerontol A Biol Sci Med Sci. 2018;73(10):1313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliveira JRS, Mohamed JS, Myers MJ, Brooks MJ, Alway SE. Effects of hindlimb suspension and reloading on gastrocnemius and soleus muscle mass and function in geriatric mice. Exp Gerontol. 2019;115:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanner RE, Brunker LB, Agergaard J et al. Age-related differences in lean mass, protein synthesis and skeletal muscle markers of proteolysis after bed rest and exercise rehabilitation. The Journal of physiology. 2015;593(18):4259–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hvid L, Aagaard P, Justesen L et al. Effects of aging on muscle mechanical function and muscle fiber morphology during short-term immobilization and subsequent retraining. Journal of Applied Physiology. 2010;109(6):1628–34. [DOI] [PubMed] [Google Scholar]
- 9.Suetta C, Hvid LG, Justesen L et al. Effects of aging on human skeletal muscle after immobilization and retraining. Journal of Applied Physiology. 2009;107(4):1172–80. [DOI] [PubMed] [Google Scholar]
- 10.English KL, Paddon-Jones D. Protecting muscle mass and function in older adults during bed rest. Current opinion in clinical nutrition and metabolic care. 2010;13(1):34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tidball JG, Wehling-Henricks M. Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J Physiol. 2007;578(Pt 1):327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng B, Wehling-Henricks M, Villalta SA, Wang Y, Tidball JG. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. Journal of immunology (Baltimore, Md. : 1950). 2012;189(7):3669–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dumont N, Frenette J. Macrophages protect against muscle atrophy and promote muscle recovery in vivo and in vitro: a mechanism partly dependent on the insulin-like growth factor-1 signaling molecule. The American journal of pathology. 2010;176(5):2228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumont NA, Frenette J. Macrophage colony-stimulating factor-induced macrophage differentiation promotes regrowth in atrophied skeletal muscles and C2C12 myotubes. The American journal of pathology. 2013;182(2):505–15. [DOI] [PubMed] [Google Scholar]
- 15.Ohira T, Wang XD, Ito T et al. Macrophage deficiency in osteopetrotic (op/op) mice inhibits activation of satellite cells and prevents hypertrophy in single soleus fibers. American journal of physiology. Cell physiology. 2015;308(10):C848–55. [DOI] [PubMed] [Google Scholar]
- 16.Tidball JG, Villalta SA. Regulatory interactions between muscle and the immune system during muscle regeneration. American journal of physiology. Regulatory, integrative and comparative physiology. 2010;298(5):R1173–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chazaud B. Macrophages: supportive cells for tissue repair and regeneration. Immunobiology. 2014;219(3):172–8. [DOI] [PubMed] [Google Scholar]
- 18.Sass FA, Fuchs M, Pumberger M et al. Immunology Guides Skeletal Muscle Regeneration. International journal of molecular sciences. 2018;19(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Santa F, Vitiello L, Torcinaro A, Ferraro E. The Role of Metabolic Remodeling in Macrophage Polarization and Its Effect on Skeletal Muscle Regeneration. Antioxid Redox Signal. 2018. [DOI] [PubMed] [Google Scholar]
- 20.Arnold L, Henry A, Poron F et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. The Journal of experimental medicine. 2007;204(5):1057–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao W, Liu Y, Chen P. Macrophage Depletion Impairs Skeletal Muscle Regeneration: the Roles of Pro-fibrotic Factors, Inflammation, and Oxidative Stress. Inflammation. 2016;39(6):2016–28. [DOI] [PubMed] [Google Scholar]
- 22.Bosurgi L, Manfredi AA, Rovere-Querini P. Macrophages in injured skeletal muscle: a perpetuum mobile causing and limiting fibrosis, prompting or restricting resolution and regeneration. Frontiers in immunology. 2011;2:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao W, Lu H, Wang X, Ransohoff RM, Zhou L. CX3CR1 deficiency delays acute skeletal muscle injury repair by impairing macrophage functions. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2016;30(1):380–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnold L, Perrin H, de Chanville CB et al. CX3CR1 deficiency promotes muscle repair and regeneration by enhancing macrophage ApoE production. Nature communications. 2015;6:8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melton DW, Roberts AC, Wang H et al. Absence of CCR2 results in an inflammaging environment in young mice with age-independent impairments in muscle regeneration. Journal of leukocyte biology. 2016;100(5):1011–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spiller KL, Koh TJ. Macrophage-based therapeutic strategies in regenerative medicine. Advanced drug delivery reviews. 2017;122:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patsalos A, Pap A, Varga T et al. In situ macrophage phenotypic transition is affected by altered cellular composition prior to acute sterile muscle injury. J Physiol. 2017;595(17):5815–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chazaud B, Brigitte M, Yacoub-Youssef H et al. Dual and beneficial roles of macrophages during skeletal muscle regeneration. Exerc Sport Sci Rev. 2009;37(1):18–22. [DOI] [PubMed] [Google Scholar]
- 29.Tonkin J, Temmerman L, Sampson RD et al. Monocyte/Macrophage-derived IGF-1 Orchestrates Murine Skeletal Muscle Regeneration and Modulates Autocrine Polarization. Molecular therapy : the journal of the American Society of Gene Therapy. 2015;23(7):1189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saclier M, Cuvellier S, Magnan M, Mounier R, Chazaud B. Monocyte/macrophage interactions with myogenic precursor cells during skeletal muscle regeneration. The FEBS journal. 2013;280(17):4118–30. [DOI] [PubMed] [Google Scholar]
- 31.Tidball JG. Regulation of muscle growth and regeneration by the immune system. Nature reviews. Immunology. 2017;17(3):165–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang W, Hu P. Hierarchical signaling transduction of the immune and muscle cell crosstalk in muscle regeneration. Cellular Immunology. 2018;326:2–7. [DOI] [PubMed] [Google Scholar]
- 33.Hardy D, Besnard A, Latil M et al. Comparative Study of Injury Models for Studying Muscle Regeneration in Mice. PLoS One. 2016;11(1):e0147198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vignaud A, Hourde C, Medja F, Agbulut O, Butler-Browne G, Ferry A. Impaired skeletal muscle repair after ischemia-reperfusion injury in mice. J Biomed Biotechnol. 2010;2010:724914–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanazawa Y, Ikegami K, Sujino M et al. Effects of aging on basement membrane of the soleus muscle during recovery following disuse atrophy in rats. Exp Gerontol. 2017;98:153–61. [DOI] [PubMed] [Google Scholar]
- 36.Arnold L, Perrin H, de Chanville CB et al. CX3CR1 deficiency promotes muscle repair and regeneration by enhancing macrophage ApoE production. Nature communications. 2015;6:8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hammers DW, Rybalko V, Merscham-Banda M, Hsieh PL, Suggs LJ, Farrar RP. Anti-inflammatory macrophages improve skeletal muscle recovery from ischemia-reperfusion. Journal of applied physiology (Bethesda, Md. : 1985). 2015;118(8):1067–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reidy PT, McKenzie AI, Mahmassani ZS et al. Aging impairs mouse skeletal muscle macrophage polarization and muscle-specific abundance during recovery from disuse. American journal of physiology. Endocrinology and metabolism. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.St Pierre BA, Tidball JG. Differential response of macrophage subpopulations to soleus muscle reloading after rat hindlimb suspension. Journal of Applied Physiology. 1994;77(1):290–7. [DOI] [PubMed] [Google Scholar]
- 40.Tidball JG. Interactions between muscle and the immune system during modified musculoskeletal loading. Clin Orthop Relat Res. 2002;(403 Suppl):S100–9. [DOI] [PubMed] [Google Scholar]
- 41.Frenette J, St-Pierre M, Côté CH, Mylona E, Pizza FX. Muscle impairment occurs rapidly and precedes inflammatory cell accumulation after mechanical loading. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2002;282(2):R351–R7. [DOI] [PubMed] [Google Scholar]
- 42.Reidy PT, Yonemura NM, Madsen JH et al. An accumulation of muscle macrophages is accompanied by altered insulin sensitivity after reduced activity and recovery. 0(0):e13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stahl EC, Brown BN. Cell Therapy Strategies to Combat Immunosenescence. Organogenesis. 2015;11(4):159–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghaly A, Marsh DR. Aging-associated oxidative stress modulates the acute inflammatory response in skeletal muscle after contusion injury. Exp Gerontol. 2010;45(5):381–8. [DOI] [PubMed] [Google Scholar]
- 45.Blau HM, Cosgrove BD, Ho ATV. The central role of muscle stem cells in regenerative failure with aging. Nature medicine. 2015;21(8):854–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paliwal P, Pishesha N, Wijaya D, Conboy IM. Age dependent increase in the levels of osteopontin inhibits skeletal muscle regeneration. Aging (Albany NY). 2012;4(8):553–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y, Wehling-Henricks M, Samengo G, Tidball JG. Increases of M2a macrophages and fibrosis in aging muscle are influenced by bone marrow aging and negatively regulated by muscle-derived nitric oxide. Aging cell. 2015;14(4):678–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Wehling-Henricks M, Welc SS, Fisher AL, Zuo Q, Tidball JG. Aging of the immune system causes reductions in muscle stem cell populations, promotes their shift to a fibrogenic phenotype, and modulates sarcopenia. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2019;33(1):1415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shavlakadze T, McGeachie J, Grounds MD. Delayed but excellent myogenic stem cell response of regenerating geriatric skeletal muscles in mice. Biogerontology. 2010;11(3):363–76. [DOI] [PubMed] [Google Scholar]
- 50.Sadeh M. Effects of aging on skeletal muscle regeneration. J Neurol Sci. 1988;87(1):67–74. [DOI] [PubMed] [Google Scholar]
- 51.Hammers DW, Merritt EK, Matheny RW Jr. et al. Functional deficits and insulin-like growth factor-I gene expression following tourniquet-induced injury of skeletal muscle in young and old rats. Journal of applied physiology (Bethesda, Md. : 1985). 2008;105(4):1274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van der Poel C, Gosselin LE, Schertzer JD et al. Ageing prolongs inflammatory marker expression in regenerating rat skeletal muscles after injury. J Inflamm (Lond). 2011;8(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sorensen JR, Skousen C, Holland A, Williams K, Hyldahl RD. Acute extracellular matrix, inflammatory and MAPK response to lengthening contractions in elderly human skeletal muscle. Exp Gerontol. 2018;106:28–38. [DOI] [PubMed] [Google Scholar]
- 54.Koh TJ, Peterson JM, Pizza FX, Brooks SV. Passive stretches protect skeletal muscle of adult and old mice from lengthening contraction-induced injury. J Gerontol A Biol Sci Med Sci. 2003;58(7):592–7. [DOI] [PubMed] [Google Scholar]
- 55.Sloboda DD, Brooks SV, Brown LA. Myeloid Cell Responses to Contraction-induced Injury Differ in Muscles of Young and Old Mice. The Journals of Gerontology: Series A. 2018;73(12):1581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patsalos A, Simandi Z, Hays TT et al. In vivo GDF3 administration abrogates aging related muscle regeneration delay following acute sterile injury. Aging cell. 2018;17(5):e12815–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu R, Pitha PM. Monocyte differentiation to macrophage requires interferon regulatory factor 7. The Journal of biological chemistry. 2001;276(48):45491–6. [DOI] [PubMed] [Google Scholar]
- 58.Rybalko V, Hsieh P-L, Merscham-Banda M, Suggs LJ, Farrar RP. The Development of Macrophage-Mediated Cell Therapy to Improve Skeletal Muscle Function after Injury. PLoS ONE. 2016;10(12):e0145550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Novak ML, Weinheimer-Haus EM, Koh TJ. Macrophage Activation and Skeletal Muscle Healing Following Traumatic Injury. The Journal of pathology. 2014;232(3):344–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Waters-Banker C, Dupont-Versteegden EE, Kitzman PH, Butterfield TA. Investigating the mechanisms of massage efficacy: the role of mechanical immunomodulation. Journal of athletic training. 2014;49(2):266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walton RG, Kosmac K, Mula J et al. Human skeletal muscle macrophages increase following cycle training and are associated with adaptations that may facilitate growth. Scientific Reports. 2019;9(1):969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ikeda S, Tamura Y, Kakehi S et al. Exercise-induced enhancement of insulin sensitivity is associated with accumulation of M2-polarized macrophages in mouse skeletal muscle. Biochemical and biophysical research communications. 2013;441(1):36–41. [DOI] [PubMed] [Google Scholar]
- 63.Butterfield TA, Zhao Y, Agarwal S, Haq F, Best TM. Cyclic compressive loading facilitates recovery after eccentric exercise. Medicine and science in sports and exercise. 2008;40(7):1289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Waters-Banker C, Butterfield TA, Dupont-Versteegden EE. Immunomodulatory effects of massage on nonperturbed skeletal muscle in rats. Journal of applied physiology (Bethesda, Md. : 1985). 2014;116(2):164–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller BF, Hamilton KL, Majeed ZR et al. Enhanced skeletal muscle regrowth and remodelling in massaged and contralateral non-massaged hindlimb. J Physiol. 2018;596(1):83–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hanson AM, Stodieck LS, Cannon CM, Simske SJ, Ferguson VL. Seven days of muscle re-loading and voluntary wheel running following hindlimb suspension in mice restores running performance, muscle morphology and metrics of fatigue but not muscle strength. Journal of muscle research and cell motility. 2010;31(2):141–53. [DOI] [PubMed] [Google Scholar]


