Figure 1.
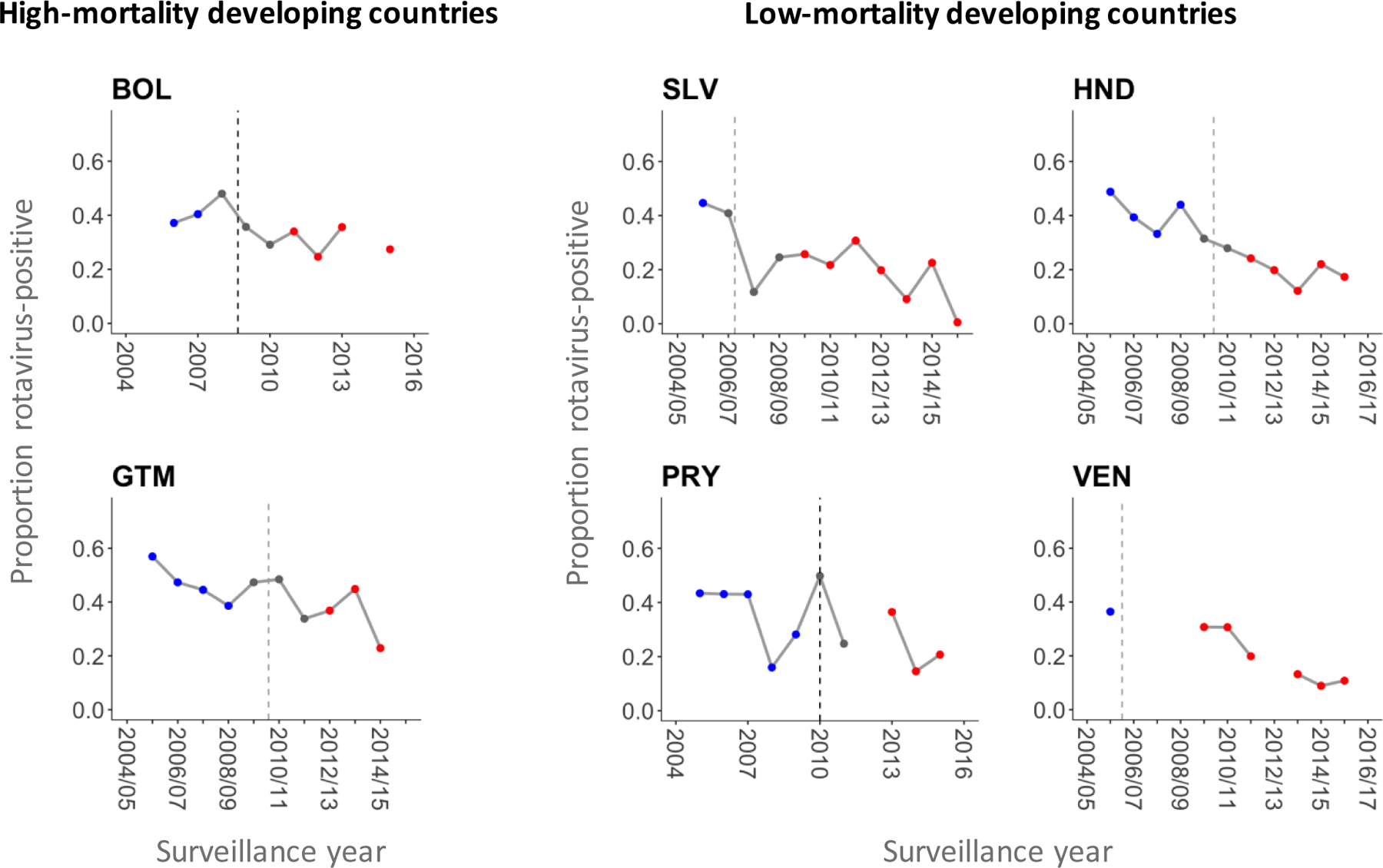
Proportion of rotavirus-positive samples among children hospitalized for acute diarrhea whose samples were collected for laboratory testing by surveillance year in six Latin American countries.
Dashed lines represent the timing of rotavirus vaccine introduction. Blue, grey, and red dots represent data points included in the pre-vaccine period, vaccine roll-out window, and post-vaccine period, respectively. Proportions were not calculated for a given surveillance year if there was more than one month in which the number of hospitalized cases with samples collected was missing. Abbreviations: BOL, Bolivia; SLV, El Salvador; GTM, Guatemala; HND, Honduras; PRY, Paraguay; VEN, Venezuela.
