Abstract
Gut health plays an important role on production and performance of broilers. This trial was undertaken with an aim to evaluate the synergistic effect of probiotic, chicory root powder and coriander seed powder on the performance and gut health of broiler chicken. For this purpose, a total of 240 day-old broiler chicks were randomly allotted to six dietary treatments with 8 replicates of 5 birds in each. Treatment groups included T1 as control i.e., basal diet (BD) without any growth promoter and T2—BD + antibiotic (BMD 0.05%). In the remaining experimental diets, T3—probiotic (@ 0.01%) + chicory root powder (@ 1.0%), T4—probiotic (@ 0.01%) + coriander seed powder (@ 1.5%), T5—chicory root powder (@ 1.0%) + coriander seed powder (@ 1.5%) and T6—probiotic (@ 0.01%) + chicory root powder (@ 1.0%) + coriander seed powder (@ 1.5%). The results indicated that supplementation of probiotic + chicory (T3), probiotic + coriander (T4), chicory + coriander (T5) and probiotic + chicory + coriander (T6) in combination resulted in significantly (P<0.05) higher weight gain and better FCR compared to control and antibiotic groups at 42 d of age. Supplementation of different dietary groups did not show any significant (P>0.05) effect on feed intake of broilers. Supplementation of all the test diets (T3 to T6) significantly (P<0.05) increased the glutathione peroxidase (GSHPx), glutathione reductase (GSHRx) and superoxide dismutase (SOD) enzyme activity when compared to control and antibiotic groups at 42 d of age. Supplementation of all the test diets (T3 to T6) significantly (P<0.05) lowered the pH in the gut, increased Lactobacillus counts, and reduced E. coli and Salmonella counts in the ileum compared to control and antibiotic groups. Supplementation of all the test diets (T3 to T6) significantly (P<0.05) increased the villus height (VH), crypt depth (CD), VH:CD ratio and villus width (VW) in the duodenum and only VH and CD in the ileum compared to control and antibiotic groups. Significantly (P<0.05) higher jejunal VH and VW and increased the goblet cell number in duodenum, jejunum and ileum was recorded in all test diets (T3 to T6) compared to control and antibiotic groups. Therefore, combinations of probiotic (0.01%), chicory root powder (1.0%) and coriander seed powder (1.5%) can be used as feed additive for improving performance and gut health of broiler chicken.
Introduction
Antibiotics are being used as growth promoters in the poultry diets all over the world. However, in recent years, there has been rising demand to reduce or abolish the use of antibiotics as growth promoters due to the detrimental human health issue of antibiotic resistance [1]. Consumers’ awareness of poultry products that do not contain antibiotic residues has increased, encouraging the use of suitable alternatives to antimicrobial compounds [2]. Among the feed additives, probiotics, prebiotics, organic acids, enzymes and medicinal plants have drawn more attention due to their prophylactic and growth promoting effects. Thus, the use of probiotics, medicinal plants, herbs and spices in poultry diets has become more popular worldwide as an alternative to antibiotics to minimize the disease incidence and achieving better performance in chicken [2].
Probiotics are single or mixed cultures of live microorganisms which beneficially affect the host by improving the balance of intestinal flora [3]. Probiotics maintain the beneficial intestinal microflora by competitive exclusion and antagonism, lowering the gut pH through acid fermentation, limiting the damage caused by pathogenic bacteria [4], improving epithelial cell integrity (villi height and width), producing bacteriocins, stimulating the gut associated immune system and increasing the production of short-chain fatty acids. Recently, herbal feed additive products like chicory root powder are gaining attention as they indirectly promote antimicrobial action by reducing the harmful bacteria in the gut. Dried chicory root powder is a good source of inulin type fructans and oligofructose chains known for having prebiotic action without any toxicity [5]. Inulin-type fructans are indigestible carbohydrates, recognized as dietary fibers that improve intestinal health and bird’s performance through their prebiotic properties [6]. The fermentation activity of inulin inhibits the growth of harmful strains, selectively stimulates the growth of beneficial bacteria by decreasing the intestinal pH through increasing the absorption of short chain fatty acids and thus promotes the growth of broiler chickens [7, 8]. Similarly, probiotic + prebiotic supplementation decreased intestinal pH and viscosity in broilers [9]. Addition of probiotics (Lactobacillus acidophilus and lactose fermenting enterobacteria) and prebiotic combinations in broilers significantly (P<0.05) increased the villus height and crypt depth of the duodenum, ileum and jejunum [10].
Coriander (Coriandrum sativum) is regarded as both herb and spice, and has been used in medicine for thousands of years. Coriander seeds possess antioxidant, diuretic, anti-diabetic, hypocholesterolemic, antimicrobial, anthelmintic and anti-mutagenic qualities [11, 12]. Coriander seed powder contains 0.5–1.0% essential oil (carvone, geraniol, limonene, borneol, camphor, elemol and linalool) having antimicrobial properties against food borne pathogen such as Salmonella species [13]. In addition, it has appetizing and stimulatory effects in the digestion process by increasing production of digestive enzymes and juices, which stimulates digestion and peristaltic motion, thus improves feed efficiency [14, 15]. Coriander seed powder as an alternative to antibiotic growth promoter has been recommended for feeding in broilers by several authors [16–18]. In view of the above, this experiment was designed to evaluate the synergistic effect of probiotic (0.01%), chicory root powder (1.0%) and coriander seed powder (1.5%) on the performance, antioxidant status and gut health of broiler chicken.
Materials and methods
To conduct the study, two hundred and forty (240) day old commercial (Vencobb 400) broiler chicks were procured, individually weighed and wing banded. The birds were distributed randomly into 6 dietary treatments, each with 8 replicates having 5 chicks in each replicate. The chicks were reared in battery brooders under standard managemental conditions. The experimental period was from day old to 42 d of age. The birds were fed with maize and soybean meal-based diets containing 2958, 3074 and 3163 kcal ME and 22.76, 21.58 and 19.68 percent crude protein, respectively during prestarter (0-14d), starter (15-28d) and finisher (28-42d) phases (Table 1). All the treatment concentrations in the feed were weight/weight basis. Treatment groups (Table 2) included T1 as control i.e., basal diet (BD) without any growth promoter and T2—BD + antibiotic (Bacitracin Methylene Disalicylate at 0.05%–manufacturer Zoetis). In the remaining experimental diets, T3—probiotic (@ 0.01%) + chicory root powder (@ 1.0%), T4—probiotic (@ 0.01%) + coriander seed powder (@ 1.5%), T5—chicory root powder (@ 1.0%) + coriander seed powder (@ 1.5%) and T6—probiotic (@ 0.01%) + chicory root powder (@ 1.0%) + coriander seed powder (@ 1.5%). The probiotic contains 109 CFU/g of lyophilized and microencapsulated Bacillus coagulans, saccharomyces boulardii, Lactobacillus acidophilus, Lactobacillus delbrueckii, Lactobacillus plantarum, Streptococcus thermophilus, Bacillus subtilis, Enterococcus faecium, Bifidobacterium bifidum. The chemical composition of chicory root powder and coriander seed powder was given in Table 3. The dose levels (Probiotic at 0.01%, chicory at 1.0% and coriander at 1.5% levels) were selected based on previous trail results conducted.
Table 1. Ingredient composition of basal diets (in kgs) fed to the commercial broilers from 0-42days.
| Ingredient | Pre-starter (0-14d) | Starter (15-28d) | Finisher (29-42d) |
|---|---|---|---|
| Maize | 55.9 | 56.4 | 60.0 |
| Oil | 2.10 | 4.0 | 5.0 |
| Soyabean meal (CP 46%) | 37.1 | 34.8 | 30.1 |
| Stone grit | 1.58 | 1.83 | 1.88 |
| Dicalcium phosphate | 1.85 | 1.90 | 1.96 |
| Salt (NaCl) | 0.45 | 0.49 | 0.49 |
| DL-Methionine | 0.22 | 0.18 | 0.16 |
| L-Lysine HCl (99%) | 0.17 | 0.15 | 0.13 |
| Trace Mineral Mixture* | 0.10 | 0.10 | 0.10 |
| Vitamin AB2D3K** | 0.020 | 0.020 | 0.020 |
| Vitamin B-Complex*** | 0.025 | 0.025 | 0.025 |
| Choline chloride (50%) | 0.15 | 0.15 | 0.15 |
| Toxin binder | 0.10 | 0.10 | - |
| Total | 100 | 100 | 100 |
| Nutrient composition (calculated values) | |||
| ME (kcal/kg) | 2964 | 3075 | 3167 |
| Crude protein (%) | 22.90 | 21.65 | 19.65 |
| Lysine (%) | 1.28 | 1.21 | 1.10 |
| Methionine (%) | 0.53 | 0.49 | 0.47 |
| Calcium (%) | 0.95 | 1.04 | 1.06 |
| Available phosphorous (%) | 0.45 | 0.45 | 0.45 |
*Trace mineral provided per kg diet (Avimin): Manganese 80mg, Zinc 70g, Iron 40g, Copper 8mg, Iodine 1gm and Selenium 0.25g.
**Vitamin AB2D3K each gram contains (Nicomix): Vitamin A 82500IU, Vitamin D3 12000IU, Vitamin B2 50mg, Vitamin K 10mg
*** Vitamin B-Complex each gram contains (Nicomix); Vitamin B1 4mg, Vitamin B6 8mg, Vitamin B12 40mcg, Niacin 60mg, Calcium Pantothenate 40mg, Vitamin E 40mg.
Table 2. Experimental diets.
| Trt | Experimental Diets |
|---|---|
| T1 | Basal diet (BD) without additive |
| T2 | BD + Antibiotic (BMD @ 0.05%) |
| T3 | BD + Probiotic (@ 0.01%) + Chicory root powder (@ 1.0%) |
| T4 | BD + Probiotic (@ 0.01%) + Coriander seed powder (@ 1.5%) |
| T5 | BD + Chicory root powder (@ 1.0%) + Coriander seed powder (@ 1.5%) |
| T6 | BD + Probiotic (@ 0.01%) + Chicory root powder (@ 1.0%) + Coriander seed powder (@ 1.5%) |
Table 3. Chemical composition of Chicory root powder.
| Composition (%) | Chicory root powder | Coriander seed powder |
|---|---|---|
| Moisture | 3.16 | 3.23 |
| Crude protein | 14.55 | 14.91 |
| Fat | 1.76 | 0.96 |
| Ash | 3.98 | 8.88 |
| Crude fiber | 30.01 | 34.21 |
| Total carbohydrates | 48.76 | 50.12 |
| Inulin | 46.89 | - |
Brooder temperature was maintained at 34 ± 1°C up to 7 days of age and then gradually reduced to 26 ± 1°C by 21 days of age after which chicks were maintained uniformly at room temperature. Feed and water were offered ad libitum throughout the experimental period. Weekly body weight, Feed intake and feed conversion ratios were calculated as feed intake per unit bodyweight gain at weekly intervals. The mortality rate was recorded throughout the experiment. The metabolic trial was conducted with one bird from each replicate to determine the retention efficiency of Dry Matter (DM), Crude Protein (CP) and energy as per the procedures described by AOAC (1997) [19]. The antioxidant enzymes such as glutathione Peroxidase (GSHPx), glutathione Reductase (GSHRx) and superoxide Dismutase (SOD) were estimated by following the methods of Paglia and Valentine (1967) [20], Carlberg and Mannervik (1985) [21] and Madesh and Balsubramanian (1998) [22] respectively.
Before slaughter, the birds were fasted overnight with free access to water and sacrificed by cervical dislocation and allowed for complete bleeding for 5 to 7 minutes. One bird from each replicate was sacrificed on 42nd day of age from each treatment group. Gut (proventriculus, gizzard, duodenum and ileum) pH was recorded immediately after collection of gastro-intestinal contents from respective part of gut. Approximately 1.0 g of sample content was suspended in 5ml distilled water, mixed vigorously with glass rod and pH was determined using digital pH meter. The electrode was rinsed with distilled water and recalibrated in between the readings [23].
Gut ecology
Eight birds from each dietary treatment were slaughtered on 42nd day and intestines were dissected at Meckel’s diverticulum. Approximately 5g of ileal digesta was collected aseptically into sterile sampling tubes and immediately transferred on ice to the laboratory for microbiological examination for E. coli, Salmonella spp and Lactobacilli spp counts. Eosin methylene blue agar (EMB) was used for E. coli growth, Salmonella-Shigella agar (SS Agar) used for Salmonella spp. and MRS agar (De Man, Rogosa and Sharpe agar) used for Lactobacilli spp growth.
Then, 9 sterile test tubes with lids containing 9mL of phosphate buffer solution (PBS, pH-7.4) as diluent were prepared. Approximately 1g of the intestinal contents taken by sterile swab and homogenized for 3 min, aseptically mixed, added to the tubes, and diluted up to 109. Later, 1ml of the contents of each test tube was transferred to one of three selective agar media on petri plates, respectively [24]. Aerobic bacterial plates (E. coli, Salmonella spp) were placed in an incubator at 37°C for 24 hours. Anaerobic (Lactobacilli spp) medium plates were placed in an anaerobic jar with an anaerobic gas pack system at 37°C for 24 hours. Finally, the intestinal bacterial colony populations formed in each plate was counted by colony counter and the number of colonies was expressed as log10 value.
Histomorphometry
On 42nd day during slaughter, 2 cm long segment of duodenum, jejunum and ileum of eight birds from each treatment were collected and then washed with physiological saline solution and fixed in 10% neutral buffered formalin solution. These samples were processed for histomorphological examination in terms of measurement of parameters like villous height (VH), cryptal depth (CD), villus width and villous height:crypt depth ratio. Histological technique involves processes like fixation of tissue, dehydration, clearing, embedding, cutting and staining. Fixation in 10% formalin with approximately 10–20 times the volume of the specimen was done. Tissues were dehydrated by using increasing strength of alcohol like 50%, 70%, 90% and 100%. Clearing was done by replacing alcohol by xylene for 0.5–1 hour. Impregnation of tissue with wax was done at melting point temperature of paraffin wax and the volume of wax was about 25–30 times the volume of tissues for a total duration of 4 hours. Impregnated tissues were placed in a mould with their labels and then fresh melted wax was poured in it and allowed to settle and solidify. These paraffin embedded tissues were sectioned at 5μm thickness and stained routinely with Hematoxylin-Eosin stain (H&E).
Histological sections were examined under 2X of light microscopy with micrometry and photographic attachment. The images were analyzed using image analyzing software (OLYMPUS cellSens Standard, version 1.13). A total of 20 intact well oriented crypt-villous units per bird were selected randomly, measured and the mean length was calculated for each sample. Villous height was measured from the tip of the villi to the base between individual villi, and crypt depth measurements were taken from the valley between individual villi to the basal membrane.
Record of temperature was maintained on daily basis where the highest daily average temperature recorded is 39.15°C and the lowest temperature is 20.8°C during the experimental period. The average relative humidity is 68.65 during the experimental period. The experiment was conducted during February and march- 2020.
Data analyzed for mean, standard errors and analysis of variance as per method of [25] and comparison of means were done [26] using software of Statistical Package for Social Sciences (SPSS) 20.0 version and significance was considered at P<0.05.
Ethical approval
All authors hereby declare that all biological trials have been examined and approved by the ethics committee of PV Narsimha Rao Telangana Veterinary University, Rajendranagar, Hyderabad, India (Institutional Animal Ethics Committee number: IV/2019-02/IAEC/CVSC, Hyderabad, India) and have therefore been performed in accordance with the ethical standards. No consent was raised by animal ethics committee while obtaining permission.
Results and discussion
Body weight gain
The results clearly indicated that supplementation of all test diets (T3 to T6) exhibited significantly (P<0.05) higher body weight gain compared to control (T1) and antibiotic (T2) groups at 42 days of age. The highest cumulative body weight gain (2185g) was recorded in probiotic + chicory (T3) combination group followed by probiotic + coriander (T4), chicory + coriander (T5) and probiotic + chicory + coriander (T6) groups. However, the lowest weight gain was recorded in control (T1) and antibiotic (T2) groups (Table 4). These results are in line with the findings of Taherpour et al. (2009) [27], who reported supplementation of probiotic and prebiotic combination improved the final body weight of broilers at 42 d of age. Similarly, supplementation of probiotic + prebiotic and probiotic + enzyme combination increased the body weight of broilers compared to control at 42 d of age [9]. Barad et al. (2017) [17] observed higher body weight gain in coriander seeds supplemented group when compared to control, turmeric powder and black pepper groups. Contrary to above results, Hofacre et al. (2003) [28] and Al-Khalaifa et al. (2019) [29] did not find positive effect on body weight in broilers fed with prebiotic + probiotic combination at 28 d of age.
Table 4. Synergistic effect of probiotic, chicory root powder and coriander powder on body weight gain (g), feed intake and feed conversion ratio of broiler chicken.
| Trt | Diets | Body weight gain | Feed intake | Feed conversion ratio |
|---|---|---|---|---|
| T 1 | Control | 1975d | 3533 | 1.79c |
| T 2 | Antibiotic | 2016c | 3566 | 1.69bc |
| T 3 | Probiotic + Chicory | 2185a | 3553 | 1.63a |
| T 4 | Probiotic + Coriander | 2149b | 3564 | 1.66ab |
| T 5 | Chicory + Coriander | 2144b | 3561 | 1.66ab |
| T 6 | Probiotic + Chicory + Coriander | 2140b | 3545 | 1.65ab |
| SEM | 10.627 | 15.869 | 0.0082 | |
| N | 8 | 8 | 8 | |
| p-value | 0.001 | 0.113 | 0.001 | |
Value bearing different superscripts within a column are significantly (P<0.05) different.
The highest mean weight gain was recorded in probiotic + chicory (T3) combination group which was significantly (P<0.05) higher among all the treatments. Similarly, Sanja et al. (2015) [30] reported addition of synbiotics (Enterococcus faecim + fructooligosaccharides) improved the body weight of broilers. Supplementation of probiotics and inulin combinations significantly (P <0.05) improved body weight gain in broilers [31]. The complimentary effect of probiotic and chicory powder on cumulative body weight gain as observed in the present study might be due to the suppression of undesirable microorganisms that lead to improved health status [32], increased nutrient digestibility, greater nutrient retention and improved gut health [33]. Similarly, increased body weight gains upon feeding diets containing probiotic + prebiotic combination [34–36] and probiotic + herb combination [37] in broiler chicken. Significant reduction in the counts of E. coli and Salmonella and reduction in gut pH by the supplementation of probiotic + chicory, probiotic + coriander, chicory + coriander and probiotic + chicory + coriander combinations in the present study is also in support with the authors. Contrary to above results, supplementation of probiotic + prebiotic combination did effect on body weight gain in broilers [10, 38, 39]
Feed Intake (FI)
The ANOVA revealed that there were no significant (P>0.05) differences in feed intake among different dietary treatments during overall experimental period (Table 4). The feed intake values at 42 d of age ranged between 3533 g to 3566 g. Similarly, supplementation of probiotic + prebiotic combination did not have significant (P>0.05) on FI of broilers [29]. In agreement with the results of this study, a series of earlier studies demonstrated that addition of probiotics + prebiotics [9, 36], chicory root powder [40, 41] and coriander seed powder [42] to the diet did not result in significant (P>0.05) effect on feed intake of broilers. On the contrary, probiotics + prebiotics [30], probiotic + herb combination [37], chicory root powder [30, 43] and coriander seed powder [17, 18] to the diets resulted in significant (P<0.05) effect on feed intake of broilers. These variations may be due to environmental factors and levels of the additives used in the experiment.
Feed conversion ratio (feed intake/ body weight gain)
Supplementation of probiotic + chicory (T3), probiotic + coriander (T4), chicory + coriander (T5) and probiotic + chicory + coriander (T6) combination groups significantly (P<0.05) improved the efficiency of feed utilization compared to control and antibiotic. However, broilers fed with the probiotic + chicory (T3) combination group was more efficient at converting feed to body mass during entire experimental period (Table 4). To stimulate the growth of beneficial bacteria in the gut using a probiotic + chicory (T3) combination was more effective than the other combinations in this study. This might be due to symbiotic relation between chicory inulin and probiotic. Chicory root powder inulin serves as a source of nutrient for the probiotic bacterial cultures for early establishing in the gut. Similar results were reported by Szakacs et al. (2015) [44] and Sanja et al. (2015) [30] who stated that probiotic + prebiotic combination improved feed efficiency in broilers. Ashayerizadeh et al. (2009) [45] reported that addition of antibiotic, probiotic + prebiotic combination improved FCR compared to control. Improved feed efficiency with probiotic 0.4% + prebiotic 0.2% was also reported by Utami and Wahyono (2019) [36]. In agreement with the results of probiotic + coriander combination in this experiment, Hedayati and Manafi (2018) [37] reported that probiotic and herbal compound supplementation significantly (P<0.05) improved feed conversion ratio compared to control and antibiotic in broilers. However, in contrary to our findings, Kirkpinar et al. (2018) [9] and Al-Khalaifa et al. (2019) [29] did not find positive effect of probiotic and prebiotic combination on FCR of broilers.
Improvement in feed conversion efficiency in treatment groups might be attributed to enhanced digestive enzymes activity and an encouraged growth of the beneficial micro-flora in the GIT induced by dietary supplementation of probiotic, chicory root powder and coriander seed powder combination [46, 47]. Mode of action of above feed additives differs from one another, but in general they are all considered as antimicrobial agents. Improvement in feed efficiency might be obtained by several factors like alteration in intestinal pH, suppression of growth of intestinal pathogens, enhancement of growth of non-pathogenic bacteria and improvement of intestinal function (increased villi height, crypt depth and integrity) and nutrient digestibility.
Nutrient utilization
Supplementation of all the test diets (T3 to T6) significantly (P<0.05) improved the energy retention, protein utilisation and dry matter digestibility compared to antibiotic, control groups (Table 5). The increased nutrient utilization in treatment groups might be due to probiotic bacteria, prebiotic properties of inulin and essential oils in coriander seed powder. Inulin-type fructan is a soluble fermentable fiber that is not digested by host digestive enzymes and serves as a substrate for beneficial like bifidobacteriae and lactobacilli in the lower part of the intestinal tract, the caeca and colon must be considered the sites of their effects on reabsorption of nutrients [48]. The mechanism by which inulin-type fructans may stimulate absorption is not well-known. The hypothesis more accepted is that the fermentation of inulin type fructans in the large intestine results in the production of short-chain fatty acids and lowers the gut pH. A lower intestinal pH facilitates absorption of nutrients [49, 50]. Similarly, Yang et al. (2008) [51] reported that supplementation of mannanoligosaccharides improved the energy and protein utilization in broilers. Justina et al. (2018) [52] indicated that supplementation of β-mannanase in broilers improved the dry matter digestibility and nutrient utilization. The enhanced dry matter digestibility and nutrient utilization may be attributed to the essential oils in coriander seed powder, which not only act as antibacterial and antioxidant, but also as stimulant of digestive enzymes in the intestinal mucosa, which might have improved the utilization of nutrients [15]. Similar results were also reported by Barad et al. (2017) [17] and Reddy et al. (2019) [53].
Table 5. Synergistic effect of probiotic, chicory root powder and coriander powder on nutrient utilization of broiler chicken.
| Trt | Diets | Energy % | Protein % | Dry matter % |
|---|---|---|---|---|
| T 1 | Control | 70.52c | 80.11c | 72.65c |
| T 2 | Antibiotic | 72.25b | 82.06b | 74.18b |
| T 3 | Probiotic + Chicory | 75.77a | 84.94a | 76.89a |
| T 4 | Probiotic + Coriander | 75.52a | 84.16a | 76.80a |
| T 5 | Chicory + Coriander | 75.21a | 84.11a | 76.02a |
| T 6 | Probiotic + Chicory + Coriander | 75.68a | 84.01a | 76.92a |
| SEM | 0.510 | 0.611 | 0.402 | |
| N | 8 | 8 | 8 | |
| p-value | 0.001 | 0.002 | 0.002 | |
Value bearing different superscripts within a column are significantly (P<0.05) different
Antioxidant enzyme activity
The glutathione peroxidase (Units/ml) enzyme activity was significantly (P<0.05) higher with all the test diets (T3 to T6) compared to control (T1) and antibiotic (T2), the highest enzyme activity being recorded in probiotic + chicory combination (T3) and probiotic + coriander combination (T4) groups. The other groups showed intermediate glutathione peroxidase enzyme activity. However, Supplementation of all test diet (T3 to T6) significantly (P<0.05) increased the glutathione reductase and superoxide dismutase enzyme activity compared to control and antibiotic (Table 6). Increased concentration of antioxidant enzymes in our study, is an indicator of better free radical scavenging of test diets. The steady state of antioxidant enzymes activity in all test groups may reflect a significant improvement in health and oxidative status of the birds. In agreement with the results, Tagang et al. (2013) [54] and Shen et al. (2014) [55] recorded increased (P<0.05) activity of serum catalase and glutathione peroxidase enzymes with probiotics in broilers. Similar results were also reported by Dong et al. (2019) [56] and Tengfei et al. (2019) [57] with probiotic supplementation in broilers.
Table 6. Synergistic effect of probiotic, chicory root powder and coriander powder on antioxidant enzyme activity of broiler chicken at 42 d of age.
| Trt | Diets | Glutathione peroxidase (Units/ml) | Glutathione reductase (Units/ml) | Superoxide dismutase (Units/mg protein) |
|---|---|---|---|---|
| T 1 | Control | 243d | 1636b | 6.62b |
| T 2 | Antibiotic | 314c | 1664b | 7.01ab |
| T 3 | Probiotic + Chicory | 397a | 1782a | 7.37a |
| T 4 | Probiotic + Coriander | 393a | 1792a | 7.25a |
| T 5 | Chicory + Coriander | 363b | 1814a | 7.24a |
| T 6 | Probiotic + Chicory + Coriander | 365b | 1835a | 7.39a |
| SEM | 8.042 | 15.67 | 0.069 | |
| N | 8 | 8 | 8 | |
| P-value | 0.001 | 0.001 | 0.005 | |
Value bearing different superscripts within a column are significantly (P<0.05) different
In agreement with the positive results of chicory root powder on antioxidant activity, Sanja et al. (2015) [30] reported that addition of synbiotics (Enterococcus faecim + fructooligosaccharides) significantly (P<0.05) increased serum glutathione peroxidase, peroxidase, glutathione reductase and catalase enzyme activities compared to the control group. Similarly, Wang et al. (2018) [58] observed increased total antioxidant capacity in prebiotics than control and antibiotic (Aureomycin) in broilers. Decreased lipid peroxidation levels and increased activity of the superoxide dismutase and catalase enzymes in broilers fed with inulin was reported by Andreia et al. (2020) [59].
The increase in activity of these antioxidant enzymes with supplementation of probiotics and chicory root powder might be due to better control of intestinal pathogens in the gut. Aerobic bacteria (Bacillus spp) use oxygen in the intestine to provide an anaerobic environment for the colonization of anaerobic bacteria, such as Lactobacilli and Bifidobacteria. Therefore, these lactic acid-producing bacteria produce a more acidic environment, which impairs the growth of opportunistic pathogens [60]. Lactobacillus acidophilus increased the hydroxyl radical and hydrogen peroxide scavenging ability. Lactic acid bacteria could produce certain factors to capture reactive oxygen species (ROS) and prohibit the cytotoxic activity of ROS [61]. Significant reduction in the gut pH, E. coli and Salmonella count and increased in Lactobacilli count in all test diets also supported by the authors. Contrary to these findings, probiotics [54, 55], chicory powder [30] did not have any positive effect on antioxidant enzyme activity in broilers.
Chitra and Leelamma (1999) [11] demonstrated that coriander had a better antioxidative effect by increasing the activity of glutathione peroxidase, glutathione reductase and superoxide dismutase enzyme compared to control. Coriander is an egregious source of phyto-chemicals and functional compounds namely polyphenols, flavonoids and ascorbic acid which ultimately constitute for its high antioxidant activity. Darughe et al. (2012) [62] demonstrated that essential oil of coriander contains camphor, cyclohexanol acetate, limonene, α-pinene and inhibited the rate of primary and secondary oxidation products formation and their effects were almost equal to BHA. The improvement in the antioxidant enzyme activity observed with the addition of coriander seed powder could be attributed to the presence of essential oils and their main components, linalool, trepene and terpenoid [63].
Gut pH
Supplementation of all test diets (T3 to T6) significantly (P<0.05) lowered the pH in duodenum, jejunum, ileum and caecum (except proventriculus) compared to control and antibiotic groups (Table 7). Probiotic bacteria produce short chain acids like lactic, acetic and other organic acids, which are responsible for reduction in the intestinal pH [64]. Aerobic bacteria (Bacillus spp) use oxygen in the intestine to provide an anaerobic environment for the colonization of anaerobic bacteria, such as Lactobacilli and Bifidobacteria. Therefore, these lactic acid-producing bacteria produce a more acidic environment, which impairs the growth of opportunistic pathogens [60]. Significant increase in the counts of Lactobacilli in test diets also support the above results. Al-Khalaifa et al. (2019) [29] reported that supplementation of probiotics and prebiotics driven the gut pH value towards acidity, but it failed to reach significance, as it was only a numerical difference. Whereas, Denli et al. (2003) [65] reported that inclusion of probiotic at 0.1% and antibiotic at 0.15% in broiler diets did not have any effect on the intestinal pH.
Table 7. Synergistic effect of probiotic, chicory root powder and coriander powder on gut pH of broiler chicken at 42 d of age.
| Trt | Diets | Proventriculus | Duodenum | Jejunum | Ileum | Caecum |
|---|---|---|---|---|---|---|
| T1 | Control | 3.54 | 6.11c | 6.43d | 6.66c | 7.39c |
| T2 | Antibiotic | 3.56 | 6.01b | 6.30c | 6.45b | 7.18b |
| T3 | Probiotic + Chicory | 3.51 | 5.89a | 6.06a | 6.23a | 6.94a |
| T4 | Probiotic + Coriander | 3.48 | 5.88a | 6.15ab | 6.30a | 6.95a |
| T5 | Chicory + Coriander | 3.54 | 5.94ab | 6.20b | 6.31a | 6.99a |
| T6 | Probiotic + Chicory + Coriander | 3.54 | 5.94ab | 6.18b | 6.25a | 7.03a |
| SEM | 0.0098 | 0.0155 | 0.0208 | 0.0270 | 0.0301 | |
| N | 8 | 8 | 8 | 8 | 8 | |
| p-value | 0.157 | 0.001 | 0.001 | 0.001 | 0.001 | |
Value bearing different superscripts within a column are significantly (P<0.05) different
Gut ecology
Supplementation of all the test diets (T3 to T6) including antibiotic group significantly (P<0.05) decreased the E. coli counts compared to control (Table 8). The lowest E. coli counts were recorded in antibiotic group (T2), probiotic + chicory (T3) and probiotic + chicory + coriander (T6) groups followed by probiotic + coriander (T4) and chicory + coriander (T5) groups. Supplementation of antibiotic (T2) significantly (P<0.05) decreased the ileal Salmonella counts compared to control and other test diets. The Salmonella count in probiotic + chicory + coriander (T6), probiotic + coriander (T4) groups and probiotic + chicory (T3) showed intermediate values, but they had significantly (P<0.05) lower Salmonella counts than the control (T1) and chicory + coriander (T5) groups. In agreement with the above results, Karwan et al. (2016) [31] observed that addition of postbiotics and inulin combinations significantly (P<0.05) reduced the Enterobacteriaceae count compared to control. Similarly, Biswas et al. (2018) [8] reported that supplementation of antibiotics (BMD) and prebiotics (MOS and FOS) reduced the total anaerobes and coliforms counts in ileum of broilers. Supplementation of antibiotic, probiotic and herbal compound significantly (P<0.05) reduced the E. coli, Salmonella and coliforms counts compared to control in broilers was reported by Hedayati and Manafi (2018) [37]. Probiotic bacteria produce short chain acids which decreases the intestinal pH and encourages the growth of Lactobacilli and Bifidobacteria [64]. Therefore, these lactic acid-producing bacteria produce a more acidic environment, which impairs the growth of opportunistic pathogens [60]. Significant increase in Lactobacilli count in all test diets was also support the above results. On contrary, Wang et al. (2018) [58] did not find any significant (P>0.05) difference in total anaerobic bacterial count in broilers with probiotic and antibiotic supplementation.
Table 8. Synergistic effect of probiotic, chicory root powder and coriander powder on gut microbiota (log10 of cfu/g count) in ileum sample of broiler chicken.
| Trt | Diets | Escherichia coli (log10 cfu/g) * | Salmonellea spp. (log10 cfu/g) ** | Lactobacillus spp. (log10 cfu/g) * |
|---|---|---|---|---|
| T 1 | Control | 8.29d | 4.23d | 7.89c |
| T 2 | Antibiotic | 7.15a | 3.23a | 6.93d |
| T 3 | Probiotic + Chicory | 7.18a | 3.92c | 8.08a |
| T 4 | Probiotic + Coriander | 7.33b | 3.90c | 7.86c |
| T 5 | Chicory + Coriander | 7.42c | 4.16d | 7.88c |
| T 6 | Probiotic + Chicory + Coriander | 7.19a | 3.81b | 7.94b |
| SEM | 0.059 | 0.049 | 0.055 | |
| N | 8 | 8 | 8 | |
| P-value | 0.001 | 0.001 | 0.001 | |
Values bearing different superscripts within a column are significantly (P<0.05) different
* Calculated as per log10 colony forming units/gram of sample (106).
** Calculated as per log10 colony forming units/gram of sample (103).
In agreement with the lowered E. coli and Salmonella counts in probiotic + coriander (T4), chicory + coriander (T5) groups, Ghazanfari et al. (2015) [66] reported that supplementation of antibiotic and coriander oil lowered the caecal E. coli counts than control in broilers. Similarly, Taha et al. (2019) [18] observed that coriander seed powder decreased the total bacterial, E. coli and C. perfringens counts in the ileum of broilers. The decreased pathogenic bacterial load in the ileum might be due to essential oils in coriander have which hydrophobic properties [67] that affect cell wall lipids of the bacteria by disturbing bacterial structures and rendering them more permeable, thus results in lower number of harmful bacteria.
The Lactobacilli counts were significantly (P<0.05) increased with the supplementation of probiotic + chicory (T3) followed by probiotic + chicory + coriander (T6) when compared to other treatment groups. Birds supplemented with antibiotic in the diet showed significantly (P<0.05) lower Lactobacilli counts. However, no significant (P>0.05) difference was recorded among control (T1), probiotic + coriander (T4) and chicory + coriander (T5) groups. Similarly, Dong et al. (2019) [56] reported that supplementation of microencapsulated probiotics significantly (P<0.05) increased the Lactobacilli counts in caecum of broilers. Similarly, Biswas et al. (2018) reported that supplementation of antibiotics (BMD) and prebiotics (MOS and FOS) increased the Lactobacilli counts in ileum of broilers. Increased faecal lactic acid bacteria in postbiotics and inulin combination groups was also reported by Karwan et al. (2016) [31] in broilers. Wang et al. (2018) [58] observed that microencapsulated probiotics and prebiotics (MEP) significantly (P<0.05) increased the Lactobacilli counts than control and antibiotic in broilers. In contrary, supplementation of probiotic did not have significant (P<0.05) effect in cecal Lactobacilli counts of broilers [29].
The increased Lactobacilli count in probiotic + chicory (T3) combination group might be due to probiotic bacteria such as Lactobacillus spp. or Bifidobacterium spp. use inulin for fermentation more efficiently than other groups of bacteria and produces short chain fatty acids on inulin to create an acidic environment which suppresses the growth of acid intolerant bacteria like Salmonellae and E. coli and enhanced the growth of acid tolerant bacteria like Lactobacilli and Bifidobacterium [68].
Gut histomorphometry
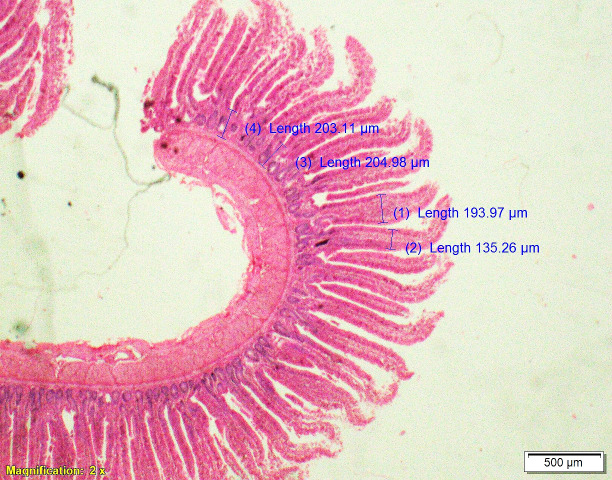
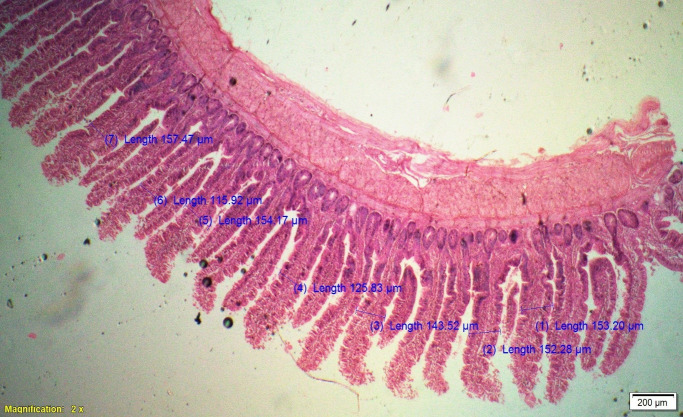
Supplementation of all the test diets (T3 to T6) significantly (P<0.05) increased the villus height (VH), crypt depth (CD), VH:CD ratio and villus width (VW) in the duodenum and only VH and CD in the ileum compared to control and antibiotic groups. Significantly (P<0.05) higher jejunal VH and VW was recorded in all test diets compared to control and antibiotic groups. Increased villus height and villus width enhances the absorptive capacity of the small intestine by reducing the digesta passage rate and therefore, optimize broiler performance. However, supplementation of different dietary groups did not show any significant (P>0.05) effect on jejunal CD and VH:CD ratio and ileal villus width at 42 d of age (Tables 9–11 and Figs 1–12).
Table 9. Synergistic effect of probiotic, chicory root powder and coriander powder on histomorphometry of duodenum of broiler chicken.
| Trt | Diets | Villus height (μm) | Crypt depth (μm) | Villus height: Crypt depth Ratio | Villus width (μm) |
|---|---|---|---|---|---|
| T 1 | Control | 1025.84c | 240.58c | 4.29c | 134.11c |
| T 2 | Antibiotic | 1174.89b | 260.43c | 4.55b | 156.05bc |
| T 3 | Probiotic + Chicory | 1598.52a | 315.58b | 5.15a | 213.64a |
| T 4 | Probiotic + Coriander | 1261.81b | 308.45b | 4.10d | 171.78b |
| T 5 | Chicory + Coriander | 1623.28a | 319.91ab | 5.07a | 168.11b |
| T 6 | Probiotic + Chicory + Coriander | 1651.62a | 343.38a | 4.87ab | 199.69a |
| SEM | 43.420 | 6.825 | 0.087 | 5.764 | |
| N | 8 | 8 | 8 | 8 | |
| P-value | 0.001 | 0.001 | 0.001 | 0.001 | |
Values bearing different superscripts within a column are significantly (P<0.05) different
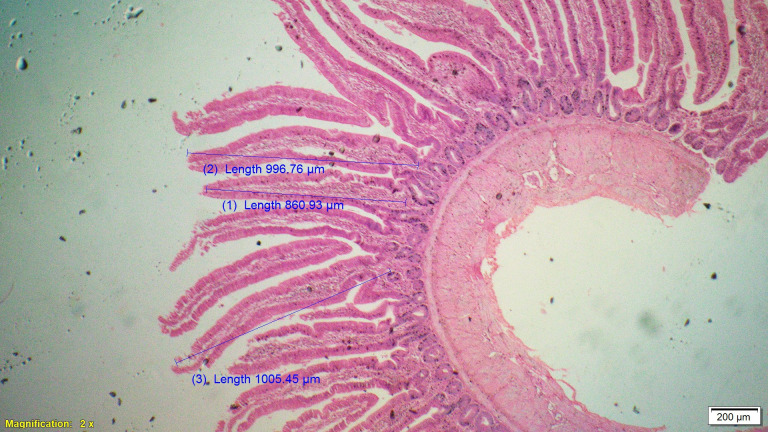
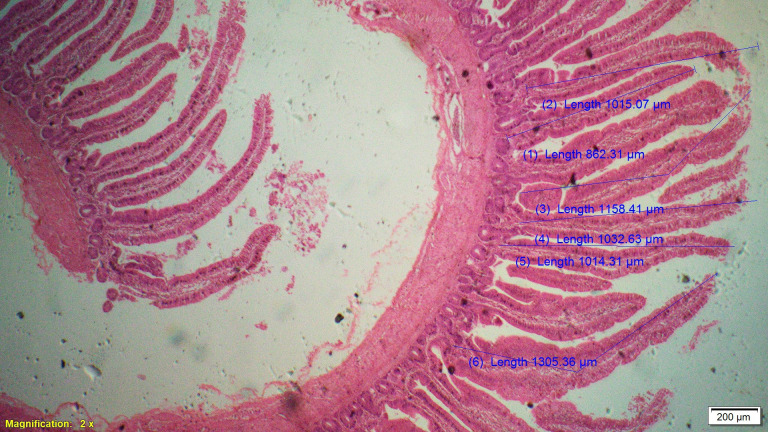
Table 11. Synergistic effect of probiotic, chicory root powder and coriander powder on histomorphometry of ileum of broiler chicken.
| Trt | Diets | Villus height (μm) | Crypt depth (μm) | Villus height: Crypt depth Ratio | Villus width (μm) |
|---|---|---|---|---|---|
| T 1 | Control | 562.15d | 172.87b | 3.28c | 131.15 |
| T 2 | Antibiotic | 668.51c | 158.67b | 4.17b | 129.05 |
| T 3 | Probiotic + Chicory | 758.38b | 160.25b | 4.63b | 136.76 |
| T 4 | Probiotic + Coriander | 1019.99a | 199.06a | 5.22a | 145.61 |
| T 5 | Chicory + Coriander | 752.38b | 166.24b | 4.47b | 131.87 |
| T 6 | Probiotic + Chicory + Coriander | 742.35b | 193.22a | 3.83c | 134.18 |
| SEM | 25.265 | 4.294 | 0.116 | 2.246 | |
| N | 8 | 8 | 8 | 8 | |
| P-value | 0.001 | 0.001 | 0.001 | 0.336 | |
Values bearing different superscripts within a column are significantly (P<0.05) different
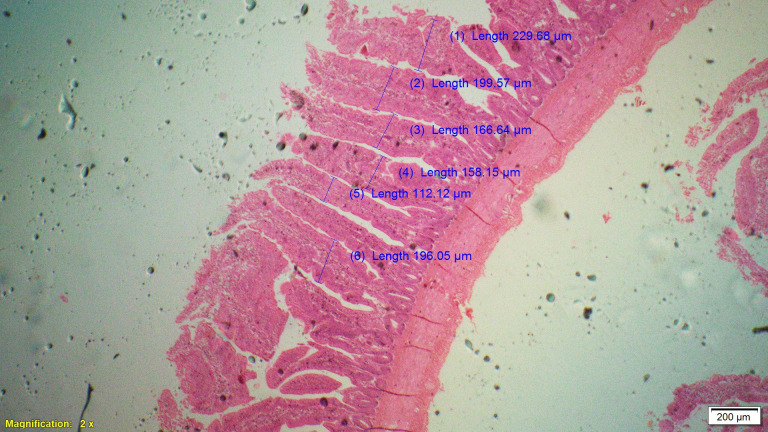
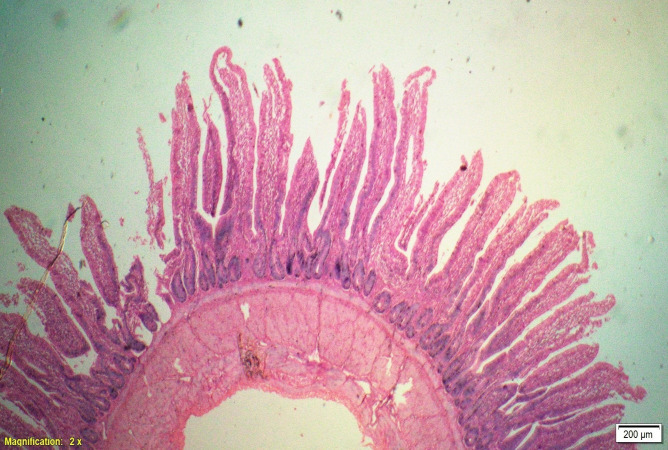
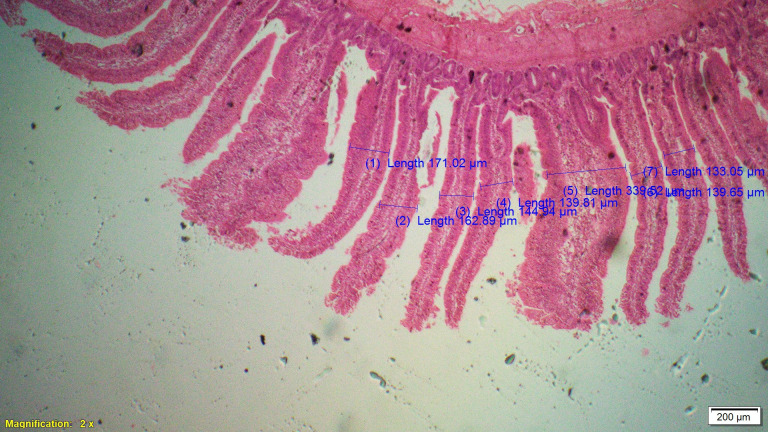
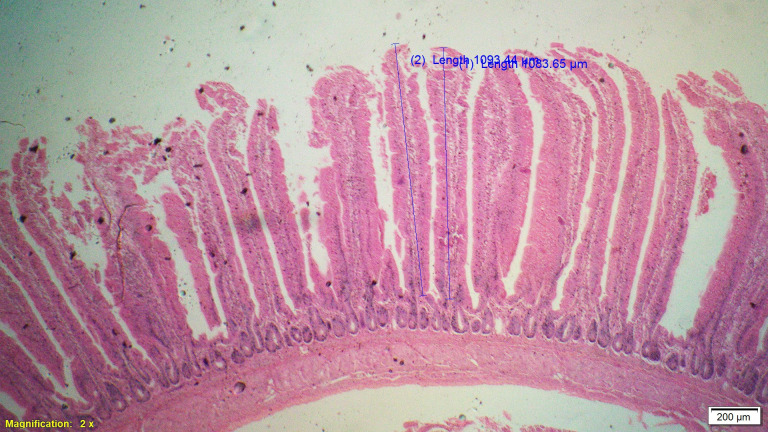
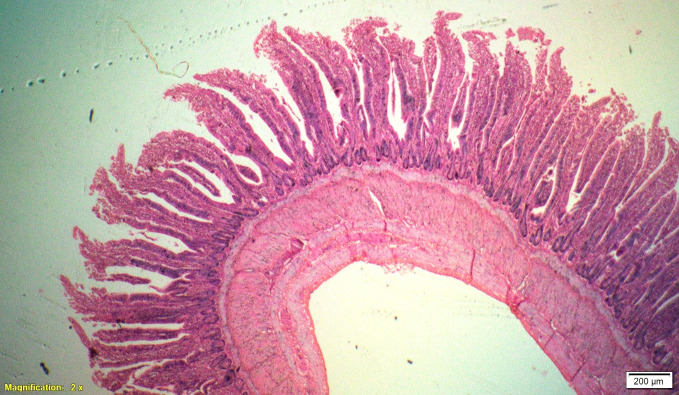
Fig 1. Photomicrograph of the cross section of Jejunum from control group (T1).
H&E, 2x.
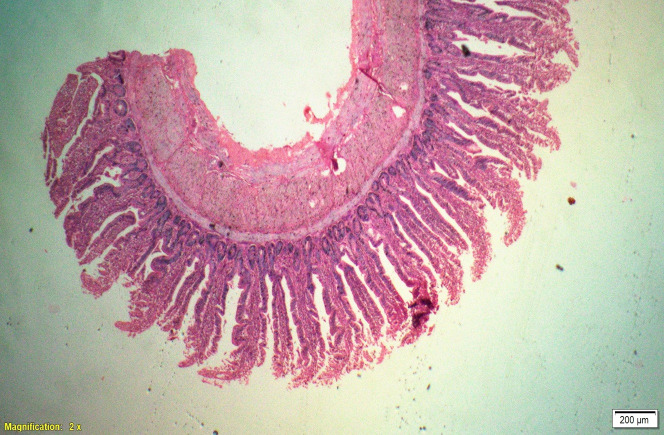
Fig 12. Photomicrograph of the cross section of ileum from probiotic + chicory + coriander group (T6), H&E, 2x.
Fig 2. Photomicrograph of the cross section of Jejunum from antibiotic group (T2).
H&E, 2x.
Fig 3. Photomicrograph of the cross section of Jejunum from probiotic + chicory group (T3), H&E, 2x.
Fig 4. Photomicrograph of the cross section of Jejunum from probiotic + coriander group (T4), H&E, 2x.
Fig 5. Photomicrograph of the cross section of Jejunum from chicory + coriander group (T5), H&E, 2x.
Fig 6. Photomicrograph of the cross section of Jejunum from probiotic + chicory + coriander group (T6), H&E, 2x.
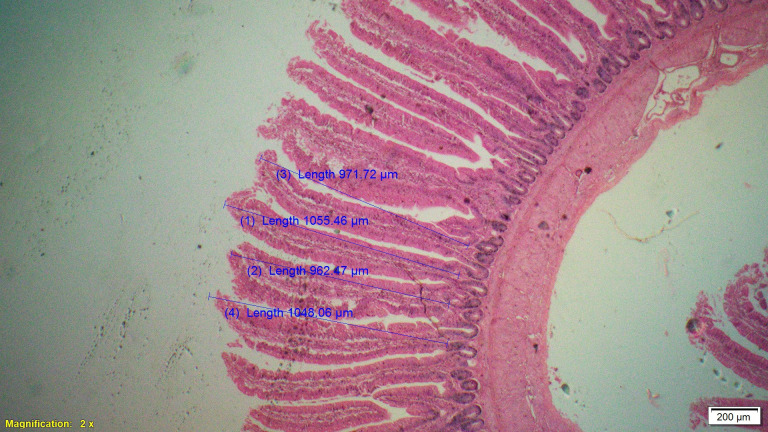
Fig 7. Photomicrograph of the cross section of ileum from control group (T1).
H&E, 2x.
Fig 8. Photomicrograph of the cross section of ileum from antibiotic group (T2).
H&E, 2x.
Fig 9. Photomicrograph of the cross section of ileum from probiotic + chicory group (T3), H&E, 2x.
Fig 10. Photomicrograph of the cross section of ileum from probiotic + coriander group (T4), H&E, 2x.
Fig 11. Photomicrograph of the cross section of ileum from chicory + coriander group (T5), H&E, 2x.
Table 10. Synergistic effect of probiotic, chicory root powder and corianderpowder on histomorphometry of jejunum of broiler chicken.
| Trt | Diets | Villus height (μm) | Crypt depth (μm) | Villus height: Crypt depth Ratio | Villus width (μm) |
|---|---|---|---|---|---|
| T 1 | Control | 949.13c | 171.25 | 5.68 | 153.23b |
| T 2 | Antibiotic | 1010.44b | 168.96 | 5.99 | 164.00b |
| T 3 | Probiotic + Chicory | 1082.86a | 165.90 | 6.69 | 210.44a |
| T 4 | Probiotic + Coriander | 1013.05b | 171.70 | 5.93 | 197.99a |
| T 5 | Chicory + Coriander | 1044.34ab | 167.88 | 6.26 | 204.35a |
| T 6 | Probiotic + Chicory + Coriander | 1061.79ab | 175.81 | 6.09 | 207.68a |
| SEM | 10.135 | 1.672 | 0.106 | 4.257 | |
| N | 8 | 8 | 8 | 8 | |
| P-value | 0.001 | 0.638 | 0.107 | 0.001 | |
Values bearing different superscripts within a column are significantly (P<0.05) different
In agreement with the above results, Karwan et al. (2016) [31, 69] reported that addition of probiotics and inulin combinations increased the villus height and crypt depth of the duodenum, ileum and jejunum compared to control. Similarly, supplementation of probiotic + organic acid combination significantly (P<0.05) increased the duodenal VH, CD and VH:CD in broilers [70]. The positive effect of probiotics and chicory root powder on the intestinal morphology mainly arose from its ability to create a favourable intestinal environment which had a better effect on intestinal morphology [71]. Probiotics and chicory root powder increase to production of short chain fatty acids and reduce intestinal pH. Hence, beneficial effects on intestinal tissue health and morphology are achieved. In contrary, Fernandes et al. (2014) [10] reported that supplementation of probiotic + prebiotic combination did not have any significant (P>0.05) effect on intestinal integrity of broiler.
Probiotic, chicory root powder and coriander seed powder may reduce the growth of many pathogenic and non-pathogenic intestinal bacteria thereby resulting in reduction in intestinal colonization and infectious process which ultimately decrease the inflammatory process of intestinal mucosa resulting in improved villus height and villus width which in turn increases secretory function, digestion, and absorption of nutrients [8]. It is hypothesised that the increase in beneficial microbial activity resulting from dietary probiotic, chicory root powder and coriander seed powder supplementation may influence gut morphology and consequently affect gut maturation.
Conclusion
Supplementation of probiotic + chicory, probiotic + coriander, chicory + coriander and probiotic + chicory + coriander combinations produced greater weight gain, improved FCR, and higher antioxidant activity compared to control and antibiotic at 42 d of age. The combination of probiotic (0.01%) with chicory root powder (1.0%) was more effective than combinations of other additives in terms of body weight gain and FCR. Supplementation of different combinations of probiotic, chicory root powder and coriander seed powder significantly lowered the gut pH, E. coli and Salmonella counts and increased the Lactobacilli counts. In addition, this treatment improved the gut morphometry parameters such as VH, CD and VW in the in the intestines. Thus, supplementation of probiotic at 0.01%, chicory root powder at 1.0%, and coriander seed powder at 1.5% combinations could be used in the diet as a potential growth promoter in broiler chickens. However, follow up large-scale studies under field conditions are necessary before recommending the compounds in the broiler diet.
Supporting information
(XLSX)
Acknowledgments
The presented manuscript is a part of the first Author’s PhD dissertation. The authors are thankful to Department of Poultry Science, College of Veterinary science, PV Narsimha Rao Telangana Veterinary University, R’nagar, Hyderabad, India.
Data Availability
All data are fully available without restriction.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Dibner JJ, Richards JD. Antibiotic growth promoters in agriculture: History and mode of action. Poultry Science 2015; 84 (4): 634–643. [DOI] [PubMed] [Google Scholar]
- 2.Huyghebaert G, Ducatelle R, Van IF. An update on alternatives to antimicrobial growth promoters for broilers. Veterinary Journal 2011; 187(2): 182–188. doi: 10.1016/j.tvjl.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 3.Fuller R. Probiotics in man and animals. Journal of Applied Bacteriology 1989; 66: 365–378. [PubMed] [Google Scholar]
- 4.Jin LZ, Ho YW, Abdullah N, Jalaludin S. Digestive and bacterial enzyme activities in broilers fed diets supplemented with Lactobacillus Cultures. Poultry Science 2000; 79: 886–891. doi: 10.1093/ps/79.6.886 [DOI] [PubMed] [Google Scholar]
- 5.Barbara MS, Nebojsa I, Alexander P, Ilya R. Toxicological evaluation of a chicory root extract. Food and Chemical Toxicology 2007; 45:1131–1139. doi: 10.1016/j.fct.2006.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saeed M, Abd EME, Alagawany M, Arain MA, Arif M, Mirza MA, et al. Chicory (Cichorium intybus) Herb: Chemical composition, pharmacology, nutritional and healthical applications. International Journal of Pharmacology 2017; 13 (4):351–360. [Google Scholar]
- 7.Rebole ALT, Ortiz ML, Rodriguez C, Alzueta J, Trevino, Velasco S. Effects of inulin and enzyme complex, individually or in combination, on growth performance, intestinal microflora, cecal fermentation characteristics and jejunal histomorphology in broiler chickens fed wheat and barley-based diet. Poultry Science 2010; 89(2): 276–286. doi: 10.3382/ps.2009-00336 [DOI] [PubMed] [Google Scholar]
- 8.Biswas A, Messam R, Kumawat M, Namit M, Mandal AB, Mir NA. Effects of prebiotics on intestinal histo-morphometry and gut microflora status of broiler chickens. Indian Journal of Animal Research 2018; 8:1–5. [Google Scholar]
- 9.Kirkpinar F, Açıkgöz Z, Mert S, Işık Ö. Effects of dietary probiotic, prebiotic and enzyme mixture supplementation on performance, carcass, organ, ileal pH and viscosity of broilers. Journal of Animal Production 2018; 59 (2):1–9. [Google Scholar]
- 10.Fernandes BCS, Martins MRFB, Mendes AA, Milbradt EL, Sanfelice C, Martins BB, et al. Intestinal integrity and performance of broiler chickens fed a probiotic, a prebiotic, or an organic acid. Brazilian Journal of Poultry Science 2014; 16 (4): 417–424. ( 10.1590/1516-635x1604417-424). [DOI] [Google Scholar]
- 11.Chithra V, Leelamma S. Hypolipidemic effect of coriander seeds (Coriandrum sativum): mechanism of action. Plant Foods for Human Nutrition 1997; 51:167–172. (doi: 10.1023/a:1007975430328). [DOI] [PubMed] [Google Scholar]
- 12.Pathak NL, Kasture SB, Bhatt NM, Rathod JD. Phytopharmacological properties of Coriandrum sativum as a potential medicinal tree: an overview. Journal of Applied Pharmacological Sciences 2011; 1(4): 20–25. [Google Scholar]
- 13.Silva F, Ferreira S, Queiroz JA, Fernanda CD. Coriander (Coriandrum sativum L.) essential oil: its antibacterial activity and mode of action evaluated by flow cytometry. Journal of Medical Microbiology 2011; 60:1479–1486. doi: 10.1099/jmm.0.034157-0 [DOI] [PubMed] [Google Scholar]
- 14.Langhout P. New additives for broiler chickens. World’s Poultry Science Journal 2000; 16(3): 22–27. [Google Scholar]
- 15.Rajeshwari U, Andallu B. Medicinal benefits of coriander (Coriandrum sativum). Spatula 2011; 1: 51–58. [Google Scholar]
- 16.Naeemasa M, Qotbi AAA, Seidavi A, Norri D, Brown D, Ginindza M. Effects of coriander (Coriandrum sativum L) seed powder and extract on performance of broiler chickens. South African Journal of Animal Science 2015; 45 (4): 125–131. [Google Scholar]
- 17.Barad NA, Savsani HH, Patil SS, Gadariya MR, Murthy KS, Fefar DT. Effect of supplementing the diet with coriander seeds, turmeric powder and black pepper on the feed intake, growth performance and carcass quality of broilers. Indian Veterinary Journal 2017; 94(11): 43–45. [Google Scholar]
- 18.Taha AE, Saber SH, Ramadan SS, Ahmed AE, Mohamed EA, Hussein E, et al. Effects of supplementing broiler diets with coriander seed powder on growth performance, blood haematology, ileum microflora and economic efficiency. Journal of Animal Physiology and Animal Nutrition 2019; 6:1–10. (doi: 10.1111/jpn.13165). [DOI] [PubMed] [Google Scholar]
- 19.AOAC. Official methods of Analysis. Association of Official Analytical Chemists, Washington D.C. USA. 2005.
- 20.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. The Journal of Laboratory and Clinical Medicine 1967; 70 (1): 158–169. [PubMed] [Google Scholar]
- 21.Carlberg I, Mannervik B. Glutathione reductase. Methods in enzymology 1985; 113: 484–490. doi: 10.1016/s0076-6879(85)13062-4 [DOI] [PubMed] [Google Scholar]
- 22.Madesh M, Balasubramanian KA. Microtiter plate assay for superoxide dismutase using MTT reduction by superoxide. Indian Journal of Biochemistry and Biophysics 1998; 35 (3)184–188. [PubMed] [Google Scholar]
- 23.Corrier DE, Hinton A Jr, Ziprin RL, Beier RC, DeLoach JR. 1990. Effect of dietary lactose on cecal pH, bacteriostatic volatile fatty acids, and Salmonella typhimurium colonization of broiler chicks. Avian Diseases 1990; 617–625. [PubMed] [Google Scholar]
- 24.Gunal M, Yayli G, Karahan N, Sulak O. The effect of antibiotic growth promoter, probiotic or organic acid supplementation on performance, intestinal microflora and tissue of broilers. International Journal of Poultry Science 2006; 5 (2): 149–155. [Google Scholar]
- 25.Snedecor GW, Cochran WG. Statistical methods 8th edn. The Iowa State University Press. Ames. Iowa, U.S.A. 1989. [Google Scholar]
- 26.Duncan DB. Multiple range and F tests. Biometrics 1955; 11: 1–42. [Google Scholar]
- 27.Taherpour K, Moravej H, Shivazad M, Adibmoradi M, Yakhchali B. Effects of dietary probiotic, prebiotic and butyric acid glycerides on performance and serum composition in broiler chickens. African Journal of Biotechnology 2009; 8 (10): 2329–2334. [Google Scholar]
- 28.Hofacre CL, Beacorn T, Collet S, Mathis G. Using competitive exclusion, mannan-oligosaccharide and other intestinal products to control necrotic enteritis. Journal of Applied Poultry Research 2003; 12: 60–64. [Google Scholar]
- 29.Al-Khalaifa H, Al-Nasser A, Al-Surayee T, Al-Kandari S, Al-Enzi N, Al-Sharrah T, et al. Effect of dietary probiotics and prebiotics on the performance of broiler chickens. Poultry Science 2019; 98(10):4465–4479. ( 10.3382/ps/pez282) [DOI] [PubMed] [Google Scholar]
- 30.Sanja JP, Ljiljana MK, Nikola MP, Jovanka DL, Olivera MD, Bojana MK, et al. Effect of synbiotic on growth and antioxidant status of blood in broiler chicken. Food and Feed Research 2015; 42 (2): 163–169. (doi: 10.5937/FFR1502163P). [DOI] [Google Scholar]
- 31.Kareem KY, Loh TC, Foo HL, Akit H, Samsudin AA. Effects of dietary postbiotic and inulin on growth performance, IGF1 and GHR mRNA expression, faecal microbiota and volatile fatty acids in broilers. Bio Medical Central Veterinary Research 2016; 12(1):1–10. (DOI: 10.1186/s12917-016-0790-9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anjum MI, Khan AG, Azim A, Afzal M. Effect of dietary supplementation of multi-strain probiotic on broiler growth performance. Pakistan Veterinary Journal 2005; 25 (1): 25–29. [Google Scholar]
- 33.Jeong JS, Kim IH. Effect of Bacillus subtilis C-3102 spores as a probiotic feed supplement on growth performance, noxious gas emission, and intestinal microflora in broilers. Poultry Science 2014; 93:1–7. [DOI] [PubMed] [Google Scholar]
- 34.Bozkurt M, Küçükyılmaz K, Çatlı AU, Çınar M. The effect of single or combined dietary supplementation of prebiotics, organic acid and probiotics on performance and slaughter characteristics of broilers. South African Journal of Animal Science 2009; 39 (3): 197–205. [Google Scholar]
- 35.Kumar L, Singh PK, Chandramoni, Kumar M. Effect of dietary supplementation of combination of probiotics on the growth performance and immune response of broiler chickens. Animal Nutrition and Feed Technology 2013; 13: 15–25. [Google Scholar]
- 36.Utami MMD, Wahyono ND. Supplementation of probiotic and prebiotic on the performance of broilers. Earth and Environmental Science 2019; 207:1–6. (doi: 10.1088/1755-1315/207/1/012024). [DOI] [Google Scholar]
- 37.Hedayati M, Manafi M. Evaluation of an herbal compound, a commercial probiotic, and an antibiotic growth promoter on the performance, intestinal bacterial population, antibody titers, and morphology of the jejunum and ileum of broilers. Brazilian Journal of Poultry Science 2018; 20 (2): 305–316. [Google Scholar]
- 38.Sahin T, Kaya I, Unal Yucel, Elmali Dilek Aksu. Dietary supplementation of probiotics and prebiotic combination (combiotics) on performance, carcass quality and blood parameters in growing quails. Journal of Animal and Veterinary Advances 2008; 7 (11): 1370–1373. [Google Scholar]
- 39.Yousfi Z, Kazemi FM, Rezaei M, Ansari PZ. Effect of chicory extract and probiotic on performance, caracas characteristics, blood parameters, intestinal microflora and immune response of broiler chickens. Iranian Journal of Animal Science Research 2017; 2 (9): 185–195. [Google Scholar]
- 40.Nabizadeh A. The effect of inulin on broiler chicken intestinal microflora, gut morphology, and performance. Journal of Animal and Feed Sciences 21(4):725–734. [Google Scholar]
- 41.Izadi H, Javad A, Golian A, Raji MR. Effects of chicory root powder on growth performance and histomorphometry of jejunum in broiler chicks. Veterinary Research Forum 2013; 4 (3): 169–174. [PMC free article] [PubMed] [Google Scholar]
- 42.Rashid MM, Ahammad MU, Ali MS, Rana MS, Ali MY, Sakib N. Effect of different levels of Dhania seed (Coriandrum sativum) on the performance of broiler. Bangladesh Journal of Animal Science 2014; 43 (1): 38–44. [Google Scholar]
- 43.Praveen T, Munegowda T, Indresh HC, Jayanaik. Effect of supplementation of various levels of inulin on growth performance, carcass characteristics and survivability in raja II broilers. International Journal of Current Microbiology and Applied Sciences 2017; 6 (9):1470–1475. (Doi: 10.20546/ijcmas.2017.609.179). [DOI] [Google Scholar]
- 44.Szakacs RA, Sorona M, Laura S, Rekha S, Andrian M. The impact of pre and probiotic on growing performance and haematological parameters in ross 708 broiler chickens. Bulletin UASVM Veterinary Medicine 2015; 72 (2): 330–335. [Google Scholar]
- 45.Ashayerizadeh A, Dabiri N, Ashayerizadeh O, Mirzadeh KH, Roshanfekr H, Mamooee M. Effect of dietary antibiotic, probiotic and prebiotic as a growth promoter, on growth performance, carcass charecteristics and haematological indices of broiler chickens. Pakistan Journal of Biological Sciences 2009; 12(1): 52–57. doi: 10.3923/pjbs.2009.52.57 [DOI] [PubMed] [Google Scholar]
- 46.Yeo J, Kim K. Effect of feeding diets containing an antibiotic, a probiotic, or yucca extract on growth and intestinal urease activity in broiler chicks. Poultry Science 1997; 76(2): 381–385. doi: 10.1093/ps/76.2.381 [DOI] [PubMed] [Google Scholar]
- 47.Ozduven M L, Samli H E, Koc A A O F, Akyurek H, Senkoylu N. Effects of mannanoligosaccharide and organic acid mixture on performance, blood parameters and intestinal microbiota of broiler chicks. Italian Journal of Animal Science 2009; 8: 595–602. [Google Scholar]
- 48.Roberfroid MB. Prebiotics and synbiotics: concepts and nutritional properties. British Journal of Nutrition 1998; 80 (4): 197–202. [PubMed] [Google Scholar]
- 49.Levrat MA, RéMéSy C, Demigné C. High propionic acid fermentation and mineral accumulation in the cecum of rats adapted to different levels of inulin. Journal of Nutrition 1991; 121: 1730–1737. doi: 10.1093/jn/121.11.1730 [DOI] [PubMed] [Google Scholar]
- 50.Lopez HW, Coudray C, Levrat-Verney M, Feilletcoudray C, Demigné C, RéMéSy C. Fructooligosaccharides enhance mineral apparent absorption and counteract the deleterious effects of phytic acid on mineral homeostasis in rats. Journal of Nutritional Biochemistry 2000; 11: 500–508. doi: 10.1016/s0955-2863(00)00109-1 [DOI] [PubMed] [Google Scholar]
- 51.Yang Y, Iji P, Kocher A, Thomson E, Mikkelsen L, Choct M. Effects of mannanoligosaccharide in broiler chicken diets on growth performance, energy utilisation, nutrient digestibility and intestinal microflora. British Poultry Science, 2008; 49: 186–194. doi: 10.1080/00071660801998613 [DOI] [PubMed] [Google Scholar]
- 52.Caldas Justina V., Vignale Karen, Boonsinchai Nirun, Wang Jinrong, Putsakum Monticha, England Judith A., et al. , The effect of β-mannanase on nutrient utilization and blood parameters in chicks fed diets containing soybean meal and guar gum. Poultry Science 2018; 97 (8): 2807–2817. 10.3382/ps/pey099 [DOI] [PubMed] [Google Scholar]
- 53.Reddy NBC, Srinivas KD, Raja KK, Kumari NRK. Effect of dietary incorporation of coriander seed meal on production performance of Japanese quail. Indian Journal of Animal Nutrition 2019; 36(2):198–201. [Google Scholar]
- 54.Tagang A, Mohammed K, Moshood R, Tavershima D, Felix G, Victor S, et al. Effect of yeast probiotic on growth, antioxidant enzyme activities and malondialdehyde concentration of broiler chickens. Antioxidants 2013; 2: 326–339. (doi: 10.3390/antiox2040326). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shen X, Yi D, Ni X, Zeng D, Jing B, Lei M, et al. Effects of Lactobacillus plantarum on production performance, immune characteristics, antioxidant status, and intestinal microflora of bursin-immunized broilers. Canadian Journal of Microbiology 2014; 60: 193–202. doi: 10.1139/cjm-2013-0680 [DOI] [PubMed] [Google Scholar]
- 56.Dong ZL, Wang YW, Song D, Wang WW, Liu KB, Wang L. et al. Effects of microencapsulated probiotics and plant extract on antioxidant ability, immune status and caecal microflora in Escherichia coli K88-challenged broiler chickens. Food and Agricultural Immunology 2019; 30 (1):1123–1134. (doi: 10.1080/09540105.2019.1664419). [DOI] [Google Scholar]
- 57.He T, Long S, Mahfuz S, Wu D, Wang X, Wei X, et al. Effects of probiotics as antibiotics substitutes on growth performance, serum biochemical parameters, intestinal morphology, and barrier function of broilers. Animals 2019; 9(11): 985. (doi: 10.3390/ani9110985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y, Dong Z, Song D, Zhou Hang, Weiwei W, Haijiang M, et al. Effects of microencapsulated probiotics and prebiotics on growth performance, antioxidative abilities, immune functions, and caecal microflora in broiler chickens. Food and Agricultural Immunology 2018; 29(1); 859–869. [Google Scholar]
- 59.Guaragni A, Boiago MM, Bottari NB, Morsch VM, Lopes TF, da Silva AS. Feed supplementation with inulin on broiler performance and meat quality challenged with Clostridium perfringens: Infection and prebiotic impacts. Microbial Pathogenesis 2020;13; 103889. doi: 10.1016/j.micpath.2019.103889 [DOI] [PubMed] [Google Scholar]
- 60.Sako T, Matsumoto K, Tanaka R. Recent progress on research and applications of non-digestible galacto-oligosaccharides. International Dairy Journal 1999; 9 (1): 69–80. [Google Scholar]
- 61.Lin MY, Yen CL. Antioxidative ability of lactic acid bacteria. Journal of Agricultural and Food Chemistry 1999; 47: 1460–1466. (doi: 10.1021/jf981149l). [DOI] [PubMed] [Google Scholar]
- 62.Darughe F, Barzegar M and Sahari MA. Antioxidant and antifungal activity of Coriander (Coriandrum sativum L.) essential oil in cake. International Food Research Journal 2012; 19 (3):1253–1260. [Google Scholar]
- 63.Zlatanov M, Ivanov SA. Studies on sterol composition of the seed oil representatives of the family Apiaceae. Fett Wissenschaft Technologie 1995; 97: 381–383. [Google Scholar]
- 64.Huang MK, Choi YJ, Houde R, Lee JW, Lee B, et al. Effects of Lactobacilli and an acidophilic fungus on the production performance and immune responses in broiler chickens. Poultry Science 2004; 83 (5): 788–795. doi: 10.1093/ps/83.5.788 [DOI] [PubMed] [Google Scholar]
- 65.Denli M, Okan F, Celik K. Effect of dietary probiotic, organic acid and antibiotic supplementation to diets on broiler performance and carcass yield. Pakistan Journal of Nutrition 2003; 2 (2): 89–91. (doi: 10.3923/pjn.2003.89.91). [DOI] [Google Scholar]
- 66.Ghazanfari S, Mohammadi Z, Moradi AM. Effects of coriander essential oil on the performance, blood characteristics, intestinal microbiota and histological of broilers. Brazilian Journal of Poultry Science 2015; 17 (4): 419–426. ( 10.1590/1516-635x1704419-426). [DOI] [Google Scholar]
- 67.Brul S, Coote P. Preservative agents in foods: Mode of action and microbial resistance mechanisms. International Journal of Food Microbiology 1999; 50: 1–17. ( 10.1016/S0168-1605(99)00072-0) [DOI] [PubMed] [Google Scholar]
- 68.Yusrizal Chen TC. Effect of adding chicory fructans in feed on faecal and intestinal microflora and excretory volatile ammonia. International Journal of Poultry Science 2003; 2 (3): 188–194. [Google Scholar]
- 69.Osfar S, Eko W, Halim N, Fatmaoctavia S, Riany GS. Effect of prebiotic and immunowall® as feed additive in enzyme activity, intestinal characteristic, and broiler performance. International Journal of Food Science and Agriculture 2019; 3 (4): 292–298. [Google Scholar]
- 70.Rodjan P, Soisuwan K, Thongprajukaew K, Theapparat Y, Khongthong S, Jeenkeawpieam J, et al. Effect of organic acids or probiotics alone or in combination on growth performance, nutrient digestibility, enzyme activities, intestinal morphology and gut microflora in broiler chickens. Journal of Animal Physiology and Animal Nutrition 2017; 102(2); 931–940. doi: 10.1111/jpn.12858 [DOI] [PubMed] [Google Scholar]
- 71.Xu ZR, Hu CH, Xia MS, Zhan XA, Wang MD. Effects of dietary fructo oligosaccharides on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poultry Science 2003; 82(6):1030–1036. doi: 10.1093/ps/82.6.1030 [DOI] [PubMed] [Google Scholar]