Abstract
Polydimethylsiloxane (PDMS)-based levonorgestrel intrauterine systems (LNG-IUSs) such as Mirena® are long-acting drug-device combination products designed to release LNG for contraceptive purposes up to 6 years. LNG-IUSs consist of a hollow cylindrical drug-PDMS reservoir mounted with a polyethylene frame and covered by an outer PDMS membrane. PDMS is the release-controlling excipient present in both the matrix and the outer membrane. The degree of PDMS crosslinking is a key parameter in LNG-IUS manufacturing, dictating the elasticity and mechanical strength (which are critical parameters in molding and demolding of the cylindrical reservoirs). In addition, elasticity and mechanical strength are also important to prevent deformation during insertion into the uterine cavity. The objectives of this study were to investigate the impact of PDMS crosslinking on the physicochemical properties of LNG-IUSs and to develop appropriate testing methods for characterization of their mechanical strength. Formulations with different degrees of crosslinking were prepared by varying the ratio of the PDMS elastomer base and the crosslinking agent. A novel solvent swelling and extraction method was developed to determine the degree of PDMS crosslinking. The extent of crosslinking was also characterized via FTIR, Raman, 1H NMR, DSC, TGA and dynamic mechanical analysis. As expected, formulations with higher degrees of crosslinking showed lower crystallinity. Interestingly, the less crystalline formulations showed higher Tg values and storage moduli compared to the high crystalline formulations, implying that crosslinking is the predominant parameter governing the physicochemical and mechanical properties in LNG-IUSs. Correlations were established between PDMS crosslinking and the physicochemical properties of LNG-IUSs which will be useful for quality control purposes during formulation screening and development. A better understanding of the physicochemical characteristics of these complex products will facilitate drug product development.
Keywords: Levonorgestrel, Intrauterine device, Polydimethylsiloxane, Crosslinking, Physicochemical characterization, Dynamic mechanical analysis
1. Introduction
The concept of using hormonal contraceptives stemmed from the idea of mimicking the effect of progesterone in blocking ovulation (dating back to the late 1950s), and using this mechanism to inhibit fertility (Diczfalusy, 1979). The use of a synthetic analogue of progesterone identified as Levonorgestrel (LNG) has rapidly grown since then (Sitruk-Ware et al., 2013). Tremendous progress was made in the past two decades with the development of advanced forms of hormonal contraception, known as Long-Acting Reversible Contraceptives (LARCs). LARCs provide safe, highly efficacious, easy to use, long-acting, and quickly reversible choice of contraception (Shoupe, 2016). According to the Centre for Disease Control and Prevention (CDC), LARCs are the most effective class of contraceptives with less than a 1% failure rate (Winner et al., 2012). The major benefit of LARCs over any other reversible methods of contraception is that they eliminate the need for daily involvement by the patient (such as remembering to take a contraceptive pill), and therefore offer greater patient compliance.
Intrauterine systems (IUSs), also known as intrauterine devices (IUDs) belong to the class of LARCs. Mirena® was the first levonorgestrel-intrauterine system (LNG-IUS) approved by the U.S Food and Drug Administration (FDA). Mirena® has a reservoir releasing levonorgestrel with an initial release rate of 20 μg/day, and it is available for use in >100 countries (Sitruk-Ware and Inki, 2005). Although Mirena® has been approved by the FDA for up to 6 years, the contraceptive efficacy data from randomized trials have shown that Mirena® is capable of releasing an effective dose of levonorgestrel for up to a period of 7 years (Benagiano et al., 2008). The effectiveness of LNG-IUSs is believed to occur due to several mechanisms (such as hormonally induced endometrial atrophy, physiological changes in the environment of the cervical mucus, and inhibition of sperm motility) (Benagiano et al., 2008). Currently, there are four LNG-IUSs available on the U.S. market. These include Mirena® (52 mg LNG, 6 years), Liletta® (52 mg LNG, 6 years), Kyleena® (19.5 mg LNG, 5 years), and Skyla® (13.5 mg LNG, 3 years) (FDA, 2021).
LNG-IUSs comprise of a cylindrical drug-polymer reservoir around the vertical stem of a T-shaped polyethylene frame. The drug-polymer reservoir is made of a mixture of LNG and polydimethylsiloxane (PDMS), a silicone-containing polymer. This reservoir is covered with an outer semi-opaque polymer membrane also made of PDMS (Fig. 1A). LNG-IUSs are considered long-acting complex drug products. The complexity of their design, and a lack of understanding of their physiochemical characteristics and drug release mechanisms, poses a challenge for generic drug development. PDMS has been used as a release-controlling excipient in drug delivery systems such as subdermal implants (Croxatt, 2002; Rowlands and Searle, 2014), intrauterine systems (Mansour, 2012), vaginal rings (Boyd et al., 2016; Mashak and Rahimi, 2009; Welsh et al., 2019) and transdermal devices (Sabo and Waters, 2021; Tan and Pfister, 1999). This wide range of applications is due to the physiochemical properties of PDMS, such as physiological inertness, low toxicity, thermal and oxidative stability, good film-forming ability, adhesion to different substrates, and excellent resistance to chemical and irradiation degradation (Blanco, 2018).
Fig. 1.

(A) Schematic diagram depicting the structure of levonorgestrel intrauterine systems; (B) The chemical structure of an unsubstituted PDMS polymer chain unit; and (C) Addition curing reaction of the PDMS elastomer base with the crosslinking agent to form crosslinked PDMS via an ethylene bridge.
PDMS is an organosilicon polymer which is formed by crosslinking silicone prepolymers or copolymers, through a process known as curing. PDMS crosslinking can be achieved by three major mechanisms - addition curing, condensation curing, and peroxide curing (Lambert, 2006). Peroxide curing and condensation curing are associated with problems such as generation of free radicals and unwanted by-products. Accordingly, addition curing is commonly employed in controlled drug delivery systems using a PDMS matrix. Crosslinking by addition curing takes place through a hydrosilylation addition between the vinyl-terminated groups of the PDMS elastomer base and the methylhydrogensiloxane group of the crosslinker in the presence of a platinum-based catalyst at high temperature (Fig. 1C). Commercial PDMS polymers based on addition curing are available as two-part systems, supplied as Part A (prepolymer A) and Part B (prepolymer B). Prepolymer A mainly contains the vinyl-terminated PDMS elastomer base along with the catalyst, and prepolymer B contains the elastomer base with the crosslinking agent. Different additives such as silica may be incorporated along with these prepolymers to achieve high tensile strength of the crosslinked PDMS polymer.
PDMS crosslinking is essential to achieve good mechanical properties. During the preparation of LNG-IUSs, drug-polymer reservoirs with high mechanical strength are required to maintain their structure while they are mounted onto the polyethylene frame. Mechanically strong formulations are also required to maintain device integrity and prevent deformation during insertion into the uterine cavity. Crosslinked PDMS polymers offer elasticity and high tensile strength, which is essential for molding and demolding during processing. In addition, crosslinking improves the thermal stability, resistance to degradation and chemical non-reactivity. For all these reasons, the degree of PDMS crosslinking is a crucial parameter in LNG-IUS manufacturing.
Previous reports have detailed: the manufacturing and characterization of qualitatively (Q1) and quantitatively (Q2) equivalent LNG-IUSs using Mirena® as the reference listed drug (RLD) (Bao et al., 2018); real-time and accelerated in vitro release testing methods (Bao et al., 2019); and the effect of product design parameters on the performance of LNG-IUSs (Bao et al., 2020). The purpose of the current study was to understand the impact of PDMS crosslinking on the physicochemical and mechanical properties of LNG-IUSs. Formulations with varying ratios of prepolymers A and B were prepared to achieve different degrees of PDMS crosslinking in LNG-IUSs. A novel method was developed to determine the degree of PDMS crosslinking using solvent swelling and extraction. Solid state techniques such as FTIR, Raman, and NMR were used to characterize these formulations. DSC and TGA were used to study the thermal changes induced by crosslinking and the degradation behavior of LNG-IUSs. Correlations were established between the mechanical properties of LNG-IUSs and the degree of PDMS crosslinking using a durometer hardness tester, a texture analyzer, and DMA. The current study is the first report detailing the effect of prepolymer ratios on the degree of PDMS crosslinking and how this impacts the physicochemical and mechanical properties of LNG-IUSs. The correlations established between the PDMS crosslinking and the physicochemical properties will be useful during the formulation screening and quality control.
2. Materials and methods
2.1. Materials
Levonorgestrel with a mean particle size of 16 μm was purchased from Tecoland (Irvine, CA, USA). Polydimethylsiloxane-based liquid silicone rubber supplied as two prepolymers (MED-4840 Part A and Part B) were purchased from Nusil™ (Carpinteria, CA, USA). Acetonitrile (ACN, HPLC grade), hexane (HPLC grade), and tetrahydrofuran (THF, HPLC grade) were purchased from Fischer Scientific (Fair Lawn, NJ, USA). Deuterated chloroform (CDCl3, NMR grade) was purchased from Sigma-Aldrich (St. Louis, MO, USA). The PDMS silicone tubing (Silastic®) was purchased from Dow Corning (Midland, MI, USA). Unless otherwise specified, all other materials used were of analytical grade.
2.2. Preparation of levonorgestrel intrauterine systems
2.2.1. Formulation of drug-polymer reservoirs with different prepolymer ratios
The drug-polymer reservoirs were prepared using a customized extrusion device as described previously (Bao et al., 2018). Briefly, a twin-syringe assembly composed of a polycarbonate mold, two 3-mL polypropylene syringes with the needle hub removed, a metal rod with a centering device A and a centering device B were used as shown in Fig. 2. Prepolymer A and prepolymer B were mixed in different stochiometric ratios of B:A (0.2:1, 0.25:1, 0.33:1, 0.5:1, 1:1, 2:1, 3:1, 4:1, and 5:1) in the presence of LNG to obtain nine different drug-polymer reservoirs. The batch size of all the drug-polymer reservoirs was 300 mg with a drug loading of 50% w/w LNG. After blending, the drug-polymer reservoirs were dried under vacuum for 5 h to remove any air bubbles. Each of these drug-polymer reservoirs were transferred into the twin syringe assembly and extruded to form hollow cylindrical drug-polymer reservoirs which were then cured at 80 °C for 20 h. The cured drug-polymer reservoirs were removed from the mold and used for further fabrication.
Fig. 2.

Pictorial representation of the preparation process of LNG-IUSs: (A) Devices used for the preparation of drug-polymer reservoirs; (B) The processes of extrusion of the PDMS polymer and the LNG mixture to form the drug reservoirs; (C) The resultant LNG drug reservoir with steel rod; (D) 100 mg standard LNG reservoir with nylon rod; and (E) LNG-IUS (with outer membrane) (with permission from (Bao et al., 2018)).
2.2.2. Preparation of LNG-IUSs with different prepolymer ratios
The prepared drug-polymer reservoirs with nine different ratios of prepolymers B:A were cut into 100 mg pieces and enveloped onto nylon rods (diameter: 1.57 mm). Silastic® silicone tubing was used as the outer polymer membrane and was cut into 24 mm long pieces. The silicone tubing segments were soaked in hexane at room temperature for 5 min to swell, and then coated onto the drug-polymer reservoirs in a symmetrical manner. The resultant LNG-IUSs, with different prepolymer ratios, were placed overnight in a desiccator under vacuum at room temperature to remove the residual hexane.
2.3. UPLC analytical method for LNG
The concentration of LNG was determined using an Ultra Performance Liquid Chromatography (UPLC) system (Acquity H-class, Waters). The mobile phase (a mixture of 0.05% trifluoracetic acid in water and acetonitrile (95:5) (Mobile phase A) and 0.05% trifluoracetic acid in water and acetonitrile (5:95) (Mobile phase B)) was used in a gradient condition at a flow rate of 0.4 mL/min for 5 min. A C18 BEH column (50 mm × 2.1 mm, 1.7 μm, Waters®) was used at 30 °C. The injection volume was 1 μL and the detection wavelength was set at 242 nm. The chromatograms were analyzed using Empower® 3.0 software.
2.4. Drug loading and content uniformity
The drug loading and content uniformity of the prepared drug-polymer reservoirs were determined using a tetrahydrofuran extraction method. The reservoirs were cut into small pieces of approximately 12 mg from three different regions and each piece was extracted in 25 mL of THF in tightly sealed volumetric flasks at room temperature for 48 h. An appropriate volume of THF was then added to achieve a total volume of 25 mL and the samples were then vortexed for 2 min. The resultant samples were diluted with acetonitrile: water (60:40) prior to being injected into the UPLC.
2.5. Determination of the degree of polymer crosslinking
A solvent swelling and extraction method was developed to determine the degree of polymer crosslinking achieved in the LNG-IUSs (Fig. 3). The drug-polymer reservoirs were cut into pieces of approximately 12 mg, accurately weighed and transferred into tightly sealed glass vials containing 25 mL of THF. LNG was allowed to extract completely into THF for 48 h and the amount of LNG extracted was determined using the developed UPLC analytical method. The extraction time was optimized to ensure the complete extraction of LNG in THF. The reservoir pieces were then removed from the THF and dried in a vacuum oven at room temperature for 20 h. The weight of the reservoirs before and after drying was recorded.
Fig. 3.

Pictorial representation of the solvent extraction method for the determination of polymer crosslinking in LNG-IUSs.
Further, the reservoirs were allowed to swell in 20 mL of hexane for 48 h at room temperature in tightly sealed glass vials. The time for extraction of reservoirs in hexane was optimized to ensure the complete extraction of the non-crosslinked polymer. The reservoirs were then carefully separated from hexane by filtration and their weight was recorded. The reservoirs were dried under vacuum for about 18 h at room temperature and the differences in their weights after drying were recorded. The amount of non-crosslinked material in THF was calculated from the difference in the initial weight of the drug-polymer reservoir used and the final weight of the dried reservoir after extraction in THF. Appropriate correction was made to account for the drug loss during this extraction process. Similarly, the amount of non-crosslinked material in hexane was calculated from the difference in the dry weight of the reservoirs before extraction and after extraction in hexane. The degree of crosslinking (expressed as % of total weight) was determined from the difference in the total non-crosslinked material extracted during the entire process and the initial weight of the drug-polymer reservoir used. The experiments were performed in triplicate for each of the drug-polymer reservoirs. The degree of crosslinking in LNG-IUSs was calculated based on the following equation.
where,
Non crosslinked material extracted in THF = ΔWTHF − WLNG
ΔWTHF (total weight loss in THF) = W0 − W1
WLNG = Amount of LNG extracted in THF (determined by UPLC) (mg)
W1 = Weight of the reservoir after vacuum drying following extraction in THF (mg)
W0 = Initial weight of drug-polymer reservoir (mg)
Non crosslinked material extracted in hexane = W1 − W2
W2 = Weight of the reservoir after vacuum drying following complete extraction in hexane (mg)
2.6. Spectral characterization
2.6.1. Fourier-transform infrared spectroscopy (FTIR)
The nine drug-polymer reservoirs prepared using different ratios of prepolymers B:A were characterized using a Nicolet FTIR spectrophotometer (iS5 FTIR, Thermo Scientific) with an attenuated total reflectance (ATR) accessory. The samples were placed on the germanium crystal window and compressed lightly using the pressure clamp. FTIR spectra were recorded over a range of 400–4000 cm−1, for 128 parallel scans. Data analysis was carried out using KnowItAll™ informatics system (Bio-Rad Laboratories, Hercules, CA, USA).
2.6.2. 1H Nuclear magnetic resonance (NMR)
The drug-polymer reservoirs prepared by mixing various ratios of prepolymers B:A were extracted in CDCl3 and then characterized using solution state 1H NMR. To avoid interference due to the high intensity signals of LNG observed under NMR, blank polymer reservoirs with nine different ratios of prepolymers B:A were prepared in a similar manner as described previously without incorporating the drug. These reservoirs were soaked in 10 mL of CDCl3 in glass vials for 4 days to extract the non-crosslinked polymer. The solution was concentrated by evaporating the solvent at room temperature until a volume of approximately 2 mL remained. 1H NMR was performed using this extracted solution (for nine different polymer reservoirs) on a Bruker 500 MHz instrument (Bruker UK Ltd., Coventry, UK). A series of reference spectra for the prepolymer A and prepolymer B silicone elastomer components were recorded to enable the identification of characteristic signals. Chemical shifts were recorded in parts per million (ppm) with respect to the chemical shift of the internal reference of tetramethyl silane (TMS), and 7.26 ppm with respect to CDCl3. Data analysis was performed using MRestNova software (Mestrelab research, S.L, Spain).
2.6.3. Raman analysis
Raman spectra were recorded using a Renishaw Raman microscope 2000 (Renishaw Inc., UK) at 50X objective equipped with a fiber-optic probe, and capable of collecting spectra with 1 μm spatial resolution. A thin transverse section of the sample specimen was cut and mounted on the spectrometer. An argon-ion laser source operating at 785 nm was used at a laser power of 10 mW and spectra were recorded from 2750 cm−1 to 100 cm−1.
2.7. Thermal characterization
2.7.1. Differential scanning calorimetry (DSC)
DSC of the prepared drug-polymer reservoirs was performed using a TA Q20 calorimeter (TA instruments, New Castle, DE, USA) equipped with a refrigerated cooling accessory. Approximately 3–4 mg of each drug-polymer reservoir were sealed in non-hermetic aluminum pans, equilibrated at −80 °C and then heated up at 10 °C/min to 250 °C. The samples were then cooled to −80 °C to record the polymer crystallization event. Nitrogen gas was used for purging at a flow rate of 50 mL/min. Data were analyzed using TA universal analysis software. The experiments were performed in triplicate for each of the drug-polymer reservoirs.
2.7.2. Thermogravimetric analysis (TGA)
TA Q500 (TA instruments, New Castle, DE, USA) was used to analyze the thermal changes. The samples were loaded into platinum pans and heated to 900 °C at a ramp rate of 20 °C/min. Data were analyzed using TA universal analysis software. Images of the residues were captured for comparison. The experiments were performed in triplicate.
2.8. Mechanical characterization
2.8.1. Hardness test using durometer
Hardness of the drug-polymer reservoirs was measured by a Type-A durometer (Rex gauge 4000 digital, Rex instruments, IL, USA). The drug-polymer reservoirs were cut into flat rectangular pieces of approximately 5 mm × 5 mm and mounted on the platform. The indenter of the instrument was then allowed to press on the sample and the hardness value was recorded after 5 s. Five measurements were performed for each of the drug-polymer reservoirs.
2.8.2. Mechanical strength test using TA.XT texture analyzer
The mechanical strength of the drug-polymer reservoirs was determined using a TA.XT Plus Texture Analyzer (Texture Technologies Corp, Hamilton, MA, USA). Approximately 2.5 cm long pieces of each of the drug-polymer reservoirs were loaded onto modified TA-96B tensile grips (Texture Technologies Corp, Hamilton, MA, USA). The initial distance was set to 10 mm and a tensile test under tension mode was performed at 1 mm/sec with a target strain of 30%. A plot of force (N/g) against strain was recorded and the slope of the test curves were compared. Data were analyzed using the built-in software. Each of the drug-polymer reservoirs were analyzed in triplicate.
2.8.3. Dynamic mechanical analysis (DMA)
Samples were tested using a Dynamic Mechanical Analyzer (DMA 1, Mettler Toledo, Columbus, OH, USA) that had been calibrated to the manufacturer’s specifications using traceable materials. An indium check yielded a value of 156.71 °C compared to a theoretical value of 156.6 °C. The drug-polymer reservoirs were cut into thin rectangular strips of about 10 mm (L) × 2.4 mm (W) × 0.9 mm (T). All samples were tested using the tension clamp in the temperature range −150 °C to 40 °C (heated at 5 °C/minute), under a load force of 0.1 N, a displacement of 2 μm, and a frequency of 1 Hz. The data were analyzed and the storage modulus (E’), loss modulus (E”) and tan delta (tan δ) values were calculated.
2.9. Statistical analysis
The data analysis, linear regression and fitting were performed using Graphpad Prism software (Graphpad software, San Diego, CA, USA) and Excel 2016 (Microsoft Corporation, Redmond, WA, USA). The data were presented as average ± standard deviation (SD).
3. Results
3.1. Drug loading and content uniformity
The observed drug loading of the drug-polymer reservoirs, determined by the THF extraction method, was close to the target drug loading (50% w/w LNG) (Fig. 4). The RSD of the triplicate samples taken from different regions of the reservoirs was below 5% indicating good content uniformity. The highest drug loading was observed at a prepolymer ratio B:A of 1:1. The observed drug loading decreases as the amount of either the PDMS elastomer base or the crosslinking agent was increased.
Fig. 4.
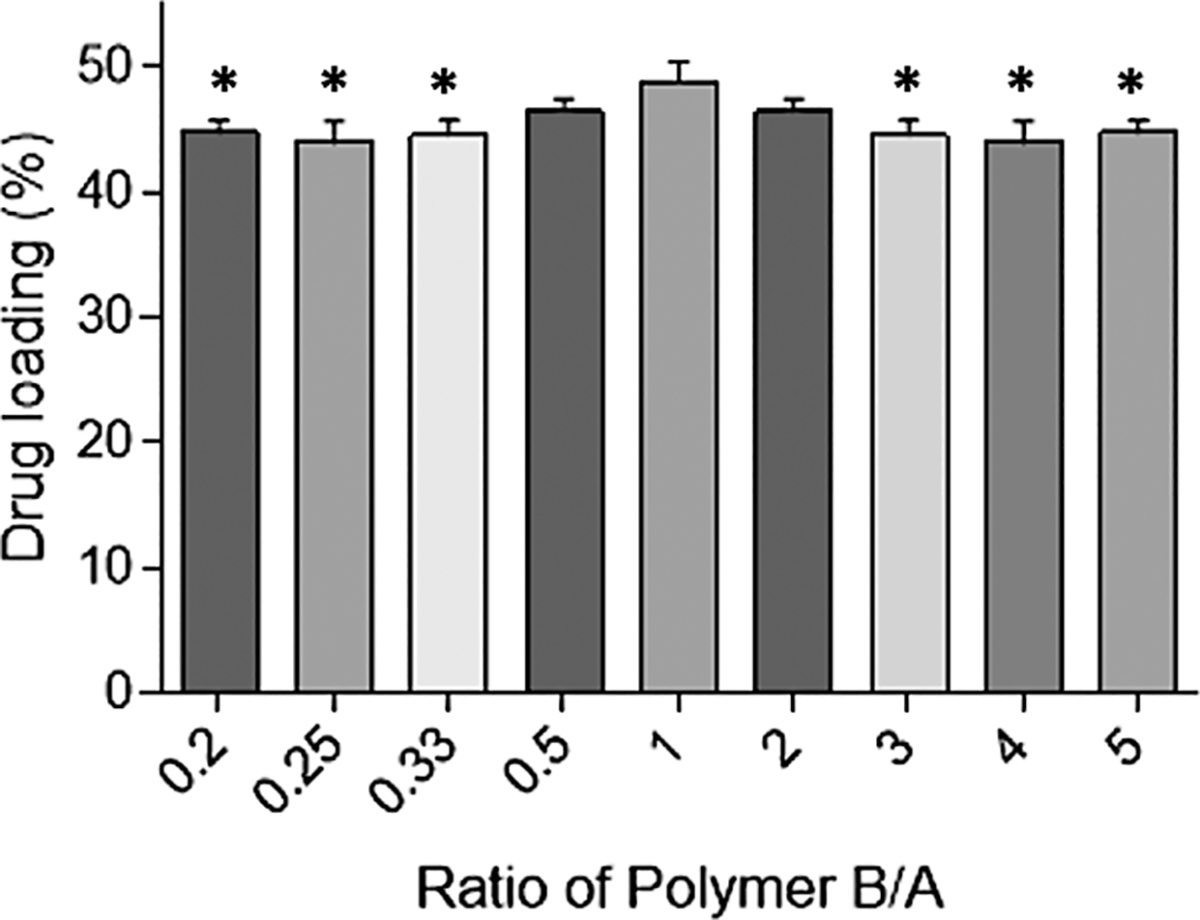
The drug loading of different drug-polymer reservoirs prepared using various ratios of the prepolymers A and B (mean ± SD, n = 3) (*The formulations were significantly different (p < 0.05) to the formulation with the prepolymer ratio B:A of 1:1).
3.2. Degree of polymer crosslinking
The degree of PDMS crosslinking in LNG-IUSs was determined by the solvent extraction method based on the total amount of non-crosslinked material extracted in THF and hexane (using the equation mentioned in Section 2.4). It was observed that the degree of PDMS crosslinking increased as the ratio of prepolymer B:A increased from 0.2 to 1. This is due to the increase in the amount of crosslinking agent in the formulations. The formulation with a prepolymer ratio of 1:1 showed the highest degree of crosslinking. However, a further increase in the amount of crosslinking agent (B:A > 1) led to a decrease in the degree of PDMS crosslinking (Fig. 5). This could be attributed to an insufficient amount of catalyst to ensure complete crosslinking.
Fig. 5.

Degree of PDMS crosslinking (%) in LNG-IUSs with different ratios of prepolymers B:A (mean ± SD, n = 3).
3.3. Spectral characterization
3.3.1. FTIR
FTIR spectrum of LNG showed characteristic peaks at 3350 cm−1 (–OH stretching), 3290 cm−1 (–C≡C stretching) and 1650 cm−1 (–C═O stretching). The characteristic peaks observed at 2962 cm−1 (–CH stretching), 1260 cm−1 (–Si–CH3 stretching), 1071 cm−1 (–Si–O–Si stretching), 1007 cm−1 (– (CH3)2Si–O stretching), 912 cm−1 (–Si–H bending) and 790 cm−1 (–Si–CH3 bending) were attributed to the PDMS polymer matrix (Fig. 6A). FTIR analysis of prepolymer B showed the presence of a characteristic peak at 912 cm−1. No variation in the intensity of the characteristic peak at 912 cm−1 was seen when the prepolymer ratio of B:A was<1. A gradual increase in the intensity was observed only when the ratio of prepolymer B:A was >1 (Fig. 6B). This peak was attributed to the -Si-H bending frequency which arises from the hydrosilyl residues present in the crosslinking agent.
Fig. 6.

(A) Representative FTIR profiles of LNG-IUS (with a 1:1 ratio of prepolymers B:A), and LNG (API); and (B) differences observed in the characteristic peak at 912 cm−1 with the changes in the ratios of the prepolymers A and B.
3.3.2. 1H NMR
The degree of PDMS crosslinking due to variation in the prepolymer ratios was characterized using solution state 1H NMR. The intensities of the characteristic peaks of LNG were very strong as compared to the intensities of the peaks attributed to the prepolymers (due to the very high drug loading in LNG-IUSs). It was observed that the drug loading in LNG-IUSs did not significantly impact the degree of polymer crosslinking (data in supplementary information). Therefore, blank polymer reservoirs (without incorporating the drug) were used to study the NMR spectral profiles for a better comparison of signals at low intensity.
Solution state 1H NMR analysis of all the reservoirs was carried out by extracting them in CDCl3. During this process, the non-crosslinked polymer was extracted in the solvent. As shown in Fig. 7, the formulation with a prepolymer ratio of 1:1 did not show any significant residual amount of non-crosslinked polymer. Formulations with prepolymer ratios of B:A > 1 showed a gradual increase in the intensity of signals at 4.75 ppm. This signal was observed due to the protons of the hydrosilyl groups in the crosslinking agent. An increase in the intensities of the multiplets between 5.7 ppm and 6.3 ppm were observed as the prepolymer ratio of B:A was decreased from 1:1 to 0.2:1. These multiplets were attributed to the characteristic signals arising due to the protons of the vinyl-terminated PDMS prepolymer.
Fig. 7.

1H NMR spectrum of the solvent extract of LNG-IUSs with different degrees of polymer crosslinking (Zoomed-in view of the spectrum is shown to display the low intensity signals of the non-crosslinked polymer).
3.3.3. Raman analysis
The Raman spectra of the drug-polymer reservoirs with different prepolymer ratios are shown in Fig. 8. Characteristic peaks at 2100 cm−1 (–C≡C stretching), 1658 cm−1 (–C═C stretching), 1680 cm−1 (–C═O stretching), and 1608 cm−1 (–C–C aromatic stretching) were attributed to LNG. Characteristic peaks in the region of 1440–1470 cm−1 were attributed to –CH3 bending in the PDMS and LNG. Characteristic peaks for PDMS were observed at 1105 cm−1 (–Si–O stretching), 710 cm−1 and 790 cm−1 (–Si–C stretching in PDMS), and 490 cm−1 (–Si–O–Si stretching).
Fig. 8.

Representative Raman spectra of the drug-polymer reservoirs of LNG-IUSs prepared using different ratios of prepolymers B:A.
3.4. Thermal characterization
3.4.1. DSC
The thermal properties of LNG and PDMS in the drug-polymer reservoirs were investigated using DSC. As the samples were heated from −80 °C at 10 °C/min, melting of PDMS was observed at −44.8 °C. The melting of LNG was observed as a sharp endotherm at ~241 °C, and the melting enthalpy (76.07 J/g) of the formulation with a 1:1 ratio of the prepolymers (50% w/w drug loading) was consistent with a previous report (Bao et al., 2018). An exothermal peak at −72.5 °C was observed during the cooling cycle from 250 °C to −80 °C. The exothermal peak was attributed to the crystallization of the polymer (Fig. 9A). The crystallization enthalpy and the melting enthalpy of PDMS for the different reservoirs were recorded. It was observed that the crystallization and melting enthalpy of the polymer decreases as the prepolymer ratio of B:A was increased. The lowest enthalpies were recorded for the formulation with the prepolymer ratio of 1:1. Further, an increase in the ratio of prepolymer B:A>1, resulted in increasing values of the crystallization and melting enthalpies of PDMS (Fig. 9B and 9C). It was shown that the crystallization and melting enthalpies decreased as the degree of PDMS crosslinking increases (Fig. 10).
Fig. 9.

(A) Representative DSC profile depicting the thermal events of the drug and PDMS in LNG-IUSs; (B) variation in the crystallization enthalpy (J/g) of PDMS with differences in the ratio of prepolymers B:A (mean ± SD, n = 3); and (C) variation in the melting enthalpy (J/g) of PDMS with differences in the ratio of prepolymers B:A (mean ± SD, n = 3).
Fig. 10.

(A) Relationship between the crystallization enthalpy of PDMS and the degree of crosslinking; and (B) relationship between the melting enthalpy of PDMS and the degree of crosslinking (n = 3).
3.4.2. TGA
The TGA profiles of the formulations with different ratios of prepolymers B:A are shown in Fig. 11 A. The melting and decomposition of the API begins above 200 °C, and degradation is complete at ~350 °C. Formulations with varying ratios of the prepolymers revealed differences in their profiles above 350 °C which was attributed to the degradation of PDMS (Fig. 11B). Polymer degradation occurred in two phases and a significant difference in weight loss (%) for both degradation phases (350–750 °C) was recorded. A lower weight loss (%) was recorded as the ratio of prepolymer B:A was increased (Fig. 12).
Fig. 11.

(A) Representative TGA profiles of LNG-IUSs with different ratios of prepolymers B:A (30 °C to 900 °C); and (B) derivative of weight loss against temperature (150 °C to 800 °C) showing differences in the degradation profiles of LNG-IUSs with different ratios of prepolymers B:A.
Fig. 12.

Weight loss (%) of the formulations with different ratios of prepolymers (recorded using TGA) (mean ± SD, n = 3).
3.5. Mechanical characterization
3.5.1. Hardness
Durometer hardness is a standardized method to measure the hardness of elastomers and plastics. The hardness of the formulations with different ratios of prepolymers B:A were determined using a Type A durometer measuring the extent of depth of indentation caused in the material due to the applied force. The lowest value of hardness was recorded for the formulation with a prepolymer B:A ratio of 0.2:1. As the ratio of prepolymers B:A was increased, the hardness of the formulations also increased. The formulation with a 1:1 ratio of the prepolymers showed the maximum hardness due to the highest degree of crosslinking achieved. The formulations prepared with a prepolymer ratio of B:A > 1 showed a lower value of hardness (Fig. 13 A). It was shown that the hardness of the formulations was directly dependent on the degree of PDMS crosslinking in LNG-IUSs (Fig. 13 B).
Fig. 13.

(A) Hardness of LNG-IUSs with different ratios of prepolymers B:A tested using a type-A durometer hardness tester (mean ± SD, n = 5); and (B) relationship between the durometer hardness (mean ± SD, n = 5) and the degree of PDMS crosslinking (mean ± SD, n = 3).
3.5.2. Mechanical strength using TA.XT texture analyzer
The mechanical strength of the drug-polymer reservoirs prepared using various ratios of the prepolymers were tested under 0–30% strain. The highest force was recorded at a given strain (%) for the formulation with a prepolymer B:A ratio of 1:1 indicating the highest tensile strength (Fig. 14A). This was consistent with the results obtained using durometer hardness. The slope (unit: g/%) of the force-strain curve for each of the formulations was calculated and its correlation with the prepolymer ratio of B:A was established. The slope (g/%) of the force-strain curve initially increased as the ratio of prepolymers B:A was increased, and it reached a maximum value for the formulation with a prepolymer ratio of 1:1. For formulations with prepolymer ratios of B:A > 1, the slope decreased drastically (Fig. 14B). The values obtained for the slope of force (g) vs strain (%) curve were indicative of the tensile strength of the formulations. A relationship between the calculated slope (g/%) and the degree of PDMS crosslinking was established which shows that the values of the slope were directly proportional the degree of crosslinking achieved in the formulations (Fig. 14 C).
Fig. 14.

(A) Representative plot of force against strain (using a texture analyzer) for LNG-IUSs with different prepolymer ratios; (B) relationship between the slope (g/%) (force against strain) and the prepolymer ratios of LNG-IUSs (mean ± SD, n = 3); and (C) relationship between the slope (g/%) (force against strain) and the degree of PDMS crosslinking (mean ± SD, n = 3).
3.5.3. Dynamic mechanical analysis (DMA)
DMA is a thermomechanical technique that measures the properties of materials as they are deformed under periodic stress. A temperature sweep from −150 °C to 40 °C at a frequency of 1 Hz was applied to the formulations with different prepolymer ratios, and properties such as glass transition temperature (Tg), storage modulus (E’), loss modulus (E”) and damping factor (tan δ) were measured.
The polymer exists in a glassy state at a temperature of −150 °C. As the temperature was increased, the Tg of PDMS was recorded at approximately −125 °C (Fig. 15). Continued heating above the Tg caused only a slight decrease in the storage modulus and loss modulus, until a second transition was observed around −53 °C. The transition from a stiff viscous elastomer to a soft elastomer occurred in this region which was accompanied by a drastic decline in E’ and E”. Therefore, a sharp peak in tan δ (ratio of E” and E’) was observed at this temperature. A higher glass transition temperature was observed for the formulation with a prepolymer B:A ratio of 1:1. Formulations with a prepolymer ratio (B:A) of 1:1, 2:1, 3:1 and 0.5:1 showed relatively larger values of E’, and lower values of E” and tan δ. Data for the formulation with a prepolymer B:A ratio of 0.2 could not be collected as the sample was too soft and could not sustain the operating conditions of the instrument.
Fig. 15.

Representative profile of a DMA scan (prepolymer B:A ratio of 1:1) showing the viscoelastic behavior in LNG-IUS with temperature-dependent stress transitions.
A correlation between the Tg and moduli properties of LNG-IUSs, and the degree of PDMS crosslinking was established (Fig. 16). It was observed that the Tg and the storage modulus at the rubbery plateau (Ep’) were directly proportional to the degree of PDMS crosslinking (Fig. 16 A and C). The tan δ values decreased as the degree of PDMS crosslinking in LNG-IUSs increased (Fig. 16 D).
Fig. 16.

(A) Effect of the degree of PDMS crosslinking in LNG-IUSs on its glass transition temperature; (B) variation in the storage moduli (E’ at the transition temperature in the viscous region) with the degree of PDMS crosslinking (%); (C) correlation between the storage modulus at the rubbery plateau (Ep’) and the degree of PDMS crosslinking in LNG-IUSs; and (D) correlation between the tan δ values and the degree of PDMS crosslinking in LNG-IUSs (n = 3).
4. Discussion
4.1. Degree of PDMS crosslinking in LNG-IUSs
The properties of PDMS which makes it suitable for application in controlled release are attributed to the crosslinked form of the polymer. Introducing crosslinks between the polymer chains affects the hydrophobicity, surface reactivity, swelling properties, tensile strength, and elasticity of the polymer which may subsequently affect performance. The presence of a high amount of residual non-crosslinked PDMS may lead to toxicity in the cells, particularly for drug products designed for long-term release in the body. Therefore, ensuring a high degree of PDMS crosslinking is not only essential to achieve good physicochemical and mechanical properties, but it is also crucial for physiological inertness in the body.
The impact of prepolymer ratios, used in the formulation of LNG-IUSs, on the degree of PDMS crosslinking was investigated. The prepolymers used for the formation of a crosslinked PDMS matrix are complex mixtures which involve an interplay of several components affecting the degree of PDMS crosslinking achieved in the final product. Prepolymer A contains dimethylvinyl-terminated PDMS (elastomer base) along with the catalyst, while prepolymer B contains methylhydrogen silane terminated PDMS (crosslinking agent) along with the elastomer base. Apart from these major constituents, the prepolymers also contain other hydrolytic products of silicones, and contain additives such as silica which act as reinforcing agents. Small amounts of moderators or inhibitors are also added to inhibit the curing process at room temperature (Lukin et al., 2020).
A simple and reliable solvent swelling and extraction method using THF and hexane was developed to determine the degree of PDMS crosslinking (expressed as %). THF was used as a solvent to extract LNG from the drug-polymer reservoirs due to the high solubility of LNG in THF and the extracted LNG was quantified using UPLC. During the process, some non-crosslinked monomers are also extracted into THF. In the subsequent step, hexane was used to ensure complete extraction of the non-crosslinked PDMS from these reservoirs. Hexane acts as a strong solvent for the non-crosslinked PDMS by inducing greater swelling in the PDMS matrix. The total amount of non-crosslinked polymer was the sum of the non-crosslinked amount extracted in THF and hexane. This value was expressed in percentage of the crosslinked material with respect to the initial weight of the drug-polymer reservoir to obtain the degree of PDMS crosslinking in LNG-IUSs. The developed solvent swelling and extraction method provided a single tool for quantifying the drug, monitoring drug stability, and determining the non-crosslinked polymer from LNG-IUSs thus providing accurate mass balance during the extraction process.
The results of FTIR and 1H NMR provided a better understanding of the observed trend in the degree of crosslinking by varying the amount of prepolymers in the formulation. At the prepolymer ratio B:A of 0.2:1, an excess of elastomer base is present, and the amount of crosslinking agent is insufficient to achieve complete crosslinking of the polymer matrix. This was observed from the results of 1H NMR which showed a decreasing intensity of the dimethylvinyl peak (5.7 ppm to 6.3 ppm) as the ratio of prepolymer B:A increases from of 0.2:1 to 0.5:1. As the ratio of prepolymer B:A increases, a higher amount of crosslinking agent becomes available to react with the elastomer base leading to a higher degree of crosslinking in the polymer chains. No peaks for the dimethylvinyl protons or methylhydrogen silane protons in the 1H NMR spectrum were observed for the formulation with a prepolymer B:A ratio of 1:1 indicating that non-crosslinked polymer was absent, or below the sensitivity limit of the instrument. On increasing the prepolymer ratio of B:A > 1, a reduction in the degree of crosslinking was seen. This was due to the presence of excess crosslinking agent in the formulations as evident from the gradual increase in the intensity of the peak corresponding to the methylhydrogen silane (-Si-H) groups (4.75 ppm) at prepolymer ratios of B:A > 1.
The FTIR results corroborated the observations of 1H NMR. FTIR spectra of the formulations with prepolymer ratios B:A > 1 showed a gradual increase in the intensity of the peak observed at 912 cm−1 which can be attributed to the -Si-H groups in the crosslinking agent. The increasing intensity of the peak observed in FTIR suggested the presence of residual crosslinking agent in the formulations which is unable to crosslink due to either insufficient elastomer base or insufficient catalyst to induce the crosslinking reaction. In addition to FTIR, investigation of the formulations with various degrees of PDMS crosslinking using Raman spectroscopy was carried out which showed strong signals for the formulation with a prepolymer B:A ratio of 1:1 (high degree of PDMS crosslinking). Based on the observed differences in FTIR, the formulations were also expected to show significant differences in Raman (which is sensitive to homonuclear molecular bonds). However, the signals arising due to LNG caused interference with the characteristic PDMS signals in the Raman spectra. Thus, FTIR proved to be a better technique than Raman to characterize the differences in PDMS crosslinking.
4.2. Impact of PDMS crosslinking on the physicochemical and mechanical properties of LNG-IUSs
4.2.1. Effect of PDMS crosslinking on the crystallinity and thermal degradation profiles
A high degree of PDMS crosslinking resulted in a low degree of crystallinity in the LNG-IUSs. There are two plausible explanations for this behavior. First, a higher degree of crosslinking leads to the formation of tight junctions at the site of crosslinks as a network of PDMS chains is formed. These crosslink junctions act as constraints to the topologically neighboring polymer chain units and reduce their mobility thus preventing the polymer chain units from incorporating back into the crystal phase. As a result, there will be a reduction in the extent and thermodynamic stability of the crystalline phase with an increase in the degree of crosslinking. These results are consistent with the findings of Roland et al., who observed the suppression of crystallinity in end-linked polydimethylsiloxane networks (Roland and Aronson, 2000). Apart from crosslinking, the second plausible explanation for the observed trend in crystallinity is the presence of additives or fillers which are incorporated in the prepolymers. Several studies have reported the use of fillers such as silica to add tensile strength to the formulations (Camenzind et al., 2010; Gong and He, 2020). Dollase et al. showed experimental evidence that solid additives such as silica and porous glass increase the crystallization temperature and lower the time for crystallization to occur (Dollase et al., 2003). PDMS is a semi-crystalline polymer with a large amount of amorphous phase, and the presence of additives increases its crystallinity. An excess amount of prepolymer A or B (lower degree of crosslinking) may contain additives in a large amount and thus promote the crystallization of PDMS. The influence of the degree of crosslinking on the crystallinity and melting of PDMS may have an impact on the drug solubility and subsequent drug release from LNG-IUSs.
The degradation behavior of PDMS in LNG-IUSs was shown to be dependent on the degree of crosslinking. The thermal degradation of PDMS mainly occurs through depolymerization into cyclic oligomers and the rate of degradation is highly dependent on the heating rate. Two competing mechanisms exist for the thermal degradation of PDMS (Camino et al., 2002; Camino et al., 2001). A molecular mechanism which causes –Si–O– bond scission leading to the formation of cyclic oligomers, primarily occurs at low temperatures and low heating rates. The other mechanism is a radical mechanism which occurs at higher temperatures and involves homolytic –Si–CH3 bond scission. The formulations with a low degree of crosslinking are believed to depolymerize into cyclic oligomers of different molecular weights, due to the rearrangement of the siloxane bond. This occurs at a relatively low temperature as evident from the TGA degradation profiles of the formulations (Fig. 11). The first degradation phase observed in TGA corresponds to the formation of cyclic oligomers which is usually seen at temperatures below 500 °C. This is accompanied by partial oxidation of the silicone chain groups into silica, with the generation of carbon dioxide and carbon monoxide.
On the other hand, crosslinking creates additional bonds and hinders the splitting of PDMS chains by the formation of cyclic oligomers. The second degradation phase observed in TGA, which occurs at higher temperatures, is due to the formation of linear oligomers of PDMS (Camino et al., 2002). The formulations with a prepolymer B:A ratio of 1:1, 2:1 and 3:1 undergo degradation at temperatures higher than 650 °C which indicate that the major pathway of polymer degradation in these formulations is by the formation of linear oligomers (Fig. 11). It was also observed that residues of different color were formed after complete degradation (at 900 °C) of the formulations with different degrees of crosslinking. The formation of silicon oxycarbide from crosslinked PDMS leads to the generation of residues that are black in color. The formulations with prepolymer B:A ratios of 1:1 and higher, formed black colored residues after complete degradation. The formulations with prepolymer ratios of B:A < 1 formed white residues which could be attributed to their high silica content.
4.2.2. Hardness and mechanical strength
A high degree of crosslinking in PDMS is necessary to achieve a high elasticity and tear resistance of the product. PDMS elastomers have a high flexibility due to the characteristic siloxane bonds in their backbone structure. Increase in the chain length due to a high degree of crosslinking imparts mechanical strength to PDMS (Tolinski, 2009). Currently, there are no standardized testing methods available for characterizing the mechanical strength of LNG-IUSs. Hardness testing by durometer and texture analyzer can provide a quality control tool for the mechanical testing of LNG-IUSs during formulation screening and optimization. Formulations showed a decrease in the hardness when an excess of either silicone elastomer base or the crosslinking agent was present. The observed trend in the hardness was attributed to the differences in the degree of PDMS crosslinking in LNG-IUSs. Similarly, the correlation between the slope (g/%) of the force-strain curve with the degree of PDMS crosslinking suggested that crosslinking imparts tensile strength, which was the highest for the formulation with a prepolymer B:A ratio of 1:1. Formulations with very low amounts of crosslinking agent and those with an excess of crosslinking agent were soft and did not retain their shape while they were demolded during processing. These formulations were difficult to assemble onto the polyethylene rods to form the final product. LNG-IUSs may also undergo mechanical abrasion inside the uterine cavity during long-term use. Therefore, a high mechanical strength is critical for drug-device products like LNG-IUSs during manufacturing, storage, and use.
4.2.3. Glass transition and dynamic moduli properties
The temperature scan in DMA is particularly well-suited to understand the viscoelastic properties and obtain information on the polymer network structure such as crosslinking density and polymer chain heterogeneity (Dyamenahalli et al., 2015; Tamareselvy and Rueggeberg, 1994). In addition, DMA allowed monitoring the thermal properties of the material at a lower temperature range which was difficult to achieve using DSC. The DMA scan of LNG-IUSs showed three distinct phases. Significant rheological differences were observed in the glass transition region, viscous transition region and the rubbery plateau region for the LNG-IUSs with different degrees of crosslinking. The extremely low Tg of PDMS (~−125 °C) makes it suitable for use as an elastomer at a wide range of processing temperatures. As shown in Fig. 16 A, the Tg was dependent on the degree of PDMS crosslinking in LNG-IUSs. Increase in the degree of crosslinking causes a decrease in the free volume which leads to restricted molecular motion of the polymer chains. Therefore, higher energy is needed and a shift in the Tg to higher temperatures was observed. Surprisingly, these results are in contrast to the expected impact of crystallinity on the Tg (based on the DSC results).
The higher storage modulus (E’) of the formulations with a higher degree of PDMS crosslinking indicated a greater mechanical strength (Fig. 16 B). It was observed that the onset temperature, onset storage modulus, and the rubbery plateau increased as the degree of crosslinking in the formulations was increased. Crystallinity had a competing effect with the degree of crosslinking on E’ and E”. The crystallinity in the formulations with a low degree of crosslinking is higher than the formulations with a high degree of crosslinking (according to the DSC studies). The crystal phase locks the polymer chains together and a higher thermal energy is required for the polymer backbone to become mobile and dissipate energy. This could be the reason for the observed deviation from linearity when the storage modulus at the viscous transition was plotted against the degree of PDMS crosslinking (Fig. 16 B). In addition to the degree of crosslinking and crystallinity, the presence of fillers in the formulations with varying prepolymer ratios may also contribute to the viscoelastic behavior and moduli properties.
For most polymers, melting is observed at the terminal region where the polymer chains can slide past each other and the material continues to flow. However, for a crosslinked thermoset polymer such as PDMS, a distinct melting would not occur since the crosslinks prevent chain slippage. Instead, a plateau was observed (known as the rubbery plateau) and the values of E’ were greater than that of E”. The viscosity of the polymer in the rubbery plateau region is dependent on the molecular weight between the crosslinks. The rubbery plateau modulus is proportional to the number of crosslinks or the chain length between entanglements of a polymer (Menard and Menard, 2015). It was observed that the formulation with a higher PDMS crosslinking exhibited a higher E’ in the rubbery plateau region. A correlation between the storage modulus in the plateau region (Ep’) and the degree of PDMS crosslinking was established (for formulations with a relatively higher degree of crosslinking) (Fig. 16 C).
The loss modulus (E”) is an indication of the viscous nature of polymeric materials. The formulations with a lower degree of crosslinking revealed a higher value of E”. As the loss modulus increases, the polymer chain units exhibit lower resistance to motion which suggests a loose internal network structure. During this, the mechanical energy (input) is dissipated as heat due to the internal friction caused by movement of the polymer chains, which is identified as the phenomenon of damping (denoted by tan δ). The intensity of tan δ maxima reflects the extent of polymer chain mobility at the transition temperature. A lower tan δ value and a smaller peak height suggested an increased elastic behavior and a greater degree of crosslinking (Fig. 16 D).
Overall, the methods discussed above can be used as tools to characterize the degree of PDMS crosslinking in LNG-IUSs, which will provide a better understanding of the product properties. The effect of polymer crosslinking on drug release is important and we are in the process of investigating drug release from these LNG-IUSs with different extents of PDMS crosslinking.
5. Conclusions
This is the first report discussing the impact of prepolymer ratios on the degree of PDMS crosslinking, which significantly influences the physicochemical and mechanical properties of LNG-IUSs. A new method based on solvent swelling and extraction was successfully developed to determine the degree of PDMS crosslinking in LNG-IUSs. The formulations with a higher degree of crosslinking exhibited low crystallinity and a high thermal stability. Despite the low crystallinities, these formulations showed a high glass transition temperature, high storage moduli and low tan δ values. All of these studies revealed that the physicochemical and mechanical properties of LNG-IUSs were predominated by PDMS crosslinking. The observed differences in the thermal properties may have potential implications on drug release from LNG-IUSs. Correlations between the mechanical properties and the degree of PDMS crosslinking were established using three different techniques (durometer hardness tester, texture analyzer, and DMA). Accordingly, controlling the degree of crosslinking allows tuning of the mechanical properties of PDMS for the intended application.
The current research has improved the understanding of the influence of unintended variations in the mixing of PDMS prepolymers during the manufacturing of LNG-IUSs on its properties. This will aid in controlling the manufacturing process variability, ensure robustness, and facilitate generic drug product development. In addition, the present study will guide in developing other PDMS-based formulations with suitable properties by modulating the degree of polymer crosslinking.
Supplementary Material
Acknowledgements
Funding for this project was made possible by a U.S. Food and Drug Administration grant (1U01FD005443-01). This manuscript reflects the views of the authors and should not be construed to represent FDA’s views or policies.
Abbreviations:
- LNG
Levonorgestrel
- LARC
Long-acting reversible contraceptives
- IUS
Intrauterine system
- IUD
Intrauterine device
- LNG-IUS
Levonorgestrel intrauterine system
- PDMS
Polydimethylsiloxane
- FDA
Food and Drug Administration
- RLD
Reference listed drug
- Q1/Q2 equivalent
Qualitatively and quantitatively equivalent
- DSC
Differential scanning calorimetry
- TGA
Thermogravimetric analysis
- FTIR
Fourier-transform infrared spectroscopy
- NMR
Nuclear magnetic resonance
- DMA
Dynamic mechanical analysis
- Tg
Glass transition temperature
Footnotes
CRediT authorship contribution statement
Suraj Fanse: Conceptualization, Methodology, Validation, Investigation, Formal analysis, Data curation, Writing – original draft, Writing – review & editing. Quanying Bao: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing. Yuan Zou: Writing – review & editing, Supervision, Project administration. Yan Wang: Writing – review & editing, Project administration. Diane J. Burgess: Conceptualization, Writing – original draft, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpharm.2021.121192.
References
- Bao Q, Gu B, Price CF, Zou Y, Wang Y, Kozak D, Choi S, Burgess DJ, 2018. Manufacturing and characterization of long-acting levonorgestrel intrauterine systems. Int. J. Pharm. 550 (1–2), 447–454. 10.1016/j.ijpharm.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Q, Zou Y, Wang Y, Choi S, Burgess DJ, 2020. Impact of product design parameters on in vitro release from intrauterine systems. Int. J. Pharm. 578, 119135. 10.1016/j.ijpharm.2020.119135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Q, Zou Y, Wang Y, Kozak D, Choi S, Burgess DJ, 2019. Drug release testing of long-acting intrauterine systems. J. Control. Release 316, 349–358. 10.1016/j.jconrel.2019.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benagiano G, Gabelnick H, Farris M, 2008. Contraceptive devices: intravaginal and intrauterine delivery systems. Expert Rev. Med. Devices 5 (5), 639–654. 10.1586/17434440.5.5.639. [DOI] [PubMed] [Google Scholar]
- Blanco I, 2018. Polysiloxanes in Theranostics and Drug Delivery: A Review. Polymers 10, 755. 10.3390/polym10070755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd P, Fetherston SM, McCoy CF, Major I, Murphy DJ, Kumar S, Holt J, Brimer A, Blanda W, Devlin B, Malcolm RK, 2016. Matrix and reservoir-type multipurpose vaginal rings for controlled release of dapivirine and levonorgestrel. Int. J. Pharmac. 511, 619–629. 10.1016/j.ijpharm.2016.07.051. [DOI] [PubMed] [Google Scholar]
- Camenzind A, Schweizer T, Sztucki M, Pratsinis S, 2010. Structure and strength of silica-PDMS nanocomposites. Polymer 51, 1796–1804. 10.1016/j.polymer.2010.02.030. [DOI] [Google Scholar]
- Camino G, Lomakin SM, Lageard M, 2002. Thermal polydimethylsiloxane degradation. Part 2. The degradation mechanisms. Polymer 43 (7), 2011–2015. 10.1016/S0032-3861(01)00785-6. [DOI] [Google Scholar]
- Camino G, Lomakin SM, Lazzari M, 2001. Polydimethylsiloxane thermal degradation Part 1. Kinetic aspects. Polymer 42 (6), 2395–2402. [Google Scholar]
- Croxatt HB, 2002. Progestin implants for female contraception. Contraception 65, 15–19. 10.1016/S0032-3861(00)00652-2. [DOI] [PubMed] [Google Scholar]
- Diczfalusy E, 1979. Gregory Pincus and steroidal contraception: a new departure in the history of mankind. J. Steroid Biochem. 11 (1), 3–11. 10.1016/0022-4731(79)90271-1. [DOI] [PubMed] [Google Scholar]
- Dollase T, Wilhelm M, Spiess HW, Yagen Y, Yerushalmi-Rozen R, Gottlieb M, 2003. Effect of interfaces on the crystallization behavior of PDMS. Interface Sci. 11, 199–209. 10.1023/A:1022174712707. [DOI] [Google Scholar]
- Dyamenahalli K, Famili A, Shandas R, 2015. 3 – Characterization of shape-memory polymers for biomedical applications. In: Yahia LH (Ed.), Shape Memory Polymers for Biomedical Applications. Woodhead Publishing, pp. 35–63. 10.1016/B978-0-85709-698-2.00003-9. [DOI] [Google Scholar]
- FDA, 2021. FDA Orange Book https://www.accessdata.fda.gov/scripts/cder/ob/index.cfm (accessed on 04.30.21).
- Gong X, He S, 2020. Highly durable superhydrophobic polydimethylsiloxane/silica nanocomposite surfaces with good self-cleaning ability. ACS Omega 5 (8), 4100–4108. 10.1021/acsomega.9b0377510.1021/acsomega.9b03775.s001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JM, 2006. The nature of platinum in silicones for biomedical and healthcare use. Journal of biomedical materials research. Part B, Appl. Biomater. 78B (1), 167–180. 10.1002/(ISSN)1552-498110.1002/jbm.b.v78b:110.1002/jbm.b.30471. [DOI] [PubMed] [Google Scholar]
- Lukin RY, Kuchkaev AM, Sukhov AV, Bekmukhamedov GE, Yakhvarov DG, 2020. Platinum-catalyzed hydrosilylation in polymer chemistry. Polymers 12 (10), 2174. 10.3390/polym12102174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour D, 2012. The benefits and risks of using a levonorgestrel-releasing intrauterine system for contraception. Contraception 85 (3), 224–234. 10.1016/j.contraception.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Mashak A, Rahimi A, 2009. Silicone polymers in controlled drug delivery systems: a review. Iranian Polym. J. (Engl.) 18, 279–295. [Google Scholar]
- Menard KP, Menard NR, 2015. Dynamic mechanical analysis in the analysis of polymers and rubbers. Encycl. Polym. Sci. Technol. 1–33. 10.1002/0471440264.pst102.pub2. [DOI] [Google Scholar]
- Roland CM, Aronson CA, 2000. Crystallization of polydimethylsiloxane end-linked networks. Polym. Bull. 45 (4–5), 439–445. 10.1007/s002890070019. [DOI] [Google Scholar]
- Rowlands S, Searle S, 2014. Contraceptive implants: current perspectives. Open Access J. Contracept. 5, 73–84. 10.2147/OAJC.S55968. [DOI] [Google Scholar]
- Sabo S, Waters LJ, 2021. Poly(dimethylsiloxane): a sustainable human skin alternative for transdermal drug delivery prediction. J. Pharm. Sci. 110 (3), 1018–1024. 10.1016/j.xphs.2020.11.028. [DOI] [PubMed] [Google Scholar]
- Shoupe D, 2016. LARC methods: entering a new age of contraception and reproductive health. Contracept. Reprod. Med. 1, 4. 10.1186/s40834-016-0011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitruk-Ware R, Inki P, 2005. The levonorgestrel intrauterine system: long-term contraception and therapeutic effects. Women’s Health (London, England) 1, 171–182. [DOI] [PubMed] [Google Scholar]
- Sitruk-Ware R, Nath A, Mishell DR Jr., 2013. Contraception technology: past, present and future. Contraception 87, 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamareselvy K, Rueggeberg FA, 1994. Dynamic mechanical analysis of two crosslinked copolymer systems. Dent. Mater. 10 (5), 290–297. 10.1016/0109-5641(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Tan HS, Pfister WR, 1999. Pressure-sensitive adhesives for transdermal drug delivery systems. Pharm. Sci. Technol. Today 2 (2), 60–69. 10.1016/S1461-5347(99)00119-4. [DOI] [PubMed] [Google Scholar]
- Tolinski M, 2009. Chapter 15 – Crosslinking. In: Tolinski M (Ed.), Additives for Polyolefins. William Andrew Publishing, Oxford, pp. 215–220. 10.1016/B978-0-323-35884-2.00015-6. [DOI] [Google Scholar]
- Welsh NR, Malcolm RK, Devlin B, Boyd P, 2019. Dapivirine-releasing vaginal rings produced by plastic freeforming additive manufacturing. Int. J. Pharm. 572, 118725. 10.1016/j.ijpharm.2019.118725. [DOI] [PubMed] [Google Scholar]
- Winner B, Peipert JF, Zhao Q, Buckel C, Madden T, Allsworth JE, Secura GM, 2012. Effectiveness of long-acting reversible contraception. New Engl. J. Med. 366, 1998–2007. 10.1056/NEJMoa1110855. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


