Abstract
Background
Immunosuppressive treatments inhibit vaccine-induced immunity against SARS-CoV-2. We evaluated whether a 2-week interruption of methotrexate treatment immediately after the COVID-19 vaccine booster improved antibody responses against the S1 receptor-binding domain (S1-RBD) of the SARS-CoV-2 spike protein compared with uninterrupted treatment in patients with immune-mediated inflammatory diseases.
Methods
We did an open-label, prospective, two-arm, parallel-group, multicentre, randomised, controlled, superiority trial in 26 hospitals in the UK. We recruited adults from rheumatology and dermatology clinics who had been diagnosed with an immune-mediated inflammatory disease (eg, rheumatoid arthritis, psoriasis with or without arthritis, axial spondyloarthritis, atopic dermatitis, polymyalgia rheumatica, and systemic lupus erythematosus) and who were taking low-dose weekly methotrexate (≤25 mg per week) for at least 3 months. Participants also had to have received two primary vaccine doses from the UK COVID-19 vaccination programme. We randomly assigned the participants (1:1), using a centralised validated computer randomisation program, to suspend methotrexate treatment for 2 weeks immediately after their COVID-19 booster (suspend methotrexate group) or to continue treatment as usual (continue methotrexate group). Participants, investigators, clinical research staff, and data analysts were unmasked, while researchers doing the laboratory analyses were masked to group assignment. The primary outcome was S1-RBD antibody titres 4 weeks after receiving the COVID-19 booster vaccine dose, assessed in the intention-to-treat population. This trial is registered with ISRCT, ISRCTN11442263; following the pre-planned interim analysis, recruitment was stopped early.
Findings
Between Sept 30, 2021 and March 3, 2022, we recruited 340 participants, of whom 254 were included in the interim analysis and had been randomly assigned to one of the two groups: 127 in the continue methotrexate group and 127 in the suspend methotrexate group. Their mean age was 59·1 years, 155 (61%) were female, 130 (51%) had rheumatoid arthritis, and 86 (34%) had psoriasis with or without arthritis. After 4 weeks, the geometric mean S1-RBD antibody titre was 22 750 U/mL (95% CI 19 314–26 796) in the suspend methotrexate group and 10 798 U/mL (8970–12 997) in the continue methotrexate group, with a geometric mean ratio (GMR) of 2·19 (95% CI 1·57–3·04; p<0·0001; mixed-effects model). The increased antibody response in the suspend methotrexate group was consistent across methotrexate dose, administration route, type of immune-mediated inflammatory disease, age, primary vaccination platform, and history of SARS-CoV-2 infection. There were no intervention-related serious adverse events.
Interpretation
A 2-week interruption of methotrexate treatment for people with immune-mediated inflammatory diseases resulted in enhanced boosting of antibody responses after COVID-19 vaccination. This intervention is simple, low-cost, and easy to implement, and could potentially translate to increased vaccine efficacy and duration of protection for susceptible groups.
Funding
National Institute for Health and Care Research.
Introduction
A key challenge at this stage in the COVID-19 pandemic is to improve the vaccine-induced immunity of immunosuppressed individuals. Methotrexate, the most commonly prescribed disease-modifying anti-rheumatic drug, is the first-line treatment for rheumatic diseases such as rheumatoid arthritis and is often the first-line systemic therapy for skin diseases such as psoriasis.1, 2 The broad immunosuppressive effects of methotrexate attenuate the vaccine-induced response against COVID-19.3, 4, 5, 6, 7, 8 Interrupting methotrexate treatment for 2 weeks after vaccination against seasonal influenza resulted in enhanced immunity to vaccination in patients with rheumatoid arthritis, with no effect of interrupting treatment for up to 4 weeks before the vaccination, in a study done in South Korea. However, this study might have limited generalisability to other conditions and for people in other parts of the world.9, 10
Research in context.
Evidence before this study
Methotrexate impairs COVID-19 vaccine-induced immunity. We searched PubMed for randomised controlled trials published between database inception and April 12, 2022, using the terms ([methotrexate] AND [vaccin*] AND [influenza OR covid-19 OR SARS-CoV-2]) AND (Therapy/Broad[filter]) with no language restrictions, to identify trials that evaluated the impact of interrupting methotrexate treatment around vaccination on vaccine responses. We identified two published reports of clinical trials in South Korea, evaluating 4-week and 2-week interruptions in methotrexate treatment around the time of vaccination for seasonal influenza. These trials showed that interrupting methotrexate treatment for 2 weeks after vaccination against seasonal influenza resulted in enhanced vaccine immunity, but there was no effect on vaccine-induced immunity of interrupting treatment for up to 4 weeks before vaccination. A 4-week treatment interruption after vaccination did not result in any further improvement in vaccine response compared with a 2-week interruption. We also identified a small (n=92), single-centre, tertiary hospital-based trial conducted in Mexico and limited to patients with well controlled rheumatoid arthritis without previous SARS-CoV-2 infection. The authors reported that a 2-week methotrexate interruption after each of the two doses of the CoronaVac vaccine (Sinovac Biotech) improved the S1-RBD antibody response. However, the study was at high risk of bias due to exclusion of participants after randomisation for previous SARS-CoV-2 infection and disease flare-up, a 33% dropout rate, and twice as many dropouts in the suspend methotrexate group than in the continue treatment group. CoronaVac elicits less immunity than mRNA-based and adenoviral platforms, and results derived from this study cannot be extrapolated to inform health policy in countries using different vaccine platforms.
Added value of this study
To our knowledge, this study was the first randomised trial of interrupting methotrexate treatment around the time of vaccination with additional COVID-19 vaccine doses in people that had received at least two previous vaccinations against COVID-19. The study showed that a 2-week interruption of methotrexate treatment immediately after COVID-19 booster vaccination resulted in a 2·19-fold increase in the S1-RBD antibody response after 4 weeks. The enhanced antibody response was maintained at 12 weeks. The treatment effect was present across groups of varying ages, methotrexate doses, diseases, and history of SARS-CoV-2 infection. Interrupting methotrexate for 2 weeks did not affect quality of life or general health. There was a temporary deterioration in self-reported disease activity and disease control at 4 weeks that resolved by week 12. More participants in the suspend methotrexate group than in the continue methotrexate group self-reported disease flare-up in the first 4 weeks, but most self-managed with no appreciable difference in seeking health-care input for flares across the two groups.
Implications of all the available evidence
At this stage in the COVID-19 vaccination programme, it is important to optimise durable vaccine protection in those who are potentially susceptible through immune suppression. Evidence from this study will be useful for policy makers, national immunisation advisory committees, and specialist societies formulating recommendations on the use of methotrexate around the time of COVID-19 vaccination. This evidence will help patients and clinicians make informed choices about the risks and benefits of interrupting methotrexate treatment around the time of COVID-19 vaccination, with implications for the potential to extend such approaches to other therapeutics.
A 2-week interruption strategy immediately after vaccination against COVID-19 has not been formally evaluated. Consequently, there is conflicting advice on whether to continue or interrupt methotrexate treatment after COVID-19 vaccination.11, 12, 13 Understanding the effectiveness and safety of this simple intervention would provide valuable and timely guidance to achieve enhanced, durable immunity following COVID-19 vaccination in this clinically susceptible population, informing clinical practice and public health policy when additional vaccinations are being considered globally. This understanding is especially important given the high impact that the COVID-19 pandemic has had on the physical, social, and psychological wellbeing of this patient group, the current high transmission rates of SARS-CoV-2 worldwide, and the reduced vaccine efficacy against the omicron (BA.1.1.529) and BA.2 variants due to attenuated cross-reactive neutralising antibody potency.14 We analysed the impact of a 2-week interruption in methotrexate treatment after COVID-19 booster vaccination on antibody responses against the receptor-binding domain of the SARS-CoV-2 spike protein (S1-RBD) in adults with immune-mediated inflammatory disease. We hypothesised that a 2-week treatment interruption would enhance immunity following vaccination, without substantial deterioration of disease activity.
Methods
Study design and participants
The Vaccine Response On/Off Methotrexate (VROOM) study15 was an open-label, prospective, two-arm, parallel-group, multicentre, randomised, controlled, superiority trial. Participants were recruited from rheumatology and dermatology clinics in 26 National Health Service (NHS) hospitals in the UK. The study was approved by Leeds West Research Ethics Committee and Health Research Authority (REC Reference: 21/HRA/3483, IRAS: 303827). The study protocol is available online.
To be eligible, participants had to be aged 18 years and older and diagnosed with an immune-mediated inflammatory disease (eg, rheumatoid arthritis, psoriasis with or without arthritis, axial spondyloarthritis, atopic dermatitis, polymyalgia rheumatica, and systemic lupus erythematosus), who had been prescribed methotrexate (≤25 mg/week oral or subcutaneous injection) for at least the previous 3 months, with or without hydroxychloroquine. Participants also had to be able to suspend methotrexate treatment for 2 weeks with the approval of their hospital team, have received two primary vaccine doses from the UK COVID-19 vaccination programme, and be eligible for an additional vaccine dose after Sept 14, 2021.
Exclusion criteria included immune-mediated inflammatory diseases for which treatment cannot be interrupted safely; recent (ie, within 18 months) or planned rituximab infusion; use of glucocorticoid-sparing drugs other than methotrexate in the past 2 months; use of prednisolone of more than 7·5 mg/day within the previous month; radiotherapy or cancer chemotherapy in the previous 6 months; and visceral cancer (see the protocol for full eligibility criteria). Written informed consent was obtained from all participants.
Randomisation and masking
Participants were randomised using a centralised validated computer randomisation program through a secure (encrypted) web-based service provided by the Oxford Clinical Trials Research Unit. The randomisation system used a minimisation algorithm to ensure balanced allocation across treatment groups, and a 1:1 ratio to allocate to either suspend methotrexate use for 2 weeks (suspend methotrexate group) or continue treatment as usual (continue methotrexate group). The trial used immune-mediated inflammatory disease type (rheumatic disease with or without skin disease or skin disease alone); age group (<40 years, 40–64 years, ≥65 years); and primary vaccination technology (mRNA, vector, or combination) as minimisation factors. The minimisation factors were chosen to balance immune-mediated inflammatory diseases and key prognostic factors that affect the response to the COVID-19 vaccine across the trial groups.16, 17, 18 Previous SARS-CoV-2 infection was not controlled for despite it being a strong modifier of serological response to COVID-19 vaccination17, 18 due to the unreliability of self-reporting, particularly at the start of the pandemic when diagnostic PCR testing was not widely available. Previous SARS-CoV-2 infection status was established by measuring N-serology at baseline and used in the statistical analysis.
The allocation sequence was generated using minimisation with a probabilistic element (following initial simple randomisation seeding) accounting for age, disease type, and primary COVID-19 vaccination type. Participants were enrolled by research staff at sites and were randomised via an online randomisation interface. Research staff at sites carried out the research visits. Participants, investigators, clinical research staff, and data analysts were unmasked to the group assignment given the nature of the study. Laboratory analyses were performed masked to group allocation.
Procedures
The VROOM study evaluated temporarily suspending versus continuing methotrexate treatment immediately after the COVID-19 vaccine booster (predominantly full-dose BNT162b2 [Pfizer–BioNTech], half-dose [50 μg] or full-dose [100 μg] mRNA-1273 [Moderna]; and AZD1222 [AstraZeneca–Oxford University]) delivered through the UK's COVID-19 vaccination programme.
For the suspend methotrexate group, methotrexate dosing was interrupted for 2 weeks immediately after receiving the COVID-19 vaccine. Participants who were vaccinated on the day on which they usually took their weekly methotrexate treatment were asked to miss methotrexate on the day of vaccination and another dose 1 week later; for all others, the advice was to suspend the weekly methotrexate doses for 2 weeks immediately after vaccination. For the continue methotrexate group, methotrexate was continued at the same dose after receiving the COVID-19 vaccine.
Any concomitant treatment (eg, folic acid or hydroxychloroquine) was continued and disease flare-ups treated by standard clinical care during the study period. Participants could also stop or take methotrexate against trial allocation if clinically indicated, for example, if there was an intercurrent infection or disease flare-up. Participants were assessed 4 and 12 weeks after the date of the booster vaccination.
Outcomes
The primary outcome was S1-RBD antibody titres, as measured at the UK Health Security Agency19 with the fully quantitative Elecsys immunoassay (Roche Diagnostics; Basel, Switzerland) 4 weeks after receiving the COVID-19 booster vaccine dose. Secondary outcomes were S1-RBD antibody titres 12 weeks after the COVID-19 vaccine booster dose; self-reported disease activity at weeks 2, 4, and 12, with a 1-week recall on an 11-point (0–10) numeric rating scale (higher scores reflecting better general health); self-reported disease flare-up and actions taken to manage them; quality of life using the EQ-5D-5L questionnaire;20 self-reported 5-point ordinal patient global assessment of disease activity, ranging from none or inactive to very severe activity with a 1-week recall; disease control since vaccination using a 5-point ordinal scale, ranging from much better to much worse at weeks 4 and 12; adherence with trial allocation; and serious adverse events.
S1-RBD antibody titres were chosen as the primary outcome as S1-RBD antibody binding correlates with the half-maximal inhibitory concentration (IC50) of neutralisation antibody against the ancestral Wuhan Hu-1 live virus and is considered a correlate of protection from COVID-19.17, 18, 21, 22, 23 Additionally, it can be measured rapidly in a large number of participants.
Statistical analysis
Statistical analyses were based on the randomised population (intention to treat), unless otherwise stated (ie, per protocol). The study was powered to detect an antibody response that was at least 25% lower in the continue methotrexate group (Cohen's d effect 0·29) with 90% statistical power at a two-sided 5% significance level. Using S1-RBD antibody response elicited by a third dose of COVID-19 vaccine,18, 24 this effect size translates to a target difference in S1-RBD antibody titres of about 5000 U/mL.
Antibody data were log-transformed (base 10) to normalise the distribution before analysis. The difference in S1-RBD antibody titres between study groups at weeks 4 and 12 was estimated using a multilevel mixed-effects regression model, allowing for repeated measures clustered within participants. The model was adjusted for minimisation factors, with previous SARS-CoV-2 infection assessed using N-serology and COVID-19 vaccine technology received as a booster dose as fixed effects. A treatment-by-time interaction was included. Adjusted geometric mean ratios (GMRs) between the groups are presented, together with 95% CIs and p values without correcting for the interim analysis given the stopping guideline used.
Consistency of treatment effect for prognostic subgroups (ie, age, rheumatic and skin disease, methotrexate dose, administration route, primary vaccination platform, and previous SARS-CoV-2 infection status) was explored at the 4-week and 12-week outcome timepoints using treatment-by-subgroup interactions. The effect of non-compliance with the randomly assigned intervention was explored using the per-protocol population but, given the very high level of compliance, a complier-average causal effects analysis was not carried out as originally planned. Similarly planned analyses to assess the impact of missing data were not carried out due to the very few missing data on S1-RBD antibody titres. Two additional analyses not envisioned in the statistical analysis plan were done and are indicated as post-hoc analyses; these analyses assessed sensitivity to adjustment for booster type and methotrexate dose in the S1-RBD mixed-effects model. Other secondary outcomes were analysed using generalised linear models for binary and continuous data, as appropriate, with model adjustment as described above. The number and details of serious adverse events were presented by treatment group.
A pre-planned interim analysis was performed when baseline and week 4 S1-RBD antibody titres were available for at least 250 participants. On the basis of the strong evidence of efficacy with a large effect size meeting the pre-specified Haybittle-Peto stopping boundary (p≤0·0001 for the primary endpoint) and enrolment of a representative study population and sufficient participant recruitment into prognostic subgroups, the independent Data Monitoring and Trial Steering Committees recommended to stop recruitment and complete the follow-up of existing participants. Thus, the study stopped recruiting on March 8, 2022. In this Article, we present the results of the analyses of the primary outcome and key secondary outcomes, including 12-week S1-RBD antibody responses and disease outcomes of those included in the interim analysis. Data on neutralising antibodies and methotrexate bioassay will be published after the ongoing laboratory analyses are completed. Data were analysed using Stata 17.0. This trial is registered with ISRCT, ISRCTN11442263.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, writing of the report, or the decision to submit for publication.
Results
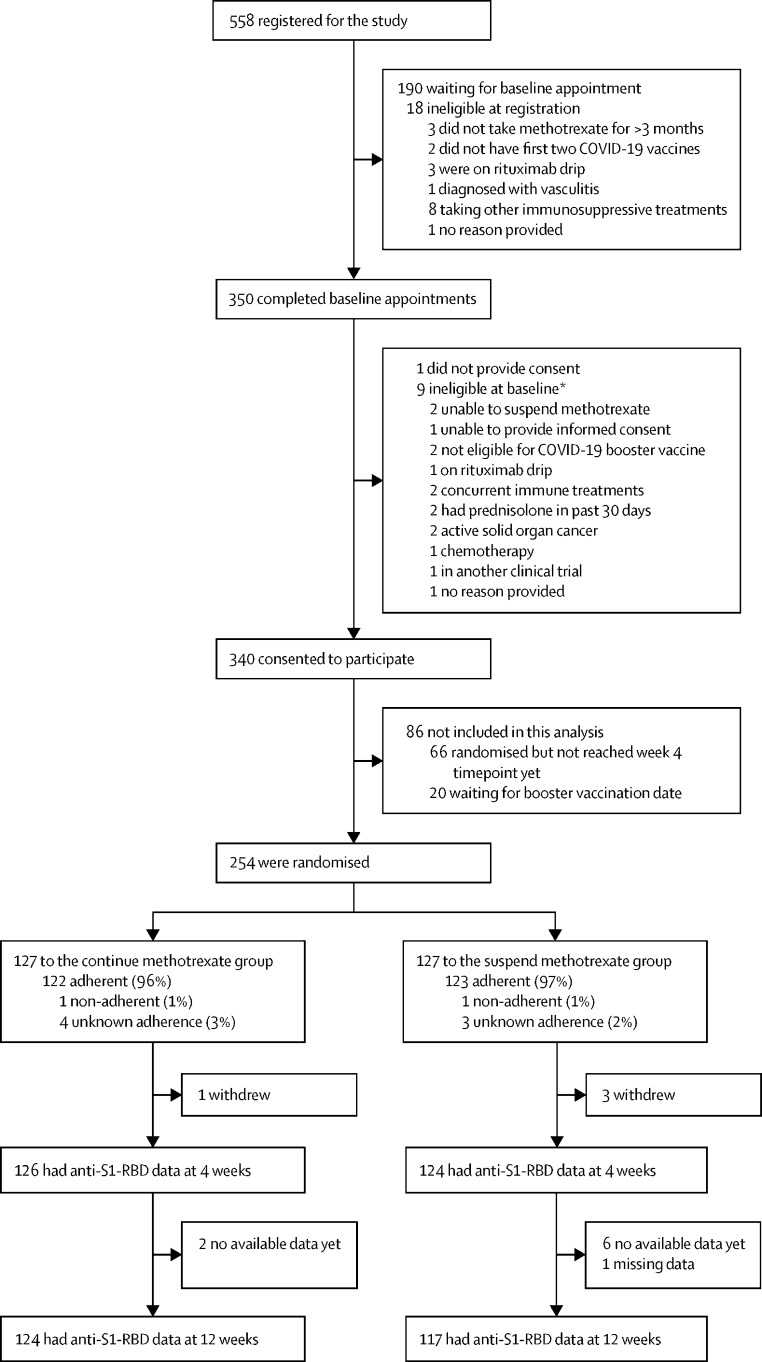
Between Sept 30, 2021 and March 3, 2022, we recruited 340 participants, of whom 254 were included in the interim analysis and randomly assigned to one of the two groups: 127 in the continue methotrexate group and 127 in the suspend methotrexate group (figure 1 ). Three participants in the suspend methotrexate group and one participant in the continue methotrexate group withdrew consent before their 4-week visit (appendix p 2). The baseline characteristics of participants were well balanced between the groups (table 1 ). The cohort mean age was 59·1 years and mean body-mass index was 29·6 kg/m2. 155 (61%) participants were female, 130 (51%) had rheumatoid arthritis, 86 (34%) had psoriasis with or without arthritis, and 51 (20%) had a skin condition alone. The median methotrexate dose was 20 mg/week. 239 (94%) received an mRNA vaccine for their booster, after a mean of 192·7 days from the second dose of the primary vaccination.
Figure 1.
Trial profile
Anti-S1-RBD=antibody for S1 subunit of SARS-CoV-2 spike protein receptor-binding domain. *Some patients had more than one stated reason for ineligibility.
Table 1.
Baseline characteristics
| Continue methotrexate (n=127) | Suspend methotrexate (n=127) | ||
|---|---|---|---|
| Age, years | 59·2 (11·0) | 59·0 (11·9) | |
| <40 years | 8 (6%) | 8 (6%) | |
| 40–64 years | 73 (57%) | 73 (57%) | |
| ≥65 years | 46 (36%) | 46 (36%) | |
| Sex | |||
| Male | 47 (37%) | 52 (41%) | |
| Female | 80 (63%) | 75 (59%) | |
| BMI, kg/m2 | 29·0 (6·5) | 30·3 (5·6) | |
| Ethnicity | |||
| White | 120 (94%) | 118 (93%) | |
| Other | 7 (6%) | 8 (6%) | |
| Missing data | 0 | 1 (1%) | |
| Smoking status | |||
| Never smoked | 67 (53%) | 68 (54%) | |
| Ex-smoker | 46 (36%) | 49 (39%) | |
| Current smoker | 14 (11%) | 10 (8%) | |
| Serum creatinine concentration, μmol/L | 71·7 (14·8) | 75·8 (13·2) | |
| Type of immune-mediated inflammatory disease | |||
| Rheumatic disease (with or without skin disease) | 101 (80%) | 102 (80%) | |
| Skin disease alone | 26 (20%) | 25 (20%) | |
| Immune-mediated inflammatory disease* | |||
| Rheumatoid arthritis | 68 (54%) | 62 (49%) | |
| Psoriasis with arthritis | 24 (19%) | 28 (22%) | |
| Psoriasis without arthritis | 15 (12%) | 19 (15%) | |
| Seronegative (axial) spondyloarthritis | 3 (2%) | 1 (1%) | |
| Reactive arthritis | 0 | 0 | |
| Atopic eczema | 6 (5%) | 6 (5%) | |
| Polymyalgia rheumatica | 2 (2%) | 3 (2%) | |
| Systemic lupus erythematosus | 2 (2%) | 0 | |
| Other rheumatic disease | 6 (5%) | 9 (7%) | |
| Other skin disease | 6 (5%) | 3 (2%) | |
| Methotrexate use | |||
| Weekly dose ≤5 mg | 55 (43%) | 51 (40%) | |
| Weekly dose >15 mg | 72 (57%) | 76 (60%) | |
| Median dose | 20 (15–20) | 20 (15–20) | |
| Oral administration | 79 (62%) | 75 (59%) | |
| Subcutaneous administration | 48 (38%) | 52 (41%) | |
| Folic acid supplementation | |||
| Yes | 124 (98%) | 125 (98%) | |
| No | 2 (2%) | 2 (2%) | |
| Missing data | 1 (1%) | 0 | |
| Hydroxychloroquine | |||
| Yes | 21 (17%) | 26 (20%) | |
| No | 106 (83%) | 101 (80%) | |
| Median dose | 200 (200–200) | 200 (200–400) | |
| Concomitant systemic medications* | |||
| Non-steroidal anti-inflammatory drugs | 22 (17%) | 23 (18%) | |
| Oral glucocorticoid | 3 (2%) | 2 (2%) | |
| Insulin | 3 (2%) | 1 (1%) | |
| Missing data | 1 (1%) | 0 | |
| Parenteral glucocorticoid in past 3 months | |||
| Intra-articular glucocorticoid | 0 | 2 (2%) | |
| Intramuscular glucocorticoid | 1 (1%) | 3 (2%) | |
| None | 125 (98%) | 122 (96%) | |
| Missing data | 1 (1%) | 0 | |
| Current use of topical glucocorticoid | |||
| Yes | 17 (13%) | 20 (16%) | |
| No | 110 (87%) | 107 (84%) | |
| Comorbidities* | |||
| Diabetes | 16 (13%) | 10 (8%) | |
| Hypertension | 30 (24%) | 28 (22%) | |
| Ischaemic heart disease | 1 (1%) | 3 (2%) | |
| Congestive cardiac failure | 0 | 0 | |
| Asthma | 18 (14%) | 16 (13%) | |
| Chronic obstructive pulmonary disease | 3 (2%) | 5 (4%) | |
| High cholesterol | 12 (9%) | 16 (13%) | |
| Stroke (including transient ischaemic attack) | 2 (2%) | 3 (2%) | |
| None of the above | 71 (56%) | 77 (61%) | |
| Primary COVID-19 vaccination type | |||
| mRNA (BNT162b2 or mRNA-1273) | 42 (33%) | 43 (34%) | |
| Viral vector (AZD1222) | 85 (67%) | 82 (65%) | |
| Combination | 0 | 2 (2%) | |
| Days between two doses | 76 (11·0) | 76 (12·0) | |
| COVID-19 disease history | |||
| COVID-19 disease leading to hospitalisation | 1 (1%) | 3 (2%) | |
| COVID-19 disease without hospitalisation | 16 (13%) | 21 (17%) | |
| No COVID-19 disease | 110 (87%) | 103 (81%) | |
| COVID-19 booster vaccine | |||
| BNT162b2 | 106 (83%) | 102 (80%) | |
| AZD1222 | 6 (5%) | 4 (3%) | |
| mRNA-1273 | 13 (10%) | 18 (14%) | |
| None | 2 (2%) | 3 (2%) | |
| Booster as 3rd dose† | 124 (99%) | 124 (100%) | |
| Booster as 4th dose† | 1 (1%) | 0 | |
| Days between previous vaccination and booster | 189·9 (31·0) | 195·4 (22·1) | |
Data are n (%), mean (SD), or median (IQR).
Participants can have more than one category.
Percentages are of those who received a booster vaccine.
Adherence to the trial regimen was high, with 123 (97%) self-reported compliance in the suspend methotrexate group and 122 (96%) in the continue methotrexate group. One participant in each of the trial groups was partially compliant with trial allocation (taking one weekly dose during the 2-week period) and compliance data were missing for seven participants (appendix p 2). Participants were not excluded from the main analysis for non-compliance. Six participants had one protocol deviation each in terms of attending study visits outside the planned window for week 4 or week 12, or both (figure 1; appendix p 1).
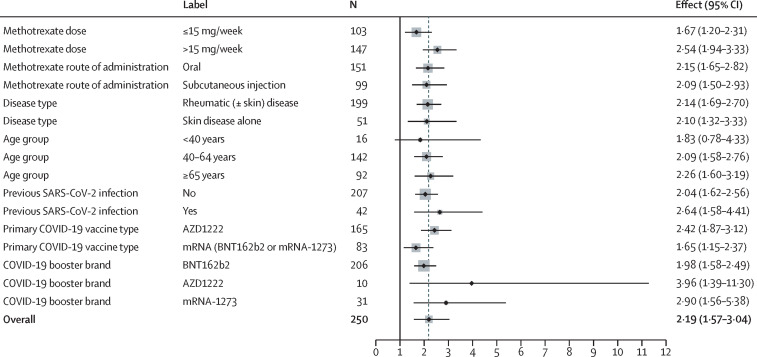
The S1-RBD antibody response was higher in the suspend methotrexate group than in the continue methotrexate group at week 4 (geometric mean titre [GMT] 22 750 U/mL [95% CI 19 314–26 796] vs 10 798 U/mL [8970–12 997]). In a mixed-effect model adjusted for baseline value, randomisation factors, previous SARS-CoV-2 infection, and booster vaccine platform used, the GMR of S1-RBD antibody responses between the two groups was 2·19 (95% CI 1·57–3·04; p<0·0001; table 2 ). Sensitivity analyses and pre-planned exploratory subgroup analyses (figure 2 ; appendix pp 3–7) revealed consistent treatment effects in the per-protocol population, with modification to variable adjustment, and across methotrexate dose, administration route, rheumatic and skin disease, age, primary vaccination platform, and previous SARS-CoV-2 infection status.
Table 2.
Key primary and secondary outcomes at week 4 and 12
| Continue methotrexate (n=127) | Suspend methotrexate (n=127) | Mixed-effects model | ||
|---|---|---|---|---|
| Anti-S1-RBD titres, U/mL | ||||
| Absolute titres (mean [SD]) | ||||
| Baseline | 3448 (11649; n=125) | 4011 (18 325; n=124) | .. | |
| 4 weeks | 17 682 (20 872; n=126) | 34 556 (38 323; n=124) | .. | |
| 12 weeks | 14 060 (14 698; n=124) | 27 407 (35 665; n=117) | .. | |
| Log10 values, U/mL (geometric mean titre [95% CI]) | ||||
| Baseline | 546 (394 to 757; n=125) | 530 (385 to 729; n=124) | .. | |
| 4 weeks | 10 798 (8970 to 12 997; n=126) | 22 750 (19 314 to 26 796; n=124) | GMR 2·19 (95% CI 1·57 to 3·04; p<0·0001)* | |
| 12 weeks | 8094 (6587 to 9946; n=124) | 16 520 (13 787 to 19 794; n=117) | GMR 2·11 (95% CI 1·51 to 2·94; p<0·0001)* | |
| EQ-5D-5L utility (mean [SD]) | ||||
| Baseline | 0·81 (0·17; n=127) | 0·77 (0·20; n=126) | .. | |
| 4 weeks | 0·79 (0·17; n=124) | 0·75 (0·20; n=122) | Mean difference −0·006 (95% CI −0·039 to 028) | |
| 12 weeks | 0·78 (0·19; n=125) | 0·75 (0·21; n=120) | Mean difference −0·005 (95% CI −0·038 to 0·029) | |
| EQ-VAS (mean [SD]) | ||||
| Baseline | 79·3 (16·5; n=127) | 77·4 (16·1; n=127) | .. | |
| 4 weeks | 79·0 (14·1; n=124) | 73·8 (19·3; n=122) | Mean difference −4·26 (95% CI −8·10 to −0·42) | |
| 12 weeks | 75·6 (18·5; n=125) | 71·2 (20·5; n=119) | Mean difference −4·08 (95% CI −7·93 to −0·24) | |
| Disease impact (general health; mean [SD]) | ||||
| Baseline | 8·00 (1·91; n=127) | 7·38 (2·01; n=127) | .. | |
| 2 weeks | 7·37 (1·78; n=123) | 6·97 (2·00; n=124) | Mean difference 0·06 (95% CI −0·40 to 0·52) | |
| 4 weeks | 7·61 (1·90; n=120) | 6·97 (2·11; n=118) | Mean difference −0·17 (95% CI −0·63 to 0·29) | |
| 12 weeks | 7·33 (2·02; n=124) | 6·93 (2·10; n=120) | Mean difference 0·09 (95% CI −0·36 to 0·55) | |
| Experienced ≥1 flare-up | ||||
| 0–4 weeks | 38/124 (31%) | 69/123 (56%) | OR 3·10 (95% CI 1·78 to 5·40)† | |
| 0–12 weeks | 56/125 (45%) | 85/120 (71%) | OR 2·83 (95% CI 1·64 to 4·88)† | |
Logistic regression models at 4 and 12 weeks were adjusted by baseline value, randomisation factors (ie, age, inflammatory condition, vaccine platform), previous infection, and booster platform. Mixed-effects model were adjusted by baseline value, randomisation factors (ie, age, inflammatory condition, vaccine platform), previous infection, and booster platform (main analysis), and include a treatment-by-time interaction. Missing information on booster vaccine received by three participants means total numbers in the model are lower at 4 weeks. GMR=geometric mean ratio. OR=odds ratio. S1-RBD=S1 subunit of spike protein in receptor-binding domain.
Main analysis.
Calculated using a logistic regression model; a flare-up was counted if it was reported at either 4 or 12 weeks for the 0–12 weeks outcome.
Figure 2.
Treatment effects (geometric mean ratio) for different subgroups at 4 weeks
Vertical black line indicates no effect. Dotted grey line indicates the overall effect.
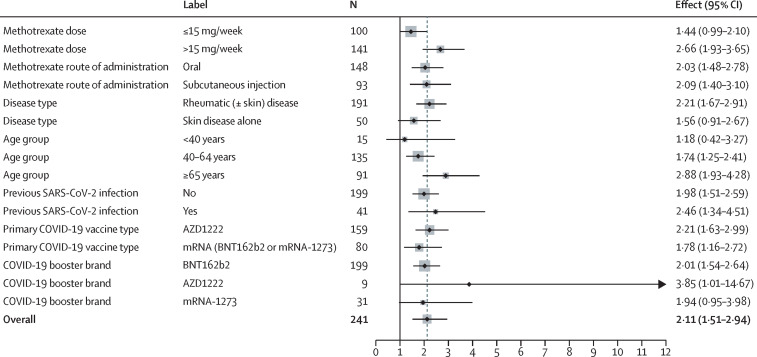
S1-RBD antibody titres remained significantly higher in the suspend methotrexate group than in the continue methotrexate group at week 12 (GMT 16 520 U/mL [95% CI 13 787–19 794] vs 8094 U/mL [6587–9946]; table 2). In a mixed-effect model, the GMR for S1-RBD antibody response between the two groups was 2·11 (95% CI 1·51–2·94; p<0·0001). At week 12, subgroup results were similar except for methotrexate dose (figure 3 ; appendix pp 4–7), which indicated a differential treatment effect (interaction GMR effect 1·85 [1·12–3·04]).
Figure 3.
Treatment effects (geometric mean ratio) for different subgroups at 12 weeks
Vertical black line indicates no effect. Dotted grey line indicates the overall effect.
Self-reported general health due to disease and EQ-5D-5L utility values were similar between the two groups at all timepoints with no significant differences (table 2). Self-reported disease activity and disease control since vaccination were significantly worse at 4 weeks in the suspend methotrexate group than in the continue methotrexate group but were similar in the two groups by week 12 (appendix pp 8–9). EQ-VAS scores were slightly lower in the suspend methotrexate group than in the continue methotrexate group.
Significantly more participants self-reported at least one disease flare in the suspend methotrexate group than in the continue methotrexate group over the 12-week follow-up period (85 [71%] of 120 vs 56 [45%] of 125; odds ratio 2·83 [95% CI 1·64–4·88]). The number of participants that self-reported at least one disease flare was higher in the suspend methotrexate group than in the continue methotrexate group between weeks 0 to 4 (69 [56%] of 123 vs 38 [31%] of 124) and between weeks 5 to 12 (68 [57%] of 120 vs 46 [37%] of 125). Most flares were self-managed with only a small proportion of participants (17 [14%] in the suspend methotrexate group vs 14 [11%] in the continue methotrexate group) seeking medical or specialist-nurse help for flare management over the 12 weeks (appendix 10–12). In addition, over the 12 weeks, a similar number of participants in the two groups self-reported using non-steroidal anti-inflammatory drugs or analgesics for managing disease flare-ups (60 [50%] in the suspend methotrexate group vs 58 [46%] in the continue methotrexate group). However, more participants who suspended methotrexate self-reported using glucocorticoids (21 [18%] vs 15 [12%]) and topical treatments for skin diseases (38 [32%] vs 28 [22%]) for managing disease flare-ups than those who continued methotrexate as usual over the 12-week period. There were no intervention-related serious adverse events, and no participants died during follow-up (appendix p 10).
Discussion
A 2-week interruption of methotrexate treatment immediately after COVID-19 vaccination with an booster vaccine dose resulted in a 2·19-fold increase in the S1-RBD antibody response at 4 weeks. This enhanced antibody response was maintained at 12 weeks. Of interest, the S1-RBD antibody titre in the suspend methotrexate group at 12 weeks was greater than that in the continue methotrexate group at 4 weeks. The treatment effect was present across a range of prognostic factors. High compliance with the intervention indicated patient acceptability. Interrupting methotrexate for 2 weeks did not impact quality of life or general health. A temporary deterioration of self-reported disease activity and self-reported disease control at week 4 was apparent, with resolution by week 12. More participants in the suspend methotrexate group self-reported disease flare-up in the first 4 weeks, but most self-managed with no appreciable difference in seeking health-care input for flares across the two groups.
Immunosuppression attenuates immunity following vaccination against COVID-198 and antibody waning results in reduced vaccine efficacy, particularly against SARS-CoV-2 variants.25, 26 The first dose of the BNT162b2 mRNA COVID-19 vaccine seroconverted 47–72% of patients treated with low-dose weekly methotrexate, and although this increased to 87–100% with the second dose,4, 5, 6, 7, 27 the S1-RBD antibody titres were about half of that observed in healthy controls even after two doses of the BNT162b2 mRNA COVID-19 vaccine.4 Booster vaccination against COVID-19 is a single vaccine dose, and the reduced vaccine response from it might potentially reduce the vaccine efficacy. Strategies to boost vaccine response are urgently needed to facilitate optimal benefit from vaccination in terms of longevity of protection and protection against current and future variants of concern (VOCs). The current vaccines were designed using the spike sequence from Wuhan Hu-1, and so have reduced efficacy with diminished immune responses against VOCs.14
Most patients in the VROOM study received prime doses of AZ1222 and were boosted with BNT162b2. The S1-RBD antibody responses in the suspend methotrexate group at 4 weeks in this study were similar to the S1-RBD antibody responses at 4 weeks in participants primed with the AZ1222 vaccine and boosted with a full dose of the BNT162b2 vaccine in the COV-BOOST trial (GMT 22 750 U/mL in the VROOM study vs 20 517 U/mL in the COV-BOOST trial),28 thus showing similar S1-RBD vaccine responses following the intervention of a 2-week break in methotrexate treatment.
A 2-week break in methotrexate treatment immediately after vaccination is a simple, low-cost, easy-to-implement, and effective intervention, which could potentially translate to greater vaccine efficacy and longer duration of protection for vulnerable groups. However, there was an associated increased risk of disease flare-up in the initial 4 weeks, which was mostly self-managed. Clinicians and patients will have to balance the possible risk of flare-ups versus the benefit of enhanced protection against COVID-19. The decision to suspend methotrexate treatment in the context of COVID-19 vaccination would be best made following an informed discussion between patient and clinician, taking into account patient preference, disease stability, and previous experience with treatment interruptions.
The evidence from this study will be useful for policy makers, such as the Joint Committee on Vaccination and Immunisation (JCVI) in the UK, similar bodies around the world, and specialist societies, in formulating recommendations on the use of long-term immunosuppression around the time of COVID-19 vaccination. Neutralising antibody IC50 is considered a correlate of protection against COVID-19.21, 22, 23 There is a strong positive correlation between the ancestral Wuhan Hu-1 live virus neutralising antibody IC50 and S1-RBD antibody binding (r=0·6–0·8), and early studies, which pre-dated the emergence of VOCs such as B.1.1.529 (omicron), reported a dose–response association between S1-RBD antibody binding, vaccine efficacy, and protection against symptomatic and severe COVID-19 after primary vaccination schedules.21, 22, 23
This study showed excellent concordance with the advice to interrupt methotrexate treatment for 2 weeks, although in a trial setting. This finding suggests easy implementation in the real world for patients who are willing to pause treatment for 2 weeks and for whom the clinician managing the condition feels it is reasonable to interrupt treatment without adverse impact on the disease course.
Methotrexate has a short elimination half-life (5–8 h) and accumulates as polyglutamates with prolonged half-lives.1, 2 A 2-week suspension in treatment would prevent T and B lymphocytes from getting exposed to the high circulating concentrations of methotrexate that occur within 24 h of weekly dosing. These findings suggest a crucial role of brief periods of methotrexate exposure on vaccine response. Indeed, complete absence of T-cell boosting after a second dose of the BNT162b2 mRNA COVID-19 vaccine was reported in patients treated with methotrexate that continued treatment as usual around the time of vaccination.27 Cross-protection against VOCs in the face of antibody escape mutations has highlighted the importance of unperturbed, primed, T-cell immunity.
Increased neutralising antibody capacity was previously reported in older patients with immune-mediated inflammatory diseases that had changed their methotrexate schedule and extended the dosing interval by more than 7 days at the time of their first or second COVID-19 vaccine dose in a non-randomised study with several limitations: potential recall bias, variability in timing of blood sample collection (1–16 weeks after vaccination), and absence of safety data.8 Another small (n=92; immunogenicity analysis set), single-centre, tertiary hospital-based trial limited to people with well controlled rheumatoid arthritis without previous SARS-CoV-2 infection reported that a 2-week methotrexate interruption after each of the two doses of the CoronaVac vaccine improved the S1-RBD antibody response.29 The previous study had several limitations, such as excluding participants after randomisation for past SARS-CoV-2 infection and disease flare-ups, and a 33% overall dropout rate with almost twice as many dropouts in the suspend methotrexate group than in the continue treatment group (45% vs 23%).29 CoronaVac elicits reduced T-cell, spike antibody binding, and neutralising antibody immunity compared with mRNA and adenovirus platforms,30 and results derived from this study cannot be extrapolated to inform health policy in countries using different vaccine platforms.
In this study, there was a suggestion of greater difference in antibody response in those that received the adenovirus AZ1222 vaccine for their first and second primary doses, followed by an mRNA vaccine, compared with those that had received an mRNA vaccine throughout, indicating a possible enhanced response following heterologous boosting but further research is needed in a larger patient group to confirm this finding.28
Strengths of our study included broad eligibility criteria making the results generalisable, excellent adherence to the intervention, minimal attrition, and assessment of outcomes at two timepoints. Limitations included no participant masking, which could result in potential bias of self-reported disease activity and flare outcomes. It was not possible to mask participants in this study without using a matching placebo, which would have increased trial costs, set-up time, and complexity, making this time-critical study unfeasible during the pandemic. The pragmatic trial design reflected real-world practice and patient experience, making the results useful to clinicians and patients alike. Concurrent or recent use of other glucocorticoid-sparing drugs might minimise the immunological boost provided by a 2-week break in methotrexate treatment and formed part of the exclusion criteria. Similarly, participants with active disease that were unable to suspend treatment were excluded from the trial, which limits to an extent the generalisability of the findings. Condition-specific disease activity measures were not used as this study recruited patients with a range of diseases, many without validated outcome measures. This study had a fairly modest sample size and was not designed to detect impact on clinical outcomes such as hospitalisation or death due to COVID-19, but instead focused on the immune response to vaccination in terms of S1-RBD antibody binding. Additionally, early termination of the study impacted on the ability to detect differences in secondary outcomes and subgroup analyses. T-cell and memory B-cell immune responses were not evaluated in this study. However, S1-RBD antibody binding has been put forward as a potential correlate of protection following vaccination.21, 22, 23 Data relating to whether VROOM study participants elected to suspend methotrexate before their first two COVID-19 vaccination doses of their own volition or following the advice of their usual care team were not collected. This approach is unlikely to have affected the results as there was no recommendation from the JCVI or specialist societies in the UK on whether to interrupt or continue methotrexate around COVID-19 vaccination. Any difference in medication-taking behaviour would be expected to balance out between the two groups of the trial due to random allocation. We did not enquire about such behaviour as it could potentially be affected by biased recall and might have affected compliance with the study intervention. Finally, exploratory subgroup analyses were performed, with some subgroups (eg, age <40 years) having very few participants. The results of these analyses should be interpreted in light of these limitations.
In conclusion, we observed a sustained increase that was more than two-fold in S1-RBD antibody binding on interruption of methotrexate treatment for 2 weeks immediately after vaccination against COVID-19, with a short-term increase in risk of disease flare-ups that were mostly self-managed and without any adverse impact on quality of life. Adoption of this simple intervention when administering additional doses of COVID-19 vaccines in patients taking methotrexate was safe and resulted in improved vaccine-induced S1-RBD antibody binding. Further research is required to assess whether interruption in treatment with other similarly acting immunosuppressive drugs will also enhance vaccine-induced immunity.
Data sharing
A participant-level dataset and the statistical code will be made available upon reasonable request to the Oxford Clinical Trials Research Unit and AA, once the VROOM study findings have been published in full. Some specific data might not be shared to maintain participant anonymity.
Declaration of interests
The institutions of the authors received funding from the NIHR-MRC-EME programme (award number NIHR 134607) towards conducting this research. LC reports research grants from Abbvie, Amgen, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, and UCB, and personal consulting fees or lecture fees from AbbVie, Amgen, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Gilead, Galapagos, GlaxoSmithKline, Janssen, Medac, Moonlake, Novartis, Pfizer, and UCB in the past 36 months. JB reports research grants from Pfizer and travel or conference fees from UCB, Pfizer, and Eli Lilly. AA reports institutional research grants from AstraZeneca and Oxford Immunotec, personal author royalties from UpTodate and Springer, personal consulting fees from Inflazome and NGM Biopharmaceuticals, and personal payments for lectures from Menarini Pharmaceuticals and Cadilla Pharmaceuticals, in the past 36 months and unrelated to the current work. AA is also co-chair of the OMERACT CPPD Working Group and co-chair of the ACR/EULAR CPPD Classification Criteria Working Group. JSN-V-T was seconded to the Department of Health and Social Care in England until March 31, 2022.
Acknowledgments
Acknowledgments
The study was funded by the National Institute for Health Research (NIHR) Efficacy Mechanism Evaluation programme, grant number NIHR134607. Support was also received from the NIHR Oxford Biomedical Research Centre. The views expressed in this document are those of the authors and not necessarily those of the NIHR, the Department of Health and Social Care, or the Joint Committee on Vaccination and Immunisation. The study is sponsored by the University of Nottingham (Nottingham, UK) and is managed by the Oxford Clinical Trials Research Unit. The co-authors would like to acknowledge the funders and the contribution of Patient and Public Involvement volunteers in Oxford and Nottingham for their help in designing this study and members of Oxford Clinical Trials Research Unit who enabled the rapid set-up and have provided ongoing support for this study. The views expressed in this Article are those of its authors and not necessarily those of the Department of Health and Social Care or the Joint Committee on Vaccination and Immunisation.
Contributors
AA, RJB, ÁM, LCC, JB, VB, AMV, IR, DMA, JSN-V-T, HCW, and JAC were involved in study conception, and AA, RJB, ÁM, LCC, JB, VB, LC, AF, DA, LE, PJ, NP, AMV, IR, DMA, JSN-V-T, HCW, and JAC were involved in the trial design. AA, JAC, RJB, and NP wrote the first draft of the manuscript. AA, RJB, ÁM, LCC, JB, VB, LC, AF, DA, LE, PJ, NP, AMV, IR, DMA, JSN-V-T, HCW, and JAC critically revised the manuscript for intellectual content. NP, IR, and JAC provided statistical expertise. NP and JAC accessed and verified the data and performed the data analysis. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Contributor Information
VROOM study investigators:
Ira Pande, Ting Seng Tang, Gui Tran, Alison Layton, Elizabeth Price, Lindsay Whittam, Srinivasan Venkatachalam, Ashley Hawarden, Gwenan Huws, Arthur Pratt, Nick J Reynolds, David Walsh, Theresa Joseph, Rengi Mathew, Stamatios Oikonomou, Catherine Gwynne, Rory Crowder, Vadivelu Saravanan, Alaa Mustafa, Cristina Tacu, Thomas Batty, Emmanuel George, Anushka Soni, Sarah Horton, Ayesha Madan, Karl Gaffney, Agnieszka Lapin, Sarah Bingham, Nick Levell, Edwin Lim, Nicola Gullick, Chris Holroyd, Salema Khalid, May Lwin, Mike Green, Laura Hunt, Nicola Alcorn, Rob Ellis, Samantha Hider, Alaa Hassan, Taryn Youngstein, Karen Douglas, Gen Nen Ho, Kirsty Levasseur, Sara Treacy, Myrto Cheila, John Pradeep, Ceril Rhys-Dillon, and Catrin Jones
Supplementary Material
References
- 1.Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2021;73:924–939. doi: 10.1002/acr.24596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445–1486. doi: 10.1016/j.jaad.2020.02.044. [DOI] [PubMed] [Google Scholar]
- 3.Mahil SK, Bechman K, Raharja A, et al. The effect of methotrexate and targeted immunosuppression on humoral and cellular immune responses to the COVID-19 vaccine BNT162b2: a cohort study. Lancet Rheumatol. 2021;3:e627–e637. doi: 10.1016/S2665-9913(21)00212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haberman RH, Herati R, Simon D, et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann Rheum Dis. 2021;80:1339–1344. doi: 10.1136/annrheumdis-2021-220597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun-Moscovici Y, Kaplan M, Braun M, et al. Disease activity and humoral response in patients with inflammatory rheumatic diseases after two doses of the Pfizer mRNA vaccine against SARS-CoV-2. Ann Rheum Dis. 2021;80:1317–1321. doi: 10.1136/annrheumdis-2021-220503. [DOI] [PubMed] [Google Scholar]
- 6.Ruddy JA, Connolly CM, Boyarsky BJ, et al. High antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021;80:1351–1352. doi: 10.1136/annrheumdis-2021-220656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee ARYB, Wong SY, Chai LYA, et al. Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ. 2022;376 doi: 10.1136/bmj-2021-068632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arumahandi de Silva AN, Frommert LM, Albach FN, et al. Pausing methotrexate improves immunogenicity of COVID-19 vaccination in elderly patients with rheumatic diseases. Ann Rheum Dis. 2022;81:881–888. doi: 10.1136/annrheumdis-2021-221876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2018;77:898–904. doi: 10.1136/annrheumdis-2018-213222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JK, Lee MA, Lee EY, et al. Effect of methotrexate discontinuation on efficacy of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2017;76:1559–1565. doi: 10.1136/annrheumdis-2017-211128. [DOI] [PubMed] [Google Scholar]
- 11.National Psoriasis Foundation COVID-19 Task Force guidance statement. 2019. https://www.psoriasis.org/covid-19-task-force-guidance-statements/
- 12.Curtis JR, Johnson SR, Anthony DD, et al. American College of Rheumatology guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 1. Arthritis Rheumatol. 2021;73:1093–1107. doi: 10.1002/art.41734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landewé RBM, Kroon FPB, Alunno A, et al. EULAR recommendations for the management and vaccination of people with rheumatic and musculoskeletal diseases in the context of SARS-CoV-2: the November 2021 update. Ann Rheum Dis. 2022 doi: 10.1136/annrheumdis-2021-222006. published online Feb 23. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt F, Muecksch F, Weisblum Y, et al. Plasma neutralization of the SARS-CoV-2 omicron variant. N Engl J Med. 2022;386:599–601. doi: 10.1056/NEJMc2119641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abhishek A, Boyton RJ, McKnight Á, et al. Effects of temporarily suspending low-dose methotrexate treatment for 2 weeks after SARS-CoV-2 vaccine booster on vaccine response in immunosuppressed adults with inflammatory conditions: protocol for a multicentre randomised controlled trial and nested mechanistic substudy (Vaccine Response On/Off Methotrexate (VROOM) study) BMJ Open. 2022;12 doi: 10.1136/bmjopen-2022-062599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Müller L, Andrée M, Moskorz W, et al. Age-dependent immune response to the BioNtech/Pfizer BNT162b2 coronavirus disease 2019 vaccination. Clin Infect Dis. 2021;73:2065–2072. doi: 10.1093/cid/ciab381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prendecki M, Clarke C, Brown J, et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet. 2021;397:1178–1181. doi: 10.1016/S0140-6736(21)00502-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds CJ, Pade C, Gibbons JM, et al. Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose. Science. 2021 doi: 10.1126/science.abh1282. published online April 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duggan J, Otter A, Andrews N, Brooks T. Evaluation of Roche Elecsys anti-SARS-CoV-2 S serology assay for the detection of anti-SARS-CoV-2 S antibodies. May 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/989460/Evaluation_of_Roche_Elecsys_anti_SARS_CoV_2_S_assay_PHE.pdf
- 20.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 22.Feng S, Phillips DJ, White T, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilbert PB, Montefiori DC, McDermott AB, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375:43–50. doi: 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynolds CJ, Gibbons JM, Pade C, et al. Heterologous infection and vaccination shapes immunity against SARS-CoV-2 variants. Science. 2022;375:183–192. doi: 10.1126/science.abm0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall V, Foulkes S, Insalata F, et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med. 2022;386:1207–1220. doi: 10.1056/NEJMoa2118691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feikin DR, Higdon MM, Abu-Raddad LJ, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399:924–944. doi: 10.1016/S0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahil SK, Bechman K, Raharja A, et al. Humoral and cellular immunogenicity to a second dose of COVID-19 vaccine BNT162b2 in people receiving methotrexate or targeted immunosuppression: a longitudinal cohort study. Lancet Rheumatol. 2022;4:e42–e52. doi: 10.1016/S2665-9913(21)00333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munro APS, Janani L, Cornelius V, et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398:2258–2276. doi: 10.1016/S0140-6736(21)02717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Araujo CSR, Medeiros-Ribeiro AC, Saad CGS, et al. Two-week methotrexate discontinuation in patients with rheumatoid arthritis vaccinated with inactivated SARS-CoV-2 vaccine: a randomised clinical trial. Ann Rheum Dis. 2022;81:889–897. doi: 10.1136/annrheumdis-2021-221916. [DOI] [PubMed] [Google Scholar]
- 30.McDonald I, Murray SM, Reynolds CJ, Altmann DM, Boyton RJ. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. NPJ Vaccines. 2021;6:74. doi: 10.1038/s41541-021-00336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
A participant-level dataset and the statistical code will be made available upon reasonable request to the Oxford Clinical Trials Research Unit and AA, once the VROOM study findings have been published in full. Some specific data might not be shared to maintain participant anonymity.