Abstract
Mismatch negativity (MMN) amplitude has been widely shown to be diminished in schizophrenia and, more recently, in other psychotic disorders. While there is considerable evidence linking MMN reduction to cognitive and functional deficits in schizophrenia, there is little evidence of associations with specific psychotic symptoms. Further, it is unclear if MMN reductions relate to specific symptoms, cognitive, and functional deficits transdiagnostically across different psychotic disorders. The present study examines MMN amplitude in a large cohort of cases diagnosed with psychotic disorders including schizophrenia and schizoaffective disorder (N=116); bipolar disorder and major depressive disorder (N=75); and other psychotic disorders (N=25), as well as individuals with no psychotic disorder diagnoses (N=248). Furthermore, we examined the association of MMN with symptoms, cognitive functioning, and real-world functioning to determine whether these relationships differ by diagnosis. Results showed that MMN amplitude was reduced in cases overall compared to never-psychotic individuals, with no differences between psychotic disorders. Furthermore, there were transdiagnostic associations of reduced MMN-D with worse auditory hallucinations (r=.14) and disorganization (r=.14), MMN-F with real-word functioning (r=.20) and episodic memory (r=−.22), and both components with executive functioning (MMN-D: r= −.17; MMN-F: r= −.15). Our findings relating MMN reductions with cognitive and real-world functioning replicate earlier research in schizophrenia and extend these relationships to other psychotic disorders. Furthermore, our correlations with MMN-D are consistent with computational modeling research and theoretical proposals that view MMN reduction, cognitive dysfunction, and psychotic symptoms as reflecting underlying predictive coding deficits. However, differences in relationships with MMN-F suggest that additional work is warranted on this topic.
Keywords: Schizophrenia, Hallucinations, ERPs, Mismatch Negativity, Predictive Coding
General Scientific Summary:
Mismatch negativity (MMN) amplitude has been shown to be diminished in schizophrenia and other psychotic disorders. We show that MMN reductions are associated with worse auditory hallucinations, disorganization, as well as cognitive and real-world functioning transdiagnostically across psychotic disorders. These findings highlight the importance of MMN in tracking both symptom and functioning related impairment across psychosis-spectrum illnesses. Furthermore, associations with MMN-D are in line with theoretical and computational modeling research that attributes MMN reductions, cognitive dysfunction and psychotic symptoms to underlying predictive coding deficits, although importantly MMN-F findings could be argued to contradict a straightforward interpretation within the predictive coding model.
It is well-established that mismatch negativity (MMN) amplitude is reduced in schizophrenia (Umbricht & Krljes, 2005) and occasionally seen to be reduced in other psychotic disorders (Erickson, Ruffle, & Gold, 2016). The MMN is an event-related potential (ERP) elicited by the presentation of a deviant stimulus differing in a property such as duration (MMN-D) or frequency (MMN-F) from a more frequently presented standard stimulus (Näätänen, 1995). It is most commonly studied in auditory paradigms, though it can also be elicited in other sensory modalities (Pazo-Alvarez, Cadaveira, & Amenedo, 2003). While there is considerable evidence linking reduced MMN amplitude to cognitive and real-world functioning in schizophrenia (Erickson et al., 2016; Kim et al., 2014; Light & Braff, 2005; Light et al., 2015; Toyomaki et al., 2008; Wynn, Sugar, Horan, Kern, & Green, 2010), it is unclear whether MMN reduction is associated with specific psychotic symptoms in schizophrenia, and if these relationships exist across different psychotic disorders. Here, we use a large, well-characterized cohort of individuals with diverse psychotic disorders as well as never psychotic (NP) individuals to examine MMN relationships with symptoms and functioning in schizophrenia and across psychotic disorders.
While several theoretical and computational models have linked MMN deficits to psychotic symptom dimensions (Adams, Stephan, Brown, Frith, & Friston, 2013; Wacongne, Changeux, & Dehaene, 2012), empirical studies provide largely null findings. A recent meta-analysis showed that across studies, the MMN bears no significant relationship to either positive or negative symptoms (Erickson et al., 2017). However, a majority of the studies in this meta-analysis examined broadly defined heterogeneous symptoms dimensions, such as total severity of positive symptoms, rather than more narrowly-defined symptoms such as hallucinations and delusions which may yield different findings. One large study (from the Consortium on Genetics in Schizophrenia; COGS-2) by Light and colleagues (2015) showed a small but significant correlation between MMN and positive symptoms (r=.08, p<.05), however, this study also did not examine which specific positive symptoms were driving this association. The few additional studies that show an association between MMN and positive symptoms do so in very circumscribed samples, such as acutely psychotic patients or patients with persistent auditory hallucinations (Fisher et al., 2011; Fisher, Labelle, & Knott, 2008, 2012), non-prodromal adolescents reporting psychotic experiences (Murphy et al., 2013), and ketamine-induced psychosis in healthy individuals (Heekeren et al., 2008; Umbricht, Koller, Vollenweider, & Schmid, 2002).
Overall, it remains unclear to what extent MMN reductions relate to specific psychotic symptom domains across psychosis-spectrum illness. Psychotic symptoms are present not only in schizophrenia but also in other psychotic disorders (APA, 2013), suggesting that any relationship between MMN amplitude and symptoms may be transdiagnostic. Though some studies have reported diminished MMN in schizophrenia compared to bipolar disorder (Erickson et al., 2016; Kaur et al., 2011), this finding is not consistent (Salisbury, Kuroki, Kasai, Shenton, & McCarley, 2007) and based predominantly on relatively small sample sizes. Finally, no study to our knowledge has examined the relationship between MMN and psychotic symptoms across the full range of psychotic disorders. In contrast to the scarcity of studies relating MMN deficits to specific psychotic symptoms, there is more research showing associations between MMN in schizophrenia and poor work/daily functioning, social functioning, and broad cognitive impairment (Baldeweg, Klugman, Gruzelier, & Hirsch, 2004; Erickson et al., 2016; Javitt, Doneshka, Grochowski, & Ritter, 1995; Kim et al., 2014; Light & Braff, 2005; Light et al., 2015; Toyomaki et al., 2008; Wynn et al., 2010). However, like psychotic symptoms, cognitive and functional impairments are seen across a range of psychotic disorders (Green, 2006; Latalova, Prasko, Diveky, & Velartova, 2011), and their potential transdiagnostic relationships with MMN remain largely unexplored.
Thus, we investigate reduction in MMN-D and MMN-F amplitude as a transdiagnostic markers of specific symptom and functioning dimensions in psychotic disorders broadly defined. To accomplish this, we use a large, well-characterized sample of psychosis-spectrum and never-psychotic individuals and a dimensional approach recommended by the National Institute of Mental Health (NIMH) research diagnostic criteria (RDoC) initiative. The goals of the present study are three-fold. First, we seek to replicate the expected MMN deficit in schizophrenia and examine to what degree this deficit is present in cases with other psychotic disorders; we hypothesize that MMN-D and MMN-F will be reduced in cases compared to NP individuals. Second, we seek to clarify links between these MMN measures and psychotic symptoms. In line with research showing that the predictive coding (PC) framework can be used to explain MMN (Baldeweg, 2007; Garrido et al., 2008; Garrido, Kilner, Stephan, & Friston, 2009; Heilbron & Chait, 2017; Stefanics, Heinzle, Horváth, & Stephan, 2018; Wacongne et al., 2012), MMN reduction in schizophrenia (McCleery et al., 2019; Rentzsch, Shen, Jockers-Scherübl, Gallinat, & Neuhaus, 2015; Wacongne, 2016), and psychotic symptoms (Adams et al., 2013; Horga, Schatz, Abi-Dargham, & Peterson, 2014), we hypothesize that reduced MMN-D and MMN-F amplitude will be associated with worse psychotic symptoms and that this relationship will be transdiagnostic in nature. Finally, we examine the relationship of MMN measures with real-world functioning and neurocognitive deficits. Among neurocognitive deficits, we focus on executive functioning, episodic memory, and processing speed, because they tend to be most impaired in schizophrenia (Barch, 2005; Reichenberg et al., 2008). In line with previously discussed literature, we expect that reduction in MMN-D and MMN-F amplitude will be associated with deficits in daily functioning and cognitive abilities across psychotic disorders.
Methods
Participants
Study participants were assessed at 20-year follow up of the Suffolk County Mental Health Project (Bromet et al., 2011), an epidemiological study of first-admission psychosis. This sample included cases with a history of schizophrenia and schizoaffective disorder (SZ group; n=116); Mood Disorder with Psychosis (i.e., bipolar disorder and major depressive disorder; MDP group; n=75); and Other Psychotic Disorders (drug-induced psychosis, psychotic disorders not otherwise specified, and other psychoses; OPD group; n=25). These groupings are based on previous research with this sample showing a distinction in course and severity of SZ vs MDP (Kotov et al., 2013; Reichenberg et al., 2008), and our aim to compare SZ to OPD. Also, 248 NP individuals were recruited from zip codes where cases resided and matched on demographics. Additional information regarding study inclusion is presented in the supplement.
Clinical, Functional and Neuropsychological Measures
Clinical measures were obtained for each participant concurrent with the MMN task and included the structured clinical interview for DSM-IV (SCID; First, Spitzer, Gibbon, & Williams, 2002) as well as scales for the assessment of positive (SAPS; Andreasen, 1984) and negative (SANS; Andreasen, 1989) symptoms, psychosocial functioning as assessed through the Social and Occupational Functioning Assessment Scale (SOFAS; Rybarczyk, 2011), and occupational functioning assessed through consensus ratings of staff psychiatrists (Schwartz et al., 2000). Symptoms were scored into four factor-analytically derived scales: reality distortion, disorganization, avolition, and inexpressivity (Kotov et al., 2016). We also specifically examined auditory hallucinations given their putative link in the predictive coding framework. While several neuropsychological tests were administered (see supplement), we focused analyses on tests of executive functioning (Stroop: Trenerry, 1989; Trails B: Reitan, 1955), processing speed (Trails A; Reitan, 1955), episodic memory (visual and auditory; indexed by Verbal Paired Associates and Visual Reconstruction components of WMS-R; Wechsler, 1987), and overall academic abilities (as measured by the WRAT-3; Wilkinson, 1993). Antipsychotic medication status for each participant was determined by asking participants to bring their pill bottles or a list of all medications and dosages. See supplemental information for further details regarding measures.
Task
Stimuli were a total of 2458 tones presented every 500ms at 78 dB with 10ms rise/fall. Tones consisted of 80% standard tones with a 50ms duration at 633 Hz and 20% deviant tones, out of which 10% percent deviated in duration (100ms; MMN-D) and 10% deviated in frequency (1000 Hz; MMN-F) from the standard. Because the MMN is elicited in a pre-attentive manner, tones were presented in the background while participants completed a picture/word matching task (described in detail by Mathalon, Roach, & Ford, 2010).
Psychophysiological Data Acquisition, Preprocessing, and Analyses
EEG data were acquired at 34 scalp electrode sites placed according to the international 10/20 system using an ActiveTwo BioSemi system (BioSemi, Amsterdam, Netherlands). Signal was digitized at 24-bit resolution with a sampling rate of 1024 Hz, and data were referenced to a common mode sense active electrode forming a monopolar channel. Electrooculogram were recorded from auxiliary electrodes placed above and below (vertical EOG) and at the outer canthi (horizontal EOG) of each eye.
Brain Vision Analyzer (Brain Products, Munich, Germany) was used for offline data analyses. Data were referenced to linked mastoids and a band-pass filter of .1 to 30 Hz was applied. Eyeblinks were removed using ocular correction, and artifacts were rejected trial-wise based on a step of 50 μV between trials or a 75.0 μV difference within a trial. ERPs were stimulus-locked to onset of tones, corrected relative to a 200-ms pre-stimulus baseline, and the MMN was computed as two difference waves: duration deviant minus standard (MMN-D) and frequency deviant minus standard (MMN-F). Duration deviants were quantified at 180-230 ms and frequency deviants at 110-160 ms from tone onset at electrode Fz, based on grand average waveforms. Standard tones were scored separately at the same time windows with respect to deviant type.
Statistical analyses were performed using IBM SPSS Statistics (Version 24.0, IBM, Armonk NY). Differences between groups in age, gender, race/ethnicity, and use of antipsychotic medication were examined using independent samples t-tests and chi-square goodness of fit tests. Though MMN-D and MMN-F have been found to be impaired in psychotic disorders (Umbricht & Krljes, 2005), the level of impairment has been shown to differ for the two. Hence, we conducted all analyses separately using MMN-D and MMN-F as dependent variables.
First, to examine MMN amplitude differences between cases and NP, we conducted an analysis of variance (ANOVA) with group (cases vs NP) as the between-subjects factor and MMN-D/MMN-F as the dependent variable, followed up by an a priori comparison of MMN amplitude across diagnosis (SZ, OPD, MDP) utilizing a one-way ANOVA. Next, we examined whether reductions in MMN amplitude were associated with psychotic symptom dimensions transdiagnostically across cases. To do so, we conducted bivariate correlations of symptom dimensions with MMN-D and MMN-F, in cases only due to a lack of variance in psychotic symptoms in the NP group. Where correlations were significant with either MMN-D or MMN-F, Fishers r to z was used to examine the significance of differences between correlations with MMN-D versus MMN-F. Symptoms correlating significantly with MMN amplitude in cases were retained for hierarchical regression analyses, with psychotic symptoms, diagnosis, and their interaction predicting MMN-D or MMN-F amplitude. A non-significant symptom x diagnosis interaction implied the presence of a transdiagnostic relationship that did not differ by diagnosis (Sheffield et al., 2017). Finally, this hierarchical regression analysis was repeated controlling for demographic variables and medications when diagnostic groups differed on them. We similarly tested hypotheses regarding the transdiagnostic relationship of MMN-D and MMN-F reductions with occupational, social, and neuropsychological functioning across cases. Finally, in instances in which bivariate correlations were significant in both cases and the NP group, similar hierarchical regression analyses were conducted with functioning as the first-level, followed by group (cases vs NP), and the group x functioning interaction term.
Results
Sample Characteristics
Sample demographics and clinical ratings are presented in Table 1. Groups (cases vs NP) did not differ in gender but differed in age, race, ethnicity, antipsychotic medication, and severity of symptoms, as well as functional impairment. Diagnostic groups (SZ vs MDP vs OPD) differed demographically only in gender and antipsychotic medication usage, as well as in symptoms and functioning; across measures, the SZ group showed the most impairment.
Table 1.
Clinical and Demographic Factors
| Cases | NP | Diagnosis | Group | |||
|---|---|---|---|---|---|---|
| SZ | MDP | OPD | N(%) | SZ vs MDP vs OPD |
Cases vs NP | |
| N(%) | N(%) | N(%) | ||||
| Gender | — | — | — | — | X2=10.3** | X2=.19 |
| Female | 43(37.1) | 42(56.0) | 6(24.0) | 110(44.4) | — | — |
| Male | 72(62.1) | 33(44.0) | 19(76.0) | 138(55.6) | — | — |
| Race1 | — | — | — | — | X2=15.1 | t=3.100** |
| Asian | 4(3.4) | 0(0) | 1(4.0) | 2(.8) | — | — |
| African American | 13(11.2) | 4(5.3) | 6(24.0) | 14(5.6) | — | — |
| White | 85(73.3) | 66(88.0) | 17(68.0) | 221(89.1) | — | — |
| More than one race | 8(6.9) | 1(1.3) | 0(0) | 4(1.6) | — | — |
| Other/Unknown | 6(5.2) | 4(5.3) | 1(4.0) | 7(2.8) | — | — |
| Ethnicity | — | — | — | — | X2=2.5 | X2=4.01* |
| Hispanic/Latino | 20(17.2) | 8(10.7) | 2(8.0) | 20(8.1) | — | — |
| Antipsychotic Medication | 93(80.2) | 27(36.0) | 6(24.0) | 4(1.6) | F=32.3*** | t=17.40*** |
| Mean (SD) |
Mean (SD) |
Mean (SD) |
Mean (SD) |
Group Comparison |
Group Comparison |
|
| Age (years) | 47.3(7.9) | 48.4(9.0) | 49.0(10.9) | 50.5 (8.8) | F=.63 | t= −.3.186** |
| Symptoms | — | — | — | — | — | — |
| Avolition | 17.5(8.6) | 8.4(7.7) | 7.8(7.6) | 2.9 (3.9) | F=33.9*** | t=15.83*** |
| Inexpressivity | 10.5(10.5) | 2.9(5.2) | 3.3(5.2) | .84 (2.6) | F=20.0*** | t=10.18*** |
| Reality Distortion | 7.8(9.9) | .55(1.7) | 1.4(2.5) | .08 (.50) | F=24.4*** | t=8.6*** |
| Disorganization | 6.6(7.4) | 2.2(4.0) | 4.0(4.9) | .92 (2.4) | F=11.8*** | t=8.7*** |
| Occupational Functioning | 4.2(1.4) | 2.8(5.2) | 2.8(2.1) | 1.9 (1.8) | F=18.2*** | t=9.4*** |
| Psychosocial Functioning | 3.5(.72) | 2.2(1.0) | 2.1(1.2) | 1.1 (.39) | F=62.3*** | t=9.5*** |
Indicates a significant difference (p<.05) between groups
p<.01
p<.001
Due to the small number of individuals identifying as non-white, group differences were examined as white vs other.
Group differences in MMN amplitude
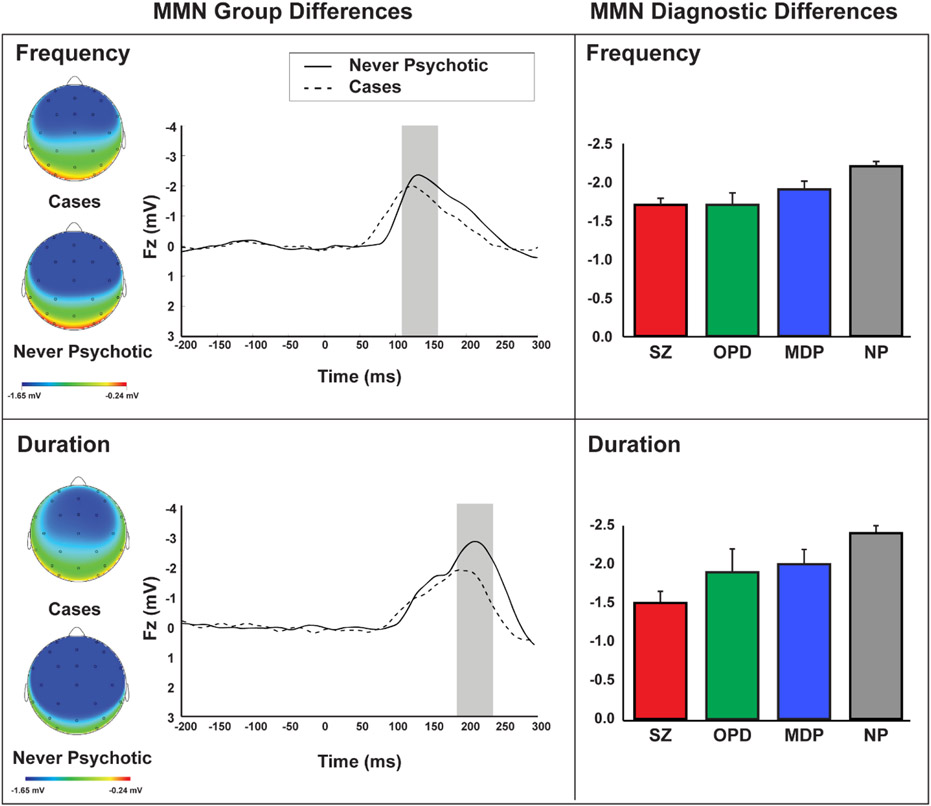
Examination of group differences (Figure 1) revealed a significant effect of group on MMN-F (F(1,462)=8.60, p=.004) and MMN-D (F(1,46)=16.114, p<.001), such that cases showed lower amplitude than NP; however, there was no significant difference among cases by diagnosis (MMN-D: F(2,215)=1.82, p=.169; MMN-F: F(2,215)=.82, p=.441).
Figure 1.
In the top panel, scalp distributions and wave forms depict MMN amplitude in response to frequency deviants in cases vs. never psychotic groups (left) while bar graphs depict MMN amplitude for each diagnostic group: schizophrenia spectrum (SZ), mood disorder (MDP), other psychotic disorders (OPD), and never psychotic (NP). The bottom panel shows corresponding effects for MMN in response to duration deviants.
Links between MMN amplitude, clinical, and functioning measures
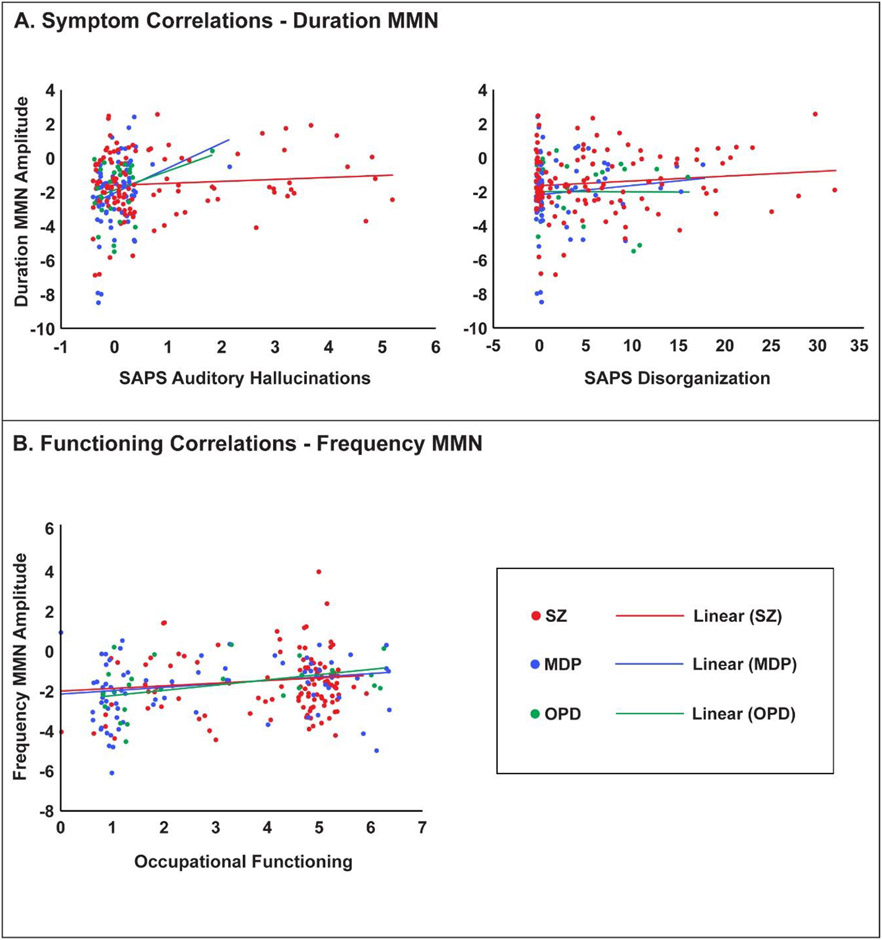
More severe disorganization and auditory hallucinations were associated with reduced MMN-D amplitude in cases (Table 2; Figure 2A). These symptoms were not associated with MMN-F and the differences between symptom correlations with MMN-D and MMN-F were significant (hallucinations p=.015, disorganization p=.048; Supplement Table 1). Hierarchical regression analyses showed that these relationships did not vary by diagnosis as indicated by the non-significant diagnosis x symptom interactions (Table 4). The relationship with disorganized symptoms and auditory hallucinations remained significant and marginally significant, respectively, after controlling for gender and antipsychotic usage (Supplement Table 3).
Table 2.
Clinical Correlations
| Cases | NP | |||
|---|---|---|---|---|
| Frequency MMN |
Duration MMN |
Frequency MMN |
Duration MMN |
|
| Symptoms | ||||
| SAPS-P | −.09 | .08 | — | — |
| Auditory Hallucinations | −.03 | .14* | — | — |
| SAPS-D | .01 | .14* | .00 | .04 |
| SANS-A | .10 | .10 | .05 | .05 |
| SANS-E | .04 | .05 | −.02 | .06 |
| Real-world Functioning | ||||
| Occupational Functioning | .20 * 1 | .10 | .01 | .07 |
| Social/Occupational Functioning |
−.13 | −.12 | −.04 | −.06 |
| Neuropsychological Functioning | ||||
| Executive Functioning | - | - | - | - |
| Stroop | −.15 * | −.17 * | −.21 * | −.26 * |
| Trails B | .15 * | .15 * | .19 * | .08 |
| Processing Speed | - | - | - | - |
| Trails A | .12 | .03 | .14 * | .14 * |
| Episodic Memory | - | - | - | - |
| Visual | −.22 * | −.11 | −.21 * | .16 * |
| Auditory | −.15 * | −.04 | −.14 * | −.20 * |
| Cognitive/Academic Ability | - | - | - | - |
| WRAT3 | −.16 * | −.11 | −.07 | −.03 |
p<.05
Underlined values survive correction for multiple comparisons, calculated using a false discovery rate of 10% applied to each domain (symptoms, real-world functioning, and neuropsychological functioning) separately for cases and for neighbors.
Figure 2.
(A) Correlations across cases (different colors for different diagnostic groups) between MMN-D and auditory hallucinations (left) and disorganization (right), in which higher scores represent increased frequency/severity. (B) Correlations between MMN-F and occupational functioning. A randomized jitter (−.4,4) was added to each ordinal value on the x-axis for display purposes in order to demonstrate overlapping data points.
Table 4.
Hierarchical Regression – Duration MMN
| Model | Predictors | R 2 | ΔR2 | p value |
|---|---|---|---|---|
| Symptoms | ||||
| SAPS-D | SAPS-D | .02 | .02 | .041* |
| Diagnosis | .03 | .01 | .407 | |
| Interaction | .03 | .00 | .705 | |
| Auditory | AH | .02 | .02 | .039* |
| Hallucinations | Diagnosis | .03 | .01 | .448 |
| Interaction | .04 | .01 | .371 | |
| Cognition | ||||
| Trails B | Trails B | .02 | .02 | .031* |
| Diagnosis | .03 | .01 | .482 | |
| Interaction | .03 | .00 | .823 | |
| Stroop | Stroop | .03 | .03 | .014* |
| Diagnosis | .04 | .01 | .407 | |
| Interaction | .04 | .00 | .963 | |
p<.05
p<.01
Worse occupational functioning was associated with reduced MMN-F amplitude (Table 2; Figure 2B) in cases. Relationships did not vary by diagnosis, as evidenced by the non-significant diagnosis x functioning interaction (Table 3) and remained significant after controlling for gender and antipsychotic usage (Supplement Table 2). Social functioning was marginally associated with MMN-F (p=.057) and MMN-D (p=.085) amplitude (Table 2); however, because this association was not statistically significant regression analyses were not performed.
Table 3.
Hierarchical Regression – Frequency MMN
| Model | Predictors | R 2 | ΔR2 | p value |
|---|---|---|---|---|
| Functioning | ||||
| Occupational | Functioning | .04 | .04 | .004** |
| Functioning | Diagnosis | .04 | .00 | .696 |
| Interaction | .04 | .00 | .595 | |
| Cognition | ||||
| Auditory EM | Verbal Paired | .02 | .02 | .03* |
| Diagnosis | .03 | .00 | .781 | |
| Interaction | .05 | .02 | .084 | |
| Visual EM | Visual EM | .05 | .05 | .002** |
| Diagnosis | .05 | .00 | .812 | |
| Interaction | .06 | .01 | .312 | |
| Trails B | Trails B | .02 | .02 | .036* |
| Diagnosis | .02 | .00 | .907 | |
| Interaction | .02 | .00 | .857 | |
| Stroop | Stroop | .04 | .04 | .038* |
| Diagnosis | .02 | .00 | .870 | |
| Interaction | .03 | .00 | .796 | |
| WRAT 3 | WRAT 3 | .03 | .03 | .018* |
| Diagnosis | .04 | .00 | .797 | |
| Interaction | .04 | .01 | .286 | |
p<.05
p<.01
Worse performance on measures of executive functioning, episodic memory, and processing speed were associated with reduced MMN-F and MMN-D amplitude (Table 2) in both cases and NP groups. When examined in cases, these relationships did not vary by diagnosis (Tables 3 & 4) and largely remained significant after controlling for gender and antipsychotic medications (Supplement Tables 2 & 3). These relationships did not vary by group, indicating that they extend to normal populations (Supplement Table 4). In no case did differences between correlations of real-world or neuropsychological functioning and MMN-D vs MMN-F prove to be significant (Supplement Table 1).
Discussion
Though MMN deficits have been linked through theoretical and computational models to symptoms such as hallucinations and delusions (Adams et al., 2013; Wacongne et al., 2012), there is a relative lack of empirical evidence linking MMN reductions to such dimensions. Using a well-characterized and large cohort that included a variety of psychotic disorders, we examined the hypotheses that MMN-D and MMN-F would be reduced not only in schizophrenia but across diagnoses, and that this reduction in amplitude would be associated with psychotic symptoms and functioning across psychosis-spectrum illness. Our results showed that MMN-D and MMN-F amplitude were reduced in cases overall, and this reduction did not differ by diagnosis. We further observed transdiagnostic associations of MMN-D with symptom domains, MMN-F with functional outcomes, and both MMN components with worse cognitive functioning.
Our study replicates relationships between MMN reduction and auditory hallucinations seen in acutely psychotic or hallucinating individuals with schizophrenia in a small sample (Fisher et al., 2011; Fisher et al., 2008, 2012), as well as MMN-D reduction and positive symptoms more broadly in larger samples (Light et al., 2015). Importantly, our study extends these findings to a more heterogeneous sample transdiagnostically across psychotic disorders. These findings are contrary, at face value, to the lack of association between positive symptoms and MMN amplitude seen in a meta-analysis of previous studies (Erickson et al., 2017). However, it is important to note that this meta-analysis examined relationships with overall psychotic symptom score, rather than specific positive symptoms. Furthermore, this study collapsed across MMN-F and MMN-D components, possibly obscuring the differential relationships that these components bear with symptoms and functioning, as seen in our sample. While we expected MMN reductions measured via both MMN components to be related to psychotic symptoms, the presence of a differential relationship may be a reflection of differences in the neuroanatomical substrates that support the generation of these components. MMN-F has been shown to correlate primarily with voxels in core auditory regions, while MMN-D has been shown to be related to activation in the somatomotor networks broadly (Lee et al., 2017). Furthermore, MMN-F was found to engage default mode regions, while MMN-D is related to activation in attention networks (Lee et al., 2017). While further research is needed to explore these differences, evidence showing that MMN-D and MMN-F may arise from different cortical processes may provide clues into why we see differential correlations of symptoms and functioning with these two components.
The relationship between MMN reduction and symptoms in our sample is in line with previous computational modeling research and the predictive coding framework. Within this framework, the auditory MMN is hypothesized to result from a failure to predict sensory input (i.e., deviant tone) and thereby suppress prediction error (Friston, 2005; Garrido et al., 2009). Specifically, when subsequent events are repeated (e.g., standards in case of the MMN oddball paradigm), there is a suppression of prediction error and the MMN is reduced; however, it appears on the presentation of the unpredicted deviant stimulus (Garrido et al., 2009). Hence, a relative decrease in neural response to deviant auditory stimuli in psychotic disorders may derive from weak prior expectations set by standard stimuli. While some studies have suggested that hallucinations may arise from strong precision of priors (Powers, Mathys, & Corlett, 2017), studies within this framework also suggest that auditory hallucinations arise from decreased precision of prior expectations and increased precision of sensory information (e.g., inner speech) which shifts the posterior toward the sensory information, resulting in maladaptive inferences (Sterzer et al., 2018). Although this framework provides one among many ways of conceptualizing psychotic symptoms and the MMN, our findings suggest predictive coding dysfunction as a common substrate that does not respect nosological categories.
Interestingly, in the present sample we also found that reduced auditory MMN-D was associated with disorganized symptoms. The PC framework has been used to explain not only perceptual aberrations but also cognitive symptoms such as delusions. Hence, it is possible that the association in the present sample is driven by a common underlying aberrant inferential mechanism that can apply to disorganized thought, language, and behavior. Additionally, reduced MMN-D and MMN-F amplitudes were associated with worse cognitive functioning, specifically episodic memory and executive functioning. This is consistent with the notion that a predictive coding framework can explain not only a range of low-level perceptual processes but also high-level cognitive abilities (Spratling, 2016). Computational modeling studies show that predictive coding can simulate several cognitive functions such as categorization, the impact of context on perception and behavioral control, recall and reasoning regarding conceptual knowledge, and task-switching (Spratling, 2010, 2016).
Interestingly, we find that not all of our cognitive measures are associated with MMN reductions. While MMN reductions correlated with tasks of executive functioning (Stroop and Trails B), and episodic memory (visual and auditory), they did not correlate with Trails A. It is possible this pattern of relationships is because MMN generation involves a higher level of cognition, rather than processing speed as measured by Trails A. Furthermore, Knowles & colleagues (Knowles, David, & Reichenberg, 2010) showed processing speed abnormalities in psychosis to be moderated by antipsychotic medication dosage, suggesting that these effects may not solely represent deficits underlying the disorder, and in fact may also be impacted by medication usage. This may explain the lack of correlation in the present sample between reduced MMN and scores on measures of cognitive processing speed. Finally, our study showed that MMN-F reductions were associated with worse social and occupation functioning in cases, replicating a large number of previous studies in schizophrenia (Erickson et al., 2016; Kim et al., 2014; Light & Braff, 2005; Light et al., 2015; Toyomaki et al., 2008; Wynn et al., 2010) and extending these findings to the broad spectrum of psychotic illnesses.
Overall, the present study uses a large and well-characterized sample to demonstrate relationships between MMN amplitude reduction and psychotic symptoms. The presence of a large heterogeneous cohort allowed us to examine transdiagnostic effects while also maximizing power to detect smaller effects. These findings are consistent with previously theorized relationships between MMN and symptoms in the predictive coding framework (Adams et al., 2013; Wacongne, 2016). However, it should be noted that the predictive coding framework is one among several other theoretical frameworks used to explain MMN. According to the model adjustment hypothesis, the MMN is generated when the current auditory input deviates from a learned regularity or a memory trace of previous standard inputs and captures attention automatically (Näätänen, Tervaniemi, Sussman, Paavilainen, & Winkler, 2001). The alternative adaptation hypothesis posits that the MMN results from a delayed N1 response to standard inputs, attenuated due to similarity with preceding standard inputs, reflecting adaptation of auditory cortex neurons (Jääskeläinen et al., 2004). The predictive coding framework unifies these competing hypotheses by proposing that repetition of standard events leads to suppression of prediction error and the disappearance of the MMN, and that prediction error is generated when there is a mismatch between the predicted and the actual sensory input (Garrido et al., 2009). Furthermore, computational modeling research shows that this framework has been shown to parsimoniously explain both the generation of the MMN and psychotic symptoms (Wacongne, 2016).
Finally, several limitations in our study should be addressed in future research. Though our sample of cases was large in total (N=216), the sizes of our diagnostic groups varied (SZ = 116, MDP = 75, OPD = 25), suggesting a need for future studies with similar sample sizes across groups. It is also important to note that associations with MMN in the present sample are small. This is in line with a substantial literature showing that variability in neuroimaging measures accounts for a small percentage of variance in clinical phenotypes (Patrick et al., 2013; Paulus & Thompson, 2019). While this small effect size indicates that MMN alone may not be sufficiently strong in making useful single-event predictions, such effects may be consequential in the long-term, and may be informative about the etiology of psychotic disorders (Funder & Ozer, 2019; Paulus & Thompson, 2019). It may also be the case that this association is one which represents a specific phenotypic pathway of dysfunction, requiring either large, heterogeneous sample sizes or more targeted recruitment; such as the recruitment of actively hallucinating patients by Fisher and colleagues (Fisher et al., 2011; Fisher et al., 2008, 2012). Additionally, while predictive coding theory lends preliminary support to the utility of the MMN as an index of prediction error, this theory cannot in its current form explain differences in correlates between MMN-D and MMN-F. Future research should focus on examining these patterns more closely, in order to provide a more complete understanding of the practical and theoretical significance of this component, as well as the utility of this theoretical framework.
Supplementary Material
Acknowledgements
The authors would like to thank Evelyn Bromet, cohort founder. This work was supported by an NIH grant from the National Institute of Mental Health to Dr. Kotov (MH094398).
This work was supported by an NIH grant from the National Institute of Mental Health to Dr. Kotov (MH094398). This study was formally approved by the Institutional Review Board (IRB) at Stony Brook University (Project 102354).
References
- Adams RA, Stephan KE, Brown HR, Frith CD, & Friston KJ (2013). The computational anatomy of psychosis. Frontiers in psychiatry, 4, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC (1984). Scale for the assessment of positive symptoms (SAPS): University of Iowa; Iowa City. [Google Scholar]
- Andreasen NC (1989). The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. The British Journal of Psychiatry, 155(S7), 49–52. [PubMed] [Google Scholar]
- APA. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®): American Psychiatric Pub. [DOI] [PubMed] [Google Scholar]
- Baldeweg T (2007). ERP repetition effects and mismatch negativity generation: A predictive coding perspective. Journal of Psychophysiology, 21(3-4), 204. [Google Scholar]
- Baldeweg T, Klugman A, Gruzelier J, & Hirsch SR (2004). Mismatch negativity potentials and cognitive impairment in schizophrenia. Schizophrenia research, 69(2), 203–217. [DOI] [PubMed] [Google Scholar]
- Barch DM (2005). The cognitive neuroscience of schizophrenia. Annu. Rev. Clin. Psychol, 1, 321–353. [DOI] [PubMed] [Google Scholar]
- Bromet EJ, Kotov R, Fochtmann LJ, Carlson GA, Tanenberg-Karant M, Ruggero C, & Chang S.-w. (2011). Diagnostic shifts during the decade following first admission for psychosis. American Journal of Psychiatry, 168(11), 1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson MA, Albrecht M, Ruffle A, Fleming L, Corlett P, & Gold J (2017). No association between symptom severity and MMN impairment in schizophrenia: A meta-analytic approach. Schizophrenia research: cognition, 9, 13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson MA, Ruffle A, & Gold JM (2016). A meta-analysis of mismatch negativity in schizophrenia: from clinical risk to disease specificity and progression. Biological psychiatry, 79(12), 980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JBW (2002). Structured clinical interview for DSM-IV-TR axis I disorders-patient edition (SCID-I/P, 11/2002 revision). New York: Biometrics Research Department, New York State Psychiatric Institute [Google Scholar]
- Fisher DJ, Grant B, Smith DM, Borracci G, Labelle A, & Knott VJ (2011). Effects of auditory hallucinations on the mismatch negativity (MMN) in schizophrenia as measured by a modified ‘optimal’multi-feature paradigm. International Journal of Psychophysiology, 81(3), 245–251. [DOI] [PubMed] [Google Scholar]
- Fisher DJ, Labelle A, & Knott VJ (2008). The right profile: mismatch negativity in schizophrenia with and without auditory hallucinations as measured by a multi-feature paradigm. Clinical neurophysiology, 119(4), 909–921. [DOI] [PubMed] [Google Scholar]
- Fisher DJ, Labelle A, & Knott VJ (2012). Alterations of mismatch negativity (MMN) in schizophrenia patients with auditory hallucinations experiencing acute exacerbation of illness. Schizophrenia research, 139(1-3), 237–245. [DOI] [PubMed] [Google Scholar]
- Friston K (2005). A theory of cortical responses. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 360(1456), 815–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funder DC, & Ozer DJ (2019). Evaluating effect size in psychological research: Sense and nonsense. Advances in Methods and Practices in Psychological Science, 2(2), 156–168. [Google Scholar]
- Garrido MI, Friston KJ, Kiebel SJ, Stephan KE, Baldeweg T, & Kilner JM (2008). The functional anatomy of the MMN: A DCM study of the roving paradigm. NeuroImage, 42(2), 936–944. doi: 10.1016/j.neuroimage.2008.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido MI, Kilner JM, Stephan KE, & Friston KJ (2009). The mismatch negativity: a review of underlying mechanisms. Clinical neurophysiology, 120(3), 453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF (2006). Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. The Journal of clinical psychiatry, 67, 3–8; discussion 36-42. [PubMed] [Google Scholar]
- Heekeren K, Daumann J, Neukirch A, Stock C, Kawohl W, Norra C, … Gouzoulis-Mayfrank E (2008). Mismatch negativity generation in the human 5HT 2A agonist and NMDA antagonist model of psychosis. Psychopharmacology, 199(1), 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbron M, & Chait M (2017). Great expectations: is there evidence for predictive coding in auditory cortex? Neuroscience. [DOI] [PubMed] [Google Scholar]
- Horga G, Schatz KC, Abi-Dargham A, & Peterson BS (2014). Deficits in predictive coding underlie hallucinations in schizophrenia. Journal of Neuroscience, 34(24), 8072–8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jääskeläinen IP, Ahveninen J, Bonmassar G, Dale AM, Ilmoniemi RJ, Levänen S, … Stufflebeam S (2004). Human posterior auditory cortex gates novel sounds to consciousness. Proceedings of the National Academy of Sciences, 101(17), 6809–6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Doneshka P, Grochowski S, & Ritter W (1995). Impaired mismatch negativity generation reflects widespread dysfunction of working memory in schizophrenia. Archives of General Psychiatry, 52(7), 550–558. [DOI] [PubMed] [Google Scholar]
- Kaur M, Battisti RA, Ward PB, Ahmed A, Hickie IB, & Hermens DF (2011). MMN/P3a deficits in first episode psychosis: comparing schizophrenia-spectrum and affective-spectrum subgroups. Schizophrenia research, 130(1-3), 203–209. [DOI] [PubMed] [Google Scholar]
- Kim M, Kim SN, Lee S, Byun MS, Shin KS, Park HY, … Kwon JS (2014). Impaired mismatch negativity is associated with current functional status rather than genetic vulnerability to schizophrenia. Psychiatry Research: Neuroimaging, 222(1-2), 100–106. [DOI] [PubMed] [Google Scholar]
- Knowles EE, David AS, & Reichenberg A (2010). Processing speed deficits in schizophrenia: reexamining the evidence. American Journal of Psychiatry, 167(7), 828–835. [DOI] [PubMed] [Google Scholar]
- Kotov R, Foti D, Li K, Bromet EJ, Hajcak G, & Ruggero CJ (2016). Validating dimensions of psychosis symptomatology: Neural correlates and 20-year outcomes. Journal of abnormal psychology, 125(8), 1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Leong SH, Mojtabai R, Erlanger ACE, Fochtmann LJ, Constantino E, … Bromet EJ (2013). Boundaries of schizoaffective disorder: revisiting Kraepelin. JAMA psychiatry, 70(12), 1276–1286. [DOI] [PubMed] [Google Scholar]
- Latalova K, Prasko J, Diveky T, & Velartova H (2011). Cognitive impairment in bipolar disorder. Biomedical Papers of the Medical Faculty of Palacky University in Olomouc, 155(1). [DOI] [PubMed] [Google Scholar]
- Lee M, Sehatpour P, Hoptman MJ, Lakatos P, Dias EC, Kantrowitz JT, … Javitt DC (2017). Neural mechanisms of mismatch negativity dysfunction in schizophrenia. Molecular psychiatry, 22(11), 1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light GA, & Braff DL (2005). Stability of mismatch negativity deficits and their relationship to functional impairments in chronic schizophrenia. American Journal of Psychiatry, 162(9), 1741–1743. [DOI] [PubMed] [Google Scholar]
- Light GA, Swerdlow NR, Thomas ML, Calkins ME, Green MF, Greenwood TA, … Nuechterlein KH (2015). Validation of mismatch negativity and P3a for use in multi-site studies of schizophrenia: characterization of demographic, clinical, cognitive, and functional correlates in COGS-2. Schizophrenia research, 163(1-3), 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon DH, Roach BJ, & Ford JM (2010). Automatic semantic priming abnormalities in schizophrenia. International Journal of Psychophysiology, 75(2), 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleery A, Mathalon DH, Wynn JK, Roach BJ, Hellemann GS, Marder SR, & Green MF (2019). Parsing components of auditory predictive coding in schizophrenia using a roving standard mismatch negativity paradigm. Psychological medicine, 49(7), 1195–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JR, Rawdon C, Kelleher I, Twomey D, Markey PS, Cannon M, & Roche RA (2013). Reduced duration mismatch negativity in adolescents with psychotic symptoms: further evidence for mismatch negativity as a possible biomarker for vulnerability to psychosis. BMC psychiatry, 13(1), 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näätänen R (1995). The mismatch negativity: a powerful tool for cognitive neuroscience. Ear and hearing, 16(1), 6–18. [PubMed] [Google Scholar]
- Näätänen R, Tervaniemi M, Sussman E, Paavilainen P, & Winkler I (2001). ‘Primitive intelligence’in the auditory cortex. Trends in neurosciences, 24(5), 283–288. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Venables NC, Yancey JR, Hicks BM, Nelson LD, & Kramer MD (2013). A construct-network approach to bridging diagnostic and physiological domains: Application to assessment of externalizing psychopathology. Journal of abnormal psychology, 122(3), 902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, & Thompson WK (2019). The challenges and opportunities of small effects: the new normal in academic psychiatry. JAMA psychiatry, 76(4), 353–354. [DOI] [PubMed] [Google Scholar]
- Pazo-Alvarez P, Cadaveira F, & Amenedo E (2003). MMN in the visual modality: a review. Biological psychology, 63(3), 199–236. [DOI] [PubMed] [Google Scholar]
- Powers AR, Mathys C, & Corlett P (2017). Pavlovian conditioning–induced hallucinations result from overweighting of perceptual priors. Science, 357(6351), 596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Harvey PD, Bowie CR, Mojtabai R, Rabinowitz J, Heaton RK, & Bromet E (2008). Neuropsychological function and dysfunction in schizophrenia and psychotic affective disorders. Schizophrenia Bulletin, 35(5), 1022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM (1992). Trail Making Test: Manual for administration and scoring: Reitan Neuropsychology Laboratory. [Google Scholar]
- Reitan RM (1955). The relation of the trail making test to organic brain damage. Journal of consulting psychology, 19(5), 393. [DOI] [PubMed] [Google Scholar]
- Rentzsch J, Shen C, Jockers-Scherübl MC, Gallinat J, & Neuhaus AH (2015). Auditory mismatch negativity and repetition suppression deficits in schizophrenia explained by irregular computation of prediction error. PloS one, 10(5), e0126775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybarczyk B (2011). Social and occupational functioning assessment scale (SOFAS). Encyclopedia of clinical neuropsychology, 2313–2313. [Google Scholar]
- Salisbury DF, Kuroki N, Kasai K, Shenton ME, & McCarley RW (2007). Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Archives of General Psychiatry, 64(5), 521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JE, Fennig S, Tanenberg-Karant M, Carlson G, Craig T, Galambos N, … Bromet EJ (2000). Congruence of diagnoses 2 years after a first-admission diagnosis of psychosis. Archives of General Psychiatry, 57(6), 593–600. [DOI] [PubMed] [Google Scholar]
- Sheffield JM, Kandala S, Tamminga CA, Pearlson GD, Keshavan MS, Sweeney JA, … Barch DM (2017). Transdiagnostic associations between functional brain network integrity and cognition. JAMA psychiatry, 74(6), 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratling MW (2010). Predictive coding as a model of response properties in cortical area V1. Journal of Neuroscience, 30(9), 3531–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratling MW (2016). Predictive coding as a model of cognition. Cognitive processing, 17(3), 279–305. [DOI] [PubMed] [Google Scholar]
- Stefanics G, Heinzle J, Horváth AA, & Stephan KE (2018). Visual mismatch and predictive coding: a computational single-trial ERP study. Journal of Neuroscience, 38(16), 4020–4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterzer P, Adams RA, Fletcher P, Frith C, Lawrie SM, Muckli L, … Corlett PR (2018). The predictive coding account of psychosis. Biological psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyomaki A, Kusumi I, Matsuyama T, Kako Y, Ito K, & Koyama T (2008). Tone duration mismatch negativity deficits predict impairment of executive function in schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 32(1), 95–99. [DOI] [PubMed] [Google Scholar]
- Trenerry MR, Crosson B, DeBoe J, & Leber WR (1989). Stroop neuropsychological screening test. Psychological Assessment Resources. [Google Scholar]
- Umbricht D, Koller R, Vollenweider FX, & Schmid L (2002). Mismatch negativity predicts psychotic experiences induced by NMDA receptor antagonist in healthy volunteers. Biological psychiatry, 51(5), 400–406. [DOI] [PubMed] [Google Scholar]
- Umbricht D, & Krljes S (2005). Mismatch negativity in schizophrenia: a meta-analysis. Schizophrenia research, 76(1), 1–23. [DOI] [PubMed] [Google Scholar]
- Wacongne C (2016). A predictive coding account of MMN reduction in schizophrenia. Biological psychology, 116, 68–74. [DOI] [PubMed] [Google Scholar]
- Wacongne C, Changeux J-P, & Dehaene S (2012). A neuronal model of predictive coding accounting for the mismatch negativity. Journal of Neuroscience, 32(11), 3665–3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1987). Manual for Wechsler Memory Scale-Revised. Psychological Corporation. San Antonio, Tex, USA. [Google Scholar]
- Wilkinson GS (1993). WRAT-3: Wide range achievement test administration manual: Wide Range, Incorporated. [Google Scholar]
- Wynn JK, Sugar C, Horan WP, Kern R, & Green MF (2010). Mismatch negativity, social cognition, and functioning in schizophrenia patients. Biological psychiatry, 67(10), 940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.