Abstract
Confocal scanning laser microscopy (CSLM) was used to demonstrate the attachment of Escherichia coli O157:H7 transformed with a plasmid encoding for green fluorescent protein (GFP) to the surface and within the internal structures of nonwaxed Red Delicious cv. apples. Apples at 2 or 25°C were inoculated with an E. coli O157:H7 cell suspension at 2 or 25°C. The effect of a negative temperature differential (cold inoculum, warm apple), a positive differential (warm inoculum, cold apple), and no differential (warm inoculum, warm apple), in combination with a pressure differential (atmospheric versus 10,130 Pa), on the attachment and infiltration of cells was determined. CSLM stereo images of external surfaces of apples subjected to all combinations of test parameters showed preferential cellular attachment to discontinuities in the waxy cuticle on the surface and to damaged tissue surrounding puncture wounds, where the pathogen was observed at depths up to 70 μm below the skin surface. Attachment to lenticels was sporadic but was occasionally observed at depths of up to 40 μm. Infiltration through the floral tube and attachment to seeds, cartilaginous pericarp, and internal trichomes were observed in all apples examined, regardless of temperature differential during inoculation. The pressure differential had no effect on infiltration or attachment of E. coli O157:H7. Image analysis to count cells at various depths within tissues was used to quantitatively compare the extent of infiltration into various apple structures as well as the effects of the temperature differential. Puncture wounds harbored greater numbers of the pathogen at greater depths than did other sites examined. Attachment or infiltration of cells was greater on the intact skin and in lenticels, russet areas, and the floral tube of apples inoculated under a negative temperature differential compared to those inoculated under no temperature differential. The results suggest that E. coli O157:H7 attached to internal core structures or within tissues of apples may evade decontamination treatments. Interventions designed to deliver disinfectants to these locations or to remove viable cells of E. coli O157:H7 and other pathogens from apples by other means need to be developed and validated.
Escherichia coli O157:H7 infections associated in recent years with the consumption of nonpasteurized apple juice have raised interest in developing efficacious methods to kill human pathogens that may be present on raw apples and other produce (8, 9, 10, 18, 23). Among the obstacles in achieving this goal is the probability that pathogens infiltrate tissues within produce, giving them protection against chemical sanitizers, physical methods of removal such as brushing or high-pressure spraying, or other commonly used interventions for cleaning and sanitizing (1, 5, 19, 21).
Infiltration of internal structures and tissues of fruits and vegetables by pathogenic bacteria is thought to occur when produce surfaces come in contact with cells suspended in water. In the field, this may occur when rain, dew, or irrigation water collects on the surface of produce or, in the event that fruit falls from trees, as a result of contact with ground water. After harvest, wash and flume waters used to clean fruits and vegetables may provide a vehicle to facilitate the infiltration of microbial cells (2, 3, 27). The potential for infiltration of viable cells is highest if the water is contaminated and antimicrobial agents such as chlorine are ineffective due to low concentration or pH (15).
The U.S. Food and Drug Administration has recommended that packers consider the effects of water temperature when attempting to remove field heat, which is a primary consideration in maintaining the quality of many types of produce (15). The problem of bacterial ingress is exacerbated by differences in water and produce temperatures (2, 6). Several researchers have demonstrated that using wash water at a temperature cooler than that of produce (i.e., a negative temperature differential) will result in the absorption of water into tissues (2, 3, 6, 17, 27). This phenomenon is predicted from the general gas law. As the temperature of fruits and vegetables decreases, gases in their tissues exert a reduced pressure, which causes the combined atmospheric and hydrostatic forces on the immersed produce to equilibrate with the internal pressure, thus facilitating ingress of water (2). Bartz and Showalter (3) demonstrated that tomatoes submerged in a suspension of Serratia marcescens under a negative temperature differential not only contained the organism more frequently but also gained more mass than tomatoes exposed to a positive temperature differential. A negative temperature differential enhances uptake of Salmonella spp. into the stem scar tissues of tomatoes (27). Buchanan et al. (6) showed that apples immersed in an E. coli O157:H7 suspension had high populations of the pathogen in the outer core region, which afforded protection of cells against chlorine treatment. They concluded that the potential for aspirating the pathogen into the internal structures of the fruit was increased by a negative temperature differential.
To date, no research has been published investigating the potential for specific structures of apples such as lenticels, the intact epidermis, and the floral tube to harbor human pathogens. In the study described here, confocal scanning laser microscopy was used to determine and quantify the degree of infiltration and attachment of E. coli O157:H7 to specific tissues and locations on the surface and in the internal structures of intact Red Delicious cv. apples as affected by temperature and pressure differentials.
MATERIALS AND METHODS
Apples.
Red Delicious cv. apples were harvested by hand at maturity from the orchards at the University of Georgia Mountain Experiment Station (Blairsville, Ga.) and transported to our laboratory within 8 h. Apples were stored at 2°C until used.
Microorganism.
E. coli O157:H7 E318, isolated from ground beef, was transformed by Jinru Chen in Mansel W. Griffiths' laboratory, University of Guelph, Ontario, Canada, using a pGFPuv plasmid (Clontech Labs, Inc., Palo Alto, Calif.). This plasmid encodes for the cycle 3 variant of green fluorescent protein (GFP), which shares a common excitation and emission profile with the wild-type GFP, but has been optimized for maximal excitation with UV wavelengths in the range of 360 to 400 nm. The GFPuv variant can be excited at 488 nm. A stock culture was maintained at −79°C in a water-glycerol (70:30, vol:vol) mixture. The organism was transferred monthly to tryptic soy agar (TSA; Difco, Detroit, Mich.) slants containing 100 μg of ampicillin (Sigma, St. Louis, Mo.) per ml, incubated for 24 h at 37°C, and stored at 4°C.
Inoculum.
For each apple to be inoculated, one petri plate containing TSA supplemented with 100 μg of ampicillin per ml (TSAA) was streaked to give confluent growth and incubated for 24 ± 1 h at 37°C. Previous experiments in our laboratory demonstrated that fluorescence intensity of GFPuv-labeled cells is higher in cultures grown on agar media compared to liquid media as detected by epifluorescent microscopy. Bacterial suspensions were prepared by flooding each TSAA plate with 5 ml of 0.01 M sterile potassium phosphate buffer containing 0.85% saline (PBS, pH 7.2) and disrupting the colonies with a sterile bent glass rod. The suspension was removed from the surface of the agar using a pipette and transferred to a sterile 50-ml centrifuge tube. Flooding, suspending cells, and pipetting was repeated twice to produce 15 ml of cell suspension, which was centrifuged at 2,000 × g for 15 min. Because two apples were inoculated at each time of analysis, pellets from cells harvested from two plates were combined after washing in 10 ml of PBS to make 20 ml of suspension. Ten milliliters of this suspension was added to 90 ml of minimal salts medium (MSM) supplemented with 0.04% glucose (13) and 100 μg of ampicillin per ml in a sterile polyethylene bag. MSM, which consisted of K2HPO4 (7.0 g/liter), KH2PO4 (3.0 g/liter), (NH4)2SO4 (1.0 g/liter), MgSO4 · 7H2O (0.1 g/liter), and yeast extract (1 mg/liter), was autoclaved prior to use. After vigorous mixing, bacterial cell suspensions were analyzed spectrophotometrically for absorbency at 590 nm and diluted with MSM as necessary to achieve the desired value of 0.88. The inoculum was then analyzed for population of E. coli O157:H7 by serially dilution in PBS and surface plating (0.1 ml in duplicate) on TSA and TSAA. Plates were incubated for 24 h at 37°C, and presumptive E. coli O157:H7 colonies were counted. E. coli O157:H7 was confirmed by visualizing fluorescent colonies under a long-wave UV light source. The percent expression of the GFPuv plasmid was calculated by comparing counts obtained on the two media.
Inoculation of apples.
Red Delicious apples selected for inoculation were of similar size and free of obvious bruises, cuts, wounds, or other assaults. Apple cortex pH was measured with a flat surface pH electrode (Fisher Scientific, Pittsburgh, Pa.) and pH meter (Denver Instruments, Arvado, Colo.). Immediately before inoculating, apple surfaces were punctured (1 cm deep by 1 mm wide) at five locations between the apex and the base with a sterile blunt nail to represent mechanical injury that may occur during handling. Sets of two apples per analysis were exposed to one of three temperature differentials during inoculation. For apples subjected to a negative temperature differential, two polyethylene bags (18 by 31 cm), each containing 100 ml of bacterial suspension, were placed in an ice bath (2°C) for 15 min. To each bag, an apple tempered at 25°C was added. One apple from the set was subjected to a vacuum by placing one polyethylene bag in a commercial vacuum packager (Koch Model CE-95; Koch, Kansas City, Mo.) and removing 90% of the gaseous phase in the unit cavity. This resulted in a pressure of 10,130 Pa within the bag. The other apple was maintained at atmospheric pressure. Apples subjected to both treatments were maintained in the ice bath for 30 min. For apples subjected to a positive temperature differential, 100 ml of the MSM cell suspension was tempered at 25°C before being used to inoculate apples. Two apples at 2°C were separately placed in polyethylene bags containing the bacterial suspension at 25°C. Both bags (one under vacuum and one at atmospheric pressure) were maintained at 21°C for 30 min. For apples not subjected to a temperature differential, two apples tempered at 25°C were separately added to 100 ml of the MSM cell suspension at 25°C, and bags were maintained under vacuum or atmospheric pressure at 21°C for 30 min. Following the 30-min incubation period, vacuum within bags containing the apples exposed to a pressure differential was released by cutting the bag with a scissors. All apples were then incubated for 18 h at 25°C before prepared for examination using CSLM. All experiments were repeated three times.
Sample preparation.
Bacterial attachment to the intact skin, russet area surrounding the stem, lenticels, and puncture wounds of apples was examined. Apples were removed from inoculum with sterile metal tongs and placed in a sterile plastic basket under a laminar flow hood (class II, type A/B3) for 30 min to dry. Apples were then placed on a sanitized cutting board. Ten 1-by-1-cm sections (five containing puncture wounds) were removed from the surface of each apple with a sterile stainless steel scalpel and rinsed by placing them in 10 ml of filtered (0.45 μm [pore size]), autoclaved, distilled water by gentle agitation (90 rpm) on a rotary shaker for 2 min. Surface sections were then removed with a sterile forceps, placed in a sterile petri plate under the laminar flow hood, and dried for 10 min.
Bacterial attachment to four internal structures, i.e., the floral tube, ventral cavity, seed locule, and seed integument, was examined (Fig. 1). Samples were prepared by removing the core of apples with a sterile stainless steel corer and slicing the core cylinder transversely above and below the seed locules with a sterile knife. The middle section containing the seeds was then cut longitudinally, and sections of cartilaginous pericarp of locules and the ventral core cavity and seeds were removed using a sterile stainless steel blade. Internal structures were rinsed and allowed to dry for 10 min as described above.
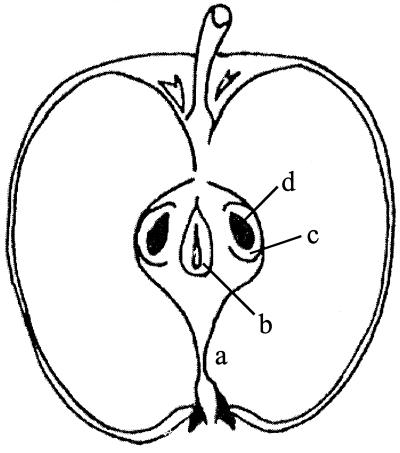
FIG. 1.
Longitudinal cross section of a Red Delicious apple showing the floral tube (a), ventral cavity (b), seed locules (c), and seeds (d).
Mounts for microscopic analysis were prepared by placing 0.3 ml of silica gel (Dow Corning, Midland, Mich.) on clean glass microscope slides. Inoculated apple sections were placed on silica gel followed by a drop of filtered (0.45 μm [pore size]) glycerol. A coverslip was then placed on the apple specimen with gentle downward pressure to facilitate adherence to the silica gel.
Visualization with CSLM.
Samples were analyzed using a Bio-Rad MRC-600 confocal scanning laser microscope (Bio-Rad, Inc., Hemel Hempstead, United Kingdom) equipped with a 50-mW argon-krypton laser. The scanning head was mounted on a Nikon Optiphot microscope (Nikon, Tokyo, Japan) fitted with a ×40 (numerical aperture = 1.30) oil immersion objective (Nikon) lens. The system was operated by the Confocal Microscope Operating Software (COMOS), version 7.1, supplied by Bio-Rad. The green fluorescence of GFPuv-labeled E. coli O157:H7 cells was detected using an excitation wavelength of 488 nm. Emitted light was collected through a 480-nm dichroic mirror, a 520-nm long-pass filter, and a 680-nm short-pass filter. Samples from random locations on each apple were examined for bacterial attachment to the eight external and internal areas and structures described above. Kalman averaging (n = 4) was used to discern attached from unattached cells. Attached cells in micrographs generated from this technique are bright and clearly defined, whereas unattached cells are less bright and blurred. Selected CSLM optical thin sections were stacked using COMOS to construct stereo projections which were formatted using CorelDRAW 8.0. Readers may attain three-dimensional views of these images by positioning a stereo viewer in front of the micrographs so that the left and right lenses are aligned above the left and right images, respectively. Alternatively, a three-dimensional view can be achieved without a viewer by separately observing the left and right figure with the left and right eyes, respectively.
Image analysis.
Digital image analysis was performed using Scion Image, a software package based on NIH Image for Macintosh created by the Scion Corporation (Frederick, Md.). The number of cells at various depths within tissues was determined to quantitatively compare attachment to the eight structures examined, as well as the effect of a negative or positive temperature differential during inoculation. Since qualitative observations revealed no influence on infiltration or attachment due to a pressure differential during inoculation of apples, quantitative analysis to determine numbers and position of cells on and in structures was not done using these apples. Representative CSLM stacks of samples prepared from apples that had not been subjected to a pressure differential were selected for quantitative examination by visualization of three-dimensional reconstructions. Cells were counted in optical slices positioned more than 3 μm apart to avoid counting the same cell more than once. Image thresholding and particle analysis were calibrated to program the software to count pixel clusters of an appropriate intensity and size. Each optical slice measured 326 by 218 μm, with a depth of resolution along the z axis of 3 μm, giving a volume of each region examined equal to approximately 2.13 × 105 μm3. Data are expressed as the number of cells per region examined, which are plotted on the y axis against depth (in microns) into tissues on the x axis.
RESULTS
Attachment and infiltration of E. coli O157:H7 to external surface structures.
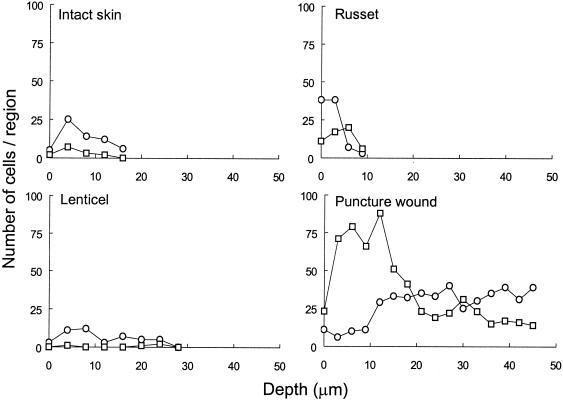
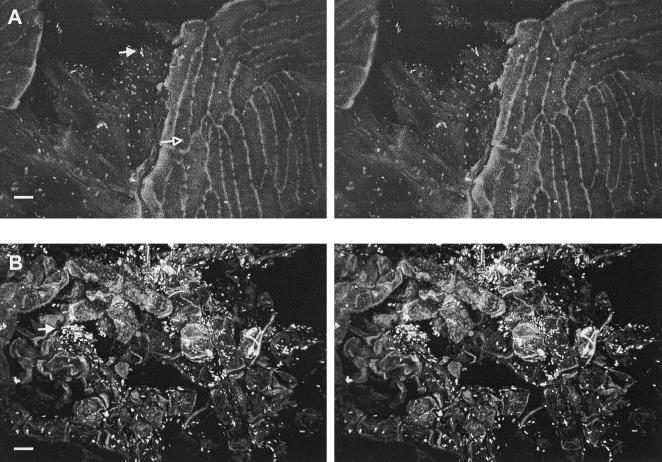
Populations of E. coli O157:H7 in the MSM used to inoculate apples ranged from 8.23 to 8.31 log10 CFU/ml throughout this study. Attachment of E. coli O157:H7 cells to intact apple skin occurred primarily at discontinuities in the waxy cuticle, including clefts and against crests located between epidermal cells (Fig. 2A). The depth of clefts ranged from 10 to 16 μm below the surrounding cuticle, and cells were attached at various depths. In general, single cells attached to the waxy cuticle. However, clumping was observed on the apple shown in Fig. 2B that was inoculated under a negative temperature differential. Although qualitative differences in number of E. coli O157:H7 cells attached to or infiltrating external structures were difficult to discern, image analysis (Fig. 3) illustrates that a higher number of cells infiltrated intact skin, russet areas, and lenticels of apples inoculated under a negative temperature differential compared to the same structures of apples inoculated under a positive temperature differential.
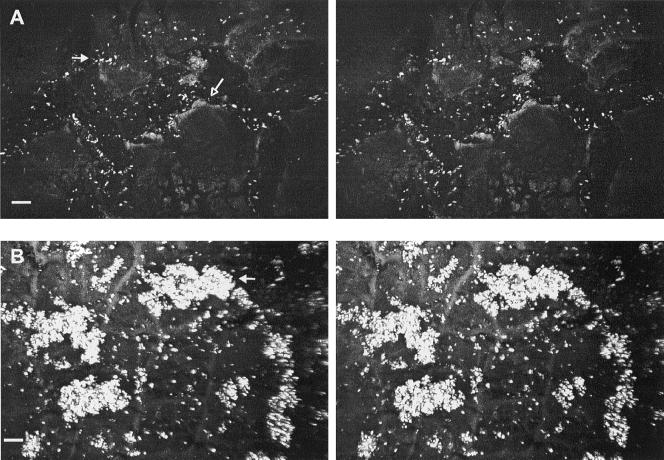
FIG. 2.
CSLM stereo images showing attachment of E. coli O157:H7 on intact apple surface. (A) Cleft (16-μm depth) in the waxy cuticle (open arrow); most cells are attached within the cleft (closed arrow). (B) Clusters of cells (arrow) on intact cuticle 34 μm in height. Cells were inoculated under a negative temperature differential. Bar, 10 μm.
FIG. 3.
Infiltration of fluorescent E. coli O157:H7 into external surface structure of apples as affected by negative (○) or positive (□) temperature differentials. The number of cells at various depths below the surface was determined by image thresholding and particle analysis in selected regions (213,000 μm3) of CSLM stacks.
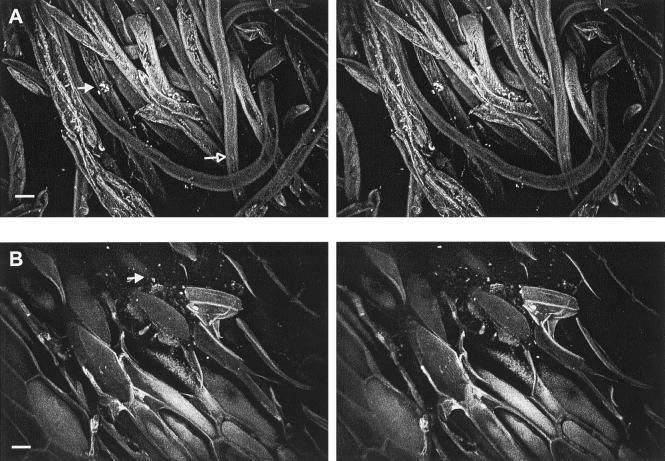
Apple lenticels and russet areas, composed of loosely packed wax platelets, typically did not attract a high number of attached E. coli O157:H7 cells. However, cuticular cracks and narrow crevices radiating from these structures were heavily colonized (Fig. 4A). Colonization of lenticels was sporadic. For example, on a given apple, most lenticels contained a few cells just inside their openings, while an occasional lenticel was heavily colonized with both attached and unattached cells (Fig. 4B). Cells in colonized lenticels were detected at depths of 40 to 50 μm below the surface with little overall influence of temperature (Fig. 3) or pressure differentials. Raised russet areas provided for moderate attachment; in general, cells were attached to russet walls and to waxy clefts within groves (Fig. 4C). Image analysis of russet areas inoculated under negative and positive temperature differentials indicate that representative samples do not vary significantly in cell numbers at depths of greater than ca. 5 μm (Fig. 3).
FIG. 4.
CSLM stereo images showing attachment of E. coli O157:H7 to apple lenticels and russet areas. (A) Narrow fissure (42-μm depth) radiating from a lenticel heavily colonized with cells (arrow). (B) Lenticel harboring attached (closed arrow) and unattached (open arrow) cells. (C) Unattached and attached cells on wax platelets of russet. Bar, 10 μm.
E. coli O157:H7 cells attached preferentially to damaged tissues surrounding puncture wounds in the skin. The pathogen heavily colonized these sites in samples subjected to all treatments and was detected at depths up to 70 μm below the tissue surface. Deep, narrow crevices radiating from torn skin tissues, in particular, held dense biofilm-like matrices of cells (Fig. 5). Infiltration of tissues within puncture wounds was influenced by temperature differential. Figure 3 illustrates the consistent presence of cells at depths up to 45 μm within the tissue of representative samples inoculated under a negative and positive temperature differentials. Greater numbers of cells at depths less than 18 μm in the puncture wound of apples inoculated under positive pressure were observed, while cell numbers in the puncture wound from apples inoculated under negative pressure increased at 18 μm and remained greater to depths of up to 45 μm.
FIG. 5.
CSLM stereo images showing attachment of E. coli O157:H7 to tissue surrounding skin puncture wounds (open arrows). Heavily colonization (closed arrows) of damaged tissue is shown to depths of 48 μm (A) and 70 μm (B) below the surface. Bar, 10 μm.
Attachment and infiltration of E. coli O157:H7 to internal structures.
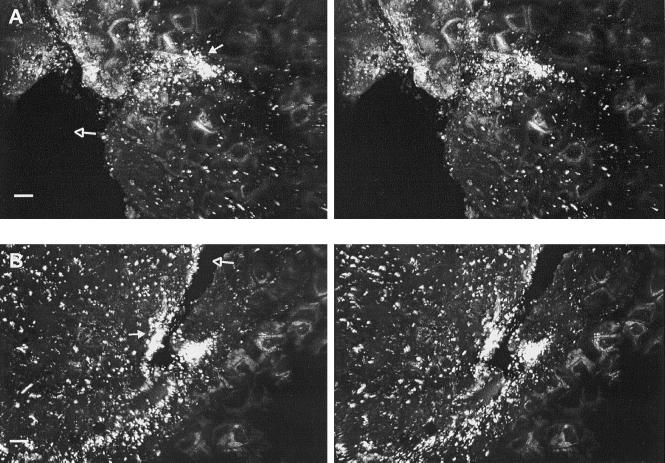
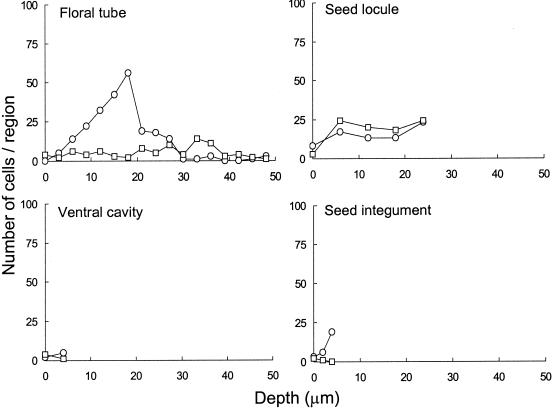
Infiltration of E. coli O157:H7 into the core of intact apples was observed in all samples, regardless of treatment. The floral tube of mature Red Delicious cv. apples remains open from the blossom to the cartilaginous pericarp of the ventral core cavity. The wall of the floral tube, composed of waxy cuticle similar to that on the skin, did not harbor high numbers of attached cells. However, the pathogen attached readily to the apple flower remnants and internal trichomes just within the floral tube (Fig. 6A). Internal trichomes typically formed dense mats, commonly entrapping cells within. Yeast cells were often observed within the floral tube, with no relation perceived between their presence and the attachment of E. coli O157:H7. Image analysis of samples representing floral tube tissues from apples inoculated under negative or positive temperature differentials revealed higher numbers of cells at depths up to 20 μm in samples inoculated under a negative temperature differential (Fig. 7).
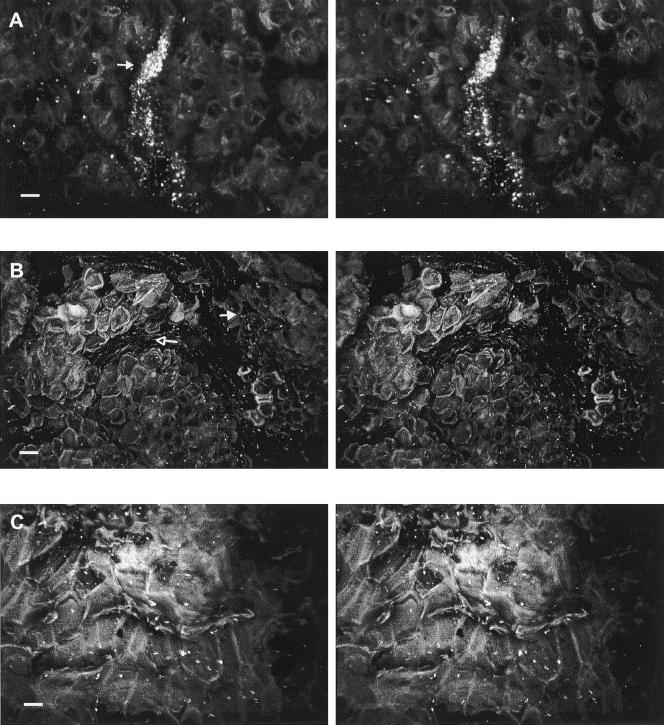
FIG. 6.
CSLM stereo images showing attachment of E. coli O157:H7 to internal trichomes (open arrow) of the floral tube at a depth of 52 μm where cells (closed arrow) are attached to or entrapped within the trichome network (A) and seed integument (18 μm depth), which harbored few attached cells (closed arrow) (B). Bar, 10 μm.
FIG. 7.
Infiltration of fluorescent E. coli O157:H7 into the internal structure of apples as affected by negative (○) or positive (□) temperature differentials. The number of cells at various depths below the surface was determined by image thresholding and particle analysis in selected regions (213,000 μm3) of CSLM stacks.
After passing through the floral tube, cells enter into the apple core, which consists of a ventral cavity and seed locules comprised of cartilaginous pericarp and seeds. The ventral core cavity of each inoculated apple contained fluid, indicating that several milliliters of the inoculum were imbibed. CSLM examination of the ventral cavity pericarp, locule pericarp, and seed integument revealed that dispersal and attachment of E. coli O157:H7 occurred throughout the apple core. Moreover, discoloration of the cortex surrounding the core suggests that some degree of infiltration occurred into the intercellular air spaces of parenchyma cells that make up the cortex. CSLM visualization of this tissue, however, was limited due to the extreme autofluorescent characteristics of the parenchyma cells. The cartilaginous pericarp of the ventral cavity and seed locules of mature apples consists of an irregular waxy tissue occasionally containing trichomes and deep crevices and ridges. Infiltration of cells into smooth regions of the cartilaginous pericarp of the ventral cavity and seed locules was minimal (Fig. 8A), whereas cells heavily colonized trichomes, crevices, and ridges (Fig. 8) associated with these structures. Image analysis of representative ventral cavity and seed locule samples revealed no influence of temperature differential during inoculation on penetration of these tissues by E. coli O157:H7 cells (Fig. 7). However, a peak in cell numbers at a depth of 5 μm was observed in the seed integument sample of apples inoculated under a negative temperature differential compared with no penetration of cells into the integument of a seed from apples inoculated under a positive temperature differential.
FIG. 8.
CSLM stereo images showing attachment of E. coli O157:H7 to the ventral cavity. (A) More attached cells (closed arrow) were observed in crevices (38-μm depth) than on the smooth regions of cartilaginous pericarp (open arrow). (B) Irregular tissue (42-μm depth) on the ventral cavity harboring many cells (closed arrow). Bar, 10 μm.
DISCUSSION
Researchers conducting an investigation to determine the cause of a 1996 outbreak of E. coli O157:H7 infection associated with apple juice concluded that apples delivered to a juice production plant harbored the pathogen (12). Of the three lots suspected to be associated with the outbreak, two were shipped directly from the same orchard. The juice company involved had issued written statements advising suppliers that it would accept only handpicked apples, but no mechanism was provided to ensure compliance. Furthermore, seasonal workers on the farm, who are paid by the number of bins harvested, were instructed not to harvest apples from the ground, but no system was in place to enforce this policy. Fresh deer feces collected from a wildlife refuge 0.25 miles from the farm was shown to contain E. coli O157:H7, although not with the same pulsed-field gel electrophoresis pattern as the isolates from patients and juice involved in the outbreak. However, it is plausible that deer, like cattle (12), may carry and excrete more than one strain. Thus, the probable use of drops in the production of juice implicated in this outbreak must be considered a possible way in which contamination occurred.
Contamination of apples may also occur at several points during pre- and postharvest handling and processing of apple juice and cider. Dingman (14) found no correlation between the frequency of isolation of E. coli and the use of drops in the production of nonpasteurized apple juice after analyzing samples from 11 cider mills in Connecticut. Fruit flies (16), dust, harvesting equipment, and irrigation water (4), however, have been noted as serving as sources of pathogenic microorganisms on produce.
Our study has shown that E. coli O157:H7 can pervade the inner core of sound Red Delicious apples, dispersing and attaching to the cartilaginous pericarp of the ventral cavity and seed locules, and to seed integument. Infiltration occurs through the blossom end of the calyx and progresses up the floral tube into the core region. The internal trichomes within the floral tube entrapped the pathogen, which may contribute to observations made by other researchers (6) that greater numbers of E. coli O157:H7 inoculated onto intact apples were recovered from the outer core regions compared to the apple skin. Our study supports the evidence presented by others that the calyx end of the apple is an area of great concern with regard to infiltration of bacteria and the resulting inaccessibility to contact with sanitizers. High numbers of attached cells were observed not only in this region but also throughout the core structures, indicating that the pathogen may evade mechanical scrubbing, treatment with chemicals, and other interventions applied to reduce or eliminate pathogens on apples. Suffusion of the liquid cell suspension within the floral tube, ventral cavity, and seed locules further decreases the effectiveness of surface treatment solutions because of their dilution in and inability to replace the moisture present on the surface of these structures. Several researchers have noted that contaminated flume and wash waters are likely vehicles of bacteria for ingress within the tissues of apples (5, 6, 15, 17). However, infiltration may be just as likely to occur on the field or in transport, since irrigation and rain water may serve as navigation systems to facilitate contact of pathogens with fruit. If in contact with the apple for sufficient time, the contaminated water may progress up the floral tube or into compromised areas on the surface by capillary action. Thus, contaminated apples entering into the processing facility would not be effectively sanitized in wash or flume waters.
E. coli O157:H7 cells attached preferentially to damaged tissues surrounding puncture wounds in the apple skin. The dense colonization of puncture wounds consistently observed throughout this study supports research by Janisiewicz et al. (16), who found that E. coli O157:H7 grew exponentially in Golden Delicious apple tissue when inoculated onto puncture wounds. Sapers et al. (20) observed higher counts of nonpathogenic E. coli associated with inoculated apple halves compared with intact apples. These workers surmised that more cells were associated with damaged surfaces than intact surfaces, a finding which is in agreement with observations made in our study. Biofilm-like matrices (Fig. 2 and 5) were visualized in narrow fissures emanating from torn skin of wounds. While the acidic (pH 4.1) environment in the wound tissue may impose a stress on E. coli O157:H7, the incubation time (18 h) and temperature (25°C) were such to enable the growth of the pathogen in compromised tissue. Thus, the formation of a biofilm may have occurred. Biofilm formation on fresh produce has been observed with confocal microscopy (7) and has ramifications in terms of surface disinfection treatments applied to fruits and vegetables because of diffusion or penetration of active disinfectants into biofilm matrices (24). However, without the use of an appropriate fluorochrome to stain bacterial exopolysaccharide, which is produced during biofilm formation, we cannot conclude that the matrices visualized in this study were indeed a biofilm.
Although attachment to the waxy cuticle layer on the surface of intact skin occurred, fewer cells were observed compared to those in puncture wounds. Other workers (22, 25) have noted similar behavior of E. coli O157:H7 on lettuce leaves, where the pathogen attached preferentially to cut edges of leaves rather than to intact surfaces. In our study, MSM with 0.04% glucose and 100 μg of ampicillin per ml was used as the suspension medium for inoculation, which may represent conditions approximating contaminated irrigation or surface water. Cells of E. coli O157:H7 cells grown in this medium were more hydrophobic than cells grown in tryptic soy broth (13), probably due to changes in bacterial membrane fatty acid composition that are brought about by the nutrient-limited environment. Thus, attachment to hydrophobic structures such as the waxy cuticle, lenticels, and russet areas would be maximized. Lenticels, however, only sporadically harbored E. coli O157:H7 cells. The development of lenticels on mature apples is a result of the formation of cutin within broken trichomes or stomata, whose guard cells remain permanently opened due to the stretching which accompanies growth. In the young fruit, stomata serve as a vehicle for gas exchange. As lenticels form and loosely packed wax platelets develop, the structure usually closes and remains impermeable to the passage of liquids and gases even under pressure (11). Approximately 5% of lenticels on the Red Delicious cultivar, however, remain open (11), which is likely to account for the infrequent and sporadic observation of cells within lenticels. The bacterial suspension suffused into the open lenticel, whereas closed lenticels remained largely void of cells as a result of the pressure differential created.
Image analysis of CSLM stacks made possible the quantification of infiltration and attachment of E. coli O157:H7 to specific structures along the z axis of samples as influenced by temperature differential. A marked effect of negative or positive temperature differential was not readily discernible, due in part to a slow diffusion of the bacterial suspension within tissues over the 18-h incubation period following inoculation. Inoculation under a negative temperature differential enhanced infiltration of the intact skin, lenticels, russet area, floral tube, and seed integument. The effect of temperature differential on the infiltration of cells into the ventral cavity was inconclusive. However, a positive temperature differential appeared to enhance infiltration into the seed locule and clearly resulted in greater infiltration at depths of up to 18 μm into the tissue surrounding puncture wounds. At depths of 20 to 45 μm, inoculation under a negative temperature differential enabled higher numbers of E. coli O157:H7 cells to infiltrate wound tissue compared to inoculation under a positive temperature differential. At the end of the 18-h incubation period at 25°C following inoculation, apples were removed from the inoculum and placed under the laminar flow hood at 21°C to dry for 30 min. Cooling of superficial air spaces in porous puncture wound tissue of the fruit during the drying process could have resulted in cell ingress, which may account for the high number of cells present within a depth of 2 to 18 μm below the surface of wound tissue of apples inoculated under positive pressure. We hypothesize that cells of E. coli O157:H7 on surfaces of apples inoculated under a positive temperature differential were less intimately associated with the surface than were cells inoculated using a negative temperature differential. It is likely that the drying step involved in the sampling process created a slight negative temperature differential, resulting in some degree of infiltration.
Infiltration of the pathogen into and throughout the core was observed in all apples analyzed, regardless of treatment. This observation may be, in part, a result of capillary action, which would draw the inoculum into the core area over the 18-h incubation period. Several researchers working with produce have demonstrated the influence of temperature differentials with the uptake of dye solutions (6, 17) and bacterial suspensions (2, 6). When exposed to a dye solution for 30 min under a negative temperature differential, 18 of 113 (15.9%) nonwaxed Golden Delicious apples imbibed the dye within core structures, whereas no apples exposed to a positive temperature differential were impregnated with the dye (6). Similar results showing dye uptake in oranges and grapefruits have been recorded (17, 26). Thus, we surmise that apples inoculated under a negative temperature differential experienced greater infiltration of E. coli O157:H7 cells into the core and surface structures within the first 30 min after exposure compared to apples inoculated under a positive temperature differential, while subsequent incubation for 18 h at 25°C under no or a positive temperature differential allowed cell ingress of apples into puncture wound and core tissues. Such phenomena would not be unlikely to occur in the field, during transport, or in the packing house.
This study clearly demonstrates that E. coli O157:H7 can infiltrate the core and subsurface structures on the skin of apples, which may reduce the efficacy of chemical sanitizers, physical treatments such as brushing, and other methods applied to remove, reduce, or eliminate pathogenic microorganisms that may be present. Interventions designed to deliver disinfectants to locations within apple tissues or to remove viable E. coli O157:H7 and other pathogens from these tissues need to be developed and validated.
ACKNOWLEDGMENTS
We are grateful to Joe Garner at the Georgia Mountain Station, University of Georgia, for the apples used in this study and to Mark Farmer at the Center for Advanced Ultrastructural Research, University of Georgia for his technical instruction and assistance with CSLM application.
REFERENCES
- 1.Adams M R, Hartley A D, Cox L J. Factors affecting the efficacy of washing procedures used in the production of prepared salads. Food Microbiol. 1989;6:69–77. [Google Scholar]
- 2.Bartz J A. Infiltration of tomatoes immersed at different temperatures to different depths in suspensions of Erwinia carotovora subsp. carotovora. Plant Dis. 1982;66:302–305. [Google Scholar]
- 3.Bartz J A, Showalter R K. Infiltration of tomatoes by aqueous bacterial suspensions. Phytopathology. 1981;71:515–518. [Google Scholar]
- 4.Beuchat L R. Pathogenic microorganisms associated with fresh produce. J Food Prot. 1996;59:204–216. doi: 10.4315/0362-028X-59.2.204. [DOI] [PubMed] [Google Scholar]
- 5.Beuchat L R, Nail B V, Adler B B, Clavero M R S. Efficacy of spray application of chlorinated water in killing pathogenic bacteria on raw apples, tomatoes, and lettuce. J Food Prot. 1998;61:1305–1311. doi: 10.4315/0362-028x-61.10.1305. [DOI] [PubMed] [Google Scholar]
- 6.Buchanan R L, Edelson S G, Miller R L, Sapers G M. Contamination of intact apples after immersion in an aqueous environment containing Escherichia coli O157:H7. J Food Prot. 1999;62:444–450. doi: 10.4315/0362-028x-62.5.444. [DOI] [PubMed] [Google Scholar]
- 7.Carmichael I, Harper I S, Coventry M J, Taylor P W J, Wan J, Hickey M W. Bacterial colonization and biofilm development on minimally processed vegetables. J Appl Microbiol Symp Suppl. 1999;85:45S–51S. doi: 10.1111/j.1365-2672.1998.tb05282.x. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Outbreak of Salmonella serotype Muenchen infections associated with unpasteurized orange juice—United States and Canada, June 1999. Morb Mortal Wkly Rep. 1999;48:582–585. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Outbreaks of Escherichia coli O157:H7 infection and cryptosporidiosis associated with drinking unpasteurized apple cider—Connecticut and New York, October 1996. Morb Mortal Wkly Rep. 1997;46:4–8. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Outbreaks of Escherichia coli O157:H7 infection associated with drinking unpasteurized commercial apple juice—British Columbia, California, Colorado, and Washington, December. Morb Mortal Wkly Rep. 1996;276:1865–1866. [PubMed] [Google Scholar]
- 11.Clements H F. Morphology and physiology of the pome lenticels of Pyrus malus. Bot Gaz. 1935;97:101–117. [Google Scholar]
- 12.Cody S H, Glynn M K, Farrar J A, Cairns K L, Griffin P M, Kobayashi J, Fyfe M, Hoffman R, King A S, Lewis J H, Swaminathan B, Bryant R G, Vugia D J. An outbreak of Escherichia coli O157:H7 infection from unpasteurized commercial apple juice. Ann Intern Med. 1999;130:202–209. doi: 10.7326/0003-4819-130-3-199902020-00005. [DOI] [PubMed] [Google Scholar]
- 13.Dewanti R, Wong A C L. Influence of culture conditions on biofilm formation by Escherichia coli O157:H7. Int J Food Microbiol. 1995;26:147–167. doi: 10.1016/0168-1605(94)00103-d. [DOI] [PubMed] [Google Scholar]
- 14.Dingman D W. Prevalence of Escherichia coli in apple cider manufactured in Connecticut. J Food Prot. 1999;62:567–573. doi: 10.4315/0362-028x-62.6.567. [DOI] [PubMed] [Google Scholar]
- 15.Food and Drug Administration. Guidance to industry: guide to minimize microbial food safety hazards for fresh fruits and vegetables. Washington, D.C.: CFSAN; 1998. [Google Scholar]
- 16.Janisiewicz W J, Conway W S, Brown M W, Sapers G M, Fratamico P, Buchanan R L. Fate of Escherichia coli O157:H7 on fresh-cut apple tissue and its potential for transmission by fruit flies. Appl Environ Microbiol. 1999;65:1–5. doi: 10.1128/aem.65.1.1-5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merker R, Edelson-Mammel S, Davis V, Buchanan R L. Preliminary experiments on the effect of temperature differences on dye uptake by oranges and grapefruits. Washington, D.C.: U.S. Food and Drug Administration, Center for Food Safety and Applied Nutrition; 1999. [Google Scholar]
- 18.Millard P S, Gensheimer K F, Addiss D G, Sosin D M, Beckett G A, Houck-Jankoski A, Hudson A. An outbreak of cryptosporidiosis from fresh-pressed apple cider. JAMA. 1994;272:1592–1596. [PubMed] [Google Scholar]
- 19.Parish M E. Public health and unpasteurized fruit juices. Crit Rev Microbiol. 1997;23:109–119. doi: 10.3109/10408419709115132. [DOI] [PubMed] [Google Scholar]
- 20.Sapers G M, Miller R L, Mattrazzo A M. Effectiveness of sanitizing agents in inactivating Escherichia coli in Golden Delicious apples. J Food Science. 1999;64:734–737. [Google Scholar]
- 21.Senkel S I, Henderson R A, Jobitado B, Meng J. Use of hazard analysis critical control point and alternative treatments in the production of apple cider. J Food Prot. 1999;62:778–785. doi: 10.4315/0362-028x-62.7.778. [DOI] [PubMed] [Google Scholar]
- 22.Seo K H, Frank J F. Attachment of Escherichia coli O157:H7 to lettuce leaf surface and bacterial viability in response to chlorine treatment as demonstrated by using confocal scanning laser microscopy. J Food Prot. 1999;62:3–9. doi: 10.4315/0362-028x-62.1.3. [DOI] [PubMed] [Google Scholar]
- 23.Steele B T, Murphy N, Arbus G S, Rance C P. An outbreak of hemolytic uremic syndrome associated with ingestion of fresh apple juice. J Pediatr. 1982;101:963–965. doi: 10.1016/s0022-3476(82)80021-8. [DOI] [PubMed] [Google Scholar]
- 24.Stewart P S, Murga R, Srinivasan R, de Beer D. Biofilm structural heterogeneity visualized by three microscopic methods. Water Res. 1995;29:2006–2009. [Google Scholar]
- 25.Takeuchi K, Frank J F. Penetration of Escherichia coli O157:H7 into lettuce tissues as affected by inoculum size and temperature and the effect of chlorine treatment on cell viability. J Food Prot. 2000;63:434–440. doi: 10.4315/0362-028x-63.4.434. [DOI] [PubMed] [Google Scholar]
- 26.Walderhaug M O, Edelson-Mammel S G, DeJesus A J, Eblen B S, Miller A J, Buchanan R L. Preliminary studies on the potential for infiltration growth and survival of Salmonella enterica serovar Hartford and Escherichia coli O157:H7 within oranges. Washington, D.C.: U.S. Food and Drug Administration, Center for Food Safety and Applied Nutrition; 1999. [Google Scholar]
- 27.Zhuang R-Y, Beuchat L R, Angulo F J. Fate of Salmonella montevideo on and in raw tomatoes as affected by temperature and treatment with chlorine. Appl Environ Microbiol. 1995;61:2127–2131. doi: 10.1128/aem.61.6.2127-2131.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]