OBJECTIVES:
The anti-α4β7 integrin antibody vedolizumab (VDZ) is successfully used for the treatment of inflammatory bowel diseases. However, only a subgroup of patients respond to therapy. VDZ is administered at a fixed dose, leading to a wide range of serum concentrations in patients. Previous work from our group showed a dose-dependent preferential binding of VDZ to effector compared with regulatory CD4+ T cells. Therefore, we aimed to determine the dose-dependent binding profile of VDZ to other leukocyte subsets.
METHODS:
We characterized α4β7 integrin expression on CD8+ T cells, CD19+ B cells, CD14+ monocytes, natural killer cells, and eosinophils from patients with inflammatory bowel disease and healthy controls. We studied the binding of VDZ to these cells at different concentrations and investigated the functional consequences for dynamic adhesion and transmigration in vitro.
RESULTS:
The expression of α4β7 differed between the analyzed leukocyte subsets and was significantly higher on eosinophils from inflammatory bowel disease patients compared with controls. Almost all α4β7-expressing cells from these subsets were bound by VDZ at a concentration of 10 μg/mL. Dynamic cell adhesion was significantly impaired in all subsets, but there were no dose-dependent differences in the inhibition of adhesion.
DISCUSSION:
Our data suggest that α4β7-expressing CD8+ T cells, CD19+ B cells, CD14+ monocytes, natural killer cells, and eosinophils are a target of VDZ. However, there do not seem to be concentration-dependent differences, regarding the effects on these cells in the clinically relevant range. Thus, the reported exposure-efficacy characteristic of VDZ can probably mainly be attributed to CD4+ T-cell subsets.
INTRODUCTION
Anti-trafficking agents with the prime example of the anti-α4β7 integrin antibody vedolizumab (VDZ) have emerged as a new pillar for the treatment of the inflammatory bowel diseases (IBDs) Crohn's disease (CD) and ulcerative colitis (UC) (1,2). The α4β7 integrin heterodimer is expressed on a plethora of leukocytes and mediates firm adhesion to and subsequent extravasation through the endothelium into the tissue (a process known as homing). This is mediated by interaction with its ligand mucosal addressin cell adhesion molecule (MAdCAM)-1, expressed on high endothelial venules of the gut (3). By binding to the integrin, VDZ blocks the homing of T cells into the gut, hence reducing the number of mucosal immune cells and attenuating inflammation.
Although the efficacy and safety of VDZ has repetitively been demonstrated, not all patients respond to therapy. VDZ is administered at a fixed dose, leading to a wide range of actual serum concentrations in patients (4,5). Drug-level monitoring studies have suggested that low serum trough levels of VDZ may at least partly contribute to nonresponse to therapy (6,7). In addition, 2 independent phase 2 trials demonstrated suboptimal outcomes at highest drug levels and suggested a bell-shaped exposure-efficacy characteristic (8,9). Consistently, previous work from our group demonstrated a dose-dependent preferential binding pattern of VDZ to CD4+ T-cell subsets that correlated with clinical remission and suggested an optimal therapeutic window for VDZ therapy in IBD (10).
However, α4β7 expression and VDZ binding have also been observed on a variety of other leukocyte subsets including innate immune cells such as eosinophils (11). Although these subsets might also contribute to the exposure-efficacy characteristics of VDZ, detailed analyses of the dose-dependent binding of VDZ to and function in these subsets are lacking so far.
In this article, we explored the expression of α4β7 on various further leukocyte subset and studied dose-dependent effects of VDZ on dynamic adhesion and transmigration. We show that the potential of VDZ to block α4β7 on different leukocytes is similar at different clinically relevant VDZ concentrations. Thus, our data argue for effects of VDZ beyond CD4+ T cells, but also support the concept that exposure-efficacy profiles are mainly driven by CD4+ T cells.
METHODS
Human blood samples
To evaluate the integrin expression as well as the dose-dependent binding of VDZ in vitro, peripheral EDTA-anticoagulated blood was collected from patients with IBD not receiving VDZ therapy (UC or CD) and from healthy donors as controls. The samples were obtained at the IBD Outpatient Clinic of the Department of Medicine 1 of the University Hospital Erlangen, Germany, after the participants' informed written consent. All procedures were approved by the Ethics Committee of the Friedrich-Alexander-University Erlangen-Nuremberg, Germany (426_20B).
Characteristics of study subjects with CD, UC, and control donors are summarized in Table S1 (see Supplementary Digital Content 2, http://links.lww.com/CTG/A811).
Isolation of human PBMCs and granulocytes
Peripheral blood mononuclear cell (PBMC) isolation was performed using density gradient centrifugation with Pancoll (PAN-Biotech) or lymphocyte separation medium (Anprotec). After separation, PBMCs were washed, counted, and stained for flow cytometry or used for subsequent cell isolation followed by functional in vitro assays.
For granulocyte isolation, the cell pellet obtained after density gradient centrifugation was suspended in 1% Dextran 500 (Roth) in PBS, and erythrocytes were left to sediment for 30 min at room temperature. The granulocyte-enriched supernatant was transferred into a fresh tube, and the remaining erythrocytes were lysed using hypotonic lysis (1 minute 0.2 M NaCl and 1 minute 1.6 M NaCl).
Magnetic-activated cell sorting
Magnetic-activated cell sorting (MACS) was used to isolate different leukocyte subsets for functional in vitro dynamic adhesion and transmigration assays. Isolation was performed according to the manufacturer's instruction. In short, for direct isolation of CD8+, CD19+, and CD14+ cells, a single cell suspension of PBMCs was incubated with magnetic beads conjugated with antibodies specific for CD8, CD19, or CD14 (Miltenyi Biotec), respectively, at 4 °C for 15 minutes, and excess beads were washed away by centrifugation. Subsequently, the cell suspension was placed onto separation columns in a magnetic field. The cells of interest bound to magnetic beads remained in the column and were eluted by flushing the columns outside the magnetic field after 3 washing steps. For indirect isolation of natural killer (NK) cells and eosinophils, single cell suspensions of PBMCs (NK) or granulocytes (eosinophils) were incubated with antibody cocktails containing biotinylated antibodies against all other cell types and secondary anti-biotin magnetic beads (Miltenyi Biotec). Subsequent loading of the cell suspension onto a column inside a magnetic field retained all cells except the target cells, which were collected with the flow-through.
Purity of the cell isolation was assessed by flow cytometry, and isolated cells were used for subsequent functional assays.
Flow cytometry
For flow cytometry, cells were stained according to standard protocols for 15 minutes at 4 °C using the following fluorochrome-conjugated extracellular antibodies: CD3 (VioGreen, REA613, Miltenyi Biotec), CD8 (PerCP/Cy5.5, RPA-T8, BioLegend; AF647, SK1, BioLegend), CD19 (VioBlue, Miltenyi Biotec), CD16 (APC/Cy7, 3G8, BioLegend), CD14 (AF488, HCD14, BioLegend, VioBlue, TÜK4, Miltenyi Biotec), CD56 (PE-Vio770, REA196, Miltenyi Biotec; FITC, HCD56, BioLegend), CCR3 (FITC, 5E8, BioLegend), Siglec 8 (PE-Dazzle594, 7C9, BioLegend), CD49d (VioBlue, MZ18-24A9, Miltenyi Biotec; PE/Cy7, 9F10, BioLegend), and integrin beta 7 (PE, FIB27, BioLegend; BV605, FIB504, BD Biosciences). Where indicated, VDZ (Entyvio, Takeda) was labeled using the Alexa Fluor Antibody Labeling Kits (AF674, Life Technologies) according to the manufacturer's instructions and used for staining. Viability staining was performed using Fixable Viability Dye eFluor 506 (Invitrogen) for 30 minutes at 4 °C.
Cells were fixed overnight using Foxp3/Transcription Factor Staining Buffer Set (eBioscience) at 4 °C.
Data were acquired on LSR Fortessa (BD Biosciences), MACSQuant 10, and MACSQuant 16 (Miltenyi Biotec) instruments and analyzed with FlowJo single cell analysis software 7.6.5 and 10.06.1 (Tree Star Inc).
Dynamic adhesion assays to MAdCAM-1
To quantify the number of cells adhering to MAdCAM-1, MACS-isolated cell subsets stained with CellTrace CFSE (Invitrogen) for 15 min at 37 °C were incubated with or without different concentrations of VDZ for 1 hour at 37 °C. Rectangle miniature capillaries (CM Scientific) were coated with 5-μg/mL rhMAdCAM-1 Fc Chimera (R&D Systems) in coating buffer (150 mM NaCl + 1 mM HEPES) and subsequently blocked with 5% BSA or 10% FBS in PBS. After incubation, cells were resuspended in adhesion buffer (150 mM NaCl, 1 mM CaCl2, and 1 mM MgCl2) with 1 mM MnCl2 at a concentration of 1.5 Mio cells/mL and perfused through MAdCAM-1–coated capillaries for 3 min at a speed of 10 μL/min using a peristaltic pump (Schenchen). Capillaries were then rinsed for 5 min at a speed of 50 μL/min to remove the remaining cell suspension, and the adherent cells in the capillaries were imaged using an inverted fluorescence microscope (Leica) by capturing 8 high-power fields at 20× magnification. Data analysis and quantification were performed using Fiji (National Institutes of Health).
Transmigration assays
For the analysis of the impact of different VDZ concentrations on MAdCAM-1–dependent transmigration of the cell subsets, MACS-isolated cells were resuspended in X-Vivo15 medium (Lonza) with 1 mM MnCl2 at a concentration of 2 Mio cells/mL and incubated with different concentrations of VDZ (0, 10, and 50 μg/mL). Inserts of a 3 μm transwell plate (Corning) were coated with 5 μg/mL rhMAdCAM-1 for 1 hour at 37 °C. The coating buffer was removed immediately before application of the cell suspension to the wells. Approximately 160,000 cells were seeded in duplicates into inserts that were then placed into wells filled with X-Vivo15 medium + 100 nM rhCCL25 (ImmunoTools). The plate was incubated at 37 °C for a transmigration period of 4 hours. Subsequently, inserts were discarded, and the number of transmigrated cells in the lower wells was quantified using flow cytometry.
Statistics
All statistical analyses were performed using Prism 8 software (GraphPad, San Diego, CA). For all data, normal distribution was assessed using the Shapiro-Wilk or Kolmogorov-Smirnov test. After outlier analysis using the Grubbs test (α = 0.05), the following statistical tests were used as specified in the figure legends: paired t-test, 1-way ANOVA with Tukey's multiple comparisons test, repeated measurement 1-way ANOVA with Tukey's multiple comparisons test, Kruskal-Wallis with Dunn's multiple comparisons test, or 2-way ANOVA with Sidak's multiple comparisons test. Results are displayed as bar graphs with SEM and single data points. P < 0.05 was considered statistically significant in all tests. Asterisks indicate the following levels of significance: *P < 0.05, **P < 0.01, and ***P < 0.001.
RESULTS
Frequency of α4β7 integrin-expressing cells in different leukocyte subsets from patients with IBD
We have previously shown that α4β7 expression varies among different leukocyte subsets, with the highest numbers detectable in eosinophils and CD4+ T cells, a very low portion of α4+β7+ cells in monocytes and virtually no positive cells in neutrophils. We could further show that the highest portion of α4β7-expressing cells evading VDZ binding at the clinically relevant concentration of 10 μg/mL VDZ was found in CD4+ T cells (10).
However, because a relevant portion of some of the other leukocyte subgroups also expressed α4β7, we set out to characterize these cells further. We stratified previous flow cytometry data (10) complemented by additional stainings for the number of α4+β7+ cells among CD8+ T cells, CD19+ B cells, CD14+ monocytes, NK cells, and eosinophils according to disease entities. We observed that expression levels were comparable between CD, UC, and healthy controls in all cell subsets, except for eosinophils (Figure 1, representative gating and controls shown in Supplementary Figures 1 and 2 (see Supplementary Digital Content 1, http://links.lww.com/CTG/A810), respectively). In this subset, we identified a significantly higher portion of α4+β7+ cells in CD and UC patients compared with healthy controls (Figure 1e).
Figure 1.
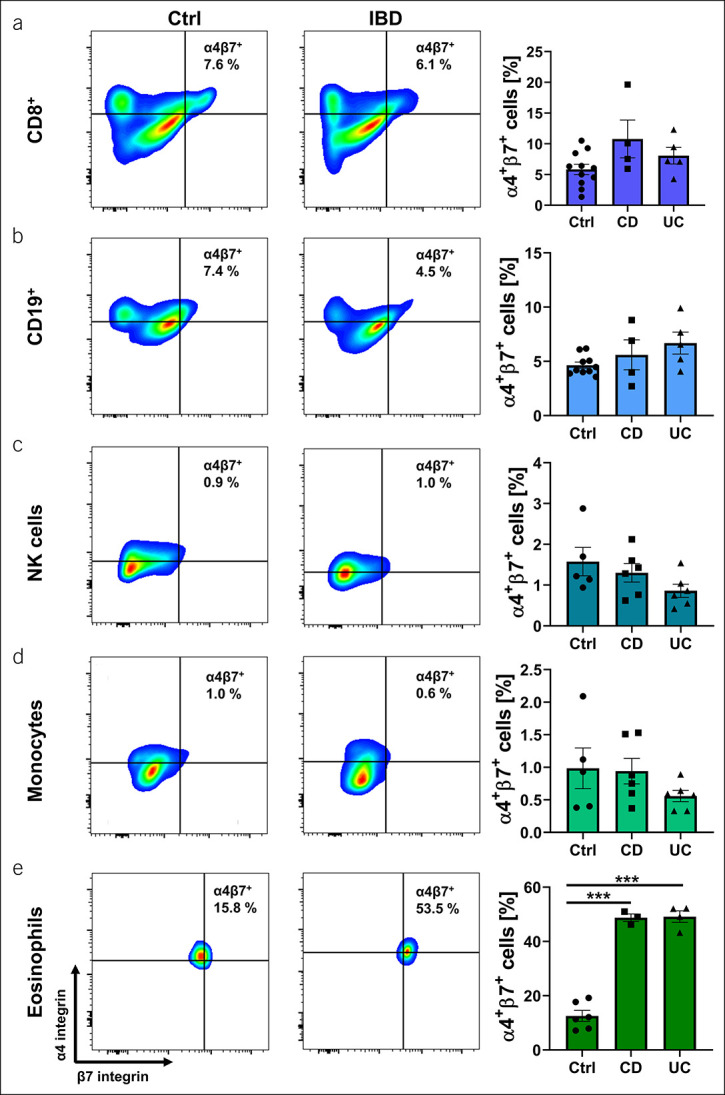
α4β7 integrin expression on different leukocyte subsets in healthy controls (Ctrl), Crohn's disease (CD), or ulcerative colitis (UC) patients. Representative (left) and quantitative (right) flow cytometry of CD8+ T cells (a), CD19+ B cells (b), natural killer (NK) cells (c), monocytes (d), and eosinophils (e). n = 3–11 per group. Results depicted as bar graphs with SEM and single data points. Significant outliers were identified using the Grubbs test (P = 0.05) and excluded from analysis. Statistical comparisons were performed using 1-way ANOVA with Tukey's multiple comparisons test. *P < 0.05; **P < 0.01; ***P < 0.001.
Binding of VDZ to leukocyte subsets at different concentrations
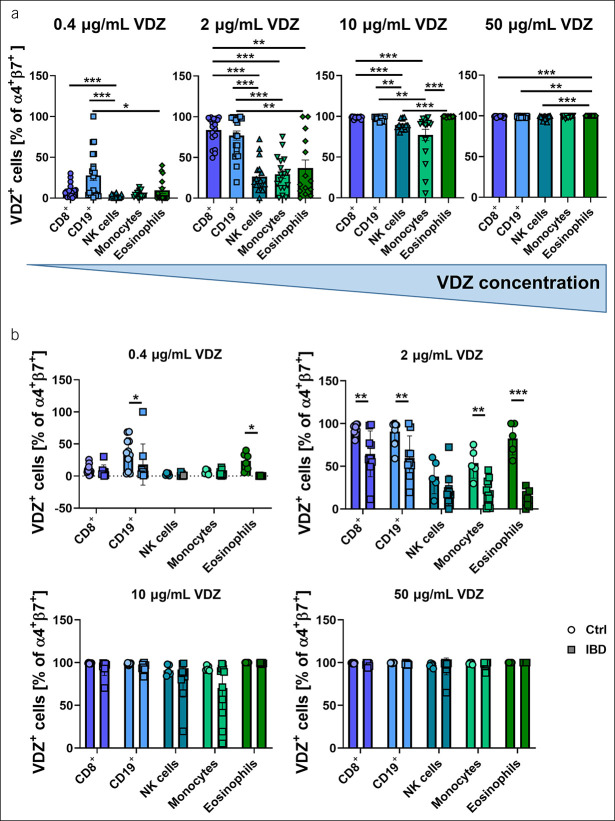
To explore whether a dose-dependent differential binding of VDZ to α4β7-expressing immune cells other than CD4+ T cells exists, we analyzed flow cytometry data from a previously established cohort of donors (10) for the frequency of VDZ+ among α4+β7+ cells after incubation with different concentrations of fluorescently labeled VDZ. At low concentrations of 0.4 and 2 μg/mL, which are clinically less relevant (12), we found a significantly higher binding of VDZ to CD8+ T cells and CD19+ B cells compared with all other cell types. After incubation with 10 μg/mL fluorescently labeled VDZ, virtually all cells expressing relevant levels of α4+β7+, namely CD8+ T cells, CD19+ B cells, and eosinophils, were fully occupied with VDZ, whereas a small portion of monocytes and NK cells was not. However, given the low expression of α4β7 in these subsets, this corresponded to very low absolute numbers of α4+β7+ monocytes and NK cells unoccupied with VDZ. At 50 μg/mL, almost all α4β7-expressing cells were bound by VDZ (Figure 2a).
Figure 2.
Dose-dependent binding of vedolizumab (VDZ) to α4β7 integrin-expressing different leukocyte subsets. (a) Quantitative flow cytometry of α4+β7+VDZ+ cells in different leukocyte subsets incubated with the indicated concentrations of fluorescently labeled VDZ. n = 15–20 per group. (b) Quantitative flow cytometry of α4+β7+VDZ+ cells in different leukocyte subsets compared between healthy controls (Ctrl) and patients with inflammatory bowel disease (IBD). n = 5–12 per group. Results depicted as bar graphs with SEM and single data points. Significant outliers were identified using the Grubbs test (P = 0.05) and excluded from analysis. Statistical comparisons were performed using Kruskal-Wallis with Dunn's multiple comparisons test (a) and 2-way ANOVA with Sidak's multiple comparisons test (b). *P < 0.05; **P < 0.01; ***P < 0.001.
We further sought to clarify whether the binding of VDZ is different to cell subsets from healthy controls in patients with IBD. We observed that at low concentrations of 0.4 and 2 μg/mL, VDZ bound to a larger portion of α4+β7+ cells from healthy controls compared with patients with IBD in almost all subsets. After treatment with 10 or 50 μg/mL VDZ, however, no differences between healthy controls and patients with IBD were detected (Figure 2b).
Together, these data suggested that among the leukocyte subsets studied, no relevant “resistance” to VDZ occurs at concentrations corresponding to the clinically notable trough levels.
Functional impact of VDZ binding on dynamic adhesion
As the main mode of action of VDZ is to inhibit adhesion of α4β7-expressing cells to MAdCAM-1, we next wanted to explore the impact of different concentrations of VDZ on the dynamic adhesion of the analyzed cell subsets. To this end, we performed dynamic adhesion assays of MACS-purified cells by perfusing cells treated with VDZ through MAdCAM-1–coated capillaries in vitro. We focused on treatment with 10 and 50 μg/mL because these are the concentrations associated with different outcomes in phase 2 trials (8) and linked to differential effects on CD4+ T cell subsets (10). Untreated cells were used as control.
These functional experiments showed a large number of untreated CD8+ T cells, CD19+ B cells, and NK cells adhering to MAdCAM-1, although only few CD14+ monocytes and eosinophils bound to MAdCAM-1 under flow conditions (Figure 3a–e, Supplementary Figure 3A [see Supplementary Digital Content 1, http://links.lww.com/CTG/A810]). We observed a substantial reduction of the number of adhering cells after treatment with VDZ compared with untreated cells for all subsets. However, the inhibition of adhesion was similar between the different VDZ concentrations, suggesting that effects on these subsets do not account for the exposure-efficacy profile of the antibody.
Figure 3.
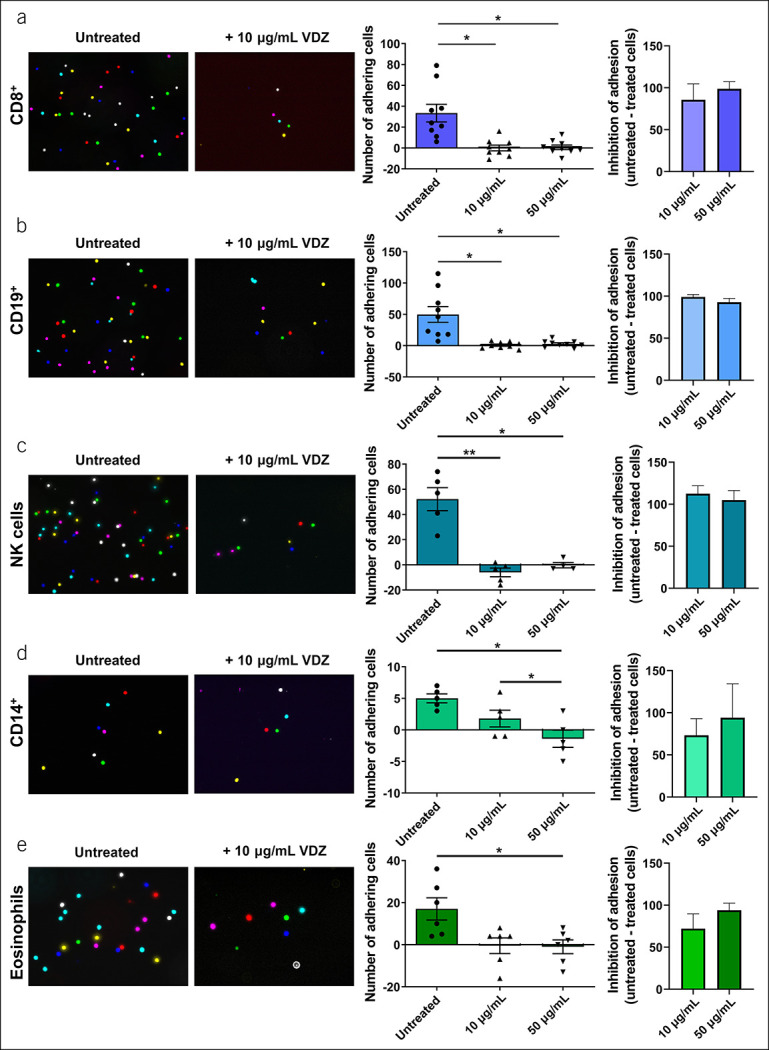
Concentration-dependent adhesion of different leukocyte subsets to mucosal addressin cell adhesion molecule (MAdCAM-1). Dynamic adhesion of magnetic-activated cell sorting (MACS) purified CD8+ T cells (a), CD19+ B cells (b), natural killer (NK) cells (c), CD14+ monocytes (d), and eosinophils (e). Left panels: Representative microscopic images of adhered cells (overlay of counted high power fields); middle panels: quantification of the background-corrected number of cells incubated with or without the indicated concentrations of vedolizumab (VDZ) (adhering to MAdCAM-1 [sum of 8 counted high power fields]; right panels: relative inhibition of adhesion of cells to MAdCAM-1 after treatment with the indicated concentrations of VDZ [background-corrected]). n = 6–12. Results depicted as bar graphs with SEM and single data points. Significant outliers were identified using the Grubbs test (P = 0.05) and excluded from analysis. Statistical comparisons were performed using repeated measurement 1-way ANOVA with Tukey's multiple comparisons test or paired t-test. *P < 0.05; **P < 0.01; ***P < 0.001.
Functional impact of VDZ binding on transmigration
MAdCAM-1 not only regulates firm adhesion but also extravasation of α4β7-expressing cells. To explore the functional relevance of VDZ treatment in this process, we analyzed the impact of in vitro treatment with different concentrations of VDZ on MAdCAM-1–dependent transmigration of different cell subsets. MACS-purified cells were incubated with different concentrations of VDZ in transwell plates coated with MAdCAM-1 and left to transmigrate toward a chemotactic gradient of CCL25. After 4 hours, transmigrated cells were quantified using flow cytometry. In isotype-treated cells (untreated), the highest transmigration was observed for CD8+ T cells, the lowest for CD19+ B cells (Figure 4, Supplementary Figure 3B [see Supplementary Digital Content 1, http://links.lww.com/CTG/A810]). VDZ treatment with all concentrations led to a reduced number of transmigrated CD8+ T cells and CD19+ B cells in comparison with untreated controls. No significant reduction of transmigration could be observed for NK cells and eosinophils, suggesting that their transmigration is not dependent on α4β7. Yet, we did not observe differences between treatment with 10 and 50 μg/mL VDZ for CD8+ T cells and CD19+ B cells, further supporting the notion that no particular dose-response characteristic applies for these cells.
Figure 4.
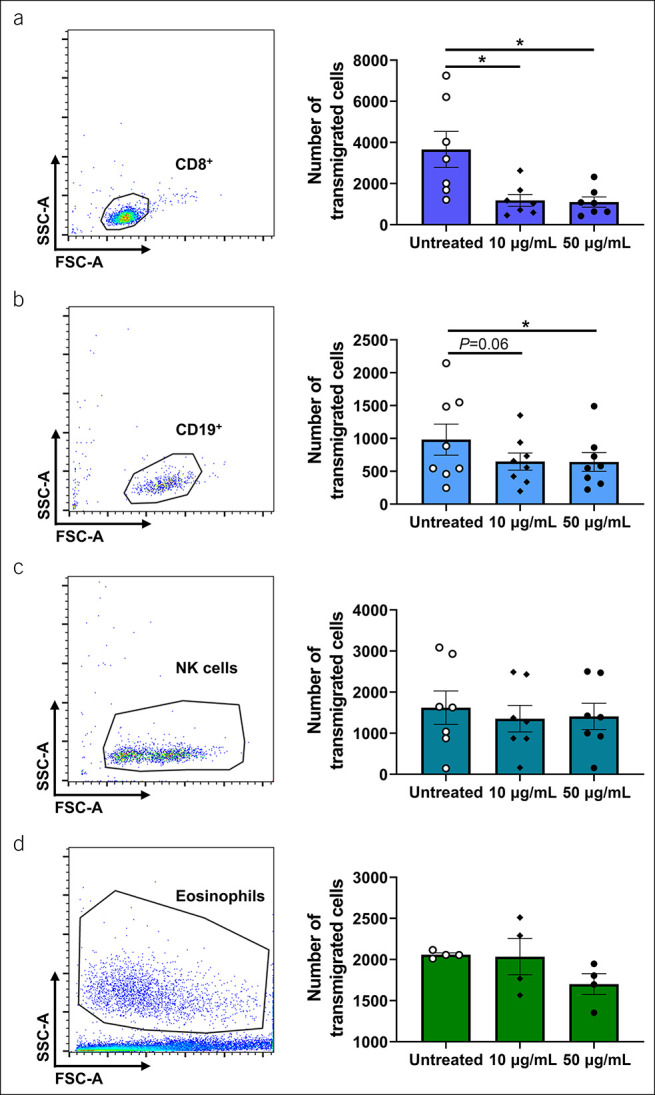
Concentration-dependent mucosal addressin cell adhesion molecule (MAdCAM-1)-driven transmigration of different leukocyte subsets. Representative (left) and quantitative (right) flow cytometry of transmigrated CD8+ T cells (a), CD19+ B cells (b), natural killer (NK) cells (c), and eosinophils (d) after treatment with the indicated concentrations of vedolizumab (VDZ) or corresponding isotype control (untreated). n = 5–8. Results depicted as bar graphs with SEM and single data points. Significant outliers were identified using the Grubbs test (P = 0.05) and excluded from analysis. Statistical comparisons were performed using repeated measurement 1-way ANOVA with Tukey's multiple comparisons test. *P < 0.05; **P < 0.01; ***P < 0.001.
DISCUSSION
Although VDZ is an established therapy for the treatment of IBD, a substantial number of patients does not sufficiently respond to treatment due to so far unknown reasons. This underscores that further efforts to understand the mechanism of action of the antibody are necessary.
One apparent approach to the problem was to interrogate the association of response to and trough levels of VDZ. In this regard, it has been repeatedly reported that achieving certain threshold exposure levels is linked to improved remission rates (13–17). At the same time, 2 independent phase 2 trials showed that exposure to very high concentrations also reduces the success rate of VDZ therapy (8,9). The mechanistic explanation for these observations supporting a bell-shaped dose-response curve remained unclear for long. Regarding differential binding efficacy of VDZ to various leukocyte subsets (11) and functional data suggesting differences between regulatory and effector T cells (18), speculations on exposure-dependent differential action of anti-adhesion molecules on different cell subsets had early been made (19). Indeed, we could recently show that VDZ displays concentration-dependent differences in its binding to regulatory and effector T cells, which lead to differential adhesion, transmigration, and in vivo homing and correlate with clinical efficacy (10). In this article, we aimed to explore whether similar mechanisms hold true for other leukocyte subsets.
In particular, our experiments covered CD8+ T cells, CD19+ B cells, NK cells, CD14+ monocytes, and eosinophils. All these subsets have been implicated in the pathogenesis of IBD: More activated CD8+ T cells were found in the peripheral blood of patients with IBD as compared with healthy controls (20), and interaction of CD8+ T cells from patients with IBD with epithelial cells led to activation (21). A large heterogeneity of CD8+ T cells in IBD was observed using single-cell RNA sequencing, with an elevated proportion of almost every subset in UC compared with healthy controls (22). Mature B cells (plasma cells) are increased in patients with IBD, and a disrupted humoral response with reduced IgA and increased autoantibodies has been reported (23,24). NK cells (innate lymphoid cells) are implicated in the orchestration of pro-inflammatory and anti-inflammatory milieus in the gut mucosa through the secretion of interferon (IFN)-γ and interleukin (IL)-22, respectively (25,26). CD14+ macrophages are increased in the intestine of patients with CD and can induce IFN-γ and IL-17 production by T cells (26). Although the role of eosinophils in IBD is still poorly understood, they are known to be involved in maintaining gut homeostasis and have also been implicated in intestinal mucosal alterations toward a proinflammatory milieu (27–29).
In a first series of experiments, we addressed the expression of α4β7 on leukocyte subsets in IBD and healthy controls and showed that, although the portion of α4β7-expressing cells is comparable between UC, CD, and healthy controls for most subsets, it is significantly elevated in eosinophils from patients with IBD. This may be part of the explanation for the accumulation of eosinophils observed in the gastrointestinal tract of patients with IBD (30) and highlights the potential pathogenic implication of eosinophils in the context of IBD.
When analyzing the concentration-dependent binding of VDZ to different leukocytes in vitro, we observed preferential binding to cells from the adaptive (CD8+ T cells and CD19+ B cells) compared with the innate (NK cells, monocytes, and eosinophils) immune system at low concentrations. At the previously described clinically relevant concentration of 10 μg/mL (8,12,31), somewhat reduced VDZ binding was found in cell subsets with very low frequencies of α4+β7+ cells, namely NK cells and monocytes. Consistently, these relative reductions corresponded to a very low absolute number of cells not occupied by VDZ. Thus, and in view of our functional data, it is questionable whether this impacts on the clinical effects. However and overall, these findings are in line with earlier data, suggesting that VDZ does not exclusively act on CD4+ T cells, but also on other lymphocyte and even innate immune cell subsets (32–34).
Functional in vitro assays with MACS-purified cells demonstrated reduced dynamic adhesion in all leukocyte subgroups studied after treatment with 10 or 50 μg/mL VDZ compared with untreated cells. However, no differences in the inhibition of adhesion were found between the concentration groups. These observations were similarly recapitulated in transmigration assays for CD8+ T cells and CD19+ B cells, where the effects of VDZ were similar on treatment with 10 or 50 μg/mL within the individual cell subsets. Thus, our data suggest that the previously reported concentration-dependent differential action of VDZ on regulatory and effector T cells (10) seems to be exclusive for the CD4+ T cell subsets and does not similarly apply for other leukocyte subsets. Importantly, this does not exclude that certain smaller subsets of CD8+ or CD19+ lymphocytes, monocytes, NK cells, or eosinophils show resistance toward VDZ in a certain concentration range.
Surprisingly, we did not observe a clear correlation of α4β7 expression with α4β7 function. For instance, NK cells expressed only low levels of α4β7 but adhered avidly to MAdCAM-1 and VDZ completely blocked this binding. Eosinophils, on the other hand, expressed high levels of α4β7 but showed clearly lower adhesion. In this regard, it was also interesting that, in contrast to lymphocytes, transmigration of NK cells and eosinophils does not seem to be regulated by α4β7-MAdCAM-1 interactions because VDZ was not able to reduce their migration over MAdCAM-1–coated membranes. Consequently, these data point at additional molecular mechanisms involved and, particularly, alternative transmigration mechanisms of NK cells and eosinophils. One explanation might be that the integrin α4β1, which is expressed on these leukocyte subsets (35–37), can also interact with MAdCAM-1 with somewhat lower affinity (38). Thus, MAdCAM-1–dependent adhesion and transmigration mediated by α4β1 (39) might occur and warrant further investigation.
We acknowledge that our study has some limitations. Although our selection of candidate leukocyte subsets was driven by previous observations, other and even very small subsets might contribute to the exposure-efficacy characteristics of VDZ. Moreover, because of recruitment opportunities and material availability, the demographic characteristics of patients and healthy controls are not perfectly matched in our study. Since Crooks et al. (40) demonstrated that frequencies of α4-expressing T cells were significantly reduced in older compared with younger cohorts, this might lead to an underestimation of the difference of α4β7 expression on eosinophils that we found between healthy donors and patients with IBD. Of note, efficacy and safety of VDZ are independent of the age of the patients (41). Similarly, the small cohort size of this study did not allow us to stratify our results for different types of treatment, which might also impact on integrin expression and function.
Yet, these data are the first to address the functional role and exposure-efficacy correlation of VDZ in leukocytes other than CD4+ T cells and in particular innate immune cells. Collectively, our data confirm that VDZ acts on a variety of immune cell subsets but also support a concept attributing dose-response characteristics mainly to CD4+ T-cell subsets. Thus, they lay the basis for better understanding the mechanisms of VDZ and might help to develop individualized future treatment strategies.
ACKNOWLEDGEMENTS
The research of SZ, RA, IA, TMM and MFN was supported by the Interdisciplinary Center for Clinical Research (IZKF) and the ELAN program of the University Erlangen-Nuremberg, the Fritz-Bender-Stiftung, the Ernst Jung-Stiftung, the Else Kröner-Fresenius-Stiftung, the Thyssen-Stiftung, the German Crohn's and Colitis Foundation (DCCV), the DFG topic program on Microbiota, the Emerging Field Initiative, the DFG Collaborative Research Centers 643, 796, 1181 and TRR241, the Rainin Foundation and the Litwin IBD Pioneers program of the Crohn's and Colitis Foundation of America (CCFA). The authors thank J. Derdau, D. Dziony, J. Marcks, J. Schuster and M. Slawik for their invaluable technical assistance.)
Study Highlights.
WHAT IS KNOWN
✓ Vedolizumab (VDZ) treatment leads to a wide range of serum concentrations.
✓ Only a fraction of patients responds to therapy.
✓ Phase 2 trials showed a bell-shaped exposure-efficacy correlation for VDZ.
✓ VDZ has differential concentration-dependent effects on regulatory and effector T cells.
WHAT IS NEW HERE
✓ VDZ binds to various leukocyte subsets and blocks their α4β7-dependent adhesion and transmigration.
✓ Transmigration of eosinophils and NK cells is not α4β7-dependent.
✓ VDZ does not show concentration-dependent effects on the leukocyte subsets studied in a clinically relevant range.
CONFLICTS OF INTEREST
Guarantor of the article: Sebastian Zundler, MD.
Specific author contributions: E.B. and A.S. performed experiments. E.B. and S.Z. designed the study. E.B., K.A.M.U., R.A., I.A., T.M.M., C.V., M.F.N., and S.Z. provided clinical samples, protocols, or reagents. E.B. and S.Z. analyzed and interpreted the data. E.B. and S.Z. drafted the manuscript; all authors critically revised the manuscript for important intellectual content.
Financial support: Funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) - ZU 377/4-1, Else Kröner-Fresenius-Stiftung (2016_A182), European Crohn's and Colitis Organization.
Potential competing interests: M.F.N. has served as an advisor for Pentax, Giuliani, MSD, AbbVie, Janssen, Takeda, and Boehringer. S.Z. received honoraria from Takeda, Roche, Janssen, Ferring, Galapagos, and Lilly. M.F.N. and S.Z. received research support from Takeda, Shire (a part of Takeda), and Roche. The other authors declare no conflicts of interest.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A810 and http://links.lww.com/CTG/A811.
Contributor Information
Anna Schweda, Email: anna.schweda@uk-erlangen.de.
Karen A. -M. Ullrich, Email: karen.ullrich@uk-erlangen.de.
Caroline Voskens, Email: Caroline.Bosch-Voskens@uk-erlangen.de.
Raja Atreya, Email: raja.atreya@uk-erlangen.de.
Tanja M. Müller, Email: tanja.mueller@uk-erlangen.de.
Imke Atreya, Email: imke.atreya@uk-erlangen.de.
Markus F. Neurath, Email: markus.neurath@uk-erlangen.de.
Sebastian Zundler, Email: sebastian.zundler@uk-erlangen.de.
References
- 1.Zundler S, Becker E, Lou SchulzeL, et al. Immune cell trafficking and retention in inflammatory bowel disease: Mechanistic insights and therapeutic advances. Gut 2019;68(9):1688–700. doi: [DOI] [PubMed] [Google Scholar]
- 2.Wiendl M, Becker E, Müller TM, et al. Targeting immune cell trafficking – insights from research models and implications for future IBD therapy. Front Immunol 2021;12:656452. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ley K, Laudanna C, Cybulsky MI, et al. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat Rev Immunol 2007;7(9):678–89. doi: [DOI] [PubMed] [Google Scholar]
- 4.Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369(8):699–710. doi: [DOI] [PubMed] [Google Scholar]
- 5.Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med 2013;369(8):711–21. doi: [DOI] [PubMed] [Google Scholar]
- 6.Pouillon L, Vermeire S, Bossuyt P. Vedolizumab trough level monitoring in inflammatory bowel disease: A state-of-the-art overview. BMC Med 2019;17(1):89–8. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Bawardy B, Ramos GP, Willrich MAV, et al. Vedolizumab drug Level correlation with clinical remission, biomarker normalization, and mucosal healing in inflammatory bowel disease. Inflamm Bowel Dis 2019;25(3):580–6. doi: [DOI] [PubMed] [Google Scholar]
- 8.Feagan BG, Greenberg GR, Wild G, et al. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med 2005;352(24):2499–507. doi: [DOI] [PubMed] [Google Scholar]
- 9.Parikh A, Leach T, Wyant T, et al. Vedolizumab for the treatment of active ulcerative colitis: A randomized controlled phase 2 dose-ranging study. Inflamm Bowel Dis 2012;18(8):1470–9. doi: [DOI] [PubMed] [Google Scholar]
- 10.Becker E, Dedden M, Gall C, et al. Residual homing of α4β7-expressing β1 + PI16 + regulatory T cells with potent suppressive activity correlates with exposure-efficacy of vedolizumab. Gut Published Online 2021:gutjnl-2021-324868. gutjnl-2021-324868. doi: 10.1136/gutjnl-2021-324868 [DOI] [PubMed] [Google Scholar]
- 11.Soler D, Chapman T, Yang LL, et al. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther 2009;330(3):864–75. doi: [DOI] [PubMed] [Google Scholar]
- 12.Rosario M, Dirks NL, Gastonguay MR, et al. Population pharmacokinetics-pharmacodynamics of vedolizumab in patients with ulcerative colitis and Crohn's disease. Aliment Pharmacol Ther 2015;42(2):188–202. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verstockt B, Mertens E, Dreesen E, et al. Influence of drug exposure on vedolizumab-induced endoscopic remission in anti-tumour necrosis factor [TNF] naïve and anti-TNF exposed IBD Patients. J Crohns Colitis 2020;14(3):332–41. doi: [DOI] [PubMed] [Google Scholar]
- 14.Osterman MT, Rosario M, Lasch K, et al. Vedolizumab exposure levels and clinical outcomes in ulcerative colitis: Determining the potential for dose optimisation. Aliment Pharmacol Ther 2019;49(4):408–18. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guidi L, Pugliese D, Panici Tonucci T, et al. Early vedolizumab trough levels predict treatment persistence over the first year in inflammatory bowel disease. United Eur Gastroenterol J 2019;7(9):1189–97. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ungaro RC, Yarur A, Jossen J, et al. Higher trough vedolizumab concentrations during maintenance therapy are associated with corticosteroid-free remission in inflammatory bowel disease. J Crohns Colitis 2019;13(8):963–9. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yarur AJ, Bruss A, Naik S, et al. Vedolizumab concentrations are associated with long-term endoscopic remission in patients with inflammatory bowel diseases. Dig Dis Sci 2019;64(6):1651–9. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer A, Zundler S, Atreya R, et al. Differential effects of α4β7 and GPR15 on homing of effector and regulatory T cells from patients with UC to the inflamed gut in vivo. Gut 2016;65(10):1642–64. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vermeire S, O'Byrne S, Keir M, et al. Etrolizumab as induction therapy for ulcerative colitis: A randomised, controlled, phase 2 trial. Lancet (London, England) 2014;384(9940):309–18. doi: [DOI] [PubMed] [Google Scholar]
- 20.Funderburg NT, Stubblefield Park SR, Sung HC, et al. Circulating CD4+ and CD8+ T cells are activated in inflammatory bowel disease and are associated with plasma markers of inflammation. Immunology 2013;140(1):87–97. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bisping G, Lügering N, Lütke-Brintrup S, et al. Patients with inflammatory bowel disease (IBD) reveal increased induction capacity of intracellular interferon-gamma (IFN-γ) in peripheral CD8+ lymphocytes co-cultured with intestinal epithelial cells. Clin Exp Immunol 2001;123(1):15–22. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corridoni D, Antanaviciute A, Gupta T, et al. Single-cell atlas of colonic CD8+ T cells in ulcerative colitis. Nat Med 2020;26(9):1480–90. doi: [DOI] [PubMed] [Google Scholar]
- 23.Uzzan M, Colombel JF, Cerutti A, et al. B cell-activating factor (BAFF)-Targeted B cell therapies in inflammatory bowel diseases. Dig Dis Sci 2016;61(12):3407–24. doi: [DOI] [PubMed] [Google Scholar]
- 24.Castro-Dopico T, Colombel JF, Mehandru S. Targeting B cells for inflammatory bowel disease treatment: Back to the future. Curr Opin Pharmacol 2020;55:90–8. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panda SK, Colonna M. Innate lymphoid cells in mucosal immunity. Front Immunol 2019;10(MAY):861. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hisamatsu T, Erben U, Kühl AA. The role of T-cell subsets in chronic inflammation in celiac disease and inflammatory bowel disease patients: More common mechanisms or More differences?. Inflamm Intest Dis 2016;1(2):52–62. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filippone RT, Sahakian L, Apostolopoulos V, et al. Eosinophils in inflammatory bowel disease. Inflamm Bowel Dis 2019;25(7):1140–51. doi: [DOI] [PubMed] [Google Scholar]
- 28.Loktionov A. Eosinophils in the gastrointestinal tract and their role in the pathogenesis of major colorectal disorders. World J Gastroenterol 2019;25(27):3503–26. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Haddad S, Riddell RH. The role of eosinophils in inflammatory bowel disease. Gut 2005;54(12):1674–5. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smyth CM, Akasheh N, Woods S, et al. Activated eosinophils in association with enteric nerves in inflammatory bowel disease. PLoS ONE 2013;8(5):e64216. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vermeire S, Lukáš M, Magro F, et al. Vedolizumab efficacy, safety, and pharmacokinetics with reduced frequency of dosing from every 4 weeks to every 8 weeks in patients with Crohn's disease or ulcerative colitis. J Crohns Colitis 2020;14(8):1066–73. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeissig S, Rosati E, Dowds CM, et al. Vedolizumab is associated with changes in innate rather than adaptive immunity in patients with inflammatory bowel disease. Gut 2019;68(1):25–39. doi: [DOI] [PubMed] [Google Scholar]
- 33.Schleier L, Wiendl M, Heidbreder K, et al. Non-classical monocyte homing to the gut via α4β7 integrin mediates macrophage-dependent intestinal wound healing. Gut 2020;69(2):252–63. doi: [DOI] [PubMed] [Google Scholar]
- 34.Binder MT, Becker E, Wiendl M, et al. Similar inhibition of dynamic adhesion of lymphocytes from IBD patients to MAdCAM-1 by vedolizumab and etrolizumab-s. Inflamm Bowel Dis 2018;24(6):1237–50. doi: [DOI] [PubMed] [Google Scholar]
- 35.Gismondi A, Morrone S, Humphries MJ, et al. Human natural killer cells express VLA-4 and VLA-5, which mediate their adhesion to fibronectin. J Immunol 1991;146(1):384–92. [PubMed] [Google Scholar]
- 36.Matsumoto K, Sterbinsky SA, Bickel CA, et al. Regulation of α4 integrin-mediated adhesion of human eosinophils to fibronectin and vascular cell adhesion molecule-1. J Allergy Clin Immunol 1997;99(5):648–56. doi: [DOI] [PubMed] [Google Scholar]
- 37.Rabinowich H, Manciulea M, Herberman RB, et al. Beta1 integrin-mediated activation of focal adhesion kinase and its association with Fyn and Zap-70 in human NK cells. J Immunol 1996;157(9):3860–8. [PubMed] [Google Scholar]
- 38.Sun H, Liu J, Zheng YJ, et al. Distinct chemokine signaling regulates integrin ligand specificity to dictate tissue-specific lymphocyte homing. Dev Cel 2014;30(1):61–70. doi: [DOI] [PubMed] [Google Scholar]
- 39.Zundler S, Schillinger D, Fischer A, et al. Blockade of αeβ7 integrin suppresses accumulation of CD8 + and Th9 lymphocytes from patients with IBD in the inflamed gut in vivo. Gut 2017;66(11):1936–48. doi: [DOI] [PubMed] [Google Scholar]
- 40.Crooks CV, Cross ML, Wall CR. Age-related differences in integrin expression in peripheral blood lymphocytes. Immun Ageing 2010;7(1):5. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yajnik V, Khan N, Dubinsky M, et al. Efficacy and safety of vedolizumab in ulcerative colitis and Crohn's disease patients stratified by age. Adv Ther 2017;34(2):542–59. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]