Abstract
Limited data is available on the coronavirus disease 2019 (COVID-19), critical illness rate, and in-hospital mortality in the African setting. This study investigates determinants of critical illness and in-hospital mortality among COVID-19 patients in Kenya. We conducted a retrospective cohort study at Kenyatta National Hospital (KNH) in Kenya. Multivariate logistic regression and Cox proportional hazard regression were employed to determine predictor factors for intensive care unit (ICU) admission and in-hospital mortality, respectively. In addition, the Kaplan-Meier model was used to compare the survival times using log-rank tests. As a result, 346 (19.3%) COVID-19 patients were admitted to ICU, and 271 (15.1%) died. The majority of those admitted to the hospital were male, 1,137 (63.4%) and asymptomatic, 1,357 (75.7%). The most prevalent clinical features were shortness of breath, fever, and dry cough. In addition, older age, male, health status, patient on oxygen (O2), oxygen saturation levels (SPO2), headache, dry cough, comorbidities, obesity, cardiovascular diseases (CVDs), diabetes, chronic lung disease (CLD), and malignancy/cancer can predicate the risk of ICU admission, with an area under the receiver operating characteristic curve (AUC-ROC) of 0.90 (95% confidence interval [CI]: 0.88–0.92). Survival analysis indicated 271 (15.1%) patients died and identified older age, male, headache, shortness of breath, health status, patient on oxygen, SPO2, headache, comorbidity, CVDs, diabetes, CLD, malignancy/cancer, and smoking as risk factors for mortality (AUC-ROC: 0.90, 95% CI: 0.89–0.91). This is the first attempt to explore predictors for ICU admission and hospital mortality among COVID-19 patients in Kenya.
Keywords: Comorbidities, Critical illness, ICU, COVID-19, SARS-CoV-2
1. Introduction
Coronavirus disease 2019 (COVID-19) is an infectious disease of the respiratory system characterized by an initial cytokine gale that can cause acute respiratory distress syndrome (ARDS), resulting in macrophage activation syndrome [1]. As of October 17, 2021, WHO reported over 2.7 million new COVID-19 infections with over 46,000 deaths globally within one week, and >240 million cases have been reported worldwide since the outbreak [2]. Africa has recorded 8.45 million COVID-19 cases, with overall death increasing by 30% as of November 23, 2021 [3]. Kenya is one of the most affected countries by COVID-19 in Africa [2]. The COVID-19 cases in Kenya are rapidly rising, and if not controlled, they may overwhelm the healthcare system in terms of resources and workforce. As of October 5, 2021, Kenya had recorded >240,000 thousand cases, and still, in most counties, the cases escalated with a positivity rate of above 20% [3].
The most common clinical features of COVID-19 recorded worldwide are fever, dry cough, general body weakness, and myalgia [4]. Low prevalence of patients with severe COVID-19 outcomes and related mortality has been documented in most African countries [5]. In Africa, COVID-19 has been characterized by epidemiological heterogeneity [5], [6]. This has prompted this study to validate the characterization findings in the Kenyan population.
Some cases of COVID-19 develop pneumonia with characteristic findings in the course of the disease [4]. Many cases are admitted to ICU [4], [7]. Epidemiological studies have reported that hospitalized patients of COVID-19 are those older and with underlying medical conditions [8], [9]. It is reported that older people with comorbidities are at a higher risk of severe outcomes compared to the younger and healthier population [8], [9], [10]. Previous studies identify chronic diseases as the risk factors for severe COVID-19 and mortality [8], [9], [10], [11]. However, the case may be different in Africa since the continent is experiencing a triple disease burden from emerging diseases, endemics, and non-communicable diseases[6]. The rapid increase of critically ill patients and those who are dying has posed a severe crisis in the public health sector of many countries [12]. Some individuals are at a higher risk of severe disease progression, which heralds increased mortality.
A retrospective cohort study was conducted to understand the epidemiological characterization of hospitalized COVID-19 patients and determine predictor factors for ICU admission and mortality.
2. Materials and methods
2.1. Study design and participants
A retrospective cohort study was conducted from March 2020 to April 2021 among COVID-19 patients admitted to Kenyatta National Hospital (KNH).
The first case of COVID-19 was documented in Kenya on 13th March 2020; The Ministry of Health in Kenya required that all infected individuals (both symptomatic and asymptomatic cases) be isolated. Therefore, all patients diagnosed with COVID-19 with positive RT-PCR confirmed diagnosis of SARS-CoV-2 (between 13th March 2020 and 30th April 2021) were recruited into the study; if only their age, sex, date of admission to hospital, and date of change of status (died or discharged) information was recorded. Patients with incomplete outcome information and vital baseline data and those transferred to other health institutions after admission were excluded from the study because of the challenge of getting their complete data.
2.2. Procedure
A structured questionnaire was used to extract data from medical records. The collected data was counter-checked with two medical doctors and the chief investigator to note the difference between the two reviewers. In summary, important patient information from the date of admission, during hospitalization to the time the patient was discharged or died was extracted.
The extracted information comprised patient demographic data, epidemiological data, clinical signs, comorbidities, and outcome data. The underlying medical conditions were identified based on the international classification of disease diagnostic codes (ICD) 10th version, while clinical outcomes were categorized to discharge from hospital or death. The patient's symptoms and medical history were also captured at admission. The patients were put into two cohorts; those stable and those severely ill. Those who were stable with oxygen saturation (SPO2) below 95% were given supplementary oxygen support, while those who were severely ill with difficulty in breathing either required invasive or non-invasive mechanical ventilation. Samples for the test were collected through nasal-pharyngeal (NP) or oral-pharyngeal (OP) swabs at government-authorized testing centres.
2.3. Data management and analysis.
2.3.1. Managing missing data
Missing data is crucial in data analysis because it can lead to problems such as statistical power loss and loss of representativeness [13]. The complete case analysis method was used to manage missing data; we dropped records of cases with missing values in some of the variables essential in the analysis. This is because Missing Completely at Random (MCAR) does not lead to biases in the result and is appropriate for missing data related to the primary outcome of interest in the study [13].
2.3.2. Data analysis
Descriptive analysis was conducted, and continuous data were tested for normality first using the Kolmogovor-Smirnov test. The data was then analyzed and was presented as mean standard deviation (SD). Categorical data were cross-tabulated and analyzed using the chi-square test or fisher’s exact test to examine the differences between survivors and non-survivors. Then it was presented as frequencies and percentages. To discriminate between survivors and non-survivors, continuous data discriminant analysis was conducted.
Different methods control confounding factors in retrospective cohort studies, including stratification [14], multivariate regression analysis [15], and propensity matching score methods [16]. We chose multivariate regression in this present study. We first conducted a bivariate logistic regression analysis. All variables whose significance was P< 0.10 were selected and entered into a multivariate logistic regression model to evaluate the association between sex, age, comorbidities, smoking status, health status during admission to hospital, symptoms, oxygen support requirement, and the dependent variable critical illness of COVID-19 patients. The odds ratio (OR) was measured at a 95% confidence interval (CI) and reported with the P-value.
Kaplan-Meier survival tool was used to compare the survival times for different groups using log-rank tests. To identify determinants of mortality from COVID-19 among hospitalized patients, we performed bivariate and multivariate analyses by fitting Cox proportional hazard regression model. Hazard ratio (HR), 95% CI, and P-values were reported. SPSS performed all the statistical analyses for windows version 26.1. All P-values reported in the study were two-tailed, and P < 0.05 was deemed statistically significant.
3. Results
3.1. Characteristics of the COVID-19 patients admitted at Kenyatta National hospital
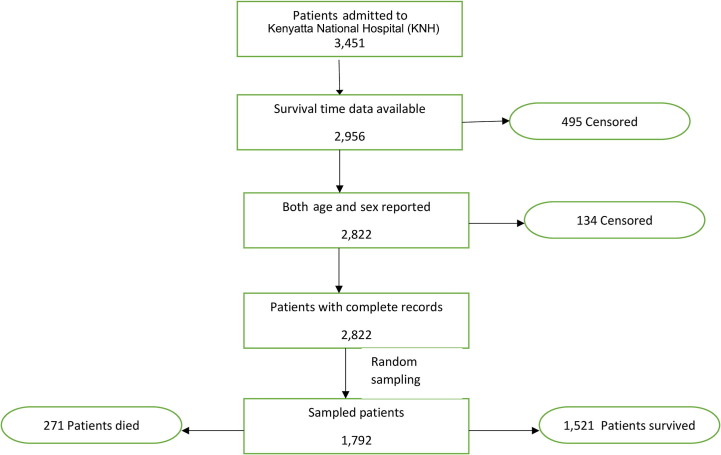
Out of 3,451 admitted patients, 2,956 patients' survival time data was available, out of which 2,822 reported gender and sex. Finally, 1,792 participants were randomly sampled from 2,822 patients with complete record data. Out of 1,792 sampled patients, 271 failed to survive while 1,521 survived (Fig. 1 ).
Fig. 1.
Flowchart for inclusion of patients in the final analysis
The participants had a mean age of 47.15 ± 15.13 years. The mean age of COVID-19 non-survivors and survivors was 60.93 ± 12.81 and 44.69 ± 14.16, respectively. Out of 1,792 sampled patients, 1,137 (63.4%) were male, while 655 (36.6%) were female.
The result indicated that 441 (24.6%) out of 1,792 patients experienced shortness of breath. On the other hand, 266 (14.8%) of patients reported to the hospital while severely ill, and only 1,526 (85.2%) reported to the hospital while in a stable condition. Furthermore, SPO2 levels among patients that were not admitted to ICU and those who survived were significantly higher compared to those admitted to ICU and non-survivors [(93.53 ± 6.51 vs 85 ± 12.05, P <0.001) (94.04 ± 5.29 vs 80.62 ± 12.85, P < 0.001)] respectively. The majority of the patients whose oxygen saturation levels were SPO2 ≤ 94% were admitted to ICU and also failed to survive. 508 (28.3%) of the patients required oxygen support, out of which 228 (44.9%) required ICU admission while 242 (47.6%) failed to survive. Shortness of breath(24.3%), fever (11.3%), dry cough (10.7%), general body weakness (9.9%), and chest pain (9.8%) were the most prevalent clinical features.
Out of 1,792 COVID-19 patients admitted, 322 (18.0%) had at least one comorbidity, with cardiovascular diseases (CVDs) including hypertension 111 (6.2%) being the most common, diabetes 97 (5.4%), HIV 71 (4.0%), chronic lung disease (CLD) 50 (2.8%), chronic liver disease 35 (2.0%), malignancy 32 (1.8%), renal disease 27 (1.5%) and obesity 27 (1.5%). The proportion of those admitted to ICU was significantly higher among those patients with obesity, CVDs including HTN, diabetes, chronic lung disease, and malignancy/cancer. On the other hand, the proportion of COVID-19 patients who had CVDs, diabetes, CLD, malignancy, and obesity was significantly higher in non-survivors than in survivors (p<0.001), The proportion of those with a history of smoking was significantly higher (P < 0.001) in no-survivors than in survivors. In contrast, the number of COVID-19 patients with HIV, chronic liver disease, and renal disease did not significantly differ between ICU admissions and non-ICU admission, survivors, and non-survivors (Table 1, Table 2 ).
Table 1.
Univariate and multivariate logistic regression analysis for predictors of ICU admission among COVID-19 patients (n = 1,792).
| Variables | ICU admission status |
COR(95% CI) | AOR(95% CI) | |
|---|---|---|---|---|
| Admitted | Not admitted | |||
| n(%) | n(%) | |||
| Age | ||||
| 0-30 (ref) | 13 (4.8) | 256 (95.2) | 1 | 1 |
| 31-60 | 169 (14.8) | 973 (85.2) | 3.42 (1.91-6.11) | 2.76 (1.49-5.11)** |
| >60 | 164 (43.0) | 217 (57.0) | 14.88 (8.23-26.93) | 6.33 (3.32-12.06)*** |
| Gender | ||||
| Male | 262 (75.7) | 875 (60.5) | 2.04 (1.56-2.66) | 1.6 (1.18-2.20)* |
| Female (ref) | 84 (24.3) | 571 (39.5) | 1 | 1 |
| Shortness of breath | ||||
| Yes | 204 (59.0) | 237 (16.4) | 7.33 (5.68-9.46) | 1.49 (0.94-2.38) |
| No (ref) | 142 (41.0) | 1,209 (83.6) | 1 | 1 |
| Health status | ||||
| Stable (ref) | 169 (48.8) | 1,357 (93.8) | 1 | 1 |
| Severely ill | 177 (51.2) | 89 (6.2) | 15.97 (11.82-21.57) | 7.20 (4.24-12.22)*** |
| 1 | ||||
| Patient on O2 | ||||
| Yes | 228 (65.9) | 280 (19.4) | 8.05 (6.22-10.41) | 3.08 (1.79-5.28)*** |
| No (ref) | 118 (34.1) | 1,166 (80.6) | 1 | 1 |
| SPO2 | ||||
| ≤ 94% | 246 (71.1) | 463 (32.0) | 5.22 (4.04-6.76) | 1.65 (1.12-2.43)* |
| ≥ 95% (ref) | 100 (28.9) | 983 (68.0) | 1 | 1 |
| Fever | ||||
| Yes | 94 (27.2) | 108 (7.5) | 4.62 (3.40-6.29) | 1.00 (0.65-1.52) |
| No (ref) | 252 (72.8) | 1,338 (92.5) | 1 | 1 |
| General weakness | ||||
| Yes | 93 (26.9) | 84 (5.8) | 5.96 (4.31-8.24) | 1.36 (0.88-2.09) |
| No (ref) | 253 (73.1) | 1,362 (94.2) | 1 | 1 |
| Headache | ||||
| Yes | 61 (17.6) | 84 (5.8) | 3.47 (2.44-4.94) | 1.73 (1.08-2.79)* |
| No | 285 (82.4) | 1,361 (94.2) | 1 | 1 |
| Dry cough | ||||
| Yes | 110 (31.8) | 82 (5.7) | 7.75 (5.64-10.65) | 2.04 (1.34-3.09)** |
| No (ref) | 236 (68.2%) | 1,364 (94.3) | 1 | 1 |
| Comorbidities | ||||
| Yes | 165 (47.7) | 157 (10.9) | 7.48 (5.72-9.79) | 2.28 (1.35-3.85)* |
| No (ref) | 181 (52.3) | 1,289 (89.1) | 1 | 1 |
| Obesity | ||||
| Yes | 18 (5.2) | 9 (0.6) | 8.76 (3.90-19.68) | 3.09 (1.05-9.08)* |
| No (ref) | 328 (94.8) | 1,437 (99.4) | 1 | 1 |
| CVDs incl HTN | ||||
| Yes | 62 (17.9) | 49 (3.4) | 6.22 (4.19-9.25) | 1.87 (1.14-3.06)* |
| No (ref) | 284 (82.1) | 1,397 (96.6) | 1 | 1 |
| HIV | ||||
| Yes | 21 (6.1) | 50 (3.5) | 1.80 (1.07-3.05) | 1.75 (0.95-3.21) |
| No (ref) | 325 (93.9) | 1,396 (96.5) | 1 | 1 |
| Diabetes | 3.30 (1.94-5.60)*** | |||
| Yes | 68 (19.7) | 29 (2.0) | 11.95 (7.60-18.81) | |
| No (ref) | 278 (80.3) | 1,417 (98.0) | 1 | |
| Chronic liver diseases | ||||
| Yes | 12 (3.5) | 23 (1.6) | 2.22 (1.10-4.51) | 1.36 (0.59-3.16) |
| No (ref) | 334 (96.5) | 1,423 (98.4) | 1 | 1 |
| Chronic lung disease | ||||
| Yes | 35 (10.1) | 15 (1.0) | 10.74 (5.79-19.90) | 3.35 (1.67-6.72)** |
| No (ref) | 311 (89.9) | 1,431 (99.0) | 1 | 1 |
| Malignancy/cancer | ||||
| Yes | 21 (6.1) | 11 (0.8) | 8.43 (4.02-17.66) | 3.55 (1.54-8.16)* |
| No (ref) | 325 (93.9) | 1,435 (99.2) | 1 | 1 |
| Renal disease | ||||
| Yes | 8 (2.3) | 19 (1.3) | 1.78 (0.77-4.09) | 1.51 (0.59-3.86) |
| No (ref) | 338 (97.7) | 1,426 (98.7) | 1 | 1 |
| Smoking | ||||
| Yes | 21 (6.1) | 36 (2.5) | 2.53 (1.46-4.39) | 1.15 (60-2.20) |
| No (ref) | 325 (93.9) | 1,410 (97.5) | 1 | 1 |
COR (95% CI): crude odds ratio at 95% confidence interval; AOR (95% CI): adjusted odds ratio, adjusted for age, gender, shortness of breath, health status, patient on O2 (oxygen), oxygen saturation(SPO2), fever, general weakness, headache, dry cough, comorbidities, obesity, CVDs incl HTN (cardiovascular diseases including hypertension), HIV, diabetes, chronic liver disease, chronic lung disease, malignancy/cancer, renal disease, and smoking. OR: Odds ratio; 95% CI: 95% confidence interval; P-value for overall association *P < 0.05, **P =0.001 and ***P < 0.0001.
Table 2.
Univariate and multivariate Cox regression analysis for predictors of mortality among the coronavirus disease 2019 (COVID-19) patients.
| Variables | Mortality status |
Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|---|---|
| Non-Survivors | Survivors | HR | 95% CI | P-value | HR | 95% CI | P-value | |
| n (%) | n (%) | |||||||
| Age | ||||||||
| 0-30 (ref) | 8 (3.0) | 261 (17.2) | 1 | 1 | ||||
| 31-60 | 96 (35.4) | 1,046 (68.8) | 2.33 | 1.13-4.80 | 0.021 | 1.35 | 0.65-2.84 | 0.423 |
| >60 | 167 (61.6) | 214 (14.1) | 11.21 | 5.51-22.80 | 0 | 2.69 | 1.28-5.68 | .009* |
| Gender | ||||||||
| Male | 208 (76.8) | 929 (61.1) | 1.85 | 1.40-2.460 | 0 | 1.54 | 1.15-2.08 | .004* |
| Female (ref) | 63 (23.2) | 592 (38.9) | 1 | 1 | ||||
| Shortness of breath | ||||||||
| Yes | ||||||||
| No (ref) | 240 (88.6) | 201 (13.2) | 21.44 | 14.75-31.18 | 0 | 2.96 | 1.61-5.43 | .000*** |
| 31 (11.4) | 1,320 (86.8) | 1 | 1 | |||||
| Health status | ||||||||
| Stable (ref) | ||||||||
| Severely ill | 58 (21.4) | 1,468 (96.5) | 1 | 1 | ||||
| 213 (78.6) | 53 (3.5) | 20.41 | 15.26-27.29 | 0 | 4 | 2.73-5.87 | .000*** | |
| Patient O2 | ||||||||
| Yes | 242 (89.3) | 266 (17.5) | 18.37 | 12.49-27.01 | 0 | 1.31 | .71-2.42 | 0.385 |
| No (ref) | 29 (10.7) | 1,255 (82.5) | 1 | 1 | ||||
| SPO2 | ||||||||
| ≤ 94% | 257 (94.8) | 452 (29.7) | 23.91 | 13.96-40.96 | 0 | 3.42 | 1.65-7.08 | .001** |
| ≥ 95% (ref) | 14 (5.2) | 1,069 (70.3) | 1 | 1 | ||||
| Fever | ||||||||
| Yes | 114 (42.1) | 88 (5.8) | 6.49 | 5.09-8.27 | 0 | 1.09 | .82-1.44 | 0.571 |
| No (ref) | 157 (57.9) | 1,433 (94.2) | 1 | 1 | ||||
| General weakness | ||||||||
| Yes | ||||||||
| No | 103 (38.0) | 74 (4.9) | 5.16 | 4.03-6.60 | 0 | 0.87 | .66-1.14 | 0.307 |
| 168 (62.0) | 1,447 (95.1) | 1 | 1 | |||||
| Headache | ||||||||
| Yes | 99 (36.5) | 46 (3.0) | 7.42 | 5.78-9.51 | 0 | 1.65 | 1.25-2.17 | .000*** |
| No (ref) | 172 (63.5) | 1,474 (97.0) | 1 | 1 | ||||
| Dry cough | ||||||||
| Yes | 119 (43.9) | 73 (4.8) | 5.68 | 4.46-7.22 | 0 | 1.08 | .82-1.42 | 0.591 |
| No (ref) | 152 (56.1) | 1,448 (95.2) | 1 | 1 | ||||
| Comorbidities | ||||||||
| Yes | 206 (76.0) | 116 (7.6) | 14.72 | 11.10-19.52 | 0 | 3.28 | 2.02-5.33 | .000*** |
| No (ref) | 65 (24.0) | 1,405 (92.4) | 1 | 1 | ||||
| Obesity | ||||||||
| Yes | 20 (7.4) | 7 (0.5) | 4.9 | 3.10-7.73 | 0 | 1.12 | .69-1.84 | 0.645 |
| No (ref) | 251 (92.6) | 1,514 (99.5) | 1 | 1 | ||||
| CVDs incl HTN | ||||||||
| Yes | ||||||||
| No (ref) | 86 (31.7) | 25 (1.6) | 8.05 | 6.22-10.43 | 0 | 2.17 | 1.60-2.94 | .000*** |
| 185 (68.3) | 1,496 (98.4) | |||||||
| HIV | ||||||||
| Yes | 15 (5.5) | 56 (3.7) | 1.4 | 0.83-2.36 | 0.207 | 1.14 | .61-2.15 | 0.685 |
| No (ref) | 256 (94.5) | 1,465 (96.3) | 1 | 1 | ||||
| Diabetes | ||||||||
| Yes | 82 (30.3) | 15 (1.0) | 8.38 | 6.46-10.88 | 0 | 2 | 1.46-2.70 | .000*** |
| No (ref) | 189 (69.7) | 1,506 (99.0) | 1 | 1 | ||||
| Chronic liver diseases | ||||||||
| Yes | ||||||||
| No (ref) | 8 (3.0) | 27 (1.8) | 1.38 | 0.68-2.79 | 0.371 | 0.72 | .34-1.51 | 0.382 |
| 263 (97.0) | 1,494 (98.2) | 1 | 1 | |||||
| Chronic lung disease | ||||||||
| Yes | ||||||||
| No (ref) | 46 (17.0) | 4 (0.3) | 7.81 | 5.68-10.74 | 0 | 3.16 | 2.18-4.58 | .000*** |
| 225 (83.0) | 1,517 (99.7) | 1 | 1 | |||||
| Malignancy/cancer | ||||||||
| Yes | 31 (11.4) | 1 (0.1) | 4.68 | 3.20-6.84 | 0 | 1.98 | 1.28-3.04 | .002* |
| No (ref) | 240 (88.6) | 1,520 (99.9) | 1 | 1 | ||||
| Renal disease | ||||||||
| Yes | 7 (2.6) | 20 (1.3) | 1.83 | 0.86-3.88 | 0.114 | 1.89 | .85-4.23 | 0.119 |
| No (ref) | 264 (97.4) | 1,500 (98.7) | 1 | 1 | ||||
| Smoking | ||||||||
| Yes | 35 (12.9) | 22 (1.4) | 4.94 | 3.46-7.05 | 0 | 2.21 | 1.52-3.21 | .000*** |
| No (ref) | 236 (87.1) | 1,499 (98.6) | 1 | 1 | ||||
HR (95% CI): Hazard ratio at 95% confidence interval for predictors of mortality-value for overall association *P < 0.05, **P =.001 and ***P < 0.0001.
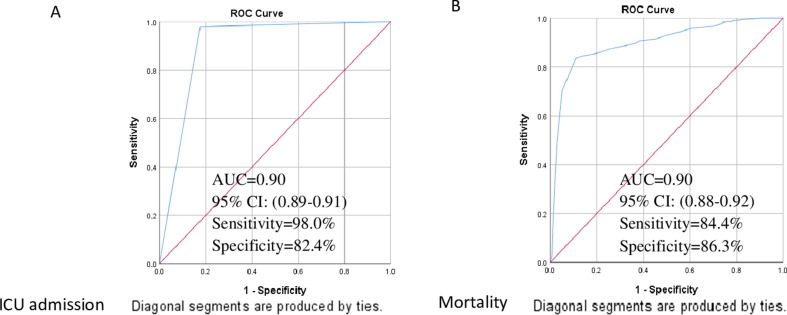
Both univariate and multivariate logistic regression model was used to define predictor variables for ICU admission among COVID-19 patients (Table 1), with (AUC-ROC: 0.90, 95% CI: 0.88–0.92) (Fig. 2 A). The model selected, older age of 31–60 and > 60 years (adjusted odds ratio (AOR): 2.76, 95% CI: 1.49–5.11, P = 0.001) and (AOR: 6.33, 95% CI: 3.32–12.06, P < 0.0001) correspondingly, male (HR: 1.59, 95% CI: 1.17–2.17, P = 0.003), health status (AOR: 7.20, 95% CI:4.24–12.22, P < 0.0001), patient on O2 (AOR: 3.08, 95% CI:1.79–5.28, P < 0.0001), SPO2 (AOR: 1.65, 95% CI: 1.12–2.43, P = 0.012), headache (AOR: 1.73, 95% CI: 1.08–2.79, P = 0.023), dry cough (AOR: 2.04, 95% CI: 1.34–3.09, P = 0.001), comorbidities (AOR: 2.28, 95% CI: 1.35–3.85, P = 0.016), obesity (AOR: 3.09, 95% CI: 1.05–9.08, P = 0.040), CVDs (AOR: 1.87, 95% CI:1.14–3.06, P = 0.013), diabetes (AOR: 3.30, 95% CI: 1.94–560, P < 0.0001), CLD (AOR: 3.35, 95% CI: 1.67–6.72, P = 0.001) and malignancy (AOR: 3.55, 95% CI: 1.54–8.16, P = 0.003) as the predictor of ICU admission among COVID-19 patients (Table 1).
Fig. 2.
Receiver operating characteristic (ROC) curves for patient charateristics to predict ICU admission (A) and mortality (B) among COVID-19 patients
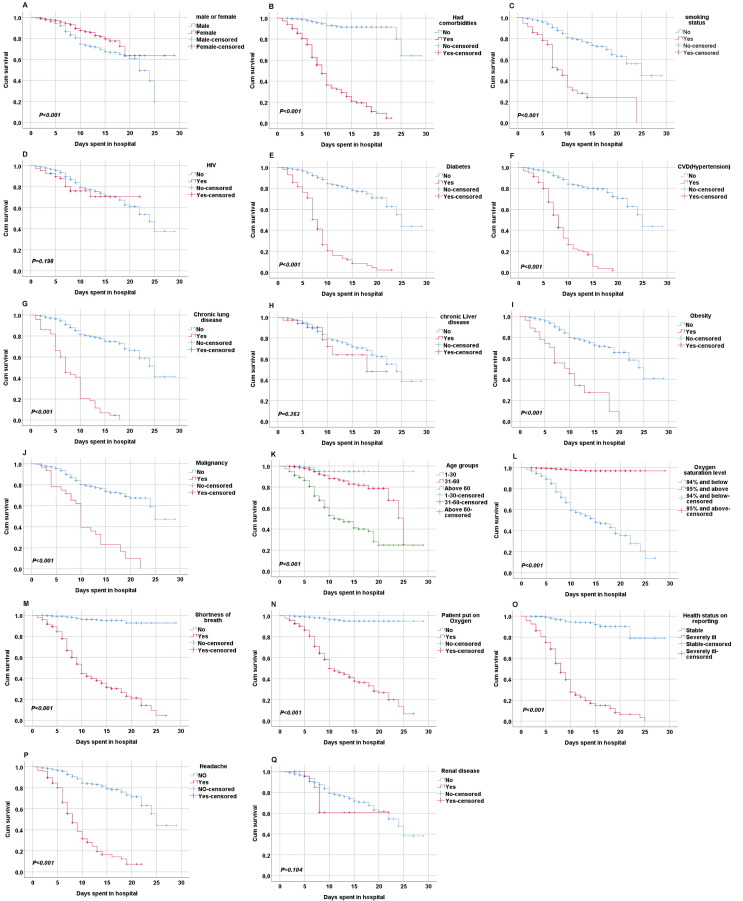
Kaplan-Meier curves were used to compare and estimate the survival times of individuals in the groups using log-rank tests. Individuals aged 31–60 and > 60 years were less likely to survive than those younger aged 0–30 years with P < 0.05. Patients who required supplementary oxygen support during hospitalization and those whose oxygen saturation levels were ≤ 94% had less survival probability and could die earlier than their counterparts. Additionally, patients who reported to have had a headache, shortness of breath, and were severely ill when admitted had fewer survival chances and could die earlier than their counterparts. There was no significant difference in the survival probability of COVID-19 patients who had HIV (P = 0.198), chronic liver disease (P = 0.363), and renal disease (P = 0.104) compared to those without these comorbidities (Fig. 2). However, the survival probability of COVID-19 patients with diabetes, CVDs, CLD, obesity, and malignancy was significantly less (P < 0.001) compared to those without the conditions (Fig. 3 ). Also, those who were male and those who had a history of smoking had a lower survival probability than their counterparts.
Fig. 3.
The Kaplan-Meier curves for both demographic and clinical variables of COVID-19 patients in Kenyatta National hospital, Kenya. A) Gender with COVID-19; B) Comorbidities with COVID-19; C) Smoking status with COVID-19; D) HIV with COVID-19; E) Diabetes patients with COVID-19; F) CVDs with COVID-19; G) CLD with COVID-19; H) Chronic liver disease with COVID-19; I) Obesity with COVID-19; J) Malignancy with COVID-19; K) Age with COVID-19; L) SPO2 with COVID-19; M) Shortness of breath withCOVID-19; N) Patient on O2 with COVID-19; O) Health status with COVID-19; P)Headache with COVID-19; Q) Renal disease with COVID-19. Abbreviations: COVID-19: coronavirus disease 2019; CVDs, cardiovascular diseases; O2, oxygen; CLD, chronic lung disease; SPO2; oxygen saturation levels
Univariate and multivariate Cox’s proportional hazard regression was performed, and results were recorded in Table 2. The final multivariate model identified the following predictor variables for mortality with ROC and areas under a curve (Fig. 2B) (AUC = 0.90, 95% CI: 0.88–0.92); age of > 60 years (hazard ratio (HR): 2.69, 95% CI: 1.28–5.68, P = 0.009), male (HR: 1.54, 95% CI: 1.15–2.08, P = 0.004), shortness of breath (HR: 2.96, 95% CI: 1.61–5.43, P < 0.0001), health status (HR: 4.00, 95% CI: 2.73–5.87, P < 0.0001), SPO2 (HR: 3.42, 95% CI: 1.65–7.08, P = 0.001) headache (HR: 1.65, 95% CI: 1.25–2.17, P < 0.0001), comorbidities (HR: 3.28, 95% CI: 2.02–5.33, P < 0.0001), CVDs (HR: 2.17, 95% CI: 1.60–2.94, P < 0.0001), diabetes (HR: 2.00, 95% CI: 1.46–2.70, P < 0.0001), CLD (HR: 3.16, 95% CI: 2.18–4.58, P < 0.0001), malignancy (HR: 1.98, 95% CI: 1.28–3.04, P = 0.002) and smoking (HR: 2.21, 95% CI: 1.52–3.21, P < 0.0001). Older COVID-19 patients above 60 years were about 3 times more likely to die than the younger patients. The patients who were smokers were 2.21 times more likely to die compared those who were non-smokers. Moreover, the hazard of death of COVID-19 patients with underlying comorbidities, CVDs, diabetes, chronic lung disease and malignancy also increased with recorded HR of 3.28, 2.17, 2.00, 3.16 and 1.98 respectively (Table 2).
When comparing simultaneously multivariate results for predictors of ICU admission and mortality simultaneously. Age groups 31–60 and > 60 years were significantly associated with increased odds of ICU admission, which are (AOR: 2.76) and (AOR: 6.33), respectively. In contrast, the mortality hazard was significantly higher in only patients above 60 years of age (HR: 2.69). In addition, male patients were at increased risk of ICU admission and mortality with (AOR: 1.61) and (HR: 1.54). The result implies male patients were 2 times more likely to be admitted to ICU or die from COVID-19. Shortness of breath significantly predicted mortality from COVID-19 but not the risk of ICU admission. Patients who were severely ill at the time of admission had SPO2 ≤ 94%, required supplementary oxygen support, had experienced headaches, had comorbidities, had CVDs, diabetes, CLD, and malignancy/cancer were more likely to require ICU admission or die from COVID-19 than their counterparts. On the other hand, those with a history of smoking were more likely to die from COVID-19 than being admitted to ICU (Tables 1&Table 2).
4. Discussion
To our limited knowledge, this is the first single-centered retrospective cohort study to explore predictors for ICU admission and hospital mortality among COVID-19 patients in Kenya. This study's most hospitalized COVID-19 patients were male, 1,137 (63.4%). Similarly, other cohort studies have reported a high prevalence of COVID-19 in men compared to females [4], [8], [9], [10], [11], [17]. For example, a study conducted at New York-Presbyterian hospital showed that 67% of those hospitalized with COVID-19 were male [18]. In the present study, 208 men died, representing (76.8%) of the total deaths due to COVID-19. This is consistent with previous studies; in China [19], men were 2.4 times more likely to succumb to COVID-19 as compared to women (70.3% vs 29.7%, P = 0.016), and in Italy, men accounted for 70% of the total death due to SARS-CoV-2 [20]. This may be attributed to the physiological fact that sex hormones are crucial in the immune system's inflammatory responses [21]. For example, estrogen stimulates both cellular and humoral immune responses [4]. This immune response favors female to attain effective resistance to microbial infection and makes them less susceptible to viral attack [4], [21]. Also, men's high prevalence of smoking and alcohol consumption makes men more vulnerable to SARS-CoV-2 than women [22].
In this present study, the risk of ICU admission and death was significantly lower in the younger population than in the older population above 60 years. However, old age has been associated with severe outcomes among COVID-19 patients [17], [23]. In our present study, older patients of COVID-19 were at risk of developing critical illness or even dying. This result can be comparable to other studies that recorded that the risk of ICU admission and death from COVID-19 increased with age, and patients above 60 years were more vulnerable [10], [17], [22], [26], [24]. The reasons surrounding this trend of older individuals experiencing severe COVID-19 might not be absolute, but they try to explain. First, old age has been associated with chronic diseases, making old individuals vulnerable to the disease [9], [25], [23], [27], [28], [26]. Moreover, old age has been associated with immune system dysfunction, making the old more susceptible and vulnerable to infections [23], [30], [29]. Additionally, older age is associated with extra inflammatory biomarkers, which damage the control of viremia and swelling, thus increasing morbidity and mortality in aged individuals [11], [30].
This present study identified shortness of breath (24.3%), fever (11.3%), dry cough (10.7%), general body weakness (9.9%) and chest pain (9.8%) as the most prevalent clinical features among admitted COVID-19 patients. This trend is consistent with other previous studies [4], [27]. For example, a cohort study at New York-Presbyterian hospitals revealed shortness of breath as the most prevalent symptom reported at 74%, followed by fever at 71% and dry cough at 66% (13).
In this study, patients admitted to ICU and non-survivors had low SPO2 levels compared to those not admitted to ICU and those who survived. These findings are consistent with other studies [31], [32], [33]. For example, a cohort study in Peru reported an increased risk of ICU admission and death among COVID-19 patients with low SPO2; patients with SPO2 < 80% were eight times more likely to die (HR: 7.74, 95% CI: 4.54–13.19). This is because oxygen is crucial in vertebrates' self-adjustment mechanism [34]. In addition, hypoxia causes acute multi-organ dysfunction, which leads to needing ICU admission [34], [35].
This study identified the co-infection rate of COVID-19 with HIV to be 4.0%, and this is almost similar to the HIV infection rate in Kenya, which is 4.8% [36]. This implies that the co-infection rate of COVID-19 among people living with HIV is similar to the general population. Similarly, most studies reported no increase in the incidence of COVID-19 among people living with HIV compared to the general population [37], [38]. The co-infection rate of COVID-19 patients with obesity, CVDs, diabetes, chronic liver disease, CLD, malignancy and renal disease was 1.5%, 6.2%, 5.4%, 2.0%, 2.8%, 1.8% and 1.5% respectively.
In our present study, ICU admission and mortality incidence among COVID-19 patients was 346 (19.3%) and 271 (15.1%), respectively. This incidence of ICU admission is relatively lower than in previous cohort studies. For instance, a study in New York reported a 22% incidence of ICU admission [18]. The lower critical illness rate among COVID-19 patients in Kenya may be due to many factors. First, the containment measures in Kenya required all individuals who tested positive for COVID-19 to be isolated. Thus giving a false impression of the total number of admitted cases. Countries with older populations experienced a higher critical illness and mortality rate due to COVID-19 [24], [39]. Kenya has a younger population whose median age is 19.7 years compared to German, Italy, Spain, the USA, and China, whose median ages are 47.1, 45.5, 42.7, 38.1 and 37.0 years, respectively [40].
In our present study, most patients admitted to ICU had underlying comorbidities. This is also in keeping records with previous cohorts [4], [11], [27]. Most chronic diseases increase inflammatory responses and consequently lower the general body immunity of individuals, thus making this group of individuals more vulnerable to COVID-19. Moreover, most of the non-survivors had comorbidities 206 (76.0%). The comorbidities that were significantly reported in non-survivors were; CVDs, diabetes, CLD, malignancy, obesity, and smoking. This is consistent with reports of other cohort studies [8], [9], [41]. Chronic diseases such as diabetes and CVDs are risk factors for morbidity and mortality. These diseases encourage viral entry and weaken immune response. This is a result of either the side effects of medication or the direct impacts of the illness [4]. In our present study, diabetes (HR: 3.30, 95% CI: 1.94–5.60) significantly increased the risk of ICU admission. Our result is consistent with the cohort study done in China [26]. The pathological mechanism behind this is not known. However, glucose control in diabetic patients is an important prognostic factor for all forms of infection [28], [30]. Poorly regulated blood glucose levels cause immune dysfunction [42]. Studies have shown that patients with diabetes have elevated CRP [43], [44], [45], which is associated with multi-organ dysfunction [21], [29].
A recent meta-analysis indicated that cardiovascular implications, including arrhythmia, hypertension, coronary heart diseases, cardiac injury, and cardiovascular diseases, increased the odds of ICU admission among COVID-19 patients [31]. In our present study, the COVID-19 patients with CVDs were about two times more likely to be admitted to ICU than those without these conditions (HR: 1.87, 95% CI: 1.14–3.06), thus keeping in record with the later meta-analysis.
In the current study, patients with comorbidities were twice more likely to die from COVID-19 than those without comorbidities. Our result agrees with other cohort studies associated comorbidities with severe COVID-19 outcomes [17], [42].
Smoking is a risk factor for most respiratory infections [46]. In addition, smoking affects the immune system and consequently weakens the ability of the host to mount suitable immune and significant inflammatory responses. In the present study, 61.4% of COVID-19 patients with a smoking history died during hospitalization. Further analysis indicated that patients with smoking history were twice more likely to die from COVID-19. Furthermore, a study conducted in China revealed that severe cases of COVID-19 had a significantly higher number of patients with a smoking history than stable cases (27.3% vs 3.0%) [47].
The major strength of this present study is that it is the first attempt to explore predictors for ICU admission and hospital mortality among COVID-19 patients in Kenya. Several studies have been done associating comorbidities and severe outcomes of COVID-19. Although there have been conflicting findings regarding the effects of HIV on COVID-19, our present study did not find any association between HIV and increased risk of ICU admission and mortality among COVID-19 patients. However, a more exclusive study should be done to validate these findings in Kenya, one of the world's high HIV prevalence regions. In addition, the study included asymptomatic, mild, and severe cases, which provided a good presentation of COVID-19 patients. However, there were several limitations to the current research. First, we collected epidemiological data retrospectively; thus, bias might have occurred. Second, there were missing data on some variables, reducing the sample's representativeness. Third, laboratory findings were not collected due to incomplete records, which might have led to the omission of other important risk factors for critical illness and mortality. Lastly, we lacked data to compare with other patients not admitted.
5. Conclusion
In conclusion, this is the first study to explore predictor factors for ICU admission and in-hospital mortality among COVID-19 patients in Kenya. However, most risk factors predicting critical illness also predict in-hospital mortality. Therefore, essential factors during the stratification of high-risk COVID-19 patients are warranted.
Ethics statement
The Institutional Ethical Review Committee approved this study of Capital Medical University in Beijing, China, approval number No. 2020SY23. In addition, the official research permit was sought from Kenya National Commission for Science, Technology and Innovation license number NACOSTI/21/8553. To access hospital data, official permission and approval were sought from the Hospital Research & Ethical Approval Board of Kenyatta National Hospital with approval number P184/03/2021.
Acknowledgements
The authors would like to acknowledge Tychicus Ogoti for editing the manuscript. We also thank the Kenyatta National Hospital administration for allowing us to access and extract data. Finally, we would like to acknowledge the institutional ethics committees of the following universities for approving this work; Capital Medical University in Beijing, China, and the University of Nairobi, Kenya.
This study projected was funded by the Beijing talent project [grant number 2020A17]. The funder did not play any role in study design formulation, data management, extraction, formal data analysis, result interpretation, and report drafting.
Conflict of interest statement
The authors declare there are no conflicts of interest.
Author contributions
Isinta M Elijah: Conceptualization, Methodology, Writing - Original Draft. Endawoke Amsalu: Software, Validation. Xuening Jian: Formal Analysis. Mingyang Cao: Data Curation. Eric K Mibei: Supervision. Danvas O Kerosi: Writing - Review & Editing. Francis G Mwatsahu: Investigation. Wei Wang: Conceptualization, Methodology. Faith Onyangore: Project Administration. Youxin Wang: Conceptualization, Methodology, Resources.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bsheal.2022.06.002.
Supplementary data
The following are the Supplementary data to this article:
References
- 1.Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., et al. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO, WHO Weekly epidemiological update. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---19-october-2021, 2021 (accessed 10 November 2021).
- 3.WHO, Weekly epidemiological update on COVID-19. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---23-november-2021, 2021 (accessed 1 December 2021).
- 4.Fouad S.H., Allam M.F., Ibrahim S., Ashraf A., Roman S.W., Hosny A., Moneer M. ICU admission of COVID-19 patients : Identification of risk factors ICU admission of COVID-19 patients : Identification of risk factors. Egypt. J. Anaesth. 2021;37:202–207. doi: 10.1080/11101849.2021.1919433. [DOI] [Google Scholar]
- 5.World Bank, Physicians (per 1,000 people). https://data.worldbank.org/indicator/SH.MED.PHYS.ZS?end=2015&locations=ZG&start=1994, 2021 (accessed 9 November 2021 ).
- 6.Tessema S.K., Nkengasong J.N. Understanding COVID-19 in Africa. Nat. Rev. Immunol. 2021;21:469–470. doi: 10.1038/s41577-021-00579-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Id E.O., Parikh A., Lopez-ruiz A., Carrilo M., Goldberg J., Cearras M., Fernainy K., Sniffen J., Herrera V., Finkler N., et al. ICU outcomes and survival in patients with severe COVID-19 in the largest health care system in central Florida. PLos One. 2021;131:1–14. doi: 10.1371/journal.pone.0249038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inciarte A., Gonzalez-Cordon A., Rojas J., Torres B., de Lazzari E., de la Mora L., Martinez-Rebollar M., Laguno M., Callau P., Gonzalez-Navarro A., et al. in a large cohort of adults living with HIV: a single-center, prospective observational study. AIDS. 2019;34(2020):1775–1780. doi: 10.1097/QAD.0000000000002643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.L. Kim, S. Garg, A. O’Halloran, M. Whitaker, H. Pham, E.J. Anderson, I. Armistead, N.M. Bennett, L. Billing, K. Como-Sabetti, et al., Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-NET), Clin. Infect. Dis. 72 (2021) e206–e214, 10.1093/cid/ciaa1012. [DOI] [PMC free article] [PubMed]
- 10.Albitar O., Ballouze R., Ping J., Maisharah S., Ghadzi S. Risk factors for mortality among COVID-19 patients Orwa. Diabetes Res. Clin. Pract. 2020;166 doi: 10.1016/j.diabres.2020.108293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lei M., Lin K., Pi Y., Huang X., Fan L., Huang J., Liu R., Liu L., Shao X., Hu K., Yang L., Qin S., He F., Saisho Y. Clinical features and risk factors of ICU admission for COVID- 19 patients with diabetes. J. Diabetes Res. 2020;2020:1–10. doi: 10.1155/2020/5237840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V., Endeman H. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb. Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papageorgiou G., Grant S.W., Takkenberg J.J.M., Mokhles M.M. Statistical primer: How to deal with missing data in scientific research ? Interact. Cardiovasc. Thorac. Surg. 2018;27:153–158. doi: 10.1093/icvts/ivy102. [DOI] [PubMed] [Google Scholar]
- 14.Z. Zhang, H. Zhang, M. Khanal, Development of scoring system for risk stratification in clinical medicine: a step-by-step tutorial, Ann. Transl. Med. 5 (2017), 436. 10.21037/atm.2017.08.22. [DOI] [PMC free article] [PubMed]
- 15.Alexopoulos E.C. Introduction to multivariate regression analysis. Hippokratia. 2010;14(Suppl 1):23–28. [PMC free article] [PubMed] [Google Scholar]
- 16.Austin P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav. Res. 2011 May;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan W.J., Liang W.H., Zhao Y.i., Liang H.R., Chen Z.S., Zheng Z.J., Li S.Y., Zhang N.-f., Zhong N.S., He J.X., et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur. Respir. J. 2020;55(5):2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M., Aaron J.G., Claassen J., Rabbani L.E., Hastie J., et al. Articles Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City : a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin J.M., Bai P., He W., Wu F., Liu X.F., Han D.M., Liu S., Yang J.K. Gender Differences in Patients With COVID-19: Focus on Severity and Mortality. Front. Public Heal. 2020;8:1–6. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borges do Nascimento I.J., Cacic N., Abdulazeem H.M., von Groote T.C., Jayarajah U., Weerasekara I., Tian M., Arcani D.M.C., O’Mathúna D.P., Marcolino M.S., et al. Novel coronavirus infection (Covid-19) in humans: A scoping review and meta-analysis. J. Clin. Med. 2020;9(4):941. doi: 10.3390/jcm9040941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taneja V. Sex hormones determine immune response. Front. Immunol. 2018;9:1–5. doi: 10.3389/fimmu.2018.01931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bwire G.M. Coronavirus: Why Men are More Vulnerable to Covid-19 Than Women? SN Compr. Clin. Med. 2020;2:874–876. doi: 10.1007/s42399-020-00341-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shahid Z., Kalayanamitra R., McClafferty B., Kepko D., Ramgobin D., Patel R., Aggarwal C.S., Vunnam R., Sahu N., Bhatt D., Jones K., Golamari R., Jain R. COVID-19 and older adults: What we know. J. Am. Geriatr. Soc. 2020;68:926–929. doi: 10.1111/jgs.16472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X., Xu S., Yu M., Wang K., Zhang Z., Renz H., Liu X., Xie J., Xie M., Zhao J., et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang E.K., Lee S.Y., Jung H., Kim M.S., Cho B., Kim Y.S. Operating protocols of a community treatment center for isolation of patients with coronavirus disease, South Korea. Emerg. Infect. Dis. 2020;26:2329–2337. doi: 10.3201/eid2610.201460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Surme S., Buyukyazgan A., Bayramlar O.F., Cinar A.K., Copur B., Zerdali E., Tuncer G., Balli H., Nakir I.Y., Yazla M., et al. pneumonia in istanbul, turkey. Jpn. J. Infect. Dis. 2019;74(2021):458–464. doi: 10.7883/YOKEN.JJID.2020.1065. [DOI] [PubMed] [Google Scholar]
- 26.Shahid Z., Kalayanamitra R., McClafferty B., Kepko D., Ramgobin D., Patel R., Aggarwal C.S., Vunnam R., Sahu N., Bhatt D., Jones K., Golamari R., Jain R. COVID-19 and older adults: What we know. J. Am. Geriatr. Soc. 2020;68:926–929. doi: 10.1111/jgs.16472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayaz A., Arshad A., Malik H., Ali H., Hussain E., Jamil B. Risk factors for intensive care unit admission and mortality in hospitalized COVID-19 patients. Acute Crit. Care. 2020;35:249–254. doi: 10.4266/ACC.2020.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanyaolu A., Okorie C., Marinkovic A., Patidar R., Younis K., Desai P., Hosein Z., Padda I., Mangat J., Altaf M. Comorbidity and its impact on patients with COVID-19. SN Compr. Clin. Med. 2020;2:1069–1076. doi: 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amber L. Mueller, Maeve S.McNamara, David A. Sinclair, Why does COVID-19 disproportionately affect older people?, Aging 12 (2020) 9959–9981, 10.18632/aging.103344. [DOI] [PMC free article] [PubMed]
- 30.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chatterjee N.A., Jensen P.N., Harris A.W., Nguyen D.D., Huang H.D., Cheng R.K., Savla J.J., Larsen T.R., Gomez J.M.D., Du-Fay-de-Lavallaz J.M., et al. Admission respiratory status predicts mortality in COVID-19. Influenza Other Respi. Viruses. 2021;15:569–572. doi: 10.1111/irv.12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bates J.H.T., Suki B. Modeling lung perfusion abnormalities to explain. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-18672-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mejía F., Medina C., Cornejo E., Morello E., Vásquez S., Alave J., Schwalb A., Málaga G., Taniyama Y. Oxygen saturation as a predictor of mortality in hospitalized adult patients with COVID-19 in a public hospital in Lima, Peru. PLoS One. 2020;15(12):e0244171. doi: 10.1371/journal.pone.0244171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marieb E.N., Hoehn K. 9th ed. Pearson Education Inc; United States of America: 2013. Human anatomy and Physiology 9th Edition. [Google Scholar]
- 35.Rahman A., Tabassum T., Araf Y., Al A., Asad N., Mohammad U., Hosen J. Silent hypoxia in COVID - 19: pathomechanism and possible management strategy. Mol. Biol. Rep. 2021;48:3863–3869. doi: 10.1007/s11033-021-06358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National AIDS Control Council, HIV Prevalence in Kenya 2021 Per County PDF, UGWIRE. https://ugwire.com/hiv-prevalence-kenya-per-county-pdf/, 2021 (accessed 12 October 2021).
- 37.Tesoriero J.M., Swain C.A.E., Pierce J.L., Zamboni L., Wu M., Holtgrave D.R., Gonzalez C.J., Udo T., Morne J.E., Hart-Malloy R., et al. COVID-19 Outcomes among Persons Living with or without Diagnosed HIV Infection in New York State. JAMA Netw. Open. 2021;4:1–14. doi: 10.1001/jamanetworkopen.2020.37069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Squillace N., Ricci E., Colella E., Bonfanti P. HIV and SARS-CoV-2 Co-Infection: What are the Risks? Infect. Drug Resist. 2021;14:3991–4014. doi: 10.2147/idr.s277899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.C. Favas, P. Jarrett, R. Ratnayake, O.J. Watson, and F. Checchi, Country differences in transmissibility, age distribution and case-fatality of SARS-CoV-2: a global ecological analysis, Int. J. Infect. Dis. 114 (2022) 210–218, 10.1016/j.ijid.2021.11.004. [DOI] [PMC free article] [PubMed]
- 40.A. O’Neill, Countries with the highest median age in 2021 (in years). https://www.statista.com/statistics/264727/median-age-of-the-population-in-selected-countries/, 2021 (accessed 10 September 2021).
- 41.Ramanathan K., Antognini D., Combes A., Paden M., Zakhary B., Ogino M., Maclaren G., Brodie D. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England) 2020;395(10229):19–21. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo L., Shi Z., Zhang Y., Wang C., Cristina N., Vale D. Comorbid diabetes and the risk of disease severitytrun or death among 8807 COVID-19 patients in China: A meta-analysis. Diabetes Res. Clin. Pract. J. 2020;166 doi: 10.1016/j.diabres.2020.108346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stringer D., Braude P., Myint P.K., Evans L., Collins J.T., Verduri A., Quinn T.J., Vilches-Moraga A., Stechman M.J., Pearce L., Moug S., McCarthy K., Hewitt J., Carter B. The role of C-reactive protein as a prognostic marker in COVID-19. Int. J. Epidemiol. 2021;50:420–429. doi: 10.1093/ije/dyab012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luan Y.Y., Yin C.H., Yao Y.M. Update advances on C-reactive protein in COVID-19 and other viral infections. Front. Immunol. 2021;12:1–10. doi: 10.3389/fimmu.2021.720363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gorgun S. A Comprehensive COVID-19 Meta-Analysis : Clinical Data of 18,450 Patients, COVID-19 Pandemic. Case Studies & Opinions. 2020;1:33–43. [Google Scholar]
- 46.Stämpfli M.R., Anderson G.P. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat. Rev. Immunol. 2009;9:377–384. doi: 10.1038/nri2530. [DOI] [PubMed] [Google Scholar]
- 47.Liu W., Tao Z.W., Wang L., Yuan M.L., Liu K., Zhou L., Wei S., Deng Y., Liu J., Liu H.G., Yang M., Hu Y., Analysis of factors associated with disease outcomes in hospitalized patients with novel coronavirus disease. Chin. Med. J. (Engl) 2019;133(2020):1032–1038. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.