Abstract
There is an art and science to performing mouse anesthesia, which is a significant component to animal research. Frequently, anesthesia is one vital step of many over the course of a research project spanning weeks, months, or beyond. It is critical to perform anesthesia according to the approved research protocol using appropriately handled and administered pharmaceutical-grade compounds whenever possible. Sufficient documentation of the anesthetic event and procedure should also be performed to meet the legal, ethical, and research reproducibility obligations. However, this regulatory and documentation process may lead to the use of a few possibly oversimplified anesthetic protocols used for mouse procedures and anesthesia. Although a frequently used anesthetic protocol may work perfectly for each mouse anesthetized, sometimes unexpected complications will arise, and quick adjustments to the anesthetic depth and support provided will be required. As an old saying goes, anesthesia is 99% boredom and 1% sheer terror. The purpose of this review article is to discuss the science of mouse anesthesia together with the art of applying these anesthetic techniques to provide readers with the knowledge needed for successful anesthetic procedures. The authors include experiences in mouse inhalant and injectable anesthesia, peri-anesthetic monitoring, specific procedures, and treating common complications. This article utilizes key points for easy access of important messages and authors’ recommendation based on the authors’ clinical experiences.
Keywords: anesthesia, animal research, animal welfare, Mus musculus, refinement
INTRODUCTION
Background
The laboratory mouse (Mus musculus) is popular in animal research because of its small size; relative ease of care; established and varied scientific procedure protocols, including genetic manipulation; and the availability to purchase established genetic lines. As such, mice commonly undergo anesthesia for experiments and surgical procedures. The wide spectrum of anesthetic experience for animal care and research personnel poses challenges for institutions responsible for training individuals of various skill levels on safe anesthetic delivery to mice. Commonly, knowledge regarding anesthesia in the research community is passed through peer-to-peer training, including veterinarians and researchers. While useful, misinterpretation of anesthesia protocols and concepts is a common occurrence. Additionally, veterinarians and researchers may be reluctant to utilize alternative anesthetic drugs, in effect limiting the potential anesthetic regimes. Given the range of anesthetic protocols utilized in mouse studies and the variations of experience and training in mouse anesthesia for both veterinarians and researchers, we sought in this paper to provide readers with fundamental mouse general anesthesia insight and practical mouse anesthetic protocols tailored for research use.
General anesthesia may be used for surgical and non-surgical procedures and is also referred to as surgical anesthesia. Properly induced and maintained general anesthesia with effective monitoring is vital to maintaining animal welfare and creating reproducible studies.1,2 It allows the performance of lengthy and otherwise potentially painful and invasive procedures, including laparotomies, orthopedic manipulations, xenograft transfers, embryo derivation, and cranial implants, by rendering an animal unconscious and immobile for a procedure. There are 4 main general anesthesia components: unconsciousness, amnesia, immobility/muscle relaxation, and analgesia. Sedation, hypnosis, and tranquilization are frequently used terms when discussing anesthesia, but are specific terms separate from the 4 main general anesthesia components.
Definitions
We define these terms as follows:
Unconsciousness: lack of awareness and perception of an animal’s surroundings.
Immobility/muscle relaxation: the inability to move where the muscles lay in a non-tensed loose state.
Analgesia: absence of pain in response to a noxious or painful stimulus.
Amnesia: inability to recall events or an experience.
Sedation: a state of central depression where the animal is drowsy and relaxed to some degree. The animal is generally unaware of its surroundings but, contrary to unconsciousness, can be aroused and stimulated by noxious stimuli.
Hypnosis: artificially induced sleep or trance from which an animal can be readily aroused.
Tranquilization: a state in which animal is relaxed and non-anxious but is aware of its surroundings.
Anesthesia
Much of our mouse anesthesia knowledge is derived from our anesthesia knowledge of humans and larger animals as the mechanisms are similar across species. In general, anesthetics produce altered states of consciousness.3–5 In animals, loss of movement (ie, movement to avoid a noxious stimulus) and the righting reflex is often used as an indicator of unconsciousness in anesthetized animals6 and is closely correlated with human loss of consciousness.7 This indicator is used across animal species8 and is especially useful in mice. In addition to an anesthetic’s ability to alter consciousness, anesthetics impact memory (ie, cause amnesia).4,5,9–11 Though we have a limited ability to assess amnesia in animals, previous studies have shown amnesia secondary to anesthesia in a variety of animal species, including mice,12,13 rats,13,14 and zebrafish.15 Thus, properly induced and conducted general anesthesia is expected to produce amnesia in laboratory animals.
Immobility during anesthesia may be the easiest method to assess anesthetic efficacy. The mechanisms in which anesthetics work to produce immobility have been discussed extensively elsewhere.8,16–18 To summarize, specialized peripheral nerve endings sensing noxious stimuli (nociceptors) relay information about a stimulus from a peripheral site on the body to an area of the spinal cord receiving sensory stimulation (dorsal horn) via connecting neurons, known as second-order neurons. This information is relayed to structures within the brain where the noxious stimulus is consciously perceived (pain). The information is then relayed back to the spinal cord through an area transmitting information for motor output (ventral horn) and subsequently sent to peripheral motor neurons, resulting in reaction to a stimulus. The ability of an anesthetic to cause immobility comes from disruption of action along this pathway and varies by the anesthetic drug utilized. At a high enough concentration, this disruption suppresses the spinal reflexes, thus preventing movement. This is discussed further in the next section and has been summarized in Figure 1.
Figure 1 .
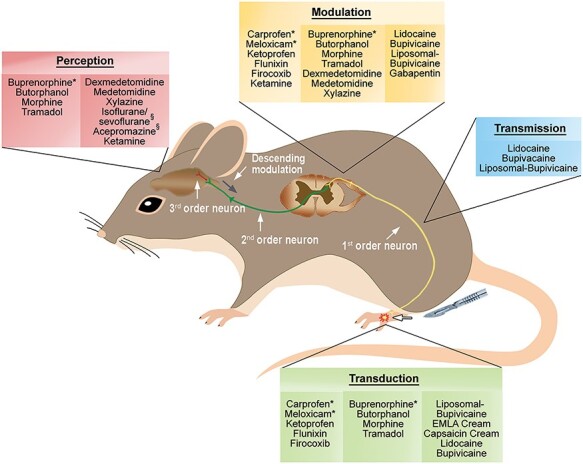
The pain pathway. A pain pathway graphic summary, including its major components. A nociceptive stimulus (injuries or surgeries) activates nociceptors (transduction). Stimulus information (ie, pain) travels through the nerve fibers such as A-δ and C fibers (transmission) to the spinal cord (modulation). Sufficient pain signals up-regulation, causing stimulus information to travel up the nervous system to the brain where pain perception occurs in conscious animals (perception). Commonly used analgesics are identified at their pain pathway action sites. *, Indicates the analgesic has both a standard and long-acting formulation available. §, Indicates the drug suppresses the perception of pain (due to a surgical anesthesia plane) but does not provide analgesia. Figure created by Janis Atuk-Jones.
Neuromuscular Blocking Agents
Neuromuscular blocking agents (NMBAs), also known as muscle relaxers or paralytics (eg, d-tubocurarine, atracurium, succinylcholine), produce immobility but not unconsciousness and can mask signs of pain, distress, or general anesthesia emergence.19 Their use must include surgical anesthesia so patients are unaware of the paralytic state. These agents cause profound muscle relaxation at first peripherally and progressing centrally, with the diaphragm affected last. NMBA reversal proceeds in the opposite direction. Due to their skeletal muscle action, NMBAs can be useful with certain types of surgery (ie, thoracotomy) to relax muscles; however, intubation and ventilation (mechanically or manually) is mandatory. Generally, NMBAs are infrequently associated with mouse anesthesia, but when needed their use must be approved by the Institutional Animal Care and Use Committee (IACUC), written in the research protocol, and frequently requires a scientific justification. Besides providing scientific rationale for NMBA use, investigators must clearly indicate what and how they will monitor animals appropriately for signs of pain and distress. Generally, this involves demonstrating the anesthetic technique’s adequacy without NMBAs. Furthermore, NMBA administration is to occur after the skin incision and should be confined exclusively to the point of the procedure where needed. During the paralytic period, the animal requires continuous monitoring for signs of pain or distress (eg, heart rate, respiratory rate [RR], blood pressure) using baseline measurements, ideally starting from the time of the skin incision.
Analgesia
Analgesia is vital to both effective anesthesia and animal welfare. Experiencing pain, mentioned above, is a conscious perception of noxious stimuli; this pathway has been summarized in Figure 1. As discussed, at a sufficiently high dose, anesthetics will render an animal unconscious, thus preventing the perception of noxious stimuli (ie, pain). Pain perception has been noted in people exposed to subanesthetic inhalant anesthesia doses.19–21 Although an anesthetized animal is not consciously perceptive of pain, it should be noted that stimuli such as surgical manipulations can yield physiologic changes, such as elevated blood pressure, increased heart rate,8,22 and brain activity changes.23 Additionally, although anesthetics are effective in inducing unconsciousness, they do not ensure analgesia24 as an animal emerges from anesthesia in the recovery period or in the post-operative period. The ability of analgesics in conjunction with anesthetics to maximize peri-anesthetic stability will be discussed later in this review.
Minimally Invasive and Refined Techniques
Whenever possible, investigators should research (eg, literature search), consider, and employ minimally invasive and refined procedures because they generally require less anesthesia, analgesia, and induce fewer postoperative complications. Additionally, highly refined procedures may be technically easier to perform and fit well into the 3Rs.25
Education, Training, and Competency
Education and training are mandatory institutional obligations set forth in the Animal Welfare Act,26 the Guide for the Care and Use of Laboratory Animals,27 and PHS Policy;28 some overseas national guidance documents outline a third related concept: competency. Broadly, education is acquiring knowledge through instruction, training is acquiring a specific technical or manual skill, and competency is the application of education and training to competently and consistently develop ability, usually through prescribed actions or requirements. Education and training (and competency) must include various elements, including anesthesia and analgesia, and how the associated items comprise an IACUC-approved protocol, regulatory compliance, and other relevant essentials.29
Each institution must develop a relevant program meeting its unique programmatic needs and providing individuals with instruction along a continuum of knowledge, skill, and ability. Specifically, anesthesia instruction will be provided to individuals with a wide variety of backgrounds, from undergraduate students with no previous experience to accomplished and respected human anesthesiologists. The resulting instruction comprises online, in-person didactic and laboratory components, with participants actively participating at least in the latter. Generally, instruction is comprised of species-specific units and a separate aseptic surgery unit; anesthesia and analgesia would be a significant component of all such training. It would be inappropriate, and quite possibly reckless, to exempt research animal users from species-specific and aseptic surgery instruction.
The resulting instruction must be dynamic towards incorporating and curating material. The IACUC would be an excellent source to suggest new material based on their interactions with research teams as they perform their regulatory compliance duties. It may be beneficial for the IACUC’s community members and non-scientists to enroll in some of the training to provide relevant feedback to the trainers and the IACUC as part of the semiannual program review. Periodic retraining deserves consideration.
ANESTHETIC DEPTH
A high anesthetic dose can blunt the autonomic nervous system, impairing an animal’s ability to respond to changing physiological conditions.
Progressive central nervous system (CNS) depression secondary to inhalant anesthetic exposure has been characterized by 4 notable stages.30 These stages are applicable for use in animals, and knowledge of these stages is valuable to anesthetists given the common use of inhalant anesthetics. Behavioral changes and loss of physiological functions occur in a predictable fashion in response to anesthetic drugs.31 Much of our knowledge comes from work in human patients and for inhalant anesthetics conveyed in terms of minimum alveolar concentration (MAC). Most veterinarians and researchers associate MAC with the inhalant anesthetic concentration whereby 50% of a species will not respond to a noxious stimulus (eg, movement) while receiving a specific anesthetic gas,32 essentially an effective dose of 50% of a group. Using this definition, isoflurane’s MAC for most species is estimated to be 1.4% to 2.0% depending on the severity of the noxious stimulus, where the stimulus is applied, and animal-specific factors (mouse strain, underling health conditions, age, etc).8,33 There are other MAC definitions corresponding to other planes of anesthetic depth that are presented in Table 1.
Table 1.
Relationship of the Different Planes of Anesthesia and MAC.
| CNS Function Lost | MAC Stage | Significance of Plane of Anesthesia | Status of CNS Functions | Functional Test in Mice | Approximate MAC Value | Vaporizer Dial Setting |
|---|---|---|---|---|---|---|
| Loss of memory | MACamnesia | Unable to form memories | Cerebral functions, spinal and autonomic reflexes intact | Difficult to assess in mice both experimentally and in clinical settings | 0.25 | 0.3–0.5% |
| Loss of consciousness | MACawake | Unable to perceive pain | Cerebral functions anesthetized; spinal and autonomic reflexes intact | Mouse can be laid on its back and will not right itself | 0.5 | 0.7–0.9% |
| Loss of motor response to a noxious stimulus | MAC | No motor response to a noxious stimulus; surgical plane of anesthesia (in veterinary medicine) | Cerebral functions and spinal reflexes anesthetized; autonomic reflexes intact | No movement in response to a noxious stimulus | 1.0 | 1.3–1.8% |
| Blunted autonomic reflexes | MACBAR | Autonomic nervous system is not responsive to physiologic changes | Cerebral functions, spinal reflexes, and autonomic reflexes anesthetized | Experimentally, no change in serum epinephrine concentrations in response to a noxious stimulus, clinically no change in HR, RR, or BP in response to noxious stimulus | 1.5 | 2.0–2.7% |
This table presents different anesthetic planes and MAC relationship, which refers to the minimum alveolar concentration where 50% of animals lose a motor response to a noxious stimulus. The MAC value is dependent on multiple factors, including the severity of the noxious stimulus, where the stimulus is applied, and animal-specific factors. In veterinary medicine, the loss of a motor response to a noxious stimulus is commonly referred to as the surgical plane of anesthesia, while this would be considered a very deep plane of anesthesia in human patients.2,6,17,321,34,322 Please note that 2% isoflurane may blunt autonomic responses; therefore, supportive care, such as warmed fluid administration and other monitoring techniques (preventing hypothermia etc ), is warranted (discussed in anesthetic monitoring). MACBAR refers to the MAC which blocks autonomic responses. HR, RR, and BP indicate heart rate, respiratory rate, and blood pressure, respectively.
In veterinary medicine, the typical definition of a surgical plane of anesthesia is that there is no movement in response to a noxious stimulus. This requires the inhibition of reflex arcs at the spinal cord level as well as higher anesthetic doses than the dose required to induce only unconsciousness, which occurs at the brain level (cerebral cortex).34 Surprisingly, these reflexes can be suppressed by delivering anesthesia to only the brain; however, very high inhalant doses are required, approximately 3 times the amount required to suppress the reflexes when the anesthesia is delivered to the spinal cord as well as the brain. It is important for researchers and veterinarians to understand that the concept of a surgical plane of anesthesia is a misnomer, because the amount of anesthetic required to inhibit the spinal reflexes is dependent on the noxious stimulus delivered. For example, during a routine canine ovariohysterectomy, the dog may be unresponsive to the noxious stimulus of the skin incision but will move in response to the pulling and dissection of the ovarian round ligament.35 In this example, the plane of anesthesia was adequate for the surgical skin incision but inadequate for round ligament manipulation. This can occur during mouse surgeries due to reported MAC value differences between experiments attributed to differences in the location or noxious stimulus severity.2,36,37
Another factor complicating anesthetic plane is that the surgical or noxious stimulation affects the anesthetic plane. A potent noxious stimulus will lighten the animal’s anesthetic plane.22 This has profound clinical importance in mouse anesthesia. For example, when a mouse is placed in a stereotaxic device, the ear bars provide an extremely noxious stimulus that must be overcome by the anesthesia. Fortunately, surgical stimulation is unlikely to bring an animal from a surgical anesthetic plane back to consciousness. This is due to the large difference in anesthetic concentrations needed to eliminate the spinal reflexes associated with a surgical incision (which would be similar to MAC for injectable anesthetics) and the anesthetic required to cause the loss of consciousness, which is approximately one-half of the MAC.33 This accounts for the generally held belief that painful stimulation is unlikely to bring an unconscious patient at a stable anesthetic level to an anesthetic plane of consciousness.
Challenges in Determining and Maintaining Anesthetic Depth in Mice
Essentially any procedure/monitoring technique available to humans is available in mice. Unfortunately, most mouse anesthetic procedures present unique challenges due to their small size and high metabolic rate, making techniques unrealistic for routine murine anesthesia. Examples include intravenous anesthetic injection, %SpO2, blood pressure, and electrocardiogram (ECG) monitoring, all being routine for other veterinary species but rare for most typical research laboratories utilizing mice. An additional concern is that mouse surgeries are commonly performed by 1 person who is responsible for performing surgery, anesthesia, and obtaining supplies. That person may have less formal anesthesia training than individuals performing anesthesia on USDA-covered veterinary species, further compounding mouse anesthesia difficulties. Ultimately, considering all factors, the education of staff performing these procedures, pre-operative preparation, and frequent focused animal monitoring are imperative to ensure successful anesthesia outcomes.
Dosing
Although there are many aspects to safe and successful anesthesia, anesthetic protocol selection is critical to the procedure’s success. For decades, our field has had limited anesthetic options and recommendations revolving around either isoflurane or a ket-/xyl-based protocol.24,38,39 Recently, a myriad of new protocols have been tested, yielding more options to accommodate special anesthetic needs. In the coming years, we expect reports on these new protocol developments, but ultimately individual groups using these protocols and conveying their published results to our field will be important to confirm their efficacy as new anesthetic options.
Many anesthetic doses are selected from previously published work studying a similar experimental design. This practice must be used with great caution. Rarely do these papers discuss the anesthetic protocol’s success (percentage of mice reaching the desired plane of anesthesia, death rate), re-dosing procedures, or confirmation of depth of anesthesia or provide reports on the anesthetic’s side effects impacting the animal’s vital parameters or research outcomes. Furthermore, there is a profoundly variable response of mice to anesthetics based on strain, age, genetic manipulations, and the skill and experience of the individual performing the procedure, all of which require consideration when evaluating potential anesthetic protocols.37,40,41 We provide a summary of commonly used anesthetic protocols in Table 2. Whenever employing a new anesthetic protocol, pilot work is invaluable to test the protocol’s efficacy for the experiment paradigm as well as to prepare for potential procedural complications.
Table 2.
Recommended Injectable Anesthetic Protocols
| Anesthetic Protocol | Route of Administration | References | |
|---|---|---|---|
| Anesthetic protocols for immobilization or imaging | Ketamine 80–100 mg/kg Xylazine 8–10 mg/kg | IP | 36,76,77,323,324 |
| Ketamine 100 mg/kg Xylazine 10 mg/kg Carprofen 4 mg/kg | IP | 36 | |
| Ketamine 100 mg/kg Xylazine 10 mg/kg Buprenorphine 0.3 mg/kg | IP | 36 | |
| Tribromoethanol 250–300 mg/kg | IP | 324–326 | |
| Anesthetic protocols for surgical plane | Ketamine 80–100 mg/kg Xylazine 8–20 mg/kg Acepromazine 1–3 mg/kg | IP | 36,75,77 |
| Males: Alfaxalone 80–120 mg/kg Xylazine 10 mg/kg Females: Alfaxalone 40–80 mg/kg Xylazine 10 mg/kg | IP if NOT doing a laparotomy or entering peritoneal cavity SQ if doing a laparotomy or entering the peritoneal cavity | 98,100 | |
| Alfaxalone 30–60 mg/kg Medetomidine 0.5–0.75 mg/kg Butorphanol 5 mg/kg | SQ | 63,99 | |
| Medetomidine 0.3 mg/kg Midazolam 4 mg/kg Butorphanol 5 mg/kg | IP | 84,85,105,106 | |
| Tribromoethanol 250–500 mg/kg | IP | 62,107,110,327 |
The table includes published anesthetic protocols and dosing for mice for either immobilization and imaging procedures, or procedures requiring a surgical plane of anesthesia. It is important to remember that there are many factors that will affect the final optimal dose for each group and experiment, including the strain, age, and gender of the mice; amount of surgical stimulation; duration of the procedure; and experience of the surgeon. We recommend giving a range of doses on the IACUC protocol to allow for doses to be easily adjusted to optimize the anesthetic protocol. IP = intraperitoneal; SQ = subcutaneous.
Inhalant Anesthesia
The first recommendation for most mouse anesthetic protocols is to use an inhalant anesthetic. The most commonly utilized inhalant is isoflurane, although sevoflurane has also been used successfully.24,38,39,42 There are several benefits to using inhalant anesthetics:
Although many of the mechanisms behind inhalant anesthetic actions have yet to be definitively determined, they are activators of both gamma aminobutyric acid and glycine receptors and inhibitors of the NMDA receptor, both of which result in CNS neural activity inhibition yielding signs of immobility, hypnosis, and amnesia associated with anesthesia.43 Because the drugs work at multiple receptors, they have an extremely steep dose response curve.44 This allows nearly 100% of animals to achieve a desired anesthetic plane (ie, a surgical plane/immobility in response to a noxious stimulus) with very few animals reaching a deeper, undesired plane (ie, blunted autonomic reflexes/death).
The low blood solubility of the commonly used inhalants result in a rapid drug uptake from the alveoli and blood brain barrier distribution.45 This activity results in a rapid anesthetic induction, CNS removal of the drug when the alveolar anesthetic concentration is decreased at the end of a procedure, or subtle anesthetic depth changes during a procedure. Such rapid CNS anesthetic changes are generally not possible following routine intraperitoneal (IP) injectable anesthetic administration, many requiring liver metabolism before excretion.46,47
There are also distinct inhalant anesthetic disadvantages in murine medicine. Rapid anesthetic depth changes are a double-edged sword, requiring very close animal monitoring due to the potential for rapid anesthetic depth changes. Also, a precision vaporizer, with periodic calibration to ensure its accuracy, is necessary to accurately deliver inhalant anesthetics, incurring an additional cost compared with injectable anesthetics. Waste anesthetic gas released into the environment is an occupational health risk for people performing anesthesia, with rodent surgeries being a particular source of personnel exposure.48
Another factor warranting consideration is the lack of analgesic properties provided by inhalant anesthetics.49 This is not a factor during a surgical procedure when the anesthetics are delivered at concentrations that completely eliminate consciousness, as consciousness is required for pain perception. It does become a factor during recovery and the smooth transition from anesthesia to analgesia. Many injectable anesthetic drugs, including ketamine, the alpha-2 adrenergic agonists, local anesthetics, and opiates, provide analgesic effects.8,50 Therefore, as the animal emerges from anesthesia to being able to perceive pain, analgesia will be present and will help bridge the animal to the full efficacy of the postoperative analgesic protocol. These effects are absent for inhalant anesthetics, and the postoperative analgesic protocol (as pre-emptive/preventive analgesia discussed below) must fully address pain from the instant the animal regains consciousness. Analgesics can provide value during the surgical procedure when using inhalant anesthesia because they can decrease the amount of inhalant anesthesia, which is addressed below.
It must always be remembered that the ideal anesthetic inhibits consciousness, but unfortunately in doing this, all anesthetics also impact the autonomic nervous system and affect many normal physiologic functions, including cardiac and respiratory function. And, in fact, anesthetic monitoring of anesthetic depth is based on assessing its effects on these systems in addition to the estimates of the animal’s consciousness and pain perception. The inhalant anesthetics are no different than the other anesthetics, causing clinically relevant, dose-dependent decreases in respiratory function, cardiac output, and blood pressure.51–55 At low doses, anesthetics inhibit the peripheral chemoreceptors monitoring blood oxygen concentrations, and as the dose increases, central chemoreceptor inhibition occurs, which is the physiologic process responsible for monitoring carbon dioxide concentrations and pH.53 The net effect is a marked decrease in RR, tidal volume, and decreased blood oxygen concentrations.53 Groeben et al (2004) demonstrated RR was significantly decreased at 1.0 MAC while remaining unchanged at 0.5 MAC.51 Additionally, general anesthesia is known to induce airway closure of the small airways and vascular shunts, resulting in ventilation-perfusion mismatch and atelectasis, further compromising respiratory function.56,57 The net result is that anesthetized mice are frequently hypoxic.51,53,58–63 The drops in oxygen concentration can be offset by using 50% to 100% oxygen as the anesthetic carrier gas.64
The inhalant anesthetics induce a dose-dependent hypotension as well, with decreases being noted at very low isoflurane concentrations of 0.25 and 0.5 MAC.65 The mechanisms behind these drops are due primarily to direct effects on the smooth muscle vasculature, stroke volume, and autonomic nervous system.54,55 This effect becomes particularly relevant when blood pressure is a key dependent variable of a study. Because of the difficulty in monitoring blood pressure in anesthetized mice (or conscious mice, for that matter), this valuable physiological parameter is rarely measured in anesthetized mice but should be addressed when anesthetizing and supporting anesthetized mice.
Depending on the noxious stimulus used to test for a motor response, the reported mouse isoflurane MAC value ranges from 1.3% to 1.8%.37,66–71 As discussed above, inhalant anesthetics have a very steep dose response curve.44 This means that when studies identify the transition point from responding to a noxious stimulus to not responding, the SD will typically be very small, in the range of 0.1%. This can then be used to provide isoflurane dosing estimates. Considering that 3 SDs from the mean will encompass greater than 99% of the total population means that MAC plus 0.3% will provide the vast majority of animals a surgical plane of anesthesia, provided the noxious stimulus for the MAC determination is similar to the surgical stimulation. Further, this will be well below the MACBAR for most animals, meaning most animals will retain the ability to mount a robust autonomic response to changing conditions at this anesthetic concentration.2 It must be remembered that there are many factors affecting the actual MAC for an experiment (surgical stimulation, mouse strain, age, etc), but typically a range of 1.5% to 2.5% isoflurane will safely anesthetize most mice requiring surgery or an invasive procedure. It should also be remembered that MAC determination is affected by atmospheric pressure, and an increased inhaled gas percentage will be required at high elevations.55 Researchers from the University of Zurich have tested sevoflurane as an inhalant anesthetic for mice, reporting MAC values of 3.25% and finding that surgery can safely be performed using 4.9% sevoflurane.42,72
A common anesthesia theme is the use of balanced, multimodal anesthesia, where multiple anesthetic drugs with complementary modes of action are used to minimize the dose of each drug required.73 This is applied universally in human and anesthesia of other veterinary species but is rarely applied when using an inhalant anesthetic in murine anesthesia. Using isoflurane as the inhalant, buprenorphine and sustained-release buprenorphine both significantly decreased MAC,66 and a combination of midazolam and butorphanol significantly decreased the isoflurane MAC values.69 Using sevoflurane, fentanyl-midazolam significantly decreased MAC, and, surprisingly, ketamine had no significant effect on MAC for sevoflurane.42,74 When all of the drugs were tested with an adjusted decreased amount of inhalant, each resulted in a higher RR of the anesthetized mice in direct response to the decreased inhalant concentration required. An additional advantage of using medications before induction is that they can decrease the stress of a tank induction for the animals, although this must be balanced against the stress of handling for the premedication given. Moving forward, future testing of multimodal anesthesia using inhalants will likely allow for decreased inhalant use and subsequent respiratory suppression and hypotension.
Ketamine/Xylazine Combinations
Before the early 2000s, pentobarbital was the “go-to” injectable mouse anesthetic. This has subsequently given way to ketamine/xylaxine (ket/xyl) based anesthesia.24,38,39 Much of this is due to the increased therapeutic index of these ketamine combinations as well as the flexibility in the dosing combinations.24,38,39 The pivot to ketamine cocktails results from their high therapeutic index and their dosing flexibility. The earliest instance of this combination was by Arras et al, which likely drove the field towards the ketamine-xylazine combinations.75 This group tested multiple ketamine-based anesthetic protocols for a surgical vasectomy and found ket/xyl, used alone at low doses, did not generate surgical anesthesia and had no deaths, whereas a high dose resulted in frequent mouse deaths. When the group added acepromazine to the ket/xyl combination, surgical anesthesia occurred in 85% of the mice with no deaths. Similar results were obtained by Buitrago et al, finding ket/xyl/ace effectively delivered a surgical plane of mouse anesthesia with minimal mortality.36 Subsequent studies have confirmed the findings.59,76–78
The efficacy of this drug combination (ie, cocktail) comes from the cocktail’s ability to minimize the side effects of any one drug. Ketamine, a dissociative anesthetic, works as an NMDA agonist. This anesthetic is considered safe because of its ability to preserve or even increase heart rate and blood pressure. The mechanism results from ketamine’s sympathomimetic effects, with the increased sympathetic tone overriding its mild cardio-depressive effects.79 Ketamine is a potent analgesic at low doses, possibly mediated, in part, through opiate receptor binding.80 Ketamine has a short half-life in mice of 13 minutes following IP injection.81 Xylazine, an alpha-2 adrenergic agonist, produces both sedation and analgesic effects. Sedation occurs at the level of the brain, whereas analgesia occurs at the level of the spinal cord. Acepromazine is a phenothiazine tranquilizer, with no analgesic activity and a long half-life.64,82,83 Although some mice will typically reach a surgical anesthesia plane with the ket/xyl cocktail, acepromazine’s addition safely increases the percentage of mice reaching a surgical plane of anesthesia to acceptable levels. It should be noted that whereas some mouse anesthetic studies report ket/xyl alone produces a surgical anesthesia plane (discussed above), there have been other studies using both ket/xyl and ket/xyl/ace that failed to inhibit movement in response to a noxious stimulus using these combinations.61,84,85 This difference in achieving a surgical plane of anesthesia may be impacted by mouse strain, which is discussed later in this review.
Much of the ket/xyl/ace cocktail’s safety is due to the different side effects associated with each drug and using multimodal anesthesia to decrease the various drug amounts required to achieve a surgical plane. Ketamine suppresses respiration, which becomes relevant at high doses in mice or when re-dosing, potentially yielding respiratory arrest, which is addressed later in this paper.60,86–89 This effect is typically not significant in other species due to the comparatively small doses used in combination with other drugs. However, significant ketamine amounts are required in most mouse protocols to produce a surgical plane of anesthesia. Xylazine and other alpha-2 adrenergic agonists induce profound bradycardia resulting from a potential early hypertensive effect, decreased sympathetic tone, and vagal activation.90 Acepromazine can cause peripheral vasodilation resulting in hypotension.64,82,90 When these drugs are used in combination, mice experience a profound bradycardia (primarily due to the xylazine), hypotension (primarily due to acepromazine), and hypoxia (due to a combination of the anesthetics). However, with appropriate dosing and monitoring, mortality is minimized in anesthetized mice.36,75,77 Concerns do arise during long procedures or procedures associated with a compromise in the animal’s condition (eg, blood loss) where compromised autonomic function will become more apparent. Monitoring in mice with use of this cocktail, post-anesthetic recovery, and treatment of arrest following its use is discussed later in this review.
Ket/xyl has been combined with other drugs to safely achieve a surgical anesthesia plane. These additional drugs have included buprenorphine, carprofen, azaperone, and lidocaine.36,76,91,92 Of these, only the highest lidocaine dose tested achieved a surgical plane of anesthesia with low mortality.91 Interestingly, many IACUCs require a scientific justification for this high lidocaine dose (16 mg/kg IP) because the dose exceeds lidocaine’s LD50 for intravenous administration. Future work may further refine the safety and efficacy of these doses, but at this time we do not recommend using most of these other drug combinations to achieve surgical anesthesia.
It is important for anesthetists to carefully assess the desired anesthetic goals. If a mouse requires immobilization for non-painful imaging, a surgical anesthesia plane is unnecessary, because this will increase the mortality risk. Ket/xyl alone, without acepromazine, effectively and safely achieves this goal.36,59,61,75–78,84,85 Multiple factors affect the selection of a dose range for these cocktails, including the mouse’s age and strain, the duration of a surgical plane required, the supportive care provided to the mouse, and the surgeon’s experience. Additionally, some articles report sex differences between ket-/xyl-based anesthetic protocols; however, these results vary between studies.76,78,93–95 Anesthetists should base their starting dosing on as much information as possible, the laboratory’s historical results, similar surgical procedures on the same mouse strain, etc. However, it is important to remember that this should be considered just a starting point for the dosing, and anesthetists should be flexible and adjust the dosing protocol as they gain experience with their specific conditions.
Injectable anesthetics typically have more duration of action variability. This variability is most pronounced with longer procedures, necessitating anesthetic re-dosing in some mice. There has been little critical evaluation of ket/xyl anesthesia IP re-dosing. The authors have demonstrated that the timing of re-dosing is critical to its success.77 Ideally, mice undergoing a surgical procedure would be re-dosed early to avoid departing the surgical plane during the procedure. Unfortunately, re-dosing mice at a set time near the time they emerged from surgical anesthesia resulted in a 50% mouse mortality after re-dosing, independent of the anesthetic dose or cocktail received. This necessitates close mouse anesthetic monitoring for the first signs of movement in response to a noxious stimulus. Fortunately, as discussed above, if this point is identified rapidly, the mice will be unlikely to reach a plane of consciousness and the ability to experience pain before re-dosing. After mice achieve a positive pedal withdrawal reflex, they can safely be re-dosed with either 50% of the initial ketamine dose or 25% of both the original ketamine and xylazine dose. Both doses successfully returned mice to the surgical plane and allowed a normal recovery. Another option for long procedures is to administer an IP infusion of ketamine or ket/xyl following ket/xyl/ace induction. Again, the advantage is that animals never leave the surgical plane of anesthesia during a 90-minute anesthetic event,94 and xyl in this combination can also be reversed at the end of the procedure.
Alfaxalone Combinations
Alfaxalone was used as an anesthetic in the 1970s and 1980s; however, the drug was withdrawn from the market due to a high frequency of allergic reactions/histamine release to the Cremaphor EL carrier.96,97 Switching the carrier to cyclodextrin made the drug more water soluble without the allergic reaction.97 Alfaxalone, a neuroactive steroid, activates the gamma aminobutyric acid receptors, which are the main inhibitory neurotransmitters in the brain and CNS. One of alfaxalone’s main advantages is its minimal cardiovascular effects despite its associated respiratory depression.97 Alfaxalone has grown in popularity in other species; 4 papers recently confirmed the efficacy of the new formulation in mice combined with either xylazine or butorphanol, and medetomidine.63,98–100 Dexmedetomidine, the purified active isomer of medetomidine, can also be used at one-half the medetomidine dose.101 These studies shared several findings; first, alfaxalone dosage combinations were identified that could reliably bring mice to a surgical plane of anesthesia. That said, both combinations showed significant dosing variability among different mouse strains. Also, in some anesthetic protocols, when given alone or in combination with other anesthetics, alfaxalone induced either myoclonic activity, jumping, or facial scratching during induction and recovery.63,100 These signs were generally alleviated by adding or changing the other drugs or doses in the anesthetic protocol. The papers were inconsistent in finding differences in responses between sex of the combinations identified in other species. Typically, females are more sensitive to alfaxalone than males and may require lower dosing to achieve an appropriate anesthetic depth and duration.63,98,100 Lastly, 1 study tested the anesthetic protocols under actual surgical conditions, finding IP administration for a laparotomy caused a 100% mouse mortality, but the anesthetic protocol was successful when administered subcutaneously.98 The researchers of this study also performed an additional study and showed the drugs could be safely administered intraperitonially for this procedure.98 As with all anesthetics, the alfaxalone combinations have profound autonomic nervous system effects. These effects included profoundly low heart rate, consistent with the use of alpha-2 adrenergic agonists, and without use of supplemental oxygen, profoundly low %SpO2.63,98 Although alfaxalone, like ketamine, cannot be reversed, pairing alfaxalone with a reversible alpha-2 adrenergic agonists (eg, xylazine, medetomidine, dexmedetomidine) permits the anesthetist to reverse the alpha-2 adrenergic agonists, thereby intervening and exerting greater control over anesthetic depth and duration as well as interceding should an overreaction to the anesthetic combination occur.90 The results of these papers are encouraging in that they provide evidence of potentially less variability between animals in regard to achieving a surgical plane of anesthesia with use of alfaxalone-based protocols, but significantly more time and usage will be required to confirm the advantages alfaxalone-based protocols over ketamine-based protocols.
Urethane, Chloral Hydrate, α-chloralose
Urethane (ethyl carbamate) is a member of a group of antiquated anesthetics experiencing continued present-day use. This list includes hypothermia, tribromoethanol/avertin, ether, and chloral hydrate. These anesthetic agents are not pharmaceutical grade, are made bench top, and their continued use in contemporary biomedical research should undergo heightened IACUC scrutiny especially regarding animal welfare concerns, sterility, pyrogenicity, batch-to-batch variability, and purity. The use of these chemicals necessitates strong scientific justification. Additionally, using these anachronistic anesthetic agents may generate significant human health concerns. One author received the following from the Office of Laboratory Animal Welfare: “This Office ...suggests that proposals to use antiquated anesthetic agents in contemporary scientific research should be justified, and pilot studies may be in order to evaluate the need for such agents.”
The anesthetics chloral hydrate and alpha-chloralose are infrequently used in biomedical research but have reported abilities to preserve autonomic function for measuring specific dependent variables (ie, autonomic reflexes). Unfortunately, because these agents are insufficient for solely and promptly inducing or eliciting a surgical anesthesia plane, inhalant anesthetics are used for induction and instrumentation (ie, placing intravenous catheters etc) and are cautiously replaced by the other, less potent anesthetics.52,102,103 This is readily possible because of the inhalant anesthetic’s fine control eliciting a surgical anesthesia plane and associated rapid pulmonary clearance. Like α-chloralose, urethane has similarly poor induction characteristics yet elicits a long-lasting (6+ hours) surgical anesthesia plane in mice.50 Urethane reportedly has minimal cardiovascular (eg, blood pressure, blood gas values, aortic blood flow) and respiratory depression effects.104 There are no reversal agents for these aforementioned agents should adverse anesthetic effects develop; furthermore, prolonged anesthetic recovery and other issues (eg, involuntary excitement, peritoneal effusion, and hemolysis) are hallmarks of using these alternative anesthetic protocols.50,104 Urethane in particular is a carcinogen; therefore, it is no longer recommended for survival procedures in mice and must be avoided when possible.
Medetomidine, Midazolam, and Butorphanol
Several Japanese groups reported on the efficacy of the medetomidine, midazolam, and butorphanol combination to reliably yield a mouse surgical anesthetic plane.63,84,85,105,106 The reports used the pedal withdrawal reflex (among others) as a defining criteria for surgical anesthesia. The authors believed the mice attained a surgical anesthesia plane due to the absence of a pedal withdrawal reflex, although some readers argue that surgical anesthetic plane was not obtained because the study lacked an actual surgery or skin incision. Of course, electroencephalogram is needed to confirm a surgical anesthetic plane as a part of any future studies.
Although none of these drugs are truly defined as a hypnotic agent (all 3 have sedative properties), the combination yields a hypnotic effect. The autonomic parameter effects are similar to ket/xyl/ace, with mice developing profound bradycardia, bradypnea, and low oxygen saturation. As with mouse alfaxalone combinations, few publications report using this combination and none of the authors in this review have used this combination, so much more work needs to be completed and reported before this mouse anesthetic cocktail can gain strong support.
TBE
2,2,2-Tribromoethanol (TBE) has a long history in laboratory animal medicine and has inspired significant and unparalleled controversy. This anesthetic was used for over a century and was a pharmaceutical-grade product under the trade name Avertin.107 It has been shown to induce a surgical plane of anesthesia in mice for short periods, although there is significant variability in the duration of action, particularly between different mouse strains. Several clinical studies and review articles published around the turn of the 20th century studied TBE in mice with varying recommendations on its use.107–109 Several articles reported peritonitis or ileus associated with IP administration, whereas others have questioned this effect. A significant factor contributing to this controversy is the drug’s unavailability in a pharmaceutical-grade formulation, meaning each laboratory must reconstitute the product from a chemical-grade product and store it, potentially introducing tremendous drug and quality variability. Since the early 2000s, there have been significantly fewer publications addressing the product, again showing profound variability in its efficacy and associated pathology.62,110–112
In 1998, Zeller et al reported various mouse strains developed microscopic lesions following TBE administration, although no gross lesions were reported,109 and in 2005, 2 articles, 1 review and 1 original research paper, both reported TBE was an effective anesthetic but the morbidity rate following its use was unacceptably high to recommend the product for survival procedures.107,108 Since this period, several articles using TBE have been published but have unfortunately not helped clarify continued product use. Cho et al demonstrated a dose-dependent inflammatory cytokine increase following TBE administration, with 200 mg/kg yielding the same cytokine profile as a control injection and 400 mg/kg increasing the cytokine concentrations after IP injection.113 Lee et al and Hill et al administered doses up to 500 mg/kg and did not report any abnormal abdominal organ histopathology lesions.62,110 An additional observation from these papers was tremendous variability in response to the drug between individuals and mouse strains, with the most extreme example reported by Hill et al, where mice failed to achieve a surgical anesthesia plane following a single administration of 500 mg/kg TBE and reached a surgical plane only when administered a second time several days later.62,110
Ultimately, the lack of a pharmaceutical-grade product and the regulatory push to eliminate chemical-grade anesthetics creates a conflicting and challenging situation for institutions, resulting in many producing TBE guidelines. These generally require a scientific justification for using TBE over other anesthetics for the procedure plus a detailed description of the drug’s reconstitution and storage.
Guaifenesin
When isoflurane or other common anesthetics (ie, ket/xyl) cannot be used, authors have used guaifenesin (glyceryl guaiacolate [GG] 5%). GG is a centrally acting muscle relaxant with an unknown mechanism of action. It is frequently used with sedatives and analgesics in large animal species.114 In general, GG causes mild cardiovascular and respiratory depression. To our knowledge, it has never been used in rodents. The authors have had success incorporating Guaifenesin in an anesthetic regimen for an imaging procedure. The regimen consisted of a combination of 5% GG starting with a loading dose (0.5–2 mL/kg intravenously [IV]) followed by a GG controlled rate infusion (CRI; 0.5-2 mL/kg/h IV) and CRI propofol (80–150 mg/kg/h IV) together with O2 supplementation. Mice were anesthetized for 1–2 hours and recovered uneventfully. In this case, 1–2 venous (tail) catheters were needed for GG and propofol. This combination may be useful in cases where other anesthetics cannot be used due to their interference with the results of a study.
Reversal Agents
Many of our injectable anesthetics have reversal agents that will counteract the anesthetic’s actions and hasten the recovery of the mouse. Reversal agents are not routinely used in many veterinary species because of the ease of monitoring the animals, ensuring that their recovery is progressing normally. This monitoring is much more difficult in mice due to their small size and rapid metabolic rate, making the routine use of reversal agents a viable option in mice to ensure a safe recovery from anesthesia. The most commonly used reversal agent in mice is atipamezole (0.1–1 mg/kg IP, SQ) which reverses the alpha-2 adrenergic agonists. This agent has been shown to effectively reverse the action of xylazine in mice better than yohimbine, both during normal recovery and in the case of advanced anesthetic arrest.77,115,116 The reversal resulted in a more rapid return of movement and the return of the righting reflex as well as the return to a normal heart rate. This return to normal physiologic function has a great value to these animals, considering that the recovery period has been shown to be the most common time of anesthetic death in other species.117 In addition, atipamezole does not alter analgesia induced by opioids (buprenorphine or butorphanol).118 Fleischmann et al reported another study demonstrating the value of reversal using an induction protocol of fentanyl, midazolam, and medetomidine.119 All 3 of these drugs have reversal agents available, and the use of all 3 reversal agents—naloxone (0.01–0.04 mg/kg IP, SQ),120 flumazenil (0.02 mg/kg IP, SQ),120 and atipamezole—resulted in a rapid return to normal body temperature and heart rate as well as eating, drinking, and nest building activity, whereas the animals that did not receive reversal agents required almost 24 hours to return to normal values.
There are potential risks to the use of reversal agents that must be considered during their usage. The alpha-2 adrenergic agonists have analgesic properties, and the reversal of this could increase the risk of the animal experiencing pain during the postoperative period. None of the studies addressing the use of the reversal agents in mice actually performed a surgery, so the risk of exposure to pain was not addressed. Additionally, when the animals are anesthetized with just ket/xyl, the reversal of the xylazine during recovery could force the animal to recover from just ketamine anesthesia, which can be an unpleasant experience for the animal and may cause convulsions.116 Increased mortality related to atipamezole administration was also reported.121 The third potential risk associated with reversal is that the reversal agents can be metabolized faster than the anesthetic agent, resulting in a return of the anesthetic effects. This was noted by Thal and Plesnila, who reported that mice receiving the same fentanyl, midazolam, and medetomidine anesthetic protocol, with reversal as Fleischmann et al used, had a larger drop in body temperature 90 minutes after reversal.122 Although this was not reported in the Fleischmann paper, it does underscore the need for monitoring recovery even after the animals have appeared to return to normal function. Although atipamezole has potential risks, based on the authors’ experiences, its effects outweigh the risks.
ANESTHETIC MONITORING
Preoperative Period
A pre-anesthetic mouse evaluation is important to detect any underlying problems that may impact the ability to provide safe anesthesia. The American Society of Anesthesiologists Physical Status (ASA PS) surgical patient classification system is used by human anesthesiologists during the preoperative assessment to assess and grade a patient’s perioperative risk.123 Veterinary patients may be assigned to 5 categories, which are outlined in Table 3.123 The human classification system serves to assess the overall patient anesthesia and surgery risk to help compare outcomes and evaluate the approaches taken.123 In veterinary medicine, ASA PS can identify an increased risk of anesthetic mortality and the potential for developing intraoperative hypothermia.117,124 Generally, when the authors apply ASA PS to mice, animals scoring ≤2 are better anesthetic candidates, whereas animals scoring ≥3 are poorer anesthetic candidates and may require anesthetic (and any adjunct procedures) postponement or cancellation. Realistically, higher scores may directly result from prior experimental procedures; therefore, conducting anesthesia and procedures under anesthesia on these animals with higher scores may be unavoidable. Therein lies the anesthesia/analgesia challenges, potentially exacerbated by the lack of veterinary anesthesiologist/veterinary input, pilot study information, or both. Pre-existing diseases can reduce the therapeutic window of anesthetics, leading to cardiopulmonary depression and depression of other physiological functions.117 The ASA PS classification does not provide a total assessment of the anesthesia and surgery risk because this will depend on the anesthetic agents chosen, surgery performed, procedure length, monitoring and supportive care equipment available, and anesthetist and surgeon skill and training.
Table 3.
American Society of Anesthesiologists Physical Status (ASA PS) Classification123
| ASA PS Classification | Definition | Examples |
|---|---|---|
| ASA 1 | Normal healthy patient | Systemically healthy |
| ASA 2 | Mild systemic disease | Disease process is controlled: obesity, pregnancy, mild lung disease, hypertension |
| ASA 3 | Severe systemic disease | Systemic disease with clinical signs: advanced heart or lung disease, anemia, dystocia, upper airway dysfunction |
| ASA 4 | Systemic disease constant threat to life | Severe uncontrolled disease process: heart failure, dyspnea, septic shock, hemorrhagic shock |
| ASA 5 | Moribund patient not expected to survive without operation | Severe disease or trauma: uncontrolled hemorrhage, advanced septic shock |
As a part of the pre-anesthetic evaluation, it is critical to collect a history of the colony, including experimental procedures or models that are being used, because this will provide context for the general health status. Newly arrived mice should be allowed to acclimate to their housing room for at least 3 days prior to undergoing any anesthetic procedures to allow for stabilization of physiological parameters following shipment.125 Unlike for humans and other veterinary species, mice typically are not fasted because they cannot vomit and prolonged fasting can lead to hypoglycemia.126–128 There can be concern that mice with a full stomach will have limited diaphragmatic function or gastric blood pooling from digestion or that the full stomach may interfere with imaging studies.126 For most anesthetic procedures, digestion of food is not a problem; however, when using positron emission tomography a fasting time of 6 hours has been suggested.50,129 For optical imaging studies, it may be necessary to consider the dietary composition because it may be a source of autofluorescence.126,130,131
At a minimum, a physical examination is crucial to identify underlying physical conditions that may negatively impact successful rodent anesthesia (see Table 4 for summary of evaluation recommendations). Start with a cage-side, home environment examination followed by an individual mouse assessment before anesthesia. Physical restraint and handling can heighten mouse stress and anxiety,132 resulting in corticosteroid, glucose, and epinephrine release that in turn leads to cardiovascular and respiratory function changes and increases in body temperature, ultimately requiring a higher anesthetic dosage.126 To minimize adverse physiological changes, restraint should be reserved for only when needed, such as anesthetic administration. Observation of general behavior in the home cage provides a good initial indication of overall health. Active and curious mice are in good condition, whereas thin, hunched, or lethargic mice are in poor health and are more likely to have poor anesthetic outcomes.129 Respiratory function can be evaluated by observing the nares and respiratory pattern, rate, and depth. Tachypnea, deep abdominal breathing, shallow rapid breathing, or gasping indicate diminished respiratory capacity, poor health, and that the mouse would be a poor anesthesia candidate.129,133 Blood circulation and perfusion can be assessed by evaluating the mucous membrane color or overall color of the ears, paw pads, and tail. Mucous membranes or skin coloration that is pale, blue, or bright red can indicate abnormal conditions such as anemia, hypoxia, infection, or circulatory failure.129,133 Poor hydration status can be assessed initially cage-side by looking for sunken eyes or piloerection and is associated with higher anesthetic mortality.129,133 Mice undergoing an anesthetic procedure should be well hydrated. Moderate dehydration can be assessed through a skin turgor test by pinching the skin between the shoulders (Figure 2); a well-hydrated mouse’s skin quickly returns to its original position after releasing the pinched skin.129,133 Body condition can be assessed by palpating the sacroiliac bones, providing a more accurate indication of health status than weight because some conditions may increase body weight while decreasing body muscle and fat (such as tumor models).129,133 A body condition score (BCS) of <2 suggests poor health and euthanasia is typically recommended, and a BCS of 5 indicates an overweight mouse (see Figure 3 for BCS).129 The mouse’s weight should be taken and recorded using a scale tarred with a container or cup. The weight will be used to calculate accurate dosages of anesthetic or analgesic drugs and can also be used to monitor mice in the immediate post-operative and recovery periods. Abnormal physical examination findings suggest the mouse has compromised health status and is a poor anesthesia candidate.
Table 4.
Physical Examination: Parameters to Evaluate on Physical Examination and the Findings That Indicate a Normal, Healthy Mouse vs Abnormal Findings Indicative of Systemic Disease and Poor Anesthetic Physical Status
| Parameters to Evaluate | Normal Findings in Healthy Mice |
Abnormal Findings, Indicating Systemic Disease and Poor Physical Status |
|---|---|---|
| Overall general appearance | Active, curious, smooth fur coat | Lethargic, hunched, ruffled fur coat |
| Respiratory function | Breaths not noticeable, no discharge from nares | Tachypnea, deep abdominal breaths, shallow breaths, open mouth breathing or gasping |
| Mucous membrane or skin coloration | Pink | Pale, blue, or bright red |
| Hydration | Normal skin turgor | Sunken eyes, piloerection, delated skin turgor test |
| Body condition scoring (BCS) | 2.5–3 | Obesity: BCS of 5; thin and in poor condition, with a bony pelvis and spine: BCS < 2 |
Figure 2 .
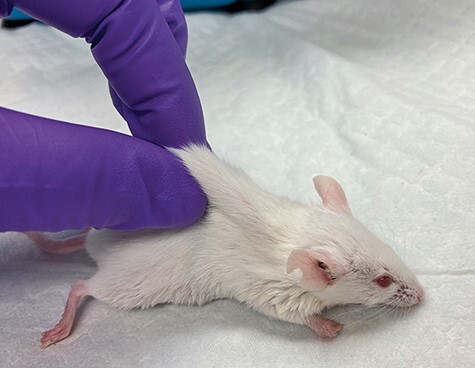
Skin tenting. Example of skin tenting. Skin tenting may be performed on an awake or anesthetized mouse. Under normal hydration, the skin should rapidly return back to the normal position. Skin tenting may indicate approximate 8–10% dehydration.319
Figure 3 .
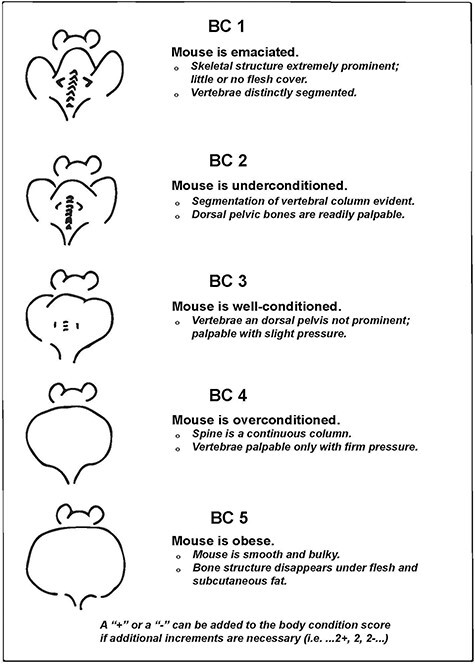
Body condition scoring. Body condition scoring (ie, BCS) uses physical attributes to indirectly assess the health status of an animal. A BCS is based on a scale of 1–5. Different strains/sexes/disease models may inherently have a lower or higher BCS (eg, strains used to study obesity). (Reprinted with permission from AALAS. Ullman-Cullere, MH and CJ Foltz. Comp Med. 49:319–323320).
Perioperative Period
Rodents have a higher risk of anesthetic-related deaths (for rats the reported anesthetic mortality rate is 2.01%).117 The majority of anesthetic-related deaths occur during the maintenance of anesthesia and the post-operative period.117 Cardiovascular and respiratory complications represent the majority of anesthetic-related deaths documented in small animals.117 Identifying major mortality risk factors can reduce the anesthesia mortality rate.
Supportive Care Measures. Proper supportive care mitigates various anesthetic complications, including fluid loss (hypovolemia), hypothermia, and respiratory depression.50 Without mitigation, these symptoms may yield prolonged anesthetic recovery, interference with experimental conditions,134–137 or death.8 During anesthesia, protective eye reflexes are lost, necessitating eye lubricant application to prevent corneal desiccation and ulcers. When performing surgical and anesthetic procedures, it is critical to estimate the total fluid loss. Fluid loss results from blood loss or evaporation. Additionally, rodents are particularly vulnerable to fluid loss because of their small body size and high metabolic rate. Minimize fluid loss by surgical site irrigation, controlling blood loss and replacing fluid loss with warmed, balanced fluids (ie, 0.9% NaCl, Normosol-R, or Plasma-Lyte A). Conscious animal core body temperature is typically tightly regulated; however, under general anesthesia, regulation is disrupted and hypothermia is a common complication.137 Skin heat loss occurs primarily through radiation, conduction, convection, and evaporation.138 Furthermore, because mice have a high surface area to body size, they rapidly lose heat. Even the simple act of applying surgical scrub has been associated with hypothermia in mice.139 Minimize heat loss with a supplemental heat source and provide both pre-warming and active warming during anesthesia and surgery.137 Recommended anesthesia heating devices include a circulating hot-water blanket or microwaveable heat pads, which maintain a surface temperature of approximately 37.5°C (Figure 4).140,141 Additionally, insulating materials or drapes over the thorax and abdomen minimize heat loss. Electric heating blankets and heat lamps are too frequently (and avoidably) associated with thermal injury and overheating.142,143 Surgical drapes144,145 and minimizing aseptic surgery prep time139 can prevent heat loss. To minimize the effects of respiratory depression and hypoxia, 100% oxygen should be delivered to the mouse. Typically, 100% oxygen is the inhalant anesthetic carrier gas, but oxygen’s value during injectable anesthesia to prevent hypoxia cannot be overstated. The authors have recently shown that mice at low doses of ket/xyl for imaging, surgical plane doses of ket/xyl/ace, and very high doses of ket/xyl/ace with no supplemental oxygen all induce a significant hypoxia as measured by %SpO2.146 This study has also shown that giving supplemental oxygen provided a significant survival effect, because all of the mice without supplemental oxygen died at the highest doses and all of the mice receiving supplemental oxygen survived. Mice on isoflurane with 21% oxygen/compressed air as the carrier gas are also profoundly hypoxic, while mice using 100% oxygen are not hypoxic; hypoxia does not affect MAC for a surgical plane.
Figure 4 .

Water circulating heating pad. During anesthesia, thermal support such as with a warm water circulating pad should be provided.
Anesthetic Monitoring and Records
Consistent and regular vital sign monitoring by trained personnel is critical to successful anesthetic outcomes. Due to the mouse’s small size and the specialized monitoring equipment, measuring vital signs can be challenging; however, both observation and monitoring equipment will improve the application and success of anesthesia. In mice, relevant vital signs to monitor include mucous membrane color, RR and pattern, and paw withdrawal response. Regular observation of vital signs every 5 minutes coupled with assessing the overall trend is imperative.77 Anesthesia problems frequently occur gradually rather than abruptly.50 Maintaining an anesthetic record (Figure 5) is essential to document parameters observed and assess the overall trend. Anesthetic records facilitate reviewing post-anesthetic complications to prevent similar, future anesthetic complications. Anesthetic records can provide a valuable training resource for new anesthetists.
Figure 5 .

Rodent surgical record. Anesthetic monitoring should be continuously monitored and the parameters recorded at least every 15 minutes.
Observational Monitoring of Mice
Tissue Perfusion and Oxygenation
Blood perfusion and oxygenation can be monitored through mucous membrane color evaluation. The mouse’s small size and use of an anesthetic nose cone impedes gum color assessment. Instead, consider assessing the overall skin color of the ears, tail, and paw pads. In white mice with normal perfusion and oxygenation levels, these areas will appear pink. Clinically relevant skin coloration changes may be attributed to a variety of causes. For example, the color may become pale with vasoconstriction, hypotension, hypovolemia and hemorrhage, or hypoxia. The skin may appear dark pink with vasodilation, hypercarbia, or toxic changes. Blue or purple coloration occurs during times of severe hypoxemia (typically oxygen saturation must fall below 50%).50 For mice with dark coat colors, the ears, tail, and paw pads are not normally pink; however, similar changes to the overall skin hue (including turning gray, pale, or pink) can be noted during anesthetic complications. Using a translucent surgical drape such as Press’N Seal Cling Film (Glad Press’n Seal, Glad Products, Oakland, CA) or Tegaderm (3M Corporation, St. Paul, MN), as opposed to fabric or medical surgical drapes, permits continuous skin coloration monitoring during surgical procedures when it is also critical to maintain a sterile surgical field (Figure 6).147 Although useful, monitoring skin coloration changes is not a sensitive lung gas exchange efficacy indicator; pulse oximetry is a more sensitive indicator.
Figure 6 .
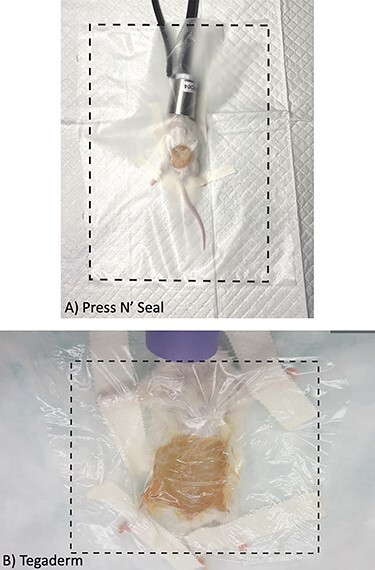
Translucent sterile surgical drape. (A) Press’nSeal as a cost-effective translucent sterile drape material. (B) Tegaderm as a translucent sterile drape material. The boundaries of the drape are illustrated by the dotted black outline. Use of a translucent surgical drape will facilitate monitoring of mucous membrane color and respiratory function during surgical procedures. Drapes can also provide additional insulation to minimize heat loss.
Respiratory Function
Normal respiration should be assessed before anesthesia so that changes in RR, pattern, and depth can be evaluated after induction of anesthesia. If monitoring equipment is unavailable, RR and pattern should be carefully monitored as indicators of anesthetic depth. The RR may be monitored by counting the rise and fall of the chest in 1 minute, resulting in the breaths per minute. Normal RR of awake adult mice is between 80 and 230 breaths per minute.148,149 Most anesthetics produce a dose-dependent depression of the respiratory system.50 The different types of anesthetics will affect RR in profoundly different ways. Mice anesthetized with inhalant anesthetics will have a low RR, ranging from 40 to 100 breaths per minute, whereas mice anesthetized with ketamine- or alfaxalone-based anesthetic protocols will have an RR between 120 and 200 breaths per minute. The respiratory effort and depth are also important to consider while monitoring anesthetized mice. It is critical to remember that an RR that is either too high or too low can be indicative of significant problems; a rate too low or very shallow breathing can indicate the anesthetic plane is too deep, and an elevated RR can potentially indicate that the animal is too light and may be experiencing pain or be at too light a plane of anesthesia.50,66,72,85,98,126,150,151
Response to Painful Stimuli and Surgical Stimulation
Paw withdrawal is commonly used to assess mouse anesthetic depth to confirm unconsciousness and a lack of pain perception.36,50 The paw withdrawal response should be confirmed just prior to making a surgical incision and be monitored regularly throughout the anesthetic and surgical procedure. To perform the paw withdrawal, firmly pinch the paw using either fingernails or atraumatic forceps. Additionally, devices such as the Touch Test device (North Coast Medical, Gilroy, CA) or the Aesthesio device (Aesthesio, DanMic Global, San Jose, CA) can be used to reliably deliver 300 g of force, which is a moderate noxious stimulus but does not result in injury to the foot, even after repeated use in a mouse.77,110 A positive reflex is indicated by retraction of the foot and indicates the mouse is outside a surgical plane of anesthesia. Samuel et al previously demonstrated there is a response difference between front and hind paw withdrawal, with the hind paw withdrawal being a more valuable indicator of a surgical anesthesia plane in mice.13 During the initial anesthesia induction (depending on the type of anesthetic used, route of administration of anesthesia, mouse age and strain), the anesthesia plane will continuously evolve into a deeper plane. For example, when using injectable ket/xyl anesthesia in mice, it can require between 6 and 20 minutes to reach a surgical anesthesia plane depending on the mouse strain and route of administration.78 It is important to consider the onset of the anesthetic used when performing the paw withdrawal evaluation so sufficient time elapses before repeat or additional anesthetic dosing occurs. In the anesthesia induction phase, the anesthetic plane may fluctuate as the anesthetic’s concentration changes the alveoli, blood stream, and brain. If a positive paw withdrawal response occurs after a sufficient anesthesia onset time, the mouse is too light and the inhalant anesthetic must be increased or the mouse re-dosed with an injectable anesthetic. Anesthetists should be aware that too frequent, forceful, or traumatic paw withdrawal testing may damage the mouse’s paw pad. If signs of traumatic injury are seen after a procedure, this can be treated with antibiotic cream in consultation with the facility veterinarian.
Monitoring Equipment
The authors recommend to start monitoring from anesthetic induction to the end of anesthesia-related procedures (not just for invasive/long procedures) and include monitoring for at least RR, paw withdrawal reflex, and mucous membrane/skin coloration. Advance anesthetic monitoring equipment can be included when needed. Any research facility recharge fees should be inclusive of all available anesthetic monitoring equipment to ensure financial bias will not negatively impact animal health and well-being. At minimum, we recommend monitoring equipment for oxygen saturation (%SpO2) and heart rate monitoring (eg, pulse oximetry) as well as rectal temperature monitoring. This equipment is not a replacement for monitoring RR/effort, mucous membrane/skin coloration, or pedal withdrawal reflex and should be used to complement these monitoring components. Furthermore, monitoring equipment requirements must be driven by the attending veterinarian or his/her designee.
Body Temperature
General anesthesia leads to vasodilation, central thermoregulatory inhibition, and an effect on sympathetic ganglia and vascular smooth muscle.152,153 Hypothermia is one of the more common complications during anesthesia, and mice are particularly susceptible because of their high surface area to mass ratio.140,154–157 Hypothermia has several negative consequences, including cardiac arrhythmias, hypercoagulability, pain, increased susceptibility to infection, prolonged recovery time, and decreased MAC for inhalant anesthetics.50,140,158 The amount of heat loss is attributed to the health status, surgical approach, exposure to fluids or air below the body temperature, and redistribution of warm blood to the periphery.154,156,157,159 Hyperthermia is less common in rodents but may occur if there is a genetic predisposition or if excessive heat sources are used. An approximate estimate of core body temperature can be measured by evaluating the skin or rectal temperature of mice during anesthetic procedures.160 Rectal thermometers can be used to easily and inexpensively monitor temperature throughout the anesthetic period (Figure 7). When placing rectal probes, care must be taken to avoid mucosal tearing that can lead to bacterial infections.161 Baseline body temperature measured rectally for C57BL6 mice at the start of isoflurane anesthetic exposure was previously found to be 36–37°C (96.8–98.6°F).141 In this study, the authors found that a 2°C change in body temperature during anesthesia prolonged recovery time.141 Infrared thermometers will quickly measure the skin temperature but will typically provide temperature measurements approximately 2.0°C lower than rectal probe measurements.162 For consistent measurements with infrared thermometers, it is critical to always aim the thermometer at the same body location.
Figure 7 .
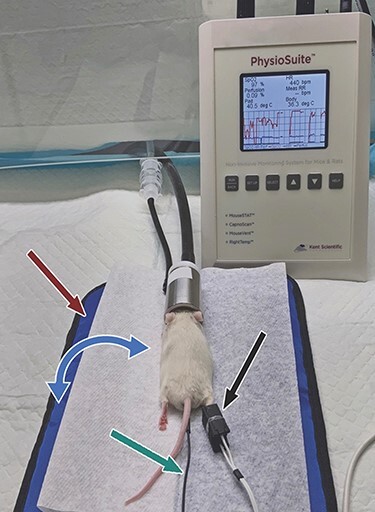
Rectal thermometer and pulse oximetry. Pulse oximetry and temperature measurements can be tracked using instruments such as the Kent Scientific PhysioSuite unit. Probe for pulse oximeter (black arrow), infrared warming pad (red arrow), and rectal thermometer (green arrow). The temperature probe relays body temperature information to the monitoring system, and the warming pad will adjust the temperature accordingly to a maintain physiologic temperature (blue arrow).
Pulse-oximetry
A pulse oximeter measures the percentage of oxygenated hemoglobin (%SpO2) in the blood and the heart rate (beats per minute [bpm]) in a non-invasive and continuous manner. Mouse pulse oximeters are available for purchase from various companies, such as Kent Scientific (MouseSTAT Pulse oximeter and heart rate monitor; see Figure 7) and Starr Life Science Corporation (MouseOx Plus). Probes are typically placed on the paws, thigh, tail, or neck (the neck may be used for awake measurements when using the MouseOx Plus). When probes are placed on the furred thigh or neck, the fur should be clipped or carefully removed with a depilatory cream in the area of the probe location (excess fur removal may lead to hypothermia) for accurate measurement. Mice breathing room air typically have measurements between 95% and 98%, whereas mice maintained on oxygen will have 100% oxygen saturation.50 Percent SpO2 values <95% indicate the onset of mild hypoxia and a reduction to 90% requires immediate action.50 Recent publications indicate that many anesthetized mice, with numerous anesthetic protocols, are in fact profoundly hypoxic unless receiving supplemental oxygen.53,56,61,72,74,75,85 Even with pulse oximetry measurements, it is critical to continue to evaluate the respiratory pattern throughout anesthesia to quickly determine when intervention is needed. A mouse maintained on room air will show changes in oxygen saturation within 30 seconds, whereas mice maintained on 100% oxygen will require 1–2 minutes to produce changes.150 Sudden changes in oxygen saturation are frequently caused by probe displacement.50 Pulse oximeters provide a continuous value for heart rate to allow for rapid identification of bradycardia or tachycardia.
Normal heart rate for mice is between 300 and 840 bpm and is dependent on the strain and age.163 As with RR, the anesthetic protocol will have a profound effect on the heart rate of the mice. Adult mice receiving an alpha-2 adrenergic agonist, either xylazine or medetomidine/dexmedetomidine, will have a heart rate of 250–350 bpm. Mice under inhalant anesthetics will have a heart rate between 350 and 450 bpm.77,94,98,150 If the anesthetic plane is too light, heart rate may increase and if the anesthetic plane is too deep, heart rate drops and can be erratic.141 An elevated heart rate can also be the result of a decreasing blood pressure due to the baroreceptor reflex.8 Oxygen saturation and heart rate are just part of the oxygen delivery, so normal values do not always mean normal perfusion.
ECG
ECGs show the real-time electrical activity of the heart, including atrial depolarization and ventricular depolarization and repolarization. Monitoring heart rate is of value because it reflects dynamic changes in cardiac electrical activity; ECG is considered to be the gold standard for monitoring heart rate.164 ECG systems in mice can be non-invasive by placing the 3 paws in contact with the electrodes for continuous recording, tethered where the wires are tunneled under the skin and exit mid scapulary, or implanted.164 Monitoring cardiac electrical activity is useful to detect arrhythmias, changes in heart rate, and alterations in electrical morphology.165 It is important to remember that the ECG only measures electrical activity and not deficiencies in circulation or the cardiac output of the heart, meaning that complications can be present in circulation without noticeable changes in the ECG.165
Blood Pressure
Blood pressure measures the pressure exerted by the blood during circulation onto vessels walls.158 It provides an estimate of cardiac output and assesses tissue perfusion.166 Blood pressure can be measured either through direct or indirect measurements. Direct blood pressure measurement involves placing a catheter in an artery that has been exposed surgically. Examples of systems available for purchase include Millar probes (Millar Mikro-Tip, Millar Sensor Systems, ADInstruments Ltd., Oxford, UK), fiber optic transducer (eg, Samba Preclin catheter, Sambra Sensors AB, Vastra Frolunda, Sweden), and pressure transducers (TSC104A blood pressure transducer; Biopac Systems, Inc., Goleta, CA, USA).167 Indirect measurements consist of an inflatable tail pressure cuff designed for use with rodents, for example the CODATM monitor from Kent Scientific (Figure 8). These systems record the cuff pressure and the pulse transducer signal from the tail artery.168 Readings will be incorrect if there is excessive motion or the cuff is an incorrect size.158 Cuff volume should be 40% of the circumference of the limb.158 Although easier to conduct, indirect monitoring will only provide intermittent information on pressure changes. Because of the high pulse rate and decreased arterial pulse, the accuracy of blood pressure measurements is lower in mice.158 Additionally, with tail cuff plethysmography, the mice need to be kept warm to ensure adequate blood flow to the tail to ensure accurate measurements.
Figure 8 .
Indirect blood pressure measurement. Blood pressure can be monitored indirectly with equipment, such as with the Kent Scientific CODA Monitor System, which uses a tail-cuff to monitor the indirect blood pressure.
Capnography
Capnography measures the exhaled CO2 concentration during the respiratory cycle and helps to assess the ventilatory and circulatory status. It provides an estimate of the partial pressure of CO2 in arterial blood, can be used to measure ventilation, and will alert the anesthetist during apnea. Examples of capnographs for use in mouse anesthesia are the CapnoScan from Kent Scientific, the Type 430 capnograph from Harvard Apparatus, and the MicroCapStar from ITIC Life Sciences Inc. These devices can provide real-time capnography and sidestream sampling on a mouse that is intubated and ventilated. End-tidal CO2 (ETCO2) is the maximum CO2 concentration during expiration, which typically should be between 30 and 45 mmHg during normal ventilation for mice.122,158 High ETCO2 or hypercapnia indicates hypoventilation, which may be caused by deep anesthetic plane, respiratory depression from opioid use, patient’s position, or obesity.158 Low ETCO2 or hypocapnia indicates hyperventilation, which may be from reduced cardiac output, blood pressure, decrease in pulmonary perfusion, or a thromboembolism.158 In mice, because of rapid respiration and small tidal volume, a capnograph waveform can be difficult to interrupt; however, changes seen in the ETCO2 are valuable to alert the anesthetist to problems with the equipment or patient.
Postoperative Period, Emergence, and Recovery
The anesthetic recovery period is a critical mouse monitoring period where consciousness and normal physiologic function return. In veterinary species, a high rate of anesthetic-related mortalities occur during the recovery period.169 Mortality risk factors include dehydration, influence of residual anesthetic drug, hypothermia, hypoglycemia, sickness, age, and respiratory complications.8,169,170 Animal research personnel should continuously monitor mice during recovery for RR normalization during recovery (slow to quick), normal breathing pattern reestablishment (deeper to more shallow), pink mucous membrane color and overall skin coloration, and eventual return of ambulation (first spinal reflexes, then righting reflex, and last, purposeful movement). Anesthetic reversal (atipamezole or yohimbine, and flumazenil for alpha-2 agonists and benzodiazepines, respectively) is recommended. However, if performed too soon after anesthetic administration, animals may become re-sedated following the rapid metabolism of the reversal agent;171 therefore, we recommend waiting 30–45 minutes after anesthesia induction before administration of reversal agents, unless extended monitoring is available.
The recovery period very roughly equates to the anesthesia time,126 though this varies between inhalant anesthetics vs injectables. Individual variations to anesthesia and the risk factors discussed may prolong the recovery period. Mice should be recovered and monitored in a specified warm and dry recovery cage with translucent sides for observation (Figure 9). To prevent overheating, no more than one-half the recovery cage needs to be warmed, so animals can freely move to the cooler side of the cage when regaining ambulation. Mice should be kept separate from other recovered/awake animals to prevent possible injury until each mouse is awake and ambulatory. These recovery recommendations also apply to neonatal mice (age <10 days). Neonates should stay in the recovery area until they regain their righting ability, which can be assessed by placing neonates on their sides or back.172 Once righting ability has returned, the neonates can be returned to the dam.
Figure 9 .

Recovery cage for mice. Example of a recovery set-up for mice after any anesthetic procedure. The cage should be clean with no bedding and transparent to allow for observation of RR, coloration, return of righting reflex, etc. Half of the cage should be placed over a warming device, such as a circulating water blanket to provide heat support during the recovery process. Recovering animals should not be overcrowded or recovered with animals that have regained ambulatory function.
Common signs of pain seen in conscious mice are unlikely to develop until recovery. However, inadequate pain control can delay recovery because mice will be reluctant to move if experiencing moderate to severe discomfort or pain. Continue to document vitals (RR, paw withdrawal reflex, and mucous membrane color) every 10–15 minutes while constantly observing the mice during recovery until the animal is fully conscious and able to ambulate. If possible, supply supplemental oxygen during the recovery period to help prevent oxygen-associated complications. If not provided prior to the procedure’s initiation, administer warmed (36–37°C; 96.8–98.6°F) balanced fluids subcutaneously or IP (0.25–0.5 mL) during recovery to replace fluids lost during surgery/long anesthetic procedures. Once ambulatory, return the mouse to the primary home cage. Soft white bedding, such as ALPHA-Dri (W.F. Fisher and Son, Branchburg, NJ), assists in monitoring for post-operative bleeding and other fluid production (eg, urination).
Supplemental care and wound care should continue for 3–7 days after the procedure. The post-operative monitoring period is dependent on institutional guidelines, the procedure’s invasiveness, and the animal’s condition. Supplemental food and fluids should be provided during this time; pellets placed on the level of the cage floor, or softened food such as moistened pellets or gelled food products, and gelled water are methods to provide this nutritional supplementation and reduce handling-associated stress (Figure 10). In our experience, mice willingly consume these products. New food products should be provided 1–2 days prior to a procedure because mice are neophobic and unlikely to readily eat a new substance. Assess the neonatal mouse’s abdomen for the presence of a “milk spot” to ensure neonates are ingesting milk and that the dam is properly caring for the neonate.172 Post-operative surgical site monitoring must include cage-side observation for dehiscence and infection, which can be secondary to surgical technique, inadequate aseptic surgical technique, and mice gnawing or licking the incision site. Proper analgesic protocols will help decrease the risk with the latter of these issues. Elizabethan and vest collars (ie, e-collars and v-collars) may prevent mice from disturbing the surgical site but must be long enough to prevent mice from reaching the incision site while still allowing access to food and water. E-collar use has been associated with mouse skin irritation173 and is likely stressful due to preventing mice from expressing normal species behavior, such as self- or allo-grooming and coprophagia. We recommend acclimating mice to any restraint device for 1–2 days prior to surgery to reduce any device-associated stress.
Figure 10 .

Nutritional supplements for mice. Shown here is an example of 1 brand that provides gelled water and nutrition for mice. These gels provide easy to access nutrition for mice needing supportive care. Additional nutritional supplements, such as EnerCal, though advertised for larger animal species, have been used anecdotally as a nutritional supplement in mice by some researchers.
An additional post-operative monitoring challenge is mouse pain assessment, because mice hide signs of pain, especially when directly observed due to their prey nature. Various mouse post-surgical health observations and assessments have been proposed, including documenting weight; noting changes in activity, food, and water intake;1 recording and assessing mouse grimace scores;174,175 nest complexity176,177 time to integrate nesting material into a nest;178,179 and burrowing behavior.180,181 Note that interpreting these assessments can be impacted by use of certain drugs, such as opioids, some of which cause mouse hyperactivity.179
A thorough and effective analgesic plan should be in place prior to the start of an experiment because post-operative pain can contribute to complications via an animal gnawing/licking/or chewing at the incision. Administer analgesics prior to anesthetic recovery to minimize the animal’s pain experience once conscious, keeping in mind analgesics require some time to be fully effective. Thus, if analgesics are administered only after an animal is fully recovered, there is potential for animals to be painful and experience unnecessary stress. A recent review of rodent clinical pain management182 recommends a pain management strategy to address post-surgical or invasive procedure pain that should assist veterinary staff and researchers in making analgesia decisions. In this strategy, a primary analgesia plan is created by anticipating the post-procedural pain expected, then readdressed with additional and/or stronger analgesics should animals exhibit signs related to persisting or increasing pain. Strategies for developing analgesic protocols are discussed in the next section.
Authors’ Recommendation
Unfortunately, hypothermia is a far-too common mouse anesthesia complication, necessitating external heat sources from induction to full recovery for all anesthetic procedures. Anesthetic monitoring for short (<30 minutes) procedures at a minimum includes RR, paw withdrawal reflex, rectal temperature, and skin color. Longer (>30 minutes) procedures may necessitate other monitoring modalities (eg, %SpO2, heart rate) and anesthesia adjunct techniques (eg, remove/clean oral secretion). Although warmed SQ/IP fluid administration is required for all surgical procedures, O2 supplementation is warranted. Based on our experiences, respiratory characteristics seem the most sensitive parameter to a mouse’s changing anesthetic plane; therefore, it is critical to frequently monitor both RR and breathing patterns.
PREVENTIVE AND MULTIMODAL ANALGESIA
This section focuses on the importance and use of analgesics during and around the anesthetic period. Thermal (heat, cold), mechanical (cutting, pinching, crushing), and chemical (inflammatory mediators, external irritant or caustic chemicals such as formalin, capsaicin, carrageenan) stimuli can cause nociception and thus pain.1 The topics of animal models of pain and the testing methods for changes in nociception threshold have been reviewed extensively elsewhere.1,183,184 Several definitions used in this pain and analgesia discussion have been defined below.
Allodynia: pain produced by a normally non-noxious stimuli.
Analgesic: a general classification for drugs that attenuate or abolish pain in a conscious animal through various molecular mechanisms.
Hyperalgesia: increased sensitivity to noxious stimuli resulting in an increased pain response.
Nociception: detection of noxious stimuli by the CNS; not equivalent to pain.
Sensitization: the process by which either peripheral or central neurons (centralized sensitization) fire more readily due to repeated stimuli of a certain threshold.
To reiterate a topic of importance, after the action of an anesthetic(s) has diminished or abated and consciousness returns, animals may experience pain unless pain has been treated or otherwise controlled. Additionally, while an animal is anesthetized, nociceptors can still be activated,185 resulting in post-procedural hyperalgesia or allodynia.186 The duration of pain post-procedure depends on the type of procedure performed and the type of pain model utilized.187 In general, the most intense pain peaks between 4 and 24 hours post-operatively.188 Untreated or inadequately treated pain can cause sensitization of pain receptors (ie, nociceptors), resulting in hyperalgesia in the primary affected tissues and in surrounding tissues (secondary hyperalgesia) as well as allodynia.188 A table of expected pain for common procedures has been provided (Table 5).
Table 5.
Expected Pain for Common Procedures in Mice
| Examples of Potentially Painful Procedures | ||
|---|---|---|
| Minimal to Mild Pain | Mild to Moderate Pain | Moderate to Severe Pain |
| Catheter implantation | Embryo transfer | Burn procedures |
| Ear notching | Hypophysectomy | Heterotopic organ transplantation |
| Intracerebral implantation Multiple ID antigen injections Ocular procedures |
Minor laparotomy incisions Orchidectomy thymectomy |
Major laparotomy/organ incision orthopedic procedures |
| Orbital sinus venotomy Superficial lymphadenectomy Superficial tumor implantation |
Thyroidectomy | Thoracotomy vertebral procedures |
| Tail clipping Vasectomy | ||
| Vascular access Port implantation |
||
Common experimental procedures performed in mice and the expected pain induced from these procedures. These are guidelines, and animals should continue to be monitoring post-procedure for signs of pain.
The most common classes of analgesics utilized in laboratory animal medicine are opioids, anti-inflammatories, alpha-2 agonists, local anesthetics (local infiltration, nerve, and epidural blocks), and the anti-convulsant/neuropathic drug gabapentin. A short overview of these different classes is provided at the end of this section. The small body size of mice means access to vasculature is technically challenging. Thus, the routes of drug administration are limited to more accessible routes, such as subcutaneous (SQ) or IP injection, or oral administration.189 IP injection has been recommended over subcutaneous injections due to variation in time for effects to occur, but the efficacy and safety of IP over SQ injections may differ depending on anesthetic protocol.78 Although oral administration is a common method of drug delivery in other veterinary species, in order to gavage mice technical expertise is required. Analgesics have been formulated for incorporation into gel,190 food,191,192 and drinking water193 for laboratory rodents. Oral (ie, drugs added to drinking water) and long-acting formulations of analgesics serve as a refinement in animal research by reducing the stress associated with repeated injections of standard analgesics, though oral formulations can result in inadequate or inconsistent dosing.191 The timing of analgesic administration, in addition to type of analgesic, is also important to consider.
Analgesics ideally should be administered prior to a procedure to prevent or mitigate anticipated nociception (ie, preventive or pre-emptive analgesia). This strategy is thought to help minimize post-operative pain. In humans, evidence for usefulness of preventive analgesia to attenuate post-operative pain is conflicting.185,194–197 However, use of this strategy in animals has shown decreased post-surgical pain in a variety of species, including post-invasive procedures in dogs and cats,198–200 post-laparotomy in pigs,201 post-formalin administration202 and post-surgically in rats,182,203–205 and post painful procedures in mice.182,206–208 Opioids and local anesthetics are common drug choices for preventive analgesia in mice due to the systemic analgesic effects of opioids and the ability to deliver analgesia locally with local anesthetics. Non-steroidal anti-inflammatory drugs (NSAIDs) have also been recommended in use for preventive analgesia for humans209 and animals,210,211 especially in cases where significant tissue trauma or inflammation is to be expected.211 There have been concerns in larger animals regarding prolonged bleeding times,212 gastrointestinal ulcerations,213 and possible renal toxicity214 secondary to hypotension during anesthesia after NSAID administration; however, these side effects are not normally noted for short-term use or short anesthetic procedures for mice.215 In our experience, buprenorphine is one of the most common analgesics used preemptively and peri-operatively in surgical and mild to moderately painful procedures in mice.206,207,216–221 Buprenorphine has a wide safety margin207 and has minimal effects on the immune system.222 Buprenorphine administration can be accompanied with a number of side effects in mice, including cardiovascular depression, constipation, decreased food and water intake, possible weight loss, and hyperactivity.207,219,36 Although discussed in the anesthetic portion of this review, alpha-2 antagonists (xylazine, dexmedetomidine) and dissociative drugs (ketamine) are also components of multimodal analgesia. The analgesic effects of ketamine are known, even at subanesthetic doses,8,223,224 though it is recommended to be used as an adjunctive analgesic and not a sole means of analgesia.
Using only preventive analgesia or only 1 analgesic agent post-operatively may be insufficient to completely prevent pain depending on the type of procedure. A common strategy to combat breakthrough pain is to either utilize a higher dose of a single drug or re-dose an animal. Even within the safety margin for some analgesics, side effects can be more pronounced at higher drug dosages. For example, opioids cause dose-dependent respiratory depression8 and dysphoria225,226 on recovery in some veterinary species. Some opioids have a ceiling effect wherein higher doses yield side effects without increased analgesia.207,227 As stated, high-NSAID doses have been associated with gastrointestinal ulceration and perforation.212,228 Therefore, lower NSAID doses combined with other analgesics should be used. This multimodal analgesia allows for analgesia to be maximized through targeting different nociception sources. This permits lower analgesic doses and decreasing the risk of side effects over those observed when using only 1 analgesic drug.210
Multimodal analgesia timing should include analgesic pain coverage during anesthetic emergence and the post-operative period. Several recently published reviews outline the impacts and benefits of analgesia in animal models post-operatively as a way to specify the impacts and address possible concerns regarding confounding results due to analgesic administration.80,222,229,230 Untreated and/or inadequate pain management can confound experimental findings. Pain related to surgery is stressful to humans and animals, which in turn impacts wound healing,80,231,232 can cause immune system perturbation,233 reduces food/water intake,234 and can change activity,235 reduce sleep and alter circadian cycles,236 change normal behaviors,178–181 and cause persistent pain due to CNS sensitization.237
There is no one-size-fits-all analgesic plan that will adequately treat pain for all surgical/invasive procedures. Researchers and veterinarians must work together to create appropriate mouse analgesic plans. Analgesics should be administered over the period where pain is expected to impact normal physiologic functions such as food and water intake, activity levels, excretory functions, and self-injurious behavior (eg, chewing, licking, or grooming at or around an incision site). Analgesic protocols should be designed for the expected procedural severity. The American College of Laboratory Animal Medicine has published guidelines to assess and manage rodent pain.238 These guidelines classify common laboratory rodent procedures into expected pain categories (minimal to mild pain, mild to moderate pain, and moderate to severe pain). We provide an overview of the different drug classes used in mice below (summarized in Table 6), with examples of preemptive and multimodal analgesic protocols for several pain categories (Table 7).
Table 6.
Commonly Used Mouse Analgesics
| Drug | Recommended Dosage and Reference | |
|---|---|---|
| Opioids | Buprenorphine-HCl (partial mu-agonist) | 0.05–0.1 mg/kg SQ q8–12238 |
| Buprenorphine (long-acting formulations; partial mu-agonist) | 0.5–1.0 mg/kg SQ (Bup-SR) q48-72 h 3.25 mg/kg SQ (Ethiqa-XR) q48-72 h | |
| Butorphanol (partial mu-agonist) | 1–5 mg/kg SQ q4h328 | |
| Morphine (full mu-agonist) | 2–5 mg/kg SQ q2-4h238 | |
| Tramadol (full mu-agonist) | 5–40 mg/kg SQ, IP (dose frequency not determined)50,182 | |
| Non-steroidal anti-inflammatories | Carprofen (COX-2 preferential) | 2.5–5 mg/kg SQ q24h328 |
| Carprofen (long-acting formulation) | 15 mg/kg SQ q24h329 | |
| Meloxicam (COX-2 preferential) | 1–5 mg/kg SQ q24h330 | |
| Meloxicam (long-acting formulation) | 6 mg/kg SQ q24h329 | |
| Ketoprofen (non-selective COX inhibitor) | 5 mg/kg SQ q12h50,83 | |
| Flunixin (non-selective COX inhibitor) | 2.5 mg/kg SQ q12h50 | |
| Firocoxib (COX-2 elective) | 10–20 mg/kg IP q24h331 | |
| Local anesthetics | Lidocaine | 2–4 mg/kg SQ over planned incision site; not to exceed 10 mg/kg50 |
| Bupivacaine | 1–2 mg/kg SQ over planned incision site; not to exceed 2 mg/kg.50 | |
| Liposomal Bupivacaine | 6 mg/kg SQ243 | |
| Other drugs | Gabapentin | 10–30 mg/kg IP182,250 |
| Maropitant | 8 mg/kg IP252 |
This table is comprised of commonly used analgesic drugs and common dosages used in mice. IP = intraperitoneal; SQ = subcutaneous.
Table 7.
Suggested Analgesic Plans for Various Pain Intensities
| Suggested Analgesic Plans for Differing Pain Intensities | ||
|---|---|---|
| Minimal to Mild Pain | Mild to Moderate Pain | Moderate to Severe Pain |
| Pre-operative: bupivacaine (1–2 mg/kg) local infiltration | Pre-operative: buprenorphine (0.05–0.1 mg/kg SQ/IP) | Pre-operative: lidocaine (2–4 mg/kg local infiltration) + long-lasting buprenorphine* once SQ |
| Post-operative: buprenorphine (0.05–0.1 mg/kg SQ/IP) every 6–12 h for 1 d, then as needed | Post-operative: carprofen (2–5 mg/kg SQ) once per day for 2 d, then as needed | |
| Pre-operative: lidocaine (2–4 mg/kg local infiltration) | Pre-operative: lidocaine (2–4 mg/kg local infiltration) + buprenorphine (0.05–0.1 mg/kg SQ/IP) | Pre-operative: bupivacaine (1–2 mg/kg local infiltration) + long-lasting buprenorphine* once SQ |
| Post-operative: carprofen (5–10 mg/kg SQ) once, then as needed | Post-operative: carprofen (2–5 mg/kg SQ) once per day for 2 d, then as needed | |
| Pre-operative: buprenorphine (0.05–0.1 mg/kg SQ/IP); effective for 6–8 h | Pre-operative: bupivacaine (1–2 mg/kg local infiltration) + long-lasting buprenorphine* once SQ | Pre-operative: long-lasting buprenorphine* once SQ |
| Post-operative: buprenorphine (0.05–0.1 mg/kg SQ/IP) if needed after initial dose has worn off | ||
| Pre-operative: carprofen (5–10 mg/kg SQ) | ||
Commonly used analgesic plans used in mice utilizing preemptive and multimodal analgesia that should be designed based on severity of pain. These can be used as a template and other drugs within a similar class utilized. IP = intraperitoneal; SQ = subcutaneous. *Long-acting buprenorphine formulations are suggested to be administered prior to surgical incision. The 2 currently available formulations of long-acting buprenorphine, Buprenorphine-SR & Ethiqa-XR, are recommended to be administered at 0.5 mg/kg and 3.25 mg/kg SQ and are advertised to provide analgesia for up to 72 hours.
Opioids
Opioid analgesics act on endogenous opioid receptors located throughout the body in a variety of tissues, including the CNS, gastrointestinal tract, joint tissue, and urinary tract.8,239 Endogenous opioid receptors play a role in pain modulation240 and are thought to play a role in emotional regulation.241 There are 3 major classes of opioid receptors: mu, kappa, and delta. The most common opioid analgesics utilized in mice target the mu-receptor. The opioid analgesics most commonly used in mice can be further classified as full or partial agonists. The efficacy to which both full and partial mu-agonists produce analgesia is dose dependent, with the maximum effects of partial agonists being less than full agonists at their peak efficacy.8 In general, opioids undergo gastrointestinal absorption and hepatic metabolism and are then excreted via the biliary and renal system.8 Opioid side effects vary by the class and type of agonist; common side effects are decreased gastrointestinal motility, decreased food and water intake, and sedation.8 Opioid use has specific legal requirements for purchasing (procurement of a Drug Enforcement Agency, i.e. DEA, license), storage (locked cabinets), and recordkeeping that may present a barrier to some researchers.
Non-Steroidal Anti-Inflammatories
NSAIDs are a drug class useful for anti-pyretic, analgesic, and anti-inflammatory effects. The anti-inflammatory effects of NSAIDs are due to inhibitory action along the arachidonic acid metabolism pathway, specifically cyclo-oxygenase enzymes, COX-1 and COX-2. Inhibition of COX enzymes interrupts production of prostanoids and prostaglandins, both involved in inflammatory processes.8 In general, NSAIDs undergo hepatic metabolism and are excreted by the kidneys. In human medicine, NSAID administration prior to surgery has been debated due to concerns over excessive bleeding secondary to the anti-thrombotic effect of COX-1 inhibition,242 but, as discussed earlier, this is not a significant concern for short-term use in mice or for short anesthetic procedures.
Local Anesthetics
Local anesthetics are sodium channel blockers. Sodium channels are essential to neural transmission. The primary usefulness of sodium channel blockers is in preventing nerve conduction from nociceptors to the CNS, thus blocking pain perception. Although an anesthetized patient will not experience pain, using local anesthetics to infiltrate the tissues around an incision site prior to the start of a procedure can prevent CNS sensitization to nociceptive stimuli and reduce the risk of a post-surgery hyperalgesia, also known as windup pain. Local anesthetic small mammal overdoses, such as in mice, occur easily and care must be taken to use less than the maximum doses listed below. There is a lack of published standardized doses for mouse local anesthetic use, but non-toxic doses that are clinically effective have been provided in tables at the end of this section. A long-acting formulation of the local anesthetic bupivacaine is currently available and has been evaluated in rats.182,243 There are few studies evaluating its use in mice,244,245 but it may be efficacious for nerve blocks.245
Other Drugs
Other drugs that have gained popularity in other veterinary species and have been utilized in mice are gabapentin and maropitant. Gabapentin is an anticonvulsant with analgesic properties for neuropathic pain used in both human and veterinary medicine.246 It provides analgesia in rats post-injury192,247,248 and in mouse models of induced nerve pain.249,250 Gabapentin’s classification as a controlled substance within the United States currently only applies to Kentucky, Michigan, and Tennessee; however this may pose as a barrier to some users.251 Maropitant is a neurokinin receptor antagonist and antiemetic, reported possibly to have anti-inflammatory effects in a study utilizing a mouse pancreatitis model.252
Authors’ Recommendation
Researchers should perform preventive and multimodal analgesia as part of the anesthesia protocol and tailor to pain severity. The addition of an opioid, that is, buprenorphine or other analgesics (lidocaine, etc), smooths out the anesthesia plane without increasing anesthetic doses, sparing hemodynamic status. Long-lasting analgesics such as Bup-SR, Bup-XR, liposomal bupivacaine, etc have been used safely, reducing stress-related handling.
FACTORS AFFECTING ANESTHESIA
Successful anesthesia administration for research mice depends on several key factors, including background (eg, strain, sex, and age), procedure (procedure performed, surgeon’s performance, length of procedure), and anesthetic plan (preventive and multimodal analgesia). Consider these factors during the anesthesia planning and implementation to ensure optimal research results and preserve research reproducibility.230,253 The consideration of these factors also allows the modern mouse researcher to move towards a more tailored anesthesia, similar to current human anesthesia trends.254
Strain
The research mouse continues to display significant advantages over other animal models in genetic research due to its small body size and ease of genetic manipulation.255–260 Mouse models are also used in genetic research because their environments can be tightly controlled, making studies on aging populations and whole lifecycles possible.261–263 In the past 25 years, mouse strain characterization,264 or “strain surveys,” have assisted research in the areas of behavior,257,265 microbiota,266 pain,267 anesthesia,68 and analgesia.230,267 Variability in anesthesia between strains should be considered when planning for research studies. For example, anesthetic sensitivity can be linked to mouse genetic loci, which can be manipulated,268 enhancing the effectiveness of select anesthetic agents.63 Similarly, gene expression profiling has revealed mouse strain differences associated with anesthetic and analgesic sensitivity.269 In addition to strain, the mouse source may be an important variable affecting research paradigms utilizing anesthesia. Recent analgesic strain surveys230 identified literature gaps where accurate strain and/or source nomenclature were absent or deficient, making comparisons between the strains and sources difficult, if not impossible. Future studies examining mouse strain and source differences between specific anesthetic agents are encouraged to better understand the role genetics play in anesthesia outcomes. Because genetically modified mouse use has expanded exponentially, it is essential to assess the background strain and the gene(s) of interest when selecting anesthesia and analgesia agents.68,230 This was demonstrated in an early study by Sonner et al, where inhalant anesthetic (isoflurane, halothane, desflurane, and ether) potency and convulsion threshold were evaluated in several inbred, outbred, wild-type, and genetically modified mice (KO PKC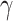 ).68 Modest differences in MAC of the anesthetic agents and convulsion threshold were demonstrated between strains, most significantly between the PKC
).68 Modest differences in MAC of the anesthetic agents and convulsion threshold were demonstrated between strains, most significantly between the PKC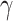 KO and their control background strain. Alzheimer’s disease research highlights another area where differences in genetically modified mouse strains can affect inhaled anesthetic potency.270 Although a complete listing of genetically modified mouse strain differences and their anesthetic potency is beyond the scope of this article, it remains essential to consider mouse strain and genetics in the anesthetic selection process.265,271
KO and their control background strain. Alzheimer’s disease research highlights another area where differences in genetically modified mouse strains can affect inhaled anesthetic potency.270 Although a complete listing of genetically modified mouse strain differences and their anesthetic potency is beyond the scope of this article, it remains essential to consider mouse strain and genetics in the anesthetic selection process.265,271
Sex
Mouse sex differences have been observed in research involving neuroimmunity,272,273 obesity,262 diabetes,262 aging,262 cardiovascular health,274 cancer,274 neuroimaging,275 euthanasia,276 pain,230,277 and anesthesia.126 One hypothesis explaining mouse anesthetic sexual dimorphism is the difference in body fat percentage (adult males > adult females), which can affect potency and/or duration of some anesthetic and analgesic agents.263 However, inbred mouse sex differences to anesthesia are more likely the result of genetic background, making variability by strain and sex critical research factors.230,278,279 And because the majority of animal subjects in mouse research studies are overwhelmingly male,280 careful interpretation of mouse study data is warranted.281 Recent examples of literature describing sex and strain differences in mice undergoing anesthesia include the comparison of IP ket/xyl and ketamine/etomidate,76 the evaluation of IP sodium pentobarbital injection pain,282 the evaluation of alfaxalone/xylazine,98 the comparison of SQ and IP ket/xyl,78 and the impact of repeated ket/xyl or isoflurane on mouse well-being.61,283
Age
Of all the physiologic factors that could affect anesthesia, age remains the least studied, especially for injectable agents. In contrast, it is well known that MAC for volatile inhalant anesthesia decreases with age in humans and animals, including mice.284 For example, Loepke et al demonstrated that in neonatal (10-day-old) C57BL/6 J and 129/SvJ mouse strains, isoflurane MAC was higher than adult controls of the same strain.284 The specific mechanism of this age-related MAC decrease is yet to be determined; however, it is likely due to a combination of physiologic factors that are altered by age.284 Advancing age can also cause cognitive impairment in elderly human patients after anesthesia285 and is hypothesized to be the result of blood brain barrier disruption caused by anesthetic agents.286 However, in mice exposed to isoflurane anesthesia, the age-related risk of anesthetic-induced cognitive impairment does not appear to occur.285 Old mice have been shown to be more sensitive to ketamine/xylazine anesthesia than young mice.95 Because the majority of anesthesia research has been conducted on inbred mouse strains, it is logical to suggest that mouse researchers also consider age a significant factor in study planning.
Surgeon’s Skill
Preparation, training, and skill of the surgeon can have a significant impact on the outcome of the procedure and the anesthetic event.287 For example, in a mouse laparoscopic adhesion model, the duration of surgery was inversely related to amount of training the surgeon received.287 It is logical to assume those with minimal experience with the procedures or surgeries performed may unintentionally inflict tissue trauma and/or pain. Similarly, the duration of the procedure and length of anesthesia period could be prolonged. Therefore, anesthetic choices should be selected wisely. A robust institutional surgical training program may enhance animal well-being and minimize surgeon variability and the negative effects of poor surgeon skill.288
Duration
Surgical or anesthesia duration will vary with the procedure or surgery performed. Human surgical site infections positively correlate with increasing procedure duration.289 In addition, hypothermia during rodent anesthesia is common137 and has recently been shown to interfere with post-operative pain assessments.290 Thus, it is widely accepted that most procedures involving rodent sedation or general anesthesia include normothermia support and the shortest duration possible. However, in cases of prolonged anesthesia duration, the anesthesia duration effects as a research variable must be addressed. For example, prolonged general anesthesia (isoflurane) can have a negative effect on the short-term and long-term anxiety response291 and intercranial CSF circulation,292 both of which should be controlled in neurological research.291 In cardiology research, prolonged anesthesia (isoflurane or sevoflurane) affects blood vessel contractility for several days after the anesthetic event.293 In addition, many research studies utilizing imaging modalities require repeated anesthetic events on the same animal. In these cases, repeated injectable61 or inhalant283 anesthetic events must be considered in the data analysis.
Authors’ Recommendations
Because of differences between mice due to sex, strain, and age, we recommend performing a pilot study with a small number of mice to determine the effect of the anesthetic protocol prior to large-scale experiments. We are perhaps years away from creating a personalized medicine approach for every mouse anesthetic event; however, identifying and addressing basic physiologic and functional factors can affect anesthetic outcome and improve research reproducibility and animal welfare.
SPECIFIC CIRCUMSTANCE ANESTHESIA
Imaging
Multiple modalities have been described to image mice, including ultrasound, electroencephalogram, (functional) magnetic resonance imaging, positron emission tomography, and computerized tomography (Figure 11).275 In contrast to the progress of these imaging modalities, optimization for safe, effective, and reproducible anesthetic paradigms that also have minimal effect on research are still necessary.50,294 Most mouse imaging studies utilize inhalant anesthesia (isoflurane, sevoflurane).295–297 Additionally, high-throughput mouse imaging has identified the need for creative solutions to provide delivery of anesthetics and gases, positioning, temperature, and anesthetic monitoring.298–300
Figure 11 .
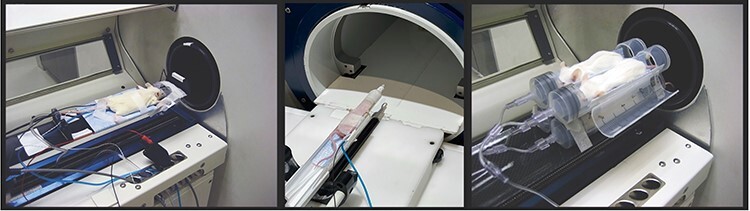
Mouse CT imaging. (Left) Individual mouse arrangement for CT imaging. The mouse can be secured with surgical tape. Care must be taken to ensure the chest is not too tightly secured, limiting respiration. A heat supply has been arranged under the mouse to prevent hypothermia. (Middle) Individual mouse prepared to enter a MRI scanner. The animal receives inhalant anesthesia via a nose cone at the end of the bed and is monitored via respiration (blue tube) and rectal temperature (black cable). The body temperature is maintained with warm air. (Right) Anesthesia can be delivered by modifying commonplace equipment. Here, slip-tip 60-mL syringes are connected to anesthetic tubing to deliver inhalant anesthesia to mice. Images courtesy of Dr Laura Pisani of Stanford Center for Innovation in in Vivo Imaging and Dr Tim Doyle and Wu Tsai Neuroscience Imaging at Stanford University.
Injectable anesthesia can be used for imaging procedure anesthesia301 and continues to be optimized for success.302 One optimization is continuous rate infusions, which permits precise dosing and eliminates inconsistent re-dosing.94 Perhaps the most progressive imaging anesthesia approach is the multidrug approach to optimize data by overcoming the limits of a single anesthesia technique.295,303 Imaging procedure durations, especially MRI, can be long. For long procedures, appropriate monitoring techniques (ie, external heat sources [Bair hugger, hot air ventilation] and body temperature, warmed fluid bags, pulse oximeter [heart rate and %SpO2], breathing rate/patterns, etc) should be performed throughout (discussed above; examples in Figure 12). In addition to monitoring, multimodal analgesia (buprenorphine, lidocaine, etc) can smooth the anesthesia plane, which is recommended when possible even for nonpainful procedures. Tables 8 and 9 provide examples of inhalant and injectable anesthetic imaging protocols. Additional consideration for mouse imaging anesthetic protocols294 has been summarized in Table 10.
Figure 12 .
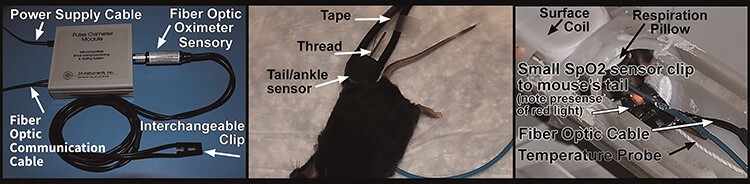
Monitoring during mouse CT imaging. (Left) An example set-up of pulse oximetry equipment for use during mouse anesthesia. (Middle) Arrangement of pulse oximeter sensor attached to mouse. (Right) Arrangement of pulse oximetry and temperature probe to monitor vitals while the mouse is undergoing imaging in a CT machine. Image courtesy of SA Instruments and Dr G. Ronald Morris.
Table 8.
Example Anesthetic Protocol for Mouse fMRI Imaging300
| Species: mouse |
| Stock or strain: C57BL/6 |
| Age: 12 wk |
| Sex: male |
| Weight: 30 g |
| Body Condition Score: 3 |
| Procedure needing anesthesia: surgical anesthesia for fMRI |
| Acclimation time: 48 h |
| Fasting time: none |
| Equipment needed: anesthetic machine, circulating water blanket, fiberoptic cable (for body temperature), foam pillow (for respiration), eye lubricant, warmed fluid |
| Pre-medication: none |
| Induction: isoflurane by induction box |
| Maintenance: etomidate CRI (0.75–1.5 mg/kg/min) after etomidate initial bolus (4 mg/kg/min over first 3 min) |
| Recovery: in home cage on circulating water blanket |
Table 9.
Example Anesthetic Protocol for Mouse Cardiac CT Imaging297
| Species: mouse |
| Stock or strain: CD1 |
| Age: 8 wk Sex: female |
| Weight: 20 g |
| Body Condition Score: 3 |
| Procedure needing anesthesia: surgical plane of anesthesia for cardiac CT |
| Acclimation time: 48 h |
| Fasting time: none |
| Equipment needed: anesthetic machine, circulating water blanket, temperature probe, eye lubricant, warmed fluid |
| Pre-medication: none |
| Induction: isoflurane by induction box |
| Maintenance: isoflurane with O2 (25–50%) and N2O (50–75%) |
| Recovery: in home cage on circulating water blanket |
Table 10.
Factors to be Considered When Developing Anesthetic Protocols for Imaging Procedures
| Considerations for Anesthetic Mouse Imaging Procedures |
| IACUC protocol requirements |
| Type of imaging |
| Length of procedure |
| Movement requirements (anesthesia plane needed) |
| Sedation vs anesthesia |
| Equipment requirements |
| MRI compatibility |
| Inhalant anesthesia |
| Intubation/ventilation |
| CRI pump |
| Physiologic monitoring |
| Carrier gases |
| Temperature maintenance |
| Positioning |
| Sedation/anesthetic agents |
| Sedation/anesthetic plan |
| Preventive/multimodal analgesia |
| Premedication |
| Neuromuscular blockade |
| Emergency drugs |
| Reproducibility |
| Training |
Stereotaxic Procedures
Stereotaxic procedures offer the neuroscience researcher a comprehensive tool to study and reproduce all aspects of the brain, including surgical lesion mapping and implantation techniques.304 Animal welfare improvements have offered refinements to this technique, further ensuring positive outcomes for neuroscience research.305 In some cases, innovative devices can be 3D printed, improving head placement, especially in mice.306 This procedure can be painful during ear bar application; therefore, mice should be at a surgical plane of anesthesia (absent paw withdrawal reflex) before placing ear bars. Coating the tips of the ear bars with lidocaine gel (Figure 13) or administration of buprenorphine at least 5–10 minutes prior to placing mice onto stereotaxic device will provide analgesia during this period. Note, pain will also be mitigated by utilizing dull/blunt-ended ear bars on all survival stereotactic procedures. Despite the fact that the animal is likely finished with the surgical procedure and is expected to be entering the recovery phase, the anesthesiologist should be prepared for the animal’s anesthetic plane to deepen after the removal of the ear bars due to the decrease in stimulation to the anesthetized animal.
Figure 13 .
Lidocaine gel for topical use. A small amount of topical lidocaine gel can be used for stereotaxic bar placement to mitigate pain in mice. The gel should be wiped off at the end of the procedure.
Neonatal Rodent Anesthesia Techniques
Neonatal mice are often used for anesthetic and survival surgical procedures; however, providing anesthesia is challenging.172 The physiology and pharmacology of anesthetic agents used in neonatal mice differs greatly from adults, affecting the pharmacodynamic adverse effects on the respiratory and cardiac systems and the propensity to develop hypothermia and hypoglycemia.172,307 Further physiological differences limiting neonatal anesthetic choices include increased blood–brain barrier permeability, higher body-water content, less mature hepatic system, and lower albumin concentrations.8
Hypothermia
This is the most commonly reported anesthetic method in neonatal mice up to 7 days of age.308 Newborn mice are poikilothermic for up to 7 days after birth and have a body temperature and metabolic rate that closely correlate with the ambient temperature until the third week of life.309,310 Because neonates have small body mass, surface cooling quickly decreases their body temperature.310 Furthermore, neonates have hypoxia tolerance in various organ systems, including the heart and brain.311 Unlike adults, where tachycardia occurs during hypoxia, in the neonate, hypoxia induces bradycardia and shunting of blood from the peripheral tissues and bradypnea.311,312 To induce hypothermia, neonatal mice can be placed on top of a latex sleeve in an ice bath and held in position until anesthesia is achieved (Figure 14).312 Latex sleeves (or another barrier material) serve to protect the pups’ skin during chilling and decrease cold-induced pain.308 Anesthesia induction takes longer than when anesthetizing adults (paw withdrawal is present until 6 minutes post-induction).312 Bradycardia, hypoventilation or apnea, and hypoxemia are all seen in the first 5–15 minutes of neonatal hypothermia anesthesia.312 Recovery time is dependent on the hypothermia anesthesia period and typically is as long or longer than the hypothermia anesthesia period.308,312 During hypothermia anesthesia recovery, it is recommended to provide gradual rewarming, because rapid warming, such as with a heating lamp, can lead to tissue damage.310 Providing supplemental 100% oxygen will facilitate hypoxia recovery. Hypothermia anesthesia is not recommended for anesthetic procedures longer than 30 minutes because there is a reported increased risk of mortality (1 in 10 postnatal day 4 rat pups died).308
Figure 14 .

Hypothermia anesthesia performed in neonatal mice. Neonate is placed on top of a latex glove in crushed ice with water.
Injectable Anesthesia
Neonatal mice have increased sensitivity and higher mortality risk when using common injectable anesthetic regimes. Typical mouse injectable anesthetics include a mixture of ketamine and sedatives or using medetomidine-based anesthesia.75,85 In adult mice, injectable anesthetic regimes necessitate careful dosage calculation, monitoring, and management to minimize cardiorespiratory depression.313 These adverse effects are more pronounced in neonatal mice. Danneman and Mandrell previously indicated ketamine, pentobarbital, and fentanyl-droperidol combinations were unsafe and were associated with a >50% mortality when used to induce a surgical anesthesia plane in 1- to 3-day-old neonatal rat pups.308 This increased mortality rate is also seen in older neonatal rodents. Tsukamoto et al evaluated ketamine-xylazine anesthesia and medetomidine-midazolam-butorphanol anesthesia in 10-day-old rat pups.313 They discovered that when using ketamine-xylazine anesthesia at a 60-mg/kg (ketamine) and 6-mg/kg (xylazine) dose, the 10-day-old pups exhibited sudden death, and when using a lower dose of 40 mg/kg (ketamine) and 4 mg/kg (xylazine), rat pups did not reach a surgical anesthesia plane. Based on these results, ketamine-xylazine anesthesia in neonatal mice is not recommended because of the narrow safety margin. The medetomidine-midazolam-butorphanol needed to reach a surgical plane in 10-day-old rat pups is higher than the adult rodent dose (0.3/4/5 mg/kg of medetomidine-midazolam-butorphanol, respectively, required for neonates).313 When using injectable anesthetic regimes, supportive care measures including maintaining an external heat source, providing 100% O2, and reversing with atipamezole (if applicable to the injectable protocol used) should be further evaluated and considered. Injectable anesthetics in neonatal mice are unpredictable, have high mortality risk, and should only be considered if gas anesthesia is not feasible or the neonates are older than 6 days of age and hypothermia cannot be used.
Inhalant Anesthetics
These are generally considered safe and effective for neonatal mice.308,312,313 When using isoflurane or sevoflurane anesthesia in neonatal mice, it is recommended to first rapidly induce pups in an induction chamber at 2 L/min of 100% O2 containing either 5% isoflurane or 8% sevoflurane.312 The MAC of isoflurane (2.3%) in 10-day-old mice is higher than in adult mice.284 A study in 3-day-old neonatal rat pups successfully maintained anesthesia using a nose cone with a glove cut to fit the animal’s snout (500 mL/min of 100% O2 with isoflurane at 3–4% or sevoflurane at 5%) while placed on top of a warm water circulating pad (Figure 15).312 With these induction methods, pups lost their righting reflex within a minute; however, the period until paw withdrawal was prolonged compared with adults (5–6 minutes).312 This study also indicated that when using inhalant anesthetic agents, RR, heart rate, and %SpO2 decrease but remain more stable than with hypothermia anesthesia.312 Recovery time for 3-day-old pups anesthetized for 15 minutes with isoflurane or sevoflurane was significantly shorter compared with hypothermia (time to recovery was 5–6 minutes shorter).312 The exact percentage of inhalant agent needed is dependent on the animal’s age because MAC decreases with age in humans and rodents.284 Increased inhalant anesthesia duration, which lasts an hour in rodent neonates, can be associated with a higher mortality rate.313 Because of an increased risk for mortality, hypothermia, bradycardia, and hypoventilation seen with increased anesthetic times, it is recommended that inhalant anesthetics are used in rodent neonates for procedures less than 30 minutes in length and that a warm water circulating pad and supplemental oxygen are provided during the recovery period. Hypoglycemia may occur with prolonged anesthetic procedures,284 and dextrose (eg, 0.25–1 mL/kg of 50% dextrose IP) may be administered.
Figure 15 .
Inhalant anesthesia performed on a neonatal mouse. Snout of the neonate is placed and sealed in a nose cone covered with a latex glove. A circular hole is cut into the latex glove to fit around the snout of the neonate.
Authors’ Recommendations
For long imaging and stereotaxic procedures, isoflurane, isoflurane with ket/xyl, or CRI including multimodal analgesia (buprenorphine, lidocaine) is recommended when possible. Appropriate monitoring, especially preventing hypothermia with appropriate external heat sources and warmed fluid administration, is highly recommended. For short anesthetic procedures (<30 minutes) on neonatal mice younger than 7 days of age, isoflurane, sevoflurane (preferred), and hypothermia anesthesia all provide a safe and reliable surgical plane of anesthesia.312 Authors have had good experiences with sevoflurane in neonatal mice with quick induction and recovery; it is critical to provide an external heat source and supplemental O2 throughout the anesthetic procedure and recovery period.
COMMON COMPLICATIONS AND TROUBLESHOOTING COMPLICATIONS
“An ounce of prevention is worth a pound a cure” is particularly apt during anesthesia. When anesthesia and surgery involve rodents, mortality can exceed 10 times the companion animal rate.117 To reduce anesthetic complications, anesthetists should be confident of the animal’s health and well-being, select appropriate anesthetic drugs/dosages, prepare anesthetic equipment, and provide procedure-specific supportive care and physiologic monitoring. Given that experience is a great teacher, it is imperative that the surgeon and anesthetist are not shared roles during training, if ever. Practically, appropriate personnel support for mouse surgical procedures includes a surgeon, an anesthetist, and, depending on a given procedure’s complexity, at least 1 assistant. Anything that can shorten a procedure’s duration is more likely to yield a given procedure’s positive outcome. Conversely, an overly hastily performed procedure may yield less than desirable outcomes. If during the course of an anesthetic procedure there are unfavorable anesthetic developments, it is imperative that the surgeon undertake the anesthetist’s direction. The first course of action would be to pause any further procedural manipulations until the anesthetist can make a proper patient assessment. During mouse anesthesia, complications typically involve the central nervous, respiratory, and cardiovascular systems; equipment set-up; and human error. The following complications will be discussed: (1) CNS: insufficient/excessive anesthetic plane; (2) respiratory system: irregular respiratory patterns/hypoventilation; (3) cardiovascular system: hypotension; and (4) other complications: hypothermia, excessive salivation, and prolonged recovery. There has been very little research or evidence-based medicine addressing the treatment of anesthetic emergencies in mice. Thus, much of the information presented in this section comes from the authors’ clinical expertise and first-hand experiences.
CNS Complications
Insufficient or Excessive Anesthetic Plane
Reaching the correct anesthetic plane is particularly challenging when using injectable anesthetics because the doses needed to reach and maintain a surgical plane of anesthesia must be titrated to the individual animal. Additionally, employing injectable anesthesia for prolonged anesthetic procedures presents additional complications associated with re-dosing an animal that is physiologically different from the animal receiving the initial (or most recent) anesthetic administration, sometimes markedly so. Other factors that must be taken into consideration for the anesthetic selection and dosing regimens include compromised animals (eg, immunocompromised, sick, pregnant, old, etc), which will require lower doses of anesthetics and/or may not be safe to be anesthetized; mouse age; and hypothermia, which will be discussed below.
Causes of complications: incorrect animal weight; improper route of administration for injectable anesthetics (such as accidental intrahepatic, intracecal, incomplete injection, etc); anesthetic underdose/overdose.
-
Prevention/treatment: monitor anesthetic plane (discussed above); if the animal is at an insufficient anesthetic plane:
Pause the procedure to assess the patient’s anesthetic plane.
Administer analgesia (eg, buprenorphine, 0.05–0.1 mg/kg SQ/IP), which may help to stabilize the anesthetic plane and be sufficient to resume the procedure.
Incrementally increase gas anesthetics in 0.25–0.5% increases, or re-dose injectable anesthetics as discussed above. Note that re-dosing injectable anesthetics may prolong the anesthetic recovery; therefore, consider administering reversal agents when the procedure ends (eg, atipamezole 0.1–1 mg/kg SQ/IP to reverse alpha-2 agonists). Caution: any analgesia provided by alpha-2 agonists will be reversed at this time.
Check the anesthetic machine connections and fittings for disconnections, misconnections, tubing kinks, or leaks. Ensure any gas anesthetic scrubber or canister system is assembled and used according to manufacturer’s recommendations, especially that gas outflow is unimpeded.
If an excessively deep anesthetic plane is determined, this complication must be treated as an emergency:
Incrementally decrease gas anesthesia (in 0.25–0.5% steps) or administer an injectable anesthetic reversal agent (eg, administer atipamezole for xylazine, medetomidine, or dexmedetomidine).
Administer naloxone if one must reverse buprenorphine.
Respiratory Complications
Irregular Respiratory Pattern (Including Hypoventilation)
Most anesthetic agents suppress the respiratory system in a dose-dependent manner. Under anesthesia, the RR and pattern should be regular and characterized by good deep breaths. The rate will depend on the anesthetic protocol being used, as discussed above in the Monitoring section. Hypoventilation is a very common general anesthetic complication. Hypercapnia is a sequela to hypoventilation and leads to respiratory acidosis and ultimately hypoxemia. Brick-red mucous membranes are suggestive of hypercapnia. If one is conducting anesthesia under room air (20% O2; ie, without oxygen supplementation), a mouse can quickly develop hypoxemia. ETCO2, or PaCO2 (the partial pressure of arterial CO2), are ideal indicators for assessing an animal’s ventilatory status:
Causes of complications: wrong anesthetic plane (ie, too light or too deep); an anesthetic overdose, resulting in a very deep anesthesia plane; anesthetic side effects (eg, induced pulmonary edema secondary to xylazine, or medetomidine administration314,315); underlying health issues (eg, hypoventilation secondary to a neoplastic chest cavity mass, or obesity, or mice infected with pulmonary infectious agents like Pneumocystis murina or Sendai virus); equipment malfunction (eg, kinked or obstructed tubing).
-
Prevention/treatment: monitor RR; keep mucous membranes pink by ensuring adequate oxygen delivery; keep %SpO2 > 95%; keep ETCO2 approximately 35–45 mmHg; if RR/pattern is too low/slow and shallow and the mouse has an absent paw withdrawal reflex or no jaw tone, the animal is too deep:
Lighten the anesthetic plane. If using injectable anesthetics, promptly complete surgery while supporting the mouse with 100% O2.316
As soon as possible, place the mouse in sternal recumbency, which increases bilateral chest excursion and reduces the gastrointestinal tract’s pressure on the diaphragm.
Administer reversal agents, such as atipamezole for alpha-2 agonists.
If RR or pattern is too high/fast:
Pause the surgical manipulations as previously indicated.
Incrementally increase inhalant anesthesia in 0.25% steps to deepen the anesthetic plane (allow 3–5 minutes between increments).
Administer injectable anesthetics or buprenorphine.
Consider practicing balanced anesthesia/multimodal analgesia, especially with longer procedures (>45 minutes).
Cardiovascular Complications
Hypotension (Blood Pressure < 60 mmHg)
Cardiac output consists of heart rate and stroke volume. Blood pressure is a product of cardiac output and systemic vascular resistance. Most anesthetics suppress the cardiovascular system in a dose-dependent manner. Without blood pressure monitoring, it is difficult to identify the problem eliciting hypotension. Prolonged capillary refill time may indicate hypotension; however, capillary refill time is difficult to assess in mice. A deeper anesthetic plane or higher anesthetic doses are more likely to suppress the cardiovascular system leading to hypotension. Hypotension leads to decreased blood flow, resulting in decreased O2 and nutrient delivery to all tissues:
1) Causes of complications: anesthetic overdose; mice with impaired cardiac pump function/cardiac contractility (ie, dilated cardiomyopathy models), decreased vascular tone, or reduced circulating volume (dehydration, hemorrhage etc); bradycardia; hypothermia; and compromised health status mice.
-
2) Prevention/treatment: If hypotension is evident:
Lighten the anesthetic plane. Inhalant anesthetics can cause hypotension by vasodilation and reduced cardiac contractility in a dose-dependent manner.
Administer balanced, warmed fluids at 5–20 mL/kg/h (IP, SC, IV). Appropriate fluid support is essential to optimize surgical outcomes and minimize complications. Isoflurane-anesthetized mice should be supported with fluid administration regardless of surgical blood loss or insensible fluid losses (ie, evaporated fluid loss). Fluid loss can also occur secondary to injectable anesthetic cocktails containing alpha-2 agonists, because these can cause elevated blood glucose levels, resulting in osmotic polyuria. The associated urinary (warm fluid) loss can profoundly affect body weight, hydration status, blood glucose, and electrolyte levels.
If the heart rate drops below normal ranges, which will depend on the anesthetic protocol used, as discussed above, atropine (0.04 mg/kg SC, IP, IV) may be indicated.50 Note that hypothermia may lead to bradycardia. Therefore, body temperature should be monitored and an external heat source must be provided.317
Monitor blood pressure (if possible) and body temperature.
If the mouse has notable blood loss (ie, hypovolemia):
Blood loss >20% can cause hypovolemic shock. If blood loss occurs, an estimated blood loss can be replaced with warmed balanced fluid (3 times the blood volume lost), anesthesia plane should be lightened, and promptly finish the procedure. Mouse blood volume 1.6–3.2 mL (6–8% body weight; approximately 80 mL blood/kg).318
Other Complications
Hypothermia
The mouse’s core body temperature is 36.5–38°C (97.7–100.4°F). Consequences of hypothermia are discussed above but include prolonged recovery, shivering, discomfort, coagulopathy and platelet dysfunction, increased incidence of wound infection, altered drug metabolism, cardiovascular system suppression, impaired tissue perfusion, and respiratory compromise.50
Causes of complications: cold ambient temperature; cold surgical prep solutions; wide shaving or cleansing area; long anesthesia; exposure to dry carrier gas, that is, O2.
Prevention/treatment: use active (external heating sources) and passive (fluid administration) cutaneous warming systems;317 reduce O2 flow to approximately 0.5 L/min with a non-rebreathing breathing circuit; wrap extremities (ie, paws and tail) and exposed surfaces with products like bubble wrap; administer warm, balanced fluids (IP, IV, SQ) at 5–20 mL/kg/h (IP, IV, SQ); monitor core body temperature periodically, especially for procedures longer than 15 minutes. A non-burning, infrared temperature feedback system is recommended. Avoid using heat lamps and electric heating pads. Note: Do not use supplemental heat sources that are not designed for anesthetized animals because they can easily cause thermal injuries. If the procedure is long, researchers may consider using a humidifier connected to the anesthetic machine.
Authors’ Recommendation
Circulating warm water heating pads can effectively control mouse body temperature during anesthesia. Such an external heat source should be provided from gas induction or immediately after injectable anesthetic administration to full ambulatory period.
Excessive Salivation
Excessive salivation can occur with ketamine and prolonged anesthesia. Without tracheal intubation, partial or total tracheal occlusion may occur, causing anesthetic emergencies. Clinical signs indicating tracheal occlusion secondary to saliva accumulation are labored breathing, poor mucous membrane or skin color (due to low O2 saturation), or agonal breathing.
Causes of complications: ketamine use or prolonged anesthesia.
Prevention/treatment: check airway patency, if possible; clean/dry saliva with a Q-tip or cotton swab; administer an anticholinergic agent (ie, glycopyrrolate, 0.01 mg/kg SC, IP); provide 100% O2 via a mask; discontinue/complete surgery as soon as possible; position animal in sternal recumbency; monitor %SpO2.
Prolonged Recovery
Prolonged recovery is commonly seen with injectable anesthesia, long anesthetic procedures, and in compromised animals. Depending on anesthetic doses, techniques, or duration, mice should recover within approximately 30–45 minutes of anesthetic discontinuation. As discussed earlier, provide aggressive supportive care in recovery:
Causes of complications: hypothermia, anesthetic overdose; long anesthesia (regardless of technique/protocol); lack of appropriate peri-operative monitoring (resulting in hypotension, hypoxemia, hypoventilation etc).
Prevention/treatment: if animals are compromised, procedures should be postponed and the animal provided supportive care until they are fully ambulatory. A veterinary consultation should be performed, and the veterinarian provided the anesthetic and procedure-related records. Appropriate peri-operative monitoring must be provided. Injectable anesthetics should be reversed or gas anesthesia incrementally decreased over time, when possible. If prolonged recovery occurs, close monitoring and supportive care (100% O2, warmed fluid administration, warm environment etc) should be provided until full recovery.
Authors’ Recommendation
It is better to prevent anesthetic complications than have to treat them. Vigilant monitoring, effective interpretation of monitoring parameters, and appropriate supportive care are crucial to prevent anesthetic complications. Researchers should closely monitor anesthetic plane, administer warmed balanced fluid with all procedures, provide 100% O2, prevent hypothermia, and administer reversal agents for injectable anesthesia at the procedure’s end while ensuring appropriate postoperative analgesia has been administered before the reversal agent. Balanced anesthesia/multimodal analgesia should always be practiced. This technique allows lower doses of anesthetics/analgesics to be used, leading to lower side effects. The anesthesia plane can be adjusted easiest and more safely with gas anesthesia.
CONCLUSIONS
Mouse anesthesia can be challenging even for those with anesthesia experience. Although inhalant anesthesia receives our top recommendation, inhalant anesthesia combined with injectable anesthetics can be used safely, including for longer procedures. If injectable anesthesia must be used, careful re-dosing may be required. To prevent anesthetic morbidity/mortality, appropriate monitoring/interpretation and supportive care must be provided. The authors strongly address the importance of anesthetic monitoring throughout any anesthetic event, no matter how mundane (eg, RR, mucous membrane color, paw withdrawal reflex, and heating pads for short procedures; those techniques with short procedures together with advance techniques/equipment [%SpO2, ECG, etc] for longer procedures). Some anesthetic complications lead to emergencies; therefore, as complications occur, they should be treated immediately and appropriately. Properly annotated anesthetic records are invaluable to adjust anesthetic protocols, prevent mishaps, and assist consultation with scientific colleagues, including veterinary colleagues. Finally, preventive and multimodal analgesia should be “factory installed” into anesthetic plans (whether it is for sedation, general anesthesia, or restraint) when possible, especially those where the procedural outcome is likely to yield a high pain severity.
Key Points
Introduction
Anesthesia is both an art and science. Although there are multitudes of texts regarding how to perform safe and effective rodent anesthesia, experience is the best training.
Mouse anesthesia is challenging due to the mouse’s unique biology and the wide range of experience in performing mouse anesthesia for both veterinarians and animal researchers.
Anesthesia and analgesia instruction is a vital element to ensure there is a minimum level of understanding towards ensuring the institutional regulatory mandate for education and training is met and the introduction of institutional resources to assist those conducting anesthesia.
Anesthetic Depth
General anesthesia causes CNS depression and can be characterized by 4 notable stages: amnesia, loss of consciousness, loss of response to noxious stimuli, and blunted autonomic responses. These 4 stages are associated with an inhalant anesthetic’s specific minimum alveolar concentration (MAC).
Loss of consciousness can be estimated by the loss of the righting reflex.
Loss of movement in response to a noxious stimulus indicates the spinal reflexes have been anesthetized.
Dosing
When selecting an anesthetic protocol, consider the anesthetic depth and duration required and the anesthetic’s impact on experimental variables before using an anesthetic protocol.
Inhalant anesthetics are strongly recommended in mice because of the steep dose-response curves and the ability to finely regulate and rapidly change the animal’s anesthetic depth.
Ketamine/xylazine (ket/xyl) and ketamine/xylazine/acepromazine (ket/xyl/ace) injectable protocols can be safely used; however, careful and frequent monitoring of the animal’s anesthetic plane is necessary to ensure adequate anesthesia for the procedure performed, while not being too deep, risking anesthetic-related death.
New mouse anesthetic protocols are being developed, including the use of alfaxalone and a combination of medetomidine, midazolam, and butorphanol, which may expand our arsenal of options to safely use different anesthetic protocols while minimizing the effects on experimental variables. Using these new protocols and discussing the results within our field will be important in the continued development of these drugs.
Anesthetic Monitoring
Appropriate monitoring techniques (pre-, peri-, and postoperative periods) are crucial to reduce mortality/morbidity.
The monitoring summary comprises an anesthetic monitoring record that includes supportive care measures, vital signs, and all administered drugs. The resulting anesthetic monitoring record helps anesthetists assess trends in vital signs and identify potential problems during an anesthetic procedure.
Prolonged recovery may occur in hypothermic mice, mice with metabolic or respiratory complications, or in painful animals. Supportive care is essential in the mouse recovery and post-operative periods and includes providing supplemental heat, warmed parenteral fluids, and easily available food and water.
Institutional veterinarian consultation is recommended for animals with an abnormal physical/health status.
Preventive and Multimodal Analgesia
Preventive and multimodal analgesia maximizes analgesia while minimizing side effects. They should be designed as a part of the anesthesia plan. This technique combines multiple anesthetics/analgesic classes into a single anesthetic protocol.
The most intense pain after a surgical procedure is expected to occur in the first 24 hours after the procedure but will vary depending on the procedure.
Mice are unlikely to show signs of pain post-operatively when directly observed, so indirect assessments are required (body weight, fecal production, grimace scores, nest building, activity level, etc).
Factors Affecting Anesthesia
Important factors affecting anesthetic choices are strain, gender, age, surgeon’s skill, and anesthesia duration/plane.
Imaging and stereotaxic procedures: if the procedure is long (>45 minutes), consider a combination of isoflurane and CRI anesthetics. Perform appropriate monitoring techniques, including body temperature measurement.
Hypothermia and inhalant anesthetics are the most common anesthetic methods for neonatal mice.
Common Complications and Troubleshooting Complications
The best way to handle anesthetic complications/emergencies is to prevent them.
Unfamiliarity with mouse anesthesia and monitoring modalities may increase the occurrence of anesthetic complications.
Anesthetic monitoring (discussed above) and the anesthetist’s familiarity with anesthesia techniques are crucial components towards preventing or reducing anesthetic complications and patient morbidity or mortality.
Preventing anesthetic-associated morbidity/mortality requires vigilance and appropriate monitoring, understanding and interpreting monitoring modalities, and relevant and rapid intervention.
Acknowledgments
We thank Janis Atuk-Jones for her assistance with formatting this manuscript, figures, and tables, and Benjamin Franco and Eden Alamaw for their assistance with taking photos for this manuscript.
Potential conflicts of interest. All authors: No reported conflicts.
Contributor Information
Kaela L Navarro, Department of Comparative Medicine, Stanford University, Stanford, California, USA.
Monika Huss, Department of Comparative Medicine, Stanford University, Stanford, California, USA.
Jennifer C Smith, Bioresources Department, Henry Ford Health System, Detroit, Michigan, USA.
Patrick Sharp, Office of Research and Economic Development, University of California, Merced, California, USA; Animal Resources Authority, Murdoch, Australia; School of Veterinary and Life Sciences, Murdoch University, Murdoch, Western Australia, Australia.
James O Marx, Department of Pathobiology, School of Veterinary Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, USA.
Cholawat Pacharinsak, Department of Comparative Medicine, Stanford University, Stanford, California, USA.
References
- 1. Council NR . Recognition and Alleviation of Pain in Laboratory Animals. Washington, DC: The National Academies Press; 2009. [PubMed] [Google Scholar]
- 2. Roizen M, Horrigan R, Frazer B. Anesthetic doses blocking adrenergic (stress) and cardiovascular responses to incision—MAC BAR. Anesthesiology 1981; 54(5):390–398. [DOI] [PubMed] [Google Scholar]
- 3. Krnjević K, Puil E.. Cellular mechanisms of general anesthesia. In: Bittar E, Bittar N, eds. Principles of Medical Biology: Molecular and Cellular Pharmacology . Vol 8. Stamford, CT: Jai Press. 1997; 811–828. [Google Scholar]
- 4. Alkire M, Hudetz A, Consciousness TG. anesthesia. Science 2008; 322(5903):876–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. MacIver M. Loss of recall and the hippocampal circuit effects produced by anesthetics. In: Hudetz A, Pearce R, eds. Suppressing the Mind: Anesthetic Modulation of Memory and Consciousness. New York, NY: Humana Press; 2010. [Google Scholar]
- 6. Antognini J, Barter L, Carstens E. Overview movement as an index of anesthetic depth in humans and experimental animals. Comp Med 2005; 55(5):413–418. [PubMed] [Google Scholar]
- 7. Franks N. General anesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci 2008; 9(5):370–386. [DOI] [PubMed] [Google Scholar]
- 8. Tranquilli, W. and Grimm, KA. Introduction: Use, Definitions, History, Concepts, Classification, and Considerations for Anesthesia and Analgesia. In: Grimm KA, Lamont LA, Tranquilli WJ, Greene SA, Robertson SA. eds. Veterinary Anesthesia and Analgesia: The 5th edition of Lumb and Jones. Ames, IA: Wiley-Blackwell; 2015: Chapter 1. [Google Scholar]
- 9. Glannon W. Anaethesia, amnesia and harm. J Med Ethics 2014; 40:651–657. [DOI] [PubMed] [Google Scholar]
- 10. Voss L, Sleigh J. Monitoring consciousness: the current status of EEG-based depth of anaesthesia monitors. Best Pract Res Clin Anaesth. 2007; 21(3):313–325. [DOI] [PubMed] [Google Scholar]
- 11. Garcia-Larrea L, Bastuji H. Pain and consciousness. Prog Neuropharm Bio Psych 2018; 87:193–199. [DOI] [PubMed] [Google Scholar]
- 12. Rosman E, Quartermain D, Turndorf H. Retrograde and anterograde amnesia in mice undergoing halothane anesthesia (abstract). Anesth Analg 1990; 70(2):pS333. [Google Scholar]
- 13. Samuel N, Taub A, Paz Ret al. . Implicit aversive memory under anaesthesia in animal models: a narrative review. Br J Anaesth 2018; 121(1):219–232. [DOI] [PubMed] [Google Scholar]
- 14. Rosman E, Quartermain D, Pang Ret al. . Halothane anesthesia causes state-dependent retrieval failure in mice. Physio Behav 1992; 52(3):449–453. [DOI] [PubMed] [Google Scholar]
- 15. Alkire M, Nathan S. Does the amygdala mediate anesthetic-induced amnesia? Basolateral amygdala lesions block sevoflurane-induced amnesia. Anesthesiology 2005; 102(4):754–760. [DOI] [PubMed] [Google Scholar]
- 16. Antognini J, Jinks S, Carstens E. The spinal cord, anesthesia and immobility: a re-examination. Int Cong Ser 2005; 1283:125–131. [Google Scholar]
- 17. Sonner J, Antognini J, Dutton Ret al. . Inhaled Anesthetics and immobility: mechanisms, mysteries, and minimum alveolar anesthetic concentration. Anesth Analg 2003; 97(3):718–740. [DOI] [PubMed] [Google Scholar]
- 18. Yamamoto T, Schindler E. Where and how do anaesthetics act? Mechanisms of action in the central nervous system. Anaesthesiol Intensive Ther 2017; 49(4):288–293. [DOI] [PubMed] [Google Scholar]
- 19. Roth D, Petersen-Felix S, Bak Pet al. . Analgesic effect in humans of subanaesthetic isoflurane concentrations evaluated by evoked potentials. Br J Anaesth 1996; 76:38–42. [DOI] [PubMed] [Google Scholar]
- 20. Petersen-Felix S, Arendt-Neilsen L, Bak Pet al. . Analgesic effect in humans of subanaesthetic isoflurane concentrations evaluated by experimentally induced pain. Br J Anaesth 1995; 75:55–60. [DOI] [PubMed] [Google Scholar]
- 21. Houghton I, Cronin M, Redfern Pet al. . The analgesic effect of halothane. Br J Anaesth 1973; 45:1105–1110. [DOI] [PubMed] [Google Scholar]
- 22. Zbinden A, Maggiorini M, Petersen-Felix Set al. . Anesthetic depth defined using multiple noxious stimuli during isoflurane/oxygen anesthesia. I Motor reactions. Anesthesiology 1994; 80(2):253–260. [DOI] [PubMed] [Google Scholar]
- 23. Lichtner G, Auksztulewicz R, Velten Het al. . Nociceptive activation in spinal cord and brain persists during deep general anaesthesia. Br J Anaesth 2018; 121(1):291–302. [DOI] [PubMed] [Google Scholar]
- 24. Carbone L, Pain AJ. Laboratory animals: publication practices for better data reproducibility and better animal welfare. PLoS One 2016; 11(5):e0155001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khajuria DK, Razdan R, Mahapatra DR. Description of a new method of ovariectomy in female rats. Rev Bras Reumatol 2012; 52(3):462–470. [PubMed] [Google Scholar]
- 26. Animal Welfare Act (PL-91-579) . U. States Department of Agriculture, & Animal and Plant Health Inspection Service. USDA, ed1970. Last accessed: 25 Apr. 2021. https://www.aphis.usda.gov/aphis/ourfocus/animalwelfare/sa_publications/ct_publications_and_guidance_documents. [Google Scholar]
- 27. National Research Council NRCU . Guide for the Care and Use of Laboratory Animals .8th ed. Washington, DC: The National Academies Press; 2011. [Google Scholar]
- 28. OLAW . Public Health Service Policy on Humane Care and Use of Laboratory Animals. Bethesda, MD: NIH-Department of Health and Human Services; 2002. [Google Scholar]
- 29. National Research Council NRCU . Education and Training in the Care and Use of Laboratory Animals: A Guide for Developing Institutional Programs. Washington, DC: The National Academies Press; 1991. [PubMed] [Google Scholar]
- 30. Siddiqui B, Kim P. Anesthesia stages. StatPearls [Internet]. Treasure Island (FL): StatePearls Publishing. e1–19. (Last accessed: 25 Apr. 2021). https://www.ncbi.nlm.nih.gov/books/NBK557596/. [Google Scholar]
- 31. Campagna J, Miller K, Forman S. Mechanisms of actions of inhaled anesthetics. NEJM 2003; 348:2110–2124. [DOI] [PubMed] [Google Scholar]
- 32. Eger IIE, Saidman L, Brandstater B. Minimum alveolar anesthetic concentration: a standard of anesthetic potency. Anesthesiology 1965; 26(6):756–763. [DOI] [PubMed] [Google Scholar]
- 33. Aranake A, Mashour G, Avidan M. Minimum alveolar concentration: ongoing relevance and clinical utility. Anesthesia 2013; 68(5):512–522. [DOI] [PubMed] [Google Scholar]
- 34. Antognini J, Schwartz K. Exaggerated anesthetic requirements in the preferentially anesthetized brain. Anesthesiology 1993; 79(6):1244–1249. [DOI] [PubMed] [Google Scholar]
- 35. Boscan P, Monnet E, Mama Ket al. . A dog model to study ovary, ovarian ligament and visceral pain. Vet Anaesth Analg 2011; 38(3):260–266. [DOI] [PubMed] [Google Scholar]
- 36. Buitrago S, Martin T, Tetens-Woodring Jet al. . Safety and efficacy of various combinations of injectable anesthetics in BALB/c mice. JALAAS. 2008; 47(1):11–17. [PMC free article] [PubMed] [Google Scholar]
- 37. Mogil J, Smith S, O’Reilly Met al. . Influence of nociception and stress-induced antinociception on genetic variation in isoflurane anesthetic potency among mouse strains. Anesthesiology 2005; 103(4):751–758. [DOI] [PubMed] [Google Scholar]
- 38. Richardson CA, Flecknell PA. Anaesthesia and post-operative analgesia following experimental surgery in laboratory rodents: are we making progress? Altern Lab Anim 2005; 33(2):119–127. [DOI] [PubMed] [Google Scholar]
- 39. Stokes EL, Flecknell PA, Richardson CA. Reported analgesic and anaesthetic administration to rodents undergoing experimental surgical procedures. Lab Anim 2009; 43(2):149–154. [DOI] [PubMed] [Google Scholar]
- 40. Rudeck J, Vogl S, Heinl Cet al. . Analgesic treatment with buprenorphine should be adapted to the mouse strain. Pharmacol Biochem Behav 2020; 191:1–12, 172877. [DOI] [PubMed] [Google Scholar]
- 41. Griebel G, Belzung C, Perrault Get al. . Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharm 2000; 148(2):164–170. [DOI] [PubMed] [Google Scholar]
- 42. Lipiski M, Arras M, Jirkof Pet al. . Premedication with fentanyl-midazolam improves sevoflurane anesthesia for surgical intervention in laboratory mice. Exp Biol Med (Maywood) 2017; 242(12):1287–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Miller R. Miller’s Anesthesia . 9th ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2019. [Google Scholar]
- 44. de Jong R. Eger II E. MAC expanded: AD50 and AD95 values of common inhalation anesthetics in man. Anesthesiology 1975; 42(4):384–389. [PubMed] [Google Scholar]
- 45. Forman S, Ishizawa Y. Inhaled anesthetic uptake, distribution, metabolism, and toxicity. In: Gropper M, Cohen N, Eriksson Let al., eds. Miller’s Anesthesia. Philadelphia, PA: Elsevier; 2020: Chapter 20, 509–539. [Google Scholar]
- 46. Njoku DB, Chitilian HV, Kronish K.Hepatic physiology KK. pathophysiology, and anesthetic considerations. In: Gropper MA, Cohen NH, Eriksson LIet al., eds. Miller's Anesthesia. 9th. Philadelphia, PA: Elsevier; 2020: Chapter 16, 420–443. [Google Scholar]
- 47. Whittem T, Beths T, Bauquier SH. General pharmacology of anesthetic and analgesic drugs. In: Grimm KA, Lamont LA, Tranquilli WJ, Greene SA, Robertson SA. eds. Veterinary Anesthesia and Analgesia: The 5th edition of Lumb and Jones. Ames, IA: John Wiley & Sons; 2015: Chapter 7. Pg 147–177. [Google Scholar]
- 48. Newcomer D, Chopra I. Evaluation of waste anesthetic gas surveillance program and isoflurane exposures during animal and human surgery. J Occup Environ Hyg 2019; 16(8):544–556. [DOI] [PubMed] [Google Scholar]
- 49. Miller A, Theodore D, Widrich J. Inhalational anesthetic. StatPearls; 2020. (Last accessed date: 25 Apr. 2021). https://www.ncbi.nlm.nih.gov/books/NBK554540/.
- 50. Flecknell PA. Laboratory Animal Anaesthesia. 3rd ed.: Burlington, MA: Academic Press; 2016:135–137. [Google Scholar]
- 51. Groeben H, Meier S, Tankersley Cet al. . Influence of volatile anaesthetics on hypercapnoeic ventilatory responses in mice with blunted respiratory drive. Br J Anaesth 2004; 92(5):697–703. [DOI] [PubMed] [Google Scholar]
- 52. Low LA, Bauer LC, Klaunberg BA. Comparing the effects of isoflurane and alpha chloralose upon Mouse Physiology. PLoS One 2016; 11(5):e0154936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Massey CA, Richerson GB. Isoflurane, ketamine-xylazine, and urethane markedly alter breathing even at subtherapeutic doses. J Neurophysiol 2017; 118(4):2389–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rödig G, Keyl C, Wiesner Get al. . Effects of sevoflurane and isoflurane on systemic vascular resistance: use of cardiopulmonary bypass as a study model. Br J Anaesth 1996; 76(1):9–12. [DOI] [PubMed] [Google Scholar]
- 55. Steffey EP, Mama KR, Brosnan RJ. Inhalation anesthetics. In: Grimm KA, Lamont LA, Tranquilli WJ, Greene SA, Robertson SA. eds. Veterinary Anesthesia and Analgesia: The 5th edition of Lumb and Jones. Ames, IA: Wiley-Blackwell; 2015: Chapter 16. Pg 297–331. [Google Scholar]
- 56. Wilding LA, Hampel JA, Khoury BMet al. . Benefits of 21% oxygen compared with 100% oxygen for delivery of isoflurane to mice (Mus musculus) and rats (Rattus norvegicus). JALAAS 2017; 56(2):148–154. [PMC free article] [PubMed] [Google Scholar]
- 57. Kavanagh B, Hedenstierna G. Respiratory physiology and pathophysiology. In: Gropper M, Cohen N, Eriksson Let al., eds. Miller’s Anesthesia. Philadelphia, PA: Elsevier; 2020: Chapter. 13. 354–383. [Google Scholar]
- 58. Alves H, Valentim A, Olsson Iet al. . Intraperitoneal propofol and propofol fentanyl, sufentanil and remifentanil combinations for mouse anaesthesia. Lab Anim 2007; 41(3):329–336. [DOI] [PubMed] [Google Scholar]
- 59. Burnside W, Flecknell P, Cameron A. Thomas a. a comparison of medetomidine and its active enantiomer dexmedetomidine when administered with ketamine in mice. BMC Vet Res 2013; 9(48):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Groeben H, Meier S, Tankersley Cet al. . Heritable differences in respiratory drive and breathing pattern in mice during anaesthesia and emergence. Br J Anaesth 2003; 91(4):541–545. [DOI] [PubMed] [Google Scholar]
- 61. Hohlbaum K, Bert B, Dietze Set al. . Impact of repeated anesthesia with ketamine and xylazine on the well-being of C57BL/6JRj mice. PLoS One 2018; 13(9):e0203559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lee M, Suh H, Kim Met al. . Comparison of the anesthetic effects of 2,2,2-tribromoethanol on ICR mice derived from three different sources. Lab Anim Res 2018; 34(4):270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tsukamoto Y, Yamada N, Miyoshi Ket al. . Anesthetic effect of a mixture of alfaxalone, medetomidine, and butorphanol for inducing surgical anesthesia in ICR, BALB/c, and C57BL/6 mouse strains. J Vet Med Sci 2019; 81(6):937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zuurbier CJ, Koeman A, Houten SMet al. . Optimizing anesthetic regimen for surgery in mice through minimization of hemodynamic, metabolic, and inflammatory perturbations. Exp Biol Med (Maywood) 2014; 239(6):737–746. [DOI] [PubMed] [Google Scholar]
- 65. Kehl F, Krolikowski J, Mraovic Bet al. . Kersten J. Is isoflurane-induced preconditioning dose related? Anesthesiology 2002; 96(3):675–680. [DOI] [PubMed] [Google Scholar]
- 66. LaTourette P II, David E, Pacharinsak Cet al. . Effects of standard and sustained-release buprenorphine on the minimum alveolar concentration of Isolurane in C57BL/6 mice. JALAAS. 2020; 59(3):298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mazze RI, Rice SA, Baden JM. Halothane, isoflurane, and enflurane MAC in pregnant and nonpregnant female and male mice and rats. Anesthesiology 1985; 62(3):339–341. [DOI] [PubMed] [Google Scholar]
- 68. Sonner J, Gong D, Li Jet al. . Mouse strain modestly influences minimum alveolar anesthetic concentration and convulsivity of inhaled compounds. Anesth Analg 1999; 89(4):1030–1034. [DOI] [PubMed] [Google Scholar]
- 69. Tsukamoto A, Iimuro M, Sato Ret al. . Effect of midazolam and butorphanol premedication on inhalant isoflurane anesthesia in mice. Exp Anim 2015; 64(2):139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhou C, Liang P, Liu Jet al. . HCN1 channels contribute to the effects of amnesia and hypnosis but not immobility of volatile anesthetics. Anesth Analg 2015; 121(3):661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Roelofs S, Manjeri GR, Willems PHet al. . Isoflurane anesthetic hypersensitivity and progressive respiratory depression in a mouse model with isolated mitochondrial complex I deficiency. J Anesth 2014; 28(6):807–814. [DOI] [PubMed] [Google Scholar]
- 72. Cesarovic N, Nicholls F, Rettich Aet al. . Isoflurane and sevoflurane provide equally effective anaesthesia in laboratory mice. Lab Anim 2010; 44(4):329–336. [DOI] [PubMed] [Google Scholar]
- 73. Brown E, Pavone K, Naranjo M. Multimodal general anesthesia: theory and practice. Anesth Analg 2018; 127(5):1246–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cesarovic N, Jirkof P, Rettich Aet al. . Combining sevoflurane anesthesia with fentanyl-midazolam or s-ketamine in laboratory mice. JALAAS. 2012; 51(2):209–218. [PMC free article] [PubMed] [Google Scholar]
- 75. Arras M, Autenried P, Rettich Aet al. . Optimization of intraperitoneal injection anesthesia in mice: drugs, dosages, adverse effects, and anesthesia depth. Comp Med 2001; 51(5):443–456. [PubMed] [Google Scholar]
- 76. Gergye C, Zhao Y, Moore Ret al. . A comparison of ketamine or etomidate combined with xylazine for intraperitoneal anesthesia in four mouse strains. JALAAS 2020; 59(5):519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jaber S, Hankenson F, Heng Ket al. . Dose regimens, variability, and complications associated with using repeat-bolus dosing to extend a surgical plane of anesthesia in laboratory mice. JALAAS. 2014; 53(5):684–691. [PMC free article] [PubMed] [Google Scholar]
- 78. Levin-Arama M, Abraham L, Waner Tet al. . Subcutaneous compared with intraperitoneal ketamine-xylazine for anesthesia of mice. JALAAS. 2016; 55(6):794–800. [PMC free article] [PubMed] [Google Scholar]
- 79. Peltoniemi MA, Hagelberg NM, Olkkola KTet al. . Ketamine: a review of clinical pharmacokinetics and pharmacodynamics in anesthesia and pain therapy. Clin Pharmacokinet 2016; 55(9):1059–1077. [DOI] [PubMed] [Google Scholar]
- 80. Huss M, Felt S, Pacharinsak C. Influence of pain and analgesia on orthopedic and wound-healing models in rats and mice. Comp Med. 2019; 69(6):535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Maxwell CR, Ehrlichman RS, Liang Yet al. . Ketamine produces lasting disruptions in encoding of sensory stimuli. J Pharmacol Exp Ther 2006; 316(1):315–324. [DOI] [PubMed] [Google Scholar]
- 82. Richter W. Estimation of vasodilator drug effects in mice by measurements of paw skin temperature. Acta Pharmacol Toxicol (Copenh) 1964; 21:91–104. [DOI] [PubMed] [Google Scholar]
- 83. Plumb D. Plumb’s Veterinary Drug Handbook. Blackwell Pub: Electronic-App; 2019. (Last accessed: 24 Apr. 2021). https://www.plumbsveterinarydrugs.com/#!/monograph/InADFktnHk. [Google Scholar]
- 84. Kawai S, Takagi Y, Kaneko Set al. . Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Exp Anim 2011; 60(5):481–487. [DOI] [PubMed] [Google Scholar]
- 85. Tsukamoto A, Serizawa K, Sato Ret al. . Vital signs monitoring during injectable and inhalant anesthesia in mice. Exp Anim 2015; 64(1):57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bourke D, Malit L, Smith T. Respiratory interactions of ketamine and morphine. Anesthesiology 1987; 66(2):153–156. [DOI] [PubMed] [Google Scholar]
- 87. Jaspar N, Mazzarelli M, Tessier Cet al. . Effect of ketamine on control of breathing in cats. J Appl Physiol Respir Environ Exerc Physiol 1983; 55(3):851–859. [DOI] [PubMed] [Google Scholar]
- 88. Sarton E, Teppema LJ, Olievier Cet al. . The involvement of the mu-opioid receptor in ketamine-induced respiratory depression and antinociception. Anesth Analg 2001; 93(6):1495–1500table of contents. [DOI] [PubMed] [Google Scholar]
- 89. Teppema LJ, Baby S. Anesthetics and control of breathing. Respir Physiol Neurobiol 2011; 177(2):80–92. [DOI] [PubMed] [Google Scholar]
- 90. Rankin DC. Sedatives and tranquilizers. In: Grimm KA, Robertson SA, Lamont LA, Tranquilli WJ, Greene SA, eds. Veterinary Anesthesia and Analgesia: The Fifth Edition of Lumb and Jones. 5th ed. Ames, IA: John Wiley & Sons; 2015. Chapter 10. p. 196–206. [Google Scholar]
- 91. Dholakia U, Clark-Price S, Keating Set al. . Anesthetic effects and body weight changes associated with ketamine-xylazine-lidocaine administered to CD-1 mice. PLoS One 2017; 12(9):e0184911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Olson ME, Renchko P. Azaperone and azaperone-ketamine as a neuroleptic sedative and anesthetic in rats and mice. Lab Anim Sci 1988; 38(3):299–304. [PubMed] [Google Scholar]
- 93. Cruz J, Loste J, Burzaco O. Observations on the use of medetomidine/ketamine and its reversal with atipamezole for chemical restraint in the mouse. Lab Anim 1998; 32(1):18–22. [DOI] [PubMed] [Google Scholar]
- 94. Erickson R, Terzi M, Jaber Set al. . Intraperitoneal continuous-rate infusion for the maintenance of anesthesia in laboratory mice (Mus musculus). JALAAS. 2016; 55(5):548–557. [PMC free article] [PubMed] [Google Scholar]
- 95. Schuetze S, Manig A, Ribes Set al. . Aged mice show an increased mortality after anesthesia with a standard dose of ketamine/xylazine. Lab Anim Res. 2019; 35:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. File S, Simmonds M. Myoclonic seizures in the mouse induced by alphaxalone and related steroid anaesthetics. J Pharm Pharmacol 1988; 40(1):57–59. [DOI] [PubMed] [Google Scholar]
- 97. Chiu K, Robson S, Devi Jet al. . The cardiopulmonary effects and quality of anesthesia after induction with alfaxalone in 2-hydroxypropyl-beta-cyclodextrin in dogs and cats: a systematic review. J Vet Pharmacol Therap 2016; 39(6):525–538. [DOI] [PubMed] [Google Scholar]
- 98. Erickson R, Blevins C, Souza Dyer Cet al. . Alfaxalone-xylazine anesthesia in laboratory mice (Mus musculus). JALAAS. 2019; 58(1):30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Higuchi S, Yamada R, Hashimoto Aet al. . Evaluation of a combination of alfaxalone with medetomidine and butorphanol for inducing surgical anesthesia in laboratory mice. Jpn J Vet Res 2016; 64(2):131–139. [PubMed] [Google Scholar]
- 100. Siriarchavatana P, Ayers JD, Kendall LV. Anesthetic activity of Alfaxalone compared with ketamine in mice. J Am Assoc Lab Anim Sci 2016; 55(4):426–430. [PMC free article] [PubMed] [Google Scholar]
- 101. Kuusela E, Raekallio M, Vaisanen Met al. . Comparison of medetomidine and dexmedetomidine as premedicants in dogs undergoing propofol-isoflurane anesthesia. Am J Vet Res 2001; 62(7):1073–1080. [DOI] [PubMed] [Google Scholar]
- 102. Hablitz L, Vinitsky H, Sun Qet al. . Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci Adv 2019; 5(2):eaav5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wang Z, Schuler B, Vogel Oet al. . What is the optimal anesthetic protocol for measurements of cerebral autoregulation in spontaneously breathing mice? Exp Brain Res 2010; 207(3–4):249–258. [DOI] [PubMed] [Google Scholar]
- 104. Field KJ, Lang CM. Hazards of urethane (ethyl carbamate): a review of the literature. Lab Anim 1988; 22(3):255–262. [DOI] [PubMed] [Google Scholar]
- 105. Kirihara Y, Takechi M, Kurosaki Ket al. . Anesthetic effects of a mixture of medetomidine, midazolam and butorphanol in two strains of mice. Exp Anim 2013; 62(3):173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kirihara Y, Takechi M, Kurosaki Ket al. . Anesthetic effects of a three-drugs mixture--comparison of administrative routes and antagonistic effects of atipamezole in mice. Exp Anim 2015; 64(1):39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Meyer RE, Fish RE. A review of tribromoethanol anesthesia for production of genetically engineered mice and rats. Lab Anim (NY) 2005; 34(10):47–52. [DOI] [PubMed] [Google Scholar]
- 108. Lieggi CC, Artwohl JE, Leszczynski JKet al. . Efficacy and safety of stored and newly prepared tribromoethanol in ICR mice. Contemp Top Lab Anim Sci 2005; 44(1):17–22. [PubMed] [Google Scholar]
- 109. Zeller W, Meier G, Burki Ket al. . Adverse effects of tribromoethanol as used in the production of transgenic mice. Lab Anim 1998; 32(4):407–413. [DOI] [PubMed] [Google Scholar]
- 110. Hill W, Tubbs J, Carter Cet al. . Repeated administration of tribromoethanol in C57BL/6NHsd mice. JALAAS. 2013; 52(2):176–179. [PMC free article] [PubMed] [Google Scholar]
- 111. Maejima Y, Yokota S, O'Hashi Ret al. . The effect of avertin anesthesia and a mixture of three types of anesthetic agents on food intake and body weight in high fat-induced obese male and female mice. Exp Anim 2019; 68(1):57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Oh SS, Hayes JM, Sims-Robinson Cet al. . The effects of anesthesia on measures of nerve conduction velocity in male C57Bl6/J mice. Neurosci Lett 2010; 483(2):127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Cho Y, Lee Y, Lee Jet al. . Kinetics of proinflammatory cytokines after intraperitoneal injection of tribromoethanol and a tribromoethanol/xylazine combination in ICR mice. Lab Anim Res. 2011; 27(3):197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Brosnan RJ, Steffey EP, Escobar Aet al. . Anesthetic induction with guaifenesin and propofol in adult horses. Am J Vet Res 2011; 72(12):1569–1575. [DOI] [PubMed] [Google Scholar]
- 115. Mees L, Fidler J, Kreuzer Met al. . Faster emergence behavior from ketamine/xylazine anesthesia with atipamezole versus yohimbine. PLoS One 2018; 13(10):e0199087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Janssen CF, Maiello P, Wright MJ Jret al. . Comparison of atipamezole with yohimbine for antagonism of xylazine in mice anesthetized with ketamine and xylazine. JALAAS. 2017; 56(2):142–147. [PMC free article] [PubMed] [Google Scholar]
- 117. Brodbelt D. Perioperative mortality in small animal anaesthesia. Vet Journal 2008; 182(2):152–161. [DOI] [PubMed] [Google Scholar]
- 118. Izer JM, Whitcomb TL, Wilson RP. Atipamezole reverses ketamine-dexmedetomidine anesthesia without altering the antinociceptive effects of butorphanol and buprenorphine in female C57BL/6J mice. JALAAS. 2014; 53(6):675–683. [PMC free article] [PubMed] [Google Scholar]
- 119. Fleischmann T, Jirkof P, Henke Jet al. . Injection anaesthesia with fentanyl-midazolam-medetomidine in adult female mice: importance of antagonization and perioperative care. Lab Anim 2016; 50(4):264–274. [DOI] [PubMed] [Google Scholar]
- 120. Papich M. Saunders Handbook of Veterinary Drugs. Elsevier Saunders: St. Louis, MO; 2007. [Google Scholar]
- 121. Johnston NA, Bosgraaf C, Cox Let al. . Strategies for refinement of abdominal device implantation in mice: s train, carboxymethylcellulose, thermal support, and atipamezole. JALAAS. 2007; 46(2):46–53. [PubMed] [Google Scholar]
- 122. Thal SC, Plesnila N. Non-invasive intraoperative monitoring of blood pressure and arterial pCO2 during surgical anesthesia in mice. J Neurosci Methods 2007; 159(2):261–267. [DOI] [PubMed] [Google Scholar]
- 123. Marian A, Bayman E, Gillett Aet al. . The influence of the type and design of the anesthesia record on ASA physical status scores in surgical patients: paper records vs. electronic anesthesia records. BMC Med Inform Decis Mak 2016; 2016(16):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Portier K, Ida K. The ASA physical status classification: what is the evidence for recommending its use in veterinary anesthesia?—A systematic review. Front Vet Sci 2018; 5:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Obernier JA, Baldwin RL. Establishing an appropriate period of acclimatization following transportation of laboratory animals. ILAR J 2006; 47(4):364–369. [DOI] [PubMed] [Google Scholar]
- 126. Gargiulo S, Greco A, Gramanzini Met al. . Mice anesthesia, analgesia, and care, part I: anesthetic considerations in preclinical research. ILAR J 2012; 53(1):E55–E69. [DOI] [PubMed] [Google Scholar]
- 127. Rao S, Verkman AS. Analysis of organ physiology in transgenic mice. Am J Physiol Cell Physiol 2000; 279(1):C1–C18. [DOI] [PubMed] [Google Scholar]
- 128. Horn CC, Kimball BA, Wang Het al. . Why can't rodents vomit? A comparative behavioral, anatomical, and physiological study. PLoS One 2013; 8(4):e60537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Foltz C, Ullman-Cullere M. Guidelines for assessing the health and condition of mice. Lab Animal 1999; 28(4):28–32. [PubMed] [Google Scholar]
- 130. Hildebrandt I, Su H, Weber W. Anesthesia and other considerations for in vivo imaging of small animals. ILAR J 2008; 49(1):17–26. [DOI] [PubMed] [Google Scholar]
- 131. McNally JB, Kirkpatrick ND, Hariri LPet al. . Task-based imaging of colon cancer in the Apc(min/+) mouse model. Appl Opt 2006; 45(13):3049–3062. [DOI] [PubMed] [Google Scholar]
- 132. Gouveia K, Hurst JL. Improving the practicality of using non-aversive handling methods to reduce background stress and anxiety in laboratory mice. Sci Rep 2019; 9(1):20305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Burkholder T, Foltz C, Karlsson Eet al. . Health evaluation of experimental laboratory mice. Curr Protoc Mouse Biol. 2012; 2:145–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Bagis H, Odaman Mercan H, Dinnyes A. Exposure to warmer postoperative temperatures reduces hypothermia caused by anaesthesia and significantly increases the implantation rate of transferred embryos in the mouse. Lab Anim 2004; 38(1):50–54. [DOI] [PubMed] [Google Scholar]
- 135. Kober F, Iltis I, Cozzone PJet al. . Myocardial blood flow mapping in mice using high-resolution spin labeling magnetic resonance imaging: influence of ketamine/xylazine and isoflurane anesthesia. Magn Reson Med 2005; 53(3):601–606. [DOI] [PubMed] [Google Scholar]
- 136. Noel-Morgan J, Muir WW. Anesthesia-associated relative hypovolemia: mechanisms, monitoring, and treatment considerations. Front Vet Sci 2018; 5:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Rufiange M, Leung VS, Simpson Ket al. . Prewarming followed by active warming is superior to passive warming in preventing hypothermia for short procedures in adult rats (Rattus norvegicus) under isoflurane Anesthesia. JALAAS. 2020; 59(4):377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Guyton AC & Hall JE. Textbook of Medical Physiology. Philadelphia, PA: Elsevier-Saunders; 2006. [Google Scholar]
- 139. Skorupski AM, Zhang J, Ferguson Det al. . Quantification of induced hypothermia from aseptic scrub applications during rodent surgery preparation. JALAAS. 2017; 56(5):562–569. [PMC free article] [PubMed] [Google Scholar]
- 140. Taylor DK. Study of two devices used to maintain normothermia in rats and mice during general anesthesia. JALAAS. 2007; 46(5):37–41. [PubMed] [Google Scholar]
- 141. Caro AC, Hankenson FC, Marx JO. Comparison of thermoregulatory devices used during anesthesia of C57BL/6 mice and correlations between body temperature and physiologic parameters. JALAAS. 2013; 52(5):577–583. [PMC free article] [PubMed] [Google Scholar]
- 142. Rembert MS, Smith JA, Hosgood G. A comparison of a forced-air warming system to traditional thermal support for rodent microenvironments. Lab Anim 2004; 38(1):55–63. [DOI] [PubMed] [Google Scholar]
- 143. Waynforth H, Flecknell P.. Experimental and Surgical Technique in the Rat . 2nd ed: Cambridge, MA: Academic Press; 1992. [Google Scholar]
- 144. Nuntnarumit P, Swatesutipun B, Udomsubpayakul Uet al. . A randomized controlled trial of plastic drape for prevention of hypothermia during umbilical catheterization. Am J Perinatol 2013; 30(10):839–842. [DOI] [PubMed] [Google Scholar]
- 145. Young VL, Watson ME. Prevention of perioperative hypothermia in plastic surgery. Aesthet Surg J 2006; 26(5):551–571. [DOI] [PubMed] [Google Scholar]
- 146. Blevins C, Celeste N, Marx J. Effects of oxygen supplementation on injectable and inhalant anesthesia in C57BL/6 mice. JALAAS 2021; 60(3). (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Emmer KM, Celeste NA, Bidot WAet al. . Evaluation of the sterility of Press'n seal cling film for use in rodent surgery. JALAAS. 2019; 58(2):235–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Adams S, Pacharinsak C. Mouse anesthesia and analgesia. Curr Protoc Mouse Biol 2015; 5(1):51–63. [DOI] [PubMed] [Google Scholar]
- 149. Danneman P, Suckow, M, Brayton, C.. The Laboratory Mouse . 2nd ed. Boca Raton, FL: CRC Press; 2013. [Google Scholar]
- 150. Ewald AJ, Werb Z, Egeblad M. Monitoring of vital signs for long-term survival of mice under anesthesia. Cold Spring Harb Protoc 2011; 2011(2): pdb.prot5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Matsuda Y, Ohsaka K, Yamamoto Het al. . Comparison of newly developed inhalation anesthesia system and intraperitoneal anesthesia on the hemodynamic state in mice. Biol Pharm Bull 2007; 30(9):1716–1720. [DOI] [PubMed] [Google Scholar]
- 152. Sessler Daniel I, McGuire J, Moayeri Aet al. . Isoflurane-induced vasodilation minimally increases cutaneous heat loss. Anesthesiology 1991; 74(2):226–232. [DOI] [PubMed] [Google Scholar]
- 153. Støen R, Sessler Daniel I. The thermoregulatory threshold is inversely proportional to isoflurane concentration. Anesthesiology 1990; 72(5):822–827. [DOI] [PubMed] [Google Scholar]
- 154. Rufiange M, Leung VSY, Simpson Ket al. . Pre-warming before general anesthesia with isoflurane delays the onset of hypothermia in rats. PLoS One 2020; 15(3):e0219722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Rose N, Kwong GP. Pang DS. A clinical audit cycle of post-operative hypothermia in dogs. J Small Anim Pract 2016; 57(9):447–452. [DOI] [PubMed] [Google Scholar]
- 156. Redondo JI, Suesta P, Gil Let al. . Retrospective study of the prevalence of postanaesthetic hypothermia in cats. Vet Rec. 2012; 170(8):206. [DOI] [PubMed] [Google Scholar]
- 157. Redondo JI, Suesta P, Serra Iet al. . Retrospective study of the prevalence of postanaesthetic hypothermia in dogs. Vet Rec 2012; 171(15):374. [DOI] [PubMed] [Google Scholar]
- 158. Pacharinsak C, Smith J. Handbook of Laboratory Animal Anesthesia and Pain Management: Rodents. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2017. [Google Scholar]
- 159. Matsukawa T, Sessler DI, Sessler AMet al. . Heat flow and distribution during induction of general anesthesia. Anesthesiology 1995; 82(3):662–673. [DOI] [PubMed] [Google Scholar]
- 160. Hershey J and Miller S. Correlation between surface temperature and core body temperature and the effect of exogenous heat during anesthesia in rats. Lab Anim Sci Prof .2016(March):40–43.
- 161. Newsom DM, Bolgos GL, Colby Let al. . Comparison of body surface temperature measurement and conventional methods for measuring temperature in the mouse. Contemp Top Lab Anim Sci 2004; 43(5):13–18. [PubMed] [Google Scholar]
- 162. Kawakami Au - Y, Sielski Au - R, Kawakami Au -Mouse body T.. temperature measurement using infrared thermometer during passive systemic anaphylaxis and food allergy evaluation. JoVE .2018(139):e58391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Whary MT, Baumgarth N, Fox JG, Barthold SW. Biology and diseases of mice. In: Fox JG, Anderson LC, Otto GM, Pritchett-Corning KR, Whary MT, eds. Laboratory Animal Medicine (Third Edition).Boston, MA: Academic Press; 2015:43–149. [Google Scholar]
- 164. Ho D, Zhao X, Gao Set al. . Heart rate and electrocardiography monitoring in mice. Curr Protoc Mouse Biol 2011; 1:123–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Flegal MC, Kuhlman SM. Anesthesia monitoring equipment for laboratory animals. Lab Animal. 2004; 33(7):31–36. [DOI] [PubMed] [Google Scholar]
- 166. Wagner AE, Brodbelt DC. Arterial blood pressure monitoring in anesthetized animals. JAVMA 1997; 210(9):1279–1285. [PubMed] [Google Scholar]
- 167. Tremoleda JL, Kerton A, Gsell W. Anaesthesia and physiological monitoring during in vivo imaging of laboratory rodents: considerations on experimental outcomes and animal welfare. EJNMMI Res 2012; 2(1):44–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Krege JH, Hodgin JB, Hagaman JR. Smithies O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension 1995; 25(5):1111–1115. [DOI] [PubMed] [Google Scholar]
- 169. Brodbelt D, Pfeiffer D, Young Let al. . Results of the confidential enquiry into perioperative small animal fatalities regarding risk factors for anesthetic-related death in dogs. JAVMA. 2008; 233(7):1096–1104. [DOI] [PubMed] [Google Scholar]
- 170. Flecknell P. Anesthesia and Analgesia in Laboratory Animals . 1st ed. San Diego, CA: Academic Press. 1997. [Google Scholar]
- 171. Young L, Brearly J, DL Ret al. . Medetomidine as a premedicant in dogs and its reversal by atipamezole. J Sm Anim Pract 1990; 31:554–559. [Google Scholar]
- 172. National Research Council NRCU . Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research.Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- 173. Jang Y, Park Y, Yun Cet al. . The vest-collar as a rodent collar to prevent licking and scratching during experiments. Lab Anim 2016; 50(4):296–304. [DOI] [PubMed] [Google Scholar]
- 174. Langford D, Bailey A, Chanda Met al. . Coding of facial expressions of pain in the laboratory mouse. Nat Methods 2010; 7(6):447–449. [DOI] [PubMed] [Google Scholar]
- 175. Miller A, Leach M. The mouse grimace scale: a clinically useful tool? PLoS One 2015; 10(9):e0136000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Jirkof P, Fleischmann T, Cesarovic Net al. . Assessment of postsurgical distress and pain in laboratory mice by nest complexity scoring. Lab Anim 2013; 47(3):153–161. [DOI] [PubMed] [Google Scholar]
- 177. Gaskill B, Karas A, Garner Jet al. . Nest building as an indicator of health and welfare in laboratory mice. J Vis Exp 2013; 82:51012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Rock M, Karas A, Gartrell Rodriguez Ket al. . The time-to-integrate-to-nest test as an Indicator of wellbeing in laboratory mice. JAALAS 2014; 53(1):24–28. [PMC free article] [PubMed] [Google Scholar]
- 179. Gallo M, Karas A, Pritchett-Corning Ket al. . Tell-tale TINT: does the time to incorporate into nest test evaluate postsurgical pain or welfare in mice? JALAAS. 2020; 59(1):37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Jirkof P, Cesarovic N, Rettich Aet al. . Burrowing behavior as an indicator of post-laparotomy pain in mice. Front Behav Neurosci 2010; 4:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Deacon R. Burrowing in rodents: a sensitive method for detecting behavioral dysfunction. Nat Protoc 2006b; 1:118–121. [DOI] [PubMed] [Google Scholar]
- 182. Foley P, Kendall L, Turner P. Clinical management of pain in rodents. Comp Med. 2019; 69(6):468–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Hogan Q. Animal pain models. Reg Anesth Pain Med 2002; 27(4):385–401. [DOI] [PubMed] [Google Scholar]
- 184. Gregory N, Harris A, Robinson Cet al. . An overview of animal models of pain: disease models and outcome measures. J Pain 2013; 14(11):1255–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Woolf C, Chong M. Preemptive analgesia - treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg 1993; 77:362–279. [DOI] [PubMed] [Google Scholar]
- 186. Gottschalk A, Smith D. New concepts in acute pain therapy: preemptive analgesia. Am Fam Physician 2001; 63(10):1979–1984. [PubMed] [Google Scholar]
- 187. Brennan T. Postoperative models of nociception. ILAR J 1999; 40(3):129–136. [DOI] [PubMed] [Google Scholar]
- 188. National Research Council NRCU . Recognition and Alleviation of Pain in Laboratory Animals. Washington, DC: National Academies Press; 2009. [PubMed] [Google Scholar]
- 189. Turner P, Brabb T, Pekow Cet al. . Administration of substances to laboratory animals: routes of administration and factors to consider. JALAAS. 2011; 50(5):600–613. [PMC free article] [PubMed] [Google Scholar]
- 190. Seymour T, Adams S, Felt Set al. . Postoperative analgesia due to sustained-release buprenorphine, sustained-release meloxicam, and carprofen gel in a model of incisional pain in rats (Rattus norvegicus). JALAAS. 2016; 55(3):300–305. [PMC free article] [PubMed] [Google Scholar]
- 191. Leach M, Forrester A, Flecknell P. Influence of preferred foodstuffs on the antinociceptive effects of orally administered buprenorphine in laboratory rats. Lab Anim 2010; 44:54–58. [DOI] [PubMed] [Google Scholar]
- 192. Zude B, Jampachairsri K, Pacharinsak C. Use of flavored tablets of gabapentin and carprofen to attenuate postoperative hypersensitivity in an incisional pain model in rats (Rattus norvegicus). JALAAS. 2020; 59(2):163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Goldkuhl R, Hau J, Abelson K. Effects of voluntarily-ingested buprenorphine on plasma corticosterone levels, body weight, water intake, and behaviour in permanently catherterised rats. In Vivo 2010; 24:131–135. [PubMed] [Google Scholar]
- 194. Bagul A, Taha R, Metcalfe Met al. . Pre-incision infiltration of local anesthetic reduces postoperative pain with no effects on bruising and wound cosmesis after thyroid surgery. Thyroid 2005; 15(11):1245–1248. [DOI] [PubMed] [Google Scholar]
- 195. Ejlersen E, Andersen H, Eliasen Ket al. . comparison between preincisional and postincisional lidocaine infiltration and postoperative Pain. Anesth Analg 1992; 74:495–498. [DOI] [PubMed] [Google Scholar]
- 196. Grape S, Tramér M. Do we need preemptive analgesia for the treatment of postoperative pain? Best Pract Res Clin Anaesth 2007; 21(1):51–63. [DOI] [PubMed] [Google Scholar]
- 197. Filos K, Vagianos C. Pre-emptive analgesia: how important is it in clinical reality? Eur Surg Res 1999; 31:122–132. [DOI] [PubMed] [Google Scholar]
- 198. Watanabe R, Monteiro B, Evangelista Met al. . The analgesic effects of buprenorphine (Vetergesic or Simbadol) in combination with carprofen in dogs undergoing ovariohysterectomy: a randomized, blinded, clinical trial. BMC Vet Res 2018; 14(1):304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Brodbelt D, Taylor P, Stanway G. A comparison of preoperative morphine and buprenorphine for postoperative analgesia for arthrotomy in dogs. J Vet Pharmacol Therap 1997; 20:284–289. [DOI] [PubMed] [Google Scholar]
- 200. Gassel A, Tobias K, Egger Cet al. . Comparison of oral and subcutaneous administration of buprenorphine and meloxicam for preemptive analgesia in cats undergoing ovariohysterectomy. JAVMA. 2005; 227(12):1937–1944. [DOI] [PubMed] [Google Scholar]
- 201. Lykkegaard K, Lauritzen B, Tessem Let al. . Local anesthetics attenuates spinal nociception and HPA-axis activation during experimental laparotomy in pigs. Res Vet Sci 2005; 79:245–251. [DOI] [PubMed] [Google Scholar]
- 202. Coderre T, Vaccarino A, Melzack R. Central nervous system plasticity in the tonic pain response to subcutaneous formalin injection. Brain Res 1990; 535:155–158. [DOI] [PubMed] [Google Scholar]
- 203. González-Darder J, Barberá J, Abellán M. Effects of prior anaesthesia on autotomy following sciatic transection in rats. Pain 1986; 24:87–91. [DOI] [PubMed] [Google Scholar]
- 204. Page G, McDonald J, Ben-Eliyahu S. Pre-operative versus postoperative administration of morphine: impact on the neuroendocrine, behavioural, and metastatic-enhancing effects of surgery. Br J Anaesth 1998; 81:216–223. [DOI] [PubMed] [Google Scholar]
- 205. Abram S, Yaksh T. Morphine, but not inhalational anesthesia, block post-injury facilitation: the role of preemptive suppression of afferent transmission. Anesthesiology 1993; 78:713–721. [DOI] [PubMed] [Google Scholar]
- 206. Jirkof P, Tourvieille A, Cinelli Pet al. . Buprenorphine for pain relief in mice: repeated injections vs sustained-release depot formulation. Lab Anim 2015; 49(3):177–187. [DOI] [PubMed] [Google Scholar]
- 207. Roughan J, Flecknell P. Buprenorphine: a reappraisal of its antinociceptive effects and therapeutic use in alleviating post-operative pain in animals. Lab Anim 2002; 36:322–343. [DOI] [PubMed] [Google Scholar]
- 208. Reichert J, Daughters R, Rivard Ret al. . Peripheral and preemptive opioid antinociception in a mouse visceral pain model. Pain 2001; 89(2–3):221–227. [DOI] [PubMed] [Google Scholar]
- 209. O’Hanlon J, Lowry D. Improved postoperative analgesia with preoperative piroxicam. Can J Anaesth 1996; 43(2):102–105. [DOI] [PubMed] [Google Scholar]
- 210. Slingsby L. Multimodal analgesia for postoperative pain relief. In Practice 2008; 30:208–212. [Google Scholar]
- 211. Corletto F. Multimodal and balanced analgesia. Vet Res Comm 2007; 31:59–63. [DOI] [PubMed] [Google Scholar]
- 212. Luna S, Basílio A, Steagall Pet al. . Evaluation of adverse effects of long-term oral administration of carprofen, etodolac, flunixin meglumine, ketoprofen, and meloxicam in dogs. Am J Vet Res 2007; 68(3):258–264. [DOI] [PubMed] [Google Scholar]
- 213. Forsyth S, Guilford W, Haslett Set al. . Endoscopy of the gastroduodenal mucosa after carprofen, meloxicam, and ketoprofen administration in dogs. J Sm Anim Pract. 1998; 39:421–424. [DOI] [PubMed] [Google Scholar]
- 214. Forsyth S, Guilford W, Pfeiffer D. Effect of NSAID administration on creatinine clearance in healthy dogs undergoing anaesthesia and surgery. J Sm Anim Pract. 2000; 41:547–550. [DOI] [PubMed] [Google Scholar]
- 215. Liles J, Flecknell P. The use of non-steroidal anti-inflammatory drugs for the relief of pain in laboratory rodents and rabbits. Lab Anim 1992; 26:241–255. [DOI] [PubMed] [Google Scholar]
- 216. Jirkof P, Durst M, Klopfleisch Ret al. . Administration of tramadol or buprenorphine via the drinking water for post-operative analgesia in a mouse-osteotomy model. Sci Rep 2019; 9:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Healy J, Tonkin J, Kamarec Set al. . Evaluation of an improved sustained-release buprenorphine formulation for use in mice. AJVR 2014; 75(7):619–625. [DOI] [PubMed] [Google Scholar]
- 218. Carbone E, Lindstrom K, Diep Set al. . Duration of action of sustained-release buprenorphine in 2 strains of mice. JALAAS. 2012; 51(6):815–819. [PMC free article] [PubMed] [Google Scholar]
- 219. Lutfy K, Cowan A. Buprenorphine: a unique drug with complex pharmacology. Curr Neuropharmacol 2004; 2(4):395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Gades N, Danneman P, Wixson Set al. . The magnitude and duration of the analgesic effect of morphine, butorphanol, and buprenorphine in rats and mice. Contemp Top Lab Anim Sci 2000; 39(2):8–13. [PubMed] [Google Scholar]
- 221. Christoph T, Kögel B, Schiene Ket al. . Broad analgesic profile of buprenorphine in rodent models of acute and chronic pain. Eur J Pharmacol 2005; 507:87–98. [DOI] [PubMed] [Google Scholar]
- 222. DeMarco G, Nunamaker EA. Review of the effects of pain and analgesia on immune system function and inflammation: relevance for preclinical studies. Comp Med. 2019; 69(6):520–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Wang J, Goffer Y, Xu Det al. . A single sub-anesthetic dose of ketamine relieves depression-like behaviors induced by neuropathic pain in rats. Anesthesiology 2012; 115(4):812–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Huang C, Long H, Shi Yet al. . Ketamine enhances the efficacy to and delays the development of tolerance to electroacupuncture-induced antinociception in rats. Neuro Let 2005; 375(2):138–142. [DOI] [PubMed] [Google Scholar]
- 225. Becker W, Mama K, Rao Set al. . Prevalence of dysphoria after fentanyl in dogs undergoing stifle surgery. Vet Surg 2015; 42(3):302–307. [DOI] [PubMed] [Google Scholar]
- 226. Guedes A, Meadows J, Pypendop Bet al. . Evaluation of tramadol for treatment of osteoarthritis in geriatric cats. JAVMA. 2018; 252(5):565–571. [DOI] [PubMed] [Google Scholar]
- 227. Malamed S. Pharmacology. In: Malamed S, ed. Sedation: A Guide to Patient Management .6th ed. St. Louis, MO: Elsevier; 2017. [Google Scholar]
- 228. Reed S. Nonsteroidal anti-inflammatory drug-induced duodenal ulceration and perforation in a mature Rottweiler. Can Vet J 2002; 43(12):971–972. [PMC free article] [PubMed] [Google Scholar]
- 229. Peterson N, Nunamaker E, Turner P. To treat or not to treat: the effects of pain on experimental parameters. Comp Med. 2017; 67(6):469–482. [PMC free article] [PubMed] [Google Scholar]
- 230. Smith JCA. review of strain and sex differences in response to pain and analgesia in mice. Comp Med. 2019; 69(6):490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Christian L, Graham J, Padgett Det al. . Stress and wound healing. Neuroimmunomodulation 2007; 13:337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Carr D, Goudas L. Acute pain. Lancet 1999; 353:2051–2058. [DOI] [PubMed] [Google Scholar]
- 233. Padgett D, Glaser R. How stress influences the immune response. Trends Immuno 2003; 24(8):444–448. [DOI] [PubMed] [Google Scholar]
- 234. Hayes J, Flecknell P. A comparison of pre-and post-surgical administration of bupivacaine or buprenorphine following laparotomy in the rat. Lab Anim 1998; 33:16–23. [DOI] [PubMed] [Google Scholar]
- 235. Flecknell P, Liles J. The effects of surgical procedures, halothane anaesthesia and nalbuphine on the locomotor activity and food and water consumption in rats. Lab Anim 1991; 25:50–60. [DOI] [PubMed] [Google Scholar]
- 236. Palada V, Gilron I, Canlon Bet al. . The circadian clock at the intercept of sleep and pain. Pain 2019; 161(5):894–900. [DOI] [PubMed] [Google Scholar]
- 237. Curtin L, Grakowsky J, Suarez Met al. . Evaluation of buprenorphine in a postoperative pain model in rats. Comp Med 2009; 59(1):60–71. [PMC free article] [PubMed] [Google Scholar]
- 238. Kohn D, Martin T, Foley Pet al. . Public statement: guidelines for the assessment and management of pain in rodents and rabbits. JAALAS. 2007; 46(2):97–108. [PubMed] [Google Scholar]
- 239. Pathan H, Williams J. Basic opioid pharmacology: an update. Br J Pain 2012; 6(1):11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Benarroch E. Endogenous opioid systems: current concepts and clinical correlations. Neurology 2012; 79(8):807–814. [DOI] [PubMed] [Google Scholar]
- 241. Peciña M, Karp J, Mathew Set al. . Endogenous opioid system dysregulation in depression: implications for new therapeutic approaches. Molec Psych 2018; 24:576–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Mathiesen O, Wetterslev J, Kontinen Vet al. . Adverse effects of perioperative paracetamol, NSAIDs, glucocorticoids, gabapentinoids and their combinations: a topical review. Acta Anaesth Scand 2014; 58(10):1182–1198. [DOI] [PubMed] [Google Scholar]
- 243. Kang S, Jampachairsri K, Seymour Tet al. . Use of liposomal bupivacaine for postoperative analgesia in an incisional pain model in rats (Rattus norvegicus). JALAAS. 2017; 56(1):63–68. [PMC free article] [PubMed] [Google Scholar]
- 244. Grant G, Vermeulen K, Langerman Let al. . Prolonged analgesia with liposomal bupivacaine in a mouse model. Reg Anesth Pain Med 1994; 19(4):264–269. [PubMed] [Google Scholar]
- 245. Markova L, Umek N, Horvat Set al. . Neurotoxicity of bupivacaine and liposome bupivacaine after sciatic nerve block in healthy and streptozotocin-induced diabetic mice. BMC Vet Res 2020; 16:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Cheng J, Chiou L. Mechanisms of the antinociceptive action of gabapentin. J Pharmacol Sci 2006; 100:471–486. [DOI] [PubMed] [Google Scholar]
- 247. Hayashida K, DeGoes S, Curry Ret al. . Gabapentin activates spinal noradrenergic activity in rats and humans and reduces hypersensitivity after surgery. Anesthesiology 2007; 106(3):557–562. [DOI] [PubMed] [Google Scholar]
- 248. McKeon G, Pacharinsak C, Long Cet al. . Analgesic effects of tramadol, tramadol-gabapentin, and buprenorphine in an incisional model of Pain in rats (Rattus norvegicus). JALAAS. 2011; 50(2):192–197. [PMC free article] [PubMed] [Google Scholar]
- 249. Takasaki I, Andoh T, Nojima Het al. . Gabapentin Antinociception in mice with acute herpetic pain induced by herpes simplex virus infection. J Pharm Exp Therap. 2001; 296(2):270–275. [PubMed] [Google Scholar]
- 250. Nishiyori M, Ueda H. Prolonged gabapentin analgesia in an experimental mouse model of fibromyalgia. Mol Pain 2008; 4:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Buscaglia M, Brandes H, Cleary J. Special report: the abuse potential of gabapentin and pregabalin. Practical pain management. https://www.practicalpainmanagement.com/treatments/pharmacological/non-opioids/special-report-abuse-potential-gabapentin-pregabalin. Date Last Accessed: 25 Apr 2021. [Google Scholar]
- 252. Tsukamoto A, Ohgoda M, Haruki Net al. . The anti-inflammatory action of maropitant in a mouse model of acute pancreatitis. J Vet Med Sci 2018; 80(3):492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Smith J. Management of anesthesia. In: Pacharinsak C, Smith J, eds. Handbook of Laboratory Animal Anesthesia and Pain Management Rodents. Boca Raton, FL: CRC Press; 2017. p. 55–78. [Google Scholar]
- 254. McKinstry-Wu AR, Wasilczuk AZ, Harrison BAet al. . Analysis of stochastic fluctuations in responsiveness is a critical step toward personalized anesthesia. Elife 2019; 8:e40143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Kest B, Wilson SG, Mogil JS. Sex differences in supraspinal morphine analgesia are dependent on genotype. J Pharm Exp Therap 1999; 289(3):1370–1375. [PubMed] [Google Scholar]
- 256. Allayee H, Andalibi A, Mehrabian M. Using inbred mouse strains to identify genes for complex diseases. Front Biosci 2006; 11:1216–1226. [DOI] [PubMed] [Google Scholar]
- 257. Bryant CD, Zhang NN, Sokoloff Get al. . Behavioral differences among C57BL/6 substrains: implications for transgenic and knockout studies. J Neurogenet 2008; 22(4):315–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Mogil JS. Pain genetics: past, present and future. Trends Genet 2012; 28(6):258–266. [DOI] [PubMed] [Google Scholar]
- 259. Liu ET, Bolcun-Filas E, Grass DSet al. . Of mice and CRISPR: the post-CRISPR future of the mouse as a model system for the human condition. EMBO Rep 2017; 18(2):187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Tuttle AH, Philip VM, Chesler EJet al. . Comparing phenotypic variation between inbred and outbred mice. Nat Methods 2018; 15(12):994–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Lariviere WR, Mogil JS. The genetics of pain and analgesia in laboratory animals. Methods Mol Biol 2010; 617:261–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Rodgers HM, Liban S, Wilson LM. Attenuated pain response of obese mice (B6.Cg-lep(Ob)) is affected by aging and leptin but not sex. Physiol Behav 2014; 123:80–85. [DOI] [PubMed] [Google Scholar]
- 263. Greenspan JD, Craft RM, LeResche Let al. . Studying sex and gender differences in pain and analgesia: a consensus report. Pain 2007; 132(Suppl 1):S26–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Mogil JS, Wilson SG, Bon Ket al. . Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain 1999; 80(1–2):67–82. [DOI] [PubMed] [Google Scholar]
- 265. Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science 1999; 284(5420):1670–1672. [DOI] [PubMed] [Google Scholar]
- 266. Franklin CL, Ericsson AC. Complex microbiota in laboratory rodents: management considerations. ILAR J 2020; 60(2):289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Mogil JS. The genetic mediation of individual differences in sensitivity to pain and its inhibition. PNAS 1999; 96(14):7744–7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Crowder CM. Mapping anesthesia genes: why and how? Anesthesiology 1998; 88(2):293–296. [DOI] [PubMed] [Google Scholar]
- 269. Wijnvoord N, Albuquerque B, Haussler Aet al. . Inter-strain differences of serotonergic inhibitory pain control in inbred mice. Mol Pain 2010; 6:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Xu H, Lu B, Zheng Bet al. . Smaller sized inhaled anesthetics have more potency on senescence-accelerated prone-8 mice compared with senescence-resistant-1 mice. J Alzheimers Dis 2014; 39(1):29–34. [DOI] [PubMed] [Google Scholar]
- 271. Moloney RD, Dinan TG, Cryan JF. Strain-dependent variations in visceral sensitivity: relationship to stress, anxiety and spinal glutamate transporter expression. Genes Brain Behav 2015; 14(4):319–329. [DOI] [PubMed] [Google Scholar]
- 272. Rosen SF, Ham B, Haichin Met al. . Increased pain sensitivity and decreased opioid analgesia in T-cell-deficient mice and implications for sex differences. Pain 2019; 160(2):358–366. [DOI] [PubMed] [Google Scholar]
- 273. Rosen S, Ham B, Mogil JS. Sex differences in neuroimmunity and pain. J Neurosci Res 2017; 95(1–2):500–508. [DOI] [PubMed] [Google Scholar]
- 274. Miaskowski C GR, and Levine JD.. Sex-related differences in analgesic responses. In: Fillingim RB, ed. Progress in Pain Research and Managment . Vol 17. Seatle, WA: IASP Press; 2000:209–230. [Google Scholar]
- 275. Da Silva JT, Seminowicz DA. Neuroimaging of pain in animal models: a review of recent literature. Pain Rep 2019; 4(4):e732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Creamer-Hente MA, Lao FK, Dragos ZPet al. . Sex- and strain-related differences in the stress response of mice to CO(2) euthanasia. JALAAS. 2018; 57(5):513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Andrews NA, Latremoliere A, Basbaum AIet al. . Ensuring transparency and minimization of methodologic bias in preclinical pain research: PPRECISE considerations. Pain 2016; 157(4):901–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Boerner KE, Chambers CT, Gahagan Jet al. . Conceptual complexity of gender and its relevance to pain. Pain 2018; 159(11):2137–2141. [DOI] [PubMed] [Google Scholar]
- 279. Mogil JS. Interactions between sex and genotype in the mediation and modulation of nociception in rodents. In Mogil JS ed. Sex, Gender, and Pain .Seattle, WA: IASP Press; 2000:25–40. [Google Scholar]
- 280. Rice AS, Cimino-Brown D, Eisenach JCet al. . Animal models and the prediction of efficacy in clinical trials of analgesic drugs: a critical appraisal and call for uniform reporting standards. Pain 2008; 139(2):243–247. [DOI] [PubMed] [Google Scholar]
- 281. Mogil JS, Bailey AL. Sex and gender differences in pain and analgesia. Prog Brain Res 2010; 186:141–157. [DOI] [PubMed] [Google Scholar]
- 282. Dutton JW 3rd, Artwohl JE, Huang Xet al. . Assessment of pain associated with the injection of sodium pentobarbital in laboratory mice (Mus musculus). JALAAS. 2019; 58(3):373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Hohlbaum K, Bert B, Dietze Set al. . Severity classification of repeated isoflurane anesthesia in C57BL/6JRj mice-assessing the degree of distress. PLoS One 2017; 12(6):e0179588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Loepke A, McCann J, Kurth Cet al. . The physiologic effects of isoflurane anesthesia in neonatal mice. Anesth Analg 2006; 102(1):75–80. [DOI] [PubMed] [Google Scholar]
- 285. Butterfield NN, Graf P, Ries CRet al. . The effect of repeated isoflurane anesthesia on spatial and psychomotor performance in young and aged mice. Anesth Analg 2004; 98(5):1305–1311table of contents. [DOI] [PubMed] [Google Scholar]
- 286. Yang S, Gu C, Mandeville ETet al. . Anesthesia and surgery impair blood-brain barrier and cognitive function in mice. Front Immunol 2017; 8:902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Corona R, Verguts J, Binda MMet al. . The impact of the learning curve on adhesion formation in a laparoscopic mouse model. Fertil Steril 2011; 96(1):193–197. [DOI] [PubMed] [Google Scholar]
- 288. Kelly RR, McCrackin MA, Russell DLet al. . Teaching surgical model development in research by using situated learning and instructional scaffolding. JALAAS. 2019; 58(3):321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Cheng H, Chen BP, Soleas IMet al. . Prolonged operative duration increases risk of surgical site infections: a systematic review. Surg Infect 2017; 18(6):722–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Klune CB, Robbins HN, Leung VSet al. . Hypothermia during general anesthesia interferes with pain assessment in laboratory rats (Rattus norvegicus). JALAAS. 2020; 59(6):719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Toyama K, Spin JM, Abe Yet al. . Controlled isoflurane anesthesia exposure is required for reliable behavioral testing in murine surgical models. J Pharmacol Sci 2019; 140(1):106–108. [DOI] [PubMed] [Google Scholar]
- 292. Gakuba C, Gaberel T, Goursaud Set al. . General anesthesia inhibits the activity of the "glymphatic system". Theranostics 2018; 8(3):710–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Terp R, Mieritz HB, Hansen PB. Long-term vascular effects of anaesthesia in mice - what does it mean for our data and our patients? Acta Physiol (Oxf) 2016; 218(4):231–233. [DOI] [PubMed] [Google Scholar]
- 294. Jensen J. Special techniques and species. In: Pacharinsak C, Smith JC, eds. Handbook of Laboratory Animal Anesthesia and Pain Managment Rodents .Boca Raton, FL: CRC Press; 2017:89–100. [Google Scholar]
- 295. Vesce GMF, Chiavaccini L. Preclinical imaging anesthesia in rodents. Q J Nucl Med Mol Imaging 2017; 61(1):1–18. [DOI] [PubMed] [Google Scholar]
- 296. Gargiulo S, Greco A, Gramanzini Met al. . Mice anesthesia, analgesia, and care, part II: anesthetic considerations in preclinical imaging studies. ILAR J 2012; 53(1):E70–E81. [DOI] [PubMed] [Google Scholar]
- 297. Constantinides C, Mean R, Janssen BJ. Effects of isoflurane anesthesia on the cardiovascular function of the C57BL/6 mouse. ILAR J 2011; 52(3):e21–e31. [PMC free article] [PubMed] [Google Scholar]
- 298. Greenwood HE, Nyitrai Z, Mocsai Get al. . High-throughput PET/CT imaging using a multiple-mouse imaging system. J Nucl Med 2020; 61(2):292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Suckow C, Kuntner C, Chow Pet al. . Multimodality rodent imaging chambers for use under barrier conditions with gas anesthesia. Mol Imaging Biol 2009; 11(2):100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Petrinovic MM, Hankov G, Schroeter Aet al. . A novel anesthesia regime enables neurofunctional studies and imaging genetics across mouse strains. Sci Rep 2016; 6:24523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Shim HJ, Jung WB, Schlegel Fet al. . Mouse fMRI under ketamine and xylazine anesthesia: robust contralateral somatosensory cortex activation in response to forepaw stimulation. NeuroImage 2018; 177:30–44. [DOI] [PubMed] [Google Scholar]
- 302. Nasrallah FA, Tay HC, Chuang KH. Detection of functional connectivity in the resting mouse brain. NeuroImage 2014; 86:417–424. [DOI] [PubMed] [Google Scholar]
- 303. Bascunana P, Thackeray JT, Bankstahl Met al. . Anesthesia and preconditioning induced changes in mouse brain [(18)F] FDG uptake and kinetics. Mol Imaging Biol 2019; 21(6):1089–1096. [DOI] [PubMed] [Google Scholar]
- 304. Cetin A, Komai S, Eliava Met al. . Stereotaxic gene delivery in the rodent brain. Nat Protoc 2006; 1(6):3166–3173. [DOI] [PubMed] [Google Scholar]
- 305. Fomari RV. Rodent stereotaxic surgery and animal welfare outcome improvements for behavioral neuroscience [video article]. JoVE 2012; 59:1–4. doi: 10.3791/3528. Published January 30, 2012 Accessed November 12, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Yoshida S, Morimoto Y, Tonooka Tet al. . An inhalation anesthetic device for stereotaxic operation on mouse pups. J Neurosci Methods 2015; 243:63–67. [DOI] [PubMed] [Google Scholar]
- 307. Clowry GJ, Flecknell PA. The successful use of fentanyl/fluanisone ('Hypnorm') as an anaesthetic for intracranial surgery in neonatal rats. Lab Anim 2000; 34(3):260–264. [DOI] [PubMed] [Google Scholar]
- 308. Danneman PJ, Mandrell TD. Evaluation of five agents/methods for anesthesia of neonatal rats. Lab Anim Sci 1997; 47(4):386–395. [PubMed] [Google Scholar]
- 309. Lagerspetz KYH. Postnatal development of thermoregulation in laboratory mice. Helgoländer Meeresun 1966; 14(1):559–571. [Google Scholar]
- 310. Phifer CB, Terry LM. Use of hypothermia for general anesthesia in preweanling rodents. Physiol Behav 1986; 38(6):887–890. [DOI] [PubMed] [Google Scholar]
- 311. Singer D. Neonatal tolerance to hypoxia: a comparative-physiological approach. Comp Biochem Physiol A Mol Integr Physiol 1999; 123(3):221–234. [DOI] [PubMed] [Google Scholar]
- 312. Huss MK, Chum HH, Chang AGet al. . The physiologic effects of isoflurane, sevoflurane, and hypothermia used for anesthesia in neonatal rats (Rattus norvegicus). JALAAS. 2016; 55(1):83–88. [PMC free article] [PubMed] [Google Scholar]
- 313. Tsukamoto A, Konishi Y, Kawakami Tet al. . Pharmacological properties of various anesthetic protocols in 10-day-old neonatal rats. Exp Anim 2017; 66(4):397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Amouzadeh HR, Qualls CW Jr, Wyckoff JH 3rdet al. . Biochemical and morphological alterations in xylazine-induced pulmonary edema. Toxicol Pathol 1993; 21(6):562–571. [DOI] [PubMed] [Google Scholar]
- 315. Amouzadeh HR, Sangiah S, Qualls CW Jret al. . Xylazine-induced pulmonary edema in rats. Toxicol Appl Pharmacol 1991; 108(3):417–427. [DOI] [PubMed] [Google Scholar]
- 316. Mechelinck M, Kupp C, Krüger JCet al. . Oxygen inhalation improves postoperative survival in ketamine-xylazine anaesthetised rats: an observational study. PLoS One 2019; 14(12):e0226430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Hankenson FC. Critical Care Management for Laboratory Mice and Rats. Boca Raton, FL: CRC Press; 2014. [Google Scholar]
- 318. McClure DE. Clinical pathology and sample collection in the laboratory rodent. Vet Clin North Am Exot Anim Pract 1999; 2(3):565–590vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Yagi K. Veterinary fluid therapy update: calculating the rate and choosing the correct solution. DVM360. https://www.dvm360.com/view/veterinary-fluid-therapy-update-calculating-rate-and-choosing-correct-solution. Published 2019. Accessed Nov 25, 2020.
- 320. Ullman-Culleré MH, Foltz CJ. Body condition scoring: a rapid and accurate method for assessing health status in mice. Lab Anim Sci 1999; 49(3):319–323. [PubMed] [Google Scholar]
- 321. Dwyer R, Bennett H, Eger IIEet al. . Effects of isoflurane and nitrous oxide in subanesthetic concentrations on memory and responsiveness in volunteers. Anesthesiology 1992; 77(5):888–892. [DOI] [PubMed] [Google Scholar]
- 322. Dwyer R, Bennett H, Eger IIEet al. . Isoflurane anesthesia prevents unconscious learning. Anesth Analg 1992; 75(1):107–112. [DOI] [PubMed] [Google Scholar]
- 323. Chaves AA, Dech SJ, Nakayama Tet al. . Age and anesthetic effects on murine electrocardiography. Life Sci 2003; 72(21):2401–2412. [DOI] [PubMed] [Google Scholar]
- 324. Pachon RE, Scharf BA, Vatner DEet al. . Best anesthetics for assessing left ventricular systolic function by echocardiography in mice. Am J Physiol Heart Circ Physiol 2015; 308(12):H1525–H1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Chu DK, Jordan MC, Kim JKet al. . Comparing isoflurane with tribromoethanol anesthesia for echocardiographic phenotyping of transgenic mice. J Am Assoc Lab Anim Sci 2006; 45(4):8–13. [PubMed] [Google Scholar]
- 326. Schaefer A, Meyer GP, Brand Bet al. . Effects of anesthesia on diastolic function in mice assessed by echocardiography. Echocardiography 2005; 22(8):665–670. [DOI] [PubMed] [Google Scholar]
- 327. Papaioannou VE, Fox JG. Efficacy of tribromoethanol anesthesia in mice. Lab Anim Sci 1993; 43(2):189–192. [PubMed] [Google Scholar]
- 328. Mayer J, Mans C. Rodents. In: Carpenter J, Marion C, eds. Exotic Animal Formulary .5th ed.St. Louis, MO: Elsevier; 2018; 459–493. [Google Scholar]
- 329. Kendall L, Hansen R, Dorsey Ket al. . Pharmacokinetics of sustained-release analgesics in mice. JALAAS. 2014; 53(5):478–484. [PMC free article] [PubMed] [Google Scholar]
- 330. Morrisey J, Carpenter J. Ferrets, Rabbits, and Rodents: Clinical Medicine and Surgery .3rd ed.St. Louis, MO: Saunders/Elservier; 2012. [Google Scholar]
- 331. Reddyjarugu B, Pavek T, Southard Tet al. . Analgesic efficacy of firocoxib, a selective inhibitor of cyclooxygenase 2, in a mouse model of incisional pain. JALAAS. 2015; 54(4):405–410. [PMC free article] [PubMed] [Google Scholar]


