Abstract
The complete nucleotide sequence of pRGO1, a cryptic plasmid from Propionibacterium acidipropionici E214, was determined. pRGO1 is 6,868 bp long, and its G+C content is 65.0%. Frame analysis of the sequence revealed six open reading frames, which were designated Orf1 to Orf6. The deduced amino acid sequences of Orf1 and Orf2 showed extensive similarities to an initiator of plasmid replication, the Rep protein, of various plasmids of gram-positive bacteria. The amino acid sequence of the putative translation product of orf3 exhibited a high degree of similarity to the amino acid sequences of DNA invertase in several bacteria. For the putative translation products of orf4, orf5, and orf6, on the other hand, no homologous sequences were found. The function of these open reading frames was studied by deletion analysis. A shuttle vector, pPK705, was constructed for shuttling between Escherichia coli and a Propionibacterium strain containing orf1 (repA), orf2 (repB), orf5, and orf6 from pRGO1, pUC18, and the hygromycin B-resistant gene as a drug marker. Shuttle vector pPK705 successfully transformed Propionibacterium freudenreichii subsp. shermanii IFO12426 by electroporation at an efficiency of 8 × 106 CFU/μg of DNA under optimized conditions. Transformation of various species of propionibacteria with pPK705 was also performed at efficiencies of about 104 to 107 CFU/μg of DNA. The vector was stably maintained in strains of P. freudenreichii subsp. shermanii, P. freudenreichii, P. pentosaceum, and P. freudenreichii subsp. freudenreichii grown under nonselective conditions. Successful manipulation of a host-vector system in propionibacteria should facilitate genetic studies and lead to creation of genes that are useful industrially.
Propionibacteria, which have a wide range of probiotic activity, are used in making dairy foods, such as cheese, for the production of vitamin B12, tetrapyrrole compounds, and propionic acid (8, 15, 21), in bread baking, as starters for ensilage, and in some pharmaceutical preparations (35). To elucidate the biosynthetic pathways of vitamin B12 and siroheme in Propionibacterium, we previously identified several genes coding for the enzymes involved in production of tetrapyrrole derivatives (hemYHBXRL) (11, 12) and vitamin B12 (cobA, cbiO) (30).
Development of genetic manipulation in propionibacteria has progressed slowly due to a lack of detailed information on the genetics of the bacteria and a lack of an appropriate plasmid that can serve as a possible transformation vector. A number of plasmids from Propionibacterium acidipropionici, P. freudenreichii, and P. jensenii, ranging in size from 4.4 to more than 119 MDa, have been described (19, 24). However, neither analysis of a plasmid DNA sequence nor construction of a vector for propionibacteria has been reported. To establish a versatile vector system to facilitate genetic analysis and to allow the transfer of a gene of interest, we investigated the development of a host-vector system in propionibacteria.
We succeeded in determining the complete nucleotide sequence of plasmid pRGO1 from P. acidipropionici E214 (24). This is the first report of the complete nucleotide sequence of an endogenous plasmid from propionibacteria. On the basis of the sequence information obtained, we were able to develop a host-vector system in propionibacteria.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The Propionibacterium spp. strains used in this study were obtained from the Hiroshima University Type Culture Collection (HUT), the Institute for Fermentation, Osaka (IFO), the American Type Culture Collection (ATCC), and the Iowa State University Culture Collection. Plasmid pRGO1, prepared from P. acidipropionici E214, was kindly provided by B. A. Glatz. Fragments of pRGO1 were cloned in pUC18/19 (37), pMW218 (3), or pBluescriptII KS+ (Stratagene, La Jolla, Calif.). Escherichia coli JM109 (37) or JM110 (37) was used as the host strain. PSMT3, a plasmid containing the hygromycin B gene (hygB), was provided by A. Jaworfsky.
Culture conditions, media, and reagents.
Propionibacteria were grown anaerobically at 32°C in sodium lactate broth (NLB) containing 1% sodium lactate, 1% yeast extract, and 1% Trypticase soy broth (13). E. coli was grown at 37°C in Luria broth (28). Ampicillin and kanamycin were added to appropriate media at concentrations of 100 and 30 μg/ml, respectively. When required, hygromycin B (Wako Pure Chemical Industries, Osaka, Japan) was added at 250 μg/ml for propionibacteria or at 100 μg/ml for E. coli. Restriction enzymes and T4 DNA ligase, obtained from either Takara Shuzo Co. (Kusatsu, Japan) or Toyobo Co. (Osaka, Japan), were used according to the manufacturer's instructions. Reagent grade chemicals were obtained from Nacalai Tesque, Inc. (Kyoto, Japan) or Sigma Chemical Co. (St. Louis, Mo.).
Manipulation of DNA.
Plasmid DNA from propionibacteria was prepared from an overnight culture by the alkali-sodium dodecyl sulfate procedure (5), with a slight modification. The propionibacterial cells were lysed with solution I containing lysozyme (20 mg/ml) or were disrupted with 0.5 g of glass beads (diameter, 0.1 mm) per 10 ml of culture by vortexing. After isopropanol precipitation, RNA was precipitated with LiCl2 (2.5 M), and then plasmid DNA from the soluble extract was precipitated with isopropanol. Samples were treated with DNase-free RNase A (1 mg/ml) at 37°C for 30 min. The plasmid DNA was then precipitated with polyethylene glycol (28). E. coli plasmid DNA was prepared by the alkaline lysis method (28).
Transformation of Propionibacterium species.
Transformation was performed on the bench top without any special anaerobic conditions. An overnight culture of propionibacteria was inoculated into fresh NLB to obtain an optical density at 600 nm of about 0.05. The cells were grown at 32°C and harvested when the optical density at 600 nm reached about 0.8. Cells were washed once with 0.5 volume of 1 mM 2-(4-[2-hydroxyethyl]-1 piperazinyl)ethylene sulfonic acid (HEPES) buffer (pH 7.0). The cell pellet was suspended in 0.1 volume of 10% glycerol and incubated on ice for 30 min. After centrifugation, the pellet was suspended in 0.02 volume of 10% glycerol to obtain a cell concentration of about 1.8 × 1010 cells/ml. This suspension could be frozen in 100-μl aliquots and stored at −80°C. For transformation by electroporation, 100 μl of the cell suspension was mixed with 1.0 μl (1 μg) of plasmid DNA in MilliQ water and transferred to a sterile cold 0.2-cm-gap cuvette. Transfer of plasmid DNA to propionibacteria was accomplished by electroporation by using an Electrocell Manipulator 600 (BTX Inc., San Diego, Calif.) and the following conditions: electric field strength, 6.0 kV/cm; capacitance, 25 μF; and resistance, 129 Ω. After the pulse, the cells were diluted with 800 μl of NLB and then incubated at 32°C for 8 h. An appropriate portion of the cell suspension was plated on NLB agar with a suitable antibiotic and incubated at 32°C for 4 days. E. coli cells were transformed by the method of Hanahan (10).
DNA sequencing.
To determine the complete nucleotide sequence of plasmid pRGO1, overlapping fragments were subcloned in pUC18/19. The nucleotide sequences of both strands were determined by the dideoxy chain termination method (29) with an ABI PRISM 310 genetic analyzer (Perkin-Elmer, Foster City, Calif.) or an ALF DNA sequencing system (Amersham Pharmacia Biotech, Uppsala, Sweden). Sequence data were assembled and analyzed by using the GENETYX MAC program, version 8 (Software Development Co., Tokyo, Japan). Homology searches were carried out by using the BLAST (Basic Local Alignment Search Tool) program (1) and the DNA Data Bank of Japan (DDBJ).
Stability of the plasmid in propionibacteria.
Propionibacteria carrying the plasmid were grown for 2 days in NLB without antibiotics (nonselective medium). The culture was diluted 1/50 with fresh NLB and propagated at 32°C for 2 days. After 10 serial transfers, cells were plated at an appropriate dilution on NLB solid medium and incubated for 4 days. Several hundred colonies were then picked randomly and replicated on NLB with and without hygromycin B. The plasmid DNAs in these colonies were analyzed by restriction enzyme digestion and gel electrophoresis. Percentages of stability were determined as follows: number of colonies grown on the selective medium/number of colonies grown on the nonselective medium × 100.
Nucleotide sequence accession number.
The nucleotide sequence data reported here have been deposited in the DDBJ database under accession no. AB007909.
RESULTS
Nucleotide sequence of pRGO1.
To develop a vector system for propionibacteria, we searched various strains of Propionibacterium species for appropriate small plasmids. From among 50 strains obtained from stock cultures, including cultures from HUT, IFO, and the Iowa State University Culture Collection, plasmid pRGO1 (24) was selected, and its complete nucleotide sequence was determined (Fig. 1). Plasmid pRGO1 is 6,868 bp long. Its G+C content is 65.0%, which is within the range of G+C contents previously reported for the genus Propionibacterium (65 to 68%) (32). Six open reading frames (ORFs), designated orf1 to orf6, were predicted by a computer frame analysis based on the G+C content of the three triplet positions of the genes of microorganisms with genomes rich in G+C (4) (data not shown). Two of the ORFs, orf4 and orf6, have opposite orientations. The six ORFs have G+C contents ranging from 61.5 to 73.7%. The G+C content in the third position of the codon is 90 to 93% in orf1, orf2, and orf6 and 81 to 83% in orf3, orf4, and orf5. These third-position G+C percentages are similar to those of several genes in P. freudenreichii (12). Predicted ribosome binding sites (Shine-Dalgarno [SD] sequences) (31) were found upstream from each initiation codon (ATG). Promoter consensus sequences (−35 and −10) similar to those of E. coli (26) were found upstream from each initiation codon except in the case of orf2.
FIG. 1.
Complete nucleotide sequence of pRGO1 and predicted amino acid sequences of orf1, orf2, orf3, orf4, orf5, and orf6. The potential promoter region (−10, −35) and SD sequences are underlined. The stop codons are indicated by asterisks.
orf1 (nucleotides 2696 to 3604) is 909 nucleotides long and encodes a putative 33.9-kDa polypeptide with a calculated isoelectric point of 7.05. orf2 (nucleotides 3604 to 3945) is 347 nucleotides long and encodes a putative 13.0-kDa polypeptide with a calculated isoelectric point of 10.26. The ATG initiation codon of orf2 overlaps the TGA termination codon of orf1. The initiation codon of orf2 is preceded by a predicted SD sequence, GGAGG, inside the orf1 coding sequence, but no promoterlike sequence was found. This arrangement suggests that orf1 and orf2 are transcriptionally coupled. Such overlapping of termination with initiation is observed in many bacteria (12, 18). The most likely initiation codon of orf3 (nucleotides 6132 to 6674) is preceded by a potential promoter and SD sequence. orf3 is 543 nucleotides long and encodes a putative 18.9-kDa polypeptide with a calculated isoelectric point of 11.34. orf4 (nucleotides 21 to 611) and orf5 (nucleotides 1075 to 1305) may code for putative 22.1- and 8.5-kDa polypeptides, respectively. A potential SD sequence and −35 and −10 promoter sequences are located upstream from the ATG initiation codon of these ORFs. orf6 (nucleotides 1623 to 2258) may encode a 22.1-kDa polypeptide. A putative SD sequence, GGAGG, is located at an appropriate distance upstream of the initiation codon.
Similarities of Orf1 and Orf2 to proteins encoded by other plasmids.
An analysis of amino acid sequence homology revealed high levels of similarity between Orf1 and the replication proteins (RepA) of several plasmids from gram-positive bacteria, including pRBL1 from Brevibacterium linens (level of similarity, 63%) (2) (accession no. U39878), pXZ10142 from Corynebacterium glutamicum (69%) (accession no. X72691), and pAL5000 from Mycobacterium fortuitum (56%) (17, 23) (Fig. 2A). Orf1 also exhibited significant homology with the Rep proteins of plasmid pMB1 from Bifidobacterium longum (48%) (27) (accession no. X84655) and pBL8 from B. linens (15a) (accession no. Y11902), as well as with Rep proteins from ColE type plasmids (24 to 29%) (ColE2imm-K317, ColE2-CA42, ColE3-CA38, ColE4-CT9, ColE5-099, ColE6-CT14, ColE7-K317, ColE8-J, and ColE9-J), which are trans-acting factors required for autonomous replication (38).
FIG. 2.
Comparison of the amino acid sequences of pRGO1 Orf1 (A) and Orf2 (B) with the corresponding amino acid sequences deduced from pRBL1 (accession no. U39878), pXZ10142 (accession no. X72691), pAL5000 (accession no. M23557), and pMB1 (accession no. X84655). Amino acids common to all replication proteins are indicated by asterisks. Gaps have been introduced to optimize the homology. The overline in panel B indicates the HTH motif.
The amino acid sequence deduced from orf2 (Fig. 2B) is similar to the amino acid sequences of RepB proteins from pAL5000 (level of similarity, 39%), pXZ10142 (38%), and pMB1 (27%). The deduced Orf2 amino acid sequence also shows the characteristic helix-turn-helix (HTH) motif typically found in DNA-binding proteins (6, 20). The predicted helix 1 is homologous to lambda cII, and helix 2 is homologous to Nul-lambda (Fig. 3). The value calculated by the Dodd-Egan weight matrix method (7) had a high standard deviation (4.4), indicating that the amino acid sequence probably forms an HTH motif (18).
FIG. 3.
HTH motif of Orf2. Asterisks indicate the conserved amino acids which are crucial in the structure. Numbers indicate amino acid positions in the protein sequence. Helix 1 and helix 2 are overlined. Amino acids found at the same position in the motif in Orf2 and cII or in Orf2 and Nul (6) are underlined.
Similarity of Orf3 to invertase/recombinase-like protein.
On the basis of the amino acid sequence alignment data, Orf3 most closely resembles DNA invertases involved in DNA rearrangement (9), such as Pin from E. coli (22) and plasmid pTF5 from Thiobacillus ferrooxidans (16) (accession no. U73041), PaeR7IN from Pseudomonas aeruginosa (34), and Cin from bacteriophage P7 (25). The levels of similarity of Orf3 to these DNA invertases range from 50 to 55%. The significant similarities include conserved regions of recombinases, such as the regions of recombinase 1 (YARVSTAEQ) and recombinase 2 (GDTLMVTRIDRLG) at amino acid positions 6 to 14 and 56 to 67, respectively (Fig. 4). As in many DNA-binding proteins (6), the amino acid sequence of the putative Orf3 DNA invertase forms an HTH motif (18) (Fig. 4), since the value calculated by the Dodd-Egan weight matrix method (7) had a high standard deviation, 4.48.
FIG. 4.
Comparison of the amino acid sequence of Orf3 of pRGO1 with the corresponding deduced amino acid sequences of PinTF from T. ferrooxidans (accession no. U73041), PaeR7IN from P. aeruginosa (accession no. S78872), PinEc from E. coli (accession no. K03521 and X01805), and CinP7 from bacteriophage P7 (accession no. X07224). The putative HTH motifs are enclosed in a box. Asterisks indicate the conserved amino acids.
Shuttle vector construction.
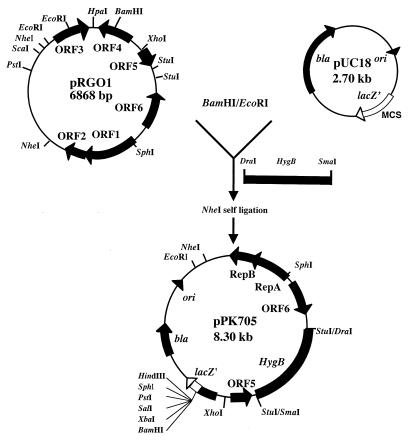
The high levels of homology between Orf1 and Orf2 of pRGO1 and the RepA and RepB proteins, which have been reported to play an important role in plasmid replication (2, 27, 33), led us to construct a shuttle vector for shuttling between Propionibacterium sp. and E. coli. A large fragment containing orf1, orf2, orf5, and orf6 was obtained from pRGO1 by digestion with EcoRI and BamHI, and the fragment was ligated to EcoRI-BamHI-digested pUC18. The resulting plasmid, digested with StuI, was ligated to the hygB fragment from plasmid pSMT3 digested with DraI-SmaI. The noncoding region downstream from orf2 of pRGO1 was deleted by digestion with NheI, and self-ligation created shuttle vector pPK705 (Fig. 5).
FIG. 5.
Scheme for vector pPK705 construction. Abbreviations: HygB, hygromycin B resistance gene; RepA, gene coding for RepA protein; RepB, gene coding for RepB protein. Unique cleavage sites of restriction enzymes are indicated. bla, β-lactamase; lacZ′, β-galactosidase; ori, colE1 replication origin; MCS, multicloning site.
Development of transformation system in propionibacteria.
When erythromycin- or chloramphenicol-resistant genes in a vector derived from pRGO1 were used, no transformant of propionibacteria was obtained. When the hygB gene was used as a marker, however, transformants were obtained. Since small colonies were sometimes observed in the background on plates containing 100 μg of HygB per ml, 250 μg of HygB per ml was used on the selective plate. Plasmid pPK705 prepared from a transformant of P. freudenreichii subsp. shermanii IFO12426 was used for optimization of transformation. Transformation was performed by electroporation under the following conditions: amount of plasmid DNA, 0.1 to 6 μg; electric field strength, 6 to 12 kV/cm; and resistance, 129, 246, 480, or 720 Ω (Table 1). From the growth rate of strain IFO12426, the postincubation time after pulse treatment of the cells was estimated to be 8 h at 32°C. The highest transformation efficiency (1.0 × 106 CFU/μg of DNA) was obtained when competent cells and 1.0 μg of plasmid DNA were exposed to an electric field strength of 10.0 kV/cm with a resistance of 129 or 246 Ω. The transformation efficiency decreased when the cells were exposed to a high resistance or high field strength or both due to an increase in the time constant leading to cell death (less than 30% survival) after the pulse treatment. Transformants were confirmed to carry plasmid pPK705 by restriction enzyme digestion and gel electrophoresis (data not shown).
TABLE 1.
Effects of resistance, field strength, and time constant on transformation efficiency of P. freudenreichii subsp. shermanii IFO12426a
| Electroporation conditions
|
Transformation efficiency (CFU/μg of DNA) | ||
|---|---|---|---|
| Resistance (Ω) | Field strength (kV/cm) | Time constant (ms) | |
| 129 | 10.0 | 5.1 | 1.0 × 106 |
| 246 | 10.0 | 5.0 | 1.0 × 106 |
| 480 | 6.0 | 8.8 | 1.1 × 106 |
| 480 | 8.0 | 8.9 | 1.0 × 106 |
| 480 | 10.0 | 8.9 | 5.0 × 105 |
| 480 | 12.0 | 8.8 | 4.0 × 105 |
| 720 | 10.0 | 22.5 | 5.0 × 105 |
As described in Materials and Methods, 100 μl of cells (about 1.8 × 1010 cells) in electroporation solution was mixed with 1 μg of pPK705 DNA prepared from P. freudenreichii subsp. shermanii IFO12426 and electroporated. After pulse treatment, addition of 800 μl of NLB, and incubation for 8 h at 32°C, transformants were selected on NLB agar with 250 μg of hygromycin B per ml.
Expansion of transformation host range.
The source of plasmid DNA usually influences the efficiency of transformation, presumably due to differences in restriction and modification of the DNA. This effect was investigated by employing shuttle vector pPK705 prepared from E. coli JM109 or P. freudenreichii subsp. shermanii IFO12426 (Table 2). Plasmid DNA prepared from each of these strains was used for transformation to strain IFO12426. We found 103-fold more transformants when plasmid DNA prepared from Propionibacterium cells was used than when plasmid DNA prepared from E. coli was used. When pPK705 prepared from E. coli JM110 (dam dcm) was used, no increase in the transformation efficiency was observed. Since both dam and dcm are associated with methylation of DNA, another methylation modification system may exist in Propionibacterium. In light of this result, plasmid pPK705 prepared from strain IFO12426 was used to transform other propionibacteria. High transformation efficiencies, ranging from about 106 to 107 CFU/μg of DNA, were obtained with P. pentosaceum HUT8606, P. freudenreichii subsp. shermanii IFO12426 and HUT8612, and P. freudenreichii ATCC 4915. P. freudenreichii subsp. freudenreichii IFO12424 and P. freudenreichii subsp. shermanii P93 were also transformed but showed 102- to 103-fold lower efficiency. These results suggest that the restriction-modification systems of strains HUT8606, HUT8612, and ATCC 4915 are more similar to that of strain IFO12426 than to those of strains IFO12424 and P93.
TABLE 2.
Efficiency of transformation and stability of pPK705 in Propionibacterium strainsa
| Strain | Transformants/ μg of DNA | Stability (%)e |
|---|---|---|
| P. freudenreichii subsp. shermanii IFO12426b | 8 × 103 | |
| P. freudenreichii subsp. shermanii IFO12426c | 6 × 103 | |
| P. freudenreichii subsp. shermanii IFO12426d | 8 × 106 | 83 |
| P. pentosaceum HUT8606d | 1.8 × 107 | 80 |
| P. freudenreichii subsp. shermanii HUT8612d | 1.4 × 106 | 85 |
| P. freudenreichii ATCC 4915d | 7 × 107 | 73 |
| P. freudenreichii subsp. freudenreichii IFO12424d | 5 × 105 | 83 |
| P. freudenreichii subsp. shermanii P93d | 4 × 104 | 48 |
Competent cells were prepared for transformation as described in Materials and Methods. Electroporation was carried out by using an electric field strength of 6.0 kV/cm, a capacitance of 25 μF, a resistance of 129 Ω, and 1.0 μg of plasmid DNA in 100 μl of competent cells.
Plasmid pPK705 prepared from E. coli JM109 (dam+ dcm+) was used for transformation.
Plasmid pPK705 prepared from E. coli JM110 (dam dcm) was used for transformation.
Plasmid pPK705 prepared from P. freudenreichii subsp. shermanii IFO12426 was used for transformation.
Stability was calculated as follows: number of colonies grown on NLB with hygromycin B/number of colonies grown on NLB without the antibiotic × 100.
Stability of pPK705 in propionibacteria.
To assess plasmid stability, Propionibacterium strains carrying plasmid pPK705 were grown in a nonselective medium. After 10 serial transfers and cultivation in the nonselective medium, 400 colonies were randomly picked and replicated on the selective and nonselective media, and percentages of stability were calculated. Twenty colonies grown on the selective medium were analyzed for plasmid DNA by gel electrophoresis. All of the hygromycin B-resistant colonies tested possessed plasmid pPK705. As shown in Table 2, plasmid pPK705, which has the pRGO1 replicon, was stably maintained in all of the Propionibacterium strains except strain P93 (percentage of stability, 48%). These results indicate that pPK705 can be replicated and maintained with good stability in propionibacteria.
DISCUSSION
To develop a host-vector system in propionibacteria, a preferred replication origin from a multicopy propionibacterial plasmid and an appropriate selective marker are required. In order to locate the region where foreign DNA can be inserted without impairing the replication properties, the nucleotide sequence of the plasmid whose replication origin is used also needs to be determined. pRGO1 is the first plasmid from propionibacteria whose DNA has been completely sequenced. Comparison of the nucleotide sequence with the restriction endonuclease cleavage map previously described (24) revealed two more unique restriction sites, ScaI and PstI, and one more cleavage site for SalI. Analysis of the DNA sequence of pRGO1 showed that the putative proteins Orf1 and Orf2 are very similar to replication proteins RepA and RepB, respectively, in a number of plasmids from B. linens, B. longum, C. glutamicum, and M. fortuitum. These bacteria are gram positive and belong to the subdivision whose members have G+C contents greater than 55% (36). The base composition corresponds to the range of G+C values determined for propionibacteria (65 to 68%) (14). The significant similarity of Orf1 of pRGO1 to the Rep proteins of ColE type plasmids strongly suggests that Orf1 is involved in plasmid replication via a theta type of replication. The existence in the plasmid from P. acidipropionici of tandem genes encoding Orf1 and Orf2 which are highly homologous to genes in plasmids from B. longum, C. glutamicum, and M. fortuitum suggests that these two genes are essential for plasmid replication. On the basis of this information concerning pRGO1, we succeeded in constructing a useful shuttle vector for shuttling between Propionibacterium cells and E. coli using regions including orf1, orf2, orf5, and orf6 of pRGO1 and pUC18. Although several attempts have been made to develop a transformation system in propionibacteria, a successful transformation system suitable for practical use has not been reported previously (35). This may be due to the selection marker and the existence of a strong restriction-modification system in propionibacteria. These problems were overcome by using the hygB gene as a selective marker and a plasmid prepared from a Propionibacterium strain. The result of transformation of Propionibacterium cells by the pPK705 vector suggests that the replicon of pRGO1, which consists of Orf1 (RepA), Orf2 (RepB), Orf5, and Orf6, is functional in propionibacteria, although the functions of Orf5 and Orf6 remain unknown. The characteristic HTH motif of DNA-binding regulatory proteins (20) suggests that Orf2 (RepB) may be involved in initiation of plasmid replication, since RepB in pAL5000 was shown to be involved in initiation of replication by binding to the ori region (33). Similarly, a shuttle vector constructed to shuttle between Bifidobacterium animalis and E. coli by using pMB1 from B. longum showed that both orf1 and orf2 are necessary for plasmid replication in Bifidobacterium cells (27). Usually, the plasmid ori region is located in the AT-rich region. In pRGO1, there are two AT-rich regions, which probably contain the ori sequence and are located upstream of orf1 and downstream of orf2. Deletion of the region downstream of orf2 had no effect on replication of pPK705 in propionibacteria (data not shown). This result suggests that the ori region is not located downstream of orf2. Since orf6, located upstream from orf1, contains AT-rich and repeated sequences, orf6 may play a role in plasmid replication, as reported in the case of a shuttle vector used for shuttling between Mycobacterium and E. coli cells (33).
orf4 and Orf4 showed no DNA or amino acid sequence homology to any of the database sequences. The amino acid sequence homology of the predicted protein product of orf3 to various DNA invertases suggests that orf3 of pRGO1 encodes a putative recombinase. Since pPK705 does not contain orf3 or orf4, these ORFs are not essential for plasmid replication, but they might improve the process.
The high transformation efficiency of pPK705 containing the pRGO1 replicon in various strains of Propionibacterium spp. suggests that shuttle vector pPK705 has a broad host range and is fairly stably maintained in Propionibacterium cells. Even in P. freudenreichii subsp. shermanii P93, which was reported to carry two endogenous plasmids, pRG07 and pRG03 (24), transformations were obtained; however, the stability of pPK705 was rather low. Successful manipulation of a host-vector system should facilitate genetic studies and lead to creation of some useful genes in propionibacteria that are likely to be industrially important.
ACKNOWLEDGMENTS
We thank B. A. Glatz and H. Y. Hsieh of Iowa State University for their kind gift of P. acidipropionici E214 and P. freudenreichii subsp. shermanii P93. We also thank A. Jaworfsky and J. Dziadek for their preliminary work with the hygB gene.
Part of this work was supported by grant-in-aid 10556019 from the Ministry of Education, Science, Sports and Culture of Japan.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Ankri S, Bouvier I, Reyes O, Predalu F, Leblon G. A Brevibacterium linens pRBL1 replicon functional in Corynebacterium glutamicum. Plasmid. 1996;36:36–41. doi: 10.1006/plas.1996.0029. [DOI] [PubMed] [Google Scholar]
- 3.Bernadi A, Bernadi F. Complete sequences of pSC101. Nucleic Acids Res. 1984;12:9415–9426. doi: 10.1093/nar/12.24.9415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bibb M J, Findlay P R, Johnson M W. The relationship between base composition and codon usage in bacterial genes and its use for simple and reliable identification of protein-coding sequences. Gene. 1984;30:157–166. doi: 10.1016/0378-1119(84)90116-1. [DOI] [PubMed] [Google Scholar]
- 5.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan R G, Matthews B W. The helix-turn-helix DNA binding motif. J Biol Chem. 1989;264:1903–1906. [PubMed] [Google Scholar]
- 7.Dodd I B, Egan J B. Improved detection of helix-turn-helix DNA binding motifs in protein sequences. Nucleic Acids Res. 1990;18:5019–5026. doi: 10.1093/nar/18.17.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Florent J, Ninet L. Vitamin B12. In: Peppler H J, Perlman D, editors. Microbial technology. 2nd ed. I. New York, N.Y: Academic Press; 1979. pp. 497–519. [Google Scholar]
- 9.Glasgow A C, Hughes K T, Simon M I. Bacterial DNA inversion systems. In: Berg D E, Howe M M, editors. Mobile DNA. Washington, D.C.: American Society for Microbiology; 1989. pp. 637–660. [Google Scholar]
- 10.Hanahan D. Studies on transformation of E. coli with plasmid. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto Y, Yamashita M, Ono H, Murooka Y. Characterization of the hem gene encoding δ-aminolevulinic acid dehydratase from Propionibacterium freudenreichii. J Ferment Bioeng. 1996;82:93–100. [Google Scholar]
- 12.Hashimoto Y, Yamashita M, Murooka Y. The Propionibacterium freudenreichii hem YHBXRL gene cluster, which encodes enzymes and a regulator involved in the biosynthetic pathway from glutamate to protoheme. Appl Microbiol Biotechnol. 1997;47:385–392. doi: 10.1007/s002530050945. [DOI] [PubMed] [Google Scholar]
- 13.Hofherr L A, Glatz B A. Mutagenesis of strains of Propionibacterium to produce cold sensitive mutants. J Dairy Sci. 1983;66:2482–2487. [Google Scholar]
- 14.Johnson J L, Cummins C S. Cell wall composition and deoxyribonucleic acid similarities among the anaerobic coryneforms, classical propionibacteria, and strains of Arachnia propionica. J Bacteriol. 1972;109:1047–1066. doi: 10.1128/jb.109.3.1047-1066.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langsrud T, Reinbold G W. Flavor development and microbiology of Swiss cheese—a review. II. Starters, manufacturing processes and procedures. J Milk Food Technol. 1973;36:531–542. [Google Scholar]
- 15a.Leret V. Ph.D. thesis. Rennes, France: Université de Rennes I; 1995. [Google Scholar]
- 16.Leschziner A E, Boocock M R, Grindley N D F. The tyrosin-6 hydroxyl of γδ resolvase is not required for the DNA cleavage and rejoining reactions. Mol Microbiol. 1995;15:865–870. doi: 10.1111/j.1365-2958.1995.tb02356.x. [DOI] [PubMed] [Google Scholar]
- 17.Mardis A E, Roe B A, Wallace R J., Jr Cloning and DNA sequencing of Mycobacterium fortuitum var. fortuitum plasmid pAL5000. Plasmid. 1992;27:130–140. doi: 10.1016/0147-619x(92)90013-z. [DOI] [PubMed] [Google Scholar]
- 18.Molnar I, Murooka Y. Helix-turn-helix DNA-binding protein motifs of Streptomyces, a cautionary note. Mol Microbiol. 1993;8:783–787. doi: 10.1111/j.1365-2958.1993.tb01621.x. [DOI] [PubMed] [Google Scholar]
- 19.Naud A I, Legault-Demare J, Ryter A. Induction of stable morphological change in Propionibacterium freudenreichii. J Gen Microbiol. 1988;134:283–293. doi: 10.1099/00221287-134-2-283. [DOI] [PubMed] [Google Scholar]
- 20.Pabo C O, Sauer R T. Protein-DNA recognition. Annu Rev Biochem. 1984;53:293–321. doi: 10.1146/annurev.bi.53.070184.001453. [DOI] [PubMed] [Google Scholar]
- 21.Playne M J. Propionic and butyric acids. In: Moo-Young M, editor. Comprehensive biotechnology. Vol. 3. Oxford, United Kingdom: Pergamon Press; 1985. pp. 731–759. [Google Scholar]
- 22.Plasterk R H A, Brinkman A, Van de Putte P. DNA inversions in the chromosome of Escherichia coli and in bacteriophage Mu: relationship to other site-specific recombination systems. Proc Natl Acad Sci USA. 1983;80:5355–5358. doi: 10.1073/pnas.80.17.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rauzier J, Moniz-Pereira J, Giquel-Sanzey B. Complete nucleotide sequence of pAL5000, a plasmid from Mycobacterium fortuitum. Gene. 1988;71:315–321. doi: 10.1016/0378-1119(88)90048-0. [DOI] [PubMed] [Google Scholar]
- 24.Rehberger T G, Glatz B A. Characterization of Propionibacterium plasmids. Appl Environ Microbiol. 1990;56:864–871. doi: 10.1128/aem.56.4.864-871.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ritthaler W, Kamp D. DNA sequence of the site-specific recombination function cin of phage P7. Nucleic Acids Res. 1988;16:6246–6246. doi: 10.1093/nar/16.13.6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenberg M, Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- 27.Rossi M, Brigidi P, Gonzalez Varay Rodriguez A, Matteuzzi D. Characterization of the plasmid pMB1 from Bifidobacterium longum and its use for shuttle vector construction. Res Microbiol. 1996;147:133–143. doi: 10.1016/0923-2508(96)80213-0. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 29.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sattler I, Roessuner C A, Stolowich N J, Hardin S H, Harris-Haller L W, Yokubaitis N T, Murooka Y, Hashimoto Y, Scott A I. Cloning, sequencing, and expression of the uroporphyrinogen III methyltransferase cobA gene of Propionibacterium freudenreichii (shermanii) J Bacteriol. 1995;177:1564–1569. doi: 10.1128/jb.177.6.1564-1569.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shine J, Dalgarno L. The 3-terminal sequence of Escherichia coli 16S ribosomal RNA: complementary to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci USA. 1974;76:1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergy's manual of systematic bacteriology. Vol. 2. Baltimore, Md: Williams & Wilkins; 1986. [Google Scholar]
- 33.Stolt P, Stoker N G. Protein-DNA interactions in the ori region of the Mycobacterium fortuitum plasmid pAL5000. J Bacteriol. 1996;178:6693–6700. doi: 10.1128/jb.178.23.6693-6700.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaisvila R, Vilkaitis G, Janulaitis A. Identification of a gene encoding a DNA invertase-like enzyme adjacent to the PaeR7I restriction-modification system. Gene. 1995;157:81–84. doi: 10.1016/0378-1119(94)00793-r. [DOI] [PubMed] [Google Scholar]
- 35.Vorobjeva L I. Propionibacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1999. [Google Scholar]
- 36.Woese C R. Bacterial evolution. Microbiol Rev. 1987;51:221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13m18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 38.Yasueda H, Horii T, Itoh T. Structural and functional organization of ColE2 and ColE3 replicons. Mol Gen Genet. 1989;244:41–48. doi: 10.1007/BF00339719. [DOI] [PubMed] [Google Scholar]